Abstract
Craniomaxillofacial reconstructive surgery is a challenging field. First it aims to restore primary functions and second to preserve craniofacial anatomical features like symmetry and harmony. Three-dimensional (3D) printed biomodels have been widely adopted in medical fields by providing tactile feedback and a superior appreciation of visuospatial relationship between anatomical structures. Craniomaxillofacial reconstructive surgery was one of the first areas to implement 3D printing technology in their practice. Biomodeling has been used in craniofacial reconstruction of traumatic injuries, congenital disorders, tumor removal, iatrogenic injuries (e.g., decompressive craniectomies), orthognathic surgery, and implantology. 3D printing has proven to improve and enable an optimization of preoperative planning, develop intraoperative guidance tools, reduce operative time, and significantly improve the biofunctional and the aesthetic outcome. This technology has also shown great potential in enriching the teaching of medical students and surgical residents. The aim of this review is to present the current status of 3D printing technology and its practical and innovative applications, specifically in craniomaxillofacial reconstructive surgery, illustrated with two clinical cases where the 3D printing technology was successfully used.
Keywords: 3D printing, rapid prototyping, craniomaxillofacial defects, implants, prosthesis, reconstructive surgery
Reconstruction of the craniomaxillofacial defects, whether secondary to trauma, tumor resection, iatrogenic lesion, infection, congenital defects, or genetic disorders, is a particularly challenging and very demanding field. These injuries seriously affect not only the patient's basic functions like vision, breathing, speech, mastication, and swallowing but also his physical appearance with a major impact in the quality of life and social role. Surgical indications and approaches should be strictly selected, based on the expected surgical outcome. Restoration of oral and craniofacial functions is the main goal, but never forgetting aesthetic facial features, seeking for facial harmony and the most perfect symmetry.1 2 3
Nowadays, autologous reconstruction techniques, namely, free flaps (fibula and iliac crest), are the gold standard for craniomaxillofacial complex osseous reconstructive surgery because of the limitations and morbidity of regional flaps (pectoralis major muscle with ribs, trapezius, temporalis muscle with calvaria), despite their advantage in tissue matching, concerning the final result. However, the use of free flaps may be limited by the availability of suitable donor sites, especially for large defects, further expensive surgeries, and tissue harvesting problems; donor site morbidity with an additional patient discomfort; chances of infection at both the recipient and donor sites; and increased surgical time.1 3
Thus, ongoing researches on both biological and nonbiological alternatives continue, with a major contribution from the field of three-dimensional (3D) printed biomedical models. They have the ability to replicate the morphology of a biological structure—a process called biomodeling.3 3D printing can be used to print implantable hard- and soft-tissue prostheses, surgical guides for intraoperative use, biocell printing of 3D tissue/organ, or to create scaffolds for tissue engineering.4 5 6
The special complexity of craniofacial anatomy, the presence or close relationship with vital structures (nerves, blood vessels, muscles, ligaments, cartilage, bone, lymph nodes, and glands), the uniqueness of each defect and chances of infection require a precise preoperative planning followed by a highly individualized and careful execution of the plan. Advanced imaging techniques have become an essential component of preoperative planning in reconstructive surgery. Image-guided improves preoperative planning by delineating safety margins during ablative tumor surgery, for example, and outlining the intended reconstructive outcome.7 However, conventional modalities, including 3D reconstructions of different organs, are limited by their representation on two-dimensional (2D) computer monitors preventing to interact physically with a model.5 8 9
3D printing technology, also known as rapid prototyping technology, additive manufacturing, or solid free-form technology, is not a new concept. Described for the first time by Charles Hull, it was introduced in the 1990s in the field of medicine by the production of physical models via computer-aided design (CAD), but it has been utilized in industrial design since 1980s.9 10 11 12 Once introduced in the biomedical field, several applications were identified as suitable for the production of biomodels to enhance surgical planning and simulation in implantology, neurosurgery, and orthopedics, as well as for the production of maxillofacial prostheses.2 13
Graspable 3D objects overcome the limitations of 3D visualization, displayed on flat screens. 3D objects can be produced based on computed tomography (CT) or magnetic resonance imaging (MRI) volumetric medical images. Using dedicated post-processing algorithms, a spatial model can be extracted from image datasets and exported to machine- readable data. This spatial model data are utilized by special printers for generating the final rapid prototype model.8 16
Significant improvements in clinical imaging and user-friendly 3D software with the progression of open source platforms, associated with the recent hardware developments (e.g., decreasing the size of 3D printers to affordable desktop 3D printers), reducing time consumption and costs, have enabled computer-aided 3D modeling of 3D biomodels using 3D printing techniques, such as stereolithography (SL), multijet modeling, selective laser sintering (SLS), binder jet technique, and fused deposition modeling (FDM).5 9
Providing tactile feedback and a superior appreciation of visuospatial relationship between anatomical structures using these 3D models, the potential advantages are enormous in various medical fields, and craniomaxillofacial reconstructive surgery was one of the areas that pioneered the use of the 3D printing technology.9 14
The emphasis of this article is on practical and innovative uses, illustrated with two clinical cases where the 3D printing technology was successfully employed for craniomaxillofacial reconstruction, so as to stimulate further growth of ideas and advancement of the technology in the surgical field.
Materials and Methods
To investigate the current status of 3D printing technology and its clinical applications in the surgical field, particularly in the craniomaxillofacial reconstructive surgery, a review of the recent literature was conducted using PubMed, Web of science, and other reliable sources. A systematic search on National Library of Medicine was performed for related articles, based on the title of the abstract, and using the following key words: 3D printing, rapid prototyping, craniomaxillofacial defects, implants, prostheses, and reconstructive surgery.
We initially found 73 results, which were narrowed down to 54 after being reviewed according to the inclusion and exclusion criteria to form a set of 39 full-text articles selected for review.
Our inclusion criteria involved original articles, written in English, with no more than 15 years old, in which the 3D printing technology and its practical applications in craniomaxillofacial reconstruction was the key point, along with future perspectives of this technique and how it can affect the daily surgical practice.
The exclusion criteria included animal studies, non-English articles, and conference abstracts.
Results
3D-printed biomodels, as previously mentioned, can be used as an accurate, tactile visualization tool and a surgical simulation device to reproduce complex, patient-unique pathologies that facilitate the surgeons to preoperatively predict potential intraoperative challenges and postoperative outcomes as well as reduce risk of complications.2 9
Depending on the production technique, it is also possible to combine materials of different elasticity or color in one model, which can be useful to create more realistic models for educational or research purposes, or for naturally looking implant. The production time depends on the method used, and also on the size and complexity of the model.8 16
The dynamic process of production of 3D biomodels, generally known as reverse engineering, consists of the following four main sequential steps1 2 3 10 12:
Acquisition of high-quality volumetric 3D image data of the anatomical structure by CT or MRI for modeling. Less than a 1-mm CT slice thickness is recommended.
3D image processing to extract the region of interest from the surrounding tissues, which requires two types of software: first, a “3D modeling” software that translates the digital imaging and communications in medicine (DICOM) files from CT/MRI scans into a CAD file highlighting the region of interest, and second, a “3D slicing” software that divides the CAD file into thin data slices suitable for 3D printing.17
Model building by machining a block of material (subtractive manufacturing) or more often by adding material layer by layer and fusion of the layers (additive manufacturing).1
Quality assurance of the model and its dimensional accuracy.
It is essential that biomedical models created by 3D printing technology are subject to rigorous quality assurance at all steps of the process of production.15 18
Review of 3D Printing Techniques
The type of 3D printer chosen for an application often depends on the material available and how the layers in the finished product are bonded. In general, 3D printer accuracy would depend directly on the accuracy of CT scans, especially, of which thickness should be as thin as possible.10
We described a brief overview of the most common 3D printing technologies used for craniomaxillofacial reconstruction, with mention to their major advantages and disadvantages. The selection of the technique depends on the materials of interest, machine limitations, and the specific requirements of the final 3D biomodel, for example, the clinical application.15 18 19
Liquid-Based 3D Printing Technology
Stereolithography
The stereolithography (SL) is the most widely used 3D technique for craniofacial surgery, where a layer of liquid photopolymer or epoxy resin in a model-building platform is cured by a low-power ultraviolet (UV) laser. A mirror, computer controlled, is used to guide the UV laser focus onto the surface of the resin and cure the resin on a layer-by-layer basis. Each of these layers corresponds to the equivalent of a slice of an axial image on CT/MRI scan.2 8 The layers are cured sequentially and bind together to form a solid object, beginning from the bottom of the model and building upward.17 18 19 20 21 The final model, after being removed from the supporting structures, is cured in a UV chamber.9
Currently, SL is considered the gold standard in 3D biomedical model production, with the best smoother surface finishing, fast processing, and the greatest accuracy (0.025 mm).4 9 22 Though, this technique has also few disadvantages—for example, it requires extensive post-production manual handling and high cost related to the materials, the printer, and maintenance.17 22
Recently, a new technique called continuous liquid interface production (CLIP) was developed based on the conventional SL but increasing the production speed by harnessing oxygen inhibition of UV. However, this technique has not been yet evaluated in reconstructive surgery.23
PolyJet Modeling
PolyJet modeling (PM) is performed by jetting liquid photopolymer materials in ultra-thin layers (16 µm) onto a build tray layer by layer, until the model is completed. The advantage of this technique is that each photopolymer layer is immediately cured by UV light immediately after it is jetted, avoiding the time-consuming post-processing in the UV chamber.19 It is also possible with this technique to print using different materials according to the desired degree of tensile strength and durability. The main limitation is the high cost of these printers, which can limit their use more often.9
Powder-Based 3D Printing Technology
Selective Laser Sintering
The SLS technique can be used to create metal, plastic, and ceramic models, by multiple steps, which require long fabrication time and high cost. First, the 2D slice data are fed into the SLS machine that directs the exposure path of the laser over a thin layer of powder, such as nylon or metals like titanium, previously deposited on the build tray and leveled with a roller. The CO2 laser beam heats the powder particles, fusing them to form a solid layer, and then moves along the x- and y-axes to design the structures according to the CAD data. After the first layer fuses, the build tray moves downward, and a new layer of powder is deposited and sintered and the process is repeated until the model is completed, without support during manufacturing. The prototype surface is opaque, usually abrasive and porous, and finished by sandblasting.19 20
3D Printing (BinderJet)
The BinderJet system uses a print head to selectively disperse a binder onto powder layers. A thin layer of powder is spread over a tray using a roller similar to that used in the SLS system. The print head scans the powder tray and delivers a continuous jet of a solution that binds the powder particles as it touches them.21
No support structures are required while the model is being produced, because the surrounding powder supports the unconnected parts. When the process is complete, the surrounding powder is aspirated. It has a lower cost and can be used for formation of complex geometrical structures.
Solid-Based 3D Printing Technology
Fused deposition modeling (FDM) uses a similar principle to SL in that it builds models on a layer-by-layer basis. The main difference is that the layers are deposited as a thermoplastic that is extruded from a fine nozzle by computer control. The 3D model is constructed by extruding the heated thermoplastic material onto a foam surface along a path indicated by the model data.19
As each layer of plastic cools, it hardens, gradually creating the final model with stability, durability, and mechanical properties.2 12 Depending on the complexity and cost of an FDM printer, it may have enhanced features such as multiple print heads. FDM printers can use a variety of plastics; the most common material used for this procedure is acrylonitrile butadiene styrene.22 24
Clinical Applications of 3D Printing Technology in Craniomaxillofacial Reconstructive Surgery
Cranioplasty
Craniofacial anomalies are among the most common human birth defects/genetic disorders and have considerable functional, aesthetic, and social consequences that require complex clinical and surgical management.24 Cranioplasty is the procedure of choice for correction of cranial defects commonly caused by trauma, after tumor resection, birth or congenital defects, or after decompressive craniotomy.1 2
Until recently, when reconstructing craniofacial defects, the accuracy of modeling the missing part depended mainly on the sculpting skills or the surgeon.2 Biomodeling changed this reality. Being able to visualize the model from various angles facilitated an intuitive understanding of the anatomical relationship between the structures.9 The creation of customized implants for reconstruction of craniomaxillofacial defects is now a reality with a precise adaptation to the region of implantation, which reduces surgical times and costs, in turn leading to less chances for infection, faster recovery, and better aesthetics results.1
A 3D digital model of the cranium is generated from the CT data. In primary reconstructions, this model is used to define the exact osteotomies sites, designing a surgical guide that fits perfectly the defect. In secondary reconstructions, the virtual model is then used to create the implant design either by mirroring from the contralateral side or by generation curves based on the anatomical region with CAD-based devices.3
The ideal cranial implant material would fit the cranial defect and achieve complete closure, (e.g., radiolucent for postoperative imaging), resistant to infections, strong to biomechanical processes, easy to shape, not expensive, and ready to use.1 25 26 27
Maxillary Reconstruction
Maxillectomy defects become more complex when critical structures such as the orbit, globe, and cranial base are involved. The reconstruction of midfacial defects can be classified into the following different types, according to the modified classification system of maxillectomies, proposed by Costa et al28:
Type I: Limited maxillectomy
Type Ia: Excluding the nasomaxillary structure, with resection of the horizontal plate, preserving the maxillary arch
Type Ib: Including the nasomaxillary structure with preservation of the anterior maxillary arch and palate
Type Ic: Including the nasomaxillary structure with resection of the anterior maxillary arch and palate
Type II: Subtotal or infrastructural maxillectomy
Type IIa: With resection of less than 50% of the palate
Type IIb: With resection of greater than 50% of the palate
Type III: Total maxillectomy
Type IIIa: With preservation of the orbital contents
Type IIIam: With orbital contents preservation and mandible resection
Type IIIb: With orbital contents exenteration
Type IIIbm: With extraction of the orbital contents and mandible resection
Type IV: Orbital or suprastructural maxillectomy
» Type IVa: With preservation of the orbital roof
» Type IVb: With resection of the orbital roof
In maxillary surgery, after imaging and virtual reconstruction of the maxilla, surgeons use the technology to carry out their digitally planned maxillary osteotomies and subsequent reconstruction with iliac crest or fibula bone. Digitally mirroring the healthy maxilla to the abnormal side helps guide the ideal skeletal geometry.1 3
In maxillofacial surgery, this has been successfully implemented in the form of interchangeable jigs that constrain operative placement to a specific area. As a result, planned osteotomies and bone movements are accurately defined and tailored to patient anatomy.29 These guides have been extensively used in maxillary and particularity mandibular reconstructive surgery, to replace what was previously considered a gold standard in treatment with fibular osseous vascular free flaps.30 31 32 33
Case 1: Maxillectomy Type IIb
This is a case of a 33-year-old female patient, diagnosed with chronic osteomyelitis, involving the right maxilla. She had previously been submitted to multiple curettages, hyperbaric oxygen sessions, and different trials of antibiotics unsuccessfully.
Surgical planning included virtual planning (Fig. 1) based on 3D CT scan data (1 mm resolution) and imported into a CAD file. Thus, simulation of the surgical procedure with a 3D bio model (Fig. 2c), produced by SL technique, allowed better visualization of the skeletal structures and the generation of custom-made surgical guides to better restore facial symmetry.
Fig. 1.
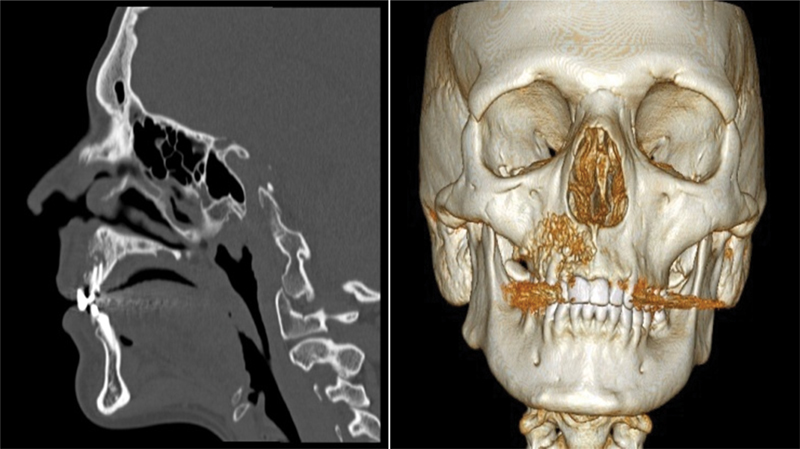
Virtual planning using 3D reconstruction software (Anatomics Pro, Anatomics TM, Melbourne, Australia) based on 3D CT scan data.
Fig. 2.
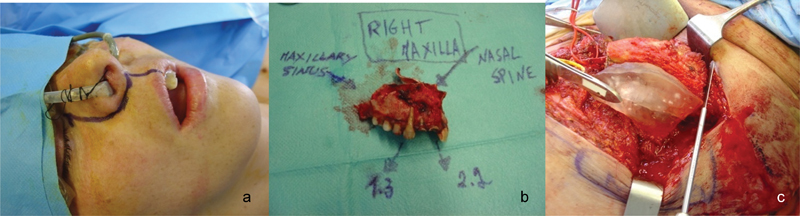
Clinical image of the patient before surgery (a), right maxilla specimen (b), and 3D biomedical model and surgical guides to dissect the iliac crest (c).
She was submitted to a right subtotal maxillectomy followed by immediate reconstruction with a vascularized chimeric myosseous iliac crest with a patch of internal oblique muscle (Fig. 3a–d).
Fig. 3.
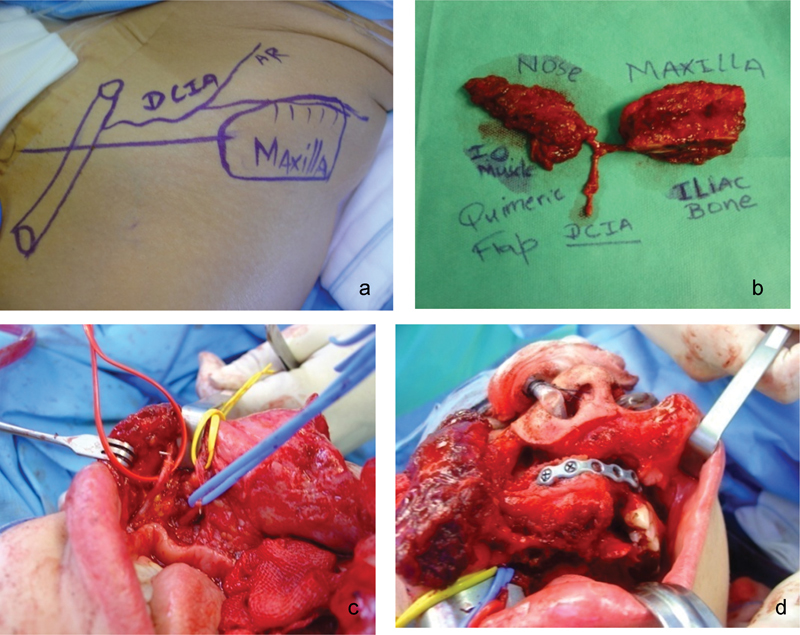
Subtotal maxillectomy with resection of greater than 50% of the palate. (a and b) Chimeric in muscle and bone flap. (c and d) Myosseous iliac crest flap implantation.
The required volume, length, and general morphology of the free flap were obtained with high accuracy according to the 3D biomedical model and the custom fitting surgical guides. This optimized the reconstructive surgery as incisions could be smaller, with less blood loss and significant improvement in the surgeon's overall visibility.
This clinical case illustrates how the use of 3D-printed models in maxillofacial surgery can determinate the final result (Fig. 4), combining a successful resection of the infected right maxilla with a very positive outcome and superior aesthetic result.
Fig. 4.
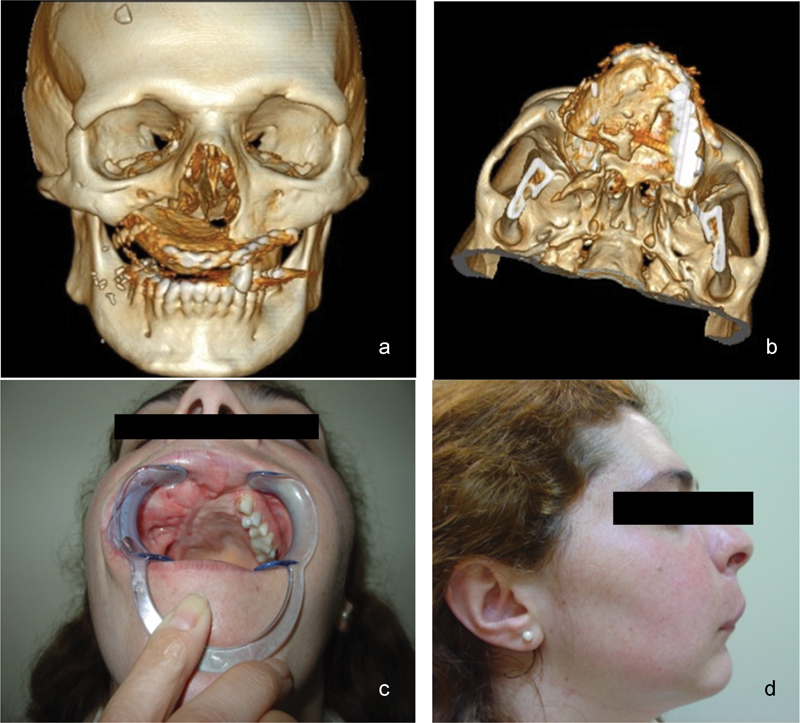
One-year postoperative images: (a and b) 3D virtual reconstructions; (c and d) inside and lateral views.
Mandibular Reconstruction
When planning facial reconstructive procedures, the ultimate goals of mandibular reconstruction are to restore speech, masticatory function, swallowing and respiration, and preserve facial features. Current reconstruction procedures combine mandible reconstruction plate fixation and use of microvascular flaps.1 In complex mandibular reconstructions using free flaps, to achieve a good functional and aesthetic outcome, knowing the precise three-dimensional characteristics of the flap is determinant to restore symmetry and structural integrity.34
Thus, by using specific software, it is possible to calculate the exact contours, angles, length, and morphology of the new mandible.3 Digitally mirroring the healthy mandible to the abnormal side helps guide the ideal skeletal geometry. Then, by using 3D-printed biomodels, there is an enhancement in the positioning of mandibular segments, shorter operative time by no intraoperative repeated bending and adapting of plates, and also less chances of postoperative plate breakage. The use of the original surface of the cortical bone as a template for adapting the reconstruction plate, facilitation of the preoperative surgical simulation, and restoration of centric occlusion of the patient were some of the benefits of virtual preoperative planning.1
However, to apply the 3D-printed titanium implant, the surgical cut or ostectomy should be matched precisely with the preoperative planning, because the 3D-printed implants are so solid that they are not easy to cut or bend. Therefore, a surgical osteotomy guide should be made.10
In some cases, a combination of custom implants and other corrective surgical procedures, such as fixation of salvageable large chunks of fractured bone as in blown-out midfacial fractures, is performed to restore the facial structure.1
Case 2: Left Hemimandibulectomy Sparing the Condyle
This is a case of a 46-year-old male patient with past history of advanced oral squamous cell carcinoma (T3N2bM0) previously submitted to radical excision with ipsilateral radical neck dissection and adjuvant radiotherapy (Fig. 5).
Fig. 5.
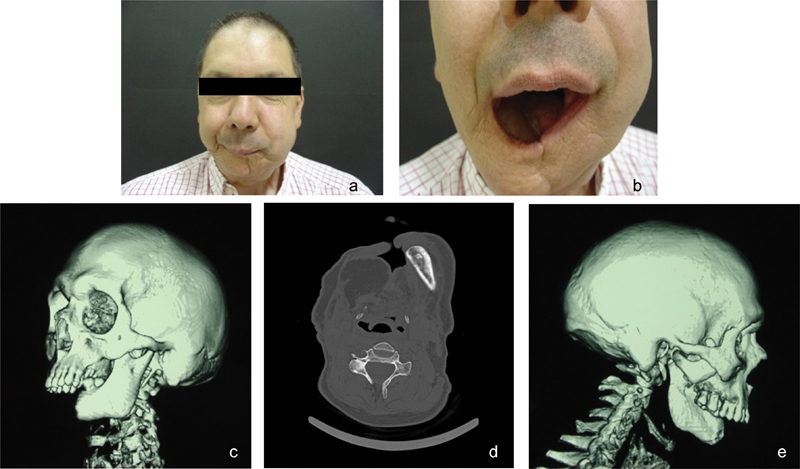
(a and b) Preoperative images of the patient, frontal view. (c–e) Preoperative CT scan data with 3D reconstruction.
Preoperative planning by 3D biomodeling technique (SL) was adopted to restore the facial symmetry of the patient and restore/improve the biofunctionality, like correction of the occlusal plan and other bone structures. The unaffected mandible was laterally mirrored or inverted and positioned to obtain the most accurate and precise result. A surgical guide for bone and plate modeling was then generated (Fig. 6).
Fig. 6.
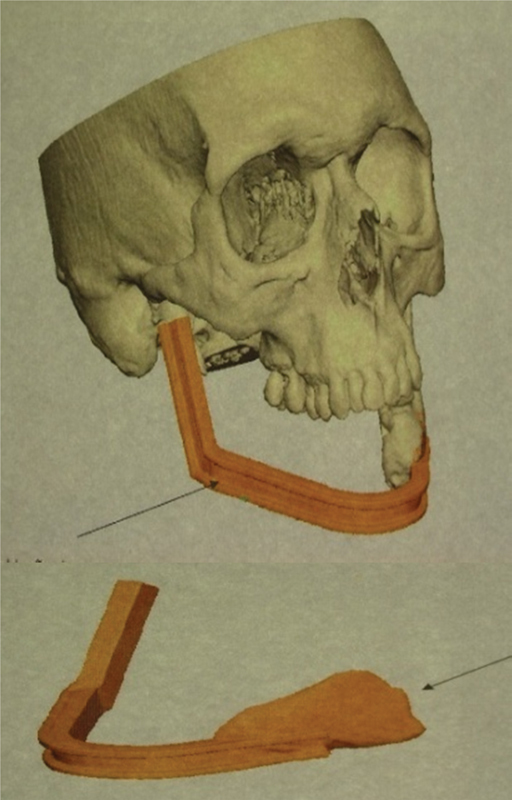
Virtual preoperative planning by 3D biomodeling technique with definition of exact bone defect and generation of a patient-specific surgical guide for bone and osteosynthesis plate modeling.
The patient underwent reconstruction with scaffold-guided 3D biomodeled osteomuscular iliac crest free flap (Fig. 7).
Fig. 7.
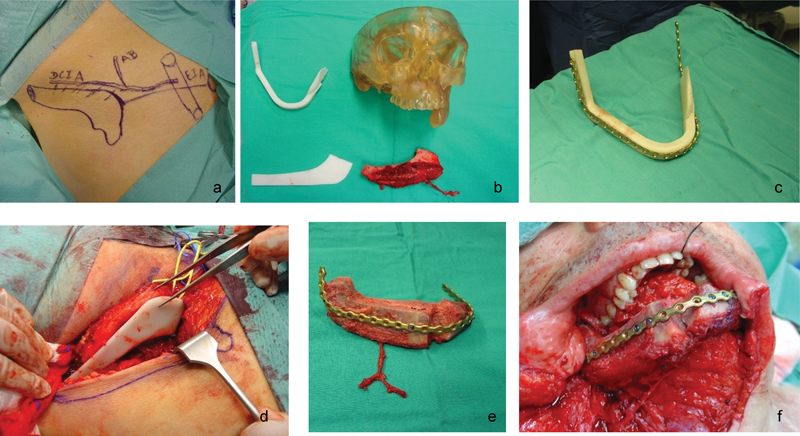
(a) Preoperative iliac crest free flap planning; (b–d) 3D biomodel of patient anatomy and custom fitting surgical guides; (e) free flap tailored in accordance with patient's defect morphology, volume, length, and angle; (f) Iliac crest free flap implantation.
Nine months after the surgery, as we can see in the postoperative images (Fig. 8), there is a significant improvement in patient's facial features by restoring the mandibular defect and biofunctionality, enabling the possibility for dental osteointegrated implant-based rehabilitation.
Fig. 8.
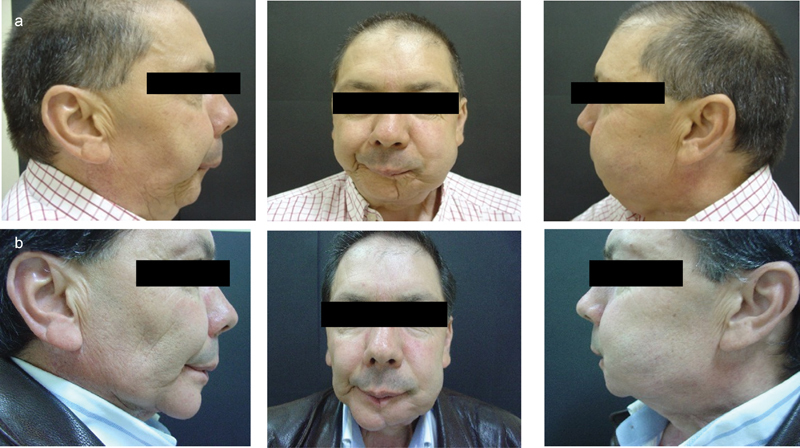
(a and b) Before and 9 months after reconstructive surgery.
Discussion
3D printing technology is innovating medicine, which furthermore challenges the surgical practice in terms of patient-specific individualized approach. It can provide an individual product in a short period of time, which suits the goal of individualized medicine, where each patient requires a specific, tailored, therapeutic approach.
The ultimate goal of any reconstructive surgical procedure is to reproduce or improve preoperative form and function. Application of 3D printing in craniomaxillofacial reconstructive surgery is changing the way surgeons are planning their surgeries and the designers are designing custom 3D biomedical models.
No matter which specific technique is used to produce the 3D biomodel, the following are proven advantages of using 3D printing for reconstructive surgery4 9 10 15 17 35:
By direct visualization of anatomic structures and their spatial relationships, there is an improved understanding of complex underlying conditions which significantly enhances the quality of diagnosis and treatment planning.
With patient's prior consent, plastic surgeons may better provide preoperative counseling to their patients utilizing 3D modeling.
There is improvement in preoperative surgical planning by designing incisions and surgical resection margins. 3D biomodel also allows the assessment of bony defects for grafting and the adaptation/prebending of reconstruction plates.
It helps in the development of intraoperative guidance tools, and improve communication among surgeons. This can translate into shorter operative time; reduced time under general anesthesia; shorter duration of wound exposure; and reduced intraoperative blood loss, errors, and risks.
It also helps in the production of patient-specific implants/prosthetics in everyday surgical practice such as TMJ prostheses, distraction devices, and fixation devices; improves aesthetic outcomes as a result of individual fitting; and complements individual anatomical needs. Furthermore, customized implants avoid the need to intraoperatively modify and adjust as occurs with the standard implants, which can directly lead to improved clinical outcomes and a decreased risk of complications, for example, infections.
In comparison to a standard implant, a custom-made implant is more likely to yield superior functional and esthetic outcomes. Typical 3D printing materials can be sterilized using chemicals, such as Food and Drug Administration–approved glutaraldehyde protocols, steam, and gas for intraoperative handling. In the last decade, investigators have reported 3D-printed prostheses of nose, ears, eyes, face, and hand.9
It provides an educational tool for medical students and residents. Physical models can be realistically held and rotated, can be interactively manipulated regardless of complexity, and are accessible without the need for computers or advanced training.4 36
The 3D printing is more predictable and provides accurate surgical outcomes.
The 3D printing is beneficial not only for bone reconstruction but also for replacing soft tissues, as it is possible to use a variety of materials.8
3D printing as an innovating technique has also some limitations to consider. The main limitations include high costs and complexity, as well as the need for specialized equipment and consumables such as photo-resistant resins.3
Rapid prototyping can only be applied to structure not exceeding certain dimensions, as 3D printers are not able to produce extremely large, whole-body models.
The time needed for producing a 3D model also limits its use in surgery to elective cases and makes it unsuitable for emergency cases (oncology and acute trauma).8 34
Another disadvantage is the higher radiation dosage to which the patient has to be exposed due to specific CT scanning criteria, comparing with a regular CT scan for diagnosis.3 34
These limitations might be overcome by future technological developments.
Conclusion
Cranial and maxillofacial structures are not only complex but also unique among individuals. The use of these 3D biomedical models in cranial and maxillofacial reconstructive surgery proved to be very useful in the design and fabrication of custom prosthesis and sizing of bone grafts, and it also allowed for manufacturing of scaffolds for bone regeneration as well as other aspects of medical education and research. According to Zenha et al,34 the use of these 3D biomedical models is particularly useful in major secondary defects (oncological, osteoradionecrosis, trauma) with relevant distortion of craniofacial structure; congenital malformations, and primary tumor surgery, when the dimensions of the tumor have altered normal anatomy significantly. Custom-made implants for the reconstruction of craniofacial defects, preserving patients' original anatomy, have gained importance due to better performance—function and aesthetic—over their generic counterparts.1 3
However, despite the reported results, additional progress for 3D printing technologies is needed for increasing resolution without sacrificing shape, strength, and hand ability of the 3D biomodels. The National Institutes of Health established the 3D Print Exchange (3dprint.nih.gov) in 2014 to promote open-source sharing of 3D print files for medical and anatomical models and custom.36 37 The integration of patient-derived tissue and induced pluripotent stem cells with rapid advances in 3D printing of biological tissues and materials holds great for bioprinting organs by using multiple print heads which will deposit different cell types (organ specific, blood vessel, and muscle cells), necessary feature for fabricating whole heterocellular tissues and organs, as potential solution to the lack of organs for transplantation.38
Over the last decade, image-guided production of 3D biomedical models has been recognized as useful tool in different fields of medicine with multiple applications. Recently, a fourth dimension was introduced to 3D Printing: time. With this fourth dimension of time, 4D printing delivers complex spatiotemporal anatomical details effortlessly and may improve even more the preoperative planning comparing with the 3D printing.9 39
Acknowledgments
The authors express their gratitude to all the members of the Plastic, Reconstructive and Craniomaxillofacial Surgery Department of the Centro Hospitalar Vila Nova de Gaia/Espinho (medical doctors, nursing staff, and secretaries) for their support in the treatment of these patients.
References
- 1.Parthasarathy J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann Maxillofac Surg. 2014;4(1):9–18. doi: 10.4103/2231-0746.133065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller A, Krishnan K, Uhl E, Mast G. The application of rapid prototyping techniques in cranial reconstruction and preoperative planning in neurosurgery. J Craniofac Surg. 2003;14(6):899–914. doi: 10.1097/00001665-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Zenha H, Barroso M L, Costa H. InTech; 2012. Advanced 3-D biomodelling technology for complex mandibular reconstruction; pp. 217–232. [Google Scholar]
- 4.Oliveira M, Hussain N S, Dias A G. et al. 3-D biomodelling technology for maxillofacial reconstruction. Mater Sci Eng C. 2008;28:1347–1351. [Google Scholar]
- 5.Naftulin J S, Kimchi E Y, Cash S S. Streamlined, inexpensive 3D printing of the brain and skull. PLoS One. 2015;10(8):e0136198. doi: 10.1371/journal.pone.0136198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michalski M H, Ross J S. The shape of things to come: 3D printing in medicine. JAMA. 2014;312(21):2213–2214. doi: 10.1001/jama.2014.9542. [DOI] [PubMed] [Google Scholar]
- 7.Hohlweg-Majert B, Schön R, Schmelzeisen R, Gellrich N C, Schramm A. Navigational maxillofacial surgery using virtual models. World J Surg. 2005;29(12):1530–1538. doi: 10.1007/s00268-005-0091-0. [DOI] [PubMed] [Google Scholar]
- 8.Rengier F, Mehndiratta A, von Tengg-Kobligk H. et al. 3D printing based on imaging data: review of medical applications. Int J CARS. 2010;5(4):335–341. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 9.Chae M P, Rozen W M, McMenamin P G, Findlay M W, Spychal R T, Hunter-Smith D J. Emerging applications of beside 3D printing in plastic surgery. Front Surg. 2015;2(25):25. doi: 10.3389/fsurg.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi J W, Kim N. Clinical application of three-dimensional printing technology in craniofacial plastic surgery. Arch Plast Surg. 2015;42(3):267–277. doi: 10.5999/aps.2015.42.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerstle T L, Ibrahim A M, Kim P S, Lee B T, Lin S J. A plastic surgery application in evolution: three-dimensional printing. Plast Reconstr Surg. 2014;133(2):446–451. doi: 10.1097/01.prs.0000436844.92623.d3. [DOI] [PubMed] [Google Scholar]
- 12.Ventola C L. Medical applications for 3D printing: current and projected uses. P&T. 2014;39(10):704–711. [PMC free article] [PubMed] [Google Scholar]
- 13.Goiato M C, Santos M R, Pesqueira A A, Moreno A, dos Santos D M, Haddad M F. Prototyping for surgical and prosthetic treatment. J Craniofac Surg. 2011;22(3):914–917. doi: 10.1097/SCS.0b013e31820f7f90. [DOI] [PubMed] [Google Scholar]
- 14.Bibb R, Eggbeer D, Evans P, Bocca A, Sugar A. Rapid manufacture of custom fitting surgical guides. Rapid Prototyping J. 2009;15(5):346–354. [Google Scholar]
- 15.Mehra P, Miner J, D'Innocenzo R, Nadershah M. Use of 3-d stereolithographic models in oral and maxillofacial surgery. J Maxillofac Oral Surg. 2011;10(1):6–13. doi: 10.1007/s12663-011-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsuragi Y, Kayano S, Akazawa S. et al. Mandible reconstruction using the calcium-sulphate three-dimensional model and rubber stick: a new method, ‘mould technique’, for more accurate, efficient and simplified fabrication. J Plast Reconstr Aesthet Surg. 2011;64(5):614–622. doi: 10.1016/j.bjps.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Winder J, Bibb R. Medical rapid prototyping technologies: state of the art and current limitations for application in oral and maxillofacial surgery. J Oral Maxillofac Surg. 2005;63(7):1006–1015. doi: 10.1016/j.joms.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Chia H N, Wu B M. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9(4):4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olszewski R, Reychler H. Belgium: InTech; 2011. Clinical applications of rapid prototyping models in cranio- maxillofacial surgery; pp. 173–206. [Google Scholar]
- 20.Malik H H, Darwood A R, Shaunak S. et al. Three-dimensional printing in surgery: a review of current surgical applications. J Surg Res. 2015;199(2):512–522. doi: 10.1016/j.jss.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 21.Wu G H, Hsu S H. Review: polymeric-based 3D printing for tissue engineering. J Med Biol Eng. 2015;35(3):285–292. doi: 10.1007/s40846-015-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinn D P, Cillo J E Jr, Miles B A. Stereolithography for craniofacial surgery. J Craniofac Surg. 2006;17(5):869–875. doi: 10.1097/01.scs.0000230618.95012.1d. [DOI] [PubMed] [Google Scholar]
- 23.Tumbleston J R Shirvanyants D Ermoshkin N et al. Additive manufacturing. Continuous liquid interface production of 3D objects Science 2015347(6228):1349–1352. [DOI] [PubMed] [Google Scholar]
- 24.Hoy M B. 3D printing: making things at the library. Med Ref Serv Q. 2013;32(1):94–99. doi: 10.1080/02763869.2013.749139. [DOI] [PubMed] [Google Scholar]
- 25.Trainor P A, Richtsmeier J T. Facing up to the challenges of advancing Craniofacial Research. Am J Med Genet A. 2015;167(7):1451–1454. doi: 10.1002/ajmg.a.37065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendez B M, Chiodo M V, Patel P A. Customized “in-office” three-dimensional printing for virtual surgical planning in craniofacial surgery. J Craniofac Surg. 2015;26(5):1584–1586. doi: 10.1097/SCS.0000000000001768. [DOI] [PubMed] [Google Scholar]
- 27.Kelley D J, Farhoud M, Meyerand M E. et al. Creating physical 3D stereolithograph models of brain and skull. PLoS One. 2007;2(10):e1119. doi: 10.1371/journal.pone.0001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa H, Zenha H, Sequeira H. et al. Microsurgical reconstruction of the maxilla: Algorithm and concepts. J Plast Reconstr Aesthet Surg. 2015;68(5):e89–e104. doi: 10.1016/j.bjps.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Ayoub A F, Rehab M, O'Neil M. et al. A novel approach for planning orthognathic surgery: the integration of dental casts into three-dimensional printed mandibular models. Int J Oral Maxillofac Surg. 2014;43(4):454–459. doi: 10.1016/j.ijom.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 30.López-Arcas J M, Arias J, Del Castillo J L. et al. The fibula osteomyocutaneous flap for mandible reconstruction: a 15-year experience. J Oral Maxillofac Surg. 2010;68(10):2377–2384. doi: 10.1016/j.joms.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 31.Schepers R H, Raghoebar G M, Vissink A. et al. Fully 3-dimensional digitally planned reconstruction of a mandible with a free vascularized fibula and immediate placement of an implant-supported prosthetic construction. Head Neck. 2013;35(4):E109–E114. doi: 10.1002/hed.21922. [DOI] [PubMed] [Google Scholar]
- 32.Levine J P, Patel A, Saadeh P B, Hirsch D L. Computer-aided design and manufacturing in craniomaxillofacial surgery: the new state of the art. J Craniofac Surg. 2012;23(1):288–293. doi: 10.1097/SCS.0b013e318241ba92. [DOI] [PubMed] [Google Scholar]
- 33.Rotaru H, Schumacher R, Kim S G, Dinu C. Selective laser melted titanium implants: a new technique for the reconstruction of extensive zygomatic complex defects. Maxillofac Plast Reconstr Surg. 2015;37(1):1–6. doi: 10.1186/s40902-015-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zenha H, Azevedo L, Rios L. et al. The application of 3-D biomodeling technology in complex mandibular reconstruction – experience of 47 clinical cases. Eur J Plast Surg. 2011;34(4):257–265. [Google Scholar]
- 35.AlAli A B, Griffin M F, Butler P E. Three-dimensional printing surgical applications. Eplasty. 2015;15:e37. [PMC free article] [PubMed] [Google Scholar]
- 36.Wurm G, Tomancok B, Holl K, Trenkler J. Prospective study on cranioplasty with individual carbon fiber reinforced polymer (CFRP) implants produced by means of stereolithography. Surg Neurol. 2004;62(6):510–521. doi: 10.1016/j.surneu.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 37.3D Print Exchange National Institutes of Health Available at: http://3dprint.nih.gov. Accessed January 25, 2016
- 38.Ozbolat I T, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013;60(3):691–699. doi: 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 39.Chae M P, Hunter-Smith D J, De-Silva I, Tham S, Spychal R T, Rozen W M. Four-dimensional (4D) printing: a new evolution in computed tomography-guided stereolithographic modeling. Principles and application. J Reconstr Microsurg. 2015;31(6):458–463. doi: 10.1055/s-0035-1549006. [DOI] [PubMed] [Google Scholar]


