Graphical abstract
Keywords: Ceratonia siliqua, Volatiles, SPME, Chemometrics, Roasting, GC-MS, Carob
Abstract
Ceratonia siliqua is a legume tree of considerable commercial importance for the flavor and sweets industry cultivated mostly for its pods nutritive value and or several health benefits. Despite extensive studies on C. siliqua pod non-volatile metabolites, much less is known regarding volatiles composition which contributes to the flavor of its many food products. To gain insight into C. siliqua aroma, 31 volatile constituents from unroasted and roasted pods were profiled using headspace solid-phase micro extraction (HD-SPME) analyzed via quadruple mass spectrometer followed by multivariate data analyses. Short chain fatty acids amounted for the major volatile class at ca. (71–77%) with caproic acid (20%) and pentanoic acid (15–25%) as major components. Compared to ripe pod, roasted ripe pod was found less enriched in major volatile classes i.e., short chain fatty acids and aldehydes, except for higher pyranone levels. Volatiles mediating for unheated and hot carob fruit aroma is likely to be related to its (E)-cinnamaldehyde and pyranone content, respectively. Such knowledge is expected to be the key for understanding the olfactory and taste properties of C. siliqua and its various commercial food products.
Introduction
Ceratonia Siliqua (Carob) is a legume tree of a well-known commercial and medicinal importance owing to its fruit (pod) enrichment in carbohydrates, dietary fibers, tannins, and phenolics. In the Mediterranean region, carob pod is consumed as animal or human food [1]. In terms of its health benefits, C. siliqua exhibits a myriad of biological effects including antibacterial, antidiarrheal, antidiabetic, anti-hypercholestrolemic, and hepatoprotective [2], [3], [4], [5]. Additionally, Carob pods, roasted and unroasted are widely used in manufacturing of sugar syrups, molasses, and beverage [6] or as a cocoa substitute in candy products and cakes [7]. Roasting of carob pod along with sugar is thought to enhance or intensify the aroma. Since the flavor and the aroma are important aspects in the carob products, our goal was to profile its volatiles, which has scarcely been reported in the literature [8]. Steam distillation of carob fruit essential oil analyzed using GC-MS revealed for its enrichments in fatty acid and fatty acyl esters amounting for 77% of its volatile composition [8], [9]. Other volatile classes found in C. siliqua prepared using hydro-distillation include aromatics, hydrocarbons and terpenoids [9], [10].
Headspace solid phase micro-extraction (SPME) is a relatively novel technique used for volatiles extraction found superior to steam distillation, being solvent free and involving no heat application [11]. Additionally, SPME enables the enrichment of volatiles from gas or liquid samples, over a fused-silica fiber then subsequent desorption of these analytes leads to detection of less abundant volatiles [12]. One powerful feature of SPME volatiles sampling lies in preserving the true aroma without development of artifacts that might be generated with heating as in the case of steam distillation [13]. SPME has been previously applied for volatiles profiling in carob flowers revealing for its enrichment in mono- and sesquiterpenes [10]. Nevertheless, the technology has yet to be further employed for volatiles profiling in the more economical used part “pod”.
Continuing our studies on Mediterranean foods flavor makeup [14], [15], a report is presented herein on volatiles analysis from C. siliqua using SPME. The main aim of this work was to explore carob aroma using a cold SPME method for volatiles extraction and to further determine the impact of processing i.e., roasting on volatile composition. To reveal for roasting effect in an untargeted manner, multivariate data analysis was applied. This study provides the most complete map for volatiles distribution in C. siliqua pod using SPME and its roasted product.
Experimental
Plant material, SPME, and chemicals
Ceratonia siliqua trees were grown in the semi-arid “Siwa” Oasis, Egypt and pods were collected in the full ripe stage during the month of May 2016. A voucher specimen code “6-4-2017” was kept in the Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Egypt. Roasting was accomplished by heating pods in an oven set at 120 °C for 30 min. Three to 4 biological replicates were analyzed for each sample. The fruits were stored at −20 °C till further analysis. SPME holder and fiber coated with 50 μm/30 μm Divinyl benzene/Carboxen/Polydimethylsiloxane (DVB–CAR–PDMS) was supplied by Supelco (Oakville, ON, Canada). All volatile standards i.e., (E)-cinnamaldehyde, α-farnesene, hexanoic and benzoic acids used in the analyses were purchased from Sigma Aldrich (St. Louis, Mo., U.S.A.).
SPME volatiles isolation
The headspace volatiles analysis using SPME was explained in details as in Ref. [15], [16] with few modifications. Briefly, a carob pod was dried and grounded yielding 100 mg. The grounded pod was placed inside 1.5 mL clear glass vials. (Z)-3-hexenyl acetate used as an internal standard (IS) being absent from the sample, dissolved in water and added to each vial at a concentration of 1 µg/vial. The vials were then immediately capped and placed on a temperature controlled tray for 30 min at 50 °C with the SPME fiber inserted into the headspace above the fruit sample. Adsorption time was 30 min. A system blank containing no fruit material was run as a control.
GC-MS volatile analysis
Three to four biological replicates for each specimen were extracted and analyzed in parallel under identical conditions to assess for biological variance SPME fibers were desorbed at 210 °C for 1 min in the injection port of a Shimadzu Model GC-17A gas chromatograph interfaced with a Shimadzu model QP-5000 mass spectrometer (Tokyo, Japan). Volatiles were separated on a DB5-MS column (30 m length, 0.25 mm inner diameter, and 0.25 μm film (J&W Scientific, Santa Clara, CA, USA). Injections were made in the splitless mode for 60 s. The gas chromatograph was operated under the following conditions: injector 220 °C, column oven 38 °C for 3 min, then programmed at a rate of 12 °C min−1 to 180 °C, kept at 180 °C for 5 min, and finally ramped at a rate of 40 °C min−1 to 220 °C and kept for 2 min, He carrier gas at 1 mL min−1. The transfer line and ion–source temperatures were adjusted at 230 and 180 °C, respectively. The HP quadrupole mass spectrometer was operated in the electron ionization mode at 70 eV. The scan range was set at m/z 40–500. Volatile components were identified using the procedure fully described as in Ref. [16] and peaks were first deconvoluted using AMDIS software (www.amdis.net) and identified by its retention indices (RI) relative to n-alkanes (C6-C20), mass spectrum matching to NIST, WILEY library database with matching score above 800 and with authentic standards when available.
Multivariate data analyses
Principal component analysis (PCA) and partial least squares-discriminant analysis (OPLS-DA) were performed with the program SIMCA-P Version 13.0 (Umetrics, Umeå, Sweden). Markers were subsequently identified by analyzing the S-plot, which was declared with covariance (p) and correlation (pcor). All variables were mean centered and scaled to Pareto variance. The PCA was run for obtaining a general overview of the variance of metabolites, and OPLS-DA was performed to identify markers for distinguishing roasted and unroasted pods.
Statistical analysis
Paired t-test analysis was performed using Microsoft Excel 2013 (Microsoft Office, VA, USA) for the analysis of volatiles data. Data are represented as mean ± standard deviation SD. P value ≤ 0.05 was considered statistically significant.
Results and discussion
Volatiles analysis
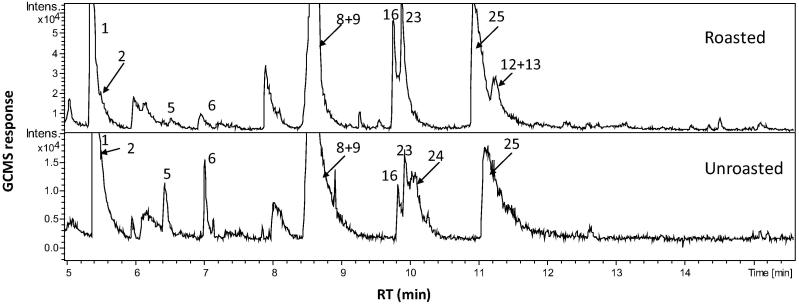
The objective of this study was to assess Carob roasted pod aroma and to compare it with the unroasted pod using SPME. GC-MS analysis of C. siliqua samples led to the identification of 31 different volatile constituents, presented in Table 1. Detected volatiles amounted for 93% of the total volatile composition. GC chromatogram (Fig. 1) displays representative volatile profile of the roasted and unroasted pod. The qualitative volatiles composition of unroasted and roasted pods was relatively comparable, and suggesting for rather quantitative differences. Generally, C. siliqua volatile profiles were dominated by 7 different volatile groups viz. aliphatic acids, esters, furans/pyrans, aldehydes/ketones, alcohols, sesquiterpenoids and aliphatic hydrocarbons, with acids as the major class amounting for ca. 71–77% of pods volatile blend. A total of 31 volatiles were identified compared to 160 previously reported using steam distillation from carob fruit. Discrepancy in results are likely as heating might have produced several volatile artifacts [8]. Indeed, many of the identified volatiles are not commonly generated in planta including xylenes, pyrazines and halogenated compounds which warrant more for the development of artifact less prone method of volatiles analysis in carob fruit.
Table 1.
Relative percentage of volatile compounds (100%) in C. siliqua pods analyzed using SPME-GC-MS (n = 4). Significant differences between roasted and unroasted fruit specimens is presented with P value less than 0.05 calculated using paired t-test.
| Molecular | Roasted | Unroasted | |||||
|---|---|---|---|---|---|---|---|
| Peak | rt (min) | KI | Name | Formula | Average ± SD | Average ± SD | P-value |
| 1 | 5.832 | 844 | Unknown acid | 2.74 ± 0.65 | 5.36 ± 1.19 | 0.03⁎ | |
| 2 | 5.916 | 849 | Pyruvic acid | C3H4O3 | 4.49 ± 0.54 | 12.52 ± 1.28 | 0.0005⁎ |
| 3 | 6.146 | 857 | Isobutyric acid | C4H8O2 | 5.38 ± 3.43 | 5.49 ± 3.83 | – |
| 4 | 6.318 | 869 | Butyric acid | C4H8O2 | 0.49 ± 0.25 | 0.24 ± 0.01 | – |
| 5 | 7.053 | 904 | Unknown fatty acid | 0.46 ± 0.29 | 0.24 ± 0.14 | – | |
| 6 | 8.858 | 1008 | Pentanoic acid | C5H10O2 | 15.57 ± 11.19 | 24.90 ± 1.13 | – |
| 7 | 9.3 | 1037 | Hexanoic acida | C6H12O2 | 20.49 ± 1.55 | 20.44 ± 3.97 | – |
| 8 | 10.17 | 1096 | Heptanoic acid | C7H14O2 | 0.98 ± 0.29 | 0.39 ± 0.13 | 0.03⁎ |
| 9 | 11.313 | 1172 | Octanoic acid | C8H16O2 | 3.19 ± 1.68 | 4.18 ± 0.71 | – |
| 10 | 11.417 | 1175 | Benzoic acida | C7H6O2 | 0.35 ± 0.35 | 0.23 ± 0.15 | – |
| 11 | 11.431 | 1180 | Unknown fatty acid | 17.15 ± 12.94 | 3.04 ± 1.52 | – | |
| Total acids (%) | 71.29 | 77.03 | |||||
| 12 | 11.433 | 1182 | Myrcenol | 0.38 ± 0.34 | 0.05 ± 0.02 | – | |
| Total alcohol (%) | 0.38 | 0.05 | |||||
| 13 | 12.542 | 1263 | (E)-cinnamaldehydea | C9H8O | 0.49 ± 0.51 | 7.93 ± 3.01 | – |
| 14 | 9.242 | 1033 | Benzeneacetaldehyde | C8H18O | 0.65 ± 0.84 | 0.10 ± 0.04 | – |
| 15 | 9.883 | 1075 | Pineapple ketone | C6H8O3 | 0.28 ± 0.26 | 0.05 ± 0.01 | – |
| Total aldehyde/ketone (%) | 1.43 | 8.08 | |||||
| 16 | 21.257 | 1819 | Octadecanea | C18H38 | 0.37 ± 0.64 | 0.04 ± 0.04 | – |
| 17 | 22.249 | 1880 | Unknown hydrocarbon | 0.98 ± 1.69 | 0.06 ± 0.05 | – | |
| Total hydrocarbons (%) | 1.35 | 0.10 | |||||
| 18 | 5.518 | 829 | Glycolic acid, acetate | C7H15O4 | 3.47 ± 1.11 | 10.02 ± 3.80 | 0.045⁎ |
| 19 | 7.075 | 905 | Methyl butyrate | C5H10O2 | 0.49 ± 0.37 | 1.54 ± 1.28 | – |
| 20 | 10.02 | 1093 | Oxalic acid, diallyl ester | C8H10O4 | 11.40 ± 1.09 | 1.30 ± 0.32 | 0.0001⁎ |
| Total esters (%) | 15.36 | 12.86 | |||||
| 21 | 5.45 | 825 | Furfural | C5H4O2 | 0.34 ± 0.29 | 0.08 ± 0.01 | – |
| 22 | 7.914 | 953 | Furfural, 5-methyl- | C6H6O3 | 0.28 ± 0.30 | 0.07 ± 0.04 | – |
| 23 | 9.752 | 1066 | 5,6-Dihydro-2-pyranone | C5H6O2 | 0.53 ± 0.34 | 0.69 ± 0.39 | |
| 24 | 9.848 | 1072 | 2-Acetylpyrrole | C6H7NO | 1.57 ± 1.88 | 0.32 ± 0.13 | |
| 25 | 10.983 | 1141 | Pyranone | C5H4O2 | 3.65 ± 0.63 | 0.06 ± 0.02 | 0.03⁎ |
| Total furan/pyran (%) | 6.38 | 1.21 | |||||
| 26 | 13.7 | 1355 | α-Cubebene | C15H24 | 0.47 ± 0.44 | 0.15 ± 0.14 | – |
| 27 | 14.307 | 1414 | β-(E)-Farnesene | C15H24 | 0.56 ± 0.72 | 0.17 ± 0.19 | – |
| 28 | 15.047 | 1465 | α-Farnesenea | C15H24 | 0.81 ± 0.92 | 0.10 ± 0.06 | – |
| 29 | 15.14 | 1473 | Unknown sesquiterpene | 0.58 ± 0.71 | 0.10 ± 0.01 | – | |
| 30 | 15.163 | 1475 | α-(Z,E)-Farnesene | C15H24 | 1.48 ± 1.25 | 0.09 ± 0.02 | – |
| 31 | 15.376 | 1492 | Unknown sesquiterpene | 0.57 ± 0.38 | 0.06 ± 0.02 | 0.08⁎ | |
| Total sesquiterpenes (%) | 4.48 | 0.67 | |||||
Compounds were identified by comparison of kovat index (KI) and mass spectral data with those of authentic compounds and by comparison of mass spectral data with those of NIST library.
P < 0.05.
Represents volatiles confirmed by running authentic standard.
Fig. 1.
Representative SPME-GC-MS chromatogram of roasted and unroasted C. siliqua pod. Assigned peaks number follow that listed in Table 1.
Volatile short chain fatty acids viz., pentanoic acid (15–25%) and hexanoic acid (caproic acid) at ca. 20% were the chief components in both roasted and unroasted pods. Several other less abundant acids were detected including pyruvic, isobutyric, butyric, heptanoic acid, octanoic and benzoic acids. Volatile low molecular weight esters comprised (13–15%) of the total identified volatiles, with glycolic acid acetate and oxalic acid diallyl ester the main volatiles found at 3 and 10%, 11 and 1% in roasted and unroasted pods, respectively. Such enrichment of fatty acid and acyl esters in C. siliqua volatiles profile might not essentially account for its pod sweet, date-like aroma and suggesting that other less abundant constituents with lower vapor pressure that might contribute for pods overall smell. Interestingly, our work on characterizing date fruit aroma revealed for the enrichment in (E)-cinnamaldehyde [12] also detected herein in C. siliqua at 8% which might mediate for the date like odor of carob pod. (E)-cinnamaldehyde is the aldehyde that gives cinnamon spice its flavor and odor [17]. This is the first report for (E)-cinnamaldehyde in carob fruit. With regards to aldehyde/ketone volatiles abundance, unroasted pod volatile blend was found more enriched in aldehydes (6.7%) vs. only (1.3%) in roasted one. Samples of roasted pod revealed a slightly higher level of benzeneacetaldehyde and pineapple ketone (1%), whereas unroasted pod possessed a much higher content of (E)-cinnamaldehyde (8%).
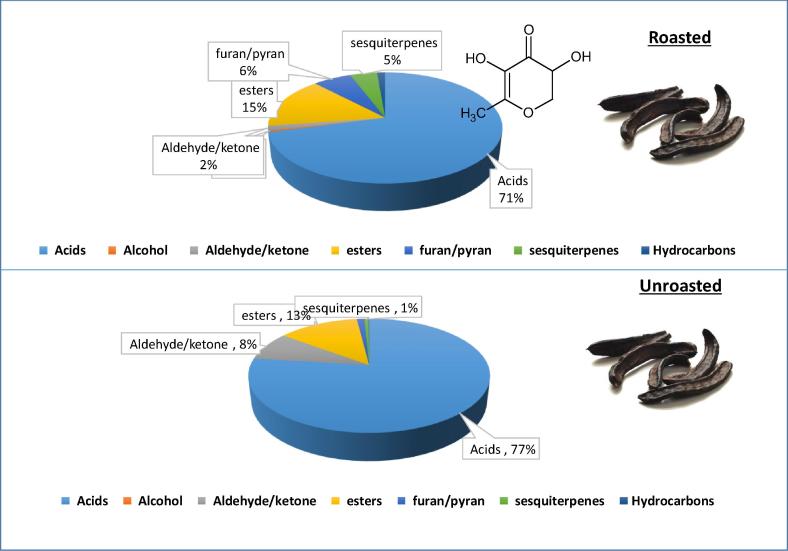
In contrast, furan/pyrans were notably more predominant in roasted pod (6.3%) versus unroasted (1.2%), with pyranone detected almost exclusively in roasted pod (3.7%) and found at trace levels in unroasted one (0.06%) suggesting that it can be used as marker to distinguish heat treated from cold carob powder. Pyranone is of considerable organoleptic characteristics as a Maillard-derived product in fermented malt syrup [18] that could explain among other furans the characteristic malt and sweet odor of heated carob food preparations. Sesquiterpene hydrocarbons percentile amounted for 4.5% in roasted pod versus ca. 1% in unroasted one with α-cubebene and α/β farnesene isomers as major components. The exclusive presence of terpenoid hydrocarbons suggests that in C. siliqua, oxygenated terpene biosynthesis is much less activated. Only, one monoterpenoid alcohol was detected in both roasted and unroasted fruit identified as “myrcenol” at levels ranging from 0.05 to 0.4%. In contrast to C. siliqua flower aroma predominated by mono- and sesquiterpenes [10], fruit aroma is found less enriched in terpenoids (Table 1). With regards to other less abundant volatile classes in C. siliqua, aliphatic hydrocarbons were detected at trace levels (0.1–1%) with octadecane and another unknown hydrocarbon (peak 18). “Siwa” oasis from where the fruit was harvested is an isolated oasis in western Egypt desert and hence has been less interbred with other trees and it is of interest to determine using SPME whether its aroma is distinct from Carob grown in Spain. In general, higher levels of volatiles were recorded in unroasted samples for most volatile classes compared to roasted which might not be reflected in (Table 1). A pie chart representing the major groups of volatile class percentile levels in roasted versus unroasted pods is represented in (Fig. 2) and showing the abundance of furans/pyrans in roasted pod (6%) versus enrichment of aldeydes/ketones in unroasted pod (8%). Acids, which amount for the major volatile class in both specimens was found at ca. 71% and 77% in roasted and unroasted pods, respectively. Considering that results presented herein shows a relative percentile volatile levels within each specimen and to reveal for impact of heat on C. siliqua aroma in an untargeted manner, multivariate data analyses were further employed on the volatile data (raw abundance levels of volatile compounds).
Fig. 2.
Pie distribution chart showing volatile class distribution in roasted and unroasted C. siliqua pods and with structure of pyranone found enriched in roasted pod aroma as determined via SPME GC/MS.
PCA and OPLS multivariate data analysis of C. siliqua volatiles
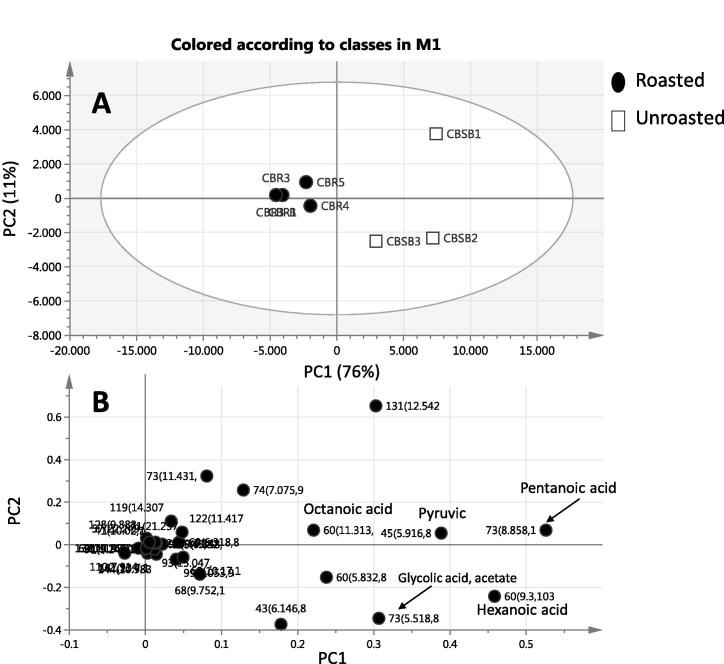
As a well-known highly consumed beverage, the impact of roasting on carob fruits volatiles was evaluated using both PCA and OPLS. Fruit roasting is routinely employed during carob beverage preparation in Egypt. PCA is an unsupervised clustering method requiring no knowledge of the dataset and acts to reduce the dimensionality of multivariate data [19]. The PCA score plot brought out that roasted and unroasted specimens could be differentiated to a good extent (Fig. 3A) along PC1 accounting for 76% of the total variance. The metabolite loading plot for PC1 (Fig. 3B), which clears the significant components with respect to scattering behavior, showed higher volatile levels in unroasted pod and with no detection of novel peaks in roasted specimen. Our results fall in agreement with previous report on roasting effect on C. siliqua analyzed using steam distillation and revealing a steep decrease in its volatiles [6]. Pentanoic and hexanoic acid (caproic acid) contributed the most positively along PC1, being more fortified in unroasted fruit. Next to pentanoic and hexanoic acids, MS signals for pyruvic acid, octanoic acid and glycolic acid-acetate (Table 1) contributed for segregation in PCA loading plots along PC1, albeit to less extent.
Fig. 3.
Score Plot of PC1 vs. PC2 scores. Principal component analyses of roasted (●) and unroasted (□) analyzed by SPME-GC-MS (n = 4). The metabolome clusters are located at the distinct positions in two-dimensional space described by two vectors of principal component 1 (PC1) = 76% and PC2 = 11%. (A) Score Plot of PC1 vs. PC2 scores. (B) Loading plot for PC1 and PC2 contributing mass peaks and their assignments, with each volatile denoted by its mass/rt (min) pair.
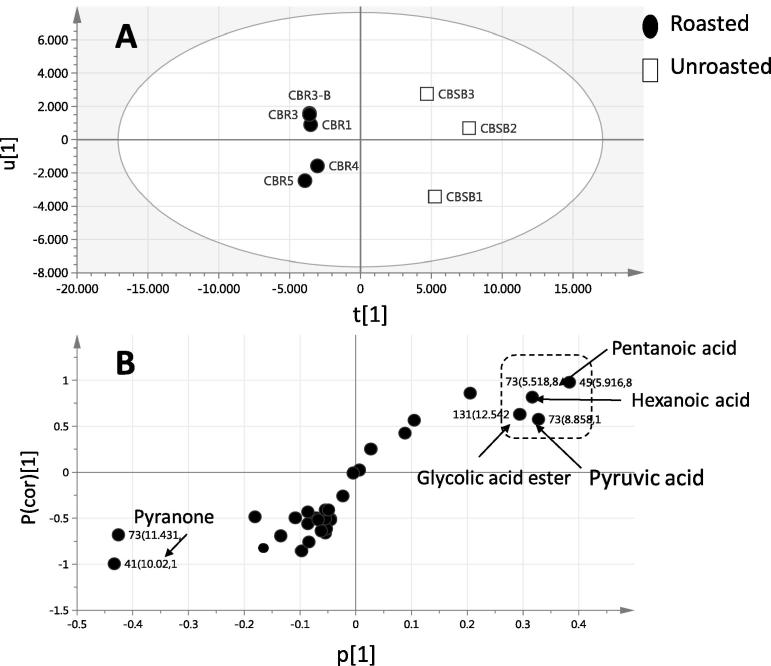
Supervised orthogonal projection to latent structures-discriminant analysis (OPLS-DA) was then employed to build a classification model for discriminating between roasted and unroasted pods; OPLS-DA also capable in the identification of markers by providing the most relevant variables for the discrimination between two sample groups. Roasted and unroasted fruit powder samples were modeled against each other using OPLS-DA with the derived score plot showing a clear segregation between both samples (Fig. 4A). The OPLS score plot described 90% of the total variance (R2 = 0.90) with the prediction goodness parameter Q2 = 0.88. An important tool that compares the variable magnitude against its reliability in OPLS charts is the S-plot and presented in (Fig. 4B), where axes plotted from the predictive component are the covariance p [1] against the correlation p(cor)[1]. For the indication of plots with retention time m/z values, a cut-off value of P < 0.05 was used. Upon comparing to roasted pod, unroasted one exhibited a richer aroma profile containing more short fatty acids, viz., pentanoic and hexanoic (caproic) acids which falls in agreement with PCA results (Fig. 2B). The enrichment of pyranone in roasted pod as revealed from S-loading plot (Fig. 3B) underlies a Maillard type degradation products which results from the interaction of the reduced sugar-amino acids upon roasting the fruits at elevated temperature, typical of the roasting process. The profiling of changes in Carob fruit non-volatile metabolites composition i.e. polyphenols in response to roasting has yet to be reported. The low volatiles level in roasted pod suggest that odor intensification of C. siliqua might be more incurred from heated sugar added to the fruit during beverage preparation yielding other flavored milliard type volatiles. In this study, no sugar was added during the roasting process of Carob fruit to help determine the impact of heat on the fruit itself aroma makeup.
Fig. 4.
(A) OPLS-DA score plot and (B) loading S-plots derived from modelling roasted (●) and unroasted pods (□) analyzed by SPME-GC-MS. The S-plot shows the covariance p [1] against the correlation p(cor) [1] of the variables of the discriminating component of the OPLS-DA model. Cut-off values of P < 0.01 were used; variables selected are highlighted in the S-plot with m/z retention time in minutes.
Conclusions
SPME used for the extraction of C. siliqua and aroma profile then further analyzed by GC-MS. A total of 31 volatile components were detected with fatty acids, esters and aldehydes counted as the major volatile classes in both roasted and unroasted Carob pod. In general, higher volatiles levels were detected in unroasted pod. The most evident difference was the higher levels of short chain fatty acids viz. caproic and pentanoic acid in unroasted compared versus high pyrans abundance i.e. pyranone in roasted pod. Roasting at elevated temperature could be critical on the aroma and flavor of the pods as a result of the accumulation of Maillard volatile products. Volatiles accounting for cold and hot carob fruit characteristic aroma is likely to be related to (E)-cinnamaldehyde and pyranone, respectively. Such knowledge could be critical in understanding the odor and taste properties of raw C. siliqua and its commercial food products or beverages. Our volatiles profiling approach accompanied with multivariate data analyses provided the true aroma profile in C. siliqua growing in Egypt, which can be further applied for investigating other factors such as geographical origin, ripening stage, and or analyzing its various commercial food products.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgments
Dr. Mohamed Ali Farag acknowledges the funding received by Alexander von Humboldt foundation, Germany.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Marakis S. Carob bean in food and feed: current status and future potentials – a critical appraisal. J Food Sci Tech MYS. 1996;33(5):365–383. [Google Scholar]
- 2.Al-Olayan E.M., El-Khadragy M.F., Alajmi R.A., Othman M.S., Bauomy A.A., Ibrahim S.R. Ceratonia siliqua pod extract ameliorates Schistosoma mansoni-induced liver fibrosis and oxidative stress. BMC Complem Altern Med. 2016;16(1):434. doi: 10.1186/s12906-016-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Rodriguez R., Navarro-Alarcon M., Rodriguez-Martinez C., Fonolla-Joya J. Effects on the lipid profile in humans of a polyphenol-rich carob (Ceratonia siliqua L.) extract in a dairy matrix like a functional food; a pilot study. Nutr Hosp. 2013;28(6):2107–2114. [PubMed] [Google Scholar]
- 4.Rached I., Barros L., Fernandes I.P., Santos-Buelga C., Rodrigues A.E., Ferchichi A. Ceratonia siliqua L. hydroethanolic extract obtained by ultrasonication: antioxidant activity, phenolic compounds profile and effects in yogurts functionalized with their free and microencapsulated forms. Food Funct. 2016;7(3):1319–1328. doi: 10.1039/c6fo00100a. [DOI] [PubMed] [Google Scholar]
- 5.Rtibi K., Selmi S., Grami D., Saidani K., Sebai H., Amri M. Ceratonia siliqua L. (immature carob bean) inhibits intestinal glucose absorption, improves glucose tolerance and protects against alloxan-induced diabetes in rat. J Sci Food Agric. 2017;97(8):2664–2670. doi: 10.1002/jsfa.8091. [DOI] [PubMed] [Google Scholar]
- 6.Cantalejo M.J. Effects of roasting temperature on the aroma components of carob (Ceratonia siliqua L.) J Agric Food Chem. 1997;45(4):1345–1350. [Google Scholar]
- 7.Papagiannopoulos M., Wollseifen H.R., Mellenthin A., Haber B., Galensa R. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MS. J Agric Food Chem. 2004;52(12):3784–3791. doi: 10.1021/jf030660y. [DOI] [PubMed] [Google Scholar]
- 8.Macleod G., Forcen M. Analysis of volatile components derived from the carob bean Ceratonia siliqua. Phytochemistry. 1992;31(9):3113–3119. [Google Scholar]
- 9.Ben Hsouna A., Trigui M., Ben Mansour R., Jarraya R.M., Damak M., Jaoua S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int J Food Microbiol. 2011;148(1):66–72. doi: 10.1016/j.ijfoodmicro.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Custodio L., Serra H., Nogueira J.M.F., Goncalves S., Romano A. Analysis of the volatiles emitted by whole flowers and isolated flower organs of the carob tree using HS-SPME-GC/MS. J Chem Ecol. 2006;32(5):929–942. doi: 10.1007/s10886-006-9044-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhu F., Xu J.Q., Ke Y.Y., Huang S.M., Zeng F., Luan T.G. Applications of in vivo and in vitro solid-phase microextraction techniques in plant analysis: a review. Anal Chim Acta. 2013;794:1–14. doi: 10.1016/j.aca.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Khalil M.N.A., Fekry M.I., Farag M.A. Metabolome based volatiles profiling in 13 date palm fruit varieties from Egypt via SPME GC-MS and chemometrics. Food Chem. 2017;217:171–181. doi: 10.1016/j.foodchem.2016.08.089. [DOI] [PubMed] [Google Scholar]
- 13.Nicolotti L., Cordero C., Cagliero C., Liberto E., Sgorbini B., Rubiolo P. Quantitative fingerprinting by headspace-Two-dimensional comprehensive gas chromatography-mass spectrometry of solid matrices: some challenging aspects of the exhaustive assessment of food volatiles. Anal Chim Acta. 2013;798:115–125. doi: 10.1016/j.aca.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 14.Farag M.A., Ezzat S.M., Salama M.M., Tadros M.G., Serya R.A.T. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z Naturforsch C. 2016;71(11–12):393–402. doi: 10.1515/znc-2016-0030. [DOI] [PubMed] [Google Scholar]
- 15.Farag M.A., Rasheed D.M., Kamal I.M. Volatiles and primary metabolites profiling in two Hibiscus sabdariffa (roselle) cultivars via headspace SPME-GC-MS and chemometrics. Food Res Int. 2015;78:327–335. doi: 10.1016/j.foodres.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Farag M.A., Wessjohann L.A. Volatiles profiling in medicinal licorice roots using steam distillation and solid-phase microextraction (SPME) coupled to chemometrics. J Food Sci. 2012;77(11) doi: 10.1111/j.1750-3841.2012.02927.x. C1179-C184. [DOI] [PubMed] [Google Scholar]
- 17.Li Y.Q., Kong D.X., Wu H. Analysis and evaluation of essential oil components of cinnamon barks using GC-MS and FTIR spectroscopy. Ind Crops Prod. 2013;41:269–278. [Google Scholar]
- 18.Salmeron I., Rozada R., Thomas K., Ortega-Rivas E., Pandiella S.S. Sensory characteristics and volatile composition of a cereal beverage fermented with Bifidobacterium breve NCIMB 702257. Food Sci Technol Int. 2014;20(3):205–213. doi: 10.1177/1082013213481466. [DOI] [PubMed] [Google Scholar]
- 19.Goodacre R., Shann B., Gilbert R.J., Timmins É.M., McGovern A.C., Alsberg B.K. Detection of the dipicolinic acid biomarker in Bacillus spores using Curie-point pyrolysis mass spectrometry and Fourier transform infrared spectroscopy. Anal Chem. 2000;72(1):119–127. doi: 10.1021/ac990661i. [DOI] [PubMed] [Google Scholar]