1. Introduction
In order to interact with the outer world, organisms have developed sensory systems that are able to integrate information from different spatio-temporal domains. This integration allows distinct sensory stimuli to be placed in a unified context but also, serves to preserve both spatial and temporal resolution. To perform such tasks, there are similar computational processes across different sensory pathways that allow stimuli to be integrated for further processing into a conscious perception. For example, the shaping of afferent information by lateral inhibition has long been known to be a critical process in multiple sensory systems including vision and somatosensation [1; 10; 22]. Such interactions between neighboring neurons in early sensory processing provide a computational mechanism to maximize spatial contrast between stimuli.
In the nociceptive system, little is known about the role of lateral inhibition. However, indirect lines of evidence suggest that it plays a significant role in the processing of afferent information. For example, localization of single point noxious thermal stimuli is accomplished with errors as small as approximately 1cm [12; 19; 31]. This accuracy exceeds that which would be predicted by the receptive field sizes (1.7cm diameter) of C polymodal nociceptive afferents [32; 33], and accordingly, would likely require additional processing centrally. Similarly, although spatial summation of pain is frequently noted, the effects are most often sub-additive in that an increase in the stimulus size typically results in a disproportionately small increase in pain [4; 6; 8; 15; 18; 20; 23; 27; 30; 34; 35]. Moreover, spatial summation of pain is clearly influenced by the configuration of stimuli. Discontiguous stimuli frequently elicit more pronounced spatial summation than contiguous stimuli, such that spatial summation of heat pain is maximal at distances of approximately 10cm [27; 30].
In contrast to other sensory modalities, delivering spatially complex nociceptive stimuli to test interactions that would be indicative of lateral inhibition has long remained challenging. However, the development of a computer targeted laser stimulator now provides the opportunity to test psychophysical responses to stimuli that can be delivered in different patterns while keeping the energy delivered to any given spot of skin constant [20]. Thus, the aim of the present investigation was to identify lateral inhibition during nociceptive processing by delivering stimuli in the form of lines of different lengths and comparing responses with two-point stimuli delivered with separation distances equal to the line lengths. Specifically, we tested the hypothesis that line stimuli would be subject to greater surround inhibition, and would therefore be perceived as less painful than two-point stimuli. Given previous findings on increased spatial summation of pain with increasing separation distances (up to 10–20 cm), we further hypothesized that stimuli which are delivered in the closest proximity will be subject to the greatest surround inhibition, but that the balance between inhibition and facilitation will shift towards summation as stimulus separation distances increase.
2. Methods
2.1 Subjects
All subjects participating in this study (11 males and 4 females) were healthy, pain and drug-free volunteers between 19 and 36 years old (average age 26.4). All participants gave written, informed consent acknowledging that they would experience experimental painful stimuli, that all methods and procedures were clearly explained, and that they were free to withdraw from the experiment at any time without prejudice. All of the procedures were approved by the local ethics committee of Northern Jutland, Denmark (ref. no N-20070029).
2.2 General stimulation methods
All thermal stimuli were delivered by a 100W CO2 laser (Synrad 57-1). A scanner head (GSI Lumonics General Scanning XY10A) containing two mirrors mounted on galvanometers rapidly, accurately, and reproducibly directed the 4 mm diameter laser beam over the skin. A 1 mm circular dithering was added to the laser trace resulting in a 5 mm diameter beam. The velocity of the laser movement was kept constant at 1525 mm/sec across all patterns of stimulation. The skin temperature during stimulation was assessed by infrared video thermography (Agema 900, FLIR Systems). Single pixel peak temperature values during the stimulated period were recorded.
All stimuli were applied to the abdomen as this body structure is relatively flat in relation to the more typically targeted arms and/or legs. Absorption of the laser energy by the skin is determined in part by the angle at which the laser beam hits the skin, since progressively oblique angles are associated with greater beam spread, potential reflection of the beam, and hence, less energy deposition.
2.3 Stimuli used to assess spatial integration of pain
Three different patterns of stimulation were employed to assess spatial integration of pain. Each pattern was presented 3–4 times during the experimental phase. All stimulus patterns were 5 seconds in duration. A minimum interval of 30 seconds between any two consecutive stimuli was maintained throughout the study to avoid long-term suppression or sensitization [26]. Each stimulated site was used only one time and stimuli were sequentially delivered using coordinates pre-programmed by computer software.
Single point stimuli: These stimuli consisted of heating a circular 0.5cm diameter area of skin and served as a control to assess spatial interactions occurring during other spatial configurations of stimuli.
Two-point stimuli: This stimulation pattern consisted of heating two circular 0.5cm diameter skin regions. The centers of these points were separated by 4, 8, and 12cm distances (Fig. 1). These stimuli were chosen to reproduce spatial summation that has been previously documented using similarly separated stimuli [20].
Line stimuli: This stimulation pattern consisted of heating skin in a contiguous 0.5cm width line. These lines were 4, 8, and 12cm in length (Fig. 1). These stimuli were chosen to assess how stimulus pattern recruits modulatory activity relative to two-point stimuli of the same separation distance. In contrast to our previous study [20] where numbers were traced onto the skin using a slow, readily perceptible movement of the laser beam over the skin (35mm/s), the entire length of the line was delivered effectively simultaneously (e.g., “stamped” rather than “traced”) by repetitively sweeping the laser beam rapidly across the skin at 1525 mm/s.
Figure 1. Schematic illustration of the stimulus configuration (drawn to scale).
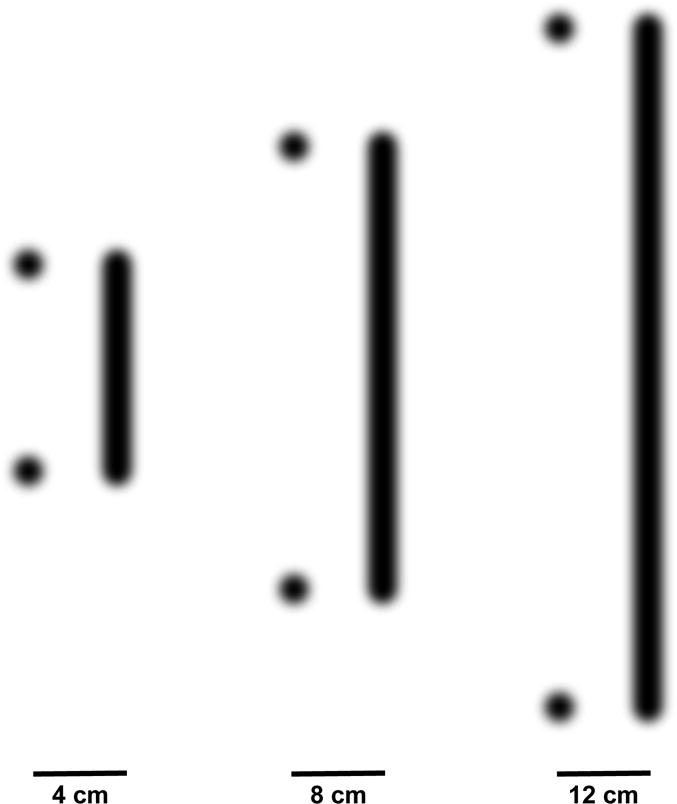
Two point and line stimuli were delivered at separation distances/lengths of 4, 8, and 12 cm. These stimuli were applied in a randomized order to the abdomen.
All different stimulus patterns (lines, pairs of stimuli, or single stimulus) were delivered to the abdomen in a horizontal orientation unilaterally (within dermatomes) and bilaterally (across the midline). Across all stimulation patterns and distances, the laser heat stimulus was applied to the skin with the exact same parameters (application frequency, velocity) to keep both energy deposition per skin area as well as stimulus duration constant. This was operationally accomplished by dynamically targeting the laser along each given line length. However, in the case of the one and two-point stimuli, the laser firing only occurred at the appropriate points, while for the line stimuli it fired along the entire length of the line. To further minimize potential confounds, different distances and stimulus patterns were presented in a random order.
The determination of stimulus intensity was accomplished by individual titration of laser power, using a two-step process. First, stepwise increases in the power of a single point stimulus were used to detect pain threshold using the method of constant stimuli. Next, a range of supra-threshold intensities was used to determine a laser power that evoked pain at an intensity that was rated between 1 and 3 visual analog units for the same single point stimulus. This relatively modest level of stimulation was chosen to provide ample margins of safety to protect research participants from tissue injury.
2.4 Psychophysical assessment and training
Subjects were in the supine position during sensory testing. In order to control for multi-sensory and attention interactions, subjects were required to focus their gaze on a point straight ahead and concentrate on the given rating task.
Following each stimulus, pain intensity was rated with mechanical visual analog scales (VAS) [24]. These 15cm sliding scales were anchored with the words “no pain sensation”-“the most intense pain imaginable”. After subjects slid the scale to the appropriate level that corresponded to their actual pain perception, the ratings were quantified by a labeled numeric index (0–10 range) on the back of the scale that was out of the subjects’ view. Qualitative ratings of each stimulus were also obtained. After providing a VAS rating, subjects were queried as to whether they perceived 1 point, 2 points, or a line.
Two series of training stimuli were used before starting the experiment. The first training series was used for stimulus intensity titration, while the second series of training stimuli exposed subjects to different stimulus patterns (lines, 2 point stimuli, 1 point stimuli) and provided them with practice rating each configuration.
A single overall VAS rating was obtained from each stimulus to evaluate pain intensity and spatial interactions (facilitation and inhibition). This single rating requires pain integration over large body areas and is typically used in studies of spatial summation [27; 28]. Subjects reported that they were able to perform the rating task after a few trials without any problems. This relatively fast and easy learning suggests that the difficulty associated with rating different stimulus patterns contributed minimally to alterations in spatial integration.
2.5 Statistical Analyses
Stimulus temperatures were compared between 2 point and line stimuli using a repeated measures analysis of variance (ANOVA) with planned post-hoc comparisons between each stimulation pattern at each stimulation distance. This was done to identify potential temperature differences that could confound interpretation of differences between stimulus patterns.
During analyses of psychophysical data, VAS ratings were first averaged across the 3–4 presentations of each condition for each subject. Using a repeated measures ANOVA, pain ratings from both lines and pairs were separately compared with those of single control stimuli to assess spatial summation of pain (SSP). Next, a two factor repeated measures ANOVA was used to determine the influence of stimulus pattern across stimulation distances.
Differences in the frequency of reports about the perceived spatial distribution of the stimuli were assessed with a Chi2 across stimulation distance and across stimulus type.
3. Results
3.1 Stimulation Temperatures
Infrared video thermography revealed that within both points and line stimuli, skin temperatures were homogeneous and uniformly distributed. A repeated measures ANOVA revealed that stimulus temperatures differed significantly between two-point stimuli and lines (F(3,42)=17.2016, p<.0001). Post-hoc pairwise comparisons between points and lines of equal distance revealed that this difference was restricted to the 12cm distance where lines were greater than points (F(1,14)=62.67, p<0.0001), while no differences were detected at the 4cm (F(1,14)=1.38, p<0.25), or the 8cm (F(1,14)=0.45, p<0.51) distances (Fig. 2). Thus, since heat deposition was greater for lines vs. points stimuli at 12 cm, data from the 12cm distance were excluded from all analyses of pain intensity.
Figure 2. Stimulus temperatures (°C) for two-point vs. line stimuli (means±SEM).
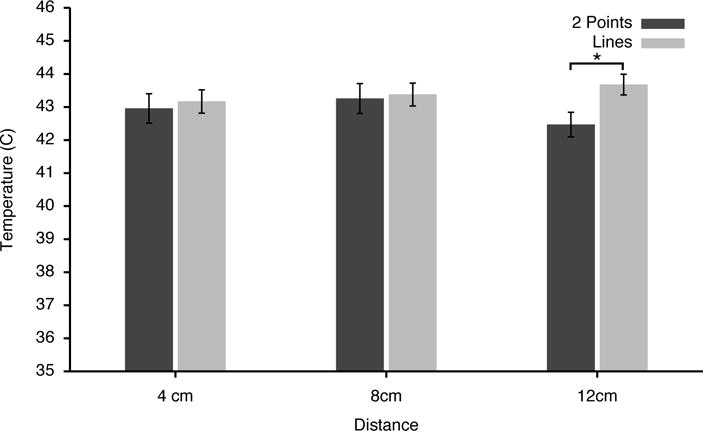
Infrared video thermography revealed that stimulus temperatures were not different between 2 points and line patterns at 4cm and 8cm distances. However, at 12cm distances, the temperatures of the two-point stimuli were significantly lower than those of the lines. Accordingly, the 12cm psychophysical data have been excluded from analyses.
3.2 Unilateral vs. Bilateral stimulation
A single factor repeated measures ANOVA revealed no statistically significant differences in pain intensity ratings between unilateral vs. bilateral stimuli (F(3,54)=0.089, p<0.98). To simplify data presentation, these conditions were pooled together for subsequent analyses.
3.3 Spatial Summation of Pain during Two-Point and Line Patterns
Pain intensity from two-point stimuli exhibited statistically significant spatial summation (F(2,28)=4.0669, p<0.0281, Fig. 3). Two-point stimuli separated by 4cm were rated as more intense than single point stimuli (p<0.047), while ratings of those separated by 8cm exhibited a strong trend to be more intense (p<0.0593). In sharp contrast to the spatial summation observed for two point stimuli, no significant spatial summation was observed for line stimuli (F(2,28)=1.4960, p<0.2414, Fig. 3). The absence of spatial summation of line stimuli is remarkable in light of the marked differences in stimulus areas between two-point and line stimulation patterns (Fig. 3). For example, during the 8cm line, the area of skin stimulated was more than 500% larger than that of a single point stimulus.
Figure 3. Pain intensity ratings by stimulus distance for two-point stimuli and lines (means±SEM).
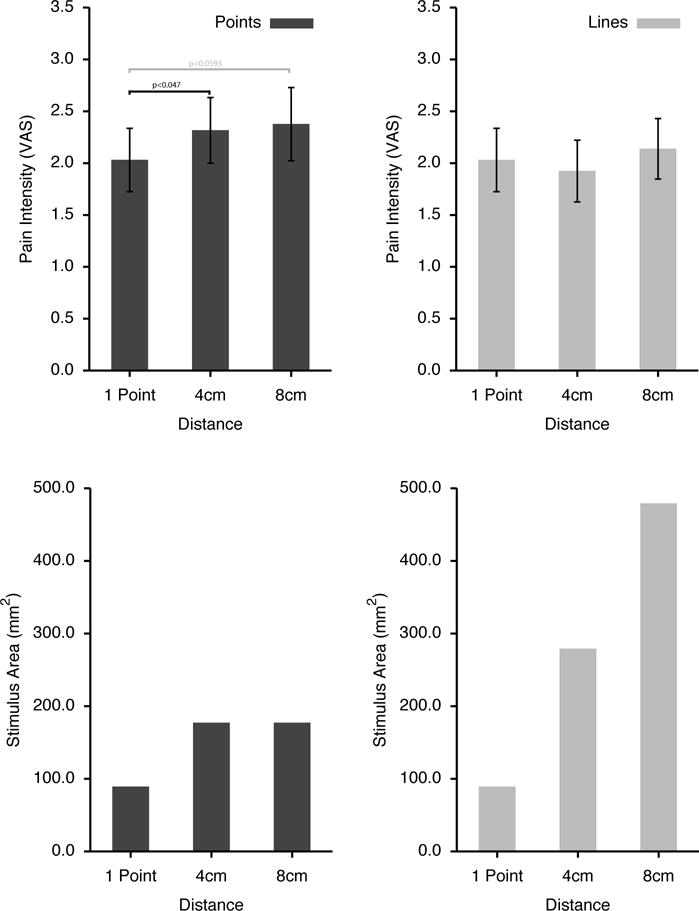
Significant spatial summation was observed for two-point stimuli, but was not detected during the line stimuli (upper panels). The absence of detectable spatial summation during the line stimuli is notable due to the substantial increase in stimulus areas of the line stimuli as the lines grew longer (lower panels).
3.5 Pain Intensity Ratings during Two-Point vs. Line Patterns
The crucial test of the main hypothesis of this study is comparison of pain from two-point stimuli vs. line stimuli. In this comparison, pain from two-point stimuli was perceived as significantly more intense than that from line stimuli (F(1,14)=14.56, p<0.0019; Fig. 4). Perceived pain intensity also increased as stimulation distance increased from 4 to 8 cm (F(1,14)= 9.82, p<0.0073), however the difference between 2 point vs. line patterns did not vary as a function of distance (F(1,14)=2.77, p<0.1185).
Figure 4. Pain intensity ratings for two-point vs. line stimuli (means±SEM. upper panel) and individual responses (lower panel).
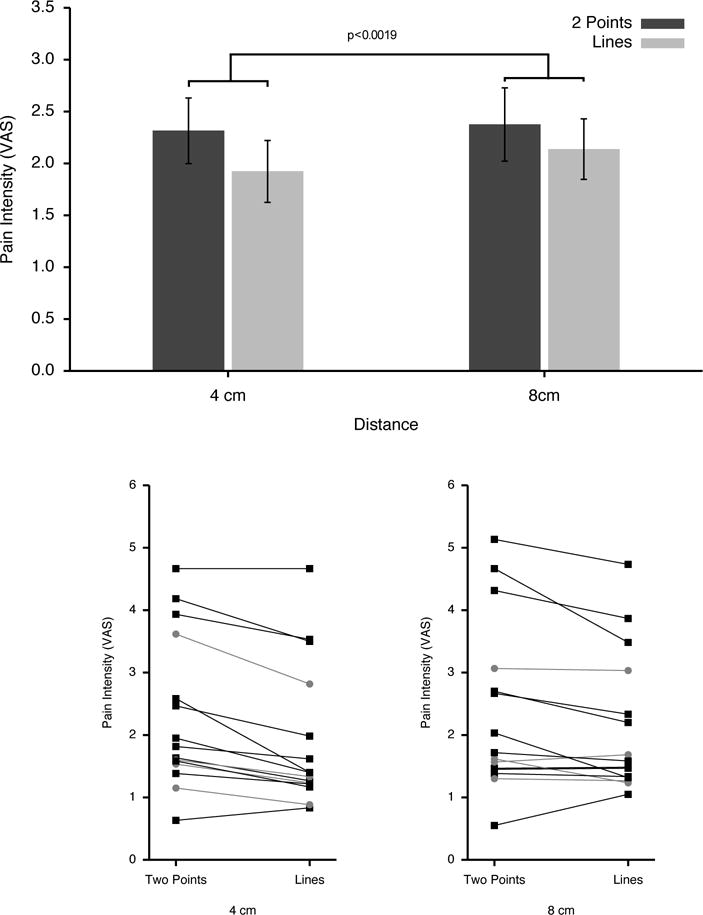
Despite involving markedly larger stimulation areas than the two-point stimuli, the line stimuli were perceived as significantly less painful than two-point stimuli. This effect remained consistent over the two different stimulation distances. Inspection of individual ratings (lower panel) reveals some individual variation in this response, however, female subjects (grey circles) did not appear to exhibit responses that varied systematically from those of male subjects (black squares). Nevertheless, this discordance between stimulus area and perceived pain intensity indicates that the pattern of stimulus application represents a critical variable that shapes the balance of facilitory vs. inhibitory interactions between nociceptive inputs arising from multiple body regions. Thus, the greater pain experienced during the two-point vs. the line stimuli provides evidence for lateral inhibition during nociceptive processing.
3.6 Qualitative reports about the perceived spatial distribution of stimuli
The frequency of qualitative reports about the spatial distribution of stimuli varied across stimulus type (Fig. 5). At 4cm distances the frequency of reports of the perception of 1 point vs. 2 points vs. line was not significantly different across stimulus type (line vs. 2 points) (Chi2=0.289, p<NS, Fig. 5). As stimulation distance increased to 8cm, the frequency of reports significantly differed according to stimulus type (Chi2=26.043, p<0.001). For two-point stimuli, subjects more frequently reported perceiving two points than either lines or 1 point. For line stimuli, subjects reported perceiving lines most frequently, although a substantial portion of responses were two points (Fig. 5).
Figure 5. Frequency of reports of different perceived spatial configurations.
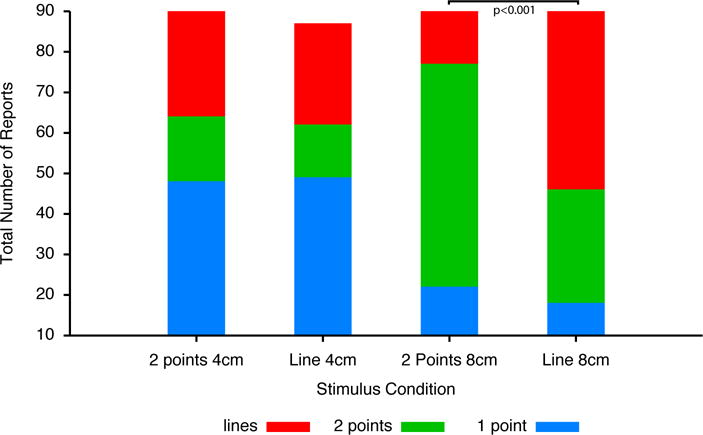
At 4 cm stimulation distances, the frequency of reports of different spatial configurations did not differ between 2 point stimuli and line stimuli. Both stimuli were characterized by frequent reports of “1 point” regardless of the stimulus type. However, at 8 cm distances subjects correctly reported “two points” most frequently during two-point stimuli, and “lines” most frequently during the line stimuli.
4. Discussion
While remote inhibitory processes associated with descending control of pain and diffuse noxious inhibitory control of pain have received a great deal of attention, more locally mediated inhibition of pain has remained poorly explored. The present psychophysical data provide strong, yet indirect, evidence that locally mediated inhibitory processes contribute substantially to interactions among afferent inputs from noxious stimuli that are in close spatial proximity. The balance between these inhibitory processes and facilitatory interactions serves to importantly shape the processing of afferent nociceptive information and the subsequent perceptual experience.
4.1 Spatial summation of pain and facilitatory interactions
Spatial summation is a classic example of interaction between multiple stimuli. The vast majority of studies of SSP surprisingly find that the degree of SSP is sub-additive [4; 6; 8; 18; 20; 23; 27; 30; 34; 35]. Classical studies examining the relationship between stimulus area and perceived pain even concluded that SSP did not exist [9]. However, factors related to the stimulus characteristics such as distance between stimuli [28] and their spatial pattern (present data) provide an explanation for both the sub-additivity as well as discrepancies between studies.
SSP is critically dependent on the spatial distribution of the stimuli and the populations of neurons that they recruit. Summation of pain from stimuli that are in close proximity is relatively minimal, but increases to be near maximal at separation distances of approximately 10cm [27; 28]. In the case of stimuli that are separated by an optimal distance for maximal summation, the populations of neurons activated by each stimulus may overlap to a degree. The facilitatory processes that occur in this overlap thus may result in a perception of a larger area being stimulated [30]. This phenomenon has been reported as the “filling-in phenomenon” in other sensory modalities [13] and can influence the magnitude of pain that is perceived during multiple stimuli [30].
The limited SSP when stimuli are in close proximity may, in part, be related to the relatively large receptive field sizes of nociceptive neurons in the CNS, especially wide dynamic range neurons in the deep dorsal horn [25]. Thus, two noxious stimuli in relatively close proximity may activate largely the same neuronal population that is activated by one stimulus alone, and thereby, produce only limited facilitatory interactions.
Interestingly, many modern investigations of SSP have typically used multiple, spatially separated thermodes to deliver noxious stimuli, while the classic studies used spatially contiguous stimuli produced by the application of increasing diameters of radiant heat [9]. Moreover, several modern studies using contiguous stimuli do not detect spatial summation of pain beyond the summation of pain observed at threshold [5; 14], or see a rapid plateau in summation despite dramatic increases in stimulus area [35]. Accordingly, contiguous stimuli used in investigations of spatial summation may be less effective at evoking facilitatory interactions than spatially separated stimuli. Conversely, lateral inhibition may also limit summation of stimuli in close proximity, such that the final percept represents a balance of facilitation and inhibition [18]. This effect may be particularly evident where stimulating progressively larger, contiguous areas of the body surface results in minimal spatial summation of pain [18].
4.2 Evidence for multiple types of lateral inhibition
Inhibition can profoundly shape interactions between stimuli and may limit summation to sub-additivity [8; 15; 18; 35]. In the present study, there was no SSP during the 4cm and 8cm line stimuli compared to the single point stimuli, despite the fact that areas 313% and 538% larger were stimulated during the line stimuli. More importantly, subjects rated pain intensity significantly lower for the line stimuli than for equidistant pairs of stimuli. When taken together with the limited spatial summation seen when stimuli are in close proximity, these findings strongly suggest that the line stimuli are engaging one or more inhibitory processes.
Lateral inhibition is a common neurocomputational function and may occur at multiple levels of the nociceptive neuraxis, ranging from the spinal cord, to thalamus, to SI, and beyond. However, the strongest and most extensive evidence for lateral inhibition lies within the spinal cord. Spinal cord nociceptive neurons have long been known to have large inhibitory surround receptive fields [7]. These inhibitory fields may occupy nearly the entire body outside of the excitatory zone. Moreover, this inhibition is not dependent on descending processes as it is largely preserved after spinal cord transection [7]. However, such large fields would be predicted to exert nearly equal influence on stimuli that were in close proximity as well as those that were widely separated. Thus, these large inhibitory surrounds may account for the sub-additive excitation produced by stimuli that are separated by ~10cm or more [29].
Large, nearly whole body inhibitory fields cannot, however, explain the progressive reductions in spatial summation that occur as stimulus separation distances are decreased below ~10cm. Although (as noted above) a large overlap in the populations of recruited neurons may explain a portion of this reduced spatial summation, neither large inhibitory fields nor diminished population recruitment can explain why line stimuli were perceived as less painful than two-point stimuli. Accordingly, an additional inhibitory mechanism appears to be required to explain spatial interactions below a ~10cm radius.
Lateral inhibition between two punctate stimuli has been reported for tactile and non-nociceptive warm stimuli delivered in close proximity [1; 2] and can provide insight into the dependence of spatial summation of pain on stimulus configuration. During pairs of stimuli, the sensory magnitude increased as the distance between the two stimuli increased (from 0 to 2cm). However, after a certain separation distance (around 3–4.5 cm), the perceived intensity of the stimuli became smaller for two-point stimulation than for a single stimulus [2]. The term “neural units” was used to describe the receptive field of an individual neuron involving a combination of sensation area (excitatory zone) and refractory area (inhibitory surround zone). These early studies on lateral inhibition suggested that it would be attributed to central interconnections [1].
In the spinal cord, both widespread and local primary afferent depolarization has been identified [16]. Such inhibition may contribute importantly to the dynamic regulation of the receptive field sizes of spinal cord neurons [38]. The local form of primary afferent depolarization may reflect lateral inhibitory processes that are of a spatial scale sufficient to account for the reduced pain observed during the line stimuli. Such primary afferent depolarization may be supported to a substantial degree by the action of GABA-ergic interneurons [38]. A large population of interneurons in laminae I–II (30–45%) is GABA-ergic neurons [36] and during thermal stimulation of the receptive field, activation of these interneurons can inhibit neurons in the deeper laminae of the spinal cord. In the case of nociceptive stimuli, a circuit in which nociceptive C-fiber input regulates the influence of other C-afferents by a GABA-ergic mechanism has been identified in substantial gelatinosa of the dorsal horn of the spinal cord [17]. Such a circuit would be well-positioned to support the local inhibition that occurs during both line stimuli as well as stimuli that are in close proximity. Finally, in vivo patch clamp recordings of nociceptive neurons indicate that local inhibitory receptive fields of nociceptive neurons paradoxically exert maximal inhibition at the center of the excitatory receptive field and that these inhibitory receptive fields are broader than corresponding excitatory receptive fields [11]. Accordingly, contiguous stimuli would be subject to more local inhibition than spatially separated stimuli. Thus, spatial summation of pain would be greater for two-point stimuli than line stimuli.
4.3 Lateral Inhibition, Spatial Discrimination, and Chronic Pain
Lateral inhibition is a process that is critical for spatial discrimination. During stimulation of a spatially discrete single point, lateral inhibitory mechanisms suppress input arising from surrounding areas to enhance single point localization to equal or exceed that predicted by receptive field organization [1; 12; 19; 22; 31]. During two-point stimulation, similar lateral inhibitory mechanisms allow two points to be correctly identified as separate stimuli. During 8 cm stimulation, subjects could reliably distinguish two-point stimuli from lines or single points. In contrast, during 4 cm stimulation, subjects could not reliably distinguish two-point stimuli or line stimuli from single point stimuli.
Lateral inhibition may be critically important in chronic pain, particularly in keeping pain localized to a given distribution. Spread of pain outside of the territory of the affected nerve is frequently noted during complex regional pain syndrome [37], suggesting that lateral inhibition may be diminished. Furthermore, many chronic pain syndromes are characterized by diminished tactile acuity [3], again suggesting disruption of lateral inhibition. Finally, training with spatial localization paradigms results in improvement of complex regional pain syndrome that parallels improvement of two-point discrimination [21], suggesting that the recovery of lateral inhibition can contribute importantly to recovery from some forms of chronic pain.
4.4 Conclusions
In summary, despite substantially larger stimulus areas, line stimulus patterns were perceived as less painful than two-point stimuli. This finding indicates that the spatial configuration of noxious stimulation may critically influence the balance of local excitatory and inhibitory activity, and underscores the importance of inhibition during nociceptive processing.
Acknowledgments
IASP/Scan|Design International Collaborative Grant, The Danish Research Council for Technology and Production, National Institute of Neurological Disorders and Stroke (NS39426), Danish National Research Foundation (DNRF121).
Footnotes
Conflict of Interest
The authors have no conflicts of interest.
References
- 1.Bekesy GV. Neural inhibitory units of the eye and skin. Quantitative description of contrast phenomena. Journal of the Optical Society of America. 1960;50:1060–1070. doi: 10.1364/josa.50.001060. [DOI] [PubMed] [Google Scholar]
- 2.Bekesy GV. Lateral inhibition of heat sensations on the skin. Journal of applied physiology. 1962;17(6):1003–1008. doi: 10.1152/jappl.1962.17.6.1003. [DOI] [PubMed] [Google Scholar]
- 3.Catley MJ, O’Connell NE, Berryman C, Ayhan FF, Moseley GL. Is Tactile Acuity Altered in People With Chronic Pain? A Systematic Review and Meta-analysis. J Pain. 2014;15(10):985–1000. doi: 10.1016/j.jpain.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Coghill RC, Mayer DJ, Price DD. The roles of spatial recruitment and discharge frequency in spinal cord coding of pain: a combined electrophysiological and imaging investigation. Pain. 1993;53(3):295–309. doi: 10.1016/0304-3959(93)90226-F. [DOI] [PubMed] [Google Scholar]
- 5.Defrin R, Urca G. Spatial summation of heat pain: a reassessment. Pain. 1996;66(1):23–29. doi: 10.1016/0304-3959(96)02991-0. [DOI] [PubMed] [Google Scholar]
- 6.Douglass DK, Carstens E, Watkins LR. Spatial summation in human thermal pain perception: comparison within and between dermatomes. Pain. 1992;50(2):197–202. doi: 10.1016/0304-3959(92)90161-4. [DOI] [PubMed] [Google Scholar]
- 7.Gerhart KD, Yezierski RP, Giesler GJ, Jr, Willis WD. Inhibitory receptive fields of primate spinothalamic tract cells. J Neurophysiol. 1981;46(6):1309–1325. doi: 10.1152/jn.1981.46.6.1309. [DOI] [PubMed] [Google Scholar]
- 8.Greenspan JD, Thomadaki M, McGillis SL. Spatial summation of perceived pressure, sharpness and mechanically evoked cutaneous pain. Somatosens Mot Res. 1997;14(2):107–112. doi: 10.1080/08990229771105. [DOI] [PubMed] [Google Scholar]
- 9.Hardy JD, Wolff HG, Goodell H. Studies on Pain. A New Method for Measuring Pain Threshold: Observations on Spatial Summation of Pain. J Clin Invest. 1940;19(4):649–657. doi: 10.1172/JCI101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartline HK, Ratliff F. Inhibitory interaction of receptor units in the eye of limulus. JGenPhysiol. 1957;40:1357–1376. doi: 10.1085/jgp.40.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato G, Kosugi M, Mizuno M, Strassman AM. Separate inhibitory and excitatory components underlying receptive field organization in superficial medullary dorsal horn neurons. J Neurosci. 2011;31(47):17300–17305. doi: 10.1523/JNEUROSCI.4474-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koltzenburg M, Handwerker HO, Torebjork HE. The ability of humans to localise noxious stimuli. Neurosci Lett. 1993;150(2):219–222. doi: 10.1016/0304-3940(93)90540-2. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu H. The neural mechanisms of perceptual filling-in. Nat Rev Neurosci. 2006;7(3):220–231. doi: 10.1038/nrn1869. [DOI] [PubMed] [Google Scholar]
- 14.Lautenbacher S, Nielsen J, Andersen T, Arendt-Nielsen L. Spatial summation of heat pain in males and females. Somatosens Mot Res. 2001;18(2):101–105. doi: 10.1080/135578501012006192-1. [DOI] [PubMed] [Google Scholar]
- 15.Lautenbacher S, Prager M, Rollman GB. Pain additivity, diffuse noxious inhibitory controls, and attention: a functional measurement analysis. Somatosens Mot Res. 2007;24(4):189–201. doi: 10.1080/08990220701637638. [DOI] [PubMed] [Google Scholar]
- 16.Lidierth M. Local and diffuse mechanisms of primary afferent depolarization and presynaptic inhibition in the rat spinal cord. J Physiol. 2006;576(Pt 1):309–327. doi: 10.1113/jphysiol.2006.110577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23(25):8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchand S, Arsenault P. Spatial summation for pain perception: interaction of inhibitory and excitatory mechanisms. Pain. 2002;95(3):201–206. doi: 10.1016/S0304-3959(01)00399-2. [DOI] [PubMed] [Google Scholar]
- 19.Moore CE, Schady W. Cutaneous localisation of laser induced pain in humans. Neurosci Lett. 1995;193(3):208–210. doi: 10.1016/0304-3940(95)91313-7. [DOI] [PubMed] [Google Scholar]
- 20.Morch CD, Andersen OK, Quevedo AS, Arendt-Nielsen L, Coghill RC. Exteroceptive aspects of nociception: insights from graphesthesia and two-point discrimination. Pain. 2010;151(1):45–52. doi: 10.1016/j.pain.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moseley GL, Zalucki NM, Wiech K. Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain. 2008;137(3):600–608. doi: 10.1016/j.pain.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Mountcastle VB, Powell TP. Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bulletin of the Johns Hopkins Hospital. 1959;105:201–232. [PubMed] [Google Scholar]
- 23.Nielsen J, Arendt-Nielsen L. Spatial summation of heat induced pain within and between dermatomes. Somatosens Mot Res. 1997;14(2):119–125. doi: 10.1080/08990229771123. [DOI] [PubMed] [Google Scholar]
- 24.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56(2):217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 25.Price DD, Hayes RL, Ruda M, Dubner R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. JNeurophysiol. 1978;41:933–947. doi: 10.1152/jn.1978.41.4.933. [DOI] [PubMed] [Google Scholar]
- 26.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3(1):57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 27.Price DD, McHaffie GJ, Larson MA. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. JNeurophysiol. 1989;62:1270–1279. doi: 10.1152/jn.1989.62.6.1270. [DOI] [PubMed] [Google Scholar]
- 28.Quevedo AS, Coghill RC. Attentional modulation of spatial integration of pain: evidence for dynamic spatial tuning. J Neurosci. 2007;27(43):11635–11640. doi: 10.1523/JNEUROSCI.3356-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quevedo AS, Coghill RC. An illusion of proximal radiation of pain due to distally directed inhibition. J Pain. 2007;8(3):280–286. doi: 10.1016/j.jpain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Quevedo AS, Coghill RC. Filling-in, spatial summation, and radiation of pain: evidence for a neural population code in the nociceptive system. J Neurophysiol. 2009;102(6):3544–3553. doi: 10.1152/jn.91350.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlereth T, Magerl W, Treede R. Spatial discrimination thresholds for pain and touch in human hairy skin. Pain. 2001;92(1–2):187–194. doi: 10.1016/s0304-3959(00)00484-x. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt R, Schmelz M, Ringkamp M, Handwerker HO, Torebjork HE. Innervation territories of mechanically activated C nociceptor units in human skin. J Neurophysiol. 1997;78(5):2641–2648. doi: 10.1152/jn.1997.78.5.2641. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt R, Schmelz M, Weidner C, Handwerker HO, Torebjork HE. Innervation territories of mechano-insensitive C nociceptors in human skin. J Neurophysiol. 2002;88(4):1859–1866. doi: 10.1152/jn.2002.88.4.1859. [DOI] [PubMed] [Google Scholar]
- 34.Staud R, Koo E, Robinson ME, Price DD. Spatial summation of mechanically evoked muscle pain and painful aftersensations in normal subjects and fibromyalgia patients. Pain. 2007;130(1–2):177–187. doi: 10.1016/j.pain.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staud R, Vierck CJ, Robinson ME, Price DD. Spatial summation of heat pain within and across dermatomes in fibromyalgia patients and pain-free subjects. Pain. 2004;111(3):342–350. doi: 10.1016/j.pain.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I–III of the mammalian spinal dorsal horn. Prog Neurobiol. 1993;41(5):609–645. doi: 10.1016/0301-0082(93)90045-t. [DOI] [PubMed] [Google Scholar]
- 37.Veldman PHJM, Goris RJ. Multiple reflex sympathetic dystrophy. Which patients are at risk for developing a recurrence of reflex sympathetic dystrophy in the same or another limb. Pain. 1996;64:463–466. doi: 10.1016/0304-3959(95)00160-3. [DOI] [PubMed] [Google Scholar]
- 38.Wall PD, Lidierth M, Hillman P. Brief and prolonged effects of Lissauer tract stimulation on dorsal horn cells. Pain. 1999;83(3):579–589. doi: 10.1016/S0304-3959(99)00170-0. [DOI] [PubMed] [Google Scholar]


