Abstract
Objective
The aim of this systematic review and meta-analysis was to provide evidence that biomarkers of large artery atherosclerosis, including arterial stenosis and greater carotid intima-media thickness (cIMT), may serve as clinical markers of subclinical haemorrhage-prone cerebral small vessel disease, reflected by cerebral microbleeds (CMBs).
Methods
We searched PubMed, MEDLINE, Web of Science, EMBASE and the Cochrane Library to identify relevant studies published before 1 July 2016. The association between arterial stenosis and CMBs was estimated by the OR and 95% CI. The association of cIMT and CMBs was calculated using the standardised mean difference (SMD). Heterogeneity and publication bias were explored.
Results
8 studies including a total of 7160 participants were pooled in the meta-analysis. 6 of the included studies were cross-sectional, except that 2 were prospective. We found a significant association between arterial stenosis >50% and the presence of CMBs (OR 1.95, 95% CI 1.13 to 3.36, I2=56.1%). A fixed-effects model suggested that patients with CMBs were more likely to have a greater cIMT (SMD 0.20, 95% CI 0.11 to 0.28, I2=24.7%).
Conclusions
This systematic review and meta-analysis found that there is a relationship between large artery atherosclerosis and CMBs. Future studies are needed to confirm the impact of atherosclerosis on the CMBs, which may have potential therapeutic implications.
Keywords: cerebral microbleeds, intima-media thickness, Atherosclerosis, arterial stenosis
Introduction
Cerebral small vessel disease (CSVD) contributes to about a fifth of all strokes worldwide, and is considered a major cause of disability and vascular cognitive impairment.1 2 Prominent manifestations of CSVD on neuroimaging include white matter hyperintensity (WMH), lacunar infarction, enlarged perivascular spaces and cerebral microbleeds (CMBs).1 2 The most common causes of CSVD are diseases that affect the cerebral perforating arteries, arterioles, capillaries and venules. However, recent studies have shown a link between CSVD and the markers of large artery atherosclerosis, including arterial stenosis and greater carotid artery intima-media thickness (cIMT).3–7
Large artery atherosclerosis is considered a systemic and chronic inflammatory disease that may lead to both cardiovascular and cerebrovascular diseases.8 Recent studies found a positive association between large artery atherosclerosis and CSVD.9 To the best of our knowledge, treatments for large artery atherosclerosis such as antihypertensive therapy are also effective in patients with CSVD.10 Although the aetiology and pathogenesis of CSVD remain unclear, large artery atherosclerosis may become a potential therapeutic target for the prevention of CVSD. Therefore, it is meaningful to clarify the relationship between CSVD and large artery atherosclerosis.
CMBs are regarded as small areas of signal loss on T2*-weighted gradient echo (GRE) MRI sequences, pathologically representing bleeding-prone microangiopathies, including hypertensive arteriopathy and cerebral amyloid angiopathy.2 11–13 The presence of CMBs is considered a strong predictor of future stroke, particularly haemorrhagic stroke.14 15 There is evidence that large artery atherosclerosis may be associated with CMBs, but the results are controversial. Older age, hypertension and diabetes mellitus are common vascular risk factors that have an impact on both large arteries and small vessels.9 Chronic cerebral hypoperfusion could be a mechanism by which CMBs are associated with large artery atherosclerosis.16 17 To determine whether an association exists between large artery atherosclerosis and CMBs, we conducted a systematic review and meta-analysis of the literature, hoping that this would throw light on the pathogenesis and therapeutic strategy for CMBs.
Methods
Search strategy
We searched PubMed, MEDLINE, Web of Science, EMBASE and the Cochrane Library to identify relevant studies published before 1 July 2016 using the following search terms: ‘microbleeds', or ‘microhaemorrhage’, or ‘gradient-echo’, or ‘susceptibility weighted imaging’ in association with ‘artery atherosclerosis’, or ‘artery stenosis’, or ‘artery plaques’, or ‘artery calcification’, or ‘carotid intima-media thickness’. Related articles and reference lists from all included articles were also searched to identify additional studies.
Selection criteria
Two independent reviewers selected all studies. Inclusion criteria for the studies were: (1) cross-sectional or longitudinal in design; (2) investigated the association of large artery atherosclerosis and CMBs; (3) artery atherosclerosis including: cerebral artery stenosis >50% (internal carotid artery (ICA) or common carotid artery, intracranial or extracranial arteries) measured by digital subtraction angiography, CT angiography (CTA), MR angiography or carotid duplex ultrasound (CDUS); cIMT measured by CDUS; (4) studies published in English; (5) full paper could be obtained; (6) the participants were humans. Consensus was reached through discussion.
Quality assessment
The quality assessment standards for observational studies were recommended by the Agency for Healthcare Research and Quality (AHRQ). Quality assessments of cohort studies were performed using the Newcastle Ottawa Scale (NOS), and quality of cross-sectional studies was assessed using an 11-item instrument.18 19 Two independent researchers evaluated the included studies. Any disagreements were resolved after discussion with another author.
Data extraction
Two authors independently went through each eligible study and extracted information on the following items: first author's name, publication year, study design, sample size, sex and age distribution, prevalence of CMBs, MRI parameters, incidence rate of stenosis >50%, mean cIMT, detection methods, definition of large artery atherosclerosis and other principal findings associated with CMBs (p<0.05). The missing data and information of included studies were obtained by contacting the authors if possible.
Statistical analysis
The association of arterial stenosis and CMBs was estimated by the OR. The association of mean cIMT and microbleeds was calculated using the standardised mean difference (SMD). A random-effects model was employed when heterogeneity was found (p<0.10 or I2 >50%); otherwise, a fixed-effects model was used. The heterogeneity among studies was assessed by the Higgins I2 statistic and Cochran's Q test. An I2 > 50% and (or) a Q test p value <0.10 indicated statistical heterogeneity. Funnel plots and Egger's linear regression test were used to evaluate publication bias. A sensitivity analysis was investigated by omitting a single study in each turn. The meta-analysis was performed using Stata V.14.1 (Stata Corporation LP, College Station, Texas, USA). All p values were two-sided and p<0.05 was considered statically significant. We prepared this report according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) proposal.20
Results
Selection process and study characteristics
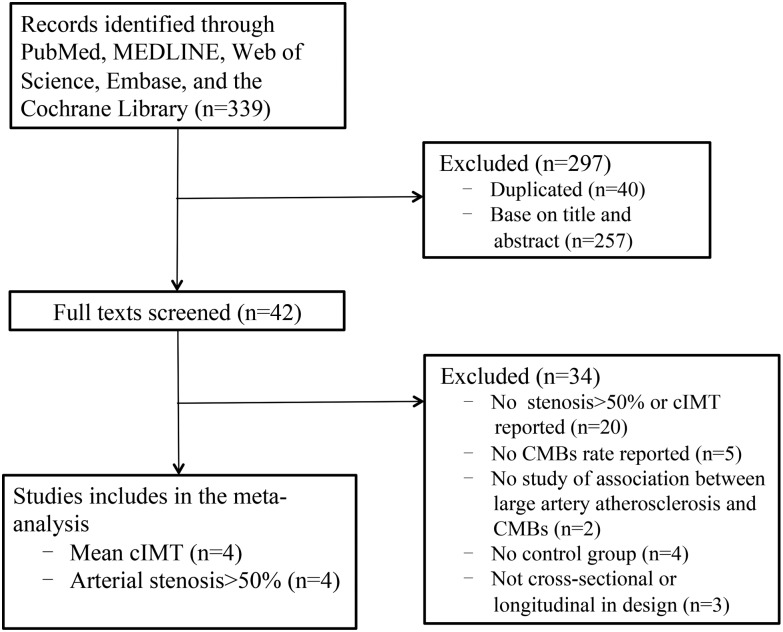
A total of 339 studies were identified. Case reports, letters and reviews were excluded. Only 42 studies were included for review of the full text based on the titles and abstracts. Of these, 34 were subsequently excluded: no stenosis>50% or mean cIMT reported (n=20), no CMBs rate reported (n=5), no study of association between large artery atherosclerosis and CMBs (n=2), no control group (n=4), not cross-sectional or longitudinal in design (n=3). Finally, eight studies including a total of 7160 participants were pooled in this meta-analysis (figure 1).21–28 Four of the eight studies reported an incidence rate of stenosis>50%,23–25 28 and four studies reported mean cIMT.21 22 26 27 Six of the included studies were cross-sectional, except that two were prospective.27 28 The main characteristics of included studies are shown in table 1.
Figure 1.
Flow chart of the study selection process. cIMT, carotid intima-media thickness; CMBs, cerebral microbleeds.
Table 1.
Characteristics of included Studies
| Author | Year | Study design | Population | Country | Patient number | Sex Men (%) |
Age, Mean, y | CMBs | Large artery atherosclerosis | Quality Score |
Other principal findings associated with CMBs | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRI | Prevalence n (%) |
Stenosis>50% n (%) | Mean cIMT mm (mean±SD) |
Method | Definition | ||||||||||
| Ochi | 2009 | Cross-sectional | Outpatients | Japan | 443 | 36 | 67.1 | 3 T, GRE | 22 (5.0%) | – | CMB(+):0.85±0.14 CMB(−):0.81±0.15 |
B-mode ultrasound | Far wall of the CCA | 11 items (8) | Age, hypertension, baPWV |
| Miwa | 2011 | Cross-sectional | Outpatients, age ≥45 years | Japan | 431 | 52 | 69.3 | 1.5 T, GRE | 65 (15.0%) | – | CMB(+):1.14±0.5 CMB(−):0.99±0.5 |
Ultrasound | Near and far walls, bilateral distal CCA, carotid bifurcation, and ICA | 11 items (8) | Age, fasting glucose, hsCRP, IL-6, IL-18 |
| Chung | 2014 | Cross-sectional | Acute ischaemic stroke or TIA | South Korea | 834 | 58 | 66.8 | 3 T, SWI | 335 (40.2%) | 468 (28.1%) | – | CTA | ICA Thick or contiguous calcification ≥50% of vessel diameter and/or ≥1 cm |
11 items (8) | Age, hypertension, hs-CRP |
| Peng | 2014 | Cross-sectional | Acute ischaemic stroke | China | 90 | 61 | 66.4 | 1.5 T, GRE | 30 (33.3%) | 46 (51.1%) | – | TCD, CDUS | ICA,intracranial and extracranial arteries; A visible narrowing (>50%) or significant haemodynamic changes |
11 items (8) | SBP, DBP |
| Song | 2015 | Cross-sectional | Ischaemic stroke | Korea | 220 | 60 | 64.0 | 1.5 T, GRE | 46 (20.9%) | 93 (42.3%) | – | MRA, CTA, DSA | Intracranial and extracranial arteries; Stenosis≥50% | 11 items (8) | Age |
| Tabara | 2015 | Cross-sectional | Healthy middle-aged to elderly individuals | Japan | 1387 | 39 | 67.0 | 3 T, GRE | 92 (6.6%) | – | CMB(+):0.84±0.14 CMB(−):0.79±0.14 |
B-mode ultrasound | Far wall, bilateral carotid arteries | 11 items (8) | Age, hypertension, T2DM |
| Ding | 2015 | Prospective | Population-based, age >65 | Iceland | 2512 | 42 | 74.6 | 1.5 T, GRE | 463 (18.4%) | – | CMB(+):0.98±0.14 CMB(−):0.96±0.14 |
B-mode ultrasound | Near and far walls, bilateral distal CCA | NOS scale (9) | Age, DBP, MAP, carotid arterial strain, DC, YEM |
| Romero | 2016 | Cohort | Framingham Offspring Study | USA | 1243 | 47 | 56.9 | 1.5 T, GRE | 101 (8.2%) | 20 (1.6%) | – | CDUS | Distal CCA, carotid artery bulb and ICA; Stenosis≥50% | NOS scale (9) | – |
baPWV, brachial-to-ankle pulse wave velocity; CCA, common carotid artery; CDUS, carotid duplex ultrasound; cIMT, carotid intima-media thickness; CTA, CT angiography; DBP, diastolic blood pressure; DC, distensibility coefficient; DSA, digital subtraction angiography; GRE, gradient-recalled echo sequences; hsCRP, high-sensitivity C reactive protein; ICA, internal carotid artery; IL, interleukin; MAP, mean arterial pressure; MRA, MR angiography; NOS scale, Newcastle–Ottawa scale; SBP, systolic blood pressure; SWI, susceptibility-weighted imaging; T2DM, type 2 diabetes mellitus; TCD, transcranial Doppler; TIA, transient ischaemic attack; YEM, Young elastic modulus.
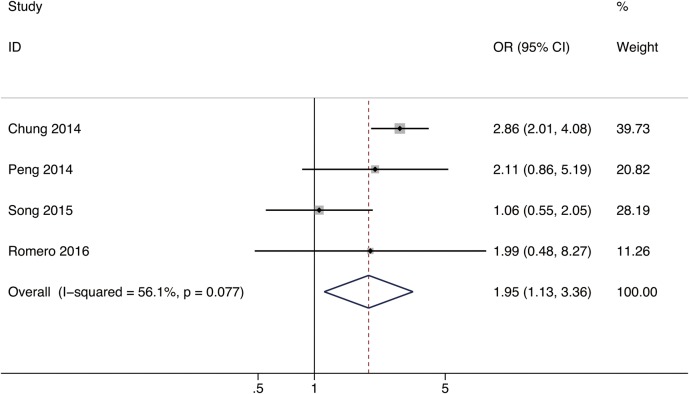
Four studies including a total of 2387 patients were pooled in the meta-analysis of the association between large artery stenosis and CMBs.23–25 28 The mean age of the study participants was 61.4 years (range, 56.9 to 66.8 years). The overall prevalence of CMBs on MRIs was 21.4% (range, 8.2–40.2%). ORs (95% CI) and adjustments are reported in table 2. Only one study demonstrated a significant relationship between cerebral large artery stenosis and CMBs presence.23 We calculated the pooled OR by most adjusted OR using a random-effects model. The combined OR was 1.95 (95% CI 1.13 to 3.36) for the association between large artery stenosis >50% and CMBs (figure 2).
Table 2.
ORs of studies included in the meta-analysis
| Author | Year | OR | 95% CI | Adjustment |
|---|---|---|---|---|
| Chung | 2014 | 4.22 | 3.19 to 5.59 | Unadjusted |
| 2.86 | 2.01 to 4.08 | Age, hypertension, previous stroke history, hs-CRP, total cholesterol, homocysteine and ICA calcification | ||
| Peng | 2014 | 2.11 | 0.86 to 5.19 | Unadjusted |
| Song | 2015 | 1.06 | 0.55 to 2.05 | Unadjusted |
| Romero | 2016 | 1.38 | 0.38 to 5.06 | Age, sex, time to MRI |
| 1.16 | 0.30 to 4.49 | Age, sex, time to MRI, diabetes, smoking, hypertension, systolic blood pressure, prevalent cardiovascular disease and statin use | ||
| 1.99 | 0.48 to 8.27 | Age, sex, time to MRI, diabetes, smoking, hypertension, systolic blood pressure, prevalent cardiovascular disease and statin use, baseline carotid IMT |
hsCRP, high-sensitivity C reactive protein; ICA, internal carotid artery; IMT, intima-media thickness.
Figure 2.
Forest plot for the association between large artery stenosis and CMBs. CMBs, cerebral microbleeds.
Risk of bias and heterogeneity between studies
The I2 statistics and Cochran's Q test indicate evidence of substantial heterogeneity among studies (p=0.077, I2=56.1%). Furthermore, visual inspection of the funnel plot and Egger's test (p=0.48) indicated no evidence of publication bias.
We conducted a sensitivity analysis by excluding a single study each time to explore the robustness of the combined results. The range of the combined ORs was from 1.41 (95% CI 0.86 to 2.32) to 2.70 (95% CI 1.96 to 3.72). The result showed no significant relationship between large artery stenosis >50% and CMBs presence when excluding Chung's study,23 with an OR 1.41 (95% CI 0.86 to 2.32). The I2 statistics and Cochran's Q test indicated the reduction of heterogeneity (p=0.423; I2=0.0%). This absolute difference may be due to different detection methods. First, Chung et al23 used CTA to detect the degree of intracranial ICA stenosis; however, three other studies mainly defined arterial stenosis by ultrasound scan, which might lead to heterogeneity. Second, the incidence rate of CMBs in Chung's study was much higher than in other studies. Chung et al assessed CMBs on susceptibility-weighted imaging (SWI), which is more sensitive. This may explain part of the heterogeneity.
Association between cIMT and CMBs
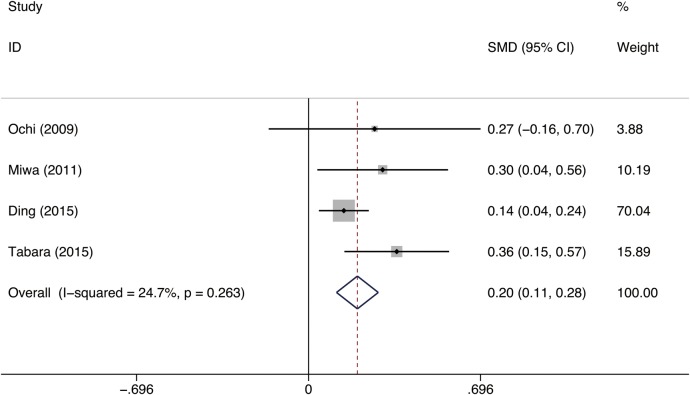
Four studies including 4773 patients provided data on the association between mean cIMT and CMBs were pooled in the meta-analysis.21 22 26 27 The mean age of the study participants was 71.2 years (range, 67 to 74.6 years), with fewer participants being men (41.3%; range, 39.4–52%). The overall prevalence of CMBs on MRI was 13.5% (range, 5% to 18.4%). A fixed-effects model was applied to estimate a pooled SMD of 0.20 (95% CI 0.11 to 0.28), which demonstrated that patients with CMBs were more likely to have a higher cIMT (p<0.001; figure 3). Three of the four studies demonstrated a statistically significant association between cIMT and cerebral microbleeds. Only one study found no association between cIMT and CMBs in healthy individuals free from a history of stroke.21
Figure 3.
Forest plot for the association between cIMT and CMBs. cIMT, carotid intima-media thickness; CMBs, cerebral microbleeds; SMD, standardised mean difference.
Risk of bias and heterogeneity between studies
The I2 statistics and Cochran's Q test indicate low heterogeneity (p=0.263; I2=24.7%). A funnel plot and Egger's test were used to assess the publication bias. The funnel plot seemed asymmetric, and the asymmetry was detected by Egger's test (coefficient=1.59, p=0.23). This demonstrated that asymmetry of the funnel was unlikely due to publication bias.
A sensitivity analysis was performed through excluding a single study each time. The result by excluding one study showed an increased SMD borderline (SMD 0.326, 95% CI 0.17 to 0.48), with no heterogeneity (p=0.909; I2=0.0%), because of the greater weight in this study.27 However, this did not change the outcome statistically. The analysis was consistent when using a random-effects model.
Discussion
CSVD is an important public health problem that has attracted increasing attention, and may coexist with large artery atherosclerosis.9 The aim of this systematic review and meta-analysis was to provide evidence that biomarkers of large artery atherosclerosis, such as greater cIMT and arterial stenosis, may serve as clinical markers of subclinical haemorrhage-prone CSVD, reflected by CMBs. Previous studies have suggested that the incidence rate of CMBs in patients with large artery atherosclerosis was 9–41.3%.24 28 29 Different populations and detection methods may lead to different incidence of CMBs. Kwon et al reported 14 of 313 patients (9.0%) with intracranial arterial stenosis (ICAS) presented with CMBs on baseline MRI in the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. However, they found no evidence linking CSVD with an increased risk of stroke in patients with ICAS.29
Large artery atherosclerosis and CSVD may share disease mechanisms, which are likely to be mediated via common vascular risk factors, such as older age, hypertension and diabetes mellitus.29 The prevalence of CMBs gradually increased with age.30 Advanced age and long-term hypertension may cause structural changes in microvessels, represented by fibrinoid necrosis and lipohyalinosis, thus increasing the risk of rupture and bleeding.31 Histopathological analyses have found hypertensive vasculopathy was specially associated with CMBs in the thalamus, basal ganglia, brainstem and cerebellum.32 Otherwise, Qiu et al33 indicated that diabetes was associated with markers of both cerebral macrovascular and microvascular diseases. Older age, hypertension and diabetes mellitus are risk factors that may lead to large artery atherosclerosis, thus giving rise to CMBs.
Previous studies have found that carotid stenosis, carotid plaque and cIMT were likely to be associated with WMH and cognitive decline in elderly people, even after accounting for vascular risk factors.7 34 In addition to the common risk factors, other mechanisms may explain the correlation. Factors that may influence cerebral blood flow especially at the origin of perforator arteries may also be involved. Gregg et al16 reported that the presence of incidental cortical CMBs is associated with significant and widespread reduction in resting-state cerebral blood flow (CBF), especially in the frontal, parietal and precuneus cortices. Moreover, Hashimoto et al17 found reduced CBF in the centrum semiovale in patients with five or more CMBs compared with those with <5 CMBs in patients with CSVD. Individuals with cortical or deep or infratentorial CMBs may be exposed to chronic cerebral hypoperfusion.16 17 35 Long-term hypoperfusion could accelerate the development of age-related ultrastructural aberrations of capillaries and cause blood-brain barrier (BBB) damage.36 37 Disruption of BBB has been suggested as a main initial pathogenic mechanism in CSVD.22 24 However, another possibility is that both CMBs and hypoperfusion are markers of small vessel diseases, and that no causal relationship between them should be considered. Further studies are needed before a conclusion can be drawn.
In this meta-analysis, we found a significant association between large artery stenosis and microbleeds in the included studies, with an OR 1.95 (95% CI 1.13 to 3.36). The results from the Framingham Heart Study demonstrated that carotid stenosis ≥25% was associated with presence of CMBs overall (OR 2.20, 95% CI 1.10 to 4.40), especially at deep and mixed locations (OR 3.60, 95% CI 1.23 to 10.5). Paradoxically, the study found that carotid stenosis ≥50% was not associated with CMBs. The study observed carotid stenosis ≥50% in only 1.5% of 1243 participants and the incidence rate of CMBs was 8.3%, which might be an underestimate.28 The evidence relating arterial stenosis to CMBs was inconsistent. This may be attributed to different inclusion criteria, different sample size, differences in demographic characteristics and different methodology for cerebral artery stenosis estimation. Some of the included studies defined arterial stenosis by ultrasonography, which may limit accuracy. In our study, we found evidence that arterial stenosis >50% was related to the incidence of CMBs. Large artery stenosis may lead to hypoperfusion, thus resulting in CMBs. Further research is needed for confirmation.
There may be other potential mechanisms that cause CMBs in patients with large artery atherosclerosis. CIMT is a non-invasive ultrasound marker of early atherosclerosis, and is increasingly used as a predictor of future clinical cardiovascular events including myocardial infarction and stroke.38–40 Previous studies considered cIMT as a marker of large-artery damage rather than CSVD. The Second Manifestations of Arterial disease study showed that mean cIMT was greater in patients with large vessel disease (1.08 mm) than in those with small vessel disease (0.92 mm) (SMD 0.11 mm, 95% CI 0.05 to 0.18).41 However, new research showed that patients with CSVD had greater cIMT compared with normal participants.42 The present systematic review and meta-analysis identified four studies focusing on the relationship between cIMT and CMBs. The result demonstrated that patients with CMBs were more likely to have a greater cIMT (SMD 0.20, 95% CI 0.11 to 0.28). Ding et al27 conducted a prospective population-based cohort study that included individuals aged >65 years without dementia and found that an increase in mean cIMT as a marker of arterial atherosclerosis was associated with an increased risk of CMBs, especially in the deep and infratentorial brain regions. All these findings suggest that there is a significant relationship between higher cIMT and CMBs risk.
One of the included studies in this review also explored the relationship between inflammation and CMBs in addition to increased cIMT. The results indicated that higher levels of circulating inflammatory markers, such as high-sensitivity C reactive protein (hsCRP), interleukin-6 (IL-6) and IL-18 were associated with CMBs, suggesting the involvement of inflammation.22 Inflammation is also implicated in the pathogenesis and development of atherosclerosis. Several studies have demonstrated that high levels of some inflammatory cytokines are important determinants in the pathogenesis of increased cIMT. Patients with higher cIMT have increased circulating levels of fibrinogen, tumour necrosis factor α, white cell count, hsCRP and IL-6.43 44 Moreover, Chung et al45 detected underlying intracranial atheroma in 60% of patients with lacunar infarction by high-resolution MRI and found potential intraplaque inflammation, suggesting the involvement of inflammation in both cerebral artery atherosclerosis and CSVD. We speculated that the link between CMBs and large artery atherosclerosis might be inflammation. Further studies are needed to confirm these findings.
There were some limitations in our study. First, only studies published in English were included, which may introduce publication bias. Second, the participants in the cross-sectional studies were consecutive patients, which may have introduced selection bias. Third, some of the studies were subject to bias because they did not involve blinded assessment of large artery atherosclerosis or blinded identification of CMBs. Fourth, the included studies varied in many aspects, such as study population, which resulted in a wide range of CMBs incidence rate (5% to 40.2%). Fifth, only one study demonstrated a significant relationship between cerebral large artery stenosis >50% and CMBs,23 using CTA to define arterial stenosis. The included studies used CDUS or transcranial Doppler to detect arterial stenosis may introduce bias due to limited accuracy. Moreover, most of the included studies assessed CMBs using T2*GRE or 1.5 T MRI machines. The use of higher-field MRI and SWI are known to increase CMBs detection. The variety of different techniques used in the assessment of artery atherosclerosis or CMBs in the included studies should be considered a source of heterogeneity. The accuracy of the methodology needs to be established in future studies.
Conclusions
In conclusion, the results of this systematic review and meta-analysis suggest that there is a relationship between large artery atherosclerosis and CMBs. Whether the occurrence of CMBs in patients with large artery atherosclerosis can predict the future risk of haemorrhagic cerebral vascular events is unclear. Longitudinal studies in larger populations are needed to confirm the impact of atherosclerosis on the bleeding-prone CMBs, which may have potential therapeutic implications.
Footnotes
Contributors: BP designed the study, and revised the manuscript. LD collected and extracted data, and drafted the manuscript. YH collected and extracted data.
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81471206) and the Beijing Natural Science Foundation (grant number 7152121).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. doi:10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. doi:10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- 3.Chutinet A, Biffi A, Kanakis A, et al. Severity of leukoaraiosis in large vessel atherosclerotic disease. AJNR Am J Neuroradiol 2012;33:1591–5. doi:10.3174/ajnr.A3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demirtas H, Ozkaynak C, Durmaz MS. Leukoaraiosis and carotid artery stenosis: evaluation with CT angiography. J Comput Assist Tomogr 2013;37:327–32. doi:10.1097/RCT.0b013e318284604e [DOI] [PubMed] [Google Scholar]
- 5.Kandiah N, Goh O, Mak E, et al. Carotid stenosis: a risk factor for cerebral white-matter disease. J Stroke Cerebrovasc Dis 2014;23:136–9. doi:10.1016/j.jstrokecerebrovasdis.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 6.Klein IF, Lavallee PC, Mazighi M, et al. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke 2010;41:1405–9. doi:10.1161/STROKEAHA.110.583534 [DOI] [PubMed] [Google Scholar]
- 7.Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, ageing, and cognitive impairment: the Framingham study. Stroke 2009;40:1590–6. doi:10.1161/STROKEAHA.108.535245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldassarre D, De Jong A, Amato M, et al. Carotid intima-media thickness and markers of inflammation, endothelial damage and hemostasis. Ann Med 2008;40:21–44. doi:10.1080/07853890701645399 [DOI] [PubMed] [Google Scholar]
- 9.Jung KW, Shon YM, Yang DW, et al. Coexisting carotid atherosclerosis in patients with intracranial small- or large-vessel disease. J Clin Neurol 2012;8:104–8. doi:10.3988/jcn.2012.8.2.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. doi:10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 11.Van Sandwijk MS, Ten Berge IJ, Majoie CB, et al. Cognitive changes in chronic kidney disease and after transplantation. Transplantation 2016;100:734–42. doi:10.1097/TP.0000000000000968 [DOI] [PubMed] [Google Scholar]
- 12.van Es AC, van der Grond J, de Craen AJ, et al. Risk factors for cerebral microbleeds in the elderly. Cerebrovasc Dis 2008;26:397–403. doi:10.1159/000151680 [DOI] [PubMed] [Google Scholar]
- 13.Kim BJ, Lee SH. Cerebral microbleeds: their associated factors, radiologic findings, and clinical implications. J Stroke 2013;15:153–63. doi:10.5853/jos.2013.15.3.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim JS, Hong KS, Kim GM, et al. Cerebral microbleeds and early recurrent stroke after transient ischemic attack: results from the Korean Transient Ischemic Attack Expression Registry. JAMA Neurol 2015;72:301–8. doi:10.1001/jamaneurol.2014.3958 [DOI] [PubMed] [Google Scholar]
- 15.Soo YO, Siu DY, Abrigo J, et al. Risk of intracerebral hemorrhage in patients with cerebral microbleeds undergoing endovascular intervention. Stroke 2012;43:1532–6. doi:10.1161/STROKEAHA.111.626853 [DOI] [PubMed] [Google Scholar]
- 16.Gregg NM, Kim AE, Gurol ME, et al. Incidental cerebral microbleeds and cerebral blood flow in elderly individuals. JAMA Neurol 2015;72:1021–8. doi:10.1001/jamaneurol.2015.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto T, Yokota C, Koshino K, et al. Cerebral blood flow and metabolism associated with cerebral microbleeds in small vessel disease. Ann Nucl Med 2016;30:494–500. doi:10.1007/s12149-016-1086-7 [DOI] [PubMed] [Google Scholar]
- 18.Rostom A, Dube C, Cranney A, et al. Celiac disease. Rockville, MD: Agency for Healthcare Research and Quality (US); (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. http://www.ncbi.nlm.nih.gov/books/NBK35156/ [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. doi:10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. doi:jst00003 [DOI] [PubMed] [Google Scholar]
- 21.Ochi N, Tabara Y, Igase M, et al. Silent cerebral microbleeds associated with arterial stiffness in an apparently healthy subject. Hypertens Res 2009;32:255–60. doi:10.1038/hr.2009.13 [DOI] [PubMed] [Google Scholar]
- 22.Miwa K, Tanaka M, Okazaki S, et al. Relations of blood inflammatory marker levels with cerebral microbleeds. Stroke 2011;42:3202–6. doi:10.1161/STROKEAHA.111.621193 [DOI] [PubMed] [Google Scholar]
- 23.Chung PW, Park KY, Kim JM, et al. Carotid artery calcification is associated with deep cerebral microbleeds. Eur Neurol 2014;72:60–3. doi:10.1159/000358513 [DOI] [PubMed] [Google Scholar]
- 24.Peng Q, Huang Y, Sun W, et al. Associations among cerebral microbleeds, cerebral large-artery diseases and endothelial function. Chin Med J 2014;127:3204–8. [PubMed] [Google Scholar]
- 25.Song TJ, Chang Y, Shin MJ, et al. Low levels of plasma omega 3-polyunsaturated fatty acids are associated with cerebral small vessel diseases in acute ischemic stroke patients. Nutr Res 2015;35:368–74. doi:10.1016/j.nutres.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 26.Tabara Y, Okada Y, Ohara M, et al. Association of postural instability with asymptomatic cerebrovascular damage and cognitive decline: the Japan Shimanami health promoting program study. Stroke 2015;46:16–22. doi:10.1161/STROKEAHA.114.006704 [DOI] [PubMed] [Google Scholar]
- 27.Ding J, Mitchell GF, Bots ML, et al. Carotid arterial stiffness and risk of incident cerebral microbleeds in older people: the Age, Gene/Environment Susceptibility (AGES)-Reykjavik study. Arterioscler Thromb Vasc Biol 2015;35:1889–95. doi:10.1161/ATVBAHA.115.305451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero JR, Preis SR, Beiser A, et al. Carotid atherosclerosis and cerebral microbleeds: the Framingham Heart Study. J Am Heart Assoc 2016;4:e002377 doi:10.1161/JAHA.115.002377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon HM, Lynn MJ, Turan TN, et al. Frequency, risk factors, and outcome of coexistent small vessel disease and intracranial arterial stenosis: results from the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Trial. JAMA Neurol 2016;73:36–42. doi:10.1001/jamaneurol.2015.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke 2010;41(10 Suppl):S103–6. doi:10.1161/STROKEAHA.110.595181 [DOI] [PubMed] [Google Scholar]
- 31.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–4. doi:10.1161/01.HYP.0000168052.00426.65 [DOI] [PubMed] [Google Scholar]
- 32.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999;20:637–42. [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu C, Sigurdsson S, Zhang Q, et al. Diabetes, markers of brain pathology and cognitive function: the Age, Gene/Environment Susceptibility-Reykjavik Study. Ann Neurol 2014;75:138–46. doi:10.1002/ana.24063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Sloten TT, Protogerou AD, Henry RM, et al. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev 2015;53:121–30. doi:10.1016/j.neubiorev.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Li Y. Cortical cerebral blood flow, oxygen extraction fraction, and metabolic rate in patients with middle cerebral artery stenosis or acute stroke. AJNR Am J Neuroradiol 2016;37:607–14. doi:10.3174/ajnr.A4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Jong GI, Farkas E, Stienstra CM, et al. Cerebral hypoperfusion yields capillary damage in the hippocampal CA1 area that correlates with spatial memory impairment. Neuroscience 1999;91:203–10. doi:S0306-4522(98)00659-9 [DOI] [PubMed] [Google Scholar]
- 37.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 2007;54:162–80. doi:10.1016/j.brainresrev.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 38.Bauer M, Caviezel S, Teynor A, et al. Carotid intima-media thickness as a biomarker of subclinical atherosclerosis. Swiss Med Wkly 2012;142:w13705 doi:10.4414/smw.2012.13705 [DOI] [PubMed] [Google Scholar]
- 39.Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459–67. doi:10.1161/CIRCULATIONAHA.106.628875 [DOI] [PubMed] [Google Scholar]
- 40.Nambi V, Pedroza C, Kao LS. Carotid intima-media thickness and cardiovascular events. Lancet 2012;379:2028–30. doi:10.1016/s0140-6736(12)60652-7 [DOI] [PubMed] [Google Scholar]
- 41.Pruissen DM, Gerritsen SA, Prinsen TJ, et al. Carotid intima-media thickness is different in large- and small-vessel ischemic stroke: the SMART study. Stroke 2007;38:1371–3. doi:10.1161/01.STR.0000260220.37016.88 [DOI] [PubMed] [Google Scholar]
- 42.Huang X, Kang X, Xue J, et al. Evaluation of carotid artery elasticity changes in patients with cerebral small vessel disease. Int J Clin Exp Med 2015;8:18825–30. [PMC free article] [PubMed] [Google Scholar]
- 43.Rundek T, Blanton SH, Bartels S, et al. Traditional risk factors are not major contributors to the variance in carotid intima-media thickness. Stroke 2013;44:2101–8. doi:10.1161/STROKEAHA.111.000745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rundek T, Elkind MS, Pittman J, et al. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes: the Northern Manhattan Prospective Cohort Study. Stroke 2002;33:1420–3. doi:10.1161/01.str.0000015558.63492.b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung JW, Kim BJ, Sohn CH, et al. Branch atheromatous plaque: a major cause of lacunar infarction (high-resolution MRI study). Cerebrovasc Dis Extra 2012;2:36–44. doi:10.1159/000341399 [DOI] [PMC free article] [PubMed] [Google Scholar]