Abstract
The hippocampus is crucial for long-term memory; its involvement in short-term or immediate expressions of memory is more controversial. Rodent hippocampus has been implicated in an expression of memory that occurs on-line during exploration termed “vicarious trial-and-error” (VTE) behavior. VTE occurs when rodents iteratively explore options during perceptual discrimination or at choice points. It is strategic in that it accelerates learning and improves later memory. VTE has been associated with activity of rodent hippocampal neurons, and lesions of hippocampus disrupt VTE and associated learning and memory advantages. Analogous findings of VTE in humans would support the role of hippocampus in active use of short-term memory to guide strategic behavior. We therefore measured VTE using eye-movement tracking during perceptual discrimination and identified relevant neural correlates with fMRI. A difficult perceptual-discrimination task was used that required visual information to be maintained during a several-second trial, but with no long-term memory component. VTE accelerated discrimination. Neural correlates of VTE included robust activity of hippocampus and activity of a network of medial prefrontal and lateral parietal regions involved in memory-guided behavior. This VTE-related activity was distinct from activity associated with simply viewing visual stimuli and making eye movements during the discrimination task, which occurred in regions frequently associated with visual processing and eye-movement control. Subjects were mostly unaware of performing VTE, thus further distancing VTE from explicit long-term memory processing. These findings bridge the rodent and human literatures on neural substrates of memory-guided behavior, and provide further support for the role of hippocampus and a hippocampal-centered network of cortical regions in the immediate use of memory in on-line processing and the guidance of behavior.
Keywords: vicarious trial-and-error behavior, eye-movement tracking, hippocampus, fMRI, short-term memory
Introduction
The necessary role of hippocampus in long-term declarative memory is widely accepted, although various theoretical accounts differ as to specific hypothesized processing mechanisms and representational qualities (Eichenbaum & Cohen, 2004, 2014b; Kumaran & Maguire, 2009; Poppenk, Evensmoen, Moscovitch, & Nadel, 2013; Ranganath, 2010; Squire, Stark, & Clark, 2004). Hippocampal damage in humans has long been thought to produce few if any short-term impairments (Scoville & Milner, 1957). However, recent findings suggest disruptions even on the timescales typically associated with short-term or working memory (Hannula, Tranel, & Cohen, 2006; Owen et al., 1995; Olson et al., 2006; Nichols, Kao, Verfaellie, & Gabrieli, 2006; Ryan & Cohen, 2004; Watson, Voss, Warren, Tranel, & Cohen, 2013), even for relatively simple stimuli maintained for very brief delays (Warren, Duff, Cohen, & Tranel, 2014; Warren, Duff, Jensen, Tranel, & Cohen, 2012; Warren, Duff, Tranel, & Cohen, 2010). One account of these findings suggests that hippocampus is required whenever memory involves binding together of multiple, arbitrarily related features (e.g., relational binding), as is necessary when novel stimuli are used as memoranda and also for episodic memory generally, irrespective of factors such as retention interval (Hannula, Tranel, & Cohen, 2006; Henke, 2010; Konkel & Cohen, 2009). However, other accounts continue to emphasize selective involvement of hippocampus in long-term memory (Jeneson & Squire, 2012).
One set of experimental observations that stands in contrast to the traditionally proposed selective role of hippocampus in long-term memory comes from studies of rodent behavior during memory-guided decisions. When discriminating objects and at decision points in mazes, rodents generate a back-and-forth exploration pattern that has been termed “vicarious trial-and-error” behavior (VTE) (Muenzinger, 1938; Tolman, 1938). VTE in rodents is strategic in that its frequency is greater for more difficult discriminations and declines with learning success (Hu, Xu, & Gonzalez-Lima, 2006; Muenzinger, 1938; Tolman, 1938). Surprisingly, VTE correlates with rodent hippocampal activity (Hu, et al., 2006; Johnson & Redish, 2007), and hippocampal ablation reduces VTE and concomitantly impairs discrimination [(Hu & Amsel, 1995), although effects might be more pronounced in memory rather than perceptual-discrimination tasks (Bett, et al., 2012)]. A view of hippocampus as being involved in long-term memory processing exclusively does not offer an account of its role in VTE and related behaviors. One account of VTE emphasizes the active query of memory, as to support predictions regarding the outcomes that would occur if one versus another option were selected (Buckner, 2010; Wang, Cohen, & Voss, 2014). VTE is thus likely an active state of memory use that transpires over the course of seconds. The establishment of functional neuroanatomical homologues between rodent and human models could thus be particularly illuminating for exploring the role of hippocampus and other relevant regions in the active use of memory in the immediate/on-line guidance of behavior.
The goal of the current experiment was to identify the neural correlates of VTE in a perceptual discrimination task. We used eye-movement tracking and fMRI to test for hippocampal involvement in VTE during a perceptual discrimination task involving novel shapes with high perceptual similarity. This task was designed to limit any possible involvement of long-term memory. We reasoned that subjects should use VTE when maintaining perceptual features across saccades in order to discriminate targets from perceptually similar foils, such that the relevant memory processing occurred over the course of seconds or less. Notably, this paradigm is not identical to that used in rodents to study VTE, either with respect to the early studies visual-discrimination apparati (Muenzinger, 1938; Tolman, 1938) or the more recent studies using T-maze, Y-maze, and similar spatial memory apparati (e.g., Johnson & Redish, 2007). We reasoned that these paradigms would be of limited utility for investigating VTE in humans, given that performance under such testing circumstances is governed by different processes in humans compared to rodents, owing to radically different behavioral and cognitive repertoires across species. Instead of replicating superficial task features, we focused on capturing similar memory and decision-making processes in humans as those thought to produce VTE in rodents. That is, VTE in rodents occurs when a conflict between two or more behavioral options must be solved using memory (either short-term or long-term). We reasoned that such conflict occurs when planning forthcoming eye movements during exploration, and that subjects can use memory for objects encountered previously during the trial to help plan the most effective eye movements needed to sample visual information relevant to the discrimination decision. That is, memory for previously viewed objects could prompt visual comparison strategies observed as VTE, thereby providing a behavioral measure of memory-based resolution of response conflict.
Given the nature of our paradigm, we reasoned that hippocampal activity related to VTE would reflect an incredibly immediate expression of the active use of memory to guide behavior. Furthermore, we reasoned that activity in prefrontal and parietal regions would also be relevant for VTE, given recent theorizing that these regions interact with hippocampus to support VTE and similar examples of memory-guided behavior (Johnson, Varberg, Benhardus, Maahs, & Schrater, 2012; Wang, et al., 2014). Furthermore, we assessed awareness of VTE using self-report measures in order to determine whether subjects had the subjective experience of performing VTE, either deliberately or unintentionally. Individuals are aware of explicit memory processing and also of deliberate behavioral strategies that depend on and enhance memory (e.g., deliberate study), and therefore lack of awareness of VTE would suggest an implicit expression of memory-based behavior in VTE.
Materials and Methods
Subjects
Behavioral and eye-tracking data were obtained from 30 individuals (13 males), ranging in age from 18 to 26 years (mean = 22 years). Of these 30 individuals, 12 performed the task during fMRI scanning. All subjects were without known neurological or psychiatric disorders and did not report current use of psychoactive drugs. All subjects for the fMRI component were right-hand dominant and did not require corrective eyeglasses or contact lenses.
Testing procedures
VTE was studied during a visual discrimination task. On each trial, either three or four stimuli were presented on the screen. All stimuli were identical except for one (the target), and the subject was instructed to identify the unique target stimulus from among the identical foils. Discrimination was difficult because targets and foils were highly perceptually similar and were arranged to permit foveal viewing of only one object at a time, such that subjects were required to make saccadic eye movements among objects. Each of the stimuli subtended approximately 9° of visual angle with approximately 16° of visual angle spacing from center-to-center for all stimuli during a trial. All objects were roughly equidistant from one another and from central fixation. Each trial began with a 2-s blinking fixation cross that appeared at the center of the screen, followed by a 1-s blank screen then 9-s presentation of visual stimuli, with a variable 10–16-s intertrial interval. Subjects attempted to locate the unique object, and maintained fixation on it upon identification. After the trial ended, objects disappeared and a number appeared at each of the three previous object locations (1–3, randomly assigned) for 3 s. Subjects reported the location of the unique object using a button-press response corresponding to the numbered position.
Three categories of stimuli were used as well as three- and four-object stimulus arrays in order to ensure that observed properties of VTE were not specific to any stimulus set or physical arrangement of stimuli on the computer screen. The stimuli used on each trial were drawn from three distinct sets involving high target/foil perceptual similarity: object silhouettes, kaleidoscope images, and real-world object photographic images. There were 16 trials involving three-object discrimination with object silhouettes, 16 trials involving three-object discrimination with kaleidoscope images, 16 trials involving four-object discrimination with object silhouettes, 16 trials involving four-object discrimination with kaleidoscope images, and 32 trials involving three-object discrimination with object photographs (96 trials total). All stimuli were trial-unique. The five trial categories were presented in 6 separate blocks (16 trials each), with short breaks between each block. Object silhouettes were selected from the set reported by (Westerberg, et al., 2006). Kaleidoscope images were selected from the set reported by (Voss & Paller, 2009). Object photographs were selected from the set reported by (Migo, Montaldi, & Mayes, 2013). All images were presented in grayscale on an LCD computer screen (behavior-only testing) or on a back-projected screen (MRI testing), both set to 60 Hz refresh. For the 12 MRI subjects, only three-object displays were used during fMRI scanning because four-object displays required presentation of stimuli at the screen borders, and eye-tracking calibration was lost when fixations were made to these regions. The four-object trials were administered following fMRI scanning.
Mixed repeated-measures ANOVA was used to test for performance differences due to the stimulus category used for each block (3 or 4 objects each of silhouettes, kaleidoscopes, or object photographs; within-subjects) and to whether subjects were in the MRI sample (MRI or non-MRI; between-subjects). VTE proportions averaged for all trials within each of these conditions did not vary according to the type of stimulus (main effect of stimulus block F(5,140)=1.39; P=0.23) or to whether subjects were in the MRI portion of the experiment (main effect of group F(8,140)=0.54; P=0.83). Data were therefore pooled across these parameters for all behavioral and eye-tracking analyses. Notably, primary analyses concern VTE proportions analyzed as a function of the number of fixations per trial, as VTE likelihood increases along with increasing overall fixations, but the high between-subject and between-trial variability in overall fixation counts precluded tests of whether VTE proportions normalized by fixation count differed according to these conditions. Nonetheless, these control analyses demonstrate no gross differences in VTE prevalence due to stimulus type or group.
Eye-movement tracking and VTE quantification
Viewing behavior was measured via eye tracking during visual discrimination in all subjects. For non-MRI testing, eye movements were recorded at 500 Hz using an EyeLink 1000 remote desktop tracking system (SR Research, Ontario, Canada). During fMRI scanning, eye movements were recorded from the right eye at 60 Hz using an ASL Eye-Trac6 long-range optic system (Applied Science Laboratories, Bedford, MA). Continuous eye-movement records for each trial were transformed into a timeseries of fixations, saccades, and blinks. Motion (0.15°), velocity (30°/s), and acceleration (8000°/s2) thresholds were used to identify saccades. Events in which the pupil size was very small were classified as blinks. Otherwise, eye-movement events falling below the saccade detection thresholds were categorized as fixation events.
Continuous records of the duration and timecourse of fixations made during discrimination were calculated for regions of interest (ROIs) using custom scripts in Matlab (The Mathworks, Inc.). ROIs were rectangles that tightly bounded objects in each display. We measured the duration of each fixation within an ROI, the number of within-ROI fixations made before transitioning (via saccade) to the next ROI, and the order of ROI-to-ROI saccadic transitions. VTE was quantified based on the order of ROI-to-ROI (i.e., object-to-object) transitions, with back-and-forth transitions (i.e., A-B-A) scored as VTE, as opposed to forward-looking (i.e., A-B-C) transitions. On average, fixations lasted for 272 ms (SE=12 ms) and 3.1 (SE=0.15) within-object fixations were made between each object-to-object transition. VTE was scored by the Matlab scripts and confirmed by visual inspection versus animated plots of the continuous eye movement records. The final object-to-object transition was excluded from analysis of VTE, in order to focus VTE analysis on the search portion of the trial. Discrimination response time was calculated using the onset of the final fixation to the unique object, for trials with correct responses only.
Assessment of subjective intentionality and awareness of VTE
Awareness and intentionality in eye-movement control and VTE was assessed for each subject immediately following the experiment via structured interview. Subjects were asked six questions that progressively measured increasing levels of awareness and intentionality, based on yes/no answers and open-ended justifications. The score was based on the highest question receiving a “yes” response. Scores of 0 to 3 represented increasing general awareness of intentional eye movements, yet absolutely no reported awareness of performing VTE. Scores of 4 indicated awareness of having performed VTE, yet no feeling of intentionality, whereas scores of 5 indicated intentional VTE. The questions were as follows: (1) “Did you use any strategies when attempting to find the unique object?”, (2) “Can you describe any strategy that you used?”, (3) “Did you look at the objects in any particular order when attempting to identify the unique object?”, (4) “Can you describe the order that you used?”, (5) “Did you make the back-and-forth pattern when looking from one object to the next”, (6) “Did you try to make the back-and-forth pattern; that is, did you use this pattern as a strategy to attempt to identify the unique object?”. Subjects were given an example VTE pattern before question 5 (an affirmative answer yielding a score of 4 or higher). Answering “no” to question 1 in addition to “no” on question 2 resulted in a score of 0, whereas an affirmative answer to question 2 resulted in a score of 1, etc. Subjects provided justifications for all answers, which were manually recorded.
MRI procedures and fMRI analysis
MRI used a Siemens 3T Trio with a 32-channel head coil. BOLD MR images were collected during the visual discrimination task (TR=2,000 ms; TE=25 ms; voxel size = 2.5 × 2.5 × 3.0 mm; FOV=22 cm), acquired perpendicular to the long axis of the hippocampus. Images were not collected and the paradigm did not begin until after the scanner reached steady state. The task was divided into four runs (16 trials each), which were concatenated offline for analysis. A structural MR image was collected after the functional runs (MP-RAGE T1-weighted scan, voxel size = 0.5 mm isotropic, 522 axial slices). fMRI analysis utilized the AFNI software package (Cox, 1996). Preprocessing steps included volume registration through time (motion correction), correction of slice-timing discrepancies, co-registration of functional and structural images, transformation to stereotactic space (MNI-305), removal of linear signal drift, and spatial smoothing of functional data with a 5-mm FWHM Gaussian kernel.
Estimates of brain activity related to task performance were obtained in each subject via a deconvolution approach within a general linear model. Nuisance variables were entered into the regression model, including the T1* and T0 components of the MR signal, as well as six-parameter movement estimates. Task periods were modeled using a regressor generated by convolving the blocked rectangular function corresponding to 9-s discrimination periods with a hemodynamic response function (HRF). The regression model allowed for independent assessment of (1) activity related to the main effect of task performance (discrimination trial activity versus the implicit baseline of non-task periods) and (2) activity that tracked linearly with trial-by-trial variations in the proportion of transitions involved in VTE (i.e., parametric analysis). Brain activity could thus be assessed based on covariation with the VTE behavioral metric independent from activity related to overall task performance. A second parametric analysis was performed to investigate saccade-related activity, using the number of saccades made per trial irrespective of VTE and without inclusion of the main effect of task versus implicit baseline. This analysis was used to identify regions nonspecifically associated with making saccades, irrespective of VTE. For both analyses, regions exhibiting significant activity at the group level were identified via random-effects analysis with a combined voxel-wise and spatial-extent threshold method incorporating Monte Carlo simulation. A stringent voxel-wise threshold was set to P<0.001 to guard against false positives during cluster correction (e.g., Ecklund et al., 2016), and the spatial-extent threshold was identified as 35 contiguous suprathreshold voxels to obtain a combined corrected threshold of P<0.05 (the smallest cluster identified was 47 voxels). The analysis was unsigned (i.e., both positive and negative effects were tested, 2-tailed), but only positive relationships between activity and conditions were identified.
Results
Properties of VTE
VTE was measured during perceptual discrimination (Figure 1). Performance was highly accurate (mean = 91% correct target selections, SE = 2.4%). Inspection of eye-movement records indicated that subjects generally started each trial by viewing the uppermost stimulus or upper-leftmost stimulus and then moving from stimulus to stimulus in a clockwise direction. This is consistent with viewing patterns for similar multiple-object displays (e.g., Hannula, et al., 2006)), with only infrequent performance of VTE. As shown in Figure 2A, the likelihood of making at least one VTE per trial increased by chance (i.e., if transitions were made randomly) as the number of transitions per trial increased. VTE was significantly less likely to occur at least once than would be expected by chance (Figure 2A, Hotelling’s T2(10,20)=36.3; P=0.039). Likewise, the overall number of transitions involved in VTE increased by chance (i.e., if transitions were made randomly) as the number of transitions per trial increased (Figure 2B). Again, the proportion of transitions involved in VTE was significantly less than would be expected by chance (Figure 2B; Hotelling’s T2(10,20)=114.7; P<0.001). This suggests that VTE did not occur by chance, but instead was generated occasionally as part of active target search during a stereotypical clockwise pattern of viewing. Furthermore, VTE transitions were no more likely than chance to include the target object (pairwise comparison vs. chance t(29)=1.0, P=0.33), also indicating that VTE occurred during search and was not merely a ramification of target identification.
Figure 1. Example visual displays and VTE.
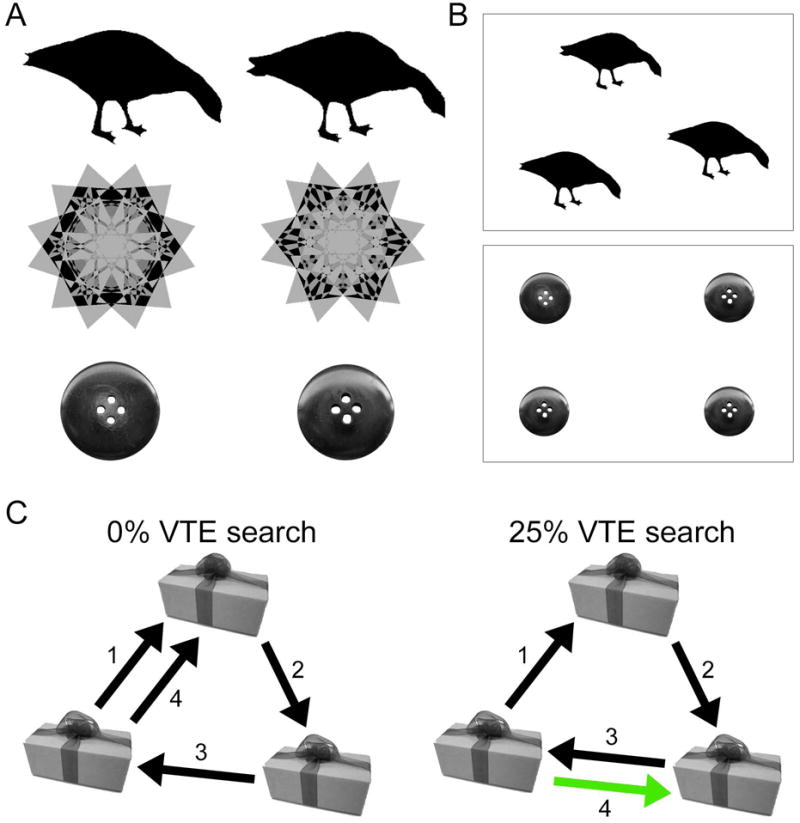
(A) Visual stimuli included targets and foils with high perceptual similarity drawn from three stimulus sets, including object silhouettes, kaleidoscope images, and object photographs. (B) Each trial used either a three-image or four-image display, with one unique target among two or three perceptually identical foils. Subjects were required to identify the unique target. (C) Two hypothetical object-to-object transition patterns are given for an example display, involving either no VTE or one VTE transition, shown in green.
Figure 2. VTE and its neural correlates.
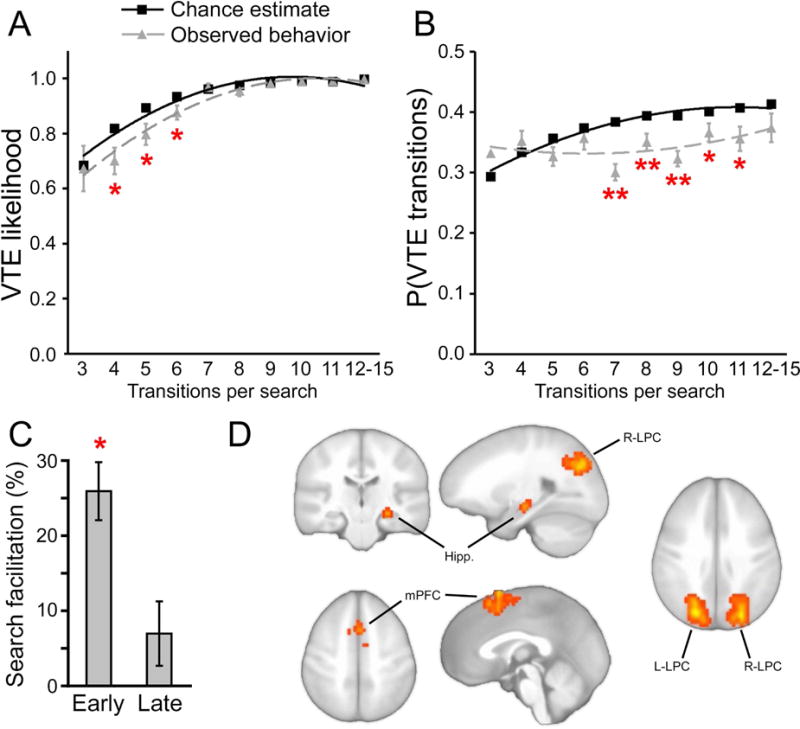
(A) The cumulative likelihood of performing at least one VTE during a trial. (B) The overall proportion of VTE transitions for trials including at least one VTE transition. (C) The unique target was identified faster for trials including at least one VTE transition versus trials with no VTE. The ~25% reduction in search duration when VTE was generated early corresponds to a reduction of ~1.1s (the mean search duration for no-VTE trials was 5.2 s). (D) fMRI activity increases that covaried with trial-by-trial fluctuations in the proportion of VTE transitions are shown superimposed on a template brain. * P<0.05 for observed versus chance. ** P<0.01 for observed versus chance or versus zero. Hipp., hippocampus.
In order to evaluate the strategic value of VTE, we compared overall search time for trials with versus without VTE, taking into account the timing during the trial when the first VTE event occurred (in order to account for possible differences in earlier-search VTE and later-search VTE). Trials with any VTE were categorized as early-search VTE or late-search VTE based on median split of the first transition included in VTE, with early-search VTE including trials with VTE within the first 5 transitions. Subjects identified targets more quickly for trials with any VTE compared to searches with no VTE (Figure 2C; t(29)=10.2, P<0.001). Furthermore, this VTE facilitation was especially pronounced for trials with early-search VTE (Figure 2C; t(29)=8.4, P<0.001), and was marginally significant for trials with late-search VTE (Figure 2C; P=0.09). This relationship between VTE and faster discrimination is similar to what has been observed in rodents (as described above), and demonstrates a strategic value for VTE in humans during perceptual discrimination.
fMRI correlates of VTE
fMRI correlates of VTE were identified as activity showing trial-by-trial fluctuation in relation to trial-by-trial fluctuations in VTE (i.e., parametric analysis), assessed independently from activity related merely to performing the discrimination task (Materials and Methods; Table 1). Activity of the right hippocampus body was positively related to VTE (Figure 2D), consonant with neural activity measures in rodents (Hu, et al., 2006; Johnson & Redish, 2007). Furthermore, activity of medial prefrontal cortex (mPFC) and bilateral lateral-parietal cortex (LPC) was also identified (Figure 2D).
Table 1.
Summary of fMRI VTE activity and stimulus-evoked non-VTE activity (task) for the main analysis, and activity related to total fixation count in the secondary/control analysis, including MNI coordinates (mm), volume (mm3), and approximate Brodmann Area(s) for the cluster centroid (Cent. BA).
| MNI Coordinates | Volume | Cent. BA | |||
|---|---|---|---|---|---|
| X | Y | Z | |||
|
|
|||||
| VTE activity | |||||
| Medial prefrontal cortex (superior) | −1 | +4 | +64 | 7,444 | 6/8 |
| Left superior parietal lobule | −23 | −73 | +35 | 6,150 | 7 |
| Right superior parietal lobule | +23 | −74 | +33 | 5,494 | 7 |
| Right hippocampus (body) | +25 | −18 | −15 | 881 | – |
| Task activity | |||||
| Left fusiform/lingual gyrus | −29 | −74 | −19 | 22,688 | 18/19 |
| Right fusiform/lingual gyrus | +32 | −70 | −18 | 21,150 | 18/19 |
| Left inferior/superior parietal lobule | −30 | −63 | +37 | 7,856 | 39 |
| Right inferior/superior parietal lobule | +33 | −54 | +35 | 6,338 | 39 |
| Medial prefrontal cortex and anterior cingulate | −4 | +3 | +51 | 4,369 | 6/32 |
| Right middle/superior frontal gyrus | +28 | −10 | +55 | 2,250 | 8 |
| Left middle/superior frontal gyrus | −25 | −8 | +59 | 2,175 | 8 |
| Total fixation count activity | |||||
| Left middle/superior frontal gyrus | −23 | −15 | +51 | 1,894 | 8 |
| Right middle/superior frontal gyrus | +31 | +9 | +61 | 1,838 | 8 |
These VTE-related regions were mostly distinct from regions involved nonspecifically in the discrimination task (i.e., trial-evoked activity independent of the parametric effect of VTE). These regions included object-sensitive ventral visual cortex, lateral prefrontal cortex corresponding approximately to frontal eye fields (FEF), and similar SEF and IPS regions that overlapped partially with the VTE-related portions of the same regions (Figure 3; Table 1). Thus, consistent with our predictions, hippocampal activity was specifically associated with VTE and was not identified nonspecifically in relation to task performance (i.e., not a generic neural correlate of viewing or learning these stimulus displays). Furthermore, a control parametric analysis was performed for overall number of saccades made during each trial (irrespective of VTE). This analysis identified positive association between saccades and activity of approximately the same lateral prefrontal regions (FEF) identified nonspecifically related to task performance (Table 1), but activity of VTE-sensitive regions was not identified by this control analysis.
Figure 3. VTE- and task-related brain activity.
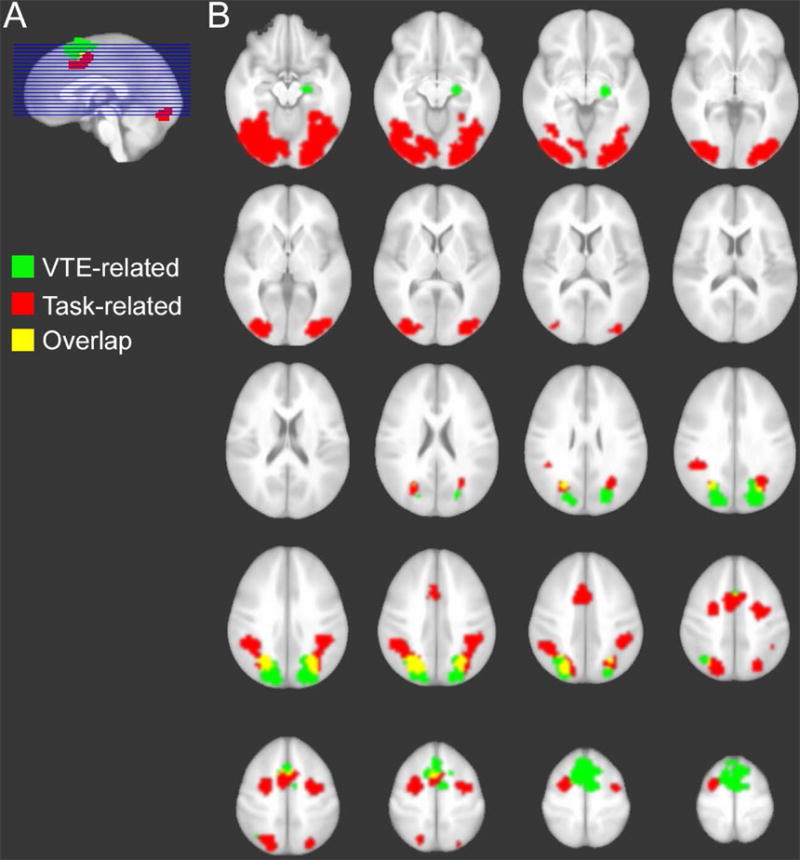
Regions are colorized according to whether activity was significantly VTE-related, task-related, or identified for both of these conditions (overlap). Images are displayed in neurological convention (right is subject’s right).
Intention and Awareness of VTE
To the extent that VTE is independent from explicit memory processing, we would expect minimal intention and/or awareness of VTE performance. That is, if VTE were to merely reflect an intentional memory-based study strategy used by subjects, then VTE-related activity could reflect heightened encoding, given that strong encoding and awareness/intentionality are often coupled (Yonelinas, 2001). Of 30 subjects, 67% were reportedly unaware of having performed VTE (Figure 4A). Further analysis indicated that awareness differed for early-search versus late-search VTE, with greater awareness associated with late-search VTE. Early-search VTE was negatively associated with awareness scores (r = −0.25) whereas late-search VTE was positively associated with awareness scores, (r = 0.44) (Figure 4B), with a significant interaction between these relationships (Z=2.7, P = 0.008). Every subject that reported awareness of VTE (scores of 4 or 5 on the awareness scale) exhibited more late-search VTE than early-search VTE (Figure 4B; 38% vs. 31%, respectively, P=0.02), and also more late-search VTE than did subjects who were unaware (Figure 4B; 38% vs. 33%, respectively, P=0.003).
Figure 4. Relative lack of intention and awareness of VTE.
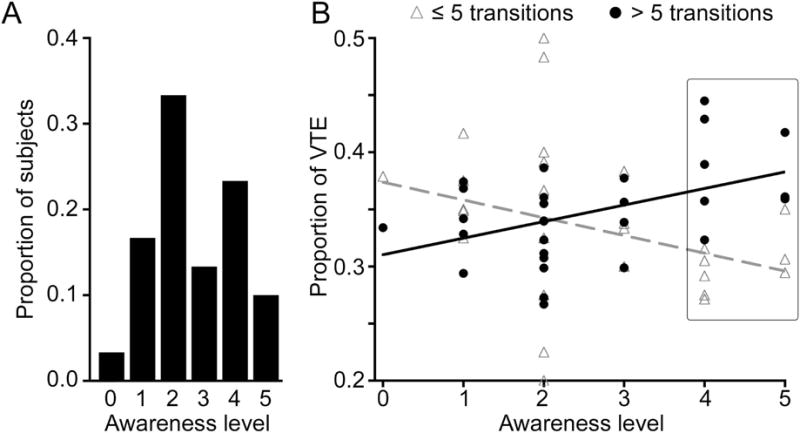
(A) The majority of subjects indicated no awareness of VTE performance (scores 0 – 3). (B) Awareness scores were negatively related to early-trial VTE and positively related to late-trial VTE. Late-trial VTE predominated for subjects with scores of 4 – 5 (highlighted), which indicated awareness of unintentional or intentional VTE, respectively, indicating that awareness and intentionality were inversely related to advantageous early-trial VTE (Figure 2C).
Interestingly, all subjects scoring a 4 (indicating intentional performance of some viewing strategy, but not necessarily VTE) described looking first at one of the objects (usually the top-most or upper-left object) and then inspecting the remaining objects in clockwise (or occasionally counterclockwise) order. These reports were confirmed by inspection of their eye-movement records, indicating a general introspective ability that stands in contrast to the general lack of introspective ability regarding VTE. In sum, awareness and intentionality measures were thus relatively unrelated to VTE, and especially unrelated to the early-search VTE that was particularly helpful in terms of its relationship with faster discrimination performance (Figure 2C). Instead, late-search VTE was associated with some intentionality and awareness.
Discussion
We found that individuals strategically generated VTE during discrimination of perceptually similar novel objects. Notably, the task did not have any overt long-term memory demands (i.e., each trial involved discrimination of novel stimuli without any across-trial repetition). The nature of the stimulus displays required subjects to make saccadic eye movements from one object to the next in order to support visual comparison and discrimination. VTE occurred less frequently than would be expected by chance and thus represented an occasional visual-comparison behavior that occurred against a dominant pattern of viewing objects serially in a clockwise fashion. VTE was strategic in that discrimination occurred more rapidly on trials with VTE versus without VTE, especially when VTE was generated within the first several saccades (Figure 2C). Given that novel visual stimuli with high perceptual similarity were used, discrimination required a holistic representation of the specific visual details and their relative configurations (Figure 1A); i.e., relational representation. VTE in this task can be considered as an expression of a strategy for visual comparison and discrimination of relational memory information over a very brief timescale.
These findings do not readily fit with the traditionally proposed exclusive role of hippocampus in long-term declarative memory. Hippocampal activity tracked the frequency of VTE performed on a given trial, whereas long-term memory encoding of stimuli would presumably occur for all trials relatively independent from VTE. Instead, the pattern of hippocampal activity related to VTE is consistent with the interpretation that this activity reflected the short-term/immediate use of stimulus representations to guide effective visual comparison (Olsen, Moses, Riggs, & Ryan, 2012). Furthermore, subjects were generally unaware of having performed VTE. Our findings are therefore consistent with the hypothesis that hippocampus is critically involved in supporting relational representation of complex, multi-feature stimuli and episodes, irrespective of any specific retention interval (i.e., both long-term and short-term/immediate) and irrespective of any necessary role of conscious awareness (see also (Hannula & Greene, 2012; Henke, 2010; Konkel & Cohen, 2009). Notably, had stimuli been perceptually or conceptually dissimilar items, discrimination would not have required relational representation among complex stimulus features and likely have been independent of the hippocampus (Mayes et al., 2007).
The current findings are consonant with and extend those from a collection of experiments that compared self-guided versus passive visual exploration (Voss, Gonsalves, Federmeier, Tranel, & Cohen, 2011; Voss, Warren, et al., 2011). Those previous studies provided fMRI and lesion evidence for the role of hippocampus in effective exploration strategies when learning objects and their locations in arrays. Correlation among hippocampus and regions of anterior and posterior hippocampal-cortical networks increased during advantageous self-guided exploration, and bilateral hippocampal lesions eliminated the boost in memory associated with self-guided (versus passive) exploration (Voss, Gonsalves, et al., 2011). Furthermore, hippocampal and medial prefrontal activity was associated with a specific exploration strategy whereby subjects looked back and forth from one object to the next (Voss, Warren, et al., 2011). This viewing pattern, which we called “revisitation”, predicted better subsequent memory but only when it was self-generated (i.e., not in the passive condition). Furthermore, although revisitation unfolded over the course of seconds, it was associated with hippocampal activity and was severely disrupted by bilateral hippocampal lesions. These findings suggested “in-the-moment” memory processing by hippocampus, with many striking parallels between “revisitation” measured during intentional learning and the patterns of VTE viewing behavior measured here during visual discrimination. However, it is notable that revisitation occurred during intentional memorization in these previous experiments and therefore could have been somewhat related to long-term memory processing. Likewise, Yee and colleagues (2014) identified inefficient visual exploration for hidden targets in individuals with hippocampal damage. Although such deficits suggest disruption of information integration over very brief intervals, the specific interval over which integration was problematic was not specified. These limitations do not apply to the current experiment, in which hippocampal activity was associated with VTE without any considerable long-term memory component.
Of course, we cannot rule out the possibility that VTE fixations could have been related to better long-term memory formation, because long-term memory was not tested. Despite this limitation and the limitations imposed by including a relatively small sample size, our findings provide a salient demonstration that hippocampal fMRI activity can robustly associate with characteristics of viewing behavior that are defined without any explicit relationship to long-term memory.
A recent study using eye-movement tracking while subjects studied faces in a long-term memory experiment found that the number of visual fixations correlated with hippocampal activity, such that trials with greater fixation counts had greater hippocampal fMRI activity (Liu et al., 2017). This finding is in apparent contrast to the relationship reported here between hippocampal activity and VTE, but lack of relationship between hippocampal activity and overall fixation counts (Table 1). However, it is important to note that Liu et al. (2017) did not attempt to isolate fixations reflecting the process that we hypothesize is reflected by VTE, namely the use of short-term memory to direct fixations to portions of the stimulus that require additional attentional resources. Because subjects were engaged in intentional memorization in that study, it is likely that this process occurred frequently (given that it is associated with enhanced memory performance; e.g., Voss, Warren, et al., 2011) and could have been particularly robust for trials with higher fixation counts. Thus, although our results diverge superficially from those of Liu et al. (2017) we consider it likely that hippocampal fMRI activity reflected similar viewing-related memory processing in both studies.
In addition to hippocampus, VTE was associated with increased activity of mPFC and LPC. Indeed, an emerging body of work has implicated these regions as part of a network for memory-guided control of behavior and strategic decision-making (Ranganath & Ritchey, 2012; Wang, et al., 2014). Indeed, the influence of memory on decisions that guide ongoing behavior have been associated with a functional network of hippocampus and mPFC (Kumaran, Summerfield, Hassabis, & Maguire, 2009), and the mPFC area identified here is highly consonant with results from our prior experiment on VTE using a long-term memory paradigm (Voss, Warren et al., 2011). We have recently provided an account of hippocampal-cortical network involvement in decisions that guide ongoing behavioral control, even when this control is not thought to involve much effortful deliberation, such as during visual exploration (Wang, et al., 2014). This proposal suggests that conflict regarding the selection of one behavior among several options prompts covert and rapid generation of the possible plans for action by prefrontal cortex. These potential action plans then cue hippocampal-based memory retrieval, thus providing simulations of possible behavioral outcomes, which can be selected based on their proximity to the desired goal outcome. This account of Covert Rapid Action-Memory Simulation (CRAMS) for behavioral decisions (Wang, et al., 2014) makes the novel claim that hippocampus, in addition to other regions of its cortical network, will be involved even in simple decisions that have little obvious connection to long-term memory functions, such as in the current experiment. Likewise, mechanistic accounts of mPFC-hippocampal interactions in rodent models of VTE-like behaviors emphasize mPFC-cued generation of predictions and mental simulations based on hippocampal representations (Bird & Burgess, 2008; Buckner, 2010; Johnson, et al., 2012). These positions are supported by the current findings.
It is striking that subjects were mostly unaware of the fact that they made VTE viewing patterns, despite relatively accurate awareness of general aspects of viewing behavior (i.e., explicit knowledge that they first viewed the topmost or leftmost object and then proceeded from object to object clockwise). Furthermore, neural correlates of intentional saccade planning and execution consistently include activity of frontal eye fields (Gaymard, Ploner, Rivaud, Vermersch, & Pierrot-Deseilligny, 1998), and activity of this region was associated with task performance generally and with the number of saccades made irrespective of VTE, but not specifically with VTE (Figure 3). Thus, fMRI activity is consistent with subjects’ lack of ability to introspect regarding VTE, despite its strategic value. Our findings thus extend others that have demonstrated implicit cognition mediated by lateral prefrontal cortex in strategic control [e.g., (Lau & Passingham, 2007; van Gaal, Ridderinkhof, Fahrenfort, Scholte, & Lamme, 2008; van Gaal, Ridderinkhof, Scholte, & Lamme, 2010)] by showing that mPFC regions associated with the online use of memory to guide behavior (Kumaran, et al., 2009) can similarly demonstrate task-related activity not strongly linked to explicit awareness [see also (Powers, 1973) for a description of control systems operating without necessary awareness/intention]. Notably, previous studies on mPFC contributions to memory focus on ventral mPFC (Preston & Eichenbaum, 2013), whereas the area identified here and in our previous fMRI study on VTE (Voss, Warren et al., 2011) was dorsal mPFC, corresponding approximately to the pre-SMA. This area has been implicated in internally guided action planning and in eye-movement control (Lau et al. 2006; Tehovnik et al., 2000) and could be involved in evaluating multiple competing internally and externally cued action plans with respect to current coals and intentions (Nachev et al., 2007; reviewed in Wang et al., 2015).
To summarize, our results extend theoretical accounts of hippocampal function by showing that hippocampal activity can reflect involvement in the immediate use of memory from stimuli experienced just moments before to guide behavior. Further, subjects were generally unaware of this immediate use of memory, and thus hippocampal activity was relatively unrelated to explicit/declarative memory constructs. These findings underscore the need to consider representational qualities of hippocampus rather than broad psychological constructs that are used to describe specific testing circumstances or specific stimulus domains, such as short-term/long-term and spatial/non-spatial. Furthermore, our findings highlight the situating of hippocampus within a network for memory-guided behavior that includes the cortical network of the hippocampus (Ranganath & Ritchey, 2012; Wang, et al., 2014; Wang & Voss, 2014). Such an emphasis on the role of hippocampus and memory in guiding on-line processing and behavioral choices helps to bridge what has been a divide between the literature on rodent spatial navigation and the human memory literature (Eichenbaum & Cohen, 2014a). Critically, the current findings document that hippocampal involvement in relational processing can be seen despite no spatial memory demands, no long-term memory demands, no demands for intentional encoding, and using incredibly brief intervals well within the timescale of short-term/immediate memory that is normally thought to be unrelated to hippocampus.
Acknowledgments
Research was supported by award R00-NS069788 from the National Institute of Neurological Disorders and Stroke and by award R01-MH062500 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no conflicts of interest.
References
- Bett D, Allison E, Murdoch LH, Kaefer K, Wood ER, Dudchenko PA. The neural substrates of deliberative decision making: contrasting effects of hippocampus lesions on performance and vicarious trial-and-error behavior in a spatial memory task and a visual discrimination task. Front Behav Neurosci. 2012;6:70. doi: 10.3389/fnbeh.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: Insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48. C21–28. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. Oxford; Oxford University Press; 2004. [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014a;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014b;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud S, Vermersch AI, Pierrot-Deseilligny C. Cortical control of saccades. Exp Brain Res. 1998;123:159–163. doi: 10.1007/s002210050557. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Greene AJ. The hippocampus reevaluated in unconscious learning and memory: At a tipping point? Front Hum Neurosci. 2012;6:80. doi: 10.3389/fnhum.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Hu D, Amsel A. A simple test of the vicarious trial-and-error hypothesis of hippocampal function. Proc Natl Acad Sci U S A. 1995;92:5506–5509. doi: 10.1073/pnas.92.12.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Xu X, Gonzalez-Lima F. Vicarious trial-and-error behavior and hippocampal cytochrome oxidase activity during Y-maze discrimination learning in the rat. Int J Neurosci. 2006;116:265–280. doi: 10.1080/00207450500403108. [DOI] [PubMed] [Google Scholar]
- Jeneson A, Squire LR. Working memory, long-term memory, and medial temporal lobe function. Learn Mem. 2012;19:15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Varberg Z, Benhardus J, Maahs A, Schrater P. The hippocampus and exploration: Dynamically evolving behavior and neural representations. Front Hum Neurosci. 2012 doi: 10.3389/fnhum.2012.00216. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Front Neurosci. 2009;3:166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Novelty signals: a window into hippocampal information processing. Trends Cogn Sci. 2009;13:47–54. doi: 10.1016/j.tics.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HC, Passingham RE. Unconscious activation of the cognitive control system in the human prefrontal cortex. J Neurosci. 2007;23:5805–5811. doi: 10.1523/JNEUROSCI.4335-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H, Rogers RD, Passingham RE. Dissociating response selection and conflict in the medial frontal surface. Neuroimage. 2006;29:446–451. doi: 10.1016/j.neuroimage.2005.07.050. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Shen K, Olsen RK, Ryan JD. Visual sampling predicts hippocampal activity. J Neurosci. 2017;37:599–609. doi: 10.1523/JNEUROSCI.2610-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Migo EM, Montaldi D, Mayes AR. A visual object stimulus database with standardized similarity information. Behav Res Methods. 2013;45:344–354. doi: 10.3758/s13428-012-0255-4. [DOI] [PubMed] [Google Scholar]
- Muenzinger KF. Vicarious trial and error at a point of choice: A general survey of its relation to learning efficiency. J Genet Psychol. 1938;53:75–86. [Google Scholar]
- Nachev P, Wydell H, O’Neil K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36:155–163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols EA, Kao YC, Verfaellie M, Gabrieli JD. Working memory and long-term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RK, Moses SN, Riggs L, Ryan JD. The hippocampus supports multiple cognitive processes through relational binding and comparison. Front Hum Neurosci. 2012;6:146. doi: 10.3389/fnhum.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci. 2006;26:4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after termporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33:1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Powers WT. Feedback: Beyond behaviorism. Science. 1973;179:351–356. doi: 10.1126/science.179.4071.351. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764–773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20:1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. The nature of change detection and online representations of scenes. J Exp Psychol Hum Percept Perform. 2004;30:988–1015. doi: 10.1037/0096-1523.30.5.988. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Rev. 2000;32:413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Tolman EC. The determiners of behavior at a choice point. Psychol Rev. 1938;45:1–41. [Google Scholar]
- van Gaal S, Ridderinkhof KR, Fahrenfort JJ, Scholte HS, Lamme VA. Frontal cortex mediates unconsciously triggered inhibitory control. J Neurosci. 2008;28:8053–8062. doi: 10.1523/JNEUROSCI.1278-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaal S, Ridderinkhof KR, Scholte HS, Lamme VA. Unconscious activation of the prefrontal no-go network. J Neurosci. 2010;30:4143–4150. doi: 10.1523/JNEUROSCI.2992-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Hippocampal brain-network coordination during volitional exploratory behavior enhances learning. Nat Neurosci. 2011;14:115–120. doi: 10.1038/nn.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. An electrophysiological signature of unconscious recognition memory. Nat Neurosci. 2009;12:349–355. doi: 10.1038/nn.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Warren DE, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Spontaneous revisittion during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proc Natl Acad Sci U S A. 2011;108:E402–409. doi: 10.1073/pnas.1100225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Cohen NJ, Voss JL. Covert rapid action-memory simulation (CRAMS): A hypothesis of hippocampal-prefrontal interactions for adaptive behavior. Neurobiol Learn Mem. 2015;117:22–33. doi: 10.1016/j.nlm.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Voss JL. Brain networks for exploration decisions utilizing distinct modeled information types during contextual learning. Neuron. 2014;82:1171–1182. doi: 10.1016/j.neuron.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Cohen NJ, Tranel D. Hippocampus contributes to the maintenance but not the quality of visual information over time. Learn Mem. 2014;22:6–10. doi: 10.1101/lm.037127.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Jensen U, Tranel D, Cohen NJ. Hiding in plain view: lesions of the medial temporal lobe impair online representation. Hippocampus. 2012;22:1577–1588. doi: 10.1002/hipo.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ. Medial temporal lobe damage impairs representation of simple stimuli. Front Hum Neurosci. 2010;4:35. doi: 10.3389/fnhum.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, Cohen NJ. Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus. 2013;23:570–580. doi: 10.1002/hipo.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, Reber PJ. When memory does not fail: familiarity-based recognition in mild cognitive impairment and Alzheimer’s disease. Neuropsychology. 2006;20:193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Yee LT, Warren DE, Voss JL, Duff MC, Tranel D, Cohen NJ. The hippocampus uses information just encountered to guide efficient ongoing behavior. Hippocampus. 2014;24:154–164. doi: 10.1002/hipo.22211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Consciousness, control, and confidence: the 3 Cs of recognition memory. J Exp Psychol Gen. 2001;130:361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]


