Abstract
Bidirectional interactions between neurons and myelinating glial cells result in formation of axonal domains along myelinated fibers. Loss of axonal domains leads to detrimental consequences on nerve structure and function resulting in reduced conductive properties and the diminished ability to reliably transmit signals to the targets they innervate. Thus, impairment of peripheral myelinated axons that project to the surface of muscle fibers and form neuromuscular junction (NMJ) synapses leads to muscle dysfunction. The goal of our studies was to determine how altered electrophysiological properties due to axonal domains disorganization lead to muscle pathology, which is relevant to a variety of peripheral neuropathies, demyelinating diseases and neurodegenerative disorders. Using conventional Contactin-Associated Protein 1 (Caspr1) and Caspr2 single or double mutants with disrupted paranodal, juxtaparanodal or both regions, respectively, in peripheral myelinated axons, we correlated defects in NMJ integrity and muscle pathology. Our data show that loss of axonal domains in Caspr1 and Caspr2 single and double mutants primarily alters distal myelinated fibers together with presynaptic terminals, eventually leading to NMJ denervation and reduction in postsynaptic endplate areas. Moreover, reduction in conductive properties of peripheral myelinated fibers together with NMJ disintegration leads to muscle atrophy in Caspr1 mutants or muscle fiber degeneration accompanied with mitochondrial dysfunction in Caspr1/Caspr2 double mutants. Together our data indicate that proper organization of axonal domains in myelinated fibers is critical for optimal propagation of electrical signals, NMJ integrity and muscle health, and provide insights into a wide range of pathologies that result in reduced nerve conduction leading to muscle atrophy.
Keywords: Myelinated fibers, Axonal Domains, Nerve Conduction, Neuromuscular Junction, Axonal Degeneration, Muscle Atrophy
Graphical Abstract
In order to effectively communicate with muscles to allow muscle contraction and to maintain muscle health, neurons send electrical signals along myelinated axons, which are organized into distinct molecular domains. Using CASPR1 and CASPR2 mouse mutants, which have disrupted paranodal and juxtaparanodal domains, we demonstrate that disruption of these domains differentially affects electrical properties of myelinated fibers and consequently the health of muscles they innervate.

Introduction
The transmission of nerve impulses to distant targets in a fast and efficient manner requires myelination of axons by glial cells. Myelinating Schwann cells in the PNS and oligodendrocytes in the CNS tightly wrap their membranes around axons to form compact myelin to allow establishment of highly organized molecular domains along axons, which include the nodes of Ranvier, paranodes (PN), juxtaparanodes (JPN) and internodes (IN) (Buttermore et al., 2013; Sherman and Brophy, 2005). Each of these domains is assembled with distinct molecular complexes; furthermore, perturbations that alter the structure and function of the nerves or the myelinating glial cells affect axonal integrity leading to various neuropathologies (Waxman, 2000; Suter and Scherer, 2003; Waxman, 2006). Progressive muscle wasting and sensory deficits are common features for the heterogeneous group of disorders termed peripheral neuropathies, caused by inability of affected axons to deliver signals to the target muscles and to transmit sensory information from periphery back to the CNS (Griffin and Sheikh, 1999; Krajewski et al., 2000; Auer-Grumbach et al., 2003). Extensive studies over the years identified candidate genes and other risk factors, and have provided a better understanding of neuropathies with underlying mechanisms. Interestingly, several studies have shown antibody-mediated immune attack against axolemma can be involved in the pathogenesis of inflammatory demyelinating neuropathies (Armati and Mathey, 2014). Surprisingly, examination of patients’ sera reactivity revealed its high binding activity to the nodal and paranodal regions of myelinated axons, and presence of autoantibodies recognizing extracellular domains of nodal proteins Neurofascin 186 and Gliomedin, as well as paranodal Caspr1, Contactin, and the glial 155 kDa Neurofascin (Nfasc 155) (Devaux et al., 2012; Ng et al., 2012; Doppler et al., 2016). These findings suggest that axonal domains could potentially become a primary target in the pathogenesis of peripheral neuropathies, and the term nodo-paranodopathy has been recently used to characterize autoimmune neuropathies that target these regions in myelinated fibers (Uncini et al., 2013). However, little is known about the downstream effects and mechanisms of how disrupted axonal domains can lead to neuromuscular system pathologies.
Increasing evidence from animal studies also suggests that compromised axonal domain integrity can lead to PNS myelinated fiber dysfunction and muscle pathology. For example in the model of experimental auto-immune neuropathy, immunization of rats with peripheral myelin was accompanied with severe motor dysfunctions at the peak of disease progression and led to disruption of voltage-gated sodium channels clusters at the nodes of Ranvier in sciatic nerves, with subsequent detection of Nfasc 186 and gliomedin associated autoantibodies (Lonigro and Devaux, 2009). Additionally, numerous studies have proved that ablation of key nodal or paranodal proteins dramatically alters conductive properties of myelinated axons (Bhat et al., 2001; Pillai et al., 2009; Thaxton et al., 2011; Susuki et al., 2013), which potentially can modify synaptic transmission at the NMJ level and disrupt the cross-talk between motor neurons and the muscles they innervate. However, knowledge of changes at the NMJs after disruption of specific axonal domains is limited to very few studies and to date no further detailed investigations of possible muscle pathology have been carried out. For instance, loss of paranodal Nfasc 155, but not Caspr1, was found to alter peripheral postnatal synaptic remodeling and result in delayed postsynaptic elimination at the NMJ (Roche et al., 2014). Moreover ablation of protein 4.1B, which is highly enriched at the paranodal, juxtaparanodal and internodal areas, revealed axonal swellings close to synaptic terminals of NMJs in knockout animals (Cifuentes-Diaz et al., 2011). Most importantly, recent reports have linked mutations in human CNTNAP1, the gene encoding paranodal CASPR1, to arthrogryposis multiplex congenita, which is accompanied with severe neuromuscular dysfunction (Laquerriere et al., 2014). These studies suggest that loss of CASPR1 or other axonal domain proteins that alter nerve conduction may have severe consequences on the neuromuscular integrity and health.
Here we report the consequences of paranodal and juxtaparanodal axonal domain disorganization due to loss of Caspr1 and/or Caspr2 on the integrity of neuromuscular apparatus. We show that disruption of PN alone or in combination with JPN due to Caspr1 and Caspr2 loss is accompanied with the widening of the nodal gap and dramatic changes in in vivo nerve conduction velocity (NCV) along myelinated fibers. We also show that single or double ablation of these genes leads to degeneration of distal axons, as well as pre- and postsynaptic changes at the NMJs, resulting in muscle pathology. Together our data indicate that intact axonal domains are critical to maintain neuromuscular system integrity and muscle health. Our data provide insights into how myelinated axon pathologies, which are accompanied with decline in nerve conduction, can cause muscle atrophy due to NMJ disintegration.
Materials and Methods
Animals
Cntnap1 (Caspr1) knockout mice used in these studies were characterized previously (Bhat et al., 2001). Cntnap2 (Caspr2) knockout mouse line was obtained from The Jackson Laboratory (RRID:IMSR_JAX:017482) and has been described previously (Poliak et al., 2003). Caspr1/2 heterozygous mice were mated to obtain Caspr1/2 double knockout animals. Primers used for the genotyping PCR were as follows: Caspr1, 5′-GGG TCC AGT TCA TAG ACC TCG-3′ with 5′-GCG TCC TCG GCG TGG AAA GCC-3′ for the wild type 580 bp band and 5′-CCC ATG GCG ATG CCT GCT TGC-3′ with 5′-GTC GAG CCC CAG CTG GTT CTT-3′ for the mutant 550 bp band; Caspr2, 5′-CTG CCA GCC CAG AAC TGG-3′ as a common forward primer together with 5′-ACA CCA GGG GCA AGA ATT G-3′ for the 389 bp wild type band or 5′-CGC TTC CTC GTG CTT TAC GGT AT-3′ for the 350 bp mutant band. Mouse lines used in these studies were on a mixed C57BL/6 and 129/Sv genetic background. Animals were analyzed at various age of postnatal (P) development: 20, 40, 60 and 180. Equal number of males and females in control and knockout groups were used for each experiment. All animal experiments were performed according to NIH and Institutional Animal Care and Use Committee approved guidelines for ethical treatment of laboratory animals at the University of Texas Health Science Center at San Antonio.
Antibodies
Anti-cytoskeletal adaptor protein 4.1B (4.1B) and anti-βIV Spectrin (βIV Spec) antibodies have been described previously (Buttermore et al., 2011; Saifetiarova et al., 2017). Other primary antibodies used for experiments are mouse anti-potassium voltage-gated channel subfamily A member 2 (KV1.2) (UC Davis/NIH NeuroMab Facility Cat# 75-008 RRID:AB_2296313), mouse anti-Dystrophin (Sigma-Aldrich Cat# D8043 RRID:AB_259241), mouse anti-Synaptotagmin (Syt) (Zebrafish International Resource Center Cat# znp-1 RRID:AB_10013783), mouse anti-Neurofilament (Nf) (Covance Research Products Inc Cat# SMI-312R RRID:AB_2315329), rabbit anti-myelin basic protein (MBP) (Abcam Cat# ab40390 RRID:AB_1141521), mouse anti-αTubulin (DSHB Cat# 12G10 anti-alpha-tubulin RRID:AB_1157911). Alexa Fluor 488 (B13422) and 594 (B13423) conjugated α-Bungarotoxin (α-BTX) and secondary antibodies for immunostaining (Alexa Fluor- 488, 568, 647) were purchased from Invitrogen (USA). Infrared (IR) conjugated secondary antibodies used for immunoblotting were from LI-COR.
Tissue preparation and immunostaining
Animals were anesthetized with i.p. injection of Avertin (400 mg/kg mouse body weight) and transcardially perfused with phosphate-buffered saline (PBS) (pH 7.2–7.4) followed by ice-cold 1% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2–7.4). The cervical region of the spinal cords (SCs) and tibialis anterior (TA) muscles were dissected out and postfixed in the same fixative for 2 hours at 4°C and then immersed in 30% sucrose in 0.1M phosphate buffer until they settled to the bottom. The tissue was rinsed several times in PBS, frozen in Tissue-Tek O.C.T. Compound (Sakura Finetek USA, Inc). Longitudinal 14 μm sections for SCs, 20 μm cross-sections and 70 μm longitudinal sections from TA muscle for NMJ immunostaining were cut with a cryostat (Leica), mounted on slides and processed for immunostaining. Distal sciatic nerves (SNs) were dissected out from anesthetized animals, fixed in 4% paraformaldehyde for 30 minutes, washed in PBS and teased into individual nerve fibers, dried overnight at room temperature and stored at −80°C.Immunostaining of all samples was carried out as previously described (Thaxton et al., 2011).
Immunoblotting
SCs and SNs were harvested and stored at −80°C until processing. Tissues were homogenized using glass mortar and pestle on ice-cold lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 10 mM ethylenediaminetetraacetic acid, 1% Triton X-100, 1% SDS, and a protease cocktail tablet). The lysates were incubated for 30 min on ice and then centrifuged at 15,000 rpm for 30 min at 4°C. Supernatants were collected, and protein concentrations were estimated using a BCA protein assay Kit (Thermo Scientific). Equal amounts of protein were resolved by SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked [5% non-fat dry milk with 0.05% Tween-200 in PBS (PBS-T)] for 1 hour following incubation with primary antibodies in blocking buffer overnight at 4°C. After washes in PBST, membranes were incubated with IR-conjugated secondary antibodies for 1 hour and scanned using Li-COR Odyssey infrared imager. KV1.2 and 4.1B bands intensities standardized to α-tubulin were quantified and averaged from 3 independent experiments.
In vivo nerve conduction recordings
Animals were anesthetized by continuous isoflurane (5% aerosolized) for in vivo NCV recordings using a Nicolet Teca Synergy system (Natus Neurology Inc., Middleton, WI). In order to maintain body temperature to 33–34°C during recordings, a warming lamp was kept above mice. NCVs and amplitudes were recorded from the tail and SN of anesthetized mice as previously reported (Oh et al., 2010; Taylor et al., 2017). Briefly for tail recordings, an electrical stimulus (0.02ms, 4mA) was applied to the base of the tail and recorded 30mm distally. For SN recording, two separate recordings were made in the dorsum of the foot with a stimulus first given at the ankle (0.02ms, 3mA) and secondly at the sciatic notch (0.02ms, 8mA). Once traces were acquired, NCV was calculated by the distance divided by the latency and amplitude was measured as the height of the peak. For sciatic NCV, the distance between the notch and ankle was divided by the latency between the notch and ankle.
Transmission Electron Microscopy
For transmission electron microscopy (TEM), all tissue was processed as previously described (Green et al., 2013). All solutions were freshly prepared on the day of perfusion. In brief, animals were anesthetized and transcardially perfused with normal saline followed by 2.5%glutaraldehyde/4% paraformaldehyde EM fixative (dissolved in 0.16 M NaH2PO4/0.11M NaOH buffer, final pH 7.2–7.4) for 30 mins. After perfusion, entire mouse carcasses were post-fixed for another 2 weeks in the same EM fixative. SNs, SCs, TA muscles were dissected out and incubated overnight in 0.1M sodium cacodylate buffer followed by incubation in 2% OsO4 solution and gradient ethanol dehydration. Samples were incubated in propylene oxide, left in 100% PolyBed resin for 36 hours and embedded in flat molds at 55°C for 36 hours. After embedding, the molds were processed and imaged on a JEOL 1230 electron microscope at the UTHSCSA Electron Microscopy Lab. EM Images were analyzed by Image J software (NIH).
Analysis of Myelinated Axons
All immunofluorescent images were acquired by a Zeiss LSM 710 confocal microscope with 40× magnification objective using identical settings. On average, 5 confocal images were taken from SN and SC per animal. Localization of KV1.2 channels was analyzed per field of view and was defined as following: at the JPN – the gap in-between βIV Spec and KV1.2 is preserved; at the PN - KV1.2 is boarding βIV Spec; undetectable – no detectable KV1.2 in the vicinity of βIV Spec.
The G-ratios of SNs and SCs myelinated fibers from TEM images were calculated by dividing inner axonal diameter to outer diameter of corresponding myelinated fiber using ImageJ software. Axonal diameter was calculated by dividing measured perimeter by π.
Analysis of NMJ Innervation and Muscle Fibers Cross Sectional Area
For the NMJ innervation, junctions with completely overlapping pre- and postsynaptic immunostaining were counted as fully innervated; those with fragmental colocalization of nerve and acetylcholine (ACh) receptor plaques were defined as partially innervated; and those without associated presynaptic staining were considered as denervated. At least 10 images per animal were taken. For TA muscle fibers cross sectional area (CSA), three images were acquired per animal and quantified by measuring the area within positive dystrophin immunostaining using ImageJ software.
Statistics
Data measurements and analysis were performed by one examiner in a non-blinded manner. All data are presented as mean ± S.E.M and n represents the number of animals (unless otherwise stated). The number of animals per group analyzed for each experiment was as following: KV1.2 localization, 4.1B and KV1.2 immunoblot analysis, NMJ innervation/endplate area and ultrastructural morphology, TA muscle CSA – 3; ultrastructural analysis of myelinated axons – 3 (100 axons from each animal were plotted); NCV and weight analysis – 7–10. Statistically significant differences between mutant and control groups were determined by 1- or 2-way ANOVA with Bonferroni’s post hoc test using GraphPad Prism5 software and are represented by *P<0.05; **P<0.01; ***P<0.001.
Results
Loss of Paranodal Caspr1 and/or Juxtaparanodal Caspr2 Leads to Redistribution of KV1.2 Channels Along Myelinated Axons
Caspr1 and Caspr2 have been well characterized for their role in the organization of specific axonal domains in myelinated axons. Caspr1 is a component of the paranodal complex and participates in the formation of paranodal axo-glial junctions to allow segregation of nodal and juxtaparanodal ion channel complexes (Bhat et al., 2001), while Caspr2 is enriched at the JPN and is required for proper clustering of KV1.2 and integrity of this region (Poliak et al., 2003). Recent studies show important role of these proteins in maintaining proper localization of KV1.2 in the internodal region of the PNS myelinated axons (Gordon et al., 2014), revealing formation of KV1.2 aggregates along mesaxonal line in the Caspr1/2 double mutants. While the role of Caspr1/Caspr2 at their respective domains in myelinated axons is established, several questions still remain to be addressed: (i) what is the developmental timeline for the axonal domain disintegration when these proteins are ablated separately or together; and (ii) whether loss of Caspr1 and/or Caspr2 has similar consequences in the PNS and CNS overtime.
In order to address these questions, we performed immunostaining of PNS and CNS myelinated fibers at different developmental time points (P20, P40, P40 and P180). Double immunostaining of SNs from wt, Caspr1, Caspr2 and Caspr1/Caspr2 double (Caspr1/2) knockout mice with antibodies against juxtaparanodal KV1.2 (green) and nodal marker βIV Spec (in red) revealed altered axonal distribution of KV1.2 in single and double mutants (Fig. 1A–D). While in the wt SNs, KV1.2 is localized at the juxtaparanodal region throughout all developmental time points analyzed (Fig. 1Aa–Da); in Caspr1 mice, KV1.2 immunostaining is invading paranodal region (Fig. 1Ab–Db). On the other hand, Caspr2 mutants show either undetectable levels of KV1.2 (Fig. 1Ac–Dc) or still preserved juxtaparanodal staining (Fig. 1Ad–Dd). Finally, Caspr1/2 double mutants have a combination of myelinated fiber either with diffuse, undetectable KV1.2 levels (Fig. 1Ae–De), as in Caspr2 knockout animals, or with KV1.2 positive paranodal immunostaining flanking βIV Spec positive nodes, similar to the observation in Caspr1 mice (Fig. 1Af–Df). Moreover, formation of KV1.2 positive aggregates along internodal regions in PNS myelinated fibers was not found at P20, P40 or P60, but was clearly observed in P180 Caspr1/2 double mutants but not in wt, Casp1, or Caspr2 mice (Fig. 1H marked with asterisks compared to Fig. 1E,F,G, respectively).
Figure 1. Caspr1 and Caspr2 Are Required for the Retention of KV1.2 Channels at the Juxtaparanodes.
(A–D) Immunostaining of SN fibers from P20 (A), P40 (B), P60 (C), P180 (D) wt (a), Caspr1 (b), Caspr2 (c,d) and Caspr1/2 (e,f) mice with antibodies against KV1.2 (green) and βIV Spec (red). Note that Caspr2 knockout animals have two prevalent phenotypes with KV1.2 either undetectable (c) or preserved at the JPN (d). Whereas Caspr1/2 double mutants have a combination of undetectable (e) and KV1.2 at the PN (f).
(E–H) Teased SN fibers from P180 wt (E), Caspr1 (F), Caspr2 (G) and Caspr1/2 (H) mice were immunostained with antibodies against KV1.2 (green) and βIV Spec (red). Arrowheads indicate KV1.2 localization at the Schmidt-Lanterman incisures; while asterisks mark large aggregates of KV1.2 along myelinated axons found only in Caspr1/2 double knockout mice
(I–L) Immunostaining of SC fibers from P20 (I), P40 (J), P60 (K), P180 (L) wt (a), Caspr1 (b), Caspr2 (c) and Caspr1/2 (d) mice with antibodies against KV1.2 (green) and βIV Spec (red).
(M, N) Quantification of changes in KV1.2 localization at P20 and P180 in SN fibers (M) and SCs (N) of wt, Caspr1, Caspr2 and Caspr1/2 mice (n=3, comparison of diffused KV1.2 between different genotypes within the same age group is performed by 1-way ANOVA with Bonferroni’s post hoc test; *P<0.05, **P<0.01, ns-not significant)
(O, P) Immunoblot analysis of SN (O) and SC (P) lysates from P60 wt, Caspr1, Caspr2 and Caspr1/2 mice with antibodies against KV1.2 and α-tubulin (Tub).
Scale bar, 2 μm. All data are represented as mean ± SEM.
Immunolabeling of CNS myelinated fibers with KV1.2 and βIV Spec revealed KV1.2 positive JPN in the wt SCs, and similar to PNS altered localization of KV1.2 in all single and double mutants throughout all the developmental time points analyzed (Fig. 1I–L). Moreover, prominent elongation of the nodal gap using βIV Spec immunostaining was observed in Caspr1/2 double mutants compared to wt, Caspr1, and Caspr2 animals. To further characterize the progression of KV1.2 mislocalization in the PNS and CNS myelinated axons, we quantified KV1.2 localization pattern at P20 and P180 for each genotype (Fig. 1M,N). Quantifications revealed increased percentage of myelinated fibers with undetectable levels of KV1.2 from P20 to P180 in the PNS of Caspr2 and Caspr1/2 animals, as well as in the CNS of all mutant genotypes. Moreover, the gradation of KV1.2 undetectable levels in all cases was Caspr1/2>Caspr2>Caspr1.
To further address whether undetectable levels of KV1.2 resulted from changes in total protein levels, we performed immunoblot analysis of SN and SC lysates at P60 (Fig. 1O,P). While there were no changes in KV1.2 levels between wt and single mutants in both PNS and CNS, quantifications of 80 kDa KV1.2 band intensity in Caspr1/2 double mutants revealed significant reduction in comparison to wt (100±6.72% wt vs. 67.85±5.33% Caspr1/2 in the PNS; 100± 3.94 % wt vs. 57.90± 8.66% Caspr1/2 in the CNS). Together, our data show that combined loss of Caspr1 and Caspr2 have similar dramatic consequences on the localization and levels of KV1.2 in the both PNS and CNS, leading to progressive diffusion of KV1.2 along myelinated fibers, indicating that KV1.2 retention at the JPN is dependent on having intact axonal domains.
Cytoskeletal Scaffolding Protein 4.1B Localization is Altered in the Absence of Caspr1 and Caspr2
To further investigate the role of Caspr1 and Caspr2 in axonal domains integrity we analyzed localization of 4.1B, which has binding sites for both Caspr1 and Caspr2 and co-localizes with each of them at the PN or JPN, respectively (Denisenko-Nehrbass et al., 2003; Horresh et al., 2010; Buttermore et al., 2011). Even though it has been previously reported that localization of 4.1B is not compromised in the PNS of Caspr1 or Caspr2 mutants (Horresh et al., 2010; Gordon et al., 2014), no detailed analysis has been performed on CNS myelinated fibers or over time in PNS regarding 4.1B localization when Caspr1 and Caspr2 are simultaneously ablated. To address these questions, we performed double immunolabeling of SN and SC myelinated fibers with antibodies against 4.1B (red) and nodal βIV Spec at P20, P40, P60 and P180 in wt, Caspr1, Caspr2 and Caspr1/2 animals.
As expected in the PNS of the wt, Caspr1 and Caspr2 mutants, 4.1B localization is not altered and evenly present in paranodal, juxtaparanodal and internodal regions (Fig. 2Aa,b,c–Dabc). However, in Caspr1/2 double mutants, 4.1B immunoreactivity is consistently undetectable at the paranodal region, flanking both sides of βIV Spec positive nodes starting from P20 (Fig. 2Ad–Dd), suggesting that in the absence of both binding partners 4.1B fails to remain stable at the former paranodal region and moves out towards the juxtaparanodal/internodal space.
Figure 2. Caspr1 and Caspr2 Ablation Leads to 4.1B Mislocalization in Myelinated Axons.
(A–D) Immunostaining of SN fibers from P20 (A), P40 (B), P60 (C), P180 (D) wt (a), Caspr1 (b), Caspr2 (c) and Caspr1/2 (d) mice with antibodies against 4.1B (red) and βIV Spec (green). Arrowheads indicate the absence of 4.1B at the paranodal area in Caspr1/2 mutants.
(E–H) Immunostaining of SCs from P20 (A), P40 (B), P60 (C), P180 (D) wt (a), Caspr1 (b), Caspr2 (c) and Caspr1/2 (d) mice with antibodies against 4.1B (red) and βIV Spec (green).
(I, J) Immunoblot analysis of SN (O) and SC (P) lysates from P60 wt, Caspr1, Caspr2 and Caspr1/2 mice with antibodies against 4.1B and α-tubulin (Tub).
(K) Immunoblot quantification of 4.1B 125 and 145 kDa isoforms level from Caspr1, Caspr2, Caspr1/2 mice SCs compared to wt, normalized to Tub levels (n=3, comparison between genotypes is performed by 1-way ANOVA with Bonferroni’s post hoc test;*P<0.05).
Scale bar, 2 μm. All data are represented as mean ± SEM.
In the CNS wt, Caspr1 and Caspr2 mutants show 4.1B distribution along the IN, JPN and PN with significantly higher immunoreactivity in the paranodal area (Fig. 2Ea,b,c–Ha,b,c). However, in Caspr1/2 animals this well-defined paranodal localization was absent; and instead, 4.1B was distributed along the entire axonal length, excluding the nodal gap (Fig. 2Ed–Hd). Immunoblotting of SNs and SCs lysates against 4.1B and quantifications of corresponding bands intensities revealed no changes in protein level in SNs among all four genotypes (Fig. 2I, quantification data are not shown); nevertheless, the 4.1B 145- and 125-kDa band intensities were decreased in Caspr1/2 SCs (Fig. 2J–K). Together, these data indicate that loss of Caspr1 and Caspr2 alone does not affect 4.1B localization in myelinated axons most likely due to their interchangeable compensation in 4.1B stabilization; however, simultaneous loss of both proteins leads to alterations in 4.1B localization at the paranodal region and may also affect 4.1B stability in myelinated axons.
Paranodal and Juxtaparanodal Domains are Necessary for Proper Propagation of Action Potentials by Myelinated Axons
The organization of myelinated axons into distinct domains is critical for their ability to conduct action potentials in a fast saltatory manner. Previously nerve conduction studies had been performed on single Caspr1 and Caspr2 knockout animals (Bhat et al., 2001; Poliak et al., 2003). While Caspr1 mice show a dramatic decrease in conduction properties of myelinated axons, no changes in nerve impulse propagation were reported for Caspr2 mutants. SNs of Caspr1/2 knockouts displayed reduced conduction velocity that was not significantly different from the Caspr1 mutants (Gordon et al., 2014). However, there are no data on how disruption of both PN and JPN, from loss of Caspr1 and Caspr2, would affect nerve conduction properties over time with only nodes remaining along the axons. To investigate these questions, we performed in vivo NCV recordings in the sciatic and tail nerves of the wt, Caspr1, Caspr2 and Caspr1/2 animals at different developmental time points (representative curves in Fig. 3A,B). As wt, Caspr1 and Caspr2 animals age, their NCV values increase in both sciatic (Fig. 3C P20 compared to P180) and tail nerves (Fig. 3E P40 compared to P180). However, the nerve conduction parameters from P20/40 to P180 in Caspr1/2 double mutants did not change significantly (Fig. 3C,E). Moreover, by P180 Caspr1/2 animals showed the slowest tail NCV in comparison to wt, Caspr1 or Caspr2 mice (Fig. 3E). Quantifications of compound action potential (CAP) amplitudes in the SNs revealed a trend towards increased values over time in wt, Caspr1 and Caspr2 animals (which was not significant due to high variations), whereas in Caspr1/2 mice the trend was opposite with reduced CAP amplitudes by P180 compared to P20 (Fig. 3D). In the tails similar to NCV quantifications, values of CAP amplitudes of wt, Caspr1 and Caspr2 mice were doubled by P180 compared to P40, but not in Caspr1/2 animals (Fig. 3F). Furthermore, by P180 Caspr1/2 CAP amplitudes were smaller than in any other three genotypes in both sciatic and tail nerves. Overall our data indicate that loss of Caspr1 alone or in combination with Caspr2 leads to alterations in electrophysiological features of myelinated axons. Moreover, loss of Caspr2 in addition to Caspr1 has synergetic effect leading to more severe reduction in conductive properties of myelinated fibers over time.
Figure 3. Peripheral Nerve Conduction Properties Decline Overtime after Loss of Caspr1 and Caspr2.
(A–B) Representative electrophysiological profiles of CAPs from SNs (A) and tails (B) of P180 wt, Caspr1, Caspr2 and Caspr1/2 mice.
(C–F) Quantification of NCV (C, E) and amplitude (D, F) respectively from SNs and tails of P20, P40, P60, P180 wt, Caspr1, Caspr2 and Caspr1/2 mice (n=7–10, comparisons between genotypes or between different age within the same genotype performed by 2-way ANOVA with Bonferroni’s post hoc test; black star – compared to P20 wt; red star – compared to P20 Caspr1; blue star - compared to P20 Caspr2; green star- compared to P20 Caspr1/2; *P<0.05, **P<0.01, ***P<0.001, ns-not significant). All data are represented as mean ± SEM.
Loss of Paranodes and Juxtaparanodes Leads to Axonal Pathology in Myelinated Fibers
To investigate if axonal domain disorganization observed in Caspr1, Caspr2 and Caspr1/2 mutants can lead to ultrastructural changes, we performed TEM analysis of PNS and CNS myelinated axons. Proximal and distal parts of SNs were analyzed at P180 in wt, Caspr1, Caspr2 and Caspr1/2 animals. For all genotypes, the proximal part of the SNs showed proper myelinated fibers, with no differences in G-ratio values (Fig. 4A,E). However, plotted distribution of axonal diameter values revealed a shift towards reduced axonal diameter in mutant animals (4–5 μm for wt vs. 3–4 for Caspr1, Caspr2 and Caspr1/2 mice) (Fig. 4F). TEM from P180 distal SNs revealed advanced pathology of myelinated fibers in Caspr1/2 mice (shrinking axons, disintegrated myelin ensheathment) (denoted by arrowheads), as well as, some moderate changes in axonal morphology in single Caspr1 and Caspr2 mutants (infolding of the myelin sheath); while, wt myelinated axon anatomy remained preserved (Fig. 4B).
Figure 4. Loss of Caspr1 and Caspr2 Results in Myelinated Axon Pathology.
(A) TEM of cross sections from proximal SNs of P180 wt, Caspr1, Caspr2 and Caspr1/2 mice.
(B) TEM of cross sections from distal SNs of P180 wt, Caspr1, Caspr2 and Caspr1/2 mice. Black arrowheads indicate axons with degeneration and myelin abnormalities.
(C) Immunostaining of SC fibers from P180 wt, Caspr1, Caspr2 and Caspr1/2 mice with antibodies against pan-Nf (green) and MBP (red). White arrowheads indicate axons with preserved MBP but missing pan-Nf immunostaining.
(D) Electron micrograph of SC axons from P180 wt, Caspr1, Caspr2 and Caspr1/2 mice. Black arrowheads indicate degenerating axons.
(E–H) Morphometric analysis of myelinated axons in proximal SNs (E,F) and SCs (G,H) of P180 wt, Caspr1, Caspr2 and Caspr1/2 mice, showing G-ratio values and axonal size distribution frequency (n=300 axons for each group).
In the CNS we first performed double immunolabeling of P180 SCs cross-sections with antibodies against pan-Nf (green) and MBP (red). While wt, Caspr1 and Caspr2, Nf positive axons were surrounded by MBP immunostaining; in Caspr1/2 double mutants cases of MBP positive areas with missing Nf positive axons were also found, suggesting axonal degeneration (Fig. 4C, arrowheads). The presence of axonal pathology in SCs of Caspr1/2 animals was further confirmed by TEM imaging, and to a milder extent in Caspr1 mice; however, such axonal pathology was not detected in the wt or Caspr2 SC myelinated fibers (Fig. 4D). G-ratio quantifications did not reveal any differences in myelination (Fig. 4G), and similar distribution of axonal diameter was observed between all studied genotypes (Fig. 4H). Together, our data indicate that ultrastructural changes at the distal part of the peripheral axons primarily happens across all studied mutant genotypes, suggesting potential ‘dying back’ degeneration; while the myelination of axons in that timeline remains relatively unaffected.
Single or Combined Ablation of Caspr1 and Caspr2 is Accompanied by Pre- and Postsynaptic Changes at the Neuromuscular Junctions
The proper molecular and ultrastructural organization of peripheral myelinated axons is not only critical for conducting neuronal impulses but also for delivering these signals to muscle fibers at NMJ. To assess whether the NMJ properties are altered by changes in axonal domain organization and thus reduced nerve transmission, we examined the NMJs of TA muscle, a fast-twitch muscle which is innervated by the SN. At P20 and P180, TA muscle NMJs in wt, Caspr1, Caspr2 and Caspr1/2 mutant animals were immunostained using antibodies to label presynaptic terminals (with a combination of anti-Nf and anti-Syt antibodies) together with fluorophore-conjugated α-BTX, which binds to postsynaptic ACh receptors (Balass et al., 1997). At P20, NMJs of all studied genotypes are properly developed and fully innervated (Fig. 5A–D); however, quantifications reveal significantly reduced postsynaptic motor endplates in Caspr1 and Caspr1/2 mutants with much smaller α-BTX positive area (Fig. 5J). Further examination of the NMJs at P180 revealed the presence of pre-synaptic together with post-synaptic changes in Caspr1, Caspr2 and Caspr1/2 animals (Fig. 5E–H). By P180, a significant percentage of NMJs in single and double mutants were fully denervated (25.92±5.23%; 13.35±3.11%; 25.99±6.75% in Caspr1, Caspr2 and Caspr1/2 animals, respectively) (Fig. 5I), together with smaller post-synaptic endplate areas (Fig. 5J) in mutants compared to wt control littermates. Together these data indicate that reduction of nerve conduction due to disruption of PN and JPN alone or in combination eventually alters NMJ integrity, leading to changes at both pre- and postsynaptic areas.
Figure 5. Loss of Caspr1 and Caspr2 Affect Pre- and Postsynaptic Integrity at the Neuromuscular Junctions.
(A–H) TA muscle NMJ from P20 (A–D) and P180 (E–H) wt, Caspr1, Caspr2 and Caspr1/2 mice immunostained with antibodies against pan-Nf and Syt (green) together with α-BTX (red). Arrowheads indicate denervated NMJs.
(I) Analysis of TA muscle NMJ innervation at P180 wt, Caspr1, Caspr2 and Caspr1/2 mice (n=3, comparison of denervated NMJs between genotypes is performed by 1-way ANOVA with Bonferroni’s post hoc test; *P<0.05, **P<0.01).
(J) Quantification of α-BTX-positive endplate area from P20 and P180 wt, Caspr1, Caspr2 and Caspr1/2 mice (n=3, comparison of endplate areas between genotypes within the same age group is performed by 1-way ANOVA with Bonferroni’s post hoc test; *P<0.05, ***P<0.001).
Scale bar, 50 μm. All data are represented as mean ± SEM.
Ultrastructural Changes at Neuromuscular Junctions in Caspr1 and Caspr2 Single and Double Mutants
Considering the altered NMJ morphology in Caspr1 and Caspr2 single and double mutants as observed by immunohistochemistry, we next carried out electron microscopic analyses to determine whether reduced nerve conduction leads to ultrastructural changes at the NMJs. Since NMJs have a well-defined presynaptic area and regularly folded postsynaptic areas, any changes in the overall NMJ structure can be identified by TEM. We analyzed TA muscle NMJs of P180 wt, Caspr1, Caspr2 and Caspr1/2 animals. In general, NMJs of single and double mutant animals were characterized by smaller presynaptic terminal areas together with thinner postsynaptic sites (Fig. 6). Moreover, the presynaptic terminals in all knockout mice were less packed with significantly smaller synaptic vesicles, which are reflected in a reduced vesicle density and vesicle diameter in Caspr1, Caspr2 and Caspr1/2 animals (Fig. 6I). These findings indicate possible alteration of neuromuscular transmission in mutants, where decreased synaptic pool (observed in Caspr1, Caspr2, Caspr1/2 mice) potentially may lead to reduced ACh release at the NMJs. Additionally, recent reports suggest that a presynaptic mitochondria pool is required for the regulation of neurotransmission (Guo et al., 2005; Verstreken et al., 2005). Our electron microscopy analysis allowed quantifications of mitochondria in the electron micrographs; however, we did not observe significant changes in the amount of mitochondria between genotypes, although Caspr1/2 mutants consistently revealed abnormal mitochondria. Caspr1/2 double mutant terminals showed highly electron-dense mitochondria which is reflective of mitochondrial dysfunction and oxidative stress at the presynaptic site of these animals (Fig. 6G,H, arrowheads). Together, our ultrastructural analyses suggest that proper maintenance of axonal domains along peripheral myelinated fibers is not only critical for nerve conduction but also for the maintenance of NMJ terminals and their ultrastructure in order to allow optimal synaptic transmission at the NMJ.
Figure 6. Neuromuscular Junction Ultrastructural Organization is Compromised in Caspr1 and Caspr2 Mutants.
(A–H) Electron micrographs of representative NMJs from TA muscle of P180 wt (A,B), Caspr1 (C,D), Caspr2 (E,F) and Caspr1/2 (J,H) mice. Black arrowheads indicate NMJs with abnormal mitochondria.
(I) Ultrastructural analysis of TA muscle NMJs from P180 wt, Caspr1, Caspr2 and Caspr1/2 mice with quantifications of presynaptic terminal area, vesicles size/density and number of mitochondria. Scale bar, 200nm (n=3, comparison between genotypes is performed by 1-way ANOVA with Bonferroni’s post hoc test; *P<0.05, ***P<0.001).
All data are represented as mean ± SEM.
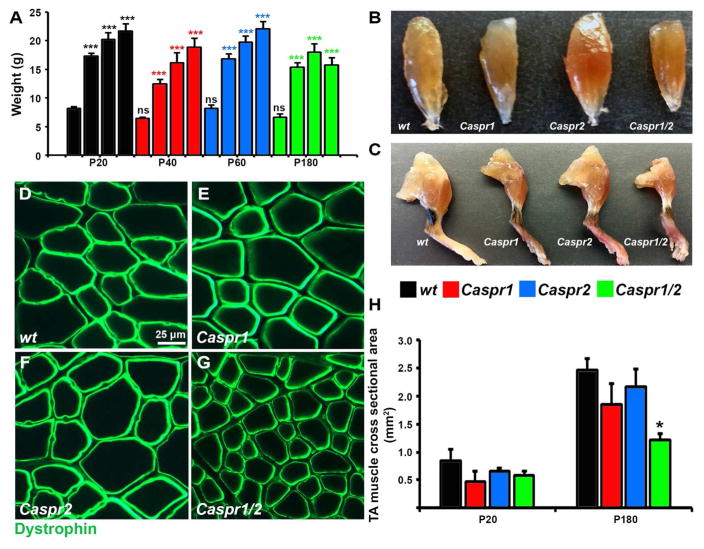
Muscle Mass is Severely Compromised as Nerve Conduction Declines in Myelinated Axons
To address whether changes in NMJ morphology caused by Caspr1 and/or Caspr2 loss can result in muscle pathology, gross muscle morphology was compared between wt, Caspr1, Caspr2 and Caspr1/2 double mutant animals. Evaluation of age-dependent weight changes at P20, P40, P60 and P180 revealed that Caspr1 and Caspr1/2 animals are consistently smaller than their age-matched wt and Caspr2 littermates (Fig. 7A). Wt, Caspr1 and Caspr2 animals from P20 to P180 demonstrated weight gain, however, from P40 to P180, the body weight increase of Caspr1/2 animals was less pronounced compared to other genotypes (Fig. 7A). On visual analysis, loss of hindlimb muscle mass, as well as atrophy of TA muscle was clearly evident in Caspr1 and to a significant extent in Caspr1/2 mutants (Fig. 7B,C). To confirm whether there is muscle atrophy in Caspr1 and Caspr1/2 animals, we performed immunostaining of TA muscle cross sections with dystrophin antibodies (Blake et al., 2002) and sequential quantification of CSA of dystrophin-positive fibers at P20 and P180. In cross-sectional views of P180 age-matched wt, Caspr1, Caspr2 and Caspr1/2 mice, the fiber diameters of Caspr1/2 animals are significantly reduced compared to other genotypes (Fig. 7G compared to D,E,F). Quantifications of TA muscle CSA revealed no significant changes at P20 between genotypes (Fig. 7H); however, by P180 Caspr1/2 mutant muscle fibers were significantly smaller by 51% in size compared to wt. Moreover, even though Caspr1 TA CSA at P180 was reduced by 25%, the difference compared to the wt did not reach significance. These data demonstrate that reduced nerve conduction by myelinated axons with disrupted axonal domains overtime leads to progressive reduction in muscle mass.
Figure 7. Proper Function of Myelinated Axons is Crucial for Muscle Mass Maintenance.
(A) Age-dependent changes in body weight of wt, Caspr1, Caspr2 and Caspr1/2 mice (n=7–10, comparisons between genotypes or between different ages within the same genotype were performed by 2-way ANOVA with Bonferroni’s post hoc test; black star – compared to P20 wt; red star – compared to P20 Caspr1; blue star - compared to P20 Caspr2; green - compared to P20 Caspr1/2; ***P<0.001, ns-not significant).
(B–C) Representative images of TA muscle (B) and right hind limbs from 1 year old wt, Caspr1, Caspr2 and Caspr1/2 mice.
(D–G) Representative images of cross-sections from TA muscle of P180 wt, Caspr1, Caspr2 and Caspr1/2 mice immunostained with antibodies against dystrophin.
(H) Mean CSA of TA fibers from P20 and P180 wt, Caspr1, Caspr2 and Caspr1/2 mice (n=3, comparison between genotypes within the same age group is performed by 1-way ANOVA with Bonferroni’s post hoc test; *P<0.05).
Caspr1 and Caspr2 Ablation Leads to Muscle Atrophy with Mitochondria Pathology
To complement our studies and to examine whether the reduced muscle mass was associated with any ultrastructural changes in muscle fibers after axonal domain disorganization, we performed TEM on P180 TA muscles of wt, Caspr1, Caspr2 and Caspr1/2 dko animals. On the cross- and longitudinal sections electron micrographs of wt, Caspr1 and Caspr2 myofibrils look similar in size and appear normal (Fig. 8A–C,E–G). However, myofibrils and contractile apparatus in Caspr1/2 muscle are visibly disorganized with the presence of degeneration (Fig. 8D,H). Moreover, Caspr1/2 mutant muscles display striking mitochondrial pathology with the presence of numerous swollen and malformed mitochondria containing disintegrated cristae (Fig. 8L compared to I,J,K). Interestingly, abnormal mitochondria in the muscles of Caspr1/2 knockout mice, were detected already at P40 (data are not shown), suggesting that mitochondrial changes might precede and be a hallmark of the further muscle atrophy. Our data indicate that disorganized PN and JPN in peripheral myelinated fibers eventually lead to muscle atrophy accompanied with mitochondrial pathology.
Figure 8. Ablation of Caspr1 and Caspr2 Leads to Degeneration of Muscle Fibers.
(A–D) TEM of TA muscle cross-sections from P180 wt, Caspr1, Caspr2 and Caspr1/2 mice.
(E–H) Electron micrographs representing TA muscle longitudinal-sections from P180 wt, Caspr1, Caspr2 and Caspr1/2 mice.
(I–L) Representative electron micrographs of mitochondria morphology in TA muscle from P180 wt, Caspr1, Caspr2 and Caspr1/2 mice.
Asterisks mark mitochondria with disrupted cristae, arrows indicate degenerating muscle fibers.
Discussion
Myelinated fibers in the PNS transmit motor information to the peripheral tissues and carry sensory information from the periphery to the CNS. In doing so they play a critical role in ensuring faithful transmission of impulses for motor coordination and sensation. Peripheral axons establish bi-directional interactions with Schwann cells that culminate in the establishment of unique axonal domains along myelinated axons, where specific proteins assemble into unique macromolecular complexes (Waxman and Ritchie, 1985; Salzer, 2003; Buttermore et al., 2013). For myelinated axons to conduct and deliver signals in a highly efficient and coordinated way, proper myelin insulation and maintenance of axonal domains is essential (Waxman, 1997). Similarly, the terminals of peripheral myelinated axons that form part of specialized structures, such as NMJ synapses, must also remain organized to ensure smooth synaptic transmission and coordination of muscle functions. The arrival of action potentials at the NMJ triggers release of ACh and activation of contractile mechanisms of muscle fibers (Takamori, 2012; Tintignac et al., 2015). Any loss of axonal integrity in the PNS has common detrimental consequences on neuromuscular performance, causing muscle wasting and atrophy, leading to the long-term disabilities in patients with peripheral neuropathies (Coleman, 2005). In our current study, we addressed the role of axonal proteins Caspr1 and Caspr2 for the nerve-muscle unit integrity. Our data show that single or double ablation of these proteins leads to pre- and postsynaptic pathological changes at the NMJs and their denervation. These neuromuscular deficits overtime lead to muscle atrophy or muscle fiber degeneration.
Caspr1 and Caspr2 in Axonal Domain Organization and Neuromuscular Pathology
Caspr1 and Caspr2 are two members of the Neurexin superfamily which are crucial for axonal domain organization along myelinated axons (Buttermore et al., 2013). Most critically both Caspr1 and Caspr2 bind to the paranodal/juxtaparanodal cytoskeletal scaffolding protein, Band 4.1B, to ensure stable interactions between the membrane proteins and axonal cytoskeleton. Loss of Caspr1/2 severely compromises these domains and clustering of juxtaparanodal Kv channels, and thus affects myelinated axon functions. Although their loss will affect nerve function and nerve conduction properties, little is known about their possible implications in neuromuscular disorders. Recent studies have linked human CASPR1 mutations to a severe form of arthrogryposis multiplex congenita, which is accompanied with muscle weakness, respiratory distress and premature death (Laquerriere et al., 2014). Human CASPR2 has been reported as a genetic risk factor for autism spectrum disorders and might also be crucial for proper muscle function, as recent reports reveal lower muscle strength in some children with autism (Kern et al., 2011). These associations raise interesting possibilities whether beyond their role in axonal domain organization, Caspr1 and Caspr2 have synaptic functions that might affect NMJs. This potential autonomous role at the mammalian NMJ for Caspr1 and/or 2 is supported by the Drosophila Neurexin superfamily member Neurexin I, which is a well-known cell-adhesion molecule at the larval NMJ (Banerjee et al., 2016). Most importantly, synaptic function of Caspr2 in the CNS has been reported in several recent studies (Anderson et al., 2012; Varea et al., 2015). However, despite our best efforts, we were not able to detect Caspr1 or Caspr2 at the mammalian NMJs by standard immunostaining techniques (data not shown).
Myelinated Axons and NMJ Integrity
PNS myelinated fibers and their terminals as part of the presynaptic machinery play an important role in the maintenance of NMJ structure and function. Pathological changes in peripheral axonal morphology and their electrophysiological properties are often accompanied with NMJ dysfunction, leading to impaired synaptic transmission and eventually NMJ denervation and degeneration (Maggs et al., 2008). Interestingly, a variety of “dying back” neurological disorders such as peripheral neuropathies, amyotrophic lateral sclerosis, and spinal muscular atrophy are characterized by initial degeneration of the distal axons with the presynaptic terminal being the most vulnerable part of this process (Cavanagh, 1979; Pollari et al., 2014). Light and electron microscopic analyses of myelinated fibers from Caspr1 and Caspr2 single or combined mutants demonstrate phenotypic similarities and pathologies with changes at the distal SN and presynaptic terminals. These axonal pathologies arise from disorganized axonal domains as well as disorganization of the axonal cytoskeleton and altered axonal transport. These phenotypes are consistent with previous observations in Caspr1 mutants, which showed impairment of fast axonal transport and accumulation of misoriented microtubules and Nfs together with accumulation of mitochondria leading to axonal swellings (Garcia-Fresco et al., 2006). In addition, axonal transport defects with reduced levels of light Nf subunits at the distal part of peripheral nerves with subsequent delay in NMJ elimination has been recently reported after ablation of Nfasc 155, which is the glial Caspr1-binding protein at the PN (Roche et al., 2014). Although axonal transport and NMJ defects at peripheral myelinated fibers were not observed at P10 in Caspr1 mutants, one can speculate that axonal transport pathology will eventually develop in older mutant animals, since ablation of Caspr1 protein inevitably leads to loss of Nfasc 155 from the PN (Bhat et al, 2001).
The ability of myelinated fibers to transmit action potentials to nerve terminals is critical in maintaining synaptic neurotransmission and postsynaptic architecture. Altered conductive properties can subsequently weaken cholinergic transmission leading to reduced strength and frequency of neurotransmitter release, which has been reported in mouse models of Charcot-Marie-Tooth disease (Spaulding et al., 2016). Our ultrastructural analysis of NMJ together with functional recordings from myelinated fibers support the idea that changes in electrophysiological properties affect neurotransmission at the NMJ after loss of Caspr1 and Caspr2 proteins. Even though our in vivo data show nerve conduction impairment only in Caspr1 and Caspr1/2 double mutant animals, it is suggested that Caspr2 loss could potentially alter repolarization phase of the action potential due to disorganization of KV1.2 channels at the JPN (Rasband and Shrager, 2000; Poliak et al., 2003; Verkerk et al., 2003). Since all mutant genotypes revealed a reduced vesicular pool (decreased vesicles size and density per terminal area) at the presynaptic terminals of the NMJs, it is suggestive of potential reduction in the overall synaptic transmission. Progressive denervation observed at P180 in Caspr1 and Caspr2 single and double mutant animals provides additional evidence of significantly reduced nerve impulses being received by the postsynaptic apparatus from the presynaptic terminal, even though initial innervation at P20 did not seem to be severely compromised.
At the NMJ, the assembly and maintenance of ACh receptor-rich postsynaptic apparatus is highly coordinated by peripheral nerves (Sanes and Lichtman, 2001). In Caspr1 and Casp1/2 double mutants, there was significant reduction in NMJ endplate areas as early as P20 which increased in severity by P180 indicating that altered axonal domains and their disorganization combined with reduced NCV may underlie changes at the postsynaptic site of the NMJ. Interestingly disruption of the juxtaparanodal domain as a result of Caspr2 loss did not show early endplate changes but showed changes as animals aged by P180 suggesting that cumulative effect of presynaptic nerve pathology leads to postsynaptic dysfunction overtime. Several studies have emphasized the important role of presynaptic mitochondrial organization for the proper NMJ function (Guo et al., 2005; Verstreken et al., 2005). Interestingly, our studies revealed electron-dense and swollen mitochondria in Caspr1/2 mutant nerve terminals suggesting potential secondary consequences with mitochondrial dysfunction in muscle fibers. Mitochondrial dysfunction has been linked with peroxidation due to increased ROS production by terminal mitochondria as a response to oxidative stress caused by axonal dysfunction, which could also contribute to axonal and muscle pathology observed in Caspr1/2 culminating in muscle atrophy. Thus, our results indicate that disruption of axonal domains, such as PN and JPN is a high-risk factor for NMJ disintegration, where axonal transport defects and potentially impaired synaptic transmission could become the underlying causes for disorganization of pre- and postsynaptic apparatus.
Axonal Domains Integrity is Crucial for Muscle Health
In various peripheral nerve disorders, like Charcot-Marie-Tooth disease or Guillain-Barré syndrome, structural abnormalities in the myelin sheath (dysmyelination) or changes in the axon structure affect nerve integrity leading to muscle weakness or severe muscle atrophy (Krajewski et al., 2000; Auer-Grumbach et al., 2003). Many studies have used peripheral nerve crush (Magill et al., 2007; Gigo-Benato et al., 2010) or nerve transection (Sakuma et al., 2016) as a model to determine the impact of axonal damage on muscle health. It has been well established that in these models rapid denervation with subsequent disintegration of the NMJ structure leads to muscle health deterioration. However, often in human neurological disorders decline in the electrical properties of myelinated axons occurs over an extended period, progressively altering muscle performance. Thus, mutant animal models are more preferable in order to determine what changes occur in muscle as nerve conduction declines overtime. Our results indicate that loss of muscle mass and muscle atrophy accompanied with mitochondria pathology is associated with disorganized axonal domains while myelination remains preserved. Our studies allow us to determine correlation between nerve conduction and muscle condition when axonal domains are disrupted. Thus, loss of PN after Caspr1 ablation leads to reduced conductive properties of myelinated fibers, visual changes in muscle morphology and modest muscle atrophy in Caspr1 mutants. Loss of JPN has no effect on nerve conduction or muscle morphology; however, this does not exclude development of muscle pathology involving functional or structural impairment overtime, since there are ultrastructural changes and elevated denervation at the NMJs of P180 Caspr2 mutants. In the most severe case of NCV reduction, after combined loss of PN and JPN, visually noticeable atrophic muscles, reduced in size myofibers and myofibrils degeneration accompanied with mitochondrial pathology was observed. Important conclusions can be drawn from our studies that there is a certain threshold in NCV reduction that needs to be reached in order for muscles to (i) start developing atrophy (Caspr1 case); and (ii) undergo degeneration (Caspr1/2 double knockout mutants). Moreover, our studies support the manifestation of mitochondrial dysfunction as a sign or even cause of muscular degeneration after axonal domains disruption. It would be of significant interest to determine additional cellular and molecular changes in muscles and nerves as a result of declined nerve conduction. Such information would be relevant to identifying the key mechanisms responsible for the loss of muscle strength and quality not only in pathological conditions but also during the normal aging process.
CONCLUSIONS
Our studies demonstrate that intact axonal domain organization is central for proper neuron-muscle communication. Loss of PNS axonal domain integrity primarily affects the most distal part of the myelinated fibers together with presynaptic terminals, sequentially leading to postsynaptic changes at the NMJ. Thus, defective myelinated axons in combination with altered conductive properties and NMJ disorganization ultimately results in severe muscle dysfunction and atrophy associated with motor disability.
Table 1.
List of Commercial Antibodies Used in this study
| Antibody | Immunogen | Manufacturer | Concentration |
|---|---|---|---|
| anti-potassium voltage-gated channel subfamily A member 2 (Kv1.2) | amino acids QYLQ-LTDV, cytoplasmic domain | UC Davis/NIH NeuroMab Facfility Cat# 75-008 RRID:AB_2296313 mouse monoclonal |
1:200 (IHC) 1:1000 (WB) |
| anti-Dystrophin | fusion protein containing the C-terminal 485 amino acids (3200–3684) of human dystrophin. | Sigma-Aldrich Cat# D8043 RRID:AB_259241 mouse monoclonal |
1:200 (IHC) |
| anti-Synaptotagmin | Zebrafish Syt2 | Zebrafish International Resource Center Cat# znp-1 RRID:AB_10013783 mouse monoclonal |
1:200 (IHC) |
| anti-Neurofilament | phosphorylated axonal epitopes on NF-M and NF-H | Covance Research Products Inc Cat# SMI-312R RRID:AB_2315329 mouse monoclonal |
1:200 (IHC) |
| anti-Myelin basic protein | residues 150 to the C-terminus of Mouse Myelin Basic Protein | Abcam Cat# ab40390 RRID:AB_1141521 mouse monoclonal |
1:200 (IHC) |
| anti-αTubulin | full length | DSHB Cat# 12G10 anti-alpha-tubulin RRID:AB_1157911 mouse monoclonal |
1:10000 (WB) |
IHC- Immunohistochemistry
WB – Western Blot
SIGNIFICANCE STATEMENT.
Functional interactions between neurons and muscles are crucial for the maintenance of the neuromuscular synapses. Propagation of nerve impulses along myelinated axons is required to allow muscle contraction and relaxation. Perturbations in these interactions as a result of nerve or muscle dysfunction underlie a variety of neuromuscular disorders including muscle atrophy. We demonstrate that disruption of selective axonal domains in myelinated axons differentially affects the electrical properties of these axons and neuromuscular synaptic integrity leading to progressive muscle atrophy.
Acknowledgments
Funding
This work was supported by grants from NIH NIGMS GM063074, National Multiple Sclerosis Society, the Zachry Foundation and the Morrison Trust. X. L. was an exchange medical student and supported by the Xiangya School of Medicine of the Central South University, China. A.M.T. was supported by the NIH NINDS Postdoctoral Fellowship (F32NS092448).
We thank Bhat Laboratory members for valuable suggestions and many helpful discussions; as well as, Jeff Dupree (Virginia Commonwealth University) for his advice on electron microscopy, which was performed at the UTHSCSA facility.
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
Author Contributions
J.S.: designed and performed experiments, and wrote the manuscript
X.L., A.M.T, J.L.: designed and performed experiments
M.A.B.: designed experiments and wrote the manuscript.
Data Accessibility
The following tools and software were used in the current study: ImageJ, RRID:SCR_003070 for images analysis (https://imagej.nih.gov/ij/); Graphpad Prism, RRID:SCR_002798 for statistical analysis (http://graphpad.com/).
References
- Anderson GR, Galfin T, Xu W, Aoto J, Malenka RC, Sudhof TC. Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development. Proc Natl Acad Sci U S A. 2012;109:18120–18125. doi: 10.1073/pnas.1216398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armati PJ, Mathey EK. Clinical implications of Schwann cell biology. J Peripher Nerv Syst. 2014;19:14–23. doi: 10.1111/jns5.12057. [DOI] [PubMed] [Google Scholar]
- Auer-Grumbach M, De Jonghe P, Verhoeven K, Timmerman V, Wagner K, Hartung HP, Nicholson GA. Autosomal dominant inherited neuropathies with prominent sensory loss and mutilations: a review. Arch Neurol. 2003;60:329–334. doi: 10.1001/archneur.60.3.329. [DOI] [PubMed] [Google Scholar]
- Balass M, Katchalski-Katzir E, Fuchs S. The alpha-bungarotoxin binding site on the nicotinic acetylcholine receptor: analysis using a phage-epitope library. Proc Natl Acad Sci U S A. 1997;94:6054–6058. doi: 10.1073/pnas.94.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Venkatesan A, Bhat MA. Neurexin, neuroligin and wishful thinking coordinate synaptic cytoarchitecture and growth at neuromuscular junctions. Mol Cell Neurosci. 2016 doi: 10.1016/j.mcn.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- Buttermore ED, Thaxton CL, Bhat MA. Organization and maintenance of molecular domains in myelinated axons. J Neurosci Res. 2013;91:603–622. doi: 10.1002/jnr.23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttermore ED, Dupree JL, Cheng J, An X, Tessarollo L, Bhat MA. The cytoskeletal adaptor protein band 4.1B is required for the maintenance of paranodal axoglial septate junctions in myelinated axons. J Neurosci. 2011;31:8013–8024. doi: 10.1523/JNEUROSCI.1015-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JB. The ‘dying back’ process. A common denominator in many naturally occurring and toxic neuropathies. Arch Pathol Lab Med. 1979;103:659–664. [PubMed] [Google Scholar]
- Cifuentes-Diaz C, Chareyre F, Garcia M, Devaux J, Carnaud M, Levasseur G, Niwa-Kawakita M, Harroch S, Girault JA, Giovannini M, Goutebroze L. Protein 4.1B contributes to the organization of peripheral myelinated axons. PLoS One. 2011;6:e25043. doi: 10.1371/journal.pone.0025043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Denisenko-Nehrbass N, Oguievetskaia K, Goutebroze L, Galvez T, Yamakawa H, Ohara O, Carnaud M, Girault JA. Protein 4.1B associates with both Caspr/paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres. Eur J Neurosci. 2003;17:411–416. doi: 10.1046/j.1460-9568.2003.02441.x. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Odaka M, Yuki N. Nodal proteins are target antigens in Guillain-Barre syndrome. J Peripher Nerv Syst. 2012;17:62–71. doi: 10.1111/j.1529-8027.2012.00372.x. [DOI] [PubMed] [Google Scholar]
- Doppler K, Appeltshauser L, Villmann C, Martin C, Peles E, Kramer HH, Haarmann A, Buttmann M, Sommer C. Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathy. Brain. 2016;139:2617–2630. doi: 10.1093/brain/aww189. [DOI] [PubMed] [Google Scholar]
- Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, Dupree JL, Bhat MA. Disruption of axo-glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proc Natl Acad Sci U S A. 2006;103:5137–5142. doi: 10.1073/pnas.0601082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigo-Benato D, Russo TL, Geuna S, Domingues NR, Salvini TF, Parizotto NA. Electrical stimulation impairs early functional recovery and accentuates skeletal muscle atrophy after sciatic nerve crush injury in rats. Muscle Nerve. 2010;41:685–693. doi: 10.1002/mus.21549. [DOI] [PubMed] [Google Scholar]
- Gordon A, Adamsky K, Vainshtein A, Frechter S, Dupree JL, Rosenbluth J, Peles E. Caspr and caspr2 are required for both radial and longitudinal organization of myelinated axons. J Neurosci. 2014;34:14820–14826. doi: 10.1523/JNEUROSCI.3369-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Yang J, Grati M, Kachar B, Bhat MA. Whirlin, a cytoskeletal scaffolding protein, stabilizes the paranodal region and axonal cytoskeleton in myelinated axons. BMC Neurosci. 2013;14:96. doi: 10.1186/1471-2202-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, Sheikh K. Schwann cell-axon interactions in Charcot-Marie-Tooth disease. Ann N Y Acad Sci. 1999;883:77–90. [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Horresh I, Bar V, Kissil JL, Peles E. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J Neurosci. 2010;30:2480–2489. doi: 10.1523/JNEUROSCI.5225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Adams JB, Troutman MR, Davis G, King PG, Young JL, Geier MR. Autism severity and muscle strength: A correlation analysis. Res Autism Spectr Disord. 2011;5:1011–1015. [Google Scholar]
- Krajewski KM, Lewis RA, Fuerst DR, Turansky C, Hinderer SR, Garbern J, Kamholz J, Shy ME. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain. 2000;123( Pt 7):1516–1527. doi: 10.1093/brain/123.7.1516. [DOI] [PubMed] [Google Scholar]
- Laquerriere A, et al. Mutations in CNTNAP1 and ADCY6 are responsible for severe arthrogryposis multiplex congenita with axoglial defects. Hum Mol Genet. 2014;23:2279–2289. doi: 10.1093/hmg/ddt618. [DOI] [PubMed] [Google Scholar]
- Lonigro A, Devaux JJ. Disruption of neurofascin and gliomedin at nodes of Ranvier precedes demyelination in experimental allergic neuritis. Brain. 2009;132:260–273. doi: 10.1093/brain/awn281. [DOI] [PubMed] [Google Scholar]
- Maggs AM, Huxley C, Hughes SM. Nerve-dependent changes in skeletal muscle myosin heavy chain after experimental denervation and cross-reinnervation and in a demyelinating mouse model of Charcot-Marie-Tooth disease type 1A. Muscle Nerve. 2008;38:1572–1584. doi: 10.1002/mus.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill CK, Tong A, Kawamura D, Hayashi A, Hunter DA, Parsadanian A, Mackinnon SE, Myckatyn TM. Reinnervation of the tibialis anterior following sciatic nerve crush injury: a confocal microscopic study in transgenic mice. Exp Neurol. 2007;207:64–74. doi: 10.1016/j.expneurol.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JK, et al. Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology. 2012;79:2241–2248. doi: 10.1212/WNL.0b013e31827689ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SS, Hayes JM, Sims-Robinson C, Sullivan KA, Feldman EL. The effects of anesthesia on measures of nerve conduction velocity in male C57Bl6/J mice. Neurosci Lett. 2010;483:127–131. doi: 10.1016/j.neulet.2010.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AM, Thaxton C, Pribisko AL, Cheng JG, Dupree JL, Bhat MA. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J Neurosci Res. 2009;87:1773–1793. doi: 10.1002/jnr.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollari E, Goldsteins G, Bart G, Koistinaho J, Giniatullin R. The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis. Front Cell Neurosci. 2014;8:131. doi: 10.3389/fncel.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Shrager P. Ion channel sequestration in central nervous system axons. J Physiol. 2000;525(Pt 1):63–73. doi: 10.1111/j.1469-7793.2000.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche SL, Sherman DL, Dissanayake K, Soucy G, Desmazieres A, Lamont DJ, Peles E, Julien JP, Wishart TM, Ribchester RR, Brophy PJ, Gillingwater TH. Loss of glial neurofascin155 delays developmental synapse elimination at the neuromuscular junction. J Neurosci. 2014;34:12904–12918. doi: 10.1523/JNEUROSCI.1725-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma M, Gorski G, Sheu SH, Lee S, Barrett LB, Singh B, Omura T, Latremoliere A, Woolf CJ. Lack of motor recovery after prolonged denervation of the neuromuscular junction is not due to regenerative failure. Eur J Neurosci. 2016;43:451–462. doi: 10.1111/ejn.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifetiarova J, Taylor AM, Bhat MA. Early and Late Loss of the Cytoskeletal Scaffolding Protein, Ankyrin G Reveals its Role in Maturation and Maintenance of Nodes of Ranvier in Myelinated Axons. J Neurosci 2017. 2017 Feb 1; doi: 10.1523/JNEUROSCI.2661-16.2017. pii: 2661–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Spaulding EL, Sleigh JN, Morelli KH, Pinter MJ, Burgess RW, Seburn KL. Synaptic Deficits at Neuromuscular Junctions in Two Mouse Models of Charcot-Marie-Tooth Type 2d. J Neurosci. 2016;36:3254–3267. doi: 10.1523/JNEUROSCI.1762-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susuki K, Chang KJ, Zollinger DR, Liu Y, Ogawa Y, Eshed-Eisenbach Y, Dours-Zimmermann MT, Oses-Prieto JA, Burlingame AL, Seidenbecher CI, Zimmermann DR, Oohashi T, Peles E, Rasband MN. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron. 2013;78:469–482. doi: 10.1016/j.neuron.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U, Scherer SS. Disease mechanisms in inherited neuropathies. Nat Rev Neurosci. 2003;4:714–726. doi: 10.1038/nrn1196. [DOI] [PubMed] [Google Scholar]
- Takamori M. Structure of the neuromuscular junction: function and cooperative mechanisms in the synapse. Ann N Y Acad Sci. 2012;1274:14–23. doi: 10.1111/j.1749-6632.2012.06784.x. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Saifetyarova J, Bhat MA. Postnatal Loss of Neuronal and Glial Neurofascins Differentially Affects Node of Ranvier Maintenance and Myelinated Axon Function. Front Cell Neurosci. 2017;11:11. doi: 10.3389/fncel.2017.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton C, Pillai AM, Pribisko AL, Dupree JL, Bhat MA. Nodes of Ranvier act as barriers to restrict invasion of flanking paranodal domains in myelinated axons. Neuron. 2011;69:244–257. doi: 10.1016/j.neuron.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintignac LA, Brenner HR, Ruegg MA. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol Rev. 2015;95:809–852. doi: 10.1152/physrev.00033.2014. [DOI] [PubMed] [Google Scholar]
- Uncini A, Susuki K, Yuki N. Nodo-paranodopathy: beyond the demyelinating and axonal classification in anti-ganglioside antibody-mediated neuropathies. Clin Neurophysiol. 2013;124:1928–1934. doi: 10.1016/j.clinph.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Varea O, Martin-de-Saavedra MD, Kopeikina KJ, Schurmann B, Fleming HJ, Fawcett-Patel JM, Bach A, Jang S, Peles E, Kim E, Penzes P. Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proc Natl Acad Sci U S A. 2015;112:6176–6181. doi: 10.1073/pnas.1423205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, Oostra BA Tourette Syndrome Association International Consortium for G. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003;82:1–9. doi: 10.1016/s0888-7543(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Axon-glia interactions: building a smart nerve fiber. Curr Biol. 1997;7:R406–410. doi: 10.1016/s0960-9822(06)00203-x. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Do ‘demyelinating’ diseases involve more than myelin? Nat Med. 2000;6:738–739. doi: 10.1038/77450. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci. 2006;7:932–941. doi: 10.1038/nrn2023. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Ritchie JM. Organization of ion channels in the myelinated nerve fiber. Science. 1985;228:1502–1507. doi: 10.1126/science.2409596. [DOI] [PubMed] [Google Scholar]