NITROGENASES
The nitrogenase enzyme catalyzes the reductive breakage of the very strong triple bond of N2 to generate NH3 in a process known as biological nitrogen fixation. Biological nitrogen fixation is an essential step in the nitrogen cycle in the biosphere, and it is a major contributor to the nitrogen available to agricultural crops. Nitrogenases are composed of two proteins that can be purified separately: dinitrogenase and dinitrogenase reductase (8, 31). Dinitrogenase, also referred to as the MoFe protein or component I, is a 220- to 240-kDa tetramer of the nifD and nifK gene products that contains two pairs of two complex metalloclusters known as the P-cluster and the iron molybdenum cofactor (FeMo-co) (10, 43, 74). Each αβ pair of subunits of NifD and NifK contains one P-cluster and one molecule of FeMo-co. FeMo-co is composed of homocitrate and a MoFe3-S3 cluster bridged to a Fe4-S3 cluster by three sulfur ligands. The Mo atom is coordinated to the C-2 carboxyl and hydroxyl groups of R-homocitrate. Recently, an electron-dense area has been observed within the core of Fe atoms of FeMo-co and has been proposed to be a low-atomic-weight species (O or N) (Fig. 1) (21). The P-cluster is a [8Fe-7S] cluster with a structure similar to that of FeMo-co, which consists of two [4Fe-3S] cubanes connected by a central S atom (Fig. 1). The P-clusters are located at the αβ subunit interface and are coordinated by cysteinyl residues from both subunits. Dinitrogenase reductase, also referred to as the Fe protein or component II, is a 60-kDa dimer of the product of the nifH gene, which contains a single [4Fe-4S] cluster at the subunit interface and two Mg-ATP-binding sites, one at each subunit (28). Hereafter in this minireview, we will use the Fe protein and MoFe protein nomenclature when referring to the nitrogenase components. The Fe protein is the obligate electron donor to the MoFe protein; electrons are transferred from the [4Fe-4S] cluster of the Fe protein to the P-cluster of the MoFe protein and in turn to FeMo-co, the site for substrate reduction (9, 14). In addition, the Fe protein functions in the biosynthesis of FeMo-co and in the maturation of apo-MoFe protein (18, 67).
FIG. 1.
Metal clusters of the nitrogenase MoFe protein. (A) FeMo-co and (B) the P-cluster in the reduced state. The amino acid ligands to FeMo-co are shown (α-Cys275 and α-His442). The central atom in the cavity of FeMo-co is colored as nitrogen. For the P-cluster, only the bridging cysteinyl ligands are shown (α-Cys88 and β-Cys95). Atoms are colored as follows: iron, magenta; molybdenum, grey; sulfur, yellow; carbon, green; oxygen, red; and nitrogen, blue.
It was assumed for a long time that the ability to fix nitrogen was a metabolic specialization carried out by a small group of organisms; however, the fact is that this capability is very widely spread among bacterial genera, in both the eubacteria and the archaeobacteria (79). On the other hand, to date, no eukaryotic organisms with the ability to fix nitrogen have been reported. Although the Mo-containing nitrogenase is the most commonly found nitrogenase in nature, there are two homologous alternative nitrogenases that are genetically distinct but have similar cofactor and subunit composition: the V-containing nitrogenase and the Fe-only nitrogenase, which are encoded by the vnf and anf genes, respectively (4, 51). One difference is that the alternative dinitrogenases in their mature forms are hexameric proteins encoded by the vnfDGK and anfDGK operons, with the δ subunits being the products of the vnfG and anfG genes, respectively (see below) (13, 39, 66). The Mo-containing, V-containing, and Fe-only nitrogenases are not equally distributed in nature. Some organisms, like Azotobacter vinelandii, possess all three types of nitrogenases (6, 13, 56). Other organisms, like Rhodobacter capsulatus and Rhodospirillum rubrum, carry the Mo-containing and Fe-only nitrogenases (17, 73), while some organisms, like Anabaena variabilis, carry the Mo- and V-containing nitrogenases (76). Finally, Klebsiella pneumoniae possesses only the Mo-containing nitrogenase (2). Interestingly, wild-type organisms carrying only an alternative nitrogenase have not yet been found in nature. Most of what is known about the maturation of nitrogenase components comes from studies on the Mo-containing nitrogenase. In addition, a fourth type of nitrogenase has been recently discovered which is not phylogenetically related to the above ones (59). This nitrogenase, found only in Streptomyces thermoautotrophicus, carries a Mo-molybdopterin cytosine dinucleotide cofactor (Mo-MCD) as active site and exhibits different protein composition. This type of nitrogenase will not be reviewed here.
The P-cluster and FeMo-co are among the most complex metalloclusters known. Thus, biosynthesis of FeMo-co and maturation of the MoFe protein have become model systems for the study of synthesis of complex metalloenzymes with significance to all of biology. It should be noted that a very large number of enzymes carry some type of [Fe-S] clusters that are involved in processes as diverse as electron transfer, catalysis, and redox sensing (3).
The structural genes for the MoFe protein, nifD and nifK, are not required for the biosynthesis of FeMo-co (36, 64, 77). It is accepted that FeMo-co is assembled separately in the cells and is finally incorporated into a FeMo-co-deficient apo-MoFe protein. A number of nitrogen fixation (nif) genes are required for the biosynthesis of FeMo-co and maturation of the nitrogenase component proteins from folded polypeptides to their metallocluster-containing catalytically active forms. Roles for the nifB, nifQ, nifX, nifN, nifE, nifV, and nifH genes in FeMo-co biosynthesis have been described (Table 1) (for recent reviews, see references 18 and 67). The nifY, nifH, nifW, nifZ genes, and the non-nif nafY gene (named for nitrogenase accessory factor Y) seem to be involved in MoFe protein maturation. In this minireview, we will define nif genes as nifA and the genes under the control of the transcriptional regulator NifA that are responsible for the production of a functional Mo nitrogenase. On the other hand, the nafY gene is involved in the formation of a functional Mo nitrogenase, but its expression is not coregulated with nif expression. Other nif genes whose roles have been defined are nifM, which seems to be required for the maturation of the Fe protein (35), and nifU and nifS genes, which are proposed to have a general role in the biosynthesis of [Fe-S] clusters for the nitrogenase components (80). No nif genes whose function is solely the synthesis of the P-clusters have been described yet.
TABLE 1.
nif gene products and their role (known or proposed) in nitrogen fixation
| Gene> | Identity and/or role of gene product |
|---|---|
| nifH | Fe protein. Obligate electron donor to MoFe protein during nitrogenase turnover. NifH is also required for FeMo-co biosynthesis and apo-MoFe protein maturation. |
| nifD | α subunit of MoFe protein. Forms an α2β2 tetramer with the β subunit. The site of substrate reduction, FeMo-co, is within the α subunit of MoFe protein. |
| nifK | β subunit of MoFe protein. P-clusters are present at each αβ subunit-interface. |
| nifT | Unknown. |
| nifY/nafY | Chaperone for the apo-MoFe protein. NafY is also a FeMo-co carrier and is proposed to aid in the insertion of FeMo-co into apo-MoFe protein. |
| nifE | Forms α2β2 tetramer with NifN. Required for FeMo-co synthesis. Proposed to function as a scaffold on which FeMo-co is synthesized. |
| nifN | Required for FeMo-co synthesis. Tetramer with NifE. |
| nifX | Involved in FeMo-co synthesis. Accumulates an FeSMo-containing precursor. |
| nifU | Molecular scaffold for the formation of Fe-S cluster for nitrogenase components. |
| nifS | Involved in mobilization of S for Fe-S cluster synthesis and repair. |
| nifV | Homocitrate synthase, involved in FeMo-co synthesis. |
| nifW | Involved in stability of MoFe protein. |
| nifZ | Unknown. |
| nifM | Required for the maturation of NifH. |
| nifF | Flavodoxin. Physiological electron donor to NifH in K. pneumoniae. |
| nifL | Negative regulatory element. |
| nifA | Positive regulatory element. |
| nifB | Required for FeMo-co synthesis. Its metabolic product, NifB-co, is a specific Fe and S donor to FeMo-co. |
| fdxN | Ferredoxin. In R. capsulatus, it serves as electron donor to nitrogenase. |
| nifQ | Involved in FeMo-co synthesis. Proposed to function in early MoO42− processing. |
| nifJ | Pyruvate: flavodoxin (ferredoxin) oxidoreductase. Electron donor to Fe protein in K. pneumoniae. |
A number of vnf genes (named for vanadium nitrogen fixation) have been implicated in the biosynthesis of FeV-co (vnfN, vnfE, vnfX, and vnfH) and in the maturation of the VFe protein (vnfG and vnfY) in A. vinelandii (Table 2) (56, 70). Very little is known about the role of anf-specific genes (named for alternative nitrogen fixation) in the biosynthesis of FeFe-co. It is interesting that no anfN or anfE genes have yet been found, suggesting that NifNE may also serve as a scaffold for the biosynthesis of FeFe-co. The roles of the vnf and anf genes from the alternative nitrogenase systems are supposed to be similar to those of their nif counterparts. For example, NifNE and VnfNE are believed to serve as scaffolds for the biosynthesis of FeMo-co and FeV-co, respectively. In addition to the vnf and anf genes, five nif genes (nifU, nifS, nifB, nifM, and nifV) are also required for the biosyntheses of FeV-co and FeFe-co of the alternative nitrogenases (5, 40, 41, 56).
TABLE 2.
vnf gene products and their role (known or proposed) in nitrogen fixation
| Gene> | Identity and/or role of gene product |
|---|---|
| vnfA | Positive regulatory element. |
| vnfE | Proposed to function in FeV-co biosynthesis. Analogous to NifE. |
| vnfN | Proposed to function in FeV-co biosynthesis. Analogous to NifN. |
| vnfX | Involved in FeV-co biosynthesis. Accumulates an FeSV precursor to FeV-co. |
| vnfH | vnf-Fe protein. Obligate electron donor to VFe protein, also involved in FeV-co biosynthesis. |
| vnfFd | Ferredoxin-like protein. Putative electron donor to VnfH. |
| vnfD | α subunit of VFe protein. |
| vnfG | δ subunit of mature VFe protein. Possibly involved in the insertion of FeV-co into apo-VFe protein. |
| vnfK | β subunit of VFe protein. |
| vnfY | Involved in FeY-co biosynthesis or insertion |
OVERVIEW OF NITROGENASE STRUCTURE
A brief description of the structural features of the MoFe and Fe proteins will be provided, because it will help the reader understand the nature of the protein environment around the metal clusters and the protein conformational changes needed to accommodate the FeMo-cofactor during the maturation of the nitrogenase components. It will become evident that large conformational changes occur within the MoFe protein along its maturation.
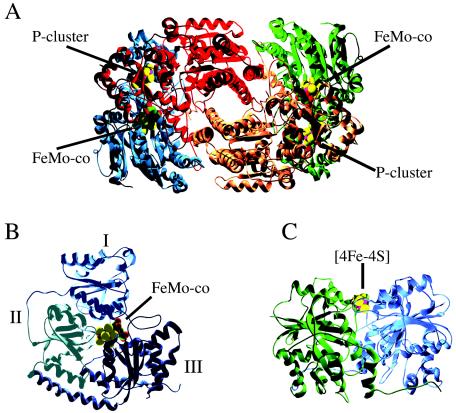
The molecular structures of the MoFe proteins from A. vinelandii (21, 43, 44), K. pneumoniae (52), and Clostridium pasteurianum (7, 45) have been solved and in all cases shown to exist as α2β2 tetramers composed of two pairs of αβ dimers related by a twofold symmetry (Fig. 2). Each polypeptide chain in the tetramer contains three parallel β-sheet/α-helix folding domains designated I, II, and III and I′, II′, and III′ for the α and β subunits, respectively. The tetramer interface is mainly stabilized by the interactions of the α-helices from domains II′ and III′. The FeMo-cofactors are located 10 Å beneath the protein surface at the interface of the three domains of the α subunits in a primarily hydrophilic environment. The protein ligands to FeMo-co are the thiol group of a cysteine, which coordinates to the distal Fe atom, and the side chain nitrogen atom of a histidine residue, which binds to the Mo atom at the other end of the cofactor (these are residues α-Cys275 (Cys275 in the α subunit) and α-His442 in the A. vinelandii MoFe protein). The Mo atom is also coordinated to the C-2 carboxyl and the hydroxyl groups of R-homocitrate. Homocitrate is surrounded by a pool of about 10 water molecules that participate in hydrogen-bonding interactions. Each P-cluster is located 10 Å beneath the protein surface in a hydrophobic protein environment formed at the interface of the domains I and I′ from the α and β subunits, respectively. In the reduced state, the P-cluster is coordinated by three cysteine residues from the α subunit and three cysteine residues from the β subunit (residues α-Cys62, α-Cys88, α-Cys154, β-Cys70, β-Cys95, and β-Cys153 in the A. vinelandii MoFe protein). The structure of the P-clusters and the coordination by protein ligands change significantly depending on the oxidation state of the MoFe protein.
FIG. 2.
Nitrogenase component proteins. (A) α2β2 tetrameric A. vinelandii nitrogenase MoFe protein. α subunits are blue and green, and β subunits are red and orange. (B) Detail of one of the α subunits of the MoFe protein showing the positions of domains I (blue), II (light blue), and III (dark blue). (C) A. vinelandii nitrogenase Fe protein (subunits in blue and green). Atoms are colored as follows:: iron, magenta; molybdenum, grey; sulfur, yellow; carbon, green; and oxygen, red.
The Fe protein of A. vinelandii is a dimer with twofold symmetry in which each subunit folds into an αβ domain formed by a core of eight β-sheets that is surrounded by nine α-helices (28) (Fig. 2). The fold of the Fe protein is similar to that of other nucleotide-binding proteins, such as Ras and RecA. The Fe protein binds two MgATP molecules at the dimer interface and undergoes conformational changes upon nucleotide hydrolysis. The [4Fe-4S] cluster of the Fe protein is located near the protein surface and is coordinated by two cysteine residues from each subunit (residues α-Cys97, α-Cys132, β-Cys97, and β-Cys132 in the A. vinelandii Fe protein).
MoFe PROTEIN MATURATION
Different forms of the apo-MoFe protein.
As noted above, mutant strains impaired in FeMo-co biosynthesis accumulate the MoFe protein as an apoprotein. apo-MoFe protein has been generally referred to as the form of the enzyme that lacks FeMo-co but contains the P-clusters. As we will show below, this is true only for certain forms of apo-MoFe proteins. Therefore, we will use apo-MoFe protein for any form of the protein that is not fully mature and catalytically active. To date, five different types of apo-MoFe proteins in A. vinelandii strains with different genetic backgrounds have been reported: (i) the form found in strains in which nifH was deleted (referred to here as ΔnifH apo-MoFe protein) (22, 26, 62, 75, 78); (ii) the form found in an A. vinelandii strain carrying an E146D variant of the Fe protein (61); (iii) the form present in nifB, nifN, and nifE mutant strains (all these forms are believed to be identical and are referred to here as ΔnifB apo-MoFe protein) (32, 54); (iv) the form found in a nifB nafY double mutant strain (referred to here as ΔnifBnafY apo-MoFe protein) (68); and (v) the His-tagged variant generated on a nifB genetic background (referred to here as ΔnifB His-apo-MoFe protein) (15). The discovery of such a variety of apo-MoFe proteins was initially puzzling, because it was not known whether the basis for that variety was simply genetic or due to experimental handling (apo-MoFe proteins are very unstable, oxygen-labile proteins that may be damaged when subjected to a long purification process). That biochemical puzzle is slowly being solved by the efforts of several laboratories. The properties of the different forms of apo-MoFe proteins will be discussed separately in the following sections, and the information obtained from a comparative analysis will be used to develop a systematic model of apo-MoFe protein maturation.
Model for apo-MoFe protein maturation.
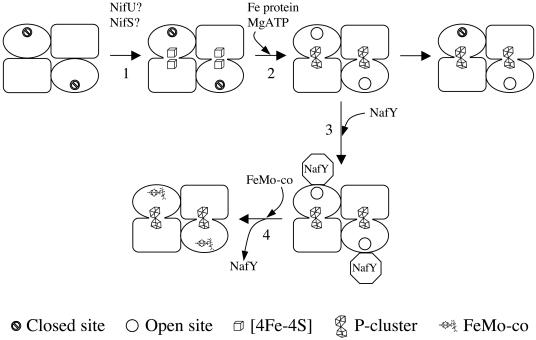
Available evidence supports a model in which the maturation of apo-MoFe protein occurs in several steps (Fig. 3). First, an α2β2 NifDK polypeptide tetramer must be synthesized and then loaded with some form of [Fe-S] cluster. Although this has not been definitely proved, on the basis of the work of Frazzon and Dean, it is presumed that NifU and NifS are responsible for the synthesis and transfer of the [Fe-S] clusters to the apo-MoFe protein (23, 24) (see “Maturation of the Fe Protein” section below for details on the roles of NifU and NifS). Recent evidence points to the existence of two pairs of adjacent but separate [4Fe-4S] clusters, one pair at each αβ dimer interface (16). This form has an inaccessible FeMo-co site. Second, each pair of [4Fe-4S] clusters is maturated to generate a functional P-cluster in a reaction that requires the presence of Fe protein and MgATP. The Fe protein-dependent maturation results in a conformational change of the apo-MoFe protein that makes the FeMo-co sites accessible and promotes the binding of a third subunit. The third subunit, named γ in the A. vinelandii apo-MoFe protein, has been shown to be the product of the nafY gene. (In K. pneumoniae, the third subunit is the product of the nifY gene.) Third, NafY (NifY) binds to the apo-MoFe protein and stabilizes the otherwise labile FeMo-co insertion site. Fourth, FeMo-co is inserted, and the NafY(NifY) subunit disassociates from the mature MoFe protein. Since NafY is also capable of binding FeMo-co, it has been proposed that it may also function as a FeMo-co insertase. To further complicate the scheme, there is increasing evidence indicating that the maturation of each αβ dimer within the apo-MoFe protein tetramer is not completely independent from the other. In the following paragraphs, we will analyze every step of the maturation of the MoFe protein.
FIG. 3.
Model for the maturation of the nitrogenase MoFe protein. The α (NifD), β (NifK), and γ (NafY) subunits of the apo-MoFe protein are represented as oval, rectangle, and octagon cartoons, respectively. Step 1 (indicated by the arrow labeled 1) is the initial loading of the α2β2 tetramer with two pairs of [4Fe-4S] clusters. Note that the FeMo-co sites within the α subunits are not accessible. Step 2 is the formation of the P-clusters and “opening” of the FeMo-co-binding sites. Step 3 is the attachment of NafY and stabilization of the open conformation. The arrow to the right of step 2 indicates that in the absence of NafY and FeMo-co, a significant percentage of the open sites is lost (50% in A. vinelandii and 95% in K. pneumoniae apo-MoFe proteins). Step 4 is the FeMo-co insertion, dissociation of NafY, and generation of mature MoFe protein.
The Fe protein is required for the maturation of the P-clusters.
Soon after the discovery by Ugalde and coworkers that strains with impaired FeMo-co biosynthesis accumulated a FeMo-co-deficient form of the MoFe protein (77), several research groups observed that the ΔnifH apo-MoFe protein had different biochemical properties than the ΔnifB, ΔnifN, or ΔnifE apo-MoFe proteins. In cell extracts, the ΔnifH apo-MoFe protein is very heat unstable, it migrates differently on anoxic native gels during electrophoresis (75, 78), and its activation by FeMo-co requires a preincubation with the Fe protein and MgATP (1, 65). Purified ΔnifH apo-MoFe protein was shown to be an α2β2 tetramer lacking FeMo-co but having enough Fe to account for the P-clusters, although these clusters showed unusual spectroscopic features (26, 62). It was also shown that the purified ΔnifH apo-MoFe protein was able to interact with the Fe protein and promote MgATP hydrolysis but was unable to accept electrons from the Fe protein and therefore was catalytically inactive (62). More importantly, once purified, the ΔnifH apo-MoFe protein is not competent for FeMo-co activation even when purified Fe protein and MgATP are also included in the reaction mixture.
It is now known that the Fe protein is involved in the maturation of the P-clusters of the apo-MoFe protein to their catalytically active forms. The precise mechanism for the maturation of the P-clusters is yet to be elucidated, but it is known that the features of the Fe protein required to be active in apo-MoFe protein maturation are different from those required in electron transfer during turnover or in FeMo-co biosynthesis. For example, the Fe protein must be able to bind MgATP and to complex with the MoFe protein; however, MgATP hydrolysis, electron transfer, and even the presence of the [4Fe-4S] cluster of the Fe protein are not required for the protein to be competent in apo-MoFe protein maturation (48, 57, 58, 63). These results rule out an obligate redox role for the Fe protein during apo-MoFe protein maturation. It has been shown that VnfH (the Fe protein counterpart of the V-containing nitrogenase) is as effective as the Fe protein in the maturation of the apo-MoFe protein in vitro (11). The Fe proteins from the Mo- and V-containing nitrogenases exhibit 91% amino acid sequence identity, and the domain(s) involved in maturation of the apo-MoFe protein is likely to be highly conserved in both.
After synthesis and folding of the NifD and NifK polypeptides, the α2β2 MoFe protein is able to coordinate a pair of [4Fe-4S] clusters at each of the two αβ subunit interfaces (illustrated by step 1 in Fig. 3). Each pair of [4Fe-4S] clusters is a precursor to a P-cluster, and their coordination involves cysteinyl and noncysteinyl ligands from both subunits (16). By a mechanism different from electron transfer, the Fe protein promotes the reassembly and condensation of the two [4Fe-4S] clusters to form one P-cluster at each αβ subunit interface (step 2 in Fig. 3). It has been suggested that the presence of P-clusters could be a prerequisite for FeMo-co insertion (78). Some MoFe protein variants with substitutions at the cysteine residues that are the ligands to the P-cluster consistently lack P-clusters and FeMo-co (42). In any event, the consequence of the Fe protein-dependent maturation is a conformational change in the apo-MoFe protein that makes the FeMo-co site accessible and promotes the binding of NafY (1, 49). Although the three-dimensional molecular structure of the ΔnifH MoFe protein is not available, thiol reactivity experiments suggest that maturation by the Fe protein causes a rearrangement of the region around residue α-Cys275 of the MoFe protein and leaves its thiol group exposed to solvent (49). It is important to stress here that the thiolate of α-Cys275 is the ligand to the distal Fe atom of FeMo-co in mature MoFe protein.
Recent work suggests a more complicated dual role of the Fe protein in the maturation of the apo-MoFe protein. Evidence supporting this proposal is found in the analysis of an A. vinelandii strain that carries an E146D Fe protein variant (61). The E146D Fe protein is competent for electron transfer to nitrogenase, biosynthesis of FeMo-co, and maturation of apo-MoFe protein P-clusters. This Fe protein variant is, however, partially unable to assist during FeMo-co insertion in vivo, and the apo-MoFe protein from such a strain lacks 50% of its FeMo-co complement. Further, compared to wild-type Fe protein, the E146D variant supports the activation of ΔnifH apo-MoFe protein by FeMo-co only to 50% of the maximal theoretical value. This can be explained if the Fe protein is required not only for the maturation of the P-clusters but additionally at a later step of apo-MoFe protein maturation that would be specifically impaired in the E146D variant.
ΔnifB apo-MoFe protein.
The ΔnifB apo-MoFe protein reflects the last step in the maturation of the MoFe protein; it lacks only FeMo-co, and therefore, it is fully activated by the addition of the FeMo-cofactor with no other requirements. As mentioned above, it was early recognized that the apo-MoFe proteins that accumulated in cell extracts of A. vinelandii or K. pneumoniae nifB, nifN, and nifE mutant strains were similar to each other but different from the apo-MoFe protein that accumulated in extracts of nifH mutant strains. The most readily apparent differences between the ΔnifB and ΔnifH apo-MoFe proteins were the different mobilities when they were subjected to electrophoresis on anoxic native gels and the ability of the first protein to be fully activated by the simple addition of isolated FeMo-co in contrast to the requirement of the ΔnifH apo-MoFe for Fe protein and MgATP (54, 75). When purified, the ΔnifB apo-MoFe protein from A. vinelandii was shown to be a hexameric protein containing a stably bound extra subunit that was designated γ (54). The γ subunit was later identified as the product of the non-nif gene nafY (68). Likewise, the ΔnifB apo-MoFe protein from K. pneumoniae contains a third type of subunit that is the product of the nifY gene (34). In both cases, the third subunit dissociates from the MoFe protein after FeMo-co insertion takes place and mature MoFe protein accumulates as a α2β2 tetramer.
A His-tagged version of the A. vinelandii ΔnifB apo-MoFe protein has been purified and shown to contain intact P-clusters, to be able to interact with the Fe protein and support ATP hydrolysis, and to be capable of accepting electrons from the Fe protein (15). Curiously, this ΔnifB apo-MoFe protein variant lacks the NafY subunit and exhibits an α2β2 subunit composition. The lack of NafY may be due to a technical detail of the purification of the His-tagged protein, because we have found that imidazole is effective in separating NafY from the apo-MoFe protein (L. M. Rubio and P. W. Ludden, unpublished results). Imidazole is used in the isolation of the His-tagged apo-MoFe protein and may dissociate NafY from the protein complex. Yet, as is the case of the standard ΔnifB apo-MoFe, the His-tagged ΔnifB apo-MoFe protein is fully activated by the simple addition of FeMo-co. This demonstrates that the presence of NafY is not essential to insert FeMo-co into the MoFe protein when excess purified FeMo-co is provided.
The crystal structure of the His-tagged ΔnifB apo-MoFe protein has been solved and compared to the structure of mature MoFe protein (72). The structural comparison highlights three important differences that may have mechanistic relevance during FeMo-co insertion (18, 72). The first important difference is that the apo-MoFe protein exhibits a positively charged funnel that is absent in the mature MoFe protein and is large enough to allow for the entry of the negatively charged FeMo-co. At the bottom of this funnel is the α-His442 residue that would coordinate the Mo atom of FeMo-co in mature MoFe protein. Second, while most of the protein conformation remains unchanged before and after FeMo-co insertion, there is a conformational change that involves a rearrangement of domain III of the α subunit and the closure of the funnel. The residue α-His442 is within domain III, and its position shifts 5Å during such rearrangement. Third, although the α-Cys275 residue is part of domain II of the α subunit and its relative position remains unaltered in the structures of the apo- and holo-MoFe proteins, the displacement of domain III in the apo-MoFe protein causes α-Cys275 to be very exposed to solvent. These domain rearrangements are consistent with the observed changes in reactivity of α-Cys275 towards the alkylating agent iodoacetamide at different stages of apo-MoFe protein maturation (49). The thiol group of α-Cys275 is nonreactive in the ΔnifH apo-MoFe protein, very reactive in the ΔnifB apo-MoFe protein after the maturation induced by the Fe protein takes place, and nonreactive again in the mature MoFe protein after FeMo-co insertion.
Thus, a mechanistically oriented comparison of the ΔnifH and ΔnifB apo-MoFe proteins reveals that the most important differences between these two stages of maturation are (i) the presence of mature P-clusters in the ΔnifB apo-MoFe protein, (ii) a structural rearrangement that leaves residue α-Cys275 exposed to solvent and ready for FeMo-co insertion, and (iii) the attachment of NafY, probably because the FeMo-co insertion site is labile and needs to be stabilized.
Stabilization of the ΔnifB apo-MoFe protein by NafY (NifY).
In A. vinelandii and K. pneumoniae, the third subunits of the hexameric ΔnifB apo-MoFe proteins are the products of the nafY and nifY genes, respectively. In fact, NifY and NafY exhibit amino acid sequence similarity to each other and are part of a family of small proteins, which we propose to name the NifX family of proteins, and whose common role seems to be the carriage of FeMo-co, FeV-co, and their biosynthetic precursors for cofactor assembly and insertion (Table 3) (20, 33, 55, 69-71). In A. vinelandii, some of the proteins from the NifX family exhibit a certain degree of cross-functionality in vivo (68). In addition to nafY, A. vinelandii carries a nifY gene, but the role of its protein product remains unknown (37, 68). Interestingly, A. vinelandii nafY or nifY mutants are capable of fixing nitrogen.
TABLE 3.
Cluster-binding capabilities of members of the NifX family of proteins
| Protein | Ability of protein to bind to:
|
||
|---|---|---|---|
| NifB-coa | FeMo-co | FeMo(V)-co precursorb | |
| NafY | Yes | Yes | ?c |
| NifY | ? | ? | ? |
| NifX | Yes | Yes | Yes |
| VnfX | Yes | Yes | Yes |
| VnfY | ? | ? | ? |
NifB-co is an inorganic cluster of unknown structure that serves as a biosynthetic precursor to FeMo-co, FeV-co, and FeFe-co of the Mo- and V-containing and Fe-only-nitrogenases, respectively. NifB-co contains Fe and S, but it does not contains either heterometal (Mo or V) or homocitrate.
Biosynthetic precursors of FeMo-co or FeV-co containing Fe, S, and Mo (or V) but lacking homocitrate.
?, unknown.
Since NafY was shown to be able to bind independently to FeMo-co or to the apo-MoFe protein, a model in which NafY had a dual role as a chaperone (to stabilize the apo-MoFe protein) and as a FeMo-co insertase was proposed (33). Several lines of evidence support the hypothesis that the presence of the third subunit stabilizes a conformation of apo-MoFe protein that is competent for FeMo-co insertion. First, while the apo-MoFe protein present in an extract of a nifB mutant of K. pneumoniae can be activated in vitro by the addition of FeMo-co, the apo-MoFe protein of a nifY nifB double mutant cannot. Because a single mutation in nifY does not have a significant effect in MoFe protein activity when placed in a wild-type background, this result is interpreted as the inability of the nifB nifY strain to maintain an activatable apo-MoFe protein (34). Second, nafY mutants of A. vinelandii exhibit very low MoFe protein activity compared to the wild type when grown under stressing conditions of high temperature and Mo limitation, in which the rate of FeMo-co biosynthesis is predicted to be lower and the newly synthesized MoFe protein would largely accumulate as an apoprotein. Further, the apo-MoFe-protein accumulated in extracts of nafY mutants grown under stress conditions cannot be activated in vitro by adding extra FeMo-co (68). Third, even when grown under standard nonstressing conditions, only 50% of the apo-MoFe protein that accumulated in extracts of the nifB nafY strain can be activated by FeMo-co (68). Whatever the damage to the ΔnifBnafY apo-MoFe protein might be, the addition of NafY to the in vitro reconstitution mixture does not reverse it, implying that the presence of NafY is not required at the time of FeMo-co insertion. It is not clear whether one of the two sites in each apo-MoFe protein tetramer is not activatable in the absence of NafY or if a distribution of competent and noncompetent apo-MoFe protein molecules is present in the extract of a ΔnifBnafY strain. Another interesting feature is that the physical presence of the Fe protein and MgATP are not required at the time of FeMo-co insertion (1), but it is not known whether they are required at the time of association between NafY and apo-MoFe protein.
The role of NafY as a FeMo-co insertase is less clear. Because NafY binds FeMo-co and apo-MoFe protein, it seems reasonable to think that NafY is also serving as a FeMo-co insertase. However, the ability to partially reconstitute the apo-MoFe protein from nafY mutant strains in vivo and in vitro indicates that the presence of NafY is not an absolute requirement when FeMo-co is abundant. Moreover, as noted above, a NafY-deficient His-tagged ΔnifB apo-MoFe protein can be fully activated by FeMo-co without any other requirement (15).
Involvement of the general chaperone GroEL.
The first reports that the general chaperone GroEL was involved in the cellular accumulation of the nitrogenase component proteins appeared in the early 1990s when Govezensky and coworkers found that GroEL had transient associations with newly synthesized NifH, NifD, and NifK polypeptides (29, 30). It was also established that GroEL is required for the correct folding of NifA (the nif-specific transcriptional activator). Later on, Ribbe and Burgess found that GroEL was required for the full activation by FeMo-co of the apo-MoFe protein present in cell extracts of an A. vinelandii strain expressing the E146D Fe protein variant, which points to a specific role during FeMo-co insertion (60). Their results can be interpreted in two different ways. (i) GroEL is directly involved in the proper folding of the ΔnifH apo-MoFe protein, which allows FeMo-co insertion to its completion. (ii) GroEL has an indirect role through the maturation of some other protein that in turn would be required for FeMo-co insertion. It was mentioned above that while the ΔnifH apo-MoFe protein present in cell extracts of A. vinelandii can be reconstituted by a preincubation with Fe protein and MgATP prior to FeMo-co addition, this is not the case for purified ΔnifH apo-MoFe protein, which is practically nonactivatable. The researchers reported that the addition of purified GroEL, Fe protein, and MgATP to a reconstitution reaction mixture was not sufficient to support FeMo-co insertion into the purified ΔnifH apo-MoFe protein, suggesting that additional factors required for its maturation were still missing in those experiments.
Is there a role for NifW and NifZ in apo-MoFe protein maturation?
Though neither the nifW nor the nifZ gene is strictly required for nitrogen fixation, there is some evidence for the involvement of their protein products in the maturation or stability of the MoFe protein. Cultures of A. vinelandii or K. pneumoniae strains carrying nifW and nifZ mutations had lower levels of MoFe protein activity but normal levels of Fe protein activity compared to the wild-type strain (38, 46, 53). The exact role of NifW is not known. The MoFe protein purified from a nifW mutant strain of A. vinelandii has slightly different substrate reactivity properties that are consistent with an alteration at the homocitrate site of FeMo-co (46). Other studies in R. capsulatus also suggest that NifW has a role in homocitrate transport or processing (50).
There are three lines of evidence suggesting that NifW and NifZ may act in concert in vivo. (i) nifW and nifZ mutant strains show similar decreases in MoFe protein activity compared to that of the wild type. (ii) There is an apparent translational coupling of the nifW and nifZ genes in the chromosome of A. vinelandii (38). (iii) It has been shown that NifW and NifZ are capable of interacting with each other by the use of the yeast two-hybrid assay (47).
MATURATION OF THE VFe PROTEIN
The most striking difference between the mature MoFe and VFe proteins is the presence in the latter of VnfG (also called δ subunit). This subunit is unique to alternative nitrogenases (a VnfG homolog, AnfG, is also found as a structural component of the Fe-only nitrogenase). The VnfG subunit has been shown to be loosely associated with apo-VFe protein in extracts of a strain incapable of synthesizing FeV-co. When subjected to electrophoresis, the three subunits comigrate on anoxic native gels, but the VnfG subunit separates from the complex by gel filtration chromatography (12). The α2β2δ2 apo-VFe protein (lacking FeV-co but containing the P-clusters) present in these cell extracts is competent to activation by partially purified FeV-co. The α2β2 complex lacking VnfG, however, is activated by FeV-co only if VnfG is also added to the reaction mixture. Similar to the specific association of FeMo-co with NafY, the association of FeV-co with VnfG has been observed. However, unlike NafY, which does not stay attached to the mature MoFe protein, VnfG stays bound to the active VFe protein. Therefore, while the subunit composition of the mature MoFe protein is α2β2, that of VFe protein is α2β2δ2. Although it is possible that NafY and VnfG perform similar functions, they do not exhibit amino acid sequence similarity or immunological cross-reactivity. A difference between FeV-co insertion into apo-VFe protein and FeMo-co insertion into apo-MoFe protein is the absence of a VnfH requirement for FeV-co insertion compared to the NifH requirement for FeMo-co insertion.
MATURATION OF THE Fe PROTEIN
NifM and NifH are the only nif-specific gene products required for the expression of a catalytically active Fe protein (35, 53). The indication that NifM plays a role in the maturation of the Fe protein comes from the analysis of K. pneumoniae and A. vinelandii nifM mutants, which exhibit dramatically lower Fe protein activity but accumulate substantial amounts of the Fe protein polypeptide (38, 63). Although NifM is required for proper maturation of the Fe protein, nifM strains accumulate active MoFe protein, indicating that FeMo-co biosynthesis and apo-MoFe protein maturation proceeds normally (53). Thus, it seems that the nonmature Fe protein is still functional in FeMo-co synthesis and apo-MoFe protein maturation. The exact role of NifM in the maturation of the Fe protein is still uncertain. The C-terminal domain of NifM has sequence similarity to peptidyl prolyl cis-trans isomerases (PPIases), and on this basis, it has been suggested that the role of NifM would be the isomerization of a Pro residue of the Fe protein (27).
As noted above, the mature Fe protein contains a single [4Fe-4S] cluster bridging its two subunits. Regarding the synthesis of this cluster and on the basis of the analysis of the phenotypes of A. vinelandii nifU and nifS mutant strains, it was early proposed that the nifU and nifS gene products were involved in the mobilization of Fe and S for the nitrogenase component proteins (37). The purification of nifS and nifU gene products confirmed the original hypothesis, demonstrating that NifS is a cysteine desulfurase able to provide S for the assembly of [Fe-S] clusters (81, 82), whereas NifU acts as a molecular scaffold in which [Fe-S] clusters are assembled in order to be transferred to target proteins (25, 80). Indeed, very recently, the transfer of a [4Fe-4S] cluster from NifU to the Fe protein has been demonstrated in an in vitro system containing only purified components (19).
CONCLUDING REMARKS
When synthesized, the nitrogenase components are not immediately competent for nitrogen fixation. Rather, they become mature by the actions of several nif and non-nif gene products to achieve catalytic competency. In this minireview, we have tried to summarize what is currently known about the maturation of the nitrogenase component proteins from the polypeptide folding to the formation of the protein forms that are catalytically active. Due to its enormous complexity, the study of the maturation of the apo-MoFe protein is especially challenging to biochemists. Many pieces of this puzzle have been already placed. We know that the Fe protein is required for the synthesis of functional P-clusters of the apo-MoFe protein and that the reaction directed by the Fe protein promotes a conformational change within the apo-MoFe protein that leaves the FeMo-co insertion site accessible. We know that apo-MoFe protein that has been matured by the Fe protein is activatable by FeMo-co with no other requirements, but it is labile unless stabilized by a third subunit that acts as a chaperone (NafY or NifY). The insertion of FeMo-co causes another conformational change that promotes the dissociation of the third subunit and ends up with the cofactor buried well inside the mature MoFe protein. In addition, there is a piece of evidence involving the general chaperone GroEL in the final insertion of FeMo-co into the apo-MoFe protein. On the other hand, the maturation of the Fe protein requires the activity of NifM (suggested to consist of a cis- to trans-isomerization of a Pro residue within the Fe protein) and the acquisition of a [4Fe-4S] cluster that would be directly donated by NifU.
At this point in time, several important questions remain to be solved and are likely to be the subject of future intense research. For example, more X-ray crystallography studies are needed to elucidate the structural differences between the ΔnifH apo-MoFe protein and the mature MoFe protein or to determine the NafY-binding site in the hexameric ΔnifB apo-MoFe protein. A biochemical approach would be needed to finally establish whether NafY is a FeMo-co insertase. Purified NafY, FeMo-co, and NafY-deficient apo-MoFe protein are now available and will facilitate the analysis of FeMo-co insertion into the MoFe protein in vitro. Likewise, it has not yet been demonstrated that NifU and NifS are involved in the formation of the [4Fe-4S] clusters of the ΔnifH apo-MoFe protein. Molecular biology tools, such as the generation of MoFe protein variants able to coordinate FeMo-co in only one αβ half of the tetramer, should be used to determine whether there is a different maturation for the two halves of the MoFe protein tetramer or whether the maturation of one half affects the maturation of the other. Finally, the role of NifM in the maturation of the Fe protein needs to be conclusively established.
Acknowledgments
We thank Enrique Flores and Gary Roberts for critically reading the manuscript.
This work was supported in part by the National Institute of General Medical Sciences, National Institutes of Health grant 35332 (to P.W.L.).
REFERENCES
- 1.Allen, R. M., M. J. Homer, R. Chatterjee, P. W. Ludden, G. P. Roberts, and V. K. Shah. 1993. Dinitrogenase reductase- and MgATP-dependent maturation of apodinitrogenase from Azotobacter vinelandii. J. Biol. Chem. 268:23670-23674. [PubMed] [Google Scholar]
- 2.Arnold, W., A. Rump, W. Klipp, U. B. Priefer, and A. Puhler. 1988. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J. Mol. Biol. 203:715-738. [DOI] [PubMed] [Google Scholar]
- 3.Beinert, H., R. H. Holm, and E. Munck. 1997. Iron-sulfur clusters: nature's modular, multipurpose structures. Science 277:653-659. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, P. E., D. M. L. Jarlenski, and D. R. Hetherington. 1980. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc. Natl. Acad. Sci. USA 77:7342-7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop, P. E., and R. D. Joerger. 1990. Genetics and molecular biology of alternative nitrogen fixation systems. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41:109-125. [Google Scholar]
- 6.Bishop, P. E., R. Premakumar, D. R. Dean, M. R. Jacobson, J. R. Chisnell, T. M. Rizzo, and J. Kopczynski. 1986. Nitrogen fixation by Azotobacter vinelandii strains having deletions in structural genes for nitrogenase. Science 232:92-94. [DOI] [PubMed] [Google Scholar]
- 7.Bolin, J. T., A. E. Ronco, T. V. Morgan, L. E. Mortenson, and N.-H. Xuong. 1993. The unusual metal clusters of nitrogenase: structural features revealed by X-ray anomalous diffraction studies of the MoFe protein from Clostridium pasteurianum. Proc. Natl. Acad. Sci. USA 90:1078-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulen, W. A., and J. R. LeComte. 1966. The nitrogenase system from Azotobacter: two enzyme requirements for N2 reduction, ATP dependent H2 evolution and ATP hydrolysis. Proc. Natl. Acad. Sci. USA 56:979-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess, B. K., and D. J. Lowe. 1996. Mechanism of molybdenum nitrogenase. Chem. Rev. 96:2983-3011. [DOI] [PubMed] [Google Scholar]
- 10.Chan, M. K., J. Kim, and D. C. Rees. 1993. The nitrogenase FeMo-cofactor and P-cluster pair: 2.2 Å resolution structures. Science 260:792-794. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee, R., R. M. Allen, P. W. Ludden, and V. K. Shah. 1997. In vitro synthesis of the iron-molybdenum cofactor and maturation of the nif-encoded apodinitrogenase. Effect of substitution of VNFH for NIFH. J. Biol. Chem. 272:21604-21608. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee, R., P. W. Ludden, and V. K. Shah. 1997. Characterization of VNFG, the delta subunit of the vnf-encoded apodinitrogenase from Azotobacter vinelandii. Implications for its role in the formation of functional dinitrogenase 2. J. Biol. Chem. 272:3758-3765. [DOI] [PubMed] [Google Scholar]
- 13.Chisnell, J. R., R. Premakumar, and P. E. Bishop. 1988. Purification of a second alternative nitrogenase from a nifHDK deletion strain of Azotobacter vinelandii. J. Bacteriol. 170:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christiansen, J., D. R. Dean, and L. C. Seefeldt. 2001. Mechanistic features of the Mo-containing nitrogenase. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52:269-295. [DOI] [PubMed] [Google Scholar]
- 15.Christiansen, J., P. J. Goodwin, W. N. Lanzilotta, L. C. Seefeldt, and D. R. Dean. 1998. Catalytic and biophysical properties of a nitrogenase Apo-MoFe protein produced by a nifB-deletion mutant of Azotobacter vinelandii. Biochemistry 37:12611-12623. [DOI] [PubMed] [Google Scholar]
- 16.Corbett, M. C., Y. Hu, F. Naderi, M. W. Ribbe, B. Hedman, and K. O. Hodgson. 2004. Comparison of iron-molybdenum cofactor deficient nitrogenase MoFe proteins by X-ray absorption spectroscopy: implications for P-cluster biosynthesis. J. Biol. Chem. 279:28276-28282. [DOI] [PubMed] [Google Scholar]
- 17.Davis, R., L. Lehman, R. Petrovich, V. K. Shah, G. P. Roberts, and P. W. Ludden. 1996. Purification and characterization of the alternative nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J. Bacteriol. 178:1445-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dos Santos, P. C., D. R. Dean, Y. Hu, and M. W. Ribbe. 2004. Formation and insertion of the nitrogenase iron-molybdenum cofactor. Chem. Rev. 104:1159-1173. [DOI] [PubMed] [Google Scholar]
- 19.Dos Santos, P. C., A. D. Schmid, J. Frazzon, V. L. Cash, M. K. Johnson, and D. R. Dean. 2004. Iron-sulfur cluster assembly. NifU-directed activation of the nitrogenase Fe protein. J. Biol. Chem. 279:19705-19711. [DOI] [PubMed] [Google Scholar]
- 20.Dyer, D. H., L. M. Rubio, J. B. Thoden, H. M. Holden, P. W. Ludden, and I. Rayment. 2003. The three-dimensional structure of the core domain of NafY from Azotobacter vinelandii determined at 1.8-A resolution. J. Biol. Chem. 278:32150-32156. [DOI] [PubMed] [Google Scholar]
- 21.Einsle, O., F. A. Tezcan, S. L. Andrade, B. Schmid, M. Yoshida, J. B. Howard, and D. C. Rees. 2002. Nitrogenase MoFe-protein at 1.16 A resolution: a central ligand in the FeMo-cofactor. Science 297:1696-1700. [DOI] [PubMed] [Google Scholar]
- 22.Filler, W. A., R. M. Kemp, J. C. Ng, T. R. Hawkes, R. A. Dixon, and B. E. Smith. 1986. The nifH gene product is required for the synthesis or stability of the iron-molybdenum cofactor of nitrogenase from Klebsiella pneumoniae. Eur. J. Biochem. 160:371-377. [DOI] [PubMed] [Google Scholar]
- 23.Frazzon, J., and D. R. Dean. 2003. Formation of iron-sulfur clusters in bacteria: an emerging field in bioinorganic chemistry. Curr. Opin. Chem. Biol. 7:166-173. [DOI] [PubMed] [Google Scholar]
- 24.Frazzon, J., J. R. Fick, and D. R. Dean. 2002. Biosynthesis of iron-sulphur clusters is a complex and highly conserved process. Biochem. Soc. Trans. 30:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Fu, W., R. F. Jack, T. V. Morgan, D. R. Dean, and M. K. Johnson. 1994. nifU gene product from Azotobacter vinelandii is a homodimer that contains two identical [2Fe-2S] clusters. Biochemistry 33:13455-13463. [DOI] [PubMed] [Google Scholar]
- 26.Gavini, N., L. Ma, G. Watt, and B. K. Burgess. 1994. Purification and characterization of a FeMo cofactor-deficient MoFe protein. Biochemistry 33:11842-11849. [DOI] [PubMed] [Google Scholar]
- 27.Gavini, N., and L. Pulakat. 2001. Presented at the 101st General Meeting of the American Society for Microbiology, Orlando, Fla.
- 28.Georgiadis, M. M., H. Komiya, P. Chakrabarti, D. Woo, J. J. Kornuc, and D. C. Rees. 1992. Crystallographic structure of the nitrogenase iron protein from Azotobacter vinelandii. Science 257:1653-1659. [DOI] [PubMed] [Google Scholar]
- 29.Govezensky, D., E. S. Bochkareva, A. Zamir, and A. S. Girshovich. 1994. Chaperonins as potential gene regulatory factors. In vitro interaction and solubilization of NifA, the nif transcriptional activator, with GroEL. J. Biol. Chem. 269:14003-14006. [PubMed] [Google Scholar]
- 30.Govezensky, D., T. Greener, G. Segal, and A. Zamir. 1991. Involvement of GroEL in nif gene regulation and nitrogenase assembly. J. Bacteriol. 173:6339-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hageman, R. V., and R. H. Burris. 1978. Nitrogenase and nitrogenase reductase associate and dissociate with each catalytic cycle. Proc. Natl. Acad. Sci. USA 75:2699-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawkes, T. R., and B. E. Smith. 1983. Purification and characterization of the inactive MoFe protein (NifB-Kpl) of the nitrogenase from nifB mutants of Klebsiella pneumoniae. Biochem. J. 209:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homer, M. J., D. R. Dean, and G. P. Roberts. 1995. Characterization of the gamma protein and its involvement in the metallocluster assembly and maturation of dinitrogenase from Azotobacter vinelandii. J. Biol. Chem. 270:24745-24752. [DOI] [PubMed] [Google Scholar]
- 34.Homer, M. J., T. D. Paustian, V. K. Shah, and G. P. Roberts. 1993. The nifY product of Klebsiella pneumoniae is associated with apodinitrogenase and dissociates upon activation with the iron-molybdenum cofactor. J. Bacteriol. 175:4907-4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard, K. S., P. A. McLean, F. B. Hansen, P. V. Lemley, K. S. Koblan, and W. H. Orme-Johnson. 1986. The Klebsiella pneumoniae nifM gene product is required for stabilization and activation of nitrogenase iron protein in Escherichia coli. J. Biol. Chem. 261:772-778. [PubMed] [Google Scholar]
- 36.Imperial, J., V. K. Shah, R. A. Ugalde, P. W. Ludden, and W. J. Brill. 1987. Iron-molybdenum cofactor synthesis in Azotobacter vinelandii Nif− mutants. J. Bacteriol. 169:1784-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobson, M. R., K. E. Brigle, L. T. Bennett, R. A. Setterquist, M. S. Wilson, V. L. Cash, J. Beynon, W. E. Newton, and D. R. Dean. 1989. Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J. Bacteriol. 171:1017-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobson, M. R., V. L. Cash, M. C. Weiss, N. F. Laird, W. E. Newton, and D. R. Dean. 1989. Biochemical and genetic analysis of the nifUSVWZM cluster from Azotobacter vinelandii. Mol. Gen. Genet. 219:49-57. [DOI] [PubMed] [Google Scholar]
- 39.Joerger, R. D., T. M. Loveless, R. N. Pau, L. A. Mitchenall, B. H. Simon, and P. E. Bishop. 1990. Nucleotide sequences and mutational analysis of the structural genes for nitrogenase 2 of Azotobacter vinelandii. J. Bacteriol. 172:3400-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy, C., and D. Dean. 1992. The nifU, nifS and nifV gene products are required for activity of all three nitrogenases of Azotobacter vinelandii. Mol. Gen. Genet. 231:494-498. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy, C., R. Gamal, R. Humphrey, J. Ramos, K. Brigle, and D. Dean. 1986. The nifH, nifM and nifN genes of Azotobacter vinelandii. Characterization by Tn5 mutagenesis and isolation from pLAFR1 gene banks. Mol. Gen. Genet. 205:318-325. [Google Scholar]
- 42.Kent, H. M., I. Ioannidis, C. Gormal, B. E. Smith, and M. Buck. 1989. Site-directed mutagenesis of the Klebsiella pneumoniae nitrogenase. Effects of modifying conserved cysteine residues in the α and β-subunits. Biochem. J. 264:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim, J., and D. C. Rees. 1992. Crystallographic structure and functional implications of the nitrogenase molybdenum-iron protein from Azotobacter vinelandii. Nature (London) 360:553-560. [DOI] [PubMed] [Google Scholar]
- 44.Kim, J., and D. C. Rees. 1992. Structural models for the metal centers in the nitrogenase molybdenum-iron protein. Science 257:1677-1682. [DOI] [PubMed] [Google Scholar]
- 45.Kim, J., D. Woo, and D. C. Rees. 1993. X-ray crystal structure of the nitrogenase molybdenum-iron protein from Clostridium pasteurianum at 3.0-A resolution. Biochemistry 32:7104-7115. [DOI] [PubMed] [Google Scholar]
- 46.Kim, S., and B. K. Burgess. 1994. Purification and characterization of nitrogenase from a ΔnifW strain of Azotobacter vinelandii. J. Biol. Chem. 269:4215-4220. [PubMed] [Google Scholar]
- 47.Lee, S. H., L. Pulakat, K. C. Parker, and N. Gavini. 1998. Genetic analysis on the NifW by utilizing the yeast two-hybrid system revealed that the NifW of Azotobacter vinelandii interacts with the NifZ to form higher-order complexes. Biochem. Biophys. Res. Commun. 244:498-504. [DOI] [PubMed] [Google Scholar]
- 48.Ludden, P. W., P. Rangaraj, and L. M. Rubio. 2004. Biosynthesis of the iron-molybdenum and iron-vanadium cofactors of the nif- and vnf-encoded nitrogenases, p. 219-253. In B. E. Smith, R. L. Richards, and W. E. Newton (ed.), Catalysts for nitrogen fixation: nitrogenases, relevant chemical models and commercial processes. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 49.Magnuson, J. K., T. D. Paustian, V. K. Shah, D. R. Dean, G. P. Roberts, D. C. Rees, and J. B. Howard. 1997. Nitrogenase iron-molybdenum cofactor binding site: protein conformational changes associated with cofactor binding. Tetrahedron 53:11971-11984. [Google Scholar]
- 50.Masepohl, B., S. Angermuller, S. Hennecke, P. Hubner, C. Moreno-Vivián, and W. Klipp. 1993. Nucleotide sequence and genetic analysis of the Rhodobacter capsulatus ORF6-nifUISVW gene region: possible role of NifW in homocitrate processing. Mol. Gen. Genet. 238:369-382. [DOI] [PubMed] [Google Scholar]
- 51.Masepohl, B., K. Schneider, T. Drepper, A. Muller, and W. Klipp. 2002. Alternative nitrogenases, p. 191-222. In G. J. Leigh (ed.), Nitrogen fixation at the millenium. Elsevier, Amsterdam, The Netherlands.
- 52.Mayer, S. M., D. M. Lawson, C. A. Gormal, S. M. Roe, and B. E. Smith. 1999. New insights into structure-function relationships in nitrogenase: a 1.6 A resolution X-ray crystallographic study of Klebsiella pneumoniae MoFe-protein. J. Mol. Biol. 292:871-891. [DOI] [PubMed] [Google Scholar]
- 53.Paul, W., and M. Merrick. 1989. The roles of the nifW, nifZ and nifM genes of Klebsiella pneumoniae in nitrogenase biosynthesis. Eur. J. Biochem. 178:675-682. [DOI] [PubMed] [Google Scholar]
- 54.Paustian, T. D., V. K. Shah, and G. P. Roberts. 1990. Apodinitrogenase: purification, association with a 20-kilodalton protein, and activation by the iron-molybdenum cofactor in the absence of dinitrogenase reductase. Biochemistry 29:3515-3522. [DOI] [PubMed] [Google Scholar]
- 55.Rangaraj, P., C. Rüttimann-Johnson, V. K. Shah, and P. W. Ludden. 2001. Accumulation of 55Fe-labeled precursors of the iron-molybdenum cofactor of nitrogenase on NifH and NifX of Azotobacter vinelandii. J. Biol. Chem. 276:15968-15974. [DOI] [PubMed] [Google Scholar]
- 56.Rangaraj, P., C. Rüttimann-Johnson, V. K. Shah, and P. W. Ludden. 2000. Biosynthesis of the iron-molybdenum and iron-vanadium cofactors of the nif- and vnf-encoded nitrogenases, p. 55-79. In E. W. Triplett (ed.), Prokaryotic nitrogen fixation: a model system for analysis of a biochemical process. Horizon Scientific Press, Wymondham, United Kingdom.
- 57.Rangaraj, P., M. J. Ryle, W. N. Lanzilotta, P. W. Ludden, and V. K. Shah. 1999. In vitro biosynthesis of iron-molybdenum cofactor and maturation of the nif-encoded apodinitrogenase. Effect of substitution for NifH with site-specifically altered forms of NifH. J. Biol. Chem. 274:19778-19784. [DOI] [PubMed] [Google Scholar]
- 58.Rangaraj, P., V. K. Shah, and P. W. Ludden. 1997. ApoNifH functions in iron-molybdenum cofactor synthesis and apodinitrogenase maturation. Proc. Natl. Acad. Sci. USA 94:11250-11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ribbe, M., D. Gadkari, and O. Meyer. 1997. N2 fixation by Streptomyces thermoautotrophicus involves a molybdenum-dinitrogenase and a manganese-superoxide oxidoreductase that couple N2 reduction to the oxidation of superoxide produced from O2 by a molybdenum-CO dehydrogenase. J. Biol. Chem. 272:26627-26633. [DOI] [PubMed] [Google Scholar]
- 60.Ribbe, M. W., and B. K. Burgess. 2001. The chaperone GroEL is required for the final assembly of the molybdenum-iron protein of nitrogenase. Proc. Natl. Acad. Sci. USA 98:5521-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ribbe, M. W., E. H. Bursey, and B. K. Burgess. 2000. Identification of an Fe protein residue (Glu146) of Azotobacter vinelandii nitrogenase that is specifically involved in FeMo cofactor insertion. J. Biol. Chem. 275:17631-17638. [DOI] [PubMed] [Google Scholar]
- 62.Ribbe, M. W., Y. Hu, M. Guo, B. Schmid, and B. K. Burgess. 2002. The FeMoco-deficient MoFe protein produced by a nifH deletion strain of Azotobacter vinelandii shows unusual P-cluster features. J. Biol. Chem. 277:23469-23476. [DOI] [PubMed] [Google Scholar]
- 63.Roberts, G. P., T. MacNeil, D. MacNeil, and W. J. Brill. 1978. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J. Bacteriol. 136:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson, A. C., B. K. Burgess, and D. R. Dean. 1986. Activity, reconstitution, and accumulation of nitrogenase components in Azotobacter vinelandii mutant strains containing defined deletions within the nitrogenase structural gene cluster. J. Bacteriol. 166:180-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson, A. C., T. W. Chun, J. G. Li, and B. K. Burgess. 1989. Iron-molybdenum cofactor insertion into the Apo-MoFe protein of nitrogenase involves the iron protein-MgATP complex. J. Biol. Chem. 264:10088-10095. [PubMed] [Google Scholar]
- 66.Robson, R. L., R. R. Eady, T. H. Richardson, R. W. Miller, M. Hawkins, and J. R. Postgate. 1986. The alternative nitrogenase of Azotobacter chroococcum is a vanadium enzyme. Nature 322:388-390. [Google Scholar]
- 67.Rubio, L. M., and P. W. Ludden. 2002. The gene products of the nif regulon, p. 101-136. In G. J. Leigh (ed.), Nitrogen fixation at the millenium. Elsevier, Amsterdam, The Netherlands.
- 68.Rubio, L. M., P. Rangaraj, M. J. Homer, G. P. Roberts, and P. W. Ludden. 2002. Cloning and mutational analysis of the gamma gene from Azotobacter vinelandii defines a new family of proteins capable of metallocluster binding and protein stabilization. J. Biol. Chem. 277:14299-14305. [DOI] [PubMed] [Google Scholar]
- 69.Rubio, L. M., S. W. Singer, and P. W. Ludden. 2004. Purification and characterization of NafY (apodinitrogenase gamma subunit) from Azotobacter vinelandii. J. Biol. Chem. 279:19739-19746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruttimann-Johnson, C., L. M. Rubio, D. R. Dean, and P. W. Ludden. 2003. VnfY is required for full activity of the vanadium-containing dinitrogenase in Azotobacter vinelandii. J. Bacteriol. 185:2383-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rüttimann-Johnson, C., C. R. Staples, P. Rangaraj, V. K. Shah, and P. W. Ludden. 1999. A vanadium and iron cluster accumulates on VnfX during iron-vanadium-cofactor synthesis for the vanadium nitrogenase in Azotobacter vinelandii. J. Biol. Chem. 274:18087-18092. [DOI] [PubMed] [Google Scholar]
- 72.Schmid, B., M. W. Ribbe, O. Einsle, M. Yoshida, L. M. Thomas, D. R. Dean, D. C. Rees, and B. K. Burgess. 2002. Structure of a cofactor-deficient nitrogenase MoFe protein. Science 296:352-356. [DOI] [PubMed] [Google Scholar]
- 73.Schneider, K., A. Muller, U. Schramm, and W. Klipp. 1991. Demonstration of a molybdenum- and vanadium-independent nitrogenase in a nifHDK-deletion mutant of Rhodobacter capsulatus. Eur. J. Biochem. 195:653-661. [DOI] [PubMed] [Google Scholar]
- 74.Shah, V. K., and W. J. Brill. 1973. Nitrogenase. IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim. Biophys. Acta 305:445-454. [DOI] [PubMed] [Google Scholar]
- 75.Tal, S., T. W. Chun, N. Gavini, and B. K. Burgess. 1991. The ΔnifB (or ΔnifE) FeMo cofactor-deficient MoFe protein is different from the ΔnifH protein. J. Biol. Chem. 266:10654-10657. [PubMed] [Google Scholar]
- 76.Thiel, T. 1993. Characterization of genes for an alternative nitrogenase in the cyanobacterium Anabaena variabilis. J. Bacteriol. 175:6276-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ugalde, R. A., J. Imperial, V. K. Shah, and W. J. Brill. 1984. Biosynthesis of iron-molybdenum cofactor in the absence of nitrogenase. J. Bacteriol. 159:888-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.White, T. C., G. S. Harris, and W. H. Orme-Johnson. 1992. Electrophoretic studies on the assembly of the nitrogenase molybdenum-iron protein from the Klebsiella pneumoniae nifD and nifK gene products. J. Biol. Chem. 267:24007-24016. [PubMed] [Google Scholar]
- 79.Young, J. P. W. 1992. Phylogenetic classification of nitrogen-fixing organisms, p. 43-86. In G. Stacey, R. H. Burris, and H. J. Evans (ed.), Biological nitrogen fixation. Chapman and Hall, New York, N.Y.
- 80.Yuvaniyama, P., J. N. Agar, V. L. Cash, M. K. Johnson, and D. R. Dean. 2000. NifS-directed assembly of a transient [2Fe-2S] cluster within the NifU protein. Proc. Natl. Acad. Sci. USA 97:599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng, L., and D. R. Dean. 1994. Catalytic formation of a nitrogenase iron-sulfur cluster. J. Biol. Chem. 269:18723-18726. [PubMed] [Google Scholar]
- 82.Zheng, L., R. H. White, V. L. Cash, R. F. Jack, and D. R. Dean. 1993. Cysteine desulfurase activity indicates a role for NIFS in metallocluster biosynthesis. Proc. Natl. Acad. Sci. USA 90:2754-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]