Abstract
Aim
The aim of this study was to explore the difference between haemorrhagic events among those patients on either aspirin or aspirin plus clopidogrel who were enrolled in the Clopidogrel in High-Risk Patients with Acute Non-disabling Ischemic Cerebrovascular Events (CHANCE) trial.
Methods
This was an ad hoc analysis of the CHANCE trial; data on all patients with any haemorrhagic event were reviewed and analysed. Cox proportional hazards regression was used to determine factors association with any bleeding.
Results
In the CHANCE trial, there were a total of 101 (2%) haemorrhagic events reported from 50 different hospitals. The clopidogrel–aspirin group had 60 (2.3%) cases and the aspirin group had 41 (1.6%, p=0.09). Moderate or severe haemorrhagic events occurred in 7 patients (0.3%) in the clopidogrel–aspirin group and in 8 (0.3%) in the aspirin group (p=0.73). Of 36 (0.7%) cases of intracranial haemorrhages, 20 (0.4%) were in the clopidogrel–aspirin group and 16 (0.3%) in the aspirin group. Each group had 8 (0.3%) cases of symptomatic haemorrhagic strokes. Other common haemorrhagic events included 24 (0.5%) cases of skin bruises, 13 (0.3%) gastrointestinal haemorrhages, 9 (0.2%) gum haemorrhages and 8 (0.2%) intraocular haemorrhages.
Conclusions
There was no overall significant difference in haemorrhagic events (p=0.29), especially in the rate of intracranial haemorrhages between the 2 treatment groups. However, patients enrolled with minor strokes had an increased risk of haemorrhagic events regardless of treatment group, not seen in patients with high-risk transient ischaemic attacks. Being elderly, of male gender and with a history of aspirin or proton pump inhibitor usage were associated with increased risk of haemorrhage. Patients with higher body mass index had lower risk of haemorrhagic events.
Trial registration number
Keywords: Hemorrhage, dual antiplatelet, minor stroke, TIA, stroke prevention
Introduction
Dual antiplatelet therapy with aspirin and clopidogrel is commonly used in patients with coronary artery disease or post intra-arterial stenting. Prior to the publication of the results from the Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial, routine use of dual antiplatelet therapy was not supported by any evidence and, furthermore, risk of haemorrhagic events outweighed the benefit.1 2 Since publication of results of the CHANCE trial, the finding of the benefit of dual antiplatelet therapy for stroke prevention in patients with minor stroke or high-risk transient ischaemic attack (TIA) has been incorporated into the 2014 American Heart Association (AHA)/American Stroke Association (ASA) stroke prevention guidelines.3 Of 5170 patients enrolled in the CHANCE trial, 101 patients had haemorrhagic events. The overall rate of haemorrhagic complication in CHANCE was 2%, which showed no statistical differences between the two treatment groups. The purpose of this manuscript is to provide a post hoc analysis of all haemorrhagic events in patients who participated in the CHANCE trial.
Methods
Details of the CHANCE trial have been published elsewhere. Briefly, CHANCE was a prospective, multicentre, double-blind, randomised, placebo-controlled trial conducted at 114 centres in China. The trial compared the combination therapy of clopidogrel and aspirin (clopidogrel at an initial dose of 300 mg, followed by 75 mg/day for 90 days, plus aspirin at a dose of 75 mg/day for the first 21 days) versus placebo plus aspirin (75 mg/day for 90 days). Of 5170 patients enrolled who were 40 years or older and able to start the study drug within 24 hours after the onset of a minor ischaemic stroke (defined by a score of 3 or less at the time of randomisation on the National Institutes of Health Stroke Scale), or high-risk TIA (defined as a score of ≥4 at the time of randomisation on the ABCD2), 2584 patients were randomised to the clopidogrel–aspirin group and 2586 to the aspirin group. All patients received open-label aspirin at a clinician-determined dose of 75–300 mg on the first day and had a 90-day follow-up visit in the clinic. The primary efficacy end point has been reported. The primary safety end point was any moderate-to-severe haemorrhagic event according to the definition used in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) trials.4 In GUSTO, severe haemorrhage was defined as fatal or intracranial haemorrhage (ICH) or other haemorrhage causing haemodynamic compromise that required blood or fluid replacement, inotropic support, or surgical intervention. Moderate haemorrhage was defined as bleeding that required transfusion of blood but did not lead to haemodynamic compromise requiring intervention. In this analysis, we used any bleeding as the primary outcome. Brain scans of all ICHs were reviewed and compared with the baseline scans. The size and location of haematoma were described. Haemorrhagic stroke was defined as a haemorrhage that took place in the area where the fresh ischaemic infarction was. All other haemorrhagic events were tabulated and described.
Statistical methods
We compared the baseline characteristics of all patients with and without haemorrhage, and those with minor stroke or TIA. Proportions were used for categorical variables and medians with IQRs were used for continuous variables. A non-parametric Kruskal-Wallis test was used to compare group differences for nominal variables and χ2 tests were used for dichotomous variables. Differences in the rate of any bleeding during the 90-day follow-up period were assessed using Cox proportional hazards regression. Backward selection was used to determine factors associated with any bleeding. Whether the treatment effect differed in stroke subtypes (TIA or minor stroke) was assessed by testing the treatment-by-stroke subtype interaction effect with the use of Cox models. Kaplan-Meier survival curves were also used to illustrate such differences. We used logistic regression to examine whether any bleeding was associated with worsening of patients’ functional outcome, measured by deterioration on the modified Rankin Score (mRS). To evaluate the impact of missing values of mRS, sensitivity analysis was also performed assuming all the missing values of mRS change (mRS at visit3–mRS at visit2) either as ≤0 or ≥1. All tests were two-sided, and a p value of 0.05 was defined as being statistically significant. All statistical analyses were performed using SAS software, V.9.4 (SAS Institute, Cary, North Carolina, USA).
Results
Among 5170 patients enrolled within 24 hours after onset of a minor ischaemic stroke or high-risk TIA, a total of 101 (2%) haemorrhagic events were reported, from 50 different hospitals. Table 1 shows baseline characteristics of all patients recruited to CHANCE, randomised to dual or mono antiplatelet groups—those with versus those without haemorrhages. With only a total of 101 haemorrhagic events in the CHANCE trial, there was no difference in the overall rate of ICHs between the two treatment groups. However, univariate analysis showed an increased risk of haemorrhagic events in patients with minor strokes but not in those with TIAs (p=0.03, for the interaction effect). The corresponding Kaplan-Meier survival risk curves demonstrated findings consistent with the analyses using Cox models and showed that most bleeding events occurred in the first 30 days (figure 1). In addition, older age, male gender, and history of aspirin and proton pump inhibitor (PPI) usage were associated with increased risk of haemorrhage regardless of treatment group (survival figure). On the contrary, patients with high body mass index (BMI) had lower risk of haemorrhagic events. Multivariable regression analysis showed that history of aspirin usage and concomitant usage of PPI could predict increased risk of haemorrhage independently. Furthermore, sensitivity test showed that, in patients who entered into CHANCE, diagnosis of minor stroke and experiencing a haemorrhagic event were likely associated with worsening of functional outcome (high mRS, table 2). This worsening of functional outcome was not observed in patients diagnosed with high-risk TIAs.
Table 1.
Baseline characteristics of patients on dual antiplatelet therapy—with versus without haemorrhages
| Summary | Minor stroke | TIA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No bleed (n=5069) | Any bleed (n=101) | p Value | No bleed | Any bleed | p Value | No bleed | Any bleed | p Value | |
| All | 2524 (49.8%) | 60 (59.4%) | 0.0557 | 1821 (49.8%) | 46 (66.7%) | 0.0055 | 703 (49.8%) | 14 (43.8%) | 0.5019 |
| Age | 62.3 (54.6–71.2) | 64.5 (57.5–74.0) | 0.0114 | 62.0 (54.7–71.2) | 65.8 (56.3–76.4) | 0.0212 | 62.5 (54.6–71.5) | 62.1 (59.9–71.5) | 0.3213 |
| Gender | 3364 (66.4%) | 56 (55.4%) | 0.0217 | 2455 (67.1%) | 41 (59.4%) | 0.1761 | 909 (64.3%) | 15 (46.9%) | 0.0420 |
| BMI | 24.5 (22.8–26.5) | 23.9 (22.0–25.4) | 0.0056 | 24.5 (22.7–26.4) | 23.7 (22.0–25.6) | 0.0162 | 24.6 (22.9–26.6) | 24.0 (22.1–25.1) | 0.1643 |
| SBP | 150 (136–161) | 150 (133–160) | 0.7271 | 150 (140–165) | 150 (133–163) | 0.5608 | 145 (130–j160) | 146 (135–160) | 0.6827 |
| DBP | 90 (80–100) | 87 (80–99) | 0.4787 | 90 (80–100) | 90 (80–100) | 0.4947 | 87 (80–95) | 82.5 (80–97.5) | 0.8785 |
| Medical history | |||||||||
| Stroke | 1019 (20.1%) | 14 (13.9%) | 0.1204 | 769 (21.0%) | 12 (17.4%) | 0.4615 | 250 (17.7%) | 2 (6.3%) | 0.0916 |
| TIA | 170 (3.4%) | 4 (4.0%) | 0.7378 | 70 (1.9%) | 2 (2.9%) | 0.5565 | 100 (7.1%) | 2 (6.3%) | 0.8566 |
| MI | 94 (1.9%) | 2 (2.0%) | 0.9261 | 72 (2.0%) | 2 (2.9%) | 0.5837 | 22 (1.6%) | 0 (0.0%) | 0.4769 |
| Hypertension | 3334 (65.8%) | 65 (64.4%) | 0.7665 | 2352 (64.3%) | 44 (63.8%) | 0.9227 | 982 (69.5%) | 21 (65.6%) | 0.6383 |
| Diabetes | 1069 (21.1%) | 24 (23.8%) | 0.5147 | 749 (20.5%) | 16 (23.2%) | 0.5821 | 320 (22.6%) | 8 (25.0%) | 0.7533 |
| Hyperlipidaemia | 564 (11.1%) | 9 (8.9%) | 0.4825 | 370 (10.1%) | 4 (5.8%) | 0.2365 | 194 (13.7%) | 5 (15.6%) | 0.7583 |
| Smoking | 2186 (43.1%) | 35 (34.7%) | 0.0886 | 1606 (43.9%) | 27 (39.1%) | 0.4262 | 580 (41.0%) | 8 (25.0%) | 0.0677 |
| ETOH use | 1565 (30.9%) | 35 (34.7%) | 0.4159 | 1140 (31.2%) | 27 (39.1%) | 0.1585 | 425 (30.1%) | 8 (25.0%) | 0.5352 |
| mRS at discharge | 4174 (82.3%) | 89 (88.1%) | 0.2710 | 2942 (80.5%) | 61 (88.4%) | 0.1587 | 1232 (87.2%) | 28 (87.5%) | 0.7475 |
| NIHSSS at discharge | 1485 (29.3%) | 35 (34.7%) | 0.6061 | 381 (10.4%) | 9 (13.0%) | 0.6131 | 1104 (78.1%) | 26 (81.3%) | 0.2900 |
| History of aspirin use | 565 (11.1%) | 19 (18.8%) | 0.0160 | 355 (9.7%) | 14 (20.3%) | 0.0036 | 210 (14.9%) | 5 (15.6%) | 0.9045 |
| Taking proton pump inhibitors | 39 (0.8%) | 7 (6.9%) | <0.0001 | 30 (0.8%) | 6 (8.7%) | <0.0001 | 9 (0.6%) | 1 (3.1%) | 0.0932 |
BMI, body mass index; DBP, diastolic blood pressure; ETOH, alcohol; MI, myocardial infarction; mRS, modified Rankin Score; NIHSSS, NIH Stroke Scale Score; SBP, systolic blood pressure; TIA, transient ischaemic attack.
Figure 1.
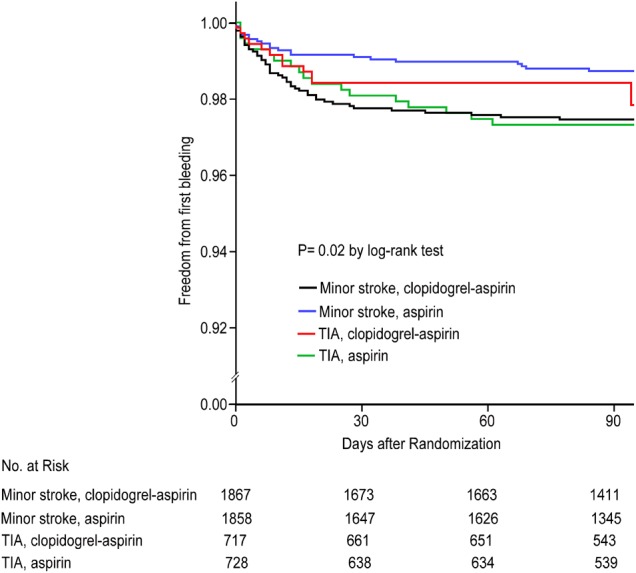
Kaplan-Meier survival curves demonstrate cumulative hemorrhagic events by treatment assignment for TIA and minor stroke. TIA, transient ischaemic attack.
Table 2.
Worsening of mRS once a haemorrhagic event has taken place
| Population | Covariate | No bleeding | Any bleeding | OR (95% CI) |
|---|---|---|---|---|
| Minor stroke | ||||
| Analysis 1 | 197/3280 (6.0%) | 10/43 (23.3%) | 4.29 (2.03 to 9.03) | |
| Analysis 2 | 197/3656 (5.4%) | 10/69 (14.5%) | 2.77 (1.38 to 5.55) | |
| Analysis 3 | 573/3656 (15.7%) | 36/69 (52.2%) | 5.30 (3.21 to 8.75) | |
| TIA | ||||
| Analysis 1 | 60/1278 (4.7%) | 2/28 (7.1%) | 1.76 (0.40 to 7.80) | |
| Analysis 2 | 60/1413 (4.2%) | 2/32 (6.3%) | 1.55 (0.35 to 6.80) | |
| Analysis 3 | 195/1413 (13.8%) | 6/32 (18.8%) | 1.49 (0.60 to 3.69) | |
| Overall | ||||
| Analysis 1 | 257/4558 (5.6%) | 12/71 (16.9%) | 3.19 (1.69 to 6.01) | |
| Analysis 2 | 257/5069 (5.1%) | 12/101 (11.9%) | 2.36 (1.28 to 4.37) | |
| Analysis 3 | 768/5069 (15.2%) | 42/101 (41.6%) | 3.70 (2.45 to 5.60) | |
Analysis 1: using data with mRS change information available.
Analysis 2: sensitivity analysis, assuming the missing values of mRS change as 0 (≤0).
Analysis 3: sensitivity analysis, assuming the missing values of mRS change as 1 (≥1).
mRS change: mRS at visit 3−mRS at visit 2.
All estimates were adjusted by age, gender, BMI, aspirin usage before randomisation, PPI usage.
BMI, body mass index; mRS, modified Rankin Score; PPI, proton pump inhibitor; TIA, transient ischaemic attack.
Detailed analysis showed that the clopidogrel–aspirin group had 60 (2.3%) cases and aspirin group had 41 (1.6%, p=0.09) cases of haemorrhagic events. Table 3 summarises all non-ICH events; the timeline to the first haemorrhagic event is shown in figure 1. The sum of haemorrhagic events from the groups in minor stroke or TIA showed that moderate or severe haemorrhage occurred in 7 patients (0.3%) in the clopidogrel–aspirin group and 8 (0.3%) in the aspirin group (p=0.73). There were totally 35 (0.7%) cases of ICHs, 20 (0.4%) in the clopidogrel–aspirin group and 16 (0.3%) in the aspirin group. These included 12 cases of microhaemorrhages (figure 1), 10 cases of intracerebral haematoma, 8 cases of haemorrhagic transformation and 5 cases of haemorrhagic infarctions. Among them, 8 (0.3%) cases in each group were symptomatic. Other common haemorrhagic events included 24 (0.5%) cases of skin bruises/petechia; 13 (0.3%) gastrointestinal (GI) haemorrhages; 9 (0.2%) gum haemorrhages; 8 (0.2%) intraocular haemorrhages; and 1 each of vaginal, oral, puncture site and upper respiratory bleeding. Details are listed in table 3. In addition, figure 1 shows the timing of the first haemorrhagic event in patients enrolled in each of the four treatment groups.
Table 3.
Analysis of non-intracranial haemorrhagic events
| Minor stroke | TIA | Overall | ||||
|---|---|---|---|---|---|---|
| Covariate | Aspirin (n=1858) | Clopidogrel–aspirin (n=1867) | Aspirin (n=728) | Clopidogrel–aspirin (n=717) | Aspirin (n=2586) | Clopidogrel–aspirin (n=2584) |
| Total | 23 (1.24%) | 46 (2.46%) | 18 (2.47%) | 14 (1.95%) | 41 (1.59%) | 60 (2.32%) |
| Epistaxis | 1 (0.05%) | 3 (0.16%) | 2 (0.27%) | 1 (0.14%) | 3 (0.12%) | 4 (0.15%) |
| Gastrointestinal bleeding | 4 (0.22%) | 6 (0.32%) | 1 (0.14%) | 2 (0.28%) | 5 (0.19%) | 8 (0.31%) |
| Gum bleeding | 1 (0.05%) | 4 (0.21%) | 4 (0.55%) | 0 (0.00%) | 5 (0.19%) | 4 (0.15%) |
| Haemoptysis | 0 (0.0%) | 0 (0.0%) | 0 (0.00%) | 1 (0.14%) | 0 (0.00%) | 1 (0.04%) |
| Intraocular haemorrhage | 1 (0.05%) | 4 (0.21%) | 2 (0.27%) | 1 (0.14%) | 3 (0.12%) | 5 (0.19%) |
| Intracranial haemorrhage | 12 (0.65%) | 17 (0.91%) | 4 (0.55%) | 3 (0.42%) | 16 (0.62%) | 20 (0.77%) |
| Oral haemorrhage | 1 (0.05%) | 0 (0.00%) | 0 (0.0%) | 0 (0.0%) | 1 (0.04%) | 0 (0.00%) |
| Puncture site bleeding | 0 (0.0%) | 0 (0.0%) | 1 (0.14%) | 0 (0.00%) | 1 (0.04%) | 0 (0.00%) |
| Skin bruises | 3 (0.16%) | 11 (0.59%) | 4 (0.55%) | 6 (0.84%) | 7 (0.27%) | 17 (0.66%) |
| Vaginal bleeding | 0 (0.00%) | 1 (0.05%) | 0 (0.0%) | 0 (0.0%) | 0 (0.00%) | 1 (0.04%) |
TIA, transient ischaemic attack.
Discussions
Based on the results of the CHANCE trial, the current AHA secondary stroke prevention guideline has recommended dual antiplatelet therapy for 21 days in patients with minor strokes or high-risk TIAs. While efficacy of dual antiplatelet therapy for secondary coronary event or stroke prevention has been proven, the issue of elevated haemorrhagic events has always been of concern. Through a literature search (PubMed, MEDLINE, Google Scholar, EMBASE), eight clinical trials were found that had, prior to the publication of the results of the CHANCE trial, tested either the combination of clopidogrel and aspirin, cilostazol and aspirin versus clopidogrel alone, or aspirin alone, for coronary artery disease or stroke/TIA prevention. The overall rate of haemorrhagic complications ranged from 1.8% to 8.1%. Please see table 4 for details.5–13
Table 4.
Summary of the haemorrhagic risk of eight trials that tested dual versus single antiplatelet agent
| Trials | Antiplatelet agent | Major or moderate haemorrhage | Haemorrhagic event | Haemorrhagic complication | Minor haemorrhage |
|---|---|---|---|---|---|
| CURE | Clopidogrel 300 mg load+ various doses of ASA (%) for 3–12 months |
3.7 | 2.1 | ||
| ASA 75–325 mg (%) for 3–12 months | 2.7 | 1.8 | |||
| SPS3 | Clopidogrel 75 mg+ ASA 325 mg for 8 years (%) |
2.1 | |||
| ASA 325 mg for 8 years (%) | 1.1 | ||||
| CHARISMA | Clopidogrel 75 mg+ASA 75–162 mg for 28 months | 2.1 | |||
| ASA 75–162 mg for 28 months | 1.3 | ||||
| MATCH | Clopidogrel 75 mg+ ASA75 mg (%) for 18 months |
8.1 | |||
| Clopidogrel 75 mg for 18 months | 3.5 | ||||
| CLAIR | Clopidogrel 300 loading, then 75 mg+ ASA 75–160 mg for 7 days |
2 cases | |||
| ASA 75–160 mg for 7 days | none | ||||
| Korean | Cilostazol 100 mg twice daily+ ASA 75–160 mg for 7 months (%) |
0.9 | |||
| Clopidogrel 75 mg+ ASA 75–160 mg for 7 months (%) |
2.6 | ||||
| SAMMPRIS | Recent stroke or TIA (within 30 days) attributable to severe stenosis (70–99%) of a major intracranial artery, clopidogrel 75 mg+ASA 325 mg for 90 days | 1.8 | |||
| CARESS | Clopidogrel 300 mg loading followed by 75 mg for 7 days | 2 of 52 cases | |||
| ASA 75 mg for 7 days | none | ||||
| CHANCE | Clopidogrel 300 mg load followed by 75 mg for 90 days +ASA 75 mg for the initial 21 days (%) |
2.3 | |||
| ASA 75 mg for the initial 21 days (%) | 1.6 | ||||
ASA, American Stroke Association; CARESS, Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis; CHANCE, Clopidogrel in High-Risk Patients with Acute Nondisabling Ischemic Cerebrovascular Events; CHARISMA, Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance; CURE, Clopidogrel in the Unstable Angina to Prevent Recurrent Events; SPS3, Secondary Prevention of Small Subcortical Strokes; TIA, transient ischaemic attack.
There are a few possible explanations for why patients of older age, males and those with a history of aspirin or PPI usage would have increased risk of haemorrhage regardless of treatment group. First, both aspirin and clopidogrel would increase the risk of haemorrhagic event used either alone or in combination. This increased risk of haemorrhage was mainly from GI bleeding (table 3). A slightly increased risk of haemorrhage was seen in the Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) trial. CAPRIE compared aspirin 325 mg to clopidogrel 75 mg, and the rate of haemorrhagic event was 2.69% in patients on aspirin 325 mg daily and 2.19% in patients on clopidogrel 75 mg daily. In the aspirin group, 1.55% had some bleeding disorder, 0.43% had ICHs and 0.71% had GI bleeding. In the clopidogrel group, 1.38% had some bleeding disorder, 0.32% had ICHs and 0.49% had GI bleeding.14 In the CHANCE trial, it is possible that these patients may already have had different degree of gastritis or peptic ulcer disease prior to enrolment and therefore were at risk after entering into the trial regardless of the treatment group. Those who had GI bleeding were perhaps not compliant with the PPI treatment and, therefore, the gastritis was not adequately treated.
Second, the interaction between aspirin and clopidogrel may play a role in potentiating haemorrhagic risk. Salicylic acid is extensively bound to plasma albumin, and causes displacement of other drugs from plasma protein.15 Clopidogrel is converted to a minor (10–15%) active thiol metabolite and major inactive carboxyl metabolite by hepatic cytochrome P450 enzymes. Both metabolites of clopidogrel are extensively protein bound.16 It is possible that salicylic acid may displace the active metabolite of clopidogrel from its protein binding site, which would increase its potency and lead to enhanced inhibition of platelet aggregation.17 Such aspirin interaction with clopidogrel might not be as prominent when aspirin is combined with cilostazol. As discussed above, cilostazol plus aspirin had less chance of haemorrhagic event (0.9%) versus clopidogrel plus aspirin (2.9%).14
Third, it has been reported that higher doses of aspirin correlate to higher chance of haemorrhagic event. With Clopidogrel in the Unstable Angina to Prevent Recurrent Events (CURE) trial, major haemorrhagic event rates in the dual therapy group were dose-dependent on the aspirin: <100 mg=2.6%; 100 to 200 mg=3.5%; >200 mg=4.9%. Major bleeding event rates for aspirin alone were dose dependent on aspirin too: <100 mg=2.0%; 100 to 200 mg=2.3%; >200 mg=4.0%. However, it was unclear if giving a loading dose of clopidogrel in addition to aspirin could cause more haemorrhagic events in dual therapy. Since neither the platelet function nor aggregation of these large antiplatelet trials was actually tested, it is possible that some patients or section of the population are very sensitive to antiplatelet agents and developing haemorrhagic events.
Last, advanced age alone has been related to an increased haemorrhagic event rate. Our analysis confirmed the findings from the CURE trial and another trial adding clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).18 In the CURE trial, major haemorrhage event rates for dual antiplatelet therapy by age were: <65 years=2.5%, ≥65 to <75 years=4.1%, ≥75 years=5.9%. Major bleeding event rates for aspirin alone by age were: <65 years=2.1%, ≥65 to <75 years=3.1%, ≥75 years=3.6%. A similar trend was seen in the COMMIT trial. Haemorrhagic rates for dual antiplatelet therapy by age were: <60 years=0.3%, ≥60 to <70 years=0.7%, ≥70 years=0.8%. Haemorrhagic rates for aspirin alone by age were: <60 years=0.4%, ≥60 to <70 years=0.6%, ≥70 years=0.7%. There was also likely an interaction between the rate of haemorrhagic events and severity of stroke plus duration of dual therapy as seen in the aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or TIA in high-risk patients (MATCH) trial, but not in the CHANCE trial. The CHANCE trial proved that those patients having TIAs and minor strokes would benefit from 21 days of dual therapy in secondary stroke prevention without increased risk of haemorrhage. In other trials, as summarised above, such benefit was offset by increased rate of haemorrhage if patients with all kinds of strokes were included and the duration of therapy was longer.
It is perceivable that patients with high BMI would have less drug–drug interaction or drug displacement. Therefore, it is likely that protein binding alterations in the active thiol metabolite could explain a significant amount of interindividual variability associated with clopidogrel.19 Higher BMI with higher protein binding of ASA and less displacement of thiol metabolite could possibly explain why higher BMI had less haemorrhagic events. That is also why taking aspirin or clopidogrel separately would have a lower rate of haemorrhagic events.14 Taking both together likely had an additive effect that potentiated the risk of developing haemorrhage. The mechanism accounting for the trend of more haemorrhagic events in males is unclear. It is contrary to the published results stating the female gender has increased haemorrhagic events in acute coronary syndrome. More research is needed to examine this phenomenon.20
It is difficult to explain why those patients who entered into the CHANCE trial—with a diagnosis of minor stroke but not high-risk TIA, and who experienced a haemorrhagic event—had worsening of functional outcome. It could be related to the use of mRS to assess functional outcome. Since patients with TIA had no neurological deficit at baseline, a non-ICH event would not cause any neurological deficit, while in patients with minor stroke, their baseline mRS was either at one or two. This worsening therefore was a reflection of the difference in the baseline mRS of patients with either TIA or minor stroke at enrolment.
The assessment of clinical significance of haemorrhagic events by GUSTO classification may have its limitations. The use of the GUSTO classification can be very subjective. The classification was not specific for type of haemorrhage but, rather, for severity. A more detailed description of the type of haemorrhage may be useful, as was provided in this analysis. A minor or moderate haemorrhagic event can evolve into a severe event if bleeding continues. For example, GI bleeding could be classified as a minor event but, if a large amount of blood loss continues, it could be classified as a major bleeding event. On the other hand, an intraocular haemorrhagic event may not be classified as a severe bleeding event but certainly could be very disabling. Our analysis of the 101 patients with haemorrhagic events in the CHANCE trial showed that even short-term dual therapy would increase the risk of future haemorrhagic events (not ICHs) in patients with a diagnosis of minor strokes but not TIAs. These events were likely minor, as classified by GUSTO classification, but could be clinically important, such as in the event of intraocular haemorrhage, GI bleeding or skin bruises.
It is unclear why in CHANCE patients with higher BMI had lower rate of haemorrhagic events.
Conclusion
Aspirin plus clopidogrel therapy in the CHANCE trial did not increase the risk of ICH. However, dual antiplatelet therapy demonstrated a trend of developing other types of haemorrhagic events. Such a trend could be potentiated if the patient is an older male with a minor stroke and has been on aspirin and/or PPI in the past. When considering dual antiplatelet therapy for secondary stroke prevention in patients with minor stroke or TIA, the lower the dose of aspirin, likely the less chance of having a haemorrhagic event. In future designing of antiplatelet clinical trials that test antiplatelet agents, a more detailed description of the types of haemorrhage and testing of platelet aggregation may provide us with better understanding of the pharmacological and biological impact of these agents, assisting clinicians in selecting antiplatelet drugs for their patients with stroke.
Acknowledgments
The authors would like to express appreciation to all participating institutes and clinicians. This study was sponsored by grants (2008ZX09312-008, 200902004, 2011BAI08B02, 2012ZX09303 and 2013BAI09B03) from the Ministry of Science and Technology of the People's Republic of China (PRC). In addition, the study was supported by the Excellence in Young Investigator Projects (81322019, 81301015 and 81371274) of the Ministry of Science and Technology of the PRC and the Beijing Institute for Brain Disorders (BIBD-PXM2013_014226_07_000084).
Footnotes
Twitter: Follow Yilong Wang at @yilong
Funding: Ministry of Science and Technology of China; Beijing Institute for Brain Disorders.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Tiantan Hospital Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Wang Y, Wang Y, Zhao X, et al. . Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. doi:10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Johnston SC, CHNANCE Investors. Rationale and design of a randomized, double-blind trial comparing the effects of a 3-month clopidogrel-aspirin regimen versus aspirin alone for the treatment of high-risk patients with acute nondisabling cerebrovascular event. Am Heart J 2010;160:380–6.e1. doi:10.1016/j.ahj.2010.05.017 [DOI] [PubMed] [Google Scholar]
- 3.Kernan WN, Ovbiagele B, Black HR, et al. , American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. [DOI] [PubMed] [Google Scholar]
- 4.An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med 1993;329:673–82. doi:10.1056/NEJM199309023291001 [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Zhao F, Mehta SR, et al. , Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Fox KA, Hacke W. et al. , CHARISMA Investigators. Clopidogrel and aspirin vs aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–17. doi:10.1056/NEJMoa060989 [DOI] [PubMed] [Google Scholar]
- 7.Benavente OR, Hart RG, McClure LA, et al. , SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012;367:817–25. doi:10.1056/NEJMoa1204133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy J, Hill MD, Ryckborst KJ, et al. . Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007;6:961–9. doi:10.1016/S1474-4422(07)70250-8 [DOI] [PubMed] [Google Scholar]
- 9.Diener HC, Bogousslavsky J, Brass LM, et al. . Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331–7. doi:10.1016/S0140-6736(04)16721-4 [DOI] [PubMed] [Google Scholar]
- 10.Wong KS, Chen C, Fu J, et al. . Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489–97. doi:10.1016/S1474-4422(10)70060-0 [DOI] [PubMed] [Google Scholar]
- 11.Kwon SU, Hong KS, Kang DW. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke 2011;42:2883–90. doi:10.1161/STROKEAHA.110.609370 [DOI] [PubMed] [Google Scholar]
- 12.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. , SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003. doi:10.1056/NEJMoa1105335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markus HS, Droste DW, Kaps M, et al. . Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 2005;111:2233–40. doi:10.1161/01.CIR.0000163561.90680.1C [DOI] [PubMed] [Google Scholar]
- 14.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996;16:1329–39. [DOI] [PubMed] [Google Scholar]
- 15.Miners JO. Drug interactions involving aspirin (acetylsalicylic acid) and salicylic acid. Clin Pharmacokinet 1989;17:327–44. doi:10.2165/00003088-198917050-00003 [DOI] [PubMed] [Google Scholar]
- 16.Bhindi R, Ormerod O, Newton J, et al. . Interaction between statins and clopidogrel: is there anything clinically relevant? QJM 2008;101:915–25. doi:10.1093/qjmed/hcn089 [DOI] [PubMed] [Google Scholar]
- 17.Ganesan S, Williams C, Maslen CL, et al. . Clopidogrel variability: role of plasma protein binding alterations. Br J Clin Pharmacol 2013;75:1468–77. doi:10.1111/bcp.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen ZM, Jiang LX, Chen YP, et al. , COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1607–21. doi:10.1016/S0140-6736(05)67660-X [DOI] [PubMed] [Google Scholar]
- 19.Vogel HG, Maas J, Gebauer A. Drug discovery and evaluation: methods in clinical pharmacology. New York, NY: Springer, 2010. [Google Scholar]
- 20.Cruden NL, Morch K, Wong DR, et al. . Clopidogrel loading dose and bleeding outcome in patient undergoing urgent coronary artery bypass grafting. Am Heart J 2011;161:404–10. doi:10.1016/j.ahj.2010.10.037 [DOI] [PubMed] [Google Scholar]


