Abstract
Purpose: The literature on physical activity (PA) in adults with cystic fibrosis, particularly in those with cystic fibrosis–related diabetes (CFRD), is limited. PA may be an important part of blood glucose management in CFRD. The purpose of this study was to describe PA levels in adults with CFRD and determine their adherence to the Canadian Diabetes Association (CDA) aerobic exercise training guidelines. Methods: Adults with CFRD were recruited from a hospital-based CF clinic. PA was measured using the Seven-Day Physical Activity Recall (telephone interview), adherence to CFRD management with the Self-Care Inventory–Revised (questionnaire), and blood glucose control from glycated hemoglobin levels documented in participants' medical chart within 3 months. Results: Eighteen adults (mean age 41 [SD 9] y) with diagnosed CFRD participated in the study. They varied in volume of PA (range 13,080–17,362 metabolic equivalent min/wk). Of the study participants, 12 (67%) met the CDA guidelines of 150 minutes of moderate to vigorous PA per week with no more than 2 consecutive days without exercise. No differences were found in clinical factors between those who met the aerobic exercise guidelines and those who did not. Conclusion: The majority of individuals with CFRD are meeting the recommended amount of aerobic PA. The factors influencing PA and blood glucose control in adults with CFRD require further investigation.
Key Words: cystic fibrosis; diabetes; exercise; physical activity, blood glucose; survey
Abstract
Objectif : peu d'études ont été publiées sur l'activité physique (AP) chez les adultes atteints de fibrose kystique, particulièrement chez ceux atteints du diabète associé à la fibrose kystique (DAFK). Or, l'AP jouerait chez ceux-ci un rôle important dans le contrôle de la glycémie. L'objectif de cette étude était de décrire le niveau d'AP chez les adultes atteints de DAFK et de déterminer leur observance des lignes directrices de l'Association canadienne du diabète (ACD) en matière d'exercice aérobique. Méthodologie : des adultes atteints de DAFK ont été recrutés dans la clinique de fibrose kystique d'un centre hospitalier. Le niveau d'AP a été mesuré au moyen d'entrevues téléphoniques (AP rapportée au cours des 7 derniers jours) et du questionnaire révisé sur la prise en charge autonome du diabète. Le contrôle de la glycémie a été évalué en fonction des niveaux d'hémoglobine glyquée relevés dans le dossier médical des participants au cours des 3 derniers mois. Résultats : au total, 18 adultes ayant reçu un diagnostic de DAFK (âge moyen=41 [ÉT=9]) ont participé à l'étude. Leur volume d'AP variait d'un minimum de 13 080 à 17 362 équivalents métaboliques par semaine. Parmi les participants à l'étude, 12 (67 %) suivaient les lignes directrices de l'ACD, soit 150 minutes d'exercice modéré à intense par semaine sans plus de 2 jours consécutifs sans exercice. Aucune différence n'a été constatée dans les facteurs cliniques entre ceux qui suivent les lignes directrices et les autres. Conclusion : la majorité des personnes atteintes de DAFK font le volume recommandé d'AP aérobique. Il faudra étudier davantage les facteurs influant sur l'AP et le contrôle de la glycémie chez les adultes atteints de DAFK.
Mots clés : fibrose kystique; diabète; exercice; activité physique, glycémie
Cystic fibrosis (CF) is a genetic disease that primarily affects the pulmonary and digestive systems.1 As medical treatment advances, individuals with CF are living longer, and the onset of cystic fibrosis–related diabetes (CFRD) is becoming more prevalent.2 CFRD is a complex chronic condition that results in part from fibrosis and fatty infiltration of the pancreas. Type 1 and type 2 diabetes risk factor genes, which are associated with inflammation, peripheral insulin resistance, and amyloid deposition, also play a role in the development of CFRD.3 CFRD shares some clinical features of type 1 and 2 diabetes but also has unique characteristics.4 CFRD is primarily associated with insulin deficiency, but unlike type 1 diabetes, the cause is not autoimmune but rather the result of mucus build-up in the pancreas.3 Thick viscous secretions result in obstructive damage to the exocrine pancreas, which leads to destruction of pancreatic islet cells and subsequently a reduction in insulin-producing cells.3 Similar to type 2 diabetes, CFRD is associated with insulin resistance but at a more modest level.3 In contrast to both type 1 and type 2 diabetes, CFRD does not typically include macrovascular complications. Management of CFRD generally follows the Canadian Diabetes Association (CDA) guidelines and involves insulin administration, but CFRD treatment also has unique aspects, including increased nutritional intake through a high-energy diet. The prevalence of CFRD increases with age, and the disease affects 2% of children, 19% of adolescents, and 40%–50% of adults.5 CFRD is the most common comorbidity in individuals with CF.2
Regular physical activity (PA) and exercise are important aspects of disease management for individuals with CF. Exercise has been shown to slow the rate of decline in pulmonary function, improve aerobic capacity, improve quality of life, increase participation in activities of daily living, and reduce hospital admissions.6 Furthermore, PA is a cornerstone of the management of type 1 and 2 diabetes and has been shown to have positive effects on the reduction of cardiovascular risk and overall mortality rates in both type 1 and 2 diabetes.7–11 Proper blood glucose control in people with CF is vital because hyperglycemia has a negative impact on their pulmonary function.6 Therefore, exercise and PA may be an important aspect of the management of CFRD, addressing issues related to both CF and blood glucose control.6
The literature investigating PA levels in individuals with CF is limited. One study showed that adults with CF performed similar levels of moderate and vigorous PA as their healthy peers. However, the study also found that adults with CF accumulated less habitual PA associated with work and transportation than adults without CF.12 To date, no studies have specifically examined levels of PA in people with CFRD. Furthermore, because PA is known to be beneficial for blood glucose control in individuals with Type 1 or 2 diabetes, regular PA of adequate intensity has potential benefits for individuals with CFRD.
The primary objective of this study was to describe PA levels in people with CFRD in terms of frequency, duration, intensity, and types of activity. Second, we wanted to determine the proportion of adults with CFRD who met the Canadian Diabetes Association (CDA) aerobic exercise guidelines.11 As an exploratory aim, we compared clinical and demographic factors (blood glucose control, lung function, adherence to CFRD management, body mass index [BMI], and age) between adults with CFRD who did and did not meet the CDA aerobic exercise guidelines.
Methods
Study design
A cross-sectional study of adults with CFRD was conducted. The study used a telephone-based interviewer-administered questionnaire for PA, the Seven-Day Physical Activity Recall (7DPAR), and a mailed self-report questionnaire, the Self Care Inventory–Revised (SCI–R), to determine adherence to CFRD management. The study protocol was approved by the St. Michael's Hospital Research Ethics Board and the University of Toronto Research Ethics Board.
Participants
Participants were recruited from the Toronto Adult Cystic Fibrosis Program at St. Michael's Hospital from February 2014 to July 2014. Participants were included if they were aged 18 years or older and had documented diagnoses of CF and CFRD in their medical chart. Participants were excluded if they met any of the following criteria: liver, lung, or kidney transplant recipient; unable to speak or understand English; use of intravenous (IV) antibiotics or corticosteroid therapy (IV or oral) within 3 months of recruitment; documented liver disease; pregnancy; or an inpatient at the time of the study. Informed consent was obtained from all participants.
Study protocol
Demographics and clinical data
Pertinent clinical information was obtained from the participants' medical charts including age, sex, weight, height, BMI, genotype, pancreatic status, Burkholderia cepacia and Pseudomonas aeruginosa status,13 forced expiratory volume in 1 second (FEV1),14 and glycated hemoglobin (HbA1c) within 3 months of study participation.
Seven-Day Physical Activity Recall
Telephone-based interviews were conducted using the 7DPAR. The 7DPAR is a structured, standardized interview to measure PA levels that has previously been validated in adults with CF.15 Two investigators administered the 7DPAR over the telephone.16 Before data collection, these investigators underwent training with a clinical expert to administer the questionnaire using a standardized script. The 7DPAR has a test–retest reliability of 0.99 and an interrater reliability of 0.86.17
To administer the 7DPAR, the investigator asks the participant to recall the physical activities he or she has done during the past 7 days. The participant is asked to categorize the intensity of the activity into one of four categories: light, moderate, hard, or very hard. To assist with recall of PA, each day is broken down into three segments (morning, afternoon, and evening). When recording PA, a minimum of duration of 10 minutes needs to be met. Participants are also asked the number of hours they spent sleeping per week, defined as from the time they got into bed until the time they got out of bed. From the data, intensity of each activity is estimated using the following metabolic equivalent (MET) values: sleep=1 MET, light activity=1.5 METs, moderate activity=4 METs, hard activity=6 METs, and very hard activity=10 METs.16,18 Weekly MET minutes is then calculated from frequency (number of days), intensity (MET level), and time engaged in PA over the 7-day period. Strength and flexibility exercises are recorded on the 7DPAR but are not included in the calculation of volume of PA. Finally, the 7DPAR allows the participant to state whether the recorded week's activities are reflective of a typical week and whether any special considerations should be taken into account, such as injury or illness.16
The results of the 7DPAR were used to group participants into those who met versus those who did not meet the CDA aerobic training guidelines. The CDA has no specific exercise guidelines for individuals with CFRD, but it does have a general aerobic exercise guideline for individuals with diabetes (type 1 or type 2) of 150 minutes of moderate to vigorous PA per week with no more than 2 consecutive days without exercise.11 Because limited information on strength training was available from the 7DPAR, the CDA guidelines for strength training twice per week were not included as part of the analysis.
Self Care Inventory–Revised
After completing the 7DPAR, each participant was mailed a copy of the SCI–R to complete and return within 2 weeks. The SCI–R is a self-report questionnaire consisting of 15 items that assess participants' perceptions of their own self-care regarding diabetes management with a 5-point Likert scale.19 The SCI–R captures four areas of diabetes management: blood glucose regulation, insulin and food regulation, exercise, and emergency precautions.19 Of the 15 items on the SCI–R, 7 that were associated with improved diabetes management were summed to produce a final score of 35. These 7 items focus on blood glucose regulation, insulin and food regulation, and exercise; items related to emergency precautions were excluded.19 The SCI–R has been validated in adults with diabetes and has an internal consistency of α=0.87.20
Statistical analysis
Data were analyzed using SPSS version 21.0 (SPSS Inc., Chicago, IL). Demographics, volume of PA, and SCI–R scores were described using mean and standard deviation. The Shapiro–Wilk test was used to test for normality. An unpaired t test, assuming unequal variance, was used to compare the volume of PA, blood glucose control (HbA1c), lung function (FEV1), adherence to CFRD management (SCI–R score), BMI, and age between participants who did and did not meet the CDA aerobic exercise guidelines. Significance was set at p≤0.05.
Results
Study sample
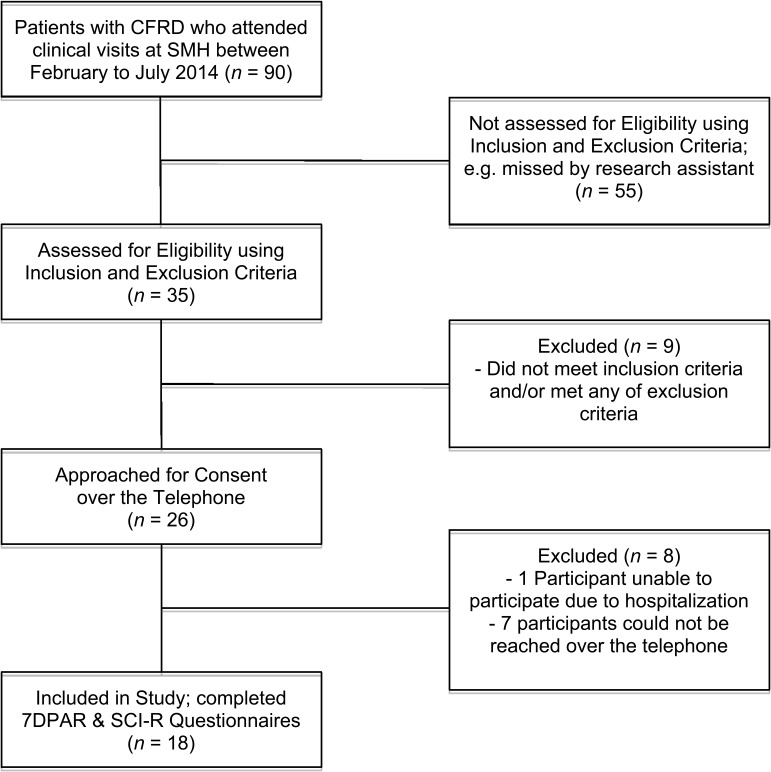
The study sample consisted of 18 adults with CFRD. The participant flow through the study is shown in Figure 1. Demographic and clinical characteristics are summarized in Table 1. Participants ranged in age from 22 to 55 years. The genetic variants of CF included homozygous variant F, F (delta F508; 8 participants), and 1 participant each had variant R347P and 2752–2A>G, 2789+5G>A, (5T), G85E, G542X and N1303K, 1898+1G>A, G542X and R560T, and 621+1G>T. Two participants had unknown genotypes. Two participants were pancreatic sufficient, defined as having sufficient pancreatic enzyme level in the intestinal lumen required to maintain normal digestion.21 Lab values showed that 4 participants (22%) grew Burkholderia cepacia and 13 participants (72%) grew Pseudomonas aeruginosa.
Figure 1.
Flow of participant recruitment. CFRD=cystic fibrosis–related diabetes; 7DPAR=Seven-Day Physical Activity Recall; SCI–R=Self Care Inventory–Revised.
Table 1.
Summary of Participant Characteristics (n=18)
| Variable | Mean (SD)* |
| Age, y | 41 (9) |
| Sex | |
| Male | 10 (55.6) |
| Female | 8 (44.4) |
| Weight, kg | 65.5 (13.1) |
| Height, cm | 167.2 (10.4) |
| BMI | 23.2 (2.5) |
| HbA1c (%) | 7.0 (1.1) |
| SCI–R score† | 22.4 (3.3) |
| FEV1, L | 2.1 (0.8) |
| FEV1, % predicted | 57.9 (20.9) |
Unless otherwise indicated.
Out of 35.
BMI=body mass index; HbA1c=glycated hemoglobin; SCI–R=Self Care Inventory–Revised; FEV1=forced expiratory volume in 1 s.
Physical activity
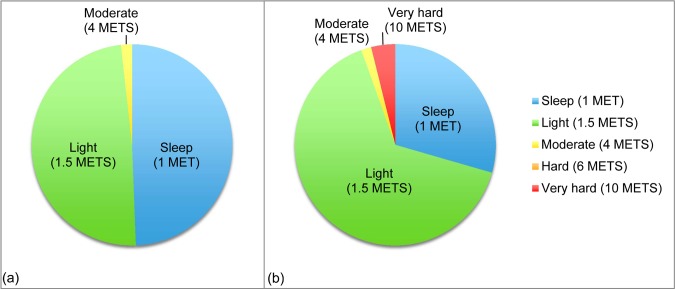
The volume of PA per week varied, ranging from 13,080 to 17,362 MET minutes per week (mean 14,370 [SD 997] MET min/wk). Three participants reported illness or injury all week affecting their 7DPAR. One participant's illness affected his or her ability to participate in gardening activities, and another participant's injury affected participation in Pilates. The last person who had an illness reported doing more PA during the week than usual. Figure 2 shows the distribution of activity in the most and least active participants in terms of volume of PA. Neither of these participants reported any illness or injury affecting their reported PA. The least active participant spent approximately half of her total weekly time either in bed or sleeping. Most of her activity was light intensity, and she spent only a small amount of time doing moderate PA, which was walking. Conversely, the participant with the highest volume of PA spent several hours per week participating in “very hard,” high-intensity activities. In contrast to the least active participant, the most active participant spent much less than half of his total hours per week sleeping (Figure 2).
Figure 2.
Distribution of physical activity time and intensity over 1 week in (a) the participant with the lowest volume of physical activity and (b) the participant with the highest volume of physical activity. METs=metabolic equivalents.
Time spent in PA for each intensity category is summarized in Table 2. Participants spent the most amount of active time engaged in light PA such as household and occupational tasks, desk work, and so forth. More than half of the participants (10; 56%) reported doing PA at a moderate intensity (4 METs) 3 or more days per week, with the most common activity being walking. Three participants reported doing hard PA (6 METs) at least 3 days per week, and activities included swimming, brisk walking on the treadmill, stationary biking, and shoveling snow. Four participants reported doing very hard PA (10 METs) at least 3 days per week, including running, playing soccer, and heavy yard work. Time spent sleeping ranged from 47 to 83 hours per week. One third of participants (6; 33%) engaged in intentional strengthening or flexibility exercises for as much as 3.5 hours per week with reported activities including weight training, Pilates, and yoga.
Table 2.
Time Spent in Physical Activity per Week in Each Intensity Category
| Intensity of activity | Total hours, mean (SD) | % of waking time, mean (SD) | Hours, range | No. participating in each activity |
| Light (1.5 METs) | 103.3 (9.4) | 96.1 (2.8) | 82–118.2 | 18 |
| Moderate (4 METs) | 2.9 (2.7) | 2.5 (2.6) | 0–8.8 | 15 |
| Hard (6 METs) | 0.8 (0.9) | 0.5 (0.8) | 0–3.0 | 8 |
| Very hard (10 METs) | 1.0 (1.9) | 1.0 (1.7) | 0–6.5 | 7 |
METs=metabolic equivalents.
Adherence to CFRD management
SCI–R scores ranged from 15 to 29 out of 35 (mean 22.4 [SD 3.3]). Approximately two thirds of participants recorded “usually” or “always” checking blood glucose levels (61%) and taking medication or insulin (66%) at the recommended time. Almost all of the participants reported “never” or “rarely” keeping food records (83%) and also reported “usually” or “always” exercising (78%).
Comparison of clinical and demographic factors
Of 18 participants, 12 (67%) met the CDA aerobic exercise guidelines.11 Comparisons between individuals who did and did not the aerobic guidelines are summarized in Table 3. In comparing individuals who met the CDA guidelines with those who did not, only a borderline difference in the weekly volume of PA (MET mins) was noted (p=0.05), with no significant difference noted in amount of sleep between the two groups (p=0.07).
Table 3.
Comparison of Participants Who Met and Did not Meet CDA Aerobic Training Guidelines: Demographic and Clinical Characteristics
| Variable | Met CDA guidelines (n=12), mean (SD) |
Did not meet CDA guidelines (n=6), mean (SD) |
p-value |
| Age, y | 41.8 (10.4) | 40.5 (7.3) | 0.72 |
| BMI, kg/m2 | 23.4 (2.3) | 23.0 (3.2) | 0.78 |
| SCI–R score | 22.2 (3.8) | 23.0 (2.0) | 0.55 |
| FEV1, % | 55.8 (19.5) | 62.3 (24.6) | 0.58 |
| HbA1c, % | 6.8 (0.7) | 7.5 (1.5) | 0.34 |
| Weekly PA, MET-min | 14,636 (1,094) | 13,840 (492) | 0.05* |
| Weekly sleep, hr | 63.2 (9.2) | 55.4 (4.4) | 0.07 |
p≤0.05 denotes a statistically significant difference.
CDA=Canadian Diabetes Association; BMI=body mass index; SCI–R=Self Care Inventory–Revised; FEV1=forced expiratory volume in 1 s; HbA1c=glycated hemoglobin; PA=physical activity; METs=metabolic equivalents.
Discussion
CFRD is a growing health concern in individuals with CF. As for individuals with type 1 and 2 diabetes, the management for CFRD may include PA.10 Our study is the first to evaluate PA levels in adults with CFRD. We found that adults with CFRD engaged in PA across all intensity categories and that 67% met the CDA aerobic training guidelines.11 We found no significant difference in blood glucose control between adults with CFRD who met the CDA guidelines and those who did not, but larger studies examining the multiple factors affecting blood glucose control are needed to examine this question further.
This study is the first to describe PA levels in adults with CFRD. It demonstrated relatively high PA levels, with 67% of the participants reaching the CDA guidelines. Although the research on PA in people with CF is limited, previous studies have also shown good participation in PA. Rasekaba et al.12 examined habitual PA patterns in adults with CF using a self-report PA questionnaire, the International Physical Activity Questionnaire. The study demonstrated that patients with CF participated in an equal amount of moderate and vigorous PA as a healthy group without CF, but adults with CF performed less PA associated with work and transport-related activities.12
Another study by Ruf et al.15 looked at PA levels in people with CF (n=41) using the 7DPAR and hip accelerometry. These authors reported PA measurements obtained from the 7DPAR in time (minutes per day). Similar to our study, they found that participants spent the least amount of time in hard or very hard activities, although they appeared to be more active per day than our sample.15 It is important to note that Ruf et al. included mostly children and those with low disease severity, which may explain the higher PA levels.15 In comparison, only about 30% of adults with type 2 diabetes achieve adequate PA levels,22 and those who follow the CDA guidelines for PA have a higher level of motivation for PA than those who do not meet the guidelines.23 Examining behavioural regulation in people with CFRD may assist us in understanding how to encourage these individuals to participate in adequate PA.
Because there are no specific PA guidelines for patient populations with CF or CFRD, and considering the similarities between CFRD and type 1 and type 2 diabetes, we used the CDA exercise guidelines for people living with diabetes. The evidence supporting these guidelines suggests that moderate to high levels of PA are effective in improving glycemic control in people with type 2 diabetes.11 We did not observe a significant difference in blood glucose control in participants who met the CDA guidelines, but those who did not meet the CDA guidelines had an average HbA1c that was over the recommended limit (<7%), which may be clinically meaningful. We should also note that multiple factors affect an individual's measure of HbA1c, including adherence to insulin administration and use of other medications for CFRD. With regard to CFRD management adherence, we found no significant difference between the SCI–R scores of the participants who met the CDA guidelines and those who did not. We also found only 66% adherence to taking diabetes medication at the recommended time, which is a similar rate of adherence to respiratory medications reported in adults with CF.24 This suboptimal level of adherence may also have affected blood glucose control in some of our participants. Further studies with a larger sample size, greater variance in HbA1c, and more specific measures of PA, such as accelerometry, are needed to elucidate this relationship. In addition, multiple regression analysis investigating different determinants of HbA1c may further our understanding of the importance of PA as a factor in the management of CFRD.
Several limitations in study methodology should be noted. We had a small sample size of participants who were volunteers and therefore may have been more interested in exercise than others with CFRD. This may have led to a sampling bias and limits the generalizability of our findings. A post hoc power analysis revealed that 76 participants would be required to detect a difference in HbA1c between those who did and did not meet the CDA guidelines. We did not include sociodemographic variables such as education level, income, marital status, or employment status, which may influence PA level.25 We did not look at the strength training aspect of the CDA guidelines because the 7DPAR groups resistance and flexibility exercises into one category. Thus, there was insufficient detail for this analysis. Furthermore, the 7DPAR is a self-report measure, which can lead to overestimation of levels of PA.26 Some participants also noted difficulty with recalling activities from the previous week, indicating that recall bias may have played a role in the accuracy of PA levels reported by participants. The use of accelerometry in future studies would allow for more accurate measures of PA and eliminate biases associated with self-report.15 Last, because participant recruitment took place from February to July, seasonal variations in PA would have been a factor in participants' reported levels of PA. For example, some of the reported activities were specific to winter such as hockey and shoveling snow.
Conclusion
This study demonstrates that many individuals with CFRD are participating in frequent PA, and the majority of our sample was meeting the recommended amount of aerobic PA to achieve optimal health for people living with diabetes. Considering that individuals with CFRD experience exercise limitation because of respiratory disease and systemic effects (e.g., muscle weakness, osteoporosis), it is encouraging to see that individuals with CFRD are able to meet the CDA guidelines. Although the role of PA as a management strategy for blood glucose levels in people with CFRD needs to be further studied, PA has known benefits for pulmonary function in individuals with CF, and it is important for clinicians to encourage PA as part of a general management strategy for individuals with CFRD.
Key Messages
What is already known on this topic
Evidence supports the importance of exercise and physical activity for both patients with cystic fibrosis CF and patients with diabetes, but the research on physical activity (PA) levels specifically in people with cystic fibrosis–related diabetes (CFRD) is limited. The role of PA in managing blood glucose control in people with CFRD has not yet been investigated.
What this study adds
This is the first study to describe PA levels in individuals with CFRD and to compare their levels of PA with the Canadian Diabetes Association–recommended guidelines for aerobic training. The results of this study add meaningful information to the literature regarding the types, volume, and frequency of PA in adults with CFRD. The majority of our sample met the recommended aerobic exercise guidelines for diabetes management, suggesting that despite potential barriers to PA related to their disease, many individuals with CFRD are capable of achieving a relatively active lifestyle.
References
- 1. Cystic Fibrosis Canada. Canadian Cystic Fibrosis Registry: 2013 annual report. Toronto: Cystic Fibrosis Canada; 2015. [Google Scholar]
- 2. Moran A, Becker D, Casella SJ, et al. ; CFRD Consensus Conference Committee. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes Care. 2010;33(12):2677–83. http://dx.doi.org/10.2337/dc10-1279. Medline:21115770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly A, Moran A. Update on cystic fibrosis-related diabetes. J Cyst Fibros. 2013;12(4):318–31. http://dx.doi.org/10.1016/j.jcf.2013.02.008. Medline:23562217 [DOI] [PubMed] [Google Scholar]
- 4. Moran A, Brunzell C, Cohen RC, et al. ; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–708. http://dx.doi.org/10.2337/dc10-1768. Medline:21115772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moran A, Dunitz J, Nathan B, et al. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32(9):1626–31. http://dx.doi.org/10.2337/dc09-0586. Medline:19542209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dwyer TJ, Elkins MR, Bye PT. The role of exercise in maintaining health in cystic fibrosis. Curr Opin Pulm Med. 2011;17(6):455–60. Medline:21881514 [DOI] [PubMed] [Google Scholar]
- 7. Konrad K, Scheuing N, Badenhoop K, et al. Cystic fibrosis-related diabetes compared with type 1 and type 2 diabetes in adults. Diabetes Metab Res Rev. 2013;29(7):568–75. Medline:23704008 [DOI] [PubMed] [Google Scholar]
- 8. Herbst A, Bachran R, Kapellen T, et al. Effects of regular physical activity on control of glycemia in pediatric patients with type 1 diabetes mellitus. Arch Pediatr Adolesc Med. 2006;160(6):573–7. http://dx.doi.org/10.1001/archpedi.160.6.573. Medline:16754817 [DOI] [PubMed] [Google Scholar]
- 9. De Feo P, Di Loreto C, Ranchelli A, et al. Exercise and diabetes. Acta Biomed. 2006;77(suppl 1):14–7. Medline:16921608 [PubMed] [Google Scholar]
- 10. O'Shea D, O'Connell J. Cystic fibrosis related diabetes. Curr Diab Rep. 2014;14(8):511 [DOI] [PubMed] [Google Scholar]
- 11. Sigal RJ, Armstrong MJ, Colby P, et al. ; Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Physical activity and diabetes. Can J Diabetes. 2013;37(suppl 1):S40–4. http://dx.doi.org/10.1016/j.jcjd.2013.01.018. Medline:24070962 [DOI] [PubMed] [Google Scholar]
- 12. Rasekaba TM, Button BM, Wilson JW, et al. Reduced physical activity associated with work and transport in adults with cystic fibrosis. J Cyst Fibros. 2013;12(3):229–33. http://dx.doi.org/10.1016/j.jcf.2012.09.003. Medline:23058656 [DOI] [PubMed] [Google Scholar]
- 13. Lee TW, Brownlee KG, Conway SP, et al. Evaluation of a new definition for chronic pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2(1):29–34. http://dx.doi.org/10.1016/S1569-1993(02)00141-8. Medline:15463843 [DOI] [PubMed] [Google Scholar]
- 14. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. http://dx.doi.org/10.1183/09031936.05.00035205. Medline:16264058 [DOI] [PubMed] [Google Scholar]
- 15. Ruf KC, Fehn S, Bachmann M, et al. Validation of activity questionnaires in patients with cystic fibrosis by accelerometry and cycle ergometry. BMC Med Res Methodol. 2012;12(43):43 http://dx.doi.org/10.1186/1471-2288-12-43. Medline:22471343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sallis JF, Haskell W, Wood P, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106 [DOI] [PubMed] [Google Scholar]
- 17. Gross LD, Sallis JF, Buono MJ, et al. Reliability of interviewers using the Seven-Day Physical Activity Recall. Res Q Exerc Sport. 1990;61(4):321–5. http://dx.doi.org/10.1080/02701367.1990.10607494. Medline:2132889 [DOI] [PubMed] [Google Scholar]
- 18. Pescatello LS, Arena R, Riebe D, et al., editors. ACSM's guidelines for exercise testing and prescription. 9th ed. Baltimore: Lippincott, Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 19. LaGreca AM. Manual for the Self Care Inventory [Internet]. Miami: Author; 2004. [cited 2014 Jul 28]. Available from: https://www.psy.miami.edu/media/college-of-arts-and-sciences/psychology/documents/faculty/alagreca/SCI_manual_2004.pdf [Google Scholar]
- 20. Weinger K, Butler HA, Welch GW, et al. Measuring diabetes self-care: a psychometric analysis of the Self-Care Inventory—Revised with adults. Diabetes Care. 2005;28(6):1346–52. http://dx.doi.org/10.2337/diacare.28.6.1346. Medline:15920050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalivianakis M, Verkade HJ. The mechanisms of fat malabsorption in cystic fibrosis patients. Nutrition. 1999;15(2):167–9. Medline:9990588 [DOI] [PubMed] [Google Scholar]
- 22. Plotnikoff RC, Johnson ST, Loucaides CA, et al. Population-based estimates of physical activity for adults with type 2 diabetes: a cautionary tale of potential confounding by weight status. J Obes. 2011;2011:561432 http://dx.doi.org/10.1155/2011/561432. Medline:20871829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miquelon P, Castonguay A. Motives for participation in physical activity and observance of physical activity recommendations among adults with type 2 diabetes. Can J Diabetes. Epub 2016 Apr 6 http://dx.doi.org/10.1016/j.jcjd.2016.02.009. Medline:27062109 [DOI] [PubMed] [Google Scholar]
- 24. Hogan A, Bonney MA, Brien JA, et al. Factors affecting nebulised medicine adherence in adult patients with cystic fibrosis: a qualitative study. Int J Clin Pharm. 2015;37(1):86–93. http://dx.doi.org/10.1007/s11096-014-0043-6. Medline:25432693 [DOI] [PubMed] [Google Scholar]
- 25. Bauman AE, Reis RS, Sallis JF, et al. ; Lancet Physical Activity Series Working Group. Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380(9838):258–71. http://dx.doi.org/10.1016/S0140-6736(12)60735-1. Medline:22818938 [DOI] [PubMed] [Google Scholar]
- 26. Adams SA, Matthews CE, Ebbeling CB, et al. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol. 2005;161(4):389–98. http://dx.doi.org/10.1093/aje/kwi054. Medline:15692083 [DOI] [PMC free article] [PubMed] [Google Scholar]