Summary
Sickle cell anaemia (SCA) is a progressive vascular disease characterized by episodic vaso-occlusive pain. Despite the broad impact of inflammation on acute and chronic clinical manifestations of SCA, no directed anti-inflammatory therapies currently exist. Statins are cholesterol-lowering agents shown to confer protection from vascular injury by suppressing inflammation. We previously documented a reduction in soluble biomarkers of inflammation in patients with sickle cell disease treated with simvastatin. To determine the potential clinical efficacy of simvastatin, we treated 19 SCA patients with single daily dose simvastatin for 3 months and assessed changes from baseline in the frequency and intensity of diary-reported pain and levels of circulating nitric oxide metabolites (NOx), high sensitivity C-reactive protein (hs-CRP), vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), ICAM-3, E-selectin, and vascular endothelial growth factor (VEGF). Treatment with simvastatin resulted in a significant reduction in the frequency of pain (p=0.0003), oral analgesic use (p=0.003) and circulating hs-CRP (p=0.003), soluble (s)E-selectin (p=0.01), sICAM-1 (p=0.02), sICAM-3 (p=0.02) and sVEGF (p=0.01). Simvastatin had no effect on pain intensity or levels of NOx, sP-selectin and sVCAM-1. The observed reductions in pain rate and markers of inflammation were greatest in subjects receiving hydroxycarbamide (HC), suggesting a synergistic effect of simvastatin. These results provide preliminary clinical data to support a larger trial of simvastatin in SCA.
Keywords: sickle cell anaemia, simvastatin, vaso-occlusive pain, inflammation, clinical effect
Introduction
Vaso-occlusive pain crises (VOC) are the clinical hallmark and the leading cause of morbidity in sickle cell anaemia (SCA), accounting for the vast majority of sickle cell-related hospital admissions. Up to 30% of adults and 10% of children with SCA experience sickle cell pain on a daily basis, suggesting that the pathology of vaso-occlusion is ongoing and unremitting, even during so-called “steady-state” periods (Aisiku, et al 2009).
The pathophysiology of vaso-occlusion involves multiple interrelated processes that have been increasingly linked to inflammation (Owusu-Ansah, et al 2016). Erythrocyte sickling and haemolysis trigger acute inflammation marked by monocyte activation, elaboration of inflammatory cytokines and abnormal expression of endothelial adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), E-selectin and P-selectin (Brown, et al 2001, Hebbel and Vercellotti 1997, Shiu, et al 2000). These circulating mediators of inflammation and adhesion have been shown to exacerbate sickling in experimental models and correlate with clinical disease severity (Dworkis, et al 2010, Kaul, et al 2004, Krishnan, et al 2010, Makis, et al 2006, Qari, et al 2012). Repeated cycles of ischaemia reperfusion promote a chronic inflammatory state that results in progressive vascular injury and multi-organ dysfunction (Hebbel 2004, Kato, et al 2009, Okpala, et al 2002, Osarogiagbon, et al 2000).
Treatment with hydroxycarbamide (HC, also termed hydroxyurea [HU]) is recommended in children and adults to reduce the frequency of acute VOC, but has not been shown to prevent disease progression and the chronic morbid complications associated with SCA (Yawn, et al 2014). Given the complex pathobiology of vaso-occlusion, a multimodal approach to treatment that includes agents with complementary mechanisms of action is warranted.
Statins (3-Hydroxy-3-methyl-glutaryl CoA (HMG-CoA) reductase inhibitors) have been shown to improve endothelial function independent of their lipid-lowering effects, by suppressing inflammation and restoring nitric oxide (NO) production (De Caterina, et al 1995, Laufs 2003, Nohria, et al 2006, O’Driscoll, et al 1997, Takemoto, et al 2002). The anti-inflammatory properties of statins exert their effects via several mechanisms involved in the pathology of sickle cell vaso-occlusion (Antonopoulos, et al 2012, Holschermann, et al 2006, Jain and Ridker 2005, Piechota-Polanczyk and Jozkowicz 2016, Tousoulis, et al 2014, van der Meij, et al 2013, Wang, et al 2005). In sickle cell mice, statins inhibited pulmonary endothelial tissue factor (TF) and VCAM-1 expression after hypoxic challenge and resulted in prolonged survival following pneumococcal infection (Rosch, et al 2010). Simvastatin inhibited ICAM-1 expression and sickle neutrophil adhesion to stimulated endothelial cells in vitro (Canalli, et al 2008, Canalli, et al 2011, Silveira, et al 2013, Solovey, et al 2004). The mechanistic role of statins in modulating endothelial nitric oxide synthase (eNOS) expression is supported by pilot studies showing improvement in NOS-dependent blood flow after atorvastatin treatment in adult SCA patients with underlying endothelial dysfunction (Bereal-Williams, et al 2012). We previously demonstrated clinical safety and improvement in soluble markers of inflammation in sickle cell disease (SCD) patients treated with short-term simvastatin (Hoppe, et al 2011). The primary objective of this study was to determine the potential clinical efficacy of simvastatin in reducing the frequency and intensity of vaso-occlusive pain in SCA.
Methods
Study Subjects
The study included patients with SCA (Hb SS or Hb S/β0 thalassaemia genotype) who were at least 10 years of age and had a history of ≥ 3 vaso-occlusive pain episodes requiring treatment with a prescribed oral or parenteral analgesic in the preceding year. Patients were recruited at their baseline status without acute pain or other SCA-related symptoms. Patients receiving treatment with HC at a stable dose for ≥ 3 months were eligible.
Laboratory exclusion criteria were defined as a pre-treatment serum total cholesterol < 2.3 mmo/l or triglycerides (TG) 0.34 mmol/l, creatine kinase (CK) greater than upper normal limit (UNL) (>232 u/l), serum creatinine greater than 1.5-fold UNL, alanine transaminase (ALT) greater than 2-fold UNL. Clinical exclusion criteria were pregnancy/lactation, red cell transfusion or hospitalization within the 30 days prior to enrolment, current treatment with statins, amiodarone or other drugs with known metabolic interactions with statins (e.g. cytochrome P450 3A4 metabolism), an underlying musculoskeletal disorder, a positive urine toxicology screen or known history of cocaine or amphetamine use. The study was approved by the Institutional Review Board at University of California, San Francisco (UCSF) Benioff Children’s Hospital Oakland (BCHO). All subjects or their parents provided written informed consent.
Study Design
The study was a single centre, open label, non-randomized trial assessing the efficacy of simvastatin (ClinicalTrials.gov Identifier: NCT00508027) in reducing daily vaso-occlusive pain events in paediatric and adult patients with SCA. All subjects received simvastatin in a single oral dose 40 mg (weight >60 kg), 30 mg (weight 45–60 kg), 25 mg (weight 35–44 kg) daily for 3 months. The primary outcome measure was the change from baseline in the frequency and intensity of diary-reported vaso-occlusive pain after 3 months of treatment with simvastatin. Baseline pain was assessed using diary data collected over a period of one month prior to initiation of simvastatin. A web-based electronic diary (eDiary) accessed by smartphone was used to report daily sickle cell-related pain, pain intensity, use of oral or parenteral analgesics and visits to a medical facility. The eDiary was previously developed for use in SCD and included well-validated metrics to assess pain specifically attributed to SCA-related vaso-occlusion, including mild daily pain as well as more severe pain associated with an acute VOC (Jacob, et al 2013a, Jacob, et al 2014, Jacob, et al 2013b, Jacob, et al 2012). The eDiary also included a section for comments using free text to describe pain that was not considered to be sickle cell-related pain.
Vaso-occlusive pain was defined as the occurrence of sickle cell-related pain within the preceding 24 h. Study participants were asked to report the intensity of their sickle cell pain, on a scale from 0 (none) to 9 (worst) in the preceding 24 h, specific pain medications used, and visits to a medical provider for sickle cell-related pain. The smartphone was pre-programmed with automated reminders prompting subjects to complete the daily diary. Subjects took an average of 3 minutes to complete the eDiary and submitted daily diaries for one month prior to treatment and for 3 months during treatment with simvastatin.
Pain rate was defined as the sum of days a subject reported sickle cell-related pain divided by the total number of daily diaries completed, expressed as a percentage. Days of hospitalization for an acute VOC were excluded from the analysis. Pain intensity was evaluated by a visual analogue scale (VAS) comprised of a horizontal line anchored by the words “none” and “worst pain” at each end that was automatically quantified on a 9-unit metric to generate a score. Pain intensity was measured as the sum of a subject’s highest daily pain score divided by the total number of pain days reported. While all medications used were recorded in the e-diary, only oral or parenteral opioids and non-steroidal anti-inflammatory drugs (NSAIDS) were included in the analysis. The frequency of analgesic use was defined as the sum of days a subject reported use of either opioid or NSAID to manage sickle cell-related pain divided by the total number of daily diaries completed, expressed as a percentage.
Clinical and laboratory assessments were performed at baseline, at monthly visits during treatment and at follow-up one month after discontinuation of simvastatin. Serial non-fasting blood samples for clinical safety studies and for plasma biomarkers were collected at each visit. Lipid profiles, complete blood counts and routine chemistries were conducted using standard laboratory techniques in the clinical laboratory at UCSF BCHO. Plasma high sensitivity C-reactive protein (hs-CRP) was determined by a latex particle-enhanced immunochemistry assay (Vitros Model 5, 1 FS Chemistry Systems, Johnson & Johnson Clinical Diagnostics Inc., Rochester, NY). Blood samples for the remaining biomarker studies were centrifuged at 3000g at room temperature and the plasma aliquots were frozen and stored at −80 °C until analysis. Plasma samples were assayed for the concentration of NO metabolites, nitrite/nitrate (NOx), using an NO chemiluminescence analyser (Sievers Model 280 NOA; Sievers Instruments, Boulder, Colorado, USA), as previously described (Braman and Hendrix 1989); VCAM-1 and vascular endothelial growth factor (VEGF) were measured by enzyme-linked immunosorbent assay (ELISA; Invitrogen, Camarillo, CA) according to the manufacturer’s instructions; ICAM-1, ICAM3, P-selectin and E-selectin were analysed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) using a bead-based immunoassay (eBioscience, San Diego, CA), as previously described (Elshal & McCoy 2006). The minimum detection limit of the ELISA assay for VCAM-1 was 0.5 ng/mL. The sensitivity of the immunoassay for E-selectin was1.2 ng/ml; ICAM-1, 5.3 ng/ml; ICAM-3, 4.8 ng/ml and for P-selectin it was 5.7 ng/ml.
Monitoring for adverse events, including muscle pain, weakness and gastrointestinal symptoms, as well as adherence to simvastatin was performed by review of the daily e-diary record and by direct questioning at each visit. A pill count was performed monthly and at the final study visit. The total study duration with follow-up was 5 months.
Statistical Analysis
Descriptive statistics were used to summarize baseline patient characteristics, expressed as mean ± SD or median with interquartile range. The effects of simvastatin treatment were assessed by comparing the frequency and intensity of pain within subjects before and after 3 months of treatment. Changes from baseline in pain measures, opioid analgesic use and biomarker levels were assessed using Wilcoxon matched-pairs signed rank test for non-parametric data. A two-tailed P <0.05 was considered significant. Statistical analysis was performed with GraphPad Prism statistical software (version 5.01; GraphPad software Inc., San Diego, CA, USA).
Results
Subject characteristics
Twenty-four subjects met the eligibility criteria for the study. Of these, 2 subjects were lost to follow-up prior to study initiation, 3 subjects were unable to adhere to the protocol and 19 subjects completed the study. All study participants had SCA (17 HbSS, 2 Hb S/β0 thalassaemia) with a mean age of 18.5 years (range 10–34 years), 13 (68%) were female and 10 (53%) were receiving stable treatment with hydroxycarbamide.
Overall adherence to treatment with simvastatin, as assessed by verbal report and monthly pill counts, was 82% (range, 70%–100%). A total of 5 serious adverse events (SAE) were reported in 3 subjects who were hospitalized for VOC requiring hospitalization. In one subject, the hospitalization was complicated by severe hyperhaemolysis following transfusion. This subject was withdrawn from the study during the treatment period, but was included and contributed a total of 74 study days to the analysis. There was one AE, possibly related to simvastatin, in a subject who developed a facial rash after one month of treatment. None of the study subjects developed clinical symptoms of myopathy or hepatitis during the treatment period.
Simvastatin effects on lipid profiles and clinical laboratory measurements
Routine laboratory values (complete blood count, serum chemistry panel and hepatic transaminase) and creatine kinase levels remained stable on serial follow-up with no observed differences from baseline (Table I).
Table I.
Effect of simvastatin treatment on lipid profiles and clinical laboratory parameters
| Laboratory | Baseline | Treatment | % change | p-value |
|---|---|---|---|---|
| Total cholesterol (mmo/l) | 2.97 (2.69, 3.36) | 2.71 (2.28, 2.90) | −9 | 0.0002 |
| LDL-C (mmo/l) | 1.37 (1.16, 1.71) | 1.01 (0.83, 1.45) | −26 | 0.0003 |
| VLDL-C (mmo/l) | 0.62 (0.38, 0.69) | 0.39 (0.31, 0.52) | −38 | 0.0021 |
| HDL-C (mmo/l) | 0.98 (0.88, 1.09) | 0.93 (0.88, 1.09) | −5 | 0.6864 |
| Triglycerides (mmo/l) | 1.06 (0.82, 1.41) | 1.01 (0.78, 1.11) | −5 | 0.0405 |
| WBC count (x 109/l) | 8.5 (6.4, 11.3) | 7.1 (5.9, 9.8) | −16 | 0.0584 |
| Hb (g/l) | 94 (85, 111) | 93 (87, 108) | −1 | 0.7944 |
| ARC (x 109/l) | 0.29 (0.18, 0.37) | 0.21 (0.14, 0.32) | −28 | 0.0732 |
| Platelet count (x 109/l) | 331 (284, 445) | 301 (269, 385) | −10 | 0.3838 |
| Total bilirubin (μmol/l) | 35.9 (23.9, 56.4) | 34.2 (17.1, 46.2) | −5 | 0.1270 |
| ALT (u/l) | 25.0 (13.0, 30.0) | 22.0 (18.0, 31.0) | −12 | 0.9133 |
| AST (u/l) | 54.0 (41.0, 81.0) | 53.0 (37.0, 80.0) | −2 | 0.7601 |
| Creatinine (μmol/l) | 44.2 (35.4, 61.9) | 44.2 (35.4, 53.0) | 0 | 0.7339 |
| Creatine kinase (U/L) | 43.0 (34.0, 52.0) | 44.0 (37.0, 55.0) | 2 | 0.3437 |
Values are expressed as median (25th, 75th percentile)
ALT, alanine transaminase; ARC, absolute reticulocyte count; AST, aspartate transaminase; Hb haemoglobin; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; VLDL-C, very low-density lipoprotein-cholesterol; WBC, white blood cell.
Simvastatin led to a significant decrease from baseline in serum lipid levels, with a decrease in total cholesterol by 9% and in low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) by 26% and 38%, respectively. There was a modest 5% decrease in non-fasting triglyceride levels. As expected, high-density lipoprotein (HDL) levels did not change following treatment with simvastatin.
Simvastatin effects on vaso-occlusive pain
The median diary completion rate (number of diaries completed/total number of study days) was 74% (range, 53%–97%). Diary adherence was similar during the baseline (median 76%, range 40%–94%) and treatment (median 73%, range 47%–99%) periods. Pain related to SCD was reported on 20% of total diary days, the majority (91%) of which were rated as mild (>0–4, 61%) to moderate (>4–7, 31%). Severe pain (>7) was reported by 7 subjects on 8% of days and led to an emergency department visit or hospitalization in 4 of these subjects. Subject enrolment was evenly distributed across the months of the year. There was no apparent seasonal effect on the frequency or intensity of pain.
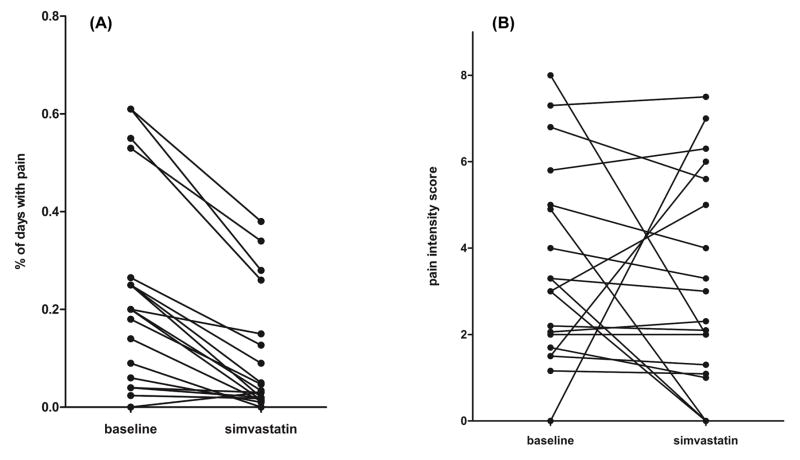
Treatment with simvastatin led to an 85% reduction in the overall rate of pain, from a baseline of 20% to 3% (p=0.0003) (Figure 1A). Oral analgesic use also decreased by 67%, from 9% to 3% of pain days after treatment (p=0.003). However, pain intensity did not change in response to simvastatin (Figure 1B). In a separate analysis examining the effect of simvastatin by HC use, the reduction in both pain rate and oral analgesic use remained significant in both subgroups of patients treated with either simvastatin alone or in combination with HC (Table II). A numerical decrease in pain intensity was observed in the subgroup on HC therapy, but did not reach significance.
Figure 1.
Effect of simvastatin on patient reported days with pain and pain intensity. (A) Within-subject changes in the proportion of total study days with reported pain. (B) Within-subject changes in the intensity of reported sickle cell pain. Pain intensity was evaluated using the numerical rating score from the visual analogue scale. Results are expressed as median (25, 75th percentile).
Table II.
Changes in pain frequency, intensity and analgesic use after 3 months of simvastatin treatment in total study group and by concomitant hydroxycarbamide use
| Total group | On HC (n=10) | Not on HC (n=9) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Simvastatin | % change | p-value | Baseline | Simvastatin | % change | p-value | Baseline | Simvastatin | % change | p-value | |
| Pain rate | 0.20 (0.06, 0.27) | 0.03 (0.02, 0.15) | −85 | 0.0003 | 0.15 (0.04, 0.32) | 0.03 | −80 | 0.006 | 0.25 (0.16, 0.41) | 0.047 | −81 | 0.013 |
| Analgesic use | 0.09 (0, 0.22) | 0.03 (0, 0.09) | −67 | 0.003 | 0.08 (0, 0.2) | 0.03 (0, 0.09) | −63 | 0.04 | 0.09 (0.03,0.23) | 0.03 (0.01,0.09) | −67 | 0.04 |
| Pain intensity | 3.0 (1.7, 5.0) | 2.3 (1.1, 5.6) | −23 | 0.2762 | 2.7 (1.5, 5.2) | 2.2 (1.3,4.5) | −19 | 0.43 | 3.0 (2.0,5.9) | 3.0 (0.5,6.3) | 0 | 0.57 |
Values are expressed as median and 25–75% interquartile range
Analgesic medications included oral or parenteral opioids and non-steroidal anti-inflammatory drugs.
HC, hydroxycarbamide
Simvastatin effects on soluble biomarkers of inflammation
Plasma levels of hs-CRP, NOx and VEGF were evaluated in all subjects. Soluble (s)ICAM-1, ICAM-3, VCAM-1, E-selectin and P-selectin levels were measured in only 14 of the 19 subjects, due to a change in availability of the adhesion marker assay kit. Baseline levels of these biomarkers were consistent with previous studies showing elevated levels in SCA patients compared to healthy, age- and race-matched control subjects (Al Najjar, et al 2016, Bhagat, et al 2012, Blann, et al 2008, Conran, et al 2004, Duits, et al 1996, Gurkan, et al 2005, Hatzipantelis, et al 2013, Kanavaki, et al 2012, Kato, et al 2005, Krishnan, et al 2010, Makis, et al 2006, Mohan, et al 2005, Qari, et al 2012, Rees, et al 1995, Saleh, et al 1999).
Simvastatin-associated changes in biomarker levels are shown in Table II. Treatment with simvastatin led to a significant reduction in plasma levels of hs-CRP, sE-selectin, sICAM-1, sICAM-3 and sVEGF. The greatest change was in hs-CRP with a 37% decrease from baseline (p=0.003). More modest changes were found in the levels of sVEGF, sE-selectin, sICAM-1 and sICAM-3, which decreased by 10–20% from baseline following simvastatin. Neither plasma nitric oxide (NOx) metabolites nor sP-selectin levels changed significantly after treatment.
In a limited sub-analysis comparing biomarker changes in subjects on HC therapy with those who were not on HC, the decreases in hs-CRP, sE-selectin, sICAM-1, sICAM-3 and sVEGF observed in response to simvastatin were localized exclusively to the subgroup on HC therapy. The treatment effect of simvastatin alone was no longer significant in subjects who were not on HC. The persistent and more dramatic reduction in biomarker levels found in the combined treatment group suggests that simvastatin and HC may act synergistically (Table III, Table IV). Notably, serum biomarker levels were similar in the HC and non-HC subgroups at baseline.
Table III.
Change in soluble biomarker levels after 3 months of simvastatin treatment
| Biomarker | Baseline | Simvastastin treatment | % change | P-value |
|---|---|---|---|---|
| NOx (μM) | 27.5 (15.4, 34.6) | 28.2 (22.1, 36.0) | +2.5 | 0.40 |
| hs-CRP (mg/l) | 1.90 (1.70, 9.90) | 1.20 (0.90, 2.30) | −37 | 0.003 |
| sE-selectin (ng/ml) | 302.3 (132.3, 542.5) | 273.2 (114.5, 417.4) | −10 | 0.01 |
| sP-selectin (ng/ml) | 474.5 (304.9, 705.9) | 360.5 (269.8, 635.1) | −24 | 0.32 |
| sVEGF (ng/ml) | 122.3 (83.0, 195.0) | 98.3 (81.8, 163.8) | −20 | 0.01 |
| sICAM-1 (ng/ml) | 511.6 (381.3, 631.9) | 428.0 (361.6, 629.2) | −16 | 0.02 |
| sICAM-3 (ng/ml) | 154.2 (86.7, 176.8) | 128.8 (76, 156) | −16 | 0.02 |
| sVCAM-1 (ng/ml) | 992.4 (796.8, 1778) | 903.6 (706.8, 1664) | −9 | 0.12 |
Values are expressed as median and 25–75% interquartile range
hs-CRP, high sensitivity C-reactive protein; NOx, nitrite/nitrate; sE-selectin, soluble E-selectinsICAM-1, -3, soluble intercellular adhesion molecule 1, 3; sP-selectin, soluble P-selectin; sVCAM-1, vascular cell adhesion molecule 1; sVEGF, soluble vascular endothelial growth factor.
Table IV.
Comparison of changes in soluble (s) biomarker levels after simvastatin by hydroxycarbamide use.
| Biomarker | HC (n=10*) | No HC (9*) | ||||||
|---|---|---|---|---|---|---|---|---|
| baseline | simvastatin | %change | p-value | baseline | simvastatin | %change | p-value | |
| NOx (μM) | 21.4 | 28 | +31 | 0.39 | 30 | 28.2 | −6 | 0.82 |
| hs-CRP (mg/l) | 1.8 (1.2,9.9) | 1.05 (.58,1.7) | −42 | 0.008 | 3 | 2.2 | −27 | 0.15 |
| sE-selectin (ng/ml) | 232.4 | 177.6 | −24 | 0.03 | 370.3 | 355.3 | −4 | 0.44 |
| sP-selectin (ng/ml) | 405.8 | 352.2 | −13 | .94 | 487.3 | 586 | 20 | 0.25 |
| sVEGF (ng/ml) | 148.9 | 96.0 | −36 | .03 | 98.6 | 116.9 | 19 | 0.13 |
| sICAM-1 (ng/ml) | 462.9 | 377.4 | −86 | .008 | 537.6 | 573.3 | 7 | 0.56 |
| sICAM-3 (ng/ml) | 160.4 | 128.8 | −20 | .008 | 151.8 | 124.2 | −18 | 0.85 |
| sVCAM-1 (ng/ml) | 822.1 | 749.6 | −9 | 0.58 | 1110 | 1129 | 2 | 0.20 |
Analyses of sE-selectin, sP-selectin, ICAM-1, ICAM-3 and VCAM-1 was limited to 14 subjects (8 on HC, 6 not on HC)
HC, hydroxycarbamide; hs-CRP, high sensitivity C-reactive protein; NOx, nitrite/nitrate; sE-selectin, soluble E-selectinsICAM-1, -3, soluble intercellular adhesion molecule 1, 3; sP-selectin, soluble P-selectin; sVCAM-1, vascular cell adhesion molecule 1; sVEGF, soluble vascular endothelial growth factor.
Discussion
In this single centre pilot study of simvastatin in adolescents and adults with SCA, treatment for up to 3 months led to a dramatic reduction in the rate of sickle cell related pain and oral analgesic use, improvement in soluble biomarkers of inflammation and an acceptable safety profile.
Study participants reported a baseline frequency of sickle cell pain on 20% of days and used an analgesic medication on 50% of these days, consistent with mounting evidence that pain in SCA is more common than previously believed (Dampier, et al 2004, Smith, et al 2011, Smith, et al 2008).
Simvastatin was associated with an 85% reduction in the frequency of self-reported pain events and a parallel reduction in analgesic use. Despite the marked decline in pain rate, pain intensity did not change with simvastatin. Although several well-validated measures of sickle cell-related pain were included in the eDiary, analysis of pain intensity was limited to VAS scores, which may not have fully captured the multi-dimensional nature of pain intensity in this disease. Not surprisingly, pain intensity scores were highest prior to an acute VOC requiring management in the emergency department or hospital. However, there were too few VOC requiring a visit to a medical facility in this relatively short study to assess an effect of simvastatin on health care utilization.
In addition to a decline in the rate of pain, treatment with simvastatin led to a decrease in several circulating mediators of inflammation including hs-CRP, sICAM-1, sICAM-3, sE-selectin and sVEGF. These markers of inflammation and endothelial adhesion are elevated in SCD and other vascular disorders characterized by endothelial dysfunction and indicate a state of sustained low-grade inflammation (Hatzipantelis, et al 2013, Mohan, et al 2005). The reduction in hs-CRP and other inflammatory mediators following treatment with simvastatin are consistent with our prior study in SCD patients and several multi-centre studies in non-SCD populations (Blake and Ridker 2000, Hoppe, et al 2011, Kinlay, et al 2008, Koh 2000, Sacks, et al 1996, Shah and Newby 2003). Notably, in individuals with low or normal cholesterol, but high hs-CRP levels, statins significantly reduced the risk of stroke, disease progression and mortality (Albert, et al 2001).
Mechanistic studies have corroborated an independent anti-inflammatory effect of statins through NO-mediated regulation of nuclear transcription factors NFkB and Akt (Bustos, et al 1998, Devaraj, et al 2006, Grip, et al 2002, Ortego, et al 1999). In experimental models of ischaemia/reperfusioninjury including sickle mice, statins have been shown to prevent vaso-occlusion and improve microvascular blood flow through inhibition of NFκB-dependent adhesion molecules, VCAM, ICAM and selectins (Bao, et al 2004, Belcher, et al 2003, De Caterina, et al 1995, Kollander, et al 2010, Liuni, et al 2010, Palmer, et al 1988, Selvaraj, et al 2003, Solovey, et al 2001). Collectively, these studies provide a plausible explanation for the reduction in soluble markers of inflammation (hs-CRP) and endothelial activation (sICAM-1, sICAM-3, sE-selectin, VEGF) observed following simvastatin treatment in this study.
Remarkably, the greatest changes in biomarker levels were found in the subgroup of patients who were also receiving HC therapy. The observed decrease in hs-CRP, sICAM-1, sICAM-3, sE-selectin and VEGF levels remained significant in patients treated with simvastatin alone, but was even more dramatic in patients receiving concomitant HC therapy. Hydroxycarbamide has proven efficacy in reducing the frequency of VOC and has been shown to inhibit expression of several adhesion molecules including E- and P-selectin, ICAM and VCAM-1 (Almeida, et al 2015, Conran, et al 2004, Kato, et al 2005, Saleh, et al 1999, Verger, et al 2014). Localization of the treatment effect to the HC subgroup suggests that simvastatin may augment the established effect of HC in reducing sICAM-1, sICAM-3 and sE-selectin levels in subjects receiving combined treatment. Despite the small number of subjects in this analysis, it is conceivable that SCA patients treated with HC may derive greater benefit from co-treatment with simvastatin. These results must be considered in light of the potential bias introduced as a result of participation in the study, including improvement in HC compliance. If confirmed, these results have promising therapeutic implications, in that simvastatin may potentially benefit patients who are already on HC, as well as those who are intolerant or have a suboptimal response to HC.
As demonstrated in our previous study, sVCAM-1 and sP-selectin levels did not change in response to treatment with simvastatin in this study. These results are not altogether surprising, as circulating levels may not reflect the differential temporal and tissue-specific expression of these markers during vaso-occlusion. In sickle mice, treatment with statins inhibited TF and VCAM-1 expression in pulmonary endothelium, but not monocytes (Chi, et al 2003, Hebbel and Mohandas 1994, Solovey, et al 2004, Solovey, et al 2001). Kato et al (2005) previously reported a reduction in sVCAM-1 levels in SCA patients following treatment with HC. It is thus conceivable that simvastatin had no additional effect in lowering sVCAM-1 levels beyond that achieved by HC in this study.
In contrast to our previous study showing an increase in NOx following simvastatin, simvastatin had no direct effect on levels of plasma NOx. However, this effect was eliminated in patients who developed acute VOC (Hoppe, et al 2011). The lack of a treatment effect on plasma NOx levels in this study may have reflected the greater disease severity in subjects with SCA enrolled in this study. An alternative and perhaps more likely explanation is that measurement of plasma NOx may not accurately estimate endogenous NO bioavailability in SCA patients, as suggested by other studies showing conflicting results (Akinsheye and Klings 2010, Lamarre, et al 2014, Lopez, et al 1996, Lopez, et al 2000). Aside from hs-CRP, there are no clinically approved assays for the biomarkers in this study and measurements may have been affected by several factors, including circadian variation, short half-life, post-prandial effects and assay stability (Moshage 1997).
Safety
There were no clinical safety concerns or simvastatin-related adverse events in this study. As in our prior study, simvastatin resulted in a relatively modest and stable decrease in total cholesterol and LDL-C levels. Moreover, simvastatin had no effect on haematological parameters, arguing against the hypothetical risk of red blood cell underproduction due to excessive cholesterol lowering. These results extend the safety profile found in our previous study and are supported by data from large clinical trials documenting the overall safety of simvastatin in diverse populations (Florentin and Elisaf 2012, Giorgi, et al 2011, Jiang, et al 2014).
Limitations
Despite the significant effects of simvastatin observed in this study, a larger, controlled trial is necessary to confirm these results. Given the variability in clinical symptoms within and among patients with SCA, a period of one month may not have adequately reflected subjects’ baseline status. Environmental factors may also contribute to the clinical variability of SCD, including the frequency of acute pain. This single institution study was conducted in a geographic area with less seasonal variation and fluctuations in climate than other regions, thus limiting the generalizability of the treatment effect observed. Variable compliance with daily diary submissions ranging between 47% and 97% may have influenced the results of this study. Missing data limited the analysis to pre- and post-measurements rather than multiple measurements over time. However, this pilot study was intended to collect preliminary efficacy data needed for future studies.
Conclusions
The results of this study, showing a reduction in the frequency of vaso-occlusive pain and a corresponding decrease in serum markers of inflammation, suggest that simvastatin alone or in combination with HC may show promise as a core preventative therapy for SCA. These findings provide preliminary clinical data to support a larger trial evaluating the long-term safety and efficacy of simvastatin in SCA.
Acknowledgments
This work was supported by the Doris Duke Charitable Foundation (ClinicalTrials.gov ID NCT) and Grant #UL1 RR024131-01 from the National Center for Research Resources (NCRR)
CH performed the research; CH and LS designed the research study; EJ contributed essential tools; FK and SL contributed essential reagents and performed the laboratory analyses; CH, LS and EJ analysed the data; CH and EV wrote the paper.
Funding Source
This work was supported by the DDCF Innovations in Clinical Research Award (grant to CCH), National Heart, Lung, and Blood Institute (NHLBI, #RC1 HL100301 to EJ) and National Center for Research Resources (NCRR, #UL1 RR024131-01)
Footnotes
Disclosure of Interests
The authors have no competing interests.
References
- Aisiku IP, Smith WR, McClish DK, Levenson JL, Penberthy LT, Roseff SD, Bovbjerg VE, Roberts JD. Comparisons of high versus low emergency department utilizers in sickle cell disease. Annals of Emergency Medicine. 2009;53:587–593. doi: 10.1016/j.annemergmed.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Akinsheye I, Klings ES. Sickle cell anemia and vascular dysfunction: the nitric oxide connection. Journal of Cellular Physiology. 2010;224:620–625. doi: 10.1002/jcp.22195. [DOI] [PubMed] [Google Scholar]
- Al Najjar S, Adam S, Ahmed N, Qari M. Markers of endothelial dysfunction and leucocyte activation in Saudi and non-Saudi haplotypes of sickle cell disease. Annals of Hematology. 2016 doi: 10.1007/s00277-016-2823-7. [DOI] [PubMed] [Google Scholar]
- Albert MA, Staggers J, Chew P, Ridker PM. The pravastatin inflammation CRP evaluation (PRINCE): rationale and design. American Heart Journal. 2001;141:893–898. doi: 10.1067/mhj.2001.115297. [DOI] [PubMed] [Google Scholar]
- Almeida CB, Souza LE, Leonardo FC, Costa FT, Werneck CC, Covas DT, Costa FF, Conran N. Acute hemolytic vascular inflammatory processes are prevented by nitric oxide replacement or a single dose of hydroxyurea. Blood. 2015;126:711–720. doi: 10.1182/blood-2014-12-616250. [DOI] [PubMed] [Google Scholar]
- Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Current Pharmaceutical Design. 2012;18:1519–1530. doi: 10.2174/138161212799504803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, Ma XL, Willette RN, Yue TL. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovascular Research. 2004;61:548–558. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- Bereal-Williams C, Machado RF, McGowan V, 2nd, Chi A, Hunter CJ, Kato GJ, Hunter L, Dalby CK, Hauser KP, Tailor A, Cannon RO., 3rd Atorvastatin reduces serum cholesterol and triglycerides with limited improvement in vascular function in adults with sickle cell anemia. Haematologica. 2012;97:1768–1770. doi: 10.3324/haematol.2011.054957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat S, Patra PK, Thakur AS. Association of Inflammatory Biomarker C-Reactive Protein, Lipid Peroxidation and Antioxidant Capacity Marker with HbF Level in Sickle Cell Disease Patients from Chattisgarh. Indian Journal of Clinical Biochemistry. 2012;27:394–399. doi: 10.1007/s12291-012-0231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. Are statins anti-inflammatory? Current Controlled Trials in Cardiovascular Medicine. 2000;1:161–165. doi: 10.1186/cvm-1-3-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blann AD, Mohan JS, Bareford D, Lip GY. Soluble P-selectin and vascular endothelial growth factor in steady state sickle cell disease: relationship to genotype. Journal of Thrombosis and Thrombolysis. 2008;25:185–189. doi: 10.1007/s11239-007-0177-7. [DOI] [PubMed] [Google Scholar]
- Braman RS, Hendrix SA. Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium (III) reduction with chemiluminescence detection. Analytical Chemistry. 1989;61:2715–2718. doi: 10.1021/ac00199a007. [DOI] [PubMed] [Google Scholar]
- Brown MD, Allen JC, Van Stavern GP, Newman NJ, Wallace DC. Clinical, genetic, and biochemical characterization of a Leber hereditary optic neuropathy family containing both the 11778 and 14484 primary mutations. American Journal of Medical Genetics. 2001;104:331–338. [PubMed] [Google Scholar]
- Bustos C, Hernandez-Presa MA, Ortego M, Tunon J, Ortega L, Perez F, Diaz C, Hernandez G, Egido J. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. Journal of the American College of Cardiology. 1998;32:2057–2064. doi: 10.1016/s0735-1097(98)00487-2. [DOI] [PubMed] [Google Scholar]
- Canalli AA, Franco-Penteado CF, Saad ST, Conran N, Costa FF. Increased adhesive properties of neutrophils in sickle cell disease may be reversed by pharmacological nitric oxide donation. Haematologica. 2008;93:605–609. doi: 10.3324/haematol.12119. [DOI] [PubMed] [Google Scholar]
- Canalli AA, Proenca RF, Franco-Penteado CF, Traina F, Sakamoto TM, Saad ST, Conran N, Costa FF. Participation of Mac-1, LFA-1 and VLA-4 integrins in the in vitro adhesion of sickle cell disease neutrophils to endothelial layers, and reversal of adhesion by simvastatin. Haematologica. 2011;96:526–533. doi: 10.3324/haematol.2010.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conran N, Fattori A, Saad ST, Costa FF. Increased levels of soluble ICAM-1 in the plasma of sickle cell patients are reversed by hydroxyurea. American Journal of Hematology. 2004;76:343–347. doi: 10.1002/ajh.20129. [DOI] [PubMed] [Google Scholar]
- Dampier C, Ely E, Eggleston B, Brodecki D, O’Neal P. Physical and cognitive-behavioral activities used in the home management of sickle pain: a daily diary study in children and adolescents. Pediatric Blood & Cancer. 2004;43:674–678. doi: 10.1002/pbc.20162. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. Journal of Clinical Investigation. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Chan E, Jialal I. Direct demonstration of an antiinflammatory effect of simvastatin in subjects with the metabolic syndrome. Journal of Clinical Endocrinology and Metabolism. 2006;91:4489–4496. doi: 10.1210/jc.2006-0299. [DOI] [PubMed] [Google Scholar]
- Duits AJ, Pieters RC, Saleh AW, van Rosmalen E, Katerberg H, Berend K, Rojer RA. Enhanced levels of soluble VCAM-1 in sickle cell patients and their specific increment during vasoocclusive crisis. Clinical Immunology and Immunopathology. 1996;81:96–98. doi: 10.1006/clin.1996.0163. [DOI] [PubMed] [Google Scholar]
- Dworkis DA, Klings ES, Solovieff N, Li G, Milton JN, Hartley SW, Melista E, Parente J, Sebastiani P, Steinberg MH, Baldwin CT. Severe sickle cell anemia is associated with increased plasma levels of TNF-R1 and VCAM-1. American Journal of Hematology. 2010;86:220–223. doi: 10.1002/ajh.21928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentin M, Elisaf MS. Simvastatin interactions with other drugs. Expert Opin Drug Saf. 2012;11:439–444. doi: 10.1517/14740338.2012.670633. [DOI] [PubMed] [Google Scholar]
- Giorgi MA, Caroli C, Arazi HC, Di Girolamo G. Pharmacogenomics and adverse drug reactions: the case of statins. Expert Opinion on Pharmacotherapy. 2011;12:1499–1509. doi: 10.1517/14656566.2011.563734. [DOI] [PubMed] [Google Scholar]
- Grip O, Janciauskiene S, Lindgren S. Atorvastatin activates PPAR-gamma and attenuates the inflammatory response in human monocytes. Inflammation Research. 2002;51:58–62. doi: 10.1007/BF02684000. [DOI] [PubMed] [Google Scholar]
- Gurkan E, Tanriverdi K, Baslamisli F. Clinical relevance of vascular endothelial growth factor levels in sickle cell disease. Annals of Hematology. 2005;84:71–75. doi: 10.1007/s00277-004-0935-y. [DOI] [PubMed] [Google Scholar]
- Hatzipantelis ES, Pana ZD, Gombakis N, Taparkou A, Tzimouli V, Kleta D, Zafeiriou DJ, Garipidou V, Kanakoudi F, Athanassiou M. Endothelial activation and inflammation biomarkers in children and adolescents with sickle cell disease. International Journal of Hematology. 2013;98:158–163. doi: 10.1007/s12185-013-1392-y. [DOI] [PubMed] [Google Scholar]
- Hebbel R, Mohandas N. Sickle cell adherence. Raven Press; New York: 1994. [Google Scholar]
- Hebbel RP. Special issue of microcirculation: examination of the vascular pathobiology of sickle cell anemia. Microcirculation. 2004;11:99–100. [PubMed] [Google Scholar]
- Hebbel RP, Vercellotti GM. The endothelial biology of sickle cell disease. Journal of Laboratory and Clinical Medicine. 1997;129:288–293. doi: 10.1016/s0022-2143(97)90176-1. [DOI] [PubMed] [Google Scholar]
- Holschermann H, Schuster D, Parviz B, Haberbosch W, Tillmanns H, Muth H. Statins prevent NF-kappaB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis. 2006;185:240–245. doi: 10.1016/j.atherosclerosis.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Kuypers F, Larkin S, Hagar W, Vichinsky E, Styles L. A pilot study of the short-term use of simvastatin in sickle cell disease: effects on markers of vascular dysfunction. British Journal of Haematology. 2011;153:655–663. doi: 10.1111/j.1365-2141.2010.08480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Stinson J, Duran J, Gupta A, Gerla M, Ann Lewis M, Zeltzer L. Usability testing of a Smartphone for accessing a web-based e-diary for self-monitoring of pain and symptoms in sickle cell disease. Journal of Pediatric Hematology/Oncology. 2012;34:326–335. doi: 10.1097/MPH.0b013e318257a13c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Duran J, Stinson J, Lewis MA, Zeltzer L. Remote monitoring of pain and symptoms using wireless technology in children and adolescents with sickle cell disease. J Am Assoc Nurse Pract. 2013a;25:42–54. doi: 10.1111/j.1745-7599.2012.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Pavlish C, Duran J, Stinson J, Lewis MA, Zeltzer L. Facilitating pediatric patient-provider communications using wireless technology in children and adolescents with sickle cell disease. Journal of Pediatric Health Care. 2013b;27:284–292. doi: 10.1016/j.pedhc.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Mack AK, Savedra M, Van Cleve L, Wilkie DJ. Adolescent pediatric pain tool for multidimensional measurement of pain in children and adolescents. Pain Management Nursing. 2014;15:694–706. doi: 10.1016/j.pmn.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Gong RR, Qiu L, Wang Q, Su M, Liu XJ, Hu MS, Lin J, Fang DZ. Efficacy and safety of pitavastatin versus simvastatin: a meta-analysis of randomized controlled trials. Clinical Drug Investigation. 2014;34:599–608. doi: 10.1007/s40261-014-0215-0. [DOI] [PubMed] [Google Scholar]
- Kanavaki I, Makrythanasis P, Lazaropoulou C, Kattamis A, Tzanetea R, Kalotychou V, Rombos I, Papassotiriou I. Adhesion molecules and high-sensitivity C-reactive protein levels in patients with sickle cell beta-thalassaemia. European Journal of Clinical Investigation. 2012;42:27–33. doi: 10.1111/j.1365-2362.2011.02551.x. [DOI] [PubMed] [Google Scholar]
- Kato GJ, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, Brennan ML, Hazen SL, Gladwin MT. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. British Journal of Haematology. 2005;130:943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. American Journal of Hematology. 2009;84:618–625. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul DK, Liu XD, Choong S, Belcher JD, Vercellotti GM, Hebbel RP. Anti-inflammatory therapy ameliorates leukocyte adhesion and microvascular flow abnormalities in transgenic sickle mice. American Journal of Physiology: Heart and Circulatory Physiology. 2004;287:H293–301. doi: 10.1152/ajpheart.01150.2003. [DOI] [PubMed] [Google Scholar]
- Kinlay S, Schwartz GG, Olsson AG, Rifai N, Szarek M, Waters DD, Libby P, Ganz P. Inflammation, statin therapy, and risk of stroke after an acute coronary syndrome in the MIRACL study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:142–147. doi: 10.1161/ATVBAHA.107.151787. [DOI] [PubMed] [Google Scholar]
- Koh KK. Effects of HMG-CoA reductase inhibitor on hemostasis. International Journal of Cardiology. 2000;76:23–32. doi: 10.1016/s0167-5273(00)00325-9. [DOI] [PubMed] [Google Scholar]
- Kollander R, Solovey A, Milbauer LC, Abdulla F, Kelm RJ, Jr, Hebbel RP. Nuclear factor-kappa B (NFkappaB) component p50 in blood mononuclear cells regulates endothelial tissue factor expression in sickle transgenic mice: implications for the coagulopathy of sickle cell disease. Translational Research: The Journal of Laboratory and Clinical Medicine. 2010;155:170–177. doi: 10.1016/j.trsl.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Setty Y, Betal SG, Vijender V, Rao K, Dampier C, Stuart M. Increased levels of the inflammatory biomarker C-reactive protein at baseline are associated with childhood sickle cell vasocclusive crises. British Journal of Haematology. 2010;148:797–804. doi: 10.1111/j.1365-2141.2009.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarre Y, Romana M, Lemonne N, Hardy-Dessources MD, Tarer V, Mougenel D, Waltz X, Tressieres B, Lalanne-Mistrih ML, Etienne-Julan M, Connes P. Alpha thalassemia protects sickle cell anemia patients from macro-albuminuria through its effects on red blood cell rheological properties. Clinical Hemorheology and Microcirculation. 2014;57:63–72. doi: 10.3233/CH-131772. [DOI] [PubMed] [Google Scholar]
- Laufs U. Beyond lipid-lowering: effects of statins on endothelial nitric oxide. European Journal of Clinical Pharmacology. 2003;58:719–731. doi: 10.1007/s00228-002-0556-0. [DOI] [PubMed] [Google Scholar]
- Liuni A, Luca MC, Gori T, Parker JD. The endothelial-protective effects of HMG-CoA reductase inhibition in the setting of ischemia and reperfusion injury. Clinical Hemorheology and Microcirculation. 2010;45:161–167. doi: 10.3233/CH-2010-1294. [DOI] [PubMed] [Google Scholar]
- Lopez BL, Barnett J, Ballas SK, Christopher TA, Davis-Moon L, Ma X. Nitric oxide metabolite levels in acute vaso-occlusive sickle-cell crisis. Academic Emergency Medicine. 1996;3:1098–1103. doi: 10.1111/j.1553-2712.1996.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Lopez BL, Davis-Moon L, Ballas SK, Ma XL. Sequential nitric oxide measurements during the emergency department treatment of acute vasoocclusive sickle cell crisis. American Journal of Hematology. 2000;64:15–19. doi: 10.1002/(sici)1096-8652(200005)64:1<15::aid-ajh3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Makis AC, Hatzimichael EC, Stebbing J, Bourantas KL. C-reactive protein and vascular cell adhesion molecule-1 as markers of severity in sickle cell disease. Archives of Internal Medicine. 2006;166:366–368. doi: 10.1001/archinte.166.3.366. [DOI] [PubMed] [Google Scholar]
- Mohan JS, Lip GY, Wright J, Bareford D, Blann AD. Plasma levels of tissue factor and soluble E-selectin in sickle cell disease: relationship to genotype and to inflammation. Blood Coagulation and Fibrinolysis. 2005;16:209–214. doi: 10.1097/01.mbc.0000164431.98169.8f. [DOI] [PubMed] [Google Scholar]
- Moshage H. Nitric oxide determinations: much ado about NO.-thing? Clinical Chemistry. 1997;43:553–556. [PubMed] [Google Scholar]
- Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circulation Research. 2006;99:1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- Okpala I, Daniel Y, Haynes R, Odoemene D, Goldman J. Relationship between the clinical manifestations of sickle cell disease and the expression of adhesion molecules on white blood cells. European Journal of Haematology. 2002;69:135–144. doi: 10.1034/j.1600-0609.2002.02775.x. [DOI] [PubMed] [Google Scholar]
- Ortego M, Bustos C, Hernandez-Presa MA, Tunon J, Diaz C, Hernandez G, Egido J. Atorvastatin reduces NF-kappaB activation and chemokine expression in vascular smooth muscle cells and mononuclear cells. Atherosclerosis. 1999;147:253–261. doi: 10.1016/s0021-9150(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–320. [PubMed] [Google Scholar]
- Owusu-Ansah A, Ihunnah CA, Walker AL, Ofori-Acquah SF. Inflammatory targets of therapy in sickle cell disease. Translational Research: The Journal of Laboratory and Clinical Medicine. 2016;167:281–297. doi: 10.1016/j.trsl.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Piechota-Polanczyk A, Jozkowicz A. The role of statins in heme oxygenase-1 activation in cardiovascular diseases. Current Drug Targets. 2016 Apr 1; doi: 10.2174/1389450117666160401123600. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Qari MH, Dier U, Mousa SA. Biomarkers of inflammation, growth factor, and coagulation activation in patients with sickle cell disease. Clinical and Applied Thrombosis/Hemostasis. 2012;18:195–200. doi: 10.1177/1076029611420992. [DOI] [PubMed] [Google Scholar]
- Rees DC, Cervi P, Grimwade D, O’Driscoll A, Hamilton M, Parker NE, Porter JB. The metabolites of nitric oxide in sickle-cell disease. British Journal of Haematology. 1995;91:834–837. doi: 10.1111/j.1365-2141.1995.tb05397.x. [DOI] [PubMed] [Google Scholar]
- Rosch JW, Boyd AR, Hinojosa E, Pestina T, Hu Y, Persons DA, Orihuela CJ, Tuomanen EI. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. Journal of Clinical Investigation. 2010;120:627–635. doi: 10.1172/JCI39843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. New England Journal of Medicine. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- Saleh AW, Hillen HF, Duits AJ. Levels of endothelial, neutrophil and platelet-specific factors in sickle cell anemia patients during hydroxyurea therapy. Acta Haematologica. 1999;102:31–37. doi: 10.1159/000040964. [DOI] [PubMed] [Google Scholar]
- Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood. 2003;102:1515–1524. doi: 10.1182/blood-2002-11-3423. [DOI] [PubMed] [Google Scholar]
- Shah SH, Newby LK. C-reactive protein: a novel marker of cardiovascular risk. Cardiology in Review. 2003;11:169–179. doi: 10.1097/01.CRD.0000077906.74217.6E. [DOI] [PubMed] [Google Scholar]
- Shiu YT, Udden MM, McIntire LV. Perfusion with sickle erythrocytes up-regulates ICAM-1 and VCAM-1 gene expression in cultured human endothelial cells. Blood. 2000;95:3232–3241. [PubMed] [Google Scholar]
- Silveira AA, Dominical VM, Lazarini M, Costa FF, Conran N. Simvastatin abrogates inflamed neutrophil adhesive properties, in association with the inhibition of Mac-1 integrin expression and modulation of Rho kinase activity. Inflammation Research. 2013;62:127–132. doi: 10.1007/s00011-012-0579-7. [DOI] [PubMed] [Google Scholar]
- Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- Smith WR, Ballas SK, McCarthy WF, Bauserman RL, Swerdlow PS, Steinberg MH, Waclawiw MA Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell A. The association between hydroxyurea treatment and pain intensity, analgesic use, and utilization in ambulatory sickle cell anemia patients. Pain Medicine. 2011;12:697–705. doi: 10.1111/j.1526-4637.2011.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovey A, Kollander R, Shet A, Milbauer LC, Choong S, Panoskaltsis-Mortari A, Blazar BR, Kelm RJ, Jr, Hebbel RP. Endothelial cell expression of tissue factor in sickle mice is augmented by hypoxia/reoxygenation and inhibited by lovastatin. Blood. 2004;104:840–846. doi: 10.1182/blood-2003-10-3719. [DOI] [PubMed] [Google Scholar]
- Solovey AA, Solovey AN, Harkness J, Hebbel RP. Modulation of endothelial cell activation in sickle cell disease: a pilot study. Blood. 2001;97:1937–1941. doi: 10.1182/blood.v97.7.1937. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Psarros C, Demosthenous M, Patel R, Antoniades C, Stefanadis C. Innate and adaptive inflammation as a therapeutic target in vascular disease: the emerging role of statins. Journal of the American College of Cardiology. 2014;63:2491–2502. doi: 10.1016/j.jacc.2014.01.054. [DOI] [PubMed] [Google Scholar]
- van der Meij E, Koning GG, Vriens PW, Peeters MF, Meijer CA, Kortekaas KE, Dalman RL, van Bockel JH, Hanemaaijer R, Kooistra T, Kleemann R, Lindeman JH. A clinical evaluation of statin pleiotropy: statins selectively and dose-dependently reduce vascular inflammation. PloS One. 2013;8:e53882. doi: 10.1371/journal.pone.0053882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger E, Schoevaert D, Carrivain P, Victor JM, Lapoumeroulie C, Elion J. Prior exposure of endothelial cells to hydroxycarbamide alters the flow dynamics and adhesion of sickle red blood cells. Clinical Hemorheology and Microcirculation. 2014;57:9–22. doi: 10.3233/CH-131762. [DOI] [PubMed] [Google Scholar]
- Wang HR, Li JJ, Huang CX, Jiang H. Fluvastatin inhibits the expression of tumor necrosis factor-alpha and activation of nuclear factor-kappaB in human endothelial cells stimulated by C-reactive protein. Clinica Chimica Acta. 2005;353:53–60. doi: 10.1016/j.cccn.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, Lanzkron SM, Lottenberg R, Savage WJ, Tanabe PJ, Ware RE, Murad MH, Goldsmith JC, Ortiz E, Fulwood R, Horton A, John-Sowah J. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]