Abstract
B lymphopoiesis arrests precipitously in rabbits such that by 2 to 4 months of age, prior to sexual maturity, little to no B lymphopoiesis occurs in the bone marrow (BM). Previously, we showed that in mice, adipocytes inhibit B lymphopoiesis in vitro by inducing inflammatory myeloid cells which produce IL-1β. Here, we characterized rabbit BM after the arrest of B lymphopoiesis and found a dramatic increase in fat, increased CD11b+ myeloid cells, and upregulated expression of the inflammatory molecules, IL-1β and S100A9 by the myeloid cells. We added BM fat, CD11b+ myeloid cells, and recombinant S100A9 to B lymphopoiesis cultures and found that they inhibited B lymphopoiesis and enhanced myelopoiesis. Unlike IL-1β which inhibits B lymphopoiesis by acting on early lymphoid progenitors, S100A9 inhibits B lymphopoiesis by acting on myeloid cells and promoting the release of inflammatory molecules, including IL-1β.
Many molecules produced by adipocytes activate the NLRP3 inflammasome, and the NLRP3 inhibitor, glybenclamide restored B lymphopoiesis and minimized induction of myeloid cells induced by adipocyte-conditioned medium in vitro. We suggest that fat provides an inflammatory microenvironment in the BM, and promotes/activates myeloid cells to produce inflammatory molecules such as IL-1β and S100A9, which negatively regulate B lymphopoiesis.
Introduction
Aging leads to changes that affect immune cell development and function, resulting in decreased immune responses to pathogens and vaccines in the elderly (1–3). Aged mouse and human bone marrow (BM) exhibit a decline in B lymphopoiesis, and hematopoiesis shifts more toward myelopoiesis (4–9). By microarray analysis, lymphoid-lineage gene expression decreases in aged HSCs, whereas the expression of myeloid-lineage genes is increased (10, 11). With aging, B lineage progenitors decline, and HSCs are functionally more likely to differentiate to myeloid cells than to B lineage cells (10, 11). In addition to changes in hematopoietic progenitors, changes to the BM microenvironment also contribute to impaired B cell development in aged individuals (5, 12–15). In rabbits, allotype suppression experiments (16), as well as the nearly complete loss of B lineage progenitors and B cell recombination excision circles (BRECs) in the BM suggested that B lymphopoiesis arrests by 2–4 months of age (17). The transfer of hematopoietic progenitors from adult rabbits into young irradiated recipients showed that the decline in B lymphopoiesis is likely due primarily to changes in the BM microenvironment rather than to hematopoietic progenitors (18). However, further characterization of the BM microenvironment is needed to identify key changes responsible for the decline in B lymphopoiesis.
Aging in mice and humans is associated with increased BM fat and an inflammatory environment referred to as inflammaging (12, 15, 19–21). Increased levels of inflammatory cytokines, including IL-1, IL-6, and TNFα, are found in aged BM, and all of these are known to negatively regulate B lymphopoiesis, and enhance myelopoiesis (22–28). We previously showed that adipocytes inhibit B lymphopoiesis in vitro, and that this inhibition is mediated in part, by IL-1 (23, 29). Adipocytes also express other inflammatory molecules, such as S100A8 and S100A9 (30, 31), and their expression is increased in multiple tissues during aging in mice and humans (32). Whether these inflammatory factors increase in aged BM, and inhibit B lymphopoiesis similar to IL-1, IL-6, and TNFα, is not known.
Here we report that the early arrest of B lymphopoiesis in rabbit BM corresponds with increases in fat and myeloid cells, both of which inhibit B lymphopoiesis in vitro. In addition we show that expression of the inflammatory molecules IL-1β and S100A9, produced by myeloid cells is increased in BM at the time B lymphopoiesis arrests, and that in vitro, these cytokines inhibit B lymphopoiesis. Further, the inhibition of B lymphopoiesis by adipocytes can be partially prevented in vitro, by an inhibitor of the NLRP3 inflammasome, implicating inflammation as a negative regulator of B lymphopoiesis. We suggest that the accelerated decline in B lymphopoiesis makes the rabbit a viable model to study inflammaging in BM of humans.
Materials and Methods
Mice, reagents, and flow cytometry
New Zealand White rabbits were maintained at Loyola University Chicago. C57BL/6 breeding pairs were from the Jackson Laboratory (Bar Harbor, ME). All rabbits and mice were used in compliance with protocols approved by the Loyola University Chicago Institutional Animal Care and Use Committee. All base media and supplements were purchased from GIBCO (Grand Island, NY). Antibodies to rabbit CD11b (M1/70) and murine B220 (RA3-6B2), CD19 (6D5), CD11b (M1/70), Gr1 (RB6-8C5), CD3ε (145-2C11), CD4 (GK1.5), CD8α (53-6.7), TER-119 (TER-119), CD49b (DX5), CD16/32 (93), Sca1(D7), CD117 (2B8), CD127 (SB/199), and CD135(A2F10) were from Biolegend (San Diego, CA). Antibodies to rabbit S100A8/A9 (MAC387) were from R&D systems (Minneapolis, MN) and to rabbit CD79a were from BD Biosciences (San Jose, CA). BD Horizon fixable viability stain (BD Biosciences, San Jose, CA) or Fixable Viability Dye eFluor 450 (eBioscience, San Diego, CA), was used to exclude dead cells from flow cytometric analysis. Flow cytometry was performed using a LSR Fortessa flow cytometer (BD Biosciences San Jose, CA). FlowJo software (Tree Star Ashland, OR) was used for all flow cytometric analyses. Murine recombinant IL-7, stem cell factor (SCF), Flt3-Ligand (Flt3-L), IL-1β, and human IL-1RA were purchased from Peprotech (Rocky Hill, NJ). Murine recombinant S100A8 was from Abcam (Cambridge, MA) and S100A9 was purchased from R&D systems.
Generation of BM fat-conditioned medium (BM fat-CM), Adipocyte-conditioned medium (ACM), and myeloid-derived suppressor cell-conditioned medium (MDSC-CM)
BM was isolated from rabbit femurs, and fat was separated from the adipocyte-free BM pellet by centrifugation. BM fat was then cultured overnight to generate conditioned medium. Adipocyte-conditioned medium (ACM) was obtained from 3T3.L1 pre-adipocytes which were differentiated into mature adipocytes using isobutyl-methylxanthine (1μM), dexamethasone (1μM), and insulin (10μg/ml) (33). Mature adipocytes were cultured in serum free DMEM for three days to generate ACM. To generate MDSCs, BM cultures were treated with ACM; CD11b+Gr1+ MDSCs were sorted by flow cytometry and cultured for three days in serum free αMEM to generate MDSC-CM.
B lymphopoiesis Cultures
All cultures were performed in 96 well plates coated with 1,000 OP9 cells per well, plated on day −1. For rabbit B lymphopoiesis cultures, 20,000 BM cells from >2-month-old rabbits were added to OP9-coated wells in the presence of human IL-7, SCF, and Flt3L (10ng/ml). BM fat-CM and ACM were typically used at 1:4–1:6 dilutions. On day 4, fresh cytokines were added to each well; cells were prepared for flow cytometry between days 7 to 10. In experiments with added effector cells, 10,000 CD11b− cells or total BM cells were used as a source of BM progenitors. In mouse B lymphopoiesis cultures, B220− BM cells (40,000), purified HSCs (500-1,-000), MPPs (500-1,000), or CLPs (500-1,000) were cultured with OP9 cells in the presence of murine IL-7, SCF, and Flt3L (10ng/ml). Mouse cultures were treated as indicated in the figure legends and analyzed by flow cytometry between days 7 and 10.
qRT-PCR
RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA), and cDNA was synthesized. qRT-PCR was performed with a C1000 thermal cycler with CFX96 real-time detection system (Bio-Rad, Hercules, CA). Expression for each target gene was normalized to a housekeeping gene, HGPRT for rabbit experiments and β-actin for mouse experiments. The following primer sets were used (forward and reverse): rabbit HGPRT 5′-AGCCCCAGCGTTGTGATTAGT-3′, 5′-CATCTCGAGCAAGCCTTTCAG-3′; rabbit IL-1β 5′-AGGTCTTGTCAGTCGTTGTG-3′, 5′-GTAGTCATCCCAGTGTGCAG-3′; rabbit S100A9 5′-ATCATCAACGTCTTCCACCAG-3′, 5′-TTATGGCTTTCTCATCCCTCG-3′; mouse β-actin 5′-GGCTGTATTCCCCTCCATCG-3′, 5′-CCAGTTGGTAACAATGCCATGT-3′; mouse IL-1β 5′-AAGAGCTTCAGGCAGGCAGTATCA-3′, 5′-ATGAGTCACAGAGGATGGGCTCTT-3′; mouse NLRP3 5′-AGAGCCTACAGTTGGGTGAAATG-3, 5′-CCACGCCTACCAGGAAATCTC-3′; mouse TNFα 5′-ACGGCATGGATCTCAAAGACAACC-3′, 5′-TGAGATAGCAAATCGGCTGACGGT-3′; mouse IL-6 5′-AGACAAAGCCAGAGTCCTTCAGAG-3′, 5′-TTGGTCCTTAGCCACTCCTTCTGT-3′.
Bone marrow sections and microscopy
For BM sectioning, the upper bone surface of rabbit femurs was removed with a surgical blade, and intact BM was embedded and frozen in OCT (VWR, Batavia, IL). BM sections were cut with a cryostat microtome, photographic images of stained sections were captured with a Leica DM IRB microscope (Leica Microsystems, Buffalo Grove, IL) and Magnafire 2.1C camera system and software.
Isolation of cells from BM fat
BM fat obtained after centrifugation of total BM at 300g for 10min, was cultured overnight, and then pipetted up and down to release cells. Red blood cells were lysed and the remaining cells were used in in vitro experiments. CD11b+ and CD11b− cells were separated using an autoMACs Pro separator.
Statistical Analysis
Data were obtained and displayed as indicated in the figure legends. Statistical analysis of all experiments was performed using Prism software (GraphPad Software; La Jolla, Ca). Statistical tests performed include unpaired two-tailed Student’s t test or analysis of variance (ANOVA) in combination with Dunnet’s or Bonferroni’s test for multiple comparisons. * P≤0.05, ** P≤0.01, *** P≤0.001, **** P≤0.0001.
Results
Developmental changes in rabbit bone marrow fat and myeloid cells
B lymphopoiesis in rabbit BM arrests by 2 to 4 months of age (17), and in mice, we found in vitro, that adipocytes inhibit B lymphopoiesis and also enhance myelopoiesis (23, 34). Rabbit BM contains large amounts of fat (35), and we sought to determine if the time of B lymphopoiesis arrest (17) correlates with the accumulation of fat. Analysis of H&E-stained BM sections from 3 wk-old rabbits, when B lymphopoiesis is ongoing, and from 2 to 13-month-old rabbits, after the arrest of B lymphopoiesis, revealed a dramatic increase in fat spaces by 2–3 months of age (Figure 1A). This large amount of fat was apparent in all rabbits (n=5) >2 months of age. To test if the BM of >2-month-old rabbits with large fat deposits display an increase in myeloid lineage cells, we stained BM for CD11b+ cells. By flow cytometry, we found significantly more CD11b+ cells in rabbits >2 months of age than in younger rabbits (Figure 1B). Further analysis of these cells revealed that the expression of CD11b was higher in the >2-month-old rabbits (Figure 1C), similar to the myeloid cells (CD11bhiGr1+) generated in mouse BM cultures treated with adipocyte factors (23). Immunofluorescent analysis of BM sections revealed that the CD11bhi cells in older rabbits are localized near large fat spaces (Supplementary Figure 1). We conclude that at the time B lymphopoiesis arrests, the BM is filled with fat and with increased CD11bhi myeloid cells in close proximity to the fat.
Figure 1. Comparison of fat and myeloid cells in rabbit BM before and after the decline in B lymphopoiesis.
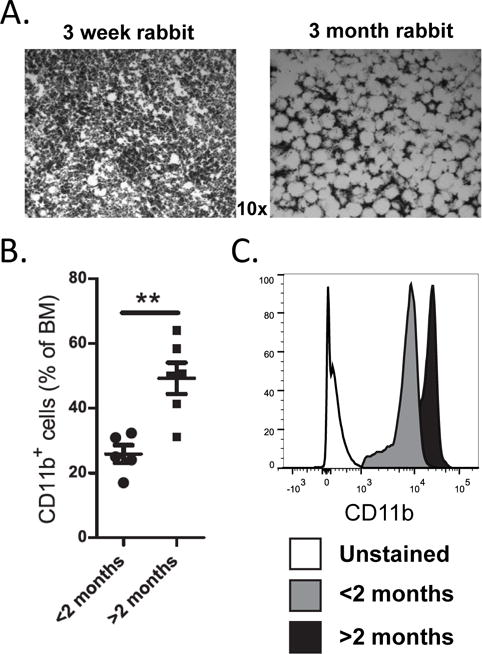
(A) Representative H&E-stained BM sections from femurs of 3-week-old (ongoing B lymphopoiesis) and 3-month-old (arrested B lymphopoiesis) rabbits. Fat is visualized as white spaces. (B and C) BM was analyzed by flow cytometry for CD11b+ cells. (B) Percentage of CD11b+ cells in BM of <2-month-old rabbits (circles) and >2-month-old rabbits (squares) n=11. (C) Representative flow cytometric analysis of gated CD11b+ cells from a <2-month-old rabbit (ongoing B lymphopoiesis) and a >2-month-old rabbit (arrested B lymphopoiesis). Significance in (B) was determined by Student’s t test. Error bars represent the average ±SD.
IL-1β expression in BM of >2-month-old rabbits
IL-1 is a downstream effector molecule responsible for the adipocyte-driven inhibition of B lymphopoiesis, in vitro (23). To test if expression of IL-1β increases with age in rabbit BM, we performed qRT-PCR analysis of BM from <2- and >2-month-old rabbits. As expected, BM from >2-month-old rabbits exhibited highly increased expression of IL-1β in CD11b+ cells, but not in CD11b− cells (Figure 2). We conclude that at the time B lymphopoiesis arrests, rabbit BM exhibits increased levels of IL-1β derived from the myeloid compartment.
Figure 2. qRT-PCR analysis of IL-1β expression in rabbit BM.
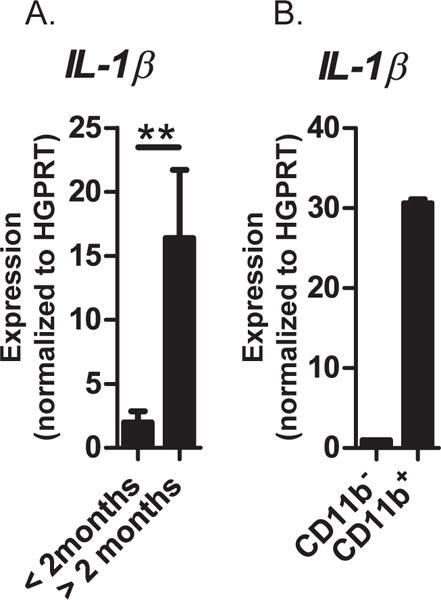
(A) BM from rabbits <2- and >2-month-old; Data are from n = 7; significance was determined by Student’s t test. Error bars represent the average ±SD. (B) CD11b+ and CD11b− cells isolated from BM of >2-month-old rabbits. Data are the average of 3 independent experiments. Expression for each sample was normalized to the HGPRT housekeeping gene.
Inhibition of B lymphopoiesis by BM fat
In vitro differentiated adipocytes produce factors that inhibit B lymphopoiesis (23), and we tested if the fat that accumulates in the BM also inhibits B lymphopoiesis. Fat from >2-month-old rabbits was cultured to generate conditioned medium (BM fat-CM) (Figure 3A), and was added to rabbit B lymphopoiesis cultures of BM cells with OP9 stromal cells in the presence of IL-7, SCF, and Flt3L. Compared to untreated cultures, BM fat-CM-treated cultures produced significantly fewer CD79a+ B lineage cells (Figure 3B–D), demonstrating that BM fat from >2-month-old rabbits indeed inhibits B lymphopoiesis. In mouse BM cultures, the inhibition of B lymphopoiesis by adipocytes is accompanied by an increase in CD11b+ myeloid cells (23), and here, we find that fat from rabbit BM also induces the generation of CD11b+ cells (Figure 3C&E). Because CD11b+ myeloid-derived suppressor cells (MDSCs) inhibit B lymphopoiesis (23), and because large numbers of CD11b+ cells are associated with fat in the bone marrow (Supplementary Figure 1), we tested if the CD11b+ cells associated with fat inhibit B lymphopoiesis. We disrupted BM fat to release associated cells, and by flow cytometry, most of the cells (~60%) were CD11b+ myeloid cells (data not shown). Purified CD11b+ and CD11b− cells were added to BM cultures, and compared to cultures containing CD11b− cells, significantly fewer B lineage (CD79a+) cells developed in the cultures with CD11b+ cells isolated from either BM fat or the BM adipocyte-free pellet (Figure 3F). We conclude show that CD11b+ myeloid cells within the BM inhibit rabbit B lymphopoiesis in vitro.
Figure 3. Inhibition of B lymphopoiesis and induction of myelopoiesis by BM fat-derived molecules.
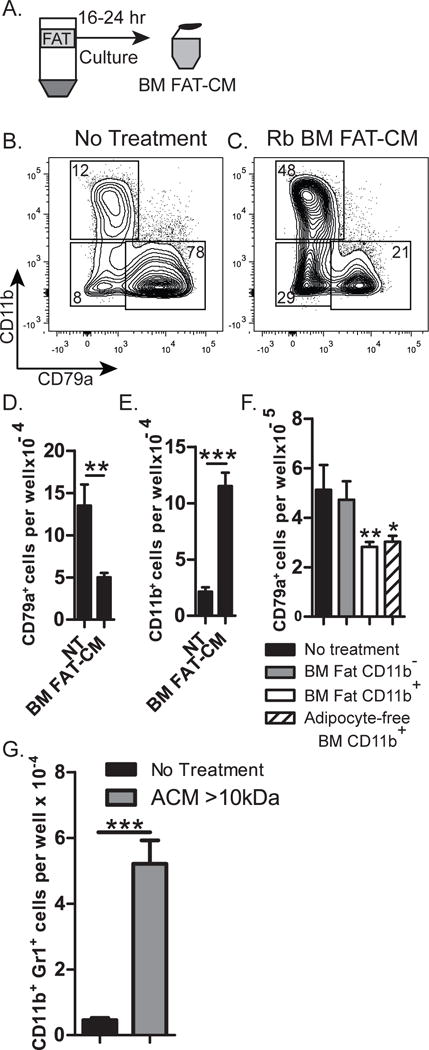
(A) Diagram of adipocyte layer isolated from centrifuged BM of >2-month-old-rabbits, and cultured for 16–24 hr to produce BM FAT-CM, which was added to BM cultures. (B and C) Flow cytometric analysis of cells from untreated cultures (no treatment) or cultures treated with BM FAT-CM, and stained for expression of CD79a and CD11b. (D&E) Number of (D) CD79a+ or (E) CD11b+ cells from untreated cultures (NT) or cultures treated with BM FAT-CM. Data are representative of four independent experiments. Statistical significance was determined using Student’s t test. Error bars represent the average of triplicate wells ±SD. (F) Rabbit BM progenitors (10,000) were cultured on OP9 stromal cells in the presence of IL-7, SCF, and Flt3L, with 10,000 of the following effector cells from BM of >2-month-old rabbits: CD11b− or CD11b+cells from BM fat; or CD11b+ cells from the adipocyte free pellet. Statistical significance compared to untreated samples was determined by ANOVA coupled with the Dunnet multiple comparison test (p<0.0001). Data are representative of 3 independent experiments. Error bars represent the average of triplicate wells ±SD. (G) ACM >10kDa was added to cultures of mouse B220− BM cells on OP9 stromal cells, and the number of CD11b+Gr1+ cells was assessed at the end of culture. Data are representative of three independent experiments. Statistical significance was determined using Students t test. Error bars represent the average of triplicate wells ±SD.
Inflammasome activation promotes development of CD11b+ MDSCs
In mouse BM cultures, adipocyte conditioned-medium (ACM) induces the accumulation of CD11b+ MDSCs, which inhibit B lymphopoiesis (23). Due to a lack of reagents to further study this in rabbit, we used mouse BM cultures to elucidate how adipocytes induce inhibitory MDSCs. ACM was fractionated using a 10kDa filter, and we found that ACM >10kDa induced MDSCs (Figure 3G), whereas ACM <10kDa did not (data not shown), indicating that the adipocyte molecules responsible for inducing MDSCs are >10kDa. Adipocytes produce numerous inflammatory molecules, including S100A8, S100A9 (30, 31), complement C3a (36), and lipid products which act as danger-associated molecular patterns (DAMPS) (37–41), and are implicated in NLRP3 inflammasome activation. We tested if blocking the NLRP3 inflammasome could prevent MDSC induction by adding glybenclamide, an inhibitor of NLRP3 inflammasome activation (42–47), to BM cultures with ACM (>10kDa). The cultures with glybenclamide had fewer CD11b+Gr1+ cells than cultures with ACM alone (Figure 4A&C), suggesting that the development of MDSCs is mediated through the NLRP3 inflammasome. Interestingly, the reduction in MDSCs in glybenclamide-treated cultures resulted in an increase in B220+ B lineage cells (Figure 4 B&D). The restoration of B lymphopoiesis to control levels was not complete in all experiments, which could be due to the presence of adipocyte factors that act directly on B lineage progenitors. Alternatively, higher concentrations of glybenclamide may be needed to completely restore B lymphopoiesis. We conclude that in vitro, glybenclamide inhibits the development of MDSCs by ACM, leading to enhanced B cell development.
Figure 4. Effect of glybenclamide treatment on ACM induction of CD11b+ Gr1+ MDSCs and inhibition of B lymphopoiesis.
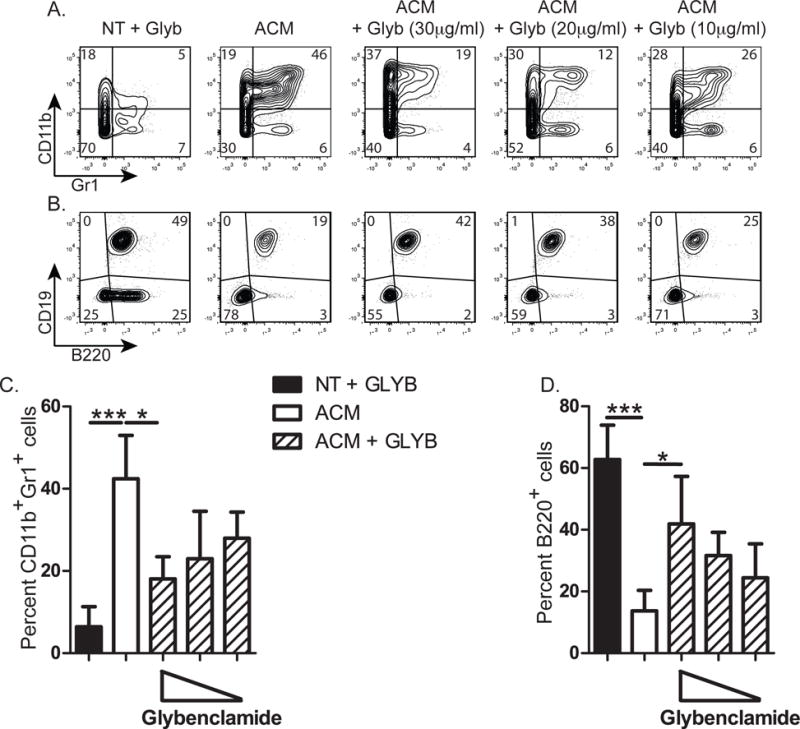
Untreated (NT) or ACM (>10kDa)-treated mouse B lymphopoiesis cultures were performed in the presence or absence of glybenclamide, and the production of CD11b+Gr1+ and B220+ cells was assessed. Representative flow cytometry profiles of cells collected at the end of culture and stained for expression of (A) CD11b and Gr1 or (B) CD19 and B220. (C and D) Percentage of (C) CD11b+Gr1+ or (D) B220+ B lineage cells resulting from the indicated treatments. Data are representative of 3 to 4 individual experiments per condition. Significance was determined by ANOVA in combination with a Bonferroni multiple comparison test (p=0.0005 and p=0.0001 for (C) and (D) respectively). Error bars represent the average ±SD.
S100A9 as an inflammatory molecule that inhibits B lymphopoiesis
Several inflammatory molecules produced by adipose tissue, including IL-1, IL-6, and TNFα are described as negative regulators of B lymphopoiesis in mammals (22–28). Two other known inflammatory molecules, S100A8 and S100A9 are produced by adipose tissue and we tested if they also negatively regulate B lymphopoiesis by treating mouse B lymphopoiesis cultures with recombinant S100A8 or S100A9. We found a significant decrease in B220+ B lineage cells compared to untreated cultures in the presence of either S100A8 or S100A9 (Figure 5 A & B). In addition, significantly more CD11b+Gr1+ myeloid cells were found in cultures with S100A8 and S100A9 (Figure 5 C&D), similar to cultures treated with the inflammatory molecule, IL-1 (23). We conclude that both S100A8 and S100A9 inhibit B lymphopoiesis and promote myeloid cell development/survival in vitro.
Figure 5. Inhibitory effect of S100A8 and S100A9 on B lymphopoiesis.
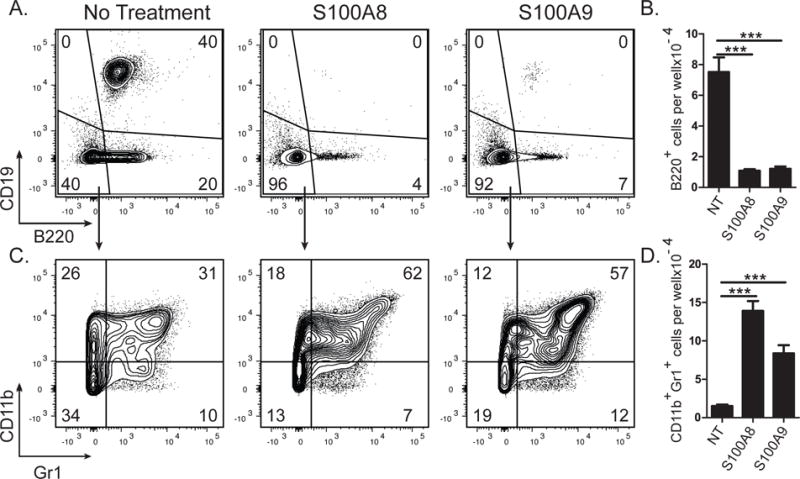
S100A8 or S100A9 was added to mouse B lymphopoiesis cultures. Representative flow cytometric analysis of cells collected after no treatment, S100A8, or S100A9 (5μg/ml) treatment, stained for B220 and CD19 (A) or CD11b and Gr1 (C). Number of (B) B220+ or (D) CD11b+Gr1+ cells. Data are representative of 3 independent experiments. Statistical significance in (B&D) was determined by ANOVA coupled with the Dunnet multiple comparison test (p<0.0001 and p<0.0001 respectively). Error bars represent the average of triplicate wells ±SD.
If the adipocyte-produced molecules, S100A8 and S100A9, contribute to the loss of B lymphopoiesis in rabbits, we expected to find elevated levels of these factors in fatty BM from >2-month-old rabbits which have arrested B lymphopoiesis. We profiled rabbit BM by flow cytometry using an anti-S100A8/S100A9 Ab and found an increase in CD11b+S100A8/S100A9+ myeloid cells in BM of older rabbits (Figure 6 A&B). Because the anti-S100A8/S100A9 Ab does not discriminate between S100A8 and S100A9, we performed qRT-PCR and found that expression of S100A9, but not S100A8 was increased in BM of rabbits >2 months old (Figure 6 C&D), suggesting that S100A9 may contribute to the arrest of B lymphopoiesis in rabbits >2 months-of-age.
Figure 6. Quantitation of S100A8 and S100A9 in BM of rabbits before and after the arrest of B lymphopoiesis.
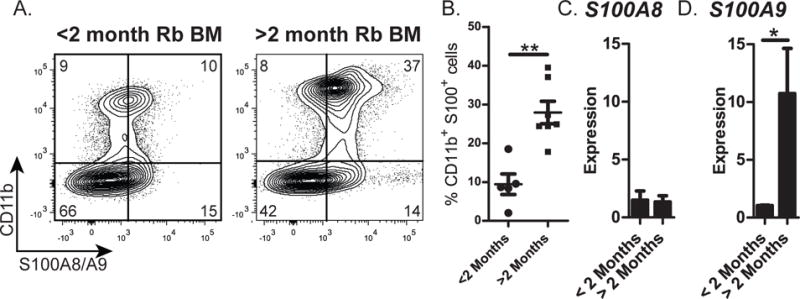
Rabbit BM nucleated cells were obtained from the adipocyte-free pellet and analyzed for the expression of S100A8 and S100A9. (A) Representative flow cytometry profiles of BM cells stained for the expression of CD11b (surface) and S100A8/A9 (intracellular). (B) Percentage of CD11b+S100A8/A9+ cells in BM of <2-month-old and >2-month-old rabbits. Data were from n=12. Error bars represent the average ±SD. (C and D) qRT-PCR of BM cells from rabbits >2- and < 2-months-of-age for expression of (C) S100A8 and (D) S100A9. Expression was normalized to HGPRT housekeeping gene. Data are the average from three independent experiments. Error bars represent the average ±SD. (B-D) Statistical significance was determined by Student’s t test.
Myeloid cells as target of S100A9
IL-1 inhibits B cell development in vitro at the multi-potent progenitor (MPP) stage, and we tested if S100A9 also inhibits B cell development and promotes myeloid cell development/survival by targeting the MPP stage. FACS-sorted HSCs, MPPs, or CLPs (data not shown) were cultured on OP9 cells in the presence or absence of S100A9, and unlike IL-1, S100A9 did not inhibit B lymphopoiesis in any of the cultures (data not shown). We conclude that S100A9 does not directly target progenitors with B-lineage potential, i.e., HSCs, MPPs, or CLPs. Because S100A9 inhibited B lymphopoiesis in BM cultures (Figure 5) which contained not only B-lineage progenitors, but also other hematopoietic lineage cells, including CD11b+Gr1+ myeloid cells, we hypothesized that S100A9 targeted a non-B-lineage cell. Mature myeloid cells are known to produce pro-inflammatory cytokines in response to S100A9 (48), and we hypothesized that the target of S100A9 is myeloid cells. We tested this by culturing CD11b+Gr1+ myeloid cells from mouse BM (Figure 7A) with S100A9 and used qRT-PCR to quantify the expression of IL-1β, NLRP3, TNFα and IL-6, inflammatory mediators known to inhibit B lymphopoiesis. Indeed, S100A9 treatment induced an early burst in expression of all four of these inflammatory mediators, with increases observed as early as 2 hr after treatment, and generally peaked at 4 hr. We conclude that S100A9 functions through myeloid lineage cells to produce molecules that inhibit B lymphopoiesis.
Figure 7. Cytokine expression by CD11b+Gr1+ myeloid progenitors after treatment with S100A9.
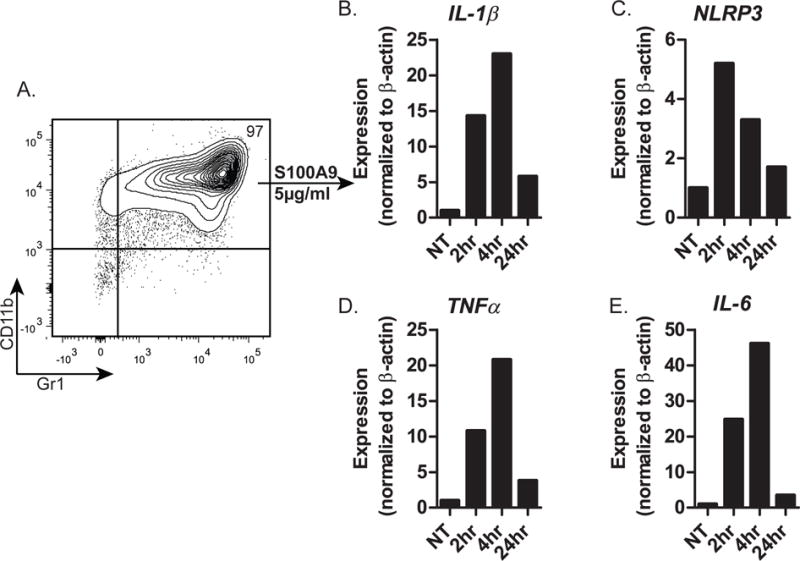
(A) Flow cytometric profile (CD11b and Gr1 expression) of magnetic bead-purified CD11b+ mouse BM cells. (B-E) qRT-PCR of CD11b+Gr1+ cells for expression of (B) IL-1β, (C) NLRP3, (D) TNFα, and (E) IL-6 at the indicated time after treatment of CD11b+Gr1+ myeloid cells with S100A9. Data are representative of 3 independent experiments.
Because IL-1 producing myeloid cells potently inhibit B lymphopoiesis (23) we tested if the inhibitory effect of S100A9 is mediated solely through IL-1 activity. B lymphopoiesis cultures were treated with S100A9 in the presence or absence of IL-1RA to block IL-1 activity. Compared to S100A9 alone, cultures containing IL-1RA had an increased percentage and number of B lineage cells (Figure 8A &B). While neutralizing IL-1 activity increased B lymphopoiesis, it did not fully restore B lineage numbers to untreated controls, suggesting that IL-1 partially mediates the inhibitory effect of S100A9. We conclude that S100A9 inhibits B lymphopoiesis by inducing the production of IL-1, as well as other inflammatory molecules.
Figure 8. Effect of IL-1 in S100A9 treated cultures.
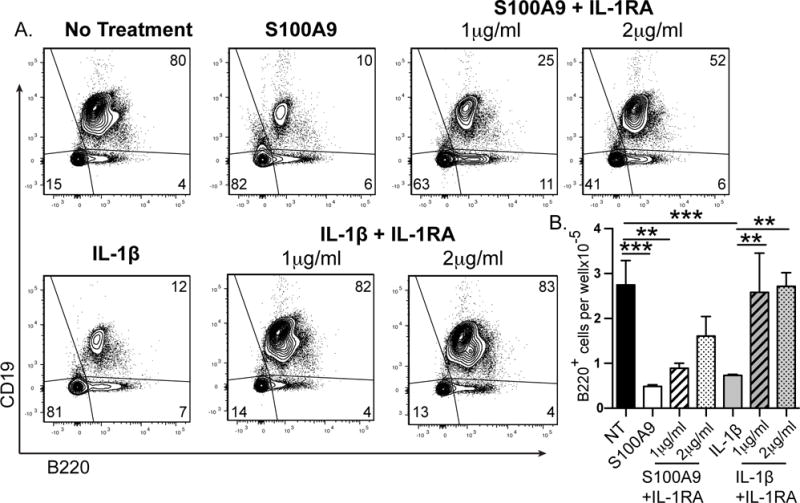
S100A9 was added to mouse B lymphopoiesis cultures in the presence or absence of IL-1RA. Representative flow cytometric analysis of cells collected after no treatment or S100A9 (1μg/ml) treatment with or without IL-1RA, stained for B220 and CD19 (A). Number of (B) B220+ cells. Data are representative of 2 independent experiments. Statistical significance in (B) was determined by ANOVA coupled with the Bonferroni multiple comparison test (p<0.0001). Error bars represent the average of triplicate wells ±SD.
Discussion
Rabbit B lymphopoiesis declines at an accelerated rate compared to that of elderly humans and aged mice (29, 49–56). In this study, we found that the arrest of B lymphopoiesis in young rabbits is associated with increased fat, myeloid cells, and inflammatory molecules, conditions similar to those seen in BM of aged humans and mice (4, 10–15, 19, 21, 57–59). From in vitro studies, we suggest that BM fat induces and activates myeloid cells to produce inflammatory molecules, which in turn, inhibit B lymphopoiesis (Figure 9). Previously, we found that in vitro-differentiated adipocytes produce factors that inhibit B lymphopoiesis in BM cultures (23, 60), and because the loss of B lymphopoiesis correlates with an increase in BM fat, we tested if the fat directly inhibited B lymphopoiesis in vitro. Not only did the fat inhibit B lymphopoiesis, but it also promoted myelopoiesis, recapitulating the BM landscape after B lymphopoiesis declines. Large numbers of CD11b+ myeloid cells reside within the fat, and these cells inhibit B lymphopoiesis, as might be expected since adipocyte factors induce inhibitory myeloid cells.
Figure 9. Depiction of rabbit BM before and after the arrest of B lymphopoiesis.
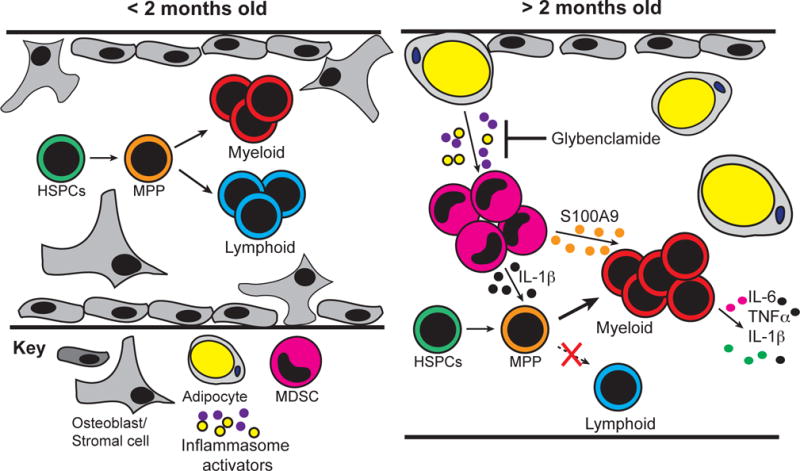
BM from <2-month-old rabbits is characterized by the presence of osteoblasts (support B lymphopoiesis) and B lineage progenitors. By 2–4 months-of-age the BM microenvironment transforms, and no longer supports B lineage development. The BM of >2-month-old rabbits fills with adipocytes and myeloid cells, which produce inflammatory mediators that specialize the BM for myelopoiesis.
The idea of inhibitory cells in BM was first discussed in the 1980’s when Soderberg and colleagues identified two types of “suppressor cells” in rabbit BM. One of these was adherent, and had macrophage-like morphology (61), similar to the suppressive cells that we found within the CD11b+ myeloid fraction, potentially MDSCs. The other suppressor cells were described as non-adherent, FcRγ+, complement receptor negative, and were thought to be suppressor lymphocytes (61, 62). If so, based on current knowledge, these cells could be Treg cells, IL-10-producing Bregs (63), or inflammatory TNF-producing aged B cells (ABCs) (27). We suggest that the 2nd “suppressor cell” of Soderberg (61) is likely an ABC because these cells are known to inhibit B cell development and to be increased in BM of aged mice (27). MDCSs are also increased in aged BM (4) and if they produce IL-1, we expect this would synergize with TNF (28) to skew the BM toward myelopoiesis instead of lymphopoiesis. These studies highlight the importance of regulatory cells in the BM which, in addition to stromal cells can regulate the microenvironment and B lymphopoiesis.
Our study suggests that the arrest of B lymphopoiesis in rabbits is primarily mediated through extrinsic changes to the BM microenvironment. Analysis of BM sections from rabbits of various ages showed that adipocytes accumulate by 3 months of age, the same time frame that B lymphopoiesis arrests in rabbits (two to four months of age) (17). This microenvironmental change was found to impair B lymphopoiesis, as molecules from BM fat inhibited B lymphopoiesis in vitro. Consistent with this finding, hematopoietic progenitor cells from older rabbits developed into B lineage cells after transfer into the microenvironment of young irradiated recipients (18). Although intrinsic defects in hematopoietic progenitors appear to contribute to the decline of B lymphopoiesis in aged mice (11, 64, 65), several studies show that changes to the BM microenvironment in aged mice can impair B lymphopoiesis (5, 6, 66, 67). Because in rabbits, the arrest of B lymphopoiesis is accelerated at a young age, we suggest that the rabbit is a valuable model for studying how changes in the BM microenvironment affect hematopoiesis during aging. Also, because the accumulation of BM fat in rabbits appears to mimic that of aged humans, where 40–50% of the proximal femur and 70% of the tibia fill with adipose tissue (58), rabbits should be a good model to understand microenvironmental changes in elderly humans.
Analysis of BM sections clearly showed that myeloid cells are in close contact with adipocytes in older rabbits, suggesting that these cells may be influenced by adipocyte products. In fact, these myeloid cells express IL-1 and inhibit B lymphopoiesis, characteristics similar to inhibitory myeloid cells generated in mouse (MDSCs) and rabbit BM cultures after treatment with adipocyte factors (23). To elucidate the adipocyte-derived molecules that induce inhibitory myeloid cells, we studied mice instead of rabbits because of the plethora of reagents available for mice. As others have found (68), we identified several molecules, including IL-6, IL-12, G-CSF, and TNFα that induce/activate MDSCs (data not shown). Many of these factors are inflammatory molecules, and rabbit BM adipocytes likely produce similar inflammatory molecules. The large adipocytes in BM of older rabbits are similar to the large adipocytes found in obesity, cells known to induce/activate myeloid cells to produce inflammatory molecules (69–71). If fact, we found that the BM myeloid cells from older rabbits produce high levels of inflammatory IL-1β and S100A9, molecules that contribute to an inflammatory environment.
Our study raises the possibility that by preventing the generation of inhibitory myeloid cells and inflammation, B lymphopoiesis could be enhanced. A major pathway that contributes to inflammation in aging and obesity is through inflammasome activation (40, 72). The NLRP3 inflammasome, for example, is critical for integrating danger signals that accumulate during aging and obesity, and for triggering active IL-1 production by myeloid lineage cells. Since adipocyte-derived molecules induce IL-1 producing myeloid cells (23), it is likely that inflammasome activation is involved in the adipocyte-mediated induction of MDSCs and the loss of B lymphopoiesis. We found that blocking the NLRP3 inflammasome with glybenclamide indeed, inhibited MDSC accumulation, and boosted B lymphopoiesis in vitro. Similarly, in mice, deletion of NLRP3 prevented thymic atrophy and the decline of T lymphopoiesis (41). These data, in combination with our study, implicate NLRP3 inflammasome activation in the negative regulation of both B and T lymphopoiesis and suggest that targeting the inflammasome pathway could be an effective means to enhance production of naïve B cells. We did not test glybenclamide in vivo because the doses needed to block the inflammasome could result in hypoglycemia if used on non-diabetic hosts (73) However, alternative strategies to reduce NLRP3 inflammasome activation or the use of glybenclamide in elderly patients with type 2 diabetes could prove useful as a treatment to prevent the induction of inflammasome-activated MDSCs, and lead to enhanced B cell development.
Two recently appreciated inflammatory cytokines, S100A8 and S100A9 are produced by adipose tissue and their expression increases during aging and obesity (30, 32). We found that the expression of S100A9 in rabbit BM increased as fat and myeloid cells accumulated and B lymphopoiesis declined. While the expression of S100A8 did not appear to change, this could be because we only analyzed the adipocyte-free BM pellet, and S100A8 is produced by mature adipocytes (30, 31). The capacity of both S100A8 and S100A9 to inhibit B lymphopoiesis further supports the idea that inflammatory factors negatively regulate B cell development. S100A9 did not act on HSCs or MPPs as did IL-1, but instead induced the expression of IL-6, TNF, and IL-1β in BM myeloid cells. One interpretation of these data is that S100A9 acts to amplify inflammation, a known role of S100A9 in inflammatory processes (74). In fact, one study found that while S100A9 monomers are relatively unstable, stimulation of cells with the inflammatory factors IL-1β and TNFα resulted in the formation of S100A9 homodimers that were highly stable and resistant to proteolytic digestion (75). In combination with our findings, these data suggest that S100A9 inhibits B lymphopoiesis indirectly by acting through BM myeloid cells to amplify the production of inflammatory factors, which in turn, inhibit B cell development.
Concluding statement
The rapid decline of B lymphopoiesis in rabbits corresponds with dramatic changes to the BM microenvironment. The large increase in fat and myeloid cells seen at the time B lymphopoiesis arrests, results in an inflammatory environment that appears to skew hematopoiesis toward myelopoiesis and to impair B lymphopoiesis. We suggest that rabbits can be used as an accelerated model to study BM changes in humans that lead to skewing of hematopoiesis toward myelopoiesis.
Supplementary Material
Footnotes
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number AI068390, and by the National Institute On Aging of the National Institutes of Health under award number F31AG047817.
References
- 1.Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10:330–335. doi: 10.1016/j.arr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enioutina EY, Bareyan D, Daynes RA. A role for immature myeloid cells in immune senescence. J Immunol. 2011;186:697–707. doi: 10.4049/jimmunol.1002987. [DOI] [PubMed] [Google Scholar]
- 5.Miller JP, Allman D. Linking age-related defects in B lymphopoiesis to the aging of hematopoietic stem cells. Semin Immunol. 2005;17:321–329. doi: 10.1016/j.smim.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 7.Rego EM, Garcia AB, Viana SR, Falcao RP. Age-related changes of lymphocyte subsets in normal bone marrow biopsies. Cytometry. 1998;34:22–29. doi: 10.1002/(sici)1097-0320(19980215)34:1<22::aid-cyto4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Nunez C, Nishimoto N, Gartland GL, Billips LG, Burrows PD, Kubagawa H, Cooper MD. B cells are generated throughout life in humans. J Immunol. 1996;156:866–872. [PubMed] [Google Scholar]
- 9.McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–2507. doi: 10.1182/blood.v98.8.2498. [DOI] [PubMed] [Google Scholar]
- 10.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–552. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuljapurkar SR, McGuire TR, Brusnahan SK, Jackson JD, Garvin KL, Kessinger MA, Lane JT, O’Kane BJ, Sharp JG. Changes in human bone marrow fat content associated with changes in hematopoietic stem cell numbers and cytokine levels with aging. J Anat. 2011;219:574–581. doi: 10.1111/j.1469-7580.2011.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskinazi DP, Knight KL, Dray S. Kinetics of escape from suppression of Ig heavy chain allotypes in multiheterozygous rabbits. Eur J Immunol. 1979;9:276–283. doi: 10.1002/eji.1830090406. [DOI] [PubMed] [Google Scholar]
- 17.Jasper PJ, Zhai SK, Kalis SL, Kingzette M, Knight KL. B lymphocyte development in rabbit: progenitor B cells and waning of B lymphopoiesis. J Immunol. 2003;171:6372–6380. doi: 10.4049/jimmunol.171.12.6372. [DOI] [PubMed] [Google Scholar]
- 18.Kalis SL, Zhai SK, Yam PC, Witte PL, Knight KL. Suppression of B lymphopoiesis at a lymphoid progenitor stage in adult rabbits. Int Immunol. 2007;19:801–811. doi: 10.1093/intimm/dxm048. [DOI] [PubMed] [Google Scholar]
- 19.Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17:7–19. doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 21.Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longev Healthspan. 2013;2:8-2395-2-8. doi: 10.1186/2046-2395-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorshkind K. IL-1 inhibits B cell differentiation in long term bone marrow cultures. J Immunol. 1988;141:531–538. [PubMed] [Google Scholar]
- 23.Kennedy DE, Knight KL. Inhibition of B Lymphopoiesis by Adipocytes and IL-1-Producing Myeloid-Derived Suppressor Cells. J Immunol. 2015;195:2666–2674. doi: 10.4049/jimmunol.1500957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirayama F, Clark SC, Ogawa M. Negative regulation of early B lymphopoiesis by interleukin 3 and interleukin 1 alpha. Proc Natl Acad Sci U S A. 1994;91:469–473. doi: 10.1073/pnas.91.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda K, Baba Y, Nagai Y, Miyazaki K, Malykhin A, Nakamura K, Kincade PW, Sakaguchi N, Coggeshall KM. IL-6 blocks a discrete early step in lymphopoiesis. Blood. 2005;106:879–885. doi: 10.1182/blood-2005-02-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda K, Malykhin A, Teague-Weber BN, Sun XH, Farris AD, Coggeshall KM. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6.Sle1.Yaa animals. Blood. 2009;113:4534–4540. doi: 10.1182/blood-2008-12-192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In senescence, age-associated B cells secrete TNFalpha and inhibit survival of B-cell precursors. Aging Cell. 2013;12:303–311. doi: 10.1111/acel.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy DE, Witte PL, Knight KL. Bone marrow fat and the decline of B lymphopoiesis in rabbits. Dev Comp Immunol. 2016;58:30–39. doi: 10.1016/j.dci.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekimoto R, Kishida K, Nakatsuji H, Nakagawa T, Funahashi T, Shimomura I. High circulating levels of S100A8/A9 complex (calprotectin) in male Japanese with abdominal adiposity and dysregulated expression of S100A8 and S100A9 in adipose tissues of obese mice. Biochem Biophys Res Commun. 2012;419:782–789. doi: 10.1016/j.bbrc.2012.02.102. [DOI] [PubMed] [Google Scholar]
- 31.Hiuge-Shimizu A, Maeda N, Hirata A, Nakatsuji H, Nakamura K, Okuno A, Kihara S, Funahashi T, Shimomura I. Dynamic changes of adiponectin and S100A8 levels by the selective peroxisome proliferator-activated receptor-gamma agonist rivoglitazone. Arterioscler Thromb Vasc Biol. 2011;31:792–799. doi: 10.1161/ATVBAHA.110.221747. [DOI] [PubMed] [Google Scholar]
- 32.Swindell WR, Johnston A, Xing X, Little A, Robichaud P, Voorhees JJ, Fisher G, Gudjonsson JE. Robust shifts in S100a9 expression with aging: a novel mechanism for chronic inflammation. Sci Rep. 2013;3:1215. doi: 10.1038/srep01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neal JW, Clipstone NA. Calcineurin mediates the calcium-dependent inhibition of adipocyte differentiation in 3T3-L1 cells. J Biol Chem. 2002;277:49776–49781. doi: 10.1074/jbc.M207913200. [DOI] [PubMed] [Google Scholar]
- 34.Bilwani FA, Knight KL. Adipocyte-derived soluble factor(s) inhibits early stages of B lymphopoiesis. J Immunol. 2012;189:4379–4386. doi: 10.4049/jimmunol.1201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bigelow CL, Tavassoli M. Fatty involution of bone marrow in rabbits. Acta Anat (Basel) 1984;118:60–64. doi: 10.1159/000145823. [DOI] [PubMed] [Google Scholar]
- 36.Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Kohl J, Cook HT, Kemper C. C3a modulates IL-1beta secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122:3473–3481. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- 37.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 38.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, Munzberg H, Rosen CJ, Ingram DK, Salbaum JM, Dixit VD. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youm YH, Kanneganti TD, Vandanmagsar B, Zhu X, Ravussin A, Adijiang A, Owen JS, Thomas MJ, Francis J, Parks JS, Dixit VD. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 2012;1:56–68. doi: 10.1016/j.celrep.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henriksbo BD, Lau TC, Cavallari JF, Denou E, Chi W, Lally JS, Crane JD, Duggan BM, Foley KP, Fullerton MD, Tarnopolsky MA, Steinberg GR, Schertzer JD. Fluvastatin causes NLRP3 inflammasome-mediated adipose insulin resistance. Diabetes. 2014;63:3742–3747. doi: 10.2337/db13-1398. [DOI] [PubMed] [Google Scholar]
- 44.Laliberte RE, Eggler J, Gabel CA. ATP treatment of human monocytes promotes caspase-1 maturation and externalization. J Biol Chem. 1999;274:36944–36951. doi: 10.1074/jbc.274.52.36944. [DOI] [PubMed] [Google Scholar]
- 45.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, Corradin G, Tschopp J. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen CC, Tsai SH, Lu CC, Hu ST, Wu TS, Huang TT, Said-Sadier N, Ojcius DM, Lai HC. Activation of an NLRP3 inflammasome restricts Mycobacterium kansasii infection. PLoS One. 2012;7:e36292. doi: 10.1371/journal.pone.0036292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tavares AH, Magalhaes KG, Almeida RD, Correa R, Burgel PH, Bocca AL. NLRP3 inflammasome activation by Paracoccidioides brasiliensis. PLoS Negl Trop Dis. 2013;7:e2595. doi: 10.1371/journal.pntd.0002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simard JC, Cesaro A, Chapeton-Montes J, Tardif M, Antoine F, Girard D, Tessier PA. S100A8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS-dependent activation of NF-kappaB(1.) PLoS One. 2013;8:e72138. doi: 10.1371/journal.pone.0072138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jasper PJ, Zhai SK, Kalis SL, Kingzette M, Knight KL. B lymphocyte development in rabbit: progenitor B cells and waning of B lymphopoiesis. J Immunol. 2003;171:6372–6380. doi: 10.4049/jimmunol.171.12.6372. [DOI] [PubMed] [Google Scholar]
- 50.McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–2507. doi: 10.1182/blood.v98.8.2498. [DOI] [PubMed] [Google Scholar]
- 51.Stephan RP, V, Sanders M, Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. Int Immunol. 1996;8:509–518. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- 52.Kirman I, Zhao K, Wang Y, Szabo P, Telford W, Weksler ME. Increased apoptosis of bone marrow pre-B cells in old mice associated with their low number. Int Immunol. 1998;10:1385–1392. doi: 10.1093/intimm/10.9.1385. [DOI] [PubMed] [Google Scholar]
- 53.Riley RL, Kruger MG, Elia J. B cell precursors are decreased in senescent BALB/c mice, but retain normal mitotic activity in vivo and in vitro. Clin Immunol Immunopathol. 1991;59:301–313. doi: 10.1016/0090-1229(91)90026-7. [DOI] [PubMed] [Google Scholar]
- 54.Sherwood EM, Blomberg BB, Xu W, Warner CA, Riley RL. Senescent BALB/c mice exhibit decreased expression of lambda5 surrogate light chains and reduced development within the pre-B cell compartment. J Immunol. 1998;161:4472–4475. [PubMed] [Google Scholar]
- 55.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 56.Miller JP, Allman D. Linking age-related defects in B lymphopoiesis to the aging of hematopoietic stem cells. Semin Immunol. 2005;17:321–329. doi: 10.1016/j.smim.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 58.Li GW, Xu Z, Chen QW, Chang SX, Tian YN, Fan JZ. The temporal characterization of marrow lipids and adipocytes in a rabbit model of glucocorticoid-induced osteoporosis. Skeletal Radiol. 2013;42:1235–1244. doi: 10.1007/s00256-013-1659-7. [DOI] [PubMed] [Google Scholar]
- 59.Lecka-Czernik B, Rosen CJ, Kawai M. Skeletal aging and the adipocyte program: New insights from an “old” molecule. Cell Cycle. 2010;9:3648–3654. doi: 10.4161/cc.9.18.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bilwani FA, Knight KL. Adipocyte-derived soluble factor(s) inhibits early stages of B lymphopoiesis. J Immunol. 2012;189:4379–4386. doi: 10.4049/jimmunol.1201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soderberg LS. Differential activities of rabbit bone marrow suppressor cells. Int Arch Allergy Appl Immunol. 1984;74:341–346. doi: 10.1159/000233570. [DOI] [PubMed] [Google Scholar]
- 62.Soderberg LS. Regulation of constitutive bone marrow cell proliferation by bone marrow suppressor cells. Int Arch Allergy Appl Immunol. 1984;74:305–310. doi: 10.1159/000233565. [DOI] [PubMed] [Google Scholar]
- 63.Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194:1395–1401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
- 64.Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–1609. [PubMed] [Google Scholar]
- 65.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J, Astle CM, Harrison DE. Development and aging of primitive hematopoietic stem cells in BALB/cBy mice. Exp Hematol. 1999;27:928–935. doi: 10.1016/s0301-472x(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 67.Labrie JE, 3rd, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 70.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 71.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. 2015;8:15–27. doi: 10.2147/JIR.S51250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cesaro A, Anceriz N, Plante A, Page N, Tardif MR, Tessier PA. An inflammation loop orchestrated by S100A9 and calprotectin is critical for development of arthritis. PLoS One. 2012;7:e45478. doi: 10.1371/journal.pone.0045478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riva M, He Z, Kallberg E, Ivars F, Leanderson T. Human S100A9 protein is stabilized by inflammatory stimuli via the formation of proteolytically-resistant homodimers. PLoS One. 2013;8:e61832. doi: 10.1371/journal.pone.0061832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


