Abstract
The Chlamydia trachomatis divalent cation-dependent regulator (DcrA), encoded by open reading frame CT296, is a distant relative of the ferric uptake regulator (Fur) family of iron-responsive regulators. Chlamydial DcrA specifically binds to a consensus Escherichia coli Fur box and is able to complement an E. coli Fur mutant. In this report, the E. coli Fur titration assay (FURTA) was used to locate chlamydial genomic sequences that are recognized by E. coli Fur. The predictive regulatory regions of 28 C. trachomatis open reading frames contained sequences functionally recognized by E. coli Fur; targets include components of the type III secretion pathway, elements involved in envelope and cell wall biogenesis, predicted transport proteins, oxidative defense enzymes, and components of metabolic pathways. Selected FURTA-positive sequences were subsequently examined for recognition by C. trachomatis DcrA using an electrophoretic mobility shift assay. The resultant data show that C. trachomatis DcrA binds to native chlamydial genomic sequences and, overall, substantiate a functional relationship between chlamydial DcrA and the Fur family of regulators.
The leading bacterial agent of sexually transmitted infections, Chlamydia trachomatis, infects and grows within genital mucosal epithelial cells. In menstruating women, endometrial epithelial cells are subject to cyclic fluctuations of estrogen and progesterone, which, in turn, affect the concentrations and availability of micronutrients such as iron (2, 13, 32). Numerous studies have shown that obligate intracellular pathogens are highly sensitive to iron deprivation or overload; however, it is also clear that each pathogen exhibits different responses reflective of their unique metabolic requirements and intracellular habitats (reviewed in references 18 and 27).
Previous studies in our laboratory using a polarized endometrial epithelial cell model of C. trachomatis infection have shown that iron limitation causes (i) a significant decrease in chlamydial infectivity, (ii) a delay in chlamydial development, (iii) the formation of abnormal morphological chlamydial forms, (iv) an increased quantity of membranous blebs within chlamydial inclusions, and (v) quantitative changes in the expression levels of specific chlamydial proteins (19). Related studies with Chlamydia pneumoniae concur with our findings and, importantly, indicate that this respiratory pathogen is exquisitely sensitive to iron availability in direct comparison with C. trachomatis (1). Overall, the chlamydiae have a demonstrated requirement for iron that is supported by inferred metabolic processes from whole-genomic-sequence analyses (16, 22).
Altered expression of specific bacterial proteins and virulence factors in response to iron limitation is commonly, but not exclusively, regulated at the level of transcription by metal-dependent repressors related to the Escherichia coli ferric uptake regulator (Fur) (6, 12, 26) or the Corynebacterium diphtheriae diphtheria toxin regulator (DtxR) (24). Although initial analysis of the chlamydial genome sequence did not reveal a Fur or DtxR homolog, we identified a distant relative of Fur that is encoded by open reading frame (ORF) CT296 (28). Chlamydial divalent cation-dependent regulator A (DcrA), which shares 37% amino acid similarity with E. coli Fur, specifically binds to a synthetic E. coli iron-responsive promoter sequence, or Fur box, and is also able to functionally complement an E. coli fur mutant.
The objective of this study was to identify chlamydial genomic sequences that serve as substrates for chlamydial DcrA. The use of consensus Fur box sequences, chlamydial genome sequences, and alignment algorithms is not an expeditious approach and is confounded by the promiscuity among the native substrate recognition patterns of Fur-like regulators in bacterial pathogens (10, 21, 25). It is also noteworthy that chlamydial genomes characteristically have short noncoding or intergenic sequences between putative ORFs (20). Therefore, we used the functional Fur titration assay (FURTA) to locate chlamydial genomic sequences that are bound by E. coli Fur (23). FURTA-positive sequences were subsequently examined for their direct interaction with chlamydial DcrA to substantiate our hypothesis that DcrA is a member of the Fur-like family of regulators.
MATERIALS AND METHODS
Construction of a FURTA library, screening, and sequencing.
Whole genomic DNA from C. trachomatis serovar E/UW-5CX was isolated from 2 × 1010 purified elementary bodies with a genomic-DNA kit from QIAGEN (Valencia, Calif.) according to the instructions of the manufacturer. DNA was partially digested with restriction endonuclease Sau3A1, and fragments of 1,000 bp or less were purified from preparative 0.5% agarose gels. High-copy-number plasmid pGEM3Zf(+) (Promega, Inc., Madison, Wis.) was completely digested with the compatible restriction enzyme BamHI and dephosphorylated with HK phosphatase (Epicentre Technologies, Madison, Wis.), and phosphatase activity was subsequently inactivated by heating the mixtures at 65°C for 30 min. After overnight incubation of pGEM3Zf(+) with chlamydial chromosomal fragments at 16°C with T4 DNA ligase, the resultant ligation mixtures were transformed into calcium-chloride-competent E. coli H1717 (23) and plated on MacConkey agar containing 50 μg of ampicillin per ml and 25 μM ferric ammonium sulfate. The plasmid vectors pGEM3Zf(+) (alone) and pGEM3Zf(+) (containing a synthetic E. coli Fur box [5′-GATAATGATAATCATTATC-3′]) in the multiple-cloning site were transformed into E. coli H1717 as negative and positive controls, respectively.
Plasmid preparations were obtained from recombinant E. coli cells exhibiting strong lactose hydrolysis on MacConkey agar plates by using the Concert kit from Invitrogen, Inc. (Carlsbad, Calif.). Automated DNA sequencing of each plasmid insert was conducted in both directions with the T7 and SP6 primers. The chromosomal location of each plasmid insert was determined by searching the C. trachomatis serovar D database (http://chlamydia-www.berkeley.edu:4231/and http://www.stdgen.lanl.gov/) using BLASTN 2.2.1 (http://www.ncbi.nlm.nih.gov). As a control, sequences were also scanned against the Mycoplasma pneumoniae genome; all sequences were negative for contamination by Mycoplasma.
Purification of DcrA.
Recombinant C. trachomatis serovar E DcrA was purified from E. coli LMG194(pJER1) using nondenaturing conditions and affinity chromatography as generally described previously (28). Cultures were incubated at 37°C to mid-log phase (A600 = 0.5) in reduced medium containing 0.2% (vol/vol) glucose, washed, and resuspended in prewarmed glucose-free reduced medium containing arabinose (0.02%, wt/vol) for a 3-h induction of recombinant protein expression. Induced whole-cell pellets were stored at −20°C. Upon removal from the freezer, cell pellets were kept on ice and suspended in 10 ml of a buffer containing 20 mM sodium phosphate (pH 7.8), 500 mM NaCl, 1 mg of lysozyme, 50 μg of RNase A (QIAGEN, Inc.), 100 μl of protease inhibitor cocktail (Sigma), and 1 g of Chelex-100. The cell slurry was then sonicated on ice 10 times for 10 s each minute using a W-385 Probe-tip sonicator (Heat-Systems Ultrasonics, Inc.) and subsequently centrifuged at 3,000 × g for 15 min at 4°C. The supernatant containing soluble DcrA was kept on ice prior to chromatography.
A nickel chloride affinity resin (ProBond; Invitrogen Co.) was used for purification and was regenerated between preparations by washing it twice in 50 mM EDTA (pH 8) and then by washing it once in 0.5 N NaOH, washing it generously in sterile deionized water, and recharging it in 5 mg of nickel chloride hexahydrate (Sigma Co.) per ml. After being washed twice with sterile, deionized water, the resin was equilibrated in 20 mM sodium phosphate buffer (pH 7.8) and 500 mM NaCl (PBS).
Soluble-DcrA-containing supernatants were applied to the nickel affinity resin at a ratio of 10 ml per 5 ml of packed resin and gently mixed at 4°C for 20 min for adsorption in a batch format. The resin with bound protein was allowed to settle by gravity, and the supernatant containing unbound proteins was gently removed with a glass pipette. The resin was washed four times with cold PBS.
Proteins that bound to the nickel resin were eluted using an increasing stepwise gradient of 50 mM to 1 M imidazole in cold PBS (pH 6.0). The resin was sequentially exposed to 1 ml of 50 mM (once), 1 ml of 250 mM (once), 1 ml of 350 mM (once), 1 ml of 0.5 M (once), 1 ml of 0.75 M (four times), and 1 ml of 1 M (three times) imidazole. Highly purified recombinant DcrA was present in fractions following elution with 1 M imidazole. Those fractions were pooled, dialyzed overnight against 10 mM phosphate buffer (pH 7.5), and stored at −20°C in the presence of 20% (vol/vol) glycerol. Fifty-microliter aliquots were examined for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting and to determine protein concentrations as described previously (28).
EMSA.
Chlamydial genomic inserts were obtained from the FURTA-positive plasmids pJER123, pJER178, pJER406, pJER408, and pJER435 by restriction endonuclease digestion and purified by agarose gel electrophoresis. The DNA substrates were subsequently labeled with biotin by using the Biotin 3′-end DNA-labeling kit (Pierce). Prior to the addition of substrates for electrophoretic mobility shift assay (EMSA), purified DcrA was equilibrated in EMSA binding buffer (20 mM Tris-HCl [pH 8.0], 50 mM KCl, 5% [vol/vol] glycerol, 0.5 mM dithiothreitol, and 0.1 mg of bovine serum albumin per ml) for 15 min at room temperature. Approximately 50 fmol of labeled DNA was incubated with increasing concentrations of DcrA (0, 400 nM, 800 nM, or 1.2 μM) in EMSA buffer containing 100 μM MnCl2 and 2 μg of salmon sperm DNA per ml for 30 min at room temperature; unlabeled substrate DNA was included in some reaction mixtures to examine competitive binding. Reaction mixtures were resolved on 6% (wt/vol) nondenaturing acrylamide gels in 40 mM Tris acetate-0.5 mM dithiothreitol, and products were transferred to positively charged nylon membranes and fixed by exposure to UV light. The detection of chemiluminescence was done using the LightShift chemiluminescent EMSA kit (Pierce), and signals were visualized by autoradiography.
Transmission electron microscopy.
Polarized human endometrial epithelial cells (HEC-1B) were infected with C. trachomatis serovar E and cultured under iron-sufficient and iron-deficient conditions as previously described in detail (19). At 24 h postinoculation, samples were washed, fixed in 2% (vol/vol) glutaraldehyde plus 0.5% (vol/vol) paraformaldehyde, processed, embedded in Epon-Araldite resin, and stained for high-contrast morphology as described previously (29). Ultrathin sections were examined with a Philips Tecnai 10 transmission electron microscope (FEI Company, Hillsboro, Oreg.) operating at 80 kV.
RESULTS AND DISCUSSION
C. trachomatis genome sequences are functionally recognized by E. coli Fur.
In order to initiate studies of the interaction of chlamydial DcrA with native chlamydial genome sequences, a selective approach was needed to identify candidate regulatory regions within the 1.04-Mbp C. trachomatis chromosome (22). It was determined that a stepwise search for Fur boxes in chlamydial genomic regulatory sequences, using alignment algorithms, would not yield useful information for several reasons. Logistically, a sequence of 20 residues or less imposes limitations for standard searches and generally results in no significant similarities. Short sequences can be used with recent derivations of BLAST, but the E (or expect) value is generally increased to lengthen the list of expectations; this maneuver also increases background noise. Scientifically, not only do optimal consensus sequences recognized by Fur proteins show variation among different bacterial genera (10, 21, 25), the traditional 19-bp palindromic E. coli Fur box has recently been redefined in more flexible terms. In one model, a Fur box is defined as having three hexameric repeats of NAT(A/T)AT —each of which may be present in either orientation within the regulatory regions of iron-responsive genes (7, 14). Other investigators have defined Fur boxes as consisting of a 15-bp inverted repeat sequence that occurs twice within a 19-bp contiguous sequence (3). For C. trachomatis, the actual structures of chlamydial promoters are intrinsically weak and difficult to recognize without direct experimental testing (20).
FURTA has been used on several gram-negative bacteria, and some gram-positive bacteria, to identify candidate genes for regulation by iron (8, 23, 25); the availability of entire genomic sequences makes this functional system an attractive approach to begin to localize potential iron-responsive genes in organisms for which amenable genetic systems have not yet been developed. FURTA strain E. coli H1717 contains a chromosomal fhuF::lacZ fusion, and fhuF is preceded by a low-affinity Fur box; the introduction of high-copy-number plasmids that contain Fur-binding sequences in the multiple cloning site causes removal of Fur from the fhuF Fur box (23). The resultant expression of LacZ is visualized as lactose-positive colonies on MacConkey agar plates. In this study, a C. trachomatis serovar E genomic library, consisting of approximately 5,000 ampicillin-resistant colonies, was generated in E. coli H1717. Four hundred forty-four isolates were mildly lactose positive, and 50 isolates were strongly lactose positive, representing 9 and 1% of this library, respectively. The mildly lactose-positive isolates likely contain less-conserved substrates for E. coli Fur, whereas recombinant plasmids from strongly lactose-positive isolates contain chlamydial genomic inserts with a high binding affinity for E. coli Fur. As a precautionary note, false-positive reactions can occur in FURTA when recombinants express a metal-binding protein (or domain) that causes the removal of iron from Fur and, in turn, the removal of Fur from the fhuF promoter (23).
E. coli Fur recognition sequences are broadly distributed throughout the C. trachomatis genome.
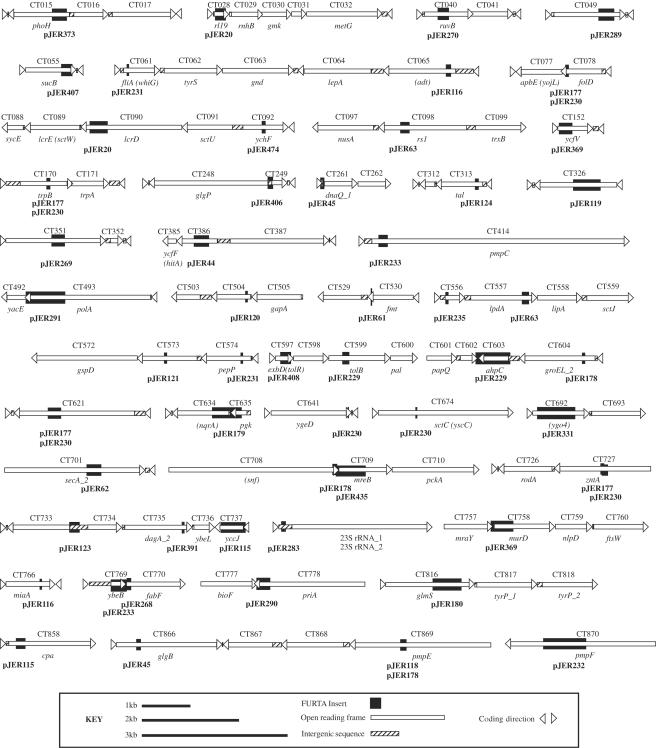
Bidirectional DNA sequencing was conducted on the 50 chlamydial plasmid inserts that showed strong binding affinity by E. coli Fur. The chromosomal location of each sequence was determined by searching the C. trachomatis serovar D database. The resultant location of FURTA-positive sequences (Fig. 1) revealed a broad distribution among predicted chlamydial ORFs within the entire genome. Sizes of the Fur-binding sequences ranged from 37 bp (pJER61) to 864 bp (pJER230); two plasmids contained identical genomic inserts, and two plasmids (pJER177 and pJER230) showed overlapping, but nonidentical, genomic inserts. Nine plasmids (data not shown) contained randomly ligated fragments of chlamydial DNA, and no contiguous sections of the chromosome greater than 18 bp were represented.
FIG. 1.
Distribution of FURTA-positive sequences throughout the C. trachomatis serovar D chromosome. Chlamydial ORFs are represented as open bars, with arrowheads indicating the coding direction. ORF numbers and predicted gene designations are provided above and below each bar, respectively. Recombinant plasmid designations are provided in bold type below each FURTA-positive insert. The data were assembled using Adobe PageMaker 7.0, embedded, and exported in Adobe Illustrator 10 as EPS and TIFF files.
Figure 1 and the chlamydial genomic websites illustrate the notably small representation of intergenic sequences in the C. trachomatis chromosome, indicating that regulatory sequences for many genes are present within the coding region of upstream genes. We view the FURTA data as belonging to one of two groups (Table 1). The first group consists of chlamydial ORFs for which the positioning of FURTA-positive sequences is within 300 bp upstream of the predicted translational start codon of a specific gene. A second group consists of chlamydial ORFs with a FURTA-positive sequence located less than 100 bp downstream of the start codon. Gene repression by E. coli Fur has been reported to involve sequences greater than 300 bp upstream, as well as sequences downstream, of the translational start codon (5). Neisseria gonorrhoeae Fur binds to sequences downstream of the −10 promoter box in the opa multigene family (21). Therefore, it is conceivable that iron-responsive genes in chlamydiae may involve binding of repressors at sites considerably removed from the start codon. Fur is also known to exhibit cooperative binding after interaction with a minimum substrate, and transmission electron microscopy of naturally occurring iron-regulated promoters shows multimers of Fur wrapping around the DNA helix (9, 15). These data help explain why FURTA-positive results were observed for Salmonella enterica serovar Typhimurium promoters having as little as 12 bp of the 19-bp traditional consensus Fur box sequence (25).
TABLE 1.
Description of FURTA-positive chlamydial genomic plasmid inserts
| Plasmid designation | Insert size (bp) | Presence of ≥2 separate regionsa | Target ORF(s) (I or II)b | Descriptionc |
|---|---|---|---|---|
| pJER20 | 430 | Yes | CT089 lcrE (I) (sctW copN) | Regulatory protein, type III secretion |
| CT028 rl19 (II) | Ribosomal protein | |||
| pJER45 | 223 | Yes | CT261 dnaQ_I (I) | DNA polymerase III alpha or epsilon chain |
| pJER61 | 37 | No | CT529 (I) | Chlamydia-specific hypothetical protein |
| pJER63 | 420 | Yes | CT558 lipA (I) | Lipoic acid synthase |
| pJER115 | 771 | Yes | CT737 yccJ (I) | SET domain DNA binding protein |
| pJER120 | 62 | No | CT504 (II) | Chlamydia-specific hypothetical protein |
| pJER123 | 237 | No | CT733 (I) | Chlamydia-specific hypothetical protein |
| CT734 (I) | ||||
| pJER177 | 724 | Yes | CT077 apbE (I) (yojL) | Inner membrane lipoprotein |
| pJER178 | 359 | Yes | CT709 mreB (I) | Shape-determining protein |
| pJER179 | 739 | No | CT634 nqrA (I) | NADH-ubiquinone oxidoreductase |
| pJER180 | 646 | No | CT817 tyrP_1 (I) | Tyrosine/tryptophan transport protein |
| pJER229 | 782 | Yes | CT603 aphC (I) | Antioxidant peroxidase |
| pJER230 | 864 | Yes | CT077 apbE (I) (yojL) | Inner membrane lipoprotein |
| pJER233 | 771 | Yes | CT769 ybeB (I) | Hypothetical protein |
| CT770 fabF (I) | Beta-ketoacyl ACP-synthase | |||
| CT414 pmpC (II) | Outer membrane protein | |||
| pJER235 | 84 | No | CT556 (II) | Chlamydia-specific hypothetical protein |
| pJER268 | 178 | No | CT770 fabF (I) | Beta-ketoacyl ACP-synthase |
| pJER291 | 787 | No | CT492 yacE (I) | Probable ATPase or kinase |
| pJER369 | 846 | Yes | CT152 ycfV (II) | ABC transporter ATPase |
| CT758 murD (I) | N-Acetylmuramyl l-alanine d-glutamate ligase | |||
| pJER373 | 394 | No | CT015 phoH (II) | Phosphate starvation-inducible ATPase |
| CT016 (I) | Chlamydia-specific hypothetical protein | |||
| pJER391 | 66 | No | CT736 ybeL (I) | Hypothetical protein |
| pJER406 | 186 | No | CT248 glgP (I) | Alpha glycan phosphorylase |
| CT249 (I) | Hypothetical protein | |||
| pJER408 | 211 | No | CT597 tolR (II) | Transport protein |
| CT598 (I) | Chlamydia-specific hypothetical protein | |||
| pJER435 | 581 | No | CT709 mreB (I) | Shape-determining protein |
The plasmid insert contains two or more genomic fragments that have ligated together from separate, noncontiguous regions of the C. trachomatis chromosome.
Target ORFs in group I indicate that the FURTA-positive sequence is less than 300 bp upstream of the predicted translational start codon; target ORFs in group II indicate that the FURTA-positive sequence is less than 100 bp downstream of the predicted translational start codon.
ACP, acyl carrier protein.
The first group of 21 FURTA-positive target ORFs (Table 1) encompasses six enzymes for basic metabolic processes and replication, including a predicted NADH-ubiquinone oxidoreductase (CT634 nrqA). ORF CT603 (aphC) is a predicted antioxidant peroxidase that may participate in oxidative defense mechanisms; the bacterial response to iron deprivation is closely coupled with oxidative stress (30). Nine ORFs are hypothetical proteins of unknown function. CT089 (lcrE) encodes a putative regulatory protein for the type III secretion pathway, and several genes downstream of FURTA-positive sequences are putative type III secretion components. Four ORFs participate in envelope biogenesis and transport; CT077 (apbE) is a predicted membrane lipoprotein, CT817 (tyrP_1) is involved in amino acid transport, CT758 (murD) is a predicted N-acetylmuramyl l-alanine d-glutamate ligase, and CT709 (mreB) is a shape-determining protein regulated by Fur in E. coli (17).
Seven ORFs have a FURTA-positive sequence positioned less than 100 bp downstream of the predicted start codon; four ORFs may be involved in membrane transport mechanisms (CT015 phoH, CT152 ycfV, CT414 pmpC, and CT597 tolR). Additional targets include a 50S ribosomal protein and two hypothetical proteins. Although operon arrangements or polycistronic messages are not yet known for many chlamydial genes, certain ORFs downstream of FURTA-positive sequences are reasonable targets for regulation by iron; examples include CT312, a possible ferredoxin, and CT415 (yebL), a predicted solute-binding protein for chelated metals.
Although the chlamydial genes identified by the FURTA system are noteworthy, the data should be viewed in the appropriate context. The FURTA data are not comprehensive or absolute, and continued sequencing of the 444 isolates with lower affinities for E. coli Fur would likely unveil additional genomic targets for chlamydial DcrA. Moreover, recent data for other bacterial pathogens illustrate that as much as 50% of iron-responsive genes are not regulated by Fur and that Fur does not bind to their upstream promoters (10). FURTA allowed us to meet the objective of locating reasonable targets for DcrA from among 1.04 Mbp of genome sequence.
Chlamydial DcrA specifically interacts with FURTA-positive chlamydial genome sequences.
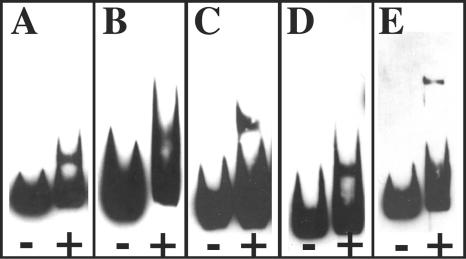
The primary objective of this study was to determine whether or not DcrA interacts with native C. trachomatis sequences. Therefore, five FURTA-positive sequences were utilized in an EMSA. DNA templates (and sizes) originated from the following plasmids: pJER123 (237 bp), pJER178 (359 bp), pJER406 (186 bp), pJER408 (211 bp), and pJER435 (581 bp). Selection was based on sequences that are positioned less than 300 bp upstream of the predicted translational start codon of the chlamydial ORF. As shown in Fig. 2, all selected FURTA-positive templates are bound by DcrA.
FIG. 2.
Binding of chlamydial DcrA to FURTA-positive chlamydial genomic DNA templates. Biotin-labeled inserts (50 fmol) from pJER123 (A), pJER178 (B), pJER406 (C), pJER408 (D), and pJER435 (E) were incubated with (+) or without (−) 800 nM purified DcrA in the presence of 100 μM MnCl2 as a cofactor. Resultant EMSA images were scanned into Adobe Photoshop 7.0 using a Microtek Scan Maker 5, embedded, assembled, and exported in Adobe Illustrator 10 as EPS and TIFF files.
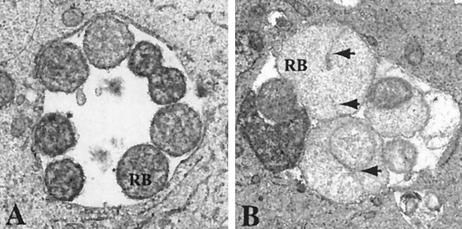
Two genomic templates were selected for further analysis. First, the 359-bp insert in plasmid pJER178 was mapped to the putative upstream regulatory region of C. trachomatis ORF709, which encodes a protein termed MreB; MreB is (i) a shape-determining protein and (ii) regulated by Fur in E. coli (17). As illustrated in Fig. 3, C. trachomatis serovar E reticulate bodies growing in iron-deficient endometrial epithelial cells exhibit unusual septum formation as one of several morphological aberrations due to iron deprivation (19). Second, the 186-bp insert in plasmid pJER406 was selected based on its exclusive positioning in the intergenic spacing region between C. trachomatis ORF248 (glgP, glycogen phosphorylase) and ORF249 (hypothetical protein). This 186-bp template contains the contiguous sequence [TAATAT] [TAATAT] T [TCTTAT], representing two convincing hexameric repeats and one degenerate hexamer, similar to what has been found with well-defined substrates for E. coli Fur (7).
FIG. 3.
Iron limitation induces unusual septum formation in C. trachomatis serovar E reticulate bodies (RB). The morphology of RB growing within polarized endometrial epithelial cells under iron-sufficient conditions (A) and iron-deficient conditions (B) is shown at 24 h postinoculation. Arrowheads indicate the unusual septa in panel B. Electron micrographs were saved in Adobe Photoshop 7.0, embedded, assembled, and exported in Adobe Illustrator 10 as EPS and TIFF files.
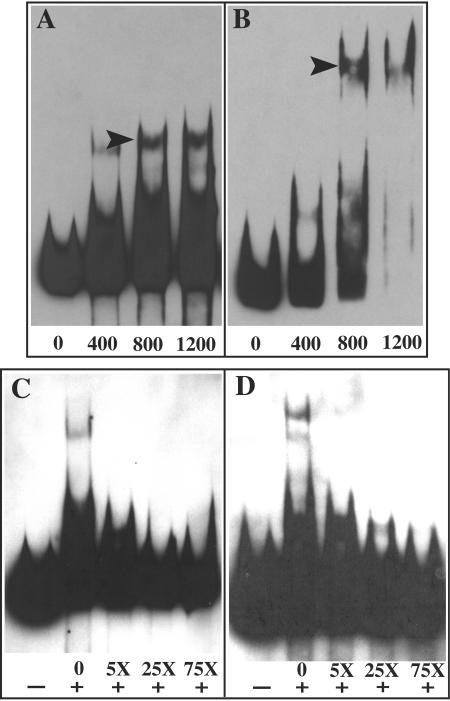
The selected templates were incubated with increasing concentrations of DcrA (Fig. 4A and B), and the results show a concentration-dependent increase in the amount of the shifted DNA template. Concentration ratios in excess of 800 nM DcrA to 50 fmol of DNA reveal notable increases in multimeric DcrA complexes or supershifted DNA (Fig. 4). To examine the specificity of interaction, increasing concentrations of unlabeled template from pJER178 and pJER406 were added to reaction mixtures prior to resolution by EMSA. When unlabeled template was present at a fivefold-higher concentration than that of the labeled template, the majority of multimeric DcrA complexes were eliminated (Fig. 4C and D); concentrations of unlabeled template in excess of 25-fold virtually eliminated the electrophoretic shift of labeled DNA to confirm the specificity of the DcrA interaction with the FURTA-positive sequences.
FIG. 4.
Binding specificity of chlamydial DcrA to selected DNA templates by EMSA. Increasing concentrations of purified DcrA (400, 800, and 1,200 nM) were added to 50 fmol of biotin-labeled plasmid inserts from pJER178 (A) and pJER406 (B) to illustrate a concentration-dependent increase in binding. Arrowheads indicate multimeric DcrA-DNA complexes. To examine competitive inhibition of chlamydial DcrA binding to selected DNA templates, 800 nM purified DcrA was absent (−) or present (+) in EMSA reaction buffer and 50 fmol of biotin-labeled substrate from pJER178 (C) and pJER406 (D) was incubated with a 5-, 25-, or 75-fold molar excess of unlabeled substrate. EMSA images were scanned into Adobe Photoshop 7.0 with a Microtek Scan Maker 5, embedded, assembled, and exported in Adobe Illustrator 10 as EPS and TIFF files.
Overall, continuing studies should provide intriguing new insights into the functionality of Fur-related proteins. The chlamydiae are unique in their capacity to express eukaryote-like nucleic binding proteins, such as histone homologs (4, 11) and integration host factor (31); thus, DNA bending may also influence the ability or inability of chlamydial regulatory factors to interact with their substrates. Other investigators have suggested that chlamydial promoters may be intrinsically weak to allow accessory transcription factors to regulate the complexity of chlamydial growth and development within eukaryotic cells (20). Our continuing studies should provide additional understanding of the functional conservation or differences between two evolutionarily isolated metal-dependent repressors—E. coli Fur and C. trachomatis DcrA.
Acknowledgments
We thank the directors of the James H. Quillen College of Medicine Core Facilities in Molecular Biology and Electron Microscopy for sequencing and use of the transmission electron microscope, respectively.
This work was supported by a grant from the National Institutes of Health (RO1AI040915) to J.E.R.
REFERENCES
- 1.Al-Younes, H. M., T. Rudel, V. Brinkmann, A. J. Szczepek, and T. F. Meyer. 2001. Low iron availability modulates the course of Chlamydia pneumoniae infection. Cell. Microbiol. 3:427-437. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, N. C. 2000. Iron homeostasis: insights from genetics and animal models. Nat. Rev. 1:208-216. [DOI] [PubMed] [Google Scholar]
- 3.Baichoo, N., and J. D. Helmann. 2002. Recognition of DNA by Fur: a reinterpretation of the Fur box consensus sequence. J. Bacteriol. 184:5826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry, C. E., III, S. F. Hayes, and T. Hackstadt. 1992. Nucleoid condensation in Escherichia coli that express a chlamydial histone homolog. Science 256:377-379. [DOI] [PubMed] [Google Scholar]
- 5.Boyer, A. E., and P. C. Tai. 1998. Characterization of the cvaA and cvi promoters of the colicin V export system: iron-dependent transcription of cvaA is modulated by downstream sequences. J. Bacteriol. 180:1662-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 2000. Evidence of an unusually long operator for the fur repressor in the aerobactin promoter of Escherichia coli. J. Biol. Chem. 275:24709-24714. [DOI] [PubMed] [Google Scholar]
- 8.Fassbinder, F., A. H. M. van Vliet, V. Gimmel, J. G. Kusters, M. Kist, and S. Bereswill. 2000. Identification of iron-regulated genes of Helicobacter pylori by a modified Fur titration assay (FURTA-Hp). FEMS Microbiol. Lett. 184:225-229. [DOI] [PubMed] [Google Scholar]
- 9.Frechon, D., and E. Le Cam. 1994. Fur (ferric uptake regulation) protein interaction with target DNA: comparison of gel retardation, footprinting and electron microscopy analyses. Biochem. Biophys. Res. Commun. 201:346-355. [DOI] [PubMed] [Google Scholar]
- 10.Grifantini, R., S. Sebastian, E. Frigimelica, M. Draghi, E. Bartolini, A. Muzzi, R. Rappuoli, G. Grandi, and C. A. Genco. 2003. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc. Natl. Acad. Sci. USA 100:9542-9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackstadt, T., W. Baehr, and Y. Ying. 1991. Chlamydia trachomatis developmentally regulated protein is homologous to eukaryotic histone H1. Proc. Natl. Acad. Sci. USA 88:3937-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hantke, K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4:172-177. [DOI] [PubMed] [Google Scholar]
- 13.Kelver, M. E., A. Kaul, B. Nowicki, W. E. Findley, T. W. Hutchens, and M. Nagamani. 1996. Estrogen regulation of lactoferrin expression in human endometrium. Am. J. Reprod. Immunol. 36:243-247. [DOI] [PubMed] [Google Scholar]
- 14.Lavrrar, J. L., and M. A. McIntosh. 2003. Architecture of a Fur binding site: a comparative analysis. J. Bacteriol. 185:2194-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Cam, E., D. Frechon, M. Barray, A. Fourcade, and E. Delain. 1994. Observation of binding and polymerization of Fur repressor onto operator-containing DNA with electron and atomic force microscopes. Proc. Natl. Acad. Sci. USA 91:11816-11820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClarty, G. 1999. Chlamydial metabolism as inferred from the complete genome sequence, p. 69-100. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 17.Oh, M., S. H. Chai, and S. Wee. 1999. Isolation and identification of Fur binding genes in Escherichia coli. Mol. Cells 9:517-525. [PubMed] [Google Scholar]
- 18.Oppenheimer, S. J. 2001. Iron and its relation to immunity and infectious disease. J. Nutr. 131:616S-635S. [DOI] [PubMed] [Google Scholar]
- 19.Raulston, J. E. 1997. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect. Immun. 65:4539-4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaumburg, C. S., and M. Tan. 2003. Mutational analysis of the Chlamydia trachomatis dnaK promoter defines the optimal −35 promoter element. Nucleic Acids Res. 31:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastian, S., S. Agarwal, J. R. Murphy, and C. A. Genco. 2002. The gonococcal fur regulon: identification of additional genes involved in major catabolic, recombination, and secretory pathways. J. Bacteriol. 184:3965-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 23.Stojiljkovic, I., A. J. Baumler, and K. Hantke. 1994. Fur regulon in Gram negative bacteria: identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J. Mol. Biol. 236:531-545. [DOI] [PubMed] [Google Scholar]
- 24.Tao, X., N. Schiering, H.-Y. Zeng, D. Ringe, and J. R. Murphy. 1994. Iron, DtxR, and the regulation of diphtheria toxin expression. Mol. Microbiol. 14:191-197. [DOI] [PubMed] [Google Scholar]
- 25.Tsolis, R. M., A. J. Baumler, I. Stojiljkovic, and F. Heffron. 1995. Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J. Bacteriol. 177:4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasil, M. L., and U. A. Ochsner. 1999. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol. Microbiol. 34:399-413. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg, E. D. 1992. Iron depletion: a defence against intracellular infection and neoplasia. Life Sci. 50:1289-1297. [DOI] [PubMed] [Google Scholar]
- 28.Wyllie, S., and J. E. Raulston. 2001. Identifying regulators of transcription in an obligate intracellular pathogen: a metal-dependent repressor in Chlamydia trachomatis. Mol. Microbiol. 40:1027-1036. [DOI] [PubMed] [Google Scholar]
- 29.Wyrick, P. B., J. Choong, C. H. Davis, S. T. Knight, M. O. Royal, A. S. Maslow, and C. R. Bagnell. 1989. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect. Immun. 57:2378-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong, J., A. L. Douglas, and T. P. Hatch. 2001. Characterization of integration host factor (IHF) binding upstream of the cysteine-rich protein operon (omcAB) promoter of Chlamydia trachomatis LGV serovar L2. Mol. Microbiol. 41:451-462. [DOI] [PubMed] [Google Scholar]
- 32.Zhu, L. J., M. K. Bagchi, and I. C. Bagchi. 1995. Ferritin heavy chain is a progesterone-inducible marker in the uterus during pregnancy. Endocrinology 136:4106-4115. [DOI] [PubMed] [Google Scholar]