Abstract
Studies of the activation of FXII in both platelet poor plasma and in neat buffer solutions were undertaken for a series of mixed thiol self-assembled monolayers spanning a broad range of water wettability. A wide spectrum of carboxyl/methyl-, hydroxyl/methyl-, and amine/methyl-thiol modified surfaces were prepared, characterized, and then utilized as the procoagulant materials in a series of FXII activation studies. X-ray photoelectron spectroscopy was utilized to verify the sample surface's thiol composition and contact angles measured to determine the sample surface's wettability. These samples were then used in in vitro coagulation assays using a 50% mixture of recalcified plasma in phosphate buffered saline. Alternatively, the samples were placed into purified FXII solutions for 30 min to assess FXII activation in neat buffer solution. Plasma coagulation studies supported a strong role for anionic surfaces in contact activation, in line with the traditional models of coagulation, while the activation results in neat buffer solution demonstrated that FXIIa production is related to surface wettability with minimum levels of enzyme activation observed at midrange wettabilities, and no statistically distinguishable differences in FXII activation seen between highly wettable and highly nonwettable surfaces. Results demonstrated that the composition of the solution and the surface properties of the material all contribute to the observation of contact activation, and the activation of FXII is not specific to anionic surfaces as has been long believed.
I. INTRODUCTION
It is well accepted that blood and blood plasma coagulates (clots) in hydrophobic plastic test tubes more slowly than it does in hydrophilic glass test tubes.1–3 This common knowledge together with the uncommon observation that blood of a particular patient, John Hageman, did not clot quickly in glass tubes led Oscar Ratnoff to discover the contact activation system of blood plasma coagulation.4 Intense research following Ratnoff's discoveries continuing through to this writing led to elaboration of the biochemistry of the plasma coagulation cascade and formulation of a biochemical mechanism for contact activation.5–9 This so-called autoactivation mechanism proposed that the zymogen Hageman factor (FXII) binds to anionic hydrophilic surfaces together with the allosteric proteins prekallikrein and high-molecular weight kininogen to form an “activation complex” through chemically specific interactions.10 Assembly of the activation complex on the activating surface leads to production of enzymatically active protease αFXIIa. In the presence of prekallikrein, further cleavage of αFXIIa results in activated FXII-fragment, βFIIa. Both αFXIIa and βFXIIa subsequently potentiate the series of zymogen-enzyme conversions that comprise the plasma coagulation cascade.11,12 Thus, it was explained that the activation complex does not assemble onto hydrophobic plastic tubes bearing no surface-resident anionic functionalities and do not efficiently activate FXII, causing blood and blood plasma to clot more slowly than blood or blood plasma contained in hydrophilic glass tubes with surface-resident anionic groups (see Ref. 13 and the citations therein for a review that supports this introductory section). In addition, FXII activation has also been linked to procogulant platelets in plasma, and multiple studies have shown that activated platelets promote coagulation in a FXII-dependent manner.14–18
Work in our laboratories developed mathematical models of plasma coagulation that could be statistically fit to experimental data measuring a decrease in plasma coagulation time (CT) as a function of activator surface area (so-called surface area titrations). A variable parameter of these models quantified the “catalytic potential” of activator materials or procoagulants.19–22 Using oxidized polymers and silanized glass particles spanning a range of surface energies (water wettability), it was shown that catalytic potential in plasma followed a systematic trend with surface energy, with low potential for poorly water-wettable (hydrophobic) materials and rising sharply with increasing hydrophilicity. These findings were in accord with the idea that the activation complex bound to hydrophilic anionic surfaces through chemically specific interactions. Similar data obtained using glass disks bearing silane self-assembled monolayers (SAMs) generally followed this trend but with some interesting departures.20 Notably, carboxyl-terminated SAMs were much more activating than anticipated on the basis of the general surface-energy trend and cationic ammonium-terminated SAMs were less activating than anticipated on a purely surface-energy basis. Technical difficulties involved in producing activators with different well-defined chemistry that were suitable for use in surface-area-titration studies prevented a thorough study of surface-chemical specificity in contact activation of blood plasma.
The matter of surface-chemical specificity was effectively dropped from our hands until activation experiments performed in buffer solutions of purified FXII showed that FXII activation was not, in fact, chemically specific for anionic hydrophilic surfaces.23 Indeed, it has been shown that FXII activation in buffer solution exhibits a parabolic response to activator surface energy, with high activation at both hydrophobic and hydrophilic extremes of water wettability and falling through a minimum over a range of intermediate surface energy.24 On the one hand, this finding is consistent with the well-known hematology fact that blood clots rapidly in hydrophilic glass tubes. On the other hand, this finding is completely inconsistent with the well-known hematology fact that blood clots slowly in hydrophobic plastic tubes because hydrophobic surfaces were found to be efficient contact activators of FXII in buffer. This apparent conundrum was explained by the “adsorption-dilution effect” asserting that a plethora of plasma proteins adsorb to hydrophobic surfaces from plasma, effectively diluting FXII surface contacts and thus retarding autoactivation at hydrophobic surfaces immersed in plasma (but not buffer solutions of FXII).13 No such adsorption-dilution effect occurs at hydrophilic surfaces that do not adsorb blood proteins and autoactivation is thus more efficient. The traditionally accepted specificity of contact activation for anionic hydrophilic surfaces was thus explained as an apparent specificity arising from the moderating influence of plasma proteins unrelated to the coagulation cascade. Similar reasoning was used to explain low-activation properties of cationic activators and other functionalities exhibiting ion-exchange properties.25
Thus, the role of surface chemistry in contact activation continued to appear subordinate to surface energy until it was recently discovered that autoactivation of FXII in buffer by glass particles bearing different silanized surface chemistries with different surface energy produced an ensemble of protein fragments that depended on activator surface chemistry/energy.24,26 It was found that contact activation of FXII appears produce a multiplicity of protein fragments,27 some of which have amidolytic (cleaves amino acid bonds of s-2302 chromogen) but not procoagulant properties (cause plasma to coagulate). More surprisingly, it was shown that some of these protein fragments actually suppress autoactivation. Unidentified suppression protein(s) are possibly related to autoinhibition of autoactivation wherein autoactivation is observed to be a self-limiting reaction. The extent to which this ensemble of protein fragments is produced by autoactivation in plasma is unknown as is the role of suppression proteins in the putative adsorption-dilution effect.
This paper further investigates the role of surface chemistry by measuring activation of both blood plasma and FXII in buffer solution by activator surfaces bearing well-defined surface chemistry. Activator surfaces were prepared using thiol adsorption onto gold substrata, creating SAMs with mixed surface chemistry by coadsorption of two different thiols with different terminal functional groups over a broad range of binary-pair compositions. Measurements reveal a heretofore unobserved chemical specificity in surface activation of FXII, which may lead to new surface engineering routes to hemocompatible biomaterials.
II. EXPERIMENT
This work measured activation of FXII and blood plasma by SAM surfaces supported on gold-coated rectangular glass coverslips. Single coverslips were immersed in solutions contained in a plastic UV-Vis spectrophotometer cuvette. A drawback of this approach compared to previous studies using either silanized glass particles or silane-SAM glass coverslips was that activator surface area was comparatively low. However, the advantage of this system was the fine control over surface chemistry afforded by the thiol-on-gold system and the ability to engineer surfaces with a controlled gradient of surface chemistries using mixed thiols.28–31 Sections II A–II F are organized to detail methods of surface engineering and measurement of calibration curves that quantified coagulation time results in terms of FXIIa yield. Section II C characterizes activator surfaces and quantifies limits of detection.
A. Plasma and proteins
Human platelet poor plasma (PPP) was prepared by pooling five units of anticoagulated recovered human plasma (outdated less than two days) from the Blood Bank at the Pennsylvania State University Milton S. Hershey Medical Center. Plasma was centrifuged at 1500 g for 10 min, and the resulting plasma supernatant was aliquoted in 15 ml polypropylene tubes and stored at −20 °C. Prior to use in assays, plasma was thawed for ∼40 min in a 37 °C water bath.
Human factor XII (Hageman factor) and human activated factor XII (αFXIIa) were obtained from Enzyme Research Laboratories (ERL) (South Bend, IN) and Haematologic Technologies, Inc. (HTI) (Essex Junction, VT). All proteins were thawed upon receipt and stored as aliquots at −80 °C. Prior to use, proteins were thawed at room temperature and diluted with phosphate buffered saline (PBS) (0.01 M, pH 7.4, Sigma–Aldrich).
B. Gold substrate and SAMs formation
Dodecanethiol (Sigma-Aldrich), 11-mercaptoundecanoic acid (Sigma-Aldrich), and 11-mercaptoundecanol (Sigma-Aldrich) were used as received. The cleaned borosilicate glass coverslips with dimension of 10.5 × 22 mm (4.6 cm2 total surface area) were sputtered coated onto both sides with a 10 nm titanium adhesion layer and 200 nm gold layer (Thin Films, Inc., NJ). These gold substrates were used for the preparation of all thiol self-assembled monolayers.
Thiol solutions (1 mM) in anhydrous ethanol were used as mixtures by varying the ratios of the single-component solutions. Stock solutions of 1 mM 11-mercaptoundecanoic acid and 11-amino-1-undecanethiol (Sigma-Aldrich) were prepared as noted above with the addition of trifluoroacetic acid or triethylamine, respectively, to 2.5% (v/v).32 For preparation of SAM surfaces, gold substrates were cleaned extensively in gold cleaning solution (Sigma-Aldrich) for 3 min. This gold cleaning solution contains sulfuric acid (5%–10%) and thiourea (0.1%–1%) and is an effective surface activator and adhesion promoter for gold substrates prior to bonding processes. This initial cleaning step was followed by rinsing thrice using deionized water (Millipore, 18.2 MΩ) and thrice using ethanol, and then the samples were further air-plasma cleaned for 15 min per side (100 W, Harrick) followed by rinsing thrice with ethanol. The samples were allowed to remain in ethanol for at least 1 h to allow the gold surface to become reduced.33,34 Finally, the samples were immersed in a 0.125% (v/v) of butyltrichlorosilane (Gelest) in chloroform for 15 min, and then rinsed with chloroform and subsequently ethanol. This final step with silane was used to block any exposed glass due to insufficient gold coating of the glass coverslip edges or inadvertent scratching from handling the samples, and that otherwise dominated the activation process.
The cleaned and prepared gold substrates were immersed in 1 mM thiol solutions at room temperature for 20 h, protected from light. After incubation, the samples were rinsed with ethanol, with the exception of samples exposed to the 11-mercaptoundecanoic acid or 11-amino-1-undecanethiol solutions, for which a solution of 0.1 M NaOH or 0.1 M HCl, respectively, in deionized water was used in lieu of ethanol as an intermediate rinse. All samples were dried under N2 and stored under vacuum, protected from light, for subsequent use.
C. SAM-surface characterization
The water wettability of SAM surface was determined by sessile drop measurements of the advancing water contact angle using a Krüss contact angle goniometer. All measurements were made using water as a probe liquid. Advancing contact angles were measured by a minimum of five independent measurements and are presented as mean ± standard deviation. The water contact angles were converted to water adhesion tension: , where mJ/m2 is the interfacial tension of water at 20 °C and θa is the observed advancing contact angle of water on the SAM surface.
The chemical compositions of SAM-surfaces were analyzed by x-ray photoelectron spectroscopy (XPS). The XPS spectra were collected by AXIS Ultra-x-ray photoelectron spectrometer (Kratos Analytical) at hybrid mode using monochromatic Al Kα radiation at a base pressure below 1 × 10−8 Torr. Scanning was carried out over a wide binding energy range (0–1350 eV) with a scan step size of 0.5 eV. A pass energy of 80 eV was used for survey spectra while a pass energy 23.5 eV and step size 0.10 eV were used to acquire carbon high resolution spectra. The high resolution O(1s), S(2p), and Au(4f) spectra were taken with a 40.0 eV pass energy. The binding energy scale was referenced by setting the C-H peak maximum in the C 1 s spectrum to 285.0 eV. The spectra were analyzed by casaxps software (version 2.3.12 Dev9).
Surface topography and features were characterized by an atomic force microscopy (AFM) (Nanoscope IIIa, Veeco, Santa Barbara, CA) utilizing a silicon nitride probe in air. The height and friction images of the mixed-SAM surfaces were acquired in an effort to detect single-component domains.
D. In vitro plasma coagulation assay
An in vitro assay was used to measure the plasma coagulation activity in terms of CT, defined as the time required from activation of the coagulation cascade to the appearance of visible clot. The assay has been described in previous publications.35,36 Briefly, 0.5 ml of PPP was recalcified with 0.1 ml of 0.1 M CaCl2 and mixed with a known dose of procoagulants (FXIIa or solid thiol SAM-surfaces) in a 2.5 ml polystyrene semi-micro-cuvette (12.5 × 12.5 × 45 mm) (BRAND+CO KG, Germany). The volume was adjusted by adding 0.01 M PBS to obtain a 1 ml solution in a 1:1 dilution of plasma in buffer. The order in which reagents and solid materials were added was varied depending on the assay type, with recalcification of plasma always occurring last to ensure a common zero time CT. The cuvettes were capped with parafilm and rotated at 8 rpm on a hematology mixer, and the corresponding CT was recorded.
Plasma coagulation time was used to quantify FXIIa activity in solution using FXIIa titration calibration curves relating [FXIIa]eq, in PEU/ml, to CT. A mathematical model developed in a previous investigation19 was fitted to the resulting plot of coagulation times to the amount of exogenous FXIIa added.
For assaying the substrate-induced contact activation in PPP, a single thiol-modified substrate (nominal surface area of 472 mm2) as the solid procoagulant was added in cuvette and CT was measured. All CT measurements were performed in PPP with n ≥ 3 for each.
E. FXII activation in neat buffer solutions
Purified FXII activation in the presence of thiol-modified surfaces was measured by recording the CT of PPP upon addition of an aliquot of the test solution and referencing the corresponding FXIIa titration calibration curve. A single thiol-modified substrate was placed in a 2.5 ml polystyrene semi-micro-cuvette containing a test solution of 30 μg/ml purified FXII in PBS and tested in triplicate for each sample (n = 3). The cuvette was gently rocked for 30 min, after which 100 μl aliquots were taken to measure the CT indicating FXII activation. The coagulation time was used to calculate the equivalent FXIIa activity by referencing back to the FXIIa-titration curve described in Sec. II D.
F. Data analysis
Statistical analyses were performed by parametric ANOVA (Holm-Sidak test) or nonparametric ANOVA (Student-Newman-Kuels test) using SigmaPlot software. Means of experimentally determined [FXIIa]eq for each surface type were compared pairwise and the differences were considered statistically significant for p-values less than the critical p-value as determined by the Holm-Sidak test (α = 0.05).
III. RESULTS AND DISCUSSION
A. Thiol-SAMs activator surface characterization
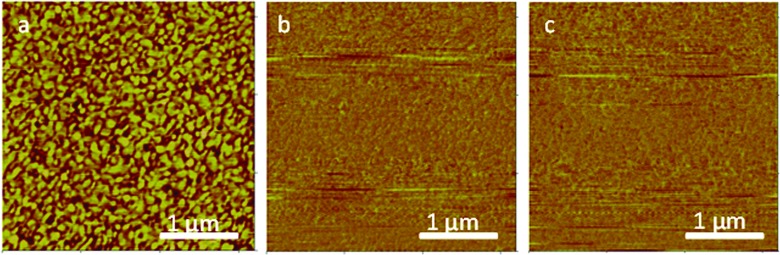
The topography of thiol-SAMs modified gold surfaces was characterized by AFM (Fig. 1). The height images of bare gold surfaces exhibited features between 50 and 100 nm in size and <10 nm in height, typical of the grain structure of sputter-coated gold. The gold surface showed an RMS roughness (Rq, root mean square) of 1.982 nm and Ra of 1.565 nm. Friction force AFM images of mixed thiol-SAMs did not detect any discernible domains that might be indicative of phase separation [Figs. 1(b) and 1(c)]. In a previous study, ∼400 nm islands of an amine-terminated silane dispersed in a butyltrichlorosilane background on glass substrates were easily visualized by these same types of friction mode atomic force microscopy images.37 Whereas islands were detectable in silanes using friction mode imaging, other investigators indicate that phase separated domains in mixed thiol-adsorbed SAMs of comparable chain length are 15 nm2 and undetected using friction force microscopy.38,39 Thus, whereas no gross domains were observed by AFM, the presence of smaller domains <15 nm2 cannot be completely excluded from the data at hand.
Fig. 1.
AFM images of thiol-modified gold surface, (a) topographic image (z scale, 20 nm), (b) friction mode trace image, and (c) friction mode retrace image. Scan size: 3.2 × 3.2 μm.
The chemical composition of thiol-surfaces was analyzed by XPS. Results show that thiol SAMs presented only Au, C, O, and S on the carboxyl-methyl and hydroxyl-methyl thiol mixed surfaces, while additional N was detected on the amine-methyl surfaces, as expected. Notably, no Si or Ti peaks were observed, consistent with an infinite thickness of a contiguous coating of Au on the glass SiOx substratum. Small oxygen peaks were detected for methyl-terminated SAMs, consistent with findings from other groups.40–42 The detailed atomic compositions of C, N, O, and Au in various SAMs surfaces are tabulated in supplementary material, Table I.49 High resolution S2p peaks corresponded to a doublet centered at 162.2 and 163.5 eV with a 2:1 peak-area ratio, consistent with published work.41 No peaks were detected in with the range of 164–169 eV, indicating the absence of oxidized sulfur species or unbound thiols. High resolution C1s spectra for methyl-terminated SAMs show peaks centered at 284.6 eV after charge correction. Hydroxyl- and carboxyl-terminated SAMs exhibited additional peaks at 286.7 and 289.5 eV, respectively, corresponding to C–O and C=O functionalities, respectively. The C1s and N1s spectra of amine-terminated SAMs exhibited peaks at 286.8 and 400.6 eV, respectively, which confirm the presence of C–N bond in SAMs.
Table I.
Practical limits of quantification for the two lots of plasma used in this study. Control coagulation time—the mean coagulation time (± standard deviation) of the controls to which no exogenous FXIIa was added; practical quantification limit—the mean control coagulation time minus ten times the standard deviation; [FXIIa]eq—the equivalent amount of exogenous FXIIa needed to elicit the practical quantification limit coagulation time.
| Plasma lot | Control coagulation time (min, mean ± SD, n = 3) | Practical quantification limit (min) | [FXIIa]eq (PEU/ml) |
|---|---|---|---|
| A | 58.20 ± 1.64 | 41.80 | 3.59 × 10−4 |
| B | 56.87 ± 0.99 | 46.97 | 3.50 × 10−4 |
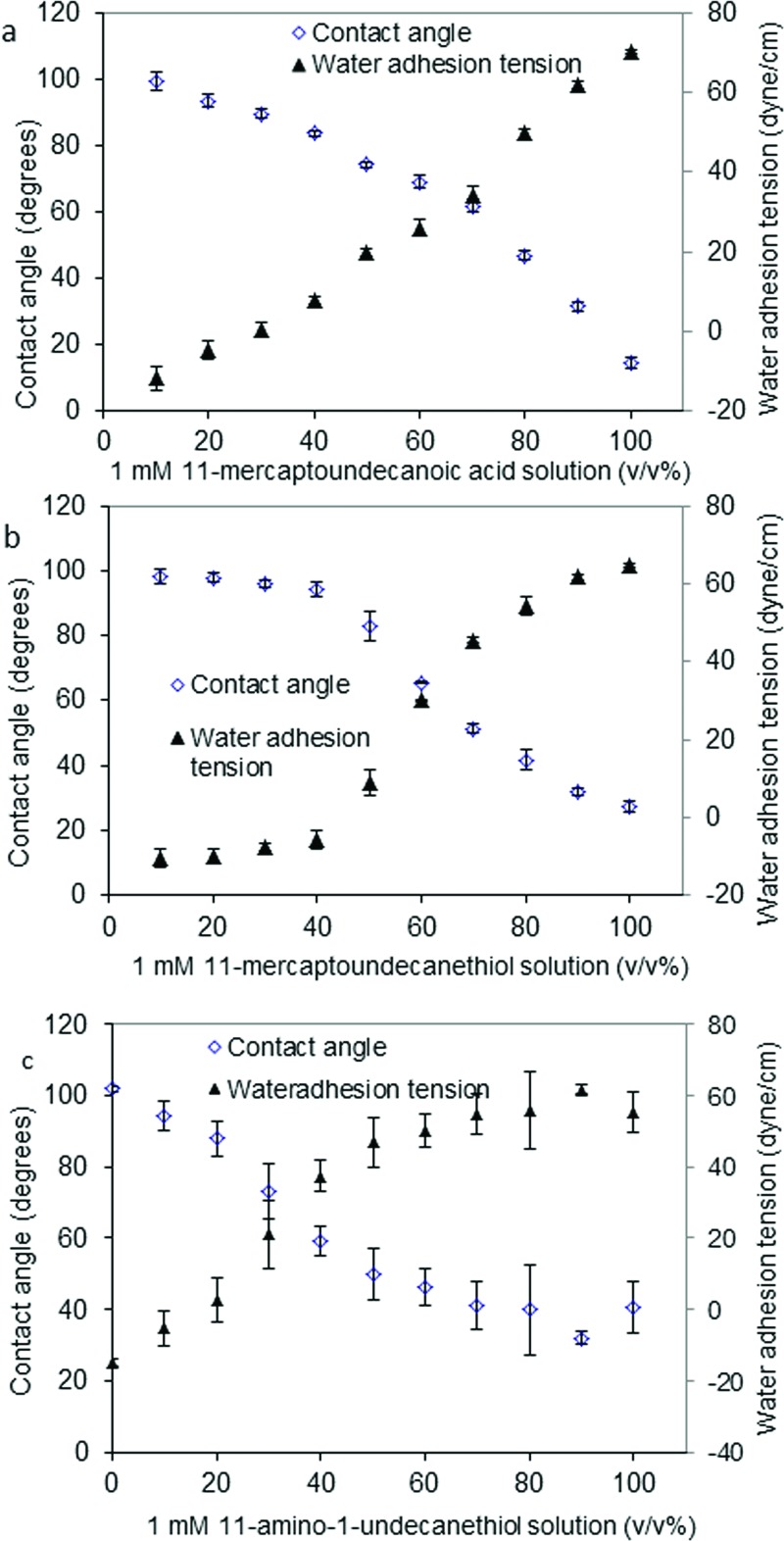
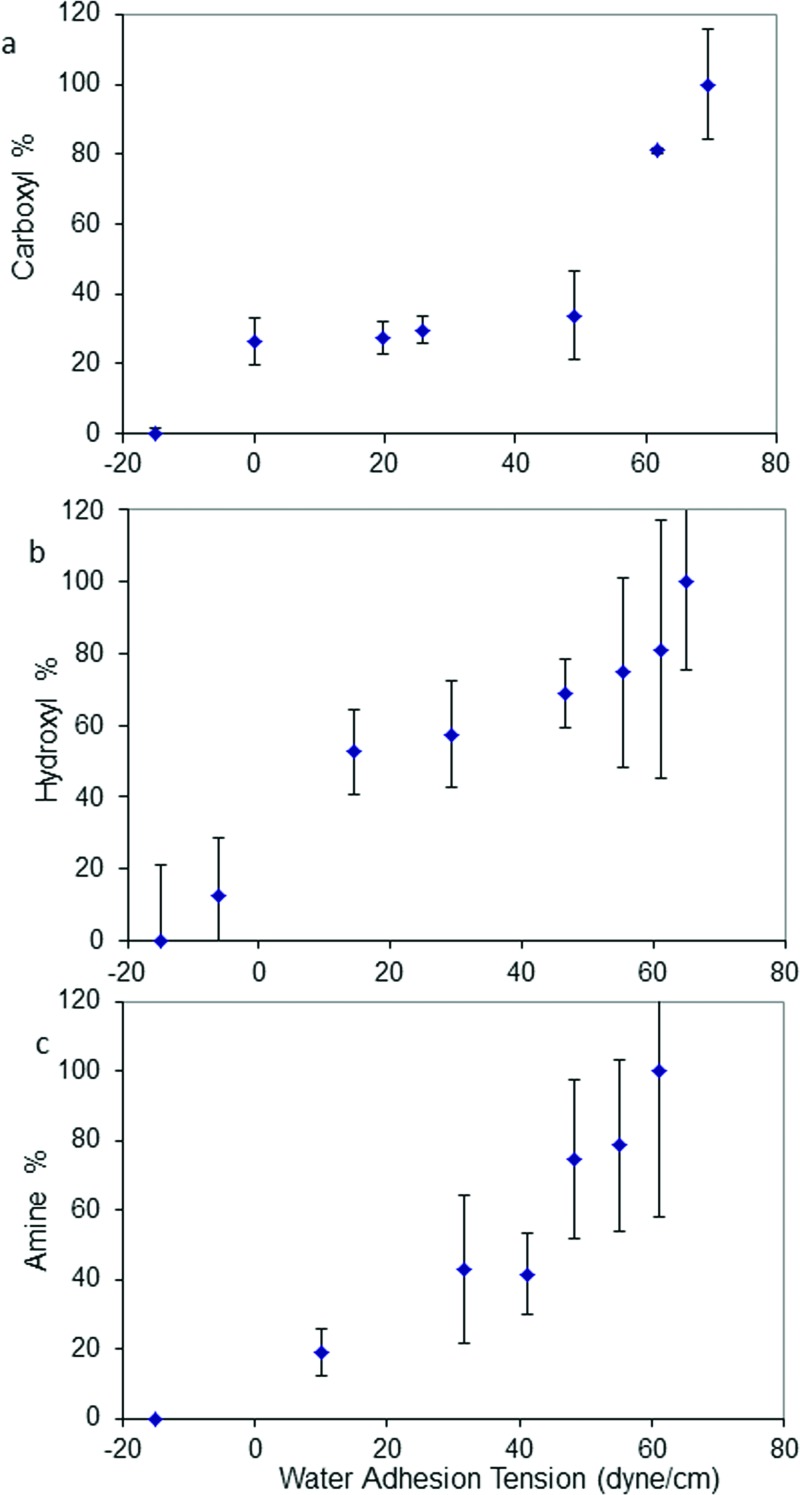
Water wettability of mixed SAMs was observed to change as a function of mixed thiol solution composition as shown in Figs. 2(a)–2(c) (carboxyl-, hydroxyl-, and amino/methyl-terminated SAMs, respectively). Generally, wetting characteristics of mixed thiols were a smooth function of thiol solution composition, and the water contact angle increased with increases in the methyl surface composition. Furthermore, results show that wetting characteristics of the hydroxyl/methyl-terminated SAMs [Fig. 2(b)] was a sharper function of solution composition than the carboxyl- [Fig. 2(a)] or amino-/methyl-terminated mixed SAMs [Fig. 2(c)] due to the solubility difference between methyl- and hydroxyl-terminated thiols, and consistent with the results previously published.40 Water wettability is related to concentration of thiol functional groups in the SAMs. Figure 3 plots the relationships between the water adhesion tension of surface and the corresponding concentrations of carboxyl-, hydroxyl-, or amine- in SAMs as determined from XPS data. Results showed that the water adhesion tension shows a strong relationship with the relative concentrations of terminal functional groups. These chemically well-defined and molecularly smooth surfaces provide activators varying only in terminal functional-group density, and represent an improvement in surface engineering over silianized glass particulate activators used in our previous work.24,26,43 However, the surface area of the SAM activators (4.6 cm2) was nearly 54× smaller than that used in particulate activation studies (∼2.5 × 102 cm2).27 FXII activation was commensurately reduced but detectable well above estimated detection limits.
Fig. 2.
Contact angles and water adhesion tension (mean ± SD, n = 3) of thiol modified substrates corresponding to the solution composition. (a) Contact angles of mixed carboxyl-/methyl-terminated thiol-modified substrates corresponding to the solution percentage of 1 mM 11-mercaptoundecanoic acid in ethanol. (b) Contact angles of mixed hydroxyl-/methyl-terminated thiol-modified substrates corresponding to the solution percentage of 1 mM 11-mercaptoundecanol (11-MU-OH) in ethanol. (c) Contact angles of mixed amino-/methyl-terminated thiol-modified substrates corresponding to the solution percentage of 1 mM 11-amino-1-undecanethiol in ethanol.
Fig. 3.
Water adhesion tension of thiol modified substrates corresponding to the terminal functional group composition in SAM surfaces as analyzed by XPS (mean ± SD, n = 3). (a) Mixed carboxyl-/methyl-terminated thiol-modified substrates. (b) Mixed hydroxyl-/methyl-terminated thiol-modified substrates. (c) Mixed amino-/methyl-terminated thiol-modified substrates.
B. FXIIa titration assay in PPP
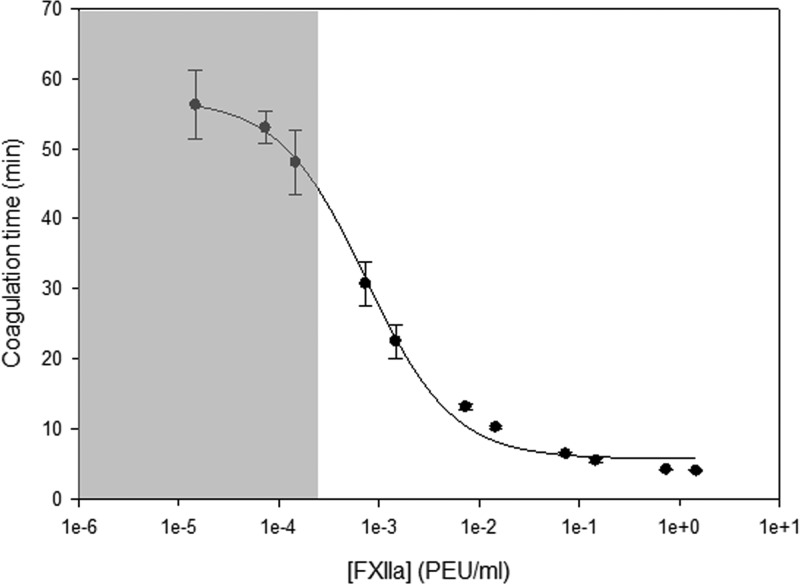
Coagulation properties of human PPP were characterized by a FXIIa titration assay. In this study, we used two lots of human PPP and each lot of plasma was titrated with FXIIa. Figure 4 shows the decreasing CT of recalcified human PPP with increasing FXIIa doses. Table I lists the coagulation times for the controls in which no exogenous FXIIa were added. A practical quantification limit was set at 10× the standard deviation of the control coagulation times subtracted from the mean coagulation time of the controls and the corresponding [FXIIa]eq. Alpha or beta errors (probability of a false positive or false negative, respectively) are extremely unlikely for a sample with a coagulation time greater than this practical limit.
Fig. 4.
FXIIa titration curves for the plasma showing coagulation times vs concentrations of exogenous FXIIa. The smooth curves result from a least squares fitting of a mathematical model developed in Guo et al. (Ref. 19). The shaded areas represent exogenous concentrations of FXIIa, and their corresponding coagulation times with respect to the modeled curve, which fall outside of the practical limit of quantification for this assay.
C. Contact activation of PPP induced by thiol-SAMs surfaces
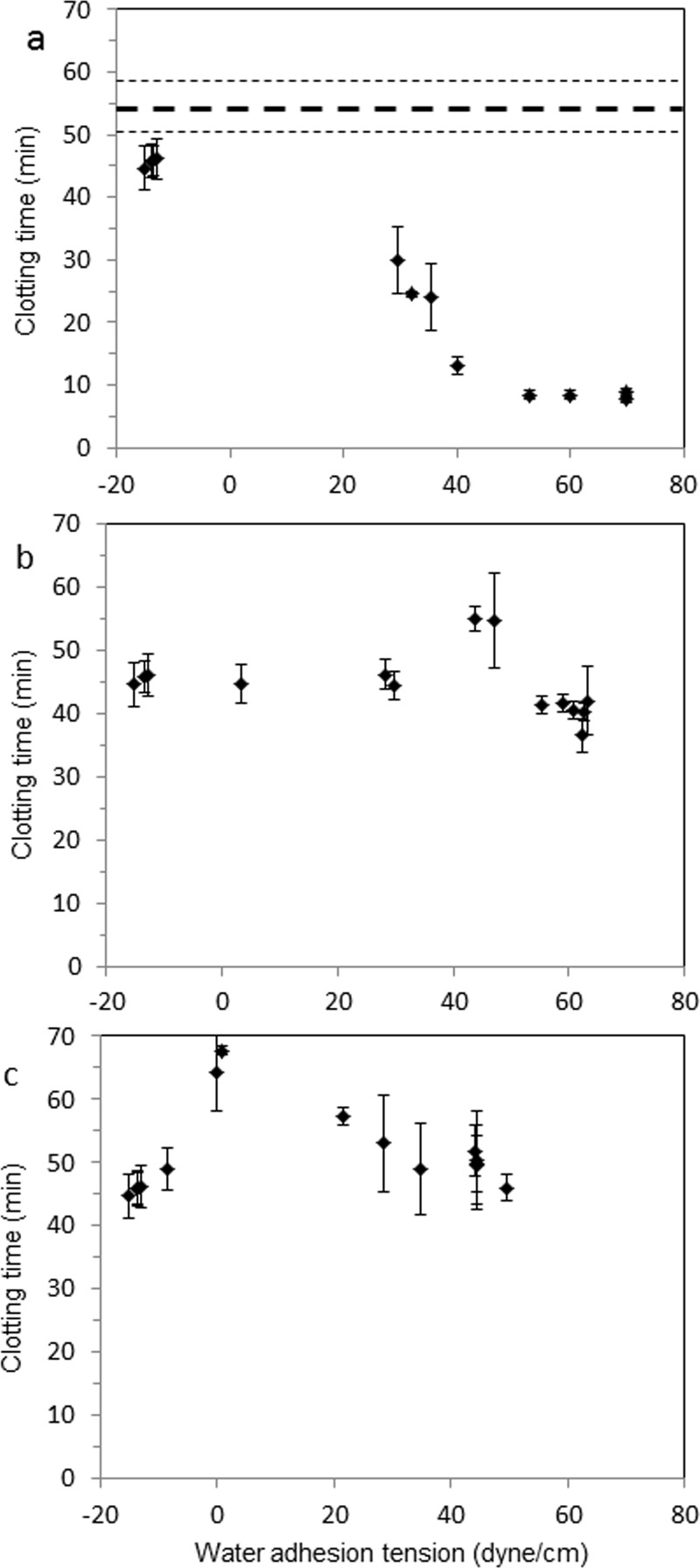
Contact activation of blood plasma by mixed-thiol SAM surfaces was measured using a coagulation-time assay. Figure 5 shows the clotting time of plasma in contact with mixed carboxyl/methyl-, hydroxyl/methyl-, and amine/methyl-terminated thiol surfaces scaled by water wettability (shown as water adhesion tension), respectively. For comparison, the mean clotting time and standard deviation of plasma without addition of any thiol-modified substrate are shown as dotted lines in Fig. 5(a). The mixed carboxy-/methyl-thiol surfaces decreased the clotting time with increasing water adhesion tension and came to a plateau after the adhesion tension reached 50 dyn/cm [Fig. 5(a)]. This trend is consistent with expectations drawn from numerous studies which demonstrated that hydrophobic activators are all but inert in plasma whereas anionic hydrophilic activators are potent activators of the extrinsic pathway of plasma coagulation (see Ref. 13 and citations therein). Furthermore, the trend in activation pivots from high-to-low clot time (low-to-high activation) near the ∼30 dyn/cm water adhesion tension where other biological responses are likewise observed to pivot from low-to-high or high-to-low, depending on the specific circumstance under investigation.44–46
Fig. 5.
Coagulation times (CT) of thiol modified substrates (mean ± SD, n = 3), (a) carboxyl-/methyl-terminated thiol-modified substrates, (b) hydroxyl-/methyl-terminated thiol-modified substrates, (c) amine-/methyl-terminated thiol-modified substrates. The dashed and dotted lines indicate the mean baseline coagulation time ± standard deviation (n > 3) in which no thiol-modified substrate has been added to the sample cuvette.
In sharp contrast, coagulation induced by -OH and -NH2-terminated mixed thiol surfaces show curves that are effectively flat, exhibiting no significant activation of plasma coagulation at any mixture composition or resulting hydrophilicity. The clotting time of plasma in contact with mixed hydroxyl/methyl-thiol surfaces were essentially constant across all water adhesion tension values with the exception of a small drop in the clotting time of plasma seen at water adhesion tension ∼62 dyn/cm [Fig. 5(b)]. The mixed amino/methyl-thiol surfaces show a peak clotting time near a water adhesion tension of 0 dyn/cm [Fig. 5(c)].
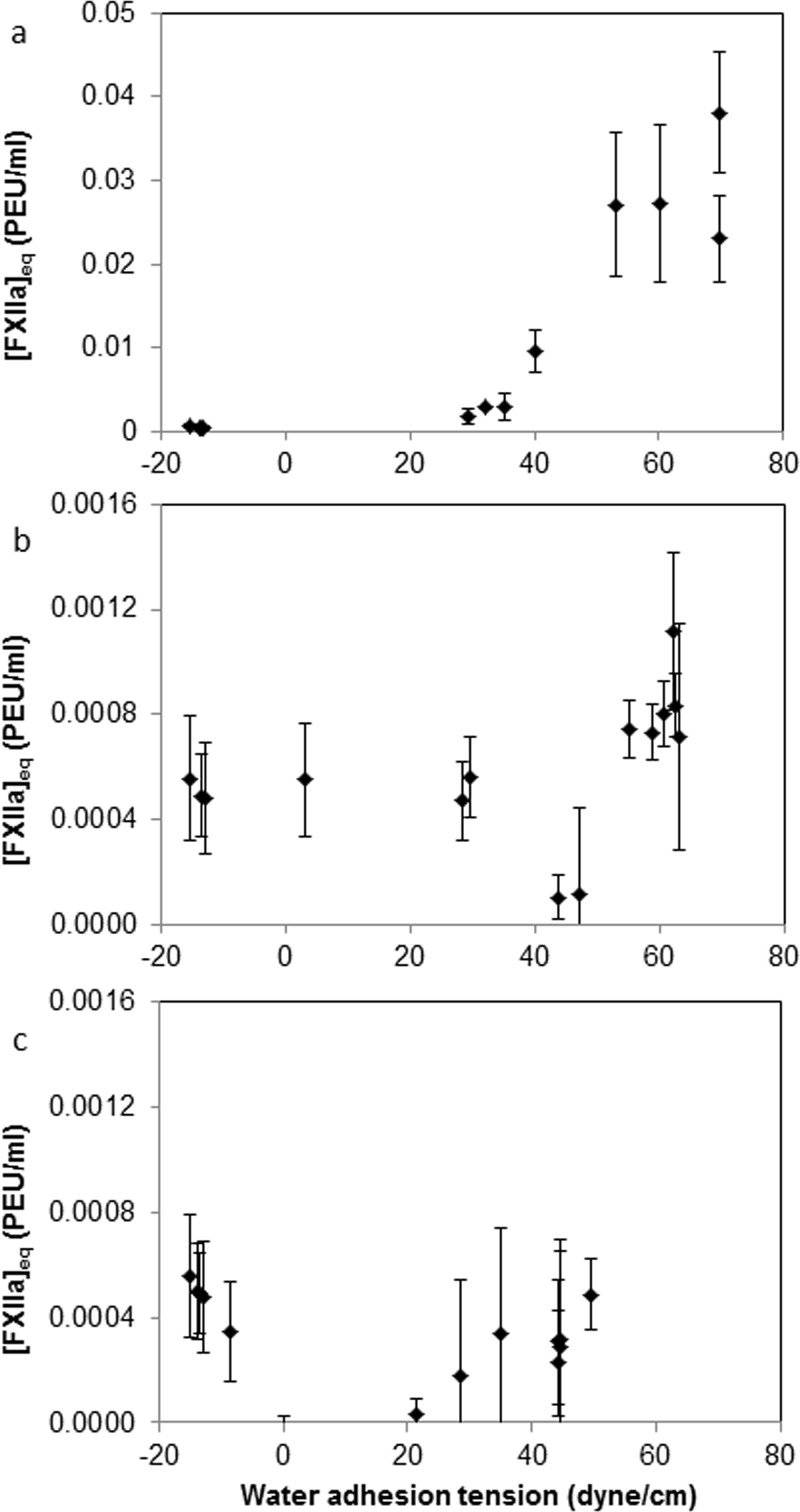
The measured coagulation time was converted to equivalent FXIIa activity [FXIIa]eq using a FXIIa titration calibration curve (Fig. 4) and is illustrated in Fig. 6. Specifically, coagulation time induced by known concentrations of exogenous FXIIa permitted estimation of an equivalent FXIIa concentration produced by the contact activation process. It is stressed that the actual FXIIa concentration was not measured but was estimated by a plasma coagulation assay, and that other activated factors such as FXIIf might have been produced in the activation process.13,24
Fig. 6.
[FXIIa]eq, calculated from coagulation times of (a) carboxyl-/methyl-terminated thiol-modified substrates, (b) hydroxyl-/methyl-terminated thiol-modified substrates, and (c) amine-/methyl-terminated thiol-modified substrates (mean ± standard error, n = 3).
It is interesting to see that there is near 40-fold increase in the amount of [FXIIa]eq produced in plasma by the pure carboxyl-terminated surface than hydroxyl- or amino-terminated thiol-surfaces. Although both surfaces are hydrophilic and have similar water contact angles, the carboxyl-terminated surface appears to be a much stronger activator in PPP, consistent with the widespread view that anionic-hydrophilic surfaces are specific activators of FXII. However, these data show that the hydroxyl-terminated surface is also an activating surface in PPP, and to an even greater extent than a protein-adsorbent methyl-terminated surface, consistent with the idea of the protein adsorption dilution effect in plasma. The observation of FXII contact activation on hydroxyl-terminated surface induced plasma coagulation suggests that the mechanisms behind surface-induced blood plasma coagulation are much more complicated than simply activation by anionic surfaces. The mechanism for FXII activation and plasma coagulation certainly must include other factors, such as surface wettability and protein adsorption properties of the materials, which are assuredly interrelated.
Comparing the coagulation times of mixed amine- and hydroxyl-thiol surfaces, no significant difference was observed in PPP, as materials having intermediate wettability appear to have little to no coagulation activity in plasma. The coagulation parameters produced by these materials are statistically indistinguishable from the background parameters, and in fact for samples with adhesion tension of approximately 0 dyn/cm (corresponding to a 90° water contact angle), the coagulation time appears to be even slightly greater than background. As seen in Fig. 6(c), this increased coagulation time would represent a “negative” value for [FXIIa]eq, and while a negative value for this term is impossible, this value may in fact be indicative of activation of an inhibitory pathway or activation of suppression proteins. However, despite an extensive search for an appropriate mechanism, we currently do not have sufficient data to identify which pathway might be activated in these materials.
For both the hydroxyl and amine series, we also found that there was a decreased level of FXII activation for surfaces with adhesion tension of approximately 45 dyn/cm that is statistically indistinguishable from the control coagulation activation, suggesting a possible minimum for FXII activation at midrange wettabilities, consistent with the results previously published by Golas et al.24 An important difference between the work of Golas et al. and this study is the means of surface preparation; in the previous work, an array of different silane chemistries was used to create surfaces with varying wettability versus the limited number of thiol chemistries mixed in different ratios in this work. It is of interest to note that the carboxyl series did not show this same minimum in activation in plasma near 45 dyn/cm, particularly in light of FXII activation results which are discussed later in this manuscript.
Combined results in Figs. 5 and 6 suggest that contact activation of plasma coagulation is correlated with activator surface energy (water adhesion tension) only for mixed carboxyl-methyl terminals, but not directly correlated with surface energy on thiol-SAM surfaces comprised of either –OH or –NH2 terminal functionalities in –CH3 background. Methyl- and amino-terminated mixed thiol chemistries apparently do not autoactivate endogenous plasma FXII in a manner that potentiates the plasma coagulation cascade. It is possible that -OH and -NH2-terminated mixed thiol chemistries might be inherently nonactivating. Alternatively, -OH and -NH2-terminated mixed thiol chemistries could be subject to the adsorption–dilution effect across the full range of hydrophilicity or perhaps FXIIa activity is suppressed. The first of the possibilities cannot be dismissed out of hand by the observation that -OH and -NH2-terminated mixed thiol chemistries activate FXII in neat buffer solution because plasma is vastly more complex than purified protein solutions. Even so, this supposition only invites the alternatives to explain how autoactivation in plasma can be so different than autoactivation in buffer. We do not observe the adsorption–dilution effect at hydrophilic surfaces,13,47 so this cannot explain why hydrophilic -OH and -NH2-terminated compositions do not activate plasma coagulation. The most viable explanation of Figs. 5 and 6 data thus appears to be that hydrophilic -OH and -NH2-terminated chemistries do, in fact, activate FXII, but also produce suppression proteins in the manner described by Golas et al.27 that effectively shuts off contact activation of plasma coagulation.
D. Contact activation of FXII induced by thiol-SAMs surfaces in neat buffer solutions
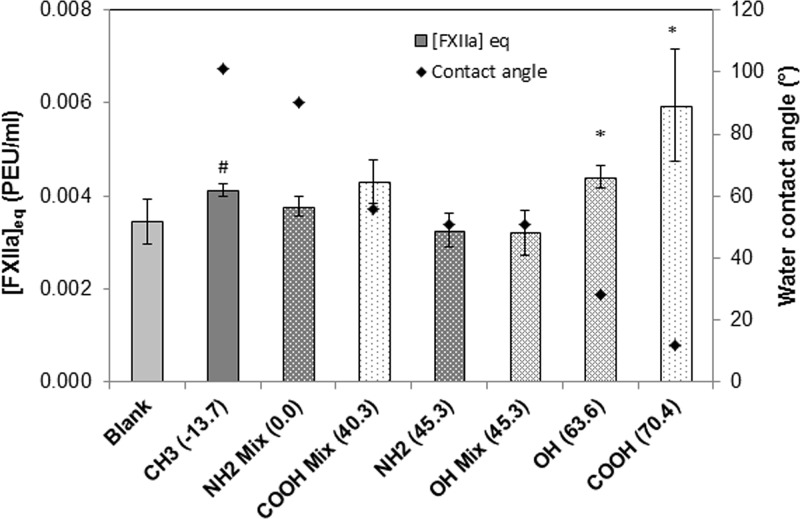
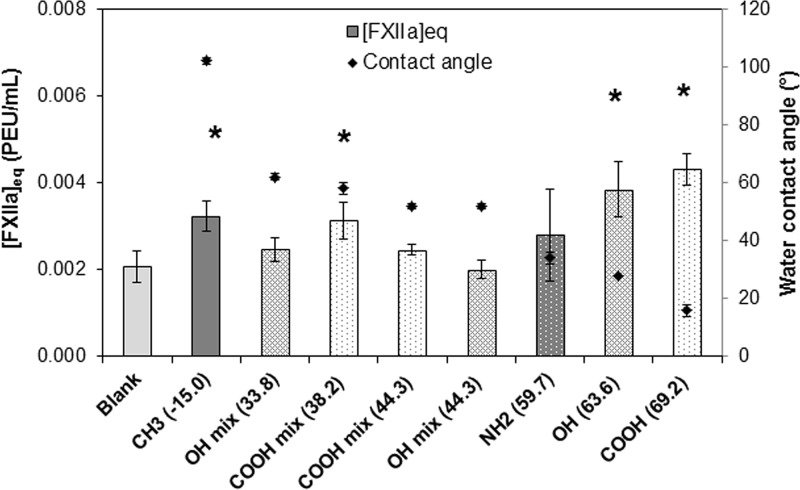
FXII activation by thiol-SAMs surfaces was measured by plasma coagulation time and conversion to [FXIIa]eq based on corresponding titration curve. Since we used two different lots of FXII and pooled human plasma, FXII activation by thiol-mixed surfaces is illustrated in Fig. 7 (FXII from ERL) and Fig. 8 (FXII from HTI), respectively. Note that the [FXIIa]eq produced by the empty-tube blanks for the two FXII lots were different by nearly 50% because manufacturer-specified FXII lot activities were different by approximately 44% (40.31 PEU/mg and 28 PEU/mg for the ERL and HTI supplied FXII, respectively). Although the exact amount of [FXIIa]eq produced in two lots of FXII were different, the trends in results are consistent between these two lots of FXII, and the levels of FXII activation products were well within the practical limits of quantification. Results show that mixed SAM surfaces activate FXII in buffer solution at approximately 10× greater than the conservative detection limit of 3.50 × 10−4 PEU/mL (Table I). These results are consistent with our previous studies showing that autoactivation is not specific to anionic hydrophilic surfaces.13
Fig. 7.
FXII (from ERL) activation (bar, mean ± SD, n ≥ 5) in neat buffer solution. The water adhesion tension values (dyn/cm) are listed in the parentheses behind each thiol functional group. The corresponding water contact angle was shown in the figure as symbol (♦). * denotes statistical significance as compared to OH mix (45.3) and NH2 mix (45.3), to the control blank and determined via the Holm-Sidak method with α = 0.05. # denotes statistical significance as compared to OH mix (45.3) only.
Fig. 8.
FXII (HTI) activation (bar, mean ± SD, n ≥ 5) on varied surfaces in neat buffer solution. The water adhesion tension values (dyn/cm) were listed in the parentheses behind each thiol functional groups. The corresponding water contact angle is shown in the figure as symbol (♦). * denotes statistical significance as compared to OH mix (44.3) and the control blank and determined via the Holm-Sidak method with α = 0.05.
The statistical analysis showed that the single component materials at the extremes of wettability (both hydrophobic and hydrophilic) produced statistically greater amounts of [FXIIa]eq, while with just the exception of the COOH-methyl mixture at 56° and 58° water contact angles (corresponding water adhesion tensions at 38.2 and 40.3 dyn/cm), none of the mixed substrates having midlevel wettabilities produce [FXIIa]eq statistically greater than the blank. This curve traces out a weak parabolic dependence on surface energy that is similar to the curve obtained with silanized glass particulate activators although that curve much more prominently obtained because of the much higher surface area of the particulate activators.27
The FXII activation studies performed in neat buffer solution provide an interesting contrast to the plasma studies discussed previously. The neat buffer studies show that the pure carboxyl-terminated surface produced a statistically significant greater [FXIIa]eq than either the purely methyl-terminated surface or the control blank, but the pure methyl-terminated surface also produced a statistically significantly greater [FXIIa]eq than the control blank, indicative of FXII activation by the seemingly “inert” methyl surface. Most interestingly, a mixed carboxyl/methyl surface with a water contact angle 56° (water adhesion tension 40.3 dyn/cm) for data in Fig. 7, or 52° (water adhesion tension 44.3 dyn/cm) for data in Fig. 8, produce levels of [FXIIa]eq that were only slightly higher than the background levels, showing that in this case, the addition of the “highly activating” anionic COOH groups to the methyl-terminated materials at middle-level surface wettability had no significant effect on FXII activation. However, the plasma coagulation studies for the mixed carboxyl series [see Figs. 5(a) and 6(a)] did not indicate any such minimum in [FXIIa]eq at these midlevel water adhesion tension values. In fact, it is not until samples have water contact angles greater than 60° that coagulation times in plasma begin to notably increase, and at pure methyl-terminated samples, a minimum [FXIIa]eq is measured. Thus, although both the pure methyl-terminated and pure carboxyl-terminated surfaces produced [FXIIa]eq above control values in buffer, this activation potential did not translate to plasma as the hydrophobic surface appears to be a highly inefficient activator of blood coagulation in plasma. Furthermore, a second COOH/methyl mixture having a water contact angle of 58° (Fig. 8) showed increased levels of FXII activation, comparable to the background methyl material with water contact angle over 100°. There is evidence that there is a very steep transition between hydrophobic (type I) and hydrophilic (type II) activators near 60° water contact angle, and it would appear that the two different carboxyl/methyl materials presented in Fig. 8 might actually straddle that transition line.48
The hydroxyl mixture series presents further data for reduced FXII activation at midrange water wettabilities. In neat buffer solution, the hydroxyl/methyl series show a similar trend to the carboxyl/methyl series. The pure hydroxyl-terminated surface produces a statistically significant greater [FXIIa]eq than either the purely methyl-terminated surface or the control blank, and again the pure methyl-terminated surface produced a statistically significant greater amount than the control blank. However, when hydroxyl is added to the pure methyl materials to produce a mixed surface having water contact angle of 51° or 52° for the two FXII lots, the data show reduced levels of FXII activation in pure buffer. That is, adding a highly activating component to an only slightly less activating surface produces a unique material having an activating potential that is less than either of them alone. When slightly less hydroxyl component is added, yielding a material with a water contact angle of 62°, one finds an increased amount of [FXIIa]eq produced, again suggesting a very sensitive transition region that occurs near 60° water contact angle.
Despite the similarities in [FXIIa]eq produced in buffer by the carboxyl/methyl and hydroxyl/methyl series, the plasma coagulation studies with these two materials are quite different. Within the hydroxyl series, a maximum coagulation time, statistically indistinguishable from the “baseline” coagulation time of the control blank, is noted near 45 dyn/cm, with slightly but not markedly reduced coagulation times seen at each of the two extremes. Furthermore, as noted previously, the [FXIIa]eq produced by the pure hydroxyl material in plasma is nearly 40× less than that of the pure carboxyl material, despite these two materials producing similar amounts of [FXIIa]eq in buffer. It is unclear at this point what factors could lead to this observation, although activation of additional pathways is high on the list of suspected mechanisms. However, to date, we have not identified which of the potential mechanisms is responsible, keeping in mind that there may be different pathways in play for different activating materials. It was observed that Kal-mediated FXII activation (reciprocal activation) is the principal pathway for FXII activation, producing 75% of the FXIIa yield within the intrinsic pathway, and the amount of activated enzyme is proportional to the initiating amount of FXIIa produced through autoactivation regardless of activating surface chemistry,35 and so it seems doubtful that this falls into a kalikrein-mediated mechanism. Other studies indicate that the role of the surface in contact activation may simply be confined to the autoactivation of FXII.43 These observations raise questions as to the role of factors outside of the proteins of contact activation (FXII, kallikrein, high-molecular-weight-kininogen, and FXI) in the plasma coagulation response to surfaces.
IV. CONCLUSION
Thiol-based SAMs offer an approach to create a set of surfaces having well-defined chemistries that differ in the proportion of terminal functional groups and water wettability. A series of thiol-mixed surfaces were studied and demonstrated that surfaces with midrange water wettabilites (∼20–40 dyn/cm) exhibit minimal activation of FXII in buffer solutions. Plasma coagulation studies appear to support a clear role for anionic surfaces in FXII activation, with a continuously increasing degree of activation with increasing COOH content. However, these same carboxyl/methyl mixed surfaces also showed a minimal amount of FXII activation at midrange wettabilities in neat buffer solution that was not reflected in plasma. Hydroxyl/methyl and amine/methyl materials showed minimum levels of activation at midrange wettability in neat buffer solution. Taken together, the results suggest that the degree of plasma coagulation initiated by a material is not simply a function of its ability to activate FXII alone. Taken in whole, these results demonstrate that the composition of the solution and the surface properties of the material all contribute to the observation of contact activation, and clearly show that activation of FXII is not specific to anionic surfaces as has been long believed.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health, Grant No. R01HL069965. The authors appreciate the support from the Materials Research Institute at Penn State University.
References
- 1. Brash J. L., Ann. N. Y. Acad. Sci. 283, 356 (1977). 10.1111/j.1749-6632.1977.tb41781.x [DOI] [Google Scholar]
- 2. Bowen R. A. R. and Remaley A. T., Biochem. Med. 24, 31 (2014). 10.11613/BM.2014.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vroman L., Materials 2, 1547 (2009). 10.3390/ma2041547 [DOI] [Google Scholar]
- 4. Ratnoff O. D. and Margolius A., Trans. Assoc. Am. Physicians 68, 149 (1955). [PubMed] [Google Scholar]
- 5. Schmaier A. H., Thromb. Haemostasis 78, 101 (1997). [PubMed] [Google Scholar]
- 6. Schmaier A. H., Thromb. Res. 133, S41 (2014). 10.1016/j.thromres.2014.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naudin C., Burillo E., Blankenberg S., Butler L., and Renne T., Semin. Thromb. Hemostasis 27, 0036 (2017). 10.1055/s-0036-1598003 [DOI] [PubMed] [Google Scholar]
- 8. Zhu S. and Diamond S. L., Thromb. Res. 134, 1335 (2014). 10.1016/j.thromres.2014.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tankersley D. L. and Finlayson J. S., Biochemistry 23, 273 (1984). 10.1021/bi00297a016 [DOI] [PubMed] [Google Scholar]
- 10. Griffin J. H., Proc. Natl. Acad. Sci. 75, 1998 (1978). 10.1073/pnas.75.4.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Kamp K. W. H. J. and van Oeveren W., J. Biomed. Mater. Res. 28, 349 (1994). 10.1002/jbm.820280309 [DOI] [PubMed] [Google Scholar]
- 12. Bjorkqvist J., Nickel K. F., Stavrou E., and Renne T., Thromb. Haemostasis 112, 868 (2014). 10.1160/TH14-04-0311 [DOI] [PubMed] [Google Scholar]
- 13. Vogler E. A. and Siedlecki C. A., Biomaterials 30, 1857 (2009). 10.1016/j.biomaterials.2008.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reviakine I., Clin. Hemorheol. Microcirc. 60, 133 (2015). 10.3233/CH-151942 [DOI] [PubMed] [Google Scholar]
- 15. Fischer M., Sperling C., and Werner C., J. Mater. Sci.: Mater. Med. 21, 931 (2010). 10.1007/s10856-009-3912-0 [DOI] [PubMed] [Google Scholar]
- 16. Sperling C., Fischer M., Maitz M. F., and Werner C., Biomaterials 30, 4447 (2009). 10.1016/j.biomaterials.2009.05.044 [DOI] [PubMed] [Google Scholar]
- 17. Bäck J., Sanchez J., Elgue G., Ekdahl K. N., and Nilsson B., Biochem. Biophys. Res. Commun. 391, 11 (2010). 10.1016/j.bbrc.2009.10.123 [DOI] [PubMed] [Google Scholar]
- 18. Johne J., Blume C., Benz P. M., Pozgajova M., Ullrich M., Schuh K., Nieswandt B., Walter U., and Renne T., Biol. Chem. 387, 173 (2006). 10.1515/BC.2006.023 [DOI] [PubMed] [Google Scholar]
- 19. Guo Z., Bussard K. M., Chatterjee K., Miller R., Vogler E. A., and Siedlecki C. A., Biomaterials 27, 796 (2006). 10.1016/j.biomaterials.2005.06.021 [DOI] [PubMed] [Google Scholar]
- 20. Vogler E. A., Graper J. C., Harper G. R., Sugg H. W., Lander L. M., and Brittain W. J., J. Biomed. Mater. Res. 29, 1005 (1995). 10.1002/jbm.820290813 [DOI] [PubMed] [Google Scholar]
- 21. Vogler E. A., Graper J. C., Sugg H. W., Lander L. M., and Brittain W. J., J. Biomed. Mater. Res. 29, 1017 (1995). 10.1002/jbm.820290814 [DOI] [PubMed] [Google Scholar]
- 22. Zhuo R., Miller R., Bussard K. M., Siedlecki C. A., and Vogler E. A., Biomaterials 26, 2965 (2005). 10.1016/j.biomaterials.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 23. Zhuo R., Siedlecki C. A., and Vogler E. A., Biomaterials 27, 4325 (2006). 10.1016/j.biomaterials.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 24. Golas A., Parhi P., Dimachkie Z. O., Siedlecki C. A., and Vogler E. A., Biomaterials 31, 1068 (2010). 10.1016/j.biomaterials.2009.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeh J. C.-H., Dimachkie Z. O., Golas A., Cheng A., Parhi P., and Vogler E. A., Biomaterials 33, 9 (2012). 10.1016/j.biomaterials.2011.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Golas A., Yeh C.-H. J., Siedlecki C. A., and Vogler E. A., Biomaterials 34, 607 (2013). 10.1016/j.biomaterials.2012.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Golas A., Yeh J. C.-H., Siedlecki C. A., and Vogler E. A., Biomaterials 32, 9747 (2011). 10.1016/j.biomaterials.2011.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirata I., Hioko Y., Toda M., Kitazawa T., Murakami Y., Kitano E., Kitamura H., Ikada Y., and Iwata H., J. Biomed. Mater. Res., Part A 66, 669 (2003). 10.1002/jbm.a.10067 [DOI] [PubMed] [Google Scholar]
- 29. Martins M. C. L., Ratner B. D., and Barbosa M. A., J. Biomed. Mater. Res., Part A 67, 158 (2003). 10.1002/jbm.a.10096 [DOI] [PubMed] [Google Scholar]
- 30. Rodrigues S. N., Goncalves I. C., Martins M. C. L., Barbosa M. A., and Ratner B. D., Biomaterials 27, 5357 (2006). 10.1016/j.biomaterials.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 31. Ulman A., Chem. Rev. 96, 1533 (1996). 10.1021/cr9502357 [DOI] [PubMed] [Google Scholar]
- 32. Wang H., Chen S. F., Li L. Y., and Jiang S. Y., Langmuir 21, 2633 (2005). 10.1021/la046810w [DOI] [PubMed] [Google Scholar]
- 33. Ron H., Matlis S., and Rubinstein I., Langmuir 14, 1116 (1998). 10.1021/la970785v [DOI] [Google Scholar]
- 34. Ron H. and Rubinstein I., Langmuir 10, 4566 (1994). 10.1021/la00024a030 [DOI] [Google Scholar]
- 35. Chatterjee K., Guo Z., Vogler E. A., and Siedlecki C. A., J. Biomed. Mater. Res., A 90, 27 (2009). 10.1002/jbm.a.32076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chatterjee K., Thornton J. L., Bauer J. W., Vogler E. A., and Siedlecki C. A., Biomaterials 30, 4915 (2009). 10.1016/j.biomaterials.2009.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller R., Guo Z., Vogler E. A., and Siedlecki C. A., Biomaterials 27, 208 (2006). 10.1016/j.biomaterials.2005.05.087 [DOI] [PubMed] [Google Scholar]
- 38. Kakiuchi T., Iida M., Gon N., Hobara D., Imabayashi S., and Niki K., Langmuir 17, 1599 (2001). 10.1021/la0014757 [DOI] [Google Scholar]
- 39. Brewer N. J. and Leggett G. J., Langmuir 20, 4109 (2004). 10.1021/la036301e [DOI] [PubMed] [Google Scholar]
- 40. Bain C. D., Evall J., and Whitesides G. M., J. Am. Chem. Soc. 111, 7155 (1989). 10.1021/ja00200a039 [DOI] [Google Scholar]
- 41. Castner D. G., Hinds K., and Grainger D. W., Langmuir 12, 5083 (1996). 10.1021/la960465w [DOI] [Google Scholar]
- 42. Martins M. C. L., Fonseca C., Barbosa M. A., and Ratner B. D., Biomaterials 24, 3697 (2003). 10.1016/S0142-9612(03)00244-8 [DOI] [PubMed] [Google Scholar]
- 43. Chatterjee K., Vogler E. A., and Siedlecki C. A., Biomaterials 27, 5643 (2006). 10.1016/j.biomaterials.2006.07.025 [DOI] [PubMed] [Google Scholar]
- 44. Berg J. M., Eriksson L. G. T., Claesson P. M., and Borve K. G. N., Langmuir 10, 1225 (1994). 10.1021/la00016a041 [DOI] [Google Scholar]
- 45. Vogler E. A., J. Biomater. Sci.-Polym. Ed. 10, 1015 (1999). 10.1163/156856299X00667 [DOI] [PubMed] [Google Scholar]
- 46. Xu L. C. and Siedlecki C. A., Biomaterials 28, 3273 (2007). 10.1016/j.biomaterials.2007.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhuo R., Siedlecki C. A., and Vogler E. A., Biomaterials 28, 4355 (2007). 10.1016/j.biomaterials.2007.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vogler E. A., in Water in Biomaterials Surface Science, edited by Morra M. ( Wiley, New York, 2001), p. 269. [Google Scholar]
- 49.See supplementary material at http://dx.doi.org/10.1116/1.4983634 E-BJIOBN-12-332702 for the relative atomic compositions of elements in SAMs by XPS analysis.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1116/1.4983634 E-BJIOBN-12-332702 for the relative atomic compositions of elements in SAMs by XPS analysis.