Abstract
Angiogenesis is considered an important issue in the development of biomaterials for the successful regeneration of tissues including bone. While growth factors are commonly used with biomaterials to promote angiogenesis, some ions released from biomaterials can also contribute to angiogenic events. Many silica-based biomaterials have been widely used for the repair and regeneration of tissues, mainly hard tissues such as bone and tooth structure. They have shown excellent performance in bone formation by stimulating angiogenesis. The release of silicate and others (Co and Cu ions) has therefore been implicated to play critical roles in the angiogenesis process. In this short review, we highlight the in vitro and in vivo findings of angiogenesis (and the related bone formation) stimulated by the various types of silicon-containing biomaterials where silicate ions released might play essential roles. We discuss further the possible molecular mechanisms underlying in the ion-induced angiogenic events.
Keywords: Angiogenesis, silicate ions, silicon-containing biomaterials, bone stimulation
Introduction
A challenging issue in bone tissue engineering is the development of angiogenic/osteogenic biomaterials which can effectively promote both bone formation and vascularization after implantation.1 During bone regeneration process, endothelial progenitor and stem cells are initially stimulated to enable angiogenesis, with the secretion of angiogenic factors; also, the stem cells are further committed to osteogenic differentiation and maturation to form vascularized bone structure.2–4 Therefore, biomaterials that are developed for bone regeneration should initially stimulate endothelial cells to form vascular networks in the damaged area and then stimulate osteoprogenitor or bone-forming cells to synthesize the extracellular matrix for bone.5,6
Much effort has been exerted to incorporate growth factors within biomaterials such as vascular endothelial growth factor (VEGF) and bone morphogenetic proteins (BMPs) to enhance the angiogenesis and osteogenesis, respectively. For example, the loading of VEGF to poly(lactic-co-glycolic acid) (PLGA) scaffolds significantly stimulated blood vessel formation and bone regeneration in irradiated osseous defects.7 However, the growth factor approach holds several drawbacks such as high cost, denaturation, or degradation of protein structure during scaffold fabrication and adverse side effects when overdosed.8,9
As another category of therapeutic molecules, ions have also been incorporated within biomaterials, and the ions used thus far include cobalt, copper, silver, strontium, zinc, iron, phosphate, and silicate.3,10,11 This ionic approach has several advantages, including simplicity, low cost, stability during scaffold processing, and effectiveness at low concentrations. In fact, many different forms of biomaterials have been developed to incorporate those ions for diverse biological effects, such as angiogenesis, osteogenesis, or antibacterial activity.3,11–13
Among the biomaterials that can incorporate ions, silica-based bioactive glasses (SBGs) have been widely explored for the repair and regeneration of hard and soft tissues.12,14 SBGs can induce the deposition of a mineral phase onto the surface which mimics the inorganic mineral of natural bone. Moreover, they have the ability to induce vascular network formation without utilizing vascular-stimulating growth factors,12,15 thus enhancing the tissue repair processes.10,12,16 Early findings showed that 45S5 Bioglass® particles enhanced vessel formation by stimulating the secretion of angiogenic growth factors such as VEGF and basic fibroblast growth factor (bFGF) from endothelial cells,17,18 which was ultimately helpful for bone formation. Similarly, calcium silicate (CS) bioceramics releasing silicate ions also showed significantly stimulated angiogenesis through the upregulated VEGF in endothelial cells and the activated kinase insert domain receptor (KDR).3,14
As witnessed in the previous studies, many silicon-containing biomaterials have the potential for bone regeneration through the angiogenic stimulation, and the role of silicate ions in the events can thus be envisaged. This mini review focuses on the silicon-containing biomaterials and highlights their ability to release silicate ions at various concentration levels and the biological role in promoting angiogenesis. We scrutinize the recent in vitro and in vivo findings related to silicate ionic roles and discuss possible mechanisms in the angiogenic stimulation.
Silicon-containing biomaterials
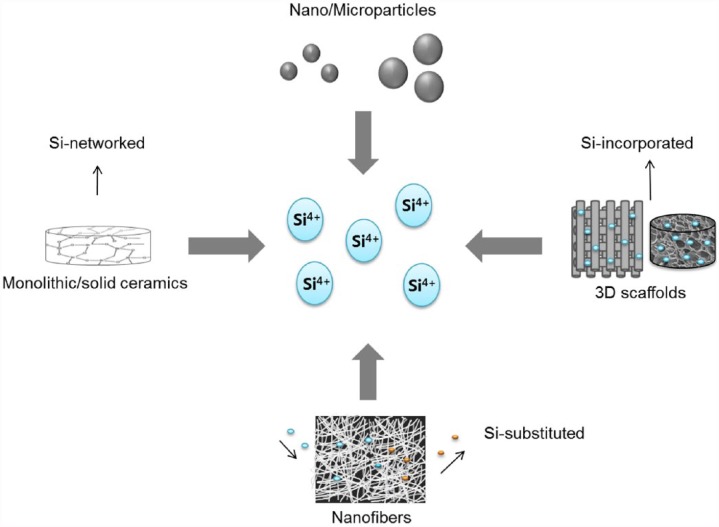
Silicate ions that can release during the degradation of silica-based biomaterials (glasses, crystalline ceramics, or composites/hybrids) have been found to influence cell functions and to accelerate osteogenesis and angiogenesis.10,12 Silicate ions release at different levels due to the different degradation rates of biomaterials which depend on the composition, size, and shape.16 A diverse array of silica-networked, silicon-substituted, or silicon-incorporating biomaterials has thus been developed with different forms, such as monoliths/solid blocks, porous scaffolds, nano/microfibers, and nano/microparticles, as presented in Figure 1. In the following sections, these biomaterials are described in detail.
Figure 1.
Various forms of silicon-containing biomaterials that can release silicate ions at proper therapeutic doses (silicate ions are symbolized as Si4+ for simplicity).
Glasses
SBGs are amorphous materials that contain SiO2 as the main glass network former. The most well-known SBG in biomedical fields is 45S5 Bioglass which has a bioactive and biodegradable silicate composition (see Table 1). This was discovered by Hench19 and Jones and Hench20 in 1969, and since then, many bioactive glass/glass ceramics (melt or sol-gel derived) have been introduced for bone repair and grafting.21 The main characteristic of the bioactive glasses is the well-known ionic reactions and the subsequent formation of hydroxyapatite (HA) layer on their surface in contact with physiological body fluid.22,23 The apatite layer is very similar to that of bone mineral.19,22,23 Furthermore, the dissolved silicate ions from bioactive glasses have stimulating effects on osteoblasts, regulating the expression of several genes including key osteoblastic markers and extracellular matrix proteins.
Table 1.
Summary of Si ion–releasing silica-based biomaterials and the biological effects observed in vitro and in vivo.
| Biomaterials | Compositions | Si ion (mM) | In vitro/in vivo | Findings | References |
|---|---|---|---|---|---|
| Glasses | |||||
| 45S5 Bioglass® | 45SiO2-24.5Na2O-24.5 CaO-6P2O5 | ~0.59 | In vitro | Ionic dissolution induces osteoblast proliferation by mediating IGF-II | Xynos et al.24 |
| mBGn | 75SiO2-25 CaO | ~3.9–6 | Induction of the bone mineral–like formation | El-Fiqi and Kim26 | |
| 58S and 80S-Nano BG | 60 SiO2-36CaO-4P2O5
80 SiO2-15CaO-5P2 O5 |
In vitro | Upregulation of angiogenic gene expression | Mao et al.48 | |
| Crystalline ceramics | |||||
| Akermanite | Ca2MgSi2O7 | 0.03–4.4 | In vitro | Stimulation of the osteoblast cell functions | Wu and Chang62 |
| Akermanite | Ca2MgSi2O7 | 0.03–4.4 | In vitro/in vivo | Induction of the proangiogenic expression | Zhai et al.12 |
| Bredigite | Ca7MgSi4O16 | ~0.08–6.98 | In vitro | Stimulation of the osteoblast cell functions | Wu and Chang62 |
| Bredigite | Ca7MgSi4O16 | ~0.08–6.98 | In vitro | Promotion of the cell growth and spreading | Wu et al.63 |
| Diopside | CaMgSi2O6 | ~0.02–1.89 | In vitro | Induction of the osteoblast cell proliferation | Wu and Chang62 |
| Si-doped HA | Ca10(PO4)6−x (SiO4)x(OH)2−x | ~0.178–0.61 | Introduction to ion delivery vehicles | Lindahl et al.64 | |
| Composites and hybrids | |||||
| Si-doped vaterite | (Si)CaCO3 | ~21–50 | In vitro | Introduction to osteogenic devices | Jin et al.40 |
| Si-doped CPG | 50P2O5–40CaO–10SiO2 | ~1–25 | In vitro | MC3T3-E1 osteoblast induction | Mohammadi et al.65 |
| PCL-GEL-BGn | Nanocomposite | ~0.5–2 | In vitro/in vivo | Stimulation of the osteogenic differentiation and bone regeneration | Fi-qi et al.21 |
| COL-ALG-BGn | Nanocomposite | ~2.9–4.3 | In vitro/in vivo | Synergistic effect accelerate bone regeneration | Perez et al.66 |
| α-TCP-BGn | Nanocomposite | ~1.4–2.1 | In vitro | Upregulation of the odontogenic differentiation and angiogenesis | Lee et al.67 |
| Wollastonite-HA | Composite | ~0.35–2.5 | In vitro | Induction of the osteoblast cell functions | Fiocco et al.68 |
| Silica/PLLA | Composite | ~0.09–0.75 | – | Application for skeletal regeneration | Gowsihan et al.41 |
IGF-II: insulin-like growth factor II.
Xynos et al. studied the effects of ions extracted from Bioglass 45S5 on cell behaviors. A twofold increase in VEGF expression was observed when human primary osteoblasts were treated with the ionic products.24 Although silicate ions could be released to a level of 16.58 ppm after 24 h of incubation, calcium and phosphorus ions were also contained in the treated medium, which might complicate the interpretation of the exact role of silicate ions, which requires further studies.
The release rate of silicate ions varies according to the material shape (spheres, fibers, or irregular), size (micro or nano), and texture (porous or non-porous), which are related to surface area.25,26,27 For example, the dissolution profiles of silicate ions from powder, foam, and monolithic disk of 58S bioactive glass (60 mol% SiO2, 36 mol% CaO, 4 mol% P2O5) with the same mass was compared in simulated body fluid (SBF) for 24 h.28 The results showed that the release of silicate ions was highest for powder (~2.5 mM) and lowest for monolith (~0.178 mM),14 reflecting the role of surface area that is in contact with the medium. In another example, the silicate ions released from irregular bioactive glass (IBG) particles and bioactive glass microspheres (BGMs) showed significantly different release kinetics.29
Another type of glasses that contain silicon is the calcium phosphate glasses (CPGs). CPGs are biodegradable materials, and their degradation rate is tailored by the compositional change.30 The incorporation of silica in the glass reduced degradation rate,10 and the released silicate ions were shown to regulate key angiogenic factors.
Crystalline ceramics
Some bioceramics, for example, akermanite, bredigite, and diopside, are silicate crystallines and thus can release silicate ions in aqueous environments. Akermanite (Ca2MgSi2O7) has been shown to be bioactive and degradable, releasing silicate ions.12 Bredigite (Ca7MgSi4O16) is another bioactive silicate ceramic with excellent biocompatibility and apatite mineralization ability. The ionic products from the bredigite dissolution significantly promoted stem cell growth.31
CS bioceramics comprise another unique class of silica-based bioceramics. They have been used for bone repair materials due to bioactivity, degradability, and biocompatibility. Several studies have reported that CS in powders or coatings exhibited excellent in vitro and in vivo bioactivity.11 There are three kinds of CSs with different Ca/Si ratios: monocalcium silicate (CaSiO3), dicalcium silicate (Ca2SiO4), and tricalcium silicate (Ca3SiO5). Monocalcium silicate (MCS) is not hydraulic and has two main forms: low temperature phase, wollastonite (β-CaSiO3), and high temperature phase, pseudowollastonite (α-CaSiO3). Dicalcium silicate and tricalcium silicate can be hydrated and then hardened by mixing with liquid phases.32,33 The silicate ions released from CS were shown to provide excellent environment for stem cell proliferation and differentiation toward an osteogenic lineage and also for human umbilical vein endothelial cell (HUVEC) proliferation and angiogenesis.3,10,34
Calcium phosphate bioceramics (HA and tricalcium phosphate (TCP)) are often doped with silicon.19,20 In fact, biological apatites are non-stoichiometric and contain small levels of impurities including silicon.35 The incorporation of silicon within HA lattice was proposed to improve the bioactivity. Thus, several methods were used to produce silicon-substituted HA (Si-HA) with varying silicon content.36 Silicon can partially replaces phosphate (PO43−) groups in HA lattice to result in Ca10(PO4)6−x(SiO4)x(OH)2−x,37 and the amount incorporated is limited with ranging from 0.1% to 5% by weight. Silicate ion was also doped to α-TCP (α-Ca3(PO4)2), which is widely used as a powder for calcium phosphate cement (CPC). The α-TCP hydrolyzes in aqueous media to convert into calcium-deficient HA. The Si-doped α-TCP was shown to release silicate ions at ~1.5 mM.38
Calcium carbonate (CaCO3) was developed to incorporate silicate species. CaCO3 mineral has three polymorphs, that is, calcite, aragonite, and vaterite.39 Vaterite is the least thermodynamically stable and thus immediately dissolves, releasing calcium ions, while a part of it re-crystallizes as the most stable calcite. Vaterite particles containing aminopropyl-functionalized silsesquioxane (SixV, where x = 0, 2.6 and 4.9 wt%) were prepared. Compositions of Si2.6V and Si4.9V were shown to hydrolyze and produce soluble silicate species in the range of ~21–50 mM.39,40
Composites and hybrids
Hybrids and composites containing silicon have also been developed for improved mechanical and biological responses. The silicon-containing glasses and ceramics were either incorporated within biopolymer matrices or mixed with other inorganic materials.15,41 Composite scaffolds of poly(d,l-lactic acid) (PDLLA) with bioactive wollastonite could release silicate ions over 3 weeks at levels ~1 mM with 20 wt% wollastonite and ~1.5 mM with 40 wt% wollastonite.42 Organic/inorganic sol–gel hybrid fibers made of silica/poly(l-lactic acid) (PLLA) were also developed.41 The hybrids with 10% and 20% silica released silicate ions ~5 and ~9 ppm, respectively, within a week. Furthermore, when calcium was also incorporated within the hybrids, the release of silicate ions was observed to increase to ~20 ppm.43
Nanocomposite fibrous membranes made of polycaprolactone–gelatin (PCL-GEL, 1:1 ratio) and bioactive glass nanoparticles (BGn, 75%SiO2-25%CaO) were also developed.21,26 The addition of 2.5% BGn was shown to release silicate ions ~0.5–2 mM within 28 days when immersed in deionized water (pH: 7.4, 37°C).
Highly porous wollastonite–HA composite scaffolds were prepared with two different CaO:SiO2 ratios (1.6 and 0.8).42 The scaffolds (CaO:SiO2 = 1.6) could release silicate ions about 57 and 72 ppm, respectively, for 24 and 72 h of immersion in SBF at 37°C. However, a further increase in immersion time up to 21 days showed an almost saturation.44
CPC composites based on α-TCP were recently prepared with the incorporation of BGn (85%SiO2-15%CaO) up to 10 wt%.37 The release of silicate ions from the nanocomposite cement was ~2 mM after 2 weeks of immersion in deionized water (pH: 7.4, 37°C).
Effects of Si ion release on angiogenic events (in vitro and in vivo findings)
The in vitro and in vivo findings demonstrated that silicon-containing biomaterials were excellent in promoting angiogenic events, such as endothelial (progenitor) cell homing, cell polarization, migration, angiogenic differentiation, and neo-blood vessel sprouting (as depicted in Figure 2).
Figure 2.
Silica-based biomaterials that release silicate ions, stimulating angiogenic events in vitro and in vivo, including endothelial (progenitor) cell homing, polarization, cell migration, angiogenesis, tubule-like formation, and neo-vessel sprouting.
In vitro angiogenic events (migration, gene stimulation, tubule-like formation)
Endothelial cell migration is a key attribute of the angiogenesis event.44 The cell migration is driven by three main routes: chemotaxis, haptotaxis, and mechanotaxis. Biomaterials that release ions like silicon can encourage the chemotaxis of cells, enabling a directional migration of cells toward a gradient of chemoattractant ions.45,46 Generally, the cell migration studies use a transwell membrane where the cells contained in the membrane are to sense the chemoattractive molecules released from the biomaterials placed at the bottom of the culture well.47 Our recent study used this in vitro model to demonstrate the stimulating effects of silicate ion release from silica microspheres on migration of endothelial cells (HUVECs) toward the silica microspheres.46 The cell migration motility can also be easily assessed in an in vitro cell scratch model, which is often used to assume the wound repair ability of biomaterials. Two different nanosized bioactive glass compositions, 58S and 80S, could enhance the modeled cellular scratch gap within 24 h, suggesting that the released ions might stimulate cellular motility and movement.48
Cellular tubulogenesis assay is a common tool to mimic many stages of in vivo angiogenesis, including cell migration, proliferation, vessel branching, and anastomosis processes.49,50 For this assay, HUVECs are supported on Matrigel, and the effects of biomaterials or molecules are examined on the tubular networking of cells. The nanosized bioactive glasses that could release silicate ions at 2.87–8.39 µg/mL presented significantly higher number of tubules and more stable tubular structure when compared to the glass-free control group. In the process, key angiogenic factors, such as VEGF, FGF, and endothelial nitric oxide synthase (eNOS), that were known to regulate endothelial cell functions, such as proliferation, migration, and differentiation, were expressed at significantly higher levels.48
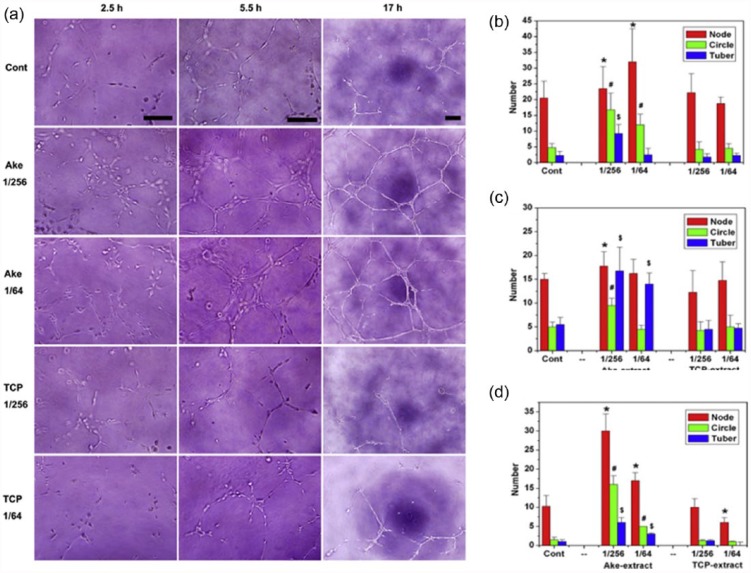
Using the model, akermanite bioceramics (containing Ca-, Mg-, and Si) were shown to drive angiogenesis by increasing proangiogenic factors and upregulating downstream signaling molecules.12,13,51,52 The akermanite extracts at 1/256 and 1/64 dilutions presented more numbers of node, circle, and tubules than the TCP extracts and control in the Matrigel assay (Figure 3). At the same time, the proangiogenic genes such as KDR, fibroblast growth factor receptor 1 (FGFR1), and eNOS were highly stimulated. The most effective concentration of silicate ions released from akermanite was found to be in the range 0.6–2 µg mL−1 based on the in vitro tubule networking.
Figure 3.
In vitro angiogenesis of HAECs cultured on ECMatrix in the presence of Ake and TCP extracts at 1/256 and 1/64 dilutions. (a) Optical images of HAECs cultured on ECMatrix in the presence of Ake and TCP extracts at 1/256 and 1/64 dilutions for 2.5 h (bar = 100 µm), 5.5 h (bar = 100 µm), and 17 h (bar = 200 µm). (b–d) The statistics of the number of nodes, circles, and tubes formed in the culture after 2.5, 5.5, and 17 h, respectively. Data represent mean values ± SD (n = 4). The symbols /, #, and $ represent p < 0.05 of node number, p < 0.01 of circle number, and p < 0.01 of tube-like number, respectively, when compared with the control. Reprint permission was obtained from Zhai et al.12
A co-culture system is often used to understand cell-to-cell interactions in the in vitro angiogenesis of cells that mimic better the in vivo multicellular conditions. In an earlier mono-culture study, the silicate ions released from CS induced HUVECs by activating VEGF and its receptor KDR to trigger the angiogenic pathway.14,34 In a co-culture of HUVECs with human dermal fibroblasts (HDFs), interestingly, the silicate ions stimulated the synthesis of abundant VEGF in HDF, which in turn activated the KDR expression in HUVECs, indicating an important role of silicate ions in driving surrounding cells to exert paracrine effects on endothelial functions. A therapeutic range for the silicate ion concentration was proposed to be 0.7–1.8 µg/mL in the co-cultures.14 Possible cellular mechanisms of the angiogenic stimulation by the released silicate ions will be detailed in section “Proposed mechanisms of silicon ionic roles in angiogenesis.”
In vivo findings
The angiogenesis of silica-based biomaterials has been witnessed in different animal models. The chorioallantoic membrane (CAM) model is considered as a simple and popular assay tool to examine in vivo angiogenesis,53 which allows a rapid growth of capillary networks in confined conditions where biomaterials come in direct contact. 45S5 Bioglass–based glass–ceramic scaffolds were used to examine the angiogenesis in the CAM model, and results showed significant differences with respect to the control polypropylene scaffold.45 When the 45S5 Bioglass scaffolds were combined with human adipose tissue–derived stem cells (hASCs), angiogenesis, including number of newly formed blood vessels and tubule networks, was substantially induced, and the ions released from the scaffolds were presumed to stimulate the hASC function or to directly induce angiogenesis.
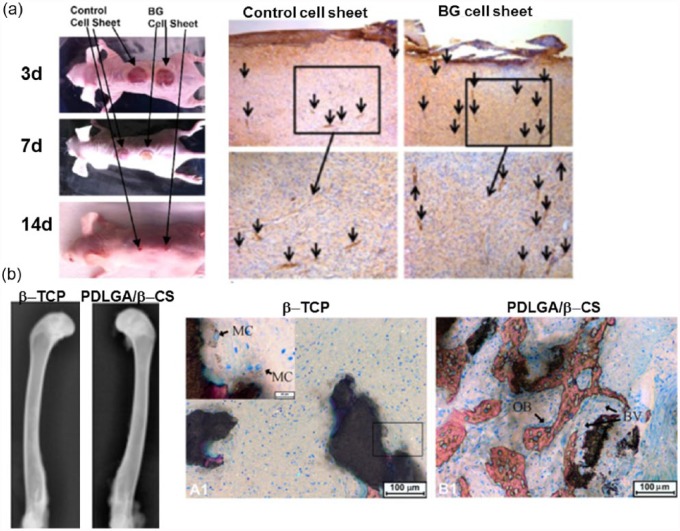
The angiogenesis event is considered critical in the wound healing in vivo as the new blood vessel formation is a key event that can provide nutrients and signaling molecules through vascular networks during the healing process.54 Therefore, the angiogenic ability of biomaterials is often incurred by the wound healing performance.55 Several silica-based biomaterials have shown excellent wound healing ability in vivo. It was56 reported that Bioglass had great potential in an in vivo wound healing model. Although the exact mechanism was not clarified, the ionic release from Bioglass might activate endothelial cells and the consequent wound healing.56,57 It was hypothesized that the silicate ions released from Bioglass (~0.88 ± 0.17 ppm) could upregulate cytokines from endothelial or other stem cells, which in turn stimulate endothelial migration toward the wound bed.34 Another in vivo study also demonstrated the effects of Bioglass in stimulating surrounding cells. When the Bioglass with fibroblast cell sheets were implanted to the wound site in rat, a successful wound healing was achieved at 14 days (Figure 4(a)).56 The wound healing and new blood vessel formation during 14 days in skin defect were higher in the Bioglass group than in the control. It was suggested that the release of ions from Bioglass significantly activated fibroblast cells to secrete proangiogenic factors such as VEGF, bFGF, and epidermal growth factor (EGF).
Figure 4.
In vivo angiogenesis stimulated by silica-based biomaterials in (a) wound healing model: wound closure at different time points and CD31 staining for the new blood vessels (arrows) at day 14 and in (b) bone defect model: β-TCP and PLDLA/β-CS implantation after 4 weeks, radiographs and Van Gieson’s picrofuchsin staining performed (BV: blood vessel; MC: multinucleate cell, and OB: osteoblast cell). Reprint permission was obtained from Wang et al.15 and Yu et al.56
Bone is one of the tissues that critically require angiogenesis in the regeneration process.13,58 Many silicate-based biomaterials are considered as excellent bone regeneratives, and the successful bone formation has often been incurred by the accelerated vascular networks.58 The CS composite with poly(d,l-lactide-co-glycolide) implanted in rabbit femur defect was able to induce better angiogenesis and osteogenesis when compared to β-TCP (Figure 4(b)). To support this, the extracts from CS substantially enhanced HUVEC proliferation which was correlated with the phosphorylation of protein kinase B (Akt) and eNOS as well as the increased nitric oxide (NO) and VEGF production.15 Moreover, earlier study showed the 45S Bioglass implanted locally in irradiated bone defects increased neovascularization based on histological and micro-computed tomographical analyses.59
In fact, the silicate ions are also known to directly stimulate the osteogenesis of cells; furthermore, the silicate ions are found to be the most highly concentrated in bone; especially, the concentration of silicon in osteoid is ~25 times higher than that in surrounding area.60,61 Mainly located at the calcification sites, silicate ions are thought to involve in the mineralization process.61 Therefore, the bone formation observed in the silica-based biomaterials may be due to the combined effects of silicate ions on angiogenesis as well as osteogenesis.6,12 Akermanite bioceramics were shown not only to upregulate osteogenic markers (BMP2, osteocalcin (OCN), and osteopontin (OPN)) but also to activate proangiogenic molecules.12,51 In the in vivo rat calvarial model, the akermanite enhanced both angiogenesis and osteogenesis, and consequently bone formation.51
Combinatory/synergistic effects with other factors
Ions are often incorporated into the silica-based biomaterials to stimulate angiogenesis. Copper is well known to induce hypoxic-mimic conditions, which is helpful for angiogenesis. The copper-doped CS (Cu-CS) was developed and the effects on angiogenesis were carried out.10 Results showed that the copper and silicate ions released could regulate the expression of hypoxia-induced factor-1α (HIF-1α), resulting in the VEGF upregulation. The optimal silicate and copper ions in the extracts were found to be 0.6 and 0.7 µg mL−1, respectively; of note, this concentration range is lower than the range known to be effective with single ion, thus suggesting a synergistic role of the dual ions in the angiogenesis.10
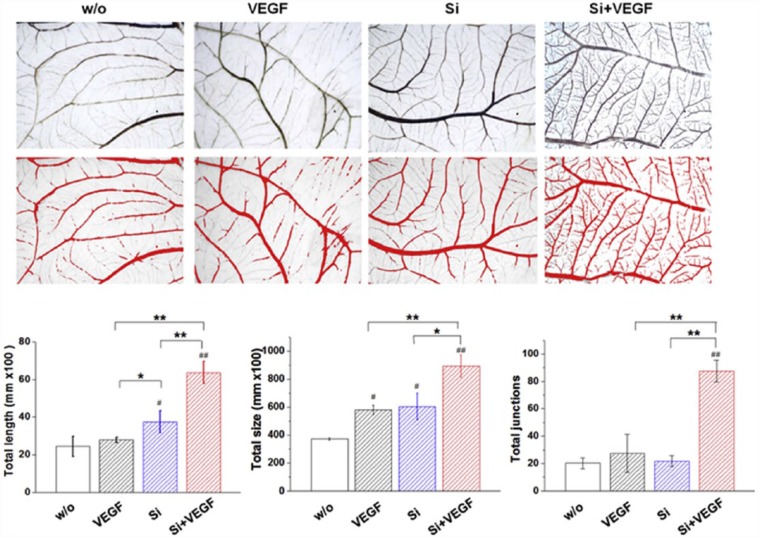
Our group has recently developed a dual factor (ion plus growth factor) delivering system using sol–gel-derived mesoporous silica microspheres.46 Along with the silicate ions intrinsically present in the silica microspheres, VEGF was extrinsically loaded onto the mesopores of microspheres. The silicate ions release was modulated at the therapeutic range (a few ppm level per day), while VEGF was released over a couple of weeks continuously. The silicate ions were shown to stabilize HIF-1α expression by blocking prolyl hydroxylase domain (PHD2) enzyme and further upregulate key angiogenic factors such as bFGF, VEGF, their receptors, FGFR and KDR, and eNOS. The VEGF/silicate ion co-delivery could demonstrate a synergism of the therapeutic molecules, such as endothelial cell migration and tubular formation, and the new blood vessel formation in the in vivo CAM model (Figure 5).
Figure 5.
Synergistic effect of the silicate ion and VEGF releasing from mesoporous silica microspheres presented excellent neovascularization from existing vessels in the CAM model. Quantification of the total length, total size, and total junctions showed significantly enhancement of each parameter. Reprint permission was obtained from Dashnyam et al.53
Proposed mechanisms of silicon ionic roles in angiogenesis
Based on recent studies,2,10,14,15,34 here, we summarize the hypothetical key points in which silicon may play roles in promoting angiogenic events in cells.
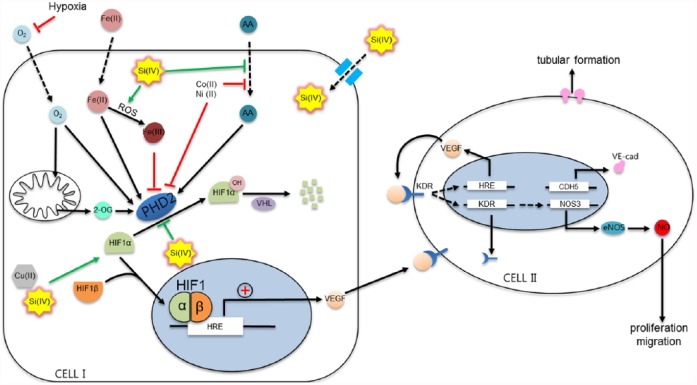
In hypoxia, cells such as fibroblasts and endothelial cells (“Cell I” in Figure 6) are able to sense reduced oxygen levels and stimulate angiogenesis mainly by the activation of HIF-1.10,14,54,69,70 HIF-1, heterodimer transcription factor composed of subunits α and β, regulates the expression of VEGF, which is responsible for the activation of angiogenesis.71 Other growth factors such as bFGF and transforming growth factor beta (TGF-β) are also upregulated and stimulate the angiogenic pathway.3,10,14,34,48,58,69,70 While HIF-1β is constitutively expressed in cells, HIF-1α is a regulatory component in angiogenesis. At low oxygen level, HIF-1α rapidly stabilizes and accumulates in the cytosol and both subunits, α and β, dimerize to form HIF-1 complex which translocates to the nucleus where it activates the hypoxia response elements (HREs).10,71,72 This activation of HRE is required for the upregulation of VEGF. The VEGF released from cells is recognized by VEGF tyrosine kinase receptors (VEGFRs) in endothelial cells (“Cell II” in Figure 6), activating the signaling cascade of angiogenesis.34,48,54,70 VEGFR2, known as KDR or fetal liver kinase 1 (Flk1), appears to be the most important receptor in the angiogenesis pathway.48 HRE is upregulated soon after KDR is activated by VEGF, and the endothelial cells express more VEGF which is then released extracellularly. In the process, autocrine (Cell II to Cell II) and paracrine (Cell I to Cell II) signaling is able to activate the angiogenic pathway.10,14,54,69,70 In addition, KDR signaling leads to upregulating eNOS and NO production, which is responsible for the proliferation and migration of endothelial cells.14,15,34,48 At the same time, VE-cadherin is overexpressed at the endothelial adherens junctions, which is essential for blood vessel formation.14,34,54,73
Figure 6.
Schematic illustration of the possible mechanisms of silicate ion in the angiogenic pathway. The key step in this diagram is the activation or inactivation of PHD2. PHD2 is in charge of the hydroxylation of HIF-1α for further degradation in the proteasome. If any cofactor (ascorbic acid, Fe2+) or substrate (oxygen or 2-oxyglutarate) is reduced or substituted by other ions, PHD2 cannot hydroxylate HIF-1α. Silicate ion may in part share the mechanisms in common with other metallic ions such as copper, cobalt, or nickel by interacting directly with PHD2 or with any of the substrates or cofactors. Besides, silicon may enhance the production of ROS and thus affecting Fe2+ role in PHD2 blocking. Modified from original article with reprint permission from Fong and Takeda.72
However, under normoxia conditions, HIF-1α is exposed to the hydroxylation at a proline residue by PHD2 protein, also referred to HIF-prolyl hydroxylase (HPH).72 This hydroxylation of HIF-1α makes it critical for the interaction with von Hippel–Lindau (VHL) protein, a component of the E3 ubiquitin ligase complex, which, by its ubiquitin ligase activity, ubiquitinizes HIF-1α. Consequently, HIF-1α is degraded at the proteasome, and angiogenesis is inhibited.10,71,72
Often, the growth factors, such as VEGF, bFGF, or TGF-β, are delivered through biomaterials to induce hypoxic-mediated angiogenic stimulation.10,34,48 Furthermore, ions involved in the stimulation of angiogenic processes (e.g. Cu2+, Co2+, and Si4+) are used with biomaterials to mimic this hypoxic condition.3 As demonstrated, those ions may be involved in the stimulation of HIF-1α through PHD2 inhibition.10,54,71,72 PHD2 uses oxygen (O2) and 2-oxyglutarate (2-OG) as co-substrates and iron (Fe(II)) and ascorbic acid (AA) as cofactors;71,72 therefore, a lack of any of these will lead to the inactivation of the protein; consequently, HIF-1α rapidly accumulates in the cytosol to induce angiogenic signaling pathway. A recent finding revealed that silicate ions released from pure mesoporous silica microspheres were able to stabilize HIF-1α in the cytosol of endothelial cells.46
Up to date, the exact molecular pathway for the inhibition of PHD2 or dehydroxylation of HIF-1α mediated by silicate ions is still unclear; thus, further investigation is needed to confirm precise mechanisms. Here, we presume the silicate interaction mechanisms based on the findings of how other ions interact in the events. Similar to other ions, silicate ions are able to flux into cells through a specific subfamily of aquaporin (AQP10), which is involved not only in the permeability of water molecules but also in the passive transport of small solutes. Specificity for silicate ion is determined by concrete residues (XX/R) in the structure domain according to the recent findings.74–77
Transition metal cations such as cobalt (Co2+) and nickel (Ni2+) resembling iron (Fe2+) can replace it in the iron-binding domain of prolyl hydroxylases;71,78 thus, PHD2 is not able to hydroxylate HIF-1α. Besides, cobalt and nickel interact directly with HIF-1α by occupying the VHL-binding domain to inhibit the interaction between VHL and hydroxylated HIF-1α.78 It has been seen that although high levels of reactive oxygen species (ROS) may result in high toxicity in cells and tissues, an initial burst of free radicals is beneficial for the initiation of angiogenesis.79 Cobalt, nickel, and also copper ions can cause oxidative damage to cells by the generation of ROS.11,71 ROS, including peroxides, superoxides, hydroxyl radicals, and singlet oxygen, is produced under normal conditions by cells as products of the oxidative phosphorylation in the mitochondria.72 Under stress environments, such as ionizing radiation, inflammation, or exposure to metals, ROS generation is enhanced leading to cellular damages.54,72 While AA mainly functions as a cofactor of enzymes, it also acts as an antioxidant by preventing the oxidation of molecules during oxidative stress. In this way, AA can reduce Fe(III) into the oxidized iron Fe(II), which is necessary for PHD2 activity. Cobalt and nickel may deplete intracellular AA levels by preventing the entrance into the cell through mechanisms of irreversible degradation of AA to smaller products.71,72,80 Free copper ion can participate in the formation of hydroxyl radicals (OH·) in the presence of AA or superoxide.11,81,82 Although the exact mechanism of silicate ionic interaction with AA still remains unknown, we hypothesize that silicate ions may also interact in a similar way, inhibiting the functions of PHD2 by chelating iron in the binding site of PHD2 and/or HIF-1α or interacting directly or indirectly with AA or even by the generation of ROS. Further studies are needed to clarify how silicate ion affects the PHD2 activity through free radicals and intracellular AA which in turn regulates ROS levels in cells.
One note with regard to the role played by silicon is the possible synergism with other ions. Copper and silicon were shown to have a synergistic effect on the co-cultured fibroblasts and endothelial cells, although silicon appeared to show lower stimulatory effects on HIF-1α expression than copper.10 It is still not clear how silicon helps copper in the angiogenic stimulation; thus, more studies are needed to elucidate the molecular signaling events of the cooperative roles of silicon and copper and also for the case with other ions, such as cobalt or nickel.
Conclusion
Many different forms of silicate-based biomaterials have shown excellent performance in the tissue repair and regeneration processes where angiogenesis is considered a key event. As witnessed in this short review, the release of silicate ions at appropriate levels (a few ppm) is thus believed to play some essential roles in the biological events of these biomaterials. Silicate ion can mimic hypoxic conditions to stimulate angiogenesis pathway by increasing the expression of proangiogenic factors, such as VEGF, FGF, and the receptors in endothelial cells, which in turn upregulates the downstream cascade of angiogenesis including the stimulation of NO. It is considered that silicate ion may inactivate PHD2 by either directly interacting or indirectly depleting intracellular AA or enhancing ROS generation, all of which possibly contribute to stabilizing HIF-1α and the consequent angiogenic events. Besides, it is needed to pay attention to the cooperative action of silicon with other angiogenic promoting ions. Still more studies are needed to clarify the exact molecular mechanisms in which silicate ion contributes to the angiogenesis of cells.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: A support from National Research Foundation (Priority Research Centers Program no. 2009-0093829), Republic of Korea.
References
- 1. Laschke MW, Harder Y, Amon M, et al. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissu Eng 2006; 12: 60913044658052. [DOI] [PubMed] [Google Scholar]
- 2. Shi M, Zhou Y, Shao J, et al. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater 2015; 21: 178–189. [DOI] [PubMed] [Google Scholar]
- 3. Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011; 32: 2757–2774. [DOI] [PubMed] [Google Scholar]
- 4. Vallet-Regí M, Ruiz-Hernández E. Bioceramics: from bone regeneration to cancer nanomedicine. Adv Mater 2011; 23: 5177–5218. [DOI] [PubMed] [Google Scholar]
- 5. Andrades JA, Narváez-ledesma L, Cerón-torres L, et al. Bone engineering: a matter of cells, growth factors and biomaterials. [Google Scholar]
- 6. Arcos D, Vallet-Regí M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater 2010; 6: 2874–2888. [DOI] [PubMed] [Google Scholar]
- 7. Kaigler D, Wang Z, Horger K, et al. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bon Miner Res: J Amer Soci Bone Miner Res 2006; 21: 735–744. [DOI] [PubMed] [Google Scholar]
- 8. Rocha FG, Sundback CA, Krebs NJ, et al. The effect of sustained delivery of vascular endothelial growth factor on angiogenesis in tissue-engineered intestine. Biomater 2008; 29: 2884–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sohier J, Moroni L, Van Blitterswijk C, et al. , Critical factors in the design of growth factor releasing scaffolds for cartilage tissue engineering. Expert Opin Drug Deliv 2008; 5: 543–566. [DOI] [PubMed] [Google Scholar]
- 10. Kong N, Lin K, Li H, et al. Synergy effects of copper and silicon ions on stimulation of vascularization by copper-doped calcium silicate. J Mater Chem B 2014; 2: 1100. [DOI] [PubMed] [Google Scholar]
- 11. Bose S, Fielding G, Tarafder S, et al. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol 2013; 31: 594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhai W, Lu H, Chen L, et al. Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater 2012; 8: 341–349. [DOI] [PubMed] [Google Scholar]
- 13. Habibovic P, Barralet JE. Bioinorganics and biomaterials: Bone repair. Acta Biomater 2011; 7: 3013–3026. [DOI] [PubMed] [Google Scholar]
- 14. Li H, Chang J. Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater 2013; 9: 6981–6991. [DOI] [PubMed] [Google Scholar]
- 15. Wang C, Lin K, Chang J, et al. Osteogenesis and angiogenesis induced by porous β-CaSiO3/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomater 2013; 34: 64–77. [DOI] [PubMed] [Google Scholar]
- 16. Rahaman MN, Day DE, Sonny Bal B, et al. Bioactive glass in tissue engineering. Acta Biomater 2011; 7: 2355–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Day RM. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng 2005; 11: 768–777. [DOI] [PubMed] [Google Scholar]
- 18. Gorustovich AA, Roether JA, Boccaccini AR. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng Part B, Rev 2010; 16: 199–207. [DOI] [PubMed] [Google Scholar]
- 19. Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc 1991; 74: 1487–1510. [Google Scholar]
- 20. Jones JR, Hench LL. Effect of surfactant concentration and composition on the structure and properties of sol-gel-derived bioactive glass foam scaffolds for tissue engineering. J Mater Sci 2003; 38: 3783–3790. [Google Scholar]
- 21. Fi-qi A, Kim JH, Kim HW. Osteoinductive Fibrous Scaffolds of Biopolymer/Mesoporous Bioactive Glass Nanocarriers with Excellent Bioactivity and Long-Term Delivery of Osteogenic Drug. ACS Appl Mater Interfaces 2015; 1140–1152. [DOI] [PubMed] [Google Scholar]
- 22. Kokubo T. Apatite formation on surfaces of ceramics, metals and polymers in body environment. Acta Mater 1998; 46: 2519–2527. [Google Scholar]
- 23. Hench LL. Bioceramics. J Am Ceram Soc 1998; 81: 1705–1728. [Google Scholar]
- 24. Xynos ID, Edgar AJ, Buttery LDK, et al. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Commun 2000; 276: 461–465. [DOI] [PubMed] [Google Scholar]
- 25. Radin S, Chen T, Ducheyne P. The controlled release of drugs from emulsified, sol gel processed silica microspheres. Biomater 2009; 30: 850–858. [DOI] [PubMed] [Google Scholar]
- 26. El-Fiqi A, Kim H-W. Mesoporous bioactive nanocarriers in electrospun biopolymer fibrous scaffolds designed for sequential drug delivery. RSC Adv 2014; 4: 4444–4452. [Google Scholar]
- 27. Arcos D, Vallet-Regí M. Sol–gel silica-based biomaterials and bone tissue regeneration. Acta Biomater 2010; 6: 2874–2888. [DOI] [PubMed] [Google Scholar]
- 28. Jones JR, Lee PD, Hench LL. Hierarchical porous materials for tissue engineering. Philos Trans A Math Phys Eng Sci 2006; 364: 263–281. [DOI] [PubMed] [Google Scholar]
- 29. Lei B, Chen X, Han X, et al. Unique physical–chemical, apatite-forming properties and human marrow mesenchymal stem cells (HMSCs) response of sol–gel bioactive glass microspheres. J Mater Chem 2011; 21: 12725. [Google Scholar]
- 30. Thein-Han W, Xu HHK. Prevascularization of a gas-foaming macroporous calcium phosphate cement scaffold via coculture of human umbilical vein endothelial cells and osteoblasts. Tissue Eng Part A 2013; 19: 1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu C, Chang J, Zhai W, et al. A novel bioactive porous bredigite (Ca7MgSi4O16) scaffold with biomimetic apatite layer for bone tissue engineering. J Mater Sci: MaterMed 2007; 18: 857–864. [DOI] [PubMed] [Google Scholar]
- 32. Lin K, Xia L, Li H, et al. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 2013; 34: 10028–10042. [DOI] [PubMed] [Google Scholar]
- 33. Saravanan S, Vimalraj S, Vairamani M, et al. Role of mesoporous wollastonite (calcium silicate) in mesenchymal stem cell proliferation and osteoblast differentiation: a cellular and molecular study. J Biomed Nanotechnol 2015; 11: 1124–1138. [DOI] [PubMed] [Google Scholar]
- 34. Li H, Chang J. Stimulation of proangiogenesis by calcium silicate bioactive ceramic. Acta Biomater 2013; 9: 5379–5389. [DOI] [PubMed] [Google Scholar]
- 35. Bigi S, Cantalamessa G, Centamore E, et al. The periadriatic basin (Marche–Abruzzi sector, Central Italy) during the Plio-Pleistocene. Geology 1997; 59: 245–259. [Google Scholar]
- 36. Murakami S, Hosono T, Jeyadevan B, et al. Hydrothermal synthesis of magnetite/hydroxyapatite composite material for hyperthermia therapy for bone cancer. J Ceram Soc Japan 2008; 116: 950–954. [Google Scholar]
- 37. Palard M, Champion E, Foucaud S. Synthesis of silicated hydroxyapatite Ca10(PO4)6-x(SiO4 )x(OH)2-x. J Solid State Chem 2008; 181: 1950–1960. [Google Scholar]
- 38. Motisuke M, Garcí-a Carrodeguas R, Zavaglia CAC, et al. A comparative study between α-TCP and Si-α-TCP calcium phosphate cement. Key Eng Mater 2009; 396–398: 201–204. [Google Scholar]
- 39. Tas AC. Vaterite bioceramics: monodisperse CaCO3 biconvex micropills forming at 7O°C in aqueous CaCl2-gelatin-urea solutions. 139–151. [Google Scholar]
- 40. Nakamura J, Poologasundarampillai G, Jones JR, et al. Tracking the formation of vaterite particles containing aminopropyl-functionalized silsesquioxane and their structure for bone regenerative medicine. J Mater Chem B 2013; 1: 4446. [DOI] [PubMed] [Google Scholar]
- 41. Poologasundarampillai G, Yu B, Jones JR, et al. Electrospun silica/PLLA hybrid materials for skeletal regeneration. Soft Matter 2011; 7: 10241. [Google Scholar]
- 42. Li H, Chang J. Preparation and characterization of bioactive and biodegradable Wollastonite/poly(D,L-lactic acid) composite scaffolds. J Mater Sci: Mater Med 2004; 15: 1089–1095. [DOI] [PubMed] [Google Scholar]
- 43. A El-Fiqi JH, Kim HK. Novel bioactive nanocomposite cement formulations with potential properties: incorporation of the nanoparticle form of mesoporous bioactive glass into calcium phosphate cements. J Mater Chem B; 7(2): 1321–1334. [DOI] [PubMed] [Google Scholar]
- 44. Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res 2007; 100: 782–794. [DOI] [PubMed] [Google Scholar]
- 45. Handel M, Hammer TR, Nooeaid P, et al. 45S5-Bioglass® -based 3D-scaffolds seeded with human adipose tissue-derived stem cells induce in vivo vascularization in the CAM angiogenesis assay. Tissue Eng Part A 2013; 19: 2703–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dashnyam K, Jin G-Z, Kim J-H, et al. Promoting angiogenesis with mesoporous microcarriers through a synergistic action of delivered silicon ion and VEGF. Biomaterials 2017; 116: 145–157. [DOI] [PubMed] [Google Scholar]
- 47. Goodwin AM. NIH Public Access. North 2009; 74: 172–183. [Google Scholar]
- 48. Mao C, Chen X, Miao G, et al. Angiogenesis stimulated by novel nanoscale bioactive glasses. Biomed Mater 2015; 10: 25005. [DOI] [PubMed] [Google Scholar]
- 49. Hong JK, Bang JY, Xu G, et al. Thickness-controllable electrospun fibers promote tubular structure formation by endothelial progenitor cells. Int J Nanomedicine 2015; 10: 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res 2007; 74: 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xia L, Yin Z, Mao L, et al. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Sci Rep 2016; 6: 22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun H, Wu C, Dai K, et al. Proliferation and osteoblastic differentiation of human bone marrow-derived stromal cells on akermanite-bioactive ceramics. Biomaterials 2006; 27: 5651–5657. [DOI] [PubMed] [Google Scholar]
- 53. Lokman NA, Elder ASF, Ricciardelli C, et al. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int J Mol Sci 2012; 13: 9959–9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hoeben ANN, Landuyt B, Highley MSM, et al. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 2004; 56: 549–580. [DOI] [PubMed] [Google Scholar]
- 55. Piraino F, Selimović Š. A current view of functional biomaterials for wound care, molecular and cellular therapies. Biomed Res Int 2015; 2015: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yu H, Peng J, Xu Y, et al. Bioglass activated skin tissue engineering constructs for wound healing. ACS Appl Mater Interfaces 2016; 8: 703–715. [DOI] [PubMed] [Google Scholar]
- 57. Li H, He J, Yu H, et al. Bioglass promotes wound healing by affecting gap junction connexin 43 mediated endothelial cell behavior. Biomaterials 2016; 84: 64–75. [DOI] [PubMed] [Google Scholar]
- 58. Jabbarzadeh E, Blanchette J, Shazly T, et al. Vasculari-zation of biomaterials for bone tissue engineering: current approaches and major challenges. Curr Angiogenesise 2012; 1: 180–191. [Google Scholar]
- 59. Leu A, Stieger SM, Dayton P, et al. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Eng Part A 2009; 15: 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jugdaohsingh R. Silicon and bone health. J Nutr Heal Aging 2007; 11: 99–110. [PMC free article] [PubMed] [Google Scholar]
- 61. Carlisle EM. Silicon: a possible factor in bone calcification. Science 1970; 167: 279–280. [DOI] [PubMed] [Google Scholar]
- 62. Wu C, Chang J. Degradation, bioactivity, and cytocompatibility of diopside, akermanite, and bredigite ceramics. J Biomed Res Part B 2007; 83B: 153–160. [DOI] [PubMed] [Google Scholar]
- 63. Wu C, Chang J, Wang J, et al. Preparation and characteristics of a calcium magnesium silicate (bredigite) bioactive ceramic. Biomaterials 2005; 26: 2925–2931. [DOI] [PubMed] [Google Scholar]
- 64. Lindahl C, Xia W, Lausmaa J, et al. Strontium and silicon co-doped apatite coating: preparation and function as vehicles for ion delivery. J Biomater and Nanobiotechnology 2012, 3, 335–334: 335–341. [Google Scholar]
- 65. Mohammadi MS, Ahmed I, Muja N, et al. Effect of Si and Fe doping on calcium phosphate glass fibre reinforced polycaprolactone bone analogous composites. Acta biomaterialia 2012; 4: 1616–1626. [DOI] [PubMed] [Google Scholar]
- 66. Perez RA, Kim JH, Buitrago JO, et al. Novel therapeutic core-shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater 2015; 295–308. [DOI] [PubMed] [Google Scholar]
- 67. Lee SI, Lee ES, El-Fiqi A, et al. Stimulation of odontogenesis and angiogenesis via bioactive nanocomposite calcium phosphate cements through integrin and VEGF signaling pathways. J Biomed Nanotechnol 2016; 12: 1048–1062. [DOI] [PubMed] [Google Scholar]
- 68. Fiocco L, Li S, Bernardo E, et al. Highly porous polymer-derived wollastonite–hydroxycarbonate apatite ceramics for bone regeneration. Biomedical Mater 2016; 11: 25016. [DOI] [PubMed] [Google Scholar]
- 69. Montesano R, Pepper MS, Orci L. Paracrine induction of angiogenesis in vitro by Swiss 3T3 fibroblasts. J Cell Sci 1993; 105(Pt 4): 1013–1024. [DOI] [PubMed] [Google Scholar]
- 70. Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care 2014; 3: 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maxwell KPS. HIF 1 an oxygen and metal responsive transcription factor. Cancer Biol Ther 2004; 3: 29–35. [DOI] [PubMed] [Google Scholar]
- 72. Fong G-H, Takeda K. Role and regulation of prolyl hydroxylase domain proteins. Cell death differ 2008; 15: 635–641. [DOI] [PubMed] [Google Scholar]
- 73. Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 2013; 26: 441–454. [DOI] [PubMed] [Google Scholar]
- 74. Garneau AP, Carpentier GA, Marcoux A-A, et al. Aquaporins mediate silicon transport in humans. PLOS ONE 2015; 10: e0136149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Carpentier GA, Garneau AP, Marcoux A-A, et al. Identification of key residues involved in Si transport by the aquaglyceroporins. J Gen Physiol 2016; 148: 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Takata K, Matsuzaki T, Tajika Y. Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem 2004; 39: 1–83. [DOI] [PubMed] [Google Scholar]
- 77. Verkman AS. Mammalian aquaporins: diverse physiological roles and potential clinical significance. Exp Rev Mole Med 2008; 10: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yuan Y, Hilliard G, Ferguson T, et al. Cobalt inhibits the interaction between hypoxia-inducible factor- and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor. J Biol Chem 2003; 278: 15911–15916. [DOI] [PubMed] [Google Scholar]
- 79. Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med Radic Biol Med 2002; 33: 1047–1060. [DOI] [PubMed] [Google Scholar]
- 80. Salnikow K, Donald SP, Bruick RK, et al. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J Biol Chem 2004; 279: 40337–40344. [DOI] [PubMed] [Google Scholar]
- 81. Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003; 189: 147–163. [DOI] [PubMed] [Google Scholar]
- 82. Jomova K, Baros S, Valko M. Redox active metal-induced oxidative stress in biological systems. Transit Met Chem 2012; 37: 127–134. [Google Scholar]