Abstract
INTRODUCTION
Little is known about the relationship between disability and mode of delivery. Prior research has indicated elevated risk of cesarean delivery among women with certain disabilities, but has not examined patterns across multiple types of disability or by parity.
OBJECTIVE
To determine whether physical, sensory, or intellectual and developmental disabilities are independently associated with primary cesarean delivery.
METHODS
We conducted a retrospective cohort study of all deliveries in California 2000–2010 using linked birth certificate and hospital discharge data. We identified physical, sensory, and intellectual and developmental disabilities using ICD-9 codes. We used logistic regression to examine the association of these disabilities and primary cesarean delivery, controlling for socio-demographic characteristics and co-morbidities and stratified by parity.
RESULTS
In our sample, 0.45% (20,894/4,610,955) of deliveries were to women with disabilities. A larger proportion of women with disabilities were nulliparous, had public insurance, and had co-morbidities (e.g., gestational diabetes) compared with women without disabilities (p<0.001 for all). The proportion of primary cesarean in women with disabilities was twice that in women without disabilities (32.7% versus 16.3%, p<0.001; aOR = 2.05; 95% CI = 1.94–2.17). The proportion of deliveries by cesarean was highest among women with physical disabilities due to injuries compared with women without disabilities (57.8% versus 16.3%, p<0.001; aOR = 6.83; 95%CI = 5.46–8.53).
CONCLUSION
Women across disability subgroups have higher odds of cesarean delivery, and there is heterogeneity by disability type. More attention is needed to this population to ensure better understanding of care practices that may impact maternal and perinatal outcomes.
INTRODUCTION
An estimated 12% of women of reproductive age have a disability (Brault, Hootman, Helmick, Theis, & Armour, 2009). Recent research indicates that pregnancy is as common among women with disabilities as among women without disabilities (Horner-Johnson, Darney, Kulkarni-Rajasekhara, Quigley, & Caughey, 2016). However, utilization of prenatal care is lower among women with disabilities (Gavin, Benedict, & Adams, 2006). While limited evidence suggests that many women with disabilities have favorable pregnancy outcomes (Signore, et al., 2011), studies have shown higher rates of preterm birth and low birth weight in this population (Mitra, Clements, et al., 2015). Further, increased cesarean delivery has been documented among women with specific types of physical disability, including spinal cord injury, rheumatoid arthritis, multiple sclerosis, cerebral palsy, spina bifida, and neuromuscular disorders (Arata, Grover, Dunne, & Bryan, 2000; Argov & de Visser, 2009; Chakravarty, Nelson, & Krishnan, 2006; Chambers, Johsnon, & Jones, 2004; Kelly, Nelson, & Chakravarty, 2009; Rudnik-Schoneborn & Zerres, 2004; Skomsvoll, Ostensen, Irgens, & Baste, 1998; Winch, Bengston, McLaughlin, Fitzsimmons, & Budden, 1993). Research to date also suggests that women with intellectual and developmental disabilities are at elevated risk for cesarean delivery (Brown, et al., 2016; Mitra, Parish, Clements, Cui, & Diop, 2015; Parish, et al., 2015).
Small sample sizes in some studies and different approaches to measuring disability make it difficult to draw conclusions to guide clinical practice. Moreover, examination of more than one type of disability within a single study is rare, so very little is known about how disability sub-populations differ from each other. Cesarean delivery and surgical recovery may be more complicated in women with disabilities (Jackson, Lindsey, Klebine, & Poczatek, 2004) and it is therefore important to understand the utilization of cesarean delivery among a range of disability types -- including physical, sensory, and intellectual and developmental disabilities (IDD) -- in comparison to the non-disabled population.
The purpose of this study was to describe primary cesarean delivery among women with and without disabilities and disaggregated by disability subgroups, and to test the association between disability status and cesarean delivery. We hypothesized that disability status would be independently associated with cesarean delivery, controlling for socio-demographics and health care utilization.
METHODS
We conducted a retrospective cohort study using linked hospital discharge and vital records data (birth certificates and death files (California Department of Health Services, 2006)) for all births in the state of California between 2000 and 2010 (N=5,772,198). The dataset contains linked birth and delivery records with de-identified information for a mother and neonate pair from the neonatal and maternal hospital discharge record and the birth certificate. The study was approved by the California Office of Statewide Health Planning and Development, and the Institutional Review Board of Oregon Health & Science University.
Consistent with prior obstetric literature (e.g. Darney, et al., 2013), we excluded women with prior cesarean deliveries from our analytic sample because prior cesarean is strongly associated with subsequent cesarean delivery. We also excluded multiple gestations and breech presentation because these are indications for cesarean delivery and could confound the relationship between disability and cesarean delivery. Figure 1 shows the number of cases excluded to arrive at our final analytic sample.
Figure 1.
Exclusion flow diagram.
We identified our key independent variables -- disability status and type -- using the International Classification of Diseases, 9th revision, clinical modification (ICD-9) diagnosis and procedure codes from the patient discharge data file. Conceptually, disability is a complex interplay of body structures, functions, activities, and participation as impacted by contextual factors (World Health Organization, 2001). Diagnoses alone cannot provide information about restrictions in functioning or participation and are an imperfect means of attempting to identify disability. While acknowledging the limitations of diagnosis codes, various authors have published lists of codes likely to be associated with broad functional categories of disability, which can be used when no other data on disability are available. We built on these prior efforts in creating our algorithm (see Appendix A), as described below.
Khoury et al. (2013) developed a list of conditions associated with mobility disability and validated the list through review by a disability epidemiologist and a physician. This list served as the starting point for our physical disability codes. In consultation with clinicians and disability researchers, we added several other conditions (e.g. cystic fibrosis) that may be associated with some level of physical disability, although not necessarily a mobility restriction. We also removed some codes for acute injuries that may not have lasting impact (e.g. fracture of the spinal column without spinal cord injury). Our list of hearing disability codes was drawn from Mann, Zhou, McKee, and McDermott (2007) to which we added “other specified forms of hearing loss”, “congenital anomalies of ear causing impairment of hearing”, and “Deaf, nonspeaking, not elsewhere classifiable”. Javitt, Zhou, and Willke (2007) categorized vision loss codes by severity and tested their classification in relation to Medicare costs associated with vision care. We used all codes associated with moderate and severe vision loss and blindness, and added codes for vision conditions that often lead to vison loss (e.g. macular degeneration and other retinal disorders). Lin et al. (Lin et al., 2013) consulted with clinicians and policy makers to create a list of codes for identifying intellectual/developmental disabilities, consistent with criteria for service eligibility; we used their list without alteration.
Our dataset was limited to diagnoses coded at or near the time of delivery as opposed to a woman’s entire medical record. Therefore, we erred on the side of inclusivity in deciding what codes to categorize as “disability”, incorporating some milder conditions that we assumed must have been deemed salient if they were coded in the delivery discharge file. Appendix A contains a full list of ICD-9 codes included in our definition, along with sample frequencies.
We classified disability in several ways. First, we created a binary indictor of presence of any of our target disability types versus none. Second, we created broad disability subgroups: physical, hearing, vision, and intellectual/developmental disabilities (IDD). Finally, given the heterogeneity of conditions within the physical disability group and potential differential association with cesarean delivery, we examined subgroups of physical disability: nervous system disorders, musculoskeletal disorders, injuries, and congenital anomalies. An individual woman could be in more than one group if she had multiple disability codes recorded on her discharge record.
We identified women with primary cesarean deliveries (our dependent variable) documented either on the birth certificate or using an ICD-9 procedure code in the discharge file. Similarly, prior cesarean delivery, multiple gestation, and breech presentation were identified using either the birth certificate checkboxes or discharge file ICD-9 codes. We privileged the discharge record when possible. Our procedures follow a body of literature about the validity of birth certificate versus hospital discharge data (Goff et al., 2012; Lain et al., 2012; Lydon-Rochelle, Holt, Cardenas, et al., 2005; Lydon-Rochelle, Holt, Nelson, et al., 2005).
We included several maternal-level covariates in the analysis. From the vital statistics birth record, we extracted gestational age to create a preterm birth indicator (<37 weeks gestation), parity of this current pregnancy (nulliparous or multiparous), and month of entry into prenatal care, which we used to create an indicator of entry to care in the first trimester (<13 weeks) or not. We classified maternal education on the birth certificate as those who had completed high school and were at least 16 years of age, and those who had not completed high school. Maternal age was derived from the birth certificate; a small proportion of values (0.06%) were missing, and maternal age was then derived from the patient discharge file in this circumstance. We classified race/ethnicity as non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian, and Other, from the birth certificate.
We included several comorbidities that are known to be associated with cesarean delivery and are potential confounders. Chronic hypertension was identified if documented on either the birth certificate or patient discharge file. Gestational hypertension or preeclampsia was extracted in a similar fashion. We identified women with chronic diabetes and gestational diabetes in the discharge file. We identified women with mental health diagnoses based on diagnoses in the discharge file (see Appendix A for a list of these ICD-9 codes). We chose not to exclude women with chronic and mental health conditions as has been done in previous studies (Darney et al., 2013) because the distribution of chronic conditions was higher among women with disabilities and we would have lost much of our sample, limiting generalizability of findings to the population of women with disabilities. We measured insurance (public insurance, private health insurance, or no insurance) from the discharge file. We included year of delivery as a categorical variable.
We used descriptive statistics and visualizations to characterize the sample overall, by any disability, by disability groups (physical, hearing, vision, or IDD), and by subgroups of physical disability (nervous system disorders, musculoskeletal disorders, congenital anomalies, or injuries). We tested for bivariate differences in the proportion of cesarean delivery between women with any disability and no disability and between each of the disability subgroups and women with no disability using chi square tests. We then stratified by parity and developed separate multivariable logistic regression models for each category of disability controlling for maternal covariates presented above and for year. We accounted for data clustering (non-independence of observations) at the hospital level in all models. As a sensitivity analysis, we tested models with the inclusion of number of comorbid disability codes as a proxy for severity in order to assess likelihood of functional limitation. Although there was evidence of a dose-response relationship between number of disability codes and cesarean (data not shown), we encountered small cell sizes, and overall results were unchanged. We used Stata 14 for data analysis (StataCorp. 2015. College Station, TX: StataCorp LP). Figures were created using R version 3.2.2 (R Core Team. 2015. R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Our analytic sample included 4,610,955 deliveries between 2000 and 2010 in California, 20,894 of which (0.45%) were to women with a code included in our disability algorithm (Table 1). Of these women, 3.6% (N = 761) had multiple disability codes. Women with disabilities had higher educational attainment than women without disabilities, largely driven by high educational attainment among women with physical disabilities, our largest group (Table 1). Women with disabilities were more likely to be non-Hispanic White, of advanced maternal age, nulliparous, and publicly insured. They were also more likely to have gestational diabetes, hypertension, and gestational hypertension or eclampsia compared with women without disabilities (p<0.001 for all), and the proportion of preterm deliveries was higher among women with disabilities (Table 1).There was heterogeneity among disability groups, with the physical disability group driving the overall disability category due to larger numbers (81.6% of the disability category consists of physical disabilities). Women with IDD were the least educated and the least likely to have initiated prenatal care in the first trimester (Table 1). Women with vision disabilities had the highest proportions of pre-gestational and gestational diabetes, hypertension and gestational hypertension, and preterm births (Table 1). Among women with physical disabilities, we examined subgroups. Women with nervous system (n=9,117) and musculoskeletal (n=5,928) disabilities accounted for the majority of the overall physical disability category (Table 2).
Table 1.
Sample characteristic by presence and type of disability, California 2000–2010*
| Cohort | Disability Type | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Cohort | No Disabilities | Any Disability | Physical | Hearing | Vision | IDD |
| N | 4,610,955 | 4,590,061 (99.55) | 20,894 (0.45) | 17,109 (0.37) | 1,772 (0.04) | 1,128 (0.02) | 1,103 (0.02) |
|
| |||||||
| Maternal Race | |||||||
| White | 1,288,542 (27.95) | 1,279,900 (27.88) | 8,642 (41.36) | 7,321 (42.79) | 604 (34.09) | 389 (34.49) | 413 (37.44) |
| Black | 242,304 (5.25) | 240,716 (5.24) | 1,588 (7.60) | 1,273 (7.44) | 107 (6.04) | 106 (9.40) | 132 (11.97) |
| Hispanic | 2,459,652 (53.34) | 2,451,148 (53.40) | 8,504 (40.70) | 6,806 (39.78) | 835 (47.12) | 483 (42.82) | 463 (41.98) |
| Asian | 506,538 (10.99) | 504,965 (11.00) | 1,573 (7.53) | 1,202 (7.03) | 196 (11.06) | 126 (11.17) | 68 (6.17) |
| Other | 86,457 (1.88) | 86,003 (1.87) | 454 (2.17) | 395 (2.31) | 24 (1.35) | 17 (1.51) | 19 (1.72) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||||
| Maternal Education | |||||||
| < High School | 1,291,018 (28.00) | 1,287,140 (28.04) | 3,878 (18.56) | 3,051 (17.83) | 367 (20.71) | 212 (18.79) | 298 (27.02) |
| Completed HS | 3,195,644 (69.31) | 3,179,317 (69.27) | 16,327 (78.14) | 13,536 (79.12) | 1,326 (74.83) | 877 (77.75) | 748 (67.82) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||||
| Advanced Maternal Age | 688,447 (14.93) | 683,996 (14.90) | 4,451 (21.30) | 3,698 (21.61) | 355 (20.03) | 260 (23.05) | 185 (16.77) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||||
| Parity | |||||||
| Nulliparous | 2,127,952 (46.15) | 2,116,458 (46.11) | 11,494 (55.01) | 9,308 (54.40) | 914 (51.58) | 709 (62.85) | 706 (64.01) |
| Multiparous | 2,477,613 (53.73) | 2,468,250 (53.77) | 9,363 (44.81) | 7,774 (45.44) | 852 (48.08) | 416 (36.88) | 396 (35.90) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||||
| Insurance | |||||||
| Private | 2,181,421 (47.31) | 2,172,267 (47.33) | 9,154 (43.81) | 7,047 (41.91) | 1,031 (58.18) | 511 (45.30) | 709 (64.28) |
| Public | 2,330,583 (50.54) | 2,319,095 (50.52) | 11,488 (54.98) | 9,853 (57.59) | 725 (40.91) | 607 (53.81) | 375 (34.00) |
| None | 98,242 (2.13) | 97,991 (2.13) | 251 (1.20) | 208 (1.22) | 16 (0.90) | 10 (0.89) | 19 (1.72) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||||
| Prenatal Care in 1st Trimester | 3,846,490 (83.42) | 3,828,718 (83.41) | 17,772 (85.06) | 14,709 (85.97) | 1,433 (80.87) | 966 (85.64) | 833 (75.52) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||||
| Diabetes | 25,186 (0.55) | 245,646 (0.54) | 622 (2.98) | 330 (1.93) | 22 (1.24) | 351 (25.80) | 15 (1.36) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||||
| Gestational Diabetes | 268,904 (5.83) | 266,820 (5.81) | 2,084 (9.97) | 1,517 (8.87) | 179 (10.10) | 351 (31.12) | 89 (8.07) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 |
|
| |||||||
| Hypertension | 40,370 (0.88) | 39,822 (0.87) | 548 (2.62) | 408 (2.38) | 23 (1.30) | 99 (8.78) | 37 (3.35) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||||
| Gestational Hypertension or Preeclampsia | 243,097 (5.27) | 241,297 (5.26) | 1,800 (8.61) | 1,302 (7.61) | 137 (7.73) | 264 (23.40) | 134 (12.15) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||||
| Mental Health Diagnosis | 48,747 (1.06) | 47,454 (1.03) | 1,293 (6.19) | 1,087 (6.35) | 66 (3.72) | 71 (6.29) | 89 (8.07) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||||
| Preterm Birth | 377,159 (8.18) | 374,546 (8.16) | 2,613 (12.51) | 2,046 (11.96) | 177 (9.99) | 281 (24.91) | 167 (15.14) |
| p-value | - | Referent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Percentages may not add up to 100% due to missingness in the data. See Appendix B for data on missing values.
Table 2.
Sample characteristic by physical disability subgroups, California 2000–2010*
| Nervous System | Musculoskeletal | Congenital Anomalies | Injuries | |
|---|---|---|---|---|
| N | 9,117 (0.20) | 5,928 (0.13) | 1,598 (0.03) | 625 (0.01) |
|
| ||||
| Maternal Race | ||||
| White | 3,724 (40.85) | 2,660 (44.87) | 781 (48.87) | 263 (42.08) |
| Black | 881 (9.66) | 295 (4.98) | 80 (5.01) | 40 (6.40) |
| Hispanic | 3,775 (41.41) | 2,208 (37.25) | 602 (37.67) | 265 (42.40) |
| Asian | 490 (5.37) | 550 (9.28) | 94 (5.88) | 46 (7.36) |
| Other | 198 (2.17) | 162 (2.73) | 32 (2.00) | 8 (1.28) |
| p-value† | <0.001 | <0.001 | <0.001 | <0.001 |
|
| ||||
| Maternal Education | ||||
| < High school (HS) | 1,961 (21.51) | 715 (12.06) | 256 (16.02) | 146 (23.36) |
| Completed HS | 6,872 (75.38) | 5,022 (84.72) | 1,300 (81.35) | 465 (74.40) |
| p-value† | <0.001 | <0.001 | <0.001 | 0.02 |
|
| ||||
| Advanced Maternal Age | 1,685 (18.48) | 1,680 (28.34) | 224 (14.69) | 131 (20.96) |
| p-value† | <0.001 | <0.001 | 0.980 | <0.001 |
|
| ||||
| Parity | ||||
| Nulliparous | 4,841 (53.10) | 3,102 (52.33) | 1,054 (65.96) | 386 (61.76) |
| Multiparous | 4,264 (46.77) | 2,815 (47.49) | 540 (33.79) | 239 (38.24) |
| p-value† | <0.001 | 0.506 | <0.001 | <0.001 |
|
| ||||
| Insurance | ||||
| Private | 4,434 (48.36) | 1,703 (28.73) | 655 (40.99) | 335 (53.60) |
| Public | 4,562 (50.04) | 4,162 (70.21) | 928 (58.07) | 277 (44.32) |
| None | 120 (1.32) | 63 (1.06) | 15 (0.94) | 13 (2.08) |
| p-value† | <0.001 | <0.001 | <0.001 | 0.018 |
|
| ||||
| Prenatal Care in 1st Trimester | 7,636 (83.76) | 5,281 (89.09) | 1,390 (86.98) | 532 (85.12) |
| p-value† | <0.001 | <0.001 | <0.001 | 0.16 |
|
| ||||
| Diabetes | 224 (2.46) | 81 (1.37) | 24 (1.50) | 8 (1.28) |
| p-value† | <0.001 | <0.001 | <0.001 | 0.013 |
|
| ||||
| Gestational Diabetes | 801 (8.79) | 555 (9.36) | 124 (7.76) | 41 (6.56) |
| p-value† | <0.001 | <0.001 | 0.001 | 0.437 |
|
| ||||
| Hypertension | 201 (2.20) | 169 (2.85) | 36 (2.25) | 7 (1.12) |
| p-value† | <0.001 | <0.001 | <0.001 | 0.684 |
|
| ||||
| Gestational Hypertension | 714 (7.83) | 452 (7.62) | 113 (7.07) | 29 (4.64) |
| p-value† | <0.001 | <0.001 | 0.005 | 0.479 |
|
| ||||
| Mental Health Diagnosis | 372 (6.28) | 634 (6.95) | 73 (4.57) | 31 (4.96) |
| p-value† | <0.001 | <0.001 | <0.001 | <0.001 |
|
| ||||
| Preterm Birth | 1,168 (12.81) | 647 (10.91) | 204 (12.77) | 67 (10.72) |
| p-value† | <0.001 | <0.001 | <0.001 | 0.036 |
Percentages may not add up to 100% due to missingness in the data. See Appendix B for data on missing values.
p-values are relative to no disability category.
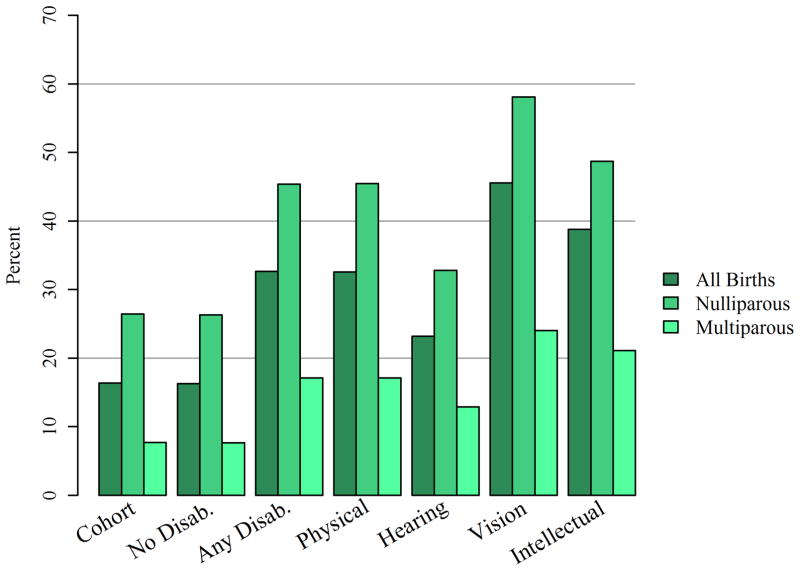
Proportions of primary cesarean delivery were higher in all disability categories compared with non-disabled women (Figure 2). The proportion of primary cesarean in women with any disability was nearly twice that in women without disabilities (32.7% versus 16.3%, p<0.001). Of the broad disability categories, women with vision disabilities had the highest proportion of cesarean deliveries (45.6% overall and 58.1% among nulliparous). Among subgroups of women with physical disabilities (Figure 3), the proportion of deliveries by cesarean was lowest among women with nervous system disabilities (28.2%, p<0.001) and highest among women with injuries (57.8% p<0.001).
Figure 2.
Primary Cesarean section rates by disability status and disability type. All comparisons within parity categories are statistically significant (p<0.001) relative to the no disabilities category.
Figure 3.
Primary Cesarean section rates by physical disability category. All comparisons within parity categories are statistically significant (p<0.001) relative to the no disabilities category.
Multivariable regression models controlling for sociodemographic characteristics, comorbidities, and prenatal care utilization support the descriptive results: women with disabilities had consistently higher odds of cesarean delivery compared with women without disabilities (nulliparous aOR = 2.03, 95% CI = 1.96 – 2.15; multiparous aOR = 2.11, 95% CI = 1.97 – 2.27; Table 3). Women with an IDD diagnosis (nulliparous aOR = 2.40, 95% CI = 2.03 – 2.83; multiparous aOR = 2.64, 95% CI = 2.07 – 3.37) and nulliparous women with a vision disability (aOR = 2.26, 95% CI = 1.91 – 2.69) had the highest odds of cesarean among the four disability subgroups (Table 3). The overall physical disability group had elevated odds of cesarean delivery (aOR = 2.10, 95% CI = 1.98 – 2.24), but odds varied markedly across physical disability subgroups. Women with injuries were the subgroup with the highest odds of cesarean delivery (nulliparous aOR = 6.19, 95% CI = 4.76 – 8.05; multiparous aOR = 7.84, 95% CI = 5.71 – 10.76) relative to women without disabilities (Table 4).
Table 3.
Adjusted odds of primary cesarean delivery by disability status or type* (aOR (95% CI)).
| Any Disability | Disability Type | ||||
|---|---|---|---|---|---|
|
| |||||
| Physical | Hearing | Vision | IDD | ||
| Model N | 4,257,884 | 4,254,568 | 4,240,452 | 4,239,906 | 4,239,813 |
|
| |||||
| All Births† | 2.05 (1.94–2.17) | 2.10 (1.98–2.24) | 1.35 (1.17–1.56) | 2.11 (1.84–2.43) | 2.43 (2.12–2.78) |
| Nulliparous | 2.03 (1.90–2.15) | 2.07 (1.93–2.21) | 1.30 (1.09–1.56) | 2.26 (1.91––2.69) | 2.40 (2.03–2.83) |
| Multiparous | 2.11 (1.97–2.27) | 2.18 (2.02–2.36) | 1.50 (1.20–1.86) | 1.80 (1.36–2.38) | 2.64 (2.07–3.37) |
Relative to those without a disability.
All models adjusted for maternal race, public insurance status, prenatal care in 1st trimester, advanced maternal age, education, year (relative to 2010), preterm birth, chronic diabetes, gestational diabetes, chronic hypertension, gestational hypertension, and mental health diagnoses.
Also adjusted for parity
Table 4.
Adjusted odds of primary cesarean delivery by physical disability category* (aOR (95% CI)).
| Nervous System | Musculoskeletal | Congenital Anomalies | Injuries | |
|---|---|---|---|---|
|
| ||||
| Model N | 4,247,191 | 4,244,361 | 4,240,363 | 4,239,463 |
| All Births† | 1.65 (1.55–1.76) | 2.49 (2.28–2.71) | 2.53 (2.06–2.91) | 6.83 (5.46–8.53) |
| Nulliparous | 1.65 (1.53–1.78) | 2.34 (2.13–2.57) | 2.63 (2.22–3.12) | 6.19 (4.76–8.05) |
| Multiparous | 1.64 (1.48–1.82) | 2.78 (2.48–3.12) | 2.32 (1.80–3.00) | 7.84 (5.71–10.76) |
Relative to those without a disability.
All models adjusted for maternal race, public insurance status, prenatal care in 1st trimester, advanced maternal age, education, year (relative to 2010), preterm birth, chronic diabetes, gestational diabetes, chronic hypertension, gestational hypertension, and mental health diagnoses.
Also adjusted for parity
DISCUSSION
A total of 20,894 deliveries in California 2000–2010 (0.45% of our sample) were to women with a diagnosis code associated with potential disability. This likely underestimates the proportion of births to women with disabilities because our data included only diagnoses coded at delivery. Previous research has reported that 12% of women of reproductive age have disabilities (Brault, et al., 2009) and 11% of reproductive age women with disabilities have been pregnant (Horner-Johnson, et al., 2016). However, our population represents a different and broader denominator: all women who gave birth in California 2000–2010. Thus, we would anticipate that a small proportion of this birth cohort would have a disability, although the true fraction may be higher than the number we were able to identify.
Our findings indicate that across disability types – physical, IDD, vision, hearing – women with disabilities were more likely to deliver by cesarean than women without disabilities. Physical disability is by far the most common type of disability, and within this group, women with injuries had the highest odds of cesarean delivery. The heterogeneity we observed across and within disability subgroups highlights the need to disaggregate disability because risks and needs vary according to disability type. Previous literature supports disaggregation (Goldacre, Gray, & Goldacre, 2015; Prilleltensky, 2003; Signore, Spong, Krotoski, Shinowara, & Blackwell, 2011), suggesting that the type of disability and range of physical abilities present diverse challenges during pregnancy and delivery which may be obscured through less nuanced measurement of disability.
Prior studies have indicated that cesarean delivery is more common in specific physical disability subtypes (Arata et al., 2000; Chakravarty et al., 2006; Chambers et al., 2004; Ghidini, Healey, Andreani, & Simonson, 2008; Jackson & Wadley, 1999; Kelly et al., 2009; Rudnik-Schoneborn & Zerres, 2004; Skomsvoll et al., 1998; Winch et al., 1993). The reasons for higher rates of cesarean delivery are not fully understood (Signore, et al., 2011). There are some particular concerns, such as risk of autonomic dysreflexia during labor among women with spinal cord lesions at or above T6 (Jackson & Wadley, 1999; Le Liepvre, et al., 2016) that may contribute to decisions to plan for cesarean delivery. Women with certain physical disabilities may also be at increased risk of placental hemorrhage or other complications that lead to emergency cesareans (Rudnick-Schoneborn & Zerres, 2004). However, some women with physical disabilities have reported that decisions about delivery were made without their input or with any consideration of the possibility that they might be able to deliver vaginally (Smeltzer, 2007).
Previous studies of cesarean delivery among women with physical disabilities have compared disability subtypes with a general population, but not with other disability groups, nor have they accounted for important factors such as co-morbidities and parity. Stratification by parity proved important in our study, revealing elevated odds of primary cesarean for women with disabilities even among women who have experienced a previous vaginal delivery. In some disability groups, this could be attributable in part to disabilities acquired between deliveries. However, the pattern was also apparent in our IDD group, which consisted exclusively of conditions that are present early in life. Further research is needed to understand possible reasons for cesarean delivery after a prior vaginal birth, and why women with disabilities appear to be at increased risk.
Our findings add to an emerging body of evidence for women with IDD. Using data from Massachusetts, Mitra, Parish, et al. (2015) reported an increased risk of cesarean among women with IDD compared with a general obstetric population (36% versus 27%; RR = 1.3, 95% CI 1.2–1.5). Data from a national sample indicated an even larger difference: 49% of women with IDD delivered by cesarean versus 33% of women without IDD; AOR=2.13, 95% CI=1.68–2.71 (Parish, et al., 2015). A Canadian study found a much smaller but statistically significant increased risk of cesarean delivery for women with IDD: 28.0% vs. 27.3%, aRR=1.09, 95% CI=1.03–1.16 (Brown et al., 2016). A study from Sweden (Höglund, Lindgren, & Larsson, 2012) also reported higher proportions of cesarean delivery in women with intellectual disabilities relative to those without intellectual disabilities (24.5% vs. 17.7%; aOR = 1.55, 95% CI 1.11–2.17). We report even greater disparities (38% among women with IDD versus 16% among women without disabilities). Despite slightly different diagnosis lists used in each of these studies, the overall pattern of disparity is similar. Cesarean delivery was more common among women with IDD than other women with disabilities in our study, with the exception of the injury subgroup of women with physical disabilities and women with vision disabilities.
There is little research in the U.S. on the experiences of women with IDD giving birth or the clinicians providing care for them. However, research in other countries indicates that women with IDD have limited understanding of the birth process and feel unsupported during pregnancy and childbirth (Walsh-Gallagher, Sinclair & Mc Conkey, 2012; Mayes, Llewellyn & McConnell, 2006; Höglund & Larsson 2013), and midwives report having insufficient training or time during appointments to provide adequate support to women with IDD (Castell & Stenfert Kroese, 2016). Women with IDD are more likely to experience pregnancy complications, such as preeclampsia, that are associated with increased risk of cesarean delivery (Brown, et al., 2016; McConnell, et al., 2008; Parish, et al., 2015). In addition to these challenges, women with IDD tend to be among the most disadvantaged women giving birth: relative to women without IDD, they are younger, less educated, poorer, less likely to be married, and considerably less likely to be able or willing to name the baby’s father on the birth certificate (Goldacre, et al., 2015; Höglund, et al., 2012; Mitra, et al., 2015). Moreover, women with IDD are more likely to be obese, more likely to smoke during pregnancy, and less likely to receive prenatal care during the first trimester (Höglund, et al., 2012; Mitra, et al., 2015). Unfortunately, we were only able to control for some of these risk factors in our analyses. Many of these factors have been included in other studies, and delivery mode differences between women with and without IDD remained significant. Nonetheless, administrative data may provide only limited insight into the full depth and breadth of disadvantages and unmet support needs associated with cesarean risk and other adverse outcomes in this population. Additional qualitative research is needed, both with women with IDD and with clinicians providing prenatal and obstetric care to these women.
Women with vision disabilities showed a much higher proportion of both pregestational and gestational diabetes than women without disability (25.8% and 31.1% vs. 0.5% and 5.8%). The high proportion of primary cesarean delivery among women with vision disabilities in our study may have been related to their diabetes status. When we controlled for diabetes in our regression models, women with vision disabilities had lower odds (within our broad disability type categories) of cesarean delivery than women with IDD. However, odds of cesarean delivery were still elevated relative to women without disabilities. Our statistical adjustments may not have fully accounted for all of the health and pregnancy risks that could be associated with vision disabilities. Additionally, women with vision disabilities may face challenges in navigating the healthcare system and obtaining information in accessible formats (O’Day, Kileen, & Iezzoni, 2004), and these challenges may compromise their childbirth preparation and outcomes.
Women with hearing disabilities had the lowest odds of cesarean delivery of any of our disability groups. While other types of disabilities may be associated with health problems that could complicate delivery, people with hearing disabilities appear to be a relatively healthy group overall (Horner-Johnson, et al., 2013). Nonetheless, women with hearing disabilities in our cohort were significantly more likely to have cesarean deliveries than women without disabilities. A recent study using similar methodology examined preterm birth and low birthweight among women with hearing limitations and found elevated odds of both, relative to women without hearing limitations (Mitra, Akobirshoev, McKee & Iessoni, 2016). Research is needed on the extent to which challenges in communicating with healthcare providers may contribute to increased risks.
Across disability groups, the higher proportion of cesarean deliveries may be related to greater prevalence of clinical indications for cesarean, or disability itself may be perceived as an indication for cesarean delivery. Indications for cesarean are beyond the scope of this study but the potential use of disability as an indication deserves further scrutiny. Cesarean deliveries may be necessary for some women with disabilities. However, anesthesia risks, scarring, shunts, or other obstructions from prior abdominal surgeries could make surgery more complicated in women with disabilities. In addition, recovery from cesarean surgery, which involves pain and activity limitations for all women, may be especially problematic for women who already have functional and movement limitations (Jackson et al., 2004; Signore et al., 2011). It is therefore important to explore ways to limit cesareans to those that are clinically necessary.
Our study shares limitations common to retrospective observational studies utilizing administrative data. We were limited to the information in the discharge record and birth certificate; the relationship between maternal disability and cesarean delivery could also be impacted by variables not available or not well-recorded in our dataset, such as smoking and marital status. Our de-identified dataset did not allow us to cluster by woman to account for multiple deliveries to the same mother. Although we did stratify by parity (nulliparous versus multiparous), any woman with more than two births during our study years was included in our multiparous analyses twice. However, it is unlikely that this meaningfully impacted our conclusions.
Importantly, we were restricted to identifying disability based on diagnosis codes, which is an imperfect science. Our measurement of disability relies on ICD-9 codes recorded at the time of delivery and may therefore miss relevant diagnoses from women’s prior medical history. It is also possible that women who deliver by cesarean are more likely to have their disabilities coded than if the same woman delivered vaginally. Moreover, ICD-9 codes do not tell us about functional limitations or impact on activities of daily living. Many of the codes we used may make a woman at risk for a disability, but she may not actually have a disability. We attempted to gain some insight into likelihood of functional limitation in our sensitivity analyses; overall results were unchanged. Further research is needed to better understand the relationship between diagnoses and function, especially in women of childbearing age.
The primary strengths of our study are rich covariate information and large numbers to be able to examine disability subgroups. Our sample size enabled us to take into account heterogeneity of parity, socio-demographic characteristics, and comorbidities across disability subgroups. Importantly, our data allowed us to ascertain that the disabilities existed around the time of delivery (temporality), and probably represent conditions perceived as serious enough to impact clinical management of the pregnancy and delivery. As such, our findings may not be generalizable to all women with disabilities but only to those with disabilities severe enough to warrant documentation during labor and delivery. Thus, our findings may overestimate the relationship between disability and cesarean delivery in the broader population of women with disabilities. However, it is useful and relevant to our area of research, obstetric outcomes, to understand how disability is captured in routinely collected clinical data on deliveries and how disability, so measured, relates to clinical care outcomes such as cesarean delivery.
IMPLICATIONS FOR PRACTICE AND POLICY
As more women with disabilities reach reproductive age and desire children, clinicians and health systems need to be prepared to care for these women. The Society for Maternal-Fetal Medicine (SMFM) and the American College of Obstetricians & Gynecologists (ACOG) have guidelines for the use of prenatal screening in relation to mother’s current disability status (Society for Maternal-Fetal Medicine Publications Committee, 2015), but limited guidance exists for management of pregnancy and delivery in women with disabilities (American Congress of Obstetricians and Gynecologists, 2016). Qualitative research suggests that pregnancy and birth in women with disabilities are best supported by a multidisciplinary team to optimize maternal and infant health outcomes (Iezzoni, Wint, Smeltzer, & Ecker, 2014; Smeltzer, 2007). For women with IDD especially, a patient advocate may be useful in facilitating effective communication between patients and physicians and helping women understand and prepare for the birth process.
CONCLUSION
Women with each type of disability we studied had higher odd of cesarean delivery compared to women with no disabilities. Increased odds of cesarean delivery were apparent for both nulliparous and multiparous women with disabilities, while controlling for sociodemographic characteristics and common risks for cesarean delivery. More attention is needed to understanding the reasons for cesarean delivery in this population and working to reduce unnecessary cesarean deliveries.
Supplementary Material
Acknowledgments
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award #R21HD081309 (Horner-Johnson, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support for Dr. Horner-Johnson’s time was provided by grant #K12HS022981 from the Agency for Healthcare Research and Quality (AHRQ) (Guise, PI). Dr. Darney is partially supported by a Junior Investigator Award from the Society of Family Planning. The funding agencies had no role in the conduct of the research or preparation of the manuscript for submission. The authors report no conflicts of interest.
Funding Sources
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (grant number R21HD081309) the Agency for Healthcare Research and Quality (grant number K12HS022981), and a Junior Investigator Award from the Society of Family Planning.
Abbreviations
- IDD
Intellectual/developmental disability
- ICD-9
International Classification of Diseases, 9th revision, clinical modification
- HS
high school
- SMFM
Society for Maternal Fetal Medicine
- ACOG
American College of Obstetrics and Gynecology
Biographies
Blair G. Darney is a health services researcher affiliated with the Oregon Health & Science University Department of Obstetrics and Gynecology and the Instituto Nacional de Salud Publica (Cuernvaca, Mexico). Her research interests include maternal morbidity and mortality, contraception, and abortion.
Frances M. Biel is an epidemiologist and research associate affiliated with Oregon Health & Science University Department of Obstetrics and Gynecology. Her research interests include maternal-child health, prenatal care, and sexually transmitted diseases.
Brian Quigley was a research associate in the Oregon Health & Science University Department of Obstetrics and Gynecology at the time of this analysis.
Aaron B. Caughey is a maternal-fetal-medicine specialist, perinatal epidemiologist, health economist, and Chair of the Oregon Health & Science University Department of Obstetrics and Gynecology. He studies complications of term pregnancies using a wide range of methodologic approaches.
Willi Horner-Johnson is an Associate Professor in the collaborative Oregon Health & Science University – Portland State University School of Public Health. Her research, based in OHSU’s Institute on Development and Disability, focuses on health and healthcare disparities impacting people with disabilities.
Footnotes
Paper presentation information
Portions of this analysis were presented at the 2016 annual meeting of the Society of Maternal Fetal Medicine (SMFM), February 1-6 2016, Atlanta, Georgia.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American College of Obstetricians and Gynecologists. Women with Disabilities. 2016 Web page. http://www.acog.org/About-ACOG/ACOG-Departments/Women-with-Disabilities.
- Arata M, Grover S, Dunne K, Bryan D. Pregnancy Outcome and Complications in Women with Spina Bifida. Journal of Reproductive Medicine. 2000;45(9):743–748. [PubMed] [Google Scholar]
- Argov Z, de Visser M. What we do not know about pregnancy in hereditary neuromuscular disorders. Neuromuscul Disord. 2009;19(10):675–679. doi: 10.1016/j.nmd.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Brault M, Hootman J, Helmick C, Theis K, Armour BS. Prevalence and Most Common Causes of Disability Among Adults -- United States, 2005. MMWR. 2009;58(16):421–426. [PubMed] [Google Scholar]
- Brown HK, Kirkham YA, Cobigo V, Lunsky Y, Vigod S. Labor and delivery interventions in women with intellectual and developmental disabilities: A population-based cohort study. J Epidemiol Comm Health. 2016;70:238–244. doi: 10.1136/jech-2015-206426. [DOI] [PubMed] [Google Scholar]
- Castell E, Stenfert Korese B. Midwives’ experiences of caring for women with learning disabilities – A qualitative study. Midwifery. 2016;36:35–42. doi: 10.1016/j.midw.2016.02.001. [DOI] [PubMed] [Google Scholar]
- California Department of Health Services. Center for Health Statistics; California Department of Health Services, editor. Birth cohort public use file: 1999–2003. Sacramento (CA): California Department of Health Services; 2006. [Google Scholar]
- Chakravarty EF, Nelson L, Krishnan E. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54(3):899–907. doi: 10.1002/art.21663. [DOI] [PubMed] [Google Scholar]
- Chambers C, Johsnon D, Jones K. Pregnancy outcome in women exposed to anti-TNF-alpha medications: the OTIS Rheumatoid Arthritis in Pregnancy study. Arthritis Rheum. 2004;50:S479–480. [Google Scholar]
- Darney BG, Snowden JM, Cheng YW, Jacob L, Nicholson JM, Kaimal A, … Caughey AB. Elective induction of labor at term compared with expectant management: maternal and neonatal outcomes. Obstet Gynecol. 2013;122(4):761–769. doi: 10.1097/AOG.0b013e3182a6a4d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin NI, Benedict MB, Adams EK. Health service use and outcomes among disabled Medicaid pregnant women. Womens Health Issues. 2006;16(6):313–322. doi: 10.1016/j.whi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Ghidini A, Healey A, Andreani M, Simonson MR. Pregnancy and women with spinal cord injuries. Acta Obstet Gynecol Scand. 2008;87(10):1006–1010. doi: 10.1080/00016340802356909. [DOI] [PubMed] [Google Scholar]
- Goff SL, Pekow PS, Markenson G, Knee A, Chasan-Taber L, Lindenauer PK. Validity of using ICD-9-CM codes to identify selected categories of obstetric complications, procedures and co-morbidities. Paediatr Perinat Epidemiol. 2012;26(5):421–429. doi: 10.1111/j.1365-3016.2012.01303.x. [DOI] [PubMed] [Google Scholar]
- Goldacre AD, Gray R, Goldacre MJ. Childbirth in women with intellectual disability: characteristics of their pregnancies and outcomes in an archived epidemiological dataset. J Intellect Disabil Res. 2015;59(7):653–663. doi: 10.1111/jir.12169. [DOI] [PubMed] [Google Scholar]
- Höglund B, Lindgren P, Larsson M. Pregnancy and birth outcomes of women with intellectual disability in Sweden: a national register study. Acta Obstet Gynecol Scand. 2012;91(12):1381–1387. doi: 10.1111/j.1600-0412.2012.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund B, Larsson M. Struggling for motherhood with an intellectual disability – A qualitiative study of women’s experiences in Sweden. 2013;29(6):698–704. doi: 10.1016/j.midw.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Horner-Johnson W, Dobbertin K, Lee JC, Andersen EM. Disparities in chronic conditions and health status by type of disability. Disab Health J. 2013;6(4):280–286. doi: 10.1016/j.dhjo.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner-Johnson W, Darney BG, Kulkarni-Rajasekhara S, Quigley B, Caughey AB. Pregnancy among US women: differences by presence, type, and complexity of disability. Am J Obstet Gynecol. 2016;214:529.e1–9. doi: 10.1016/j.ajog.2015.10.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzoni LI, Wint AJ, Smeltzer SC, Ecker JL. Effects of disability on pregnancy experiences among women with impaired mobility. Acta Obstet Gynecol Scand. 2014;94(2):133–140. doi: 10.1111/aogs.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AB, Lindsey L, Klebine P, Poczatek R. Reproductive health for women with spinal cord injury: pregnancy and delivery. Spinal Cord Inj Nurs. 2004;21(2):88–91. [PubMed] [Google Scholar]
- Jackson AB, Wadley V. A Multicenter Study of Women's Self-Reported Reproductive Health After Spinal Cord Injury. Arch Phys Med Rehabil. 1999;80:1420–1428. doi: 10.1016/s0003-9993(99)90253-8. [DOI] [PubMed] [Google Scholar]
- Javitt JC, Zhou Z, Willke RJ. Association between vision loss and higher medical care costs in Medicare beneficiaries costs are greater for those with progressive vision loss. Ophthalmology. 2007;114(2):238–245. doi: 10.1016/j.ophtha.2006.07.054. [DOI] [PubMed] [Google Scholar]
- Kelly VM, Nelson LM, Chakravarty EF. Obstetric outcomes in women with multiple sclerosis and epilepsy. Neurology. 2009;73:1831–1836. doi: 10.1212/WNL.0b013e3181c3f27d. [DOI] [PubMed] [Google Scholar]
- Khoury AJ, Hall A, Andresen E, Zhang J, Ward R, Jarjoura C. The association between chronic disease and physical disability among female Medicaid beneficiaries 18–64 years of age. Disabil Health J. 2013;6(2):141–148. doi: 10.1016/j.dhjo.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Le Liepvre H, Dinh A, Idiard-Chamois B, Chartier-Kastler E, Phé V, Even A, Robain G, Denys P. Pregnancy in spinal cord-injured women, a cohort study of 37 pregnancies in 25 women. Spinal Cord. 2016 doi: 10.1038/sc.2016.138. [DOI] [PubMed] [Google Scholar]
- Lain SJ, Hadfield RM, Rayes-Greenow CH, Ford JB, Mealing NM, Algert CS, Roberts CL. Quality of Data in Perinatal Population Health Databases. Medical Care. 2012;50(4):e7–e20. doi: 10.1097/MLR.0b013e31821d2b1d. [DOI] [PubMed] [Google Scholar]
- Lin E, Balogh R, Cobigo V, Ouellette-Kuntz H, Wilton AS, Lunsky Y. Using administrative health data to identify individuals with intellectual and developmental disabilities: a comparison of algorithms. J Intellect Disabil Res. 2013;57(5):462–477. doi: 10.1111/jir.12002. [DOI] [PubMed] [Google Scholar]
- Lydon-Rochelle MT, Holt VL, Cardenas V, Nelson JC, Easterling TR, Gardella C, Callaghan WM. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193(1):125–134. doi: 10.1016/j.ajog.2005.02.096. [DOI] [PubMed] [Google Scholar]
- Lydon-Rochelle MT, Holt VL, Nelson JC, Cardenas V, Gardella C, Easterling TR, Callaghan WM. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Pediatric and Perinatal Epidemiology. 2005;19:460–471. doi: 10.1111/j.1365-3016.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- Mann JR, Zhou L, McKee M, McDermott S. Children with hearing loss and increased risk of injury. Ann Fam Med. 2007;5(6):528–533. doi: 10.1370/afm.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes R, Llewellyn G, McConnell D. Misconception: the experience of pregnancy for women with intellectual disabilities. Scand J Disab Res. 2006;8(2):120–131. [Google Scholar]
- McConnell D, Mayes R, Llewellyn G. Women with intellectual disability at risk of adverse pregnancy and birth outcomes. J Iintellect Disabil Res. 2008;52:529–535. doi: 10.1111/j.1365-2788.2008.01061.x. [DOI] [PubMed] [Google Scholar]
- Mitra M, Clements KM, Zhang J, Iezzoni LI, Smeltzer SC, Long-Bellil LM. Maternal Characteristics, Pregnancy Complications, and Adverse Birth Outcomes Among Women With Disabilities. Med Care. 2015;53:1027–1032. doi: 10.1097/MLR.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra M, Parish SL, Clements KM, Cui X, Diop H. Pregnancy outcomes among women with intellectual and developmental disabilities. Am J Prev Med. 2015;48(3):300–308. doi: 10.1016/j.amepre.2014.09.032. [DOI] [PubMed] [Google Scholar]
- Mitra M, Akobirshoev I, McKee MM, Iezzoni LI. Birth outcomes among U.S. women with hearing loss. Am J Prev Med. 2016 doi: 10.1016/j.amepre.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Day BL, Kileen M, Iezzoni LI. Improving health care eperiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193–200. doi: 10.1177/106286060401900503. [DOI] [PubMed] [Google Scholar]
- Parish SL, Mitra M, Son E, Bonardi A, Swobada PT, Igdalsky L. Pregnancy outcomes among U.S. women with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2015;120(5):433–443. doi: 10.1352/1944-7558-120.5.433. [DOI] [PubMed] [Google Scholar]
- Prilleltensky O. A Ramp to Motherhood: The Experiences of Mothers with Physical Disabilities. Sexuality and Disability. 2003;21(1):21–47. [Google Scholar]
- Rudnik-Schoneborn S, Zerres K. Outcome in pregnancies complicated by myotonic dystrophy: a study of 31 patients and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2004;114(1):44–53. doi: 10.1016/j.ejogrb.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Signore C, Spong CY, Krotoski D, Shinowara NL, Blackwell SC. Pregnancy in women with physical disabilities. Obstet Gynecol. 2011;117(4):935–947. doi: 10.1097/AOG.0b013e3182118d59. [DOI] [PubMed] [Google Scholar]
- Skomsvoll J, Ostensen M, Irgens L, Baste V. Obstetric and neonatal outcome in pregnanct patients with rheumatic disease. Scand J Rheumatol Suppl. 1998;107:109–112. doi: 10.1080/03009742.1998.11720781. [DOI] [PubMed] [Google Scholar]
- Smeltzer SC. Pregnancy in Women With Physical Disabilities. JOGNN. 2007;36:88–96. doi: 10.1111/J.15526909.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- Society for Maternal-Fetal Medicine Publications Committee. Electronic address. SMFM Statement: clarification of recommendations regarding cell-free DNA aneuploidy screening. Am J Obstet Gynecol. 2015;213(6):753–754. doi: 10.1016/j.ajog.2015.09.077. [DOI] [PubMed] [Google Scholar]
- Society for Maternal-Fetal Medicine Publications Committee. Electronic address. #36: Prenatal aneuploidy screening using cell–free DNA. Am J Obstet Gynecol. 2015;212(6):711–716. doi: 10.1016/j.ajog.2015.03.043. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International Classification of Functioning, Disability, and Health. Geneva, Switzerland: WHO; 2001. [Google Scholar]
- Walsh–Gallagher D, Sinclair M, Mc Conkey R. The ambiguity of disabled women’s experience of pregnancy, childbirth, and motherhood: a phenomenological understanding. Midwifery. 2012;28(2):156–162. doi: 10.1016/j.midw.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Winch R, Bengston L, McLaughlin J, Fitzsimmons J, Budden S. Women with cerebral palsy: obstetric experience and neonatal outcome. Dev Med Child Neurol. 1993;35(11):974–982. doi: 10.1111/j.1469-8749.1993.tb11579.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.