Abstract
OBJECTIVES
(1) To determine the relationship of incident delirium during hospitalization with 90-day mortality; (2) to identify potential in-hospital mediators through which delirium increases 90-day mortality.
DESIGN
Analysis of data from Project Recovery, a controlled clinical trial of a delirium prevention intervention from 1995 to 1998 with follow-up through 2000.
SETTING
Large academic hospital.
PARTICIPANTS
Patients ≥70 years-old without delirium at hospital admission who were at intermediate-to-high risk of developing delirium and received usual care only.
MEASUREMENTS
(1) Incident delirium; (2) potential mediators of delirium on death including use of restraining devices (physical restraints, urinary catheters), development of hospital acquired conditions (HACs) (falls, pressure ulcers), and exposure to other noxious insults (sleep deprivation, acute malnutrition, dehydration, aspiration pneumonia); (3) death within 90 days of admission.
RESULTS
Among 469 patients, 70 (15%) developed incident delirium. These patients were more likely to experience restraining devices (37% vs. 16%, p<.001), HACs (37% vs. 12%, p<.001), other noxious insults (63% vs. 49%, p=.03), and 90-day mortality (24% vs. 6%, p<.001). The inverse probability weighted hazard of death due to delirium was 4.2 (95% CI=2.8–6.3) in bivariable analyses, increased in a graded manner with additional exposures to restraining devices, HACs, and other noxious insults, and declined by 10.9% after addition of these potential mediator categories, providing evidence of mediation.
CONCLUSION
Restraining devices, HACs, and additional noxious insults were more frequent among patients with delirium, increased mortality in a graded manner, and were responsible for a significant percentage of the association of delirium with death. Additional efforts to prevent potential downstream mediators through which delirium increases mortality may help to improve outcomes among hospitalized older adults.
Keywords: geriatrics, delirium, hospital care, quality of care, hospital-acquired conditions
INTRODUCTION
Delirium is a common, costly, and morbid condition that affects more than one in five hospitalized older adults and costs more than $160 billion per year in the United States alone.1 Patients who develop delirium while receiving hospital care for medical,2,3 neurological,4 and surgical3,5 conditions have an elevated risk of death during and after hospitalization6,7 This higher risk of death from delirium has persisted across time in different health care settings and across countries.1
While the association of delirium with death is well appreciated, there is lack of agreement as to whether delirium is directly harmful to patients or is instead a marker of their intrinsic vulnerability. Evidence supporting delirium’s direct adverse effects include observational data relating delirium to a higher risk of death after controlling for multiple patient factors associated with adverse outcomes including advanced age, cognitive impairment, functional limitations, multimorbidity, and high illness severity.3,8–10 However, residual confounding may influence these findings, as patients developing delirium generally have greater chronic disease burden and acute illness severity than patients who do not.
It is also not known if mortality from delirium is mediated by its potential downstream consequences including the use of restraining devices, development of hospital-acquired conditions (HACs) such as falls and pressure ulcers, and exposure to other noxious insults such as sleep deprivation, acute malnutrition, dehydration, and aspiration pneumonia.11–13 All of these adverse exposures are more common among sicker hospitalized patients,14–18 many of whom may have delirium.14,19 These events can result in significant harm 20–22 and have therefore become an increasing focus for quality improvement.23,24 Yet these adverse exposures still occur at unacceptably high rates to hospitalized patients.25–30 If commonly associated with the pathway from delirium to death, restraining devices, HACs, and other noxious insults during hospitalization can serve as specific targets for quality improvement in patients with delirium.
We therefore examined the relationship of incident delirium during hospitalization with the risk of short-term mortality using data from Project Recovery,31 a high quality dataset measuring delirium development, risk factors, and outcomes in older patients hospitalized with a range of medical conditions. We hypothesized that patients developing delirium during hospitalization are at higher risk of 90-day mortality after adjusting for demographic characteristics, lifestyle factors, chronic health history, acute illness severity, and potential downstream mediators linking delirium to death. We also hypothesized that adverse hospital exposures including the use of restraining devices, development of HACs, and occurrence of other noxious insults during hospitalization significantly mediate the association of delirium with death.
METHODS
Study population
We examined 469 older patients who were enrolled in the usual care arm of Project Recovery, a controlled clinical trial of a delirium prevention intervention at Yale-New Haven Hospital from 1995 through 1998, with follow up to 2000.31 Project Recovery is highly unique in its daily assessments for delirium, intercurrent illnesses, and hospital care practices, as well as its careful mortality tracking after hospital discharge. These daily clinical assessments permitted comprehensive identification of both incident delirium and adverse hospital exposures including restraining devices, HACs, and other noxious insults from hospitalization that are potential mediators of delirium on death. Project Recovery continues to be actively used for ongoing investigations related to delirium.32
Patients were included in Project Recovery if they were ≥70 years-old, did not have delirium at hospital admission, and were at intermediate-to-high risk of developing delirium. Patients were excluded if they had terminal illness, were unable to participate in interviews, or had a hospital stay of ≤48 hours.
Data collection
Study researchers performed daily patient assessments and reviewed medical records to collect data. The study team completed 99.8% of all potential daily hospital assessments. Incident delirium during hospitalization, the primary exposure of interest, was identified through daily patient assessment using cognitive screening tests and the Confusion Assessment Method (CAM), a reliable and valid scale for delirium diagnosis.33,34 The CAM has a sensitivity and specificity of 94 and 89 percent, respectively, against reference standard ratings by geriatric psychiatrists, as well as high inter-rater reliability.1 A delirium diagnosis with the CAM required evidence of acute onset and a fluctuating course of symptoms, inattention, and either disorganized thinking or an altered level of consciousness. Interrater reliability of ratings for each of these features was confirmed in 16 paired observations involving all members of the research staff (kappa, 1.0). Delirium was considered as a binary outcome (present or absent) for these analyses.
Adverse hospital exposures that are potential mediators of the relationship of delirium with death were also assessed daily. The use of physical restraints and urinary catheters (“one point restraints”35) was identified through daily examination and medical record review. Falls were identified through review of medical records and hospital incident reports. Pressure ulcers were identified by daily bedside observation of 11 pressure points and medical record review. Exposure to other noxious insults was identified through daily patient interviews (sleep deprivation) and medical record review (acute malnutrition, dehydration, aspiration pneumonia). Sleep deprivation was recognized if ≥3 of 6 items indicating sleep deprivation were endorsed during daily patient interviews, which were completed at a high rate (83%) even among patients with delirium. Dehydration was indicated by a blood urea nitrogen/creatinine ratio of ≥18. Acute malnutrition was recognized if dietary supplements were prescribed or if a patient had documented weight loss of ≥5.6 kg during hospitalization. Aspiration pneumonia was identified if respiratory suctioning was performed36 or if specifically noted in hospital records.
Other variables that were collected and used in our study included: (1) demographic characteristics including age, sex, race, education in years, and marital status; (2) lifestyle factors including current smoking status and alcohol intake; (3) chronic health status including body mass index (BMI), performance in activities of daily living (ADL) and instrumental activities of daily living (IADL) at admission (functional impairment rated as present if patient needed help to perform at least one ADL or IADL), dementia at admission (Mini Mental State Examination [MMSE, purchased from Psychological Assessment Resources, Inc.] score <24 and modified Blessed Dementia Rating Scale score ≥4, and cognitive impairment present for ≥6 months),37,38 hearing impairment at admission (use of hearing aid or positive whisper test39), vision impairment at admission (Jaeger Card Test score ≥10 with corrective lenses), depression at admission (Geriatric Depression Scale score ≥6),40 Charlson Comorbidity Index score (range 0–15), and living in nursing home; and (4) acute illness severity per the Acute Physiology and Chronic Health Evaluation II (APACHE II) score (range 7–29).
Deaths were identified through follow-up interviews with family members, daily review of obituaries, medical record review, and the Social Security Death Index. Dates of death were all confirmed by review of medical records, National Death Index, death certificates, and Medicare enrollment and claims data. Mortality tracking was complete for all patients.
Study Outcome
The primary outcome was death within 90 days of hospital admission. We conducted two major analyses, as follows: (1) the relationship of incident delirium during hospitalization with death within 90 days of the admission date in fully adjusted and propensity-weighted models; and (2) the extent to which exposure to restraining devices, HACs, and other noxious insults during hospitalization attenuated the relationship of incident delirium with death within 90 days of the admission date in fully adjusted and propensity-weighted models.
Statistical Analyses
We identified demographic, lifestyle, health history, and acute illness severity information for patients who did and did not develop delirium during hospitalization. We compared continuous variables using analysis of variance and categorical variables using chi-square tests. To account for a small amount for missing data in predictor variables (<5%), we performed multiple imputation using multivariate imputation chained equations.41,42
We balanced patient characteristics between patients who did and did not develop delirium during hospitalization by constructing regression-based propensity scores with variables for patient demographics, lifestyle factors, health history, and acute illness severity. We calculated inverse probability weights (IPWs) for each patient who developed delirium as the inverse of the predicted probability of developing delirium conditional on observed covariates that were significantly associated (p<0.05) with both delirium and death. We calculated weights for patients who did not develop delirium as the inverse of one minus the predicted probability of developing delirium conditional on observed covariates.43,44
We calculated unweighted and inverse probability weighted Cox regression models to determine the hazard of death within 90 days of the admission date due to incident delirium and adverse hospital exposures including restraining devices, HACs, and other noxious insults during hospitalization that are potential mediators of delirium on death. We first calculated the hazard of death due to incident delirium and each potential mediator variable using bivariable Cox regression models. We then calculated Cox regression models for death due to incident delirium after adjustment for potential individual mediator variables. We additionally calculated the cumulative incidence of death for persons with and without incident delirium after adjustment for all adverse hospital exposures potentially mediating delirium on death. We also calculated the cumulative incidence of death as a function of the number of adverse hospital exposures (0, 1, 2, 3, ≥4) after adjustment for delirium status.
We defined the presence of mediation as a ≥5 percent change in the parameter estimate of delirium on death with the addition of the potential mediator variable to the model. This definition of mediation is a liberal one relative to the 10% parameter estimate change typically used to define practically important levels of confounding.45 We used a liberal definition of mediation to be maximally inclusive of potential mediating effects. Our approach to testing mediation based on parameter estimate changes is conceptually equivalent to path analysis approaches that make presumed causal relationships among variables explicit in a visual manner.46 We tested adverse exposures individually and by group (e.g. any restraining device, any HAC, any hospital stressor). Our final Cox regression analysis adjusted for the presence of delirium and all hospital adverse exposure categories. We did not test for moderation, which would require that we model the statistical interaction of variables, for which we had inadequate statistical power.46
Statistical significance was set at an alpha of 0.05. Analyses were performed using STATA 13 (StataCorp LP, College Station, TX).
RESULTS
Of 469 patients, 70 (15%) developed delirium during hospitalization. Thirty-nine patients (8.3%) died within 90 days of admission. The rate of death was greater in patients who developed delirium (n=17, 24%) than in those who did not develop delirium (n=22, 6%) (p<.001). As compared to those who did not develop delirium, patients who developed delirium more often lived in a nursing home (13% vs. 5%, p=.02), more often had impairments in ADLs (56% vs. 32%, p<.001) and IADLs (94% vs. 85%, p=0.04), and had a higher prevalence of dementia (23% vs. 11%, p=0.01) (Table 1).
Table 1.
Patient Characteristics.
| Characteristic | All Patients (N=469) | Patients With Delirium (N=70) | Patients Without Delirium (N=399) | P Value for Difference Between Patients With and Without Delirium |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Average age in years (SD) | 80.1 (6.5) | 81.2 (7.2) | 79.9 (6.4) | 0.100 |
| Male, n (%) | 187 (39.9) | 29 (41.4) | 158 (39.6) | 0.773 |
| Non-white race, n (%) | 70 (14.9) | 15 (21.4) | 55 (13.8) | 0.098 |
| Average education in years, (SD) | 11.0 (3.6) | 9.8 (3.5) | 11.3 (3.6) | 0.002 |
| Married, n (%) | 161 (34.3) | 19 (27.1) | 142 (35.6) | 0.170 |
| Lifestyle factors | ||||
| Currently smoking, n (%) | 43 (9.2) | 5 (7.1) | 38 (9.5) | 0.524 |
| Average alcoholic drinks/week (SD) | 2.0 (8.1) | 1.9 (10.2) | 2.0 (7.7) | 0.985 |
| Chronic health status | ||||
| Average BMI (kg/m2) (SD) | 25.3 (6.3) | 25.5 (5.7) | 25.3 (6.4) | 0.812 |
| Impairment in ADLs,a n (%) | 165 (35.2) | 39 (55.7) | 126 (31.6) | <.001 |
| Impairment in IADLs,b n (%) | 405 (86.4) | 66 (94.3) | 339 (85.0) | 0.036 |
| Dementia,c n (%) | 60 (12.8) | 16 (22.9) | 44 (11.0) | 0.006 |
| Hearing impairment,d n (%) | 83 (17.7) | 16 (23.1) | 67 (16.8) | 0.199 |
| Visual impairment,e n (%) | 131 (27.9) | 21 (30.0) | 110 (27.6) | 0.676 |
| Depression,f n (%) | 148 (31.6) | 26 (37.1) | 122 (30.6) | 0.276 |
| Average Charlson Comorbidity Index score (SD) | 2.7 (2.2) | 3.4 (2.5) | 2.6 (2.1) | 0.003 |
| Living in nursing home, n (%) | 30 (6.4) | 9 (12.9) | 21 (5.3) | 0.017 |
| Acute illness severity | ||||
| Average Apache II score (SD) | 15.6 (4.1) | 17.2 (4.6) | 15.4 (4.0) | 0.001 |
Impairment in ADLs was defined as needing help to perform at least 1 activity of daily living
Impairment in IADLs was defined as needing help to perform at least 1 instrumental activity of daily living
Dementia was defined as Mini-Mental State Examination Score <24, modified Blessed Dementia Rating Scale score ≥4, and duration of cognitive symptoms for ≥6 months.
Hearing impairment was identified if a patient used a hearing aid or had positive Whisper test.
Visual impairment was identified if a patient scored ≥10 on Jaeger Card Test while wearing corrective lenses.
Depression was identified if Geriatric Depression Scale score ≥6 and ≥8 out of 15 items were answered.
SD=standard deviation; BMI=body mass index; ADL=activity of daily living; IADL=instrumental activity of daily living.
Restraining devices, HACs, and other noxious insults were, in most cases, more common among patients developing delirium (Supplementary Table S1). Among patients who did and did not develop delirium, respectively, physical restraints were used in 20% vs. 1% (p<.001), a fall occurred in 9% vs. 2% (p=.003), a pressure ulcer developed in 33% vs. 10% (p<.001), acute malnutrition developed in 39% vs. 13% (p<.001), and aspiration pneumonia occurred in 4% vs. 1% (p=.04). No difference between groups was found in urinary catheter use (23% vs. 15%, p=.10), sleep deprivation (46% vs. 39%, p=.26), or dehydration (3% vs. 4%, p=.71). Patients developing delirium were more likely to receive at least one restraining device (37% vs. 16%, p<.001), develop at least one HAC (37% vs. 12%, p<.001), and experience at least one additional noxious insult (63% vs. 49%, p=.03).
Incident delirium was associated with a greater risk of short-term mortality in both unweighted (Hazard Ratio [HR] 5.0, 95% confidence interval [CI]=2.6–9.4) and inverse probability weighted (HR 4.2, 95% CI=2.8–6.3) analyses. Inverse probability weights were calculated using variables for education level, functional status, dementia, Charlson Comorbidity Index score, and APACHE score, all of which were significantly associated (p’s<.05) with both incident delirium and death. In the weighted models, the hazard of death was greater in patients receiving physical restraints (HR 2.0, 95% CI=1.4–2.9) or urinary catheters (HR 1.5, 95% CI=1.1–2.3), patients who fell (HR 3.6, 95% CI=2.4–5.4), developed a pressure ulcer (HR 2.3, 95% CI=1.6–3.2), experienced sleep deprivation (HR 2.5, 95% CI=1.8–3.5), acute malnutrition (HR 3.7, 95% CI=2.7–5.1), or aspiration pneumonia (HR 8.4, 95% CI=5.6–12.6) (Table 2).
Table 2.
Risk of Death Associated with Delirium and Potential Mediators of Delirium on Death in Bivariable Analyses.
| Variable | N (%) | Unweighted Model HR (95% CI) | Inverse Probability Weighted Model HR (95% CI) |
|---|---|---|---|
| Incident delirium | 70 (14.9) | 5.0 (2.6–9.4) | 4.2 (2.8–6.3) |
| Restraining devices | |||
| Physical restraints | 17 (3.6) | 3.5 (1.2–9.8) | 2.0 (1.4–2.9) |
| Urinary catheter use | 76 (16.2) | 2.4 (1.2–4.7) | 1.5 (1.1–2.3) |
| Hospital-acquired conditions (HACs) | |||
| Fall | 14 (3.0) | 2.8 (0.9–9.0) | 3.6 (2.4–5.4) |
| Pressure ulcer | 63 (13.4) | 2.6 (1.3–5.3) | 2.3 (1.6–3.2) |
| Other noxious insults | |||
| Sleep deprivation | 186 (39.7) | 1.2 (0.7–2.4) | 2.5 (1.8–3.5) |
| Acute malnutrition | 77 (16.4) | 4.9 (2.6–9.3) | 3.7 (2.7–5.1) |
| Dehydration | 17 (3.6) | 1.4 (0.3–5.9) | 0.3 (0.1–1.2) |
| Aspiration pneumonia | 7 (1.5) | 4.2 (1.0–17.4) | 8.4 (5.6–12.6) |
Data are based on unweighted and inverse probability weighted Cox regression models.
HR= Hazard ratio; CI= Confidence interval.
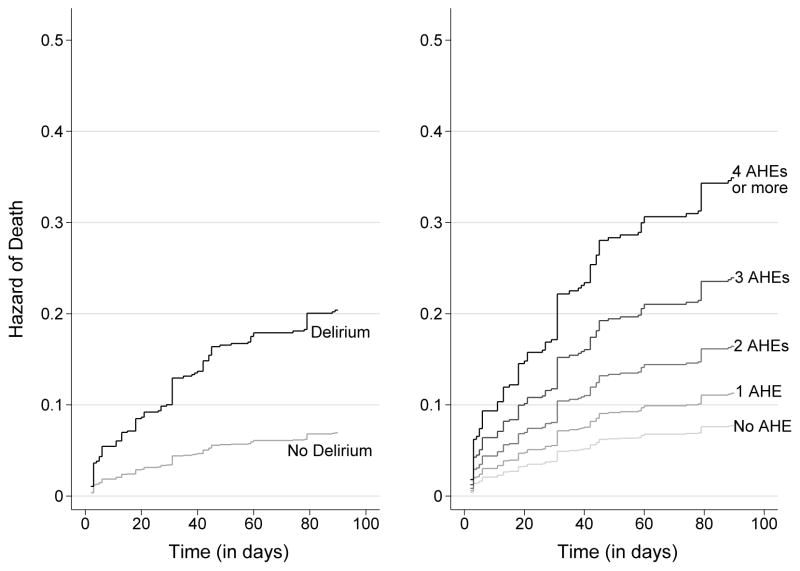
The cumulative incidence of death within 90 days of the admission date was higher in persons who developed delirium and in persons experiencing adverse hospital exposures including restraining devices, HACs, and other noxious stimuli. A greater number of adverse hospital exposures (0, 1, 2, 3, ≥4) was associated with a higher risk of death in a graded manner after controlling for the effect of delirium (Figure 1, right panel).
Figure 1. Cumulative Hazard of Death Associated with Delirium and the Number of Adverse Hospital Exposures.
The left panel shows data for the cumulative hazard of death associated with incident delirium in the 90 days after hospital admission. Results are adjusted for the presence of adverse hospital exposures, which include the use of physical restraints, use of a urinary catheter, occurrence of a fall, occurrence of a pressure ulcer, occurrence of sleep deprivation, occurrence of acute malnutrition, occurrence of dehydration, and occurrence of aspiration pneumonia. The right panel shows data for the cumulative hazard of death associated with the number of adverse hospital exposures, which include the use of physical restraints, use of a urinary catheter, occurrence of a fall, occurrence of a pressure ulcer, occurrence of sleep deprivation, occurrence of acute malnutrition, occurrence of dehydration, and occurrence of aspiration pneumonia. Results are adjusted for the presence of incident delirium. AHE=Adverse Hospital Exposure.
In inverse probability weighted models with delirium and one other adverse hospital exposure, the occurrence of falls, pressure ulcers, acute malnutrition, and aspiration pneumonia changed the parameter estimate of delirium on death by more than 5%, suggesting that these adverse exposures may be mediators on the pathway from delirium to death within 90 days of hospital admission (Table 3). When risk factor categories were grouped, the use of any restraining device and development of any HAC also changed the parameter estimate of delirium on death by more than 5% (Table 3).
Table 3.
Potential Mediation Effects of Delirium on Death After Adjustment for Individual Adverse Hospital Exposures.
| Risk Factor | HR for Delirium (95% CI) | HR for Adverse Hospital Exposure (95% CI) | Percent Change in Parameter Estimate of Delirium |
|---|---|---|---|
| Delirium only | 4.2 (2.8–6.3) | ||
| Restraining devices | |||
| Physical restraints | 4.0 (2.6–6.1) | 1.2 (0.8–1.8) | 3.6% |
| Urinary catheter use | 4.2 (2.8–6.3) | 1.5 (1.0–2.2) | 0.3% |
| Any restraining device | 3.9 (2.5–5.9) | 1.4 (1.0–2.0) | 5.6% |
| Hospital-acquired conditions (HACs) | |||
| Fall | 3.7 (2.5–5.7) | 2.6 (1.7–3.9) | 8.0% |
| Pressure ulcer | 3.7 (2.4–5.7) | 1.6 (1.1–2.3) | 8.9% |
| Any HAC | 3.9 (2.5–5.9) | 1.3 (0.9–1.9) | 5.6% |
| Other noxious insults | |||
| Sleep deprivation | 4.2 (2.8–6.3) | 2.5 (1.8–3.5) | 0.3% |
| Acute malnutrition | 3.3 (2.1–5.0) | 2.8 (2.0–3.9) | 17.5% |
| Dehydration | 4.2 (2.8–6.4) | 0.3 (0.1–1.1) | −0.7% |
| Aspiration pneumonia | 3.6 (2.4–5.5) | 6.4 (4.2–9.7) | 9.9% |
| Any noxious insult | 3.9 (2.6–5.9) | 2.3 (1.6–3.3) | 4.9% |
Data are based on results of inverse-probability weighted Cox regression models. Hazard ratios for death within 90 days of admission are shown for delirium only and for delirium plus one additional adverse hospital exposure. The final column shows the percentage change in parameter estimate of delirium on death with the addition of individual adverse hospital exposure variables. A change in the parameter estimate of delirium of more than 5% provides evidence for mediation.
HR=hazard ratio; CI=confidence interval.
The final inverse probability weighted multivariable model with delirium and all adverse hospital exposure categories (restraining devices, HACs, and other noxious stimuli) resulted in a 10.9% reduction in the parameter estimate of delirium on death compared to the bivariable model with delirium only, suggesting that these adverse hospital exposures are mediators on the pathway between delirium and death (Table 4). In this final model, incident delirium remained independently associated with a greater risk of mortality within 90 days of the admission date (HR 3.6, 95% CI=2.3–5.5).
Table 4.
Potential Mediation Effects of Delirium on Death After Adjustment for All Adverse Hospital Exposure Categories.
| Risk Factor | HR (95% CI) | Percent Change in Parameter Estimate of Delirium |
|---|---|---|
| Bivariable model | ||
| Delirium | 4.2 (2.8–6.3) | -- |
| Multivariable model | ||
| Delirium | 3.6 (2.3–5.5) | 10.9% |
| Restraining devices | 1.1 (0.8–1.6) | |
| Hospital-acquired conditions | 1.2 (0.8–1.9) | |
| Other noxious insults | 2.2 (1.5–3.2) | |
Data are based on results of inverse-probability weighted Cox regression models. Hazard ratios for death within 90 days of admission are shown for delirium only and for delirium plus all adverse hospital exposure categories. The final column shows the percentage change in parameter estimate of delirium on death with the addition all adverse hospital exposure categories to the Cox regression model. A change in the parameter estimate of delirium of more than 5% provides evidence for mediation. HR=hazard ratio; CI=confidence interval.
DISCUSSION
New-onset delirium during hospitalization is strongly predictive of mortality within 90 days of the admission date even after adjusting for baseline characteristics and potential downstream mediators of delirium on death including the use of restraining devices, development of hospital-acquired conditions, and occurrence of additional noxious insults during hospitalization. Almost all of these adverse exposures were more than three-times as likely to occur among patients with delirium, increased mortality in a graded manner, and were responsible for a significant percentage (10.9%) of the association of delirium with death. These findings suggest that additional efforts to prevent use of restraining devices, hospital-acquired conditions, and additional noxious insults that are common during hospitalization may be worthy targets to improve outcomes and reduce mortality for the many million older patients with delirium.
Our work extends the literature in two ways. Firstly, it strengthens evidence that delirium itself may be directly harmful to patients. We build upon previous studies linking incident delirium with death3,8–10 by demonstrating a persistent relationship after balancing baseline characteristics of patients who did and did not develop delirium during hospitalization through the use of regression-based propensity scores. In contrast with previous studies, we also adjusted for potential downstream mediators through which delirium may cause harm. Despite these adjustments, the hazard of death for older patients developing delirium during hospitalization is almost four times greater than that for persons who do not develop delirium. Secondly, our work applies research on adverse hospital exposures to the study of delirium and suggests that efforts to minimize use of restraining devices, prevent the occurrence of falls and pressure ulcers, and reduce the likelihood of sleep disturbance, insufficient food intake, and aspiration events may reduce mortality from delirium by a significant extent, as delirium is both common and strongly associated with death. Almost all of these adverse exposures are much more likely to occur among patients with delirium and increase mortality in a graded manner, suggesting that even partially successful preventative efforts can improve outcomes.
This analysis has a number of strengths. We used data from Project Recovery, which included daily ascertainment for delirium and multiple adverse hospital exposures using rigorous, validated approaches. Only 0.2% of daily interviews and assessments were missed. In addition, detailed data were collected on a wide range of clinically relevant variables that potentially confound the relationship of delirium with death including cognition, functional status, sensory impairments, and acute illness severity. Ascertainment of post-hospital mortality was complete and validated through multiple data sources including the Social Security Death Index, National Death Index, and Medicare databases. Project Recovery therefore continues to be used to examine delirium and its outcomes.32 We also used regression-based propensity scores to minimize differences in observed characteristics between patients who did and did not develop delirium while hospitalized. This technique is especially useful when examining conditions like delirium that more commonly affect patients with greater vulnerability to adverse outcomes.
Results should also be interpreted in the context of the following potential limitations. Firstly, Project Recovery does not have contemporary data, as data collection occurred between 1995 and 1998, with follow up to 2000. However, our primary relationship of interest between delirium and death would not be expected to be different today, as no interventions have been shown to reduce mortality from delirium.1 In addition, the CAM remains the tool of choice to identify delirium,34 and the adverse hospital exposures examined in our study continue to occur at sub-optimally high rates.25–30 Secondly, we cannot eliminate all sources of bias influencing the relationship of delirium with death. Thirdly, we cannot confirm that restraining devices, HACs, and additional noxious insults during hospitalization were downstream consequences of delirium. However, our finding that these adverse exposures explain a significant proportion of the association of delirium with death and predict mortality in a graded manner suggests that they may be important targets to reduce adverse outcomes among patients with delirium even if not causally related. Fourthly, our use of respiratory suctioning as one way to identify aspiration pneumonia may have suboptimal specificity. However, we used this approach since oropharyngeal and tracheal suction are recommended treatments for witnessed aspiration.36 Fifthly, the low number of deaths limited our statistical power and precluded our fitting a single model examining the association of all adverse hospital exposures, included individually, with death. However, it is likely that many of these variables are clinically important, as we found that 90-day mortality increases in a graded manner with the number of adverse hospital exposures.
Our findings have implications for clinical practice and research. They underscore the importance of multicomponent interventions designed to prevent delirium, which can reduce rates of incident delirium by 30 to 40 percent.31 These strategies involve assessment and modification of clinical factors known to precipitate delirium during hospitalization and have lowered delirium incidence in real world practice. Our results also suggest that improved hospital care for older patients with delirium may improve patient outcomes. Targeted strategies shown to reduce urinary catheter use,47 a broad range of HACs48,49 and sleep disturbances50 may be considered once delirium is diagnosed. Future research including intervention trials can prospectively determine the value and effectiveness of proactive strategies to identify delirium and mitigate its downstream consequences within the hospital.
In summary, we found that a significant proportion of the association of delirium with death may be explained by the use restraining devices, development of hospital-acquired conditions, and occurrence of additional noxious insults during hospitalization. We also found that as the number of adverse hospital exposures increase, so does the risk of death following delirium onset. These results suggest that intensified efforts to reduce hospital-acquired insults and complications may improve health outcomes among the more than 2.6 million older Americans that develop delirium while hospitalized each year.
Supplementary Material
Supplementary Table S1. Adverse Hospital Exposures In Study Populations.
Acknowledgments
Conflict of interest disclosures (details below):
| Elements of Financial/Personal Conflicts | K. Dharmarajan | S. Swami | R. Gou | R. Jones | S. Inouye | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | |||||
| Centers for Medicare & Medicaid Services | ||||||||||
| Grants/Funds | X | X | X | X | X | |||||
| Honoraria | X | X | X | X | X | |||||
| Speaker Forum | X | X | X | X | X | |||||
| Consultant | X | X | X | X | X | |||||
| Clover Health | ||||||||||
| Stocks | X | X | X | X | X | |||||
| Royalties | X | X | X | X | X | |||||
| Expert Testimony | X | X | X | X | X | |||||
| Board Member | X | X | X | X | X | |||||
| Clover Health | ||||||||||
| Patents | X | X | X | X | X | |||||
| Personal Relationship | X | X | X | X | X | |||||
Conflict of interest disclosures for each author (detailed):
Kumar Dharmarajan: Employment or affiliation: works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures; Grants/funds: no; Honoraria: no; Speaker forum: no; Consultant: consultant for Clover Health; Stocks: no; Royalties: no; Expert testimony: no; Board member: member of a scientific advisory board for Clover Health; Patents: no; Personal relationship: no
Sunil Swami: Employment or affiliation: no; Grants/funds: no; Honoraria: no; Speaker forum: no; Consultant: no; Stocks: no; Royalties: no; Expert testimony: no; Board member: no; Patents: no; Personal relationship: no
Ray Yun Gou: Employment or affiliation: no; Grants/funds: no; Honoraria: no; Speaker forum: no; Consultant: no; Stocks: no; Royalties: no; Expert testimony: no; Board member: no; Patents: no; Personal relationship: no
Richard N. Jones: Employment or affiliation: no; Grants/funds: no; Honoraria: no; Speaker forum: no; Consultant: no; Stocks: no; Royalties: no; Expert testimony: no; Board member: no; Patents: no; Personal relationship: no
Sharon K. Inouye: Employment or affiliation: no; Grants/funds: no; Honoraria: no; Speaker forum: no; Consultant: no; Stocks: no; Royalties: no; Expert testimony: no; Board member: no; Patents: no; Personal relationship: no
Funding/support: This research was partially supported by NIH/NIA grants K23AG048331 (Dharmarajan), P30AG021342 (Dharmarajan through Yale University), R03AG045633 (Inouye), P01AG031720 (Inouye), and K07AG041835 (Inouye). The content is solely the responsibility of the authors and does not necessarily represent the official views or policies of the National Institutes of Health, which had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation of the manuscript or decision to submit it for publication.
Footnotes
At the time this research was performed, Dr. Swami was associated with the Aging Brain Center, Institute for Aging Research, Hebrew SeniorLife, Boston, MA.
Meeting Presentation: None.
Non-author contributors: none
Sponsor’s role: The sponsors had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Authors’ contributions:
Study concept and design: Dharmarajan, Swami, Jones, Inouye
Acquisition of data: Inouye
Analysis and interpretation of data: Dharmarajan, Swami, Gou, Jones, Inouye
Drafting of the manuscript: Dharmarajan
Critical revision of the manuscript for important intellectual content: Dharmarajan, Swami, Gou, Jones, Inouye
Statistical analysis: Swami, Gou, Jones
Obtained funding: Jones, Inouye
Administrative, technical, or material support: Jones, Inouye
Study supervision: Jones, Inouye
References
- 1.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eeles EM, Hubbard RE, White SV, et al. Hospital use, institutionalisation and mortality associated with delirium. Age Ageing. 2010;39:470–475. doi: 10.1093/ageing/afq052. [DOI] [PubMed] [Google Scholar]
- 3.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 4.Oldenbeuving AW, de Kort PL, Jansen BP, et al. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology. 2011;76:993–999. doi: 10.1212/WNL.0b013e318210411f. [DOI] [PubMed] [Google Scholar]
- 5.Ansaloni L, Catena F, Chattat R, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg. 2010;97:273–280. doi: 10.1002/bjs.6843. [DOI] [PubMed] [Google Scholar]
- 6.Fong TG, Jones RN, Marcantonio ER, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156:848–856. w296. doi: 10.7326/0003-4819-156-12-201206190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koster S, Hensens AG, Schuurmans MJ, et al. Consequences of delirium after cardiac operations. Ann Thorac Surg. 2012;93:705–711. doi: 10.1016/j.athoracsur.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 9.McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162:457–463. doi: 10.1001/archinte.162.4.457. [DOI] [PubMed] [Google Scholar]
- 10.Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi: 10.1371/journal.pone.0026951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–857. [PubMed] [Google Scholar]
- 12.Micek ST, Anand NJ, Laible BR, et al. Delirium as detected by the CAM-ICU predicts restraint use among mechanically ventilated medical patients. Crit Care Med. 2005;33:1260–1265. doi: 10.1097/01.ccm.0000164540.58515.bf. [DOI] [PubMed] [Google Scholar]
- 13.Lee EA, Gibbs NE, Fahey L, et al. Making hospitals safer for older adults: updating quality metrics by understanding hospital-acquired delirium and its link to falls. Perm J. 2013;17:32–36. doi: 10.7812/TPP/13-065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krauss MJ, Evanoff B, Hitcho E, et al. A case-control study of patient, medication, and care-related risk factors for inpatient falls. J Gen Intern Med. 2005;20:116–122. doi: 10.1111/j.1525-1497.2005.40171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogerty MD, Abumrad NN, Nanney L, et al. Risk factors for pressure ulcers in acute care hospitals. Wound Repair Regen. 2008;16:11–18. doi: 10.1111/j.1524-475X.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281:2013–2019. doi: 10.1001/jama.281.21.2013. [DOI] [PubMed] [Google Scholar]
- 17.Wakefield BJ, Mentes J, Holman JE, et al. Postadmission dehydration: risk factors, indicators, and outcomes. Rehabil Nurs. 2009;34:209–216. doi: 10.1002/j.2048-7940.2009.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 18.Raguan B, Wolfovitz E, Gil E. Use of Physical Restraints in a General Hospital: a Cross-Sectional Observational Study. Isr Med Assoc J. 2015;17:633–638. [PubMed] [Google Scholar]
- 19.Taylor JK, Fleming GB, Singanayagam A, et al. Risk factors for aspiration in community-acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126:995–1001. doi: 10.1016/j.amjmed.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Lyder CH, Wang Y, Metersky M, et al. Hospital-acquired pressure ulcers: results from the national Medicare Patient Safety Monitoring System study. J Am Geriatr Soc. 2012;60:1603–1608. doi: 10.1111/j.1532-5415.2012.04106.x. [DOI] [PubMed] [Google Scholar]
- 21.Evans D, Wood J, Lambert L. Patient injury and physical restraint devices: a systematic review. J Adv Nurs. 2003;41:274–282. doi: 10.1046/j.1365-2648.2003.02501.x. [DOI] [PubMed] [Google Scholar]
- 22.Platt R, Polk BF, Murdock B, et al. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307:637–642. doi: 10.1056/NEJM198209093071101. [DOI] [PubMed] [Google Scholar]
- 23.Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System Policy Changes and Fiscal Year 2016 Rates; Revisions of Quality Reporting Requirements for Specific Providers, Including Changes Related to the Electronic Health Record Incentive Program; Extensions of the Medicare-Dependent, Small Rural Hospital Program and the Low-Volume Payment Adjustment for Hospitals. Final rule; interim final rule with comment period. Fed Regist. 2015;80:49325–49886. [PubMed] [Google Scholar]
- 24.Agency for Healthcare Research and Quality. [Accesed June 22, 2016];Patient safety indicators overview (online) Available at: http://www.qualityindicators.ahrq.gov/modules/psi_resources.aspx.
- 25.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311:2169–2170. doi: 10.1001/jama.2014.3695. [DOI] [PubMed] [Google Scholar]
- 27.Attenello FJ, Wen T, Cen SY, et al. Incidence of “never events” among weekend admissions versus weekday admissions to US hospitals: national analysis. BMJ. 2015;350:h1460. doi: 10.1136/bmj.h1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoder JC, Staisiunas PG, Meltzer DO, et al. Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172:68–70. doi: 10.1001/archinternmed.2011.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler L, Yi D, Li M, et al. Impact of Inpatient Harms on Hospital Finances and Patient Clinical Outcomes. J Patient Saf. 2015 doi: 10.1097/PTS.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braunschweig C, Gomez S, Sheean PM. Impact of declines in nutritional status on outcomes in adult patients hospitalized for more than 7 days. J Am Diet Assoc. 2000;100:1316–1322. doi: 10.1016/S0002-8223(00)00373-4. quiz 1323–1314. [DOI] [PubMed] [Google Scholar]
- 31.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 32.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160:526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 34.Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304:779–786. doi: 10.1001/jama.2010.1182. [DOI] [PubMed] [Google Scholar]
- 35.Saint S, Lipsky BA, Goold SD. Indwelling urinary catheters: a one-point restraint? Ann Intern Med. 2002;137:125–127. doi: 10.7326/0003-4819-137-2-200207160-00012. [DOI] [PubMed] [Google Scholar]
- 36.Bartlett JG. Aspiration pneumonia in adults. [Accessed September 2, 2016];UpToDate Online (online) Available at: http://www.uptodate.com/contents/aspiration-pneumonia-in-adults?source=machineLearning&search=aspiration+pneumonia+treatment&selectedTitle=1~150§ionRank=1&anchor=H14 - H10.
- 37.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 39.Pirozzo S, Papinczak T, Glasziou P. Whispered voice test for screening for hearing impairment in adults and children: systematic review. BMJ. 2003;327:967. doi: 10.1136/bmj.327.7421.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 41.Rubin DB. Statistical Analysis with Missing Data. 1987. [Google Scholar]
- 42.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 43.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Rosenbaum PR, Rubin DB, Yesavage JA, et al. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 45.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 46.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 47.Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172:255–260. doi: 10.1001/archinternmed.2011.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. [Accessed May 17, 2016];National and state healthcare associated infections: progress report 2016 (online) Available at: http://www.flashalertnewswire.net/images/news/2016-03/1635/92388/2016-CDC-hai-progress-report.pdf”.
- 49.Miake-Lye IM, Hempel S, Ganz DA, et al. Inpatient fall prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:390–396. doi: 10.7326/0003-4819-158-5-201303051-00005. [DOI] [PubMed] [Google Scholar]
- 50.Van Rompaey B, Elseviers MM, Van Drom W, et al. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16:R73. doi: 10.1186/cc11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Adverse Hospital Exposures In Study Populations.