Abstract
Quassinoids often exhibit anti-oxidant and anti-proliferative activity. Emerging evidence suggests that these natural metabolites also display chemopreventive actions. In this study, we investigated the potential for the quassinoid glaucarubulone glucoside (Gg), isolated from the endemic Jamaican plant Castela macrophylla (Simaroubaceae), to display potent cytotoxicity and inhibit human cytochrome P450s (CYPs), particularly CYP1A enzymes, known to convert polyaromatic hydrocarbons (PAHs) into carcinogenic metabolites. Gg reduced the viability of MCF-7 breast adenocarcinoma cells (IC50 = 121 nM) to a greater extent than standard of care anticancer agents 5-fluorouracil, tamoxifen (IC50 > 10 μM) and the tamoxifen metabolite 4-hydroxytamoxifen (IC50 = 2.6 μM), yet was not cytotoxic to non-tumourigenic MCF-10A breast epithelial cells. Additionally, Gg induced MCF-7 breast cancer cell death. Gg blocked increases in reactive oxygen species in MCF-10A cells mediated by PAH benzo[a]pyrene (B[a]P) metabolite benzo[a]pyrene 1,6 quinone, yet down-regulated the expression of genes that promote antioxidant activity in MCF-7 cells. This implies that Gg exhibits anti-oxidant and cytoprotective actions in non-tumourigenic breast epithelial cells and pro-oxidant, cytotoxic actions in breast cancer cells. Furthermore, Gg inhibited the activities of human CYP1A according to non-competitive kinetics and attenuated the ability of B[a]P to induce CYP1A gene expression in MCF-7 cells. These data indicate that Gg selectively suppresses MCF-7 breast cancer cell growth without impacting non-tumourigenic breast epithelial cells and blocks B[a]P-mediated CYP1A induction. Taken together, our data provide a rationale for further investigations of Gg and similar plant isolates as potential agents to treat and prevent breast cancer.
Keywords: chemoprevention, natural product, cytotoxicity, breast cancer, cytochrome P450
1. Introduction
Humans and other living organisms are constantly exposed to an array of pollutants and potentially genotoxic environmental chemicals. Polycyclic aromatic hydrocarbons (PAHs) such as Benzo[a]pyrene (B[a]P) are ubiquitous chemicals produced by the incomplete pyrolysis of organic materials commonly released through the combustion of fossil fuels. PAHs are found in considerable quantities in vehicle exhaust, cigarette smoke, particulate matter in urban air, and charcoal-broiled food (Wogan et al., 2004). Lipophilic PAHs easily diffuse into cells and bind with high affinity to the cytosolic aryl hydrocarbon receptor (AHR) (Moorthy, 2008; Sissung et al., 2006). This PAH-activated transcription factor forms a nuclear complex with AHR nuclear translocator (ARNT), which transcriptionally activates genes containing the Ah locus, including the cytochrome P450 (CYP) 1 enzyme family members CYP1A1, CYP1A2 and CYP1B1 (Hanlon et al, 2005). The dihydrodiol epoxide (DDE) pathway catalyzes B[a]P-metabolism via CYPs and epoxide hydrolase (EH), to form highly mutagenic and reactive B[a]P metabolites (Baird et al., 2005; Shimada and Fujii-Kuriyama, 2004). CYP1 enzymes (particularly CYP1A1) carry out epoxidation at the 7,8 position, followed by hydrolysis to form B[a]P-trans-7,8-dihydrodiol. An additional CYP-catalysed epoxidation produces the ultimate carcinogen 7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BDPE) (Shimada, 2006; Shimada and Fujii-Kuriyama, 2004). CYP1A1 is crucial in BDPE formation in human lung cells (Uppstad et al., 2010).
Bioactivation of B[a]P via dihydrodiol dehydrogenase yields the redox active product B[a]P-7,8-dione (Shimada, 2006). The first round of CYP-mediated B[a]P bioactivation leads to quinones that can undergo redox cycling to cause continuous release of prominent reactive oxygen species (ROS): superoxide anion radicals, hydroxyl radical and hydrogen peroxide via the Fenton reaction. The genotoxic actions of PAHs such as B[a]P occur either directly or through the formation of benzoquinones. Indeed ROS are associated with DNA damage, cell damage and apoptosis (Bolt and Ross, 2008; Bolton et al., 2000). In addition, benzoquinones formed by B[a]P metabolism have been shown to increase cell proliferation, generate ROS and transactivate the epidermal growth factor receptor in MCF-10A breast epithelial cells and this provides a plausible mechanism for B[a]P-mediated tumour promotion (Burdick et al., 2003).
Oxidative stress can activate pro-inflammatory mediators that disrupt the expression and activities of drug metabolizing enzymes, including CYP1A1, in various tissues (Aitken et al., 2006). CYP1B1 activity is enhanced during inflammation to further exacerbate damage to lung cells (Smerdova et al., 2013). Thus, the manner and extent to which specific CYP1 enzymes potentially mediate the damage caused by activated B[a]P varies under differing conditions. In the hope of identifying novel leads that can successfully reduce the metabolic activation of B[a]P, our study was initiated to search for inhibitors capable of targeting cytochrome P450s and B[a]P-mediated CYP1A transcription induction. Given the exploitation of inhibitors of drug metabolizing enzymes (Francis et al, 2016) and CYP enzymes in particular, (including those of natural origin) for a plethora of inflammatory conditions (Francis and Delgoda, 2014), targeting those initiated by PAH toxins such as B[a]P is timely.
In 1975, the potent cytotoxic agent and quassinoid bruceantin was isolated (Kupchan et al., 1975). Quassinoids have been used in traditional folk medicine to treat a variety of medical conditions (Fiaschetti et al., 2011). In particular, these plant isolates have been shown to display anti-inflammatory and anti-cancer actions (Lau et al., 2009, Miyake et al., 2010, Yang et al., 2013). Previously it was demonstrated that the quassinoid 2′-(R)-O-acetylglaucarubinone, derived from the Odyendyea gabonensis (Pierre) Engler plant, demonstrated potent cytotoxicity against a number of cancer cell lines including breast cancer cells (Usami et al., 2010). More recently, quassinoids from the fruits of Brucea Javanica were found to exhibit potent inhibitory activity in breast cancer cells (Ye et al., 2015).
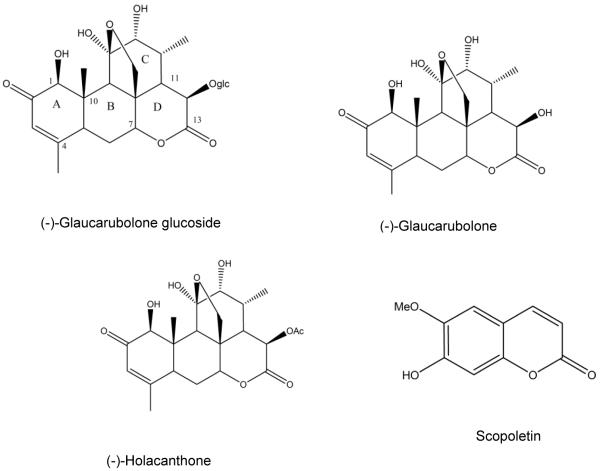
We previously demonstrated the ability of a quassinoid to bind to CYP1A1 (Shields et al., 2009). The Castela sp (Simaroubaceae) plant was reported to accumulate quassinoids (Grieco et al., 1993; Jacobs et al., 2007) and Castela macrophylla Urb. is endemic to Jamaica (Adams, 1972). Isolates from this plant showed anti-feedant activities against tobacco budworms, Plasmodium falciparum, and Plasmopara viticola (Hoffman et al., 1992), but no further biological activities have been reported. In the current study, glaucarubulone glucoside (Gg) and two related quassinoids, glaucarubulone and holacanthone, and a coumarin scopoletin (Figure 1), previously isolated from C. macrophylla (Jacobs et al., 2007), were obtained. Since quassinoids frequently display anticancer and chemopreventive actions, we hypothesized that the quassinoids isolated from the C. Macrophylla plant exhibit cytotoxic actions in breast cancer cells by modulating oxidative stress and suppress B[a]P-mediated CYP1 induction.
Figure 1.
Structures of isolated constituents from Castela macrophylla.
We evaluated the potential for Gg and other quassinoids to elicit cytotoxicity using cell viability assays. We also evaluated the ability of Gg to inhibit CYP1 enzyme activity directly using heterologously-expressed microsomes and to diminish B[a]P-mediated induction of CYP1A mRNA expression in MCF-7 cells. Furthermore, we determined the ability of Gg to inhibit the propensity of B[a]P-1,6-quinone (1,6BPQ) to increase ROS levels in non-tumourigenic MCF-10A cells. Finally, we determined the impact of Gg on anti-oxidant genes using a human oxidative stress antioxidant defence PCR array in MCF-7 cells. To the best of our knowledge, we are the first to report oxidative stress-specific gene profiling in cancer cells exposed to quassinoids. This study provides new insight into the potential for Gg and related compounds to serve as agents to impact molecular targets of toxicity and prevent or treat cancer.
2. Materials and Methods
2.1 Chemicals & Reagents
Benzo[a]pyrene (B[a]P), benzo[a]pyrene-1,6-quinone (1,6-BPQ), and all chemicals for the MTS and CYP inhibition assays were purchased from Sigma-Aldrich (St. Louis, MO, USA). B[a]P is an established pro-carcinogen and should be handled with proper protection in accordance with the MSDS which includes the use of lab coat, safety glasses and gloves. Although 1,6-BPQ is not an established carcinogen, it is recommended that care be used in handling this chemical because it is a metabolite of B[a]P, a known pro-carcinogen. Dimethyl sulfoxide (DMSO) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA), 7-amino-actinomycin D (7AAD) was purchased from eBiosciences (San Diego, CA, USA) and Annexin V-FITC was purchased from Pharmingen (San Diego, CA, USA). All CYP substrates and metabolites were purchased from Gentest Corporation (Woburn, MA, USA). All cell lines along with their respective media and supplements were purchased from ATCC (Manassas, VA, USA). ATCC certifies that the cells were authenticated prior to shipment using short tandem repeat profiling to verify that they are of the correct lineage and uncontaminated with other cell types. In addition, the cells were routinely screened to ensure they were free from mycoplasma contamination and other types of microorganisms. Escherichia coli membranes expressing human CYP1A1, CYP1A2, CYP1B1, CYP2D6, CYP3A4 and CYP2C19 co-expressed with CYP reductase were purchased from Cypex Ltd. (Dundee, UK).
2.2 Plant isolates
Castela macrophylla (Simaroubaceae) was collected in Hellshire Hills, St. Catherine, Jamaica, in June 2003, and a voucher specimen, (#UWI-Mona 34,982) was deposited in the Herbarium at the University of the West Indies, Mona, Jamaica. The leaves, twigs, and thorns were air-dried, chopped, and exhaustively extracted successively with hexane, acetone, and methanol. The resulting residues were subjected to column chromatography on silica gel. Elution with various solvent polarities (ranging between 5-100% acetone-hexane) and recrystallization yielded the pure compounds (−)-glaucarubolone, glaucarubulone glucoside (Gg), (−)-holacanthone and scopoletin, as described elsewhere (Jacobs et al., 2007). These purified and previously characterized isolates were used in the current study following reconfirmation for purity using NMR spectrometry.
2.3 Cell Culture
CCD18 Co normal colon and MCF-7 breast adenocarcinoma cells were maintained in ATCC-formulated Eagle's Minimum Essential Medium and HT29 colon carcinoma cells were maintained in McCoy's 5a Medium Modified supplemented with 10% foetal bovine serum (Atlas; Fort Collins, CO, USA), 10mM HEPES solution, 100 mM L-glutamine penicillin-streptomycin solution, 3 g/L glucose, and 1.5 g/L of sodium bicarbonate. MCF-10A human breast epithelial cells were obtained from the American Type Culture Collection (ATCC) and maintained in Dulbecco’s Modified Eagle’s Medium/nutrient mixture F-12 (Mediatech, Herndon, VA, USA) supplemented with hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA), 5% (v/v) horse serum (Invitrogen, Carlsbad, CA, USA) cholera toxin (Calbiochem, BD Biosciences, La Jolla, CA, USA) and penicillin-streptomycin antibiotics (Mediatech, Herndon, VA, USA). For the apoptosis studies and certain cell viability assays, MCF-7 cells were maintained in RPMI medium supplemented with 10% FBS and penicillin and streptomycin antibiotics. All cells were maintained at 37°C in humidified air containing 5% CO2.
2.4 Cell viability assays
CCD18 Co, HT29, MCF-10A and MCF-7 cells were treated with plant isolates or drugs before cell proliferation was evaluated using an MTS assay according to the manufacturer’s instructions (Palmari et al., 1996). All assays were performed at least three times, and their O.D. measured at 590 nm (Heusch and Maneckjee, 1999). Cell viability was measured as a percentage of the controls containing respective solvents. In some experiments, MCF-7 or MCF-10A cells were exposed to media containing DMSO (≤ 0.1 %), plant isolates or drugs (10−10 −10−4 M) and analysed using the Alamar Blue™ assay as previously described (Heusch and Maneckjee, 1999; McLean et al., 2008, McLean et al., 2015).
2.5 Annexin V-7AAD apoptosis assay
The ability of Gg to induce apoptosis in MCF-7 cells was investigated similarly to a method previously described in detail elsewhere (Lee et al., 2009; McLean et al., 2008). Briefly, cells were exposed to Gg (0.1-1 μM, 24 h). Following treatment, cells were washed with cold PBS, harvested and counted, and aliquots placed in 96-well plates. Cells were then re-suspended in assay buffer and stained with Annexin-V (which stains for cells in early apoptosis and distinguishes them from those that are viable) and 7-amino-actinomycin D (7AAD; which stains for necrotic or late apoptotic cells). Cells were then analysed using flow cytometry.
2.6 CYP inhibition assays
The test compounds were evaluated for their ability to inhibit the catalytic activity of human CYP1 enzymes by means of high throughput fluorometric inhibition assays conducted in 96-well microtitre plates, as described elsewhere (Crespi et al., 1997). 7-ethoxyresorufin (ERes) was used as a substrate for detecting activities of CYP1B1 (at 0.37 μM) and 3-cyano-7-ethoxy-coumarin (CEC) was used as a substrate for both CYPs 1A1 (at 0.5 μM) and 1A2 (at 5 μM). Furthermore, the substrates 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin (AMMC) (at 1.5 μM), 7-Benzyloxy-4-trifluoromethylcoumarin (BFC) (at 50 μM), and CEC (at 25 μM) were used as substrates for CYPs 2D6, 3A4, and 2C19, respectively. The reactions were monitored fluorometrically at 37o C, using a Varian Cary Eclipse Fluorescence spectrophotometer. All inhibitors were dissolved in a solvent of 20% acetonitrile in water and less than 0.3% of acetonitrile was used in the final assay.
2.7 RNA extraction, real-time quantitative RT-PCR and PCR array analysis
Total RNA was isolated from MCF-7 breast cancer cells treated with media containing 0.02 % DMSO, B[a]P alone or in combination with Gg for 24 h as described in detail elsewhere (McLean et al., 2015). The cDNA was used as a template for real time quantitative PCR analysis using a CFX-96 PCR instrument (Bio-Rad, Hercules, CA, USA). The primers for the reference genes GAPDH and GUSB as well as the CYP1A1 and CYP1A2 genes were obtained from SA Biosciences (Frederick, MD, USA). Alternatively, the human oxidative stress and antioxidant defence PCR array was employed to profile genes that regulate oxidative stress in MCF-7 cells, as previously described (McLean et al., 2015).
2.8 Reactive oxygen species (ROS) analysis
Intracellular ROS levels were measured in MCF-10A breast epithelial cells exposed to 0.025% DMSO (control) or 2 μM 1,6-BPQ alone or combined with 1 μM Gg for 2 h, as described previously (Burdick et al., 2003; McLean et al., 2015).
2.9 Data & Statistical analysis
IC50 and Ki values were determined by fitting the data in Sigma Plot (version 10.0) and enzyme kinetics module, using non-linear regression analysis. The apparent Ki values were determined on the basis of visual inspection of Eadie Hofstee plots and various statistics to evaluate goodness of fit, such as residual sum of squares, Akaike information criterion, and standard error (Enzyme kinetics module, version 1.3). The data listed represent the average values from three different determinations. Statistical significance between three or more groups was determined using one-way ANOVA with the Tukey-Kramer multiple comparison test. Statistical analysis was performed using GraphPad Prism 4.0 (Graph Pad Software, Inc. San Diego, CA,)
3. Results
3.1. Isolates derived from Castela macrophylla inhibit cell viability
Since quassinoids and coumarins have been shown to exhibit anti-cancer activity (Zhao et al., 2014; Tyagi et al., 2015), we reasoned that such compounds isolated from C. macrophylla would also have anticancer actions. Therefore, colorectal (CCD18Co) and MCF-10A breast non-tumourigenic cells as well as HT29 colon and MCF-7 breast cancer cells were treated with the plant isolates or established anticancer agents (tamoxifen, doxorubicin, and 5-fluorouracil) which served as positive controls for 24 h and their viability determined using the MTS or Alamar Blue assay. IC50 values (the concentration needed to inhibit cell viability by 50%) were calculated for all test isolates and standard of care anticancer agents (Table 1).
Table 1.
Cytotoxicity determination of isolates from Castela macrophylla and standard of care agents
| Compound | Cell Lines | |||
|---|---|---|---|---|
| CCD18 Co | HT29 | MCF-10A | MCF-7 | |
| (−)-Glaucarubolone | NI | NI | NI | NI |
| (−)-Holacanthone | <5 | 9.91 ± 0.52 | 0.3 ± 1.25 | 11.031 ± 1.13 |
| (−)-Glaucarubolone glucoside |
40.14 ± 3.41 | NI | NI | 8.65 ± 1.11 |
| Scopoletin | NI | 19.28 ± 0.348 | NI | NI |
| Tamoxifen | ND | ND | 38.9 ± 1.19 | 17.28 ± 0.06 |
| 5-Fluorouracil | 55.51 ± 3.71 | 23.50 ± 1.12 | NI | 78.6 ± 1.73 |
| Doxorubicin | ND | ND | 30.2 ± 1.29 | NI |
Cells were exposed to the isolates or anticancer agents (1 nM-100 μM) for 24 h before cytotoxicity analysis using the MTS or the Alamar Blue™ assay. IC50 represents the concentration needed to inhibit the growth of cancer cells by 50%. These values (μM) were obtained from the interaction of isolates with various cancer and normal cell lines along with positive controls (standard of care agents Tamoxifen, Fluorouracil and Doxorubicin), as outlined in Materials and Methods. Results are represented as the mean of at least three independent experiments ±SEM. Key: NI: No inhibition (<10% inhibition at 60 μM), ND: Not determined.
Scopoletin selectively reduced the viability of HT29 cells, and its IC50 value of 19.28 ± 0.34 μM was comparable to 5-fluorouracil (23.50 ± 1.12 μM), yet this isolate did not affect the viability of CCD18Co normal colon cells (<10% inhibition at 60 μM). 5-Fluorouracil displayed less cytotoxicity towards these cells, with an IC50 of 55.51 ± 3.71 μM (Table 1). Holacanthone reduced the viability of MCF-7 cells, with similar potency as Gg (IC50 = 11.03 ± 1.13 μM vs. 8.65 ± 1.11 μM), and more potently than tamoxifen. Holacanthone inhibited HT29 cells to a comparable degree as the MCF-7 cells (IC50 = 9.91 ± 0.52 μM vs. 11.03 ± 1.13 μM), but was also highly cytotoxic to the CCD18Co (IC50 <5 μM) and MCF-10A cells (IC50 = 0.3 ± 1.25 μM). Glaucarubolone had no impact on any of the four cell lines evaluated (Table 1). Gg was inactive in HT29 colon cancer cells in contrast to 5-fluorouracil. Gg displayed cytotoxicity in MCF-7 cells with greater potency than tamoxifen following 24 h treatment as determined by the MTS (IC50=8.65 ± 1.11μM vs. to 17.28 ± 0.06 μM) and Alamar Blue (IC50 = 3.52 ± 0.918 μM vs. 18 ± 2.21 μM) assays, respectively (Table 1, Figure 2A). Due to the superior activity of Gg in this initial screen, further bioactivity studies focused exclusively on this quassinoid. Scopoletin only demonstrated appreciable cytotoxicity in HT-29 cells. Holacanthone, tamoxifen and Doxorubicin displayed some cytotoxicity at higher micromolar concentration levels in MCF-10A breast epithelial others while Gg and 5-fluorouracil demonstrated minimal toxicity in these cells (Table 1).
Figure 2.
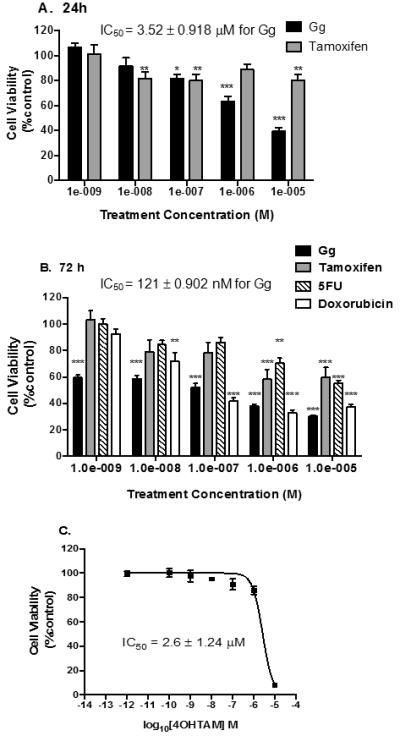
Gg demonstrates greater anticancer activity than 5-Fluorouracil, tamoxifen or its metabolite 4OH-tamoxifen in MCF-7 breast cancer cells. In A-C, cells were analysed for cell survival using the Alamar Blue™ assay following treatment, as outlined in Materials and Methods. Statistical significance as indicated by * P < 0.05, **P < 0.01 or ***P < 0.01 versus vehicle control.
3.2. Gg induces cytotoxicity and apoptosis in MCF-7 cells
We employed the Alamar Blue™ assay to determine whether Gg-mediated reductions in cell viability were more pronounced following 72 h of treatment. Indeed Gg inhibited cell viability to a greater extent at 72 h (Figure 2B). Often cytotoxicity is more evident during longer durations of exposure since the cells have more time to double. This is the most plausible reason that Doxorubicin demonstrated no inhibition following 24 h of exposure yet definitive cytotoxicity following 72 h of exposure. The disparity in cell viability between cells treated with Tamoxifen and 5-fluorouracil compared to Gg was particularly evident following 72 h of exposure (IC50=121 nM vs. >10 μM), though Doxorubicin exhibited more similar cytotoxicity in these cells particularly at higher concentrations (IC50=121 nM vs. 100 nM). We found that even the active metabolite of tamoxifen, 4-hydroxy-tamoxifen (4OH-Tam) was considerably less potent than Gg (Figure 2C, IC50 =2.6 μM). Gg demonstrated selectivity in its cytotoxicity when its cytotoxic action in MCF-7 breast cancer cells was compared to non-tumourigenic MCF-10A breast epithelial cells (IC50=121 nM vs. > 50 μM; Figure 3).
Figure 3.
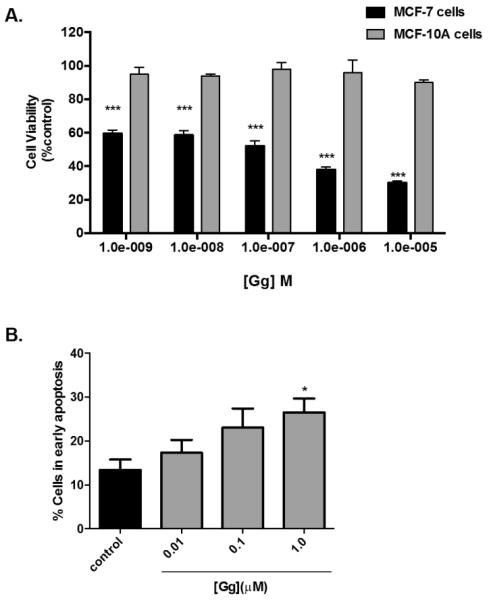
Gg displays no appreciable cytotoxicity in MCF-10A breast epithelial cells and apoptosis in MCF-7 breast cancer cells.(A) MCF-10A were exposed to Gg (1 nM-10 μM) 4 OH-Tam (1 nM-10 μM) or vehicle for 72 h before Alamar Blue™ assay analysis was employed, as outlined in Materials and Methods. Statistical significance as indicated by *** P < 0.001 versus vehicle control.(B) MCF-7 cells were exposed to media containing Gg (0.01-1.0 μM) or 0.025% DMSO for 24 h before being analysed for apoptosis using the AnnexinV-7AAD assay, as described in Materials and Methods. Data represent the mean percentage ± SEM of three independent experiments performed in triplicate. Statistical significance as indicated by * P < 0.05 versus vehicle control.
Plant isolates that diminish cell viability frequently induce apoptosis (Tyagi et al., 2015). We therefore evaluated the ability of Gg to induce apoptosis in MCF-7 cells since these cells were most sensitive to this quassinoid. The cells were treated for 24 h with media containing 0.025% DMSO or Gg. Using the AnnexinV-7AAD assay, a dose-dependent increase was detected in early apoptosis following Gg exposure (Figure 3B), which was significant at 1 μM (26.5 ± 9.5%). Interestingly, this concentration is more than 3-fold lower than the IC50 value determined after 24 h of treatment. Apoptosis and cytotoxicity were also apparent after 48 h of Gg treatment (data not shown).
3.3. Gg induces pro-oxidant activity in cancer cells
To examine the impact of Gg on the expression of genes that regulate oxidative stress a pathway-specific PCR array was used to profile such genes in MCF-7 cells treated with Gg. It was found that a number of genes were substantially down-regulated after Gg exposure (Table 2), including those encoding for aldehyde oxidase-1 (AOX1), glutathione peroxidase 1 (GPX1), metallothionein-3 (MT3), and superoxide dismutase 3 (SOD3). AOX-1 hydroxylates heterocycles and oxidizes various aldehydes and participates in myriad of cellular activities (Terao et al., 2006). GPX1 and SOD3 encode for antioxidant enzymes (Guo et al., 2003), while MT3 is known to enhance invasiveness and tumorigenesis (Kmiecik et al., 2015). Gg likely exhibits pro-oxidant actions in MCF-7 cells via down-regulation of AOX-1, GPX1, and SOD3. In addition, the data suggest Gg down-regulates MT3 to suppress the tumourigenic potential of MCF-7 cells, although additional studies are needed to confirm this.
Table 2.
Genes down-regulated in MCF-7 cells exposed to Gg using the human oxidative stress and antioxidant defence PCR array.
| Gene symbol | Gene description | Expression relative to control |
|---|---|---|
| GPX | glutathione peroxidase-1 | −24.59 |
| SOD-3 | superoxide dismutase | −10.78 |
| MT3 | metallothionein-3 | −10.48 |
| SFTPD | surfactant pulmonary associated protein-D | − 7.36 |
| AOX1 | aldehyde oxidase-1 | −6.72 |
Cells were exposed to media containing 1 μM Gg or vehicle (control) for 24 h followed by RNA extraction and real-time quantitative PCR analysis. Results represent the mean of two independent experiments performed in triplicate.
3.4. Gg inhibits cytochrome P450 (CYP) enzyme activity
Given the involvement of CYP1 enzymes (particularly CYP1A1) in pro-carcinogen activation, the ability of the test compounds to inhibit the activity of these enzymes was evaluated using fluorogenic probe substrates. Gg inhibited CYP1A1 activity (IC50 = 6.93 ± 0.31 μM; Figure 4A). Furthermore, kinetic characterisation using Eadie-Hofstee plots yielded non-competitive inhibition of CYP 1A1 (Ki = 7.1 ± 1.44 μM, data not shown). These compounds were also profiled against other drug metabolising CYP enzymes, which is a key step in predicting the potential for drug interactions by new drug entities including natural products (Murray et al, 2016; Delgoda and Westlake 2004). The results (Figure 4B), reveal that while Gg weakly inhibited the activities of CYPs 2C19 (IC50 = 19.40 ± 1.11 μM) and 2D6 (IC50 = 18.02 ± 0.51 μM), this metabolite inhibited CYPs1A1 and 1B1 more potently, and most potently inhibited CYP3A4 (IC50 = 1.31 ± 0.49μM). The latter is also critical in carcinogenic activation processes (Uppstad et al, 2010).
Figure 4.
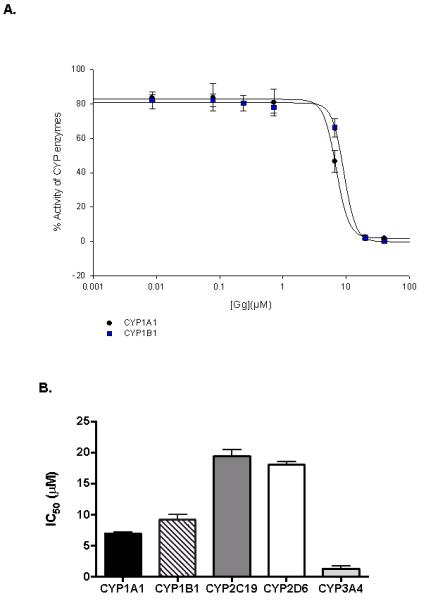
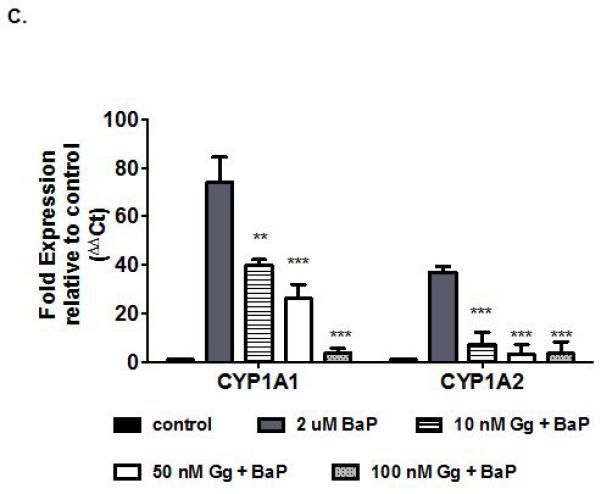
Gg inhibits CYP1 enzyme activities and suppresses B[a]P-induced CYP1A gene expression in non-invasive MCF-7 breast cancer cells. (A) Human recombinant CYP1B1-catalysed 7-ethoxyresorufin activity (0.37μM), CYPs 1A1 catalysed 7-ethoxy-3-cyanocoumarin deethylase activity (0.5μM), were determined in the presence of varying concentrations of Gg (0-20μM, as described in Materials and Methods for IC50 determinations. Control enzyme activity (mean ± SEM) for CYPs 1B1 and 1A1 were 0.34 ± 0.08, 0.86 ± 0.01 μM/min/pmol of CYP respectively. (B) Human recombinant CYP activity (as indicated by relative fluorescence) for isoforms CYP1A1, CYP1B1, CYP2C19, CYP2D6 and CYP3A4 following treatment with Gg reported as IC50 values in accordance with Materials and Methods. Results are represented as the mean of at least three independent experiments ±SEM. (C) MCF-7 cells were exposed to B[a]P alone or in combination with Gg at indicated concentrations for 24 h. Cells were harvested, RNA extracted and quantitative real-time PCR analysis performed in accordance with Materials and methods to evaluate CYP1A1 and CYP1A2 mRNA expression. Data represent the mean ± SEM of three independent experiments. Statistical significance as indicated by ** P< 0.01 or ***P < 0.001 versus treatment with B[a]P only.
To verify the accuracy of the experimental techniques employed to detect CYP inhibition, assays were performed with the known inhibitors furafylline (against CYP1A2), ketoconazole (against CYPs1A1, 1B1 and 3A4), (−)-N-3-benzyl-phenobarbital (NBPB, against CYP2C19), and quinidine (against CYP2D6). The determined IC50 values (0.8 ± 0.2, 0.04 ± 0.01, 6.3 ± 1.7, 0.06 ± 0.01, 0.3 ± 0.01, and 0.03 ± 0.01 μM, respectively) were consistent with the published values (0.99, <10, 0.06, 0.25 and 0.04 μM, respectively) (Cai et al., 2004; Cali, 2003; Powrie, 2007; Stresser et al., 2004). The Michaelis constant, KM, was determined for each marker substrate under the specified experimental conditions to determine suitable substrate concentrations for assessing the inhibitory potential of test compounds.
3.5. Gg attenuates Benzo[a]pyrene-induced CYP1A mRNA expression
B[a]P represents a PAH known to induce CYP1 through the AhR locus, thereby facilitating its own catalysis into its activated and carcinogenic forms (Shiizaki et al., 2013). Since MCF-7 cells were among the most sensitive to Gg and are malignant yet non-invasive, they were exposed to B[a]P in the presence or absence of Gg (10-100 nM) for 24 h, and the CYP1A1 and CYP1A2 mRNA expression levels evaluated. B[a]P induced robust increases in CYP1A1 and CYP1A2 mRNA expression levels, respectively relative to untreated controls (Figure 4C). On the other hand, Gg inhibited B[a]P-induced CYP1A1 mRNA expression in a dose-dependent fashion and caused sustained inhibition of B[a]P-induced CYP1A2 mRNA expression. While it is well established that B[a]P also up-regulates CYP1B1 expression (Uppstad et al., 2010), it does so to a much lesser extent than CYP1A.
3.6. Gg counteracts 1,6-BPQ-mediated increases in reactive oxygen species in MCF-10A cells
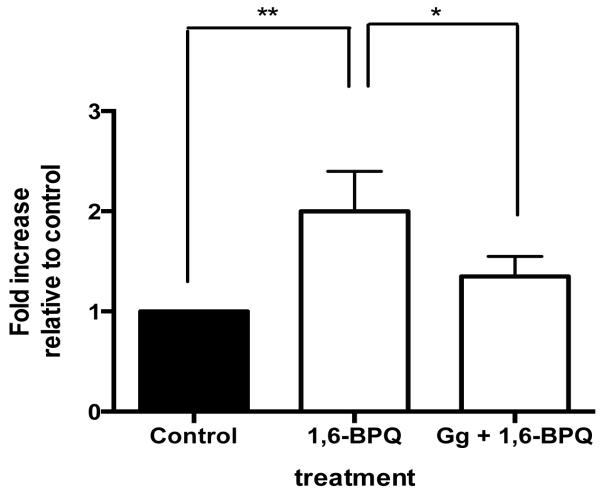
Increases in intracellular reactive oxygen species (ROS) frequently occur just before DNA becomes damaged within susceptible cells and often following metabolic activation of B[a]P (Leadon et al., 1988). Increases in ROS production within MCF-10A breast epithelial cells which are both non-invasive and non-malignant, can promote 4-hydroxyestradiol-mediated malignant transformation (Okoh et al., 2013). We chose to evaluate the ability of Gg to block 1,6-BPQ-mediated increases only in breast epithelial cells since the potential of a substance to act as a chemopreventive is best established in a non-tumorigenic cell line and because B[a]P was previously determined to increase proliferation of MCF-10A cells (Tannheimer et al.,1997). In particular, the B[a]P metabolite 1,6-B[a]P quinone (1,6-BPQ), formed through the AKR pathway following initial CYP activation, is known to increase ROS production in MCF-10A cells (Burdick et al., 2003). In order to determine whether Gg would suppress or potentiate 1,6-BPQ-mediated increases in ROS in MCF-10A cells, ROS levels were evaluated in MCF-10A cells exposed to media containing 0.01% DMSO, 1,6-BPQ (2 μM) alone or in combination with Gg (1 μM). Figure 5 indicates that 1,6-BPQ caused the expected 2-fold increase in ROS levels. However, Gg effectively suppressed the 1,6-BPQ-mediated increases in ROS production within these cells. Gg combined with 1,6-BPQ did not result in statistically significant increases in ROS as compared to control. These data suggest that Gg displays anti-oxidant actions in non-malignant cells, while displaying pro-oxidant actions in malignant cells.
Figure 5.
Gg suppresses 1,6-BPQ-mediated increases in ROS in non-malignant MCF-10A cells. MCF-10A cells were exposed to Gg (1 μM) alone or in combination with 1,6-BPQ (2 μM) for 2 hours before being analysed for ROS production using flow cytometry as described in Materials and methods. Data represent the mean ± SEM of three independent experiments. Statistical significance as indicated by * P < 0.05 or ** P < 0.01 when comparing indicated data points.
4. Discussion
Since CYP1 enzymes contribute to DDE and dione production to form DNA adducts and hence elicit the carcinogenicity of the PAH B[a]P, much attention has been focused on identifying CYP1 inhibitors as potential chemoprotectors (Badal and Delgoda, 2014). Indeed, several natural compounds, including resveratrol (Leung et al., 2007; Ren et al., 2003; Skupinska et al., 2009) and coumarins (Cai et al., 1997) have been categorised as such. In addition, CYP1A promotes the metabolism of estrogen into 2-hydroxy-estradiol which is carcinogenic and the 4-hydroxy and 16-hydroxy estradiol metabolites promote proliferation of MCF-7 cells (Gregoraszczuk et al.2008). Thus, inhibiting CYP1A provides multiple mechanisms of potential chemoprevention. In this study, the quassinoid Gg exhibited cytotoxicity in certain cancer cells with low impact on normal cells, and influenced several key stages of B[a]P activation. Gg moderately inhibited the activities of CYPs 1A1 and 1B1; the former is critical for B[a]P activation under normal conditions (Shimada and Fujii-Kuriyama, 2004) and the latter under inflammatory conditions (Smerdova et al., 2013).
In general, Gg demonstrated low cytotoxicity towards normal cells (Table 1), a desirable trait in a quest for lead compounds as chemotherapeutic and chemopreventive agents. In particular, Gg displayed selective cytotoxicity towards the MCF-7 breast cancer cell line in comparison to the non-malignant MCF-10A breast cancer cell line (Figure 3A). We found that holacanthone, scopoletin and particularly Gg displayed cancer-selective cytotoxic properties comparable to, or more potent than, known anticancer drugs (Table 1). Gluacarubolone did not impact cell viability of any of the cancer cells, although its structural relatives Gg and holacanthone did. It appears that conjugation (glycosylation or acetylation) is critical for bioactivity. Following just 24 h of treatment, Gg was twice as potent towards breast cancer cells than tamoxifen, a standard of care agent used to treat ER+ breast cancer (Osborne, 1998). After 72 h of exposure, Gg exhibited greater cytotoxicity in MCF-7 cells than 5-fluorouracil, tamoxifen and its active metabolite 4OHTam (Figure 2C) while exhibiting comparable cytotoxicity as Doxorubicin. Though we used phenol red media, it is unlikely that contaminating estrogens reduced the anticancer activity of Tamoxifen since such contaminants have negligible estrogenic effects (Moreno-Cuevas et al., 2000). In addition, the IC50 values we obtained for MCF-7 cells following 24 h exposure to tamoxifen are lower than a recent report (Sabzichi et al., 2016). Moreover, Gg (1 μM, 24 h) induced significant early apoptosis in MCF-7 breast cancer cells at a concentration more than 3-fold lower than the IC50 for this isolate. The clinically available anti-breast cancer agent tamoxifen was previously shown to induce apoptosis in MCF-7 cells (Karami-Tehrani and Salami, 2003), though it is considerably less potent than Gg in these cells.
In previous studies from this laboratory (Badal et al., 2008; Shields et al., 2009), the key residues required for the effective interactions of a quassinoid bound to the CYP1A1 active site were identified. The generalised model identified a hydrogen bond donor in position 1 which can aid binding with the backbone carbonyl of Asp313, and the oxygen atoms on rings C (2) and A (3) which can bind with the side chains of Thr111 and Ser124, respectively. Hydrophobic groups, possibly on ring A, can facilitate interactions with Phe123, Ile386, and Leu496. Gg has a glucoside moiety on ring D appended to the hydroxyl group found in quassin, thereby preventing it from acting as a hydrogen bond donor. This may explain the lack of affinity to the active site as implicated by the non-competitive binding kinetics for CYP1A1. Non-competitive inhibitors generally follow the relation IC50=Ki (Cheng and Prusoff, 1973). In these studies, the obtained IC50 value for Gg on CYPs1A1 yielded similar Ki values, further confirming the in silico suggestions.
B[a]P, like many other PAHs, binds with high affinity to the cytosolic aryl hydrocarbon receptor (AhR) (Wogan, 2004) which then complexes with the related AhR nuclear translocator (ARNT) protein in the nucleus, commencing with the transcriptional activation of CYP1A1 and CYP1A2. AhR undergoes post-translational modification for optimal transactivation potential, and whether Gg inhibits the kinases that phosphorylate AhR, as previously postulated for polyphenols (Mukai et al., 2008), remains to be determined. What is clear is that B[a]P induction of CYP1A gene expression is reduced in the presence of Gg. Yet alone, it did not appreciably affect the endogenous mRNA expression of any of the CYP1 enzymes within the breast cancer cells (data not shown). Such reductions in CYP1A, coupled with direct inhibition of the enzyme activities in the presence of Gg, may be expected to decrease the levels of carcinogenic metabolites formed through CYP1A activity. Surprisingly, Gg could not appreciably suppress B[a]P-mediated induced CYP1A1 protein expression (data not shown). This implies that Gg suppresses B[a]P-mediated actions by inhibiting CYP1A1 transcriptional activation and not by inhibiting CYP1A1 translation, nor by suppressing the effects of CYP1A1 protein turnover akin to what has been described previously (Sidorova et al., 2016).
The ability of Gg to block 1,6-BPQ-mediated increases in ROS levels in non-tumourigenic MCF-10A breast epithelial cells suggests that Gg may possess chemopreventive properties, though more extensive in vitro and in vivo studies are needed to determine the potential for Gg to serve as a chemopreventive agent. Other quassinoids were previously shown to exhibit chemopreventive actions by inhibiting Epstein-Barr virus activation (Tamura et al., 2002), rather than blocking B[a]P-mediated CYP1A induction as illustrated in this study. Gg diminished the expression of genes, such as GPX1 and SOD3, to reveal a probable mechanism for its pro-oxidant actions in cancer cells.
The loss of anti-oxidant gene activation in response to Gg may stem from cross-talk between the apoptotic and anti-oxidant cellular pathways, and the need to eliminate the protection that is conferred by anti-oxidant gene expression once the cell commits to programmed death. Grape seed extracts, and naringinen and genistein represent compounds that activate anti-oxidant gene expression, and this leads to reduced apoptosis (Chen et al., 2015; Ramprasath et al., 2014; Yu et al., 2015; Zhang et al., 2013). One key study demonstrated that di(2-ethylhexyl) phthalate induced apoptosis through mechanisms that depended on its ability to suppress antioxidant protection (Sun et al., 2015).
Gg also showed weak inhibition of CYP2D6, and since this enzyme plays a key role in the metabolism of tamoxifen, combination therapy with the pharmaceutical agent should have little risk of metabolism-based, drug-drug interactions (Bonanni et al., 2006). Although most warnings for drug-drug interactions for tamoxifen users are against CYP2D6 inhibitors, CYP3A4 is also involved in its metabolism and represents a predominant enzyme in the metabolism of a myriad of agents. Since Gg inhibits CYP3A4, adjustments may be needed when this isolate is combined with tamoxifen and/or other drugs predominantly reliant on this enzyme for bioactivation. Importantly, CYP3A4 is the most abundant hepatic cytochrome P450 enzyme and has the capacity to oxidise a dihydrodiol to a dihydrodiol epoxide. Indeed, these data show that Gg was a more potent inhibitor of CYP3A4 than CYP1. This suggests that the chemopreventive actions of Gg may be even more extrinsically linked to its ability to inhibit CYP3A4 as compared to CYP1. We speculate that Gg becomes active by a mechanism distinct from the anti-estrogenic mechanism that characterises Tamoxifen anticancer actions and this provides a plausible explanation for its superior activity in MCF-7 cells in comparison to Tamoxifen. We also suspect that Gg exhibits anticancer activity via a mechanism that differs from 5fluorouracil (an inhibitor of thymidylate synthase) and doxorubicin (a topoisomerase 2 inhibitor).
In summary, among the quassinoids, Gg demonstrated the most promising anticancer activity, which was superior to that of the clinically available agent Tamoxifen and corresponding active metabolite 4-OH tamoxifen. Gg showed growth inhibitory potency against breast cancer cells and induced apoptosis. Gg moderately inhibited the CYP1A1 enzyme with non-competitive binding kinetics, and significantly inhibited the induction of CYP1A1 by B[a]P in a dose-dependent manner. Gg thwarted 1,6-BPQ-mediated increases in ROS production in MCF-10A cells suggesting that this metabolite exhibits antioxidant actions in non-tumourigenic cells. It is plausible that Gg promotes ROS-mediated anticancer actions in tumourigenic cells since it suppressed GPX1 and SOD3 in MCF-7 cells, though additional studies are needed to confirm this speculation. Should future studies demonstrate that Gg blocks PAH-mediated DNA damage, this would strengthen the rationale to initiate in vivo studies to investigate the potential for Gg to serve as a chemopreventive and chemotherapeutic lead. This study further validates the need for an on-going search for biologically significant leads from natural products from endemic tropical biodiversity.
Acknowledgements
We thank Jennelle Jennings Suchecki for technical assistance and Kimone Stewart for administrative aid. We thank Professor Geoffrey A. Cordell, Evanston, IL (USA) for his assistance in preparing the manuscript. We are grateful to the University of the West Indies postgraduate fund, the Forest Conservation fund, the Luther Speare Scholarship, and the National Heath Fund (Jamaica) for financial support. This work was supported, in part, by intramural funds from the Loma Linda University Health School of Pharmacy Department of Pharmaceutical and Administrative Sciences, as well as NIH grants R15 AT007860-01A1 (to GMR), P20 MD006988 and R01 CA101864-8S2. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
References
- Adams C. Flowering Plants of Jamaica. University Press; Glasgow: 1972. pp. 1–848. [Google Scholar]
- Aitken AE, Richardson TA, Morgan ET. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006;46:123–49. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- Badal S, Delgoda R. Role of the modulation of CYP1A1 expression and activity in chemoprevention: A mini review. J Appl Toxicol. 2014 doi: 10.1002/jat.2968. [DOI] [PubMed] [Google Scholar]
- Badal S, Shields M, Niazi U, Jacobs H, Sutcliffe M, Delgoda R. Medimond Publishers. Saratoga Springs; New York, USA: 2008. Screening Natural Products for CYP1 Inhibitors Proceedings of the 17th International Symposium on Microsomes and Drug Oxidation; pp. 20–24. [Google Scholar]
- Baird WM, Hooven LA, Mahadevan B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ Mol Mutagen. 2005;45(2-3):106–14. doi: 10.1002/em.20095. [DOI] [PubMed] [Google Scholar]
- Bolt H, Ross P. Cytochrome P450, role in the metabolism and toxicity of drugs and other xenobiotics. RSC Publishing; Cambridge: 2008. [Google Scholar]
- Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. doi:tx9902082. [DOI] [PubMed] [Google Scholar]
- Bonanni B, Macis D, Maisonneuve P, Johansson H. Polymorphism in the CYP2D6 tamoxifen-metabolizing gene influences clinical effect but not hot flashes: Data from the Italian Tamoxifen trial. J Clin Oncol. 2006;24:3708–3715. doi: 10.1200/JCO.2006.06.8072. [DOI] [PubMed] [Google Scholar]
- Burdick AD, Davis JW, 2nd, Liu KJ, Hudson LG, Shi H, Monske ML, Burchiel SW. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003;63(22):7825–33. [PubMed] [Google Scholar]
- Cai X, Wang R, Edom R, Evans D, Shou M, Rodrigues D, Liu W, Dean D, Baillie T. Validation of (−)-N--3-benzyl-phenobarbital as a selcetive inhibitor of CYP2C19 in human liver microsomes. Drug Metab Dispos. 2004;32:584–586. doi: 10.1124/dmd.32.6.584. [DOI] [PubMed] [Google Scholar]
- Cai Y, Kleiner H, Johnston D, Dubowski A, Bostic S, Ivie W, DiGiovanni J. Effect of naturally occurring coumarins on the formation of epidermal DNA adducts and skin tumors induced by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 1997;18:1521–1527. doi: 10.1093/carcin/18.8.1521. [DOI] [PubMed] [Google Scholar]
- Cali J. Screen for CYP450 inhibitors using P450-GLOTM luminescent cytochrome P450 assays. cell notes. 2003 issue available at www.promega.com.
- Chen S, Zhu Y, Liu Z, Gao Z, Li B, Zhang D, Zhang Z, Jiang X, Liu Z, Meng L, Yang Y, Shi B. Grape Seed Proanthocyanidin Extract Ameliorates Diabetic Bladder Dysfunction via the Activation of the Nrf2 Pathway. PloS One. 2015;10(5):e0126457. doi: 10.1371/journal.pone.0126457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff W. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Crespi C, Miller V, Penman B. Microtitre plate assays for inhibition of human, drug metabolising cytochromes P450. Anal Biochem. 1997;248:188–190. doi: 10.1006/abio.1997.2145. [DOI] [PubMed] [Google Scholar]
- Delgoda R, Westlake AC. Herbal interactions involving cytochrome p450 enzymes: a mini review. Toxicol Rev. 2004;23(4):239–49. doi: 10.2165/00139709-200423040-00004. [DOI] [PubMed] [Google Scholar]
- Fiaschetti G, Grotzer MA, Shalaby T, Castelletti D, Arcaro A. Quassinoids: From traditional drugs to new cancer therapeutics. Curr Med Chem. 2011;18:316–28. doi: 10.2174/092986711794839205. [DOI] [PubMed] [Google Scholar]
- Francis S, Delgoda R. A patent review on the development of human cytochrome P450 inhibitors. Expert Opin Ther Pat. 2014;24:699–717. doi: 10.1517/13543776.2014.899583. [DOI] [PubMed] [Google Scholar]
- Francis S, Laurieri N, Nwokocha C, Delgoda R. Treatment of Rats with Apocynin Has Considerable Inhibitory Effects on Arylamine N-Acetyltransferase Activity in the Liver. Sci Rep. 2016 May 31;6:26906. doi: 10.1038/srep26906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoraszczuk EL, Rak A, Ludewig G, Gasińska A. Effects of estradiol, PCB3, and their hydroxylated metabolites on proliferation, cell cycle, and apoptosis of human breast cancer cells. Environ Toxicol Pharmacol. 2008;25(2):227–33. doi: 10.1016/j.etap.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Grieco P, Collins J, Moher E, Fleck T, Gross R. Synthetic studies on quassinoids: total synthesis of (−)-chaparrinone, (−)-glaucarubolone, and (+)-glaucarubinone. J Am Chem Soc. 1993;115:6078–6093. [Google Scholar]
- Guo Z, Higuchi K, Mori M. Spontaneous hypomorphic mutations in antioxidant enzymes of mice. Free Radic Biol Med. 2003;12:1645–52. doi: 10.1016/j.freeradbiomed.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Hanlon PR, Zheng W, Ko AY, Jefcoate CR. Identification of novel TCDD-regulated genes by microarray analysis. Tox and App Pharm. 2005;202(3):215–228. doi: 10.1016/j.taap.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Heusch WL, Maneckjee R. Effects of bombesin on methadone-induced apoptosis of human lung cancer cells. Cancer Lett. 1999;136:177–185. doi: 10.1016/s0304-3835(98)00335-8. [DOI] [PubMed] [Google Scholar]
- Hoffman J, Jolad S, Hutter L, McLaughlin S, Savage S, Cunningham S, Genet J, GR R. Glaucarubolone-glucoside, a potential fungicidal agent for the control of grape downy mildew. J Agric Food Chem. 1992;40:1056–10571. [Google Scholar]
- Jacobs H, Simpson DS, Reynolds WF. Quassinoids and a coumarin from Castela macrophylla (Simaroubacea) Biochem Syst Ecol. 2007;35:42–44. [Google Scholar]
- Karami-Tehrani F, Salami S. Cell kinetic study of tamoxifen treated MCF-7 and MDA-MB 468 breast cancer cell lines. Iranian Biomed J. 2003;7:51–56. [Google Scholar]
- Kmiecik AM, Pula B, Suchanski J, Olbromski M, Gomulkiewicz A, Owczarek T, Kruczak A, Ambicka A, Rys J, Ugorski M, Podhorska-Okolow M, Dziegiel P. Metallothionein-3 Increases Triple-Negative Breast Cancer Cell Invasiveness via Induction of Metalloproteinase Expression. PLoS One. 2015 May 1;10(5):e0124865. doi: 10.1371/journal.pone.0124865. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchan SM, Britton RW, Lacadie JA, Ziegler MF, Sigel CW. The isolation and structural elucidation of bruceantin and bruceantinol, new potent antileukemic quassinoids from Brucea antidysenterica. J Org Chem. 1975;40:648–654. doi: 10.1021/jo00893a023. [DOI] [PubMed] [Google Scholar]
- Lau ST, Lin ZX, Liao Y, Zhao M, Cheng CH, Leung PS, Bruceine D. induces apoptosis in pancreatic adenocarcinoma cell line PANC-1 through the activation of p38-mitogen activated protein kinase. Cancer Lett. 2009;281:42–52. doi: 10.1016/j.canlet.2009.02.017. PMID: 19286308. [DOI] [PubMed] [Google Scholar]
- Leadon SA, Stampfer MR, Bartley J. Production of oxidative DNA damage during the metabolic activation of benzo[a]pyrene in human mammary epithelial cells correlates with cell killing. Proc Natl Acad Sci U S A. 1988;12:4365–8. doi: 10.1073/pnas.85.12.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Adler AI, Sandhu MS, Sharp SJ, Forouhi NG, Erqou S, Luben R, Bingham S, Khaw KT, Wareham NJ. Association of C-reactive protein with type 2 diabetes:prospective analysis and meta-analysis. Diabetologia. 2009;52:1040–1047. doi: 10.1007/s00125-009-1338-3. [DOI] [PubMed] [Google Scholar]
- Leung H, Wang Y, Chan H, Leung L. Developing a high throughput system for the screening of cytochrome P450 1A1 - Inhibitory polyphenols. Toxicol in vitro. 2007;21:996–1002. doi: 10.1016/j.tiv.2007.04.005. [DOI] [PubMed] [Google Scholar]
- McLean LS, Watkins CN, Campbell P, Zylstra D, Rowland L, Amis LH, Scott L, Babb CE, Livingston WJ, Darwanto A, Davis WL, Jr, Senthil M, Sowers LC, Brantley E. Aryl Hydrocarbon Receptor Ligand 5F 203 Induces Oxidative Stress That Triggers DNA Damage in Human Breast Cancer Cells. Chem Res Toxicol. 2015;28:855–871. doi: 10.1021/tx500485v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean W, Soto U, Agama K, Francis J, Jimenez R, Pommier Y, Sowers L, Brantley E. Aminoflavone induces oxidative DNA damage and reactive oxidative species-mediated apoptosis in breast cancer cells. Int J Cancer. 2008;122:1665–1674. doi: 10.1002/ijc.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Li F, Tezuka Y, Awale S, Kadota S. Cytotoxic activity of quassinoids from Eurycoma longifolia. Nat Prod Commun. 2010;5:1009–1012. PMID: 20734929. [PubMed] [Google Scholar]
- Moorthy B. In: Cytochromes P450. Role in Drug Metabolism and Toxicity of Drugs and other Xenobiotics. Ioannides C, editor. RSC Publishing, Cambridge; UK, Cambridge: 2008. pp. 97–135. [Google Scholar]
- Moreno-Cuevas JE, Sirbasku DA. Estrogen mitogenic action. III. is phenol red a "red herring"? In Vitro Cell Dev Biol Anim. 2000;36(7):447–64. doi: 10.1290/1071-2690(2000)036<0447:EMAIIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mukai R, Fukuda I, Nishiumi S, Natsume M, Osakabe N, Yoshida K, Ashida H. Cacao polyphenol extract suppresses transformation of an aryl hydrocarbon receptor in C57BL/6 mice. J Agric Food Chem. 2008;56:10399–10405. doi: 10.1021/jf802453t. [DOI] [PubMed] [Google Scholar]
- Murray J, Picking D, Lamm A, McKenzie J, Hartley S, Watson C, Williams L, Lowe H, Delgoda R. Significant inhibitory impact of dibenzyl trisulfide and extracts of Petiveria alliacea on the activities of major drug-metabolizing enzymes in vitro: An assessment of the potential for medicinal plant-drug interactions. Fitoterapia. 2016;111:138–46. doi: 10.1016/j.fitote.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Okoh VO, Felty Q, Parkash J, Poppiti R, Roy D. Reactive oxygen species via redox signaling to PI3K/AKT pathway contribute to the malignant growth of 4-hydroxy estradiol-transformed mammary epithelial cells. PLoS One. 2013;8:e54206. doi: 10.1371/journal.pone.0054206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- Palmari J, Dussert C, Berthois Y, Penel C, Martin P. Distribution of estrogen receptor heterogeneity in growing MCF-7 cells measured by quantitative microscopy. Cytometry. 1996;27:26–35. [PubMed] [Google Scholar]
- Powrie RH. High-throughput inhibition screening of five major human cytochrome P450 enzymes using an in-vitro substrate cocktail. CXR biosciences. 2007 Available at < http://wwwcxrbiosciencescom/cmsimages/media/pdfs/Microsoft%20PowerPoint%20-%20P450%20inhibition%20posterpdf>.
- Ramprasath T, Senthamizharasi M, Vasudevan V, Sasikumar S, Yuvaraj S, Selvam GS. Naringenin confers protection against oxidative stress through upregulation of Nrf2 target genes in cardiomyoblast cells. J Physiol Biochem. 2014;70:407–15. doi: 10.1007/s13105-014-0318-3. [DOI] [PubMed] [Google Scholar]
- Sabzichi M, Samadi N, Mohammadian J, Hamishehkar H, Akbarzadeh M, Molavi O. Sustained release of melatonin: A novel approach in elevating efficacy of tamoxifen in breast cancer treatment. Colloids Surf B Biointerfaces. 2016;145:64–71. doi: 10.1016/j.colsurfb.2016.04.042. [DOI] [PubMed] [Google Scholar]
- Shields M, Niazi U, Badal S, Yee T, Sutcliffe M, Delgoda R. Inhibition of CYP1A1 by quassinoids found in Picrasma excelsa. Planta Med. 2009;75:137–141. doi: 10.1055/s-0028-1088350. [DOI] [PubMed] [Google Scholar]
- Shiizaki K, Kawanishi M, Yagi T. Dioxin suppresses benzo[a]pyrene-induced mutations and DNA adduct formation through cytochrome P450 1A1 induction and (+/-)-anti-benzo[a]pyrene-7,8-diol-9,10-epoxide inactivation in human hepatoma cells. Mutat Res. 2013;750(1-2):77–85. doi: 10.1016/j.mrgentox.2012.09.008. doi:S1383-5718(12)00290-2 [pii] [DOI] [PubMed] [Google Scholar]
- Shimada T. Xenobiotic metabolizing enzymes involved in activation and detoxification of carinogenic polycyclic aromatic hydrocarbons. Drug metab Pharmacokinet. 2006;21:257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochrome P50 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorova YA, Perepechaeva ML, Pivovarova EN, Markel AL, Lyakhovich VV, Grishanova AY. Menadione Suppresses Benzo(α)pyrene-Induced Activation of Cytochromes P450 1A: Insights into a Possible Molecular Mechanism. PLoS One. 2016;11(5):e0155135. doi: 10.1371/journal.pone.0155135. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissung TM, Price DK, Sparreboom A, Figg WD. Pharmacogenetics and regulation of human cytochrome P450 1B1: implications in hormone-mediated tumor metabolism and a novel target for therapeutic intervention. Mol Cancer Res. 2006;4:135–50. doi: 10.1158/1541-7786.MCR-05-0101. doi:4/3/135 [pii] [DOI] [PubMed] [Google Scholar]
- Skupinska K, Misiewicz-Krzeminska I, Stypulkowski R, Lubelska K, Kasprzycka-Guttman T. Sulforaphane and its analogues inhibit CYP1A1 and CYP1A2 activity induced by benzo[a]pyrene. J Biochem Mol Toxicol. 2009;23:18–28. doi: 10.1002/jbt.20259. [DOI] [PubMed] [Google Scholar]
- Smerdova L, Neca J, Svobodova J, Topinka J, Schmuczerova J, Kozubik A, Machala M, Vondracek J. Inflammatory mediators accelerate metabolism of benzo[a]pyrene in rat alveolar type II cells: the role of enhanced cytochrome P450 1B1 expression. Toxicology. 2013;314(1):30–8. doi: 10.1016/j.tox.2013.09.001. doi:S0300-483X(13)00238-2 [pii] [DOI] [PubMed] [Google Scholar]
- Stresser DM, Broudy MI, Ho T, Cargill CE, Blanchard AP, Sharma R, Dandeneau AA, Goodwin JJ, Turner SD, Erve JCL, Patten CJ, Dehal SS, Crespi CL. Highly selective inhibition of human CYP3A in vitro by azmulin and evidence that inhibition is irreversible. Drug Metab Dispos. 2004;32:105–112. doi: 10.1124/dmd.32.1.105. [DOI] [PubMed] [Google Scholar]
- Sun X, Lin Y, Huang Q, Shi J, Qiu L, Kang M, Chen Y, Fang C, Ye T, Dong S. Di(2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. J Cell Mol Med. 2015;19:581–594. doi: 10.1111/jcmm.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S, Fukamiya N, Okano M. Cancer chemopreventive effect of quassinoid derivatives. Introduction of side chain to shinjulactone C for enhancement of inhibitory effect on Epstein–Barr virus activation. Cancer Lett. 2002;185:47–51. doi: 10.1016/s0304-3835(02)00302-6. [DOI] [PubMed] [Google Scholar]
- Tannheimer SL, Barton SL, Ethier SP, Burchiel SW. Carcinogenic polycyclic aromatic hydrocarbons increase intracellular Ca2+ and cell proliferation in primary human mammary epithelial cells. Carcinogenesis. 1997;18(6):1177–82. doi: 10.1093/carcin/18.6.1177. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Tyagi M, Pachauri M, Gosh PC. Potential therapeutic applications of plant toxin-ricin in cancer:challenges and advances. Tumour Biol. 2015;36(11):8239–46. doi: 10.1007/s13277-015-4028-4. [DOI] [PubMed] [Google Scholar]
- Uppstad H, Ovrebo S, Haugen A, Mollerup S. Importance of CYP1A1 and CYP1B1 in bioactivation of benzo[a]pyrene in human lung cell lines. Toxicol Lett. 2010;192:221–228. doi: 10.1016/j.toxlet.2009.10.025. doi:S0378-4274(09)01480-5 [pii] [DOI] [PubMed] [Google Scholar]
- Usami Y, Nakagawa-Goto K, Lang JY, Kim Y, Lai CY, Goto M, Sakurai N, Taniguchi M, Akiyama T, Morris-Natschke SL, Bastow KF, Cragg G, Newman DJ, Fujitake M, Takeya K, Hung MC, Lee EY, Lee KH. Sakurai N Antitumor agents 282.2'(R)-O-Acetylgalucarubinone a quassinoid from Odeyendyea gabonensis as a potential anti breast and anti ovarian cancer agent. J Nat Prod. 73(9):1553–1558. doi: 10.1021/np100406d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. Environmental and chemical carcinogenesis. Seminars Cancer Biol. 2004;14(6):473–486. doi: 10.1016/j.semcancer.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Yang J, Li S, Xie C, Ye H, Tang H, Chen L, Peng A. Anti-inflammatory activity of ethyl acetate fraction of the seeds of Brucea Javanica. J Ethnopharmacol. 2013;147:442–446. doi: 10.1016/j.jep.2013.03.034. PMID: 23538165. [DOI] [PubMed] [Google Scholar]
- Ye QM, Bai LL, Hu SZ, Tian HY, Ruan LJ, Tan YF, Hu LP, Ye WC, Zhang DM, Jiang RW. Isolation, chemotaxonomic significance and cytotoxic effects of quassinoids from Brucea javanica. Fitoterapia. 2015;105:66–72. doi: 10.1016/j.fitote.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Yu Y, Bai F, Liu Y, Yang Y, Yuan Q, Zou D, Qu S, Tian G, Song L, Zhang T, Li S, Liu Y, Wang W, Ren G, Li D. Fibroblast growth factor (FGF21) protects mouse liver against D-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol Cell Biochem. 2015;403:287–299. doi: 10.1007/s11010-015-2358-6. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wang F, Xu HX, Yi L, Qin Y, Chang H, Mi MT, Zhang QY. Activation of nuclear factor erythroid 2-related factor 2 and PPARgamma plays a role in the genistein-mediated attenuation of oxidative stress-induced endothelial cell injury. Brit J Nutr. 2013;109:223–235. doi: 10.1017/s0007114512001110. [DOI] [PubMed] [Google Scholar]
- Zhao L, Li C, Zhang Y, Wen Q, Ren D. Phytochemical and biological activities of an anticancer plant medicine: Brucea javanica. Anticancer Agents Med Chem. 2014;14:440–458. doi: 10.2174/18715206113136660336. [DOI] [PubMed] [Google Scholar]