Abstract
Background/Objectives
To test the hypothesis that a long-term structured, moderate intensity physical activity (PA) program is more effective than a health education (HE) program in reducing the risk of s elf-reported dependency and disability in basic activities of daily living (BADLs), disability in instrumental ADLs (IADL), and mobility disability.
Design
The Lifestyle Interventions and Independence for Elders (LIFE) study was a multicenter, single-blinded randomized trial.
Setting
University-based research clinic
Participants
1,635 sedentary men and women aged 70–89 years, who had functional limitations, defined as a score ≤9 on the Short Physical Performance Battery.
Intervention
Participants were randomized to a structured, moderate intensity PA program (n=818) that included aerobic, resistance, and flexibility exercises or to a HE program (n=817).
Measurements
All outcomes were derived by self-report using periodic interviews that asked about the degree of difficulty and receipt of help during the past month. Dependency was defined as “receiving assistance” or “unable” to do ≥ 1 activities. Disability was defined as having “a lot of difficulty” or “unable” doing ≥ 1 activities. Severe disability was defined as reporting difficulty or being unable to perform ≥ 3 activities.
Results
Over an average follow-up of 2.6 years, the cumulative incidence of BADL dependency was 15.2% among PA and 15.1% among HE participants (HR=1.0, 95%CI=0.78–0.1.3). Intervention groups had similar rates of incident BADL disability, IADL disability and reported mobility disability. Reporting severe mobility disability (HR=0.78, 95%CI=0.64–0.96) and ratings of difficulty on mobility tasks were reduced in the PA group.
Conclusion
A structured physical activity intervention reduces reported severe mobility disability and difficulty on mobility tasks, but not BADL and IADL disability in older adults with functional limitations.
INTRODUCTION
Twenty-three percent of the United States population, 65+ years of age report having difficulty walking or climbing stairs and 3.6 million older adults report having difficulty with basic activities like dressing and bathing.1 Reporting difficulty with, needing assistance, or being unable to perform daily tasks lead to higher utilization of health care services and loss of physical independence.2 Considerable resources are spent on managing disability among older adults, but there is limited research that evaluates strategies to prevent disability in basic and instrumental activities of daily living. A promising prevention strategy is enhancing physical activity levels, as there is well-established epidemiological evidence that diminished physical activity is associated with onset of physical disability in older adults.3–6
The Lifestyle Interventions and Independence for Elders (LIFE) Study was a large randomized trial designed to compare the effects of a long-term, moderate-intensity physical activity (PA) program with a “successful aging” health education (HE) program.7, 8 The primary outcome of the LIFE study was major mobility disability defined as the loss in the ability to walk 400 m in 15 minutes without help. Here MMD was assessed objectively by observing the ability to walk 400 m. Self-reported measures of mobility and disability inherently capture a participant’s viewpoint of their ability within their own environment. This is a different construct and evaluation of this domain would provide additional insight about the effects of physical activity. The study enrolled inactive older adults 70–89 years of age who were at high risk of disability based on a lower extremity performance test, yet were community dwelling and able to walk 400 meters (about ¼ mile) at baseline. Therefore, the sample enrolled in the LIFE study was well-suited to evaluate the hypothesis that compared to a HE group, a long-term PA program would prevent the incidence of dependency and disability in basic ADLs (BADLs) as well as disability in instrumental ADLs (IADLs), and reported mobility disability (e.g. walking a quarter mile).
METHODS
Trial design and participants
Design, recruitment, baseline characteristics, and main outcomes of the LIFE study are published and described elsewhere.7–9 Briefly, the LIFE study was a multicenter, single-blinded, parallel randomized trial conducted at 8 field centers across the U.S. between February 2010 and December 2013. The eligibility criteria consisted of men and women aged 70–89 years who (a) were inactive (reporting <20 min/week in the past month performing regular physical activity and <125 min/week of moderate physical activity); (b) were at high risk for mobility disability based on lower extremity functional limitations measured by the Short Physical Performance Battery (SPPB)10 score ≤9 out of 12 (45% of participants were targeted to have a score ≤ 7); (c) could walk 400 m in ≤15 minutes without sitting, leaning, or the help of another person or walker; (d) had no major cognitive impairment (Modified Mini-Mental State Examination [3MSE]11 1.5 standard deviations below education- and race-specific norms); and (e) could safely participate in the intervention as determined by medical history, physical exam and resting ECG.
The study protocol was approved by the institutional review boards at all participating sites. Written informed consent was obtained from all study participants. The trial was monitored by a data and safety monitoring board appointed by the National Institute on Aging. The trial is registered at ClinicalsTrials.gov with the identifier NCT01072500.
Interventions
The physical activity intervention followed the US Department of Health and Human Services 2008 Physical Activity Guidelines for Americans, with a goal of 150 min/week of walking, in addition to strength, flexibility, and balance training 12. Exercises from a publically available book entitled, Exercise & Physical Activity: Your Everyday Guide from the National Institute on Aging was used to complement the guidelines.13 Commensurate with these guidelines, the intervention was tailored to the ability and fitness level of individual participants. For example, lower extremity exercises that exacerbated joint pain were replaced with different, but comparable exercise. Promotion activities, described elsewhere7, 14, were used to encourage participants to increase their amount of regular physical activity over time. The intervention included attendance at two center-based sessions per week in a group setting. A certified interventionist supervised the center-based sessions to ensure safety and exercise duration/intensity was met according to a manual of operations. Participants were encouraged to perform home-based exercise 3–4 times per week to reach guidelines. Interventionists collected home exercise logs as an accountability tool for the duration of the study. A protocol was in place to restart the intervention for the participants who suspended the physical activity for medical reasons. The physical activity sessions were individualized and progressed towards a goal of 30 min of walking daily at moderate intensity, 10 min of primarily lower extremity strength training by means of body weight (e.g. chair rises, toe stand) and ankle weights (e.g. standing leg curl, side hip raise, standing bent leg hip flexion, seated leg extension, standing leg circles) (2 sets of 10 repetitions) and, 10 min of balance training, and large muscle group flexibility exercises. As recommended by the Physical Activity Guidelines for Americans, participants began with lighter intensity and gradually increased intensity over the first 2–3 weeks of the intervention. The Borg’s scale of self-perceived exertion,15 with scores ranging from 6 to 20, was used to measure intensity of activity. Participants were asked to walk at an intensity of 13 (activity perception of “somewhat hard”), and perform lower extremity strengthening exercises at an intensity of 15 to 16 (activity perception of “hard”). The balance training involved five levels of complexity for meeting progressive intensity. Beginning levels included activities like hip circles, toe stands and side-stepping while holding with two hands onto a firm object for stability (e.g. kitchen counter, balance bar). Progression to higher levels involved holding with one hand and eventually no hand support. Advanced levels incorporated tandem and crossover stepping that also included head turning and lunging.
The health education (HE) group attended weekly workshops during the first 26 weeks, and then monthly sessions thereafter (bi-monthly attendance was optional). Workshops included topics, other than physical activity, relevant to older adults, such as how to effectively negotiate the health care system, how to travel safely, preventive services and screenings recommended at different ages, where to go for reliable health information, nutrition, etc. The program also included a 5- to 10-minute instructor-led program of gentle upper extremity stretching or flexibility exercises.
Outcome Assessment
Self-reported physical function was assessed with the Pepper Assessment Tool for Disability (PAT-D) 16 at baseline and 6, 12, 24 and 36 months post-randomization. The PAT-D is a 23-item measure that assesses difficulty with an array of discrete functional tasks in three domains: basic activities of daily living (BADLs), instrumental activities of daily living (IADLs), and mobility. Questions were added to the PAT-D to assess BADL dependency. Certified staff members who were masked to the intervention assignment administered the questionnaire. The participant was asked, “During the past month, how much difficulty have you had…” with each of the 23 items. Responses were made on a five-point Likert scale with options of: 1 (“No difficulty”), 2 (“A little difficulty”), 3 (“Some difficulty”), 4 (“A lot of difficulty”), 5 (“unable to do the activity”) or “did not do for other reasons” or “Don’t know/Refused”. For five items that asked about BADLs (walk across a room, chair transfer, bed transfer, use of a toilet, dressing, and bathing), an extra question was asked of all participants about the dependency, “Do you usually receive help from another person when…” performing the task, with possible responses of “Yes”, “No” or “Don’t know/Refused”. The purpose of this question was to detect difficulty that might be masked by the participant’s receipt of help. Additionally, items were categorized into total and subscale PAT-D disability domain scores that used all items, BADL disability score (moving in and out of a chair, moving in and out of a bed, using toilet, dressing, bathing, and walking across a small room), IADL disability score (doing light housework, managing money, using the telephone, taking care of a family member, visiting with relative or friends, participating in community activities such as religious services, social activities, or volunteer work) and mobility disability score (walking one block, walking several blocks, walking for a quarter of a mile, climbing one flight of stairs, climbing several flights of stairs, getting in and out of a car, lifting and carrying something as heavy as 10 pounds, lifting heavy objects). Responses were summed according to the hierarchical code and averaged across the number of questions in each domain. Higher scores equate to more disability with a score of 1 indicating no difficulty and a score of 5 being unable to do the activity. Outcomes for ADL dependency and ADL, IADL and mobility disability are described in Table 1. The severity of disability was also captured as having “a lot of difficulty” or “unable” to perform 3 or more domain-related tasks. This outcome was designed to capture participants with progressive and/or catastrophic disability. It is important to note that participants may be counted as an outcome for a disability and severe disability events and therefore the outcomes are not mutually exclusive. We note that these measures are conceptually different than the primary outcome of the LIFE study where major mobility disability was ascertained by direct observation or by a committee of experts who judged the capability of the participant to complete the 400 meter walk using available information from a participants medical record.7, 8
Table 1.
Description of self-reported dependency and disability outcomes.
| Outcomes | Description |
|---|---|
| Incident BADL dependency | First follow-up report of “receiving assistance” or “unable” to perform one or more of the following tasks: moving in and out of a chair, moving in and out of a bed, using toilet, dressing, bathing, and walking across a small room |
| Incident BADL disability | First follow-up report of having “a lot of difficulty” or “unable” to perform any of the 6 ADLs |
| Incident severe BADL disability | First follow-up report of having “a lot of difficulty” or “unable” to perform 3 or more of the ADLs. |
| Incident IADL disability | First follow-up report of having “a lot of difficulty” or “unable” to perform the following tasks: doing light housework, managing money, using the telephone, taking care of a family member, visiting with relative or friends, participating in community activities such as religious services, social activities, or volunteer work. |
| Incident severe IADL disability | First follow-up report of having “a lot of difficulty” or “unable” to perform 3 or more of the IADLs. |
| Incident reported mobility disability | Reported mobility disability outcomes operationalized the same as ADL outcomes. First follow-up report of having “a lot of difficulty” or “unable” on the following tasks: walking one block, walking several blocks, walking for a quarter of a mile, climbing one flight of stairs, climbing several flights of stairs, getting in and out of a car, lifting and carrying something as heavy as 10 pounds, lifting heavy objects. |
| Incident severe reported mobility disability | First follow-up report of having “a lot of difficulty” or “unable” to perform 3 or more of the reported mobility tasks |
Note: BADL: Basic activity of daily living. IADL: Instrumental activity of daily living. It is possible for participants to be counted as both a disability and severe disability outcome.
Additional measurements
The main baseline measures included self-reported demographic characteristics including race and ethnicity reported according to NIH requirements, medical and hospitalization history, physical activity assessed with the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire,17 and accelerometry over 7-days (GT3X, Actigraph Inc., Pensacola FL), cognitive function assessed with the Modified Mini-Mental Status Exam11, 400 m walk test, the SPPB, body mass and height. These measures are described in detail elsewhere.7–9 Adherence measures were assessed by questions on the CHAMPS and accelerometry. For CHAMPS, the time per week doing walking activities (walking playing golf, jogging or running, walking/hiking uphill, walking briskly or leisurely for exercise) and strength training activities were combined and collected every 6 months. Accelerometry was measured at 6, 12 and 24 months.
Statistical Analysis
Baseline characteristics were summarized by intervention group using mean (SD) or percentages. For BADL disability and dependency, IADL, and reported mobility disability outcomes, participants with a reported disability (or dependency) at baseline were excluded from the analysis. The effect of the intervention on the incident disability (or dependency) outcomes was tested based on a two-tailed significance of 0.05. The analysis was conducted according to randomized grouping, which is consistent with the intention to treat approach. To compare interventions, we used a likelihood ratio test from a Cox regression model, stratified by field center and sex. Failure time was measured from the time of randomization; follow-up was censored at the last questionnaire assessment. For participants who did not have outcome assessments, we assigned one hour of follow-up time, since we knew that they completed the functional assessment at baseline. Effect modification was examined using interaction terms entered into these Cox models. Here, effect modification was examined across levels of baseline pre-specified subgroups (ethnicity/race, sex, SPPB<8, age 80+, history cardiovascular disease, diabetes, walking speed <0.80 m/sec) and post-hoc subgroups of cognitive function (3MSE<90), depression (CESD≥16), pain due to arthritis and morbid obesity (BMI ≥ 35). Cumulative hazard plots stratified by treatment arm were obtained for self-reported BADL dependency, IADL disability, reported mobility disability, and reported severe mobility disability for each of the domains. Results were expressed as hazard ratio (HR) with 95% confidence intervals (CI).
Total and subscale PAT-D disability scores and PAT-D domain (ADL, IADL and mobility) disability score were analyzed using mixed effects models for repeatedly measured outcomes with an unstructured parameterization for longitudinal covariance. Models contained the following terms: field center and sex (both used to stratify randomization), baseline value of the relevant outcome, intervention, clinic visit, and intervention-by-visit interaction. Least squares means were obtained from these models and contrasts were used to estimate the average effects (95% CI) over follow-up. Statistical analyses were performed in SAS 9.4.
RESULTS
The two intervention groups were similar at baseline with respect to demographics, health conditions, cognition, physical function and self-reported disability (Table 2). The mean age was 78.9 years, 67.2% were women, 17.6% were African American, the average body mass index was 30.2, and the average SPPB score was 7.4, indicating that participants had substantial functional impairments. Intervention adherence was described previously in an earlier report8. In brief, the PA intervention maintained a 104-min/wk difference (95% CI, 92–116; P < .001) in walking and strength training activities compared with the HE group during the initial 2 years in which all participants were followed up. Objectively measured physical activity with accelerometry demonstrated a 40-min/wk difference in moderate to vigorous intensity activity; 95% CI, 29 to 52; P < 0.01.
Table 2.
Baseline characteristics of the participants
| Characteristic | Physical Activity N=818 |
Health Education N=817 |
|---|---|---|
| Age (years) | 78.7 ± 5.2 | 79.1 ± 5.2 |
| Women | 547 (66.9) | 551 (67.4) |
| Ethnicity/race | ||
| Hispanic | 31 (3.8) | 30 (3.7) |
| Caucasian | 604 (73.8) | 635 (77.7) |
| African American | 163 (19.9) | 125 (15.3) |
| SPPB score | 7.4 ± 1.6 | 7.3 ± 1.6 |
| SPPB score <8 | 353 (43.2) | 378 (46.3) |
| 400 m walking speed (m/s) | 0.83 ± 0.17 | 0.82 ± 0.17 |
| Body mass index (kg/m2) | 30.1 ± 5.7 | 30.3 ± 6.2 |
| Self-reported minutes per week in walking activities | 135.1 ± 184.6 | 120.4 ± 165.2 |
| Self-reported minutes per week in strength training activities | 5.3 ± 18.8 | 4.7 ± 17.5 |
| Accelerometry minutes per week of moderate physical activity * | 27.7 ± 25.6 | 27.4 ± 22.7 |
| 3MSE score (0–100 scale) | 91.5 ± 5.5 | 91.6 ± 5.3 |
| Health conditions a | ||
| Hypertension | 568 (69.7) | 583 (72.3) |
| Diabetes | 210 (25.7) | 208 (25.6) |
| Heart attack or myocardial infarction | 65 (8.0) | 64 (7.9) |
| Stroke | 53 (6.5) | 56 (6.9) |
| Cancer | 181 (22.2) | 189 (23.2) |
| Chronic pulmonary disease b | 133 (16.3) | 120 (14.7) |
| Severe arthritis | 152 (18.6) | 160 (19.6) |
| Depressive symptoms | 111 (14.3) | 142 (18.3) |
| Morbid obesity (BMI ≥ 35) | 152 (18.6) | 166 (20.3) |
| Disability scores c | ||
| Basic ADL subscale score | 1.3 ± 0.4 | 1.3 ± 0.4 |
| Instrumental ADL subscale score | 1.1 ± 0.3 | 1.1 ± 0.3 |
| Reported Mobility subscale score | 1.8 ± 0.7 | 1.8 ± 0.7 |
| Total disability score | 1.4 ± 0.4 | 1.4 ± 0.4 |
Data are means and standard deviations or n (%); SPPB = short physical performance battery.
Defined as “moderate physical activity” for accelerometry based on the 760 counts/minute cut-point. 45.
Values are in a subset of individuals (590 in PA and 581 in HE) with valid accelerometry data.
Some values may slightly differ from those previously published due to data updates.
BMI, body mass index calculated as weight in kilograms divided by height in meters squared; 3MSE, Modified Mini-Mental State Exam; SD, standard deviation.
Self-reported, physician diagnosed
Asthma, chronic bronchitis, emphysema, or COPD.
Scores are calculated by first assigning sequential values for each response (e.g. 1 = “nodifficulty” through 5 = “unable to do”) and averaging all the disability responses. Responses of “Did not do for other reasons” are not included in scoring.
Table 3 provides the cumulative number of events and associated hazard ratios between interventions groups across all disability domains assessed. The number of participants who reported the event at baseline is listed in Table 3 and the proportion excluded was similar between intervention groups. For BADL dependency, there were 120 events in each intervention group with an approximately equal probability of 15%. Figure 1A shows the cumulative hazards for ADL dependency were similar between PA and HE intervention groups. Incident IADL disability occurred in 141 (17.8%) and 140 (17.8%) participants in the PA and HE intervention groups, respectively (Figure 1B). Lastly, the number of participants reporting mobility disability was similar over the follow-up in the PA and HE groups [270 (46%) and 287 (49%), respectively; Figure 1C]. Likelihood ratio tests from Cox regression models showed no statistical differences between intervention groups for BADL dependency, BADL disability, IADL disability and mobility disability. Non-significant effects ranged from a 7% decrease to a 20% increase across the outcomes.
Table 3.
Overall number and percentage of events and hazard ratios for disability outcomes between physical activity and health education groups.
| Basic Activities of daily living (BADL) | Physical Activity | Health Education | HR | 95% CI | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Events | % | N | Events | % | ||||
| Incident BADL dependency1 | 793 | 120 | 15.1 | 787 | 120 | 15.2 | 1.00 | 0.78–1.29 | 0.97 |
| Incident BADL disability2 | 782 | 133 | 17.0 | 784 | 143 | 18.2 | 0.93 | 0.74–1.19 | 0.57 |
| Incident severe BADL disabilty3 | 816 | 42 | 5.1 | 817 | 40 | 4.9 | 1.10 | 0.71–1.70 | 0.66 |
| Instrumental ADL (IADL)4 | |||||||||
| Incident IADL disability | 790 | 141 | 17.8 | 787 | 140 | 17.8 | 1.00 | 0.79–1.27 | 0.97 |
| Incident severe IADL disability | 818 | 25 | 3.1 | 817 | 21 | 2.6 | 1.21 | 0.68–2.17 | 0.51 |
| Reported mobility disability5 | |||||||||
| Incident reported mobility disability | 579 | 270 | 46.6 | 577 | 287 | 49.7 | 0.96 | 0.82–1.14 | 0.70 |
| Incident severe reported mobility disability | 773 | 159 | 20.6 | 775 | 203 | 26.2 | 0.78 | 0.64–0.96 | 0.02 |
Note: For BADL outcomes, 55, 69 and 2 participants had reported the outcome of dependency, disability and severe disability, respectively, at baseline. For IADL disability, 58 participants reported having difficulty or inability to perform at least one task at baseline making an analytic sample of 1577 participants. For mobility disability, 479 (29% of the cohort) participants reporting having “a lot of difficulty” or “unable” to perform one of the eight mobility tasks on the PAT-D.
Figure 1.
Cumulative hazard plots of intervention groups for the first occurrence of self-reported A) basic activity of daily living (BADL) dependency, B) instrumental activity of daily living (IADL) disability, C) reported mobility disability and D) reported severe mobility disability. Number of events represents cumulative events, and adjusted hazard ratios and p-values are from proportional hazards regression models defined in the methods.
Fifty-five out of 276, 42 out of 281 and 184 out of 314 BADL, IADL and mobility disability events, respectively, developed severe disability by reporting “a lot of difficulty” or “unable” to perform 3 or more domain-related tasks. The remaining severe disability events were unique and did not follow an initial disability event (27 BADL, 4 IADL and 178 mobility disability events that were severe). The interventions showed similar severe BADL and IADL disability incident rates (Table 3). Incident severe mobility disability was lower in the PA (20.6%) compared to the HE (26.2%) intervention group (HR, 0.78 [95% CI, 0.64–0.96], Figure 1D).
Table 4 provides results from pre-specified subgroup analyses that were performed on the primary outcome of BADL dependency. Results did not significantly differ between subgroups of ethnicity/race, sex, baseline low physical performance according to having SPPB score < 8, history of cardiovascular disease, and baseline 400 m walking speed. There appeared to be slightly more benefit of PA on ADL dependency among those treated for diabetes (HR=0.64 for PA to HE comparison), but this intervention effect was not statistically different when compared to the other two baseline history of diabetes subgroups (p=0.08). In post-hoc analyses, the effect of PA on BADL dependency was similar in participants with a 3MSE score of less than 90 and in those with a score of 90 or higher, those with and without severe arthritis, and those with and without morbid obesity. Those with depressive symptoms (CES-D ≥ 16) showed a reduced effect of PA (interaction p-value = 0.02). Here, 23.3% of the PA group who had depressive symptoms had an ADL dependency event whereas this occurred in 12.7% of the HE group with depressive symptoms.
Table 4.
Hazard ratios of basic activity of daily living dependency for Physical Activity versus Health Education according to baseline subgroups.
| Subgroup | HE (Events / N) | PA (Events / N) | Hazard Ratio (95% CI) | Interaction p-value | ||
|---|---|---|---|---|---|---|
| N | Events | N | Events | |||
| Ethnicity/race | ||||||
| Non-Hispanic white | 609 | 90 | 591 | 91 | 1.07 (0.80–1.44) | 0.45 |
| Other | 176 | 29 | 199 | 29 | 0.85 (0.51–1.43) | |
| Sex | ||||||
| Male | 259 | 43 | 264 | 40 | 0.93 (0.61–1.44) | 0.73 |
| Female | 528 | 77 | 529 | 80 | 1.03 (0.75–1.41) | |
| SPPB score | ||||||
| 8 or 9 | 426 | 49 | 454 | 55 | 1.08 (0.73–1.59) | 0.68 |
| < 8 | 361 | 71 | 339 | 65 | 0.97 (0.69–1.36) | |
| Age | ||||||
| Age<80 | 438 | 54 | 459 | 65 | 1.20 (0.84–1.73) | 0.18 |
| Age 80+ | 349 | 66 | 334 | 55 | 0.84 (0.59–1.21) | |
| History of CVD | ||||||
| No CVD | 539 | 75 | 556 | 82 | 1.08 (0.79–1.48) | 0.45 |
| CVD | 238 | 45 | 219 | 38 | 0.88 (0.57–1.37) | |
| History of diabetes | ||||||
| None | 395 | 52 | 393 | 61 | 1.24 (0.85–1.80) | 0.08 |
| Impaired FG | 161 | 22 | 182 | 28 | 1.21 (0.68–2.13) | |
| Diabetes | 221 | 31 | 200 | 28 | 0.64 (0.40–1.02) | |
| 400 m walk speed | ||||||
| <0.80 m/sec | 486 | 83 | 462 | 87 | 1.12 (0.83–1.52) | 0.29 |
| 0.80+ m/sec | 301 | 37 | 331 | 33 | 0.83 (0.52–1.33) | |
| 3MSE | ||||||
| <90 | 244 | 78 | 244 | 77 | 0.96 (0.70–1.32) | 0.58 |
| 90+ | 533 | 42 | 531 | 43 | 1.11 (0.72–1.71) | |
| Depression Symptoms | ||||||
| CES-D < 16 | 615 | 95 | 614 | 90 | 0.90 (0.67, 1.20) | 0.02 |
| CES-D ≥ 16 | 133 | 17 | 107 | 25 | 2.03 (1.09, 3.78) | |
| Severe Arthritis | ||||||
| Absent | 638 | 98 | 351 | 96 | 0.97 (0.73, 1.29) | 0.58 |
| Present | 149 | 22 | 142 | 24 | 1.17 (0.65, 2.11) | |
| Morbid Obesity | ||||||
| BMI < 35 | 627 | 96 | 645 | 95 | 1.00 (0.75, 1.33) | 0.95 |
| BMI ≥ 35 | 160 | 24 | 148 | 25 | 1.02 (0.58,1.80) | |
SPPB: Short Physical Performance Battery; CVD: cardiovascular disease; 3MSE: Modified Mini-Mental State Exam ; Impaired FG : Impaired fasting glucose measured at baseline.
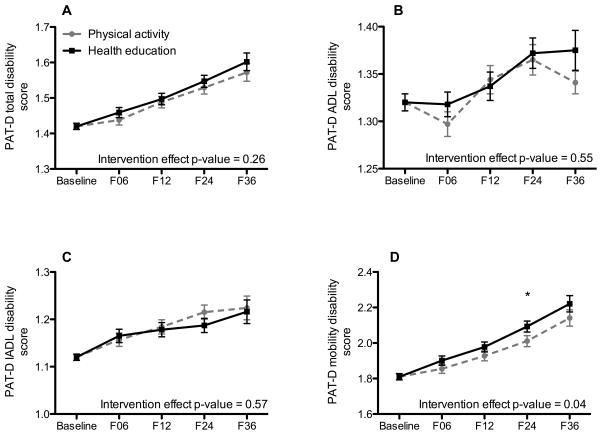
Average self-reported PAT-D disability scores steadily increased (worsened) in each domain (Figures 2A–D) over the three years of follow-up. There was no effect of physical activity on scores from the PAT-D total disability score (Figure 2A), PAT-D ADL disability score (Figure 2B) and PAT-D IADL disability score (Figure 2C). The PA intervention resulted in a significant attenuation in PAT-D mobility disability score compared to the HE intervention (overall intervention effect, p = 0.04, Figure 2D). However, the overall intervention effect was considered relatively small – Cohen’s d = 0.09.
Figure 2.
Average PAT-D disability scores for A. total disability, B. basic activities of daily living, C. instrumental activities of daily living and D. mobility domains. Each figure is shows adjusted means and standard errors for intervention groups across data collection visits. Plotted values represent least squares means (95% CI) from a mixed effects model adjusting for clinical site and sex (both used to stratify randomization) and the baseline self-reported disability score. In addition to the above-mentioned variables, the model contained a term for intervention group, follow-up clinic visit (i.e., 6, 12, 34 and 36 months) and the intervention by visit interaction.
DISCUSSION
Participants randomized to a structured, long-term moderate intensity physical activity program showed no benefit on ADL dependency, ADL disability and IADL disability outcomes compared with a HE intervention. However, consistent with the main findings of the LIFE study,8 which demonstrated that PA reduced major mobility disability assessed using an objective test, i.e., the ability to walk 400-m in ≤ 15 min, over 2.6 years compared to HE, PA had a modest effect on the incidence of self-reported severe mobility disability defined as reporting “a lot of difficulty” or “unable” to perform three or more of mobility tasks and self-reported PAT-D mobility disability scores. The results suggest that a physical activity program designed to preserve mobility is effective, but carry over benefits on ADL dependency, ADL disability and IADL disability are not achieved.
Incident ADL dependency was experienced by approximately 15% of the randomized participants over the follow-up, which is similar to what would be expected from this age group over 3 years.18 Contrary to expectations, the incidence of IADL disability was similar to BADL disability and dependency. Because LIFE participants were screened for normal cognition yet had low physical performance, their IADL disability may have been lower relative to the amount of physical impairments contributing to BADL disability and dependency. The lack of effect on incident disability domains is contrary to the epidemiological literature suggesting that individuals who engage in no or low volumes of physical activity are at elevated risk for physical disability.3–6 Additionally, this lack of benefit on ADL dependency was similar across pre-specified subgroups as indicated by non-significant interaction terms. However, when compared to HE, the PA group had an estimated 36% non-significant reduction in ADL dependency among those treated for diabetes. Interestingly, the elevated physical activity benefit on the primary outcome of major mobility disability—inability to walk 400 m—that was seen in lower functioning participants (SPPB < 8) was not seen for ADL dependency. In that regard, reported measures in the current study have psychometric qualities that often do not provide equivalent information to physical tests. This could explain discrepancies with the observed inability to walk 400 m reported in the primary paper.8, 19 One example is that reported measures ascertain function in the context of an individual’s own environment where compensatory strategies are often used to complete tasks and objective measures are performed in a standardized environment where compensation is limited.20, 21 As such, these domains are likely capturing different dimensions of the disablement process.22 However, the effect of physical activity was pronounced when reported mobility disability scores and incident severe reported mobility disability. In the long-term, this effect could have positive implications for ADL/IADL disability because loss of mobility is considered a gateway stage to more advanced disability and dependency.23
There is a substantial literature examining the effect of physical activity on physical function, physical performance, and disability in functionally impaired older adults.24, 25 Meta-analyses conducted on the topic in 2008 and 2014 found consistent positive effects of physical activity on muscle strength, mobility, physical fitness and balance, but only a few studies demonstrated positive effects that carried over to self-care disability outcomes.24, 25 There are several explanations for the lack of benefit with physical activity on self-reported disability that was observed in this study. First, there is discordance between the PA intervention program that was focused on lower extremity exercise and outcomes that assessed difficulty in upper body tasks (e.g. gripping, washing dishes). Second, the intensity of the exercise program was based on public health guidelines that used self-perceived exertion and may not have been of sufficient intensity to elicit a physiological adaptation. As seen in other studies, a program that incorporates higher intensity exercise may provide a greater adaptation stimulus to impact disability outcomes.26–28 Additionally, more successful programs were tailored to individuals after a comprehensive assessment of physical impairments that formed an intervention comprised of ADL exercise29 that targeted the mechanics of specific ADL and IADL tasks to enhance the transition to improvements in ADL/IADL function.30–32 As such, functional training or task-specific exercise programs might be needed and/or used as adjuncts to a physical activity program to reduce incidence of ADL/IADL disability.
The beneficial activities performed in the health education group could have masked the benefit of physical activity making it more difficult to detect intervention group differences. For example, convening as a group likely produced social relationships and connections that act as an informal support system has a positive impact on risk of and recovery from ADL disability.33–35 In fact, these social connections could create an informal support system that buffered the disability-depression relationship causing the HE group to have a lower rate of ADL dependency than the PA group.36, 37 These social relationships are also a hallmark component of Rowe and Kahn’s38, 39 successful aging paradigm that is an important factor in averting disability. Therefore, the HE group could impact reports of disability by giving individuals a more satisfying view of their functional disposition, particularly in those with depressive symptoms.40, 41 Health promotion activities that took place during the HE sessions could have improved competency to self-manage their comorbidities or ailments and seek out additional care, thereby reducing risk of ADL/IADL disability.42 This has been demonstrated in a randomized trial where chronic illness self-management was found to be effective in reducing major disability and hospitalizations in chronically ill older adults.43 Lastly, new social relationships created through HE sessions along with a focus on health enhancing activities could work in combination to imprint new behaviors that are associated with lower rates of disability (e.g. additional physician visits).44
The LIFE study is one of the largest prevention trials of a long-term physical activity intervention in vulnerable older adults, which makes this a particular strong study in comparison with others in the literature. However, the results are balanced with some notable weaknesses that include not being able to examine the many psychosocial, environmental and proxy-related factors that are related to disability occurrence. Additionally, no information was collected about frequency of care or other outside health promoting activities to explain factors related to disability occurrence. The potential benefits of HE were not well captured and there was not a third arm of the study in which participants received no intervention at all, both of which limited the interpretation of the results. Lastly, we chose to assess several disability constructs causing a large number of hypothesis tests, which may result in a false positive finding.
In conclusion, as compared with a HE program, a structured moderate intensity physical activity program incorporating walking, strength, flexibility and balance reduced the risk of severe mobility disability—reporting “a lot of difficulty” or “unable” to perform three or more common mobility tasks and reports of difficulty on mobility tasks. However, these positive effects on self-reported mobility did not translate to lower risk of self-reported dependency or disability in basic activities of daily living or disability in instrumental activities of daily living in older adults with functional limitations.
Supplementary Material
Supplementary Appendix S1: Research Investigators for the LIFE Study
Acknowledgments
| Elements of Financial/Personal Conflicts | Manini | Glynn | Gill | Guralnik | Marsh | Spring | Beavers | Pahor | Church | King | Folta | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Grants/Funds | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Honoraria | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Speaker Forum | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Consultant | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Stocks | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Royalties | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Expert Testimony | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Board Member | X | X | X | X | X | X | X | X | X | |||||||||||||
| Patents | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Personal Relationship | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
Grants/Funds explanation for all authors: The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332), Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744).
Sponsors role: The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332), Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744). Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1).
LIFE investigators are also partially supported by the following:
Dr. Manini is partially supported by R01AG042525, R01HL121023, SBIR HHSN261201500014C and P30AG028740.
Dr. Thomas Gill (Yale University) is the recipient of an Academic Leadership Award (K07AG3587) from the National Institute on Aging.
Dr. Carlos Fragoso (Spirometry Reading Center, Yale University) is the recipient of a Career Development Award from the Department of Veterans Affairs.
Dr. Roger Fielding (Tufts University) is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-0-014. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
Dr. Bonnie Spring is partially supported by NIH DK097364, HL075451, U54EB020404, and AHA 14SFRN20740001
Footnotes
Brief explanation for Manini: $1000 Honorarium provided for ACCME to the Texas Health, Research & Education Institute. LIFE data was presented at this event.
Brief explanation for Spring: Service on Actigraph Scientific Advisory Board
Author Contributions: All authors: study concept, data collection critical revisions of content and approval of final version for publication; TMM: initial draft of manuscript and results; DPB: statistical analyses; MP: PI of the LIFE study and obtained funding. DPB had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Erickson W, Lee C, SvS . Disability Statistics from the 2012 American Community Survey (ACS) Ithaca, NY: Cornell University Employment and Disability Institute (EDI); 2014. [Google Scholar]
- 2.Fried TR, Bradley EH, Williams CS, Tinetti ME. Functional disability and health care expenditures for older persons. Arch Intern Med. 2001;161:2602–2607. doi: 10.1001/archinte.161.21.2602. [DOI] [PubMed] [Google Scholar]
- 3.Fries JF. Physical activity, the compression of morbidity, and the health of the elderly. J R Soc Med. 1996;89:64–68. doi: 10.1177/014107689608900202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G, Pratt M, Macera CA, Zheng ZJ, Heath G. Physical activity, cardiovascular disease, and medical expenditures in U.S. adults. Ann Behav Med. 2004;28:88–94. doi: 10.1207/s15324796abm2802_3. [DOI] [PubMed] [Google Scholar]
- 5.Wu SC, Leu SY, Li CY. Incidence of and predictors for chronic disability in activities of daily living among older people in Taiwan. J Am Geriatr Soc. 1999;47:1082–1086. doi: 10.1111/j.1532-5415.1999.tb05231.x. [DOI] [PubMed] [Google Scholar]
- 6.Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 2011;11:33. doi: 10.1186/1471-2318-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: Design and Methods. The journals of gerontology Series A, Biological sciences and medical sciences. 2011 doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi: 10.1093/gerona/glt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 12.USDHHS; USDHHS, editor. Physical activity guidelines for Americans. Washington DC: USDHHS; 2008. [Google Scholar]
- 13.Aging NIo; Services DoHaH, editor. Exercise & Physical Activity: Your Everyday Guide from the National Institute on Aging. Washington, DC: 2009. [Google Scholar]
- 14.Rejeski WJ, Axtell R, Fielding R, et al. Promoting physical activity for elders with compromised function: the lifestyle interventions and independence for elders (LIFE) study physical activity intervention. Clin Interv Aging. 2013;8:1119–1131. doi: 10.2147/CIA.S49737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borg G. Borg’s perceived exertion and pain scales. Champaign: Human Kinetics; 1998. [Google Scholar]
- 16.Rejeski WJ, Ip EH, Marsh AP, Miller ME, Farmer DF. Measuring disability in older adults: the International Classification System of Functioning, Disability and Health (ICF) framework. Geriatrics & gerontology international. 2008;8:48–54. doi: 10.1111/j.1447-0594.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Sonn U. Longitudinal studies of dependence in daily life activities among elderly persons. Scand J Rehabil Med Suppl. 1996;34:1–35. [PubMed] [Google Scholar]
- 19.Bean JF, Olveczky DD, Kiely DK, LaRose SI, Jette AM. Performance-based versus patient-reported physical function: what are the underlying predictors? Phys Ther. 2011;91:1804–1811. doi: 10.2522/ptj.20100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naugle KM, Higgins TJ, Manini TM. Obesity and use of compensatory strategies to perform common daily activities in pre-clinically disabled older adults. Arch Gerontol Geriatr. 2012;54:e134–138. doi: 10.1016/j.archger.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manini TM, Cook SB, VanArnam T, Marko M, Ploutz-Snyder L. Evaluating task modification as an objective measure of functional limitation: repeatability and comparability. J Gerontol A Biol Sci Med Sci. 2006;61:718–725. doi: 10.1093/gerona/61.7.718. [DOI] [PubMed] [Google Scholar]
- 22.Higgins TJ, Janelle CM, Manini TM. Diving below the surface of progressive disability: considering compensatory strategies as evidence of sub-clinical disability. J Gerontol B Psychol Sci Soc Sci. 2014;69:263–274. doi: 10.1093/geronb/gbt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khokhar SR, Stern Y, Bell K, et al. Persistent mobility deficit in the absence of deficits in activities of daily living: a risk factor for mortality. J Am Geriatr Soc. 2001;49:1539–1543. doi: 10.1046/j.1532-5415.2001.4911251.x. [DOI] [PubMed] [Google Scholar]
- 24.Daniels R, van Rossum E, de Witte L, Kempen GI, van den Heuvel W. Interventions to prevent disability in frail community-dwelling elderly: a systematic review. BMC Health Serv Res. 2008;8:278. doi: 10.1186/1472-6963-8-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gine-Garriga M, Roque-Figuls M, Coll-Planas L, Sitja-Rabert M, Salva A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:753–769. e753. doi: 10.1016/j.apmr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002;50:655–662. doi: 10.1046/j.1532-5415.2002.50159.x. [DOI] [PubMed] [Google Scholar]
- 27.Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. Journal of Applied Physiology. 2007;102:368–373. doi: 10.1152/japplphysiol.00789.2006. [DOI] [PubMed] [Google Scholar]
- 28.Seynnes O, Fiatarone Singh MA, Hue O, Pras P, Legros P, Bernard PL. Physiological and functional responses to low-moderate versus high-intensity progressive resistance training in frail elders. J Gerontol A Biol Sci Med Sci. 2004;59:503–509. doi: 10.1093/gerona/59.5.m503. [DOI] [PubMed] [Google Scholar]
- 29.Gill TM, Baker DI, Gottschalk M, et al. A Prehabilitation Program for Physically Frail Community-living Older Persons. Arch Phys Med Rehabil. 2003;84:394–404. doi: 10.1053/apmr.2003.50020. [DOI] [PubMed] [Google Scholar]
- 30.Alexander NB, Galecki AT, Grenier ML, et al. Task-specific resistance training to improve the ability of activities of daily living-imparied older adults to rise from a bed and from a chair. Journal of the American Geriatric Society. 2001;49:1418–2001. doi: 10.1046/j.1532-5415.2001.4911232.x. [DOI] [PubMed] [Google Scholar]
- 31.de Vreede PL, Samson MM, van Meeteren NL, Duursma SA, Verhaar HJ. Functional-task exercise versus resistance strength exercise to improve daily function in older women: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:2–10. doi: 10.1111/j.1532-5415.2005.53003.x. [DOI] [PubMed] [Google Scholar]
- 32.Manini TM, Marko M, Van Arnam T, et al. Efficacy of resistance and task-specific exercise in older adults who modify tasks of everyday life. Journals of Gerontology: Medical Sciences. 2007;62:616–623. doi: 10.1093/gerona/62.6.616. [DOI] [PubMed] [Google Scholar]
- 33.Everard KM, Lach HW, Fisher EB, Baum MC. Relationship of activity and social support to the functional health of older adults. J Gerontol B Psychol Sci Soc Sci. 2000;55:S208–212. doi: 10.1093/geronb/55.4.s208. [DOI] [PubMed] [Google Scholar]
- 34.Mendes de Leon CF, Glass TA, Beckett LA, Seeman TE, Evans DA, Berkman LF. Social networks and disability transitions across eight intervals of yearly data in the New Haven EPESE. J Gerontol B Psychol Sci Soc Sci. 1999;54:S162–172. doi: 10.1093/geronb/54b.3.s162. [DOI] [PubMed] [Google Scholar]
- 35.Latham K, Clarke PJ, Pavela G. Social Relationships, Gender, and Recovery From Mobility Limitation Among Older Americans. J Gerontol B Psychol Sci Soc Sci. 2015;70:769–781. doi: 10.1093/geronb/gbu181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor MG, Lynch SM. Trajectories of impairment, social support, and depressive symptoms in later life. J Gerontol B Psychol Sci Soc Sci. 2004;59:S238–246. doi: 10.1093/geronb/59.4.s238. [DOI] [PubMed] [Google Scholar]
- 37.Chan N, Anstey KJ, Windsor TD, Luszcz MA. Disability and depressive symptoms in later life: the stress-buffering role of informal and formal support. Gerontology. 2011;57:180–189. doi: 10.1159/000314158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 39.Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- 40.Penninx BW, Guralnik JM, Bandeen-Roche K, et al. The protective effect of emotional vitality on adverse health outcomes in disabled older women. J Am Geriatr Soc. 2000;48:1359–1366. doi: 10.1111/j.1532-5415.2000.tb02622.x. [DOI] [PubMed] [Google Scholar]
- 41.Penninx BW, Guralnik JM, Simonsick EM, Kasper JD, Ferrucci L, Fried LP. Emotional vitality among disabled older women: the Women’s Health and Aging Study. J Am Geriatr Soc. 1998;46:807–815. doi: 10.1111/j.1532-5415.1998.tb02712.x. [DOI] [PubMed] [Google Scholar]
- 42.Phelan EA, Williams B, Leveille S, Snyder S, Wagner EH, LoGerfo JP. Outcomes of a community-based dissemination of the health enhancement program. J Am Geriatr Soc. 2002;50:1519–1524. doi: 10.1046/j.1532-5415.2002.50407.x. [DOI] [PubMed] [Google Scholar]
- 43.Leveille SG, Wagner EH, Davis C, et al. Preventing disability and managing chronic illness in frail older adults: a randomized trial of a community-based partnership with primary care. J Am Geriatr Soc. 1998;46:1191–1198. doi: 10.1111/j.1532-5415.1998.tb04533.x. [DOI] [PubMed] [Google Scholar]
- 44.Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. Am J Health Promot. 2000;14:362–370. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- 45.Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37:S512–522. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix S1: Research Investigators for the LIFE Study