Abstract
A widely distributed family of small regulators, called C proteins, controls a subset of restriction-modification systems. The C proteins studied to date activate transcription of their own genes and that of downstream endonuclease genes; this arrangement appears to delay endonuclease expression relative to that of the protective methyltransferase when the genes enter a new cell. C proteins bind to conserved sequences called C boxes. In the PvuII system, the C boxes have been reported to extend from −23 to +3 relative to the transcription start for the gene for the C protein, an unexpected starting position relative to a bound activator. This study suggests that transcript initiation within the C boxes represents initial, C-independent transcription of pvuIICR. The major C protein-dependent transcript appears to be a leaderless mRNA starting farther downstream, at the initiation codon for the pvuIIC gene. This conclusion is based on nuclease S1 transcript mapping and the effects of a series of nested deletions in the promoter region. Furthermore, replacing the region upstream of the pvuIIC initiation codon with a library of random oligonucleotides, followed by selection for C-dependent transcription, yielded clones having sequences that resemble −10 promoter hexamers. The −35 hexamer of this promoter would lie within the C boxes. However, the spacing between C boxes/−35 and the apparent −10 hexamer can be varied by ±4 bp with little effect. This suggests that, like some other activator-dependent promoters, PpvuIICR may not require a −35 hexamer. Features of this transcription activation system suggest explanations for its broad host range.
The genera hosting restriction-modification (RM) systems appear to comprise the majority of the prokaryotic world. RM systems play a variety of roles, ranging from the simply selfish (44, 54) through defense against bacteriophages (42, 51) to facilitating recombination (34, 36). While much remains to be learned about the functions of RM systems, our understanding of how they are regulated is even more limited. Most, if not all, RM system genes can move from cell to cell, either by residing on a plasmid or because of transduction, transformation, or Hfr-type conjugation. As a result, they all face a critical regulatory problem—how to ensure that a new host's DNA is protectively methylated before endonuclease activity appears. This problem is particularly acute for the type II RM systems, in which the methyltransferase (MTase) and endonuclease (REase) function independently.
A subset of type II RM systems includes, in addition to genes for the MTase and REase, a third gene for a conserved regulator called the C (controller) protein. C proteins were originally discovered in the PvuII (58, 59) and BamHI (22, 57) RM systems and were then noted in several other systems as well (1, 11, 53, 59). The C protein gene has no fixed orientation relative to the gene for MTase but to date has consistently been found upstream of and in the same orientation as the gene for REase. In several cases, the C protein has been shown to be a positive regulator required for expression of REase. In the PvuII RM system, the C protein (C.PvuII) has been shown to activate transcription of its own gene, with the downstream gene for REase having no independent promoter (60). The apparent regulatory logic is that when these genes enter a new host cell, only the gene for MTase is expressed immediately. In contrast the gene for the C protein, subject to autogenous activation, is initially expressed at a very low level but its transcription is predicted to ramp up dramatically over time along with that of the downstream gene for REase. Thus, REase expression is delayed relative to that of MTase. This interpretation is consistent with observations that pre-expressing C protein in a cell makes it impossible to transform that cell with the intact RM system, presumably because of premature expression of the REase (44, 60).
On the basis of sequence similarity and mutation analysis, the C proteins were predicted to be dimeric helix-turn-helix (HTH) proteins (58, 59). In fact, a newly determined high-resolution structure for C.AhdI, the first for a member of the C.PvuII family, reveals an all-α-helical homodimeric protein containing a classical HTH domain that can be assigned to the Xre family of transcriptional regulators (33; J. McGeehan and G. Kneale, personal communication).
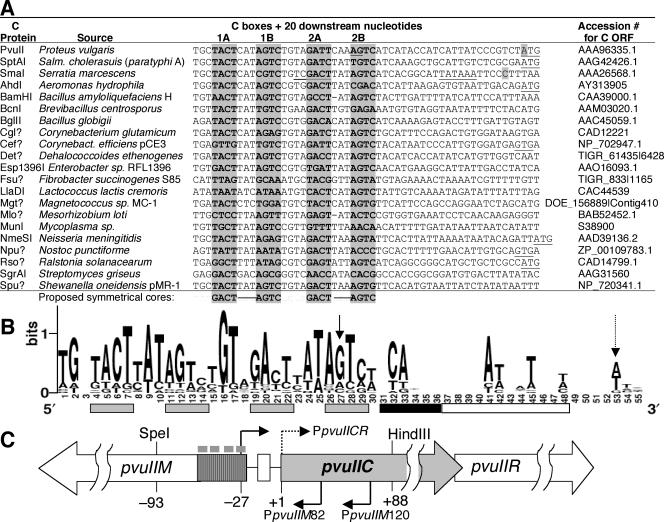
Comparison of C protein-encoding genes revealed the presence of conserved upstream sequences, called C boxes, having a consensus (53) that has since been redefined (3, 60) as shown in Fig. 1. The C boxes have been shown in several cases, including PvuII (60), EcoRV (64), EcoO109I (27), and BamHI (J. Brooks, personal communication), to be bound by the cognate C protein. For PvuII, it has been shown that mutation of the C box can abolish this binding (60). As indicated in Fig. 1A (bottom line) and B, the C box sequences appear to reflect recognition of a symmetrical core sequence (labeled 1A/1B and 2A/2B), as would be expected for homodimeric HTH proteins. The nature of the C boxes, including the strong conservation of asymmetric sequences between the box elements, has been noted previously (60).
FIG. 1.
Control region for the PvuII RM system. (A) Comparison of regions upstream of known and some hypothetical C protein-encoding genes. These genes were identified by using the Entrez BLink feature with both C.PvuII and C.SmaI as seeds and screening for proteins with predicted recognition helices similar to that of C.PvuII (HRTYI) (59). A question mark in the protein name indicates an open reading frame (ORF) that has not yet been formally named. Sequences upstream of the C open reading frames are shown; in all cases, the initiation codon is to the right (underlined where shown; others are farther downstream). The sequences are alphabetical by C open reading frame name, except for the top three: PvuII, the focus of this study; SptAI, which is very closely related to PvuII (43); and SmaI, for which the start of transcription has been determined (20). The shaded nucleotides indicate actual or predicted C boxes; the proposed symmetrical core (3, 60) is shown at the bottom. For C.PvuII (top line), the underlined nucleotides in box 2B indicate the previously identified transcript starts (60) while the shaded A of the C.PvuII initiation codon (far right) was identified in this study as a transcript start. (B) Sequence logo derived from the sequences in part A. The height of each stack reflects the extent of conservation at that position, while the height of symbols within the stack indicates the relative frequency of each nucleotide (56). The logo was generated by the server at http://weblogo.berkeley.edu. Gray bars under the numbers indicate the C boxes, the black bar delineates a region shown in this study to be dispensable for C-dependent transcription, while the white bar shows the regions found in this study to be required (at least in part) for C-dependent transcription. Vertical arrows indicate the transcript starts found previously (60) (solid) or in this study (dotted). (C) Genetic map of the PvuII RM system. Numbering is relative to the initiation codon of pvuIIC. The hatched area represents the previously identified promoter area for the pvuIICR genes, and the small gray rectangles above indicate the positions of the C boxes (the sequences shaded in panel A). The white rectangle between pvuIIM and pvuIIC corresponds to the white rectangle on the right of panel B; the two pvuIIC transcript starts (rightward bent arrows) indicate the one previously identified (solid, −27) and the one identified in this study (dotted, +1). Two promoters for the pvuIIM gene are also shown (leftward bent arrows) (60). Salm., Salmonella.
Previous transcript mapping in two systems (PvuII and SmaI, Fig. 1 and references 20 and 60) suggested that C boxes are in different positions relative to the promoters they control. The PvuII C boxes were reported to overlap the −10 hexamer and the start of transcription, where no other bacterial activators are known to bind (48), although some activators bind downstream of the −10 hexamer (40, 41). In contrast, the SmaI C boxes run from −21 to −46, entirely spanning the −35 hexamer, which is a rare but precedented location for activator binding (17, 32, 52, 63).
This study began as an attempt to determine whether or not the C box-promoter region is both necessary and sufficient for C-dependent activation of the genes for the PvuII C and REase proteins (pvuIICR). We find that the previously identified promoter is a weak, C-independent promoter that may initiate production of C.PvuII when the PvuII-encoding genes enter a new cell. We show that a portion of the pvuIIC gene between the C boxes and the initiation codon is responsible for C.PvuII-dependent expression of pvuIICR and demonstrate that this region includes the −10 hexamer for a C-dependent promoter that yields a leaderless mRNA. We also show that, surprisingly, the distance between the C boxes and the −10 hexamer of the C-activated promoter can be varied ±4 bp without reducing the level of activated transcription.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All experiments were done with Escherichia coli TOP10 (Invitrogen, Carlsbad, Calif.) unless otherwise indicated. This strain is McrBC− and thus permissive for the PvuII MTase (5, 50). Cells were grown at 37°C in lysogeny broth (LB) (37) containing antibiotics as indicated. Kanamycin A monosulfate (50 mg/liter), carbenicillin disodium salt (CB; 60 mg/liter), tetracycline hydrochloride (10 mg/liter), and chloramphenicol (CM; various concentrations) were obtained from Sigma-Aldrich (St. Louis, Mo.). TOPO TA 2.1, a cloning vector with preattached topoisomerase, was obtained from Invitrogen. pKK232-8, which contains a promoterless cat gene between strong transcription terminators (6), was obtained from Amersham Biosciences (Piscataway, N.J.). Plasmids pBR322 (2) and pACYC184 (12) were originally obtained from New England Biolabs (Beverly, Mass.).
Promoter clones and their derivatives.
Details of the construction of various plasmids used in this study, together with a table of the oligonucleotides used (Table S1), are available as supplemental material to this article.
Assays for reporter gene activity.
β-Galactosidase activity was measured at several times during exponential growth. The assay used, which was described by Miller (37) and modified by Platko et al. (49), monitors hydrolysis of o-nitrophenyl-β-d-galactopyranoside. The units of β-galactosidase activity were calculated by dividing ΔA420 by the reaction time and by the volume of permeabilized cells used. The specific activity was obtained from the slope of a plot of β-galactosidase activity versus the optical density (OD) of the culture at 420 nm.
Protein concentration.
The Micro BCA kit from Pierce (Rockford, Ill.) was used to determine protein concentrations in accordance with the manufacturer's instructions. A standard curve based on bovine serum albumin and ranging from 0.5 to 200 μg/ml was generated.
Chloramphenicol acetyltransferase (CAT) assay.
The Quan-T-CAT kit from Amersham Biosciences (unfortunately no longer available) was used to measure CAT reporter activity via transfer of acetyl groups from 3H-labeled acetyl coenzyme A. The substrate in this assay is missing the hydroxyl group of carbon 7 so that only monoacetylated CM can be produced, simplifying the kinetics, and the CM is biotinylated to allow separation from unreacted acetyl coenzyme A with streptavidin beads. A 250-μl sample was removed and pelleted for 1 min in a microcentrifuge when the culture OD at 600 nm reached 0.3, 0.4, and 0.5. The cells were resuspended in 180 μl of 100 mM Tris (pH = 7.8) and subjected to six freeze-thaw cycles. The extracts were incubated for 10 min at 65°C to decrease endogenous deacetylase activity (16). The cell extracts were centrifugally cleared, with 40 μl of supernatant taken for CAT assay, and portions of the remainder were used to determine the protein concentration. The CAT reaction mixture was incubated at 37°C for 1 min before streptavidin beads were added. 3H-labeled acetylated CM was measured in a scintillation counter after resuspending the beads in 1 ml of ScintiSafe 30% scintillant (Fisher Scientific, Chicago, Ill.). CAT activity was calculated as counts per minute per microgram of protein per minute.
Generation and analysis of library randomized for region downstream of C boxes.
Oligonucleotide DK31 (Table S1 in the supplemental material) spans the region between the C boxes and the pvuIIC initiation codon and includes nine consecutive randomized positions (IDT, Coralville, Iowa). Oligonucleotide DK32 was used to generate the complementary strand. The two oligonucleotides were hybridized, and the shorter primer was extended with Klenow polymerase. The DNA was sequentially digested with HindIII and SalI, followed by ligation into pDK156 (which contains the pvuIICR promoter from −93 to −24, relative to the initiation codon of pvuIIC, in pKK232-8) and electroporation into Electromax EC100 E. coli cells (Epicentre, Madison, Wis.). Cells were plated onto LB agar containing CB. Colonies were pooled, and plasmid DNA was recovered with the midi plasmid kit (QIAGEN). The library DNA was electroporated into an E. coli strain containing pDK74 (a pACYC184-based plasmid that specifies C.PvuII), and Cat+ colonies were selected on plates containing CB and 25 μg of CM per ml. Plasmid DNA was isolated from these colonies, and the 9-nucleotide (nt) randomized region was sequenced with primer DK5.
Nucleotide sequence analysis and transcript mapping.
All of the plasmid inserts generated in this study were sequence confirmed. DNA sequencing was done with the Big Dye termination system (Applied Biosystems, Foster City, Calif.), a variant of the dideoxy method (55). Reaction mixtures were resolved on a Prism 310 DNA analyzer (Applied Biosystems).
For total-cell RNA, 100-ml cultures were grown in LB medium to an A600 of 0.5. Ten milliliters of 5% phenol in ethanol was added to the culture to stabilize the RNA (31), and cells were pelleted. RNA was extracted by three alternative methods: (i) guanidine isothiocyanate (15); (ii) Trizol, a monophasic solution of phenol and guanidine isothiocyanate (Invitrogen; in accordance with manufacturer's protocol); and (iii) RNeasy (QIAGEN; in accordance with the manufacturer's protocol). RNase-free reagents and materials were used. To remove phenol, which affects reverse transcriptases, Phase Lock gel (heavy, 2 ml, from Eppendorf) was used to separate the organic and aqueous phases.
Nuclease S1 mapping was performed as described at http://www.fhcrc.org/labs/hahn/methods/mol_bio_meth/s1_oligo_probe.html. Annealing temperatures of 45 to 65°C were tested; the final annealing temperature for both the 77- and 51-nt probes (DK37 and DK38; Table S1 in the supplemental material) was 55°C. Total RNA was isolated from E. coli strains TOP10 and JM107MA2, each carrying the pPvuRM3.4 or pPvuRM3.4Esp19 plasmid (59), and Proteus vulgaris carrying the pPvu1 plasmid (5). Assays included 25 or 50 μg of total RNA. Samples were resolved on Tris-borate-EDTA-urea 10 to 20% acrylamide gradient gels (Invitrogen). A 10-bp ladder of size standards (Invitrogen) was loaded onto each gel.
Primer extensions were performed as recommended by the manufacturer of each reverse transcriptase used (Moloney murine leukemia virus from Amersham Biosciences and SuperScript II and Avian Thermoscript from Invitrogen). RNA preparations were those described above under S1 mapping. One picomole of 32P-end-labeled oligonucleotide (RV1, DK35, or DK36; Table S1 in the supplemental material) was mixed with 5 μg of total RNA at 70°C. Moloney murine leukemia virus and Thermoscript reaction mixtures were then placed on ice for 5 min, while Superscript II reaction mixtures were cooled to 42°C for 2 min. Reactions were allowed to proceed for 50 min and terminated via heat inactivation, and protein was precipitated with yeast tRNA and isopropanol and resuspended. Samples were resolved on a 6% polyacrylamide-Tris-borate-EDTA-urea sequencing gel along with a 10-bp DNA marker ladder.
RESULTS
C boxes are not sufficient for C-dependent activation of transcription.
C boxes are conserved sequences found upstream of C protein-encoding genes (3, 53, 60) (Fig. 1A and B). The C boxes have been shown in several cases, including PvuII (60), to be bound by the cognate C protein. Subclones containing the C boxes and just 9 downstream bp were reported to have significant promoter activity, although adding additional downstream pvuIIC DNA was found to boost this expression fourfold (60). To explore the minimal requirements for C.PvuII-activated transcription, we inserted into plasmid pKK232-8 (6) a DNA fragment that includes the pvuIICR promoter from −66 to +114 relative to the 3′ most of the two adjacent transcription starting points previously identified in E. coli (60). This corresponds to −93 to +88 relative to the initiation codon of pvuIIC, which for clarity we will henceforth use as the basis for numbering (Fig. 1C). pKK232-8 is a moderate-copy-number plasmid, and the PvuII-encoding genes naturally reside on a plasmid (5, 10). In the resulting plasmid, pDK58, the pvuIICR promoter is upstream of a promoterless gene for CAT (cat), and the promoter-reporter fusion is flanked by strong transcription terminators to minimize transcriptional readthrough from vector promoters. In some of our plasmids, the promoterless lacZ reporter gene was also inserted upstream of cat. Since these reporter plasmids contain only a portion of the pvuIIC gene, a compatible plasmid was also present that carried either the wild-type (WT) C.PvuII-encoding gene or a null mutant allele (pvuIICEsp19) in which the HTH motif has been disrupted by insertion of an additional leucine codon (59). Following deletions from the 5′ or 3′ side, promoter activity was assessed in exponential-phase cells growing in a rich medium (LB).
Deletion, from the 5′ side, of nt −93 to −60 (relative to the initiation codon of pvuIIC) replaced the putative −35 hexamer for the previously reported promoter (TCAACA, ending 6 nt upstream of the sequence shown in Fig. 1A) with the vector-derived sequence GTCGAC while leaving the 18-bp spacer region unchanged. This deletion substantially reduced the level of C-independent transcription, although that level was very low to begin with (Table 1). However, this upstream deletion had relatively little effect on stimulation of transcription by active C.PvuII (a roughly twofold reduction).
TABLE 1.
Effect of deleting the region upstream of the C boxes
| Upstream enda | pvuIIC alleleb | LacZ activityc
|
|
|---|---|---|---|
| Absolute | Normalized | ||
| −59 | WT | 3.98 (0.88) | 0.43 |
| Esp19 | 0.01 (0.91) | <0.01 | |
| −93 | WT | 9.32 (0.94) | 1.00 |
| Esp19 | 0.34 (0.94) | 0.04 | |
The number of nucleotides from the native pvuII sequence, numbered relative to the pvuIIC initiation codon. This corresponds to 8 or 42 bp of native sequence upstream of the C boxes.
C.PvuII is supplied in trans from either the WT allele or a mutant inactive allele in which an extra codon has been inserted within the predicted HTH motif.
β-Galactosidase activity was measured as described in Materials and Methods. In these plasmids, both cat and lacZ are downstream of the cloned promoter. Enzyme activity was measured at three culture densities during exponential growth, and the slope of the activity versus the culture OD is given (together with the correlation coefficient for the least-squares fit).
We next deleted from the 3′ side, and the results are shown in Table 2. The promoter clone, ending just downstream of the C boxes (−93 to −25), showed only a slight reduction in C-independent transcription compared to the longer promoter clone (−93 to +88) but showed negative activation (about threefold repression) by C.PvuII. In contrast, in the presence of C.PvuII the longer promoter yielded more than 30 times more CAT activity than the shorter promoter. The shorter clone includes DNA from −68 to +2 relative to the previously identified promoter. Thus, these results suggest that sequences downstream of the C boxes, and thus downstream of the previously reported pvuIICR transcript start (60) play a substantial role in C-dependent activation and that the C boxes are necessary but not sufficient for such activation. The background CAT activity in this system is very low for the vector control, about 50-fold below even the low activity seen for the −93 to −25 clone in the absence of C.PvuII. Thus, the results in Table 2 indicate the presence of a real but relatively weak (and C.PvuII-unactivated) promoter within or upstream of the C boxes, consistent with the location previously identified for a pvuIICR promoter (60). As C.PvuII is an autogenous activator, this low level of C-independent transcription may represent the background activity needed to initiate autoactivation.
TABLE 2.
Transcriptional effect of sequences downstream of the C boxes
| Downstream enda | pvuIIC alleleb | CAT activityc
|
|
|---|---|---|---|
| Absolute | Normalized | ||
| −24 | WT | 271 ± 20 | 0.03 |
| Esp19 | 748 ± 23 | 0.08 | |
| +88 | WT | 8,998 ± 1,117 | 1.00 |
| Esp19 | 514 ± 44 | 0.06 | |
The number of nucleotides from the native pvuII sequence between the C boxes and the reporter gene cloning junction. In both cases, the 5′ endpoints are −93 (relative to the pvuIIC initiation codon). The 3′ endpoints correspond to +3 and +114, respectively, relative to the previously reported promoter (60). In all cases, the consensus C boxes are present in the context of an otherwise WT pvuIIC sequence. In the longer clones, a short substitution immediately downstream of the 3′-most C box generates a restriction site used for clone assembly; substitutions in this area were found not to affect transcription (see text).
C.PvuII is supplied in trans from either the WT allele or a mutant inactive allele in which an extra codon has been inserted within the predicted HTH motif.
CAT activity was measured as described in Materials and Methods and is expressed (± the standard error) as mean counts per minute incorporated per minute per microgram of protein from triplicate samples. The vector control levels (not shown) were subtracted as background.
Defining the C.PvuII-activated promoter of pvuIICR.
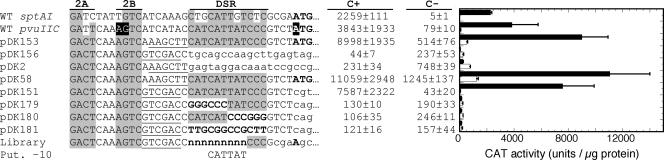
As the promoter within the C boxes appeared not to be activated by C.PvuII (it was, in fact, repressed; Table 2), we sought to define the limits of the DNA downstream of the C boxes that is required for C.PvuII-dependent transcription. We made a series of nested 3′ deletions in the pKK232-8 vector carrying the pvuIICR promoter region and assessed transcription by measuring CAT activity in the presence or absence of active C.PvuII. The shortest subclone used was equivalent to the −93 to −25 fragment described in the preceding section, meaning that its 3′ end is the 3′ end of the C boxes. The results revealed that including 22 or more native bp downstream of the C boxes is required for activation by C.PvuII (not shown). Furthermore, the first 7 bp downstream of the C boxes are not required, as they were replaced with little effect by either of two restriction sites during our ligation of the deletion series to the fixed upstream promoter region (underlined nucleotides in Fig. 2). Thus, the relevant segment includes all or part of a 16-bp region 8 to 22 nt 3′ to the C boxes (−17 to −3 relative to the pvuIIC initiation codon). We replaced portions of this 16-bp region and assessed the effects on the resulting CAT activity. Replacing nt −18 to −13, nt −12 to −7, or the 12 nt all at once abolished activation by C.PvuII (Fig. 2), defining at least the core of the segment between the C boxes and the pvuIIC initiation codon that is required for activation.
FIG. 2.
Effect of replacing DNA downstream of the C boxes on pvuIICR promoter activity. Promoter clones, all having the 5′ end at −93 relative to the pvuIIC initiation codon, were placed upstream of the promoterless cat gene in pKK232-8. Only the sequence 3′ of C box 2A is shown. To readily replace portions of the DNA, the nucleotides immediately following C box 2B were altered to generate restriction sites (underlined). Lowercase letters indicate vector DNA. These plasmids were used to transform E. coli already containing a compatible plasmid expressing either WT C.PvuII (black bars) or a defective variant (white bars), and CAT activity was determined in triplicate as described in Materials and Methods. Error bars indicate the standard deviation. For comparison, the sequence of the SptAI RM system is also shown (top line); the genes of this system are extremely closely related to those of PvuII, and their transcription is activated by C.PvuII (43), but even in this case conservation of the region between the C boxes and pvuIIC initiation codon is limited. Also for comparison, the bottom lines show the sequence context into which the randomized sequences were placed in other experiments (Library) and the location of the putative (Put.) −10 hexamer identified as a result.
Selection of functional sequences from a library randomized in the region downstream of the C boxes.
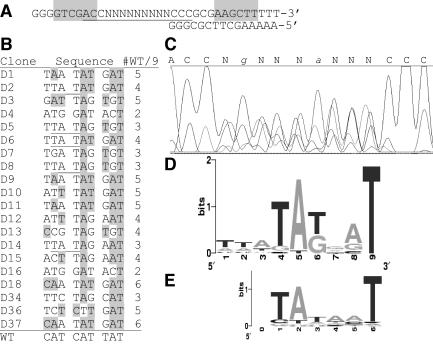
As the DNA sequence is poorly conserved among C-dependent RM systems in the region downstream of the C boxes (Fig. 1A and B), we prepared a pDK156 library in which a 9-nt segment of this region was replaced with a randomized sequence (Fig. 3A and bottom of Fig. 2) to determine the characteristics important to its function. Following electroporation into E. coli, a plasmid library was extracted from the pooled transformants; sequencing indicated the degree to which the 9-nt region had been randomized (Fig. 3C). This library was then electroporated into an E. coli strain containing a compatible plasmid expressing pvuIIC. We had previously shown that one of our CAT reporter plasmids with the DSR deleted did not confer resistance to even 24 μg of CM per ml on this strain, while the equivalent plasmid containing the WT sequence in this region formed colonies efficiently in the presence of 124 μg of CM per ml (not shown). We thus plated the transformants onto 25 μg of CM per ml and picked the resulting colonies; all such colonies were also resistant to 125 μg of CM per ml when subsequently tested in liquid medium (not shown). Plasmids were isolated from Cmr strains and introduced into strains producing either WT or inactive C.PvuII; all of the plasmids tested still showed C-dependent CAT expression (not shown). Next, for 20 of these plasmids we determined the sequence of the randomized region (Fig. 3B). The aggregate results are shown in the form of a sequence logo (56). The extent of selection is apparent from a comparison of the aligned sequence trace for the unselected library (Fig. 3C) with the logo for the Cmr transformants (Fig. 3D)—the possible biases in the starting library at positions 2 (G bias) and 6 (A bias) of the randomized segment are not reflected in the selected logo, and the universally conserved T at position 9 in the logo is not apparent in the original sequence trace. The aggregate pattern for active sequences is strikingly similar to that for −10 promoter hexamers (Fig. 3E), although some individual active variants shown in Fig. 3B seem quite different from the −10 hexamer pattern. This result, together with the results shown in Fig. 2, suggested that C.PvuII-activated pvuIICR transcription could start close to or at the pvuIIC initiation codon.
FIG. 3.
Selection of functional sequences from a randomized library. (A) Two synthetic oligonucleotides were acquired. The 28-mer includes nine consecutive positions (N) that were synthesized in the presence of an equal mixture of all four phosphorothioate nucleotides. The second strand was synthesized by using the 12-mer shown and extending with Klenow polymerase. The shaded areas indicate restriction sites used for subsequent cloning. In the resulting plasmids, the pvuIICR promoter and C boxes are upstream of the sequence shown while downstream is a promoterless cat gene. (B) The plasmid library was used to transform an E. coli strain that already carried a compatible plasmid producing C.PvuII, and the transformants were plated onto agar containing CM to select for functional sequences. Twenty of these were sequenced as described in Materials and Methods; the 9-nt randomized region is shown with the native PvuII sequence at the bottom. Shading indicates a match to the native sequence, with number of matching positions indicated to the right; underlining reveals shifted occurrences of a TAT motif. In all 20 clones, the 9-nt region was preceded by ACC and followed by CCC. (C) Sequencing trace of the pooled randomized plasmid library before selection (portion underlined in panel A). The lower strand was sequenced, so the image has been reversed to match other portions of the figure and the complementary bases indicated above the trace. In the unselected randomized region, two positions showed enough bias for the sequencing software to call them as shown. (D) Logo analysis (56) of sequences shown in panel B. The logo was generated at weblogo.berkeley.edu and is aligned with the corresponding positions of panel C. (E) Logo analysis of 350 E. coli −10 promoter hexamers generated by the Computational Genomics Research Group, Department of Plant and Microbial Biology, University of California at Berkeley, and available at weblogo.berkeley.edu.
C.PvuII-dependent transcription of pvuIICR yields a leaderless mRNA.
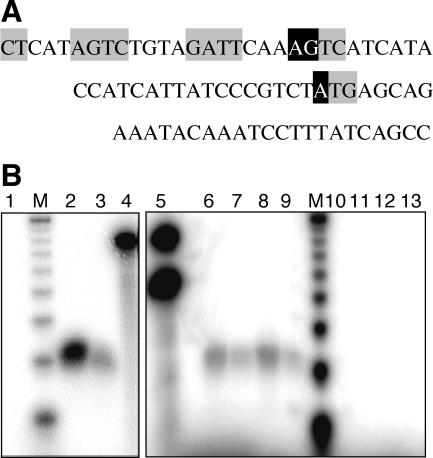
The previous analysis of pvuIICR transcript initiation used primer extension analysis with reverse transcriptase (60). This method has been reported to give artifactually long runoff products in some cases, although this is generally only 1 or 2 nt in excess length (14). Nevertheless, we repeated these experiments with different RNA preparation methods and different reverse transcriptases and also used the alternative approach of S1 nuclease mapping together with a labeled 77-nt probe oligonucleotide spanning the DSR and most of the C box region (Fig. 4A).
FIG. 4.
Determination of pvuIIC mRNA 5′ terminus via S1 nuclease digestion. (A) Sequence complementary to the 77-nt probe used in S1 mapping analyses. Gray boxes indicate the C box elements and the ATG initiation codon of pvuIIC. Transcript starts identified previously (60) and in this study are shown in black boxes. The distances from the labeled 5′ end of the probe (corresponding to the 3′ end of the sequence shown) are 30 nt to the ATG start and 56 or 57 nt to the AGTC start. In addition, a 51-nt probe was used, the 5′ end of which is complementary to the position 4 nt upstream of the ATG start codon (GTCT) and that extends 7 nt upstream of the entire sequence shown. (B) S1-digested products were resolved on acrylamide gels as described in Materials and Methods; autoradiograms are shown. The two marker lanes (M) show a 10-nt ladder, with the bands shown ranging from 20 to 100 nt. Unless noted otherwise, all reaction mixtures included the 77-nt probe. Lanes: 1, RNA from the strain used as background, E. coli TOP10; 2 and 3, 50 and 25 μg of RNA from TOP10 carrying pPvuRM3.4 (WT PvuII RM system); 4, same as lane 3 but with no nuclease S1 added; 5, same as lane 4 but with both 77- and 51-nt probes present; 6 and 7, 50 and 25 μg of RNA from TOP10 carrying pPvuRM3.4; 8 and 9, 50 and 25 μg of RNA from P. vulgaris, the native host for the PvuII RM system; 10 and 11, same as lanes 6 and 7 but with the 51-nt probe; 12 and 13, same as lanes 8 and 9 but with the 51-nt probe.
In the S1 mapping experiments, this probe should have yielded a 56- or 57-nt product on the basis of the previously characterized transcript initiation site (60). We could find no such product with two different overlapping probes, RNAs prepared via three methods, and RNAs isolated from three strains (E. coli TOP10 carrying pPvuRM3.4 [as shown in Fig. 4]; P. vulgaris, the native host for the PvuII RM system [also shown in Fig. 4]; and E. coli JM107MA2 carrying pPvuRM3.4 as used before [60] [data not shown]).
In contrast, we consistently saw a shorter 30-nt S1 digestion product, suggesting transcript initiation at the first nucleotide of the translation initiation codon (Fig. 4B). This would result in the production of a leaderless mRNA, as has been seen in several other cases (26, 39, 46), and would explain the lack of an obvious Shine-Dalgarno sequence upstream of the ATG.
Surprisingly, the primer extension analyses never showed the ATG initiation point even with the same RNA preparations that clearly showed this start in S1 mapping experiments. Two of the three RNA preparation methods also yielded no primer extension products for the C box start previously reported by Vijesurier et al. (60). However, RNAs prepared via use of guanidine isothiocyanate, which is what Vijesurier et al. had used, did reveal faint extension products including the previously reported C box starts (not shown). The reason for this disparity is unclear although, as described above, the results in Table 2 also indicate weak but significant promoter activity within the C boxes.
To verify that the pvuIICR mRNA detected by S1 mapping is leaderless, we confirmed that the assigned initiation codon is actually used for translation of pvuIIC. This codon had been implicated on the basis of being near the start of the open reading frame, and in vitro transcription and translation of this gene had yielded a product of the expected size (58). To further support this choice, we altered the initiation codon without changing the first (transcript initiation) position, ATG → AGA, and confirmed the remainder of the pvuIIC sequence. This change reduced restriction of bacteriophage λvir 106-fold (not shown), even when the entire downstream pvuIIR gene was replaced to rule out its inadvertent alteration during the site-directed mutagenesis. This loss of restriction is probably not due to transcriptional effects, since an ATG → CGT substitution at that position reduced C-dependent transcription of a cat reporter gene by only about a third (pDK151 in Fig. 2).
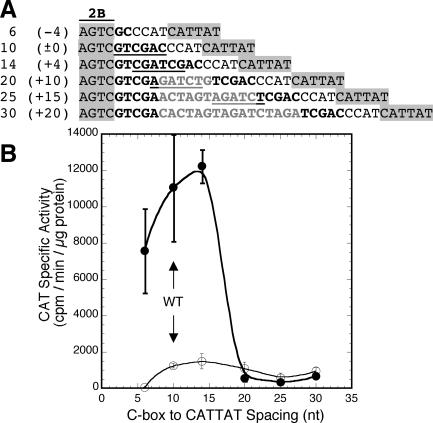
Effects of altered spacing between the C boxes and the DSR.
Our data suggest that the region downstream of the C boxes functions by contributing the −10 hexamer to a pvuIICR promoter that initiates transcription at the pvuIIC initiation codon. In this case, the −35 hexamer would overlap C box element 2A (TAGATT on the basis of 17-bp spacing from the −10 hexamer; Fig. 1A). We assessed the effects of changing the spacing between the −10 hexamer and the C box region. As shown in Fig. 5, adding or removing 4 bp in the spacer region had minimal effects on C-dependent transcription. This is inconsistent with the behavior of standard promoters, in which even 1-bp changes from the ideal 17-bp −10 to −35 spacing profoundly reduce promoter activity (4, 25, 62). These 4-bp changes shift the DNA double helix face on which the −10 hexamer is exposed by ±140° relative to the C boxes (assuming B-form DNA). In contrast, inserting 10 or 20 bp, which would change the relative phasing by just 7 or 14°, respectively, eliminated C-dependent transcription. Thus, the functional relationship between the C boxes and the −10 hexamer depends on linear distance but not, apparently, on phasing.
FIG. 5.
Effect of altered spacing between the C boxes and putative −10 hexamer on pvuIICR transcription. (A) Promoter variants tested. Each promoter began at −93 relative to the pvuIIC initiation codon; only sequences 3′ of C box 2B are shown. All include nt −23 to +6 of the native PvuII DNA and were inserted into pKK232-8 upstream of the promoterless cat gene. The nucleotides from −24 to −19 were replaced to generate a restriction site (underlined), and the spacing between the putative −10 hexamer for the ATG transcription start and the C boxes/promoter was varied by cleaving the restriction site and digesting the single-stranded tails, filling in the single-stranded regions, or inserting oligonucleotide duplexes of various lengths prior to ligation. The putative −10 hexamer, CATTAT, is −14 to −9 relative to the pvuIIC initiation codon. WT spacing of 10 nt between C box 2B and the putative −10 hexamer is indicated by ±0. (B) The sequences of all variants were confirmed, and they were used to transform E. coli already containing a compatible plasmid expressing either WT C.PvuII (closed circles) or a defective C variant (open circles); CAT activity was determined in triplicate as described in Materials and Methods. The bars indicate standard errors.
Interaction between the region downstream of the C boxes and a pvuIIM promoter.
The pvuIIM gene is oriented divergently to pvuIICR, and its transcription is not affected by C.PvuII (60); its two promoters lie within pvuIIC and are thus convergent with respect to the pvuIICR promoter (Fig. 1C). In some cases, convergent transcription interferes with one or both opposing promoters (9). However, this does not appear to be the case in the PvuII RM system. There was no effect of PpvuIIM82 on pvuIICR transcription, as judged from the nearly identical rates of pvuIICR transcription whether or not the 3′ clone endpoint included PpvuIIM82 (not shown; seven pvuIICR endpoints between +1 and +88 showed no significant change in CAT activity). Furthermore, we altered a longer segment that included both pvuIIM promoters, making synonymous pvuIIC mutations that impaired both −10 hexamers (TCGAAG → CGCCAG for PpvuIIM82 and GATAGA → GAGAGC for PpvuIIM120). In one orientation upstream of cat, these mutations abolished detectible transcription from the pvuIIM promoters, while in the opposite orientation they had no effect on C-dependent transcription of pvuIIC (not shown).
Interestingly, the −10 hexamer region of the C-activated pvuIICR promoter stimulated transcription from at least one of the two opposing pvuIIM promoters (Table 3). The upstream pvuIIM promoter M120 is truncated at position −24 in our reporter plasmids, but the complete M82 promoter (60) is present in the longer pvuIICR promoter clones (−56 to +123 relative to the M82 initiation point). The region encompassing the pvuIICR −10 hexamer is +33 to +48 relative to the PpvuIIM82 transcript start. The data in Table 3 reveal a large positive effect of the DSR on pvuIIM transcription but, as expected, no effect at all on C.PvuII. The basis for this effect has not yet been determined.
TABLE 3.
Effect of the DSR on transcription from pvuIIM promoter M82
| pvuIIC allelea | DSRb | CAT activityc
|
|
|---|---|---|---|
| Absolute | Normalized | ||
| WT | + | 1,202 ± 118 | 1.00 |
| − | 3 ± 0.5 | <0.01 | |
| Esp19 | + | 1,183 ± 440 | 0.98 |
| − | 3 ± 0.5 | <0.01 | |
C.PvuII is supplied in trans from either the WT allele or a mutant inactive allele in which an extra codon has been inserted within the predicted HTH motif.
Plus and minus signs indicate the presence and absence of the DSR, respectively.
CAT activity was measured as described in Materials and Methods and is expressed as mean counts per minute per minute incorporated per microgram of protein ± the standard error from triplicate samples. The vector control levels (not shown) were subtracted as background.
DISCUSSION
Location of the C.PvuII-activated promoter for pvuIICR.
Several of our observations combined to suggest that C.PvuII-activated pvuIICR transcription begins at a point distinct from or in addition to the point previously identified (60). First, replacing the putative originally reported −35 hexamer had no effect (Table 1), although there are precedents for such behavior (38, 47). Second, a region well downstream of the previously identified transcript start was needed for substantial transcription (Fig. 3 and data not shown), although this also has precedents (21). Third, selection for function in a randomized DSR yielded −10 hexamer-like sequences (Fig. 3). Fourth, the related SmaI RM system initiates transcription farther downstream of the C boxes (Fig. 1A and reference 20). In repeating the earlier studies, we again found the reported transcript start, but only when the exact conditions reported were again used. This gave a weak signal but is consistent with reporter gene evidence for a weak promoter within the C boxes (Table 2).
In contrast, S1 nuclease mapping consistently indicated a different starting point (Fig. 4), at the first nucleotide of the initiation codon. This starting point was not seen in primer extension analyses, even with the same RNA preparations that gave clear nuclease S1 signals. This type of result could be explained by such phenomena as stable RNA secondary structures or RNA splicing, but there is no obvious evidence that either applies in this case. It is more difficult to rule out mispriming on background mRNA in the primer extensions, although we used two species (E. coli and P. vulgaris) and multiple primers. Irrespective of the primer extensions, our results are most consistent with C-dependent production of a leaderless pvuIICR mRNA. The results in Table 2 suggest that the previously identified initiation point (within the C boxes) represents weak C-independent transcription, such as would be required early after gene transfer into a new cell to initiate the autoactivation circuit.
Implications for mechanism of transcription activation by C proteins.
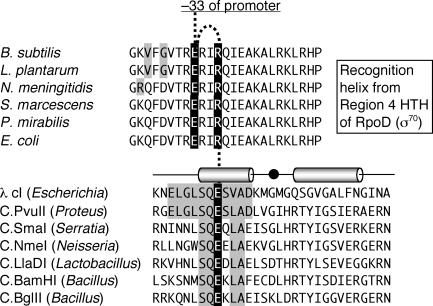
The 23-bp distance between the C boxes and the ATG transcript initiation point would place bound C.PvuII at a rare but by no means unique position for transcriptional activators (17, 32, 48, 52, 63). In addition, a fortuitous region of very high similarity between C.PvuII and the cI repressor-activator of bacteriophage λ (Fig. 6) suggests a specific activation mechanism. The match covers a region known (for λcI) (24) or predicted (for C.PvuII) (59) to include the activation patch on the upstream helix of the HTH motif. The key contact for λcI activation is between Glu34 and Arg588 in region 4 of σ70 (RpoD) (8, 29, 30, 45). Glu34 is in the middle of the 11-amino-acid segment conserved between λcI and C.PvuII, and this Glu and flanking amino acids are highly conserved among C proteins (Fig. 6). λcI activates transcription via effects on the isomerization step, although there is evidence that the same protein-protein interactions can result in activation via DNA binding or isomerization, depending on the precise spatial context of the interaction with RNA polymerase (18).
FIG. 6.
Model for interaction between C proteins and region 4 of RpoD. The lower half shows sequences of the predicted HTH motifs of selected C proteins. The sequence above C.PvuII is that of cI protein from bacteriophage lambda, which has been structurally characterized and has a known activation mechanism (24). The shading indicates the nearly complete conservation of the upstream α helix between λcI and C.PvuII (one Val → Leu substitution out of 11 consecutive amino acids), including the key activation residue (Glu shown in white on black). In the case of λcI, this Glu activates by contacting a conserved Arg in the HTH motif of region 4.2 at the carboxyl end of RpoD (σ70; upper half). This Arg, in turn, is coordinated with a conserved Glu (both shown in white on black), and the Glu contacts position −33 in the promoter −35 hexamer (see text for details). In the RpoD sequences, gray shading indicates amino acids that differ from those in the E. coli ortholog. The RpoD sequences shown were chosen to match the native hosts of the C protein sequences in the lower half. Sequences are from the GenBank database, except for RpoD of P. mirabilis, which was kindly made available from the partially complete genome sequence (http://www.sanger.ac.uk/Projects/P_mirabilis/).
The recently determined high-resolution structure of the C.PvuII ortholog C.AhdI reveals an all-α-helical dimeric protein that contains a classical HTH domain (33; McGeehan and Kneale, personal communication). Homology modeling of the structure of C.AhdI with the λcI-DNA-RNA polymerase ternary complex is entirely consistent with direct contact between C.PvuII and the σ70 subunit (McGeehan and Kneale, personal communication), although this remains to be proven.
A surprising feature of the pvuIICR promoter we report here is its relative insensitivity to changes in spacing (Fig. 5). The native sequence has a repetitive quality that might mask changes in spacing. Specifically, the WT sequence has a −10 hexamer (CATTAT) with 17-bp spacing from the putative −35 hexamer; the −4 variant might function with a TATCCC −10 hexamer having 16-bp spacing, and the +4 variant might function with a CATCAT −10 hexamer having 18-bp spacing. However, there may simply be no −35 hexamer. While the −10 hexamer lacks the TGN sequence characteristic of −35-independent, extended −10 sequences (7), in some promoters the activator functionally replaces the −35 hexamer (28). In this case the varied spacing between the C boxes and the promoter may reflect a structural feature of the C box-C.PvuII complex. Specifically, the center-to-center distance between box 1A/1B and box 2A/2B is 1.4 helical turns of the DNA (15 bp; Fig. 1A); this would likely position a pair of bound C.PvuII homodimers on opposite faces of the DNA, similar to what has been found for DtxR (13), so that a half turn of the DNA in either direction would still leave an activation surface in place to contact RNA polymerase.
Implications for host range of C-dependent RM systems.
Transcription activators often have a narrower host range than repressors, as the former have to make spatially constrained productive contacts with RNA polymerase (although these contacts can involve a very small number of amino acids). For example, a transcription activator from Bacillus could function in E. coli only when part of the E. coli RNA polymerase α subunit was replaced with the corresponding Bacillus sequence (35). However, the C proteins appear to have a broad host range (23).
We suggest three features that may contribute to the apparent host range breadth of C-dependent RM systems. First, as illustrated in Fig. 6, if the analogy to λcI is valid, then the activating contact involves a very highly conserved segment among major vegetative sigma factors. Second, the apparent independence of PpvuIICR from a −35 hexamer, suggested by the results in Fig. 5, might contribute to host range breadth. The replacement of conserved sequences having fixed spacing requirements (both the −35 hexamer and the spacer region) (61) with a more flexibly spaced site for binding an activator (47) could bypass some species-specific promoter requirements. Third, while the 3′ end of 16S rRNA is highly conserved, there is evidence that the Shine-Dalgarno element in mRNAs plays roles beyond simply serving as a hybridization tag for ribosome docking (19). If this is correct, then a leaderless mRNA such as that for pvuIICR could bypass some species-specific regulatory signals; indeed, leaderless mRNAs are present and translated in all kingdoms of life (39). Given the potentially lethal consequences of misregulating RM systems, the host range of the regulatory system may be a limiting factor for the host range of the entire RM system.
Supplementary Material
Acknowledgments
We thank Joan Brooks, John McGeehan, and Geoff Kneale for sharing unpublished results; Eric LaFontaine, Darren Sledjeski, and R. Mark Wooten for critically reviewing the manuscript; and the anonymous reviewers for important suggestions.
This research was supported by a grant from the U.S. National Science Foundation (MCB-9904523) with additional support from the Research Challenge Program of the Ohio Board of Regents via the Medical College of Ohio.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Anton, B. P., D. F. Heiter, J. S. Benner, E. J. Hess, L. Greenough, L. S. Moran, B. E. Slatko, and J. E. Brooks. 1997. Cloning and characterization of the BglII restriction-modification system reveal a possible evolutionary footprint. Gene 187:19-27. [DOI] [PubMed] [Google Scholar]
- 2.Balbas, P., X. Soberon, E. Merino, M. Zurita, H. Lomeli, F. Valle, N. Flores, and F. Bolivar. 1986. Plasmid vector pBR322 and its special-purpose derivatives—a review. Gene 50:3-40. [DOI] [PubMed] [Google Scholar]
- 3.Bart, A., J. Dankert, and A. van der Ende. 1999. Operator sequences for the regulatory proteins of restriction modification systems. Mol. Microbiol. 31:1277-1278. [DOI] [PubMed] [Google Scholar]
- 4.Beutel, B. A., and M. T. Record, Jr. 1990. E. coli promoter spacer regions contain nonrandom sequences which correlate to spacer length. Nucleic Acids Res. 18:3597-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal, R. M., S. A. Gregory, and J. S. Cooperider. 1985. Cloning of a restriction-modification system from Proteus vulgaris and its use in analyzing a methylase-sensitive phenotype in Escherichia coli. J. Bacteriol. 164:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosius, J. 1984. Plasmid vectors for the selection of promoters. Gene 27:151-160. [DOI] [PubMed] [Google Scholar]
- 7.Burns, H., and S. Minchin. 1994. Thermal energy requirement for strand separation during transcription initiation: the effect of supercoiling and extended protein DNA contacts. Nucleic Acids Res. 22:3840-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushman, F. D., C. Shang, and M. Ptashne. 1989. A single glutamic acid residue plays a key role in the transcriptional activation function of lambda repressor. Cell 58:1163-1171. [DOI] [PubMed] [Google Scholar]
- 9.Callen, B. P., K. E. Shearwin, and J. B. Egan. 2004. Transcriptional interference between convergent promoters caused by elongation over the promoter. Mol. Cell 14:647-656. [DOI] [PubMed] [Google Scholar]
- 10.Calvin-Koons, M. D., and R. M. Blumenthal. 1995. Characterization of pPvu1, the autonomous plasmid from Proteus vulgaris that carries the genes of the PvuII restriction-modification system. Gene 157:73-79. [DOI] [PubMed] [Google Scholar]
- 11.Cesnaviciene, E., G. Mitkaite, K. Stankevicius, A. Janulaitis, and A. Lubys. 2003. Esp1396I restriction-modification system: structural organization and mode of regulation. Nucleic Acids Res. 31:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, C. S., A. White, J. Love, J. R. Murphy, and D. Ringe. 2000. Methyl groups of thymine bases are important for nucleic acid recognition by DtxR. Biochemistry 39:10397-10407. [DOI] [PubMed] [Google Scholar]
- 14.Chen, D., and J. T. Patton. 2001. Reverse transcriptase adds nontemplated nucleotides to cDNAs during 5′-RACE and primer extension. BioTechniques 30:574-583. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 16.Crabb, D. W., and J. E. Dixon. 1987. A method for increasing the sensitivity of chloramphenicol acetyltransferase assays in extracts of transfected cultured cells. Anal. Biochem. 163:88-92. [DOI] [PubMed] [Google Scholar]
- 17.Dhiman, A., and R. Schleif. 2000. Recognition of overlapping nucleotides by AraC and the sigma subunit of RNA polymerase. J. Bacteriol. 182:5076-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dove, S. L., F. W. Huang, and A. Hochschild. 2000. Mechanism for a transcriptional activator that works at the isomerization step. Proc. Natl. Acad. Sci. USA 97:13215-13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuglsang, A., and J. Engberg. 2003. Non-randomness in Shine-Dalgarno regions: links to gene characteristics. Biochem. Biophys. Res. Commun. 302:296-301. [DOI] [PubMed] [Google Scholar]
- 20.Heidmann, S., W. Seifert, C. Kessler, and H. Domdey. 1989. Cloning, characterization and heterologous expression of the SmaI restriction-modification system. Nucleic Acids Res. 17:9783-9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu, L. 2002. Promoter clearance and escape in prokaryotes. Biochim. Biophys. Acta 1577:191-207. [DOI] [PubMed] [Google Scholar]
- 22.Ives, C. L., P. D. Nathan, and J. E. Brooks. 1992. Regulation of the BamHI restriction-modification system by a small intergenic open reading frame, bamHIC, in both Escherichia coli and Bacillus subtilis. J. Bacteriol. 174:7194-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ives, C. L., A. Sohail, and J. E. Brooks. 1995. The regulatory C proteins from different restriction-modification systems can cross-complement. J. Bacteriol. 177:6313-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain, D., B. E. Nickels, L. Sun, A. Hochschild, and S. A. Darst. 2004. Structure of a ternary transcription activation complex. Mol. Cell 13:45-53. [DOI] [PubMed] [Google Scholar]
- 25.Jensen, P. R., and K. Hammer. 1998. The sequence of spacers between the consensus sequences modulates the strength of prokaryotic promoters. Appl. Environ. Microbiol. 64:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, R. L., III, J. C. Jaskula, and G. R. Janssen. 1992. In vivo translational start site selection on leaderless mRNA transcribed from the Streptomyces fradiae aph gene. J. Bacteriol. 174:4753-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kita, K., J. Tsuda, and S. Y. Nakai. 2002. C.EcoO109I, a regulatory protein for production of EcoO109I restriction endonuclease, specifically binds to and bends DNA upstream of its translational start site. Nucleic Acids Res. 30:3558-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar, A., B. Grimes, N. Fujita, K. Makino, R. A. Malloch, R. S. Hayward, and A. Ishihama. 1994. Role of the sigma 70 subunit of Escherichia coli RNA polymerase in transcription activation. J. Mol. Biol. 235:405-413. [DOI] [PubMed] [Google Scholar]
- 29.Li, M., W. R. McClure, and M. M. Susskind. 1997. Changing the mechanism of transcriptional activation by phage lambda repressor. Proc. Natl. Acad. Sci. USA 94:3691-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, M., H. Moyle, and M. M. Susskind. 1994. Target of the transcriptional activation function of phage lambda cI protein. Science 263:75-77. [DOI] [PubMed] [Google Scholar]
- 31.Lin-Chao, S., and S. N. Cohen. 1991. The rate of processing and degradation of antisense RNAI regulates the replication of ColE1-type plasmids in vivo. Cell 65:1233-1242. [DOI] [PubMed] [Google Scholar]
- 32.Makino, K., M. Amemura, S. K. Kim, A. Nakata, and H. Shinagawa. 1993. Role of the sigma 70 subunit of RNA polymerase in transcriptional activation by activator protein PhoB in Escherichia coli. Genes Dev. 7:149-160. [DOI] [PubMed] [Google Scholar]
- 33.McGeehan, J. E., S. Streeter, J. B. Cooper, F. Mohammed, G. C. Fox, and G. G. Kneale. 2004. Crystallization and preliminary X-ray analysis of the controller protein C.AhdI from Aeromonas hydrophilia. Acta Crystallogr. D Biol. Crystallogr. 60:323-325. [DOI] [PubMed] [Google Scholar]
- 34.McKane, M., and R. Milkman. 1995. Transduction, restriction and recombination patterns in Escherichia coli. Genetics 139:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mencia, M., M. Monsalve, F. Rojo, and M. Salas. 1998. Substitution of the C-terminal domain of the Escherichia coli RNA polymerase alpha subunit by that from Bacillus subtilis makes the enzyme responsive to a Bacillus subtilis transcriptional activator. J. Mol. Biol. 275:177-185. [DOI] [PubMed] [Google Scholar]
- 36.Milkman, R. 1997. Recombination and population structure in Escherichia coli. Genetics 146:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Minakhin, L., and K. Severinov. 2003. On the role of the Escherichia coli RNA polymerase sigma 70 region 4.2 and alpha-subunit C-terminal domains in promoter complex formation on the extended −10 galP1 promoter. J. Biol. Chem. 278:29710-29718. [DOI] [PubMed] [Google Scholar]
- 39.Moll, I., S. Grill, C. O. Gualerzi, and U. Blasi. 2002. Leaderless mRNAs in bacteria: surprises in ribosomal recruitment and translational control. Mol. Microbiol. 43:239-246. [DOI] [PubMed] [Google Scholar]
- 40.Munson, G. P., L. G. Holcomb, and J. R. Scott. 2001. Novel group of virulence activators within the AraC family that are not restricted to upstream binding sites. Infect. Immun. 69:186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munson, G. P., and J. R. Scott. 2000. Rns, a virulence regulator within the AraC family, requires binding sites upstream and downstream of its own promoter to function as an activator. Mol. Microbiol. 36:1391-1402. [DOI] [PubMed] [Google Scholar]
- 42.Murray, N. E. 2002. Immigration control of DNA in bacteria: self versus non-self. Microbiology 148:3-20. [DOI] [PubMed] [Google Scholar]
- 43.Naderer, M., J. R. Brust, D. Knowle, and R. M. Blumenthal. 2002. Mobility of a restriction-modification system revealed by its genetic contexts in three hosts. J. Bacteriol. 184:2411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakayama, Y., and I. Kobayashi. 1998. Restriction-modification gene complexes as selfish gene entities: roles of a regulatory system in their establishment, maintenance, and apoptotic mutual exclusion. Proc. Natl. Acad. Sci. USA 95:6442-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nickels, B. E., S. L. Dove, K. S. Murakami, S. A. Darst, and A. Hochschild. 2002. Protein-protein and protein-DNA interactions of σ70 region 4 involved in transcription activation by lambda cI. J. Mol. Biol. 324:17-34. [DOI] [PubMed] [Google Scholar]
- 46.O'Donnell, S. M., and G. R. Janssen. 2002. Leaderless mRNAs bind 70S ribosomes more strongly than 30S ribosomal subunits in Escherichia coli. J. Bacteriol. 184:6730-6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pande, S., A. Makela, S. L. Dove, B. E. Nickels, A. Hochschild, and D. M. Hinton. 2002. The bacteriophage T4 transcription activator MotA interacts with the far-C-terminal region of the σ70 subunit of Escherichia coli RNA polymerase. J. Bacteriol. 184:3957-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Rueda, E., J. D. Gralla, and J. Collado-Vides. 1998. Genomic position analyses and the transcription machinery. J. Mol. Biol. 275:165-170. [DOI] [PubMed] [Google Scholar]
- 49.Platko, J. V., D. A. Willins, and J. M. Calvo. 1990. The ilvIH operon of Escherichia coli is positively regulated. J. Bacteriol. 172:4563-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raleigh, E. A., N. E. Murray, H. Revel, R. M. Blumenthal, D. Westaway, A. D. Reith, P. W. J. Rigby, J. Elhai, and D. Hanahan. 1988. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 15:1563-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redaschi, N., and T. A. Bickle. 1996. DNA restriction and modification systems, p. 773-781. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, vol. 1. ASM Press, Washington, D.C.
- 52.Rhee, K. Y., D. F. Senear, and G. W. Hatfield. 1998. Activation of gene expression by a ligand-induced conformational change of a protein-DNA complex. J. Biol. Chem. 273:11257-11266. [DOI] [PubMed] [Google Scholar]
- 53.Rimseliene, R., R. Vaisvila, and A. Janulaitis. 1995. The eco72IC gene specifies a trans-acting factor which influences expression of both DNA methyltransferase and endonuclease from the Eco72I restriction-modification system. Gene 157:217-219. [DOI] [PubMed] [Google Scholar]
- 54.Rocha, E. P., A. Danchin, and A. Viari. 2001. Evolutionary role of restriction/modification systems as revealed by comparative genome analysis. Genome Res. 11:946-958. [DOI] [PubMed] [Google Scholar]
- 55.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider, T. D., and R. M. Stephens. 1990. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 18:6097-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sohail, A., C. L. Ives, and J. E. Brooks. 1995. Purification and characterization of C.BamHI, a regulator of the BamHI restriction-modification system. Gene 157:227-228. [DOI] [PubMed] [Google Scholar]
- 58.Tao, T., and R. M. Blumenthal. 1992. Sequence and characterization of pvuIIR, the PvuII endonuclease gene, and of pvuIIC, its regulatory gene. J. Bacteriol. 174:3395-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tao, T., J. C. Bourne, and R. M. Blumenthal. 1991. A family of regulatory genes associated with type II restriction-modification systems. J. Bacteriol. 173:1367-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vijesurier, R. M., L. Carlock, R. M. Blumenthal, and J. C. Dunbar. 2000. Role and mechanism of action of C.PvuII, a regulatory protein conserved among restriction-modification systems. J. Bacteriol. 182:477-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voskuil, M. I., and G. H. Chambliss. 2002. The TRTGn motif stabilizes the transcription initiation open complex. J. Mol. Biol. 322:521-532. [DOI] [PubMed] [Google Scholar]
- 62.Warne, S. E., and P. L. deHaseth. 1993. Promoter recognition by Escherichia coli RNA polymerase. Effects of single base pair deletions and insertions in the spacer DNA separating the −10 and −35 regions are dependent on spacer DNA sequence. Biochemistry 32:6134-6140. [DOI] [PubMed] [Google Scholar]
- 63.Wood, T. I., K. L. Griffith, W. P. Fawcett, K. W. Jair, T. D. Schneider, and R. E. Wolf, Jr. 1999. Interdependence of the position and orientation of SoxS binding sites in the transcriptional activation of the class I subset of Escherichia coli superoxide-inducible promoters. Mol. Microbiol. 34:414-430. [DOI] [PubMed] [Google Scholar]
- 64.Zheleznaya, L. A., D. E. Kainov, A. K. Yunusova, and N. I. Matvienko. 2003. Regulatory C protein of the EcoRV modification-restriction system. Biochemistry (Moscow) 68:105-110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.