Abstract
In randomized controlled trials (RCTs), medication side effects may lead to beliefs that one is receiving the active intervention and enhance active treatment responses, thereby majoring drug-placebo differences. We tested these hypotheses with an experimental double-blind RCT of a nonsteroidal anti-inflammatory medication (NSAID) with and without the addition of atropine to induce side effects. One hundred healthy volunteers were told they would be randomized to either combined analgesics that might produce dry mouth or inert placebos. In reality, they were randomized double blind, double-dummy to one of 4 conditions: 1) 100 mg diclofenac+1.2 mg atropine, 2) placebo+1.2 mg atropine, 3) 100 mg diclofenac+placebo, 4) placebo+placebo, and tested with heat-induced pain. Groups did not differ significantly in demographics, temperature producing moderate pain, state anxiety, or depression. Analgesia was observed in all groups; there was a significant interaction between diclofenac and atropine, without main effects. Diclofenac alone was not better than double-placebo. The addition of atropine increased pain relief more than threefold among participants given diclofenac (d= 0.77), but did not enhance the response to placebo (d= 0.09). A mediation analysis supported the hypothesized chain from atropine to dry mouth to beliefs one had received the active medication to analgesia. There was also a direct effect of atropine on analgesia among participants who received diclofenac, suggesting the possibility of a synergistic relation between diclofenac and atropine.
Keywords: Expectancy, unblinding, placebo analgesia, induced side effects, RCT model, NSAID
Introduction
The design of placebo-controlled randomized clinical trials (RCTs) implicitly assumes that drug and placebo effects are additive, such that the drug effect is the difference between the response to the drug and the response to the placebo. Data from studies contrasting open and hidden administration of medication as well as research showing that the probability of receiving a placebo affects the response to the active drug support this assumption [4; 11; 17]. For example, according to meta-analyses, open-label or active comparator trials produce larger drug responses than placebo controlled trials [49; 54] and drug responses decrease as the number of RCT arms increases [40; 53]. Studies assessing the relationship between side effects and drug-placebo differences provide additional support for the additivity hypothesis. Side effects might lead participants in clinical trials to break blind and realize that they have been given the active medication, which, in turn, might enhance their response [43; 52]. In fact, correct guesses of treatment assignment have been linked to greater drug-placebo differences [7; 34] and the perception of side effects has been tied to treatment outcome [36; 52]. Furthermore, clinical trials of treatments for depression and dental pain indicate that patients' beliefs about treatment assignment have stronger associations with clinical outcomes than the treatment that was actually received [8; 15; 60].
Although some data support the additivity assumption, other studies point towards more complex interactions between drug and placebo effects [30]. For example, a large asthma study of 601 patients found that optimistic messages increased the effects of a placebo treatment, but not of the active medication [65]. Meta-analyses have found larger increases in responses to placebo than to active drug over time (i.e., year of publication), with consequent diminution of drug-placebo differences [45; 58; 63], contradicting earlier findings of parallel increases in drug and placebo responses [62]. Complicating the issue even further, one study reported additive effects of caffeine and information about the drug on one outcome (alertness), but an interaction on another outcome (tension) [28]. Thus the degree to which placebo and drug effects are additive remains unresolved.
Determining whether drug and placebo effects are additive or interactive would have important implications for establishing the efficacy of various treatments. Additivity would support the implicit logic of the current RCT methodology, but would also open the possibility that treatment effects may be overestimated for drugs with noticeable side effects [52]. Conversely, it has been hypothesized that large placebo responses could be masking true drug effects, rendering it increasingly difficult to establish the efficacy of new drugs [44; 48; 58].
In this experimental RCT, we evaluated whether drug and placebo effects are additive or interactive in the treatment of pain by evaluating the analgesic effects of diclofenac, a non-steroidal anti-inflammatory medication (NSAID), with and without an induced side effect (dry mouth) produced by the addition of atropine (Figure 1). We also hypothesized a mediational chain in which atropine would lead to dry mouth, dry mouth would foster the belief that one had received the active medication, and this belief would, in turn, enhance the analgesic response.
Figure 1. Study design and proceedings.
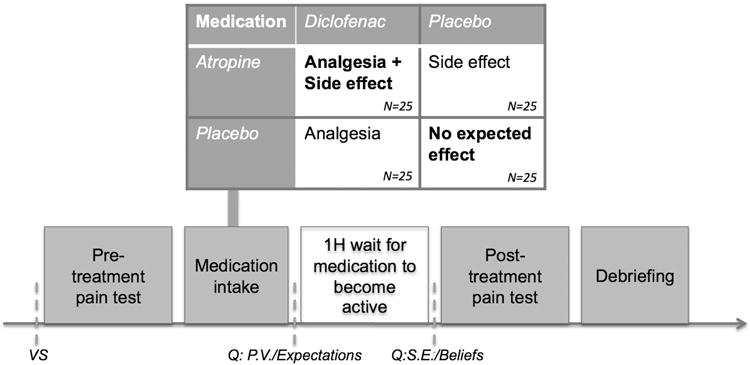
Participants were only aware of the two boldened randomization groups, ie. diclofenac+ atropine and placebo + placebo. VS= vital signs, Q= Questionnaires, P.V. = psychological variables, Expectations= measure of participant's expectations of relief, S.E.= side effects, Beliefs= beliefs about treatment assignment
2. Methods
2.1 Sample
Participants recruited from the general population in Boston, Massachusetts were screened to be healthy. Exclusion criteria were chronic intake of medication except birth control, chronic pain, psychiatric condition, or any condition (e.g. gastritis, pregnancy, high blood pressure) with increased risk for NSAIDs or atropine. Out of the 101 participants invited to the clinical test center, one female presented with exclusion criteria (hypertension) (see Consort diagram). One hundred participants (51 Female, age range 18-38 years old, Mean age = 23.82 ± 4.56) were randomized to one of the four conditions. We calculated a priori that 25 participants per cell would be sufficient to detect an effect size comparable to that obtained in a previous study of placebo analgesia [d = 0.30; 61] with 80% power.
2.2. Procedure
Healthy adults were invited to participate in “a research study on a combination of FDA-approved non-opioid pain medication that will be tested against placebo.” During the written consent procedure, participants received full information about the side effects of diclofenac and atropine. The information sheet stated “Possible side effects of atropine and/or diclofenac include: Dry mouth; Hot, red, dry skin; Blurry vision (issues with visual accommodation); Slower or faster heartbeat; High blood pressure; Stomach ulcer and bleeding; Skin rash.” This was read out to the participants, and commented: “Most of these side effects are unlikely to occur in a young, healthy person. However, most participants do experience some degree of dry mouth. This resolves within 2-4 hours of taking the drug, and is in general well tolerated.” Participants were told they would be randomized to either combined analgesics that might produce dry mouth or inert placebos. In fact, participants were randomized double blind, double-dummy to one of 4 conditions: 1) 100 mg diclofenac+1.2 mg atropine, 2) placebo+1.2 mg atropine, 3) 100 mg diclofenac+placebo, 4) placebo+placebo and tested with induced heat pain. Such a balanced placebo design allows one to test drug and expectancy effects as well as their interaction [20]. Diclofenac is an NSAID. Atropine, an antimuscarinic given to induce dry mouth, was presented deceptively as an analgesic; participants were fully debriefed at the conclusion of the session.
Testing took place at the Center for Clinical Investigations at Brigham and Women's Hospital between June and November 2014. Participants were asked to fast for 2 h prior to testing, to favor medication absorption. The IRB-approved study was registered on clinicaltrials.gov (N° 2013P-001857, Partners Healthcare IRB).
To exclude ineligible volunteers, vital signs were assessed (Figure 1), and a urinary beta-hcg test excluded pregnancy in women who were not on reliable contraception and had their periods more than 2 weeks ago (N=2). Participants then underwent baseline pain testing. Following this, a nurse dispensed the medication combination saying “this is the active medication or a placebo, it can often cause dry mouth or other mild symptoms, but this goes away quickly”. The research pharmacy was in charge of the randomization order (created through a random numbers generator), and prepared the medication for each individual participant in matching gelatin capsules labeled “diclofenac 50 mg/placebo” and “atropine 0.6 mg/placebo”. The placebo capsules were filled with microcrystalline cellulose. All of the study team in contact with participants (physicians, RA, nurses) was blinded to the randomization order. Participants took 2 capsules of each medication/placebo per mouth with liquid. Participants were then taken to a waiting area, where they first filled questionnaires (expectations about relief, anxiety, optimism, depression, anxiety sensitivity) during 15 minutes, followed by a 45 minute wait for the medication to become active. Salivary flow significantly decreases 50 min after administration of atropine [22], peak anti-inflammatory activity starts at 60 min after administration of diclofenac [55]. After 60 min waiting, participants were brought back to the examination room and reported - blindly to the researchers- a) side effects on a standardized questionnaire [42], b) beliefs about treatment assignment on Likert scales (0= definitely placebo, 1=maybe placebo, 2=fully uncertain, 3=maybe medication, 4=definitely medication), and proceeded with the post-treatment pain sequence. Finally, participants were debriefed regarding the actual randomization and the use of atropine (Figure 1).
2.3. Measures
2.3.1. Pain testing
Initial calibration identified the individual temperature eliciting moderate (Mod) pain ratings (Mean VAS rating = 58 on a 100 mm scale, SD 12.9) and ascertained that Mod -2.5°C was minimally painful (Mean VAS rating 8.7/100, SD 11.2) Mod -1.5° was perceived, on average, as low pain (Mean VAS rating = 20.6/100, SD 10.9) and Mod -0.5°C was perceived as low-moderate (Mean VAS rating = 39.2/100, SD 20.9) on an 8-second thermal stimulus applied to the left forearm (TSA-II, Medoc, Ramat Yishai, Israel) [32]. The moderate stimulus was meant as the outcome of interest, with the other temperatures intended as distracters [3]. The pre- and post-treatment sequences consisted of the same sequence of eight stimuli: low/minimal/Mod/low/Mod/near-moderate/Mod/low.
Subjects rated pain intensity of each stimulus on a 100 mm-VAS (with anchors of “no pain” and “most intense pain imaginable”) [26] during 20-35s breaks in between stimuli.
2.3.2 Baseline characteristics
In order to control for possible confounders, expectations about relief (“How much relief do you personally expect to experience from this medication?”; VAS ranging from 0= none, 100= full pain relief), state anxiety (State anxiety inventory STAI-s) [9], depression (BDI-II) [9], anxiety sensitivity index (ASI) [41], LOT optimism [50], were collected. All data were collected and managed using REDCap electronic data capture tools hosted at Partners Healthcare [23].
2.4 Statistical analysis
The primary outcome was the reduction in moderate pain intensity following treatment (i.e. analgesia, pre- minus post- treatment moderate pain ratings). We assessed the effect of diclofenac and atropine on pain reduction with a 2×2 (Diclofenac × Atropine) ANCOVA, with pre-treatment moderate pain scores as the covariate. We hypothesized that atropine-induced dry mouth would lead participants to believe they were given active medication, thereby enhancing analgesia and explored this model through a mediation analysis. We also used 2×2 (Diclofenac × Atropine) ANOVAs (for continuous variables) and chi-square (for gender) to assess age, gender, temperatures eliciting moderate pain, pre-treatment moderate pain ratings, expectations of relief, anxiety sensitivity, state anxiety, dispositional optimism, and depression, as possible confounders. We used SPSS 22.0 to conduct ANOVAs, ANCOVAs, and chi-square analyses, and Amos 22.0 to evaluate mediation (IBM, Armonk, USA).
3. Results
3.1. ANCOVA results
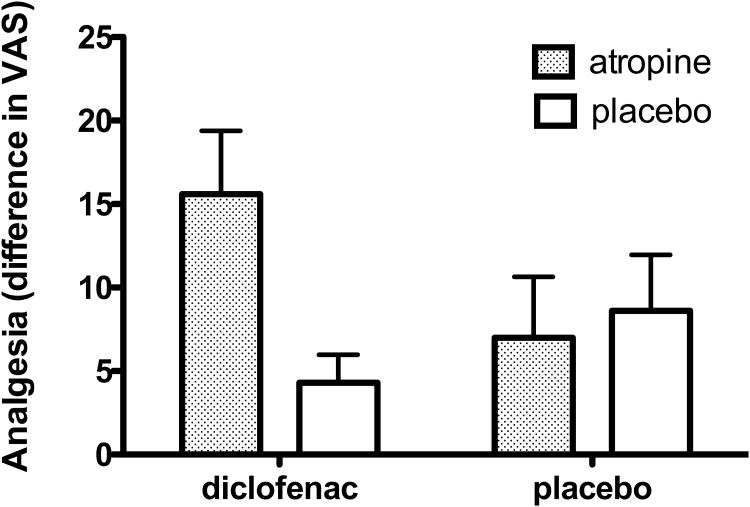
Randomized treatment groups did not differ significantly in age, gender, temperature eliciting moderate pain, pre-treatment pain ratings, expected relief, state anxiety, anxiety sensitivity, optimism or depression (see Table 1). The ANCOVA (Diclofenac × Atropine) on moderate pain ratings revealed a significant interaction, F (1, 95) = 4.754, p = .032, with no significant main effects. As illustrated in Figure 2, among participants given diclofenac, the addition of atropine increased pain relief more than threefold (d= 0.77); in contrast, the addition of atropine did not enhance the response to placebo (d= 0.09).
Table 1. Participant pre-treatment characteristics.
| Atropine + diclofenac (n = 25) M (SD) | Atropine + placebo (n = 25) M (SD) | Placebo + diclofenac (n = 25) M (SD) | Placebo + placebo (n = 25) M (SD) | Sig. | |
|---|---|---|---|---|---|
| Age, years | 24.48 (5.08) | 24.12 (4.83) | 23.52 (4.48) | 23.16 (3.94) | P > 0.5 |
| % Female | 48 | 44 | 56 | 56 | P > 0.5 |
| Calibration for moderate pain (°C) | 46.26 (1.6) | 47.16 (0.99) | 46.68 (1.28) | 46.48 (1.58) | P = 0.13 |
| Pre-treatment moderate pain ratings (VAS/100) | 59.6 (13.9) | 57.5 (11.6) | 60.9 (11.8) | 54.0 (13.9) | P = 0.25 |
| Expected relief (VAS/100) | 60.09 (23.59) | 65.48 (19.13) | 59.04 (20.08) | 60.79 (17.37) | P > 0.5 |
| ASI | 12.92 (5.64) | 13.08 (6.49) | 11.48 (6.54) | 13.28 (7.75) | P > 0.5 |
| STAI- S | 30.8 (6.08) | 33.2 (10.33) | 29.40 (7.50) | 28.44 (7.49) | P = 0.18 |
| LOT | 7.16 (4.46) | 6.88 (4.29) | 6.36 (5.86) | 7.4 (3.40) | P > 0.5 |
| BDI | 4.2 (3.83) | 3.52 (3.85) | 4.24 (5.48) | 3.96 (5.21) | P > 0.5 |
VAS, visual analogue scale; ASI, Anxiety sensitivity index; STAI-S, State-Trait Anxiety Inventory Scale; LOT, Life orientation Test (optimism); BDI, Beck Depression Index.
Figure 2. Effects of diclofenac and atropine on pain relief.
The levels of analgesia, defined as the difference between pre- minus post- treatment pain ratings, are illustrated for the four treatment groups. Diclofenac is a Non Steroidal Anti-inflammatory Drug (NSAID) reducing heat pain, atropine is an antimuscarinic agent without known analgesic effects, that was given to induce a side effect in the form of a dry mouth. Error bars = SEM.
Prior studies have reported conflicting data concerning gender differences in NSAID and placebo analgesia [3; 9; 13]. For that reason, we conducted an additional ANCOVA on pain reduction, adding gender as a factor. Neither the main effect of gender nor any interaction involving gender reached significance.
3.2. Mediation analysis
We evaluated the hypothesized mediational chain using a two-group structural equation model, as shown in Figure 3. Because we had no hypotheses about how this mediated effect might differ across the diclofenac and no-diclofenac groups, all parameters (paths and error variances) were set equal across them. This model fit extremely well, χ2 (6) = 6.476, p = .372, CFI = .983, RMSEA = .028, pclose = .523. The estimates obtained, shown on the figure, indicate that all three legs of the mediation chain were statistically significant: Atropine to Dry Mouth, Dry Mouth to Belief about Treatment, and Belief about Treatment to Analgesia. In addition, a bias-corrected bootstrap test of the indirect effect represented by the combined sequence of these three paths was also statistically significant, p = .008 (standardized indirect effect = 0.026, 95% CI = [0.006, 0.073]).
Figure 3. Structural equation model predicting analgesia.
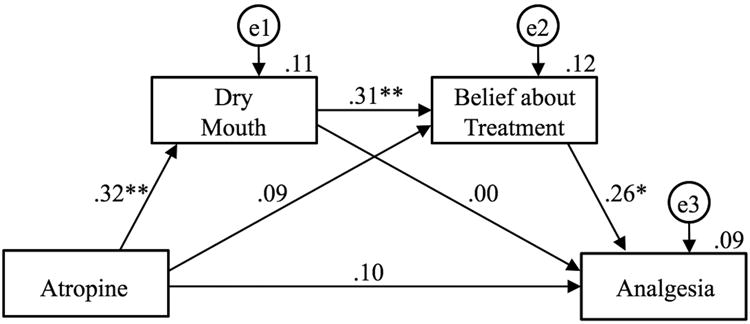
Standardized estimates are shown on the paths connecting the variables, and proportions of variance accounted for in each variable are shown above each variable box. The variables shown in circles represent unexplained variance in each measured variable. * p < 0.05. ** p < 0.01.
Given the interaction finding in the ANCOVA, which suggests there might possibly be process differences between the diclofenac and no-diclofenac groups, we also tested for a statistically significant difference between groups for each path in this model. The only difference that approached statistical significance was the direct effect of Atropine on Analgesia, χ2(1) = 3.715, p = .054. For those given diclofenac, there was a significant direct effect of Atropine on Analgesia, yielding a standardized path of .28, p = .039, whereas there was no such effect for those not given diclofenac, standardized path = -.09, p = .539. We also used the Bayesian custom-estimand approach in Amos to test for moderated mediation – that is, whether the mediational chain differed significantly across the two groups; it did not (p =.75). Thus, the two groups seem to have differed only in the direct effect of Atropine on Analgesia, and not in the mediational chain.
Discussion
To our knowledge, we provide the first experimental test of how the perception of side effects in an analgesic RCT affects beliefs about treatment assignment, which in turn increases the response to the medication. Our mediation analysis confirmed the hypothesis that atropine increased the perception of side effects, which led to enhanced beliefs that one had received the active medication, in turn enhancing analgesia. We also hypothesized an additive relation between placebo and drug effects, such that altering expectations would impact the response to both active drug and placebo, increasing the placebo response and masking drug-placebo differences [48; 58]. Instead, the ANCOVA revealed a significant interaction. In fact, the difference between diclofenac and placebo was significant only when atropine had been added. The mean difference between diclofenac+ atropine and placebo + atropine was 11.32 on a 100 mm VAS. This can be compared to the mean drug-placebo difference of 8.39 reported in a Cochrane review of the effects of NSAIDs on low back pain [46].
The tests for differences between the diclofenac and no-diclofenac groups for each path in the mediational model might provide clues to understand these findings. There was a nearly significant (p = .054) between-group difference in the direct path from atropine to analgesia. For participants given diclofenac, there was a significant direct effect of atropine on analgesia (p = .039), whereas there was no such effect for those not given diclofenac, which is consistent with the interaction observed in the ANCOVA. These data suggest that atropine and diclofenac may work synergistically in the relief of pain. These data need to be interpreted cautiously, as the significance of the between group difference was marginal. However, tests of moderator effects have notoriously low power, leading many statisticians to recommend using higher than conventional alpha levels for them [21].
How could one explain the apparent synergistic effect of diclofenac and atropine? A possible explanation can be derived from two separately well-established findings. First, placebo analgesia can be blocked by naloxone, an opioid antagonist, supporting that placebo analgesia is partially mediated by the release of endogenous opioids [1; 19; 31]. Second, an analgesic synergy between exogenous opioids and NSAIDs, including diclofenac, has been reported both in humans [35] and animal models [37; 38; 56]. Thus, we hypothesize that diclofenac could potentiate the analgesic effects of endogenous opioids in a manner that is similar to the way that NSAIDs potentiate the analgesic effects of exogenous opioids. In sum, the beliefs about treatment assignment that were indirectly induced by atropine (Figure 3) may have stimulated the release of endogenous opioids, which were then potentiated by diclofenac.
An alternative explanation could be inherent to our model: we chose a measure of beliefs about medication attribution, hence capturing one factor of expectancy. Yet, predictions about the future involve multiple cognitive processes, leaving a number unaccounted for by our model [12]. In fact, we did not collect measures for all conscious processes that could have interacted with embodied predictions, such as fear, attention, therapeutic alliance, or recall of previous somatic experiences [5; 16]. Furthermore, non-conscious processes, which are complex to measure, have been shown to induce placebo effects, and could also have been involved through the sensory nature of our expectancy manipulation [24; 25; 47]. Therefore, the unmediated effects of atropine on analgesia in the diclofenac group might be related to unmeasured processes that were affected by the presence of an active drug.
Interesting parallels can be made with recent publications. In a clinical RCT of amitriptyline for pain, expectations about treatment outcomes correlated with analgesia only in the real treatment group [57]. Also, in an experimental model of relief from heat pain using lidocaine cream, enhanced expectation-induced placebo analgesia was only found in the active treatment group, and not in the inactive cream condition [51]. For both these studies, there is direct [57] or indirect [29] evidence that participants were in fact unblinded to their treatment allocation, due to various drug effects. Interestingly, both these studies used a treatment that had shown little efficacy for the investigated pain conditions in relevant separate clinical trials [14] and experimental studies [2; 29]. This suggests that a signal of “being on active medication” could boost the efficacy of a treatment that has small effect sizes.
We found that the mediational model from atropine to dry mouth, from dry mouth to the belief that one has been given the active analgesic, and from this belief to analgesia was well supported by the mediation analysis. Somewhat surprisingly, in view of the interaction found in ANCOVA analysis, the mediational chain did not differ significantly across the two groups (p = .75). Mediation models can have substantially greater power than other types of analysis [27], and here the model detected a relation of atropine to analgesia in the no-diclofenac group that was not apparent in the ANCOVA. Instead, the two groups seem to have differed only in the direct effect of Atropine on Analgesia, and not in the mediational chain. These data support the hypothesis that the perception of side effects might artifactually enhance drug-placebo differences in conventional clinical trials. To control for this possibility, the use of active placebos to protect blinding might be considered more frequently.
Our sample included both genders, without significant effects of gender on analgesia. There are contradictory reports in the literature regarding gender differences in analgesia through NSAIDs. A large study (195F/119 M) on molar dental extraction showed no difference [6], whilst smaller studies on experimental pain models such as electrical stimuli (n=20) [13] or cold pressor test (n=50) [18] suggest gender differences in NSAID and placebo differences, yet with conflicting results. Larger samples might be needed to clarify this debate.
This study has some limitations, inherent to its design. Participants were healthy volunteers, and replication with a clinical sample would be warranted. A similar trial in a larger population, possibly with an analgesic that has stronger effects on the chosen pain model, would also be helpful. However this requirement is a challenge, knowing the limitations in potency of analgesics for acute pain [39] and the possibility for negative expectations to fully block the effects of a strong opiate such as remifentanyl [11]. A blinding of the treating physician to the deception could also be considered, although practically difficult to carry through. Finally, if the demonstrated effect is universal or only applicable to specific situations, such as NSAIDs, requires more research.
In conclusion, these findings could have important implications for the design of RCTs, because the double-blind nature of an RCT instills uncertainty in subjects regarding whether they received the active drug or placebo. Side effects may reduce uncertainty in active drug arms, thereby enhancing drug-placebo differences. This comes at a time when RCT methodology is being questioned in the light of placebo research [10; 30; 33; 59; 64]. These experimental findings warrant replication in clinical populations. The possibility of a synergy between diclofenac and endogenous opioids in the context of a placebo effect could also be investigated further.
Supplementary Material
Acknowledgments
We thank Dan Ariely, PhD, for providing data allowing calculation of the effect sizes in Waber et al. 2008, which we used in our power calculations. We thank Randy L. Gollub, MD PhD, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Martinos Center for Biomedical Imaging, Boston, USA for key technical support and James P. Rathmell, MD, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, USA for hosting CB. None of these mentors were financially compensated for their support. The Swiss National Science Foundation (P3SMP3_151710) provided support for C.B.; the Foundation for the Science of the Therapeutic Encounter (F-STE) supported S.R.Z.; T.K. and the study was supported through NCCIH (K24 AT004095). CB and IK had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was conducted with support from the BWH Research Pharmacy and Center for Clinical Investigations, Harvard Catalyst, The Harvard Clinical and Translational Science Center (NIH Award 1UL1 TR001102-01 and financial contributions from Harvard University and its affiliated academic healthcare centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Center for Advancing Translational Science or the National Institutes of Health. There is no conflict of interest to declare.
Footnotes
Declaration: This manuscript is original work that has not been previously published. The abstract has been accepted for a poster presentation at the 2016 World congress on Pain, meeting of the IASP, Yokohama, September 26-29 2016.
References
- 1.Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specifc activation of endogenous opioids. Pain. 2001;90:205–215. doi: 10.1016/S0304-3959(00)00486-3. [DOI] [PubMed] [Google Scholar]
- 2.Aslaksen PM, Zwarg ML, Eilertsen HI, Gorecka MM, Bjorkedal E. Opposite effects of the same drug: reversal of topical analgesia by nocebo information. Pain. 2015;156(1):39–46. doi: 10.1016/j.pain.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 3.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(39):12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(23):8053–8064. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atlas LY, Wielgosz J, Whittington RA, Wager TD. Specifying the non-specific factors underlying opioid analgesia: expectancy, attention, and affect. Psychopharmacology. 2014;231(5):813–823. doi: 10.1007/s00213-013-3296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Averbuch M, Katzper M. A search for sex differences in response to analgesia. Arch Intern Med. 2000;160(22):3424–3428. doi: 10.1001/archinte.160.22.3424. [DOI] [PubMed] [Google Scholar]
- 7.Baethge C, Assall OP, Baldessarini RJ. Systematic review of blinding assessment in randomized controlled trials in schizophrenia and affective disorders 2000-2010. Psychother Psychosom. 2013;82(3):152–160. doi: 10.1159/000346144. [DOI] [PubMed] [Google Scholar]
- 8.Bausell RB, Lao L, Bergman S, Lee WL, Berman BM. Is acupuncture analgesia an expectancy effect? Preliminary evidence based on participants' perceived assignments in two placebo-controlled trials. Eval Health Prof. 2005;28(1):9–26. doi: 10.1177/0163278704273081. [DOI] [PubMed] [Google Scholar]
- 9.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 10.Benedetti F, Carlino E, Piedimonte A. Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects. The Lancet Neurology. 2016 doi: 10.1016/S1474-4422(16)00066-1. [DOI] [PubMed] [Google Scholar]
- 11.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Science translational medicine. 2011;3(70):70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 12.Bubic A, Von Cramon DY, Schubotz R. Prediction, cognition and the brain. Frontiers in Human Neuroscience. 2010;4:25. doi: 10.3389/fnhum.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butcher BE, Carmody JJ. Sex differences in analgesic response to ibuprofen are influenced by expectancy: a randomized, crossover, balanced placebo-designed study. Eur J Pain. 2012;16(7):1005–1013. doi: 10.1002/j.1532-2149.2011.00104.x. [DOI] [PubMed] [Google Scholar]
- 14.Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96(3):365–373. doi: 10.1016/S0304-3959(01)00483-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen JA, Papakostas GI, Youn SJ, Baer L, Clain AJ, Fava M, Mischoulon D. Association between patient beliefs regarding assigned treatment and clinical response: reanalysis of data from the Hypericum Depression Trial Study Group. The Journal of clinical psychiatry. 2011;72(12):1669–1676. doi: 10.4088/JCP.10m06453. [DOI] [PubMed] [Google Scholar]
- 16.Clark A. Embodied Prediction. In: Metzinger TK, Windt JM, editors. Open MIND. Frankfurt am Main: MIND Group; 2015. [Google Scholar]
- 17.Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. The Lancet Neurology. 2004;3(11):679–684. doi: 10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- 18.Compton P, Charuvastra VC, Ling W. Effect of oral ketorolac and gender on human cold pressor pain tolerance. Clinical and experimental pharmacology & physiology. 2003;30(10):759–763. doi: 10.1046/j.1440-1681.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 19.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the Opioidergic Descending Pain Control System Underlies Placebo Analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Enck P, Klosterhalfen S, Weimer K, Horing B, Zipfel S. The placebo response in clinical trials: more questions than answers. Philos Trans R Soc Lond B Biol Sci. 2011;366(1572):1889–1895. doi: 10.1098/rstb.2010.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frazier PA, Tix AP, Barron KE. Testing moderator and mediator effects in counseling psychology research. Journal of counseling psychology. 2004;51(1):115. [Google Scholar]
- 22.Fry JR, Burr SA. A double-blind atropine trial for active learning of autonomic function. Advances in physiology education. 2011;35(4):438–444. doi: 10.1152/advan.00075.2011. [DOI] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen KB, Kaptchuk TJ, Chen X, Kirsch I, Ingvar M, Gollub RL, Kong J. A Neural Mechanism for Nonconscious Activation of Conditioned Placebo and Nocebo Responses. Cereb Cortex. 2015;25(10):3903–3910. doi: 10.1093/cercor/bhu275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(39):15959–15964. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 27.Kenny DA, Judd CM. Power anomalies in testing mediation. Psychol Sci. 2014;25(2):334–339. doi: 10.1177/0956797613502676. [DOI] [PubMed] [Google Scholar]
- 28.Kirsch I, Rosadino MJ. Do double-blind studies with informed consent yield externally valid results? An empirical test. Psychopharmacology. 1993;110(4):437–442. doi: 10.1007/BF02244650. [DOI] [PubMed] [Google Scholar]
- 29.Krumova EK, Zeller M, Westermann A, Maier C. Lidocaine patch (5%) produces a selective, but incomplete block of Adelta and C fibers. Pain. 2012;153(2):273–280. doi: 10.1016/j.pain.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Kube T, Rief W. Are placebo and drug-specific effects additive? Questioning basic assumptions of double-blinded randomized clinical trials and presenting novel study designs. Drug Discovery Today. 2017 doi: 10.1016/j.drudis.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Levine J, Gordon N, Fields H. The mechanism of placebo analgesia. The Lancet. 1978;312(8091):654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 32.Loggia ML, Jensen K, Gollub RL, Wasan AD, Edwards RR, Kong J. The catechol-O-methyltransferase (COMT) val158met polymorphism affects brain responses to repeated painful stimuli. PloS one. 2011;6(11):e27764. doi: 10.1371/journal.pone.0027764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lund K, Vase L, Petersen GL, Jensen TS, Finnerup NB. Randomised controlled trials may underestimate drug effects: Balanced placebo trial design. PloS one. 2014;9(1) doi: 10.1371/journal.pone.0084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margraf J, Ehlers A, Roth WT, Clark DB, Sheikh J, Agras WS, Taylor CB. How “blind” are double-blind studies? J Consult Clin Psychol. 1991;59(1):184–187. doi: 10.1037//0022-006x.59.1.184. [DOI] [PubMed] [Google Scholar]
- 35.Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology. 2005;102(6):1249–1260. doi: 10.1097/00000542-200506000-00027. [DOI] [PubMed] [Google Scholar]
- 36.Max MB, Schafer SC, Culnane M, Dubner R, Gracely RH. Association of pain relief with drug side effects in postherpetic neuralgia: a single-dose study of clonidine, codeine, ibuprofen, and placebo. Clinical pharmacology and therapeutics. 1988;43(4):363–371. doi: 10.1038/clpt.1988.44. [DOI] [PubMed] [Google Scholar]
- 37.Miranda HF, Pinardi G. Lack of effect of naltrindole on the spinal synergism of morphine and non-steroidal anti-inflammatory drugs (NSAIDS) J Physiol Pharmacol. 2009;60(2):71–76. [PubMed] [Google Scholar]
- 38.Miranda HF, Prieto JC, Pinardi G. Spinal synergy between nonselective cyclooxygenase inhibitors and morphine antinociception in mice. Brain Res. 2005;1049(2):165–170. doi: 10.1016/j.brainres.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 39.Moore RA, Derry S, McQuay HJ, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults. Cochrane Database Syst Rev. 2011;(9):Cd008659. doi: 10.1002/14651858.CD008659.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19(1):34–40. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 42.Rief W, Barsky AJ, Glombiewski JA, Nestoriuc Y, Glaesmer H, Braehler E. Assessing general side effects in clinical trials: reference data from the general population. Pharmacoepidemiol Drug Saf. 2011;20(4):405–415. doi: 10.1002/pds.2067. [DOI] [PubMed] [Google Scholar]
- 43.Rief W, Bingel U, Schedlowski M, Enck P. Mechanisms involved in placebo and nocebo responses and implications for drug trials. Clinical pharmacology and therapeutics. 2011;90(5):722–726. doi: 10.1038/clpt.2011.204. [DOI] [PubMed] [Google Scholar]
- 44.Rief W, Glombiewski JA. The hidden effects of blinded, placebo-controlled randomized trials: an experimental investigation. Pain. 2012;153(12):2473–2477. doi: 10.1016/j.pain.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Rief W, Nestoriuc Y, Weiss S, Welzel E, Barsky AJ, Hofmann SG. Meta-analysis of the placebo response in antidepressant trials. J Affect Disord. 2009;118(1-3):1–8. doi: 10.1016/j.jad.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 46.Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database Syst Rev. 2008;(1):CD000396. doi: 10.1002/14651858.CD000396.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen A, Yi J, Kirsch I, Kaptchuk TJ, Ingvar M, Jensen KB. Effects of subtle cognitive manipulations on placebo analgesia - An implicit priming study. Eur J Pain. 2016 doi: 10.1002/ejp.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutherford BR, Roose SP. A model of placebo response in antidepressant clinical trials. Am J Psychiatry. 2013;170(7):723–733. doi: 10.1176/appi.ajp.2012.12040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rutherford BR, Sneed JR, Roose SP. Does study design influence outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychother Psychosom. 2009;78(3):172–181. doi: 10.1159/000209348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheier MF, Carver CS, Bridges MW. Distinguishing Optimism From Neuroticism (and Trait Anxiety, Self-Mastery, and Self-Esteem): A Reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67(6):1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 51.Schenk LA, Sprenger C, Geuter S, Buchel C. Expectation requires treatment to boost pain relief: an fMRI study. Pain. 2014;155(1):150–157. doi: 10.1016/j.pain.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 52.Shah E, Triantafyllou K, Hana AA, Pimentel M. Adverse events appear to unblind clinical trials in irritable bowel syndrome. Neurogastroenterol Motil. 2014;26(4):482–488. doi: 10.1111/nmo.12289. [DOI] [PubMed] [Google Scholar]
- 53.Sinyor M, Levitt AJ, Cheung AH, Schaffer A, Kiss A, Dowlati Y, Lanctot KL. Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. The Journal of clinical psychiatry. 2010;71(3):270–279. doi: 10.4088/JCP.08r04516blu. [DOI] [PubMed] [Google Scholar]
- 54.Sneed JR, Rutherford BR, Rindskopf D, Lane DT, Sackeim HA, Roose SP. Design makes a difference: a meta-analysis of antidepressant response rates in placebo-controlled versus comparator trials in late-life depression. Am J Geriatr Psychiatry. 2008;16(1):65–73. doi: 10.1097/JGP.0b013e3181256b1d. [DOI] [PubMed] [Google Scholar]
- 55.Stacher G, Steinringer H, Schneider S, Mittelbach G, Winklehner S, Gaupmann G. Experimental pain induced by electrical and thermal stimulation of the skin in healthy man: sensitivity to 75 and 150 mg diclofenac sodium in comparison with 60 mg codeine and placebo. British journal of clinical pharmacology. 1986;21(1):35–43. doi: 10.1111/j.1365-2125.1986.tb02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torres-Lopez JE, Carmona-Diaz E, Cortes-Penaloza JL, Guzman-Priego CG, Rocha-Gonzalez HI. Antinociceptive synergy between diclofenac and morphine after local injection into the inflamed site. Pharmacol Rep. 2013;65(2):358–367. doi: 10.1016/s1734-1140(13)71011-9. [DOI] [PubMed] [Google Scholar]
- 57.Turner JA, Jensen MP, Warms CA, Cardenas DD. Blinding effectiveness and association of pretreatment expectations with pain improvement in a double-blind randomized controlled trial. Pain. 2002;99(1-2):91–99. doi: 10.1016/s0304-3959(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 58.Tuttle AH, Tohyama S, Ramsay T, Kimmelman J, Schweinhardt P, Bennett GJ, Mogil JS. Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain. 2015;156(12):2616–2626. doi: 10.1097/j.pain.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 59.Vase L, Amanzio M, Price DD. Nocebo vs. Placebo: The challenges of trial design in analgesia research. Clinical pharmacology and therapeutics. 2015;97(2):143–150. doi: 10.1002/cpt.31. [DOI] [PubMed] [Google Scholar]
- 60.Vase L, Baram S, Takakura N, Yajima H, Takayama M, Kaptchuk TJ, Schou S, Jensen TS, Zachariae R, Svensson P. Specifying the nonspecific components of acupuncture analgesia. Pain. 2013;154(9):1659–1667. doi: 10.1016/j.pain.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waber RL, Shiv B, Carmon Z, Ariely D. Commercial Features of Placebo and Therapeutic Efficacy. JAMA. 2008;299(9):1016–1017. doi: 10.1001/jama.299.9.1016. [DOI] [PubMed] [Google Scholar]
- 62.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 63.Weimer K, Colloca L, Enck P. Placebo effects in psychiatry: Mediators and moderators. The Lancet Psychiatry. 2015;2(3):246–257. doi: 10.1016/S2215-0366(14)00092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weimer K, Enck P. Traditional and innovative experimental and clinical trial designs and their advantages and pitfalls. Handbook of Experimental Pharmacology. 2014;225:237–272. doi: 10.1007/978-3-662-44519-8_14. [DOI] [PubMed] [Google Scholar]
- 65.Wise RA, Bartlett SJ, Brown ED, Castro M, Cohen R, Holbrook JT, Irvin CG, Rand CS, Sockrider MM, Sugar EA American Lung Association Asthma Clinical Research C. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol. 2009;124(3):436–444. 444e431–438. doi: 10.1016/j.jaci.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.