Abstract
Background
While colonoscopy screening is widely used in several European countries and the United States, no randomised trials exist to quantify its benefits. The Nordic-European Initiative on Colorectal Cancer (NordICC) is a multinational, randomized controlled trial aiming at investigating the effect of colonoscopy screening on CRC incidence and mortality. This paper describes the rationale and design of the NordICC trial.
Material and methods
Men and women age 55 to 64 years are drawn from the population registries in the participating countries and randomly assigned to either once-only colonoscopy screening with removal of all detected lesions, or no screening (standard of care in the trial regions). All individuals are followed for 15 years after inclusion using dedicated national registries.
Results
The primary endpoints of the trial are cumulative CRC-specific death and CRC incidence during 15 years of follow up. We hypothesize a 50% CRC mortality-reducing efficacy of the colonoscopy intervention and predict 50% compliance, yielding a 25% mortality reduction among those invited to screening. For 90% power and a two-sided alpha level of 0.05, using a 2:1 randomisation, 45,600 individuals will be randomised to control, and 22,800 individuals to the colonoscopy group. Interim analyses of the effect of colonoscopy on CRC incidence and mortality will be performed at 10 years follow-up.
Conclusions
The aim of the NordICC trial is to quantify the effectiveness of population-based colonoscopy screening. This will allow development of evidence-based guidelines for CRC screening in the general population.
Keywords: colorectal cancer, screening, colonoscopy, mortality, incidence
Colorectal cancer (CRC) is the most common cancer and the second most common cause of cancer-related death within the European Union in men and women combined.1 The clinical observations, that sporadic CRC invariably has a polypoid precursor lesion, with a long preclinical stage, and that survival depends on stage at detection, have led an increasing number of countries to recommend and implement CRC screening.2–4 Three randomized controlled trials have shown that screening by fecal occult blood testing (FOBT) can reduce mortality from CRC.5–7 Endoscopic screening tests facilitate CRC prevention by removal of precursor lesions.8–11 A recently published randomized controlled trial on once only flexible sigmoidoscopy screening has shown a reduction of both CRC mortality and incidence.12 The possible benefit of primary colonoscopy screening, however, has not been quantified in any randomized trial.
Although invasive, inconvenient, and expensive, colonoscopy has conceptual advantages compared to other CRC screening tools. First, the high sensitivity for both cancers and precursor lesions such as advanced adenomas,13,14 makes colonoscopy suitable both for CRC prevention and early cancer detection.4 As the most sensitive CRC screening test, colonoscopy is expected to yield the lowest cumulative incidence of interval CRC, that occur in between screening examinations.15 Second, colonoscopy examines the entire colon. This is a substantial advantage over other CRC screening tests, particularly in light of evidence for proximal shift in the distribution of CRC16,17 and the limited sensitivity of other screening modalities in detecting right-sided lesions.18,19 Third, colonoscopy is a unique one-step screening modality that allows both detection and treatment of precursors in a single session. In contrast, a positive FOBT or flexible sigmoidoscopy requires further diagnostic work-up, which remains incomplete in 5–20% of the two-step screening modalities.20,21 Fourth, colonoscopy does not need to be repeated frequently. This may improve screening uptake and simplify screening organization. Finally, unlike FOBT which generates many false positive results, colonoscopy has a high specificity.22
Notwithstanding absence of evidence of benefit from randomized controlled trials, colonoscopy is increasingly used as a primary screening method.2,23 Current recommendations for colonoscopy screening are based on assumptions derived from trials of other screening methods in which colonoscopy was used as a second screening step, usually following a positive FOBT test24, and from observational case-control and cohort studies that are prone to confounding and selection bias.10,25–27 A recent case-control study demonstrated a reduction of CRC mortality after colonoscopy only for left-sided but not right-sided CRC, questioning the effectiveness of colonoscopy in reducing CRC mortality irrespective of colonic segment.28 Unfortunately, these results were based on billing codes instead of actual endoscopy reports which precluded assessment of completion of the procedures. However more recent, population based case-control study showed that a significant reduction in CRC incidence is achievable for both sides of colonic segment.29 The decision to undergo a screening examination can be facilitated by designating a preferred strategy from a menu of competing tests.30 The American College of Gastroenterology endorses colonoscopy as the preferred screening modality.4 However, available estimates of uptake rates and mortality-reducing efficacy for FOBT (59–90% and 25–37%, respectively5–7), flexible sigmoidoscopy (32–65% and 43–59%, respectively21,31,32) and colonoscopy (10–60% and 65–69%, respectively26,27,33,34), suggest a similar effectiveness of approximately 20–25% reduction in CRC mortality for the three screening modalities. Therefore, only a randomized trial mimicking organized, population-based screening allows accurate modeling of colonoscopy effectiveness and valid comparison with other screening modalities.
A randomized controlled trial is the preferred method to quantify both benefits and adverse outcomes of screening. Potential adverse outcomes are first directly related to colonoscopy, which is the most invasive CRC screening modality, associated with notable morbidity and a small, but not negligible death rate (<0.01%).35,36 Secondly, patients who screen positive are subjected to subsequent treatments, including surgery for large precursor lesions or cancers, that could result in physical and/or psychological harm or death that otherwise would not have occurred, if the lesions had never become symptomatic. Although it is unknown how many CRC’s remain undetected during one’s lifetime, one series of 379 autopsies uncovered unsuspected CRC, which was unrelated to the cause of death, in 2.6% of patients at age over 70 years.37 Thirdly, through false reassurance, CRC screening may unfavorably impact future lifestyle choices,38 which are believed to contribute to an increase in cardiovascular deaths after screening examinations.39,40
Finally, the cancer screening guidelines of the European Union require evidence from randomised controlled studies to reduce incidence or mortality of the target disease before advocating population-wide cancer screening.41
This paper describes the design and methodological solutions of the Nordic-European Initiative on Colorectal Cancer (NordICC) study, a multicenter, multinational, population-based randomised controlled trial to investigate the effect of colonoscopy screening on CRC incidence and mortality.
Study aims
The trial is designed to evaluate the effectiveness of colonoscopy as a primary screening method for colorectal cancer.
1. The primary aim is to compare colorectal cancer mortality and incidence between the screening group and the control group under the intention-to-screen principle during 15 years of follow-up.
2. The secondary aims are: (i) to compare colorectal cancer mortality and incidence between the screening group and the control group after adjustment for imperfect adherence, and (ii) to evaluate mortality from all causes during 15 years of follow-up.
3. Further predefined study aims (in auxiliary studies) include evaluation of screening attendance, and change of lifestyle patterns in the screened group, control group and among non-attendees.
Trial design: management versus explanatory approach
The design of randomized controlled trials can be divided into explanatory or pragmatic (the latter also called management).42,43 Explanatory studies include a higher degree of control, e.g. with the study subjects enrolled, and with inclusion and exclusion criteria, while management trials are looser and more like real life.43 It may be argued that explanatory trials aim at the efficacy of an intervention, while management studies also intend to estimate the benefit of the intervention to the entire population, thereby mimicking effectiveness.
Recent evidence from randomized controlled trials on flexible sigmoidoscopy screening show a reduction of CRC incidence and mortality after 11 years of follow-up with an explanatory design12 (only randomizing those who have agreed to be enrolled in the trial) and no apparent effect after 7 years of follow-up with a management trial design (randomizing directly from the population registry without prior consent).31 This could reflect the difference in the follow-up time, or the difference in the study design of the Flexiscope and NORCAPP studies.12,31
In the screening setting, the management trial design measures the effectiveness of the screening strategy, thus taking into account not only the efficacy of the screening test but also uptake rates, self-selection and the characteristics of the attendees and the non-attendees. A drawback of the management study design includes the risk of dilution of the efficacy of the screening intervention by including non-compliers and subjects who are not eligible for the trial. Hence, the efficacy of the intervention may be underestimated. Furthermore, in case of low compliance with the intervention, self-selection and confounding by lifestyle, the interpretation of non-significant findings may become a challenge.
An explanatory study design is estimating the highest possible benefit that could be achieved with the screening intervention by random allocation of subjects who are known to be eligible and likely to be compliant.12 On the other hand, information about any hypothetic effect of the intervention may trigger opportunistic screening activity in the control group, and thereby introduce bias for the effect of screening and reduce statistical power achieved with higher compliance.
The NordICC trial may be regarded as a management study. The major reason underlying this choice is that with a management study design we intend to answer the question whether colonoscopy screening is suitable for organized, population-based programs.
Patient selection and randomization
Figure 1 shows the flowchart of the trial. Men and women 55 to 64 years of age are randomly drawn from the population registries in each of the participating countries. The eligible population is updated in three to six-month intervals to exclude recent cases of death and colorectal cancers occurring in the pre-randomization period. In Poland, the eligible population is additionally matched against the opportunistic CRC screening program database to exclude individuals who underwent colonoscopy screening within the last 10 years. Eligible subjects are randomly assigned to either the control or the screening group in a two-to-one ratio. Randomization is stratified by sex, birth year and preferably also by household (individuals living together are assigned to the same randomization group). Each subject randomised to the screening group is matched to two subjects from the control group with the same sex and birth year, to facilitate assignment of identical screening and virtual screening dates (see below). The randomization process is performed separately by independent bodies in each participating country.
Figure 1.
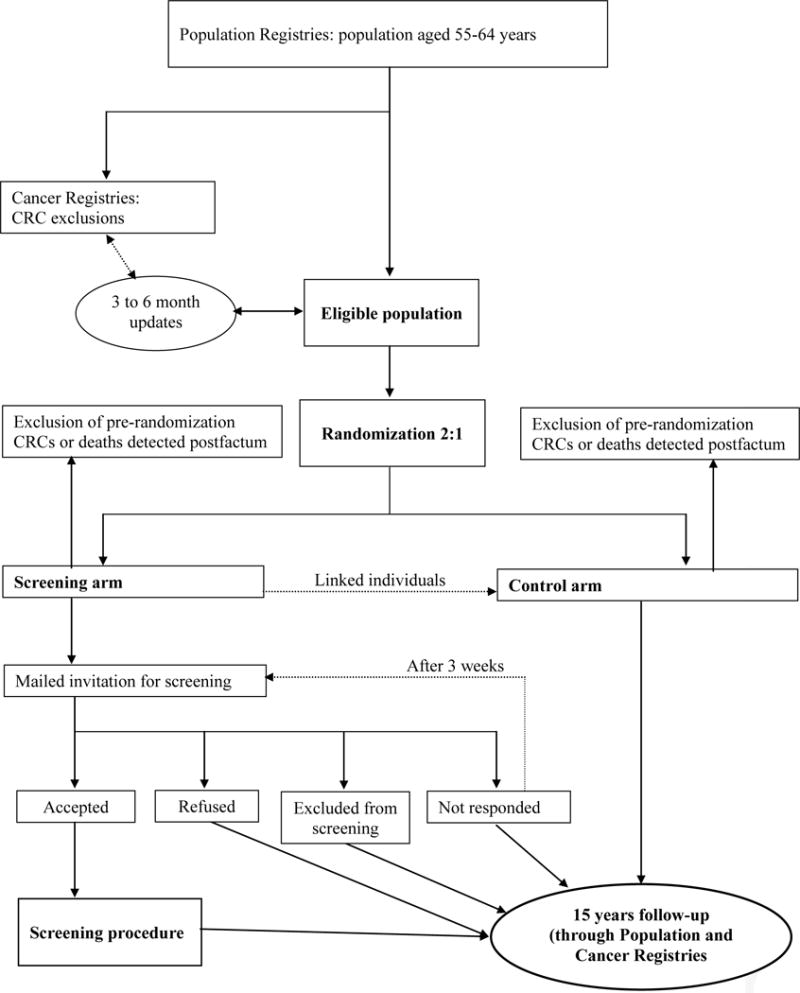
Flowchart for recruitment of study participants*
The only exclusion criteria are death or diagnosis of colorectal cancer before randomization or study entry date (see below or noted above). However, individuals randomized to the screening group (and therefore included in the intent-to-screen analysis described below) will not be offered colonoscopy if any of the following applies: (i) previous open colorectal surgery, (ii) need for long term attention and nursing services (somatic or psychosocial reasons, mental retardation), (iii) ongoing cytotoxic treatment or radiotherapy for malignant disease, (iv) severe chronic cardiac or pulmonary disease (NYHA III and IV), (v) lifelong anticoagulant treatment, (vi) a coronary or cerebrovascular incident requiring hospitalization during the last three months, (vii) residence abroad, (viii) unknown residence or message of death of the subject (which had not been updated in Population Registry), or (ix) failure to provide written informed consent). Individuals with suspicious symptoms are advised to consult their physician for further investigation.
Invitation procedures
Each individual in the screening group receives an invitation letter with the appointment date and time for a colonoscopy at the corresponding participating centre. In one country (the Netherlands) the colonoscopy appointment dates are arranged only after the center receives a response phone call from the invitee. The matched control subjects are assigned an identical date for a virtual screening appointment, but are not informed of their status as controls.
The baseline invitation schedule is designed to maximize participation and with some local variation includes (i) mailing a letter of invitation 6 to 7 weeks prior to the appointment, (ii) mailing a reminder letter to non-responders 3 weeks prior to the appointment, and (iii) mailing a re-invitation letter to non-responders, if needed. The invitation package contains (i) a brief, personalized invitation letter, (ii) a folder with extensive information on the study background and aims, colorectal cancer epidemiology, risks and benefits of colonoscopy and organization of screening procedures, (iii) a reply form for informed consent including the list of exclusion criteria and the screening centre telephone number to be used for discussing medication or diseases that may be relevant for bowel cleansing, endoscopy or polypectomy, and (iv) a pre-paid, return envelope. The colonoscopy procedure is offered free of charge. To overcome obstacles to screening and to maximize the rate of participation a number of additional methods are employed.44 Screening appointments are timed in proximity to annual milestones (e.g. birthday) when possible.45 The screening dates and hours are flexible, and modified upon request. Bowel cleansing is provided free of charge, either sent upon confirmation of attendance one week prior to the appointment date or picked up at the nearest pharmacy. For the procedure each participant is offered a free parking spot, one-day medical leave certificate, and sedation upon request. In addition, the general practitioners in the screening areas receive an informational brochure about the study and colorectal cancer screening.
Examination procedures
Bowel preparation
Polyethylene glycol (PEG) based split dose bowel preparation regimens are used as a standard in the trial (such as3 L of polyethylene glycol solution with or without two tablets of bisacodyl 5mg or 2L polyethylene glycol solution with ascorbic acid). If the proposed bowel preparation deters subjects from participation, alternative less volume-intensive regimens are offered. The preparation regimen is designed to maximize bowel cleanliness without compromising study attendance.
Endoscopic examination
All individuals in the screening group are offered ‘once only’ full colonoscopy. The examinations are performed with standard video colonoscopes using carbon dioxide for insufflation and an optional magnetic endoscope imaging system for orientation of the endoscope position. Technical aids are used to optimize cecal intubation rate and satisfaction among the screening subjects.46 At colonoscopy, all detected CRC precursor lesions are removed whenever feasible, and other pathological findings are biopsied. Insertion of the endoscope is discontinued if the subject expresses serious discomfort or a wish to stop. Post-screening follow-up or surveillance in NordICC is administered by the local health service according to local practice or guidelines. A standard protocol for follow-up of screenees with neoplasia is incorporated in the endoscopy and pathology IT software used by all participating centres. From January 2011, all centres follow the newly established polyp surveillance strategy of the European guidelines for quality assurance in colorectal cancer screening and diagnosis.41
Histopathological examination
Dedicated pathologists at the local pathology labs are responsible for tissue examination in the respective centres. Histopathological examination follows the guidelines of the World Health Organization.47 Online reporting from the pathology labs are integrated with the endoscopy IT module48 to provide continuous measurements of adenoma detection rates and to determine pending histopathology reports and issue surveillance instructions.
Endpoint ascertainment
The primary endpoints of the trial are CRC-specific death and CRC incidence. In each participating country, the entire study population will be periodically matched against Cancer Registries, Population Registries and Registries of Causes of Death. The primary analysis is scheduled at 15 years after randomization. An interim analysis of the primary endpoints is planned at 10 years of follow-up.
The endpoints of the trial will be evaluated by matching of study subjects to cause of death and cancer registries in the participating countries. In addition, independent endpoint committee49 in each of the participating countries (including one pathologist, one epidemiologist, and one surgeon or oncologist) will evaluate the standardized classification of CRC incidence and cause of death, based on patient records and/or death certificates according to the International Statistical Classification of Diseases and Related Health Problems (tenth revision, ICD-10, guidelines for mortality and morbidity coding).50 Committee members will be blinded as to the allocation to screening or control arm and all information that could identify whether a cancer was screen-detected will be withheld from them. Deaths attributable to complications from CRC diagnosis or treatment will be assigned as CRC deaths.
Statistical power considerations
Although colonoscopy is regarded as a cancer prevention test, the primary aim of each screening modality is to reduce disease specific mortality; therefore, incidence based CRC mortality is the variable used for power calculation in the trial. The number of expected CRC deaths in the control group is based on CRC incidence, expected stage distribution, the relative survival rate by stage of diagnosed CRC, and all cause mortality per country to derive an incidence based mortality for the age group 55–64 followed over 10 and 15 years. The incidence based CRC mortality estimates per participating countries and the weighted average based on number enrolled per country are shown in Table 1. The cumulative CRC incidence based mortality over 15 years of follow-up, adjusted for deaths from other causes, is expected to be approximately 1%. The estimated effect of screening colonoscopy on CRC mortality depends primarily on three variables: attendance to screening, efficacy of examination and screening contamination in the control group. The estimates of these variables are uncertain. Table 2 provides power calculations for different scenarios of efficacy, attendance and contamination. A 25% difference in the CRC mortality is our main hypothesis based on an assumption of 50% attendance, 50% screening efficacy and 0% effect reduction due to contamination in the control group. A cautious assumption of 50% screening efficacy is based on estimates of 65–69% mortality reduction in screening colonoscopy or adenoma cohorts when compared with epidemiological data.26,34 We anticipate an effect of screening colonoscopy due to early detection of CRC as well as removal of adenomas. The assumption of screening attendance is based on 10–60% participation to colonoscopy in three population-based randomized controlled trials.27,33,51 Given the use of similar invitation materials as in the Norwegian trial, which achieved the highest participation27, a 50% attendance is estimated. The randomization is performed in a 2:1 ratio (control and screening arm, respectively). To detect a 25% difference in mortality with power of 0.90 at the 5% level of significance, the study requires 22,800 individuals randomized to the screening arm and 45,600 individuals to the control arm. Therefore, we plan to screen at least 11 400 individuals, but aim for 15 000 screened individuals to achieve adequate power also with lower compliance to screening, more contamination or lower effect of the intervention (see Table 2 for the different scenarios that may be applied). Presently, the Netherlands, Poland and Norway have started to include participants in the trial, and Sweden plans to start recruiting in the near future.
Table 1.
Estimated incidence based mortality from colorectal cancer per 1000 unscreened controls in actively recruiting countries (minimum 11,400 colonoscopies).
| 10 yrs follow-up | 15 yrs follow-up | |
|---|---|---|
| Netherlands | 4.64 | 9.56 |
| Norway | 4.89 | 9.95 |
| Poland | 5.61 | 10.21 |
| Sweden | 4.89 | 9.95 |
| Weighted average | 5.23 | 10.02 |
Table 2.
Power of the trial with different scenarios of efficacy, attendance and contamination within the estimated range (11,400 – 15,000) of colonoscopy workload.*†
| Attendance rate | Contamination rate | Efficacy of intervention | Difference in mortality | No. colonoscopies | No. invited | No. controls | Power | |
|---|---|---|---|---|---|---|---|---|
| 10 yrs of follow-up | 15 yrs of follow-up | |||||||
| 50% | 0% | 50% | 25% | 11,400 | 22,800 | 45,600 | 64% | 90% |
| 40% | 0% | 60% | 24% | 11,400 | 28,500 | 57,000 | 70% | 93% |
| 50% | 0% | 60% | 30% | 11,400 | 22,800 | 45,600 | 80% | 98% |
| 40% | 0% | 70% | 28% | 11,400 | 28,500 | 57,000 | 83% | 98% |
| 50% | 0% | 50% | 25% | 15,000 | 30,000 | 60,000 | 77% | 96% |
| 40% | 0% | 60% | 24% | 15,000 | 37,500 | 75,000 | 82% | 98% |
| 50% | 0% | 60% | 30% | 15,000 | 30,000 | 60,000 | 90% | 99% |
| 40% | 0% | 70% | 28% | 15,000 | 37,500 | 75,000 | 92% | 99% |
| 50% | 5% | 50% | 23% | 15,000 | 30,000 | 60,000 | 68% | 92% |
| 40% | 5% | 60% | 22% | 15,000 | 37,500 | 75,000 | 71% | 94% |
| 50% | 5% | 60% | 28% | 15,000 | 30,000 | 60,000 | 85% | 98% |
| 40% | 5% | 70% | 25% | 15,000 | 37,500 | 75,000 | 85% | 99% |
Bolded row shows primary power calculation used in the trial.
Calculated for weighted average estimate of incidence based mortality in participating countries.
Statistical analysis
The primary analysis will use the date of randomization as the start of follow-up for each individual. A secondary analysis will use the date of scheduled screening (virtual screening for matched controls) as the start of follow-up. Both analyses are expected to yield similar results if the period between randomization and scheduled screening date is short, or if randomization to screening does not affect the probability of dropping out of the study before the scheduled screening date.31 Each individual’s follow-up will end at CRC death (CRC diagnosis for CRC incidence endpoint), loss to follow-up (i.e. emigration) or 15 years after randomization, whichever happens first.
For the primary analysis the effect of screening colonoscopy on colorectal cancer mortality will be assessed for the time to the event (CRC mortality) in an intention to treat analysis using the log rank test stratified by country. We also will perform a Cox proportional hazards model analysis, stratified by country, to assess the screening effect as well as that due to any cofactors or interaction effects on risk of CRC mortality. We also will derive the Kaplan Meier cumulative survival curves and plot the annual hazard ratio to assess the proportionality assumption for the Cox modeling. Similar analyses will be performed for the impact of screening colonoscopy on CRC incidence. We anticipated that incidence curves will be non-proportional in the years immediately following the colonoscopy intervention.
All these analyses will be repeated for per-protocol analysis adjusting for non-compliance.52
We plan one interim analysis at 10 years with the primary analysis at 15 year using the O’Brien-Fleming rule.53
NordICC structure and management
The NordICC organizational structure is presented in Figure 2. The scientific committee, consisting of at least one member from each of the participating countries, together with the head secretariat in Oslo, Norway, coordinates the management, screening, quality control, and endpoint observation and publication activity. The scientific committee reports to an external data safety and monitoring board (DSMB). The DSMB provides advice to the scientific committee on adverse events and endpoint evaluation. The DSMB monitors the primary endpoint of the trial after 10 years of follow-up. The coordinating secretariat manages administrative work and the screening activities together with national executive committees via the joint database server located at the Cancer Registry of Oslo, and connected to all participating centres. During the follow-up period, national endpoint committees report on study endpoints to the respective executive committees.
Figure 2. Organizational structure of the NordICC trial*.
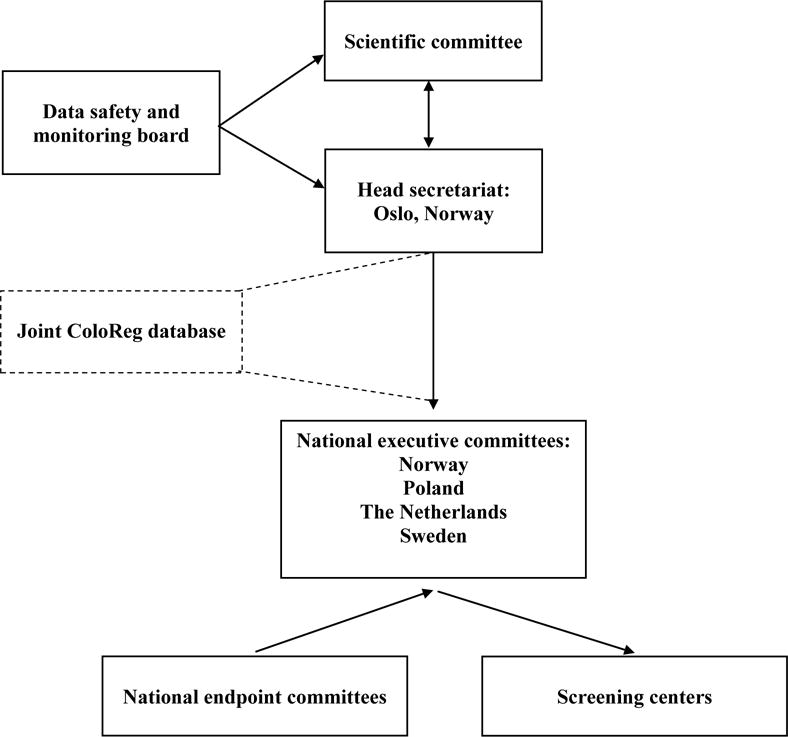
*Arrows indicate directions of relative dependency.
Quality assurance program
Indirect evidence has provided various estimations of the preventive effect of colonoscopic polypectomy on colorectal cancer incidence.8,54,55 It has been suggested that observed differences in CRC protection may reflect differences in the quality of colonoscopy performance.15 Therefore, continuous quality control and improvement is essential to ensure highly efficacious examinations. We will use dedicated IT solutions to monitor quality, using a centralized database48 and paper-based questionnaires56 to be scanned and merged with the database. These IT solutions allow (i) central registration and notification of substandard colonoscopy performance (assessed using adenoma detection rates, cecal intubation rates, perceived pain and discomfort during and after the screening examination, and satisfaction with the screening center personnel), (ii) notification of all complications and adverse effects, (iii) notification of screen-positives with no scheduled appointments for adequate follow-up, and (iv) notification of missing, inadequate or illogical registration of histological findings. In addition, video recording of all colonoscopies during withdrawal allows for subsequent analyses of quality.
Ethical issues
At all participating institutions, ethics committees or National Health Councils have approved the study protocol prior to recruiting individuals to the trial.. All participants randomized to the screening group must provide written informed consent before they are examined by colonoscopy. Individuals assigned to the control group will not be informed about their status as controls in the present trial. Our study design facilitates a truly population-based study, which allows generalization of the effect estimate of the screening intervention to the general population, because it mimics national CRC screening program.
Auxiliary studies
Attendance rates for screening colonoscopy
Limited data is available regarding colonoscopy screening attendance rates. This study utilizes available information to maximize screening uptake and measure achievable attendance rates. The auxiliary studies plan to investigate and compare (i) attendance in response to various invitation time schedules (sending the invitation letter and reminder 6 and 3 weeks vs. 4 and 2 weeks prior to appointment date, respectively), (ii) attendance in response to several versions of the invitation letter (choice of endoscopist gender vs. choice of definite endoscopist vs. standard content), and (iii) initial non-responder attendance following re-invitation or CRC screening directed educational meetings. Once the most effective invitation procedure is established, it will be applied to the individuals who remain to be invited.
Change of lifestyle in screening group, control group and non-attendees
Due to false reassurance, screening programs may have a negative impact on future lifestyle choices, promoting an unhealthy lifestyle. Such behavioral changes might reduce, eliminate or even reverse the overall benefit from screening by increasing CRC or overall mortality.38 Lifestyle changes over time with and without invitation to colonoscopy screening will be monitored in random samples assessing also potential differences in effect of screening on lifestyle according to findings at screening.
Further auxiliary studies will be considered provided that they are not regarded detrimental to attendance rates.
Conclusions
Although colonoscopy screening is recommended for the prevention of colorectal cancer in several European countries and the United States, no randomised trials have quantified its possible benefit. This randomized controlled trial has been designed to quantify the long-term effect of colonoscopy screening on colorectal cancer incidence and mortality. Accrual of participants directly from population registries mimics population-based screening, and provides an opportunity to explore the logistics and effectiveness of an organized colonoscopy screening. Results will become available after 10 and 15 year follow-up.
Acknowledgments
Financial support: The NordICC study has received research grants from each participating country organisations; Norway: the Nordic Cancer Union, the Norwegian Research Council (grant no. 197309), and the Health Fund of South-East Norway (grant no. 5135); Poland: the National Centre for Research and Development of Poland (grant no. N R13 0024 04) and the Polish Foundation of Gastroenterology; Netherlands: the Dutch Ministry of Health and Health Care Prevention Program–Implementation (ZonMw 2008).
Footnotes
Trial registration: Clinical trials NCT 00883792.
References
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39:168–73. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 3.Butruk E, Regula J, Polkowski M, Rupinski M, Przytulski K. National colorectal cancer screening programme in Poland. Endoscopy. 2002;34:939–40. [Google Scholar]
- 4.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104:739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 5.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993;328:1365–71. doi: 10.1056/NEJM199305133281901. [Erratum, N Engl J Med 1993; 329: 672.] [DOI] [PubMed] [Google Scholar]
- 6.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with fecal occult blood test. Lancet. 1996;348:1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 7.Hardcastle JD, Chamberlain JO, Robinson MHE, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 8.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 9.Atkin WS, Cuzik J, Northover JMA, et al. Prevention of colorectal cancer by once-only sigmoidoscopy. Lancet. 1993;341:736–40. doi: 10.1016/0140-6736(93)90499-7. [DOI] [PubMed] [Google Scholar]
- 10.Thiis-Evensen E, Hoff G, Sauar J, et al. Population-based surveillance by colonoscopy: Effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol. 1999;34:414–20. doi: 10.1080/003655299750026443. [DOI] [PubMed] [Google Scholar]
- 11.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 12.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicenter randomized trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 13.Rex DK, Rahmani EY, Haseman JH, et al. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology. 1997;112:17–23. doi: 10.1016/s0016-5085(97)70213-0. [DOI] [PubMed] [Google Scholar]
- 14.Pickhardt PJ, Nugent PA, Mysliwiec PA, et al. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352–9. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 15.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 16.McCallion K, Mitchell RM, Wilson RH, et al. Flexible sigmoidoscopy and the changing distribution of colorectal cancer: implications for screening. Gut. 2001;48:522–5. doi: 10.1136/gut.48.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabeneck L, Davila JA, El-Serag HB. Is there a true “shift” to the right colon in the incidence of the colorectal cancer? Am J Gastroenterol. 2003;98:1400–9. doi: 10.1111/j.1572-0241.2003.07453.x. [DOI] [PubMed] [Google Scholar]
- 18.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061–8. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 19.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129:422–8. doi: 10.1016/j.gastro.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 20.Guittet L, Bouvier V, Mariotte N, et al. Comparison of a guaiac based and an immunochemical feacal occult blood test in screening for colorectal cancer in a general average risk population. Gut. 2007;56:210–4. doi: 10.1136/gut.2006.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hol L, Van Leerdam ME, Van Ballegooijen M, et al. Screening for colorectal cancer; randomized trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut. 2009;59:62–8. doi: 10.1136/gut.2009.177089. [DOI] [PubMed] [Google Scholar]
- 22.Levin B, Lieberman D, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–72. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 24.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 25.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 26.Zauber AG, Winawer SJ, O’Brien MJ, Shi W, Bayuga S. Significant long term reduction in colorectal cancer mortality with colonoscopic polypectomy: findings of the National Polyp Study. Gastrointest Endosc. 2007;65:AB268. [Google Scholar]
- 27.Citarda F, Tomaselli G, Capocaccia R, et al. The Italian Multicenter Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–5. doi: 10.1136/gut.48.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer: a population-based, case-control study. Ann Intern Med. 2008;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 29.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based study, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 30.Inadomi J, Kuhn L, Vijan S, et al. Adherence to competing colorectal cancer screening strategies. Am J Gastroenterol. 2005;100:S387–8. [Google Scholar]
- 31.Hoff G, Grotmol T, Skovlund E, Bretthauer M, Norwegian Colorectal Cancer Prevention Study Group Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomized controlled trial. BMJ. 2009 doi: 10.1136/bmj.b1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkin WS, Hart A, Edwards R, et al. Uptake, yield of neoplasia, and adverse effects of flexible sigmoidoscopy screening. Gut. 1998;42:560–5. doi: 10.1136/gut.42.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segnan N, Senore C, Andreoni B, et al. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology. 2007;132:2304–12. doi: 10.1053/j.gastro.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770–5. doi: 10.1016/j.cgh.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–1906. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 36.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–6. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 37.Stemmermann GN. Cancer of the colon and rectum discovered at autopsy in Hawaiian Japanese. Cancer. 1966;11:1567–72. doi: 10.1002/1097-0142(196611)19:11<1567::aid-cncr2820191118>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 38.Larsen IK, Grotmol T, Almendingen K, Hoff G. Impact of colorectal cancer screening on future lifestyle choices: a three-year randomized controlled trial. Clin Gastroenterol Hepatol. 2007;5:477–83. doi: 10.1016/j.cgh.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Ahlquist DA. Fecal occult blood testing for colorectal cancer. Can we afford to do this? Gastroenterol Clin North Am. 1997;26:41–55. doi: 10.1016/s0889-8553(05)70282-x. [DOI] [PubMed] [Google Scholar]
- 40.Moayyedi P, Achkar E. Does fecal occult blood testing really reduce mortality? A reanalysis of systematic review data. Am J Gastroenterol. 2006;101:380–4. doi: 10.1111/j.1572-0241.2006.00537.x. [DOI] [PubMed] [Google Scholar]
- 41.Segnan N, Patrick J, von Karsa L, editors. European Union 2010. European guidelines for quality assurance in colorectal cancer screening and diagnosis. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chron Dis. 1967;20:637–48. doi: 10.1016/0021-9681(67)90041-0. [DOI] [PubMed] [Google Scholar]
- 43.McMahon AD. Study control, violators, inclusion criteria and defining explanatory and pragmatic trials. Stat Med. 2002;21:1365–76. doi: 10.1002/sim.1120. [DOI] [PubMed] [Google Scholar]
- 44.Harewood GC, Wiersema MJ, Melton LJ., 3rd A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol. 2002;97:3186–94. doi: 10.1111/j.1572-0241.2002.07129.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoff G, Bretthauer M. Appointments timed in proximity to annual milestones and compliance with screening: randomized controlled trial. BMJ. 2008;337:a2794. doi: 10.1136/bmj.a2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bretthauer M, Lynge AB, Thiis-Evensen E, Hoff G, Fausa O, Aabakken L. Carbon dioxide insufflation in colonoscopy: safe and effective in sedated patients. Endoscopy. 2005;37:706–9. doi: 10.1055/s-2005-870154. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton SR, Aaltonen LA, editors. World Health Organisation Classification of Tumors Pathology and Genetics of Tumors of the Digestive Tract. IARC Press; Lyon: 2000. pp. 104–19. [Google Scholar]
- 48.Hoff G, Ottestad PM, Skafløtten SR, Bretthauer M, Moritz V. Quality assurance as an integrated part of the electronic medical record - a prototype applied for colonoscopy. Scand J Gastroenterol. 2009;4:1–7. doi: 10.1080/00365520903132021. [DOI] [PubMed] [Google Scholar]
- 49.Naslund U, Grip L, Fischer-Hansen J, Gundersen T, Lehto S, Wallentin L. The impact of an end-point committee in a large multicentre, randomized, placebo-controlled clinical trial. Eur Heart J. 1999;20:771–7. doi: 10.1053/euhj.1998.1351. [DOI] [PubMed] [Google Scholar]
- 50.International Statistical Classification of Diseases and Related Health Problems. Vol. 2. World Health Organization; 2004. 10th Revision. [Google Scholar]
- 51.Lisi D, Hassan C, Crespi M, the AMOD Study Group Participation to colorectal cancer screening with FOBT and colonoscopy: an Italian, multicentre, randomized population study. Dig Liver Dis. 2009;42:371–6. doi: 10.1016/j.dld.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 52.Cuzick J, Edwards R, Segnan N. Adjusting for non-compliance and contamination in randomized clinical trials. Stat Med. 1997;16:1017–29. doi: 10.1002/(sici)1097-0258(19970515)16:9<1017::aid-sim508>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 53.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 54.Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34–41. doi: 10.1053/j.gastro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Jorgensen OD, Kronborg O, Fenger C. The Funen Adenoma Follow-up Study. Incidence and death from colorectal carcinoma in an adenoma surveillance program. Scand J Gastroenterol. 1993;28:869–74. doi: 10.3109/00365529309103127. [DOI] [PubMed] [Google Scholar]
- 56.Hoff G, Bretthauer M, Huppertz-Haus G, et al. The Norwegian Gastronet project: Continous quality improvement of colonoscopy in 14 Norwegian centres. Scand J Gastroenterol. 2006;41:481–7. doi: 10.1080/00365520500265208. [DOI] [PubMed] [Google Scholar]


