Abstract
In Bacillus subtilis, the expression of genes encoding enzymes and other proteins involved in purine de novo synthesis and salvage is affected by purine bases and phosphoribosylpyrophosphate (PRPP). The transcription of the genes belonging to the PurR regulon is negatively regulated by the PurR protein and PRPP. The expression of the genes belonging to the G-box (XptR) regulon, including the pbuE gene, is negatively regulated by a riboswitch-controlled transcription termination mechanism. The G-box regulon effector molecules are hypoxanthine and guanine. pbuE encodes a purine base efflux pump and is now recognized as belonging to a third purine regulon. The expression of the pbuE gene is positively regulated by a riboswitch that recognizes adenine. Here we show that the expression of pbuE′-lacZ transcriptional fusions are induced by adenine to the highest extent in mutants which do not express a functional PbuE pump. In a mutant defective in the metabolism of adenine, the ade apt mutant, we found a high intracellular level of adenine and constitutive high levels of PbuE. A growth test using a purine auxotroph provided further evidence for the role of PbuE in lowering the intracellular concentration of purine bases, including adenine. Purine analogs also affect the expression of pbuE, which might be of importance for the protection against toxic analogs. In a mutant that overexpresses PbuE, the expression of genes belonging to the PurR regulon was increased. Our findings provide further evidence for important functions of the PbuE protein, such as acting as a pump that lowers the purine base pool and affects the expression of the G-box and PurR regulons, including pbuE itself, and as a pump involved in protection against toxic purine base analogs.
Most of the genes in Bacillus subtilis that are involved in purine de novo synthesis, purine salvage, and transport belong to one or two regulons. The PurR regulon consists of purR, purA, glyA, guaC, pbuO, pbuG, and the pur-, yqhZ-folD, and xpt-pbuX operons (10). The G-box regulon, previously called the XptR regulon, comprises the pbuG, nupG, and pbuE genes and the pur- and xpt-pbuX operons (4). The low-molecular-weight regulatory molecules are purine bases which act indirectly through their effect on the cellular phosphoribosylpyrophosphate (PRPP) pool size or directly as the free base. The PurR regulator is a dimer that binds to a DNA operator site and inhibits transcription (13, 14). When the size of the PRPP pool is increased, PRPP binds to PurR and prevents the binding of PurR to the tandem PurBox DNA sequence. The recognition site for the PurR protein has been found to be located at various positions both up- and downstream of the transcriptional start site of the PurR regulon genes (1, 13). Uptake and metabolism of adenine from the medium result in reduction of the size of the cellular PRPP pool, while guanine has the opposite effect (12). The expression of the G-box regulon genes is repressed by hypoxanthine and guanine. In the presence of these repressor molecules, transcription of the pur- and xpt-pbuX operons terminates before RNA polymerase enters the first structural gene of the operon (2, 3). This transcriptional regulation is mediated by a riboswitch RNA structure containing a conserved so-called G-box sequence motif which recognizes hypoxanthine and guanine (5). By using transcriptional lacZ fusions, it was recently determined that pbuE, formerly known as ydhL, contains a riboswitch structure in its 5′ untranslated leader sequence that is nearly identical to the G-box riboswitch. In contrast to the genes belonging to the G-box regulon, the pbuE gene is induced by hypoxanthine and guanine (4). However, the increase in expression was only twofold for the pbuE gene, while it varied from 10- to 41-fold for the genes of the G-box regulon. The function of PbuE as a purine base and purine base analog efflux pump is based on a number of observations. Resistance to 2-fluoroadenine was caused by a mutation that resulted in overexpression of pbuE (4). In an hpt mutant defective in hypoxanthine phosphoribosyltransferase activity, the expression of the G-box regulon is down-regulated, most likely because of intracellular accumulation of hypoxanthine. Increased G-box regulon expression was observed in the double mutant hpt pbuE1, which overexpresses PbuE, most likely as a result of the increased pumping activity that reduces the pool of hypoxanthine (4). In this report we show that adenine in vivo is a much better effector molecule for pbuE induction and that expression of pbuE again affects the expression of the PurR and G-box regulons. This observation complements the findings of Breaker and Mandal, who have demonstrated that pbuE belongs to a third purine regulon (A-box regulon), which is regulated by an adenine-sensitive riboswitch mechanism (6). Furthermore, we show that PbuE is also an adenine pump and the pump function affects the uptake and utilization of exogenous purine compounds.
Induction of pbuE expression
The finding by Breaker and Mandal that the pbuE gene is regulated by an adenine-controlled riboswitch (6) prompted us to further study the effect of adenine on the induction of pbuE. The level of expression was determined using transcriptional pbuE-lacZ fusions, including one in which the pbuE gene was disrupted and another in which the gene was functional. The greatest effect of adenine was observed in strain LJ32 (Table1) with a disrupted pbuE gene (Table 2). For comparison, the level was only 7 U/mg of protein in the presence of hypoxanthine. Another way of supplying adenine is to provide the cells with adenosine that is cleaved to adenine inside the cells while at the same time the external level of adenine is low. Under these conditions the induction is significantly reduced, most dramatically in strain LJ24 containing wild-type pbuE (Table 2). These data suggest that PbuE is also an adenine efflux pump because the highest level of expression, 267 U/mg of protein, is seen in strain LJ32, which is defective in PbuE. In the absence of the efflux function, adenine is expected to accumulate inside the cell. The relatively low level of pbuE induction by adenosine may indicate that the cellular adenine pool formed from the intracellular cleavage of adenosine is smaller than the pool in cells growing with equimolar amounts of adenine.
TABLE 1.
B. subtilis strains used in this studya
| Strain | Genotype | Source or reference and/or description |
|---|---|---|
| 168 | trpC2 | C. Anagnostopoulos |
| HH443 | trpC2 amyE::[pLJ25 xpt′-lacZ (neo)] pbuE1 ydhK::pBOE335 (cat) | LJ26 transformed with DNA from LJ40 selecting for Cmr |
| KN05n | trpC2 amyE::[pKN05n glyA′-lacZ (neo)] | 10 |
| LJ24 | trpC2 amyE::[pLJ24 pbuE′-lacZ (cat)] | 4 |
| LJ25 | trpC2 amyE::[pLJ25 xpt′-lacZ (neo)] | 4 |
| LJ26 | trpC2 amyE::[pLJ25 xpt′-lacZ (neo)] pbuE1 | 4 |
| LJ27 | trpC2 thr-5 pbuE1 | 4; 2-fluoroadenine resistant |
| LJ32 | trpC2 pbuE::pLJ32 (erm) | 168 transformed with pLJ32 selecting for Err. pLJ32 is pMutin4 (16) containing an internal part of the ydhL transcriptional unit |
| LJ40 | trpC2 ydhK::pBOE335 (cat) | 168 transformed with pLJ40 selecting for Cmr. pLJ40 is pBOE335 (9) containing an internal part of the ydhK transcriptional unit |
| LJ54 | trpC2 amyE::[pLJ25 xpt′-lacZ (neo)] pbuE::pLJ42 (cat) | 4 |
| ED265 | trpC2 ade-1 | 8 |
| ED279 | trpC2 ade-1 apt-7 | 8 |
| ED181 | trpC2 purF6 pbuG1 | 11 |
| ED182 | trpC2 purF6 | 11 |
| ED463 | trpC2 purF6 pbuG1 ydhK::pBOE335 (cat) pbuE1 | ED181 transformed with DNA from HH443 selecting for Cmr |
| ED464 | trpC2 purF6 ydhK::pBOE335 (cat) pbuE1 | ED182 transformed with DNA from HH443 selecting for Cmr and 2-fluoroadenine resistance |
| ED501 | trpC2 amyE::[pLJ24 pbuE′-lacZ (cat)] ade-1 | ED265 transformed with DNA from LJ24 selecting for Cmr |
| ED502 | trpC2 amyE::[pLJ24 pbuE′-lacZ (cat)] ade-1 apt-7 | ED279 transformed with DNA from LJ24 selecting for Cmr |
| ED503 | trpC2 pbuE::pLJ32 (erm) ade-1 | ED265 transformed with DNA from LJ32 selecting for Err |
| ED504 | trpC2 pbuE::pLJ32 (erm) ade-1 apt-7 | ED279 transformed with DNA from LJ32 selecting for Err |
| ED508 | trpC2 amyE::[pKN05n glyA′-lacZ (neo)] pbuE::pLJ42 (cat) | KN05n transformed with DNA from LJ54 selecting for Cmr |
| ED509 | trpC2 thr-5 amyE::[pKN05n glyA′-lacZ (neo)] pbuE1 | LJ27 transformed with DNA from KN05n selecting for Neor |
The designation ydhL has been replaced with the novel functional designation pbuE, defined in reference 4. For selection of antibiotic resistance, antibiotics were used at the following concentrations: ampicillin, 100 mg/liter; neomycin (Neo), 5 mg/liter; erythromycin (Er), 1 mg/liter; lincomycin, 25 mg/liter; and chloramphenicol (Cm), 6 mg/liter. Isolation of DNA and basic molecular biology techniques were performed as previously described (9).
TABLE 2.
Effects of adenine and adenosine on the expression of pbuE′-lacZ transcriptional fusions in B. subtilis mutants defective in the metabolism of adenine
| Strain | Relevant genotype | PbuE status | β-Galactosidase activity (U/mg of protein) witha:
|
||
|---|---|---|---|---|---|
| No purine | 150-μg/ml adenine | 300-μg/ml adenosine | |||
| LJ24 | amyE::[pLJ24 pbuE′-lacZ (cat)] | Wild type | 0.6 | 25 | 4 |
| LJ32 | pbuE::pLJ32 (erm) | Deficient | 1.1 | 267 | 41 |
| ED501 | amyE::[pLJ24 pbuE′-lacZ (cat)] ade | Wild type | 0.4 | 32 | 12 |
| ED502 | amyE::[pLJ24 pbuE′-lacZ (cat)] ade apt | Wild type | 10 | 31 | 17 |
| ED503 | pbuE::pLJ32 (erm) ade | Deficient | 1.0 | 279 | 155 |
| ED504 | pbuE::pLJ32 (erm) ade apt | Deficient | 176 | 249 | 179 |
Values are means of results from three experiments. The variation was less than 25%. Cells were grown in minimal medium. B. subtilis was grown in Spizizen minimal salt medium (15) supplemented with 0.2% l-glutamate, 1 mg of thiamine/liter, and 0.4% glucose as a carbon source (11). Amino acids, when required, were added to give a final concentration of 40 mg/liter. Activity of β-galactosidase was determined in cell extracts as described previously (2). All enzyme determinations were repeated at least three times. One unit of enzyme activity is equal to 1 nmol of product formed per min. Total protein was determined by the Lowry method.
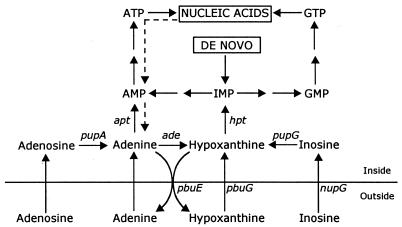
To see whether the expression of the PbuE pump was affected in cells that are limited in their metabolism of adenine, the pbuE expression was determined in ade mutants and ade apt double mutants (Fig. 1; Table 2). Adenosine was also used in these experiments because adenine uptake is reduced in an ade apt double mutant (8). In the ade mutant strains, adenine induces pbuE expression like in the wild-type strain, while the induction by adenosine was higher. In the ade apt double mutant, the basal level of expression was high. The highest level was seen in mutant strains in which the pbuE gene was disrupted. Addition of adenine or adenosine has only little effect. Most likely the cells already contained an increased intracellular level of adenine. To determine whether the studied strains (Table 2) were able to produce and accumulate adenine, the cells were grown in the presence of 67 μM 14C-labeled hypoxanthine (50 μCi per μmol) for 1 h, during which period the intracellular soluble purine compounds, including adenine (Fig. 1), would be fully labeled (7). The uptake of hypoxanthine and the appearance of adenine in the medium were monitored. Cells were harvested by filtration, and free adenine was extracted with formic acid (12). The cellular content was calculated from the known specific activity of hypoxanthine in the growth medium. After 1 h the strains had metabolized between 60 and 70% of the administered hypoxanthine, and most of the label was incorporated into nucleic acids and nucleotides. About 0.5% was recovered in the medium as adenine in the ade mutants. In the ade apt double mutants, substantial amounts of the administered hypoxanthine (4% in strain ED502 and 7% in ED504) were converted to adenine, which was excreted into the medium. Only the double mutants contained measurable adenine pools. The sensitivity of the analysis would allow us to determine an intracellular concentration of only >2 μM adenine. The concentration of adenine measured in the ade apt double mutants ranged from 20 μM in ED502 to 35 μM in strain ED504.
FIG. 1.
Map of central metabolic pathways that are involved in adenine metabolism of B. subtilis. The different enzymatic steps are represented by the corresponding gene designations. Abbreviations: pbuE, purine base efflux pump; pbuG, hypoxanthine-guanine permease; nupG, inosine-guanosine permease; pupA, adenosine phosphorylase; pupG, inosine-guanosine phosphorylase; ade, adenine deaminase; apt, adenine phosphoribosyltransferase; hpt, hypoxanthine-guanine phosphoribosyltransferase. Dashed lines indicate uncharacterized conversions.
Effects of purine analogs on growth and gene expression
Guanine analogs have been shown to bind to the G-box RNA from the xpt-pbuX 5′ untranslated leader sequence (5). Consequently, the expression of the G-box regulon is expected to be repressed. This leads to a decreased expression of the guanine transporter PbuG and a reduction of the uptake of guanine analogs. For adenine analogs the situation is similar: such analogs bind to the A box (6) and activate the efflux pump. We tested several purine analogs for their effects on growth and on the expression of genes belonging to the G- and A-box regulons, and all of the analogs had some effect. The effect of 6-thioguanine was studied in more detail in strain LJ25, with a wild-type pbuE gene, containing an xpt-lacZ fusion in a construct that does not contain the PurR binding site (2, 4). The other strain used (LJ32) contained a pbuE′-lacZ fusion construction in which the pbuE gene was disrupted. Growth of both strains was inhibited by 6-thioguanine. Following addition of 6-thioguanine, the doubling time was increased from 48 to 85 min in strain LJ25 and from 45 to 136 min in strain LJ32. The expression of the xpt′-lacZ fusion was reduced from 280 to 93 U/mg of protein in strain LJ25 and increased from 1.1 to 51 U/mg of protein in strain LJ32 as a result of growth in the presence of 6-thioguanine. Our findings suggest that increased expression of PbuE and reduced expression of the guanine transporter PbuG constitute a cellular mechanism for protection against toxic purine analogs.
Role of PbuE in purine utilization
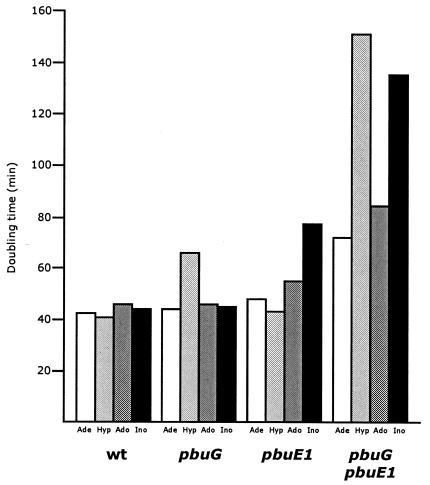
To study the role of PbuE on purine utilization in a purine auxotroph, we constructed a set of strains with different levels of PbuE activity with or without an active hypoxanthine-guanine transporter (PbuG). Cells were grown in liquid minimal medium with different purine compounds at concentrations that would allow exponential growth up to an optical density at 436 nm of 0.8 on nucleosides and 1.6 on purine bases. Inside the cells, the nucleosides are cleaved to their corresponding free bases, and part of the adenine is deaminated to hypoxanthine (Fig. 1). The purine auxotrophic reference strain (the wild type) grew with similar doubling times on the different purine sources; however, it grew slightly slower on nucleosides (Fig. 2), while the pbuG mutant derivative grew significantly slower on hypoxanthine. In a mutant defective in pbuE, the doubling time was the same on all purine sources, namely, approximately 42 min (data not shown). The growth rate of the pbuE1 strain, which overexpresses the PbuE efflux pump, was slightly reduced on all purine sources, most significantly when nucleosides served as the purine source. In the double mutant pbuG pbuE1, growth was dramatically reduced in particular on hypoxanthine and inosine, most likely because hypoxanthine is excreted due to overexpression of PbuE and is only slowly taken up due to a defective PbuG transporter. In the pbuE1 strain the overexpression of PbuE reduced the growth on inosine but did not affect the growth on hypoxanthine. This indicates, as pointed out earlier, that utilization of purine bases derived from nucleosides is reduced when the pump is expressed at high levels. When guanine and guanosine were substituted for hypoxanthine and inosine, similar results were obtained (data not shown).
FIG. 2.
Effect of the purine source on the growth rate of B. subtilis purine auxotrophs (purF), which also are defective in purine base uptake (pbuG) and express PbuE at high levels (pbuE1). The wild-type (wt) strain is ED182, the pbuG strain is ED181, the pbuE1 strain is ED464, and the pbuG pbuE strain is ED463. White columns, cells grown with adenine (Ade); light grey columns, cells grown with hypoxanthine (Hyp); dark grey columns, cells grown with adenosine (Ado); black columns, cells grown with inosine (Ino). Purine bases and nucleosides were added at a concentration of 10 μg/ml.
Is the PurR regulon affected when PbuE is overexpressed
To investigate the effect of PbuE activity on genes controlled solely by PurR and PRPP, we studied the expression of the glyA gene. In wild-type cells of strain KN05n, we observed the expected effects of purine addition, namely, that adenine lowers the PRPP pool and represses glyA expression, while guanosine had the opposite effect (Table 3). The same was found in the mutant (ED508) deficient in PbuE activity. However, in strain ED509, which overexpresses PbuE, the level of expression of the glyA gene was now high both without and with guanosine. The expression of another gene of the PurR regulon, purA encoding succinyl-AMP synthase in strain ED509, followed the same pattern of expression (Table 3). Since PurR regulon expression is controlled by the level of PRPP, we determined the PRPP pool size and found that it was increased in strain ED509 to a level that would stimulate the expression of the PurR regulon. It appears that changes in expression of the pbuE gene and of the G-box regulon cause a metabolic situation that result in a change in PRPP pool size, which increases the expression of the PurR regulon.
TABLE 3.
Expression of the PurR-controlled genes glyA and purA as affected by mutations in pbuE of B. subtilis using a glyA-lacZ transcriptional fusion and determination of succinyl-AMP synthase (PurA) activity
| Strain | Relevant genotype | PbuE status | Activity (U/mg of protein)a
|
Pool size (nmol/mg dry weight)b
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| PurA with no purine addedc | β-Galactosidase with:
|
||||||||
| No purine added | Adenine | Guanosine | ATP | GTP | PRPP | ||||
| KN05n | amyE::[pKN05n glyA′-lacZ (neo)] | Wild type | 9 | 427 | 210 | 663 | 2.4 | 1.3 | 0.7 |
| ED509 | amyE::[pKN05n glyA′-lacZ (neo)] pbuE1 | Overexpressed | 22 | 1,409 | 231 | 1,599 | 3.0 | 1.5 | 1.4 |
| ED508 | amyE::[pKN05n glyA′-lacZ (neo)] pbuE::pLJ42 (cat) | Deficient | 7 | 495 | 223 | 668 | 2.8 | 1.3 | 0.7 |
β-Galactosidase activity was determined as described in the legend to Table 2. Succinyl-AMP synthase activity was determined as described in reference 2.
Pools were determined in cells growing without purine addition (12).
Cells were grown in minimal medium, adenine was added at a concentration of 150 μg/ml, and guanosine was added at 300 μg/ml.
Summary
The level of expression of the G- and A-box and PurR regulons is regulated to ensure a balanced synthesis of purine compounds. This control is mediated via the availability and composition of purines in the growth medium and by intracellularly formed purine bases. The pbuE-encoded efflux pump of B. subtilis is responsible for the adjustment of the cellular pool of free purine bases and therefore plays a key role in the regulatory mechanism that ensures an optimal balance between purine base utilization and purine biosynthesis in B. subtilis. This system also apparently provides a means of protection against toxic purine analogs.
Acknowledgments
We thank Jenny Steno Christensen for excellent technical assistance.
This work was supported by Danish Natural Science Research Council grant 21-03-0570 and the Saxild Family Foundation.
REFERENCES
- 1.Bera, A. K., J. Zhu, H. Zalkin, and J. L. Smith. 2003. Functional dissection of the Bacillus subtilis pur operator site. J. Bacteriol. 185:4099-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christiansen, L. C., S. Schou, P. Nygaard, and H. H. Saxild. 1997. Xanthine metabolism in Bacillus subtilis: characterization of the xpt-pbuX operon and evidence for purine- and nitrogen-controlled expression of genes involved in xanthine salvage and catabolism. J. Bacteriol. 179:2540-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebbole, D. J., and H. Zalkin. 1987. Cloning and characterization of a 12-gene cluster from Bacillus subtilis encoding nine enzymes for de novo purine nucleotide biosynthesis. J. Biol. Chem. 262:8274-8287. [PubMed] [Google Scholar]
- 4.Johansen, E. L., P. Nygaard, C. Lassen, Y. Agersø, and H. H. Saxild. 2003. Definition of a second Bacillus subtilis pur regulon comprising the pur and xpt-pbuX operons plus pbuG, nupG (yxjA), and pbuE (ydhL). J. Bacteriol. 185:5200-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandal, M., B. Boese, J. E. Barrick, W. C. Winkler, and R. R. Breaker. 2003. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell 113:577-586. [DOI] [PubMed] [Google Scholar]
- 6.Mandal, M., and R. R. Breaker. 2004. Adenine riboswitches and gene activation by disruption of a transcription terminator. Nat. Struct. Biol. 11:29-35. [DOI] [PubMed] [Google Scholar]
- 7.Nygaard, P. 1993. Purine and pyrimidine salvage pathways, p. 359-378. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. American Society for Microbiology, Washington, D.C.
- 8.Nygaard, P., P. Duckert, and H. H. Saxild. 1996. Role of adenine deaminase in purine salvage and nitrogen metabolism and characterization of the ade gene in Bacillus subtilis. J. Bacteriol. 178:846-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxild, H. H., L. N. Andersen, and K. Hammer. 1996. dra-nupC-pdp operon of Bacillus subtilis: nucleotide sequence, induction by deoxyribonucleosides, and transcriptional regulation by the deoR-encoded DeoR repressor protein. J. Bacteriol. 178:424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxild, H. H., K. Brunstedt, K. I. Nielsen, H. Jarmer, and P. Nygaard. 2001. Definition of the Bacillus subtilis PurR operator using genetic and bioinformatic tools and expansion of the PurR regulon with glyA, guaC, pbuG, xpt-pbuX, yqhZ-folD, and pbuO. J. Bacteriol. 183:6175-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxild, H. H., and P. Nygaard. 1988. Gene-enzyme relationships of the purine biosynthetic pathway in Bacillus subtilis. Mol. Gen. Genet. 211:160-167. [DOI] [PubMed] [Google Scholar]
- 12.Saxild, H. H., and P. Nygaard. 1991. Regulation of levels of purine biosynthetic enzymes in Bacillus subtilis: effects of changing nucleotide pools. J. Gen. Microbiol. 137:2387-2394. [DOI] [PubMed] [Google Scholar]
- 13.Shin, B. S., A. Stein, and H. Zalkin. 1997. Interaction of Bacillus subtilis purine repressor with DNA. J. Bacteriol. 179:7394-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha, S. C., J. Krahn, B. S. Shin, D. R. Tomchick, H. Zalkin, and J. L. Smith. 2003. The purine repressor of Bacillus subtilis: a novel combination of domains adapted for transcription regulation. J. Bacteriol. 185:4087-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spizizen, J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. USA 44:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vagner, V., E. Dervyn, and D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]