Abstract
OBJECTIVE
To examine the association between long-term metformin therapy and serum vitamin B12 monitoring.
DESIGN
Retrospective cohort study.
SETTING
A single Veterans Affairs Medical Center (VAMC), 2002-2012.
PARTICIPANTS
Veterans 50 years or older with either Type 2 diabetes and long-term metformin therapy (n=3,687) or without diabetes and no prescription for metformin (n=13,258).
MEASUREMENTS
We determined diabetes status from outpatient visits, and defined long-term metformin therapy as a prescription ≥500 mg/day for at least six consecutive months. We estimated the proportion of participants who received a serum B12 test and used multivariable logistic regression, stratified by age, to evaluate the association between metformin use and serum B12 testing.
RESULTS
Only 37% of older adults with diabetes receiving metformin were tested for vitamin B12 status after long-term metformin prescription. The mean B12 concentration was significantly lower in the metformin-exposed group (439.2 pg/dL) compared to those without diabetes (522.4 pg/dL) (P=0.0015). About 7% of persons with diabetes receiving metformin were vitamin B12 deficient (<170 pg/dL) compared to 3% of persons without diabetes or metformin use (P=0.0001). Depending on their age, metformin users were 2- to 3-times more likely not to receive vitamin B12 testing compared to those without metformin exposure, after adjusting for sex, race and ethnicity, body mass index, and number of years treated at the VAMC.
CONCLUSION
Long-term metformin therapy is significantly associated with lower serum vitamin B12 concentration, yet those at risk are often not monitored for B12 deficiency. Because metformin is first line therapy for Type 2 diabetes, clinical decision support should be considered to promote serum B12 monitoring among long-term metformin users for timely identification of the potential need for B12 replacement.
Keywords: aging, metformin, Type 2 diabetes, Veterans, vitamin B12
INTRODUCTION
Diabetes and its complications are major contributors to morbidity and mortality in the U.S. (1). According to the 2011-2012 U.S. National Health and Nutrition Examination Survey, an estimated 14.3% of the adult population aged 20 years and older has diabetes (both diagnosed and undiagnosed) (2). The prevalence increases with advancing age, where 33.0% of adults aged 65-years and older are affected (2). Because the proportion of the U.S. population over the age of 65 years continues to grow, the number of people with Type 2 diabetes is expected to rise in the next 20 years (2). These trends have greater impact for the approximately 19 million U.S. Veterans, a large proportion of whom are male and older, and many of whom have diabetes and thus are at risk for its adverse health impacts (3; 4).
Metformin is recommended as first line treatment for individuals with Type 2 diabetes and normal kidney function (5; 6). Almost 70% of all U.S. Veterans with Type 2 diabetes receive metformin as a first line therapy for diabetes (3). That metformin reduces serum concentrations of vitamin B12 remains poorly appreciated, despite several studies demonstrating the effect (7-11). The clinical impact of metformin-induced serum B12 deficiency has been less well studied. One study suggested an association between cumulative metformin dose and more severe peripheral neuropathy associated with B12 deficiency (12).
Detecting and treating advanced cases of vitamin B12 deficiency in patients receiving long-term metformin therapy could reduce morbidity and related healthcare costs; however, there is limited research on the prevalence and determinants of vitamin B12 monitoring among older adults who are prescribed metformin over an extended period. For example, a chart review-based study from a single Veterans Affairs Medical Center (VAMC) found that only 40% of patients on high dose metformin therapy (≥2000 mg/day) were monitored for serum vitamin B12 concentrations; and among those that were treated with metformin for more than 10 years, 50% never received a vitamin B12 assessment (13). A recently published study using data from a health maintenance organization in Israel showed that, among diabetic patients with incident metformin prescription, only 44.5% received a vitamin B12 test referral (14).
Based on the availability of longitudinal pharmacy and laboratory data, the present study aimed to examine the association between long-term metformin therapy and serum B12 monitoring in older Veterans. We hypothesized that Type 2 diabetic patients managed only on metformin therapy for six or more months would have at least the same rate of monitoring for serum vitamin B12 concentrations as a comparison group of persons without Type 2 diabetes and unexposed to metformin. We expect our findings to inform best practices for diabetes care among older adults.
METHODS
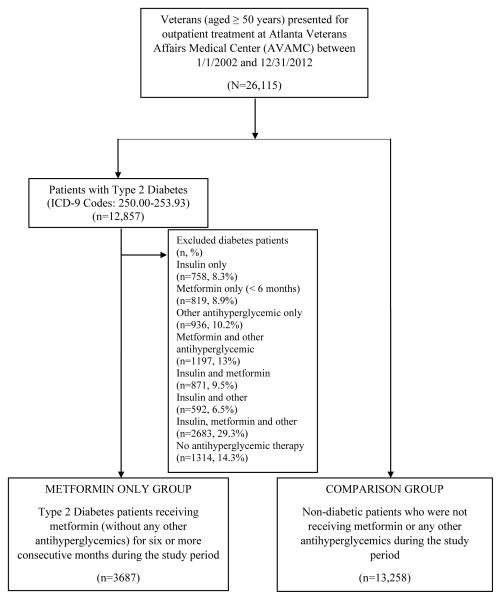
We conducted a retrospective cohort study using existing data for Veterans seen at a single VAMC between January 2002 and December 2012. Outpatient prescription and laboratory data for each patient were linked. We included all Veterans aged 50 years or older, without a history of pernicious anemia or end-stage renal disease. The exposed group consisted of patients with a diagnosis indicating Type 2 diabetes mellitus in the outpatient records, as identified by any of the following Internal Classification of Disease (ICD)-9 diagnosis codes: 250.00-250.90, 250.02-250.92 on at least one outpatient visit during the study period. Patients in the exposed group were also required to have a drug code indicating prescription filled for metformin within the electronic medical record at a dose of ≥500 mg/day, and refilled for at least six consecutive months during the study period. Diabetic patients who were prescribed other types of antihyperglycemics, including insulin, were not included to reduce confounding factors that might influence a provider’s clinical decision to order a serum B12 assessment such as more advanced diabetes with co-existing peripheral neuropathy. The comparison group included all Veterans identified in the outpatient visits of the same VAMC, but without any diagnostic code indicative of Type 2 diabetes; and without any prescription for metformin, or other antihyperglycemics, during the study period. The study was approved by the Emory University Institutional Review Board and the participating VAMC Research and Development Committee.
Study Variables
We examined selected demographic and clinical characteristics including age (continuous and categorical), sex, race and ethnicity, body mass index (BMI) (kg/m2) (continuous and categorical), and the number of years treated at the VAMC during the study period. Years treated at the VAMC were categorized into three groups: 1-3 years, 4-6 years, and 7-10 years. This grouping ensured an equal time interval to compare follow-up periods between diabetic Veterans receiving metformin and the comparison group. We examined serum vitamin B12 and creatinine concentrations using VAMC laboratory records. Patients were determined to have a serum B12 assessment if they had a valid test result any time after six months of metformin prescription. Laboratory results from the first serum B12 test any time after six consecutive months of metformin prescription were used in our analysis. Serum creatinine test results were derived from the test closest to the vitamin B12 test date. Outlier measures of vitamin B12 (defined as values >2,000 pg/dL) and creatinine (defined as values >25 mg/dL) were identified and recoded as missing. For categorical analysis, serum B12 concentrations were classified as deficient (<170 pg/dL), borderline (170-300 pg/dL), or adequate (>300 pg/dL). Serum creatinine levels were categorized as normal (<1.3 mg/dL) or elevated (≥1.3 mg/dL). Criteria for serum vitamin B12 and creatinine concentration categories were based on previously established cut-points among older Veterans (15).
Statistical Analysis
We estimated the proportion of patients who received a vitamin B12 test after six consecutive months of metformin therapy. We compared selected demographic and clinical characteristics among diabetic Veterans receiving metformin and the comparison group using Chi square tests for categorical variables and independent sample t-tests for continuous variables. The Wilcoxon Rank Sum Test was used to compare variables with non-parametric distributions (serum creatinine and index serum B12 concentration). We used multivariable logistic regression to examine the association between long-term metformin therapy and serum B12 monitoring. Potential co-factors, including sex, race, BMI, and years treated at the VAMC, were selected based on clinical relevance. These co-factors were also statistically significant as potential confounders in the multivariable model, using backward logistic regression and a 10% change-in-estimate criterion. Ultimately, we performed separate models stratified by age because age decade was a significant effect modifier (Breslow-Day Statistic P = 0.0088). All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
There were a total of 26,115 patients aged 50 years or older who met initial study eligibility criteria and received outpatient care at the VAMC during the study period. Of these, 12,857 patients (49.2%) were identified to have ICD-9 codes indicating Type 2 diabetes on at least one outpatient visit. Among the 12,857 patients, 3,687 (~30%) had a prescription filled for metformin for at least six consecutive months, and received no other antihyperglycemics. The comparison group consisted of 13,258 patients that did not have Type 2 diabetes and did not receive metformin or any other antihyperglycemics during our study period (Figure 1).
Figure 1.
Subject Selection Criteria
The mean age of patients in the metformin group was slightly younger at 60.9 years compared to 61.6 years in the comparison group (P < 0.001). Patients in both groups were predominantly male (98.4% metformin group and 96.4% comparison group; P< 0.0001). An increased proportion of metformin group patients were non-Hispanic white compared to non-Hispanic blacks (68.3% vs 54.0%, respectively; P<0.0001). The BMI measures showed a significantly increased likelihood of overweight and obesity among the exposed group compared to the unexposed group. About 60% of metformin group patients were treated at the VAMC for 7-10 years, allowing for a long follow-up period, whereas up to two-thirds of the unexposed group (57.7%) had only 1-3 years of treatment records (Table 1). Compared to included diabetic patients, diabetic patients who were ineligible were older (Mean=61.5 years), less likely to be non-Hispanic white (56.9%), had higher BMI (Mean=30.6 kg/m2) and a higher serum creatinine (Mean=1.3 mg/dL) (P < 0.001 for all) (data not shown).
Table 1.
Baseline Characteristics of Veterans with Diabetes and Metformin Use and the Comparison Group (No Diabetes and No Metformin Use) at a single Veterans Affairs Medical Center, 2002-2012
| Characteristics | Diabetes and Metformin (N=3687) |
No Diabetes No Metformina (N=13,258) |
P b | ||
|---|---|---|---|---|---|
| Age (years), Mean (SD) | 60.9 (8.2) | 61.6 (9.8) | <0.001* | ||
| Body Mass Index (kg/m2), Mean (SD) | (n=3670) 30.5 (5.9) |
(n=8864) 26.7 (6.2) |
0.0008* | ||
| Serum vitamin B12 (pg/dL), Mean (SD) | (n=1369) 439.2 (276.4) |
(n=4301) 522.4 (296.7) |
0.0015* | ||
| Serum creatinine (mg/dL), Mean (SD) | (n=3668) 1.1 (1.0) |
(n=8600) 1.2 (0.9) |
<0.0001* | ||
| n | (%) | n | (%) | ||
| Age group (years) | |||||
| 50-59 | 2101 | 57.0 | 7567 | 57.1 | <0.0001* |
| 60-69 | 965 | 26.2 | 2760 | 20.8 | |
| 70-79 | 544 | 14.8 | 2132 | 16.1 | |
| ≥80 | 77 | 2.1 | 799 | 6.0 | |
| Sex | |||||
| Male | 3629 | 98.4 | 12,778 | 96.4 | <0.0001* |
| Female | 58 | 1.6 | 480 | 3.6 | |
| Race and ethnicity | |||||
| Non-Hispanic, White | 2517 | 68.3 | 7154 | 54.0 | <0.0001* |
| Non-Hispanic, Black | 928 | 25.2 | 4938 | 37.3 | |
| Other | 242 | 6.6 | 1166 | 8.8 | |
| Body Mass Index (kg/m2) | |||||
| Low (<18.5) | 25 | 0.7 | 580 | 6.5 | <0.0001* |
| Normal (18.5-24.9) | 533 | 14.5 | 3171 | 35.8 | |
| Overweight (25.0-29.9) | 1354 | 36.9 | 2849 | 32.1 | |
| Obese (≥30) | 1758 | 47.9 | 2264 | 25.5 | |
| Number of years treated at the VAMC | |||||
| 1-3 | 397 | 10.8 | 7653 | 57.7 | <0.0001* |
| 4-6 | 1093 | 29.6 | 2530 | 19.1 | |
| 7-10 | 2197 | 59.6 | 3075 | 23.2 | |
| Received vitamin B12 assessmentc | |||||
| Yes | 1371 | 37.2 | 4325 | 32.6 | <0.0001* |
| Vitamin B12 concentration (pg/dL) | |||||
| <170 | 104 | 7.6 | 129 | 3.0 | <0.0001* |
| 170-300 | 398 | 29.1 | 822 | 19.1 | |
| >300 | 867 | 63.3 | 3350 | 77.9 | |
| Serum creatinine concentration (mg/dL) | |||||
| <1.3 | 3126 | 85.2 | 6605 | 76.8 | <0.0001* |
| ≥1.3 | 542 | 14.8 | 1995 | 23.2 | |
no other antihyperglycemics
Chi square test for categorical variables and two-sample t test for continuous variables.
Assessment any time after 6 months of consecutive metformin therapy among exposed, and anytime during the study period among unexposed.
Statistical significance set at P < 0.05; Frequencies may not equal total due to missing data.
SD, Standard Deviation; VAMC, Veterans Affairs Medical Center
Only 37% of the metformin group patients were tested for vitamin B12 status sometime after six months of metformin prescription during the study period. The mean B12 concentration was significantly lower in the metformin group (Mean=439.2 pg/dL; Standard Deviation=276.4 pg/dL) versus the comparison group (Mean=522.4 pg/dL; Standard Deviation=296.7 pg/dL) (P=0.0015) (Table 1). Overall, data on long-term metformin exposed patients who had information on B12 concentrations revealed that 7% were deficient in their serum concentrations of vitamin B12 (<170 pg/dL) compared to 3% of those who were not metformin exposed (P<0.0001). Additionally, patients in the exposed groups were also more likely to have borderline deficient B12 concentrations (170-300 pg/dL) compared to their counterparts (29.1% vs. 19.1%, respectively).
As the association between long-term metformin use and B12 testing was significantly modified by age, we stratified our multivariable analyses by age group. All models were adjusted for sex, race and ethnicity, BMI, and years of treatment at the VAMC. Our final results showed that among patients aged 50-59 years, long-term metformin use was associated with a 2.4-fold increased risk of not receiving a serum vitamin B12 test (adjusted odds ratio (aOR)=2.4; 95% confidence interval (CI)=2.2, 2.7) (Table 2). Patients 60-69 years and 70-79 years of age also had a similar, but slightly attenuated risk of being tested for serum vitamin B12 levels (aOR=1.9; 95% CI=1.6, 2.3 and aOR=2.0; 95% CI=1.6, 2.5), respectively). However, patients who were 80 years or older and categorized as long-term metformin users had the highest odds of not being tested for vitamin B12 (aOR=3.1; 95% CI=1.8, 5.4) compared to all other age groups examined in our study (Table 2).
Table 2.
Multivariable analysis to evaluate the absence of vitamin B12 testing associated with metformin use among older adults seeking outpatient care, stratified by age, at a single Veterans Affairs Medical Center, 2002-2012.
| Age Groups | |||
|---|---|---|---|
| 50-59 years | 60-69 years | 70-79 years | ≥80 years |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) |
| 2.4 (2.2,2.7) | 1.9 (1.6,2.3) | 2.0 (1.6,2.5) | 3.1 (1.8,5.4) |
Adjusted for sex, race and ethnicity, body mass index, and number of years treated at the Veterans Affairs Medical Center.
aOR, Adjusted Odds Ratio; CI, Confidence Interval
DISCUSSION
Our study demonstrates that long-term metformin therapy is not associated with timely assessment of serum B12 status and this association differs by age. Older metformin users between 50-59 years and those who are 80 and over have relatively higher odds (2.5- to 3.1 fold) of not being tested for vitamin B12 concentrations. Additionally, diabetic Veterans on long term metformin therapy had lower serum B12 concentrations on average than persons without Type 2 diabetes not taking metformin. This is particularly concerning because vitamin B12 deficiency is reversible and a potential contributor to the development of disability and peripheral neuropathy among older adults, both of which could add to the burden of morbidity and mortality associated with diabetes (12; 16-18). Whether vitamin B12 deficiency had any clinical implications in our study population was not in the scope of our study, and will be undertaken in a future analysis.
Overall, our finding that long-term metformin-exposed older adults had a lower mean serum B12 concentration compared to the unexposed group is consistent with previous studies (7-11). Additionally, those taking metformin were significantly more likely to have B12 deficiency (<300 pg/dL), which should prompt repletion and screening for other potential causes of B12 deficiency. Further, our data are consistent with smaller studies that providers fail to order a vitamin B12 assessment for their patients on long-term metformin therapy (13; 14).
Older diabetic patients are at an increased risk of peripheral neuropathy, neuropathic pain, and subsequent decreased mobility compared to those without diabetes and younger persons with diabetes (19-21). However, similar to many conditions prevalent among older adults, risk factors for peripheral neuropathy in older adults with Type 2 diabetes are usually multifactorial (22). Providers may falsely assume that complaints suggestive of neuropathy are related only to diabetic neuropathy, while potentially ignoring a reversible cause such as low B12 concentrations. This could lead to polypharmacy and potentially inappropriate medication prescribing as pharmacologic treatments for painful diabetic neuropathy are more likely to cause adverse drug effects compared to cyanocobalamin supplementation.
Multiple organizations have begun to develop guidelines for care that account for age-related physiologic changes and other individualized factors promoting personalization of health management. Updates by the American Geriatrics Society and the American Diabetes Association guidelines for the care of older diabetic patients have yet to include recommendations for routine assessment of serum B12 levels among those treated with metformin (6; 23). Serum vitamin B12 assessment is often recommended in certain clinical settings among older adults, such as initial assessment of cognitive impairment, unexplained neurologic symptoms, or macrocytic anemia (24). While B12 concentrations begin to decrease as soon as six months after initiation of metformin, it may take 2 to 5 years to deplete physiologic reserves and even longer to develop clinically apparent symptoms (11; 25).
Strengths of this analysis include the large sample size and the ability to analyze longitudinal pharmacy and laboratory linked data from the VAMC over a 10 year time-span. The large sample size enabled examination of effect modification between metformin prescription and vitamin B12 assessment by other variables. Weaknesses include the dearth of women and use of only one VAMC. However, neither variation of B12 levels by sex, nor metformin effect modification by sex have been described in the literature. While we included major confounders of the relationship between metformin prescription and vitamin B12 testing, it is possible that there are other unmeasured factors impacting the clinical decision to assess vitamin B12 status in diabetic patients receiving metformin. We were not able to assess whether additional tests related to B12 homeostasis were ordered, such as methylmelonic acid or homocysteine levels. It is possible multivitamin use impacted B12 levels in diabetic Veterans taking metformin; however, data regarding multivitamin use were not available. Lastly, we were not able to include an accurate representation of duration of metformin use (beyond six consecutive months). Some participants could have entered the cohort after starting metformin or the end dates of the study might not have included the end date for metformin use.
Our findings, showing infrequent monitoring for B12 concentrations after long-term metformin therapy, and an increased prevalence of vitamin B12 deficiency among metformin users, raise the issue of possible lack of surveillance and treatment for metformin-associated health complications among older adults. Future studies should prioritize longitudinal analyses and intervention trials to gain an understanding of the clinical progression and treatment of B12 deficiency among metformin users. These studies may inform guidelines for best practices in diabetes care among the growing population of older adults with Type 2 diabetes.
CONCLUSION
Because older adults are already at greater risk of vitamin B12 deficiency, the effect of metformin to alter B12 metabolism is magnified in older adults with diabetes. Consequently, the potential impact related to the development of disability in this already at-risk population is substantial. Because vitamin B12 deficiency is easily treatable, it is reasonable that providers screen patients prior to and within 1-2 years of initiating long-term metformin therapy. The present study showing older Veterans receiving metformin are more likely to demonstrate laboratory evidence of B12 deficiency, yet are less likely to have testing, contributes to the growing body of literature highlighting a potential gap in provision of high quality care to older adults with Type 2 diabetes. Because metformin is recommended as first line therapy for Type 2 diabetes, clinical decision support could be implemented to increase the likelihood that older adults receive timely serum B12 assessment and monitoring.
ACKNOWLEDGEMENTS
Sponsor’s Role:
This study was supported by the Department of Veterans Affairs Birmingham/Atlanta Geriatric Research, Education, and Clinical Center and a Veterans Health Administration Career Development Award (CDA-2) to Dr. Vaughan (1 IK2 RX000747-01). This work was also supported in part by the Federal Drug Administration (FDA) award RO1FD003527 (L.S.P.), Veterans Affairs Health Services Research & Development Investigator-Initiated Research (VA HSR&D IIR) award 07-138 (L.S.P.), National Institutes of Health (NIH) awards R21DK099716 (L.S.P.) DK066204 (L.S.P.), U01 DK091958 (L.S.P.), U01 DK098246 (L.S.P.), and a Cystic Fibrosis Foundation award PHILLI12A0 (L.S.P). The sponsors had no role in the design, methods, data collection, or analysis of the study and had no role in the preparation of the manuscript.
Conflict of Interest:
| Elements of Financial/Personal Conflicts |
Kancherla | Elliott | Patel | Holland | Phillips | Johnson | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
|
Employment or Affiliation |
No | No | No | No | No | No | ||||||
| Grants/Funds | No | No | No | No | No | No | ||||||
| Honoraria | No | No | No | No | No | No | ||||||
| Speaker Forum | No | No | No | No | No | No | ||||||
| Consultant | No | No | No | No | No | No | ||||||
| Stocks | No | No | No | No | No | No | ||||||
| Royalties | No | No | No | No | No | No | ||||||
| Expert Testimony | No | No | No | No | No | No | ||||||
| Board Member | No | No | No | No | No | No | ||||||
| Patents | No | No | No | No | No | No | ||||||
| Personal Relationship | No | No | No | No | No | No | ||||||
| Elements of Financial/Personal Conflicts |
Khakharia | Oakley | Vaughan | |||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
|
Employment or Affiliation |
No | No | No | |||
| Grants/Funds | No | No | No | |||
| Honoraria | No | No | No | |||
| Speaker Forum | No | No | No | |||
| Consultant | No | No | No | |||
| Stocks | No | No | No | |||
| Royalties | No | No | No | |||
| Expert Testimony | No | No | No | |||
| Board Member | No | No | No | |||
| Patents | No | No | No | |||
| Personal Relationship | No | No | No | |||
Author Contributions:
VK: Conception and design, analysis and interpretation of data, preparation of manuscript; JLE: analysis and interpretation of data, preparation and final approval of manuscript; BBP: interpretation of data, review and final approval of manuscript, NWH: interpretation of data, review and final approval of manuscript; TMJ: conception and design, interpretation of data, review and final approval of manuscript; AK: data compilation for analysis, review and final approval of manuscript; LSP: interpretation of data, review and final approval of manuscript; GPO: conception and design, interpretation of data, review and final approval of manuscript; CPV: conception and design, interpretation of data, preparation and final approval of manuscript.
Footnotes
Meeting Presentation: This research was presented in abstract form at the 2014 Annual Scientific meeting of the American Geriatrics Society in Orlando, Florida, USA.
REFERENCES
- 1.Centers for Disease Control and Preventyion [Accessed October 30, 2016];Diabetes Report Card, 2014. (online), Available at: http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf.
- 2.Menke A, Casegrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler S, Moore K, Forsberg CW, et al. Mortality among veterans with type 2 diabetes initiating metformin, sulfonylurea or rosiglitazone monotherapy. Diabetologia. 2013;56:1934–1943. doi: 10.1007/s00125-013-2958-1. [DOI] [PubMed] [Google Scholar]
- 4. [Accessed October 30, 2016];U.S. Census Beureau American Community Survey, 2016. (online), Available at: http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2014.pdf.
- 5. [Accessed October 30, 2016];Managing Older People with Type 2 Diabetes Global Guideline. 2013 (online), Available at http://www.idf.org/guidelines/managing-older-people-type-2-diabetes.
- 6.American Diabetic Association (ADA) Standards of Medical Care in Diabetes-2014. Diabetes Care. 2014;37:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Li S, Quan H, Li J. Vitamin B12 Status in Metformin Treated Patients: Systematic Review. PLoS ONE. 2014;9:e100379. doi: 10.1371/journal.pone.0100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaghan TS, Hadden DR, Tomkin GH. Megaloblastic Anaemia Due To Vitamin B12 Malabsorption Associated With Long-Term Metformin Treatment. BMJ. 1980;280:1214–1215. doi: 10.1136/bmj.280.6225.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wulffelé MG, Kooy A, Lehert P, et al. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Int Med. 2003;254:455–463. doi: 10.1046/j.1365-2796.2003.01213.x. [DOI] [PubMed] [Google Scholar]
- 10.Ting R, Szeto C, Chan M, et al. Risk factors of vitamin b12 deficiency in patients receiving metformin. Arch Int Med. 2006;166:1975–1979. doi: 10.1001/archinte.166.18.1975. [DOI] [PubMed] [Google Scholar]
- 11.De Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010;340:c2181. doi: 10.1136/bmj.c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wile DJ, Toth C. Association of Metformin, Elevated Homocysteine, and Methylmalonic Acid Levels and Clinically Worsened Diabetic Peripheral Neuropathy. Diabetes Care. 2010;33:156–161. doi: 10.2337/dc09-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce SA, Chung AH, Black KK. Evaluation of Vitamin B12 Monitoring in a Veteran Population on Long-Term, High-Dose Metformin Therapy. Ann Pharmacother. 2012;46:1470–1476. doi: 10.1345/aph.1R223. [DOI] [PubMed] [Google Scholar]
- 14.Fogelman Y, Kitai E, Blumberg G, et al. Vitamin B12 screening in metformin-treated diabetics in primary care: were elderly patients less likely to be tested? Aging Clin Exp Res. 2016 doi: 10.1007/s40520-016-0546-1. [Epub ahead of print: PMID: 26914484] [DOI] [PubMed] [Google Scholar]
- 15.Bernard MA, Nakonezny PA, Kashner TM. The effect of vitamin B12 deficiency on older Veterans and its relationship to health. J Am Geriatr Soc. 1998;46:1199–1206. doi: 10.1111/j.1532-5415.1998.tb04534.x. [DOI] [PubMed] [Google Scholar]
- 16.Oberlin B, Tangney C, Gustashaw K, et al. Vitamin B12 Deficiency in Relation to Functional Disabilities. Nutrients. 2013;5:4462–4475. doi: 10.3390/nu5114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leishear K, Boudreau RM, Studenski SA, et al. Relationship Between Vitamin B12 and Sensory and Motor Peripheral Nerve Function in Older Adults. J Am Geriatr Soc. 2012;60:1057–1063. doi: 10.1111/j.1532-5415.2012.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leishear K, Ferrucci L, Lauretani F, et al. Vitamin B12 and Homocysteine Levels and 6-Year Change in Peripheral Nerve Function and Neurological Signs. J Geron Ser A: Biol Sci Med Sci. 2012;67A:537–543. doi: 10.1093/gerona/glr202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiles NS, Phillips CL, Volpato S, et al. Diabetes, peripheral neuropathy, and lower- extremity function. J Diabetes Complications. 2014;28:91–95. doi: 10.1016/j.jdiacomp.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalli P, Chan A, Garven A, et al. Increased gait variability in diabetes mellitus patients with neuropathic pain. J Diabetes Complications. 2013;27:248–254. doi: 10.1016/j.jdiacomp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Sudore R, Karter A, Huang E, et al. Symptom Burden of Adults with Type 2 Diabetes Across the Disease Course: Diabetes & Aging Study. J Gen Int Med. 2012;27:1674–1681. doi: 10.1007/s11606-012-2132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinik AI, Strotmeyer ES, Nakave AA, et al. Diabetic Neuropathy in Older Adults. Clin Geriatr Med. 2008;24:407–435. doi: 10.1016/j.cger.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Geriatrics Society Expert Panel on the Care of Older Adults with Diabetes M Guidelines Abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 Update. J Am Geriatr Soc. 2013;61:2020–2026. doi: 10.1111/jgs.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knopman DSM, DeKosky STM, Cummings JLM, et al. Practice parameter: Diagnosis of dementia (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 25.Oh R, Brown D. Vitamin B12 Deficiency. Am Fam Physician. 2003;67:979–986. [PubMed] [Google Scholar]