Abstract
The Cpx envelope stress response mediates adaptation to potentially lethal envelope stresses in Escherichia coli. The two-component regulatory system consisting of the sensor kinase CpxA and the response regulator CpxR senses and mediates adaptation to envelope insults believed to result in protein misfolding in this compartment. Recently, a role was demonstrated for the Cpx response in the biogenesis of P pili, attachment organelles expressed by uropathogenic E. coli. CpxA senses misfolded P pilus assembly intermediates and initiates increased expression of both assembly and regulatory factors required for P pilus elaboration. In this report, we demonstrate that the Cpx response is also involved in the expression of the type IV bundle-forming pili of enteropathogenic E. coli (EPEC). Bundle-forming pili were not elaborated from an exogenous promoter in E. coli laboratory strain MC4100 unless the Cpx pathway was constitutively activated. Further, an EPEC cpxR mutant synthesized diminished levels of bundle-forming pili and was significantly affected in adherence to epithelial cells. Since type IV bundle-forming pili are very different from chaperone-usher-type P pili in both form and biogenesis, our results suggest that the Cpx envelope stress response plays a general role in the expression of envelope-localized organelles with diverse structures and assembly pathways.
Many bacterial stress responses appear to serve several cellular roles, offering protection against a variety of stresses while at the same time assisting in distinct physiological functions. The Cpx envelope stress response has been studied predominantly with laboratory strains of Escherichia coli. In this background, the Cpx envelope stress response facilitates adaptation to potentially fatal insults to the envelope, such as changes in pH (17) or membrane composition (16, 61). The response is controlled by a three-component regulatory system made up of the membrane-bound sensor kinase CpxA, the periplasmic inhibitor protein CpxP, and the cytoplasmic response regulator CpxR (17, 18, 24, 71, 74-76, 100). CpxP plays a unique role in inhibiting the Cpx envelope stress response, through an undescribed mechanism (74, 75). CpxA is thought to recognize misfolded envelope proteins that arise during stresses to the envelope, since mutations that constitutively activate the kinase rescue the cell from mutant, misfolded envelope proteins (12) and since overproduced, misfolded envelope proteins potently activate the sensor (19, 47, 87). These signals lead to the autophosphorylation of CpxA and to the subsequent phosphorylation of CpxR (76), in a fashion typical of two-component regulators. Phosphorylated CpxR likely alleviates envelope stress through transcriptional activation of genes affecting envelope protein folding and proteolysis. Some of these genes encode the periplasmic protease chaperone DegP (18, 19, 71); two peptidyl-prolyl isomerases, PpiA (71) and PpiD (20); the major envelope disulfide oxidase DsbA (18, 71); and the periplasmic stress protein Spy (74).
Recent studies indicated the involvement of the Cpx envelope stress response in multiple aspects of bacterial physiology and the cellular response to stress. Bioinformatic approaches suggest that the Cpx pathway may regulate a host of additional genes with diverse functions, many of which are part of other stress response pathways (21, 22). Additionally, the Cpx envelope stress response was recently linked to adhesion processes. In stationary phase, the Cpx response is activated by and required for efficient attachment to abiotic surfaces (67). Further, the Cpx signal transduction pathway is involved in the assembly and regulation of at least one type of pilus.
P or Pap pili are attachment organelles expressed by strains of uropathogenic E. coli (UPEC). P pili are the prototypes of the chaperone-usher class of pili, which are all thought to be expressed on the cell surface by means of a similar assembly mechanism (91). P pili consist of multiple subunits that make up a thin tip fibrillum containing the adhesin anchored to a thick, helical rod (8, 32, 53). The subunits are initially synthesized in the cytoplasm and transported to the periplasm through the general secretory machinery. Upon entry into the periplasm, the subunits are met by a P pilus-specific chaperone protein which serves to prevent premature aggregation into toxic aggregates in the periplasm, assist folding, and foster assembly into the growing pilus at an outer membrane assembly platform or usher (3, 80, 81). The platform consists of a single, pilus-specific usher protein assembled into ring-shaped oligomeric complexes containing a central pore through which pilus subunits are secreted and incorporated into mature pili (23, 92).
During P pilus expression, some subunits leave the pathway (off-pathway) or fail to interact with the periplasmic chaperone, thereby forming in the periplasmic toxic misfolded aggregates that associate with the inner membrane (39, 47). In laboratory strains of E. coli, the expression of all of the pap genes required for P pilus expression induces the Cpx envelope stress response, which in turn activates the expression of at least two genes that encode products required for efficient P pilus assembly (47). DegP degrades the otherwise toxic, off-pathway aggregated subunits (47), while DsbA is required for the proper folding of several P pilus subunits and assembly proteins (43). In the absence of the Cpx pathway, laboratory strains of E. coli that are provided with the pap gene cluster synthesize aberrant P pili that are far shorter than those synthesized by the isogenic wild-type strain (40). In addition, phosphorylated CpxR binds to the control region of the pap operon that mediates the phase-variable expression of these structures, indicating that Cpx regulators also act to affect the transcription of the pap operon (40). Accordingly, at least in laboratory strains of E. coli, one role of the Cpx pathway is to enable the proper expression of P pili.
Interestingly, a number of Cpx-regulated Dsb and DegP homologues have been implicated in the assembly of envelope-localized virulence determinants in a variety of pathogens. These include type IV pili (TFP) in Vibrio cholerae (70) and Neisseria meningitidis (93), type III secretion systems (TTSS) in Shigella flexneri (99, 107-109) and Salmonella enterica serovar Typhimurium (27, 60), and virulence determinants in Burkholderia cepacia (37), Erwinia carotovora (97), Pseudomonas aeruginosa (7, 36, 58), Bordetella pertussis (89), S. enterica serovar Typhimurium (27, 46), S. flexneri (73), and Yersinia pestis (42, 101). Further, DegP homologues have been implicated in virulence in Klebsiella pneumoniae (11) and Yersinia enterocolitica (57) and shown to be involved in the intracellular survival of Bartonella henselae (78), Y. enterocolitica (105), Legionella pneumophila (69), S. enterica serovar Typhimurium (4, 29), and Haemophilus influenzae (13). In addition, an E. coli K1 strain was defective in causing systemic disease in an infant rat model when the dsbA gene was obliterated (33). Thus, it seems possible that the Cpx envelope stress response plays a role in the expression of diverse types of envelope structures that share a common need for Cpx-regulated folding and/or degrading factors. In this study, we have tested this hypothesis by analyzing the role of the Cpx envelope stress response in the expression of the type IV bundle-forming pili (BFP) of enteropathogenic E. coli (EPEC).
EPEC is the leading cause of human infantile diarrhea (65). Although the actual causative mechanism behind the diarrheal symptoms is not clear, the events comprising infection have been well characterized at the molecular level. Initially, clumps, or microcolonies, of EPEC held together through interactions between BFP on adjacent cells loosely adhere to intestinal epithelial cells in a pattern known as localized adherence (25, 31). This event is followed by the injection of virulence determinants (E. coli secreted proteins) through a TTSS made up of E. coli secreted proteins and E. coli secretion complex proteins into the host cell cytoplasm (28, 44, 51, 52, 98, 103) and more intimate adherence mediated by the bacterial outer membrane protein intimin and an EPEC-encoded receptor known as the translocated intimin receptor (50). The translocated intimin receptor and other TTSS-translocated effector proteins mediate gross changes in the cytoskeleton of the eukaryotic cell that lead to the formation of actin-rich pedestals beneath tightly adhering EPEC bacteria, resulting in the disruption of the host cell surface (for reviews, see references 9, 26, 30, 65, and 95).
In this study, we have examined the effect of the Cpx envelope stress response on the expression of the type IV BFP. The TFP, including the BFP of EPEC, have different topologies and are thought to utilize an assembly mechanism very different from that of the chaperone-usher-type pili (104). They consist predominantly of a single pilin subunit and are thought to be assembled from pools of pilin subunits in the inner membrane by pilus-specific assembly complexes that span the entire envelope. Genetic and biochemical studies of the 14 genes found in the bfp gene cluster (90) suggest that the BFP assembly apparatus spans the envelope and is comprised of distinct subassembly complexes made up of BFP proteins that reside in the inner and outer membranes and are connected by a periplasmic component containing BfpU (1, 41, 77, 83). Recent work suggests that the inner membrane subassembly complex consists of the integral inner membrane proteins BfpE (6) and BfpC, together with the putative cytoplasmic ATPases BfpD and BfpF (88, 90), which are proposed to power pilus extension and retraction (2, 5, 15, 77), respectively. BfpB is an outer membrane secretin (82) which, together with BfpG, constitutes the outer membrane subassembly complex (77). The bfp genes that are sufficient for expression and assembly are carried on a large plasmid known as the EPEC adherence factor (EAF) plasmid (25, 31) and are subject to transcriptional regulation by the BfpTVW/PerABC regulatory locus and a variety of environmental signals (59, 72, 94).
Curiously, although a cluster of 14 bfp genes was previously shown to be sufficient for BFP expression in E. coli laboratory strain HB101 (90), we found that the same gene cluster failed to permit BFP assembly on the surface of laboratory strain MC4100 unless the Cpx pathway was constitutively activated by the presence of cpxA* gain-of-function mutations. This observation led us to investigate the role of the Cpx envelope stress response in the expression of BFP in an EPEC background. Our experiments indicate that an intact Cpx pathway is required for efficient BFP expression and attachment to epithelial cells. This is the first study to address the role of the Cpx envelope stress response directly in a pathogenic isolate of E. coli.
MATERIALS AND METHODS
Strains and plasmids.
The strains and plasmids used in this study are described in Table 1. The construction of an E2348/69 cpxR::Kn mutant is described below. All other strains were constructed by standard genetic techniques (84). To construct the CpxR overexpression vector pROX, the cpxR open reading frame was amplified from the chromosome of MC4100 by using BamHI-tagged primer cpxRpBAD (5′-CGGGATCCCGGCGACGTCTGATGACGTAATTTC-3′) and PstI-tagged primer cpxRC (5′-TGCACTGCAGATCATGAAGCAGAAACCATC-3′) (underlining indicates the tags). The PCR product was purified and cloned into the BamHI and PstI sites of pBAD18 by standard molecular biological techniques.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E2348/69 | EPEC (O127:H6) isolated from an infant with gastroenteritis | 55 |
| JPN15 | E2348/69 lacking the EAF plasmid encoding the BFP | 45 |
| ALN17 | MC4100 λRS88 (spy-lacZ)(ptrc99A) | This study |
| ALN18 | MC4100 λRS88 (spy-lacZ)(pKDS301) | This study |
| ALN19 | MC4100 λRS88 (spy-lacZ)(pKDS302) | This study |
| ALN33 | MC4100 λRS88 (spy-lacZ) cpxR1::Spc(ptrc99A) | This study |
| ALN34 | MC4100 λRS88 (spy-lacZ) cpxR1::Spc(pKDS301) | This study |
| ALN35 | MC4100 λRS88 (spy-lacZ) cpxR1::Spc(pKDS302) | This study |
| ALN40 | MC4100 λRS88 (spy-lacZ) cpxA101 zii::Tn10(ptrc99A) | This study |
| ALN41 | MC4100 λRS88 (spy-lacZ) cpxA101 zii::Tn10(pKDS301) | This study |
| ALN42 | MC4100 λRS88 (spy-lacZ) cpxA101 zii::Tn10(pKDS302) | This study |
| ALN51 | MC4100 λRS88 (spy-lacZ) cpxA106 zii::Tn10(ptrc99A) | This study |
| ALN52 | MC4100 λRS88 (spy-lacZ) cpxA106 zii::Tn10(pKDS301) | This study |
| ALN53 | MC4100 λRS88 (spy-lacZ) cpxA106 zii::Tn10(pKDS302) | This study |
| ALN54 | MC4100 λRS88 (spy-lacZ) cpxA711 zii::Tn10(ptrc99A) | This study |
| ALN55 | MC4100 λRS88 (spy-lacZ) cpxA711 zii::Tn10(pKDS301) | This study |
| ALN56 | MC4100 λRS88 (spy-lacZ) cpxA711 zii::Tn10(pKDS302) | This study |
| ALN57 | MC4100 λRS88 (spy-lacZ) cpxA24 zii::Tn10(ptrc99A) | This study |
| ALN58 | MC4100 λRS88 (spy-lacZ) cpxA24 zii::Tn10(pKDS301) | This study |
| ALN59 | MC4100 λRS88 (spy-lacZ) cpxA24 zii::Tn10(pKDS301) | This study |
| ALN88 | E2348/69 cpxR::Kn | This study |
| ALN92 | MC4100 λRS88 (spy-lacZ) cpxA101 zii::Tn10 | This study |
| ALN104 | ALN92(pKDS302) | This study |
| ALN105 | ALN88(pBAD18) | This study |
| ALN106 | ALN88(pROX) | This study |
| ALN143 | E2348/69(pBAD18) | This study |
| TR1042 | E2348/69 cpxR::Kn | This study |
| TR1043 | E2348/69 cpxR::Kn | This study |
| Plasmids | ||
| ptrc99A | Expression vector with a multiple cloning site following an IPTG-inducible trc promoter | Pharmacia |
| pKDS301 | bfpA, bfpG, and bfpB′ cloned downstream of the IPTG-inducible trc promoter | 90 |
| pKDS302 | bfpA to bfpL cloned downstream of the IPTG-inducible trc promoter | 90 |
| pBAD18 | Expression vector with a multiple cloning site following an arabinose-inducible PBAD promoter | 35 |
| pKD46 | Plasmid for arabinose-inducible expression of λ red recombination functions | 106 |
| pROX | cpxR cloned downstream of the arabinose-inducible PBAD promoter of pBAD18 | This study |
| pTP223 | Plasmid for IPTG-inducible expression of λ red recombination functions | 62 |
Media and growth conditions.
Laboratory strains of E. coli were grown in Luria-Bertani (LB) broth with appropriate antibiotics at 37°C with shaking. For analysis of BFP expression in MC4100 derivatives carrying pKDS301 or pKDS302, overnight cultures were subcultured 1:50 in LB broth containing 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Invitrogen) and grown at 37°C with shaking until bacterial aggregates were visible in positive control cultures. MC4100 derivatives carrying cpxA* alleles were grown at 30°C in the presence of 3 μg of amikacin/ml (76). For analysis of BFP expression in E2348/69 derivatives, organisms were grown in 5 ml of LB broth containing the appropriate antibiotics in test tubes at 37°C with shaking (225 rpm) overnight. Primary cultures were subcultured 1:50 in 10 ml of Dulbecco minimal essential medium-F-12 tissue culture medium (DMEM/F-12) (Gibco catalogue no. 11039-021) containing 0.1 M Tris (pH 7.4) (49) and the appropriate antibiotics in a 50-ml flask and grown at 37°C with shaking (225 rpm) to an optical density at 600 nm (OD600) of 0.2 to 0.6 (3 to 4 h). Overnight cultures required for localized-adherence assays were grown in Trypticase soy broth at 37°C without shaking. On the following day, the cultures were subcultured in DMEM/F-12 in the presence of CO2 and grown statically for 1 h to promote BFP expression before the localized-adherence assays were performed (see below).
Antibiotics were purchased from Sigma and used at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; amikacin, 3 μg/ml; spectinomycin, 20 μg/ml; and tetracycline, 25 μg/ml.
Construction of E2348/69 cpxR::Kn mutants (ALN88, TR1042, and TR1043).
The cpxR gene was amplified from the chromosome of MC4100 by using restriction enzyme-tagged primers finpho (5′-CATTAACAGGATCCTGTTCGTGCC -3′) and cpxR3′Eco (5′-CGGAATTCCGGTTAAGCTGCCTATCATG -3′) (underlining indicates the tags). The PCR product was purified by using spin columns according to the manufacturer's recommendations (Qiagen), digested with EcoRI and BamHI (Invitrogen), and cloned into the same restriction sites in pUC19. A kanamycin resistance cassette was excised from pUC4K (New England Biolabs) by using restriction enzyme SalI (Invitrogen) and cloned into the unique XhoI site in cpxR by standard techniques (79). The resultant cpxR::Kn fragment was excised from the plasmid by using BamHI and EcoRI and purified by using a GeneClean kit (QBIOgene) according to the manufacturer's instructions. The mutated cpxR::Kn allele was used to electroporate E2348/69 carrying plasmid pKD46 or pTP223, each of which encodes the λ Red recombination system, as previously described (62, 106). Transformants resistant to kanamycin were selected, and the λ Red-encoding plasmids were cured. Plasmid pKD46 was cured by virtue of its temperature-sensitive origin of replication, while transformants that had spontaneously lost plasmid pTP223 were screened by virtue of the loss of tetracycline resistance (62, 106). The newly generated mutation was confirmed by analysis of PCR products derived from amplification of the cpxR gene with primers that flanked the point of insertion, Western blot analysis (see below) and, in some experiments, Southern analysis with standard techniques and an AlkPhos direct nonradioactive labeling kit (Amersham) according to the manufacturer's instructions.
β-Galactosidase assays.
β-Galactosidase assays were performed as previously described (76, 86).
Western blot analysis.
Whole-cell lysates were made by resuspending cell pellets derived from 1 ml of culture in 50 μl of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer (125 mM Tris [pH 6.8], 20% glycerol, 10% β-mercaptoethanol, 6% sodium dodecyl sulfate, 0.2% bromophenol blue). Lysates (10 μl) were subjected to electrophoresis and Western blotting as previously described (75). The blots were reacted with a 1:30,000 dilution of rabbit polyclonal antibody directed against bundlin (a kind gift from M. Donnenberg) or a 1:5,000 dilution of rabbit polyclonal antibody directed against a maltose-binding protein (MBP)-CpxR fusion protein (75), followed by incubation with a 1:20,000 dilution of secondary anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Sigma). The blots were developed with an enhanced chemiluminescence kit according to the specifications of the manufacturer (Bio-Rad). Proteins analyzed by Western blotting were quantified with an AlphaEase software package and a FluorChem IS-5500 imaging system (Alpha Innotech, Fisher Scientific). The densities along each lane of the Western blot were measured by using the 1D-Multi autogrid function. The peak area corresponding to the level of CpxR, BfpA, or a nonspecific loading control was normalized to either E2348/69 or E2348/69(pBAD18), which was arbitrarily assigned a value of 100.
Autoaggregation assays.
Autoaggregation assays were performed as previously described (1) but with the following modifications. Briefly, overnight cultures were subcultured 1:50 in either LB broth containing 1 mM IPTG or DMEM/F-12 containing 0.1 M Tris (pH 7.4) and appropriate antibiotics and grown at 37°C with shaking to an OD600 of 0.2 to 0.6 (3 to 4 h). The OD600 of each culture was measured, the samples were allowed to remain at room temperature for 5 min and then were vortexed for 2 to 5 min, and the OD600 was remeasured (OD600V). The aggregation index was calculated as [(OD600V − OD600)/OD600] × 100. Each assay was done in triplicate and repeated at least twice.
Transmission electron microscopy.
E2348/69 and MC4100 derivatives were grown as described above for BFP expression. One drop of culture was placed on a 300-mesh Formvar-carbon-coated copper grid and allowed to dry. The grids were stained with 0.5% phosphotungstic acid in phosphate-buffered saline and examined at a magnification of ×14,000 by using a Morgagni 268 Philips transmission electron microscope. Three grids were analyzed for each strain, and representative images are shown.
Localized-adherence assays.
Analysis of the localized-adherence phenotype exhibited by E2348/69 and ALN88 was performed as previously described (96).
RESULTS
The Cpx pathway senses bfp gene overexpression.
The expression of the chaperone-usher-type P pilus in MC4100 is sensed by the Cpx envelope stress response through off-pathway misfolded pilus subunits (47). The ensuing activation of the Cpx regulatory pathway enables elevation of the levels of the envelope folding and degrading factors DsbA and DegP, which facilitate P pilus assembly (40). In order to determine whether the Cpx envelope stress response can sense the assembly of envelope structures in addition to chaperone-usher-type pili, we examined whether the expression of BFP intermediates would activate the Cpx response. To do this, we transformed plasmids expressing the major subunit BfpA (pKDS301) (90) or the entire bfp operon containing all genes necessary for BFP elaboration (pKDS302) (90) into strains of MC4100 carrying the Cpx-regulated spy-lacZ transcriptional reporter fusion (74). Both of these plasmids express the relevant bfp genes from the exogenous trc promoter and so are not subject to transcriptional regulation by the Cpx response. pKDS302 was previously shown to confer BFP expression on laboratory strain HB101 (90).
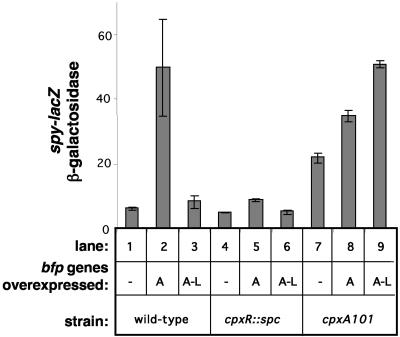
In a wild-type MC4100 background, we found that the overexpression of bundlin (BfpA), the major subunit of BFP, increased the expression of β-galactosidase from the spy-lacZ promoter approximately fivefold in a CpxR-dependent fashion (Fig. 1, compare lanes 1 and 2 to lanes 4 and 5). Similarly, in a strain carrying the cpxA101 allele, which consitutively activates the Cpx signal transduction pathway but is still sensitive to inducing cues (76), the overexpression of BfpA alone caused increased expression of spy-lacZ (Fig. 1, compare lanes 7 and 8). In the absence of the bfp genes required for the processing and incorporation of bundlin or BfpA into the mature pilus, BfpA accumulates in an unprocessed form in the inner membrane (1, 77, 90). Consequently, these observations suggest that the Cpx signal transduction pathway is sensitive to the accumulation of prebundlin in the inner membrane.
FIG. 1.
The Cpx pathway is activated by the overexpression of bfp genes. Expression from the spy promoter was measured by monitoring β-galactosidase expression from a spy-lacZ fusion harbored in MC4100 strains carrying control vector ptrc99A (−) (lanes 1, 4, and 7), plasmid pKDS301, which overexpresses bfpA (A) (lanes 2, 5, and 8), or plasmid pKDS302, which overexpresses the entire bfp gene cluster (A-L) (lanes 3, 6, and 9). Assays were performed with the wild-type strain (lanes 1 to 3), a cpxR1::Spc strain (lanes 4 to 6), and a strain carrying a cpxA101 allele (lanes 7 to 9). The strains used were as follows: lane 1, ALN17; lane 2, ALN18; lane 3, ALN19; lane 4, ALN33; lane 5, ALN34; lane 6, ALN35; lane 7, ALN40; lane 8, ALN41; and lane 9, ALN42. All assays were performed in triplicate and repeated at least twice. The data shown represent the mean and standard deviation from one experiment.
Despite these observations, in the presence of the complete bfp gene cluster, previously shown to confer BFP assembly on laboratory strain HB101 (90), no increase in the expression of spy-lacZ was observed in a wild-type MC4100 background (Fig. 1, compare lanes 1 and 3). Surprisingly, however, the presence of the same genes on pKDS302 in the cpxA101 background caused more than a twofold increase in spy-lacZ expression, above the elevated basal levels already present in this strain (Fig. 1, lanes 7 and 9). These data suggest that BFP are being elaborated and therefore detected only in the cpx* background.
Expression of BFP in MC4100 requires constitutive activation of the Cpx envelope stress response.
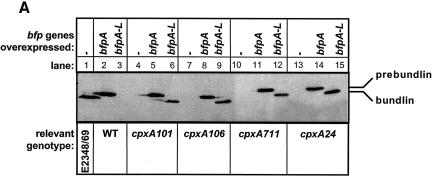
To determine whether the bfp genes present on pKDS301 and pKDS302 were expressed in the strain MC4100 background, we performed Western blot analysis of whole-cell lysates with antibody directed against bundlin or BfpA (Fig. 2A). As expected, in MC4100(pKDS301), which carries bfpA but lacks most of the bfp gene cluster, including the genes for the processing peptidase, the expression of unprocessed prebundlin was detected (Fig. 2A, lane 2). However, in MC4100 transformants carrying pKDS302, which carries the entire bfp gene cluster, we could not detect any BfpA-specific product, either processed or unprocessed (Fig. 2A, lane 3). These observations suggest that the presence of pKDS302 is unable to confer BFP elaboration in an MC4100 background.
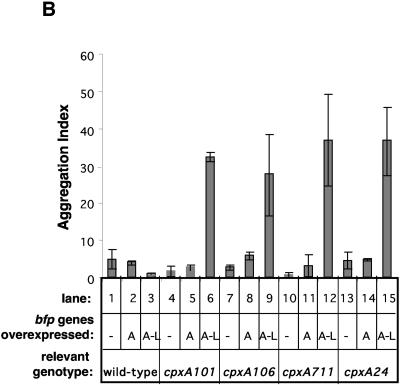
FIG. 2.
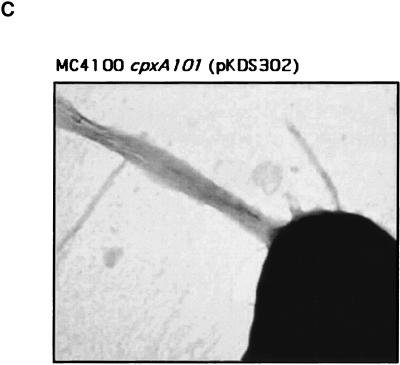
Constitutive activation of the Cpx response enables BFP expression in MC4100. BFP expression was examined in wild-type (WT) and cpxA* derivatives of MC4100 transformed with control vector ptrc99A (−), plasmid pKDS301, which overexpresses bfpA (A), or plasmid pKDS302, which overexpresses the entire bfp gene cluster (A-L). BFP expression was analyzed by Western blotting with antibundlin polyclonal antibody (A), by autoaggregation assays (B), and by transmission electron microscopy (C). The strains used were as follows: (A) lane 1, E2348/69; lane 2, ALN18; lane 3, ALN19; lane 4, ALN40; lane 5, ALN41; lane 6, ALN42; lane 7, ALN51; lane 8, ALN52; lane 9, ALN53; lane 10, ALN54; lane 11, ALN55; lane 12, ALN56; lane 13, ALN57; lane 14, ALN58; and lane 15, ALN59; (B) lane 1, ALN17; lane 2, ALN18; lane 3, ALN19; lane 4, ALN40; lane 5, ALN41; lane 6, ALN42; lane 7, ALN51; lane 8, ALN52; lane 9, ALN53; lane 10, ALN54; lane 11, ALN55; lane 12, ALN56; lane 13, ALN57; lane 14, ALN58; and lane 15, ALN59; and (C) ALN104. Western blotting was performed at least twice with independently isolated samples. Autoaggregation assays were performed in triplicate and repeated at least twice; data are reported as the mean and standard deviation. Electron microscopy was performed at least twice, and each time three separate grids were examined.
When BFP are expressed on the surface of bacteria grown in liquid cultures, they cause the cells to aggregate (5). This property can be measured by the change in optical density that the cultures undergo after vortexing to disrupt bacterial aggregates (1). In order to confirm the results of our Western blot analysis, we performed aggregation assays with the same strains (Fig. 2B). In support of the immunoblot observations, we found that MC4100(pKDS302) failed to aggregate in liquid cultures (Fig. 2B, lane 3). This was a surprising finding, since pKDS302 was previously shown to confer BFP expression on E. coli K-12 laboratory strain HB101 (90).
To rule out the possibility that a chance mutation or rearrangement of plasmid pKDS302 was responsible for our observations, we performed the same experiments with strain HB101 and were able to detect processed bundlin by Western blotting and aggregation of transformants in liquid cultures, as previously described (data not shown). Accordingly, we speculated that the lack of expression of BFP in MC4100 transformants carrying pKDS302 was due to the strain background. Since the Cpx response controls the expression of folding factors that are involved in the efficient assembly of P pili (40), we hypothesized that the increased activity of the Cpx pathway might enable BFP assembly in the strain MC4100 background.
To test this idea, we transformed pKDS301 and pKDS302 into a variety of cpx* backgrounds in which the Cpx envelope stress response is constitutively activated due to mutations in the cpxA gene, encoding the sensor kinase (76). The resulting transformants were subjected to anti-BfpA immunoblot analysis and aggregation assays to test for the presence of BFP. Western blot analysis indicated that, in the presence of four different cpx* mutations, mature, processed bundlin could be detected in strains carrying the entire bfp gene cluster (Fig. 2A, lanes 6, 9, 12, and 15). Further, these strains displayed aggregation in liquid cultures (Fig. 2B, lanes 6, 9, 12, and 15), indicating that BFP are expressed on the surface of cpx* mutants carrying plasmid pKDS302.
To confirm that BFP were expressed on the cell surface of cpx* mutants carrying pKDS302, we performed transmission electron microscopy on negatively stained preparations of an MC4100 cpxA101(pKDS302) transformant (Fig. 2C). We observed characteristic BFP on three of three grids examined (Fig. 2C), while no BFP were seen on grids fixed with negative control strain JPN15, which lacks the BFP-encoding EAF plasmid (see Fig. 4B). Together, these data suggest that BFP are expressed in MC4100 only when the Cpx pathway is constitutively activated. Further, when BFP expression is enabled in this fashion, it is sensed by the Cpx pathway, as indicated by the activation of the spy-lacZ fusion (Fig. 1, lane 9).
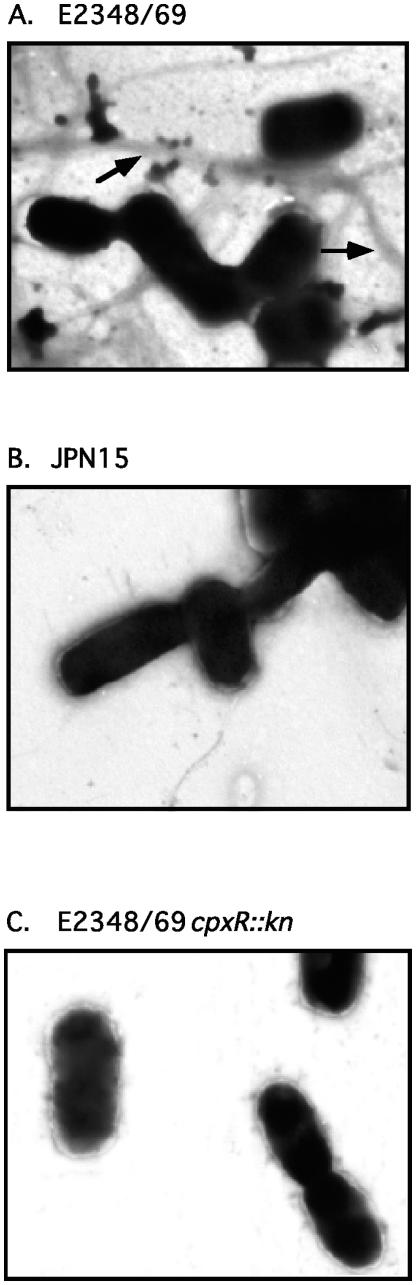
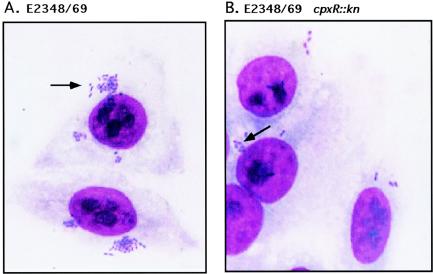
FIG. 4.
BFP are not detectable by transmission electron microscopy in EPEC cpxR-null mutants. BFP were examined by transmission electron microscopy of negatively stained samples of E2348/69 (A), JPN15 (B), and ALN88 (C) as described in Materials and Methods. Arrows indicate typical BFP seen. Samples were viewed at a magnification of ×14,000.
Construction of EPEC cpxR mutants.
Our studies suggested that in laboratory strain MC4100, the Cpx envelope stress response plays a role in the expression of BFP. To determine whether this was true for EPEC type strain E2348/69, we constructed a cpxR-null mutation by insertional inactivation. We isolated and analyzed three independent E2348/69 cpxR::Kn mutants (ALN88, TR1042, and TR1043) to confirm that any phenotypes that we observed were reproducible.
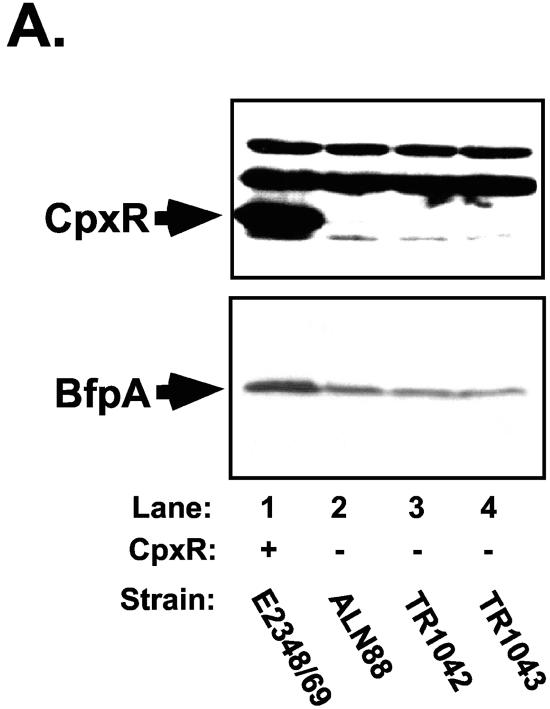
To ensure that this technique worked as expected with EPEC, we analyzed the resultant E2348/69 cpxR::Kn mutants by PCR (data not shown) and Western blot analysis (Fig. 3A). PCR analysis with primers that flanked the point of the kanamycin resistance cassette insertion showed that the selected mutants carried an insertion of the expected size in the cpxR gene (data not shown). Similarly, Southern blot analysis of one of the mutant chromosomes showed bands of the expected sizes when a cpxR probe was used for hybridization (data not shown). Finally, we performed Western blot analysis of whole-cell lysates of mutant and wild-type E2348/69 strains with polyclonal antisera directed against CpxR to confirm that we had eliminated CpxR from the mutant strains (Fig. 3A, top). As anticipated, the band corresponding to the predicted molecular weight for CpxR was absent from whole-cell lysates of the newly constructed E2348/69 cpxR::Kn mutants (Fig. 3A, top, compare lane 1 with lanes 2 to 4), indicating that we had successfully disrupted cpxR in these mutants.
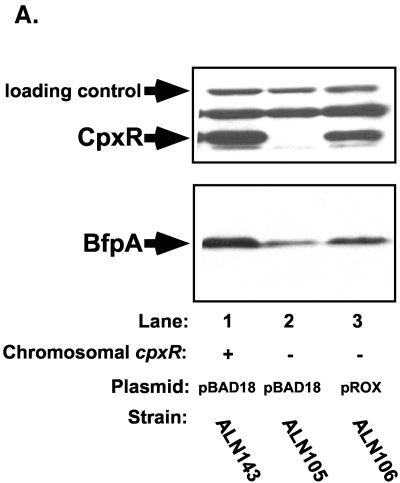
FIG. 3.
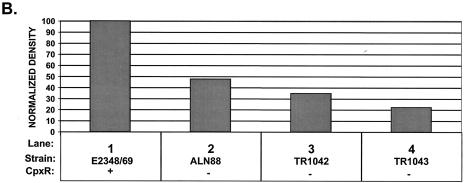
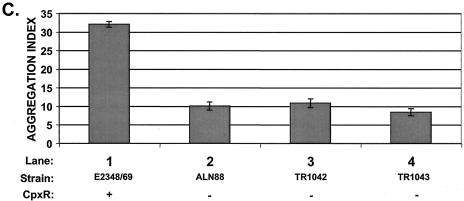
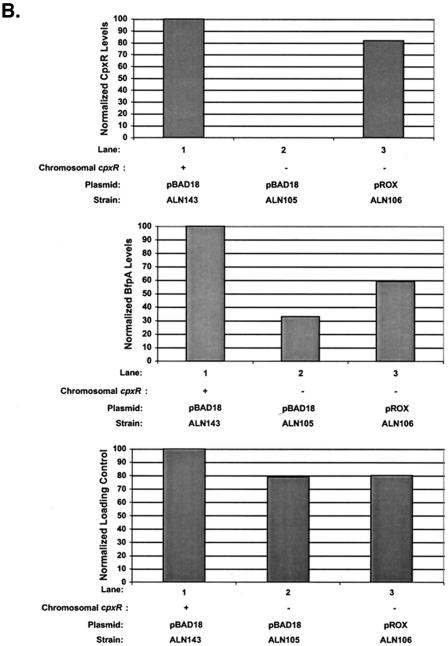
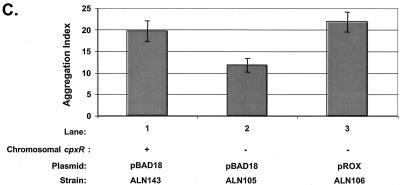
Mutation of cpxR in an EPEC strain background inhibits BFP production. CpxR and BFP levels were measured in a wild-type strain E2348/69 background (lanes 1) and in three independently isolated E2348/69 cpxR::Kn mutant strains, ALN88 (lanes 2), TR1042 (lanes 3), and TR1043 (lanes 4), by Western blotting (A and B) and autoaggregation assays (C) as described in Materials and Methods. Western blotting was performed multiple times, and one representative blot is shown. BfpA levels detected in panel A were quantitated with AlphaEase software and a FluorChem IS-5500 imaging system as described in Materials and Methods; the results are shown in panel B. BfpA levels were normalized to that of wild-type strain E2348/69, which was set at 100. Autoaggregation assays were performed in triplicate for each experiment and repeated at least twice; the data shown represent the mean and standard deviation from one experiment.
Mutation of the Cpx pathway diminishes BFP expression in EPEC.
To determine what effect elimination of the Cpx signal transduction pathway had on BFP expression in strain E2348/69, we first performed Western blot analysis of whole-cell lysates of strains grown under optimal conditions for BFP expression (Fig. 3A and B). In the absence of CpxR, the level of processed bundlin or BfpA associated with whole cells was significantly diminished (Fig. 3A and B). Quantification of the BfpA Western blot indicated that bundlin or BfpA levels were diminished between two- and fourfold relative to those in the wild-type E2348/69 control (Fig. 3A and B, compare lane 1 with lanes 2 to 4). As a negative control, whole-cell lysates collected from JPN15 grown under the same conditions were also probed. JPN15 lacks the EAF plasmid carrying the bfp gene cluster. As expected, no bundlin or BfpA was present in this strain (data not shown). The levels of two nonspecific proteins that cross-reacted with the CpxR antisera indicated that equal amounts of lysates were loaded in the lanes (Fig. 3A, top). Accordingly, differences in cell density or loading cannot account for the diminished levels of processed bundlin or BfpA observed.
To determine whether the diminished levels of processed bundlin associated with whole cells in the cpxR mutants reflected lower levels of cell surface-associated BFP, we performed aggregation assays (Fig. 3C). In agreement with the results of the Western blot analysis, we found that in liquid cultures, our three independently isolated E2348/69 cpxR::Kn mutants aggregated weakly relative to the wild-type parent strain (Fig. 3C, compare lane 1 with lanes 2 to 4), which displayed the formation of large, plentiful bacterial aggregates that were visible with the naked eye (data not shown). We observed that, similar to the BfpA levels detected by Western blot analysis, autoaggregation was diminished approximately threefold in the E2348/69 cpxR::Kn mutants relative to the wild-type parent strain (Fig. 3C).
Since Western blot analysis and aggregation assays suggested that BFP were expressed at much lower levels in the E2348/69 cpxR::Kn mutants, we examined one of the mutants, ALN88, together with parent strain E2348/69 and negative control strain JPN15 by transmission electron microscopy after negative staining for the presence of BFP on the cell surface (Fig. 4). In the wild-type E2348/69 background, BFP were present in thick, rope-like structures between bacteria on three of three grids examined (Fig. 4A). Conversely, in JPN15, although flagella and hair-like structures that were likely type I fimbriae were observed, no BFP were present (Fig. 4B). Suprisingly, even though we could detect bundlin by Western blotting, no structures reminiscent of BFP were observed on E2348/69 cpxR::Kn cells on three of three grids examined (Fig. 4C).
To confirm that the absence of BFP observed by transmission electron microscopy and the diminished levels of bundlin or BfpA revealed by Western blot analysis and autoaggregation assays were due to the absence of CpxR, we cloned MC4100 cpxR downstream of the inducible PBAD promoter on pBAD18. The resulting CpxR overexpression plasmid, pROX, was transformed into one of the E2348/69 cpxR::Kn mutants. Surprisingly, even though the PBAD promoter is tightly regulated by arabinose in laboratory strains of E. coli (35), we observed CpxR expression from plasmid pROX in the absence of arabinose (Fig. 5A and B). Thus, it appears that the regulation of PBAD by arabinose is somewhat leaky in E2348/69. At present, we do not know the basis for this leaky expression phenotype. Although the tissue culture medium that we used (DMEM/F-12) is not reported to contain arabinose, it is possible that there are trace amounts of arabinose in this medium that account for the expression that we observed. Alternatively, perhaps PBAD regulation occurs in a different manner in an EPEC strain background. Regardless, our data demonstrate that plasmid pROX permits the restoration of CpxR levels in an E2348/69 cpxR::Kn background in the absence of arabinose.
FIG. 5.
BfpA production is partially complemented in an E2348/69 cpxR::Kn background by a plasmid encoding cpxR. Levels of CpxR (A and B), bundlin (A and B), and surface-exposed BFP (C) were analyzed by Western blotting (A and B) and autoaggregation assays (C) of E2348/69 carrying control vector pBAD18 (lanes 1) or the E2348/69 cpxR::Kn mutant ALN88 transformed with pBAD18 (lanes 2) or CpxR overexpression plasmid pROX (lanes 3). Western blotting was performed at least twice. Levels of CpxR, BfpA, and a nonspecific band that cross-reacted with the CpxR antisera in panel A were quantitated with AlphaEase software and a FluorChem IS-5500 imaging system as described in Materials and Methods; the results are shown in panel B. Autoaggregation assays were performed in triplicate for each experiment and repeated at least twice; the data shown represent the mean and standard deviation from one experiment.
Consequently, E2348/69 cpxR::Kn(pROX) was grown under conditions optimal for the expression of BFP and BfpA or bundlin, and autoaggregation levels were measured (Fig. 5). As previously shown (Fig. 3), in the presence of control vector pBAD18, the E2348/69 cpxR::Kn mutant expressed no CpxR and approximately threefold less BfpA or bundlin than wild-type strain E2348/69 carrying the same plasmid (Fig. 5A and B, compare lanes 1 and 2). CpxR-encoding plasmid pROX restored CpxR levels to 80% those in parent strain E2348/69 (Fig. 5A and B, top, compare lanes 2 and 3). Similarly, BfpA or bundlin levels were increased about twofold in the cpxR::Kn background when CpxR-encoding plasmid pROX was present (Fig. 5A and B, compare lanes 2 and 3). However, in spite of this increase, BfpA or bundlin levels still approached only 60% those in parent strain E2348/69 carrying control vector pBAD18 (Fig. 5A and B, compare lanes 1 and 3). This difference is real, since the levels of a nonspecific, cross-reactive loading control were unchanged in the presence of plasmid pROX (Fig. 5A and B). Thus, CpxR-encoding plasmid pROX is capable of partial complementation of the bfp phenotype.
Elimination of the Cpx envelope stress response has deleterious effects on localized adherence.
BFP mediate the distinctive initial stage of attachment during EPEC infection that is known as localized adherence (25, 31). Localized adherence is the attachment of microcolonies of multiple bacteria held together by BFP to the surface of epithelial cells. Strains lacking BFP are defective in this stage of infection and in causing disease in human volunteers (56). Since the elimination of CpxR affected the production of BFP by EPEC grown in cultures (Fig. 3, 4, and 5), we examined whether localized adherence might also be altered. Wild-type and cpxR E2348/69 strains were incubated with HEp-2 cells and stained, and the number and size of microcolonies per cell were quantified. Visually it was obvious that, even though the same numbers of bacteria were used in the experiments, the cpxR mutant exhibited fewer microcolonies per epithelial cell and fewer microbes within each microcolony than did the wild-type parent (Fig. 6). Statistical analysis confirmed these observations, revealing an approximate 33% decrease in the number of E2348/69 cpxR::Kn microcolonies per epithelial cell relative to those of the wild-type E2348/69 parent (Table 2). Further, the microcolonies formed by the cpxR mutant were less than half the size of those formed by the wild-type E2348/69 parent (Table 2). Thus, elimination of the Cpx envelope stress response has a significant effect on the first step in EPEC pathogenesis, attachment of microcolonies to epithelial cells.
FIG. 6.
An EPEC cpxR mutant exhibits altered localized adherence. Localized-adherence assays were performed with E2348/69 (A) and ALN88 (B) as described in Materials and Methods. Arrows indicate typical clumps of adherent bacteria seen for each strain. The assays were performed in triplicate and repeated twice as described in Materials and Methods. The data represent typical fields of view. The statistical analysis is shown in Table 2.
TABLE 2.
Differences in localized adherence between E2348/69 and E2348/69 cpxR::Kn
| Strain | % of HEp-2 cells demon- strating localized adherencea | Avg no. of bacteria/ microcolonyb |
|---|---|---|
| E2348/69 | 76.4 | 15.31 |
| E2348/69 cpxR::Kn | 53.6 | 7.41 |
Results represent the average number of HEp-2 cells demonstrating localized adherence in two separate experiments. A total of 600 HEp-2 cells were counted per experiment. Student's t test was performed to determine whether the statistical difference between the means for the strains was significant. The P value was < 0.01.
Results represent the average number of bacteria per microcolony. A total of 300 microcolonies were analyzed. The P value, determined by Student's t test, was <0.01.
DISCUSSION
In this report we provide evidence that the Cpx envelope stress response is involved in the expression of the type IV BFP of EPEC. In E. coli laboratory strain MC4100, a plasmid encoding all of the bfp genes necessary for the elaboration of these structures in other strains (90) fails to confer BFP expression unless the activity of the Cpx pathway is elevated above normal background levels. Strikingly, in the presence of cpx* mutations, which constitutively activate the Cpx response, not only are BFP made on the cell surface (Fig. 2) but also their synthesis further induces the already highly active Cpx signal transduction pathway (Fig. 1). In an EPEC background, elimination of the Cpx signal transduction pathway by mutation of cpxR leads to diminished BFP elaboration, coincident with a lessened ability of bacterial cells to aggregate and the inability to detect these type IV pili on the cell surface (Fig. 3 and 4). Importantly, the cpxR mutant is significantly handicapped in its ability to adhere to epithelial cells, the first step in a productive EPEC infection (Fig. 6 and Table 2). This study suggests that the Cpx envelope stress response may play a general role in the assembly of diverse envelope-localized structures and is the first to address the role of this response directly in a pathogenic strain of E. coli.
BFP assembly intermediates are sensed by the Cpx signal transduction pathway.
In this study, we discovered that the Cpx envelope stress response is activated by the overexpression of unprocessed BfpA as well as mature, surface-expressed BFP (Fig. 1). In laboratory strain MC4100, expression of unprocessed BfpA leads to more than a fivefold increase in the Cpx-dependent activation of spy expression. Further, when the Cpx response is constitutively activated by mutation, the Cpx signal transduction pathway is further activated by expression of the entire bfp gene cluster (Fig. 1). This result indicates that other assembly intermediates, beyond unprocessed BfpA, are sensed by the CpxA sensor kinase. Currently, we do not know the nature of the CpxA activating signal. It was recently shown that CpxA recognizes the off-pathway P pilus subunit PapE in the absence of the cognate PapD chaperone in a manner that requires the PapE N-terminal extension that mediates the assembly of PapE into the pilus fibrillum (54). Since Cpx activation by PapE did not correlate with protein stability, subunit association, or levels of protein, it has been proposed that the N-terminal extension is involved in facilitating a folding intermediate in the absence of the PapD chaperone that specifically activates the Cpx stress response (54). Although the TFP share no similarities with chaperone-usher-type pili at the level of structure or assembly, all TFP subunits contain a hydrophobic N-terminal alpha helix that mediates subunit-subunit interactions to promote fiber formation (14, 38, 48, 68). In addition, electrostatic and hydrophobic interactions between the globular head domains of pilin subunits are also important in TFP architecture (14). Thus, by analogy with PapE, perhaps these domains contribute to a distinct folding intermediate in the absence of other BFP assembly components. This intermediate might be generated when BfpA is expressed alone or when BFP elaboration is up-regulated, leading to activation of the sensor kinase CpxA and the production of factors that promote efficient BFP expression. Expression of the bfp structural and assembly genes are regulated by a multitude of signals thought to reflect the host environment via a regulatory factor consisting of the perABC and bfpTVW gene products (59, 72, 94). Accordingly, upon initiation of an infection, increased elaboration of BFP may be the trigger that activates the Cpx response, reinforcing BFP expression and adherence.
The CpxA sensor may also recognize distinct signals from PerABC and BfpTVW and/or the accumulation of bundlin intermediates in vivo. Alterations in pH, which are encountered by EPEC in transit through the intestinal tract, are known to activate the Cpx response (17, 64). Further, recent experiments showed that the Cpx signal transduction cascade senses and mediates attachment to hydrophobic surfaces via the lipoprotein NlpE (67). Thus, another in vivo signal that may activate the Cpx response could be contact with hydrophobic host cell surfaces in vivo. In this respect, initial BFP binding of host cells might lead to further induction of the Cpx response via contact with hydrophobic host cell surfaces. Interestingly, a number of studies on TFP expression have implicated chemotaxis-related signal transduction proteins in this process (for a review, see reference 102). Part of the Cpx response involves the inhibition of motility and chemotaxis genes (21; unpublished data). In consequence, the Cpx envelope stress response may exert effects on BFP assembly and adherence via this route as well.
The Cpx envelope stress response is involved in the assembly of diverse envelope-localized structures.
The Cpx envelope stress response was recently shown to sense and assist in the assembly of UPEC P pili in laboratory strain MC4100 (40, 47). P pili are multisubunit pili (8, 32, 53) that are elaborated by means of a specific periplasmic chaperone, PapD, and outer membrane secretion platform, or usher, formed by the secretin PapC (3, 80, 81). CpxA is thought to detect off-pathway PapG and PapE subunits that fail to associate with PapD and thus become misfolded (47, 54). The Cpx-regulated gene products DsbA (43) and DegP (47) are involved in P pilus biogenesis, and the abolition of CpxR leads to a short-pilus phenotype (40). Consequently, activation of the Cpx envelope stress response upon pilus expression appears to be an important mechanism in ensuring efficient assembly of the P pilus, likely by providing necessary folding and degrading factors. TFP structure and assembly are significantly different from those of the chaperone-usher class of pili (104). Recent studies suggest that TFP are elaborated from inner membrane pools of the mature pilus subunit via distinct subassemblies of biogenesis proteins that span the envelope (1, 15, 41, 77, 104). Despite extensive mutational analysis of the EPEC BFP biogenesis machinery, no periplasmic assembly intermediates have been identified, and the pilus is thought to originate in the inner membrane and form a structure that spans the entire envelope (1, 77). Accordingly, since the P pilus assembly requirement for the Cpx pathway is thought to reflect the need for folding and degrading factors that act on periplasmic assembly intermediates, it is somewhat surprising that a similar requirement appears to exist for the BFP of EPEC.
Our data clearly suggest that this is the case. In laboratory strain MC4100, BFP cannot be elaborated, even when the necessary genes are expressed from an exogenous promoter that is highly expressed in this strain background, unless the Cpx envelope stress response is constitutively activated (Fig. 2). Intriguingly, although no periplasmic assembly intermediates have been identified for BFP, DsbA is absolutely required for biogenesis (110). The first step in the assembly pathway is simultaneous cleavage of the prebundlin leader by the BfpP peptidase and introduction of an essential C-terminal disulfide bond, which is catalyzed by DsbA. Thus, it is possible that the Cpx requirement reflects a need for elevated levels of DsbA. Alternatively, additional Cpx-regulated assembly factors may also be required. For example, DegP is required in P pilus biogenesis to degrade toxic, off-pathway subunits (47). At this time, we are not certain what part of the assembly process is affected by the Cpx response. Prebundlin is clearly made in the absence of other Bfp biogenesis factors (Fig. 2); however, no bundlin, mature or otherwise, is detectable when the entire bfp biosynthetic gene cluster is present in laboratory strain MC4100 (Fig. 2). Hence, in the absence of elevated levels of a critical Cpx-regulated factor(s), some step after prebundlin production is prevented, leading to the degradation of bundlin and/or the assembly apparatus. In an EPEC strain background, reduced levels of bundlin are seen when the Cpx signal transduction pathway is removed (Fig. 3). Extensive mutational analysis indicates that cell-associated bundlin levels are unaltered in the presence of any one of a number of bfp mutations (except bfpP) (1, 77), suggesting that the effects of the Cpx envelope stress response on bundlin levels may be due to altered levels of folding and/or degrading factors that work directly on bundlin. Alternatively, the Cpx response may affect BfpP stability or multiple Bfp biogenesis proteins found in one or more of the envelope-localized subassemblies that are thought to mediate BFP elaboration. Recently, it was shown that the elimination of BfpE leads to reduced levels of bundlin (77). BfpE is thought to form an inner membrane scaffold required for the movement of assembly proteins across the inner membrane (77). Since the phenotype of the cpxR mutant with respect to bundlin levels is similar, it is possible that the Cpx response affects the levels or function of BfpE in EPEC.
Curiously, even though we detected cell-associated mature bundlin and weak aggregation in the EPEC cpxR mutant (Fig. 3), we were not able to visualize BFP by electron microscopy (Fig. 4). It is tempting to speculate that, as with P pili, the cpxR mutant elaborates shorter BFP that are not visible by transmission electron microscopy but still function weakly in aggregation and localized adherence (40). Alternatively, the residual localized adherence that we saw may reflect non-BFP adhesins (10, 66).
At this point, we cannot say whether the Cpx response affects the transcription of the bfp genes. In MC4100, the Cpx pathway must be constitutively active to permit BFP expression (Fig. 2). Since the bfp genes in this experiment were expressed from an exogenous, non-Cpx-regulated promoter in MC4100, some, if not all, of the effects of the Cpx envelope stress response on BFP expression must occur at posttranscriptional steps. Given the role of the Cpx envelope stress response in P pilus assembly, we favor the idea that the posttranscriptional effects occur at the level of assembly, likely facilitated by Cpx-regulated folding factors. However, since our experiments in the EPEC strain background all examined BFP expressed from the native promoter, we cannot say whether the effects of eliminating the Cpx response in this background are partly due to effects on bfp transcription. Experiments are under way to address this question.
Whatever the mechanism, our data clearly show that the Cpx envelope stress response influences the biogenesis of type IV BFP of EPEC in addition to chaperone-usher-type pili like the UPEC P pilus. Since these pili have very different structures and assembly pathways, it will be interesting to determine whether the Cpx stress response plays a role in the elaboration of other, varied envelope-localized virulence determinants in E. coli.
An EPEC cpxR mutant is only partially complemented by a plasmid encoding CpxR.
The bfp phenotype of our EPEC cpxR::Kn mutants was only partially complemented by a plasmid encoding CpxR (Fig. 5). The EPEC cpxR mutant transformed with a plasmid carrying an intact cpxR gene expressed levels of bundlin and autoaggregation that were intermediate between those of the parent E2348/69 and the mutant E2348/69 cpxR::Kn (Fig. 5). There are several possible explanations for why we observed only partial complementation of BFP expression by pROX in our EPEC cpxR mutant. First, since cpxR is found upstream of cpxA in an operon, the cpxR::Kn mutant that we created disrupts both cpxR and cpxA expression. Therefore, it is possible that full complementation of BFP expression requires both CpxR and CpxA. Efforts to clone both genes together in the past have been unsuccessful (T. L. Raivio, unpublished data). We believe that this situation reflects the deleterious effects of the hyperactivation of the Cpx pathway, since the constitutively active cpx* mutants display many pleiotropic phenotypes (12). Perhaps in the absence of CpxA, the CpxR expressed from our complementing plasmid is not sufficiently phosphorylated to confer full complementation. Alternatively, since the cpxRA operon is autoregulated, it is possible that in uncoupling cpxR expression from its native promoter, our complementation plasmid does not express CpxR at the appropriate levels or time for full complementation. Finally, we used the cloned MC4100 cpxR gene in our complementation experiments. Our sequence analysis of the E2348/69 cpxRA operon indicates that there is a single amino acid difference between the MC4100 and E2348/69 cpxR genes (A. Z. Nevesinjac and T. L. Raivio, unpublished data). Accordingly, it is also possible that the lack of complete complementation reflects differences in the activities of the MC4100 and E2348/69 CpxR proteins. Whatever the reason, our complementation experiments clearly show that partial restoration of CpxR levels permits limited reinstatement of BFP expression. Plainly, the level, phosphorylation status, and/or presence of CpxA are important factors in the effect of Cpx on BFP elaboration.
The Cpx envelope stress response affects early events required to initiate EPEC infection.
Although other adhesins have been implicated (10, 66), BFP are thought to be the major facilitators of localized adherence to epithelial cells, a diagnostic trait of EPEC infections. Human volunteer studies show that mutants that fail to make BFP are much less effective at initiating an infection (56), emphasizing the importance of this initial step in the EPEC disease process. Our studies show that the Cpx envelope stress response is needed for the optimal expression of BFP (Fig. 3 and 4) and localized adherence (Table 2 and Fig. 6) in an EPEC background. Thus, it is probable that the activation of the Cpx envelope stress response optimizes early events in EPEC infection.
Interestingly, a role for the Cpx response in the regulation of virulence in the closely related microbe S. flexneri has been documented. Phosphorylated CpxR directly activates the transcription of the virF gene, a master regulator of virulence in Shigella spp. (63). Further, DsbA and DegP homologues are involved in the expression of TFP, TTSS, virulence, or intracellular survival in numerous pathogens (see above). These studies, together with our observations, suggest that the role of the Cpx envelope stress response in the expression of virulence determinants extends to other pili, envelope-localized virulence determinants, and regulatory proteins. Thus, as for many other stress responses, the Cpx stress response appears to play multiple roles within the cell. In addition to the regulation of virulence determinant expression, as shown here, these include adaptation to potentially lethal envelope stresses (12, 17), regulation of conjugation (34, 85), surface sensing (67), and likely others, as indicated by bioinformatics approaches (22).
BFP expression is strain dependent.
Curiously, although the bfp genes carried on pKDS302 are sufficient to confer BFP elaboration in laboratory strain HB101 (90), the same plasmid fails to permit BFP expression in MC4100 unless the Cpx signal transduction pathway is constitutively activated by mutation (Fig. 2). In addition, BFP are elaborated by the EPEC type strain E2348/69 without any manipulation of the Cpx signal transduction cascade. These observations suggest that there are fundamental differences between these strains. For EPEC, it is easy to imagine that additional factors not found in laboratory strains permit BFP expression; however, the finding that HB101 can assemble these cell surface structures confounds this idea. An alternative explanation is that there may be differences in the basal levels of activity of the Cpx signal transduction pathway in these strains, such that HB101 and EPEC contain Cpx responses that are inherently more active.
Alternative explanations for the strain differences in BFP expression exist. Perhaps some critical Cpx-regulated factor is expressed at higher levels in HB101 and EPEC, permitting the assembly of BFP in these backgrounds but not in that of MC4100. The differences between the MC4100 and EPEC strain backgrounds could also reflect the fact that in the MC4100 background, we are observing only posttranscriptional effects, since the bfp genes are expressed from the exogenous trc promoter, while in EPEC, the effects of the Cpx response on BFP expression may be the result of both transcriptional and posttranscriptional events. Perhaps Cpx-mediated transcriptional events at the bfp locus lead to a different outcome in EPEC. Studies are currently under way to distinguish these possibilities.
Conclusions.
Taken together, our data demonstrate that the Cpx envelope stress response is able to sense BFP assembly and is involved in the expression of these structures, at least at the posttranscriptional level. Additionally, we have shown that the Cpx response plays a role in maximizing the first step in EPEC infection, localized adherence to epithelial cells. This study allows us to generalize findings with regard to the role of the Cpx signal transduction pathway in UPEC P pilus assembly to other, distinct envelope structures with much different assembly pathways.
Acknowledgments
We thank Michael Donnenberg (University of Maryland) for providing plasmids pKDS301 and pKDS302 as well as anti-BfpA sera. We also thank Glen Armstrong and George Mulvey (University of Calgary) for help with performing localized-adherence assays. We are grateful to Jonathan Dennis (University of Alberta) and members of the Raivio Laboratory for critical reading of the manuscript.
This work was supported by a scholar award (T.L.R.) from the Alberta Heritage Foundation for Medical Research and an operating grant from the Canadian Institutes of Health Research.
REFERENCES
- 1.Anantha, R. P., K. D. Stone, and M. S. Donnenberg. 2000. Effects of bfp mutations on biogenesis of functional enteropathogenic Escherichia coli type IV pili. J. Bacteriol. 182:2498-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anantha, R. P., K. D. Stone, and M. S. Donnenberg. 1998. Role of BfpF, a member of the PilT family of putative nucleotide-binding proteins, in type IV pilus biogenesis and in interactions between enteropathogenic Escherichia coli and host cells. Infect. Immun. 66:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnhart, M. M., J. S. Pinkner, G. E. Soto, F. G. Sauer, S. Langermann, G. Waksman, C. Frieden, and S. J. Hultgren. 2000. PapD-like chaperones provide the missing information for folding of pilin proteins. Proc. Natl. Acad. Sci. USA 97:7709-7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumler, A. J. , J. G. Kusters, I. Stojiljkovic, and F. Heffron. 1994. Salmonella typhimurium loci involved in survival within macrophages. Infect. Immun. 62:1623-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieber, D., S. W. Ramer, C. Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 6.Blank, T. E., and M. S. Donnenberg. 2001. Novel topology of BfpE, a cytoplasmic membrane protein required for type IV fimbrial biogenesis in enteropathogenic Escherichia coli. J. Bacteriol. 183:4435-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucher, J. C., J. Martinez-Salazar, M. J. Schurr, M. H. Mudd, H. Yu, and V. Deretic. 1996. Two distinct loci affecting conversion to mucoidy in Pseudomonas aeruginosa in cystic fibrosis encode homologs of the serine protease HtrA. J. Bacteriol. 178:511-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullitt, E., and L. Makowski. 1995. Structural polymorphism of bacterial adhesion pili. Nature 373:164-167. [DOI] [PubMed] [Google Scholar]
- 9.Celli, J. , W. Deng, and B. B. Finlay. 2000. Enteropathogenic Escherichia coli (EPEC) attachment to epithelial cells: exploiting the host cell cytoskeleton from the outside. Cell. Microbiol. 2:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Cleary, J. , L. C. Lai, R. K. Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150:527-538. [DOI] [PubMed] [Google Scholar]
- 11.Cortes, G., B. de Astorza, V. J. Benedi, and S. Alberti. 2002. Role of the htrA gene in Klebsiella pneumoniae virulence. Infect. Immun. 70:4772-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosma, C. L., P. N. Danese, J. H. Carlson, T. J. Silhavy, and W. B. Snyder. 1995. Activation of the Cpx two-component signal transduction pathway in Escherichia coli suppresses envelope associated stresses. Mol. Microbiol. 18:491-505. [DOI] [PubMed] [Google Scholar]
- 13.Craig, J. E., A. Nobbs, and N. J. High. 2002. The extracytoplasmic sigma factor, final sigma(E), is required for intracellular survival of nontypeable Haemophilus influenzae in J774 macrophages. Infect. Immun. 70:708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig, L., R. K. Taylor, M. E. Pique, B. D. Adair, A. S. Arvai, M. Singh, S. J. Lloyd, D. S. Shin, E. D. Getzoff, M. Yeager, K. T. Forest, and J. A. Tainer. 2003. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol. Cell 11:1139-1150. [DOI] [PubMed] [Google Scholar]
- 15.Crowther, L. J. , R. P. Anantha, and M. S. Donnenberg. 2004. The inner membrane subassembly of the enteropathogenic Escherichia coli bundle-forming pilus machine. Mol. Microbiol. 52:67-79. [DOI] [PubMed] [Google Scholar]
- 16.Danese, P. N., G. R. Oliver, K. Barr, G. D. Bowman, P. D. Rick, and T. J. Silhavy. 1998. Accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J. Bacteriol. 180:5875-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danese, P. N., and T. J. Silhavy. 1998. CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180:831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danese, P. N., and T. J. Silhavy. 1997. The σE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183-1193. [DOI] [PubMed] [Google Scholar]
- 19.Danese, P. N., W. B. Snyder, C. L. Cosma, L. J. Davis, and T. J. Silhavy. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9:387-398. [DOI] [PubMed] [Google Scholar]
- 20.Dartigalongue, C., and S. Raina. 1998. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 17:3968-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Wulf, P., O. Kwon, and E. C. C. Lin. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J. Bacteriol. 181:6552-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Wulf, P., A. M. McGuire, X. Liu, and E. C. Lin. 2002. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277:26652-26661. [DOI] [PubMed] [Google Scholar]
- 23.Dodson, K. W., F. Jacob-Dubuisson, R. T. Striker, and S. J. Hultgren. 1993. Outer-membrane PapC molecular usher discriminately recognizes periplasmic chaperone-pilus subunit complexes. Proc. Natl. Acad. Sci. USA 90:3670-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong, J. S., S. Iuchi, S. Kwan, Z. Lu, and E. C. C. Lin. 1993. The deduced amino-acid sequence of the cloned cpxR gene suggests the protein is the cognate regulator for the membrane sensor, CpxA, in a two-component signal transduction system of Escherichia coli. Gene 136:227-230. [DOI] [PubMed] [Google Scholar]
- 25.Donnenberg, M. S., J. A. Giron, J. P. Nataro, and J. B. Kaper. 1992. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli asociated with localized adherence. Mol. Microbiol. 6:3427-3437. [DOI] [PubMed] [Google Scholar]
- 26.Donnenberg, M. S., and T. S. Whittam. 2001. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J. Clin. Investig. 107:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellermeier, C. D., and J. M. Slauch. 2004. RtsA coordinately regulates DsbA and the Salmonella pathogenicity island 1 type III secretion system. J. Bacteriol. 186:68-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott, S. J. , L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 30.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 31.Giron, J. A., A. S. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 32.Gong, M., and L. Makowski. 1992. Helical structure of P pili from Escherichia coli. Evidence from X-ray fiber diffraction and scanning transmission electron microscopy. J. Mol. Biol. 228:735-742. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez, M. D., C. A. Lichtensteiger, and E. R. Vimr. 2001. Adaptation of signature-tagged mutagenesis to Escherichia coli K1 and the infant-rat model of invasive disease. FEMS Microbiol. Lett. 198:125-128. [DOI] [PubMed] [Google Scholar]
- 34.Gubbins, M. J. , I. Lau, W. R. Will, J. M. Manchak, T. L. Raivio, and L. S. Frost. 2002. The positive regulator, TraJ, of the Escherichia coli F plasmid is unstable in a cpxA* background. J. Bacteriol. 184:5781-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ha, U. H., Y. Wang, and S. Jin. 2003. DsbA of Pseudomonas aeruginosa is essential for multiple virulence factors. Infect. Immun. 71:1590-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi, S., M. Abe, M. Kimoto, S. Furukawa, and T. Nakazawa. 2000. The dsbA-dsbB disulfide bond formation system of Burkholderia cepacia is involved in the production of protease and alkaline phosphatase, motility, metal resistance, and multi-drug resistance. Microbiol. Immunol. 44:41-50. [DOI] [PubMed] [Google Scholar]
- 38.Hazes, B., P. A. Sastry, K. Hayakawa, R. J. Read, and R. T. Irvin. 2000. Crystal structure of Pseudomonas aeruginosa PAK pilin suggests a main-chain-dominated mode of receptor binding. J. Mol. Biol. 299:1005-1017. [DOI] [PubMed] [Google Scholar]
- 39.Hung, D. L., J. S. Pinkner, S. D. Knight, and S. J. Hultgren. 1999. Structural basis of chaperone self-capping in P pilus biogenesis. Proc. Natl. Acad. Sci. USA 96:8178-8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2001. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 20:1508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang, J. , D. Bieber, S. W. Ramer, C. Y. Wu, and G. K. Schoolnik. 2003. Structural and topographical studies of the type IV bundle-forming pilus assembly complex of enteropathogenic Escherichia coli. J. Bacteriol. 185:6695-6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson, M. W., and G. V. Plano. 1999. DsbA is required for stable expression of outer membrane protein YscC and for efficient Yop secretion in Yersinia pestis. J. Bacteriol. 181:5126-5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacob-Dubuisson, F., J. Pinkner, X. Xu, R. Striker, A. Padmanhaban, and S. J. Hultgren. 1994. PapD chaperone function in pilus biogenesis depends on oxidant and chaperone-like activities of DsbA. Proc. Natl. Acad. Sci. USA 91:11552-11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarvis, K. G., J. A. Giron, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson, K., I. Charles, G. Dougan, D. Pickard, P. O'Gaora, G. Costa, T. Ali, I. Miller, and C. Hormaeche. 1991. The role of a stress-response protein in Salmonella typhimurium virulence. Mol. Microbiol. 5:401-407. [DOI] [PubMed] [Google Scholar]
- 47.Jones, C. H., P. N. Danese, J. S. Pinkner, T. J. Silhavy, and S. J. Hultgren. 1997. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 21:6394-6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keizer, D. W., C. M. Slupsky, M. Kalisiak, A. P. Campbell, M. P. Crump, P. A. Sastry, B. Hazes, R. T. Irvin, and B. D. Sykes. 2001. Structure of a pilin monomer from Pseudomonas aeruginosa: implications for the assembly of pili. J. Biol. Chem. 276:24186-24193. [DOI] [PubMed] [Google Scholar]
- 49.Kenny, B., A. Abe, M. Stein, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect. Immun. 65:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 51.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kresse, A. U., M. Rohde, and C. A. Guzman. 1999. The EspD protein of enterohemorrhagic Escherichia coli is required for the formation of bacterial surface appendages and is incorporated in the cytoplasmic membranes of target cells. Infect. Immun. 67:4834-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuehn, M. J. , J. Heuser, S. Normark, and S. J. Hultgren. 1992. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature 356:252-255. [DOI] [PubMed] [Google Scholar]
- 54.Lee, Y. M., P. A. DiGiuseppe, T. J. Silhavy, and S. J. Hultgren. 2004. P pilus assembly motif necessary for activation of the CpxRA pathway by PapE in Escherichia coli. J. Bacteriol. 186:4326-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, and S. Sotman. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet 1:1119-1122. [DOI] [PubMed] [Google Scholar]
- 56.Levine, M. M., J. P. Nataro, H. Karch, M. M. Baldini, J. B. Kaper, R. E. Black, M. L. Clements, and A. D. O'Brien. 1985. The diarrhoeal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 152:550-559. [DOI] [PubMed] [Google Scholar]
- 57.Li, S. R., N. Dorrell, P. H. Everest, G. Dougan, and B. W. Wren. 1996. Construction and characterization of a Yersinia enterocolitica O:8 high-temperature requirement (htrA) isogenic mutant. Infect. Immun. 64:2088-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malhotra, S., L. A. Silo-Suh, K. Mathee, and D. E. Ohman. 2000. Proteome analysis of the effect of mucoid conversion on global protein expression in Pseudomonas aeruginosa strain PAO1 shows induction of the disulfide bond isomerase, DsbA. J. Bacteriol. 182:6999-7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez-Laguna, Y., E. Calva, and J. L. Puente. 1999. Autoactivation and environmental regulation of bfpT expression, the gene coding for the transcriptional activator of bfpA in enteropathogenic Escherichia coli. Mol. Microbiol. 33:153-166. [DOI] [PubMed] [Google Scholar]
- 60.Miki, T., N. Okada, and H. Danbara. 2004. Two periplasmic disulfide oxidoreductases, DsbA and SrgA, target outer membrane protein SpiA, a component of the Salmonella pathogenicity island 2 type III secretion system. J. Biol. Chem. 279:34631-34642. [DOI] [PubMed] [Google Scholar]
- 61.Mileykovskaya, E., and W. Dowhan. 1997. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J. Bacteriol. 179:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy, K. C., and K. G. Campellone. 2003. Lambda Red-mediated recombinogenic engineering of enterohemorrhagic and enteropathogenic E. coli. BMC Mol. Biol. 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakayama, S.-I., and H. Watanabe. 1998. Identification of cpxR as a positive regulator essential for expression of the Shigella sonnei virF gene. J. Bacteriol. 180:3522-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakayama, S.-I., and H. Watanabe. 1995. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of the Shigella sonnei virF gene. J. Bacteriol. 177:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nougayrede, J. P., P. J. Fernandes, and M. S. Donnenberg. 2003. Adhesion of enteropathogenic Escherichia coli to host cells. Cell. Microbiol. 5:359-372. [DOI] [PubMed] [Google Scholar]
- 67.Otto, K., and T. J. Silhavy. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 99:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parge, H. E., K. T. Forest, M. J. Hickey, D. A. Christensen, E. D. Getzoff, and J. A. Tainer. 1995. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature 378:32-38. [DOI] [PubMed] [Google Scholar]
- 69.Pedersen, L. L., M. Radulic, M. Doric, and Y. Abu Kwaik. 2001. HtrA homologue of Legionella pneumophila: an indispensable element for intracellular infection of mammalian but not protozoan cells. Infect. Immun. 69:2569-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peek, J. A., and R. K. Taylor. 1992. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 89:6210-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pogliano, J. A., S. Lynch, D. Belin, E. C. C. Lin, and J. Beckwith. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11:1169-1182. [DOI] [PubMed] [Google Scholar]
- 72.Puente, J. L., D. Bieber, S. W. Ramer, W. Murray, and G. K. Schoolnik. 1996. The bundle-forming pili of enteropathogenic Escherichia coli: transcriptional regulation by environmental signals. Mol. Microbiol. 20:87-100. [DOI] [PubMed] [Google Scholar]
- 73.Purdy, G. E., M. Hong, and S. M. Payne. 2002. Shigella flexneri DegP facilitates IcsA surface expression and is required for efficient intercellular spread. Infect. Immun. 70:6355-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raivio, T. L., M. W. Laird, J. C. Joly, and T. J. Silhavy. 2000. Tethering of CpxP to the inner membrane prevents spheroplast induction of the Cpx envelope stress response. Mol. Microbiol. 37:1186-1197. [DOI] [PubMed] [Google Scholar]
- 75.Raivio, T. L., D. L. Popkin, and T. J. Silhavy. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J. Bacteriol. 181:5263-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raivio, T. L., and T. J. Silhavy. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179:7724-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramer, S. W., G. K. Schoolnik, C. Y. Wu, J. Hwang, S. A. Schmidt, and D. Bieber. 2002. The type IV pilus assembly complex: biogenic interactions among the bundle-forming pilus proteins of enteropathogenic Escherichia coli. J. Bacteriol. 184:3457-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Resto-Ruiz, S. I., D. Sweger, R. H. Widen, N. Valkov, and B. E. Anderson. 2000. Transcriptional activation of the htrA (high-temperature requirement A) gene from Bartonella henselae. Infect. Immun. 68:5970-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sambrook, J. , E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 80.Sauer, F. G., K. Futterer, J. S. Pinkner, K. W. Dodson, S. J. Hultgren, and G. Waksman. 1999. Structural basis of chaperone function and pilus biogenesis. Science 285:1058-1061. [DOI] [PubMed] [Google Scholar]
- 81.Sauer, F. G., J. S. Pinkner, G. Waksman, and S. J. Hultgren. 2002. Chaperone priming of pilus subunits facilitates a topological transition that drives fiber formation. Cell 111:543-551. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt, S. A., D. Bieber, S. W. Ramer, J. Hwang, C. Y. Wu, and G. Schoolnik. 2001. Structure-function analysis of BfpB, a secretin-like protein encoded by the bundle-forming-pilus operon of enteropathogenic Escherichia coli. J. Bacteriol. 183:4848-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schreiber, W., K. D. Stone, M. A. Strong, L. J. DeTolla, Jr., M. Hoppert, and M. S. Donnenberg. 2002. BfpU, a soluble protein essential for type IV pilus biogenesis in enteropathogenic Escherichia coli. Microbiology 148:2507-2518. [DOI] [PubMed] [Google Scholar]
- 84.Silhavy, T. J. , M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 85.Silverman, P. M., L. Tran, R. Harris, and H. M. Gaudin. 1993. Accumulation of the F plasmid TraJ protein in cpx mutants of Escherichia coli. J. Bacteriol. 175:921-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Slauch, J. M., and T. J. Silhavy. 1991. cis-Acting ompF mutations that result in OmpR-dependent constitutive expression. J. Bacteriol. 173:4039-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Snyder, W. B., L. J. B. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sohel, I., J. L. Puente, S. W. Ramer, D. Bieber, C.-Y. Wu, and G. K. Schoolnik. 1996. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J. Bacteriol. 178:2613-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stenson, T. H., and A. A. Weiss. 2002. DsbA and DsbC are required for secretion of pertussis toxin by Bordetella pertussis. Infect. Immun. 70:2297-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stone, K. D., H.-Z. Zhang, L. K. Carlson, and M. S. Donnenberg. 1996. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol. Microbiol. 20:325-337. [DOI] [PubMed] [Google Scholar]
- 91.Thanassi, D. G., and S. J. Hultgren. 2000. Assembly of complex organelles: pilus biogenesis in gram-negative bacteria as a model system. Methods 20:111-126. [DOI] [PubMed] [Google Scholar]
- 92.Thanassi, D. G., E. T. Saulino, M. J. Lombardo, R. Roth, J. Heuser, and S. J. Hultgren. 1998. The PapC usher forms an oligomeric channel: implications for pilus biogenesis across the outer membrane. Proc. Natl. Acad. Sci. USA 95:3146-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tinsley, C. R., R. Voulhoux, J. L. Beretti, J. Tommassen, and X. Nassif. 2004. Three homologues, including two membrane-bound proteins, of the disulfide oxidoreductase DsbA in Neisseria meningitidis: effects on bacterial growth and biogenesis of functional type IV pili. J. Biol. Chem. 279:27078-27087. [DOI] [PubMed] [Google Scholar]
- 94.Tobe, T., G. K. Schoolnik, I. Sohel, V. H. Bustamante, and J. L. Puente. 1996. Cloning and characterization of bfpTVW, genes required for the transcriptional activation of bfpA in enteropathogenic Escherichia coli. Mol. Microbiol. 21:963-975. [DOI] [PubMed] [Google Scholar]
- 95.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vanmaele, R. P., and G. D. Armstrong. 1997. Effect of carbon source on localized adherence of enteropathogenic Escherichia coli. Infect. Immun. 65:1408-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vincent-Sealy, L. V., J. D. Thomas, P. Commander, and G. P. C. Salmond. 1999. Erwinia carotovora DsbA mutants: evidence for a periplasmic-stress signal transduction system affecting transcription of genes encoding secreted proteins. Microbiology 145:1945-1958. [DOI] [PubMed] [Google Scholar]
- 98.Warawa, J. , B. B. Finlay, and B. Kenny. 1999. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect. Immun. 67:5538-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watarai, M., T. Tobe, M. Yoshikawa, and C. Sasakawa. 1995. Disulfide oxidoreductase activity of Shigella flexneri is required for release of Ipa proteins and invasion of epithelial cells. Proc. Natl. Acad. Sci. USA 92:4927-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weber, R. F., and P. J. Silverman. 1988. The Cpx proteins of Escherichia coli K-12: structure of the CpxA polypeptide as an inner membrane component. J. Mol. Biol. 203:467-476. [DOI] [PubMed] [Google Scholar]
- 101.Williams, K., P. C. Oyston, N. Dorrell, S. Li, R. W. Titball, and B. W. Wren. 2000. Investigation into the role of the serine protease HtrA in Yersinia pestis pathogenesis. FEMS Microbiol. Lett. 186:281-286. [DOI] [PubMed] [Google Scholar]
- 102.Winther-Larsen, H. C., and M. Koomey. 2002. Transcriptional, chemosensory and cell-contact-dependent regulation of type IV pilus expression. Curr. Opin. Microbiol. 5:173-178. [DOI] [PubMed] [Google Scholar]
- 103.Wolff, C., I. Nisan, E. Hanski, G. Frankel, and I. Rosenshine. 1998. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 28:143-155. [DOI] [PubMed] [Google Scholar]
- 104.Wolfgang, M., J. P. van Putten, S. F. Hayes, D. Dorward, and M. Koomey. 2000. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 19:6408-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamoto, T., T. Hanawa, S. Ogata, and S. Kamiya. 1997. The Yersinia enterocolitica GsrA stress protein, involved in intracellular survival, is induced by macrophage phagocytosis. Infect. Immun. 65:2190-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu, J. 1998. Inactivation of DsbA, but not DsbC and DsbD, affects the intracellular survival and virulence of Shigella flexneri. Infect. Immun. 66:3909-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu, J. , B. Edwards-Jones, O. Neyrolles, and J. S. Kroll. 2000. Key role for DsbA in cell-to-cell spread of Shigella flexneri, permitting secretion of Ipa proteins into interepithelial protrusions. Infect. Immun. 68:6449-6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu, J. , E. E. Oragui, A. Stephens, J. S. Kroll, and M. M. Venkatesan. 2001. Inactivation of DsbA alters the behaviour of Shigella flexneri towards murine and human-derived macrophage-like cells. FEMS Microbiol. Lett. 204:81-88. [DOI] [PubMed] [Google Scholar]
- 110.Zhang, H. Z., and M. S. Donnenberg. 1996. DsbA is required for stability of the type IV pilin of enteropathogenic Escherichia coli. Mol. Microbiol. 21:787-797. [DOI] [PubMed] [Google Scholar]