Abstract
Sleep has been postulated to promote brain energy restoration. It is yet unknown if increasing the energy availability within the brain reduces the sleep need. The guanidine amino acid creatine (Cr) is a well-known energy booster in cellular energy homeostasis. Oral creatine-monohydrate supplementation (CS) increases exercise performance and has been shown to have substantial effects on cognitive performance, neuroprotection and circadian rhythms. The effect of CS on cellular high energy molecules and sleep-wake-behavior is unclear.
Here, we examined the sleep-wake behavior and brain energy metabolism before and after four week-long oral administration of CS in the rat. CS significantly decreased total sleep time and non-rapid eye movement (NREM) sleep during the light (inactive) but not during the dark (active) period. NREM-sleep and NREM delta activity were significantly decreased in CS rats after 6h of sleep deprivation. Biochemical analysis of brain energy metabolites showed a tendency increase in phosphocreatine after CS, while cellular ATP level decreased. Microdialysis analysis showed that the sleep deprivation-induced increase in extracellular adenosine was attenuated after CS.
These results suggest that CS reduces sleep need and homeostatic sleep pressure in rats, thereby indicating its potential in the treatment of sleep related disorders.
Keywords: ATP, creatine, brain energy metabolism, sleep homeostasis, adenosine
Introduction
Sleep is an essential state proven by the fact that one third of our life is spent sleeping. While increasing evidence indicates that adequate sleep is essential for key brain functions, sleep loss and associated health issues are frequent among many adults and adolescents. A long-lasting hypothesis postulates that the function of sleep is to restore brain energy expended during active waking (Benington and Heller, 1995). Several direct and indirect measurements of energy metabolism indicate that during sleep brain energy expenditure decreases (Madsen and Vorstrup, 1991, Maquet, 1995). Compared with wakefulness or REM-sleep, during NREM sleep brain energy demand is reduced as indicated by a 44% reduction in the cerebral metabolic rate (CMR) of glucose and a 25% reduction in the CMR of oxygen, while glucose and ATP concentrations increase (Netchiporouk et al., 2001, Dworak et al., 2010). In contrast, prolonged sleep deprivation is associated with brain energy utilization (Kong et al., 2002, Dworak et al., 2010, Dworak et al., 2007). Energy depletion in localized brain areas enhances sleep (Kalinchuk et al., 2003).
The direct relationship between wake-related neuronal activation and increased brain energy use was also addressed in our previous reports, where the metabolic by-product of energy, adenosine (AD), increased extracellularly in a wake-active brain region, the basal forebrain, during sleep deprivation (Porkka-Heiskanen et al., 1997, Brown et al., 2012).
If brain energy depletion induces NREM sleep, and NREM sleep is essential for brain energy homeostasis, an increase in brain energy availability may reduce homeostatic NREM sleep need. Cellular energy demand and supply are tightly regulated for efficiency and economy of energy use. Cells with high and fluctuating energy requirements, such as neurons, can significantly increase the rate of ATP hydrolysis (Attwell and Laughlin, 2001, Alle et al., 2009). Under normal circumstances, intracellular ATP levels remain constant. However, under situations of increased neuronal activity such as sleep deprivation, ATP and AD concentrations vary and affect cellular signaling and vigilance via ATP receptors (P2X, P2Y) and AD receptors (Burnstock et al., 2011, Harris et al., 2012).
The guanidine amino acid Creatine (Cr) is an essential molecule in cellular ATP homeostasis, particularly in tissues subject to high metabolic demands such as brain and muscle. A growing number of reports now provide evidence for the importance of the Cr Kinase/PhosphoCreatine (CK/PCr) system and Cr metabolism for normal function of the brain, as well as under neuropathological conditions. Chronic oral Cr supplementation protects neurons in animal models of amyotrophic lateral sclerosis (ALS), Huntington disease, Parkinson disease and after cerebral ischemia and traumatic brain injuries (Klivenyi et al., 1999, Matthews et al., 1999, Matthews et al., 1998, Lensman et al., 2006, Sullivan et al., 2000), and improves overall health and cognitive performance (Bender et al., 2008, Rae et al., 2003). Furthermore, Cr has been shown to improve cognitive and psychomotor performance in sleep-deprived humans (Cook et al., 2011).
Since sleep deprivation is associated with brain energy deficit, we hypothesize that Cr supplementation has the potential to compensate these energy-demanding challenges by increasing total energy availability within the brain. Given the potential usefulness of safe supplementation for alleviating the negative effects associated with sleep deprivation, the present study examines the effects of 4 weeks oral CS on sleep-wake behavior, sleep deprivation and associated changes in brain energy metabolism in an animal model.
Experimental procedures
Animal and surgical procedures
The total of 19 male Sprague-Dawley rats (300–400g) used in this study were kept in a room with constant temperature (23°C) and 12-h light-dark cycle (lights on at 7.00 AM). Water and food were provided ad libitum. Under general anesthesia (i.m. ketamine 7.5mg/100g body weight, xylazine 0.38mg/100g, acepromazine 0.075mg/100g) rats were implanted with electroencephalogram (EEG) and electromyogram (EMG) electrodes.
EEG electrodes (stainless steel screws) were implanted epidurally over the frontal (primary motor, AP=+2.0; ML=2.0) and parietal (retrosplenial, AP=−4.0; ML=1.0) cortices. EMG recording electrodes (silver wires covered with Teflon) were implanted into neck muscles. The EEG/EMG signals were amplified and sampled at 104 Hz (filter settings for EEG: 0.3 Hz high pass and 40 Hz low pass, for EMG: 30 Hz high pass and 100 Hz low pass)For collection of microdialysis samples (N=7), an unilateral guide cannula (CMA/11 Guide, CMA/Microdialysis, Stockholm, Sweden) was implanted targeting the basal forebrain area of horizontal and diagonal band/substantia innominate/magnocellular preoptic area (HDB/SI/MCPO), using bregma co-ordinates at: AP=−0.3; ML=2.2; V=8.8 (Paxinos & Watson, 1998). Animals were treated in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care and Use Committee at Boston VA Healthcare system, Harvard University and U.S. National Institute of Health. Every effort was made to minimize animal suffering and to reduce the number of animals used.
After 1 week of the recovery period, rats were connected to EEG/EMG recording leads for adaptation which lasted 4 days. Before microdialysis probe insertion, rats were connected to the recording cables at 08.00AM and EEG/EMG was continuously recorded for at least 24h to monitor the stabilization of EEG and sleep-waking cycles.
EEG recording and analysis
EEG recordings (acquisition using Grass Gamma v4.3) were scored using the Rat Sleep Stager (version 3.2) in 10sec epochs manually for NREM sleep, REM sleep and wakefulness as described earlier (Ramesh et al., 2007). The ‘waking’ (W) was identified by sustained EMG activity and low amplitude, high frequency (desynchronized) waves accompanied by movements, grooming, eating etc, ‘NREM sleep’ by low EMG, high-amplitude low-frequency EEG with increased activity in delta range (0.5–4.5Hz) and quite inactive state often assuming a curved posture, and ‘REM sleep’ with a further reduced EMG-signal and low-amplitude EEG with high theta (5–9 Hz) activity. The amounts of wakefulness, NREM sleep, REM sleep and slow-wave EEG power in delta range during NREM episodes after 4 weeks of Cr supplementation (CS) were compared with the recordings performed before the rats were put on creatine diet (baseline). Thus the % changes of parameters in each animal were calculated.
Sleep Deprivation
Sleep deprivation (SD) was done by “gentle handling” which, according to standard protocols (Franken et al., 1991) involved presentation of new objects into the cage, tapping of the cage or gentle touching by a brush. SD for 6 h began at 7 a.m. and was ended at 1 p.m. During SD, the rats continued to have access to food and water ad libitum.
Creatine supplementation (CS) and feeding protocol
For microdialysis and EEG study all rats (N=7) were first fed standard rodent diet ad libitum for 2 weeks. Baseline experiments (EEG recordings and microdialysis sample collections for 24h baseline sleep, 6h sleep deprivation (SD) and 6h recovery sleep (RS)) were performed in all rats before the start of the CS. Then they were fed with rodent diet supplemented with 2% creatine-monohydrate (Sigma 3630) by Teklad Diets-Harlan Laboratories, Inc (Madison) for 4 weeks. For tissue biochemistry 12 rats were used, out of which 6 rats were fed with creatine supplemented diet and other 6 (controls) on standard diet. The initial body weight of 400.6 ± 7.4 g during BL recordings showed a normal trend increase in 4 weeks with Cr diet (529.8 ± 14.3 g). A comparable increase in body weight was also observed in untreated rats. The effect of Cr on sleep and extracellular adenosine was determined by comparing with the baseline measurements before the start of CS. During the whole experiment, food and water were provided ad libitum.
Tissue collection, reversed-phase HPLC determination of creatine and phosphocreatine and measurement of ATP-content
A group of 12 rats were used for tissue collection experiments. Tissue samples were collected on experimental days at 10am during the light period. The rats (Cr N=6; Control N=6) were decapitated and brains removed. Coronal slices (2 mm thick) were carefully placed on a dry-ice (−78,5°C) containing covered Petri dish for rapid freezing and subsequent dissection. Four brain regions were dissected: frontal cortex (FC) (Bregma 4.2 to 2.2), basal forebrain (BF) (Bregma −0.26 to −1.2), cingulate cortex (CCX) (Bregma −0.26 to −1.2) and the entire hippocampus (HIPP). While the brain slices freeze instantly on the Petridish, further caution was exercised to complete dissections of brain regions, with an average time of 80+9 sec for all the animals; collection times did not differ between experimental groups. The dissected regions were stored at −80° C until used for biochemical measurements. Phosphocreatine (PCr) and Cr concentrations were measured using reversed phase high performance liquid chromatography and UV-detection as described earlier (Helzberg et al., 1987, Dworak et al., 2007, Dworak et al., 2010). Determination of ATP was performed by a luciferin-luciferase based Assay (Lundin and Thore, 1975; McElroy and DeLuca, 1983), a method which allowed highly reproducible results and was described in detail earlier (Dworak et al., 2007, Dworak et al., 2010).
Tissue samples were collected on experimental days at 10am during the light period.
Microdialysis and extracellular adenosine ([AD]ex) measurements
To determine the effect of CS on the homeostatic sleep factor adenosine in the basal forebrain, microdialysis probes (CMA/11, 2 mm membrane length and 0.24mm diameter; CMA/Microdialysis) were inserted into the guide cannulae at least 20h before the start of the experiments in each rat. Artificial cerebrospinal fluid (aCSF) was perfused through the microdialysis probe at 1 μl/min. On each experimental day, microdialysis samples were collected at 30 min intervals during the course of the experiment. The sample were collected for 24h from 7am to 7pm on baseline day, followed by 6 h of sleep deprivation from 7:00 am – 1:00 pm and subsequent 6h of recovery sleep (1:00 pm – 7:00 pm). Collected samples were stored at −80° C until used for biochemical measurements. Since the CS was carried on for four weeks, the microdialysis probes were removed after baseline sample collection. A fresh set of probes were used after 4 weeks of CS, 20 h before the microdialysis sample collection. Adenosine levels were measured using high performance liquid chromatography coupled to an ultraviolet detector as described previously (Dworak et al., 2010, Porkka-Heiskanen et al., 1997). For comparing the changes in [AD]ex during SD before and after the CS we chose the 7am value for each group as 100% baseline. The following hours were calculated and compared to the individual baseline value (7am) to provide an accurate between group comparison.
Statistical Analysis
Data are presented as means ± standard errors of the mean. Statistical comparisons were performed using Students t-test or one-way ANOVA followed by Student-Neuman-Keuls post-hoc test. Pre-post comparisons (before and after treatment) were performed using paired t-test. A P-value < 0.05 indicates statistical significance. All statistical analyses were performed using SigmaPlot 11.0 for MS Windows (Systat Software Inc., Chicago, IL, USA).
Results
Creatine supplementation increases spontaneous waking time and reduces SWS
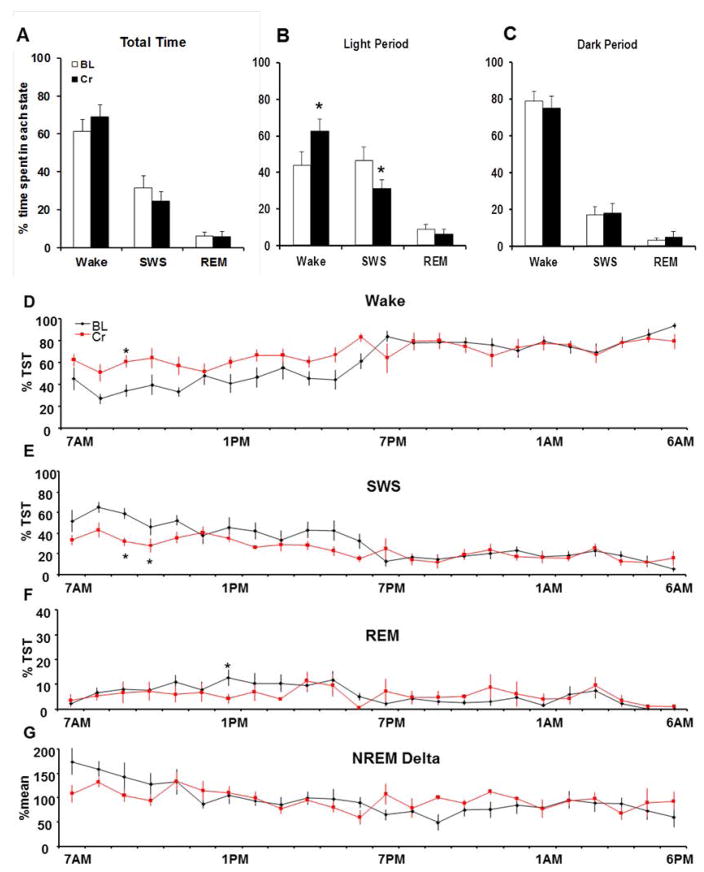
Four weeks of oral CS results in a significant increase in wake time during the light period (Cr: 61.4±6.4%; pre-diet control: 43.7±7.7%; P=0.014) but not during the dark period in rats (Cr: 75.0±6.8%; pre-diet control: 79.1±4.9%; P=0.158) (Fig. 1A). Wake time was increased in the initial hours during the light period. Significant higher levels (* P<0.05; Data for 24h measurements were tested by using one-way ANOVA followed by Student-Neuman-Keuls post-hoc test for each hour comparison) were observed following CS conditions at 9AM (Fig. 1D). Total sleep time during the light period was significantly (P=0.013) reduced after CS (Cr: 37.2±3.8%), when compared to pre-diet control measurements (54.9±5.2 %), whereas no changes were observed in the dark period (Cr. 22.9±3.9%; pre-diet control: 20.3±2.9%; P=0.642). The reductions in total sleep time during the light period are due to a significant decrease in NREM SWS (Cr: 31.1± 4.8 %; pre-diet control: 46.3±7.8 %; P=0.04), while REM sleep did not change significantly before and after CS (Cr: 6.1±2.8 %TST; pre-diet control: 8.7±2.7 %TST; P=0.445). These results indicate that four weeks of CS affect sleep-wake behavior by increased wakefulness and reduced NREM SWS. NREM delta activity did not change significantly in both groups during 24h undisrupted control conditions.
Figure 1. Creatine-supplementation increases waking time in the light not in the dark period.
Increased spontaneous waking time after creatine supplementation (CS): Four weeks of oral CS increased wake time during the light period (+ 19.10 %; P=0.014) but not during the dark (P=0.158) period. NREM SWS decreased significantly by −15.14% in the same diurnal pattern (Cr: 31.1± 4.8 %; pre-diet control: 46.3±7.8 %TST; P=0.04), while REM sleep did not change significantly before and after CS (Cr: 6.1±2.8 %; pre-diet control: 8.7±2.7 %; P=0.445).
24h EEG recordings were performed before (BL, black line) and after 4 weeks of oral Cr-supplementation (Cr, red line) in the same animals (n=6). D) Wake time was increased in the initial hours during the light period. Significant higher levels (* P<0.05) were observed under CS conditions at 9AM E) SWS was reduced during the beginning of the light period. Significant lower levels (* P<0.05) were observed at 9AM and 10 AM when compared to BL. F) REM-sleep before and after Cr-supplementation remained unchanged. Only at 1PM, REM-sleep was significantly increased after Cr-supplementation when compared to BL (* P<0.05). G) No changes in NREM Delta during spontaneous sleep-wake behavior before and after Cr-supplementation were observed.
Creatine reduces homeostatic sleep pressure after sleep deprivation
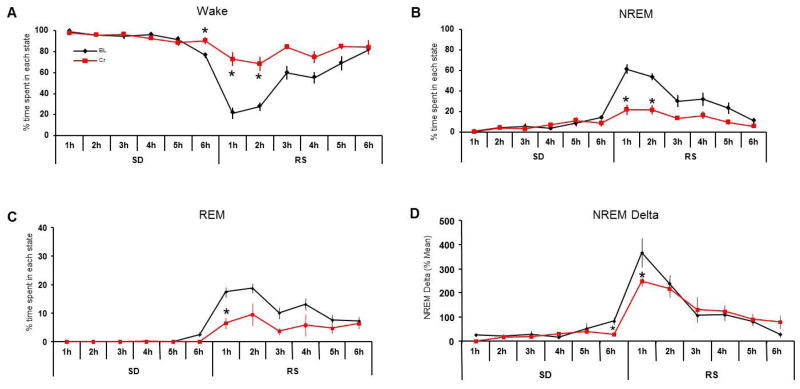
Six hours of total SD during the light period (7am–1pm) increased sleep pressure, which increased microsleep leading to some NREM sleep in hour 6 of SD (12–1PM) in our evaluations (undisrupted control, wake: 48.4+8.3%; NREM: 38.24+7.8%; hour 6 SD (12–1pm), wake: 76.6+2.5%; NREM: 14.1+1.8%). During control diet conditions rats showed increased NREM and REM sleep within the first two hours of recovery sleep which was attenuated after 4 weeks on creatine diet (NREM, pre-diet control: 61.1+4.8% and 53.7+3.2%; Cr: 21.7+4.9% and 21.6+4.2%; REM, pre-diet control: 17.4+1.6 and 18.7+1.8; Cr: 5.4+2.2 and 9.9+3.3)) (Fig 2B, C). CS rats showed a significant increase in wakefulness within the first 2 hours of RS) when compared to control conditions (wake, Cr.72.9+6.7% and 68.4+6.8%; pre-diet control: 21.5+5.4% and 27.6+4.3%; P<0.05). EEG NREM delta activity (0.5 – 4.5 Hz) was significantly lower in the first hour of RS (−66.3%; p=0.028), and lower but not statistically significant in the second hour (−32.7%) when compared to baseline conditions (Fig 2D).
Figure 2. Cr supplementation diminishes sleep pressure after 6h of sleep deprivation.
Changes in wake, NREM and REM sleep were observed during 6h of sleep deprivation followed by 6h of recovery sleep before and after 4 weeks of Cr-supplementation. Wake time was significantly higher after Cr-supplementation in the last hour of SD and the first 2h of recovery sleep (RS) (A). NREM sleep was significantly reduced in the last hour of SD and the initial hours of RS (B), when REM sleep was reduced (C). NREM delta activity (0.5–4.5 Hz) was also reduced at the end of SD (6h) and the first hour of RS (D), indicating a reduced homeostatic sleep pressure.
Effects of creatine supplementation on brain phosphocreatine and creatine levels
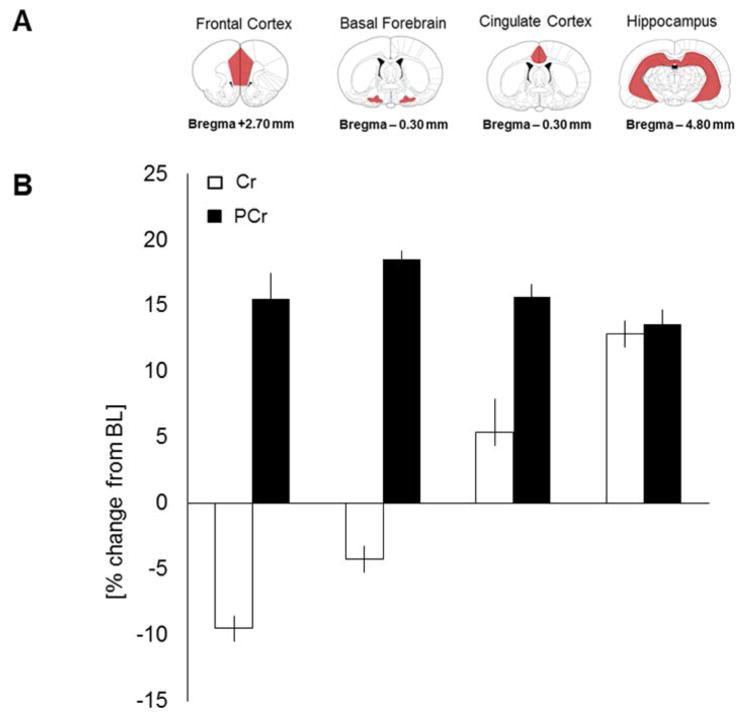
To address if four weeks of CS affect brain PCr and Cr concentrations in areas important for sleep and wake regulation, we measured both metabolites from brain tissue samples dissected from FC, BF, CCX and HIPP. Brain PCr and Cr concentrations did not differ significantly (P>0.05) before and after 4 weeks of oral CS in all 4 tested brain regions (PCr, FC: +15.5±2.0%; BF+18.5±0.7%; CCX: 15.7±0.9%; HIPP: +12.9±1.1%; Cr, FC: −9.5±0.9; BF: −4.2±1.0; CCX: +5.4±2.5; HIPP: +12.9±1.0) however, a trend for increased PCr concentrations in FC, BF, CCX and HIPP was observed in CS (Fig. 3B).
Figure 3. Effects of 4 weeks oral creatine supplementation on brain tissue creatine (Cr) and phosphocreatine (PCr) levels.
A) Coronal sections of the brain showing bregma coordinates of dissected brain regions: frontal cortex, basal forebrain, cingulate cortex and hippocampus. B) PCr/Cr-levels measured before and after 4 weeks of oral creatine supplementation. Cr levels did not differ significantly before and after creatine supplementation in all examined brain regions. No significant change in PCr levels was observed in the examined brain regions before and after the diet. There was just a tendency for increased PCr levels after the supplementation period.
Reduced brain ATP concentrations after CS
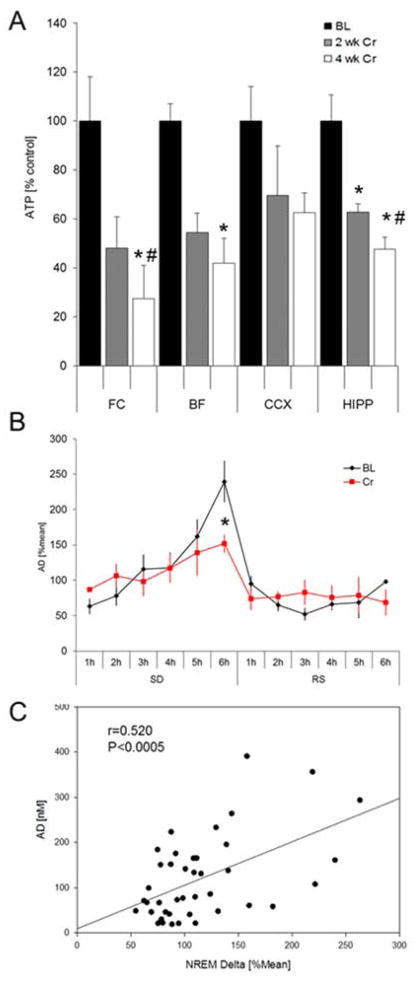
Brain ATP concentrations were measured in FC, BF, CCX and HIPP at 10 AM at baseline and after 2 weeks and 4 weeks of CS (Fig. 4). ATP-levels decreased significantly (ANOVA and Student-Newman-Keuls post-hoc, P>0.05) in FC (−51.94±12.87%), BF (−45.6±7.9%) and HIPP (−37.3±3.5%) after 2 weeks of CS when compared to BL. In FC and HIPP, ATP-levels declined further between week 2 and week 4 (FC: −72.7±13.8%; HIPP: −52.3±4.9%; P>0.05). No significant overall change was observed in CCX (ANOVA, P>0.05) (Figure 4A).
Figure 4.
A) Cellular ATP concentrations measured via a luciferin-luciferase based assay. ATP-levels decreased significantly in frontal cortex, basal forebrain and hippocampus after 2 weeks of CS when compared to BL. B) Effects of Cr-supplementation on [AD]ex during 6h SD and RS. After Cr-supplementation, [AD]ex was significantly reduced after 6h SD when compared to BL (P=0.03). No changes were observed during the RS period. C) Correlation between [AD]ex und NREM delta activity: A significant positive correlation between [AD]ex and average NREM delta activity was observed.
Creatine supplementation reduces extracellular AD concentrations in the BF
Extracellular AD [AD]ex concentrations were measured via in vivo microdialysis in the BF for 6h SD and 6h RS during the initial light period (7am–1pm). Under baseline conditions, [AD]ex increased during SD and reached highest values after 6h SD. However, after CS the increase in [AD]ex was attenuated and [AD]ex was significant lower (+151.7±12.7; P<0.05) after 6h SD when compared to pre-diet measurements (+239.4±29.4%). No significant changes in [AD]ex were observed during the RS period (Fig 4B).
[AD]ex correlates with EEG NREM delta activity
[AD]ex in the BF is known for its role in sleep-wake regulation. The levels of [AD]ex increase with increased sleepiness (Porkka-Heiskanen et al., 1997; Basheer et al., 1999; 2004) and thus [AD]ex serves as a marker of homeostatic sleep pressure. To test, if the concentrations of [AD]ex correlate with the amount of NREM delta activity, we performed a correlation analysis of hourly measures of [AD]ex with NREM sleep delta activity of the same hour for the 12h of light period. First, we found a strong positive correlation (Pearson) between the hourly [AD]ex concentrations and same hour NREM delta activity (r=0.952; P<0.0001). Also more detailed analysis of time matched [AD]ex samples and related NREM delta activity showed a significant positive correlation (Pearson) between [AD]ex level in the BF and NREM delta activity (r=0.520; P<0.0005), therefore indicating a direct relationship between the nucleoside AD and homeostatic sleep pressure (Fig. 4C).
Discussion
The current study provides evidence that prolonged oral CS increases time spent in wakefulness. The homeostatic sleep response was also attenuated in rats after 4 weeks on CS diet with a concurrent attenuation in SD-induced increase in the levels of [AD]ex in BF. Both NREM sleep and NREM delta showed significant reduction in the first hour of recovery sleep after 6h SD, when compared to the control (pre-diet) condition. The phosphocreatine levels show a tendency for increase, whereas the ATP levels decrease. Thus CS can alter the molecular components associated with cellular energy metabolism and enhance wakefulness.
Within the last decade accumulating evidence indicates that Cr may be useful in the treatment of certain diseases. Since Cr is involved in energy production and acts as an ATP shuttle from the inner mitochondria to the cytosol, Cr was theorized to be useful in diseases of mitochondria where energy production is altered (Andres et al., 2008). CS has been shown to be beneficial in diseases in which there is mitochondrial dysfunction such as Parkinson’s, Huntington’s, and myopathy, encephalopathy, ALS and some kinds of depression (Andres et al., 2008, Matthews et al., 1998, Klivenyi et al., 1999, Persky and Brazeau, 2001). Recent studies also suggest that CS significantly improved psychomotor performance and mood state following acute sleep deprivation (Cook et al., 2011, McMorris et al., 2007, McMorris et al., 2006).
The positive effects of Cr on behavioral functioning are likely due to changes in brain energy metabolism. The cellular Cr pool is maintained by nutritional intake and de novo synthesis which takes place in liver, kidney, pancreas, and to a smaller extent also in brain (Walker, 1979).
Human studies provided evidence that oral consumption of Cr yielded in a statistically significant increase in total Cr in different brain regions, which was highly reversible, as evidenced by control measurements at least 3 months after the diet (Dechent et al., 1999, Lyoo et al., 2003).
Since neuronal activity is related to ATP usage at the expense of PCr (Cadoux-Hudson et al., 1989, Chen et al., 1997), an increased PCr pool, after CS may be used to produce ATP from PCr, required for neuronal activity upon demand.
Our finding that PCr increased, though marginally, over time in the Cr group suggests that increased brain Cr levels shifted the equilibrium in the creatine kinase reaction (Cr + ATP ←→ PCr + ADP + H+) toward the right, therefore resulting in a decreased intracellular ATP and subsequent extracellular AD production. We interpret our finding of decreased ATP, not as a decrease in the total high energy phosphate reservoir, but as a reflection of a shift in metabolite concentrations to maintain equilibrium via the creatine kinase reaction.
The reduction in cellular ATP with Cr supplementation was also observed in in vivo human MRS studies, showing that the increase in brain Cr was associated with decreased ATP levels and an increase in inorganic phosphate levels (Lyoo et al., 2003).
Reduced cellular ATP can affect [AD]ex concentrations by lacking the molecules needed for further AD production. Under natural conditions AD is produced by increased breakdown of ATP by hydrolytic cascades that occurs in the intra- and extracellular medium (Basheer et al., 2004, Burnstock et al., 2011). Therefore, [AD]ex concentrations depend on both, release of intracellular AD by various transporters or breakdown of extracellular ATP, released from neurons and glia cells (Corriden and Insel, 2012). After its release ATP is rapidly broken down to ADP, AMP, and adenosine by ectonucleotidases (Burnstock and Verkhratsky, 2009). The formation and concentration of adenosine is narrowly linked with neuronal metabolic activity and [AD]ex concentration increase under conditions of increased energy demand, such as prolonged sleep deprivation.
Therefore, the observed reduction in [AD]ex during prolonged sleep deprivation in CS rats suggests an increase in the high-energy phosphate pool with a decrease in ATP levels through the CK equilibrium reaction and a subsequent reduced production and release of AD to the extracellular medium.
On the physiological level, [AD]ex is a well-known endogenous sleep-inducing substance and a homeostatic regulator of brain energy during sleep (Porkka-Heiskanen et al., 1997, Basheer et al., 2004). The levels of [AD]ex increase during wakefulness when compared to sleep and increase even further with prolonged SD. Sleepiness, indicated by NREM delta activity correlates positively with the levels of [AD]ex. Accordingly, we observed that 4 weeks of CS diet not only resulted in attenuated levels of SD-induced [AD]ex, but was also resulted in decreased homeostatic sleep response.
In conclusion, the present study suggests that chronic CS can affect sleep-wake behavior and reduce homeostatic sleep pressure after sleep deprivation through reductions in [AD]ex in the BF. Therefore, Cr might be a potential candidate to reduce the negative effects of insufficient sleep, which is very common in the general population. Further studies are needed to evaluate the effects of Cr on human sleep and behavior associated with SD.
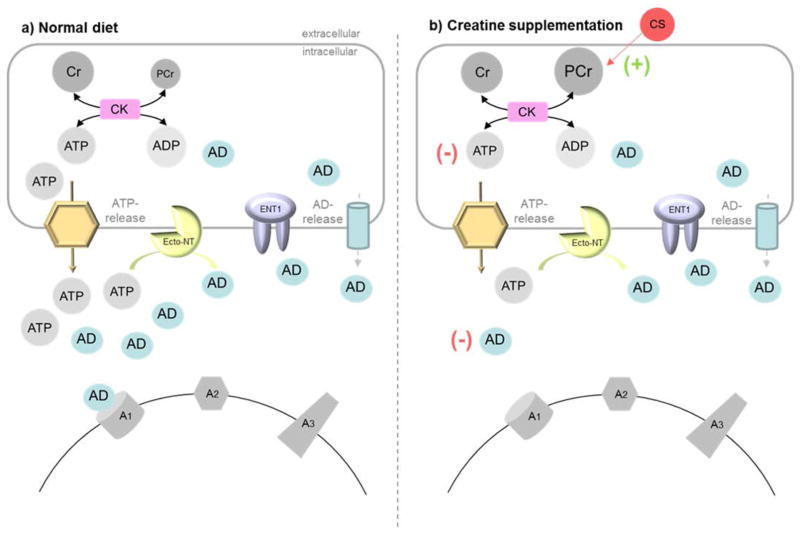
Figure 5. Model for the effects of CS on brain energy metabolism and sleep need during sleep deprivation.
This model summarizes our hypothesis about the effect of CS on [AD]ex during sleep deprivation and sleep-wake regulation. a) Under normal conditions, [AD]ex concentrations depend on AD release from intracellular sources and to a major extend on the extracellular production out of ATP via ecto-nucleotidase (ecto-NT). High [AD]ex concentrations modulate neuronal activity and increase NREM delta activity via A1 receptor binding. b) Creatine supplementation tend to increase brain PCr levels via a concomitant decrease in brain ATP through the CK reaction. Lower cellular ATP levels will result in less ATP release and reduced [AD]ex by reducing the substrate for the ecto-NT reaction. A reduced AD tone may be associated with a reduced sleep drive during sleep deprivation.
Acknowledgments
We thank Prof. Dr. T. Wallimann (ETH Zurich, Switzerland) for helpful discussions on the study design and the discussion of the MS. We gratefully acknowledge Farzana Pervin Nipa for technical assistance, and Diane Ghera and Dewayne Williams for help with animal care. This work was supported by awards from the Department of Veterans Affairs Medical Research Service Award (I01BX001404) to RB, a Deutsche Forschungsgemeinschaft fellowship (DW66/1-2) to M.D., and the National Institute of Mental Health (MH039683) to RWM.
Footnotes
Conflict of Interest: MD is currently an employee of Novartis Pharma, Germany.
References
- Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–8. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–43. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Basheer R, Porkka-Heiskanen T, Stenberg D, McCarley RW. Adenosine and behavioral state control: adenosine increases c-Fos protein and AP1 binding in basal forebrain of rats. Br Res Mol Br Res. 1999;73:1–10. doi: 10.1016/s0169-328x(99)00219-3. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, Mccarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–96. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bender A, Beckers J, Schneider I, et al. Creatine improves health and survival of mice. Neurobiol Aging. 2008;29:1404–11. doi: 10.1016/j.neurobiolaging.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, Mckenna JT, Strecker RE, Mccarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–74. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf) 2009;195:415–47. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- Cadoux-Hudson TA, Blackledge MJ, Radda GK. Imaging of human brain creatine kinase activity in vivo. FASEB J. 1989;3:2660–6. doi: 10.1096/fasebj.3.14.2629743. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhu XH, Adriany G, Ugurbil K. Increase of creatine kinase activity in the visual cortex of human brain during visual stimulation: a 31P magnetization transfer study. Magn Reson Med. 1997;38:551–7. doi: 10.1002/mrm.1910380408. [DOI] [PubMed] [Google Scholar]
- Cook CJ, Crewther BT, Kilduff LP, Drawer S, Gaviglio CM. Skill execution and sleep deprivation: effects of acute caffeine or creatine supplementation - a randomized placebo-controlled trial. J Int Soc Sports Nutr. 2011;8:2. doi: 10.1186/1550-2783-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriden R, Insel PA. New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal. 2012;8:587–98. doi: 10.1007/s11302-012-9311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechent P, Pouwels PJ, Wilken B, Hanefeld F, Frahm J. Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am J Physiol. 1999;277:R698–704. doi: 10.1152/ajpregu.1999.277.3.R698. [DOI] [PubMed] [Google Scholar]
- Dworak M, Diel P, Voss S, Hollmann W, Struder HK. Intense exercise increases adenosine concentrations in rat brain: implications for a homeostatic sleep drive. Neuroscience. 2007;150:789–95. doi: 10.1016/j.neuroscience.2007.09.062. [DOI] [PubMed] [Google Scholar]
- Dworak M, Mccarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–16. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–77. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Helzberg JH, Brown MS, Smith DJ, Gore JC, Gordon ER. Metabolic state of the rat liver with ethanol: comparison of in vivo 31phosphorus nuclear magnetic resonance spectroscopy with freeze clamp assessment. Hepatology. 1987;7:83–8. doi: 10.1002/hep.1840070118. [DOI] [PubMed] [Google Scholar]
- Kalinchuk AV, Urrila AS, Alanko L, et al. Local energy depletion in the basal forebrain increases sleep. Eur J Neurosci. 2003;17:863–9. doi: 10.1046/j.1460-9568.2003.02532.x. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Ferrante RJ, Matthews RT, et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nature medicine. 1999;5:347–50. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–7. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensman M, Korzhevskii DE, Mourovets VO, et al. Intracerebroventricular administration of creatine protects against damage by global cerebral ischemia in rat. Brain Res. 2006;1114:187–94. doi: 10.1016/j.brainres.2006.06.103. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kong SW, Sung SM, et al. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003;123:87–100. doi: 10.1016/s0925-4927(03)00046-5. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Vorstrup S. Cerebral blood flow and metabolism during sleep. Cerebrovasc Brain Metab Rev. 1991;3:281–96. [PubMed] [Google Scholar]
- Maquet P. Sleep function(s) and cerebral metabolism. Behav Brain Res. 1995;69:75–83. doi: 10.1016/0166-4328(95)00017-n. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Ferrante RJ, Klivenyi P, et al. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157:142–9. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Jenkins BG, et al. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington’s disease. J Neurosci. 1998;18:156–63. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcmorris T, Harris RC, Howard AN, et al. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol Behav. 2007;90:21–8. doi: 10.1016/j.physbeh.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Mcmorris T, Harris RC, Swain J, et al. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology (Berl) 2006;185:93–103. doi: 10.1007/s00213-005-0269-z. [DOI] [PubMed] [Google Scholar]
- Netchiporouk L, Shram N, Salvert D, Cespuglio R. Brain extracellular glucose assessed by voltammetry throughout the rat sleep-wake cycle. Eur J Neurosci. 2001;13:1429–34. doi: 10.1046/j.0953-816x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- Persky AM, Brazeau GA. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev. 2001;53:161–76. [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, Mccarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C, Digney AL, Mcewan SR, Bates TC. Oral creatine monohydrate supplementation improves brain performance: a double-blind, placebo-controlled, cross-over trial. Proc Biol Sci. 2003;270:2147–50. doi: 10.1098/rspb.2003.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000;48:723–9. [PubMed] [Google Scholar]
- Walker JB. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol. 1979;50:177–242. doi: 10.1002/9780470122952.ch4. [DOI] [PubMed] [Google Scholar]