Abstract
Objective
To examine the association between midlife cardiovascular health and physical performance 25 years later.
Design
Cohort study measured cardiovascular health in mid-life and physical function 25 years later. Multinomial logistic and logistic regression were used, adjusted for demographics and clinical measures.
Setting
Four US communities: Forsyth County, NC; Washington County, MD; Minneapolis, MN; and Jackson, MS
Participants
Atherosclerosis Risk in Communities (ARIC) Study
Measurements
Cardiovascular health was measured using Life's Simple 7 (LS7) scores (0-14) and LS7 component categories (poor, intermediate, ideal) for each risk factor defined by the American Heart Association. The Short Physical Performance Battery (SPPB) quantified physical function as an ordinal (0-12) and categorical outcome: low (0-6), fair (7-9), good (10-12).
Results
Among 15,744 participants at baseline (mean age=54.2±5.8 years, 55% female, 27% black) the mean LS7 Score was 7.9±2.4, and 6,144 (39%) returned 25 years later for the fifth exam when the SPPB was administered. Good SPPB was observed in 3,288 of 5,916 who completed the SPPB (50%). Each 1-unit increase in LS7 score was associated with a 17% higher SPPB (1.17 95% confidence interval [CI]: 1.15-1.19) and a 29% greater chance of having good SPPB versus SPPB <10 in late life (RR=1.29, 95% CI: 1.25, 1.34). Ideal glucose (RR=2.53, 95% CI 2.24, 2.87), smoking (RR=1.97, 95% CI 1.81, 2.15), blood pressure (RR=1.70, 95% CI 1.54, 1.88), body mass index (RR=1.51, 95% CI 1.37, 1.66), and physical activity (RR=1.31, 95% CI 1.20, 1.43) showed the strongest associations, adjusting for other LS7 components.
Conclusions
Better cardiovascular health during mid-life may lead to improvements in physical functioning at older ages.
Keywords: mid-life, cardiovascular, physical performance
Introduction
Maintaining optimal physical function with age is a top priority among older adults and an important contributor to quality of life.1,2,3 Poor physical function leads to adverse events such as incident disability, institutionalization, hospitalization, and mortality.4,5-10 Additionally, women and blacks have higher rates of physical disability than men or whites for unclear reasons.11,12 Relatively few studies have examined the role of modifiable risk factors during midlife and functional outcomes in older age13-16 although the benefit of interventions is probably greater if implemented earlier and maintained over the life course.
Existing studies suggest that modifiable, individual cardiovascular risk factors may contribute to poor function in older age.12-14,17-26 Few measured risk factor exposures in mid-life13,14,24-26 thus requiring recall of mid-life exposures.24 Other studies were limited by lack of objective performance measures14,15,20 and limited representation by blacks, a group that is disproportionately burdened by cardiovascular risk factors and mobility disability.12,27 Furthermore, the impact of mid-life cardiovascular risk factors, accounting for other health factors and behaviors, on late-life functional performance measures has not been defined. Finally, women with cardiac events, particularly black women, are more likely to present with atypical symptoms.28 Whether or not cardiovascular risk factors differentially affect physical performance in women and blacks is unknown.
The American Heart Association (AHA) developed measures of ideal cardiovascular health called Life's Simple 7 (LS7) which include optimal, intermediate, and poor categorization of seven established cardiovascular risk factors: smoking, body mass index (BMI), physical activity, diet, total cholesterol, blood pressure and glucose (Supplementary Table S1). LS7 metrics have been associated with incident cardiovascular disease,29 end-stage renal disease, and mortality.30 The AHA advocated ideal cardiovascular health should “encompass more than the absence of CVD” and “be based on data that suggest excellent prognosis with regard to CVD-free survival, longevity, healthy longevity, and quality of life.”31 Better physical function, a marker of healthy longevity and quality of life, thus represents an important outcome of interest associated with cardiovascular health. Identifying relationships of both individual and clusters of cardiovascular health measures to late-life function could be useful in developing and implementing targeted interventions earlier in life for reducing late-life disability. Accordingly, this study sought to examine the association of LS7 scores and individual LS7 components in midlife with objective measures of late-life physical performance in a biracial cohort.
Methods
Population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort from four US communities–Forsyth County, North Carolina; Washington County, Maryland; Minneapolis, Minnesota; and Jackson, Mississippi (blacks only)32. Designed to investigate the mechanisms of atherosclerosis and cardiovascular risk factors, the study sampled 15,792 men and women aged 45-64 years at baseline (1987-89). The current analysis used data from the baseline and the fifth examination (2011-2013). We excluded individuals reporting non-black and non-white race (n=48) leaving 15,744. Mortality ascertainment in ARIC was conducted using annual follow-up phone calls to participants or proxies, community-wide hospital surveillance, and linkage with local and national death registries. The study complied with the ethical rules for human experimentation in the Declaration of Helsinki and included institutional review board approval and informed consent from all participants.
Life's Simple Seven (LS7)
AHA LS7 components were each assigned points (poor=0, intermediate=1, and ideal=2). (Supplementary Table S1).31 The LS7 score (0-14 scale) was created by adding points from the seven LS7 components. A description of LS7 development in the ARIC cohort has been described.29 Briefly, smoking history was self-reported and categorized as never, former (smoked at least 100 cigarettes but quit >1 year ago) or current smoker (currently smoking or quit within the past year). BMI was calculated as weight (kg)/ height squared (m2); height was measured by stadiometer to the nearest centimeter and weight by balance beam scale. Physical activity was reported using the ARIC-Baecke questionnaire from which an index score for activity at work, during sports, and leisure was calculated.33 Dietary intake was assessed using a 66-item, semi-quantitative food-frequency questionnaire, a modified version of the 61-item instrument designed and validated by Willett et al.34 Blood pressure was measured three times using a random-zero sphygmomanometer. For analysis, the second and third measurements were averaged. Glucose and cholesterol were measured with standardized enzymatic methods using fasting blood samples. Clinical staff recorded medications participants used in the previous two weeks.
Covariates
Participants self-reported age, sex, race, education, and alcohol consumption at baseline. Education was categorized as <12 years, 12-16 years, and >16 years. Prior coronary heart disease and stroke35,36 were identified at baseline through self-report and electrocardiograms, and since baseline through annual phone calls (semi-annual since 2012), surveillance of local hospitals, and state and national death indexes. For potential coronary heart disease, stroke and (since 2005) heart failure events, medical records were obtained, charts abstracted, and events adjudicated by physicians. Heart failure at baseline was identified using the Gothenburg criteria.37
Physical Function
The physical function assessment was offered to all participants at visit 5 using a standardized protocol for the Short Physical Performance Battery (SPPB).11,38 The SPPB is a composite score based on performance of three tasks: balance (standing, semi-tandem, tandem stands, held up to 10 seconds each), usual gait speed over four meters, and time to rise from a seated position five times without using their arms. Gait speed was the faster of two trials walking “at your normal pace”. Each task was scored from a range of 0 (worse) to 4 (best) based on population thresholds and summed to create a composite score ranging from 0-12.11 Participants who were unable to perform a task received a score of 0. A four-category SPPB outcome was constructed as (1) poorest, defined as those deceased before exam 5, alive but did not attend visit 5, attended visit 5 but were missing SPPB; (2) low (SPPB 0-6); (3) fair (SPPB 7-9); and (4) good (SPPB 10-12).38 The poorest category was used to address biases of complete case analyses since people with poor performance are less likely to undergo functional assessments.
Data Analysis
Descriptive statistics included means and standard deviation for continuous variables and counts and percentages for categorical variables. Negative binomial regression models for SPPB (0-12) integer outcomes and multinomial logistic regression models for SPPB four-level categorical outcomes compared rates of achieving a SPPB categorical outcome versus another SPPB categorical outcome. Results from the top three categories of the four-level multinomial models are shown for brevity; full results are included in appendices. Poisson regression models were run as a sensitivity analysis. Finally a logistic regression model modeled the probability of a good SPPB outcome (SPPB=10-12) relative to SPPB scores of <10. A three-way interaction between sex, race, and LS7 along with lower level 2-way interactions were included to examine race and sex differences due to well-established associations of greater mobility difficulty with female sex and black race;11,12 a separate model with sex-LS7 interaction and race-LS7 interaction terms was examined and compared to the three-way interaction model. Marginal probabilities were calculated and plotted with 95% confidence intervals. Linearity checks were performed via fractional polynomial smoothers. Sensitivity analyses to examine influences of missing data were conducted by comparing results presented to results from a complete case analysis model. All models were adjusted for age, sex, race, site, education, heart failure, heart disease, alcohol use, and stroke. Models examining relations of individual LS7 metrics with SPPB outcomes were adjusted for other LS7 metrics. All analyses used Stata version 14 (StataCorp LP, College Station, TX).
Results
Among 15,744 participants at baseline (mean age 54.2 years, 27% black, 55% women) (Table 1), there were 5, 034 deaths (32%) over the 25 years between the first and fifth examinations, leaving 10,710 who were alive at visit 5. Of these, 4,566 did not attend visit 5; of the 6,144 who attended, (mean age 75.8 years, 24% black, 59% women), 5,916 completed the SPPB (96% of attendees; 38% of original cohort). (Supplementary Figure S1) Compared to those who returned for visit 5, participants with missing SPPB data were older and more likely to be smokers at baseline, of black race and to have lower education, and generally poorer health parameters including higher blood pressure, glucose, BMI, less physical activity and lower LS7 scores. Among those attending visit 5, participants with low SPPB were more likely to be older, female, black, and have lower education and poorer health metrics. Conversely, participants with good late-life SPPB had higher mid-life LS7 scores across all LS7 components.
Table 1.
Mid-life (visit 1) characteristics of participants overall and stratified by those who died before visit 5/or alive but missing SPPB and by SPPB categories for those who completed the examination in late-life (visit 5)
| Late-life (visit 5) Physical Function (SPPB)a | |||||
|---|---|---|---|---|---|
| Visit 1 characteristics | Visit 1 Total N=15,744 | Died/Missing N=9,828 (62%) | Low [≤6] N=858 (6%) | Fair [>6, <10] N=1,770 (11%) | Good ≥10 N=3,288 (21%) |
| Age, years | 54.17 (5.8) | 55.58 (5.7) | 54.5 (5.5) | 52.5 (5.1) | 50.8 (4.5) |
| Women | 8685 (55%) | 5257 (53%) | 583 (68%) | 1098 (62%) | 1747 (53%) |
| Black race | 4266 (27%) | 2958 (30%) | 335 (39%) | 445 (25%) | 528 (16%) |
| Education <12 years | 3754 (24%) | 2951 (30%) | 218 (25%) | 278 (16%) | 307 (9%) |
| Body Mass Index, kg/m2 | 27.7 (5.4) | 28.1 (5.7) | 29.4 (5.90) | 27.3 (4.7) | 26.3 (4.2) |
| Current smoker | 4117 (26%) | 3085 (31%) | 158 (18%) | 352 (20%) | 522 (16%) |
| Diabetes | 1558 (10%) | 1320 (14%) | 86 (10%) | 79 (4%) | 73 (2%) |
| Fasting glucose, mg/dL | 109.0 (40.61) | 113.7 (47.7) | 107.2 (35.5) | 101.6 (24.5) | 99.3 (16.5) |
| Diabetes Medication | 882 (6%) | 787 (8%) | 37 (4%) | 26 (1%) | 32 (1%) |
| Hypertension | 4640 (30%) | 3433 (35%) | 302 (35%) | 385 (22%) | 520 (16%) |
| Systolic BP, mmHg | 121.4 (19.0) | 124.4 (20.1) | 122.2 (17.4) | 116.8 (15.5) | 114.5 (15.0) |
| Diastolic BP, mmHg | 73.8 (11.3) | 74.3 (11.9) | 75.3 (11.4) | 72.9 (10.19) | 72.2 (10.1) |
| Hypertension Medication | 4833 (31%) | 3567 (36%) | 300 (35%) | 433 (24%) | 533 (16%) |
| Heart Failure | 751 (5%) | 621 (6%) | 43 (5%) | 46 (3%) | 41 (1%) |
| Heart Disease | 765 (5%) | 657 (7%) | 12 (1%) | 49 (3%) | 47 (1%) |
| Stroke | 284 (2%) | 246 (3%) | 15 (2%) | 12 (1%) | 11 (0%) |
| Current alcohol use | 8742 (56%) | 5123 (52%) | 426 (50%) | 1046 (59%) | 2147 (65%) |
| Cholesterol, mg/dL | 215.0 (42.1) | 217.8 (43.2) | 216.5 (43.0) | 212.7 (39.5) | 207.5 (38.7) |
| HDL cholesterol, mg/dL | 51.6 (17.1) | 50.7 (17.1) | 53.3 (16.6) | 53.4 (17.5) | 52.8 (16.8) |
| LDL cholesterol, mg/dL | 137.7 (39.4) | 140.1 (40.1) | 138.0 (41.8) | 135.3 (36.8) | 131.6 (37.0) |
| Triglycerides, mg/dL | 131.8 (90.5) | 138.6 (96.4) | 126.2 (71.1) | 123.6 (84.0) | 117.6 (77.0) |
| Lipid Lowering Medication | 452 (3%) | 326 (3%) | 16 (2%) | 37 (2%) | 73 (2%) |
| Physical Activity (mins/wk) | 57.6 (99.7) | 51.37 (94.2) | 45.37 (91.5) | 60.59 (102.1) | 77.7 (112.6) |
| LS 7 Score (0-14) | 7.9 (2.4) | 7.37 (2.4) | 7.53 (2.3) | 8.41 (2.3) | 9.1 (2.1) |
Values are mean (SD) or N (%). BP=blood pressure; HDL=high-density lipoprotein; LDL=low-density lipoprotein; LS7=Life's Simple 7
Note: 5,034 (32%) participants were deceased by Visit 5; 4,566 (30%) participants did not attend visit 5; 228 (4%) attended but were missing SPPB at visit 5. To convert values to mmol/L, multiply values for total, HDL, and LDL cholesterol by 0.0259; multiply values for triglycerides by 0.0113
Table 2 shows the prevalence of ideal LS7 components in the cohort and by race. The population was reasonably distributed across poor, intermediate and ideal categories in most LS7 components, but few participants achieved ideal diet (5%) and most (71%) were classified as ideal in the smoking category. Blacks were less likely than whites to achieve ideal status in all metrics except cholesterol, which was similar between racial groups.
Table 2.
Prevalence of Ideal Life Simple 7 Components Overall and by Race at Baseline (Visit 1)
| Ideal Cardiovascular Health Indicators | Total (N=15744) | White (N=11478) | Black (N=4266) | p-value |
|---|---|---|---|---|
| Smoking | 11147 (71%) | 8268 (72%) | 2879 (67%) | <0.001 |
| BMI | 5191 (33%) | 4259 (37%) | 932 (22%) | <0.001 |
| Physical Activity | 5779 (37%) | 4867 (42%) | 912 (21%) | <0.001 |
| Diet | 817 (5%) | 632 (6%) | 185 (4%) | 0.003 |
| Cholesterol | 5675 (36%) | 4089 (36%) | 1586 (37%) | 0.071 |
| Blood Pressure | 5941 (38%) | 5063 (44%) | 878 (21%) | <0.001 |
| Glucose | 7990 (51%) | 6087 (53%) | 1903 (45%) | <0.001 |
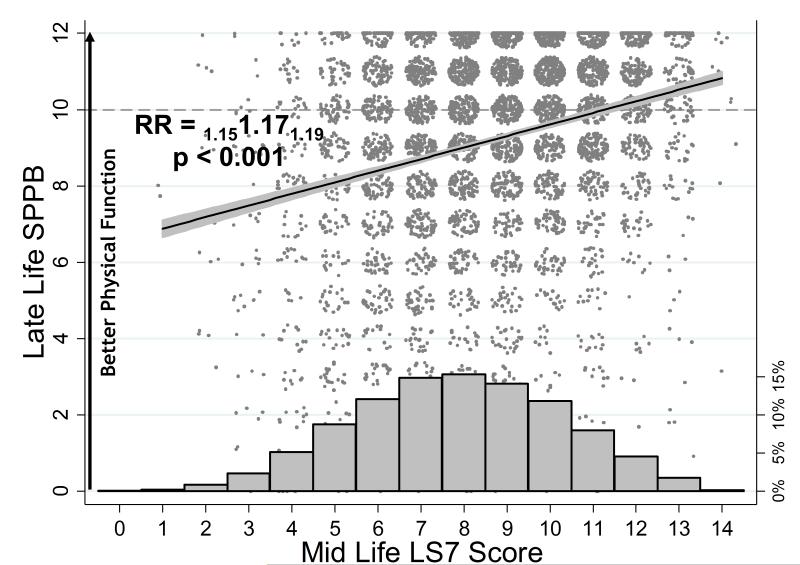
Figure 1 illustrates the mid-life LS7 distribution along with the LS7 relationship to SPPB integer outcomes. Each 1-unit increase in LS7 score was associated with a 17% higher SPPB (RR=1.17, 95% CI 1.15, 1.19; Figure 1, Table 3). All ideal LS7 metrics were associated with higher SPPB scores as an integer outcome except cholesterol (RR=1.06, 95% CI 0.97, 1.17), and the association of SPPB with diet was of borderline statistical significance (RR=1.19, 95% CI 1.00, 1.41) (Table 3). The largest effect sizes were for ideal glucose (RR=2.53, 95% CI 2.24, 2.87) and smoking (RR=1.97, 95% CI 1.81, 2.15), followed by ideal blood pressure (RR=1.70, 95% CI 1.54, 1.88), BMI (RR=1.51, 95% CI 1.37, 1.66), and physical activity (RR=1.31, 95% CI 1.20, 1.43).
Figure 1.
Relationship of mid-life Life's Simple 7 (LS7) score with late-life Short Physical Performance Battery (SPPB). Jittered datapoints, association and adjusted relative rates from negative binomial model with subscripted 95% CI shown.
Footnote: RR=relative ratio from negative binomial regression model adjusted for age, sex, race-site, education, heart failure, heart disease, alcohol use, stroke. Histogram shows distribution of LS7 scores with percentages on right y-axis.
Table 3.
Associations of late-life Short Physical Performance Battery (SPPB) outcomes with Mid-life Life Simple 7 (LS7) score or individual ideal compared to poor LS7 components
| Baseline LS7 Score and Ideal LS7 Components | SPPB (0-12)a | SPPB Categories (Low [≤6], Fair [>6, <10], Good [10-12]) RRb, p-value, 95% CI | ||
|---|---|---|---|---|
| Fair (n=1770) vs Low (n=858) | Good (n=3288 ) vs Fair (n=1770) | Good (n=3288 ) vs Low (n=858) | ||
| LS7 Score (0-14) | 1.17 p<0.001 (1.15,1.19) | 1.14 p<0.001 (1.10,1.18) | 1.13 p<0.001 (1.10,1.17) | 1.29 p<0.001 (1.24,1.34) |
| Ideal Smoking | 1.97 p<0.001 (1.81,2.15) | 1.00 p=0.997 (0.80,1.24) | 1.45 p<0.001 (1.24,1.70) | 1.45 p<0.001 (1.18,1.79) |
| Ideal Body Mass Index | 1.51 p<0.001 (1.37,1.66) | 2.07 p<0.001 (1.65,2.59) | 1.83 p<0.001 (1.55,2.16) | 3.78 p<0.001 (3.05,4.69) |
| Ideal Physical Activity | 1.31 p<0.001 (1.20,1.43) | 1.47 p<0.001 (1.20,1.80) | 1.41 p<0.001 (1.22,1.63) | 2.07 p<0.001 (1.71,2.51) |
| Ideal Diet | 1.19 p=0.052 (1.00,1.41) | 1.01 p=0.949 (0.69,1.49) | 1.14 p=0.338 (0.87,1.51) | 1.16 p=0.421 (0.81,1.66) |
| Ideal Total Cholesterol | 1.06 p=0.202 (0.97,1.17) | 1.02 p=0.827 (0.82,1.27) | 1.28 p=0.003 (1.09,1.50) | 1.31 p=0.010 (1.07,1.60) |
| Ideal Blood Pressure | 1.70 p<0.001 (1.54,1.88) | 1.81 p<0.001 (1.43,2.30) | 1.28 p=0.008 (1.07,1.54) | 2.33 p<0.001 (1.86,2.91) |
| Ideal Glucose | 2.53 p<0.001 (2.24,2.87) | 1.69 p=0.001 (1.23,2.33) | 1.54 p=0.003 (1.15,2.06) | 2.61 p<0.001 (1.91,3.57) |
Rate Ratios from negative binomial models
Relative Rate Ratios from 4-category multinomial models (results for top 3 categories reported for brevity; see methods and Supplementary Table S2 for all 4 categories)
Adjusted for age, sex, race-site, education, heart failure, heart disease, alcohol use, stroke
Table 3 also shows relations of LS7 score or ideal compared to poor LS7 components with relative rates of achieving SPPB categories. For each one-unit increase in LS7 score, participants had a 14% higher rate of achieving a fair SPPB (RR=1.14, 95% CI 1.10, 1.18) and 29% higher rate of achieving a good SPPB relative to low SPPB (RR=1.29, 95% CI 1.24, 1.34). Similarly, for a one unit increase in LS7 score, a 13% higher rate of achieving good SPPB relative to fair SPPB was observed (RR=1.13, 95% CI 1.10, 1.17).
Each ideal LS7 component, except for diet, was associated with increased likelihood of having good compared to fair or low SPPB (Table 3). Ideal mid-life BMI, physical activity, blood pressure, and glucose were each associated with higher rates of fair compared to low SPPB. Ideal smoking, cholesterol and diet, compared to poor smoking, cholesterol and diet respectively, were not associated with higher rates of fair compared to low SPPB.
Results comparing rates of being in the poorest SPPB category, which comprised those who died, those who were alive but did not participate in the fifth exam, and a small number at the exam who did not attempt the SPPB, are shown in Supplementary Tables S2 and S3. Among participants with poor smoking status, 75% were in the poorest SPPB category compared to 58% with ideal smoking status. (Supplementary Table S2) This translates to a 23% lower rate of poorest SPPB associated with ideal smoking compared to poor smoking (RR=0.77, 95% CI: 0.75,0.79). Conversely, those with midlife ideal compared to poor smoking were nearly twice as likely to have good late-life SPPB, 24% vs 13% (RR=1.89, 95% CI 1.73, 2.06). All other ideal LS7 components, compared to poor, were associated with lower rates of poorest SPPB in late life, including diet.
Sensitivity analyses demonstrated a 34% higher chance of being in the good versus poorest SPPB category with each unit higher LS7 score (RR=1.34, 95% CI: 1.31, 1.37) (Supplementary Table S3). Additionally, each individual LS7 metric classified as Ideal was associated with a higher chance of having good versus poorest SPPB, including diet (RR=1.29, 95% CI: 1.05,1.59) and cholesterol (RR=1.41, 95% CI: 1.25,1.58). Results were substantively unchanged although some attenuation was noted in sensitivity analyses using complete case analysis (Supplementary Table S4).
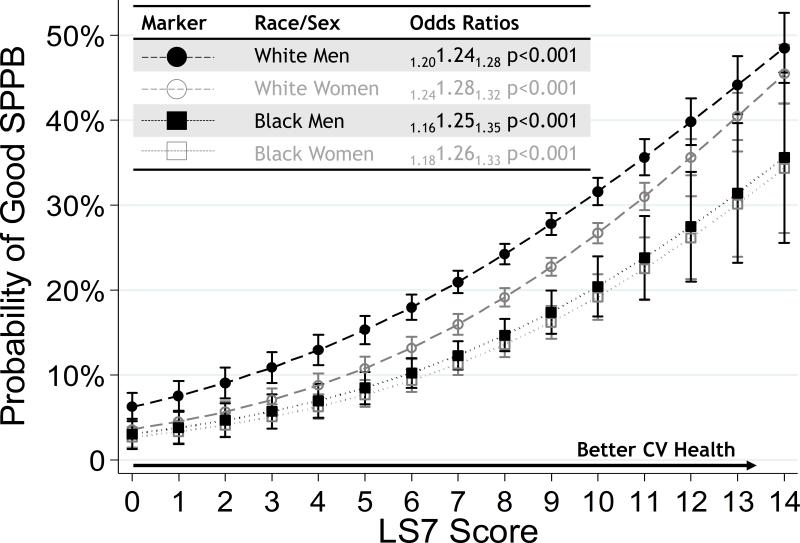
Although men and whites had better SPPB than women and blacks, the relationship of LS7 with good SPPB did not appear to differ substantially across groups (Figure 2; likelihood ratio test for pooled model vs 3-way interaction model: p=0.282). The odds of having good SPPB was 24% higher with each 1-unit increase in LS7 in white men (OR=1.24, 95% CI 1.20, 1.28), 28% higher in white women (OR=1.28, 95% CI 1.24, 1.32), 25% higher in black men (OR=1.25, 95% CI 1.16, 1.35) and 26% higher in black women (OR=1.26, 95% CI 1.18, 1.33).
Figure 2.
Race and sex-stratified adjusted odds ratios (OR) with subscripted 95% confidence intervals for having good late-life Short Physical Performance Battery (SPPB), defined as SPPB ≥10 according to mid-life Life's Simple 7 (LS7) score.
Footnote: Logistic regression models stratified by age and sex, adjusted for education, heart failure, heart disease, alcohol use, stroke.
Discussion
In this study, better midlife cardiovascular health, 25 years earlier, was strongly associated with better late life physical function in a large community cohort of blacks and whites. The associations were similar across race and sex, although blacks and women generally had poorer physical function compared to whites and men as others have reported.12,27 The strongest associations were for glucose and smoking, followed by BMI, blood pressure, and physical activity. Each of these is modifiable and the results suggest an emphasis should be placed on optimizing them across the lifespan to preserve late-life mobility. Additionally, ideal levels of glucose, obesity, and blood pressure control, which blacks in the ARIC cohort were much less likely to achieve, may be emphasis areas to target that might reduce disparities of mobility disability in blacks.
Our findings are in line with and build upon other studies of relations of cardiovascular health to late-life physical performance in several ways. First, ARIC is among a few studies that measured risk factor exposures in mid-life.13,14,24-26 These measures were complemented by objective performance measures, thus avoiding recall bias of exposures and overestimation of performance in late-life. The current study extends findings from previous studies on the relations of cardiovascular risk factors with functional outcomes, which have been limited by subjective recall of risk factors in mid-life,24 subjective reports of physical function in late life,15,20 using measures of predictors from late-life only,17,19,20,22 and limited representation by blacks, despite a greater burden of disability in blacks.12 In addition, studies conducted only in older age may fail to identify important relationships due to attenuated relationships in late-life despite strong relationships when measured earlier in life. Among studies with objective measures of mid-life risk factors, most focused on one risk factor without accounting for all cardiovascular health metrics in the LS7.13,24-26 As a result, previously reported associations could have been confounded by unmeasured and often coexisting cardiovascular risk factors. Others lacked objective performance measures,14,15 and only one reported sizeable representation of blacks.14 The ARIC data provided an extended follow-up of 25 years, adjusted for adjudicated measures of other risk factors and health conditions often lacking in other studies, and our analysis accounted for attrition. The findings from the current study examined seven cardiovascular risk factors readily available in clinical practice and included a large sample of blacks. In addition, these findings are the first to provide a comparison across modifiable risk factors on late life physical performance, accounting for each of the other risk factors. These findings therefore identified a group of risk factors in mid-life that preventive and therapeutic interventions might focus upon across the lifespan in the prevention of aging-associated mobility disability.
Dietary intake in mid-life was not associated with late-life physical function, although those with a healthy diet in mid-life were less likely to be missing late-life SPPB measures. One explanation for this lack of association could be measurement error in dietary intake, specifically under- or over-reporting of certain foods and omission of food intake due to the limited number of questions for fruit, vegetables, whole grain products, and snack food categories. Another potential explanation is lack late-life dietary intake and the inability to assess changes in dietary intake in the 25-year interval from mid- to late-life. Furthermore, diet may be correlated with other risk factors, including cholesterol, glucose, blood pressure, and BMI (all of which were associated with physical function), which could attenuate associations between diet and physical function. In addition, there could be no true association of mid-life diet with late-life function.
Associations of mid-life cholesterol with late-life physical function using the LS7 integer outcome or across lower categories of functional status were not supported. However, ideal mid-life cholesterol was associated with greater likelihood of being in the highest functional level compared to the lower levels of function. The null findings among lower functioning categories could be due to limited power in the fair and low SPPB categories.
Limitations of the current study warrant comment and include lack of LS7 exposures across all visits and therefore an inability to incorporate changes in cardiovascular health measures from mid-life to late life. Inherent to cohort studies, loss to follow-up was substantial over 25 years. Participants with poor function are more likely to drop out of studies over time.39,40 To account for informative missingness, our analysis included those with missing functional performance data in the group with poorest function. We also presented results from complete case analysis models, ignoring participants with missing functional performance, and found substantively the same although attenuated relationships. The actual relationship probably lies between estimates resulting from the two models. However, both analyses support the hypothesis that mid-life cardiovascular health is important for late-life physical function. The potential for race and site aliasing within the population exists, as most blacks in the study were from one field center. However, the findings reported, including poorer function with older age, in women, and in blacks, are consistent with other populations.12,41-43 Lastly, the observational study design is a limitation, and although findings establish associations, causality cannot be inferred. Residual confounding remains possible. Despite these confines, this unique data spanning 25 years support the importance of maintaining healthy lifestyles and cardiovascular risk factor profiles across the adult lifespan to preserve physical performance in older age.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
Financial Disclosure: The ARIC Study is carried out as a collaborative study by National Heart, Lung and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Miss Harrison was funded by the AFAR and National Institute on Aging Medical Student Training in Aging Research (MSTAR) program for part of this work.
Sponsor's Role: The sponsor played no role in the design, methods, subject recruitment, data collections, analysis, or preparation of paper
Footnotes
Author Contributions: All authors made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; provided critical revision of the final draft; and provided final approval.
Conflict of interest: Drs. Windham, Lutsey, Pompeii, Gabriel, Griswold, Mosley, Miss Harrison and Mr. Lirette have received grant funding related to this work.
REFERENCES
- 1.Fried TR, Tinetti M, Agostini J, Iannone L, Towle V. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Education and Counseling. 2011;83(2):278–282. doi: 10.1016/j.pec.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindgren A-M, Svardsudd K, Tibblin G. Factors Related to Perceived Health among Elderly People: The Albertina Project. Age and Ageing. 1994;23(4):328–333. doi: 10.1093/ageing/23.4.328. [DOI] [PubMed] [Google Scholar]
- 3.La Grow S, Yeung P, Towers A, Alpass F, Stephens C. The impact of mobility on quality of life among older persons. Journal of aging and health. 2013;25(5):723–736. doi: 10.1177/0898264313490198. [DOI] [PubMed] [Google Scholar]
- 4.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. New England Journal of Medicine. 1995;332(9):556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. Journal of Gerontology. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 7.Vasunilashorn S, Coppin AK, Patel KV, et al. Use of the Short Physical Performance Battery Score to Predict Loss of Ability to Walk 400 Meters: Analysis From the InCHIANTI Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64A(2):223–229. doi: 10.1093/gerona/gln022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volpato S, Cavalieri M, Guerra G, et al. Performance-Based Functional Assessment in Older Hospitalized Patients: Feasibility and Clinical Correlates. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63(12):1393–1398. doi: 10.1093/gerona/63.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller DK, Wolinsky FD, Andresen EM, Malmstrom TK, Miller JP. Adverse outcomes and correlates of change in the Short Physical Performance Battery over 36 months in the African American health project. The journals of gerontology. Series A, Biological sciences and medical sciences. 2008;63(5):487–494. doi: 10.1093/gerona/63.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. The journals of gerontology. Series A, Biological sciences and medical sciences. 2001;56(3):M158–166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 11.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 12.Mendes de Leon CF, Barnes LL, Bienias JL, Skarupski KA, Evans DA. Racial disparities in disability: recent evidence from self-reported and performance-based disability measures in a population-based study of older adults. The journals of gerontology. Series B, Psychological sciences and social sciences. 2005;60(5):S263–271. doi: 10.1093/geronb/60.5.s263. [DOI] [PubMed] [Google Scholar]
- 13.Chang M, Saczynski JS, Snaedal J, et al. Midlife Physical Activity Preserves Lower Extremity Function in Older Adults: Age Gene/Environment Susceptibility–Reykjavik Study. Journal of the American Geriatrics Society. 2013;61(2):237–242. doi: 10.1111/jgs.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhamoon MS, Dong C, Elkind MSV, Sacco RL. Ideal Cardiovascular Health Predicts Functional Status Independently of Vascular Events: The Northern Manhattan Study. Journal of the American Heart Association. 2015;4(2) doi: 10.1161/JAHA.114.001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinsky JL, Branch LG, Jette AM, et al. Framingham Disability Study: relationship of disability to cardiovascular risk factors among persons free of diagnosed cardiovascular disease. American journal of epidemiology. 1985;122(4):644–656. doi: 10.1093/oxfordjournals.aje.a114144. [DOI] [PubMed] [Google Scholar]
- 16.Robinson SM, Jameson KA, Syddall HE, et al. Clustering of lifestyle risk factors and poor physical function in older adults: the Hertfordshire cohort study. Journal of the American Geriatrics Society. 2013;61(10):1684–1691. doi: 10.1111/jgs.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapuri PB, Gallagher JC, Smith LM. Smoking Is a Risk Factor for Decreased Physical Performance in Elderly Women. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(1):93–99. doi: 10.1093/gerona/62.1.93. [DOI] [PubMed] [Google Scholar]
- 18.Strand BH, Mishra G, Kuh D, Guralnik JM, Patel KV. Smoking History and Physical Performance in Midlife: Results From the British 1946 Birth Cohort. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66A(1):142–149. doi: 10.1093/gerona/glq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koster A, Penninx BW, Newman AB, et al. Lifestyle factors and incident mobility limitation in obese and non-obese older adults. Obesity. 2007;15(12):3122–3132. doi: 10.1038/oby.2007.372. [DOI] [PubMed] [Google Scholar]
- 20.Gopinath B, Russell J, Flood VM, Burlutsky G, Mitchell P. Adherence to dietary guidelines positively affects quality of life and functional status of older adults. Journal of the Academy of Nutrition and Dietetics. 2014;114(2):220–229. doi: 10.1016/j.jand.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 21.De Rekeneire N, Resnick HE, Schwartz AV, et al. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the Health, Aging, and Body Composition study. Diabetes care. 2003;26(12):3257–3263. doi: 10.2337/diacare.26.12.3257. [DOI] [PubMed] [Google Scholar]
- 22.Landi F, Russo A, Cesari M, Pahor M, Bernabei R, Onder G. HDL-cholesterol and physical performance: results from the ageing and longevity study in the sirente geographic area (ilSIRENTE Study). Age and ageing. 2007;36(5):514–520. doi: 10.1093/ageing/afm105. [DOI] [PubMed] [Google Scholar]
- 23.Rosano C, Longstreth WT, Jr., Boudreau R, et al. High blood pressure accelerates gait slowing in well-functioning older adults over 18-years of follow-up. Journal of the American Geriatrics Society. 2011;59(3):390–397. doi: 10.1111/j.1532-5415.2010.03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenholm S, Rantanen T, Alanen E, Reunanen A, Sainio P, Koskinen S. Obesity history as a predictor of walking limitation at old age. Obesity. 2007;15(4):929–938. doi: 10.1038/oby.2007.583. [DOI] [PubMed] [Google Scholar]
- 25.Stenholm S, Sainio P, Rantanen T, et al. High body mass index and physical impairments as predictors of walking limitation 22 years later in adult Finns. The journals of gerontology. Series A, Biological sciences and medical sciences. 2007;62(8):859–865. doi: 10.1093/gerona/62.8.859. [DOI] [PubMed] [Google Scholar]
- 26.Strand BH, Mishra G, Kuh D, Guralnik JM, Patel KV. Smoking history and physical performance in midlife: results from the British 1946 birth cohort. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66(1):142–149. doi: 10.1093/gerona/glq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostchega Y, Harris TB, Hirsch R, Parsons VL, Kington R. The prevalence of functional limitations and disability in older persons in the US: data from the National Health and Nutrition Examination Survey III. Journal of the American Geriatrics Society. 2000;48(9):1132–1135. doi: 10.1111/j.1532-5415.2000.tb04791.x. [DOI] [PubMed] [Google Scholar]
- 28.Eastwood JA, Johnson BD, Rutledge T, et al. Anginal symptoms, coronary artery disease, and adverse outcomes in Black and White women: the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) study. J Womens Health (Larchmt) 2013;22(9):724–732. doi: 10.1089/jwh.2012.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muntner P, Judd SE, Gao L, et al. Cardiovascular risk factors in CKD associate with both ESRD and mortality. Journal of the American Society of Nephrology : JASN. 2013;24(7):1159–1165. doi: 10.1681/ASN.2012070642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association's Strategic Impact Goal Through 2020 and Beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 32.The Atherosclerosis Risk in Communities (ARIC) Study: Design and Objectives. The ARIC Investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 33.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 34.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. American journal of epidemiology. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 35.Goff DC, Jr., Howard G, Wang CH, et al. Trends in severity of hospitalized myocardial infarction: the atherosclerosis risk in communities (ARIC) study, 1987-1994. American heart journal. 2000;139(5):874–880. doi: 10.1016/s0002-8703(00)90020-6. [DOI] [PubMed] [Google Scholar]
- 36.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea - Validation of a scoring test for clinical-epidemiological use: The study of men born in 1913. European heart journal. 1987;8(9):1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 38.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. The New England journal of medicine. 1995;332(9):556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gubhaju L, Banks E, Macniven R, et al. Factors relating to participation in follow-up to the 45 and up study in Aboriginal and non-Aboriginal individuals. BMC Med Res Methodol. 2016;16:53. doi: 10.1186/s12874-016-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt JA, Gruman C, King MB, Wolfson LI. Attrition in an exercise intervention: a comparison of early and later dropouts. Journal of the American Geriatrics Society. 2000;48(8):952–960. doi: 10.1111/j.1532-5415.2000.tb06894.x. [DOI] [PubMed] [Google Scholar]
- 41.Allman R, Baker P, Maisiak R, Sims R, Roseman J. Racial similarities and differences in predictors of mobility change over eighteen months. Journal of general internal medicine. 2004;19(11):1118–1126. doi: 10.1111/j.1525-1497.2004.30239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latham K. Racial and educational disparities in mobility limitation among older women: what is the role of modifiable risk factors? The journals of gerontology. Series B, Psychological sciences and social sciences. 2014;69(5):772–783. doi: 10.1093/geronb/gbu028. [DOI] [PubMed] [Google Scholar]
- 43.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology. Series A, Biological sciences and medical sciences. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.