Receptor-like kinases and reactive oxygen species are intricately entangled and have central roles in controlling many processes in plants, but their interaction is still insufficiently understood.
Abstract
In plants, receptor-like kinases (RLKs) and extracellular reactive oxygen species (ROS) contribute to the communication between the environment and the interior of the cell. Apoplastic ROS production is a frequent result of RLK signaling in a multitude of cellular processes; thus, by their nature, these two signaling components are inherently linked. However, it is as yet unclear how ROS signaling downstream of receptor activation is executed. In this review, we provide a broad view of the intricate connections between RLKs and ROS signaling and describe the regulatory events that control and coordinate extracellular ROS production. We propose that concurrent initiation of ROS-dependent and -independent signaling linked to RLKs might be a critical element in establishing cellular responses. Furthermore, we discuss the possible ROS sensing mechanisms in the context of the biochemical environment in the apoplast. We suggest that RLK-dependent modulation of apoplastic and intracellular conditions facilitates ROS perception and signaling. Based on data from plant and animal models, we argue that specific RLKs could be components of the ROS sensing machinery or ROS sensors. The importance of the crosstalk between RLK and ROS signaling is discussed in the context of stomatal immunity. Finally, we highlight challenges in the understanding of these signaling processes and provide perspectives for future research.
INTRODUCTION
Multicellular organisms use a plethora of mechanisms to control and adjust the functions of cells to ensure coordinated and synchronized responses in tissues, organs, and throughout the entire organism. The perception of specific molecules at the cell perimeter is of crucial importance for these signaling processes. In plants, communication between cells and the extracellular environment is largely controlled by receptor-like kinases (RLKs) and receptor-like proteins. The RLKs are a large protein family with over 600 members in the model plant Arabidopsis thaliana (Shiu and Bleecker, 2003). RLKs are transmembrane proteins that are anchored to the plasma membrane. The N-terminal extracellular region, the ectodomain, extends into the apoplast where it perceives stimuli, whereas the C-terminal kinase domain resides inside the cytoplasm and relays signals into the intracellular environment. Recent studies have highlighted the roles of RLKs as central regulators of development, growth, pathogen defense, and responses to abiotic cues (Marshall et al., 2012). Since plants are constantly exposed to multiple stimuli, the large number of RLKs and the corresponding potential ligands might mediate the integration of simultaneous signals through crosstalk and the use of similar signaling components. Despite their importance, the protein complexes that coordinate receptor action remain poorly understood.
In addition to RLKs, reactive oxygen species (ROS) are important components of multiple signaling pathways. ROS include singlet oxygen (1O2), superoxide anion (O2·−), hydrogen peroxide (H2O2), and hydroxyl radical (HO·), each with distinct chemical properties and important roles as signaling molecules in all domains of life. ROS are produced in multiple subcellular locations, including chloroplasts, peroxisomes, mitochondria, and the apoplast. Importantly, localized ROS accumulation frequently affects the redox status of other subcellular compartments (Joo et al., 2005; Vahisalu et al., 2010) and even of distant cells (Gilroy et al., 2016). Intracellular ROS production is primarily associated with photorespiration and metabolic processes characterized by high redox potentials, such as photosynthetic/mitochondrial electron transport chains. By contrast, apoplastic ROS accumulation results mainly from the specific activation of plasma membrane-localized NADPH oxidases, in plants known as respiratory burst oxidase homologs (RBOHs), and cell wall peroxidases (Figure 1; Kärkönen and Kuchitsu, 2015). In plants, ROS exert control over metabolic regulation, development, pathogen defense, and responses to abiotic stimuli (Wrzaczek et al., 2013). As evident from transcriptomic responses (Vaahtera et al., 2014; Willems et al., 2016), plant cells meticulously sense ROS and trigger specific responses tailored to the type, concentration, and subcellular origin of ROS molecules. However, it is unclear how this signaling specificity is achieved.
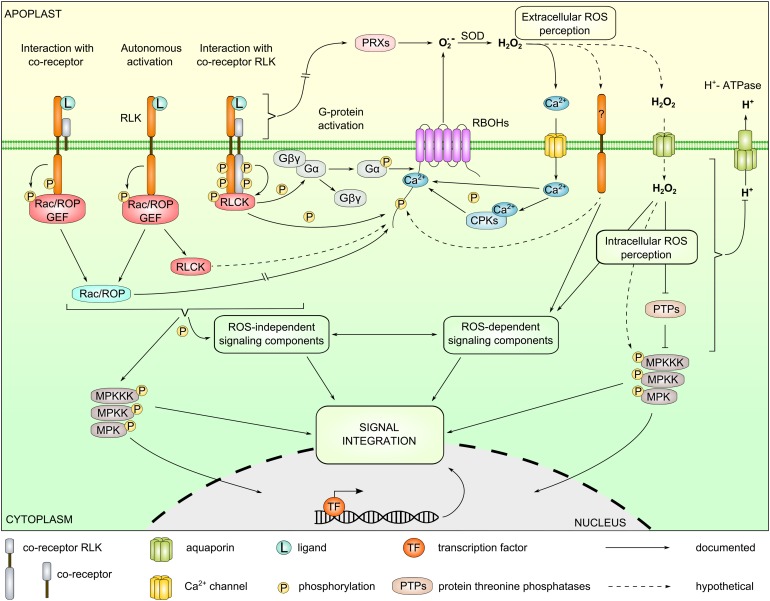
Figure 1.
RLK-Related ROS Production, Perception, and Signaling Pathways.
Apoplastic ROS are produced by the activation of plasma membrane-localized NADPH oxidases (RBOHs) and cell wall peroxidases. RLKs, in concert with coreceptors, RLCKs, small GTPases, and heterotrimeric G-proteins, control the activity of RBOHs. In addition to controlling RBOH activity, RLK complexes also regulate ROS-independent signaling components, e.g., MAPK cascades. Apoplastic ROS production leads to Ca2+ influx and Ca2+-dependent activation of RBOHs. RLK signaling can also mediate inhibition of H+-ATPase activity leading to an increase of apoplastic pH. Sensing of ROS by putative RLKs in the apoplast and following influx through aquaporins by intracellular proteins leads to activation of ROS-dependent signaling components. ROS-dependent/-independent signaling and MAPK signaling are integrated to establish signal specificity. PRXs, peroxidases; Gαβγ, heterotrimeric G-protein subunits; MPK, mitogen-activated protein kinase; MPKK, MAPK kinase; MPKKK, MAPKK kinase; SOD, superoxide dismutase. Detailed descriptions of specific regulatory mechanisms are provided in the main text.
An emerging theme associated with RLK signaling is the production of ROS in the apoplast. In this context, the question of signaling specificity gains additional importance, as functionally independent RLKs can trigger the production of the same type of ROS in the same subcellular compartment. The integration of ROS-dependent and -independent RLK signaling mechanisms likely provides specificity for local and systemic ROS signaling. While numerous reviews discuss RLK and ROS signaling separately, here, we evaluate recent progress in understanding the crosstalk between RLK and ROS signaling. We discuss how RLKs both influence and are influenced by ROS production and signaling and propose that crosstalk between RLKs and ROS signaling may play a critical role in plant development and environmental responses.
RLKs REGULATE ROS PRODUCTION AND SIGNALING (ROS DOWNSTREAM OF RLKs)
The large variety of RLKs enables perception of a wide rage of signaling molecules, which can act as ligands and activate RLKs upon binding (Greeff et al., 2012). Ligands primarily bind to their receptors in a noncovalent manner, often via hydrogen bonds or hydrophobic interactions (Hohmann et al., 2017). Ligand binding can induce the formation of multimeric complexes involving activated receptors, coreceptors, and intracellular kinases (Couto and Zipfel, 2016). This process further triggers the phosphorylation of a wide range of different substrates, such as RBOHs, which orchestrate cellular responses.
The production of O2·− molecules by RBOHs, and their subsequent dismutation into H2O2 both spontaneously and enzymatically via apoplastic superoxide dismutases has been described for numerous processes downstream of RLK activation. These processes range from the recognition of microbe-associated molecular patterns (MAMPs) and host-derived damage-associated molecular patterns (DAMPs) to the control of plant development (Table 1). The Arabidopsis genome encodes 10 RBOHs involved in different physiological processes (Suzuki et al., 2011). Two RBOH isoforms, RBOHD and RBOHF, play major roles in the responses to abiotic and biotic stimuli. RBOHs are synergistically regulated by phosphorylation and calcium (Ca2+) binding to EF-hand domains in the N-terminal cytosolic region (Kärkönen and Kuchitsu, 2015). While RLKs might stimulate extracellular ROS production via several mechanisms, genetic and biochemical analyses have highlighted two classes of signaling components by which RLK activation controls RBOH activity: receptor-like cytoplasmic protein kinases (RLCKs) and small guanine nucleotide binding proteins, the so-called Rac/Rho-like guanine nucleotide exchange factors (Rac/ROP GEFs) (Figure 1). RLCKs represent a subset of RLKs that lack the extracellular and transmembrane regions and are used by several RLKs as downstream signaling components (Table 1). Rac/ROP GEFs belong to the Ras and Rho subfamilies of the Ras superfamily of small GTPases and function as molecular switches in many signaling cascades (Wu et al., 2011; Table 1). Cell wall peroxidases also contribute to apoplastic ROS production (Daudi et al., 2012), although little is known about their regulation.
Table 1. Summary of RLKs That Are Functionally Linked to the ROS Burst.
| RLKs | Classification | Ligand/Stimuli | RLCK/GEF Interaction | ROS Bursta | Apoplast pH↑ | Stomatal Closurea | References |
|---|---|---|---|---|---|---|---|
| FLS2 | LRR | flg22 | BIK1, PCRK1?, PBL1/13? | Yes (At), FLS2 OE (↑), fls2 mutant (−), bik1 mutant (↓), pbl1 mutant (↓), pbl13 mutant (↑) | Yes (tomato, potato, N. tabacum, At) | Yes (At) | Felix et al., 1999; Gómez-Gómez and Boller, 2000; Chinchilla et al., 2006; Melotto et al., 2006; Zipfel et al., 2006; Zhang et al., 2010; Sreekanta et al., 2015 |
| EFR | LRR | elf18 | BIK1 | Yes (At), efr mutant (−) | Yes (At) | Yes (At) | Kunze et al., 2004; Zipfel et al., 2006; Desikan et al., 2008; Zhang et al., 2010 |
| PEPR1/2 | LRR | AtPep1 | BIK1, PBL1 | Yes (At), pepr1 mutant (↓), pepr2 mutant (↓), pepr1/2 double mutant (−), bik1 mutant (↓), pbl1 mutant (↓) | Yes (At) | Huffaker et al., 2006; Pearce et al., 2008; Krol et al., 2010; Yamaguchi et al., 2010; Liu et al., 2013 | |
| LYK4/5 | LysM | Chitin | Yes (tomato, At), lyk4 mutant (↓), lyk5 mutant (↓) | Yes (tomato, barley) | Yes (tomato) | Felix et al., 1993; Lee et al., 1999; Cao et al., 2014; Felle et al., 2004 | |
| CERK1 | LysM | PGN, Chitin | BIK1, PBL27 | Yes (At by PGN), cerk1 mutant (−) by chitin, bik1 mutant (↓) by chitin, pbl27 mutant (=) by chitin | Yes (At, by PGN) | Gust et al., 2007; Miya et al., 2007; Zhang et al., 2010; Liu et al., 2012; Shinya et al., 2014 | |
| LIK1 | LRR | Chitin | lik1 mutant (↑) by chitin | Le et al., 2014 | |||
| OsCERK1 | LysM | Chitin | GEF1, RLCK176/ 185 | Yes (rice), OsCERK1-RNAi (↓), OsRacGEF1 RNAi (−), OsRLCK176-RNAi (↓), OsRLCK185 RNAi (↓) | Yes (rice), OsCERK1-RNAi (↓) | Akamatsu et al., 2013; Ao et al., 2014; Yamaguchi et al., 2013 | |
| HAESA/ HLS2 | LRR | IDA | HAE OE (=), HSL2 OE (↑) (transiently expressing N. benthamiana) | Cho et al., 2008; Butenko et al., 2014 | |||
| BRI1 | LRR | BR | BIK1, BSKs | Yes (N. benthamiana) | Yes (Vicia faba) | Wang et al., 2001; Haubrick et al., 2006; Tang et al., 2008; Lin et al., 2013; Deng et al., 2015 | |
| FER | Malectin-like | RALF | GEF1/4/7/10/14, MARIS? | Yes (tomato) | Pearce et al., 2001; Berken et al., 2005; Duan et al., 2010; Boisson-Dernier et al., 2015; Haruta et al., 2014 | ||
| ANXs | Malectin-like | MARIS? | Boisson-Dernier et al., 2015 | ||||
| AtPRK2 | LRR | GEF1/12 | Zhang and McCormick, 2007; Chang et al., 2013 | ||||
| PRK5 | LRR | GRI | Wrzaczek et al., 2015 | ||||
| THE1 | Malectin-like | CWD? | the mutant (↓) by CWD | Denness et al., 2011 | |||
| IOS1 | Malectin-like | CWD? | ios mutant (=) by flg22, IOS OE (=) by flg22 | Yeh et al., 2016; Singh et al., 2012 | |||
| LORE | S-lectin | LPS | Yes (N. tabacum, At), lore mutant (−) | Yes (N. tabacum) | Yes (At) | Gerber et al., 2004; Melotto et al., 2006; Ranf et al., 2015 | |
| RLK7 | LRR | PIP | Yes (At) | Yes (At) | Hou et al., 2014 | ||
| LecRK-VI.2 | L-type lectin | lecrk-VI.2 mutant (=) by flg22 | lecrk-VI.2 mutant (↓) by flg22, elf18 | Singh et al., 2012 | |||
| LecRK-V.5 | L-type lectin | LecRK-V.5 OE (↓) by flg22, elf18, LPS | LecRK-V.5 OE (↓) by flg22, elf18, LPS | Desclos-Theveniau et al., 2012 | |||
| WAK1 | WAK1-like | OGs | Yes (grapevine, At) | Yes (grapevine) | Allègre et al., 2009; Brutus et al., 2010; Galletti et al., 2008 | ||
| GHR1 | LRR | ghr1 mutant (−) by flg22 | Hua et al., 2012 | ||||
| CRKs | DUF26 | crk2/3/13/31 mutant (↓) by flg22, crk20/23/29 mutant (↑) by flg22, CRK4/6/28/36 OE (↑) by flg22 | crk23 mutant (↑) by flg22, crk5/17/20/28 mutant (↓) by flg22 | Bourdais et al., 2015; Yeh et al., 2015; Yadeta et al., 2016 |
Receptors that have been suggested to participate in ROS-dependent processes are listed, even in the absence of direct evidence for their roles in the control of ROS production. PCRK1, PATTERN-TRIGGERED IMMUNITY (PTI) COMPROMISED RECEPTOR-LIKE CYTOPLASMIC KINASE1; HSL2, HAESA-LIKE 2; LORE, LIPOOLIGOSACCHARIDE-SPECIFIC REDUCED ELICITATION ; DUF26, Domain of Unknown Function 26; PGN, peptidoglycan; IDA, INFLORESCENCE DEFICIENT IN ABSCISSION; CWD, cell wall damage; PIP1, PAMP-INDUCED SECRETED PEPTIDE 1; At, Arabidopsis thaliana; N. tabacum, Nicotiana tabacum; N. benthamiana, Nicotiana benthamiana; OE, overexpressing lines; tomato, Solanum lycopersicum; potato, Solanum tuberosum; barley, Hordeum vulgare; grapevine, Vitis vinifera.
Phenotypes compared to the wild type: (↑) enhanced, (↓) reduced, (−) abolished, and (=) not affected.
The activation of ROS production downstream of multiple independent RLKs (Table 1) highlights the notion that ROS bursts are a central element of RLK signaling. However, the precise messages conveyed by the RLK-triggered ROS burst have yet to be decrypted.
RLCKs Connect RLKs with ROS Production
The activation of RBOHs by RLCKs is a well-recognized response to MAMPs (summarized in Table 1). Well-characterized MAMPs include bacterial flagellin (epitope flg22), elongation factor Tu (EF-Tu; epitopes elf18 and elf26), and fungal chitin. The recognition of ligands by the respective receptors FLAGELLIN-SENSITIVE2 (FLS2), EF-TU RECEPTOR (EFR) or Lysin motif (LysM)-CONTAINING RECEPTOR KINASE5 (LYK5), and CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) triggers the formation of heteromeric complexes with their respective coreceptors, ultimately leading to the activation of the RLCK BOTRYTIS-INDUCED KINASE1 (BIK1; Couto and Zipfel, 2016). Activated BIK1 phosphorylates RBOHD and thereby initiates ROS production (Kadota et al., 2014). Likewise, BIK1 plays a central role in DAMP signaling (Table 1). The receptor for the DAMP AtPEP1, PEP RECEPTOR1 (PEPR1) interacts with and phosphorylates BIK1 in vitro (Liu et al., 2013). Phosphorylation of RBOHD by BIK1 is required for AtPep1-induced ROS production (Kadota et al., 2014). These findings suggest that the binding of AtPep1 to PEPR1 triggers BIK1 activation and induces ROS production by RBOHD. These examples emphasize the essential role of BIK1 in activating ROS production downstream of MAMP/DAMP receptors.
While it is unclear whether different RLKs phosphorylate the same residues in BIK1 (Lu et al., 2010; Lin et al., 2014), the targeting of different sites could adjust the activity and selectivity of BIK1 toward different substrates. Importantly, BIK1 is not the only RLCK that interacts with RLKs (Zhang et al., 2010). For example, the RLCK PBS1-LIKE1 (PBL1) interacts with FLS2 and PEPR1 and is phosphorylated upon treatment with flg22 (Zhang et al., 2010; Liu et al., 2013). However, it is unclear whether PBL1 is directly phosphorylated by FLS2 or PEPR1. Furthermore, PBL1 interacts with RBOHD and contributes to flg22-induced ROS production (Kadota et al., 2014) but has not been shown to phosphorylate RBOHD. However, it is conceivable that PBL1 might be phosphorylated by RLKs and can itself phosphorylate RBOHs in a manner similar to that of BIK1. These different RLCKs could represent different signaling modules or could be specific to distinct cells or tissues. It is likely that RLCKs modulate the repertoire of responses by phosphorylating diverse sets of substrates, thereby ultimately helping to establish signaling specificity.
Small G-Proteins Control ROS Production Downstream of RLKs
In rice (Oryza sativa), the control of intracellular signaling events downstream of chitin perception relies on RacGEF rather than RLCKs (Table 1). Upon chitin treatment, OsCERK1 associates with CHITIN ELICITOR BINDING PROTEIN (Shimizu et al., 2010). Subsequently, OsCERK1 phosphorylates RacGEF1, which activates the small GTPase Rac1. Rac1 then stimulates ROS production by RBOHB (Akamatsu et al., 2013).
The regulation of ROS production by Rac/ROP downstream of RLK activation is also important for controlling plant growth and development. In Arabidopsis, FERONIA (FER), a member of the Catharanthus roseus RLK1-like kinase family (CrRLK1L), perceives the RAPID ALKALINIZATION FACTOR (RALF) peptide to inhibit primary root elongation (Haruta et al., 2014). Other CrRLK1L kinases are also thought to recognize polysaccharides or glycosylated proteins to monitor cell wall integrity and regulate cell expansion (Boisson-Dernier et al., 2011; Lindner et al., 2012). In roots, FER interacts with ROPGEF1, which then recruits and activates ROP2 (Duan et al., 2010), which in turn potentially activates RBOHC/ROOT HAIR DEFECTIVE2 (RHD2; Jones et al., 2007). The FER-ROPGEF-ROP module also controls ROS-mediated pollen tube reception in the female gametophyte (Duan et al., 2014). ROS accumulation is essential for pollen tube growth and relies on RBOHH and RBOHJ, as evident from the male sterility phenotype of rbohH rbohJ plants (Kaya et al., 2014). Two members of the CrRLK1L kinase family, ANXUR1 (ANX1) and ANX2, function upstream of RBOHH and RBOHJ (Boisson-Dernier et al., 2013), likely contributing to the control of their ROS production activity, as anx1 anx2 double mutants phenocopy the male sterility of rbohH rbohJ plants. Interestingly, ROPGEF1 is also phosphorylated by the leucine-rich repeat (LRR)-RLK POLLEN-SPECIFIC RECEPTOR-LIKE KINASE2 (PRK2), forming a complex with PRK2 and ROP1 that participate in the control of pollen tube growth (Chang et al., 2013). However, it is currently unclear whether PRK2-ROPGEF1-ROP1 controls ROS production. Similarly, it is unknown whether ANXs phosphorylate ROPGEFs. Activated ROP1 could be a key element in regulating the activity of RBOHH and RBOHJ downstream of ANXs and PRK2. In addition to FER, ANX1, and ANX2, the CrRLK1L kinase THESEUS1 (THE1) also appears to be involved in ROS-dependent processes (Table 1; Denness et al., 2011). However, their interactions with ROPGEFs or ROPs have not been demonstrated. It is plausible that all CrRLK1L kinases that initiate apoplastic ROS production could activate a similar set of ROPGEFs, thus making them central regulators of ROS production downstream of RLK activation.
Integration of RLCK and Rac/ROP GEF Signaling Modules
CrRLK1L kinases serve as an excellent example demonstrating that the control of ROS production by RLKs is not necessarily facilitated by a single mechanism. A suppressor mutant screen for male sterility in the anx1 anx2 double mutant identified a mutation in the RLCK MARIS (MRI) that stimulates its kinase activity. Hyperactive (but not wild type) MRI partially rescued the male sterility of anx1 anx2 and rbohH rbohJ double mutants (Boisson-Dernier et al., 2015), suggesting that MRI is capable of activating other RBOHs. This hypothesis is in line with the ability of hyperactive MRI to rescue RBOHC/RHD2-dependent root hair growth defects in fer (Boisson-Dernier et al., 2015). To gain more insight into the integration of MRI into CrRLK1L signaling, it would be interesting to assess ROS accumulation in plants with hyperactive and kinase-dead MRI. In summary, these findings suggest that RLCKs and Rac/ROP GEFs might function together. Furthermore, another class of GTPases, heterotrimeric G-proteins, also function in the receptor-RLCK system. Under resting conditions, heterotrimeric G-proteins interact with the FLS2-BIK1 complex and attenuate BIK1 degradation (Liang et al., 2016). In response to flg22, G-proteins stimulate ROS production, likely by helping to activate RBOHD.
While the regulation of RBOH through RLCKs and Rac/ROP GEF signaling modules is well described, the direct regulation of RBOH by RLKs is a largely unexplored research direction. BRI1-ASSOCIATED KINASE1 (BAK1) interacted weakly with the N terminus of RBOHD in vitro, but the in planta significance of this interaction is unclear (Kadota et al., 2014). A survey of public interactome data suggests that RLKs such as cysteine-rich receptor-like kinases (CRKs) can interact with RBOHs (Jones et al., 2014), which is in agreement with the results of a functional analysis of crk mutants. CRKs are involved in ROS-related processes, such as the regulation of MAMP-induced ROS production and stomatal closure (Bourdais et al., 2015). Furthermore, CRKs associate with other RLKs, such as FLS2 (Yeh et al., 2015) and BAK1 (Yadeta et al., 2016). Taken together, this emerging evidence supports a potential role for CRKs in the regulation of ROS production by RBOHs. However, the exact mechanism of how CRKs influence apoplastic ROS production is still unclear.
RLKs Positively and Negatively Control ROS Production
Many different receptors and processes positively regulate ROS production (Table 1). However, negative regulation is equally important for helping plants avoid the consequences of unrestricted ROS accumulation. Unlike BIK1 and PBL1, the RLCK PBL13 negatively regulates ROS production in the absence of flagellin, as evident from the enhanced MAMP-induced ROS burst in pbl13 plants and the disruption of the PBL13-RBOHD interaction by flg22 treatment (Lin et al., 2015). Similarly, Arabidopsis mutants for LysM RLK1-INTERACTING KINASE1 (LIK1), an LRR-RLK, show higher ROS production in response to chitin than the wild type (Le et al., 2014). CERK1 phosphorylates LIK1 in a chitin-independent manner (Le et al., 2014), but the relevance of this activity has not been clarified. These data suggest that LIK1 negatively regulates ROS production, but a potential interaction between LIK1 and RBOHs has not yet been demonstrated. Intriguingly, FER, which activates RBOHC/RHD2 as described above, might also negatively regulate RBOHF-mediated ROS production. FER directly interacts with ROPGEFs, which activate ROP11. Activated ROP11 interacts with ABSCISIC ACID INSENSITIVE2 (ABI2), which subsequently inhibits OPEN STOMATA1 (OST1; Yu et al., 2012). OST1 phosphorylates RBOHF and potentially regulates its activity (Sirichandra et al., 2009). Thus, FER might counteract the activation of RBOHF by OST1 via ROPGEFs, ROP11, and ABI2. FER also influences ROS production through its role as a scaffold protein. Depending on binding of different RALF peptides to its ectodomain, FER can either promote or inhibit receptor complex formation and subsequent signaling (Stegmann et al., 2017). Moreover, another RLK, LecRK-V.5, negatively affects ROS production in guard cells in response to MAMPs and the plant hormone abscisic acid (ABA; Desclos-Theveniau et al., 2012), suggesting that, depending on the context, RLKs can negatively regulate RBOH-dependent ROS production.
The positive and negative regulation of ROS production are tightly integrated to provide fine-tuned control, with RLCKs and Rac/ROP GEFs possibly functioning as a central integration node (Table 1). The brassinosteroid (BR) signaling mechanism illustrates the complexity of this regulatory balance. BRs are perceived by the LRR-RLK BRASSINOSTEROID INSENSITIVE1 (BRI1; Li and Chory, 1997) and induce an RBOH-dependent ROS burst (Nie et al., 2013). Like many MAMP/DAMP receptors, the hormone receptor BRI1 associates with its coreceptor BAK1 in response to BR, resulting in BRI1 activation (Li et al., 2002). Subsequently, BIK1 is phosphorylated by BRI1 and released from the receptor complex. In addition to BIK1, BRI1 phosphorylates BR signaling kinases (BSKs; Tang et al., 2008); these RLCKs might activate RBOH-dependent ROS production via mitogen-activated protein kinase (MAPK) cascades. However, other downstream components of BR signaling, such as the transcription factors BRI1 EMS SUPPRESSOR1 (BES1) and BRASSINAZOLE RESISTANT1 (BZR1), inhibit RBOH-dependent ROS production (Deng et al., 2016). Moreover, BRI might also counteract extracellular ROS production by negatively controlling peroxidase expression (Kim et al., 2012). In summary, it appears that BR treatment not only induces, but also counteracts, ROS production. While some questions remain, it is plausible that, considering the functions of BIK1 described above, BIK1 phosphorylates RBOHD to promote BR-induced ROS production. Other factors that control BR-induced ROS production remain to be determined. It is tempting to speculate that RBOH activation might represent the first response to BR, while responses that rely on changes in gene expression could occur later.
Taken together, multiple lines of evidence link the processes controlled by RLKs and ROS signaling (Table 1). Through “guilt-by-association,” it can be postulated that RLKs, which employ the RLCK and Rac/ROP GEF modules discussed above, might modulate ROS signaling. Thus far, the direct ROS dependency of RLK signaling has been ascertained in only a few cases. As shown in Table 1, many elements that regulate ROS production downstream of RLKs have yet to be identified or are insufficiently understood. The ubiquitous nature of ROS production in response to RLK activation strongly suggests that ROS play central but incompletely characterized roles in RLK signaling. The conservation of RLK-associated protein complexes might reflect the importance of robust ROS production, which can be adjusted through the integration of parallel signaling pathways.
Disentangling ROS-Dependent and ROS-Independent Responses
Since ROS production is not the only response to RLK activation, it is important to determine which parts of a signaling pathway and the eventual outcomes are dependent or independent of ROS. These separate downstream processes might not be readily separated from each other. Indeed, a strong overlap between responses to, e.g., elicitation with MAMPs and the direct application of ROS, is visible at the gene expression level. However, in addition, clear treatment-specific effects on gene expression patterns can be observed (Vaahtera et al., 2014; Bourdais et al., 2015). Furthermore, such signal specificity can already be observed at the level of intermediate responses, such as ROS-induced Ca2+ fluxes (Evans et al., 2005). These findings support the notion that ROS, as well as RLKs, can trigger both generic and highly specific responses.
The activation of MAPKs downstream of FLS2 serves as an excellent model illustrating the existence of ROS-dependent and -independent processes. Both pathogen attack and the direct application of ROS lead to the activation of MPK3 and MPK6 (Ahlfors et al., 2004). However, FLS2-dependent activation of RBOHD is not required for the activation of MPK3 and MPK6 in response to bacterial pathogens (Xu et al., 2014). By contrast, the Malectin-like/LRR-RLK IMPAIRED OOMYCETE SUSCEPTIBILITY1 (IOS1; Yeh et al., 2016), a component of the FLS2 receptor complex, and LecRK-VI.2 (Singh et al., 2012) are required for the activation of MPK3 and MPK6 but not for the ROS burst. While the identities of all complexes involving IOS1 remain to be elucidated, it is likely that IOS1 serves as a common node leading to MAPK activation. Since Malectin-like RLKs are thought to sense cell wall damage (Table 1), IOS1 might convey information about the status of the cell wall to control MAPK activation. Both ROS and pathogens damage the cell wall, which might be sensed by IOS1 to trigger MAPK activation. To test this hypothesis, it would be interesting to investigate the activation of MAPKs in the ios1 mutant upon ROS treatment.
As described above, BIK1 and PBL1 positively regulate RBOH-dependent ROS production, whereas PBL13 negatively regulates this process. However, the mechanisms underlying the seemingly opposite functions of RLCKs are currently unclear. These RLCKs might phosphorylate different amino acids within RBOHs. Moreover, in addition to common sets of substrates, including RBOHs, RLCKs might also phosphorylate specific substrates to establish signaling specificity downstream of RLK activation. Analogously, such selectivity might also be attributed to ROPGEFs. The mechanisms that dictate the interaction bias between RLK kinase domains and cytoplasmic signaling components remain to be elucidated. Clearly, the RLK kinase domain has a major effect on the outcome of signaling. However, kinase domains of closely related RLKs have overlapping functions. For example, swapping the kinase domains between CrRLK1L kinase family members can complement the fer mutant phenotype (Kessler et al., 2015), indicating that the kinase domains of CrRLK1Ls can substitute for each other. By contrast, evolutionarily distant kinases likely display less overlap in their substrates, as illustrated by the kinase domain of WALL-ASSOCIATED KINASE1 (WAK1), which is sufficient to mediate responses mirroring treatment with oligogalacturonides, even when fused to the ectodomain of EFR (Brutus et al., 2010). These two cases show that the kinase domain strongly contributes to downstream signaling specificity. However, kinase selectivity might be less important between closely related RLKs. A wide range of substrates might allow dynamic feedback regulation to occur between RLK signaling and other cellular processes.
THE APOPLAST: AN ARENA FOR ROS SIGNALING
The apoplastic concentrations of the major redox buffers GSH and ascorbate (AA) are orders of magnitude lower than those in the cytoplasm. While the cytoplasmic concentration of GSH is in the millimolar range (Koffler et al., 2013), apoplastic GSH is barely detectable (Vanacker et al., 1998; Koffler et al., 2013). Therefore, despite the physiological significance of apoplastic GSH metabolism (Tolin et al., 2013), it is still unclear whether apoplastic GSH serves as a donor of reducing equivalents or purely functions in signaling (Zechmann, 2014). Similarly, apoplastic AA contributes little to the total leaf AA pool (Zechmann et al., 2011). Moreover, even under standard growth conditions, most apoplastic AA is oxidized, while over 90% of total leaf AA is present in the reduced form (Veljovic-Jovanovic et al., 2001; Booker et al., 2012). As the antioxidant capacity of apoplastic AA is limited, oxidative AA derivatives are thought to function as signaling molecules (Tran et al., 2013). Apoplastic AA might also buffer “redox noise” and restrict the perception of extracellular ROS below the threshold level. Consequently, the low abundance of GSH and AA extend the half-life of H2O2, the most stable form of ROS (Mattila et al., 2015), from milliseconds (as observed in the intracellular environment) to seconds (Costa et al., 2010), thereby enabling diffusion of H2O2 through the apoplast for cell-to-cell signaling. Thus, the unique physicochemical conditions of the apoplast, i.e., low redox buffering capacity and low pH (discussed below), make it well suited for the generation and propagation of ROS signals.
ROS Are Perceived via Oxidative Posttranslational Modifications
In contrast to signaling molecules that interact with macromolecular domains within receptor proteins (Hohmann et al., 2017; Table 1), ROS directly modify amino acid residues, most prominently cysteine and methionine. This activity results in covalent oxidative posttranslational modifications (PTMs) of a large pool of potential ROS targets (Figure 2A). Most ROS signaling pathways utilize the oxidation of sulfur atoms within the thiol groups of cysteine residues as the initial perception event (Waszczak et al., 2015). In sensor proteins, cysteine reactivity is tightly controlled and depends on the ability of thiol groups (-SH) to form thiolate anions (-S−), which are prone to oxidation. Low pH favors the protonated state of thiols, while at elevated pH, reactive thiol groups are present in dissociated form. Hence, low pKa cysteine residues, i.e., residues that form thiolate anions at physiological pH (pKa < pH; Figure 2A), are the first points of ROS perception (Roos et al., 2013). However, excessive differences between the pKa and pH of the environment can reduce thiolate reactivity (Ferrer-Sueta et al., 2011). Thus, cysteines are most prone to oxidation at pH levels close to their pKa (Roos et al., 2013). The pKa of cysteine residues is determined by the local electrostatic environment, especially hydrogen bonds between thiolate and proximal amino acids (Roos et al., 2013). Therefore, the reactivity of cysteine residues can differ significantly within a single protein molecule.
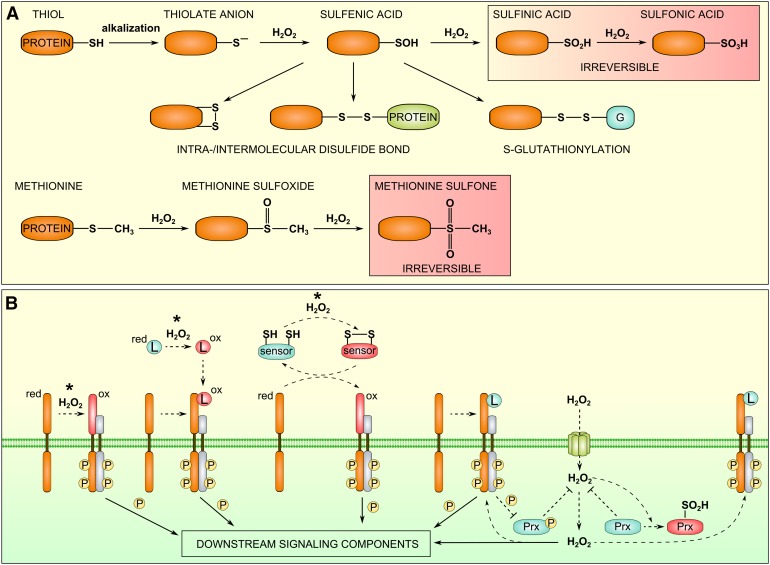
Figure 2.
Overview of ROS Perception Mechanisms.
(A) Oxidative posttranslational modifications of cysteine and methionine residues. Alkalization of the apoplast is expected to increase the availability of thiolate anions for reaction with H2O2. The oxidation of methionine residues is pH independent within the apoplastic pH range.
(B) RLK-assisted ROS perception mechanisms. Asterisks indicate pH-dependent reactions, assuming that the oxidized amino acid residues are cysteines. See the main text for a detailed description. Prx, peroxiredoxin.
In the presence of H2O2, reactive thiols undergo oxidation to sulfenic acid (-SOH), which usually serves as a gateway to further oxidative modifications (Figure 2A). These modifications include oxidation to sulfinic (-SO2H) and sulfonic (-SO3H) acid, which, with few exceptions, leads to protein damage (Waszczak et al., 2015). Overoxidation of sulfenic acid can be prevented by S-glutathionylation (-SSG) or the formation of intra- and intermolecular disulfide bonds (Figure 2A). Within the intracellular environment, multiple proteins undergo S-glutathionylation (Zaffagnini et al. 2012); however, considering the negligible amount of GSH present in the apoplast, this PTM might not be as prevalent within this compartment. Importantly, cysteine residues are not the only physiological ROS targets. Oxidation of methionine residues has recently emerged as an additional mechanism for ROS perception (Figure 2A; Jacques et al., 2015). Furthermore, the metal-catalyzed dissipation of H2O2 produces highly reactive HO·, which reacts with amino acid residues, resulting in protein carbonylation and subsequent aggregation/inactivation.
Apoplastic posttranslational modifications might also involve reactions arising in the presence of nitric oxide (NO). NO is synthesized in the apoplast during the spontaneous reduction of nitrite at acidic pH and enzymatically by nitrite reductases and polyamine oxidases (Groß et al., 2013). Within the intracellular environment, NO reacts with GSH to form S-nitrosoglutathione (GSNO), which is thought to serve as a physiological NO donor (Corpas and Barroso, 2014). Treatment with GSNO leads to S-nitrosylation of cysteine residues within multiple proteins (Astier et al., 2012); however, the significance of this PTM in the apoplast environment remains to be determined. NO might also react with O2·− to form peroxynitrite (ONOO−). Peroxynitrite can react with cysteine thiolate anions, leading to the formation of cysteine sulfenic acid (Zeida et al., 2013). Furthermore, the formation of ONOO− results in the nitration of tyrosine residues; the existence of this reaction in the plant apoplast has only recently been documented (Szuba et al., 2015).
Like other posttranslational modifications, oxidative modifications can alter protein conformation or stability, thereby enabling ROS perception (Waszczak et al., 2015). However, the mere presence of a ROS-sensitive protein does not ensure a ROS perception event. In addition to physicochemical restrictions, the reactivity of redox-sensitive amino acids also depends on their steric accessibility, with solvent-exposed residues being more prone to oxidation than other residues. This accessibility depends on protein structure, which might be subject to dynamic changes controlled by other PTMs. For example, phosphorylation might provide a priming event that orients redox-sensitive amino acids into an accessible configuration (Scotto et al., 1998). Therefore, the activation of antagonistic or synergistic signaling networks might help control ROS perception events and provide additional flexibility for the modulation of signaling outcomes.
Apoplastic pH: An Overlooked Component of ROS Signaling?
Considering the pH dependence of thiol oxidation processes, the low pH in the apoplast might be the most crucial factor controlling cysteine-based ROS perception in this compartment. The resting pH of the apoplast typically ranges between 4.5 and 5.5. Therefore, it can be expected that under such conditions, most thiol groups are protonated and not prone to oxidation. However, RLK activation and ROS accumulation frequently coincide with apoplast alkalinization (Table 1). Alkalinization of the apoplast can occur in response to a plethora of stimuli, including exposure to pathogens (Felle et al., 2005), MAMPs, and DAMPs, as well as ligands that regulate plant development (Table 1). Furthermore, salt stress (Geilfus and Mühling, 2013), auxin treatment (Gjetting et al., 2012), and mechanical cues (Monshausen et al., 2009) are potent stimulators of apoplast alkalinization.
Apoplast alkalinization is attributed to the inhibition of plasma membrane H+-ATPases, which are responsible for establishing membrane potentials. Inhibition of H+-ATPases, combined with the activation of anion efflux channels, leads to plasma membrane depolarization and the subsequent activation of voltage-dependent K+ efflux channels. H+-ATPases are regulated by phosphorylation, with specific sites inhibiting or promoting the interaction with 14-3-3 proteins, a process required for the activation of the pump (Elmore and Coaker, 2011). The activity of plasma membrane H+-ATPases has been linked to RLK signaling. For example, perception of the RALF peptide by FER triggers phosphorylation and inhibition of the H+-ATPase AHA2, leading to RALF-dependent apoplast alkalinization (Haruta et al., 2014).
As discussed above, an increase in pH might promote the dissociation of thiols, thereby increasing the availability of thiolate anions for reactions with hydrogen peroxide. The pH-dependence of the reaction rates between H2O2 and free cysteine (Luo et al., 2005), glutathione (Finley et al., 1981), and thiols in proteins (Griffiths et al., 2002) is well documented. Therefore, apoplastic alkalinization could facilitate the oxidative modification of cysteines in redox-sensitive proteins. Importantly, such a mechanism would also restrict the perception of apoplastic ROS at low pH, thereby limiting the signaling role of background ROS levels which, due to limited apoplast redox buffering capacity, are likely to arise in the apoplast, even under control conditions.
RLKs MEDIATE APOPLASTIC ROS PERCEPTION (RLKs DOWNSTREAM OF ROS)
RLK ectodomains and other signaling molecules, such as RLK ligands, are exposed to the apoplastic environment and are therefore confronted with highly oxidizing conditions. Considering the highly reactive nature of ROS, a functional interaction between ROS and RLK signaling could arise in the apoplast. While transcriptomic responses to ROS are specific to the origin and type of ROS molecules (Vaahtera et al., 2014; Willems et al., 2016), it is unlikely that the properties of ROS alone can explain this signaling specificity. Instead, ROS signaling pathways can interact with parallel signaling cascades initiated by, for instance, the activation of RLKs (Figure 1) to fine-tune the signaling outcome. Despite the evidence supporting the interplay between ROS and RLKs, little is known about how plants perceive apoplastic ROS. Consequently, a major issue in plant ROS biology is the identification of sensors that transduce ROS signals into the cell for downstream responses.
Perception of Apoplastic ROS at the Place of Origin
At least two models for the perception of apoplastic ROS signals emerge from the literature. Based on the nature of the primary ROS sensor, the models (which are not mutually exclusive) can be classified as direct (the primary ROS sensor serves as an effector) or indirect (the ROS sensor conveys the signal to the effector). The direct ROS perception model assumes that apoplast-localized soluble or membrane-associated proteins exist that continuously monitor the redox status of the apoplast and directly relay the information to downstream signaling components, simultaneously fulfilling the roles of sensor and effector. This scenario could involve the direct oxidation of RLK ectodomains and the subsequent activation of signaling functions (Figure 2B). The indirect ROS perception model assumes that extracellular peptides or metabolites exist that, upon oxidation, bind to RLKs (Figure 2B). Alternatively, the oxidized ROS sensor proteins might oxidize RLK ectodomains via a redox relay mechanism (Figure 2B).
The oxidation of RLK ectodomains or the binding of oxidized peptides or metabolites may lead to differential RLK complex formation, phosphorylation events, and subsequent signal transduction (Figure 2B). While such signaling components have not yet been identified, CRKs have frequently been associated with processes involving apoplastic ROS accumulation. Transcriptional regulation by apoplastic ROS, the presence of conserved cysteines in the ectodomain, and functional analysis of crk mutants suggest that CRKs are intricately involved in ROS signaling (Chen, 2001; Bourdais et al., 2015). The recent report of a cysteine-dependent function for CRK28 (Yadeta et al., 2016) further supports this hypothesis. However, it will be critical to investigate whether the cysteines in the CRK ectodomains fulfill signaling functions or are purely relevant for the structure of the domain.
Other data support the relevance of interactions between plant peptides and RLKs in ROS perception events (Figure 2B). One example involves the extracellular GRIM REAPER (GRI) protein (Wrzaczek et al., 2015). Upon accumulation of ROS in the apoplast, GRI is cleaved by METACASPASE9. The resulting 11-amino acid GRI peptide binds to PRK5 to initiate cell death (Wrzaczek et al., 2015). While the mechanisms controlling GRI proteolysis are currently unknown, it is unlikely that GRI/PRK5 signaling is the sole route for initiating cell death in response to apoplastic ROS. Oxidatively modified metabolites or cellular components might form another class of RLK ligands; the growing portfolio of cell wall-derived signaling molecules (Wolf et al., 2012) supports the existence of such mechanisms in the apoplast.
Multiple lines of evidence support the signaling function of antioxidant proteins; for example, ROS scavengers such as peroxiredoxins (Sobotta et al., 2015) and glutathione peroxidases (Miao et al., 2006) transduce oxidative signals to effector proteins via redox relay. These enzymes undergo H2O2-dependent oxidation. Once oxidized, the enzymes interact with and oxidize effector proteins, thereby altering their biological activity (Figure 2B). The sensitivity of such mechanisms stems from the high rate constants for reactions between H2O2 and specialized ROS scavengers (Marinho et al., 2014). Importantly, in many cases, an effector protein might not be limited to interactions with a single sensor. Such a lack of selectivity could help merge multiple inputs into a single output reaction, thus allowing for signal integration.
Despite the physiological importance of apoplastic ROS accumulation, until recently, virtually no targets for apoplastic ROS had been documented. However, the discovery of the activation of voltage-dependent STELAR K+ OUTWARD RECTIFIER (SKOR) by H2O2 (Garcia-Mata et al., 2010) provided compelling evidence for the occurrence of such signaling. In SKOR, channel opening induced by membrane depolarization exposes Cys-168 to the apoplastic environment, thereby allowing H2O2 to reversibly enhance SKOR activity (Garcia-Mata et al., 2010). Future research into apoplastic ROS perception will likely result in the identification of additional proteins that are regulated in a similar ROS-dependent manner.
Intracellular Sensing of Apoplastic ROS
The activity of apoplastic ROS might not be limited to the extracellular environment. Indeed, a growing body of evidence supports the intracellular sensing of apoplastic ROS (as depicted in Figures 1 and 2B). Import of H2O2 through aquaporins is a well-recognized mechanism in animal cells (Satooka and Hara-Chikuma, 2016). However, while the H2O2 permeability of plant aquaporins was documented a decade ago (Bienert et al., 2007), its functional significance has been described only recently (Tian et al., 2016). It remains unclear how imported H2O2 avoids intracellular ROS scavengers and how cells differentiate between imported and intracellular ROS. Conceptually, this could involve a localized decrease in ROS scavenging capacity modulated by RLKs. In animal cells, the activation of plasma membrane-localized receptors triggers the phosphorylation and inhibition of Peroxiredoxin I, allowing limited H2O2 buildup and oxidation events to occur (Woo et al., 2010). Alternatively, overoxidation of intracellular ROS scavengers, i.e., peroxiredoxins, could enable local accumulation of imported H2O2 (“floodgate theory”; Wood et al., 2003). Both mechanisms could lead to the formation of H2O2 maxima (Mishina et al., 2011). Interestingly, a number of membrane proteins, including RLKs, localize to distinct membrane microdomains in plants (Jarsch et al., 2014). The distance of a protein to the site of ROS maxima affects its likelihood of becoming a target, which allows ROS perception events to be controlled through spatial-temporal dynamics, i.e., positioning sensors in close proximity to producers. Thus, the joining of components of dedicated signaling networks together by microdomains might represent one way in which nearby effectors are modified and signals are passed from the ROS source in a concentration-dependent gradient. This model is in agreement with the recent discovery of oxidative PTMs of intracellular domains of cell surface receptors in animals (Truong et al., 2016). Conceptually, the hypothesis of local ROS maxima is well established, but the proposed model does not provide a sufficient explanation for the problem of differentiation between intracellular and apoplastic ROS sources. Considering the short lifetime of ROS, the strong cytoplasmic redox buffering capacity, and the level of customization observed in response to different forms of ROS, the apoplastic perception scenario appears more plausible. However, the ROS perception models described above are not mutually exclusive, and they all likely contribute to downstream signaling.
Are RLKs and ROS Modules Integrated during Long-Distance Signaling?
Research on both RLK and ROS signaling frequently focuses on local processes. Such approaches are justified by the physiological relevance of immediate local responses within specific cells and their immediate neighbors. However, systemic signal transduction by peptides, non-peptide plant hormones, carbohydrates, and other molecules plays important roles in relaying information between tissues and organs in a plethora of environmental and developmental responses.
During long-distance nutritional signaling, secreted peptides transported through the vasculature are perceived by RLKs (Okamoto et al., 2016). On the other hand, ROS have traditionally been considered unsuitable for systemic signaling due to their short lifetimes and high reactivity. However, in addition to local ROS production, treatments such as wounding and abiotic cues induce a rapid self-propagating “ROS wave” in the apoplast through the activation of RBOHD in each cell along a systemic path (Miller et al., 2009). It is conceivable that an RLK-dependent ROS burst might initiate a ROS wave as, for instance, the activation of PEPR signaling has been suggested to connect local and systemic responses (Ross et al., 2014). Ca2+ is a key regulator of the ROS wave (Gilroy et al., 2016). Interestingly, Nissen et al. (2016) proposed that the CrRLK1L kinase family could be involved in integrating ROS and Ca2+ signaling by monitoring the status of the cell wall, at least locally. However, since apoplastic ROS can also affect cell wall composition and integrity (Lee et al., 2013), CrRLK1L kinases might participate in the propagation of ROS waves. Furthermore, apoplastic pH changes also contribute to the systemic signaling wave (Monshausen et al., 2007; Geilfus and Mühling, 2013). As apoplastic alkalinization depends on the inactivation of plasma membrane H+-ATPases, it is conceivable that RLKs might also control this aspect of systemic signaling.
These collective data suggest that RLK and ROS signal transduction functions are integrated in long-distance signaling. However, the molecular details of whether and how RLKs might be connected to systemic ROS and other signaling elements await elucidation.
GUARD CELLS, A MODEL FOR ROS AND MAMP/DAMP PERCEPTION RESEARCH
As evident from the previous sections, many mechanisms that control RLK-ROS signaling processes are not completely understood. Research often benefits from the use of well-established model systems that offer a basic framework for future discoveries. In the context of RLK-ROS crosstalk, guard cell signaling leading to stomatal closure is particularly well suited for this purpose. Guard cells serve as a convenient system for studying apoplastic signaling, as the lack of plasmodesmata isolates their cytoplasm from that of the neighboring cells. Processes controlling stomatal aperture respond to a multitude of stimuli to balance photosynthetic CO2 uptake with water loss. Another core function of guard cells is to control stomatal immunity, which restricts the entry of pathogens into the plant (Melotto et al., 2006). The control of stomatal closure unites multiple signaling molecules/events linked to RLK signaling (Figure 3), such as apoplastic ROS (Sierla et al., 2016), Ca2+ signaling (Laanemets et al., 2013), and apoplast alkalization (Merlot et al., 2007).
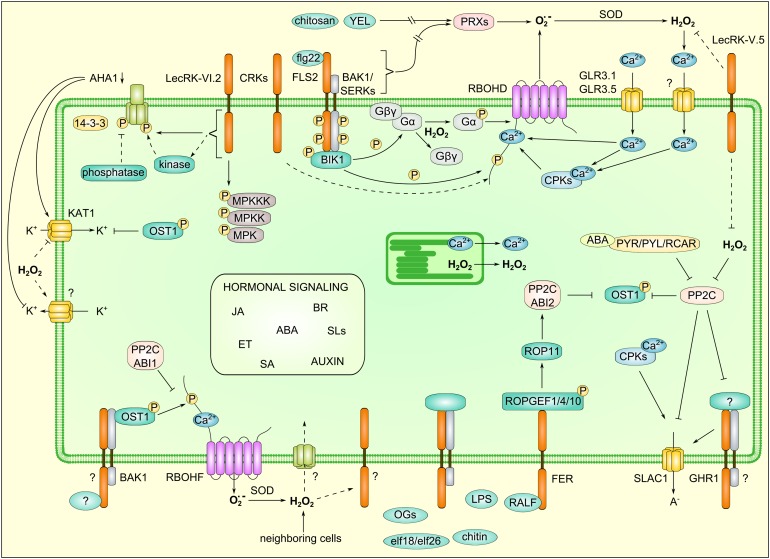
Figure 3.
Integration of RLKs and ROS Signaling in the Control of Guard Cell Closure.
Stomatal closure is controlled by a complex interplay of signaling pathways dependent on ABA and MAMP signaling that involve numerous RLK- and ROS-dependent events. The figure displays a single guard cell with signaling components that have been shown to control stomatal closure. RLKs are involved in initial MAMP perception, regulation of ROS production, and control of ion channels. Detailed descriptions of specific regulatory mechanisms are in the main text. ET, ethylene; GLR, GLUTAMATE RECEPTOR; JA, jasmonates; KAT1, POTASSIUM CHANNEL IN ARABIDOPSIS THALIANA1; PP2C, PROTEIN PHOSPHATASE 2C; PYR, PYRABACTIN RESISTANCE; PYL, PYR1-LIKE; RCAR, REGULATORY COMPONENTS OF ABA RECEPTOR; SA, salicylic acid; SERK, SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE; SLs, strigolactones; SOD, superoxide dismutase; YEL, yeast elicitor.
Stomatal Closure Requires a Plethora of Signaling Events
The initial signal perception events controlling stomatal closure in response to abiotic stimuli, such as high CO2, low humidity, darkness, and air pollutants, use different receptors. However, the downstream processes involve components of a relatively well-studied stomatal ABA signaling pathway, with the protein kinase OST1/SnRK2.6 acting as a regulatory hub (Figure 3). In response to ABA, OST1 presumably phosphorylates RBOHF (Sirichandra et al., 2009), which in turn triggers the accumulation of apoplastic ROS. In addition to phosphorylation, the activation of RBOHs depends on Ca2+ transients provided by ROS-independent Ca2+ channels (Kong et al., 2016). While RBOHF is the major RBOH isoform involved in ABA-induced stomatal closure, RBOHD also contributes to this process (Kwak et al., 2003). However, it is unclear whether OST1 also phosphorylates RBOHD. Apoplastic ROS accumulation is both necessary and sufficient to induce stomatal closure by activating ROS-dependent inward-directed Ca2+ channels (Kwak et al., 2003). The increase in cytosolic Ca2+ concentration activates a plethora of calcium-dependent protein kinases that phosphorylate RBOHD and RBOHF (among other substrates) to amplify ROS production (Sierla et al., 2016). Other key substrates of calcium-dependent protein kinases include anion channels, with SLOW ANION CHANNEL-ASSOCIATED1 (SLAC1) serving as a major executor of stomatal closure (Vahisalu et al., 2008). Under most conditions, the activation of SLAC1 requires the LRR-RLK GUARD CELL HYDROGEN PEROXIDE-RESISTANT1 (GHR1; Hua et al., 2012). However, the precise functions of GHR1 are not yet sufficiently understood. SLAC1-mediated efflux of anions, combined with the deactivation of the H+-ATPase AHA1 (Merlot et al., 2007), lead to guard cell plasma membrane depolarization and the activation of unidentified voltage-dependent K+ efflux channels, ultimately culminating in stomatal closure (Figure 3).
RLKs Play Multiple Roles in Controlling Stomatal Immunity
The control of stomatal closure in response to MAMPs and DAMPs involves the use of similar signaling components, many of which are not sufficiently understood. Despite the importance of stomatal immunity, as discussed by Arnaud and Hwang (2015), the requirement of the respective MAMP/DAMP receptors has been ascertained only for FLS2 (Melotto et al., 2006). Therefore, it would be interesting to investigate the roles of the respective RLKs (Table 1) in the initiation of stomatal closure. According to publicly available gene expression data, many RLKs necessary for MAMPs/DAMPs perception are present in guard cells. However, considering the central role of apoplastic ROS, it is conceivable that ROS produced upon MAMP/DAMP perception in neighboring epidermal pavement cells contribute to stomatal closure.
The central role of OST1 in stomatal immunity is demonstrated by the phenotypes of ost1 mutants that fail to respond to flg22 and lipopolysaccharides (LPSs; Melotto et al., 2006; Guzel Deger et al., 2015). Interestingly, BAK1, a coreceptor for many RLKs, activates OST1 in response to ABA, but this activation is inhibited by ABI1 and by treatment with BR (Shang et al., 2016). However, the identity of RLKs associated with the BAK1-OST1 complex is currently unknown. Moreover, it remains to be investigated whether the interaction between BAK1 and OST1 can be triggered by the application of MAMPs.
While RBOHF plays an important role in ABA-induced stomatal closure, RBOHD is the major isoform involved in stomatal immunity (Macho et al., 2012; Kadota et al., 2014), and multiple studies describe differential RBOHD phosphorylation in response to MAMPs (Nühse et al., 2007; Benschop et al., 2007; Kadota et al., 2014). In response to MAMP treatment, EFR and FLS2 activate BIK1, which plays a major role in stimulating ROS production by phosphorylating Ser-39, Ser-339, and Ser-343 of RBOHD (Kadota et al., 2014). Consequently, complementation of rbohD plants with RBOHDS39A, S339A, S343A does not restore stomatal responses to elf18 and flg22 (Kadota et al., 2014). However, the activation of BIK1 and apoplastic ROS production are not sufficient to complete the process of stomatal closure. Plants deficient in LecRK-VI.2, an RLK involved in the FLS2 receptor complex, cannot close their stomata in response to flg22 and elf26, despite successful activation of BIK1 and apoplastic ROS production (Singh et al., 2012). ABA-induced stomatal closure is intact in lecrk-VI.2, indicating that LecRK-VI.2 acts upstream or independently of ABA signaling. Another RLK, LecRK-V.5, also negatively regulates stomatal closure in response to bacterial pathogens and MAMPs (Desclos-Theveniau et al., 2012). The lack of LecRK-V.5 results in constitutive stomatal closure due to the accumulation of ROS within guard cells. Conversely, plants overexpressing LecRK-V.5 cannot initiate ROS production in response to MAMPs. Consequently, LecRK-V.5 overexpressors are deficient in stomatal closure in response to flg22, elf26, and LPS (Desclos-Theveniau et al., 2012). Collectively, these data position LecRK-V.5 as a negative regulator of stomatal immunity, acting through the inhibition of ROS accumulation. Interestingly, LecRK-V.5 appears to also be involved in the ABA signaling pathway, as LecRK-V.5 overexpression partially impairs stomatal closure in response to ABA treatment (Desclos-Theveniau et al., 2012). As described earlier, FER, which stimulates ROS production, can also negatively regulate ABA-induced ROS accumulation in guard cells (Yu et al., 2012). Considering the different roles of RBOHF and RBOHD, guard cells could serve as a model for further studies on the functions and modes of activation of these isoforms. However, RBOHs are not the only sources of apoplastic ROS, as the ROS burst in response to yeast elicitor (Khokon et al., 2010a), chitosan (Khokon et al., 2010b), and cytokinins (Arnaud et al., 2017) mainly depends on the activity of apoplastic peroxidases. Taken together, these findings highlight the central role of RLKs in controlling guard cell ROS production. However, despite the physiological relevance of guard cell ROS signaling, the initial ROS perception events remain elusive (Figure 3).
As is the case in the responses to many abiotic stimuli, MAMP-induced stomatal closure requires apoplast alkalinization, which is achieved by the deactivation of AHA1. Plants with constitutively active AHA1 fail to initiate stomatal immunity in response to flg22, LPS, and virulent Pseudomonas syringae (Liu et al., 2009). This finding is in agreement with the observation that rapid phosphorylation/dephosphorylation of AHAs occurs upon MAMP treatment (Nühse et al., 2007; Benschop et al., 2007). The identities of guard cell kinases and phosphatases that regulate AHA1 activity downstream of MAMP perception are currently unknown. Similarly, apart from the well-established function of apoplast alkalization in the activation of voltage-dependent K+out channels, the precise roles of apoplast alkalization are not fully understood. Considering the effect of apoplastic pH on oxidative posttranslational modifications of cysteine residues, it is conceivable that AHA1 might help regulate apoplastic ROS signaling processes.
Additionally, multiple hormones, pathogen effectors, and plant metabolites play important roles in regulating guard cell signaling, as recently reviewed by Arnaud and Hwang (2015) and Ye and Murata (2016).
PERSPECTIVES
Traditionally, ROS were viewed as toxic by-products of cellular metabolism or antimicrobial compounds. Currently, however, ROS are recognized as signaling molecules that exert control over many plant processes. Studies in the past decade have led to the identification of multiple mechanisms that plants use to modulate ROS production. RLK and ROS signaling are inextricably linked: RLKs have emerged as key regulators of apoplastic ROS bursts and ROS production downstream of RLK activation is orchestrated through a complex interplay of downstream signaling components. The functional conservation of these signaling modules highlights the central importance of the ROS burst in the responses of plants to extracellular cues. In the future, it will be important to identify mechanisms that allow plants to sense ROS signals; RLKs are emerging as potential regulators of these processes. We anticipate that the development of ROS-sensitive probes that enable high-resolution visualization of extra- and intracellular ROS will facilitate this research. Furthermore, as ROS-dependent responses are intertwined with intracellular signaling governed by RLK kinase domains, it will be equally important to identify components that function independently of ROS accumulation. In this context, it will be crucial to identify RLK phosphorylation substrates other than ROS-producing enzymes. Future studies on the interactions between these responses could increase our understanding of ROS signaling specificity. Research on the integration of RLK and ROS signaling with hormonal homeostasis at the whole-plant level will provide a holistic view of long-distance signaling processes. Ultimately, modulation of ROS-RLK crosstalk will allow adjusting plant growth and environmental responses in a dynamic environment.
Supplementary Material
Acknowledgments
All authors are members of the Centre of Excellence in the Molecular Biology of Primary Producers (2014-2019), which is funded by the Academy of Finland (decisions #271832 and #307335). C.W. is funded by the Academy of Finland (decision #294580). Research in the M.W. laboratory is funded by the Academy of Finland (decisions #275632 and #283139). We thank Adrien Gauthier for discussions and help with the conception of this manuscript. We also thank Mikael Brosché, Julia Krasensky, Luis Orlando Morales, Kirk Overmyer, Alexey Shapiguzov, Julia Vainonen, and Melinka Alonso Butenko for commenting on the manuscript. We apologize to all colleagues whose work we could not incorporate due to space constraints.
AUTHOR CONTRIBUTIONS
All authors contributed to the writing of the article.
Glossary
- RLK
receptor-like kinase
- ROS
reactive oxygen species
- RBOH
respiratory burst oxidase homolog
- MAMP
microbe-associated molecular pattern
- DAMP
damage-associated molecular pattern
- RLCK
receptor-like cytoplasmic protein kinase
- LRR
leucine-rich repeat
- CRK
cysteine-rich receptor-like kinase
- ABA
abscisic acid
- BR
brassinosteroid
- MAPK
mitogen-activated protein kinase
- AA
ascorbate
- PTM
posttranslational modification
- LPS
lipopolysaccharide
Footnotes
Articles can be viewed without a subscription.
References
- Ahlfors R., Macioszek V., Rudd J., Brosché M., Schlichting R., Scheel D., Kangasjärvi J. (2004). Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 40: 512–522. [DOI] [PubMed] [Google Scholar]
- Akamatsu A., Wong H.L., Fujiwara M., Okuda J., Nishide K., Uno K., Imai K., Umemura K., Kawasaki T., Kawano Y., Shimamoto K. (2013). An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 module is an essential early component of chitin-induced rice immunity. Cell Host Microbe 13: 465–476. [DOI] [PubMed] [Google Scholar]
- Allègre M., Héloir M.-C., Trouvelot S., Daire X., Pugin A., Wendehenne D., Adrian M. (2009). Are grapevine stomata involved in the elicitor-induced protection against downy mildew? Mol. Plant Microbe Interact. 22: 977–986. [DOI] [PubMed] [Google Scholar]
- Ao Y., Li Z., Feng D., Xiong F., Liu J., Li J.F., Wang M., Wang J., Liu B., Wang H.B. (2014). OsCERK1 and OsRLCK176 play important roles in peptidoglycan and chitin signaling in rice innate immunity. Plant J. 80: 1072–1084. [DOI] [PubMed] [Google Scholar]
- Arnaud D., Hwang I. (2015). A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol. Plant 8: 566–581. [DOI] [PubMed] [Google Scholar]
- Arnaud D., Lee S., Takebayashi Y., Choi D., Choi J., Sakakibara H., Hwang I. (2017). Regulation of reactive oxygen species homeostasis by cytokinins modulates stomatal immunity in Arabidopsis. Plant Cell 29: 543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier J., Kulik A., Koen E., Besson-Bard A., Bourque S., Jeandroz S., Lamotte O., Wendehenne D. (2012). Protein S-nitrosylation: what’s going on in plants? Free Radic. Biol. Med. 53: 1101–1110. [DOI] [PubMed] [Google Scholar]
- Benschop J.J., Mohammed S., O’Flaherty M., Heck A.J.R., Slijper M., Menke F.L.H. (2007). Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol. Cell. Proteomics 6: 1198–1214. [DOI] [PubMed] [Google Scholar]
- Berken A., Thomas C., Wittinghofer A. (2005). A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 436: 1176–1180. [DOI] [PubMed] [Google Scholar]
- Bienert G.P., Møller A.L.B., Kristiansen K.A., Schulz A., Møller I.M., Schjoerring J.K., Jahn T.P. (2007). Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282: 1183–1192. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A., Franck C.M., Lituiev D.S., Grossniklaus U. (2015). Receptor-like cytoplasmic kinase MARIS functions downstream of CrRLK1L-dependent signaling during tip growth. Proc. Natl. Acad. Sci. USA 112: 12211–12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Kessler S.A., Grossniklaus U. (2011). The walls have ears: the role of plant CrRLK1Ls in sensing and transducing extracellular signals. J. Exp. Bot. 62: 1581–1591. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A., Lituiev D.S., Nestorova A., Franck C.M., Thirugnanarajah S., Grossniklaus U. (2013). ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. 11: e1001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker F.L., Burkey K.O., Jones A.M. (2012). Re-evaluating the role of ascorbic acid and phenolic glycosides in ozone scavenging in the leaf apoplast of Arabidopsis thaliana L. Plant Cell Environ. 35: 1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdais G., et al. ; CRK Consortium (2015). Large-scale phenomics identifies primary and fine-tuning roles for CRKs in responses related to oxidative stress. PLoS Genet. 11: e1005373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutus A., Sicilia F., Macone A., Cervone F., De Lorenzo G. (2010). A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. USA 107: 9452–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko M.A., Wildhagen M., Albert M., Jehle A., Kalbacher H., Aalen R.B., Felix G. (2014). Tools and strategies to match peptide-ligand receptor pairs. Plant Cell 26: 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Liang Y., Tanaka K., Nguyen C.T., Jedrzejczak R.P., Joachimiak A., Stacey G. (2014). The kinase LYK5 is a major chitin receptor in Arabidopsis and forms a chitin-induced complex with related kinase CERK1. eLife 3: e03766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Gu Y., Ma H., Yang Z. (2013). AtPRK2 promotes ROP1 activation via RopGEFs in the control of polarized pollen tube growth. Mol. Plant 6: 1187–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. (2001). A superfamily of proteins with novel cysteine-rich repeats. Plant Physiol. 126: 473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D., Bauer Z., Regenass M., Boller T., Felix G. (2006). The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18: 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.K., Larue C.T., Chevalier D., Wang H., Jinn T.-L., Zhang S., Walker J.C. (2008). Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 105: 15629–15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas F.J., Barroso J.B. (2014). Peroxynitrite (ONOO-) is endogenously produced in arabidopsis peroxisomes and is overproduced under cadmium stress. Ann. Bot. (Lond.) 113: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A., Drago I., Behera S., Zottini M., Pizzo P., Schroeder J.I., Pozzan T., Lo Schiavo F. (2010). H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J. 62: 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto D., Zipfel C. (2016). Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16: 537–552. [DOI] [PubMed] [Google Scholar]
- Daudi A., Cheng Z., O’Brien J.A., Mammarella N., Khan S., Ausubel F.M., Bolwell G.P. (2012). The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X.-G., Zhu T., Peng X.-J., Xi D.-H., Guo H., Yin Y., Zhang D.-W., Lin H.-H. (2016). Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana. Sci. Rep. 6: 20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X.-G., Zhu T., Zhang D.-W., Lin H.-H. (2015). The alternative respiratory pathway is involved in brassinosteroid-induced environmental stress tolerance in Nicotiana benthamiana. J. Exp. Bot. 66: 6219–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denness L., McKenna J.F., Segonzac C., Wormit A., Madhou P., Bennett M., Mansfield J., Zipfel C., Hamann T. (2011). Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 156: 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclos-Theveniau M., Arnaud D., Huang T.Y., Lin G.J., Chen W.Y., Lin Y.C., Zimmerli L. (2012). The Arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathog. 8: e1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R., et al. (2008). The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS One 3: e2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q., Kita D., Johnson E.A., Aggarwal M., Gates L., Wu H.M., Cheung A.Y. (2014). Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 5: 3129. [DOI] [PubMed] [Google Scholar]
- Duan Q., Kita D., Li C., Cheung A.Y., Wu H.-M. (2010). FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. USA 107: 17821–17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore J.M., Coaker G. (2011). The role of the plasma membrane H+-ATPase in plant-microbe interactions. Mol. Plant 4: 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans N.H., McAinsh M.R., Hetherington A.M., Knight M.R. (2005). ROS perception in Arabidopsis thaliana: the ozone-induced calcium response. Plant J. 41: 615–626. [DOI] [PubMed] [Google Scholar]
- Felix G., Duran J.D., Volko S., Boller T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18: 265–276. [DOI] [PubMed] [Google Scholar]
- Felix G., Regenass M., Boller T. (1993). Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 4: 307–316. [Google Scholar]
- Felle H.H., Herrmann A., Hanstein S., Hückelhoven R., Kogel K.-H. (2004). Apoplastic pH signaling in barley leaves attacked by the powdery mildew fungus Blumeria graminis f. sp. hordei. Mol. Plant Microbe Interact. 17: 118–123. [DOI] [PubMed] [Google Scholar]
- Felle H.H., Herrmann A., Hückelhoven R., Kogel K.-H. (2005). Root-to-shoot signalling: apoplastic alkalinization, a general stress response and defence factor in barley (Hordeum vulgare). Protoplasma 227: 17–24. [DOI] [PubMed] [Google Scholar]
- Ferrer-Sueta G., Manta B., Botti H., Radi R., Trujillo M., Denicola A. (2011). Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 24: 434–450. [DOI] [PubMed] [Google Scholar]
- Finley J.W., Wheeler E.L., Witt S.C. (1981). Oxidation of glutathione by hydrogen peroxide and other oxidizing agents. J. Agric. Food Chem. 29: 404–407. [DOI] [PubMed] [Google Scholar]
- Galletti R., Denoux C., Gambetta S., Dewdney J., Ausubel F.M., De Lorenzo G., Ferrari S. (2008). The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol. 148: 1695–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C., Wang J., Gajdanowicz P., Gonzalez W., Hills A., Donald N., Riedelsberger J., Amtmann A., Dreyer I., Blatt M.R. (2010). A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. J. Biol. Chem. 285: 29286–29294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geilfus C.-M., Mühling K.-H. (2013). Ratiometric monitoring of transient apoplastic alkalinizations in the leaf apoplast of living Vicia faba plants: chloride primes and PM-H+-ATPase shapes NaCl-induced systemic alkalinizations. New Phytol. 197: 1117–1129. [DOI] [PubMed] [Google Scholar]
- Gerber I.B., Zeidler D., Durner J., Dubery I.A. (2004). Early perception responses of Nicotiana tabacum cells in response to lipopolysaccharides from Burkholderia cepacia. Planta 218: 647–657. [DOI] [PubMed] [Google Scholar]
- Gilroy S., Białasek M., Suzuki N., Górecka M., Devireddy A.R., Karpiński S., Mittler R. (2016). ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 171: 1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjetting K.S.K., Ytting C.K., Schulz A., Fuglsang A.T. (2012). Live imaging of intra- and extracellular pH in plants using pHusion, a novel genetically encoded biosensor. J. Exp. Bot. 63: 3207–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L., Boller T. (2000). FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5: 1003–1011. [DOI] [PubMed] [Google Scholar]
- Greeff C., Roux M., Mundy J., Petersen M. (2012). Receptor-like kinase complexes in plant innate immunity. Front. Plant Sci. 3: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S.W., King J., Cooney C.L. (2002). The reactivity and oxidation pathway of cysteine 232 in recombinant human α 1-antitrypsin. J. Biol. Chem. 277: 25486–25492. [DOI] [PubMed] [Google Scholar]
- Groß F., Durner J., Gaupels F. (2013). Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 4: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust A.A., Biswas R., Lenz H.D., Rauhut T., Ranf S., Kemmerling B., Götz F., Glawischnig E., Lee J., Felix G., Nürnberger T. (2007). Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J. Biol. Chem. 282: 32338–32348. [DOI] [PubMed] [Google Scholar]
- Guzel Deger A., Scherzer S., Nuhkat M., Kedzierska J., Kollist H., Brosché M., Unyayar S., Boudsocq M., Hedrich R., Roelfsema M.R.G. (2015). Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol. 208: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M., Sabat G., Stecker K., Minkoff B.B., Sussman M.R. (2014). A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrick L.L., Torsethaugen G., Assmann S.M. (2006). Effect of brassinolide, alone and in concert with abscisic acid, on control of stomatal aperture and potassium currents of Vicia faba guard cell protoplasts. Physiol. Plant. 128: 134–143. [Google Scholar]
- Hohmann U., Lau K., Hothorn M. (2017). The structural basis of ligand perception and signal activation by receptor linases. Annu. Rev. Plant Biol. 68: 10.1146/annurev-arplant-042916-040957. [DOI] [PubMed] [Google Scholar]
- Hou S., Wang X., Chen D., Yang X., Wang M., Turrà D., Di Pietro A., Zhang W. (2014). The secreted peptide PIP1 amplifies immunity through receptor-like kinase 7. PLoS Pathog. 10: e1004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D., Wang C., He J., Liao H., Duan Y., Zhu Z., Guo Y., Chen Z., Gong Z. (2012). A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker A., Pearce G., Ryan C.A. (2006). An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. USA 103: 10098–10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques S., Ghesquière B., De Bock P.-J., Demol H., Wahni K., Willems P., Messens J., Van Breusegem F., Gevaert K. (2015). Protein methionine sulfoxide dynamics in Arabidopsis thaliana under oxidative stress. Mol. Cell. Proteomics 14: 1217–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsch I.K., Konrad S.S.A., Stratil T.F., Urbanus S.L., Szymanski W., Braun P., Braun K.-H., Ott T. (2014). Plasma membranes are subcompartmentalized into a plethora of coexisting and diverse microdomains in Arabidopsis and Nicotiana benthamiana. Plant Cell 26: 1698–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.M., et al. (2014). Border control--a membrane-linked interactome of Arabidopsis. Science 344: 711–716. [DOI] [PubMed] [Google Scholar]
- Jones M.A., Raymond M.J., Yang Z., Smirnoff N. (2007). NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J. Exp. Bot. 58: 1261–1270. [DOI] [PubMed] [Google Scholar]
- Joo J.H., Wang S., Chen J.G., Jones A.M., Fedoroff N. V (2005). Different signaling and cell death roles of heterotrimeric G Protein α and β Subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17: 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y., Sklenar J., Derbyshire P., Stransfeld L., Asai S., Ntoukakis V., Jones J.D., Shirasu K., Menke F., Jones A., Zipfel C. (2014). Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54: 43–55. [DOI] [PubMed] [Google Scholar]
- Kärkönen A., Kuchitsu K. (2015). Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112: 22–32. [DOI] [PubMed] [Google Scholar]
- Kaya H., et al. (2014). Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26: 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S.A., Lindner H., Jones D.S., Grossniklaus U. (2015). Functional analysis of related CrRLK1L receptor-like kinases in pollen tube reception. EMBO Rep. 16: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokon M.A.R., Hossain M.A., Munemasa S., Uraji M., Nakamura Y., Mori I.C., Murata Y. (2010a). Yeast elicitor-induced stomatal closure and peroxidase-mediated ROS production in Arabidopsis. Plant Cell Physiol. 51: 1915–1921. [DOI] [PubMed] [Google Scholar]
- Khokon M.A.R., Uraji M., Munemasa S., Okuma E., Nakamura Y., Mori I.C., Murata Y. (2010b). Chitosan-induced stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis. Biosci. Biotechnol. Biochem. 74: 2313–2315. [DOI] [PubMed] [Google Scholar]
- Kim B.H., Kim S.Y., Nam K.H. (2012). Genes encoding plant-specific class III peroxidases are responsible for increased cold tolerance of the brassinosteroid-insensitive 1 mutant. Mol. Cells 34: 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler B.E., Bloem E., Zellnig G., Zechmann B. (2013). High resolution imaging of subcellular glutathione concentrations by quantitative immunoelectron microscopy in different leaf areas of Arabidopsis. Micron 45: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D., et al. (2016). L-Met activates Arabidopsis GLR Ca2+ channels upstream of ROS production and regulates stomatal movement. Cell Reports 17: 2553–2561. [DOI] [PubMed] [Google Scholar]
- Krol E., Mentzel T., Chinchilla D., Boller T., Felix G., Kemmerling B., Postel S., Arents M., Jeworutzki E., Al-Rasheid K.A.S., Becker D., Hedrich R. (2010). Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J. Biol. Chem. 285: 13471–13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze G., Zipfel C., Robatzek S., Niehaus K., Boller T., Felix G. (2004). The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J.M., Mori I.C., Pei Z.M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D.G., Schroeder J.I. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22: 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanemets K., Brandt B., Li J., Merilo E., Wang Y.-F., Keshwani M.M., Taylor S.S., Kollist H., Schroeder J.I. (2013). Calcium-dependent and -independent stomatal signaling network and compensatory feedback control of stomatal opening via Ca2+ sensitivity priming. Plant Physiol. 163: 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le M.H., Cao Y., Zhang X.C., Stacey G. (2014). LIK1, a CERK1-interacting kinase, regulates plant immune responses in Arabidopsis. PLoS One 9: e102245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Choi H., Suh S., Doo I.S., Oh K.Y., Choi E.J., Schroeder Taylor A.T., Low P.S., Lee Y. (1999). Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 121: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Rubio M.C., Alassimone J., Geldner N. (2013). A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412. [DOI] [PubMed] [Google Scholar]
- Li J., Chory J. (1997). A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938. [DOI] [PubMed] [Google Scholar]
- Li J., Wen J., Lease K.A., Doke J.T., Tax F.E., Walker J.C. (2002). BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222. [DOI] [PubMed] [Google Scholar]
- Liang X., Ding P., Lian K., Wang J., Ma M., Li L., Li L., Li M., Zhang X., Chen S., Zhang Y., Zhou J.M. (2016). Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 receptor. eLife 5: e13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Li B., Lu D., Chen S., Zhu N., He P., Shan L. (2014). Tyrosine phosphorylation of protein kinase complex BAK1/BIK1 mediates Arabidopsis innate immunity. Proc. Natl. Acad. Sci. USA 111: 3632–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Lu D., Gao X., Jiang S., Ma X., Wang Z., Mengiste T., He P., Shan L. (2013). Inverse modulation of plant immune and brassinosteroid signaling pathways by the receptor-like cytoplasmic kinase BIK1. Proc. Natl. Acad. Sci. USA 110: 12114–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z.-J.D., Liebrand T.W.H., Yadeta K.A., Coaker G. (2015). PBL13 is a serine/threonine protein kinase that negatively regulates Arabidopsis immune responses. Plant Physiol. 169: 2950–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner H., Müller L.M., Boisson-Dernier A., Grossniklaus U. (2012). CrRLK1L receptor-like kinases: not just another brick in the wall. Curr. Opin. Plant Biol. 15: 659–669. [DOI] [PubMed] [Google Scholar]
- Liu J., Elmore J.M., Fuglsang A.T., Palmgren M.G., Staskawicz B.J., Coaker G. (2009). RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol. 7: e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Liu Z., Song C., Hu Y., Han Z., She J., Fan F., Wang J., Jin C., Chang J., Zhou J.-M., Chai J. (2012). Chitin-induced dimerization activates a plant immune receptor. Science 336: 1160–1164. [DOI] [PubMed] [Google Scholar]
- Liu Z., Wu Y., Yang F., Zhang Y., Chen S., Xie Q., Tian X., Zhou J.-M. (2013). BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc. Natl. Acad. Sci. USA 110: 6205–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Wu S., Gao X., Zhang Y., Shan L., He P. (2010). A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl. Acad. Sci. USA 107: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Smith S.W., Anderson B.D. (2005). Kinetics and mechanism of the reaction of cysteine and hydrogen peroxide in aqueous solution. J. Pharm. Sci. 94: 304–316. [DOI] [PubMed] [Google Scholar]
- Macho A.P., Boutrot F., Rathjen J.P., Zipfel C. (2012). Aspartate oxidase plays an important role in Arabidopsis stomatal immunity. Plant Physiol. 159: 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. (2014). Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2: 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A., et al. (2012). Tackling drought stress: receptor-like kinases present new approaches. Plant Cell 24: 2262–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila H., Khorobrykh S., Havurinne V., Tyystjärvi E. (2015). Reactive oxygen species: Reactions and detection from photosynthetic tissues. J. Photochem. Photobiol. B 152: 176–214. [DOI] [PubMed] [Google Scholar]
- Melotto M., Underwood W., Koczan J., Nomura K., He S.Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980. [DOI] [PubMed] [Google Scholar]
- Merlot S., Leonhardt N., Fenzi F., Valon C., Costa M., Piette L., Vavasseur A., Genty B., Boivin K., Müller A., Giraudat J., Leung J. (2007). Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 26: 3216–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y., Lv D., Wang P., Wang X.-C., Chen J., Miao C., Song C.-P. (2006). An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18: 2749–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Schlauch K., Tam R., Cortes D., Torres M.A., Shulaev V., Dangl J.L., Mittler R. (2009). The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2: ra45. [DOI] [PubMed] [Google Scholar]
- Mishina N.M., Tyurin-Kuzmin P.A., Markvicheva K.N., Vorotnikov A.V., Tkachuk V.A., Laketa V., Schultz C., Lukyanov S., Belousov V.V. (2011). Does cellular hydrogen peroxide diffuse or act locally? Antioxid. Redox Signal. 14: 1–7. [DOI] [PubMed] [Google Scholar]
- Miya A., Albert P., Shinya T., Desaki Y., Ichimura K., Shirasu K., Narusaka Y., Kawakami N., Kaku H., Shibuya N. (2007). CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 104: 19613–19618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen G.B., Bibikova T.N., Messerli M.A., Shi C., Gilroy S. (2007). Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 104: 20996–21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen G.B., Bibikova T.N., Weisenseel M.H., Gilroy S. (2009). Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 21: 2341–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie W.F., Wang M.M., Xia X.J., Zhou Y.H., Shi K., Chen Z., Yu J.Q. (2013). Silencing of tomato RBOH1 and MPK2 abolishes brassinosteroid-induced H2O2 generation and stress tolerance. Plant Cell Environ. 36: 789–803. [DOI] [PubMed] [Google Scholar]
- Nissen K.S., Willats W.G.T., Malinovsky F.G. (2016). Understanding CrRLK1L function: cell walls and growth control. Trends Plant Sci. 21: 516–527. [DOI] [PubMed] [Google Scholar]
- Nühse T.S., Bottrill A.R., Jones A.M.E., Peck S.C. (2007). Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J. 51: 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Tabata R., Matsubayashi Y. (2016). Long-distance peptide signaling essential for nutrient homeostasis in plants. Curr. Opin. Plant Biol. 34: 35–40. [DOI] [PubMed] [Google Scholar]
- Pearce G., Moura D.S., Stratmann J., Ryan C.A. Jr. (2001). RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc. Natl. Acad. Sci. USA 98: 12843–12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G., Yamaguchi Y., Munske G., Ryan C.A. (2008). Structure-activity studies of AtPep1, a plant peptide signal involved in the innate immune response. Peptides 29: 2083–2089. [DOI] [PubMed] [Google Scholar]
- Ranf S., Gisch N., Schäffer M., Illig T., Westphal L., Knirel Y.A., Sánchez-Carballo P.M., Zähringer U., Hückelhoven R., Lee J., Scheel D. (2015). A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 16: 426–433. [DOI] [PubMed] [Google Scholar]
- Roos G., Foloppe N., Messens J. (2013). Understanding the pK(a) of redox cysteines: the key role of hydrogen bonding. Antioxid. Redox Signal. 18: 94–127. [DOI] [PubMed] [Google Scholar]
- Ross A., Yamada K., Hiruma K., Yamashita-Yamada M., Lu X., Takano Y., Tsuda K., Saijo Y. (2014). The Arabidopsis PEPR pathway couples local and systemic plant immunity. EMBO J. 33: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satooka H., Hara-Chikuma M. (2016). Aquaporin-3 controls breast cancer cell migration by regulating hydrogen peroxide transport and its downstream cell signaling. Mol. Cell. Biol. 36: 1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto C., Mély Y., Ohshima H., Garin J., Cochet C., Chambaz E., Baudier J. (1998). Cysteine oxidation in the mitogenic S100B protein leads to changes in phosphorylation by catalytic CKII-α subunit. J. Biol. Chem. 273: 3901–3908. [DOI] [PubMed] [Google Scholar]
- Shang Y., Dai C., Lin M.M., Kwak J.M., Nam K.H. (2016). BRI1-Associated Receptor Kinase 1 regulates guard cell ABA signaling mediated by Open Stomata 1 in Arabidopsis. Mol. Plant 9: 447–460. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Nakano T., Takamizawa D., Desaki Y., Ishii-Minami N., Nishizawa Y., Minami E., Okada K., Yamane H., Kaku H., Shibuya N. (2010). Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J. 64: 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya T., et al. (2014). Selective regulation of the chitin-induced defense response by the Arabidopsis receptor-like cytoplasmic kinase PBL27. Plant J. 79: 56–66. [DOI] [PubMed] [Google Scholar]
- Shiu S.H., Bleecker A.B. (2003). Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 132: 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierla M., Waszczak C., Vahisalu T., Kangasjärvi J. (2016). Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 171: 1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Kuo Y.-C., Mishra S., Tsai C.-H., Chien C.-C., Chen C.-W., Desclos-Theveniau M., Chu P.-W., Schulze B., Chinchilla D., Boller T., Zimmerli L. (2012). The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell 24: 1256–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C., Gu D., Hu H.-C., Davanture M., Lee S., Djaoui M., Valot B., Zivy M., Leung J., Merlot S., Kwak J.M. (2009). Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 583: 2982–2986. [DOI] [PubMed] [Google Scholar]
- Sobotta M.C., Liou W., Stöcker S., Talwar D., Oehler M., Ruppert T., Scharf A.N.D., Dick T.P. (2015). Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 11: 64–70. [DOI] [PubMed] [Google Scholar]
- Sreekanta S., Bethke G., Hatsugai N., Tsuda K., Thao A., Wang L., Katagiri F., Glazebrook J. (2015). The receptor-like cytoplasmic kinase PCRK1 contributes to pattern-triggered immunity against Pseudomonas syringae in Arabidopsis thaliana. New Phytol. 207: 78–90. [DOI] [PubMed] [Google Scholar]
- Stegmann M., Monaghan J., Smakowska-Luzan E., Rovenich H., Lehner A., Holton N., Belkhadir Y., Zipfel C. (2017). The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355: 287–289. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Miller G., Morales J., Shulaev V., Torres M.A., Mittler R. (2011). Respiratory burst oxidases: the engines of ROS signaling. Curr. Opin. Plant Biol. 14: 691–699. [DOI] [PubMed] [Google Scholar]
- Szuba A., Kasprowicz-Maluśki A., Wojtaszek P. (2015). Nitration of plant apoplastic proteins from cell suspension cultures. J. Proteomics 120: 158–168. [DOI] [PubMed] [Google Scholar]
- Tang W., Kim T.-W., Oses-Prieto J.A., Sun Y., Deng Z., Zhu S., Wang R., Burlingame A.L., Wang Z.-Y. (2008). BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Wang X., Li P., Wang H., Ji H., Xie J., Qiu Q., Shen D., Dong H. (2016). Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol. 171: 1635–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin S., Arrigoni G., Trentin A.R., Veljovic-Jovanovic S., Pivato M., Zechman B., Masi A. (2013). Biochemical and quantitative proteomics investigations in Arabidopsis ggt1 mutant leaves reveal a role for the gamma-glutamyl cycle in plant’s adaptation to environment. Proteomics 13: 2031–2045. [DOI] [PubMed] [Google Scholar]
- Tran D., Kadono T., Molas M.L., Errakhi R., Briand J., Biligui B., Kawano T., Bouteau F. (2013). A role for oxalic acid generation in ozone-induced signallization in Arabidopis cells. Plant Cell Environ. 36: 569–578. [DOI] [PubMed] [Google Scholar]
- Truong T.H., Ung P.M.-U., Palde P.B., Paulsen C.E., Schlessinger A., Carroll K.S. (2016). Molecular basis for redox activation of epidermal growth factor receptor kinase. Cell Chem. Biol. 23: 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaahtera L., Brosché M., Wrzaczek M., Kangasjärvi J. (2014). Specificity in ROS signaling and transcript signatures. Antioxid. Redox Signal. 21: 1422–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T., et al. (2010). Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 62: 442–453. [DOI] [PubMed] [Google Scholar]
- Vahisalu T., Kollist H., Wang Y.-F., Nishimura N., Chan W.-Y., Valerio G., Lamminmäki A., Brosché M., Moldau H., Desikan R., Schroeder J.I., Kangasjärvi J. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker H., Harbinson J., Ruisch J., Carver T.L.W., Foyer C.H. (1998). Antioxidant defences of the apoplast. Protoplasma 205: 129–140. [Google Scholar]
- Veljovic-Jovanovic S.D., Pignocchi C., Noctor G., Foyer C.H. (2001). Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol. 127: 426–435. [PMC free article] [PubMed] [Google Scholar]
- Wang Z.Y., Seto H., Fujioka S., Yoshida S., Chory J. (2001). BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410: 380–383. [DOI] [PubMed] [Google Scholar]
- Waszczak C., Akter S., Jacques S., Huang J., Messens J., Van Breusegem F. (2015). Oxidative post-translational modifications of cysteine residues in plant signal transduction. J. Exp. Bot. 66: 2923–2934. [DOI] [PubMed] [Google Scholar]
- Willems P., Mhamdi A., Stael S., Storme V., Kerchev P., Noctor G., Gevaert K., Van Breusegem F. (2016). The ROS Wheel: Refining ROS transcriptional footprints. Plant Physiol. 171: 1720–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Hématy K., Höfte H. (2012). Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 63: 381–407. [DOI] [PubMed] [Google Scholar]
- Woo H.A., Yim S.H., Shin D.H., Kang D., Yu D.-Y., Rhee S.G. (2010). Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 140: 517–528. [DOI] [PubMed] [Google Scholar]
- Wood Z.A., Poole L.B., Karplus P.A. (2003). Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300: 650–653. [DOI] [PubMed] [Google Scholar]
- Wrzaczek M., et al. (2015). GRIM REAPER peptide binds to receptor kinase PRK5 to trigger cell death in Arabidopsis. EMBO J. 34: 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzaczek M., Brosché M., Kangasjärvi J. (2013). ROS signaling loops - production, perception, regulation. Curr. Opin. Plant Biol. 16: 575–582. [DOI] [PubMed] [Google Scholar]
- Wu H.M., Hazak O., Cheung A.Y., Yalovsky S. (2011). RAC/ROP GTPases and auxin signaling. Plant Cell 23: 1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Xie J., Yan C., Zou X., Ren D., Zhang S. (2014). A chemical genetic approach demonstrates that MPK3/MPK6 activation and NADPH oxidase-mediated oxidative burst are two independent signaling events in plant immunity. Plant J. 77: 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadeta K.A., Elmore J.M., Creer A.Y., Feng B., Franco J.Y., Rufian J.S., He P., Phinney B.S., Coaker G.L. (2016). An extracellular cysteine-rich protein kinase associates with a membrane immune complex and is required for cell death. Plant Physiol. 173: 773–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., et al. (2013). A receptor-like cytoplasmic kinase targeted by a plant pathogen effector is directly phosphorylated by the chitin receptor and mediates rice immunity. Cell Host Microbe 13: 347–357. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Huffaker A., Bryan A.C., Tax F.E., Ryan C.A. (2010). PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 22: 508–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W., Murata Y. (2016). Microbe associated molecular pattern signaling in guard cells. Front. Plant Sci. 7: 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y.-H., Chang Y.-H., Huang P.-Y., Huang J.-B., Zimmerli L. (2015). Enhanced Arabidopsis pattern-triggered immunity by overexpression of cysteine-rich receptor-like kinases. Front. Plant Sci. 6: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y.-H., Panzeri D., Kadota Y., Huang Y.-C., Huang P.-Y., Tao C.-N., Roux M., Chien H.-C., Chin T.-C., Chu P.-W., Zipfel C., Zimmerli L (2016). The Arabidopsis malectin-Like/LRR-RLK IOS1 is critical for BAK1-dependent and BAK1-independent pattern-triggered immunity. Plant Cell 28: 1701–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., et al. (2012). FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc. Natl. Acad. Sci. USA 109: 14693–14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffagnini M., Bedhomme M., Marchand C.H., Morisse S., Trost P., Lemaire S.D. (2012). Redox regulation in photosynthetic organisms: focus on glutathionylation. Antioxid. Redox Signal. 16: 567–586. [DOI] [PubMed] [Google Scholar]
- Zechmann B. (2014). Compartment-specific importance of glutathione during abiotic and biotic stress. Front. Plant Sci. 5: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B., Stumpe M., Mauch F. (2011). Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta 233: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeida A., González Lebrero M.C., Radi R., Trujillo M., Estrin D.A. (2013). Mechanism of cysteine oxidation by peroxynitrite: An integrated experimental and theoretical study. Arch. Biochem. Biophys. 539: 81–86. [DOI] [PubMed] [Google Scholar]
- Zhang J., et al. (2010). Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7: 290–301. [DOI] [PubMed] [Google Scholar]
- Zhang Y., McCormick S. (2007). A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104: 18830–18835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C., Kunze G., Chinchilla D., Caniard A., Jones J.D.G., Boller T., Felix G. (2006). Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.