Abstract
Background
Perfluoroalkyl substances (PFASs) are a group of fluorinated organic substances that are widely used in consumer products and are often detectable in human tissues. Human studies on prenatal exposure to PFASs and neurodevelopment in children are few and inconsistent.
Methods
In the Taiwan Maternal and Infant Cohort Study, we collected serum samples from pregnant women during the third trimester and measured concentrations of 9 PFASs using a high performance liquid chromatography system. A subsample of their children was assessed with full scale intelligence quotient (FSIQ), verbal IQ (VIQ) and performance IQ (PIQ) at both age 5 (n = 120) and 8 years (n = 120). We used multivariate linear regression models to examine prenatal PFAS exposure in relation to IQ scores at each age period.
Results
Prenatal perfluoroundecanoic acid (PFUnDA) concentrations were inversely associated with children’s PIQ scores at age 5 years, with an adjusted coefficient (β) of −1.6 (95% confidence interval [CI]:(−3.0, −0.2). When children reached 8 years, most of the prenatal PFASs showed inverse association with children’s FSIQ, VIQ and PIQ scores. Among them, prenatal perfluorononanoic acid (PFNA) reached significance. Children with higher prenatal PFNA levels had lower VIQ with an adjusted of −2.1 (95% CI:−3.9, −0.2).
Conclusions
We found two prenatal PFAS exposure, both long-chain PFASs, in association with decreased IQ test scores in children. Our findings suggest more studies on long-chain PFASs and children’s neurodevelopment are needed.
Introduction
Perfluroalkyl Substances (PFASs) are a group of man-made, fluorinated organic substances. They all contain one or more carbon atoms on which all the hydrogen substituents are replaced by fluorine atoms. Due to their chemical and thermal stability, and their hydrophobic and lipophobic nature, they are widely used in consumer products, such as food packaging, carpets, clothing fabrics, and fire extinguishers (Lau et al., 2007). As a consequence, a broad range of these substances are detected globally in the environment, wildlife and humans. Long elimination half-lives have been observed for some PFASs in humans; for example, means of 4.5 years for perfluoroundecanoic acid (PFUnDA, an 11-carbon product) in young females and 12 years in all males and older females (Zhang et al., 2013). PFOS and PFOA, two 8-carbon products, have been investigated the most. Their potential toxicological impact led to the phase-out of production by the major global manufacturer, 3M Company, in 2000–2002 (3M Company, 2014; US Environmental Protection and Agency, 2014); however, they are still detectable in human tissues, including serum, liver, cord blood and milk (Apelberg et al., 2007;Karrman et al., 2007). Long chain substances (>8 carbons), such as perfluorononanoic acid (PFNA, a 9-carbon product), PFUnDA, perfluorododecanoic acid (PFDoDA, a 12-carbon product) were also frequently detected in human beings (Andersen et al., 2008; Calafat et al., 2007). The detection rate and blood concentrations may vary in different populations. For example, in Taiwan, serum concentrations of PFUnDA and PFDoDA were much higher than reported from studies in western countries (Lien et al., 2011; Lin et al., 2013). The toxicological activities of the long-chain PFASs and their effects on human health are not well studied, although they were reported to be more bioaccumulative and toxic than PFOS and PFOA (Zhang et al., 2013).Humans are exposed to PFASs through diet, drinking water, indoor dust, and outdoor air (Fromme et al., 2009). Fetuses can be exposed to PFASs via placental transfer (Apelberg et al., 2007),and infants through breastfeeding (Karrman et al., 2007). During the fetal and neonatal period, the brain is undergoing fundamental developmental processes, and is vulnerable to neurotoxicants. The consequences of exposure to these compounds may be permanent (Rice and Barone, 2000). Studies performed with PFOS and PFOA on experimental rats and mice indicated that PFASs may induce neurobehavioral effects, particularly in developmentally exposed animals (Mariussen, 2012). The findings on PFASs and human neurodevelopment are inconsistent. A study in Taiwan found that prenatal exposure to PFOS, but not PFOA, was negatively associated with children’s gross motor development at age2 years (Chen et al., 2013). A study of Danish children aged 7 years showed that prenatal blood levels of PFOA and PFOS were not associated with parent-reported behavioral and motor coordination problems (Fei and Olsen, 2011). In another study of children from PFOA-contaminated water districts, those in the highest quartile of estimated in utero PFOA level had increases in full scale IQ (FSIQ) and decreases in characteristics of Attention Deficit Hyperactivity Disorder (ADHD) compared with children in the lowest quartile(Stein et al., 2013). We still do not have much information on pre-natal measurement of other PFASs besides PFOA and PFOS and any association with neurodevelopment using longitudinal data. Due to children’s widespread and bioaccumulative exposure to PFASs, beginning in utero, we investigated the effects of prenatal exposure to PFASs on children’s IQ tested at two ages in early childhood.
Materials and methods
Design and study participants
This research is a part of the Taiwan Maternal and Infant Cohort Study (TMICS). The main purpose of the study was to investigate maternal exposure to environmental pollutants and several health outcomes in the pregnant women and their infants and children. Between December 2000 and November 2001, we invited all pregnant women who visited local clinics in the study area of central Taiwan to participate and enrolled those who were willing to provide baseline information and blood samples (n = 430). At the first visit, they were interviewed for demographic information, reproductive and medical history, diet, and other lifestyle characteristics before and during pregnancy. We collected blood samples only during the 3rd trimester and followed their children for growth and development as well as other health outcomes Details can be found in previous publications (Wang et al., 2005;Huang et al., 2012). The Human Ethical Committee of the National Health Research Institutes in Taiwan approved the study. Each of the participating pregnant women, mothers, and children at age 8 provided signed informed consents before the commencement of the study activities.
Exposure
Maternal serum samples collected in the third trimester were used to measure prenatal PFASs exposure. Altogether 9 PFASs were analyzed, namely perfluorohexanesulfonic acid (PFHxS), PFOA,PFOS, PFNA, perfluorodecanoic acid (PFDeA), PFUnDA, PFDoDA, perfluoroheptanoic acid (PFHpA), and perfluorohexanoic acid (PFHxA). We used an Agilent-1200 high performance liquid chromatography system (Agilent, Palo Alto, CA, USA) coupled with a triple-quadrupole mass spectrometer (Sciex API 4000, Applied Biosystems, Foster City, CA, USA). The detailed analytical method has been described in a previous publication (Lien et al., 2011). Nine calibration standard solutions were analyzed in the same way. The concentrations of specific analytes ranged from 0.25 to 125 ng/mL, with a fixed amount of internal standard (5 ng/mL). Using the standard solutions, we found that the intra-assay coefficients of variation (CVs) for PFASs concentrations ranged from 0.83 to 7.94% and the inter-assay CVs for PFASs were between 1.6 and 24.7%. The limit of quantitation (LOQ), defined as a signal-to-noise ratio of ten, ranged from 0.07 to 0.45 ng/mL for the nine PFASs (Lien et al., 2011).
Outcomes
In the current study, 120 children (28%) were followed up when they reached age 5 years (other children were lost of follow-up). We used the Chinese version of the Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) (Wechsler, 2000) to assess their intelligence. It has five subtests of verbal skill and five subtests of visual-spatial skill for 3–7 year old children. The combined scores from five subtests are called the verbal IQ (VIQ) and the performance IQ (PIQ), respectively. All ten subtests are combined to yield the FSIQ. The Chinese version of WPPSI-R was validated in Taiwan in2000 (Wechsler, 2000). When the children reached age 8 years, 120 children (28%) were followed up as well (other children were lost to follow-up) and were administered the Chinese version of Wechsler’s Intelligence Scale for Children-III (WISC-III) (Wechsler, 1997),which is designed for children ages 6–16 years, and which also gives VIQ, PIQ and FSIQ. The Chinese version of WISC-III was standardized in Taiwan. Both IQ tests have a mean of 100 and a standard deviation of 15. A total of 10 certified psychologists who were unaware of the results of PFASs measurement administered all the tests. One senior psychologist trained all testers, reviewed randomly selected forms during the assessment, and checked all completed evaluation forms. Only those validated test scores entered the data base for final analysis.
Covariates
We also collected other prenatal variables at the first interview during pregnancy, i.e., maternal age (years), maternal education(≤high school, part or full college, or >college), previous live births(0 or ≥1), family income (<$20,000 or ≥$20,000), and maternal fish consumption during pregnancy (times/week). Maternal blood lead concentrations (μg/dL) were measured in the blood samples drawn at the third trimester. Additionally, at a home visit during each follow-up, we used a Chinese version of the Home Observation for Measurement of the Environment Inventory (HOME) to identify environments that are stimulating to children (Caldwell and Bradley, 1984). The HOME was done in a conversational, low-key manner with observation, and a yes/no format was used in scoring for the HOME. For children at 5 years of age, we used the Early Childhood HOME Version, which contains 55 items clustered into eight subscales: learning materials; language stimulation; physical environment; parental responsiveness; learning stimulation; modeling of social maturity; variety of experience; and acceptance of child. At age 8 years, we used the Middle Childhood HOME Inventory, which contains 59 items clustered into eight subscales: parental responsiveness; physical environment; learning materials; active stimulation; encouraging maturing; emotional climate; parental involvement; and family participation. The total HOME scores in each version were calculated by summing the individual item scores; higher scores indicate better home environment.
Statistical analysis
The distribution of prenatal PFASs concentrations was skewed and we present the percentiles and geometric means (GMs). All the IQ test scores were normally distributed and we present means and standard deviations (SDs). To investigate the associations between prenatal PFASs exposure and the outcomes, we fit multivariate linear regression models between each prenatal PFAS and each IQ score measured at each year. In all the models, IQ scores were included as continuous dependent variables, and prenatal PFASs concentrations were treated as continuous independent variables in log 2-transformed scale. For values below LOQ of each PFAS, we imputed the corresponding expected value conditional on values being below LOQ, based on an assumed log-normal distribution of PFASs (Richardson and Ciampi, 2003). As prenatal PFASs concentrations were log2-transformed in the models, the linear coefficient(β) corresponds to the unit change in the cognitive test scores with a doubling of prenatal PFAS concentrations (ng/mL). In the models, we adjusted for children’s sex and age at IQ assessment a priori, and identified other covariates mentioned before as confounders if they were both significantly associated with any of prenatal PFAS and any of IQ test scores. We used robust regression to fit the multi-variate linear regression models, a procedure of ROBUSTREG in SAS9.3 (SAS Institute, Cary, NC), which detects outliers and provides stable results in the presence of outliers. As only a subset of children from the original birth cohort had IQ tests done, selection bias may operate. We compared children who were followed up and those who were not for demographic characteristics that we thought might introduce such bias using Student t-test or Chi-square test. We did not adjust for multiple comparisons, as suggested by Rothman (Rothman, 1990). All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and the significance level was set to p < 0.05.
Results
For the subsample of children included in the present study, Table 1 presents the prenatal characteristics of children who were assessed for IQ at 5 and 8 years old, respectively. Their mothers averaged 29 years old at delivery, and generally did not smoke or consume alcohol during pregnancy. Most of mothers had a high school education or more, and over half of the women were primiparous. The average age of children at IQ testing was 5.3 and 8.2years, respectively. There were 120 children administered IQ tests at age 5 years and 120 children at age 8 years, respectively, with 89 paired children. The mean FSIQ was 105.8 (SD = 12.8) and 109.1(SD = 11.4) at each age, respectively. Among the nine prenatal PFASs assayed, PFHxA and PFHpA concentrations were detected in less than 20% of samples and thus were not considered further. The other seven substances were detected in more than 70% of the serum samples (Table 2). PFOS had the highest median concentration, followed by PFUnDA, PFOA, PFNA, PFHxS, PFDeA and PFDoDA.
Table 1.
Prenatal characteristics and IQ test scores of children at age 5 years and 8 years.
| Characteristics | Children at age 5 years (N = 120) Mean (SD) or n (%) | Children at age 8 years (N = 120) Mean (SD) or n (%) |
|---|---|---|
| Maternal Age | ||
| Age at enrollment (years), mean (SD) | 29.1 (4.4) | 29.1 (4.2) |
| Pre-pregnancy BMI (kg/m2), mean (SD) | 20.5 (2.8) | 20.5 (2.8) |
| Fish consumption (times/week), median (range) | 4.1 (0.1, 26.0) | 4.0 (0.1, 41.3) |
| Blood lead concentrations (μg/dL), mean (SD) | 2.6 (1.7) | 2.7 (1.6) |
| Previous live births, n (%) | ||
| 0 | 64 (54) | 59 (50) |
| ≥1 Education, n (%) |
54 (46) | 58 (50) |
| <High school | 2 (2) | 3 (3) |
| High school | 45 (38) | 44 (38) |
| Part college | 50 (42) | 46 (40) |
| ≥College | 21 (18) | 22 (19) |
| Annual family income (US $), n (%) | ||
| <20,000 | 49 (42) | 44 (39) |
| ≥20,000 | 67 (58) | 70 (61) |
| Smoking during pregnancy, n (%) | 1 (1) | 1 (1) |
| Drinking alcohol during pregnancy, n (%) | 0 | 0 |
| Children | ||
| Age (years), mean (SD) | 5.3 (0.3) | 8.2 (0.2) |
| HOME score, mean (SD) | 45.4 (5.2) | 46.0 (6.1) |
| IQ scores, mean (SD) | ||
| FSIQ | 105.8 (12.8) | 109.1 (11.4) |
| VIQ | 103.3 (12.7) | 110.1 (11.8) |
| PIQ | 106.9 (13.5) | 106.9 (12.0) |
| Sex, n (%) Male | 47 (52) | 45 (50) |
Abbreviation: BMI, body mass index; FSIQ, full scale intelligence quotient; HOME, Home Observation for Measurement of the Environment Inventory; PIQ, performance intelligence quotient; SD, standard deviation; VIQ, verbal intelligence quotient. There were missing values for maternal age (n = 2), pre-pregnancy BMI (n = 11); fish consumption (n = 7); blood lead concentrations (n = 9); previous live births (n = 1); education (n = 1); annual family income (n = 3); child HOME score at age 5 years (n = 1) and child sex (n = 28). There were missing values for maternal age (n = 4), pre-pregnancy BMI (n = 10); fish consumption (n = 7); blood lead concentrations (n = 10); previous live births (n = 1); education (n = 3); annual family income (n = 4); child HOME score at age 8 years (n = 8) and child sex (n = 28).
Table 2.
Serum concentrations of prenatal PFASs (ng/mL).
| Prenatal PFASs | % > LOQ | Molecular formula | Children at age 5 years
|
|
|---|---|---|---|---|
| Median (IQR) | GM (95% CI) | |||
| PFHxS | 78 | C5 F13 CSO3 H | 0.69 (0.07–1.09) | 0.44 (0.34–0.56) |
| PFOA | 87 | C7 F15 CO2 H | 2.50 (1.54–3.35) | 2.00 (1.75–2.29) |
| PFOS | 100 | C7 F17 CSO3 H | 13.25 (9.75–17.50) | 11.93 (10.44–13.63) |
| PFNA | 96 | C8 F17 CO2 H | 1.59 (0.84–2.43) | 1.41 (1.19–1.68)) |
| PFDeA | 71 | C9 F19 CO2 H | 0.44 (0.16–0.72) | 0.40 (0.35–0.45) |
| PFUnDA | 91 | C10 F21 CO2 H | 3.42 (1.92–8.90) | 3.28 (2.57–4.19) |
| PFDoDA | 82 | C11 F23 CO2 H | 0.38 (0.22–0.52) | 0.29 (0.25–0.34) |
| Prenatal PFASs | % > LOQ | Molecular formula | Children at age 8 years
|
|
|---|---|---|---|---|
| Median (IQR) | GM (95% CI) | |||
| PFHxS | 78 | C5 F13 CSO3 H | 0.69 (0.07–1.07) | 0.45 (0.35–0.57) |
| PFOA | 87 | C7 F15 CO2 H | 2.50 (1.54–3.33) | 2.00 (1.72–2.33) |
| PFOS | 100 | C7 F17 CSO3 H | 12.28 (9.50–16.3) | 11.5 (10.2–13.07) |
| PFNA | 96 | C8 F17 CO2 H | 1.44 (0.77–2.25) | 1.33 (1.12–1.59) |
| PFDeA | 71 | C9 F19 CO2 H | 0.44 (0.16–0.70) | 0.39 (0.34–0.44) |
| PFUnDA | 91 | C10 F21 CO2 H | 3.13 (0.70–8.90) | 3.05 (2.37–3.94) |
| PFDoDA | 82 | C11 F23 CO2 H | 0.37 (0.23–0.53) | 0.29 (0.25–0.34) |
Abbreviation: CI, confidence interval; GM, geometric mean; IQR, interquartile range; LOQ, limit of quantitation; PFDeA, perfluorodecanoic acid; PFDoDA, perfluorododecanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid.
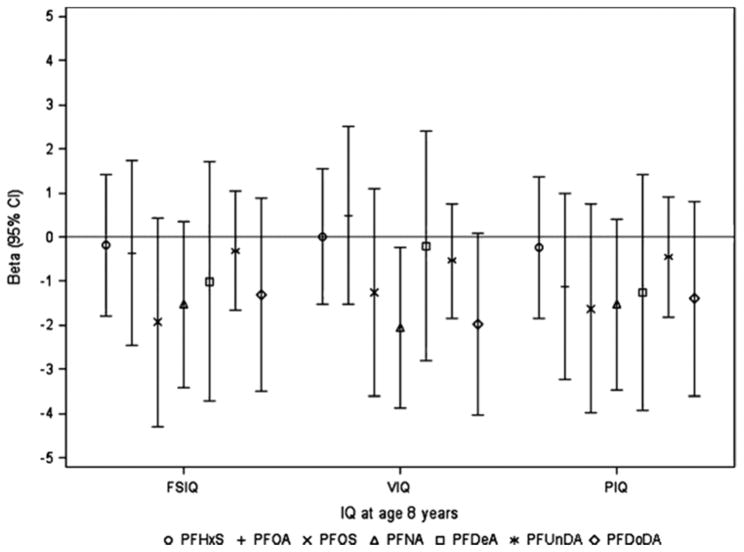
At age 5 years, we found that prenatal PFUnDA exposure was significantly inversely associated with children’s PIQ score (Fig. 1, Supplemental Table 1). The adjusted β from multivariate regression models was −1.6 (95% confidence interval [CI]: (−3.0, −0.2), which corresponds to an estimated 1.6 point decrease in children’s PIQ scores with a doubling of prenatal PFUnDA concentrations (ng/mL). Other prenatal PFASs showed similar trends with PIQ scores, but the associations with FSIQ or VIQ scores were not consistent in direction. When children reached 8 years old, almost all prenatal PFAS exposures showed inverse associations with children’s FSIQ, VIQ and PIQ scores, and prenatal PFNA reached significance (Fig. 2, Supplemental Table 1). Higher prenatal PFNA levels were associated with lower VIQ scores, with an adjusted β of −2.1 (95% CI: −3.9,−0.2), which corresponds to an estimated 2.1 point decrease in children’s VIQ score with a doubling of prenatal PFNA concentration(ng/mL).
Fig. 1.
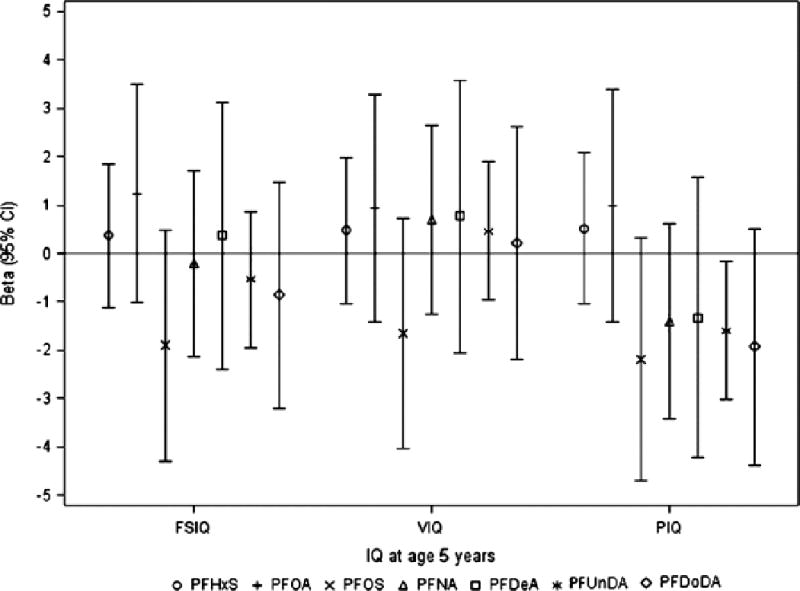
β (95% CI) from linear regression models for associations of Log 2-transformed prenatal PFASs concentrations with IQ scores in children at age 5 years (n = 89). Prenatal PFASs were log 2-transformed in all models. Models were adjusted for maternal education, family annual income, children’s age, sex and HOME score at IQ assessment.
Abbreviation: CI, confidence interval; FSIQ, full scale IQ; PFASs, perfluroalkyl substances; PFDeA, perfluorodecanoic acid; PFDoDA, perfluorododecanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid; PIQ, performance IQ; VIQ, verbal IQ.
Fig. 2.
β (95% CI) from linear regression models for associations of Log 2-transformed prenatal PFASs with IQ scores in children at age 8 years (n = 85). Prenatal PFASs were log 2-transformed in all models. Models were adjusted for maternal education, family annual income, children’s age, sex and HOME score at IQ assessment. Abbreviation: CI, confidence interval; FSIQ, full scale IQ; PFASs, perfluroalkyl substances; PFDeA, perfluorodecanoic acid; PFDoDA, perfluorododecanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid; PIQ, performance IQ; VIQ, verbal IQ
In comparison with children who did not have IQ measurements, the children analyzed in the present study were similar in prenatal characteristics, such as maternal age, education, fish consumption during pregnancy, maternal PFAS concentrations during pregnancy, and annual family income (Supplemental Table 2).
Discussion
We observed inverse associations of prenatal PFUnDA with PIQ scores in children at age 5 years and prenatal PFNA exposure with VIQ scores in children at age 8 years. The point estimates for the associations between prenatal PFASs and IQ were more consistent at age 8 years compared with age 5 years, particularly for the long-chain PFASs.
We examined four long chain PFASs in the current study. PFNA, PFDeA, PFUnDA and PFDoDA are 9-, 10-, 11-, and 12-carbon versions of PFOA, respectively. They have higher environmental persistence and longer half-lives than PFOA (Zhang et al., 2013), and may show different toxic features. Their blood concentrations were higher in East Asian populations (Harada et al., 2011) than the others (Calafat et al., 2007; Axmon et al., 2014). PFASs concentrations in Taiwan were even higher from two other studies than the present study (Lien et al., 2011; Lin et al., 2013). These PFASs were primarily manufactured in Japan via the oxidation of a linear fluorotelomer olefin mixture (Prevedouros et al., 2006), which may explain the high concentrations detected in East Asia. There is little about prenatal exposure to long chain PFASs and children’s neurodevelopment in the existing literature. We found only one study which reported an inverse association between postnatal exposure to PFNA and a measure of impulsivity in the US children aged 9–11 years (Gump et al., 2011). The biological mechanism of develop-mental neurotoxicity from long chain PFASs remains unknown.
As yet, there is a little known about PFOS or PFOA from experimental models of human intelligence. Liu et al found that prenatal exposure to PFOS and PFOA impaired spatial cognition and memory in rats, which could be related to a reduction in N-methyl-d-aspartate receptor 2B concentration in the cortex and hippocampal region of the brain (Liu et al., 2009), and changes in gene expression of calcium-dependent signal molecules in the hippocampus during gestation in their subsequent study (Liu et al., 2010). Additionally, it is possible that PFASs exhibit neurotoxic effects by interfering with thyroid function, which plays an essential role in fetal neurodevelopment. In our previous study we observed that prenatal PFNA, PFUnDA, PFDeA and PFDoDA levels were adversely associated with fetal thyroid hormones, triiodothyronine and thyroxine(Wang et al., 2014). Finally, postnatal PFOS, PFOA, and PFHxS were also found to cause altered function of the cholinergic system and reduced cognitive function in rats (Viberg et al., 2013). Future studies of the longer chain PFAS are warranted.
In the present study, we did not find any consistent association between PFOS, PFOA, and PFHxS and children’s IQ measurements, even though PFOS and PFOA exposure was captured in the peak period (2001–2002). Another study in Taiwan, however, reported that children with higher prenatal PFOS levels, but not PFOA, had lower gross motor scores at age 2 years (Chen et al., 2013). Compared with our study, they had larger sample size (n = 239), they measured prenatal PFOS in cord blood, and they assessed neurodevelopment with the Comprehensive Developmental Inventory. The C8 Health Effects study found a favorable effect of in utero PFOA exposure on children’s IQ scores (Stein et al., 2013). Their subjects’ in utero PFOA exposures were estimated based on documented PFOA releases, environmental fate and transport modeling, human exposure and excretion pharmacokinetics, and geocoded residential history. A neuroprotective effect was not anticipated, and it is difficult to interpret. In Danish children, there was no association between prenatal PFOA and PFOS plasma levels and maternal report of motor or mental development through age 18 months (Feiet al., 2008) or parent-reported behavioral and motor coordination problems at the age 7 years (Fei and Olsen, 2011). The neurobehavioral assessment in the latter two cohorts was limited and serum long chain PFAS levels were not measured. Comparisons between the different studies are limited because estimations of PFAS and neurodevelopmental outcomes were widely differing among the studies.
Our study provides first indications for associations between prenatal PFASs exposure and IQ scores in children. We measured multiple prenatal PFASs, including long-chain compounds. IQ scores were assessed at two ages in early childhood, which provide a more reliable representation of children’s IQ. However, some limitations should be noted. First, only a subset of children participated in IQ tests. Although we compared the prenatal characteristics between these children and the original cohort and did not find differences, selection bias could not be ruled out. Additionally, the small sample size may limit the limit the explanatory power. Third, IQ is also affected by other neurotoxicants, and we cannot exclude a role for other toxicants with similar exposure pathways through bioaccumulation into food, such as polychlorinated biphenyls (PCBs), or mixture effects. Finally, some potentially confounding factors were not measured, such as maternal and caregiver’s IQ.
In conclusion, the present study provides first indications of possible associations of prenatal exposure to two long-chain PFASs with lower IQ scores in children. No significant associations were found for the other examined PFASs. Our study provides data to better understand PFAS exposure and neurodevelopment in children.
Supplementary Material
Acknowledgments
Funding
The study was funded by National Health Research Institutes, Taiwan (EH-102-SP-01, EH-103-SP-02, EO-103-PP-05), Ministry of Science and Technology, Taiwan (NSC101-2325-B-400-008, MOST103-2314-B-400-006). This study was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
We thank the clinics of the Department of Obstetrics and Gynecology and Department of Pediatrics, Chung-Shan Medical University Hospital, especially for the assistance of Dr. Tsung-HoYing, Ms. Carol Huichun Chen and Chien-Wen Sun at the National Health Research Institutes, Miaoli, Taiwan, for subject recruitment, and data and specimen collections, Dr. Po-Chin Huang and Ms. Hsin-I Huang for organizing the expert team and training courses for the IQ measurements, and the Taiwan Maternal and Infant Cohort Study Group.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- β
coefficient
- CI
confidence interval
- CV
coefficients of variation
- FSIQ
full scale IQ
- GM
geometric mean
- HOME
home observation for measurement of the environment inventory
- LOQ
limit of quantitation
- IQR
interquartile range
- PCB
polychlorinated biphenyl
- PFASs
perfluroalkyl substances
- PFDeA
perfluorodecanoic acid
- PFHpA
perfluoroheptanoic acid
- PFHxA
perfluorohexanoic acid
- PFHxS
perfluorohexanesulfonic acid
- PFOS
perfluorooctane sulfonate
- PFOA
perfluorooctanoic acid
- PFNA
perfluorononanoic acid
- PFUnDA
perfluoroundecanoic acid
- PFDoDA
perfluorododecanoic acid
- PIQ
performance IQ
- SD
standard deviation
- TMICS
Taiwan maternal and infant cohort study
- VIQ
verbal IQ
- WPPSI-R
Wechsler preschool and primary scale of intelligence-revised
- WISC-III
Chinese version of Wechsler’s intelligence scale for children-III
Footnotes
Conflict of interest statement
There is no conflict of interest.
References
- 3M Company. Voluntary use and exposure information profile for perfluorooctanoic acid and salts. [accessed October 23, 2014];USEPA Administrative Record AR226-0595. http://www.regulations.gov as document EPA-HQ-OPPT-2002-0051-0009.
- Andersen ME, Butenhoff JL, Chang SC, et al. Perfluoroalkyl acids and related chemistries—toxicokinetics and modes of action. Toxicol Sci. 2008;102:3–14. doi: 10.1093/toxsci/kfm270. [DOI] [PubMed] [Google Scholar]
- Apelberg BJ, Goldman LR, Calafat AM, et al. Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. Environ Sci Technol. 2007;41:3891–3897. doi: 10.1021/es0700911. [DOI] [PubMed] [Google Scholar]
- Axmon A, Axelsson J, Jakobsson K, Lindh CH, Jönsson BA. Time trends between 1987 and 2007 for perfluoroalkyl acids in plasma from Swedish women. Chemosphere. 2014;102:61–67. doi: 10.1016/j.chemosphere.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the national health and nutrition examination survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home Observation for Measurement of the Environment (HOME), revised ed. University of Arkansas; Little Rock: 1984. [Google Scholar]
- Chen MH, Ha EH, Liao HF, et al. Perfluorinated compound levels in cord blood and neurodevelopment at 2 years of age. Epidemiology. 2013;24:800–808. doi: 10.1097/EDE.0b013e3182a6dd46. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy. Environ Health Perspect. 2008;116:1391–1395. doi: 10.1289/ehp.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, Olsen J. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ Health Perspect. 2011;119:573–578. doi: 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds—exposure assessment for the general population in western countries. Int J Hyg Environ Health. 2009;212:239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Gump BB, Wu Q, Dumas AK, Kannan K. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environ Sci Technol. 2011;45:8151–8159. doi: 10.1021/es103712g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada KH, Hitomi T, Niisoe T, et al. Odd-numbered perfluorocarboxylates predominate over perfluorooctanoic acid in serum samples from Japan, Korea and Vietnam. Environ Int. 2011;37:1183–1189. doi: 10.1016/j.envint.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Huang PC, Su PH, Chen HY, et al. Childhood blood lead levels and intellectual development after ban of leaded gasoline in Taiwan: a 9-year prospective study. Environ Int. 2012;40:88–96. doi: 10.1016/j.envint.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Karrman A, Ericson I, van Bavel B, et al. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect. 2007;115:226–230. doi: 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lien GW, Wen TW, Hsieh WS, Wu KY, Chen CY, Chen PC. Analysis of perfluorinated chemicals in umbilical cord blood by ultra-high performance liquid chromatography/tandem mass spectrometry. J Chromatogr, B: Anal Technol Biomed Life Sci. 2011;879:641–646. doi: 10.1016/j.jchromb.2011.01.037. [DOI] [PubMed] [Google Scholar]
- Lin CY, Wen LL, Lin LY, et al. The associations between serum perfluorinated chemicals and thyroid function in adolescents and young adults. J Hazard Mater. 2013;244–245:637–644. doi: 10.1016/j.jhazmat.2012.10.049. [DOI] [PubMed] [Google Scholar]
- Liu L, Jin YH, Wang L, et al. Effects of perfluorooctane sulfonate on learning and memory of rat pups. Zhonghua Yu Fang Yi Xue Za Zhi. 2009;43:622–627. [PubMed] [Google Scholar]
- Liu X, Liu W, Jin Y, Yu W, Wang F, Liu L. Effect of gestational and lactational exposure to perfluorooctanesulfonate on calcium-dependent signaling molecules gene expression in rats’ hippocampus. Arch Toxicol. 2010;84:71–79. doi: 10.1007/s00204-009-0467-2. [DOI] [PubMed] [Google Scholar]
- Mariussen E. Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance. Arch Toxicol. 2012;86:1349–1367. doi: 10.1007/s00204-012-0822-6. [DOI] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol. 2006;40:32–44. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DB, Ciampi A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am J Epidemiol. 2003;157:355–363. doi: 10.1093/aje/kwf217. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology. 2013;24:590–599. doi: 10.1097/EDE.0b013e3182944432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency. [accessed October 23];EPA and 3M Announce Phaseout of PFOS. 2014 http://yosemite.epa.gov/opa/admpress.nsf/0/33aa946e6cb11f35852568e1005246b4.
- Viberg H, Lee I, Eriksson P. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology. 2013;304:185–191. doi: 10.1016/j.tox.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Wang SL, Su PH, Jong SB, Guo YL, Chou WL, Papke O. In utero exposure to dioxins and polychlorinated biphenyls and its relations to thyroid function and growth hormone in newborns. Environ Health Perspect. 2005;113:1645–165019. doi: 10.1289/ehp.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen PC, et al. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect. 2014;122:529–534. doi: 10.1289/ehp.1306925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3. Chinese Behavioural Science Corporation; Taipei: 1997. (Chinese Version) [Google Scholar]
- Wechsler D. Manual for the Wechsler Preschool and Primary Scale of Intelligence, Revised. Chinese Behavioural Science Corporation; Taipei: 2000. (Chinese Version) [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol. 2013;47:10619–10627. doi: 10.1021/es401905e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.