Abstract
Cancer chemoprevention, a scientific term coined by Dr. Sporn in the late seventies, implies use of natural or synthetic chemicals to block, delay or reverse carcinogenesis. Phytochemicals derived from edible and medicinal plants have been studied rather extensively for cancer chemoprevention using preclinical models in the past few decades. Nevertheless, some of these agents (e.g., isothiocyanates from cruciferous vegetables like broccoli and watercress) have already entered into clinical investigations. Examples of widely studied and highly promising phytochemicals from edible and medicinal plants include cruciferous vegetables constituents (phenethyl isothiocyanate, benzyl isothiocyanate, and sulforaphane), withaferin A (WA) derived from a medicinal plant (Withania somnifera) used heavily in Asia, and an oriental medicine plant component honokiol (HNK). An interesting feature of these structurally-diverse phytochemicals is that they target mitochondria to provoke cancer cell-selective death program. Mechanisms underlying cell death induction by commonly studied phytochemicals have been discussed rather extensively and thus are not covered in this review article. Instead, the primary focus of this perspective is to discuss experimental evidence pointing to mitochondrial dysfunction in cancer chemoprevention by promising phytochemicals.
Keywords: Phytochemicals, Mitochondrial Dysfunction, Mitochondrial Dynamics, Electron Transport Chain, Chemoprevention
2. Introduction
Practicality and promise of cancer chemoprevention is demonstrated by clinical integration of selective estrogen receptor modulators and aromatase inhibitors for breast cancer and human papilloma virus vaccines for cervical cancer (Fisher et al. 1998; Goss et al. 2011; McNamara et al. 2016). Small molecule phytochemicals present in dietary and medicinal plants appear promising for cancer chemoprevention (Surh 2003; Powolny et al. 2012; Singh and Singh 2012). Cancer chemoprevention research on phytochemicals derived from the dietary plants was originally inspired by cues from the population-based epidemiological studies (Verhoeven et al. 1996; Kolonel et al. 2000; Greenwald et al. 2001). More recently, the scientific community has witnessed a surge in research focused on identification of cancer chemopreventive phytochemicals from medicinal plants (Garodia et al. 2007; Teiten et al. 2013; Vyas and Singh 2014). Isothiocyanates (ITCs) from cruciferous vegetables (e.g., watercress, broccoli, mustard, and so forth), which are naturally stored as glucosinolates in these plants, have been widely studied for cancer chemoprevention using preclinical models (Hecht 1999; Singh and Singh 2012). Examples of well-characterized cancer chemopreventive ITCs include phenethyl isothiocyanate (PEITC) from watercress, benzyl isothiocyanate (BITC) from garden cress, and sulforaphane (SFN) from broccoli (Powolny et al. 2012; Singh and Singh 2012; Sehrawat and Singh 2013). All of these ITCs have shown in vivo cancer chemopreventive activity in rodent models (Powolny et al. 2012; Singh and Singh, 2012; Sehrawat and Singh 2013). Even though the evidence for cancer chemopreventive activity of ITCs in humans is still lacking, PEITC and SFN have entered clinical arena to determine their safety, bioavailability, and biological activity (Cipolla et al. 2015; Yuan et al. 2016). Similarly, the preclinical evidence for cancer chemoprevention by some phytochemicals isolated from medicinal plants is quite persuasive. Examples of promising cancer chemopreventative phytochemicals isolated from medicinal plants include withaferin A (WA) from Withania somnifera and honokiol (HNK) from Magnolia officinalis (Arora et al. 2012; Vyas and Singh 2014). Evidence continues to accumulate to suggest that mitochondrial dysfunction is a critical event in cancer chemoprevention by ITCs, WA, and HNK. The net result of mitochondrial dysfunction triggered by these phytochemicals in cancer cells is apoptotic death that is mediated by generation of reactive oxygen species (ROS) (Arora et al. 2012; Singh and Singh 2012; Sehrawat and Singh 2013; Vyas and Singh 2014). The mechanisms by which these chemicals cause generation of ROS and the underlying pathways in cell death induction have been reviewed extensively by us and others, and therefore, are not covered in this article (Antosiewicz et al. 2008; Singh and Singh 2012; Sehrawat and Singh 2013; Hahm et al. 2014; Vyas and Singh 2014). The primary focus of this perspective is to discuss experimental evidence pointing to mitochondrial dysfunction as a critical event leading to generation of reactive oxygen species (ROS) and eventual cancer cell death by promising phytochemicals. Gaps in our knowledge and unanswered questions pertaining to the mitochondrial dysfunction in cancer chemopreventive mechanisms for selected phytochemicals (ITCs, WA, and HNK) are also highlighted.
3. Mitochondrial dysfunction and cancer
Mitochondrial function is not limited to ATP generation from oxidative phosphorylation (OXPHOS) but this organelle is implicated in numerous biochemical reactions (Nunnari and Suomalainen 2012). Mitochondrial involvement in carcinogenesis has also been reviewed extensively (Carew and Huang 2002; Gogvadze et al. 2008; Scatena 2012). Furthermore, this organelle is a target of cancer therapy because of its vital role in pro-death and pro-survival pathways (Fulda et al. 2010; Wenner 2012). Mitochondrial dysfunction is also implicated in cancer initiation and progression (Carew and Huang 2002). Defects in the mitochondrial genome in cancer cells lead to deficient respiration and ATP generation and overproduction of ROS causing damage to the mitochondria and other macromolecules (Modica-Napolitano and Singh 2004; Galluzzi et al. 2010). Germline mutations in mitochondrial DNA have been suggested to predispose to cancer development (Canter et al. 2005; Petros et al. 2005). Canter et al. demonstrated the association of the mitochondrial DNA G10398A polymorphism in susceptibility to breast cancer in African-Americans women (Canter et al. 2005). The mitochondrial DNA mutations were suggested to increase prostate cancer (Petros et al. 2005). It is interesting to note that four of the conserved mutations were detectable in multiple patients (Petros et al. 2005). Experimentally, introduction of a mitochondrial DNA mutant through cybrid transfer increased tumorigenic potential of PC-3 cells (Petros et al. 2005). Mitochondrial dysfunctions are also linked to impaired cell death (Carew and Huang 2002; Kroemer and Pouyssegur 2008). In summary, it is clear that mitochondrial dysfunction is intimately linked to cancer development.
4. Inhibition of electron transport chain (ETC) by cancer chemopreventive phytochemicals
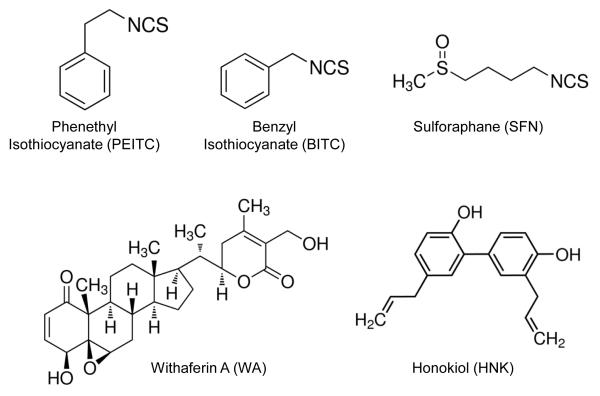
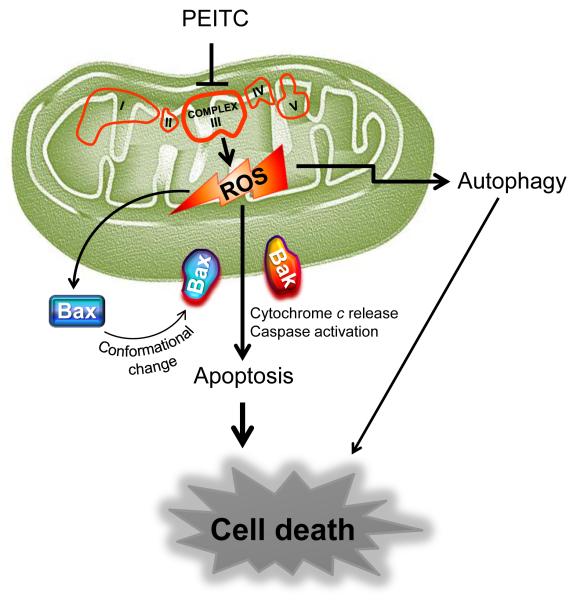
In this section, we review scientific evidence implicating inhibition of ETC by cancer chemopreventive phytochemicals shown in Figure 1. PEITC and BITC are aromatic ITCs with a minor difference, whereas SFN is a thioalkyl type ITC compound. Several other naturally-occurring thioalkyl type ITCs have also been identified in plants but SFN is the best studied member of this subclass (Fahey et al. 2001). It is important to mention that SFN occurs naturally as an L-isomer but most studies have used synthetic racemic D,L-SFN for mechanistic and efficacy studies in cancer prevention research. WA is a steroidal lactone and HNK is a phenolic compound. Despite structural difference, they all have been shown to inhibit ETC. Inhibition of ETC causes generation of ROS to trigger Bax and/or Bak-mediated apoptosis as summarized for PEITC in Figure 2 (Hahm et al., 2008; Xiao et al. 2008; Xiao et al. 2009; Xiao et al. 2010; Hahm et al. 2011; Hahm et al. 2014; Pan et al. 2014).
Figure 1.
Molecular structures of the cancer chemopreventive phytochemicals highlighted in this article.
Figure 2.
Mechanistic summary of mitochondria-mediated apoptosis induction by PEITC based on our own findings in prostate cancer cells (Xiao et al. 2010).
4.1 ETC inhibition by ITCs
Chemopreventive effect of PEITC has been shown in chemically-induced as well as transgenic rodent models of various cancers (Powolny et al. 2012; Singh and Singh 2012). Several mechanisms have been implicated in cancer chemoprevention by PEITC, including inhibition of carcinogen activation through inhibition of phase I metabolism and induction of phase II systems to boost detoxification of carcinogenic intermediates (Hecht 1999; Powolny et al. 2012; Singh and Singh 2012). Furthermore, PEITC triggers death of cancer cells by causing apoptosis and autophagy (Xiao et al. 2006a; Bommareddy et al. 2009; Cheung and Kong 2010). Various reports suggest involvement of ROS in PEITC-induced apoptosis (Trachootham et al. 2006; Zhang et al. 2008; Xiao et al. 2010; Powolny and Singh 2010). We have used prostate cancer cells to study the mechanism underlying ROS generation by PEITC (Xiao et al. 2010). Prostate cancer cells exhibit a decrease in OXPHOS after PEITC treatment that is associated with complex III inhibition (Xiao et al. 2010). This effect of PEITC seems cancer cell-selective because OXPHOS inhibition is not seen in PrEC, a normal human prostate epithelial cell line (Xiao et al. 2010). However, the mechanism for cancer cell-selective inhibition of OXPHOS, and potentially complex III is yet to be determined. We believe that the differential sensitivity is likely due to differences in uptake of PEITC by cancer cells versus normal cells. The sequential mechanism downstream of ROS in PEITC-mediated apoptosis involves activation of Bax that is observed in wild-type LNCaP (androgen-responsive) and PC-3 (androgen-independent) human prostate cancer cells but not in their Rho-0 variants (Xiao et al. 2010). The Rho-0 cells lack OXPHOS. Inhibition of complex III and oxygen consumption as well as Bax activation were also shown in a hepatoma HepG2 cell line (Rose et al. 2005). Condensed mitochondria and mitochondrial cristae structure perturbations (rounded and dilated shape) were observed in ultrastructure studies within 4 hours of PEITC treatment in prostate cancer cells (Xue et al. 2014). However, the in vivo evidence for inhibition of OXPHOS or ROS production upon PEITC treatment is still lacking. Another unanswered question in this context is precise mechanism by which PEITC inhibits complex III. Nevertheless, breast cancer chemoprevention by PEITC in a transgenic mouse model representative of human epidermal growth factor receptor 2 subtype is associated with alterations of metabolism-related proteins, including pyruvate kinase isozymes M1/M2, mitochondrial ATP synthase H+ transporting F1 complex beta subunit, hexokinase-1, isoform CRA_f, and L-lactate dehydrogenase A chain isoform 1 (Singh et al. 2012).
BITC is a close structural analog of PEITC and exhibits in vivo preventive efficacy in rodent models of cancer (Hecht 1999; Warin et al. 2009; Sehrawat and Singh 2013). The proapoptotic effect of BITC is also cancer cell-selective and linked to ROS production as exemplified by our own data in breast cancer cells (Xiao et al. 2006b; Xiao et al. 2008). BITC treatment in breast cancer cell lines MCF-7 (estrogen-responsive) and MDA-MB-231 (estrogen-independent) resulted in inhibition of complex III, ROS production, c-Jun NH2-terminal kinase-dependent activation of Bax leading to apoptosis (Xiao et al. 2008). Overexpression of antioxidative enzymes attenuated ROS production and apoptosis by BITC in these breast cancer cells (Xiao et al. 2008). Mitochondrial damage and loss of the mitochondrial membrane potential with BITC treatment were shown in rat liver epithelial cells (Nakamura et al. 2002). Similar to PEITC, the mechanism for complex III inhibition by BITC is yet to be elucidated but likely involves a common mechanism for both these agents.
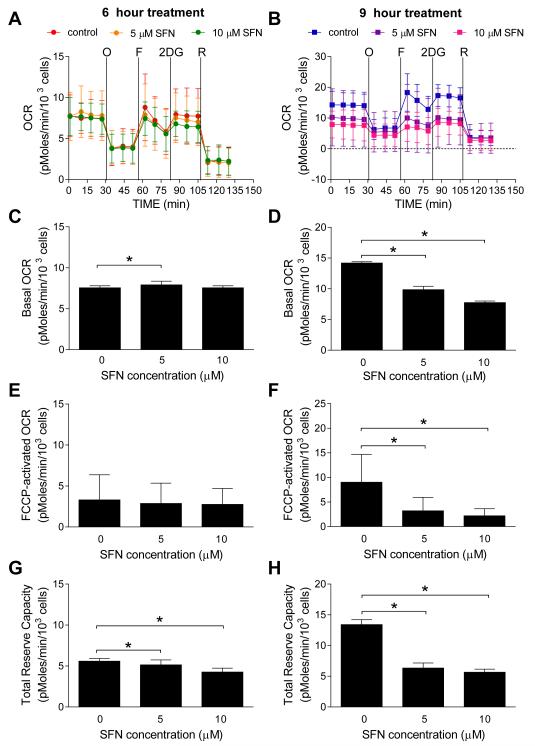
Cancer chemoprevention by SFN was first shown for 9,10-dimethyl-1,2-benzanthracene-induced breast cancer in rats (Zhang et al. 1994). A cancer chemopreventive role for SFN has also been established in other types of cancers (Clarke et al. 2008). Our own data implicated ROS in SFN-induced apoptosis in prostate cancer cells (Singh et al. 2005). In contrast to PEITC or BITC (Xiao et al. 2008; Xiao et al. 2010), the ROS production by SFN was accompanied by inhibition of complex I, II, and III of the ETC (Xiao et al. 2009). However, the relative contribution of different complexes in ROS generation by SFN is yet to be established. Nevertheless, our unpublished results show inhibition of OXPHOS in LNCaP human prostate cancer cell line after 9 h treatment with SFN (Figure 3).
Figure 3.
Inhibition of OXPHOS by SFN in LNCaP human prostate cancer cell line. (A-B) Representative pharmacologic profiling of oxygen consumption rate (OCR), indicative of OXPHOS, in LNCaP cells treated with DMSO (control) or the indicated doses of SFN for 6 hours (A) or 9 hours (B) through real-time measurements using the Seahorse Bioscience XF24 Extracellular Flux Analyzer. After measurement of basal oxygen consumption, the cells were treated with oligomycin (“O”, 1 μM), FCCP (“F”, 0.3 μM), 2-deoxyglucose (“2DG”, 100 mM), and rotenone (“R”, 1 μM) as indicated. Results shown are mean ± SD (n = 3). (C-D) Basal OCR level in LNCaP cells treated with DMSO (control) or indicated doses of SFN for 6 hours (C) or 9 hours (D). (E-F) FCCP-activated OCR level in LNCaP cells treated with DMSO (control) or the indicated doses of SFN for 6 hours (E) or 9 hours (F). (G-H) Total reserve capacity in LNCaP cells treated with DMSO (control) or the indicated doses of SFN for 6 hours (G) or 9 hours (H). Combined results (C-H) from three independent experiments are shown as mean ± SD (n = 13~14). *Statistically significant compared to DMSO-treated control by one-way ANOVA followed by Dunnett’s adjustment.
4.2 ETC inhibition by WA
WA is a steroidal lactone that is abundant in the leaf and root of Withania somnifera plant (also known as Ashwagandha or Indian winter cherry), which is a key ingredient in Ayurvedic medicine practiced in India. Cancer chemopreventive effect of WA has been demonstrated for oral, breast, and skin cancers (Manoharan et al. 2009; Hahm et al. 2013; Li et al. 2015). ROS-dependent apoptosis seems to be an important mechanism in anticancer effects of WA in leukemia (Malik et al. 2007), melanoma (Mayola et al. 2011), and breast cancer (Hahm et al. 2011). However, the mechanism for ROS generation following WA treatment has been characterized in breast cancer cells (Hahm et al. 2011). WA treatment inhibited both basal and reserve OXPHOS in MCF-7 and MDA-MB-231 human breast cancer cells (Hahm et al. 2011). Inhibition of OXPHOS after WA treatment was associated with suppression of complex III in MDA-MB-231 cells (Hahm et al. 2011). Interestingly, a modest but significant increase in complex IV activity was also discernible in WA-treated MDA-MB-231 cells, but implications of this finding are unclear (Hahm et al. 2011). Similar to the PEITC effect observed in prostate cancer cells, a normal human mammary epithelial cell line was resistant to WA-mediated ROS production and apoptosis (Hahm et al. 2011). A role for ROS in apoptosis following WA treatment was further established using mitochondrial DNA-deficient Rho-0 variants of MDAMB-231 and MCF-7 cells (Hahm et al. 2011). Because breast cancer is a heterogeneous disease broadly grouped into different subtypes (luminal, basal, HER2 amplified, and normal like breast cancer), an obvious unanswered question is whether OXPHOS and/or complex III inhibition by WA occurs in other subtypes of breast cancer. Inhibitory effect of WA on OXPHOS has been shown in luminal (MCF-7) and basal-like MDA-MB-231 cells but the experimental evidence for complex III inhibition only exists for MDA-MB-231 cells (Hahm et al. 2011). Unlike ITCs, however, we were able to demonstrate that breast cancer prevention by WA in a transgenic mouse model was associated with in vivo inhibition of complex III in the mammary tumor (Hahm et al. 2013). While involvement of ROS in anticancer effect of WA is indisputable, the mechanism for complex III inhibition by WA is still unresolved similar to ITCs. It is also important to establish if inhibition of OXPHOS and complex III by WA treatment is not specific for breast cancer cells. In this regard, a recent study using skin epidermal JB6 P+ cells showed reversal of 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced changes by WA (Li and Zhao 2013). TPA treatment inhibited mitochondrial membrane potential, complex I activity and mitochondrial respiration, which were reversed by WA (Li and Zhao 2013).
4.3 ETC inhibition by HNK
HNK is present in Magnolia tree whose bark extract is widely used in the oriental traditional medicine (Lee et al. 2011). Anticancer properties of HNK were first demonstrated in leukemia cell lines (Hirano et al. 1994). However, Bai et al (2003) were the first to show in vivo anticancer activity of HNK. Chemoprevention of cancer by HNK has also been documented (Chilampalli et al. 2010; Pan et al. 2014). For example, N-nitroso-trischloroethylurea-induced lung squamous cell carcinoma development in mice was inhibited significantly by HNK treatment (Pan et al. 2014). A role for ROS in anticancer effects of HNK has also been suggested previously (Han et al. 2009; Hahm et al. 2014; Lin et al. 2016). One group of investigators showed that HNK can induce rat liver mitochondrial swelling, decrease membrane potential and modulate respiration (Dong et al. 2013). Another study also reported inhibition of basal OXPHOS by HNK treatment in lung cancer cells that was accompanied by stimulation of extracellular acidification rate, a measure of glycolysis, in H226 cells but not in H520 cells (Pan et al. 2014). HNK is not electrophilic and thus post-translational modification of ETC complex subunits is not a likely explanation for inhibition of OXPHOS.
5. Alteration of mitochondrial dynamics by BITC in breast cancer cells
Persistent fission and fusion of mitochondria is essential for their integrity and normal physiology (Detmer and Chan 2007). A role for mitochondrial dynamics in regulation of apoptosis has been suggested (Brooks and Dong 2007; Suen et al. 2008). Generally speaking, mitochondrial fusion inhibits apoptosis and mitochondrial fission promotes release of apoptogenic factors to trigger apoptotic cell death (Brooks et al. 2007; Boland et al. 2013). In cells committed to apoptosis, the normal filamentous network of mitochondria is fragmented (punctate and spherical) due to increased fission or inhibition of fusion. We recently explored the possibility of whether mitochondrial dynamics was affected by BITC using human breast cancer cells as a model (Sehrawat et al. 2016). We showed that BITC treatment disrupted mitochondrial filamentous network and inhibited mitochondrial fusion (Sehrawat et al. 2016). On the other hand, mitochondrial integrity was preserved after BITC treatment in MCF-10A cells, which is a non-tumorigenic mammary epithelial cell line that was spontaneously immortalized (Sehrawat et al. 2016). Effect of BITC on mitochondrial dynamics was associated with a transient or sustained decrease in mitochondrial fission and fusion regulating protein levels, including Dynamin-related protein 1 (Drp1) and mitofusins. Immortalized mouse embryonic fibroblasts Drp1 knockout mice were resistant to BITC-induced apoptosis when compared with those from wild-type mice (Sehrawat et al. 2016). This protein regulates mitochondrial fission (Lennon and Salgia 2014). Besides apoptosis regulation, Drp1 may either directly or indirectly affect other anticancer functions of BITC. We found a decrease in S616 phosphorylation of Drp1 upon BITC treatment (Sehrawat et al. 2016) and this phosphorylation of Drp1 is mediated by cyclinB/cyclin-dependent kinase complex (Lennon and Salgia 2014). We have also shown previously that BITC-mediated G2/M phase cell cycle arrest in MDA-MB-231 and MCF-7 cells is associated with suppression of cyclinB/cyclin-dependent complex (Xiao et al., 2006b). Our prior work also shows inhibition of breast cancer cell migration after BITC treatment (Kim et al., 2012). Interestingly, Drp1 silencing reduces breast cancer cell migration in vitro (Zhao et al., 2013). It would be interesting to determine if Drp1 overexpression attenuates breast cancer cell migration inhibition by BITC. It is equally important to determine if the BITC-mediated inhibition of mitochondrial fusion is specific for breast cancer cells or this ITC only. Because other ITCs and WA are electrophilic and exhibit mechanistic similarities (e.g., inhibition of complex III), it is highly likely that mitochondrial fusion is also inhibited by these agents. However, it is uncertain if the mitochondrial dynamic is also affected by HNK. Future research will likely address these questions.
6. Conclusions and gaps in knowledge
Even though the experimental evidence for ETC inhibition by the highlighted cancer chemopreventive phytochemicals is compelling, the underlying mechanism is still not fully resolved. One possibility for ETC inhibition by ITCs and WA may entail covalent modification of sulfhydryl groups in critical cysteine in complex III subunits owing to their electrophilic nature. It is also plausible that the agents covered in this review and possibly other cancer chemopreventive phytochemicals cause downregulation of ETC complex subunits. Further research is needed to probe these possibilities. An equally intriguing and unresolved question relates to differences in ETC inhibition by aromatic ITCs (complex III) versus SFN (complex I, II, and III) (Xiao et al. 2008; Xiao et al. 2009; Xiao et al. 2010). Another obvious gap in our knowledge is the in vivo evidence for inhibition of ETC and mitochondrial dynamics, although we were able to demonstrate complex III inhibition in vivo in mammary tumors from WA-treated mice (Hahm et al. 2013). We have shown recently that inhibition of mitochondrial fusion is an early and critical event in cell death induction by BITC (Sehrawat et al. 2016). However, the evidence for mitochondrial dynamics alterations by PEITC, SFN, WA, and HNK is still lacking. Finally, it is imperative that these agents are tried clinically for their cancer chemopreventive activity to move the field further. Nevertheless, this article clearly establishes that mitochondrion is a critical target of structurally-diverse cancer chemopreventive phytochemicals.
Acknowledgments
Funding: The research cited from the senior author’s laboratory was supported by United States Public Health Services grants CA101753, CA115498, CA129347, and CA142604 awarded by the National Cancer Institute- National Institutes of Health.
Abbreviations
- BITC
benzyl isothiocyanate
- Drp1
dynamin-related protein 1 (also known as DNML1 and dynamin 1 like
- ETC
electron transport chain
- HNK
honokiol
- ITCs
isothiocyanates
- OXPHOS
oxidative phosphorylation
- PEITC
phenethyl isothiocyanate
- ROS
reactive oxygen species
- SFN
sulforaphane
- WA
withaferin A
Footnotes
Conflict of Interest: None of the authors declares any conflict of interest.
References
- Antosiewicz J, Ziolkowski W, Kar S, Powolny AA, Singh SV. Role of reactive oxygen intermediates in cellular responses to dietary cancer chemopreventive agents. Planta Med. 2008;74:1570–1579. doi: 10.1055/s-2008-1081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S, Singh S, Piazza GA, Contreras CM, Panyam J, Singh AP. Honokiol: a novel natural agent for cancer prevention and therapy. Curr Mol Med. 2012;12:1244–1252. doi: 10.2174/156652412803833508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommareddy A, Hahm ER, Xiao D, Powolny AA, Fisher AL, Jiang Y, Singh SV. Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Res. 2009;69:3704–3712. doi: 10.1158/0008-5472.CAN-08-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C, Dong Z. Regulation of mitochondrial morphological dynamics during apoptosis by Bcl-2 family proteins: a key in Bak? Cell Cycle. 2007;6:3043–3047. doi: 10.4161/cc.6.24.5115. [DOI] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci USA. 2007;104:11649–11654. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter JA, Kallianpur AR, Parl FF, Millikan RC. Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res. 2005;65:8028–8033. doi: 10.1158/0008-5472.CAN-05-1428. [DOI] [PubMed] [Google Scholar]
- Carew JS, Huang P. Mitochondrial defects in cancer. Mol Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilampalli S, Zhang X, Fahmy H, Kaushik RS, Zeman D, Hildreth MB, Dwivedi C. Chemopreventive effects of honokiol on UVB-induced skin cancer development. Anticancer Res. 2010;30:777–783. [PubMed] [Google Scholar]
- Cipolla BG, Mandron E, Lefort JM, Coadou Y, Della Negra E, Corbel L, Le Scodan R, Azzouzi AR, Mottet N. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev Res (Phila) 2015;8:712–719. doi: 10.1158/1940-6207.CAPR-14-0459. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Dong JX, Zhao GY, Yu QL, Li R, Yuan L, Chen J, Liu Y. Mitochondrial dysfunction induced by honokiol. J Membr Biol. 2013;246:375–381. doi: 10.1007/s00232-013-9543-x. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N, other NSABP Investigators Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Kepp O, Vitale I, Rigoni A, Vacchelli E, Michaud M, Zischka H, Castedo M, Kroemer G. Mitochondrial gateways to cancer. Mol Aspects Med. 2010;31:1–20. doi: 10.1016/j.mam.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garder JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H, the NCIC CTG MAP.3 Study Investigators Exemestane for breast-cancer prevention in postmenopausal women. N Eng J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- Greenwald P, Clifford CK, Milner JA. Diet and cancer prevention. Eur J Cancer. 2001;37:948–965. doi: 10.1016/s0959-8049(01)00070-3. [DOI] [PubMed] [Google Scholar]
- Hahm ER, Arlotti JA, Marynowski SW, Singh SV. Honokiol, a constituent of oriental medicinal herb Magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin Cancer Res. 2008;14:1248–1257. doi: 10.1158/1078-0432.CCR-07-1926. [DOI] [PubMed] [Google Scholar]
- Hahm ER, Lee J, Kim SH, Sehrawat A, Arlotti JA, Shiva SS, Bhargava R, Singh SV. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J Natl Cancer Inst. 2013;105:1111–1122. doi: 10.1093/jnci/djt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011;6:e23354. doi: 10.1371/journal.pone.0023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm ER, Sakao K, Singh SV. Honokiol activates reactive oxygen species-mediated cytoprotective autophagy in human prostate cancer cells. Prostate. 2014;74:1209–1221. doi: 10.1002/pros.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LL, Xie LP, Li LH, Zhang XW, Zhang RQ, Wang HZ. Reactive oxygen species production and Bax/Bcl-2 regulation in honokiol-induced apoptosis in human hepatocellular carcinoma SMMC-7721 cells. Environ Toxicol Pharmacol. 2009;28:97–103. doi: 10.1016/j.etap.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. J Nutr. 1999;129(suppl.):768S–774S. doi: 10.1093/jn/129.3.768S. [DOI] [PubMed] [Google Scholar]
- Hirano T, Gotoh M, Oka K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci. 1994;55:1061–1069. doi: 10.1016/0024-3205(94)00641-5. [DOI] [PubMed] [Google Scholar]
- Kim SH, Sehrawat A, Singh SV. Notch2 activation by benzyl isothiocyanate impedes its inhibitory effect on breast cancer cell migration. Breast Cancer Res Treat. 2012;134:1067–1079. doi: 10.1007/s10549-012-2043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger RS., Jr. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Lee YM, Lee CK, Jung JK, Han SB, Hong JT. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther. 2011;130:157–176. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Lennon FE, Salgia R. Mitochondrial dynamics: biology and therapy in lung cancer. Expert Opin Investig Drugs. 2014;23:675–692. doi: 10.1517/13543784.2014.899350. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang C, Du H, Huang V, Sun B, Harris JP, Richardson Q, Shen X, Jin R, Li G, Kevil CG, Gu X, Shi R, Zhao Y. Withaferin A suppresses the up-regulation of acetyl-CoA carboxylase 1 and skin tumor formation in a skin carcinogenesis mouse model. Mol Carcinog. 2015 doi: 10.1002/mc.22423. in press. [DOI] [PubMed] [Google Scholar]
- Li W, Zhao Y. Withaferin A suppresses tumor promoter 12-O-tetradecanoylphorbol 13-acetate-induced decreases in isocitrate dehydrogenase 1 activity and mitochondrial function in skin epidermal JB6 cells. Cancer Sci. 2013;104:143–148. doi: 10.1111/cas.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Chen TL, Tseng YY, Wu GJ, Hsieh MH, Lin YW, Chen RM. Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol Appl Pharmacol. 2016;304:59–69. doi: 10.1016/j.taap.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Malik F, Kumar A, Bhushan S, Khan S, Bhatia A, Suri KA, Qazi GN, Singh J. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis. 2007;12:2115–2133. doi: 10.1007/s10495-007-0129-x. [DOI] [PubMed] [Google Scholar]
- Manoharan S, Panjamurthy K, Menon VP, Balakrishnan S, Alias LM. Protective effect of Withaferin-A on tumour formation in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis in hamsters. Indian J Exp Biol. 2009;47:16–23. [PubMed] [Google Scholar]
- Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C, Lemaire C. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16:1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- McNamara M, Batur P, Walsh JM, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med. 2016 doi: 10.1007/s11606-016-3725-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica-Napolitano JS, Singh KK. Mitochondrial dysfunction in cancer. Mitochondrion. 2004;4:755–762. doi: 10.1016/j.mito.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kawakami M, Yoshihiro A, Miyoshi N, Ohigashi H, Kawai K, Osawa T, Uchida K. Involvement of the mitochondrial death pathway in chemopreventive benzyl isothiocyanate-induced apoptosis. J Biol Chem. 2002;277:8492–8499. doi: 10.1074/jbc.M109760200. [DOI] [PubMed] [Google Scholar]
- Nunnari J, Suomalainen A. Mitochondria: In sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Zhang Q, Liu Q, Komas SM, Kalyanaraman B, Lubet RA, Wang Y, You M. Honokiol inhibits lung tumorigenesis through inhibition of mitochondrial function. Cancer Prev Res (Phila) 2014;7:1149–1159. doi: 10.1158/1940-6207.CAPR-14-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, Marshall FF, Wallace DC. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci USA. 2005;102:719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powolny AA, Bommareddy A, Singh SV. Slow but steady progress in cancer chemoprevention with phenethyl isothiocyanate: fulfilled promises and translational challenges. In: Sarkar FH, editor. Nutraceuticals and Cancer. Springer Science+Business Media; 2012. pp. 231–258. [Google Scholar]
- Powolny AA, Singh SV. Differential response of normal (PrEC) and cancerous human prostate cells (PC-3) to phenethyl isothiocyanate-mediated changes in expression of antioxidant defense genes. Pharm Res. 2010;27:2766–2775. doi: 10.1007/s11095-010-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose P, Armstrong JS, Chua YL, Ong CN, Whiteman M. β-phenylethyl isothiocyanate mediated apoptosis; contribution of Bax and the mitochondrial death pathway. Int J Biochem Cell Biol. 2005;37:100–119. doi: 10.1016/j.biocel.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Scatena R. Mitochondria and cancer: a growing role in apoptosis, cancer cell metabolism and dedifferentiation. Adv Exp Med Biol. 2012;942:287–308. doi: 10.1007/978-94-007-2869-1_13. [DOI] [PubMed] [Google Scholar]
- Sehrawat A, Croix CS, Baty CJ, Watkins S, Tailor D, Singh RP, Singh SV. Inhibition of mitochondrial fusion is an early and critical event in breast cancer cell apoptosis by dietary chemopreventative benzyl isothiocyanate. Mitochondrion. 2016;30:67–77. doi: 10.1016/j.mito.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehrawat A, Singh SV. Molecular mechanisms of cancer chemoprevention with benzyl isothiocyanate. In: Kong AN, editor. Inflammation and Cancer: Mechanisms and Dietary Approaches for Cancer Prevention. CRC Press-Taylor and Francis; 2013. pp. 447–462. Chapter 24. [Google Scholar]
- Singh SV, Kim SH, Sehrawat A, Arlotti JA, Hahm ER, Sakao K, Beumer JH, Jankowitz RC, Chandra-Kuntal K, Lee J, Powolny AA, Dhir R. Biomarkers of phenethyl isothiocyanate-mediated mammary cancer chemoprevention in a clinically relevant mouse model. J Natl Cancer Inst. 2012;104:1228–1239. doi: 10.1093/jnci/djs321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012;33:1833–1842. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Teiten MH, Gaascht F, Dicato M, Diederich M. Anticancer bioactivity of compounds from medicinal plants used in European medieval traditions. Biochem Pharmacol. 2013;86:1239–1247. doi: 10.1016/j.bcp.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- Vyas AR, Singh SV. Molecular targets and mechanisms of cancer prevention and treatment by withaferin A, a naturally occurring steroidal lactone. AAPS J. 2014;16:1–10. doi: 10.1208/s12248-013-9531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warin R, Chambers WH, Potter DM, Singh SV. Prevention of mammary carcinogenesis in MMTV-neu mice by cruciferous vegetable constituent benzyl isothiocyanate. Cancer Res. 2009;69:9473–9480. doi: 10.1158/0008-5472.CAN-09-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner CE. Targeting mitochondria as a therapeutic target in cancer. J Cell Physiol. 2012;227:450–456. doi: 10.1002/jcp.22788. [DOI] [PubMed] [Google Scholar]
- Xiao D, Lew KL, Zeng Y, Xiao H, Marynowski SW, Dhir R, Singh SV. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006a;27:2223–2234. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- Xiao D, Powolny AA, Antosiewicz J, Hahm ER, Bommareddy A, Zeng Y, Desai D, Amin S, Herman-Antosiewicz A, Singh SV. Cellular responses to cancer chemopreventive agent D,L-sulforaphane in human prostate cancer cells are initiated by mitochondrial reactive oxygen species. Pharm Res. 2009;26:1729–1738. doi: 10.1007/s11095-009-9883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Powolny AA, Moura MB, Kelley EE, Bommareddy A, Kim SH, Hahm ER, Normolle D, Van Houten B, Singh SV. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J Biol Chem. 2010;285:26558–26569. doi: 10.1074/jbc.M109.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Powolny AA, Singh SV. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J Biol Chem. 2008;283:30151–30163. doi: 10.1074/jbc.M802529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006b;5:2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- Xue C, Pasolli HA, Piscopo I, Gros DJ, Liu C, Chen Y, Chiao JW. Mitochondrial structure alteration in human prostate cancer cells upon initial interaction with a chemopreventive agent phenethyl isothiocyanate. Cancer Cell Int. 2014;14:30. doi: 10.1186/1475-2867-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JM, Stepanov I, Murphy SE, Wang R, Allen S, Jensen J, Strayer L, Adams-Haduch J, Upadhyaya P, Le C, Kurzer MS, Nelson HH, Yu MC, Hatsukami D, Hecht SS. Clinical trial of 2-phenethyl isothiocyanate as an inhibitor of metabolic activation of a tobacco-specific lung carcinogen in cigarette smokers. Cancer Prev Res (Phila) 2016;9:396–405. doi: 10.1158/1940-6207.CAPR-15-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Trachootham D, Lu W, Carew J, Giles FJ, Keating MJ, Arlinghaus RB, Huang P. Effective killing of Gleevec-resistant CML cells with T315I mutation by a natural compound PEITC through redox-mediated mechanism. Leukemia. 2008;22:1191–1199. doi: 10.1038/leu.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, Abel PW, Tu Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 2013;32:4814–4824. doi: 10.1038/onc.2012.494. [DOI] [PMC free article] [PubMed] [Google Scholar]