Abstract
Purpose
Relationships of farm history and insecticide exposure at home or work with lymphohematopoietic (LH) neoplasm risk were investigated in a large prospective cohort of United States women.
Methods
In questionnaires, women self-reported history living or working on a farm, personally mixing or applying insecticides, insecticide application in the home or workplace by a commercial service, and treating pets with insecticides. Relationships with non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, plasma cell neoplasms, and myeloid leukemia were investigated using Cox proportional hazard models. Age and farming history were explored as effect modifiers.
Results
The analysis included 76,493 women and 822 NHL cases. Women who ever lived or worked on a farm had 1.12 times the risk of NHL (95% CI: 0.95–1.32) compared to those who did not. Women who reported that a commercial service ever applied insecticides in their immediate surroundings had 65% higher risk of CLL/SLL (95% CI: 1.15–2.38). Women younger than 65 who ever applied insecticides had 87% higher risk of DLBCL (95% CI: 1.13–3.09).
Conclusions
Insecticide exposures may contribute to risk of CLL/SLL and DLBCL. Future studies should examine relationships of LH subtypes with specific types of household insecticides.
Keywords: Pesticides, insecticides, women, lymphoma, leukemia
INTRODUCTION
Elevated rates of non-Hodgkin lymphoma (NHL) have been observed among farmers [1–4]. Other lymphohematopoietic (LH) neoplasms, including multiple myeloma, Hodgkin lymphoma, and some myeloid leukemias, have been associated with farming occupation [5–7]. Various exposures are hypothesized to contribute to these excess risks. Of these, pesticides have received considerable attention.
Indeed, NHL has been associated with organochlorine, organophosphate, and carbamate insecticide exposure [8–11]. Pesticides may contribute to NHL risk through a variety of mechanisms, including genotoxicity, immunotoxicity [12;13], increased cell proliferation [13], and chromosomal aberrations [14]. Nevertheless, associations with insecticides have been inconsistent and could be confounded by other exposures, including dusts, sunlight, or infectious agents [2].
Insecticide use in the general population is common. In data from the National Health and Nutrition Examination Survey (NHANES) from 2011–2012, approximately 10% of respondents with complete data reported using insecticides in the past seven days [15]. In Minnesota (1997), approximately 98% of a representative sample of households with children ages 3–13 were storing pesticide products, and 88.4% reported using pesticides in the previous year [16]. In California (2001–2006), 95% of 259 households surveyed had at least one stored pesticide product; insecticides were found in 46% of the households with any pesticides [17].
Despite widespread use, there is limited research on the relationship of LH cancer risks with pesticide use in the general population. In a population-based case control study conducted at four U.S. Surveillance, Epidemiology, and End-Results sites (1,057 cases), termite insecticides, but not other insecticide treatments, were associated with a small increased risk of NHL [18, 19]. In that same study, measured levels of DDE, a metabolite of the insecticide DDT, and chlordane in carpet dust from participants’ homes was associated with increased risk of NHL [19]. In a case-control study of women in upstate New York, USA (376 cases), residential and occupational insecticide exposures were associated with increased risk of NHL [20]. Because these two studies were retrospective, the questionnaire responses could have been subject to recall bias. Furthermore, there has been virtually no research on associations of residential insecticide exposures with other LH neoplasms, such as plasma cell neoplasms or myeloid leukemia.
The objective of these analyses was to investigate associations of LH cancer risk with farming history and insecticide use at home and/or work using data from the Women’s Health Initiative Observational Study (WHI OS), a large, prospective cohort study of women from 40 United States sites.
METHODS
Study population
From October 1, 1993 through December 31, 1998, 93,676 postmenopausal women enrolled in the WHI OS. Participants were followed through March 2005 and subsequently invited to enroll for continued follow-up in WHI extension studies [21, 22]. Women were eligible for the current analysis if they responded to a one-year follow-up questionnaire, which included questions on insecticide use. The follow-up questionnaire was mailed to participants two months before the one-year anniversary of their enrollment in the observational study.
Exclusions
In addition to being excluded if they did not participate in the one-year follow-up questionnaire (N=4,278), women were excluded if they had incident tumors but no histological information (N=2), reported history of cancer diagnosis at baseline (N=11,725), lacked cancer follow-up information (N=428), and/or were diagnosed with a cancer before the one-year follow-up questionnaire (N=750).
Cancer
Medical history was updated annually by mailed questionnaire. Reports of incident cancers were confirmed by centrally trained cancer adjudicators based upon review of medical records and pathology reports. Trained coders from the Surveillance, Epidemiology, and End-Results (SEER) program classified the case morphology using ICD-O-3 coding [23]. LH neoplasms were categorized into WHO subtypes [24] according to InterLymph Consortium recommendations [25]. Here, results for subtypes with 100 or more cases (exposed and non-exposed) are reported – specifically, NHL overall (n=822), diffuse large B-cell lymphoma (DLBCL, n=172), follicular lymphoma (n=127), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL, n=171), plasma cell neoplasms (n=156), and myeloid leukemia (acute myeloid leukemia and chronic myeloid leukemia combined, n=110).
Exposure
Relationships with the following exposures were investigated: history of living or working on a farm, personally mixing or applying insecticides, having insecticides applied at home or work by someone else, and insecticide pet treatment.
History of living or working on a farm (ever/never and duration, <5, 5–9, 10–14, 15–19, and 20+ years) was reported in the WHI OS baseline questionnaire. Information on insecticide use and pet treatment was self-reported in the one-year follow-up questionnaire. Specifically, women reported if, since age 21, they or someone else had poured, mixed, sprayed, or applied insecticides in their immediate surroundings at home or work. If yes, then women reported if they mixed or sprayed insecticides themselves, and/or if a commercial service applied them. They reported total years (duration) and average number of times per year (frequency) that they personally mixed/applied insecticides, and/or that someone else applied insecticides. They reported duration and frequency using the following categories, respectively: never or < 1 year, 1–4, 5–9, 10–14, 15–19, or 20+ years duration; and never or < 1, 1–5, 6–12, 13–24, or 25+ times/year frequency. “I don’t know” responses to the question about insecticide exposure were coded as missing and excluded from the main analyses.
Women also reported if they ever lived with a pet in their home, and if so, if they used insecticides to treat their pet(s) for fleas, mites, or ticks. If yes, they reported the method (none, flea/tick collars, powder or spray, dips, other).
Intensity measures of personal or commercial insecticide use were calculated by first assigning the mid-point value or a value equal to 50% above the highest category (eg. 30 was assigned to the 20+ year category) to the duration and frequency variables, and then multiplying the two numbers. To calculate intensity measures for women missing either duration or frequency (but not both), the missing value was assigned the value most commonly reported by non-cases in the same duration/frequency category that was non-missing.
Farm history, personal mixing of insecticides, personal application of insecticides, commercial service application of insecticides in the home, and lawn service application of insecticides were investigated using ever/never dichotomous coding; women without the exposure comprised the referent.
Additionally, dose-response relationships with duration of farm history and with duration, frequency, and intensity of personal or commercial insecticide use were investigated. Trend tests were performed by entering the categorical scored variables into models as continuous variables. Wald tests were performed and two-sided p-values were calculated.
Duration and frequency categories were based on those listed in the questionnaire and collapsed due to small cell sizes. Intensity measure categories were never/low (0–2, referent), low-moderate (3–100), and high/very high (more than 100). In analyses of duration or frequency, referent categories contained women who neither personally applied/mixed insecticides nor had a lawn or commercial service apply insecticides. In most analyses of pet insecticides, the referent contained women who had a pet but did not treat the pet. The relationship of pet insecticide treatment versus never living with a pet was also investigated.
Statistical analysis
Cox proportional hazard models were used to derive hazard ratios (HR) and 95% confidence intervals (CI). The baseline hazard function in the Cox model was stratified by age at baseline (5-year categories) and by a time-varying indicator for extension study participation. Time to incident LH neoplasm was computed as days from the year-one follow-up questionnaire to first diagnosis of a LH cancer following completion of the questionnaire. Otherwise, follow-up time was censored at the last documented follow-up contact (defined based on participation in the extension study), death, or 17 September 2012 (whichever came first). Women who were cancer free at the start of follow-up and experienced a cancer diagnosis but not a LH cancer during follow-up were censored at the last documented contact, death, or 17 September 2012.
Since the analyses were hypothesis driven, adjustment for multiple comparisons were not made [26]. Analyses were conducted using SAS system, version 9.3 (SAS Institute, Cary, NC, USA).
Covariates
The following covariates were selected a priori and included in all models: age (coded continuously), race/ethnicity (white/non-white), education (<high school, high school degree/general education diploma, school after high school, college degree or higher), USA region of residence (Northeast, South, Midwest, West), occupational type (managerial/professional, technical/sales/administration, service/labor, homemaker), body mass index (BMI: <25, 25 to <30, 30 to <35, and 35+), and smoking status (never, past, current). This information was collected in the baseline questionnaire.
Effect modification by age at enrollment (<65 versus 65+ years) and farm history (ever/never) was investigated using categorical product terms with the main exposure of interest. Likelihood ratio tests (LRT) were used to evaluate effect modification. Because insecticides permitted for residential use in the USA have changed over time, older women might have been exposed to different insecticides than younger women. Women with past farm exposures might have been exposed to different pesticides or had different exposure patterns compared to women without farming history.
RESULTS
In total, 93,676 women enrolled in the WHI OS. After exclusions, 76,493 remained for analysis. Follow-up time ranged from one to 16.4 years; the mean follow-up time was 11.5 years (standard deviation: 3.9). Of the 76,493 women included in the analysis, 53,901 (70.5%) participated in one or both WHI extension studies.
Compared to the entire cohort, LH cases were slightly older at baseline (mean ages ranged from 63.7 years for CLL/SLL to 65.9 years for DLBCL and plasma cell neoplasms versus 63.4 years for the entire cohort, Table 1). Higher proportions of LH cancer cases than the entire cohort were non-Hispanic White (proportions ranged from 85.9% of plasma cell neoplasms to 92.9% of follicular lymphoma cases versus 84.3% of the entire cohort). With the exception of myeloid leukemia and follicular lymphoma, all LH subtypes had a higher proportion of never smokers than all cohort participants (52.1–57.1% versus 50.5% of the entire cohort). LH cases were generally similar to the entire cohort in terms of occupation.
Table 1.
Demographic and lifestyle characteristics of participants of the Women’s Health Initiative observational study and lympho-hematopoietic cancer groups
| WHI OS cohort (N=76,493) |
NHL (N=822) |
DLBCL (N=172) |
Follicular lymphoma (N=127) |
CLL/SLL (N=171) |
Plasma cell neoplasms (N=156) |
Myeloid leukemia (N=110) |
|
|---|---|---|---|---|---|---|---|
| Age at baseline in years, (mean and standard deviation) | 63.4 (7.3) | 65.1 (6.8) | 65.9 (7.2) | 64.8 (6.4) | 63.7 (6.7) | 65.9 (6.2) | 65.0 (7.1) |
| Body mass index (mean and standard deviation) | 27.1 (5.8) | 26.8 (5.5) | 27.8 (6.2) | 26.9 (6.0) | 26.3 (4.8) | 26.7 (5.0) | 27.4 (6.0) |
| Race/ethnicity | |||||||
| White | 64,491(84.3) | 743 (90.4) | 156 (90.7) | 118 (92.9) | 157 (91.8) | 134 (85.9) | 99 (90.0) |
| Black | 5,573(7.3) | 39(4.7) | 4(2.3) | 1 (0.8) | 11 (6.4) | 14 (9.0) | 5 (4.6) |
| Hispanic | 2,736(3.6) | 14(1.7) | 3 (1.7) | 2 (1.6) | 2 (1.2) | 6 (3.9) | 2 (1.8) |
| Asian/Pacific Islander | 2,292(3.0) | 13(1.6) | 5 (2.9) | 2 (1.6) | 1 (0.6) | 0 | 3 (2.7) |
| Other | 1,401(1.8) | 13(1.6) | 4 (2.3) | 4 (3.2) | 0 | 2 (1.3) | 1 (0.9) |
| Education | |||||||
| Less than high school | 3,527 (4.6) | 30 (3.7) | 8 (4.7) | 6 (4.7) | 3 (1.8) | 8 (5.1) | 3 (2.7) |
| High school diploma/General education diploma | 12,330 (16.1) | 136 (16.6) | 27 (15.7) | 21 (16.5) | 34(19.9) | 23(14.7) | 16 (14.6) |
| School after high school | 27,597 (36.1) | 295(35.9) | 62 (36.1) | 46 (36.2) | 57 (33.3) | 64(41.0) | 42 (38.2) |
| College degree or higher | 32,435 (42.4) | 358 (43.6) | 74 (43.0) | 53 (41.7) | 77(45.0) | 61 (39.1) | 48 (43.6) |
| US Region of residence at the time of enrollment | |||||||
| Northeast | 17,644(23.1) | 215(26.2) | 48(27.9) | 30 (23.6) | 36 (21.1) | 40 (25.6) | 29 (26.4) |
| South | 19,081 (24.9) | 177 (21.5) | 39 (22.7) | 32 (25.2) | 44 (25.7) | 31(19.9) | 23 (20.9) |
| Midwest | 16,948 (22.2) | 188(22.9) | 35 (20.4) | 31 (24.4) | 42 (24.6) | 37 (23.7) | 24(21.8) |
| West | 22,820 (29.8) | 242(29.4) | 50 (29.1) | 34 (26.8) | 49 (28.7) | 48 (30.8) | 34 (30.9) |
| Occupational sector | |||||||
| Managerial/Professional | 31,868 (41.7) | 345 (42.0) | 76 (44.2) | 51(40.2) | 70(40.9) | 57(36.5) | 44 (40.0) |
| Technical/Sales/Administration | 20,901(27.3) | 222 (27.0) | 48 (27.9) | 35(27.6) | 52(30.4) | 38(24.4) | 32 (29.1) |
| Service/Labor | 12,267(16.0) | 130 (15.8) | 28 (16.3) | 25(19.7) | 24(14.0) | 25(16.0) | 14(12.7) |
| Homemaker | 7,839(10.3) | 88 (10.7) | 15 (8.7) | 13(10.2) | 16(9.4) | 26(16.7) | 16(14.6) |
| Cigarette smoking | |||||||
| Never | 38,620(50.5) | 433 (52.7) | 91(52.9) | 64(50.4) | 89(52.1) | 89(57.1) | 50(45.5) |
| Past | 32,255(42.2) | 347 (42.2) | 75(43.6) | 54(42.5) | 72(42.1) | 61(39.1) | 55 (50.0) |
| Current | 4,561 (6.0) | 33 (4.0) | 6 (3.5) | 8(6.3) | 8(4.7) | 3(1.9) | 4 (3.6) |
| Ever lived or worked on a farm | |||||||
| Never | 5,6300 (73.6) | 583 (70.9) | 119 (69.2) | 89 (70.1) | 124 (72.5) | 108 (69.2) | 79 (71.8) |
| Ever | 19,790 (25.9) | 232 (28.2) | 52 (30.2) | 35 (27.6) | 45 (26.3) | 48 (30.8) | 31 (28.2) |
| Exposed to insecticides | |||||||
| Never | 23,344 (30.5) | 239 (29.1) | 47 (27.3) | 37 (29.1) | 43 (25.2) | 57 (36.5) | 39 (35.5) |
| At work only | 1,310 (1.7) | 14 (1.7) | 2 (1.2) | 2 (1.6) | 6 (3.5) | 3 (1.9) | 2 (1.8) |
| At home or leisure only | 36,055 (47.1) | 426 (51.8) | 92 (53.5) | 70 (55.1) | 87 (50.9) | 71 (45.5) | 48 (43.6) |
| At work and at home or leisure | 8,317 (10.9) | 79 (9.6) | 19 (11.1) | 8 (6.3) | 21 (12.3) | 10 (6.4) | 10 (9.1) |
Abbreviations: NHL, non Hodgkin lymphoma; DLBCL, Diffuse large B cell lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma
Approximately 26% of participants ever lived or worked on a farm. There was a high prevalence of insecticide use; 30.5% of WHI OS women were never exposed to insecticides at work or home. Proportions of the LH cancer groups who were never exposed to insecticides ranged from 25.2% of CLL/SLL to 36.5% of plasma cell neoplasm cases.
Women who lived or worked on a farm had 1.12 times the risk of NHL; the estimate was relatively precise but borderline non-statistically significant (95% CI: 0.95–1.32, Figure 1). Ever applying insecticides was associated with an increased, borderline non-statistically significant risk of DLBCL (HR, 95% CI: 1.36, 0.99–1.88). Those who reported having had a commercial service apply insecticides in their home had 24% higher risk of NHL (95% CI: 1.03–1.48) and 65% higher risk of CLL/SLL (95% CI: 1.15–2.38). Other effect estimates were near unity and/or imprecise.
Figure 1.
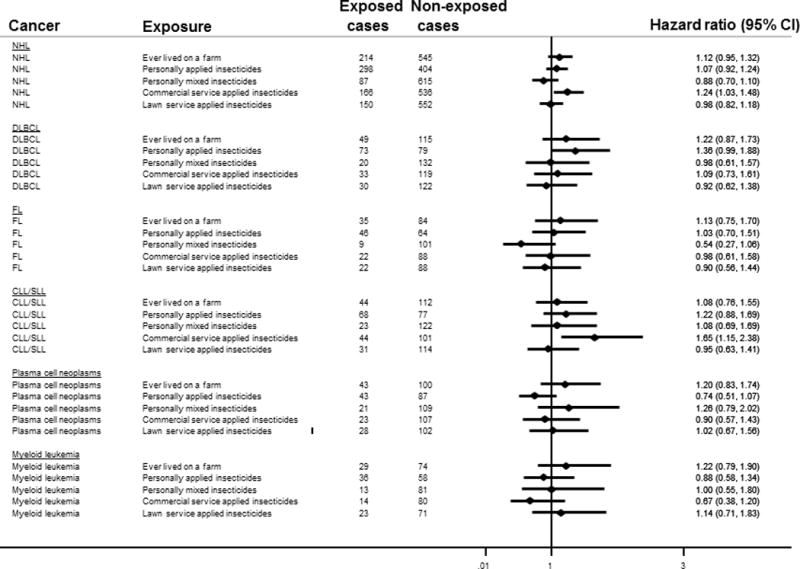
Forest plot showing hazard ratio estimates of association between living/working on a farm or household insecticide exposure and lympho-hematapoetic neoplasms. All exposures were categorized dichotomously. The referent categories for all exposures were defined as the group of women not exposed to that particular metric. Exposures were entered into the models individually. The baseline hazard functions were stratified by age at baseline and a time varying indicator for extension study participation. All models were adjusted for age, race/ethnicity, education, United States region of residence, occupational type, body mass index and smoking status.
Abbreviations: NHL, non Hodgkin lymphoma; DLBCL, Diffuse large B cell lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma
Table 2 shows HR estimates for the association of LH subtype risk with exposure frequency, duration, or intensity measures. With the exception of plasma cell neoplasms, of which there were only 3 exposed cases, HRs were highest in association with ≥20 years living/working on a farm. However, the CIs associated with these effect estimates were imprecise and crossed the null, and p-values for trend ranged from 0.16 for NHL to 0.58 for CLL/SLL. Risk of DLBCL was 49% (95% CI: 0.84–2.65, p for trend: 0.05), and 72% (95% CI: 0.97–3.05, p for trend: 0.01) higher in association with the highest categories of frequency and intensity personally mixing/applying insecticides, respectively. Otherwise, there was not strong evidence of dose-response relationships.
Table 2.
Hazard ratio estimates of dose-response relationships of lympho-hematopoietic neoplasms with living/working on a farm or household insecticide exposure1
| NHL | DLBCL | FL | CLL/SLL | Plasma cell neoplasms | Myeloid leukemia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| N | HR, 95% CI | N | HR, 95% CI | N | HR, 95% CI | N | HR, 95% CI | N | HR, 95% CI | N | HR, 95% CI | ||
| Duration of time lived/worked on a farm | |||||||||||||
| Never | 545 | Ref | 115 | Ref | 84 | Ref | 112 | Ref | 100 | Ref | 74 | Ref | |
| < 5 years | 52 | 1.08, 0.81–1.44 | 14 | 1.37, 0.79–2.40 | 8 | 1.04, 0.50–2.16 | 11 | 1.07, 0.58–2.00 | 6 | 0.69, 0.30–1.58 | 7 | 1.11, 0.51–2.41 | |
| 5–19 years | 126 | 1.12, 0.92–1.37 | 26 | 1.10, 0.71–1.71 | 18 | 1.00, 0.59–1.68 | 25 | 1.04, 0.67–1.62 | 34 | 1.62, 1.08–2.41 | 16 | 1.16, 0.67–2.01 | |
| 20+ years | 35 | 1.17, 0.83–1.66 | 9 | 1.46, 0.73–2.92 | 9 | 1.84, 0.91–3.75 | 8 | 1.30, 0.63–2.71 | 3 | 0.51, 0.16–1.62 | 6 | 1.76, 0.75–4.12 | |
| Never exposed2 | 217 | Ref | 46 | Ref | 34 | Ref | 37 | Ref | 53 | Ref | 36 | Ref | |
| Personally mixed or applied insecticides | |||||||||||||
| Duration | |||||||||||||
| <1 year | 196 | 1.11, 0.92–1.35 | 38 | 1.06, 0.69–1.63 | 32 | 1.17, 0.72–1.90 | 44 | 1.37, 0.88–2.13 | 28 | 0.67, 0.42–1.06 | 21 | 0.68, 0.40–1.18 | |
| 1–4 years | 96 | 1.27, 1.00–1.62 | 19 | 1.23, 0.72–2.10 | 14 | 1.17, 0.63–2.20 | 24 | 1.71, 1.02–2.87 | 18 | 1.02, 0.60–1.75 | 9 | 0.70, 0.33–1.45 | |
| 5–19 years | 112 | 1.23, 0.98–1.55 | 30 | 1.62, 1.02–2.59 | 19 | 1.32, 0.75–2.33 | 22 | 1.30, 0.76–2.21 | 17 | 0.79, 0.46–1.37 | 13 | 0.83, 0.43–1.57 | |
| ≥20 years | 78 | 0.92, 0.71–1.19 | 20 | 1.14, 0.67–1.94 | 11 | 0.81, 0.41–1.61 | 17 | 1.10, 0.62–1.97 | 11 | 0.53, 0.28–1.02 | 14 | 0.95, 0.51–1.77 | |
| Frequency | |||||||||||||
| <1 time per year | 197 | 1.10, 0.90–1.34 | 35 | 0.96, 0.61–1.49 | 32 | 1.14, 0.70–1.86 | 44 | 1.34, 0.86–2.09 | 31 | 0.73, 0.46–1.14 | 20 | 0.64, 0.37–1.11 | |
| 1–5 times per year | 225 | 1.15, 0.95–1.39 | 55 | 1.39, 0.93–2.07 | 36 | 1.16, 0.72–1.87 | 52 | 1.43, 0.93–2.20 | 38 | 0.82, 0.54–1.25 | 28 | 0.83, 0.50–1.36 | |
| ≥ 6 times per year | 55 | 1.07, 0.80–1.45 | 16 | 1.49, 0.84–2.65 | 6 | 0.74, 0.31–1.78 | 11 | 1.19, 0.60–2.34 | 5 | 0.40, 0.16–1.00 | 10 | 1.15, 0.57–2.34 | |
| Intensity3 | |||||||||||||
| Never/very low | 409 | Ref | 78 | Ref | 66 | Ref | 81 | Ref | 84 | Ref | 56 | Ref | |
| Low/moderate | 245 | 1.08, 0.92–1.26 | 61 | 1.44, 1.03–2.02 | 39 | 1.05, 0.70–1.56 | 54 | 1.14, 0.80–1.61 | 40 | 0.88, 0.60–1.28 | 30 | 0.95, 0.61–1.49 | |
| High/very high | 45 | 1.05, 0.77–1.44 | 14 | 1.72, 0.97–3.05 | 5 | 0.72, 0.29–1.78 | 9 | 1.04, 0.52–2.08 | 3 | 0.34, 0.11–1.08 | 8 | 1.37, 0.65–2.88 | |
| Insecticide application in home, lawn, or garden by someone else | |||||||||||||
| Duration | |||||||||||||
| < 1 year | 160 | 1.23, 1.00–1.51 | 35 | 1.30, 0.84–2.03 | 22 | 1.08, 0.63–1.85 | 37 | 1.58, 1.00–2.51 | 25 | 0.80, 0.50–1.30 | 15 | 0.67, 0.36–1.22 | |
| 1–4 years | 95 | 0.97, 0.76–1.24 | 22 | 1.10, 0.66–1.84 | 19 | 1.24, 0.71–2.19 | 20 | 1.10, 0.64–1.90 | 16 | 0.69, 0.39–1.21 | 14 | 0.82, 0.44–1.53 | |
| 5–19 year | 155 | 1.19, 0.96–1.46 | 34 | 1.28, 0.82–2.10 | 25 | 1.22, 0.72–2.05 | 33 | 1.35, 0.84–2.16 | 25 | 0.82, 0.50–1.32 | 22 | 0.96, 0.56–1.64 | |
| 20+ year | 72 | 1.03, 0.78–1.34 | 14 | 0.97, 0.53–1.77 | 9 | 0.82, 0.39–1.72 | 18 | 1.40, 0.79–2.47 | 10 | 0.60, 0.30–1.18 | 6 | 0.49, 0.20–1.16 | |
| Frequency | |||||||||||||
| <1 time per year | 162 | 1.22, 0.99–1.50 | 38 | 1.38, 0.89–2.13 | 20 | 0.96, 0.55–1.68 | 40 | 1.67, 1.07–2.62 | 24 | 0.75, 0.46–1.22 | 17 | 0.75, 0.42–1.33 | |
| 1–5 times per year | 231 | 1.02, 0.85–1.23 | 48 | 1.04, 0.69–1.57 | 38 | 1.08, 0.68–1.73 | 46 | 1.10, 0.71–1.70 | 42 | 0.79, 0.52–1.19 | 28 | 0.71, 0.43–1.17 | |
| ≥ 6 times per year | 86 | 1.29, 1.00–1.67 | 18 | 1.29, 0.74–2.24 | 16 | 1.51, 0.82–2.78 | 20 | 1.58, 0.91–2.76 | 11 | 0.70, 0.36–1.35 | 13 | 1.14, 0.59–2.17 | |
| Intensity3 | |||||||||||||
| Never/very low | 369 | Ref | 81 | Ref | 53 | Ref | 77 | Ref | 76 | Ref | 51 | Ref | |
| Low/moderate | 283 | 0.99, 0.85–1.16 | 65 | 1.07, 0.77–1.49 | 51 | 1.25, 0.85–1.84 | 55 | 0.86, 0.61–1.22 | 45 | 0.79, 0.54–1.15 | 37 | 0.91, 0.59–1.39 | |
| High/Very high | 49 | 1.13, 0.84–1.53 | 6 | 0.63, 0.27–1.44 | 5 | 0.78, 0.31–1.98 | 13 | 1.32, 0.73–2.40 | 9 | 1.06, 0.53–2.13 | 6 | 0.99, 0.42–2.32 | |
Abbreviations: NHL, non Hodgkin lymphoma; DLBCL, Diffuse large B cell lymphoma; FL, follicular lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma
Each exposure variable was entered into the model individually. The baseline hazard functions were stratified by age at baseline and a time varying indicator for extension study participation. All models were adjusted for age, race/ethnicity, education, United States region of residence, occupational type, body mass index and smoking status.
Women who never personally used insecticides and who never had pesticides applied by a commercial or lawn service make up the referent group for models investigating the relationship between lympho-hematopoietic neoplasms and frequency or duration of personal insecticide use or exposure to insecticides applied by a commercial or lawn service.
Intensity was defined as frequency × duration.
Compared to women who had pets but did not treat them, women who treated pets with insecticides had a 71% higher, borderline non-statistically significant risk of plasma cell neoplasms (95% CI: 0.96–3.01, Table 3). Compared to women without pets, the relationship between plasma cell neoplasm risk and treating pets with insecticides was weaker but more precise and still not statistically significant (HR, 95% CI: 1.09, 0.71–1.69). Use of powders or sprays on pets was associated with 62% higher risk of follicular lymphoma (95% CI: 1.06–2.48).
Table 3.
Hazard ratio estimates of relationships of lympho-hematapoietic neoplasms with insecticide pet treatments1
| NHL | DLBCL | FL | CLL/SLL | Plasma cell neoplasms | Myeloid leukemia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| N | HR, 95% CI | N | HR, 95% CI | N | HR, 95% CI | N | HR, 95% CI | N | HR, 95% CI | N | HR, 95% CI | |
| Lived with pets compared to living with no pets | ||||||||||||
| Never lived with pet | 132 | Ref | 29 | Ref | 27 | Ref | 23 | Ref | 28 | Ref | 13 | Ref |
| Never treated pets | 108 | 1.04, 0.80–1.34 | 22 | 0.99, 0.57–1.74 | 19 | 0.88, 0.49–1.60 | 19 | 1.04, 0.56–1.93 | 14 | 0.65, 0.34–1.25 | 16 | 1.52, 0.73–3.20 |
| Ever treated pets | 507 | 1.12, 0.92–1.36 | 114 | 1.19, 0.78–1.80 | 70 | 0.72, 0.45–1.13 | 113 | 1.32, 0.83–2.08 | 96 | 1.09, 0.71–1.69 | 70 | 1.48, 0.81–2.70 |
| Lived with treated pets compared to living with untreated pets | ||||||||||||
| Lived with untreated pet | 108 | Ref | 22 | Ref | 19 | Ref | 19 | Ref | 14 | Ref | 16 | Ref |
| Lived with treated pet | 507 | 1.07,0.86–1.32 | 114 | 1.19, 0.75–1.89 | 70 | 0.80, 0.48–1.34 | 113 | 1.25, 0.76–2.05 | 96 | 1.71, 0.96–3.01 | 70 | 0.95, 0.55–1.66 |
| Ever used flea or tick collar | ||||||||||||
| Lived with untreated pet | 208 | Ref | 47 | Ref | 35 | Ref | 40 | Ref | 33 | Ref | 34 | Ref |
| Lived with treated pet | 407 | 1.07, 0.90–1.27 | 89 | 1.05, 0.73–1.50 | 54 | 0.83, 0.54–1.28 | 92 | 1.23, 0.84–1.79 | 77 | 1.33, 0.88–2.01 | 52 | 0.80, 0.52–1.24 |
| Ever use flea spray or powder | ||||||||||||
| Lived with untreated pet | 324 | Ref | 69 | Ref | 40 | Ref | 75 | Ref | 56 | Ref | 53 | Ref |
| Lived with treated pet | 291 | 1.20, 1.02–1.41 | 67 | 1.33, 0.94–1.87 | 49 | 1.62, 1.06–2.48 | 57 | 0.93, 0.66–1.32 | 54 | 1.36, 0.93–1.98 | 33 | 0.80, 0.52–1.25 |
| Ever used flea dip | ||||||||||||
| Lived with untreated pet | 460 | Ref | 102 | Ref | 63 | Ref | 95 | Ref | 81 | Ref | 71 | Ref |
| Lived with treated pet | 155 | 1.11, 0.92–1.34 | 34 | 1.11, 0.74–1.65 | 26 | 1.33, 0.83–2.14 | 37 | 1.13, 0.76–1.67 | 29 | 1.30, 0.84–2.01 | 15 | 0.66, 0.37–1.16 |
Abbreviations: NHL, non Hodgkin lymphoma; DLBCL, Diffuse large B cell lymphoma; FL, follicular lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma
Each exposure variable was entered into the model individually. The baseline hazard functions were stratified by age at baseline and a time varying indicator for extension study participation. All models were adjusted for age, race/ethnicity, educational level, United States region of residence, occupational type, body mass index and smoking status.
Living or working on a farm did not modify any of the overall relationships observed (Supplemental table 1). When age was investigated as an effect modifier, the observed association of ever personally applying insecticides with risk of DLBCL was limited to younger women (HR, 95% CI: 1.87, 1.13–3.09 in women younger than 65, p for LRT: 0.10, Figure 2). Similarly, among younger but not older women, DLBCL risk increased in association with higher frequency and intensity measures of personal mixing/applying insecticides (HR, 95% CI for ≥ 6 times per year: 4.76, 1.77–12.81, p for LRT: 0.01, and HR, 95% CI for high/very high vs. never/very low: 3.15, 1.41–7.02, p for LRT: 0.07). The relationship between commercial service applied insecticides and CLL/SLL was stronger in older women (HR, 95% CI: 2.13, 1.26–3.61 in older women vs. 1.35, 0.83–2.21 in younger women, p for LRT: 0.21, Supplemental Table 2).
Figure 2.
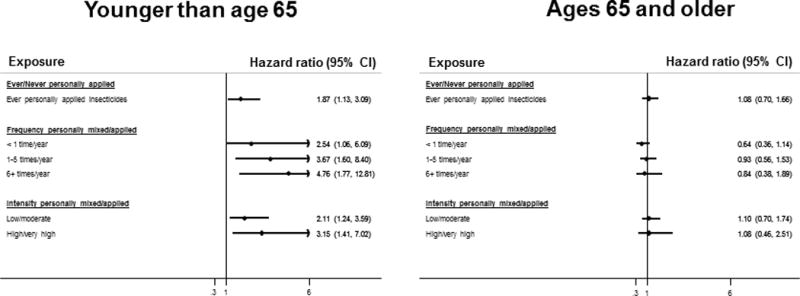
Forest plot showing hazard ratio estimates of association of personal application of insecticides with risk of diffuse large B cell lymphoma, stratified by age (< 65 versus ages 65+), derived from cox proportional hazard models that included an interaction term between the exposure and age. The referent category for ever personally applied insecticides was women who reported never personally using insecticides. The referent category for the other exposure metrics consisted of women who reported that they neither personally applied/mixed insecticides nor had a commercial or lawn service apply insecticides in their home. The baseline hazard functions were stratified by age at baseline and a time varying indicator for extension study participation. All models were adjusted for age, race/ethnicity, education, United States region of residence, occupational type, body mass index and smoking status.
DISCUSSION
In a large prospective cohort of postmenopausal women in the United States, insecticide exposure was modestly associated with increased risk of several LH subtypes. Among women younger than 65, ever personally applying insecticides and higher intensity and frequency of personal insecticide use was associated with increased risk of DLBCL. CLL/SLL risk was associated with reports of having had insecticides applied in the home by a commercial service. Treatment of pets with sprays or powders was associated with increased risk of several LH subtypes.
The 12% increase in NHL risk associated with living or working on a farm (95% CI: 0.95, 1.32) is similar in magnitude to modest, yet fairly consistent risk increases of 10% (95% CI: 1.03–1.19) [7] and 34% (95% CI: 1.17–1.55) [3] reported in farming occupation meta-analyses. In a case-control study in Nebraska, women who lived/worked on a farm and had a first degree relative with a history of LH cancer had 2.5 times the odds of NHL compared to women without either exposure [27]. In a case-control study in New York state, women who worked for 10+ years on a farm where pesticides were used had 2.12 times the odds of NHL compared to women who never worked on a farm (95% CI: 1.21–3.71) [20]. In Iowa, women living on a farm had a more than 2-fold higher risk of acute myeloid leukemia (AML) compared to women living in a city of 10,000+ residents (95% CI: 1.25–3.99, 30 exposed cases) [28]. Due to small numbers, we did not report results for AML only. There was, however, a modest but slightly elevated estimate of association between farm history and myeloid leukemia. The relationship between AML and farm residence might have been diluted by other types of leukemia, such as chronic myeloid leukemia, which may be less impacted by the exposures.
Data on WHI OS participants’ exposures to specific insecticides were unavailable. Past surveys provide insight into the chemicals to which participants might have been exposed. In an inventory of pesticide products stored in control households participating in the Northern California Childhood Leukemia Study (years 2001–2006), the most common insecticides belonged to pyrethroids, organophosphates, botanical, and unclassified chemical classes [17]. Based on interviews and inventories (May–August 1997), the most common classes of insecticides that Minnesota households with children ages 3 to 13 years stored in the home and reported using in the past year were pyrethrins, pyrethroids, organophosphates, and carbamates [16].
Relationships between NHL and insecticides, including the organochlorines, organophosphates, and carbamates, have been observed previously [29]. Most studies investigated occupational rather than household exposures and were conducted in entirely or predominantly male populations. In a case-control study of agricultural pesticide exposure among women in Nebraska (1983–1986), personal handling of organophosphate insecticides was associated with a nearly 5-fold increased odds of NHL[27]. In a pooled analysis of case-control data from several European countries, occupational exposure to organophosphates was associated with increased risk of CLL [30]. In an analysis of pooled case-control data from the Midwestern United States, farmers with organophosphate exposure had 80% higher risk of DLBCL compared to non-farmers (95% CI: 1.2–2.6) [11]. Also, carbaryl use (a carbamate insecticide) was associated with a 2.9-fold increased odds of SLL (95% CI: 1.2–7.0) and carbamate insecticide exposure was associated with 1.6 times the odds of DLBCL (95% CI: 1.0–2.7) [31]. In a prospective cohort study of pesticide applicators in Iowa and North Carolina, USA, follicular B cell lymphoma was associated with carbaryl, diazinon (organophosphorus insecticide), and lindane (organochlorine insecticide) exposure, and CLL/SLL/marginal cell lymphoma was associated with terbufos (organophosphorus insecticide) and DDT (organochlorine insecticide)[32].
Associations were stronger for personally spraying/applying insecticides than personally mixing insecticides. In an analysis of WHI OS participants, rheumatoid arthritis risk was more strongly associated with spraying/applying than mixing insecticides [33]. These findings could reflect higher percentages of the cohort having reporting applying rather than mixing insecticides.
Insecticides approved for household use have changed over time. In 1988, the USA Environmental Protection Agency banned all chlordane use[34]. In years 2001 and 2002, respectively, the agency restricted residential use of the insecticides chlorpyrifos and diazinon [35, 36]. Pyrethroid use became more common after these restrictions [37, 38]. Changes in use could help explain the restriction of relationships between DLBCL and personal insecticide use to younger women. Few studies have investigated the modifying effect of age on the relationship of residential insecticide exposure and LH cancers. In a population-based case-control study in the USA, residential treatment for termites before 1988, the year when chlordane was banned, was associated with increased risk of NHL in participants ages 65 and older (OR: 1.8, 95% CI: 1.2–2.7), but not in participants between the ages of 45 and 64 (OR: 1.0, 95% CI: 0.7–1.4) [18]. However, it is difficult to compare these results to ours since we do not have information about the time period during which residential insecticides were used.
Our results related to effect modification by age should be interpreted cautiously. These results could reflect changes in types of insecticides permitted for use over time, as discussed above. However it is possible that the stronger results in younger participants might reflect a selection bias that was induced by excluding women who already had diagnoses of cancer at the time of follow-up. That is, it is possible that by making this exclusion, we excluded older, susceptible women who already developed cancer and that the younger susceptible women remained because they had not reached the critical age at which the LH cancer appeared or was detected.
These analyses had several limitations. Detailed residential and farming history data were unavailable. Also, it was not possible to distinguish between working versus living on a farm. Since insecticide exposure was self-reported, it could have been misreported. The lack of detailed chemical information precluded investigation of relationships with specific insecticides. Investigation of associations with herbicides was impossible, since data on herbicide use was not collected.
Strengths of this analysis include the large cohort, which allowed investigation of relationships with rarer LH subtypes, and the prospective study design, which reduced the potential for recall bias. Because the WHI OS includes a sample of women from across the United States, these results are generalizable to a variety of regions. Also, the cancer diagnoses should have had high accuracy due to review by trained adjudicators [23]. Finally, due to the focus on household rather than occupational farming related insecticide exposures, many of the potential co-exposures that might confound estimates of association, such as dusts, sunlight, or infectious agents [2], are not a major concern in this analysis.
Insecticide exposures at work or home may be associated with risk of LH neoplasms, especially DLBCL and CLL/SLL. This study highlights the need for further prospective studies investigating relationships between LH subtypes and insecticide use, particularly in households, where these exposures are common.
Supplementary Material
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. This research was supported [in part] by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
List of abbreviations
- CI
Confidence interval
- CLL/SLL
Chronic lymphocytic leukemia/small lymphocytic lymphoma
- DDT
Dichlorodiphenyltrichloroethane
- DDE
Dichlorodiphenyldichloroethylene
- DLBCL
Diffuse large B cell lymphoma
- HR
Hazard ratio
- LH
Lympho hematopoietic
- NHL
Non Hodgkin lymphoma
- WHI OS
Women’s health initiative observational study
References
- 1.Blair A, Dosemeci M, Heineman EF. Cancer and other causes of death among male and female farmers from twenty-three states. American journal of industrial medicine. 1993;23(5):729–742. doi: 10.1002/ajim.4700230507. [DOI] [PubMed] [Google Scholar]
- 2.Blair A, Zahm SH. Agricultural exposures and cancer. Environmental health perspectives. 1995;103(Suppl 8):205–208. doi: 10.1289/ehp.95103s8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller-Byrne JE, Khuder SA, Schaub EA, McAfee O. A meta-analysis of non-Hodgkin’s lymphoma among farmers in the central United States. American journal of industrial medicine. 1997;31(4):442–444. doi: 10.1002/(sici)1097-0274(199704)31:4<442::aid-ajim10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Khuder SA, Schaub EA, Keller-Byrne JE. Meta-analyses of non-Hodgkin’s lymphoma and farming. Scandinavian journal of work, environment & health. 1998;24(4):255–261. doi: 10.5271/sjweh.318. [DOI] [PubMed] [Google Scholar]
- 5.Viel JF, Richardson ST. Lymphoma, multiple myeloma and leukaemia among French farmers in relation to pesticide exposure. Social science & medicine. 1993;37(6):771–777. doi: 10.1016/0277-9536(93)90371-a. [DOI] [PubMed] [Google Scholar]
- 6.Orsi L, Delabre L, Monnereau A, Delval P, Berthou C, Fenaux P, Marit G, Soubeyran P, Huguet F, Milpied N, et al. Occupational exposure to pesticides and lymphoid neoplasms among men: results of a French case-control study. OccupEnvironMed. 2009;66(5):291–298. doi: 10.1136/oem.2008.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khuder SA, Mutgi AB. Meta-analyses of multiple myeloma and farming. American journal of industrial medicine. 1997;32(5):510–516. doi: 10.1002/(sici)1097-0274(199711)32:5<510::aid-ajim11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Cantor KP, Blair A, Everett G, Gibson R, Burmeister LF, Brown LM, Schuman L, Dick FR. Pesticides and other agricultural risk factors for non-Hodgkin’s lymphoma among men in Iowa and Minnesota. Cancer research. 1992;52(9):2447–2455. [PubMed] [Google Scholar]
- 9.Eriksson M, Hardell L, Carlberg M, Akerman M. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. International journal of cancer. 2008;123(7):1657–1663. doi: 10.1002/ijc.23589. [DOI] [PubMed] [Google Scholar]
- 10.McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, Robson D, Skinnider LF, Choi NW. Non-Hodgkin’s lymphoma and specific pesticide exposures in men: cross-Canada study of pesticides and health. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10(11):1155–1163. [PubMed] [Google Scholar]
- 11.Waddell BL, Zahm SH, Baris D, Weisenburger DD, Holmes F, Burmeister LF, Cantor KP, Blair A. Agricultural use of organophosphate pesticides and the risk of non-Hodgkin’s lymphoma among male farmers (United States) Cancer causes & control: CCC. 2001;12(6):509–517. doi: 10.1023/a:1011293208949. [DOI] [PubMed] [Google Scholar]
- 12.Zahm SH, Ward MH, Blair A. Pesticides and cancer. Occupational medicine. 1997;12(2):269–289. [PubMed] [Google Scholar]
- 13.Figgs LW, Holland NT, Rothmann N, Zahm SH, Tarone RE, Hill R, Vogt RF, Smith MT, Boysen CD, Holmes FF, et al. Increased lymphocyte replicative index following 2,4-dichlorophenoxyacetic acid herbicide exposure. Cancer causes & control: CCC. 2000;11(4):373–380. doi: 10.1023/a:1008925824242. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder JC, Olshan AF, Baric R, Dent GA, Weinberg CR, Yount B, Cerhan JR, Lynch CF, Schuman LM, Tolbert PE, et al. Agricultural risk factors for t(14;18) subtypes of non-Hodgkin’s lymphoma. Epidemiology. 2001;12(6):701–709. doi: 10.1097/00001648-200111000-00020. [DOI] [PubMed] [Google Scholar]
- 15.National Health Nutrition Examiniation Survey. Pesticide Use (PUWMEC_G) [ http://wwwn.cdc.gov/nchs/nhanes/2011–2012/PUQMEC_G.htm#Component_Description]
- 16.Adgate JL, Kukowski A, Stroebel C, Shubat PJ, Morrell S, Quackenboss JJ, Whitmore RW, Sexton K. Pesticide storage and use patterns in Minnesota households with children. Journal of exposure analysis and environmental epidemiology. 2000;10(2):159–167. doi: 10.1038/sj.jea.7500078. [DOI] [PubMed] [Google Scholar]
- 17.Guha N, Ward MH, Gunier R, Colt JS, Lea CS, Buffler PA, Metayer C. Characterization of residential pesticide use and chemical formulations through self-report and household inventory: the Northern California Childhood Leukemia study. Environmental health perspectives. 2013;121(2):276–282. doi: 10.1289/ehp.1204926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colt JS, Davis S, Severson RK, Lynch CF, Cozen W, Camann D, Engels EA, Blair A, Hartge P. Residential insecticide use and risk of non-Hodgkin’s lymphoma. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(2):251–257. doi: 10.1158/1055-9965.EPI-05-0556. [DOI] [PubMed] [Google Scholar]
- 19.Colt JS, Severson RK, Lubin J, Rothman N, Camann D, Davis S, Cerhan JR, Cozen W, Hartge P. Organochlorines in carpet dust and non-Hodgkin lymphoma. Epidemiology. 2005;16(4):516–525. doi: 10.1097/01.ede.0000164811.25760.f1. [DOI] [PubMed] [Google Scholar]
- 20.Kato I, Watanabe-Meserve H, Koenig KL, Baptiste MS, Lillquist PP, Frizzera G, Burke JS, Moseson M, Shore RE. Pesticide product use and risk of non-Hodgkin lymphoma in women. Environmental health perspectives. 2004;112(13):1275–1281. doi: 10.1289/ehp.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHI extension study protocol outline. [ https://www.whi.org/researchers/studydoc/Consents/Protocol%202005–2010.pdf]
- 22.Protocol for clinical trial and observational study components. [ https://www.whi.org/researchers/studydoc/Consents/Protocol%201993–2005.pdf]
- 23.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Annals of epidemiology. 2003;13(9 Suppl):S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 24.Swerdlow SH, Campo E, Harris L, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 25.Turner JJ, Morton LM, Linet MS, Clarke CA, Kadin ME, Vajdic CM, Monnereau A, Maynadie M, Chiu BC, Marcos-Gragera R, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116(20):e90–98. doi: 10.1182/blood-2010-06-289561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 27.Zahm SH, Weisenburger DD, Saal RC, Vaught JB, Babbitt PA, Blair A. The role of agricultural pesticide use in the development of non-Hodgkin’s lymphoma in women. Archives of environmental health. 1993;48(5):353–358. doi: 10.1080/00039896.1993.9936725. [DOI] [PubMed] [Google Scholar]
- 28.Jones RR, Yu CL, Nuckols JR, Cerhan JR, Airola M, Ross JA, Robien K, Ward MH. Farm residence and lymphohematopoietic cancers in the Iowa Women’s Health Study. Environmental research. 2014;133:353–361. doi: 10.1016/j.envres.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schinasi L, Leon ME. Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis. International journal of environmental research and public health. 2014;11(4):4449–4527. doi: 10.3390/ijerph110404449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cocco P, Satta G, Dubois S, Pili C, Pilleri M, Zucca M, t Mannetje AM, Becker N, Benavente Y, de Sanjose S, et al. Lymphoma risk and occupational exposure to pesticides: results of the Epilymph study. Occupational and environmental medicine. 2013;70(2):91–98. doi: 10.1136/oemed-2012-100845. [DOI] [PubMed] [Google Scholar]
- 31.Zheng T, Zahm SH, Cantor KP, Weisenburger DD, Zhang Y, Blair A. Agricultural exposure to carbamate pesticides and risk of non-Hodgkin lymphoma. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2001;43(7):641–649. doi: 10.1097/00043764-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Alavanja MC, Hofmann JN, Lynch CF, Hines CJ, Barry KH, Barker J, Buckman DW, Thomas K, Sandler DP, Hoppin JA, et al. Non-hodgkin lymphoma risk and insecticide, fungicide and fumigant use in the agricultural health study. PloS one. 2014;9(10):e109332. doi: 10.1371/journal.pone.0109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parks CG, Walitt BT, Pettinger M, Chen JC, de Roos AJ, Hunt J, Sarto G, Howard BV. Insecticide use and risk of rheumatoid arthritis and systemic lupus erythematosus in the Women’s Health Initiative Observational Study. Arthritis care & research. 2011;63(2):184–194. doi: 10.1002/acr.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chlordane, CAS # 57-74-9. [ http://www.atsdr.cdc.gov/tfacts31.pdf]
- 35.Interim reregistration eligibility decision for chlorpyrifos: EPA 738-R-01-007. [ http://www.epa.gov/pesticides/reregistration/REDs/chlorpyrifos_ired.pdf]
- 36. (EPA 738-R-04-006).Interim reregistration eligibility decision: Diazinon. [ http://www.epa.gov/pesticides/reregistration/REDs/diazinon_ired.pdf]
- 37.Bekarian N, Payne-Sturges D, Edmondson S, Chism B, Woodruff TJ. Use of point-of-sale data to track usage patterns of residential pesticides: methodology development. Environmental health: a global access science source. 2006;5:15. doi: 10.1186/1476-069X-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horton MK, Jacobson JB, McKelvey W, Holmes D, Fincher B, Quantano A, Diaz BP, Shabbazz F, Shepard P, Rundle A, et al. Characterization of residential pest control products used in inner city communities in New York City. Journal of exposure science & environmental epidemiology. 2011;21(3):291–301. doi: 10.1038/jes.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


