Abstract
We examined in live coronal slices from rat and mouse which brain regions generate potassium-triggered spreading depolarization (SDKt). This technique simulates cortical spreading depression, which underlies migraine aura in the intact brain. An SDKt episode was evoked by increasing bath [K+]o and recorded as a propagating front of elevated light transmittance representing transient neuronal swelling in gray matter of neocortex, hippocampus, striatum, and thalamus. In contrast, SDKt was not imaged in hypothalamic nuclei or brainstem with exception of those nuclei near the dorsal brainstem surface. In rat slices, single neurons were whole-cell current clamped during SDKt. “Higher” neurons depolarized to near zero millivolts indicating SDKt generation. In contrast, seven types of neurons in hypothalamus and brainstem only slowly depolarized without generating SDKt, supporting our imaging findings. Therefore, SDKt is not a default of CNS neurons but rather displays a region-specific susceptibility, similar to anoxic depolarization, which we have proposed is correlated with a region’s vulnerability to traumatic brain injury. In the higher brain, SDKt may be a vestigial spreading depolarization that originally evolved to shut down and vasoconstrict gray matter regions more exposed to impact and contusion.
Keywords: Brainstem, ischemia, migraine aura, spreading depolarization, traumatic brain injury
Introduction
Spreading depolarization (SD) is a sudden and profound loss of membrane potential by neurons and neighboring astrocytes that migrates at 1.7–9.2 mm/min across gray matter.1,2 It is important clinically because SD can arise repeatedly following focal stroke, subarachnoid hemorrhage, or traumatic brain injury (TBI) to expand the original focal damage.3,4 In live brain slices under simulated ischemia (oxygen/glucose deprivation, OGD), a propagating wave of anoxic depolarization (AD) arises, which leaves neurons damaged in its wake.5–7 Under less metabolic stress, higher gray matter supports cortical spreading depolarization (CSD), which can be experimentally evoked in vivo and in slices by elevating [K+]o. This “potassium-triggered spreading depolarization” (SDKt) is the common technique to evoke CSD-like events.8–10 CSD is responsible for the marching sensory deficit that often dominates migraine aura although CSD itself does not induce neural injury. AD and SDKt represent extremes along a continuum of SD6,11 whereby ultimate neuronal injury is dependent upon the metabolic state of the gray matter through which the SD event propagates.11–14 Several studies indicate that more rostral brain regions are more susceptible to ischemia15,16 and generate stronger AD.17–20 In this article, we examine whether this is also true regarding SDKt and if there are regional differences in the ability to inhibit SDKt in the presence of the glutamate receptor antagonist kynurenate. Live brain slices allow study of the intrinsic propensity of neurons to generate AD because potential variability in blood flow among brain regions is not a factor. SDKt can be induced repeatedly by brief bath elevation of [K+]o in rat hippocampal slices,21 in human neocortical slices22 and in submerged slices from rat neocortex.23,24 Therefore, as observed in vivo, SDKt is not damaging to neurons in brain slices.
The propensity for SDKt and ischemic SD generation in human neocortex could be hypothesized to be a necessary consequence of higher network complexity that leads to electrical instability. However, it is important to note that SD has evolved in the insect CNS to promote survival, likely arising more than 200 million years before mammals even existed. On a gram-for-gram basis, it can be argued that the insect brain is as complex and sophisticated as a human’s brain. SD in the locust25,26 and in Drosophila27 is physiologically similar to mammals and is evoked by high temperature or by anoxia, inducing a recoverable coma as neurons depolarize to zero millivolts in a propagating fashion. Three benefits to these insects result. First, sudden electrical silence avoids uncoordinated behavior and hyperactivity that will arise from neuronal depolarization as the Na/K pump begins to fail. The only other option to induce electrical silence would be a widespread hyperpolarization, but that in itself is an energy-consuming process. Second with complete depolarization, production of reactive oxygen species is reduced because oxygen is not being consumed. But with return of oxygen, reactive oxygen species may then dramatically increase, so SD can be considered a “delay” tactic in this regard. Third, the enforced inactivity lowers the metabolic rate, so that the Na/K pump can recover. And indeed insects easily “awake” from their comatosed state after a few minutes.
Along similar lines, we have proposed that mammalian SD promotes a “lie-low” survival strategy by promoting sudden loss of consciousness following TBI whereby the chances of epileptiform activity and brain hemorrhage are reduced and metabolism is lowered.19,28 Immediately upon head trauma from a predator, from warfare or from falling out of a tree, shut-down is a good default behavior. It is better to be quiescent when injured than to be moving in a slow or epileptiform fashion which advertises injury. Somjen29 has noted that “It is easy to imagine that a blow to the head could trigger SD at once in many areas of the brain, perhaps including subcortical nuclei, rendering the victim unconscious.” A temporary shutdown of the higher gray matter with vasoconstriction would have been a reasonable default strategy to help survive head injury throughout our vertebrate evolution. In contrast, stroke and sudden cardiac arrest occur primarily in the aging population. Sudden loss of blood flow to the brain has become an issue only over the past 200 years as the human life span has increased beyond 40 years.30 In these cases, our brain may be misinterpreting lost blood flow as hemorrhage. The result is ischemia-induced failure of the Na/K pump causing a propagating electrical shutdown and vasoconstriction in higher gray matter, whether the cause is focal stroke, subarachnoid hemorrhage or global ischemia.
It has been argued that migraine pain must have been beneficial for survival to be so prevalent today.31 But because migraine pain imparts prolonged agony and incapacitation, we think that a survival advantage is questionable. Rather, as with neuropathic pain, migraine pain can be considered a natural defense mechanism that has spiraled out of control and so lacks evolutionary benefit. There is good evidence that the CSD driving migraine aura can evoke the headache that follows.9,10 Here we examine the regional susceptibility of the brain to generate SDKt and whether that susceptibility emulates the propensity of higher brain neurons to generate strong AD in comparison with lower brain gray matter.19,20 If SDKt is a weaker or residual version of SD, it follows that lower brain regions being more protected from TBI by bone and higher brain (even early in vertebrate evolution) should be less susceptible to SDKt.
Methods
Neocortical slice preparation
Male C57 Black mice or Sprague-Dawley rats, 21–50 days old (Charles River, St. Constant, Quebec) were cared for in accordance with the principles and guidelines of the Canadian Council on Animal Care as overseen by the Queen’s University Animal Care Committee. This study complied with the ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments). Rodents were housed in a controlled environment (22 ± 1℃, 12 h light:12 h dark cycle) with food (Purina rat chow) and water supplied ad libitum. Rats were placed in a rodent restrainer (DecapiCone; Braintree Scientific, Inc.) and guillotined. The brain was excised and placed in ice-cold oxygenated (95% O2–5% CO2) artificial cerebrospinal fluid (aCSF) with NaCl replaced by sucrose. Coronal slices (400 µm) were taken using a vibrating blade microtome (Leica VT1000S). Five to seven slices were transferred to a submerged net in a beaker containing regular aCSF gassed with O2–CO2. The slices were then slowly warmed over 1 h to 32℃ prior to experimentation at 33–34℃.
Experimental solutions and drugs
The aCSF contained (in mM) 120 NaCl, 3.3 KCl, 26 NaHCO3, 1.3 MgSO4 7H2O, 1.2 NaH2PO4, 1.8 CaCl2, and 11D-glucose. All were dissolved in double-distilled water at pH 7.3–7.4. The aCSF was used for incubation and as a vehicle to administer experimental solutions. For high-K+ aCSF, 9.6, 26, or 52 mM KCl replaced equimolar NaCl. Kynurenic acid (2 mM) was added to control aCSF or to elevated-KCl ACSF as required. All drugs were from the Sigma-Aldrich. During an experiment, a slice were submerged in oxygenated aCSF flowing at a rate of 3–4 ml/min at 33–34℃.
Imaging intrinsic optical signals
Changes in light transmittance (LT) through live brain slices are generated as LT is increased by brain cell swelling or reduced by dendritic beading which increases light scatter.32,33 Using previously described techniques,6,23 ΔLT was monitored and recorded. Graphical and statistical analyses of data were carried out using Excel. Images were imported and figures were prepared using CorelDraw. A ΔLT value from each brain region of interest (ROI) is an average of that region (∼500 µm2). The data from the imaging experiments were analyzed such that changes in LT of a given ROI were expressed as percent changes of the Tcont for that region, taken from the control image. This normalized the graphical data across the different regions of the neocortex, which was necessary because of the variation in opacity that causes different initial LT values (Tcont). The means were calculated and reported using a paired Student’s t-test for statistical analysis.
Electrophysiology
Micropipettes were pulled from borosilicate glass (OD 1.2 mm, ID 0.68 mm; World Precision Instruments) to a resistance of 3–6 MΩ. The internal pipette solution contained (in mM) 125 K-gluconate, 10 KCl, 2 MgCl2, 5.5 EGTA, 10 HEPES, 2 Na-ATP, and 0.1 CaCl2 (pH was adjusted to 7.3 with KOH). A 14 mV junction potential was corrected prior to achieving whole-cell configuration. Whole-cell patched neurons were analyzed if they displayed stable resting membrane potentials and if the series resistance could be sufficiently compensated. Recordings were from neurons visualized with near-infrared illumination and Dodt gradient contrast optics (Luigs and Neumann, Ratingen, Germany) through an upright microscope (Axoscope 2FS, Zeiss) with a × 40 immersion objective lens. Neurons were recorded from neocortical layers 2–3, striatum, thalamus, the hypothalamus (supraoptic nucleus, paraventricular nucleus, suprachiasmatic nucleus), the mesencephalic nucleus (MES) of midbrain-pons, locus coeruleus (LC) of midbrain-pons, dorsal nucleus solitarius (SolN) of medulla oblongata, and dorsal motor nucleus (DMV) of medulla oblongata. All recordings were acquired in current clamp mode of an Axoclamp 2 A amplifier and a Digidata 1322 A/D converter. Clampex 10 software was used for data acquisition with subsequent analysis using Clampfit 10 software. Sampling frequency was 10 kHz and low pass filtering was with an external Bessel filter (LPF 202 a) at 2 kHz (Molecular Devices). Tabulated data of the electrophysiological properties of higher and lower neurons exposed to OGD can be found at http://hdl.handle.net/1974/7578. A subset of these neurons for this study were first exposed to elevated [K+]o, and their properties are listed in Table 1. All of these neurons returned to their baseline properties following the brief K+ exposure. Electrophysiological data were presented as means ± standard deviation.
Table 1.
Whole-cell parameters during 2–3 min of [K+]o elevated to 52 mM recorded from “higher” pyramidal neurons (PYR) in neocortex and CA1 region as well as from spiny neurons in striatum (total n = 14).
| Neuron type | n | Rmp (mV) | AP ampl (mV) | Rin (MΩ) | Depol rate (mV/min) | SDKt rate (mV/s) |
|---|---|---|---|---|---|---|
| Neocortex PYR | 5 | Mean −82 | 78 | 82 | NA | 10 to 25 |
| SD ± 2.7 | 7.9 | 12.1 | ||||
| CA1 PYR | 5 | mean −73 | 82 | 97 | NA | 3 to 22 |
| SD ± 2.5 | 13.1 | 17.8 | ||||
| Striatum | 4 | mean −71 | 80 | 54 | NA | 12 to 30 |
| SD ± 4 | 10.5 | 15.2 | ||||
| Hypothal MNC | 1 | −62 | 94 | 569 | 15 | NA |
| Hypothal Parvo | 1 | −51 | 70 | 778 | 18 | NA |
| Hypothal SCN | 1 | −50 | 80 | 676 | 15 | NA |
| Midbrain MES | 1 | −54 | 77 | 52 | 20 | NA |
| Midbrain LC | 1 | −48 | 89 | 181 | 20 | NA |
| Medulla SolN | 1 | −47 | 94 | 720 | 17.5 | NA |
| Medulla DVM | 1 | −48 | 98 | 284 | 17.5 | NA |
These values are compared with “lower” neurons in hypothalamus and brainstem neurons (n = 7). All higher neurons undergo SDKt while lower neurons only slowly depolarize at a mean rate of 17.6 ± 2.0 mV/min.
Results
Imaging spreading depression
To generally assess the regional propensity of the CNS to undergo spreading depression, we exposed live coronal brain slices to elevated [K+]o while imaging changes in light transmittance (ΔLT). Elevating bath [K+]o to 26 mM for 3 to 5 min evokes a front of spreading depression (SDKt) in the mouse brain slice (n = 14 mice). The increased LT is caused by the swelling of neurons as the cells depolarize with astrocytes swelling secondarily.34 The SDKt front (Figure 1(a), arrows) propagates in neocortex (NC) and hippocampus (Hp) but much less so in the brainstem with the exception of dorsal brainstem gray specifically, the superficial superior colliculus (Figure 1(a), SC), superficial inferior colliculi (IC) and periaqueductal gray (Figure 1(a), PAG), solitary nucleus (SolN), and tegmental nucleus (TN). All regions recover from SDKt-associated swelling.
Figure 1.

Elevating bath [K+]o to 26 mM for 3 min triggers a front of spreading depolarization (SDKt) in the mouse brain slice. (a) The increased light transmittance (LT) is caused first by the swelling of neurons and then by astrocytes as they secondarily take up K+.24,34 The SDKt front (arrows) propagates in neocortex (NC) and hippocampus (Hp) but not in the brainstem with the exception of the superficial colliculi (SC) and periaqueductal Continued.
gray (PAG). All regions recover from SDKt-associated swelling. (b) Changes in light transmittance (ΔLT) plotted in different brain region of interest in response to elevating [K+]o to 26 mM. Each peak represents the SDKt front. Responses are normalized to account for regional differences in slice translucency. (c) Mean LT values plotted across three additional CNS regions, specifically cerebellar cortex (Cbell), inferior colliculus (IC), and tegmental nucleus (TN). There are no significant differences for the LT changes with kynurenic acid pre-treatment (not plotted, but see Figure 3).
SDKt was induced by exposing coronal mouse brain slices to 26 mM K+ aCSF for 3–5 min. Figure 1(b) and 1(c) illustrates several regional ΔLT time courses during simultaneous SDKt events. The CA1 region (46.5 ± 10.1%) and cerebellar cortex (54.0 ± 20.8%) had a significantly higher mean LT peak compared with other regions (*p < 0.05) (Figure 2(a)). The mean LT peak in SC was 34.9 ± 11.3% which was similar to the neocortex (33.9 ± 14.4%). PAG had a mean LT peak of 29.3 ± 17.6%. In addition, there was little difference in the mean LT peak among IC (32.4 ± 11.3%), TN (30.1 ± 7.3%), and SolN (32.0 ± 13.0%).
Figure 2.
K+-induced (26 mM) SDKt properties across different brain regions with or without 2 mM kynurenate pre-treatment. (a) SDKt–associated peak changes in light transmittance. Kynurenate blocks SDKt in PAG, tegmental nucleus (TN), and solitary nucleus (SolN) but does not affect peak signal strengths in the other regions. (b) K+-induced SDKt onset latency in minutes. Where SDKt is observed, onset latency is consistent across brain regions. Kynurenate pre-treatment does not significantly alter the time to onset. (c) The SDKt propagation rate (mm/min) in neocortex and the hippocampal CA1 region is significantly slowed by kynurenate pre-treatment (*p < 0.001) but not in other regions where it can initiate and propagate.
There was no significant difference in SDKt onset time among the responding regions (Figure 2(b)). The SDKt propagation rate in brainstem nuclei (Figure 2(c)) was 1.9 ± 1.2 mm/min in SC, 1.4 ± 1.5 mm/min in IC, 0.7 ± 0.2 mm/min in PAG, and 0.5 ± 0.2 mm/min in TN. These rates were significantly slower than the higher brain regions of neocortex (4.1 ± 0.8 mm/min), CA1 (3.4 ± 2.0 mm/min), and cerebellar cortex (2.6 ± 2.3 mm/min). SDKt spread through SC and IC at similar speeds. PAG, TN, and IC displayed significantly slower SDKt compared with neocortex (p < 0.05) (Figure 2(c)). Comparatively, the propagation rate was neocortex > CA1 > cerebellar cortex > SC > IC > PAG > TN. The small size of the solitary nuclei made it difficult to measure a rate.
Kynurenic acid did not significantly affect the mean peak LT in neocortex (n = 4), CA1 (n = 3), SC (n = 7), cerebellar cortex (n = 6), or IC (n = 4) (number of mice = 7) (Figure 2(a)). SDKt onset in the cerebellar cortex and neocortex were delayed by kynurenate compared with control (*p < 0.05). Kynurenic acid did not alter SDKt onset in CA1, SC, or IC (Figure 2(b)). In addition, the SDKt propagation rate tended to decrease in most regions including neocortex, CA1, SC, cerebellar cortex, and IC but only the propagation in neocortex and the CA1 region was significantly different (*p < 0.001) (Figure 2(c)). In some cerebellar and IC slices, we detected increases in LT but without obvious SDKt propagation (not shown). No SDKt was observed after kynurenate pre-treatment in PAG (6/6 slices) and SolN (5/5 slices). Only 1/9 TN slices displayed a signal with kynurenic acid treatment. The distinct LT increases in brainstem nuclei that emulated higher gray matter were only observed near the dorsal surface of brainstem and not in hypothalamus.
Intracellular recordings
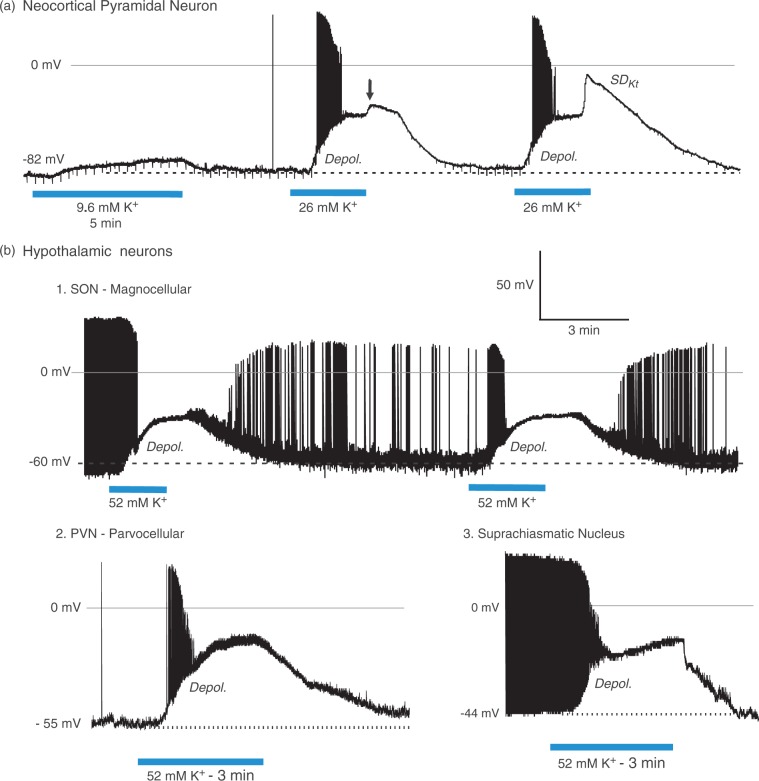
As described in numerous previous studies, pyramidal neurons in layers II–III of the neocortex depolarize in response to elevated [K+]o, leading to spreading depression (SDKt) that approaches zero millivolts (Figure 3(a)). In response to an initial 9.6 mM K+ exposure, a pyramidal cell passively depolarizes slightly and repolarizes in control aCSF. Exposure to 26 mM K+ elicits more depolarization with firing and then spike inactivation, before a plateau of −48 mV is reached. SDKt onset is aborted just as control aCSF reaches the slice (arrow). A second exposure again evokes firing and spike inactivation, reaching a plateau at −48 mV. A steep secondary depolarization signals SDKt onset. The membrane potential again returns to resting level after aCSF return. In contrast, hypothalamic neurons slowly depolarize without the active SDKt event (Figure 3(b1)). A magnocellular neuron in the supraoptic nucleus (SON) depolarizes in 52 mM K+ aCSF. No SDKt event that approaches zero millivolts is evoked. After each exposure, the cell recovers. In Figure 3(b2), a parvocellular neuron in paraventricular nucleus (PVN) depolarizes in 52 mM [K+]o aCSF. Action potentials inactivate while slowly reaching a plateau but no SDKt is rises. With return to control aCSF, it repolarizes. Similarly, a suprachiasmatic nucleus (SCN) neuron responds to 3 min of 52 mM [K+]o and depolarizes to a plateau as action potentials inactivate (Figure 3(b3)), but does not generate SDKt.
Figure 3.
Pyramidal neurons in layers II–III of the neocortex depolarize in response to elevated [K+]o, leading to SDKt that approaches zero millivolts. In contrast, hypothalamic neurons slowly depolarize without the active SDKt event. (a1) In response to 5-min 9.6 mM K+, a pyramidal cell passively depolarizes slightly to −73 mV then repolarizes in control aCSF. Exposure to 26 mM K+ elicits more depolarization with action potential firing and then spike inactivation, before a plateau of −48 mV is reached. SDKt onset is aborted just as control aCSF reaches the slice (arrow). A second exposure to 26 mM K+ again evokes firing and spike inactivation, reaching a plateau at −48 mV. A steep depolarization then coincides with SDKt onset. Membrane potential again returns to resting level after aCSF return. (b1) A magnocellular neuron in the supraoptic nucleus (SON) depolarizes to −35 and −34 mV in response to 3 - and 4-min superfusion of 52 mM K+, respectively. No full-blown SDKt event approaching zero millivolts is evoked. After each exposure the cell recovers, albeit with reduced action potential amplitude. (b2) A parvocellular neuron in paraventricular nucleus (PVN) depolarizes in response to 52 mM [K+]o exposure. Action potentials inactivate before slowly reaching a plateau at a level well below zero millivolts. No SDKt is generated. Following return of control aCSF, it repolarizes to near its original resting potential. (b3) An neuron in suprachiasmatic nucleus (SCN) responds to 3 min of 52 mM [K+]o. As in B1 and B2, it depolarizes to a plateau as action potentials inactivate, but does not generate SDKt. Back in control aCSF, the neuron returns to the original resting potential. Figure modified from literature.35,36
Exposing a neostriatal neuron to 100 s of 52 mM [K+]o results in a rapid depolarization to near zero millivolts (Figure 4(a1)). A brief plateau at −30 mV (arrow) occurs before a second more rapid depolarization to near zero millivolts, typical of SDKt. Upon return of control aCSF, the membrane potential recovers. This contrasts to the typical response to oxygen/glucose deprivation by striatal neurons (Figure 4(a2)), where a typical “higher” neuron response is a rapid, complete, and irreversible “anoxic” depolarization (AD). In midbrain slices, a 3-min exposure to 52 mM [K+]o elicited depolarization of a mesencephalic neuron (MES, n = 3) to −19 mV with loss of action potential firing (Figure 4(b1)). No SDKt is generated unlike in Figure 4(a1). Upon return of control aCSF, MES neurons return to their original resting membrane potential. Similarly in a locus ceruleus neuron, 3 min of 52 mM [K+]o elicits depolarization and spike inactivation to −11 mV but no SDKt (Figure 4(b2)). Upon return of control aCSF, the LC neuron repolarizes.
Figure 4.
(a1) Striatal neuron’s response to 52 mM [K+]o. The neuron rapidly depolarizes, reaching a brief plateau (arrow) before undergoing SD. After depolarizing to near-zero millivolts, return of control aCSF allowed repolarization to baseline potential. (a2) A different striatal neuron undergoes anoxic depolarization (AD) in response to oxygen/glucose deprivation (OGD). Typically, no recovery follows 10 min OGD by higher brain neurons. (b1) MES neuron response to 3 min of 52 mM [K+]o. The neuron depolarizes, reaching a plateau of ∼ −20 mV as action potentials inactivate. No SDKt is generated. Upon return of control aCSF, the neuron returns to near-baseline. (b2) LC neuron response to 3 min of 52 mM [K+]o. It plateaus but does not generate the additional depolarization of SDKt. Upon return to control aCSF. the neuron recovers. Figure modified from literature.36,37
Figure 5 further contrasts a higher brain region (thalamus) with brainstem recordings from slices of medulla. A thalamic neuron responds to 120 s of 52 mM [K+]o with a quick depolarization to near zero millivolts (Figure 5(a1)), typical of SDKt in higher neurons. The membrane potential returns to baseline upon return of control aCSF (not shown). As in Figure 4(a2), thalamic neurons also rapidly undergo AD when exposed to OGD (Figure 5(a2)). In lower neurons, a 3-min exposure to 52 mM [K+]o elicits slow depolarization of a dNTS neuron with loss of action potential firing, before reaching a plateau of −30 mV (Figure 5(b1)). No SDKt is generated. With return of control aCSF, the SolN neuron repolarizes. Similarly, DVM neurons depolarize during 3 min of 52 mM [K+]o, reaching a plateau of −29 ± 2.1 mV as firing stops (n = 8). Upon return of control aCSF, all DMV neurons repolarized to their original resting levels. The DMV neuron in Figure 5(b2) was subjected to two separate 52 mM [K+]o exposures (3 min) that reached respective plateaus of −32 and −28 mV. As with the hypothalamic and brainstem neurons recorded in this study, no SDKt was generated (Table 1). The rate of depolarization from baseline to plateau by seven lower neurons during 2 min of 52 mM KCl exposure was 17.5 ± 2.0 mV/min. That is exceeding slow compared with the SDKt rate generated by higher neurons, which, once underway, ranges between 3 and 30 mV/s, as shown in Table 1.
Figure 5.
(a1) Thalamic neuron’s response to 52 mM [K+]o. The neuron rapidly depolarizes, smoothly transitioning to SDKt. (a2) A second striatal neuron undergoes anoxic depolarization (AD) in response to oxygen/glucose deprivation (OGD). No recovery follows 10 min OGD. (b1) A brainstem neuron from the solitary nucleus (SolN) responds to 3 min of 52 mM [K+]o. It depolarizes to a plateau as action potentials inactivate but SDKt is not generated. Upon return of control aCSF, the neuron repolarizes to near-baseline. (b2) A DMV neuron responds to two 3-min exposures of 52 mM [K+]o. It gradually depolarizes, reaching a plateau as action potentials inactivate. No SDKt is generated. Following return to control aCSF, the neuron repolarizes and fires. During a second exposure of 52 mM [K+]o, the neuron again plateaus without generating SDKt. Back in control aCSF, it repolarizes. Figure modified from literature.35,36
Discussion
Recurring SD may impart some neuroprotection against further ischemic stress by preconditioning38 or by promoting plasticity and regeneration as reviewed by Dreier.3 However, in general, SD has been considered a pathological process by clinicians and basic researchers alike. This is understandable because migraine aura can lead to terrible pain; ischemic SD can lead to neuronal death. On a different tack, we have proposed that the initial anoxic (ischemic) SD is protective immediately following TBI, because it causes a rapid behavioral shut-down as well as a vasoconstriction. We suggest that SD has evolved distant in our vertebrate past. Because occlusive stroke and cardiac arrest have only become prevalent recently as the human lifespan has increased,19,20 sudden loss of blood flow in these non-traumatic cases will elicit SD and vasoconstriction (inverse neurovascular coupling) as though the brain region is hemorrhaging from TBI.26 This may explain why SD during ischemia is robust in higher brain regions that are most exposed to impact. It follows that ischemic SD should be weaker and recoverable in the lower brain, and indeed that appears to be the case as reported here. Although not yet shown directly, it is likely that an ischemic depolarization in the brainstem, even though delayed and weaker than in higher brain, could disrupt respiration and prove deadly.39,40
A less metabolically stressful version of SD is cortical spreading depression (CSD), which generates migraine aura.10 This event can be evoked by elevating [K+]o, termed K+-triggered spreading depolarization (SDKt).11,24,34 The current study shows that compared with high brain regions, SDKt is likewise weak or non-existent in the nuclei of hypothalamus and brainstem. This is demonstrated both by LT imaging as well as intracellular recording as discussed below.
With regard to imaging SDKt, all higher brain regions showed a robust front. Indeed, there are clinical correlates of SDKt, depending on whether it propagates in neocortex, hippocampus, striatum, or thalamus.9,10 In contrast, hypothalamic and more ventral brainstem nuclei (including regions in which we subsequently whole-cell recorded) showed no spreading signal in slices, although LT could transiently and weakly increase during elevated [K+]o. Only dorsal brainstem regions displayed a propagating LT front when [K+]o was elevated, specifically in the superficial colliculi, PAG, tegmental nuclei, and solitary nuclei. From an evolutionary perspective, these dorsal regions would have been more exposed to trauma, especially prior to expansion of the cerebral hemispheres in mammals and birds. In contrast, we found that hypothalamus and ventral brainstem do not generate SDKt. In line with the findings here, hypothalamus and brainstem gray show only weak and recoverable AD in response to OGD.19,20,35,37
Brain regions displayed K+-induced SDKt properties that varied in a rostral-caudal fashion. The most obvious difference was the propagation rate, which was faster in higher brain regions. In lower brain nuclei if a propagating signal was detected, the onset time was similar to higher regions. Also the strength of the peak signal at the propagating front was not significantly different among regions, except for the hippocampal CA1 region and cerebellar cortex where the higher signal likely reflected more concentrated neuronal packing. Pre-treatment with the general glutamate receptor antagonist kynurenate (2 mM) had no significant effect on the peak LT signal nor on the onset latency of the spreading front in all brain regions. However, the front’s propagation rate was consistently slowed in all regions if a spreading signal was observed.
Recording intracellularly from select neuronal types supported the imaging data. In the neocortex, striatum, and thalamus, 1–2 min of 26 or 52 mM [K+]o caused an initial slow depolarization over several seconds that suddenly transitioned to a rapid depolarization that quickly approached zero millivolts i.e., classic spreading depression. Lower brain neurons did not undergo this additional rapid depolarization, despite longer exposure (3 min) to 52 mM [K+]o. This is consistent with previous recordings in SON of hypothalamus and in brainstem of the adult rat35,37 where neurons did not generate SDKt
The role of swelling in SD
Both astrocytes and neurons swell during AD33,41 and SDKt.34 Astrocytic swelling is more easily explained because their surface is studded with water channels that mediate passive water flux across their plasma membranes.42 As [K+]o rises during SD, K+ and Cl− are taken up followed by osmotic water. But with CNS neurons, swelling is more complicated because their plasma membrane lacks functioning aquaporins and so they do not passively swell or shrink over many minutes33,41 across a range of osmolalities (−80 to +80 milliosmoles). Osmotic stress can acutely arise over a short period of time in a number of clinical situations.43,44 The accumulation of water within neurons under metabolic stress occurs in at least two general ways. First, many ion and amino acid co-transporters, most energetically linked to the Na/K pump, move water across plasma membrane as they carry out their normal functions,45–47 even against its concentration gradient.48 Second, water is a catabolic by-product of metabolism and if the water pumping noted above fails as ischemia develops, the lack of neuronal aquaporins means accumulating water is trapped intracellularly. The result during metabolic stress is neuronal swelling because water cotransporters are disrupted so residual water produced would remain in the neuron.
Why does SD show a regional susceptibility?
The question arises as to why SD in higher gray matter is stronger and less reversible than in the lower gray matter. There is no obvious architectural reason why higher and lower regions differ in susceptibility to SD. Also there are no data indicating that higher brain regions consume more energy per unit volume than lower regions. Nor is there any evidence that a cortical neuron requires more energy than a brainstem neuron nor that neurons from higher vs. lower regions systematically differ in their ion channel composition. We think it more likely that there is a regional difference involving the molecular biology of Na+/K+ pump failure and the ensuing depolarization that results. The physiological cause of SD is pump failure or alternately, overriding the pump by raising [K+]o. One approach is to look for regional variations in the expression of molecular transporters known to play important roles in maintaining normal metabolism.
For example, in situ hybridization data mined from the Allen Mouse Brain Atlas shows the regional expression pattern of the ATP1α1 pump and the glutamate transporter SLC17a7 (Figure 6). Gene expression primarily in higher brain regions is high for these two transporters or their related proteins, suggesting a potential specific interaction between them. Rose et al.49 proposed that the Na+/K+ pump interacts through its γ-subunit because inhibiting the ATPase also partly inhibits the glutamate transporter. Such an interaction could promote extracellular glutamate accumulation during Na/K pump failure, extending ischemic injury in higher brain regions. Therefore, regional variation in acute ischemic injury may not simply involve an up- or down-regulation of metabolic pumps but also how they interact. In comparison to a wide variety of ligand and voltage-gated channels that have been proposed to malfunction in migraine and stroke (yielding little mechanistic insight), transporters have only been minimally studied.
Figure 6.
In situ hybridization data mined from the Allen Mouse Brain Atlas shows the regional expression pattern of the ATP1α1 pump and the glutamate transporter SLC17a7. Both transporters show high expression specifically in higher brain regions. The expression images highlight those cells with the highest probability of gene expression using a heat map color scale. The Expression Energy is calculated as follows: Within a given area A (voxel or structure), expression energy = (sum of intensity of expressing pixels in A)/(sum of all pixels in A). In the centre is depicted the crystal structures of a Na/K-ATPase and a glutamate transporter embedded in the cell membrane (modified from Rose and Koo49). They proposed an interaction through the γ-subunit by showing that inhibiting the Na/K-ATPase also partly inhibits the glutamate transporter. Such an interaction could promote extracellular glutamate accumulation during Na/K pump failure, thereby increasing ischemic injury in higher brain regions where these transporters are highly expressed.
In summary, SD may contribute to survival both at behavioral and neuronal levels and so be selected during vertebrate evolution for the same reason as in insects. Both the locust25,26 and fruit-fly27 use SD to temporarily shut down their nervous system when temperature is too high or too low or when anoxia sets in. Similarly, we propose that SD arising from TBI elicits electrical silence and vasoconstriction in higher gray matter to preserve metabolic energy, to avoid epileptiform activity and to reduce hemorrhage. From a behavioral standpoint, a sudden loss of consciousness following TBI is preferable to seizure or uncoordinated movement in terms of avoiding the attention of enemies or predators. As a milder version of SD, we propose that SDKt is a vestigial response to local metabolic stress, and so it is generated in TBI-prone brain regions but is rare in hypothalamus and ventral brainstem.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
RDA planned and supervised experiments and wrote the manuscript. Y-TH and CDB carried out experiments, analyzed data, prepared initial figures, and contributed to early manuscript drafts as part of their theses work.
References
- 1.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev 2001; 81: 1065–1096. [DOI] [PubMed] [Google Scholar]
- 2.Woitzik J, Hecht N, Pinczolits A, et al. Propagation of cortical spreading depolarization in the human cortex after malignant stroke. Neurology 2013; 80: 1095–1102. [DOI] [PubMed] [Google Scholar]
- 3.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011; 17: 439–447. [DOI] [PubMed] [Google Scholar]
- 4.Lauritzen M, Dreier JP, Fabricius M, et al. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab 2011; 31: 17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obeidat AS, Andrew RD. Spreading depression determines acute cellular damage in the hippocampal slice during oxygen/glucose deprivation. Eur J Neurosci 1998; 10: 3451–3461. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis CR, Anderson TR, Andrew RD. Anoxic depolarization mediates acute damage independent of glutamate in neocortical brain slices. Cereb Cortex (New York, NY 1991) 2001; 11: 249–259. [DOI] [PubMed] [Google Scholar]
- 7.Joshi I, Andrew RD. Imaging anoxic depolarization during ischemia-like conditions in the mouse hemi-brain slice. J Neurophysiol 2001; 85: 414–424. [DOI] [PubMed] [Google Scholar]
- 8.Anderson TR, Jarvis CR, Biedermann AJ, et al. Blocking the anoxic depolarization protects without functional compromise following simulated stroke in cortical brain slices. J Neurophysiol 2005; 93: 963–979. [DOI] [PubMed] [Google Scholar]
- 9.Pietrobon D, Moskowitz MA. Pathophysiology of Migraine. Annu Rev Physiol 2013; 75: 365–391. [DOI] [PubMed] [Google Scholar]
- 10.Eikermann-Haerter K, Negro A, Ayata C. Spreading depression and the clinical correlates of migraine. Rev Neurosci 2013; 24: 353–363. [DOI] [PubMed] [Google Scholar]
- 11.Hartings JA, Shuttleworth CW, Kirov S, et al. The continuum of spreading depolarizations in acute cortical lesion development: Examining Leão’s legacy. J Cereb Blood Flow Metab. 2017; 37: 1571–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leao AAP. Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiol 1947; 10: 409–414. [DOI] [PubMed] [Google Scholar]
- 13.Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron 2015; 86: 902–922. [DOI] [PubMed] [Google Scholar]
- 14.Marshall WH. Spreading cortical depression of Leao. Physiol Rev 1959; 39: 239–279. [DOI] [PubMed] [Google Scholar]
- 15.Longstreth WT. Neurological complications of cardiac arrest. In: Aminoff MJ. (ed). Neurology and general medicine, Philadelphia: Elsevier, 2008, pp. 163–183. [Google Scholar]
- 16.Sieber FE, Palmon SC, Traystman RJ, et al. Global incomplete cerebral ischemia produces predominantly cortical neuronal injury. Stroke 1995; 26: 2091–2095. discussion 2096. [DOI] [PubMed] [Google Scholar]
- 17.Bures J, Buresová O. Cerebral [K+]e increase as an index of the differential susceptibility of brain structures to terminal anoxia and electroconvulsive shock. J Neurobiol 1981; 12: 211–220. [DOI] [PubMed] [Google Scholar]
- 18.Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg 1999; 91: 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrew RD, Hsieh Y-T, Brisson CD. The persistent vegetative state: Evidence that the lower brain survives because its neurons intrinsically resist ischemia. In: Monti MM, Sannita WG. (eds). Brain function and responsiveness in disorders of consciousness, Switzerland: Springer International Publishing, 2016, pp. 119–144. [Google Scholar]
- 20.Brisson CD, Hsieh YT, Kim D, et al. Brainstem neurons survive the identical ischemic stress that kills higher neurons: Insight to the persistent vegetative state. PLoS ONE. 9(5): e96585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somjen GG, Aitken PG, Czeh GL, et al. Mechanisms of spreading depression - A review of recent findings and a hypothesis. Can J Physiol Pharmacol 1992; 70: S248–S254. [DOI] [PubMed] [Google Scholar]
- 22.Gorji A. Spreading depression: a review of the clinical relevance. Brain Res Rev 2001; 38: 33–60. [DOI] [PubMed] [Google Scholar]
- 23.Footitt DR, Newberry NR. Cortical spreading depression induces an LTP-like effect in rat neocortex in vitro. Brain Res 1998; 781: 339–342. [DOI] [PubMed] [Google Scholar]
- 24.Anderson TR, Andrew RD. Spreading depression: imaging and blockade in the rat neocortical brain slice. J Neurophysiol 2002; 88: 2713–2725. [DOI] [PubMed] [Google Scholar]
- 25.Hou N, Armstrong GA, Chakraborty-Chatterjee M, et al. Na+/K+-ATPase trafficking induced by heat shock pretreatment correlates with increased resistance to anoxia in locusts. J Neurophysiol 2014; 112: 814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spong KE, Rochon-Terry G, Money TG, et al. Disruption of the blood-brain barrier exacerbates spreading depression in the locust CNS. J Insect Physiol 2014; 66: 1–9. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong GA, Xiao C, Krill JL, et al. Glial Hsp70 protects K+ homeostasis in the Drosophila brain during repetitive anoxic depolarization. PLoS One 2011; 6: e28994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrew RD and Brisson CD. Trauma trumps stroke: Why is our higher brain inept at dealing with blocked blood flow? In: Vasospasm 2011: The 11th international conference on neurovascular events after subarachnoid hemorrhage, 2011, pp.311–312.
- 29.Somjen GG. Ions in the Brain. New York: Oxford University Press, 2004, p.268.
- 30.Holliday R. Evolution of human longevity, population pressure and the origins of warfare. Biogerontology 2005; 6: 363–368. [DOI] [PubMed] [Google Scholar]
- 31.Loder E. What is the evolutionary advantage of migraine? Cephalalgia 2002; 22: 624–632. [DOI] [PubMed] [Google Scholar]
- 32.Obeidat AS, Jarvis CR, Andrew RD. Glutamate does not mediate acute neuronal damage after spreading depression induced by O2/glucose deprivation in the hippocampal slice. J Cereb Blood Flow Metab 2000; 20: 412–422. [DOI] [PubMed] [Google Scholar]
- 33.Andrew RD, Labron MW, Boehnke SE, et al. Physiological evidence that pyramidal neurons lack functional water channels. Cereb Cortex (New York, NY 1991) 2007; 17: 787–802. [DOI] [PubMed] [Google Scholar]
- 34.Zhou N, Gordon GRJ, Feighan D, et al. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb Cortex 2010; 20: 2614–2624. [DOI] [PubMed] [Google Scholar]
- 35.Brisson CD, Lukewich MK, Andrew RD. A distinct boundary between the higher brain’s susceptibility to ischemia and the lower brain's resistance. PLoS One 2013; 18(11): e79589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brisson CD, Andrew RD. A neuronal population in hypothalamus that dramatically resists acute ischemic injury compared to neocortex. J Neurophysiol 2012; 108: 419–430. [DOI] [PubMed] [Google Scholar]
- 37.Andrew RD, Hsieh Y-T, Brisson DC. Projection neurons in brainstem and hypothalamus intrinsically resist acute stroke injury while projection neurons in cortex, striatum and thalamus die. Program No. 449.04. Neuroscience [2012] Abstracts, New Orleans, LA: Society for Neuroscience. Online, 2012. [Google Scholar]
- 38.Gniel HM, Martin RL. Cortical spreading depression-induced preconditioning in mouse neocortex is lamina specific. J Neurophysiol 2013; 109: 2923–2936. [DOI] [PubMed] [Google Scholar]
- 39.Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med 2015; 7: 282ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richter F, Bauer R, Lehmenkühler A, et al. The relationship between sudden severe hypoxia and ischemia-associated spreading depolarization in adult rat brainstem in vivo. Exp Neurol 2010; 224: 146–154. [DOI] [PubMed] [Google Scholar]
- 41.Risher WC, Andrew RD, Kirov SA. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia 2009; 57: 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amiry-Moghaddam M, Frydenlund DS, Ottersen OP. Anchoring of aquaporin-4 in brain: Molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience 2004; 129: 999–1010. [DOI] [PubMed] [Google Scholar]
- 43.Andrew RD, Fagan M, Ballyk BA, et al. Seizure susceptibility and the osmotic state. Brain Res 1989; 498: 175–180. [DOI] [PubMed] [Google Scholar]
- 44.Andrew RD. Seizure and acute osmotic change: clinical and neurophysiological aspects. J Neurol Sci 1991; 101: 7–18. [DOI] [PubMed] [Google Scholar]
- 45.Steffensen AB, Sword J, Croom D, et al. Chloride cotransporters as a molecular mechanism underlying spreading depolarization-induced dendritic beading. J Neurosci 2015; 35: 12172–12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeuthen T. Water-transporting proteins. J Membr Biol 2010; 234: 57–73. [DOI] [PubMed] [Google Scholar]
- 47.Østby I, Øyehaug L, Einevoll GT, et al. Astrocytic mechanisms explaining neural-activity-induced shrinkage of extraneuronal space. PLoS Comput Biol 2009; 5(1): e1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeuthen T, Macaulay N. Transport of water against its concentration gradient: Fact or fiction? Wiley Interdiscip Rev Membr Transp Signal 2012; 1: 373–381. [Google Scholar]
- 49.Rose EM, Koo JCP, Antflick JE, et al. Glutamate transporter coupling to Na,K-ATPase. J Neurosci 2009; 29: 8143–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]