Summary
Acquired aplastic anaemia (AA) is an immune-mediated bone marrow failure disorder inextricably linked to clonal haematopoiesis. The majority of AA patients have somatic mutations and/or structural chromosomal abnormalities detected as early as at diagnosis. In contrast to other conditions linked to clonal haematopoiesis, the clonal signature of AA reflects its immune pathophysiology. The most common alterations are clonal expansions of cells lacking glycophosphotidylinositol-anchored proteins, loss of human leucocyte antigen alleles, and mutations in BCOR/BCORL1, ASXL1 and DNMT3A. Here, we present the current knowledge of clonal haematopoiesis in AA as it relates to aging, inherited bone marrow failure, and the grey-zone overlap of AA and myelodysplastic syndrome (MDS). We conclude by discussing the significance of clonal haematopoiesis both for improved diagnosis of AA, as well as for a more precise, personalized approach to prognostication of outcomes and therapy choices.
Keywords: clonal haematopoiesis, aplastic anaemia, bone marrow failure, MDS, CHIP
1. Introduction
Acquired aplastic anaemia (AA), an immune-mediated bone marrow aplasia, has long been linked to clonal haematopoietic disorders(Dameshek 1967). Within 10 years of receiving immunosuppression therapy (IST), approximately 10% of AA patients develop myelodysplastic syndrome (MDS) and 7% progress to acute myeloid leukaemia (AML)(de Planque, et al 1989, Frickhofen, et al 2003, Socie, et al 1993, Tichelli, et al 1988). Additionally, more than half of AA patients acquire detectable paroxysmal nocturnal haemoglobinuria (PNH) cell clones lacking surface glycosylphosphatidylinositol (GPI)-linked proteins due to somatic mutations in the PIGA gene(de Planque, et al 1989, Frickhofen, et al 2003, Sugimori, et al 2006, Tichelli, et al 1988). Morphological findings of patchy haematopoiesis, commonly seen in bone marrow biopsies of AA patients, have also been proposed to be a possible histopathological correlate of clonal haematopoietic recovery(Hotta, et al 1990, Huic, et al 2002)
Despite the early recognition of AA as a pre-leukaemic state(Marsh and Geary 1991, Socie 1996), it was only recently that our ability to detect clonal changes expanded beyond detection of gross cytogenetic alterations and PNH. Recent application of high-density genotyping with single nucleotide polymorphism arrays (SNP-A)(Afable, et al 2011, Babushok, et al 2014, Katagiri, et al 2011) and next-generation sequencing (NGS)(Babushok, et al 2015a, Heuser, et al 2014, Kulasekararaj, et al 2014, Lane, et al 2013, Yoshizato, et al 2015) uncovered a plethora of clonal somatic changes in the bone marrow of AA patients, making AA the non-neoplastic blood disease most singularly linked to clonal haematopoiesis.
In this review, we will discuss recent advances in our understanding of clonal haematopoiesis in AA. We will specifically focus on the characteristics of clonal haematopoiesis that directly reflect on its underlying immune pathogenesis. In doing so, we will compare clonal changes in AA to those seen in normal aging and in inherited bone marrow failure and will review the evidence for the increasingly difficult-to-define boundaries between AA and MDS. We will conclude by critically appraising the data for significance of acquired genetic changes in AA as they relate to the diagnosis of AA, AA-directed therapy and the patients’ overall prognosis.
2. Forces That Drive Clonal Haematopoiesis in Aplastic Anaemia
To understand clonal haematopoiesis in AA, it is important to consider the underlying currents that influence haematopoietic stem and progenitor cell (HSPC) populations over the course of AA. Under normal circumstances, humans have approximately 11,000 haematopoietic stem cells (HSCs), up to 30% of which are estimated to actively contribute to haematopoiesis, with steady-state haematopoiesis primarily maintained by long-lived multipotent progenitors and short-term (ST) HSCs (Abkowitz, et al 2002, Busch, et al 2015, Catlin, et al 2011). In AA, immune-mediated depletion of haematopoiesis-maintaining cells, probably multipotent progenitors and ST-HSCs, leads to aplasia and cytokine-driven marrow suppression (Busch, et al 2015, Catlin, et al 2011, Smith, et al 2016). It is not known whether long-term HSCs are also directly targeted by immune-mediated destruction, or whether HSC loss occurs through attrition and differentiation.
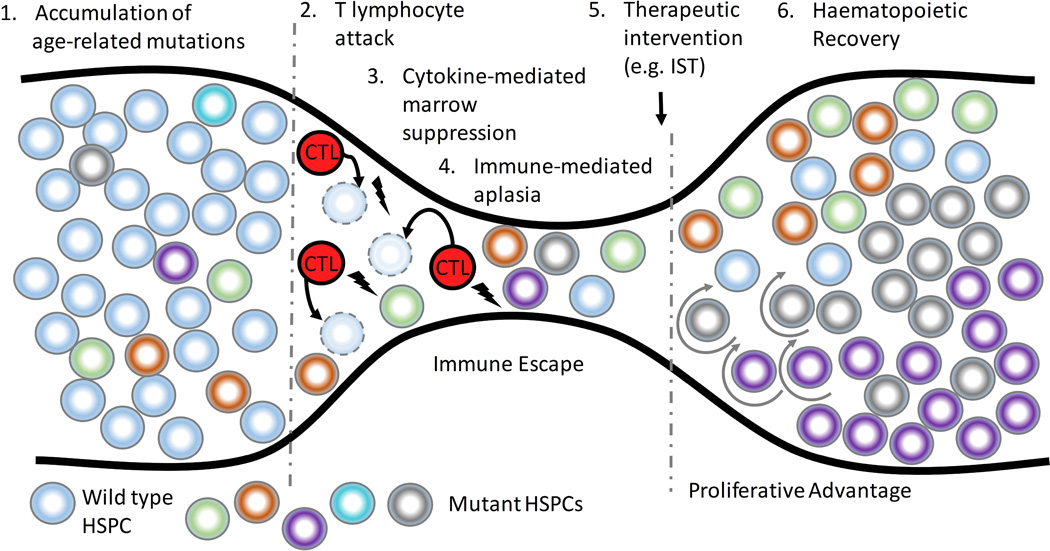
Pre-existing genetic mutations acquired as a part of normal aging can become a substrate for clonal selection at the time of haematopoietic stress, serving as an evolutionary “bottleneck” (Figure 1). Thus, in AA, HSPC-directed autoimmunity can lead to selective growth advantage for a cell that, through acquisition of somatic alterations, becomes either less immunogenic or acquires relative resistance to cytotoxicity or cytokine-mediated marrow suppression. Additionally, alterations that increase the replicative potential or promote differentiation down the myeloid lineage could lead to clonal expansion within the myeloid compartment during AA haematopoietic recovery (Figure 1).
Figure 1. Clonal Haematopoiesis in Aplastic anaemia.
In Aplastic anaemia (AA), cytotoxic T lymphocyte (CTL)-mediated attack on the haematopoietic stem and progenitor cells (HSPCs) leads to an evolutionary “bottleneck”. Pre-existing age-related genetic mutations (1), depicted as circles of different colors, serve as a substrate for clonal selection. Cells that are either less immunogenic or more resistant to CTL-mediated apoptosis (2) or cytokine-mediated marrow suppression (3) have a relative growth advantage in the setting of autoimmunity, leading to immune escape of mutant HSPCs. During haematopoietic recovery (6), genetic events that increase HSPC replicative potential (depicted by circular arrows) lead to expansion of mutant clones. IST, immunosuppressive therapy.
3. Markers of Clonal Haematopoiesis in AA
Our current understanding of clonal haematopoiesis in AA is based on a composite of data from a variety of studies evaluating different markers of clonality in AA: cytogenetic abnormalities, clonal copy number-neutral loss of heterozygosity (CN-LOH), somatic mutations, and X chromosome inactivation bias.
3.1 Structural Genomic Variants: Recurrent Cytogenetic Abnormalities and CN-LOH
Studies of structural genomic variation, with metaphase karyotyping and, more recently, single-nucleotide polymorphism arrays (SNP-A), have identified acquired cytogenetic abnormalities in up to a quarter of patients with otherwise unremarkable AA (Tables I and II). The most common cytogenetic alterations include −7/del(7q) and trisomy 8, with other recurrent abnormalities including del(13q), trisomy 6, trisomy 15 and trisomy 21. Other chromosomal abnormalities common to myeloid neoplasms, such as del(5q) and del(20q), occur less frequently in AA (Table I)(Gupta, et al 2006, Maciejewski, et al 2002, Maciejewski and Selleri 2004, Ohga, et al 2002). Notably, approximately ~1–4% (range 0–23%) of patients with newly diagnosed AA have clonal cytogenetic abnormalities detected at the time of diagnosis(Maciejewski, et al 2002); this may be an underestimate because of the not-infrequent failure of karyotyping due to poor cell growth. The rarity of AA and of cytogenetic abnormalities within the AA population have limited the ability to perform prospective studies of specific cytogenetic abnormalities in AA. However, retrospective analyses of cytogenetic changes in AA led to several important observations. Similar to its adverse prognosis in myeloid malignancies, −7/del(7q) was found to confer worse outcomes in AA, with a lower response to immunosuppression and an increased mortality and greater progression to MDS(Maciejewski, et al 2002). In contrast, trisomy 8 and deletion 13q, particularly when detected in the presence of a small PNH clone, were found to be associated with an improved response to immunosuppression and a better prognosis(Holbro, et al 2013, Hosokawa, et al 2012, Hosokawa, et al 2015, Ishiyama, et al 2002, Maciejewski, et al 2002). Additionally, there are documented cases of cytogenetic abnormalities, including cases with monosomy 7, which remit with haematopoietic recovery(Gupta, et al 2006, Maciejewski, et al 2002, Mikhailova, et al 1996).
Table I.
Recurrent Cytogenetic Abnormalities in AA
| Study | AA Patients, n |
Age, years; median (range) |
AC, n (%) | AC at Dx, n (%) |
Recurrent Abnormalities (n)** |
|---|---|---|---|---|---|
| Appelbaum et al (1987) | 176 | 24 (4–51) | 7 (4.0%) | n/a | −7 (3), +8 (2), +6 (2), +21, del (5q) |
| de Planque et al (1988) | 38 | 47 (22–72) AC | 5 (13.2%) | n/a | −7 (3) |
| Mikhailova et al (1996) § | 69 | 30 (7–70) | 18 (26.1%) | 3 (4.3%) | + 8 (8), −7 (2), del(5q) |
| Jameel et al (1997) | 31 | 20 (7–55) | 7 (22.6%) | 7 (22.6%) | +8 (4) |
| Kaito et al (1998) | 72 | 39 (17–79) | 5 (6.9%) | n/a | −7 (4), +8, +21 |
| Fuhrer et al (1998) | 114 | 9.5 (0.9 – 15.8) | 6 (5.3%) | 2 (1.8%) | −7 (2), del(5q), +8, +21 |
| Piaggio et al (1999) § | 97 | 26 (7–70) | 13 (13.4%) | 0 (0%) | +8 (4), del(7q), +15 |
| Bacigalupo et al (2000) § | 54 | 16 (1–72) | 7 (13.0%) | 1 (1.9%) | −7 (2), del(5q) |
| Ohga et al (2002) § | 159 | 11 (5–14) AC | 7 (4.4%) | 7 (4.4%) | del(5q), −7, del(13q), del(20q) |
| Maciejewski et al (2002) | 189 | 48 (8–77) | 30 (15.9%) | n/a | −7 (12), +8 (7), del(13q) (4), del(5q), +6, del(20q), +21 |
| Kojima et al (2002) | 113 | 9 (1–18) | 16 (14.2%) | 0 (0%) | −7 (7), +8 (3), +21, del(13q) |
| Tisdale et al (2002) | 31 | n/a | 3 (9.7%) | 0 (0%) | +8 (2), del(20q) |
| Rosenfeld et al (2003) | 122 | 35 (n/a) | 13 (10.7%) | 0 (0%) | −7 (9), +8 (2) |
| Gupta et al (2006) | 81 |
*33 (5–73) NC *40.5 (31–64) AC |
10 (12.3%) | 10 (12.3%) | +15 (2), +8 (2), +6 (2), −7, 20q- |
| Tripathi et al (2008) | 50 | 22 (13–71) | 7 (14.0%) | 2 (4.0%) | +8 (3), +6 (2), −7, +21 |
| Kim et al (2010) | 600 | 67 (17–87) | 28 (4.7%) | 28 (4.7%) | +8 (15), −7/del(7q) (5), +15 |
| Lee et al (2010) | 127 | 53 (25–70) | 12 (9.4%) | 6 (4.7%) | +8 (2), −7 |
| Li et al (2011) | 802 | 23 (3–74) | 42 (5.2%) | 10 (1.2%) | −7 (11), +8 (4), +21 (2), del(20q) |
| Holbro et al (2012) ǂ | 86 | 9 (n/a) | 6 (7.0%) | 4 (4.7%) | del(13q) (6) |
| Kulasekararaj et al (2014) | 131 | 44 (17–84) | 13 (9.9%) | n/a | −7 (3), +8 (3), del(13q), +15 |
| Huang et al (2015) | 138 | 30.7 (5–76) | 8 (5.8%) | n/a | +8 (3), −7(2), del(13q) (2) |
| Yoshizato et al (2015) | 417 | n/a (2–88) | 18 (4.3%) | n/a | −7 (7), del(13q) (2), +8 |
| Hosokawa et al (2015) ǂ | 754 | *58 (10–87) AC | 22 (2.9%) | n/a | +8 (22) |
Abbreviations: AA = Aplastic Anaemia, NC = Normal Cytogenetics, AC= Abnormal Cytogenetics, Dx= Diagnosis, n/a = Not available
Age at diagnosis,
Reports cytogenetic remission,
Focused solely on the presented abnormality,
isted for n>1.
Abnormalities depicted in color have a known prognostic correlation in AA: Blue, associated with response to immunosuppression and connotes a neutral or good prognosis in AA; Red, associated with poor survival and myelodysplastic syndrome transformation (see text). Referenced studies are listed in Supplemental Information.
Table II.
Acquired CN-LOH in AA
| Study | AA Patients, n |
Age, years; median (range) |
Patients with acquired CN-LOH, n (%) |
CN-LOH at Dx, n (%) |
6p CN-LOH, n (%) |
CN-LOH Regions other than 6p |
|---|---|---|---|---|---|---|
| Afable et al (2011) | 93 | 42 (5–80) | 7 (7.6%) | 0 of 33 (0%) | 3 (3.2%) | 1p, 3q, 11q, 17q, 22q |
| Katagiri et al (2011) | 306 | Newly diagnosed: 64 (9–88) Previously diagnosed: 24 (2–80) |
46 (15.0%) | 16 of 107 (15%) |
40 (13.1%) | 1q, 2p, 9q, 10q, 11p, 19q, 20p |
| Babushok et al (2013) | 31 | n/a (<1–61) | 6 (19.4%) | n/a | 4 (12.9%) | 5q, 6q, 15q |
| Kulasekararaj et al (2014) | 43 | 44 (17–84)* | 2 (4.7%) | n/a | 1 (2.3%) | 15q, 18q |
| Yoshizato et al (2015) ǂ | 417 | n/a (2–88)* | n/a | n/a | 55 (13.2%) | n/a |
| Betensky et al (2015) ǂ | 67 | n/a (0.5–67)* | n/a | n/a | 8 (11.9%) | n/a |
Abbreviations: AA= Aplastic Anaemia, CN-LOH= Copy number-neutral loss of heterozygosity, Dx= Diagnosis, n/a = Not available
Focused solely on 6p CN-LOH,
Evidence of clone disappearance,
Age data from entire patient population
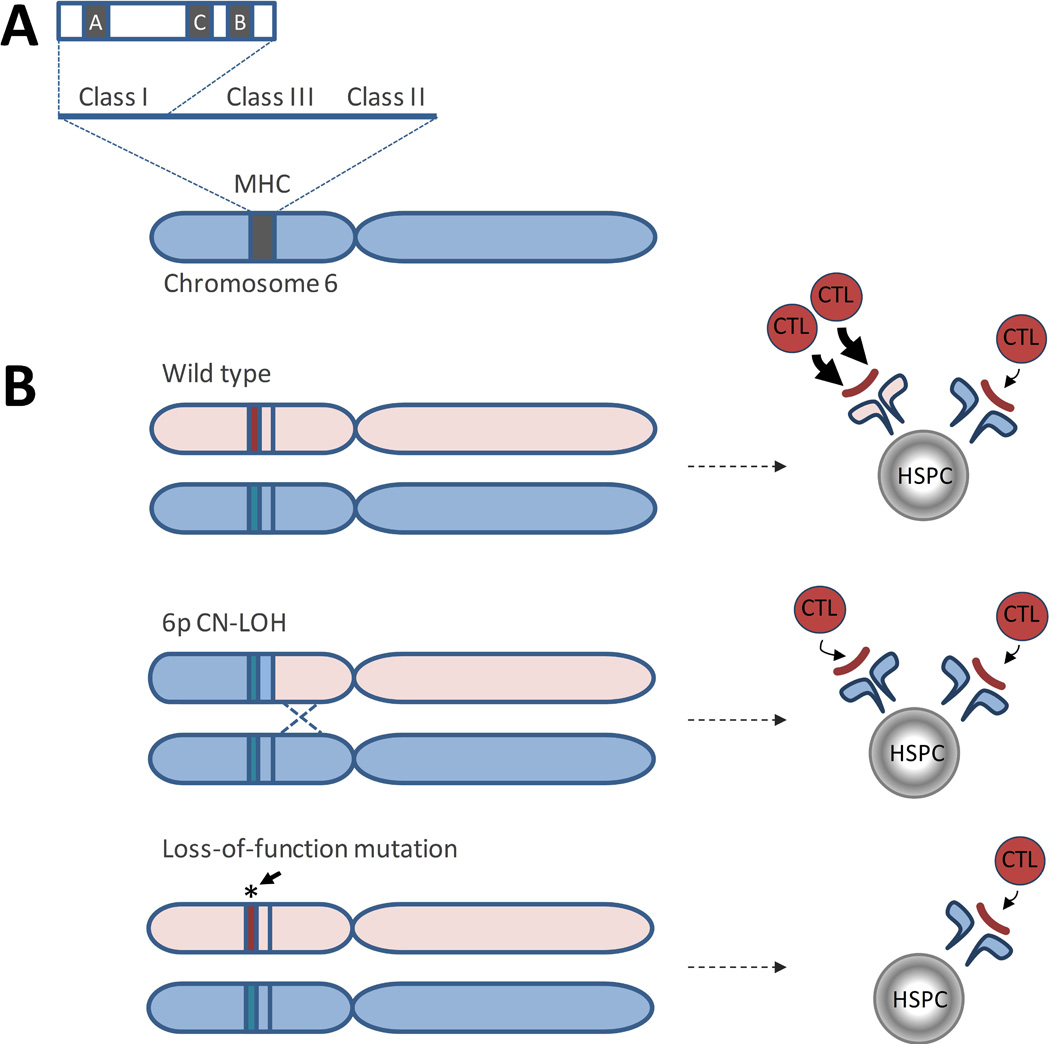
The recent advent of SNP-A genotyping and its use in clinical cytogenetic testing for patients with cancer and bone marrow failure has enabled detection of another acquired structural genetic abnormality, copy number neutral loss of heterozygosity (CN-LOH), that is not detected by conventional cytogenetic approaches. Using SNP-A genotyping, AA patients were found to have regions of acquired CN-LOH at a significantly higher frequency than patients with other forms of bone marrow failure(Babushok, et al 2014). The region most commonly involved by acquired CN-LOH in AA is the short arm of chromosome 6, at the site of the major histocompatibility complex (MHC) locus (Figure 2A), detected in ~11–13% of patients(Afable, et al 2011, Babushok, et al 2014, Betensky, et al 2016, Katagiri, et al 2011) (Table II). Importantly, acquired 6p CN-LOH is characteristic and relatively specific to AA, as compared to MDS, where it occurs at only ~1% frequency(Mohamedali, et al 2015). Additionally, 6p CN-LOH is exceedingly rare in the general population, where its prevalence is estimated at ~ 0.09% (1 of 1153)(Score, et al 2015). 6p CN-LOH appears to favour the loss of specific human leucocyte antigen (HLA) alleles, including HLA-A*02:01, -A*02:06, -A*31:01, -B*40:02 and -B*14:02, suggesting specificity to the underlying immune attack in AA(Betensky, et al 2016, Katagiri, et al 2011). Larger, prospective studies are needed to more fully evaluate the prognostic significance of 6p CN-LOH, as data thus far have been mixed (see further discussion of clinical significance below)(Betensky, et al 2016, Katagiri, et al 2011).
Figure 2. Loss of Class I Human Leucocyte Antigen (HLA) Alleles in Aplastic anaemia.
A) A diagram of the Major Histocompatibility Locus (MHC), located on the short arm of chromosome 6. B) A schematic showing two mechanisms of HLA class I allele loss in AA. In patients whose HLA alleles allow for enhanced cytotoxic T cell (CTL) immune recognition due to an immunogenic features of a particular HLA class I allele, HSPCs that undergo a somatic loss of that allele have a selective advantage compared to wild type cells. Top panel, haematopoietic stem and progenitor cell (HSPC) containing wild type HLA alleles, with differential immunogenicity (shown by arrows of different width) of an autoantigen (red line) presented in the context of two different HLA class I alleles. Middle panel, HLA allele loss due to an acquired 6p copy number-neutral loss of heterozygosity (CN-LOH) results in lessened immune recognition. Lower panel: HLA allele loss due to loss-of-function somatic mutations in HLA class I alleles can lead to decreased immune recognition by abolishing expression of the more immunogenic HLA allele.
3.2 Somatic Mutations in AA
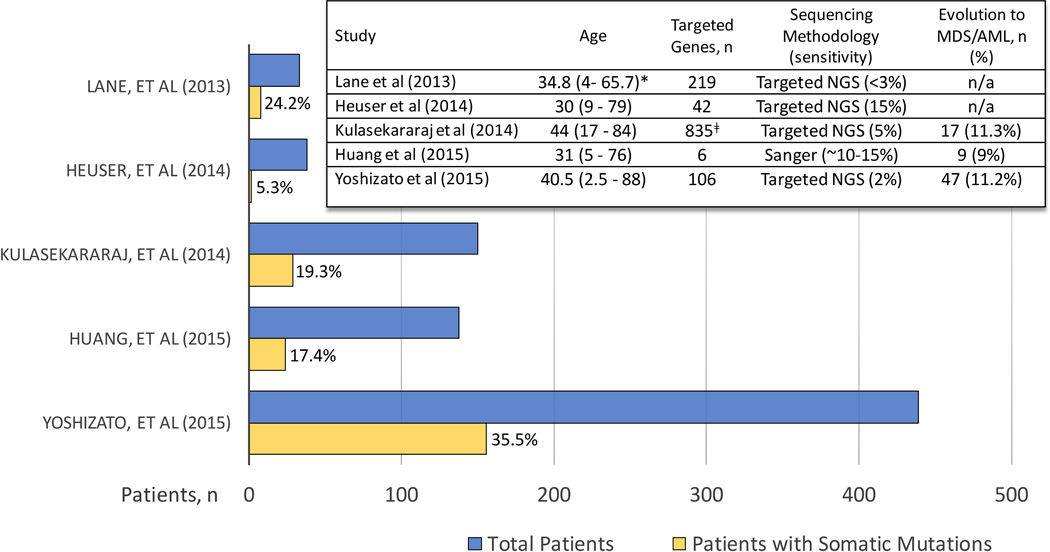
Several groups have used targeted sequencing of genes recurrently mutated in haematological malignancies to identify emergence of clonal, haematopoietic-specific mutations in MDS-associated genes in 5–36% of AA patients (Figure 3) (Heuser, et al 2014, Huang, et al 2015, Kulasekararaj, et al 2014, Lane, et al 2013, Yoshizato, et al 2015). The wide variation in mutation frequencies among studies reflects the differences in study methodologies, including compositions of targeted gene panels, detection sensitivities, as well as significant differences in study populations, both with respect to age and in inclusion of patients with post-AA MDS. Despite the methodological differences, studies have consistently identified recurrent somatic mutations in AA falling into a small cluster of genes: PIGA, ASXL1, BCOR, and DNMT3A, while other genes were mutated less frequently(Kulasekararaj, et al 2014, Lane, et al 2013, Yoshizato, et al 2015). In many cases, these mutations were present at diagnosis, albeit at a small allele burden, and expanded over time(Yoshizato, et al 2015). Compared to clones associated with MDS, mutant clones in AA were smaller, and frequently were stable and compatible with stable blood counts for a long period of time, even in cases that eventually progressed to MDS/AML(Yoshizato, et al 2015). Mutations could be found in multiple haematopoietic lineages, suggesting involvement of a multipotent primitive haematopoietic progenitor or an HSC(Lane, et al 2013, Yoshizato, et al 2015). Although additional studies in paediatric patients are needed, preliminary data from the National Institutes of Health (NIH), Japan and Philadelphia cohorts suggests that children and young adults may be less likely to carry somatic mutations in MDS-associated genes(Babushok, et al 2015b, Yoshizato, et al 2015, Young and Ogawa 2015).
Figure 3. Targeted Sequencing Studies of MDS-associated Genes in AA.
A histogram summarizing targeted sequencing studies of patients with aplastic anaemia (AA). Individual studies are listed along the Y-axis, with the number of patients included in each study plotted along the X-axis. The blue bar represents the total number of patients in the study; the yellow bar shows the number of patients with identified somatic mutations, with corresponding percentage of patients with mutations stated to the right of each yellow bar. An embedded table depicts salient characteristics of each study. Age, median age (range) at diagnosis in years). Sensitivity, minimal % mutant allele frequency detected in each study. n/a: not available.*, the listed age corresponds to a larger patient cohort; ǂ, Cohort of 57 patients was screened for 835 genes; with a validation cohort of 93 patients screened for a mutations in a smaller subset of genes. AML, acute myeloid leukaemia; MDS, myelodysplastic syndrome; NGS, next generation sequencing.
To comprehensively evaluate the landscape of somatic mutations in AA, two studies used comparative whole exome sequencing (WES) of the patients’ bone marrow or blood DNA compared to the paired germline DNA control. Both studies identified a high frequency of somatic mutations in genes not previously linked to myeloid neoplasms(Babushok, et al 2015a, Yoshizato, et al 2015). Only a small number of genes were involved multiple times, with the majority interpreted to be passenger mutations in genes of larger size (Yoshizato, et al 2015), with a notable exception of inactivating mutations in the HLA class I genes (Babushok, et al 2015a). Two patients were found to have oligoclonal loss of specific HLA alleles—HLA-A*33:03 and HLA-B*14:02 through two independent mechanisms in the same patients—6p CN-LOH and mutational inactivation (Babushok, et al 2015a) (Figure 3B). While the immune selection against specific HLA alleles was previously proposed to be a mechanism for emergence of 6p CN-LOH (Katagiri, et al 2011), strong linkage disequilibrium and the numerous other genes present within the MHC locus made it difficult to exclude other genes in the region (e.g. TNF-α and complement proteins). Thus, finding recurrent somatic mutations causing HLA class I loss provides evidence of immune specificity in AA, and underscores that immune escape is a major mechanism of AA clonal haematopoiesis.
3.3 In AA, Clonal Haematopoiesis is a Rule Rather Than an Exception
Putting together the results of cytogenetics, SNP-A and WES/NGS, clonal haematopoiesis has been detected in the vast majority (~70–85%) of all AA patients, ranging from ~60% in childhood-onset AA to almost 100% in adults (Babushok, et al 2015a, Yoshizato, et al 2015). These numbers are significantly higher than previously estimated by historical studies that used skewed X-chromosome inactivation (XCI) as a marker of clonality. The lower estimates in the XCI studies can be largely explained by the lower sensitivity of XCI that require at least 35% of the studied population to be involved in the clone to ensure detection (Ishiyama, et al 2003); co-occurrence of multiple clones with potentially disparate XCI in each clone may also confound the analysis.
4. Blurring the Line Between AA and MDS: AA/MDS Overlap
The recent discovery that clonal haematopoiesis develops in most cases of AA has brought to the forefront the long-acknowledged difficulty in distinguishing between hypoplastic MDS (hMDS) and AA. The diagnosis of MDS, based on the 2008 World Health Organization Classification Criteria, requires morphological dysplasia in 10% of one or more myeloid lineages (Vardiman, et al 2009), while, at the same time, allowing for a presumptive diagnosis of MDS in the absence of morphological dysplasia when certain cytogenetic abnormalities are identified. Although the majority of patients with MDS have a hypercellular or normocellular marrow, up to 20% of MDS patients may have a cellularity under 30%, a condition referred to as hMDS(Geary, et al 1996, Maschek, et al 1993, Tuzuner, et al 1995). Because the pathological criteria used to define dysplasia are difficult to apply to severely hypocellular marrow with only few cells to assess, distinguishing hMDS from AA on morphological grounds is often impossible. In such cases, detection of a clonal marker, whether a chromosomal abnormality or molecular markers of clonal haematopoiesis, has been traditionally used to support a diagnosis of hMDS (Gondek and DeZern 2014, Tuzuner, et al 1995). However, given the recent finding of high prevalence of clonality in AA, with the spectrum of genetic changes overlapping that of MDS, the presence of clonality cannot be used to exclude AA and extreme caution must be used in interpreting somatic mutations and chromosomal abnormalities to make the MDS diagnosis.
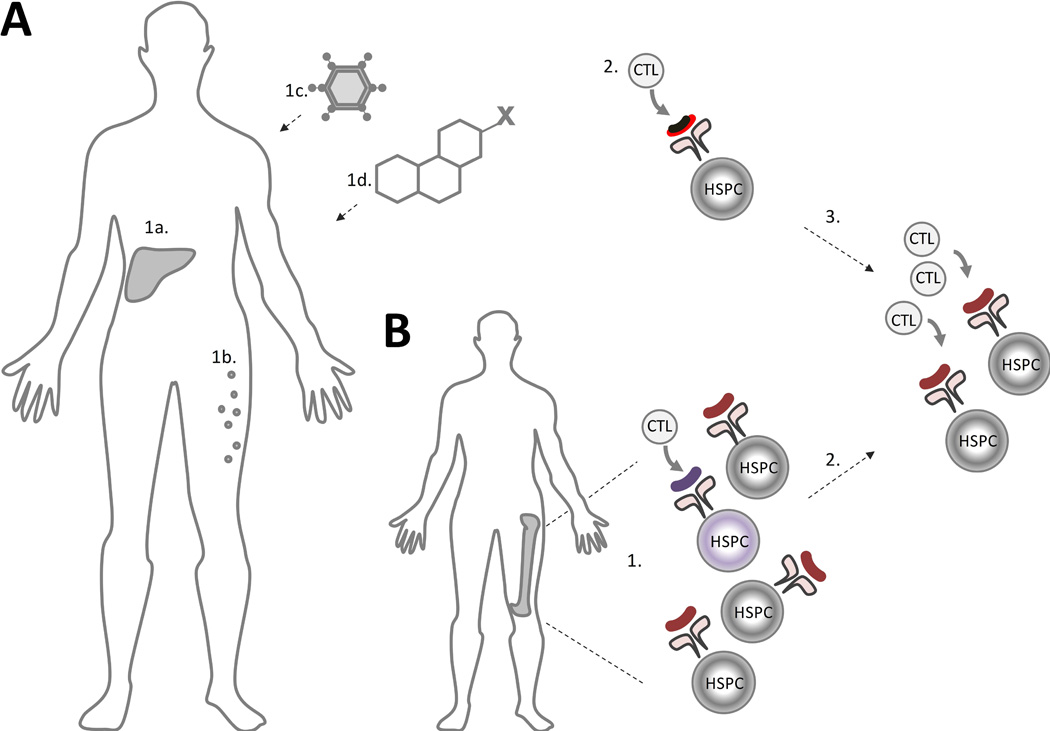
Notably, a subset of patients with AA, primarily in the elderly, can be better characterized as an overlap syndrome of AA and hMDS. Unlike the traditional model of AA, where a T-cell mediated immune response is directed at the healthy HSPCs, patients with the AA/hMDS overlap develop immune responses directed at the underlying MDS clone, which is associated with “bystander” autoimmune aplasia (Figure 4). This pathophysiological link between MDS and autoimmune aplasia is supported by several clinical observations. First, both syndromes share age-related epidemiological characteristics: AA has a well-described bimodal distribution, with the second and major peak in the elderly coincident with the rise in age-related clonal haematopoiesis and MDS (International Agranulocytosis and Aplastic Anemia Study 1987). Second, patients with both AA and MDS can respond to immunosuppressive therapy, with ~30% of patients with hMDS showing a haematological improvement with immunosuppression, including the loss of the predominant clonal T cell populations in responding patients (Kochenderfer, et al 2002, Sloand, et al 2008). One of the better examples of the role of immune system in MDS are studies of patients with MDS and trisomy 8, where Sloand and colleagues demonstrated that trisomy 8 cells are more susceptible to lymphocyte-mediated apoptosis, and have a preferential expansion in culture with T-cell depletion or cyclosporine therapy (Sloand, et al 2002, Sloand, et al 2005). Third, in the same vein, there are well described cases of MDS developing in AA patients within months of immunosuppression, suggesting that immune surveillance may be suppressing the malignant clone, which can be unmasked by immunosuppressive therapy (Nazha, et al 2015). These, together with the many commonalities in clonal haematopoiesis seen in AA and MDS, including detectable PNH cells in ~15% of MDS patients (Kaiafa, et al 2008, Wang, et al 2002, Wang, et al 2009), suggest that a subset of patients may have co-existing hMDS and secondary immune-mediated aplasia, a syndrome which can be thought of as AA/hMDS overlap.
Figure 4. Model of Autoimmune Pathogenesis of AA and of the AA/MDS Overlap Syndrome.
A) In aplastic anaemia (AA), an immune trigger, such as type-negative hepatitis (1a), eosinophilic fasciitis (1b), or an exposure to an infectious agent (1c), drug or toxin (1d) leads to immune recognition of a neoantigen or an aberrantly expressed autoantigen (black and red line) presented in the context of a patient’s human leucocyte antigen (HLA) molecules (2). (3) Immune-mediated bone marrow aplasia is caused by subsequent clonal expansion of cytotoxic T lymphocytes (CTL) with autoreactivity against the normal haematopoietic stem and progenitor cells (HSPCs) due to epitope spread. B) In patients with coexisting AA and myelodysplastic syndrome (MDS), pre-existing MDS-related mutations may lead to presentation of an aberrantly-expressed or somatically modified neoantigen in the MDS HSPC (1) (shown in purple). Unlike the traditional model of AA, where a T-cell mediated immune response is directed at the healthy HSPCs, patients with the overlap AA/MDS have the immune response triggered by an underlying MDS clone with immune-mediated aplasia to the “bystander” wild type HSPCs (2).
5. Differences in Clonal Haematopoiesis of Inherited Bone Marrow Failure Syndromes (IBMFS) and AA
While AA and hMDS are frequently considered in adults presenting with pancytopenia and hypocellular bone marrow, there is a growing awareness that inherited marrow failure can present similarly and should be included in the differential diagnosis in children and adults presenting with apparent AA and MDS (Babushok, et al 2016). A recent study evaluated the frequency of undiagnosed IBMFS in 98 patients aged ≤ 40 years transplanted for AA at Fred Hutchinson Cancer Research Center; germline mutations were identified in 5.1% of AA patients, most of whom did not have a suggestive family history or syndromic stigmata (Keel, et al 2016). The 5% prevalence of germline mutations in this cohort of AA patients is in general agreement with historical studies, which identified mutations in TERT and TERC genes in 3.4% (7/205) and ~1.4% (3/210) of AA patients, respectively (Fogarty, et al 2003, Yamaguchi, et al 2003, Yamaguchi, et al 2005). Additional syndromes, probably underdiagnosed in adults, include germline GATA2 haploinsufficiency and Fanconi Anaemia (FA), both of which have been reported at as high as a 12% and 8% frequency in selected AA and bone marrow failure cohorts(Ganapathi, et al 2015, Pinto, et al 2009). The true prevalence of occult IBMFS may actually be higher than currently appreciated, given the incomplete knowledge of and limited screening for IBMFS in the adult population. Thus, undiagnosed IBMFS may account for a subset of AA patients who fail to respond to immunosuppressive therapy.
Similar to AA, IBMFS is associated with clonal haematopoiesis, but has a significantly greater risk of transformation to MDS and AML (Abkowitz, et al 1984, Alter, et al 2010). Among the IBMFS most likely to mimic AA because of their late presentation or lack of overt extrahaematopoietic manifestations [e.g. FA, Dyskeratosis Congenita (DC), and GATA2 haploinsufficiency], all have high rates of clonal haematopoiesis with cytogenetic abnormalities and somatic mutations (Jongmans, et al 2012, Perdigones, et al 2016, Quentin, et al 2011, Soulier, et al 2005, Steele, et al 2006, Venkatraj, et al 1994, West, et al 2014, Wlodarski, et al 2016). Importantly, the differences in the underlying selective pressure in AA and IBMFS lead to distinct characteristics of the acquired genetic aberrations seen in these disorders. In AA, clonal haematopoiesis appears to be driven largely by immune escape, with the two most common abnormalities being PNH and 6p CN-LOH(Katagiri, et al 2011, Kulasekararaj, et al 2014, Yoshizato, et al 2015). In contrast, both PNH and 6p CN-LOH are exceedingly rare or absent in IBMFS (Babushok, et al 2014, DeZern, et al 2014, Oo 2014, Quentin, et al 2011). Conversely, in IBMFS, clonal haematopoiesis frequently occurs due to events that rescue the underlying inherited defect. For example, up to 15% of FA patients undergo somatic reversion in their haematopoietic compartment (Soulier, et al 2005). In DC, reversion of the underlying pathogenic mutations in TERC and DKC1 was recently identified as a driver of clonal haematopoiesis (Jongmans, et al 2012, Perdigones, et al 2016). Similarly, recurrent genetic abnormalities involving the site of the SBDS gene, i(7)(q10) or 7q CN-LOH lead to improved haematopoiesis in compound heterozygous patients with Shwachman Diamond Syndrome (SDS). This is caused by duplication of the hypomorphic mutant SBDS allele allowing for the production of some normal SBDS protein, thus increasing the mutant HSC fitness (Minelli, et al 2009, Parikh, et al 2012). It is likely that other recurrent abnormalities in IBMFS (e.g. trisomy 1q and trisomy 3q in FA, monosomy 7 in GATA2 haploinsufficiency, and del(20q) in SDS) in some way alleviate the underlying defect in these disorders; however, the mechanism for emergence of these abnormalities still remains poorly understood.
6. Age-Related Clonal Haematopoiesis Forms a Substrate for Immune Selection in AA
Healthy individuals are now known to accumulate structural genomic rearrangements and somatic mutations as a part of normal aging. Two pivotal studies used SNP-A analysis, each in over 50,000 individuals from a variety of genome-wide association cohorts, to find that although individuals under the age of 50 years had <0.5% frequency of chromosomal rearrangements, the frequency of chromosomal rearrangements reached 2–3% in the elderly(Jacobs, et al 2012, Laurie, et al 2012). Subsequently, three large whole exome sequencing studies of 2,728 control blood specimens from the Cancer Genome Atlas (TCGA)(Xie, et al 2014), 17,182 blood specimens from population-based cohorts of type 2 diabetes and heart disease (Jaiswal, et al 2014), and 12,380 blood samples from case-control studies of schizophrenia and bipolar disorder (Genovese, et al 2014) demonstrated an age-related increase in somatic mutations, with mutations found in ~10% of patients aged over 65 years. Most commonly, somatic mutations disrupted key cellular epigenetic modifiers, DNMT3A, TET2 and ASXL1, whose loss has been linked to expansion of HSCs through impaired differentiation(Challen, et al 2012), increased self-renewal(Moran-Crusio, et al 2011), or, in the case of ASXL1, to increased proliferation of haematopoietic progenitors(Wu, et al 2015). The authors found that the presence of clonal haematopoiesis was associated with an ~11- to 12-fold higher risk of developing haematological malignancies.
Importantly, subsequent studies of clonal haematopoiesis in healthy individuals highlighted new facets of age-related clonality, raising new questions as to its true prevalence, prognostic implications, and the role of the bone marrow environment in shaping clonal evolution. To evaluate the true prevalence of age-related clonal haematopoiesis, Young et al (2016) employed a novel technique of error-corrected NGS to push the limits of sensitivity in detecting somatic mutations in twenty 50- to 60-year-old women from the Nurse’s Health Study. The authors found mutations in 19 out of 20 women (95%), far more commonly than the ~10% previously reported for this age group using less sensitive NGS methodology. The mutations had a very low median allele frequency of 0.0024, with 64% of mutations occurring in the DNMT3A and TET2 genes. Approximately a quarter of the mutations were persistent at two time points a decade apart, while 14% and 59% were detected at only the first or the second sampling, respectively. This study brings to light the nearly ubiquitous, yet clinically silent clonal haematopoiesis in middle-aged adults, which contrasts with the much lower prevalence of myeloid malignancies in the population.
Focusing on the differences in mutational spectrum as a function of age, the study of 3,067 blood donors and 1,152 unselected elderly from the United Kingdom Household Longitudinal Study highlighted a role of age-related changes in the marrow environment in shaping the HSC clonal make-up (McKerrell, et al 2015). The authors observed that in addition to the expected age-related increase in mutation frequency, there were age-related differences in the types of observed mutations, with spliceosome gene mutations detected exclusively in those over 70 years of age. Such age-related differences in mutational profiles indicate that varied selective pressures within the bone marrow environment can change the clonal composition over an individual’s lifetime.
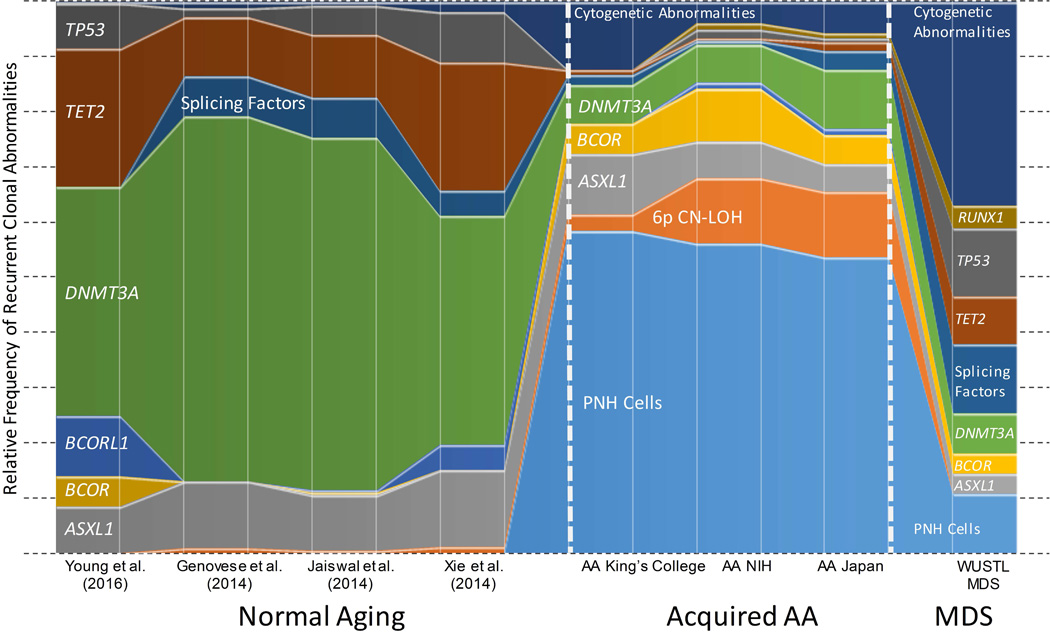
Thus, in AA, pre-existing age-related somatic mutations serve as a substrate that is then shaped by autoimmune selection and haematopoietic stress to create the unique clonal signature of AA (Figure 5). Although age-related somatic mutations are frequently detected in adult AA patients, they form a backdrop to the two most common abnormalities in all age groups: mutations in PIGA and the loss of HLA class I alleles(Kulasekararaj, et al 2014, Yoshizato, et al 2015) (Figure 5). These two prototypical markers of immune escape in AA(Gargiulo, et al 2013, Inaguma, et al 2016, Katagiri, et al 2011, Murakami, et al 2002) are closely followed by mutations in ASXL1 and BCOR/BCORL1(Kulasekararaj, et al 2014, Yoshizato, et al 2015), which are significantly over-represented in AA compared to their relative prevalence in similarly aged individuals, outcompeting the two most commonly mutated genes, DNMT3A and TET2 (Figure 5). Other age-related mutations appear to grossly track with the patients’ age, and probably represent expansion of pre-existing age-related clones. An example of such age-related differences in AA is illustrated by the differences in the mutation spectra in the two main AA cohorts in the study by Yoshizato and colleagues, where the older Japanese cohort (median age 60 years) had a significantly higher prevalence of mutations in DNMT3A(Young and Ogawa 2015), as well as qualitatively higher prevalence in mutant TET2 and spliceosome factors, as compared to the younger USA cohort (NIH cohort median age 29.5)(Yoshizato, et al 2015). Both cohorts had a significantly higher proportion of age-related mutations as compared to the primarily paediatric study published by our group (median age 18.5 (range 2–61) as well as the paediatric subset of patients within the cohort of Yoshizato and colleagues, where the recurrent age-related mutations were mainly found in ASXL1 and BCOR (Babushok, et al 2015a, Yoshizato, et al 2015). It is likely that mutations in the repressive epigenetic modifier genes BCOR, BCORL1, and ASXL1 confer a selective advantage specific to the AA bone marrow environment. Possible mechanisms could involve enhancing progenitor proliferative capacity (Cao, et al 2016, Wu, et al 2015) or reducing susceptibility to the T cell-mediated autoimmune destruction and/or cytokine marrow suppression; however, specific mechanisms have not been defined.
Figure 5. Clonal Signature of AA Compared to MDS and Normal Aging.
A schematic diagram comparing the relative frequencies of the most common clonal abnormalities found healthy individuals(Genovese, et al 2014, Jaiswal, et al 2014, Xie, et al 2014, Young, et al 2016), in three aplastic anaemia (AA)cohorts--King’s College (Kulasekararaj, et al 2014), National Institutes of Health (NIH) and Japan cohorts(Yoshizato, et al 2015), and in a myelodysplastic syndrome (MDS) cohort (Washington University in St. Louis, WUSTL) (Graubert, et al 2012, Walter, et al 2013). The prevalence of PNH cells in the healthy individuals and patients with MDS is depicted based on the published high-sensitivity PNH flow cytometry data in these populations (Kaiafa, et al 2008, Sutherland, et al 2012). The frequencies of cytogenetic abnormalities for healthy individuals are shown based on population-based studies of mosaic copy number abnormalities (Jacobs, et al 2012, Laurie, et al 2012). The prevalence of 6p copy number-neutral loss of heterozygosity (CN-LOH) in healthy individuals and in MDS is depicted based on a selection of studies (Jacobs, et al 2012, Laurie, et al 2012, Mohamedali, et al 2015).
7. Clinical Significance of Clonal Haematopoiesis in AA
The growing understanding of distinct features of clonal haematopoiesis in AA, as compared to normal aging, IBMFS and MDS, holds promise for improved patient care through more precise diagnosis and an improved ability to predict response to immunosuppression and the risk of progression to MDS/AML.
7.1 Using Clonal Haematopoiesis to Improve Diagnostic Precision in AA
Because clonal abnormalities reflect the underlying selective pressure on HSC, finding clonal changes characteristic of a particular pathogenic entity can be of diagnostic value. This is particularly applicable to AA, where the diagnosis relies on the exclusion of mimicking disorders. In this setting, detection of clonal changes reflective of immune escape, such as a small PNH clone (DeZern, et al 2014, Oo 2014), or loss of HLA alleles through 6p CN-LOH or somatic inactivation (Afable, et al 2011, Babushok, et al 2014, Betensky, et al 2016, Katagiri, et al 2011), can suggest the immune pathogenesis and support the diagnosis of AA.
Conversely, cytogenetic abnormalities that are recurrent in AA (e.g. trisomy 8, del(13q), trisomy 6, trisomy 15) should not be used as a means of establishing a diagnosis of MDS in a patient with an otherwise unremarkable AA (Table I). Approximately ~5–15% of AA patients have cytogenetic abnormalities, frequently at diagnosis, which may persist or remit with haematopoietic recovery. In contrast, finding clonal abnormalities that are characteristic of IBMFS, should prompt an evaluation for an alternative diagnosis. These include classical cytogenetic abnormalities of IBMFS, such as trisomy 1q and trisomy 3q in FA, isochromosome 7q or 7q CN-LOH in SDS, and 3q CN-LOH leading to mosaic reversion of TERC mutations in DC.
Finally, several genetic changes, including somatic mutations in DNMT3A, TET2, ASXL1 and certain chromosomal aberrations, such as monosomy 7, appear to reflect the shared pathway of HSC clonal evolution, which selects for characteristics that enhance relative HSC fitness, such as decreased differentiation and increased self-renewal. Genetic changes in this category can be observed in clonal haematopoiesis in many settings, including AA, MDS, IBMFS and, for some abnormalities, in normal aging.
7.2. Effect of Therapy on Clonal Haematopoiesis
An intriguing and important question in the clinical care of AA patients is the role of therapy on clonal evolution. It is well established that clonal complications predominantly affect individuals treated with immunosuppression, with a much lower rate of clonal complications in patients treated with allogeneic transplant (Socie, et al 1993). With appreciation that age-related somatic mutations contribute to clonal haematopoiesis in AA, it remains an open question whether paediatric patients who respond to front-line immunosuppression are at an increased risk of developing MDS. Among the different immunosuppression therapies, there do not appear to be differences in cytogenetic clonal evolution in patients treated with a cyclophosphamide-based regimen (Scheinberg, et al 2014a), nor in patients treated with a prolonged cyclosporine course (Scheinberg, et al 2014b). However, studies have identified that longer disease duration and relapsed and refractory AA may potentiate clonal evolution (Desmond, et al 2014, Kojima, et al 2002, Kulasekararaj, et al 2014), thus providing another impetus for optimizing AA therapies to achieve a prompt and durable disease control.
The question of whether growth factors adversely alter the natural history of AA remains unresolved. While there is no evidence that granulocyte colony stimulating factor (G-CSF) potentiates transformation to MDS or changes overall survival in AA (Teramura, et al 2007, Tichelli, et al 2011), this may be a reflection of the relatively short duration of G-CSF therapy given to most patients, as compared to patients with severe congenital neutropenia where long-term high dose G-CSF therapy has been definitively linked to leukemogenesis through the acquisition of CSF3R mutations (Link, et al 2007). In the case of AA, multiple groups found an association between the use and duration of G-CSF therapy and subsequent emergence of monosomy 7 (Desmond, et al 2014, Hama, et al 2015, Kaito, et al 1998, Kojima, et al 2002, Li, et al 2011). A possible mechanistic link was reported by Sloand et al (2006), where pharmacological doses of G-CSF were shown to preferentially stimulate the growth of monosomy 7 clones through abnormal signalling by the GCSF class IV receptor.
More recently, the thrombopoietin receptor agonist and HSC homeostatic agent, eltrombopag, was found to facilitate trilineage haematopoietic recovery in patients with relapsed refractory AA(Desmond, et al 2014, Olnes, et al 2012). Despite US Food and Drug Administration approval, clonal evolution remains a major concern, as 8 of 43 studied patients (18.6%) developed new cytogenetic abnormalities within 3–13 months of eltrombopag therapy, including 5 patients with monosomy 7(Desmond, et al 2014). Future prospective randomized studies are needed to more clearly determine the role of eltrombopag in clonal evolution, both in the upfront as well as in the refractory AA setting.
7.3 Prognostic Implications of Somatic Changes in AA
Using all modalities, clonal haematopoiesis can be detected in ~85% of patients with adult-onset AA and over ~60% of patients with paediatric-onset AA (Babushok, et al 2015a, Yoshizato, et al 2015). In many patients, somatic mutations, acquired CN-LOH and cytogenetic abnormalities can be detected at a low allelic burden at the time of initial presentation. Although early studies suggested that patients with any somatic mutations in MDS-related genes are at an increased risk of MDS transformation (Kulasekararaj, et al 2014), this was not validated in subsequent studies (Yoshizato, et al 2015). Additionally, the discovery of nearly universal subclinical age-related mutations in aging individuals(Young, et al 2016) adds to the uncertainty regarding the prognostic impact of age-related mutations in AA. To interpret the significance of clonal haematopoiesis at the level of an individual patient, clonal characteristics have to be considered within the context of their age, prior and current disease states, underlying immune- and other selective pressures, therapies received and clonal dynamics over time.
7.3.1 Genetic Markers of Immune Escape Can be Predictive of Improved Response to Immunosuppression
Currently, there are insufficient data to accurately characterize the prognostic impact of most mutations, with a notable exception of mutations in PIGA. Multiple studies found that PIGA mutations, as assessed by the presence of a PNH clone by high-sensitivity flow cytometry, are predictive of improved response to immunosuppression (~67–91% for PNH clone-positive as compared to ~32–65% for PNH clone-negative patients), with no effect on clonal evolution or overall survival (Kulagin, et al 2014, Maciejewski, et al 2001, Narita, et al 2015, Sugimori, et al 2006, Tutelman, et al 2014, Zhao, et al 2015). The prognostic impact of the other characteristic AA abnormality attributed to immune escape, 6p CN-LOH, is less clear. In a Japanese cohort, patients with 6p CN-LOH were more likely to have a favourable response to immune suppression therapy (IST), with apparent 100% response to therapy and ~40% complete response rate in evaluable patients (Katagiri, et al 2011). In contrast, in a North American cohort, no patient with 6p CN-LOH was found to have a complete response, and 3 patients had refractory and/or relapsed disease (Betensky et al. 2016). The difference between the two studies may reflect the underlying immunogenetic differences between the populations and requires further investigation in large prospective studies of different ethnic groups. Importantly, improved response to immunosuppression by patients with detectable PNH clones and, possibly, 6p CN-LOH, underscores that these clonal changes are de facto genetic markers of autoimmune pathophysiology.
7.3.2 Prognostic Implications of MDS-Associated Somatic Mutations in AA
Among the genes recurrently mutated in AA as well as in normal aging, mutations in BCOR, BCORL1 and ASXL1 occur at a disproportionately high frequency in AA (Figure 5), suggesting that they confer an increased fitness on the HSPC within the AA marrow environment. Indirectly, this also implies that there are differences between the marrow environment of AA and that of aging. In a recent study, patients with mutant BCOR and BCORL1 were found to have an improved response to immunosuppression (Yoshizato, et al 2015), and, together with mutations in PIGA, marked a group with “favourable” prognosis with respect to survival and progression to MDS (Yoshizato, et al 2015). In longitudinal follow-up, clones bearing PIGA and BCOR/BCORL1 mutations were more likely to remain stable or to decrease overtime (Yoshizato, et al 2015).
In contrast, the significance of mutations in ASXL1 is less clear. Huang et al (2015) analysed clinical outcomes of 138 AA patients, to find that ASXL1-mutant patients had a higher risk of MDS transformation than patients with wild type ASXL1 (33% vs 8%). However, perhaps due to insufficient statistical power, the independent negative impact of ASXL1 mutations was not confirmed in other studies (Kulasekararaj, et al 2014, Yoshizato, et al 2015). The discrepancy can be partly explained by two differences. First, unlike the cohorts reported by Kulasekararaj et al (2014) and Yoshizato et al (2015), ASXL1-mutant patients in the Huang et al (2015) cohort were more likely to be children with abnormal cytogenetics, raising a question of possible admixture of patients with occult IBMFS. Second, by virtue of using direct sequencing for mutation identification, Huang et al (2015) focused on ASXL1-mutant clones of larger allelic size than the NGS-based studies by the other two groups(Kulasekararaj, et al 2014, Yoshizato, et al 2015). Together with the finding that a large subset of ASXL1-mutant clones expand overtime (Yoshizato, et al 2015), the results reported by Huang et al (2015) may imply that ASXL1 mutations have an adverse prognostic significance when they lead to clonal dominance.
Although no other mutations were found to have prognostic significance individually, Yoshizato et al (2015) identified a set of “unfavourable mutations” (mutations in DNMT3A, ASXL1, TP53, RUNX1 and CSMD1), which was associated with worse overall survival, particularly in patients under 60 years of age. Similar to mutations in ASXL1, clones bearing these mutations frequently expanded over time (Yoshizato, et al 2015).
7.3.3. Prognostic Implications of Cytogenetic Abnormalities
Because of the small numbers of patients involved, studies of prognostic implications of cytogenetic abnormalities in AA have been limited to the three most common chromosomal changes—monosomy 7, trisomy 8 and del(13q).
Structural and numerical abnormalities of chromosome 7 were generally associated with poor overall survival and a greater risk of transformation to MDS/AML (Kaito, et al 1998, Kojima, et al 2002, Li, et al 2011, Maciejewski, et al 2002). Although the presence of monosomy 7 does not preclude the ability to respond to immunosuppressive therapy and although cases of cytogenetic remission have also been described(Gupta, et al 2006), eligible patients with monosomy 7 should be considered for allogeneic transplantation because of the potential for rapid progression to MDS/AML. Evolution to monosomy 7 in AA has been linked to the use of growth factors, including both G-CSF and eltrombopag (Desmond, et al 2014, Hama, et al 2015, Kaito, et al 1998, Kojima, et al 2002, Li, et al 2011), and, in the case of G-CSF, may be caused by the preferential stimulation of monosomy 7 clones by aberrant signalling of the G-CSF class IV receptor(Sloand, et al 2006). Long-term randomized prospective studies of these agents in the upfront and relapsed setting are needed to better characterize this association.
The impact of trisomy 8 has been extensively studied in the context of both AA and hMDS. Patients with trisomy 8 were found to respond to immunosuppression at ~56–100% response rates (Gupta, et al 2006, Hosokawa, et al 2015, Maciejewski, et al 2002, Sloand, et al 2002, Sloand, et al 2005). In a subset of patients, trisomy 8 MDS clones were suppressed by oligoclonal T cells in vitro, and clonally expanded after T cell depletion (Sloand, et al 2002, Sloand, et al 2005); this association was not validated in a Japanese cohort where trisomy 8 clones in patients responding to immunosuppression were variable and failed to show a steady increase (Hosokawa, et al 2015). In an attempt to better stratify the trisomy 8 population, Hosokawa et al (2015) identified two trisomy 8 subsets of AA patients with differing clinical outcomes; a favourable subset, representing approximately one quarter of trisomy 8 patients, had a detectable PNH clone. PNH-positive trisomy 8 patients had a higher response to immunosuppressive therapy and an improved 5-year overall survival compared to patients without PNH cells (88% and 100%, respectively, as compared to 41% and 59%) (Hosokawa, et al 2015). This preliminary observation suggests that stratifying trisomy 8 patients based on PNH cells can potentially identify patients most likely to benefit from immunosuppressive therapy.
Finally, three case series totalling 37 patients described a generally benign clinical course for AA patients with del(13q) (Holbro, et al 2013, Hosokawa, et al 2012, Ishiyama, et al 2002). None of the studied patients had overt bone marrow dysplasia. There was a 60–100% response to immunosuppression and a greater than 75% 5-year survival; several patients had cytogenetic remission post-therapy. Interestingly, the majority of del(13q) patients also had a detectable PNH clone, pointing to both abnormalities being a phenomena related to immune escape.
Conclusions and Future Directions
In summary, AA is an autoimmune bone marrow failure disorder inextricably linked to clonal haematopoiesis. Clonal haematopoiesis of AA has a unique signature of immune escape, overlaid on the fabric of age-related and MDS-associated somatic mutations shared with other bone marrow failure syndromes. The presence of characteristic clonal changes such as PNH and 6p CN-LOH helps to support the diagnosis of immune-mediated bone marrow failure, and, in some cases, can be predictive of response to immunosuppressive therapy. The implications of age- and MDS-related somatic mutations in AA are complex and require cautious interpretation. While mutations in BCOR and BCORL1 are prognostically favourable, the significance of other age-related somatic mutations is less clear and depends on the clinical status of AA, clonal dynamics, and on the potential clonal selection by therapeutic agents.
Future opportunities and challenges lie in translating the knowledge of individual patient’s haematopoietic clonal constitution into improved diagnostic precision and better prognostic stratification. An improved ability to stratify patients based on their likelihood of response and risk of progression to haematopoietic malignancies will be instrumental in enabling personalized treatment strategies aimed at recovery and maintenance of healthy haematopoiesis.
Supplementary Material
Acknowledgments
We are grateful to Dr. Monica Bessler for insightful discussions, mentorship and passion for improving the care of patients with all forms of bone marrow failure. This work was supported by the NIH/NIDDK R24DK103001 and the Buck Family to the Children’s Hospital of Philadelphia Bone Marrow Failure Center, and by the NHLBI K08 HL132101-01 and AA & MDS International Foundation Research Grant to D.V.B. and NIH/NHLBI K08 HL122306 to T.S.O.
Footnotes
Author contributions
N.S. performed systematic literature review and prepared the tables and the targeted sequencing figure, D.B. wrote the manuscript, N.S., T.O., and D.B. revised and approved the final version of the manuscript.
Conflict of interest statement: The authors have no conflicts of interests to disclose.
References
- Abkowitz JL, Fialkow PJ, Niebrugge DJ, Raskind WH, Adamson JW. Pancytopenia as a clonal disorder of a multipotent hematopoietic stem cell. The Journal of clinical investigation. 1984;73:258–261. doi: 10.1172/JCI111199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abkowitz JL, Catlin SN, McCallie MT, Guttorp P. Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood. 2002;100:2665–2667. doi: 10.1182/blood-2002-03-0822. [DOI] [PubMed] [Google Scholar]
- Afable MG, 2nd, Wlodarski M, Makishima H, Shaik M, Sekeres MA, Tiu RV, Kalaycio M, O'Keefe CL, Maciejewski JP. SNP array-based karyotyping: differences and similarities between aplastic anemia and hypocellular myelodysplastic syndromes. Blood. 2011;117:6876–6884. doi: 10.1182/blood-2010-11-314393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, Carr AG, Greene MH, Rosenberg PS. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. British journal of haematology. 2010;150:179–188. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babushok DV, Xie HM, Roth JJ, Perdigones N, Olson TS, Cockroft JD, Gai X, Perin JC, Li Y, Paessler ME, Hakonarson H, Podsakoff GM, Mason PJ, Biegel JA, Bessler M. Single nucleotide polymorphism array analysis of bone marrow failure patients reveals characteristic patterns of genetic changes. British journal of haematology. 2014;164:73–82. doi: 10.1111/bjh.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babushok DV, Perdigones N, Perin JC, Olson TS, Ye W, Roth JJ, Lind C, Cattier C, Li Y, Hartung H, Paessler ME, Frank DM, Xie HM, Cross S, Cockroft JD, Podsakoff GM, Monos D, Biegel JA, Mason PJ, Bessler M. Emergence of clonal hematopoiesis in the majority of patients with acquired aplastic anemia. Cancer Genet. 2015a;208:115–128. doi: 10.1016/j.cancergen.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babushok DV, Olson TS, Bessler M. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. The New England journal of medicine. 2015b;373:1673. doi: 10.1056/NEJMc1509703#SA1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babushok DV, Bessler M, Olson TS. Genetic predisposition to myelodysplastic syndrome and acute myeloid leukemia in children and young adults. Leukemia & lymphoma. 2016;57:520–536. doi: 10.3109/10428194.2015.1115041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betensky M, Babushok D, Roth JJ, Mason PJ, Biegel JA, Busse TM, Li Y, Lind C, Papazoglou A, Monos D, Podsakoff G, Bessler M, Olson TS. Clonal evolution and clinical significance of copy number neutral loss of heterozygosity of chromosome arm 6p in acquired aplastic anemia. Cancer Genet. 2016;209:1–10. doi: 10.1016/j.cancergen.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner SM, Reth M, Hofer T, Rodewald HR. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518:542–546. doi: 10.1038/nature14242. [DOI] [PubMed] [Google Scholar]
- Cao Q, Gearhart MD, Gery S, Shojaee S, Yang H, Sun H, Lin DC, Bai JW, Mead M, Zhao Z, Chen Q, Chien WW, Alkan S, Alpermann T, Haferlach T, Muschen M, Bardwell VJ, Koeffler HP. BCOR regulates myeloid cell proliferation and differentiation. Leukemia. 2016;30:1155–1165. doi: 10.1038/leu.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin SN, Busque L, Gale RE, Guttorp P, Abkowitz JL. The replication rate of human hematopoietic stem cells in vivo. Blood. 2011;117:4460–4466. doi: 10.1182/blood-2010-08-303537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, Bock C, Vasanthakumar A, Gu H, Xi Y, Liang S, Lu Y, Darlington GJ, Meissner A, Issa JP, Godley LA, Li W, Goodell MA. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2012;44:23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dameshek W. Riddle: what do aplastic anemia, paroxysmal nocturnal hemoglobinuria (PNH) and "hypoplastic" leukemia have in common? Blood. 1967;30:251–254. [PubMed] [Google Scholar]
- de Planque MM, Bacigalupo A, Wursch A, Hows JM, Devergie A, Frickhofen N, Brand A, Nissen C. Long-term follow-up of severe aplastic anaemia patients treated with antithymocyte globulin. Severe Aplastic Anaemia Working Party of the European Cooperative Group for Bone Marrow Transplantation (EBMT) British journal of haematology. 1989;73:121–126. doi: 10.1111/j.1365-2141.1989.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Desmond R, Townsley DM, Dumitriu B, Olnes MJ, Scheinberg P, Bevans M, Parikh AR, Broder K, Calvo KR, Wu CO, Young NS, Dunbar CE. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123:1818–1825. doi: 10.1182/blood-2013-10-534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZern AE, Symons HJ, Resar LS, Borowitz MJ, Armanios MY, Brodsky RA. Detection of paroxysmal nocturnal hemoglobinuria clones to exclude inherited bone marrow failure syndromes. European journal of haematology. 2014;92:467–470. doi: 10.1111/ejh.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty PF, Yamaguchi H, Wiestner A, Baerlocher GM, Sloand E, Zeng WS, Read EJ, Lansdorp PM, Young NS. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H German Aplastic Anemia Study, G. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003;101:1236–1242. doi: 10.1182/blood-2002-04-1134. [DOI] [PubMed] [Google Scholar]
- Ganapathi KA, Townsley DM, Hsu AP, Arthur DC, Zerbe CS, Cuellar-Rodriguez J, Hickstein DD, Rosenzweig SD, Braylan RC, Young NS, Holland SM, Calvo KR. GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood. 2015;125:56–70. doi: 10.1182/blood-2014-06-580340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargiulo L, Papaioannou M, Sica M, Talini G, Chaidos A, Richichi B, Nikolaev AV, Nativi C, Layton M, de la Fuente J, Roberts I, Luzzatto L, Notaro R, Karadimitris A. Glycosylphosphatidylinositol-specific, CD1d-restricted T cells in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121:2753–2761. doi: 10.1182/blood-2012-11-469353. [DOI] [PubMed] [Google Scholar]
- Geary CG, Marsh JC, Gordon-Smith EC. Hypoplastic myelodysplasia (MDS) British journal of haematology. 1996;94:582–583. [PubMed] [Google Scholar]
- Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, Purcell SM, Svantesson O, Landen M, Hoglund M, Lehmann S, Gabriel SB, Moran JL, Lander ES, Sullivan PF, Sklar P, Gronberg H, Hultman CM, McCarroll SA. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. The New England journal of medicine. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondek LP, DeZern AE. I walk the line: how to tell MDS from other bone marrow failure conditions. Curr Hematol Malig Rep. 2014;9:389–399. doi: 10.1007/s11899-014-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubert TA, Shen D, Ding L, Okeyo-Owuor T, Lunn CL, Shao J, Krysiak K, Harris CC, Koboldt DC, Larson DE, McLellan MD, Dooling DJ, Abbott RM, Fulton RS, Schmidt H, Kalicki-Veizer J, O'Laughlin M, Grillot M, Baty J, Heath S, Frater JL, Nasim T, Link DC, Tomasson MH, Westervelt P, DiPersio JF, Mardis ER, Ley TJ, Wilson RK, Walter MJ. Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat Genet. 2012;44:53–57. doi: 10.1038/ng.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Brooker C, Tooze JA, Yi QL, Sage D, Turner D, Kangasabapathy P, Marsh JC. Clinical relevance of cytogenetic abnormalities at diagnosis of acquired aplastic anaemia in adults. British journal of haematology. 2006;134:95–99. doi: 10.1111/j.1365-2141.2006.06105.x. [DOI] [PubMed] [Google Scholar]
- Hama A, Takahashi Y, Muramatsu H, Ito M, Narita A, Kosaka Y, Tsuchida M, Kobayashi R, Ito E, Yabe H, Ohga S, Ohara A, Kojima S. Comparison of long-term outcomes between children with aplastic anemia and refractory cytopenia of childhood who received immunosuppressive therapy with antithymocyte globulin and cyclosporine. Haematologica. 2015;100:1426–1433. doi: 10.3324/haematol.2015.128553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser M, Schlarmann C, Dobbernack V, Panagiota V, Wiehlmann L, Walter C, Beier F, Ziegler P, Yun H, Kade S, Kirchner A, Huang L, Koenecke C, Eder M, Brummendorf TH, Dugas M, Ganser A, Thol F. Genetic characterization of acquired aplastic anemia by targeted sequencing. Haematologica. 2014;99:e165–e167. doi: 10.3324/haematol.2013.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbro A, Jotterand M, Passweg JR, Buser A, Tichelli A, Rovo A. Comment to "Favorable outcome of patients who have 13q deletion: a suggestion for revision of the WHO 'MDS-U' designation". Haematologica. 2013;98:e46–e47. doi: 10.3324/haematol.2012.082875. Haematologica. 2012;97(12):1845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K, Katagiri T, Sugimori N, Ishiyama K, Sasaki Y, Seiki Y, Sato-Otsubo A, Sanada M, Ogawa S, Nakao S. Favorable outcome of patients who have 13q deletion: a suggestion for revision of the WHO 'MDS-U' designation. Haematologica. 2012;97:1845–1849. doi: 10.3324/haematol.2011.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K, Sugimori N, Katagiri T, Sasaki Y, Saito C, Seiki Y, Mochizuki K, Yamazaki H, Takami A, Nakao S. Increased glycosylphosphatidylinositol-anchored protein-deficient granulocytes define a benign subset of bone marrow failures in patients with trisomy 8. European journal of haematology. 2015;95:230–238. doi: 10.1111/ejh.12484. [DOI] [PubMed] [Google Scholar]
- Hotta T, Murate T, Inoue C, Kagami T, Tsushita K, Wang JY, Saito H. Patchy haemopoiesis in long-term remission of idiopathic aplastic anaemia. European journal of haematology. 1990;45:73–77. doi: 10.1111/j.1600-0609.1990.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Ge M, Lu S, Shi J, Li X, Zhang J, Wang M, Yu W, Shao Y, Huang Z, Zhang J, Nie N, Zheng Y. Mutations of ASXL1 and TET2 in aplastic anemia. Haematologica. 2015;100:e172–e175. doi: 10.3324/haematol.2014.120931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huic D, Ivancevic V, Aurer I, Dodig D, Nemet D, Labar B, Poropat M, Munz DL. Bone marrow immunoscintigraphy in haematological patients with pancytopenia: preliminary results. Nucl Med Commun. 2002;23:757–763. doi: 10.1097/00006231-200208000-00009. [DOI] [PubMed] [Google Scholar]
- Inaguma Y, Akatsuka Y, Hosokawa K, Maruyama H, Okamoto A, Katagiri T, Shiraishi K, Murayama Y, Tsuzuki-Iba S, Mizutani Y, Nishii C, Yamamoto N, Demachi-Okamura A, Kuzushima K, Ogawa S, Emi N, Nakao S. Induction of HLA-B*40:02-restricted T cells possessing cytotoxic and suppressive functions against haematopoietic progenitor cells from a patient with severe aplastic anaemia. British journal of haematology. 2016;172:131–134. doi: 10.1111/bjh.13464. [DOI] [PubMed] [Google Scholar]
- International Agranulocytosis and Aplastic Anemia Study. Incidence of aplastic anemia: the relevance of diagnostic criteria. Blood. 1987;70:1718–1721. [PubMed] [Google Scholar]
- Ishiyama K, Karasawa M, Miyawaki S, Ueda Y, Noda M, Wakita A, Sawanobori M, Nagai H, Nakao S. Aplastic anaemia with 13q-: a benign subset of bone marrow failure responsive to immunosuppressive therapy. British journal of haematology. 2002;117:747–750. doi: 10.1046/j.1365-2141.2002.03518.x. [DOI] [PubMed] [Google Scholar]
- Ishiyama K, Chuhjo T, Wang H, Yachie A, Omine M, Nakao S. Polyclonal hematopoiesis maintained in patients with bone marrow failure harboring a minor population of paroxysmal nocturnal hemoglobinuria-type cells. Blood. 2003;102:1211–1216. doi: 10.1182/blood-2002-12-3706. [DOI] [PubMed] [Google Scholar]
- Jacobs KB, Yeager M, Zhou W, Wacholder S, Wang Z, Rodriguez-Santiago B, Hutchinson A, Deng X, Liu C, Horner MJ, Cullen M, Epstein CG, Burdett L, Dean MC, Chatterjee N, Sampson J, Chung CC, Kovaks J, Gapstur SM, Stevens VL, Teras LT, Gaudet MM, Albanes D, Weinstein SJ, Virtamo J, Taylor PR, Freedman ND, Abnet CC, Goldstein AM, Hu N, Yu K, Yuan JM, Liao L, Ding T, Qiao YL, Gao YT, Koh WP, Xiang YB, Tang ZZ, Fan JH, Aldrich MC, Amos C, Blot WJ, Bock CH, Gillanders EM, Harris CC, Haiman CA, Henderson BE, Kolonel LN, Le Marchand L, McNeill LH, Rybicki BA, Schwartz AG, Signorello LB, Spitz MR, Wiencke JK, Wrensch M, Wu X, Zanetti KA, Ziegler RG, Figueroa JD, Garcia-Closas M, Malats N, Marenne G, Prokunina-Olsson L, Baris D, Schwenn M, Johnson A, Landi MT, Goldin L, Consonni D, Bertazzi PA, Rotunno M, Rajaraman P, Andersson U, Beane Freeman LE, Berg CD, Buring JE, Butler MA, Carreon T, Feychting M, Ahlbom A, Gaziano JM, Giles GG, Hallmans G, Hankinson SE, Hartge P, Henriksson R, Inskip PD, Johansen C, Landgren A, McKean-Cowdin R, Michaud DS, Melin BS, Peters U, Ruder AM, Sesso HD, Severi G, Shu XO, Visvanathan K, White E, Wolk A, Zeleniuch-Jacquotte A, Zheng W, Silverman DT, Kogevinas M, Gonzalez JR, Villa O, Li D, Duell EJ, Risch HA, Olson SH, Kooperberg C, Wolpin BM, Jiao L, Hassan M, Wheeler W, Arslan AA, Bueno-de-Mesquita HB, Fuchs CS, Gallinger S, Gross MD, Holly EA, Klein AP, LaCroix A, Mandelson MT, Petersen G, Boutron-Ruault MC, Bracci PM, Canzian F, Chang K, Cotterchio M, Giovannucci EL, Goggins M, Hoffman Bolton JA, Jenab M, Khaw KT, Krogh V, Kurtz RC, McWilliams RR, Mendelsohn JB, Rabe KG, Riboli E, Tjonneland A, Tobias GS, Trichopoulos D, Elena JW, Yu H, Amundadottir L, Stolzenberg-Solomon RZ, Kraft P, Schumacher F, Stram D, Savage SA, Mirabello L, Andrulis IL, Wunder JS, Patino Garcia A, Sierrasesumaga L, Barkauskas DA, Gorlick RG, Purdue M, Chow WH, Moore LE, Schwartz KL, Davis FG, Hsing AW, Berndt SI, Black A, Wentzensen N, Brinton LA, Lissowska J, Peplonska B, McGlynn KA, Cook MB, Graubard BI, Kratz CP, Greene MH, Erickson RL, Hunter DJ, Thomas G, Hoover RN, Real FX, Fraumeni JF, Jr, Caporaso NE, Tucker M, Rothman N, Perez-Jurado LA, Chanock SJ. Detectable clonal mosaicism and its relationship to aging and cancer. Nat Genet. 2012;44:651–658. doi: 10.1038/ng.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. The New England journal of medicine. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongmans MC, Verwiel ET, Heijdra Y, Vulliamy T, Kamping EJ, Hehir-Kwa JY, Bongers EM, Pfundt R, van Emst L, van Leeuwen FN, van Gassen KL, Geurts van Kessel A, Dokal I, Hoogerbrugge N, Ligtenberg MJ, Kuiper RP. Revertant somatic mosaicism by mitotic recombination in dyskeratosis congenita. Am J Hum Genet. 2012;90:426–433. doi: 10.1016/j.ajhg.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiafa G, Papadopoulos A, Ntaios G, Saouli Z, Savopoulos C, Tsesmeli N, Kontoninas Z, Chatzinikolaou A, Tsavdaridou V, Klonizakis I, Hatzitolios A. Detection of CD55- and CD59-deficient granulocytic populations in patients with myelodysplastic syndrome. Annals of hematology. 2008;87:257–262. doi: 10.1007/s00277-007-0420-5. [DOI] [PubMed] [Google Scholar]
- Kaito K, Kobayashi M, Katayama T, Masuoka H, Shimada T, Nishiwaki K, Sekita T, Otsubo H, Ogasawara Y, Hosoya T. Long-term administration of G-CSF for aplastic anaemia is closely related to the early evolution of monosomy 7 MDS in adults. British journal of haematology. 1998;103:297–303. doi: 10.1046/j.1365-2141.1998.01014.x. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Sato-Otsubo A, Kashiwase K, Morishima S, Sato Y, Mori Y, Kato M, Sanada M, Morishima Y, Hosokawa K, Sasaki Y, Ohtake S, Ogawa S, Nakao S. Frequent loss of HLA alleles associated with copy number-neutral 6pLOH in acquired aplastic anemia. Blood. 2011;118:6601–6609. doi: 10.1182/blood-2011-07-365189. [DOI] [PubMed] [Google Scholar]
- Keel SB, Scott A, Sanchez-Bonilla M, Ho PA, Gulsuner S, Pritchard CC, Abkowitz JL, King MC, Walsh T, Shimamura A. Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica. 2016;101:1343–1350. doi: 10.3324/haematol.2016.149476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Kobayashi S, Wieder ED, Su C, Molldrem JJ. Loss of T-lymphocyte clonal dominance in patients with myelodysplastic syndrome responsive to immunosuppression. Blood. 2002;100:3639–3645. doi: 10.1182/blood-2002-01-0155. [DOI] [PubMed] [Google Scholar]
- Kojima S, Ohara A, Tsuchida M, Kudoh T, Hanada R, Okimoto Y, Kaneko T, Takano T, Ikuta K, Tsukimoto I Japan Childhood Aplastic Anemia Study, G. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood. 2002;100:786–790. doi: 10.1182/blood.v100.3.786. [DOI] [PubMed] [Google Scholar]
- Kulagin A, Lisukov I, Ivanova M, Golubovskaya I, Kruchkova I, Bondarenko S, Vavilov V, Stancheva N, Babenko E, Sipol A, Pronkina N, Kozlov V, Afanasyev B. Prognostic value of paroxysmal nocturnal haemoglobinuria clone presence in aplastic anaemia patients treated with combined immunosuppression: results of two-centre prospective study. British journal of haematology. 2014;164:546–554. doi: 10.1111/bjh.12661. [DOI] [PubMed] [Google Scholar]
- Kulasekararaj AG, Jiang J, Smith AE, Mohamedali AM, Mian S, Gandhi S, Gaken J, Czepulkowski B, Marsh JC, Mufti GJ. Somatic mutations identify a subgroup of aplastic anemia patients who progress to myelodysplastic syndrome. Blood. 2014;124:2698–2704. doi: 10.1182/blood-2014-05-574889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane AA, Odejide O, Kopp N, Kim S, Yoda A, Erlich R, Wagle N, Abel GA, Rodig SJ, Antin JH, Weinstock DM. Low frequency clonal mutations recoverable by deep sequencing in patients with aplastic anemia. Leukemia. 2013;27:968–971. doi: 10.1038/leu.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie CC, Laurie CA, Rice K, Doheny KF, Zelnick LR, McHugh CP, Ling H, Hetrick KN, Pugh EW, Amos C, Wei Q, Wang LE, Lee JE, Barnes KC, Hansel NN, Mathias R, Daley D, Beaty TH, Scott AF, Ruczinski I, Scharpf RB, Bierut LJ, Hartz SM, Landi MT, Freedman ND, Goldin LR, Ginsburg D, Li J, Desch KC, Strom SS, Blot WJ, Signorello LB, Ingles SA, Chanock SJ, Berndt SI, Le Marchand L, Henderson BE, Monroe KR, Heit JA, de Andrade M, Armasu SM, Regnier C, Lowe WL, Hayes MG, Marazita ML, Feingold E, Murray JC, Melbye M, Feenstra B, Kang JH, Wiggs JL, Jarvik GP, McDavid AN, Seshan VE, Mirel DB, Crenshaw A, Sharopova N, Wise A, Shen J, Crosslin DR, Levine DM, Zheng X, Udren JI, Bennett S, Nelson SC, Gogarten SM, Conomos MP, Heagerty P, Manolio T, Pasquale LR, Haiman CA, Caporaso N, Weir BS. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat Genet. 2012;44:642–650. doi: 10.1038/ng.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li X, Ge M, Shi J, Qian L, Zheng Y, Wang J. Long-term follow-up of clonal evolutions in 802 aplastic anemia patients: a single-center experience. Annals of hematology. 2011;90:529–537. doi: 10.1007/s00277-010-1140-9. [DOI] [PubMed] [Google Scholar]
- Link DC, Kunter G, Kasai Y, Zhao Y, Miner T, McLellan MD, Ries RE, Kapur D, Nagarajan R, Dale DC, Bolyard AA, Boxer LA, Welte K, Zeidler C, Donadieu J, Bellanne-Chantelot C, Vardiman JW, Caligiuri MA, Bloomfield CD, DiPersio JF, Tomasson MH, Graubert TA, Westervelt P, Watson M, Shannon W, Baty J, Mardis ER, Wilson RK, Ley TJ. Distinct patterns of mutations occurring in de novo AML versus AML arising in the setting of severe congenital neutropenia. Blood. 2007;110:1648–1655. doi: 10.1182/blood-2007-03-081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski JP, Selleri C. Evolution of clonal cytogenetic abnormalities in aplastic anemia. Leukemia & lymphoma. 2004;45:433–440. doi: 10.1080/10428190310001602363. [DOI] [PubMed] [Google Scholar]
- Maciejewski JP, Rivera C, Kook H, Dunn D, Young NS. Relationship between bone marrow failure syndromes and the presence of glycophosphatidyl inositol-anchored protein-deficient clones. British journal of haematology. 2001;115:1015–1022. doi: 10.1046/j.1365-2141.2001.03191.x. [DOI] [PubMed] [Google Scholar]
- Maciejewski JP, Risitano A, Sloand EM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129–3135. doi: 10.1182/blood.v99.9.3129. [DOI] [PubMed] [Google Scholar]
- Marsh JC, Geary CG. Is aplastic anaemia a pre-leukaemic disorder? British journal of haematology. 1991;77:447–452. doi: 10.1111/j.1365-2141.1991.tb08608.x. [DOI] [PubMed] [Google Scholar]
- Maschek H, Kaloutsi V, Rodriguez-Kaiser M, Werner M, Choritz H, Mainzer K, Dietzfelbinger M, Georgii A. Hypoplastic myelodysplastic syndrome: incidence, morphology, cytogenetics, and prognosis. Annals of hematology. 1993;66:117–122. doi: 10.1007/BF01697619. [DOI] [PubMed] [Google Scholar]
- McKerrell T, Park N, Moreno T, Grove CS, Ponstingl H, Stephens J, Understanding Society Scientific, G. Crawley C, Craig J, Scott MA, Hodkinson C, Baxter J, Rad R, Forsyth DR, Quail MA, Zeggini E, Ouwehand W, Varela I, Vassiliou GS. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 2015;10:1239–1245. doi: 10.1016/j.celrep.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailova N, Sessarego M, Fugazza G, Caimo A, De Filippi S, van Lint MT, Bregante S, Valeriani A, Mordini N, Lamparelli T, Gualandi F, Occhini D, Bacigalupo A. Cytogenetic abnormalities in patients with severe aplastic anemia. Haematologica. 1996;81:418–422. [PubMed] [Google Scholar]
- Minelli A, Maserati E, Nicolis E, Zecca M, Sainati L, Longoni D, Lo Curto F, Menna G, Poli F, De Paoli E, Cipolli M, Locatelli F, Pasquali F, Danesino C. The isochromosome i(7)(q10) carrying c.258+2t>c mutation of the SBDS gene does not promote development of myeloid malignancies in patients with Shwachman syndrome. Leukemia. 2009;23:708–711. doi: 10.1038/leu.2008.369. [DOI] [PubMed] [Google Scholar]
- Mohamedali AM, Gaken J, Ahmed M, Malik F, Smith AE, Best S, Mian S, Gaymes T, Ireland R, Kulasekararaj AG, Mufti GJ. High concordance of genomic and cytogenetic aberrations between peripheral blood and bone marrow in myelodysplastic syndrome (MDS) Leukemia. 2015;29:1928–1938. doi: 10.1038/leu.2015.110. [DOI] [PubMed] [Google Scholar]
- Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, Perna F, Pandey S, Madzo J, Song C, Dai Q, He C, Ibrahim S, Beran M, Zavadil J, Nimer SD, Melnick A, Godley LA, Aifantis I, Levine RL. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Kosaka H, Maeda Y, Nishimura J, Inoue N, Ohishi K, Okabe M, Takeda J, Kinoshita T. Inefficient response of T lymphocytes to glycosylphosphatidylinositol anchor-negative cells: implications for paroxysmal nocturnal hemoglobinuria. Blood. 2002;100:4116–4122. doi: 10.1182/blood-2002-06-1669. [DOI] [PubMed] [Google Scholar]
- Narita A, Muramatsu H, Sekiya Y, Okuno Y, Sakaguchi H, Nishio N, Yoshida N, Wang X, Xu Y, Kawashima N, Doisaki S, Hama A, Takahashi Y, Kudo K, Moritake H, Kobayashi M, Kobayashi R, Ito E, Yabe H, Ohga S, Ohara A, Kojima S Japan Childhood Aplastic Anemia Study Group. Paroxysmal nocturnal hemoglobinuria and telomere length predicts response to immunosuppressive therapy in pediatric aplastic anemia. Haematologica. 2015;100:1546–1552. doi: 10.3324/haematol.2015.132530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazha A, Seastone D, Radivoyevitch T, Przychodzen B, Carraway HE, Patel BJ, Carew J, Makishima H, Sekeres MA, Maciejewski JP. Genomic patterns associated with hypoplastic compared to hyperplastic myelodysplastic syndromes. Haematologica. 2015;100:e434–e437. doi: 10.3324/haematol.2015.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga S, Ohara A, Hibi S, Kojima S, Bessho F, Tsuchiya S, Ohshima Y, Yoshida N, Kashii Y, Nishimura S, Kawakami K, Nishikawa K, Tsukimoto I Aplastic Anaemia Committee of the Japanese Society of Paediatric, H. Treatment responses of childhood aplastic anaemia with chromosomal aberrations at diagnosis. British journal of haematology. 2002;118:313–319. doi: 10.1046/j.1365-2141.2002.03582.x. [DOI] [PubMed] [Google Scholar]
- Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, Parikh AR, Soto S, Biancotto A, Feng X, Lozier J, Wu CO, Young NS, Dunbar CE. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. The New England journal of medicine. 2012;367:11–19. doi: 10.1056/NEJMoa1200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo TH. Presence of paroxysmal nocturnal hemoglobinuria clones does not exclude inherited bone marrow failure syndromes. European journal of haematology. 2014;93:171. doi: 10.1111/ejh.12351. [DOI] [PubMed] [Google Scholar]
- Parikh S, Perdigones N, Paessler M, Greenbaum B, Tooke LS, Biegel JA, Mason PJ, Bessler M. Acquired copy number neutral loss of heterozygosity of chromosome 7 associated with clonal haematopoiesis in a patient with Shwachman-Diamond syndrome. British journal of haematology. 2012;159:480–482. doi: 10.1111/bjh.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigones N, Perin JC, Schiano I, Nicholas P, Biegel JA, Mason PJ, Babushok DV, Bessler M. Clonal hematopoiesis in patients with dyskeratosis congenita. American journal of hematology. 2016;91:1227–1233. doi: 10.1002/ajh.24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto FO, Leblanc T, Chamousset D, Le Roux G, Brethon B, Cassinat B, Larghero J, de Villartay JP, Stoppa-Lyonnet D, Baruchel A, Socie G, Gluckman E, Soulier J. Diagnosis of Fanconi anemia in patients with bone marrow failure. Haematologica. 2009;94:487–495. doi: 10.3324/haematol.13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin S, Cuccuini W, Ceccaldi R, Nibourel O, Pondarre C, Pages MP, Vasquez N, Dubois d'Enghien C, Larghero J, Peffault de Latour R, Rocha V, Dalle JH, Schneider P, Michallet M, Michel G, Baruchel A, Sigaux F, Gluckman E, Leblanc T, Stoppa-Lyonnet D, Preudhomme C, Socie G, Soulier J. Myelodysplasia and leukemia of Fanconi anemia are associated with a specific pattern of genomic abnormalities that includes cryptic RUNX1/AML1 lesions. Blood. 2011;117:e161–e170. doi: 10.1182/blood-2010-09-308726. [DOI] [PubMed] [Google Scholar]
- Scheinberg P, Townsley D, Dumitriu B, Scheinberg P, Weinstein B, Daphtary M, Rios O, Wu CO, Young NS. Moderate-dose cyclophosphamide for severe aplastic anemia has significant toxicity and does not prevent relapse and clonal evolution. Blood. 2014a;124:2820–2823. doi: 10.1182/blood-2014-05-573642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg P, Rios O, Scheinberg P, Weinstein B, Wu CO, Young NS. Prolonged cyclosporine administration after antithymocyte globulin delays but does not prevent relapse in severe aplastic anemia. American journal of hematology. 2014b;89:571–574. doi: 10.1002/ajh.23692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Score J, Chase A, Forsberg LA, Feng L, Waghorn K, Jones AV, Rasi C, Linch DC, Dumanski JP, Gale RE, Cross NC. Detection of leukemia-associated mutations in peripheral blood DNA of hematologically normal elderly individuals. Leukemia. 2015;29:1600–1602. doi: 10.1038/leu.2015.13. [DOI] [PubMed] [Google Scholar]
- Sloand EM, Kim S, Fuhrer M, Risitano AM, Nakamura R, Maciejewski JP, Barrett AJ, Young NS. Fas-mediated apoptosis is important in regulating cell replication and death in trisomy 8 hematopoietic cells but not in cells with other cytogenetic abnormalities. Blood. 2002;100:4427–4432. doi: 10.1182/blood-2002-01-0096. [DOI] [PubMed] [Google Scholar]
- Sloand EM, Mainwaring L, Fuhrer M, Ramkissoon S, Risitano AM, Keyvanafar K, Lu J, Basu A, Barrett AJ, Young NS. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005;106:841–851. doi: 10.1182/blood-2004-05-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloand EM, Yong AS, Ramkissoon S, Solomou E, Bruno TC, Kim S, Fuhrer M, Kajigaya S, Barrett AJ, Young NS. Granulocyte colony-stimulating factor preferentially stimulates proliferation of monosomy 7 cells bearing the isoform IV receptor. Proc Natl Acad Sci U S A. 2006;103:14483–14488. doi: 10.1073/pnas.0605245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–2511. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JN, Kanwar VS, MacNamara KC. Hematopoietic Stem Cell Regulation by Type I and II Interferons in the Pathogenesis of Acquired Aplastic Anemia. Front Immunol. 2016;7:330. doi: 10.3389/fimmu.2016.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socie G. Could aplastic anaemia be considered a pre-pre-leukaemic disorder? European journal of haematology. Supplementum. 1996;60:60–63. doi: 10.1111/j.1600-0609.1996.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Socie G, Henry-Amar M, Bacigalupo A, Hows J, Tichelli A, Ljungman P, McCann SR, Frickhofen N, Van't Veer-Korthof E, Gluckman E. Malignant tumors occurring after treatment of aplastic anemia. European Bone Marrow Transplantation-Severe Aplastic Anaemia Working Party. The New England journal of medicine. 1993;329:1152–1157. doi: 10.1056/NEJM199310143291603. [DOI] [PubMed] [Google Scholar]
- Soulier J, Leblanc T, Larghero J, Dastot H, Shimamura A, Guardiola P, Esperou H, Ferry C, Jubert C, Feugeas JP, Henri A, Toubert A, Socie G, Baruchel A, Sigaux F, D'Andrea AD, Gluckman E. Detection of somatic mosaicism and classification of Fanconi anemia patients by analysis of the FA/BRCA pathway. Blood. 2005;105:1329–1336. doi: 10.1182/blood-2004-05-1852. [DOI] [PubMed] [Google Scholar]
- Steele JM, Sung L, Klaassen R, Fernandez CV, Yanofsky R, Wu J, Odame I, Silva M, Champagne J, Ali K, Brossard J, Samson Y, Abish S, Le D, Jardine L, Hand JP, Lipton JH, Charpentier K, Stephens D, Freedman M, Dror Y Canadian Inherited Marrow Failure, R. Disease progression in recently diagnosed patients with inherited marrow failure syndromes: a Canadian Inherited Marrow Failure Registry (CIMFR) report. Pediatric blood & cancer. 2006;47:918–925. doi: 10.1002/pbc.20876. [DOI] [PubMed] [Google Scholar]
- Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, Mizoguchi H, Omine M, Nakao S. Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308–1314. doi: 10.1182/blood-2005-06-2485. [DOI] [PubMed] [Google Scholar]
- Sutherland DR, Keeney M, Illingworth A. Practical guidelines for the high-sensitivity detection and monitoring of paroxysmal nocturnal hemoglobinuria clones by flow cytometry. Cytometry B Clin Cytom. 2012;82:195–208. doi: 10.1002/cyto.b.21023. [DOI] [PubMed] [Google Scholar]
- Teramura M, Kimura A, Iwase S, Yonemura Y, Nakao S, Urabe A, Omine M, Mizoguchi H. Treatment of severe aplastic anemia with antithymocyte globulin and cyclosporin A with or without G-CSF in adults: a multicenter randomized study in Japan. Blood. 2007;110:1756–1761. doi: 10.1182/blood-2006-11-050526. [DOI] [PubMed] [Google Scholar]
- Tichelli A, Gratwohl A, Wursch A, Nissen C, Speck B. Late haematological complications in severe aplastic anaemia. British journal of haematology. 1988;69:413–418. doi: 10.1111/j.1365-2141.1988.tb02382.x. [DOI] [PubMed] [Google Scholar]
- Tichelli A, Schrezenmeier H, Socie G, Marsh J, Bacigalupo A, Duhrsen U, Franzke A, Hallek M, Thiel E, Wilhelm M, Hochsmann B, Barrois A, Champion K, Passweg JR. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434–4441. doi: 10.1182/blood-2010-08-304071. [DOI] [PubMed] [Google Scholar]
- Tutelman PR, Aubert G, Milner RA, Dalal BI, Schultz KR, Deyell RJ. Paroxysmal nocturnal haemoglobinuria phenotype cells and leucocyte subset telomere length in childhood acquired aplastic anaemia. British journal of haematology. 2014;164:717–721. doi: 10.1111/bjh.12656. [DOI] [PubMed] [Google Scholar]
- Tuzuner N, Cox C, Rowe JM, Watrous D, Bennett JM. Hypocellular myelodysplastic syndromes (MDS): new proposals. British journal of haematology. 1995;91:612–617. doi: 10.1111/j.1365-2141.1995.tb05356.x. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- Venkatraj VS, Gaidano G, Auerbach AD. Clonality studies and N-ras and p53 mutation analysis of hematopoietic cells in Fanconi anemia. Leukemia. 1994;8:1354–1358. [PubMed] [Google Scholar]
- Walter MJ, Shen D, Shao J, Ding L, White BS, Kandoth C, Miller CA, Niu B, McLellan MD, Dees ND, Fulton R, Elliot K, Heath S, Grillot M, Westervelt P, Link DC, DiPersio JF, Mardis E, Ley TJ, Wilson RK, Graubert TA. Clonal diversity of recurrently mutated genes in myelodysplastic syndromes. Leukemia. 2013;27:1275–1282. doi: 10.1038/leu.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chuhjo T, Yasue S, Omine M, Nakao S. Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood. 2002;100:3897–3902. doi: 10.1182/blood-2002-03-0799. [DOI] [PubMed] [Google Scholar]
- Wang SA, Pozdnyakova O, Jorgensen JL, Medeiros LJ, Stachurski D, Anderson M, Raza A, Woda BA. Detection of paroxysmal nocturnal hemoglobinuria clones in patients with myelodysplastic syndromes and related bone marrow diseases, with emphasis on diagnostic pitfalls and caveats. Haematologica. 2009;94:29–37. doi: 10.3324/haematol.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RR, Hsu AP, Holland SM, Cuellar-Rodriguez J, Hickstein DD. Acquired ASXL1 mutations are common in patients with inherited GATA2 mutations and correlate with myeloid transformation. Haematologica. 2014;99:276–281. doi: 10.3324/haematol.2013.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarski MW, Hirabayashi S, Pastor V, Stary J, Hasle H, Masetti R, Dworzak M, Schmugge M, van den Heuvel-Eibrink M, Ussowicz M, De Moerloose B, Catala A, Smith OP, Sedlacek P, Lankester AC, Zecca M, Bordon V, Matthes-Martin S, Abrahamsson J, Kuhl JS, Sykora KW, Albert MH, Przychodzien B, Maciejewski JP, Schwarz S, Gohring G, Schlegelberger B, Cseh A, Noellke P, Yoshimi A, Locatelli F, Baumann I, Strahm B, Niemeyer CM. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127:1387–1397. doi: 10.1182/blood-2015-09-669937. quiz 1518. [DOI] [PubMed] [Google Scholar]
- Wu X, Bekker-Jensen IH, Christensen J, Rasmussen KD, Sidoli S, Qi Y, Kong Y, Wang X, Cui Y, Xiao Z, Xu G, Williams K, Rappsilber J, Sonderby CK, Winther O, Jensen ON, Helin K. Tumor suppressor ASXL1 is essential for the activation of INK4B expression in response to oncogene activity and anti-proliferative signals. Cell Res. 2015;25:1205–1218. doi: 10.1038/cr.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, Ozenberger BA, Welch JS, Link DC, Walter MJ, Mardis ER, Dipersio JF, Chen F, Wilson RK, Ley TJ, Ding L. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nature medicine. 2014;20:1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. The New England journal of medicine. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- Yoshizato T, Dumitriu B, Hosokawa K, Makishima H, Yoshida K, Townsley D, Sato-Otsubo A, Sato Y, Liu D, Suzuki H, Wu CO, Shiraishi Y, Clemente MJ, Kataoka K, Shiozawa Y, Okuno Y, Chiba K, Tanaka H, Nagata Y, Katagiri T, Kon A, Sanada M, Scheinberg P, Miyano S, Maciejewski JP, Nakao S, Young NS, Ogawa S. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. The New England journal of medicine. 2015;373:35–47. doi: 10.1056/NEJMoa1414799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7:12484. doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NS, Ogawa S. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. The New England journal of medicine. 2015;373:1675–1676. doi: 10.1056/NEJMc1509703. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang L, Jing L, Zhou K, Li Y, Peng G, Ye L, Li Y, Li J, Fan H, Song L, Yang W, Zhang F. The role of paroxysmal nocturnal hemoglobinuria clones in response to immunosuppressive therapy of patients with severe aplastic anemia. Annals of hematology. 2015;94:1105–1110. doi: 10.1007/s00277-015-2348-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.