Abstract
Cardiovascular disease is a compound name for clusters of disorders afflicting the heart and blood vessels; it is assuming an increasing role as a major cause of morbidity and mortality. Unhealthy practices such as smoking, high intake of saturated fat and cholesterol, diabetes and physical inactivity are predisposing factors. The risk factors cause alteration in vascular integrity, compromised membrane integrity, increase free radical generation and reduced endogenous antioxidant system resulting in oxidative stress. Substance with ability to maintain vascular integrity, prevent, or reduce radical formation are able to treat cardiovascular disease. Conventional drugs in use to this effect are with side effect and as alternative, medicinal plants are increasingly gaining acceptance from the public and medical professionals. Reports have shown that bioactive compounds in plants with antioxidant, anti-inflammatory, ability to protect vascular endothelium, prevent lipid oxidation, and augment endogenous antioxidant system are cardioprotective. Phenolics and flavonoids in medicinal plants have been widely reported to play these major roles. This study reviewed the role of bioactive compounds in medicinal plants using a wide range database search.
Keywords: Cardiovascular disease, bioactive compounds, medicinal plants, predisposing factors
Introduction
The burden of chronic diseases among which cardiovascular disease (CVD) is named is increasing rapidly worldwide [1]. Cardiovascular disease is a compound name for clusters of disorders afflicting the heart and blood vessels. They include high blood pressure (hypertension), coronary heart failure (heart attack) cerebrovascular disease (stroke) and heart failure [1,2].
CVD is assuming an increasing role as a major cause of morbidity and mortality [3]. About 17.3 million death was estimated because of CVD by WHO in 2013 [4] and it has been forecast to increase up to 23.3 million in 2030. Different habits and unhealthy practices have been termed predisposing factors to CVD, this include high intake of saturated fat and cholesterol [5], stress, cigarette smoking, physical inactivity and diabetes [2], atherosclerosis and hypertension [6]. The pathogenesis of CVD through these factors is by causing oxidative stress, which is characterized by upsurge in reactive oxygen species beyond the threshold of the endogenous antioxidant system [7], endothelial dysfunction [8] or alteration in the vasculature/vascular injury resulting in the mobilization of inflammatory markers [9].
Endothelial dysfunction refers to impairment of endothelium dependent vaso-relaxation caused by a loss of Nitric oxide bioavailability in the vessel wall observed in the presence of cardiovascular risk factors [10,11]. The decreased bioavailability of NO allows diminished anti-inflammatory properties of the endothelial cell, permitting the activity of growth factors on the cell surface and platelet activation to act as chemo attractants to a parade of inflammatory events [12]. However, any substance with anti-inflammatory and antioxidant activity as well as substance with protective activity on the vascular endothelium will be important in treating CVD. Bioactive compounds present in medicinal plants play these active roles [13]. The interest of this review is to know the major roles bioactive compounds in plants could play in the CVD treatment.
Commonly used conventional drugs in the treatment of CVD are with side effects [14] and are very expensive [15], hence the need for a safer, cheaper, and more potent alternative. Herbal medicine is increasingly gaining acceptance from the public and medical professionals due to advances in the understanding of the mechanisms by which herbs positively influence health and quality of life [16]. There is increasing trend in the use of medicinal plants to treat CVD [17,18]. The therapeutic properties of medicinal plants arise from the characteristics bioactive secondary metabolites present in them [19]. These secondary metabolites with biological activity in plants and other traditional nutrients that have beneficial effect on human health are termed phytochemicals [20]. Several drug lead from plants have developed and being derived for the development of commercial drug preparation [21]. Over 2000 plants have been listed in the traditional i.e. herbal/alternative systems of medicine and some of these are providing comprehensive relief to the people suffering from CVD, especially hyperlipidemia and ischemic heart disease [22]. In addition, herbal treatments have been used in patients with congestive heart failure and atherosclerosis [23].
Risk factors in the pathogenesis of CVD
Several processes underline the pathogenesis of CVD. Evidence has suggest that most risk factors of CVD increase the risk of ROS production [7]. In addition, other processes such as the expression of adhesion molecules, the proliferation, and migration of smooth muscle cells, the apoptosis of endothelial cells, the oxidation of lipids, alteration of vasomotor activity, the activation of metalloproteinases [24,25] are triggered by CVD risk factors. The factors include hypercholesterolemia, diabetes mellitus, arterial hypertension, smoking, age and nitrate intolerance [7] and cancer [26].
Atherosclerosis
Simply speaking, inflammation occupies a very important central position in all phases of atherosclerosis and this is the underlying cause of heart attack [27]. There is a general agreement regarding atherosclerosis as an inflammatory disease that is associated with lipid and protein oxidation in the vascular wall [28,29]. Furthermore, high plasma concentration of cholesterol, in particular those of low-density lipoprotein (LDL) cholesterol is one of the principal risk factors [105] and the process of atherogenesis has been considered by many to consist largely of the accumulation of lipids within the artery wall and even much more [106]. At inflammatory sites, the local cellular environment is enriched with cytokines, chemo-attractant, chemokines and reactive oxygen species such as superoxide anion that are mainly produced by the activated leukocytes adhering to the endothelium [30] which can result in atheroma buildup and consequently block the free flow of blood in the artery [31]. Oxidative stress has been implicated in atherogenesis through the oxidative modification of low-density lipoprotein in the arterial wall by ROS [7], which can result in atherosclerotic lesions [32]. Atherosclerosis, which simply means the buildup of plague in the inner lining of coronary artery [33]. The plague is an unstable collection of lipids and white blood cell (WBC) in the wall of artery [33]. When this plague ruptures, catastrophic thrombus formation occurs, occluding the artery and preventing blood flow downstream, a process that results in myocardial infarction (heart attack) [34]. The myocardial tissue is typically aerobic, meanwhile metabolic processes are almost dependent upon oxygen availability, and this is confirm by the abundance of mitochondria and myoglobin in the cardiomyocytes [35]. Because of this, the myocardial cells are highly sensitive to oxygen deficiency and so when blockage of blood flow to the myocardial tissue occur and oxygen shortage environment (ischemia) characterized by decreased energy supply and acidosis caused by anaerobic glycolysis induced by oxygen deficiency, the result in serious cardiac damage in the ischemic region [36,37] (Figure 1).
Figure 1.
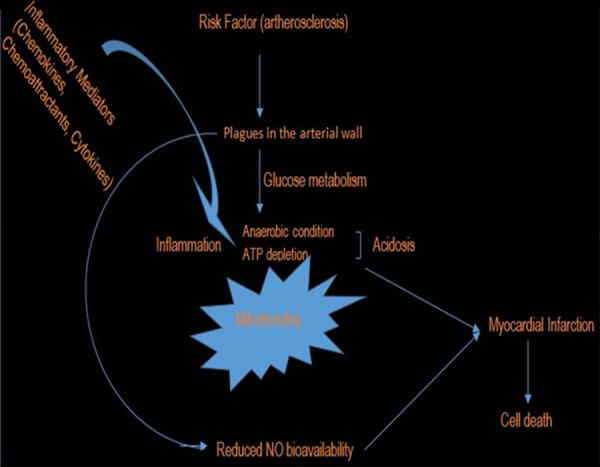
Atherosclerosis in the pathogenesis of myocardial infarction.
Endothelial dysfunction is characterize by the loss of nitric oxide bioavailability in the vessel wall. It occurs in the presence of elevated and modified LDL, increased free radical generation caused by cigarette smoking, hypertension, diabetes mellitus and genetic alterations; elevated plasma homocysteine concentrations, infectious microorganisms such as herpes viruses or Chlamydia pneumonia; and combination of these or other factors [106] have been described in the pathogenesis of CVD including atherosclerosis [6]. The consequent endothelial dysfunction is the alteration in the compensatory responses of the normal homeostatic properties of the endothelium such as increase in the adhesiveness of the endothelium with respect to leukocytes or platelets, as well as its permeability, presence of procoagulant instead of anticoagulant properties and formation of vasoactive molecules, cytokines, and growth factors [106]. Cardiovascular diseases (CVDs) like myocardial infarction (heart attack), acute coronary syndrome, or stroke arise on a background of plaques and lesions inside the arteries [107,108]. Hence, hypercholesterolemia, hypertension, and obesity are high risk factors in the progression of CVD [109]. The lesions of atherosclerosis represent a series of highly specific cellular and molecular responses that can best be described, in aggregate, as an inflammatory disease [110-112] which occur principally in large and medium-sized elastic and muscular arteries and can lead to ischemia of the heart, brain, or extremities, resulting in infarction [106], a lesions that may be present throughout lifetime.
Hyperlipidemia/hypercholesterolemia
Risk factors including elevated low-density lipoprotein cholesterol, low level of high-density lipoprotein cholesterol have been identified as primary cause of CVD [38]. Total cholesterol can be broken into diagnostic lipoprotein profile, including high-density lipoprotein (HDL), low-density lipoprotein (LDL), intermediate density lipoprotein (IDL), very low-density lipoprotein (VLDL), chylomicron remnants, and triglycerides [9]. Hypercholesterolemia stimulates the production of superoxide anion radicals from the smooth muscle cells of vessels, an event that leads to increased oxidation of LDL (Figure 2), consequently, mobilization of macrophages for oxidized-LDL uptake. LDL as an atherogenic lipoprotein with access to the sub-endothelial space undergoes oxidative modification when trapped in the intracellular matrix [39]. The uptake of oxidized LDL by macrophages is easy compared to non-oxidized lipoprotein [7]. The LDL uptake by the macrophage begins to develop into foam cells between the basal lamina of the endothelium and the smooth muscle layer [40,42]. These foam cells lead to the production of numerous inflammatory and oxidative stress markers, cytokines, chemokines, and growth factors, which aggravate the balance of endothelial equilibrium leading to vascular dysfunction [42]. Harrison [43] report that leukocytes (macrophages) that are recruited due to endothelial injury signal and inefficiencies within smooth muscle cell mitochondrial metabolism are one of the major source of oxidant within the vasculature.
Figure 2.
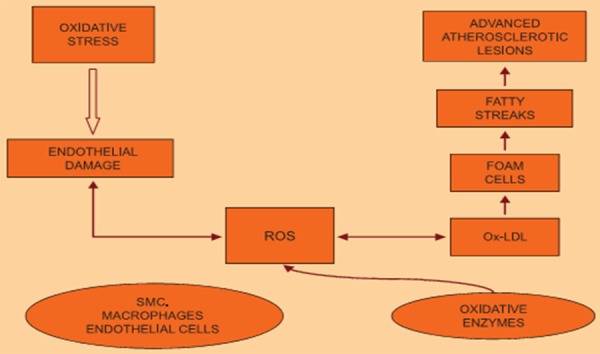
Hypercholesterolemia induced oxidative stress in the pathogenesis of CVD. Source; [7].
Studies showed hypercholesterolemia leads to an inflammatory response within the vasculature, reflected by endothelial cell activation, leukocyte recruitment, rolling and adherence as well as platelet activation and adhesion [44,45]. Leukocyte activation can subsequently obstruct capillary networks and thus reduce pumping [46] i.e. atherosclerosis (Figure 2). In hyperlipidemia/atherosclerotic patients, adaptation to oxidative stress is poor; this is due to the impairment in the endogenous mechanisms against myocardial stress [47]. The role of cholesterol in the interruption and alteration of vascular structure and function as it builds within the lining of the vascular wall leading to lesions, plagues, occlusion, reduction in healing, recovery and appropriate management of ischemia/reperfusion injury has been described [9,48].
The overall activity of high cholesterol level has been described as induction of hyperlipidemia consequently resulting in the plaguing of arterial wall, alteration of vascular function resulting in the decreased bioavailability of nitric oxide, diminishing of the anti-inflammatory properties of the endothelial cell.
Drugs
Chemotherapy as a method for treating cancer has yielded significant clinical benefits although its full therapeutic effectiveness is mask with severe side effects [49] one of which includes cytotoxicity. Conventional drugs in cancer treatment have been implicated in cardio toxicity induction [50,51] through the generation of highly cytotoxic free radicals [51,52].
In many models, Doxorubicin and isoprotenol are commonly in use for the induction of cardiovascular disease. Several research described the method by which the two anticancer drugs induce cardio toxicity [34,50]. Isoprotenol generates highly cytotoxic free radicals that stimulate peroxidation of membrane phospholipids leading to myocardial membrane damage [16].
Reactive oxygen species (ROS) in the progression of CVD
Since CVD is typically progressive and often associated with inter-related disease states (i.e. atherosclerosis, hypertension) several recent studies have demonstrated that altered oxygen utilization and/or increased formation of reactive oxygen species (ROS) contribute to CVD progression [53]. ROS as well as RNS are normal cellular metabolic products [30]. They participate in normal cell signaling as mediators that regulate vascular function [54-56]. The endothelium smooth muscles and adventitia layers of the vascular wall produce ROS [57]. Under physiological condition, production of free radicals is in low concentration and play regulatory roles in the vascular system [59,60]. For example, studies in animal models have described the pathway in the etiology of MI and implicated it to increase free radical activity with consequent lipid peroxidation [113-115].
Vascular risk factors such as hypertension, diabetes, smoking, dyslipidemia, and atherosclerosis are associated with a marked increase in vascular ROS production [7,60]. In oxidative stress (i.e. increase production of free radicals or deficiency of enzymatic or non-enzymatic antioxidants), oxidant metabolites exert their toxic effect because of altered cellular mechanism of protection [61] consequently, modification of LDL to oxidized LDL or cellular lipids, proteins and DNA [30]. Reduced production and increased consumption of nitric oxide with possible vascular impairment is implicative of oxidative stress [62]. The consequent impairment of NO production results in vasoconstriction, platelet aggregation, and leukocyte-endothelium adhesion [63], which is the hallmark of cardiovascular risk and even progression of atherosclerotic disease [64].
Alteration in the vascular tone (vascular homeostasis) from the toxic effect of free radicals as a consequent of increased production of ROS, leads to the accumulation of damage in various cellular locations and to the deregulation of sensitive metabolic and signaling pathway [30].
Nitric oxide is a key signaling messenger in the cardiovascular system [65], in addition to its function as endothelial derived relaxing factor, other functions in cardiovascular physiology include maintenance of vascular integrity by inhibiting platelet aggregation [66,67], leukocyte-endothelium adhesion [69] and vascular smooth muscle proliferation [69]. Endothelial nitric oxide synthase activity on L-arginine results in NO production [24].
Evidence has shown that the protective role of nitric oxide (NO) can turn around to be pro-atherosclerotic elements, producing several vascular contracting pro-aggregating and pro-inflammatory factors including cyclooxygenase derived products and ROS which in turn causes NO breakdown in the presence of CVD risk factors [70]. In other words, endothelial dysfunction in impaired NO availability is considered an early and major promoter for thrombosis and atherosclerosis [64]. When endothelial nitric oxide synthase (eNOS) is uncoupled, NO and superoxide radical can react together to produce peroxynitrite anion. The anion is a potent oxidizing agent with ability to causing oxidative damage to biomolecules with subsequent inhibition of their biological function [71].
Conclusively, increased ROS generation impairs NO bioavailability and consequently endothelium dysfunction that has been describe in the pathogenesis of cardiovascular disease.
Role of bioactive compounds in medicinal plants investigated as cardio-protective
Research has reported the cardio-protective properties of different medicinal plants and the roles are link to bioactive compounds that are present in these plants.
The use of medicinal plant as remedies for human disease has been known over centuries [14]. Medicinal plants throughout their existence contributed immensely to the health needs of human categorically due to the presence of component with therapeutic value in them [14]. The use of conventional drugs in the treatment of cardiovascular disorders has proved effective but not without side effects, making their use limited [14] and necessitating alternative search.
Medicinal plants considered cheap and safe are gaining attention and their cardio-protective action is by providing nutritional substances mainly phytochemicals with potentials of restoring and maintaining balanced body system [72]. Different research has reported the cardio-protective activity of medicinal plants pointing to the antioxidant potentials as possible mechanism. Epidemiological, clinical and experimental studies have provided evidence that myocardial infarction is largely preventable by suppression of free radical generation and augmentation of endogenous antioxidant [50]. However, several investigation has provided evidence justifying medicinal plants to play important roles.
Aside the antioxidant role played by medicinal plants, anti-inflammatory potential plants has been reported with cardioprotective effect.
Antioxidant role
Normal aerobic metabolic processes as well as exposure to radiation, redox cycling materials and other environmental substances result in free radical generation in the body [73]. ROS such as superoxide anion, hydrogen peroxide, and hydroxyl radical are produced in-vivo due to successive reduction of oxygen [74]. ROS have been reported to play important role as mediator of the progression of cardiovascular disorder and other diseases such as diabetes and neurodegenerative disease [75]. When the production of free radicals exceeds the threshold of the antioxidant defense system, oxidative stress occurs with successive oxidation of macromolecules such as DNA, protein, and lipids [76]. The antioxidant defense system in human includes enzymes such as glutathione peroxidase, superoxide dismutase, catalase, and reduced glutathione [77]. However, exogenous antioxidants such as vitamin E, ascorbic acid can augment the effects of the endogenous antioxidant system.
Research has shown a reduction in the endogenous antioxidant enzyme in cardiovascular disease [18,34,50]. Increased lipid peroxidation often accompany the increased ROS generation in the pathogenesis of CVD with resultant vascular membrane damage [34,79]. Since the formation of ROS play a key role in cardiac pathophysiology, targeting oxidative stress will substantially improve the treatment of CVD (Figure 3). Furthermore, with oxidative stress as an underlying factor, antioxidants may decrease cellular injury and apoptosis through a radical-scavenging mechanism [116]. Therapeutic intervention via suppression of free radical generation and/or augment action of endogenous antioxidant enzymes may for example attenuate myocardial dysfunction [51]. The use of antioxidants in pharmacology is intensively studied. Antioxidants have gained popularity recently for their many health benefits. They have been shown to lower the risk of heart disease [117].
Figure 3.
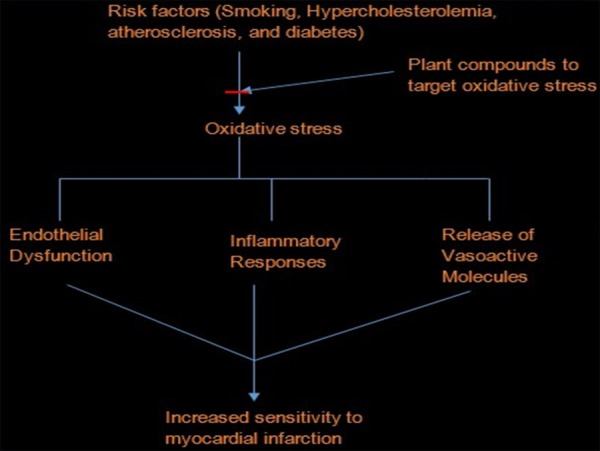
Interruption of oxidative stress by plant compounds, prevent cardiovascular development.
These antioxidant compounds are present in plants. Nowadays, interest has grown towards the use of natural antioxidants as protective strategy against cardiovascular related problems such as ischemia reperfusion [118].
In a study by Jahan et al. [15], Terminela arjuna was reported to regulate the activities of antioxidant enzymes in Isoprotenol induced myocardial infarction. The authors demonstrated the cardio protective activity of the polyphenolic rich extract of T. arjuna in both curative and preventive studies. The result shows that isoprotenol significantly increased the level of serum cardiac marker enzymes like LDH, AST, and ALT reflecting the severity of iso-induced myocardial cell necrosis. The myocardial cell necrosis can be due to increase in lipid peroxidation, but treatment with the polyphenolic rich extract lowered the serum cardiac marker enzymes demonstrating the plant’s ability to maintain membrane integrity consequently constrain the leakages of enzymes. In addition, the activity of the myocardial antioxidant enzymes such as SOD, CAT and Peroxidase that were lowered by isoprotenol induction were restored to near normal when treated with T. arjuna. The activity of the plant extracts were compared with the combined activity of propranolol and gemfibrazole standard drug and was found to be comparable. They related the potentials of the plant to the polyphenols fraction of the extract and its antioxidant activity. The presence of flavonol (quercetin, myricetin), kaempferol, phenolic acids (garlic and ferulic acid) (Figure 4) was higher in T. arjuna as observed in the HPLC analysis.
Figure 4.
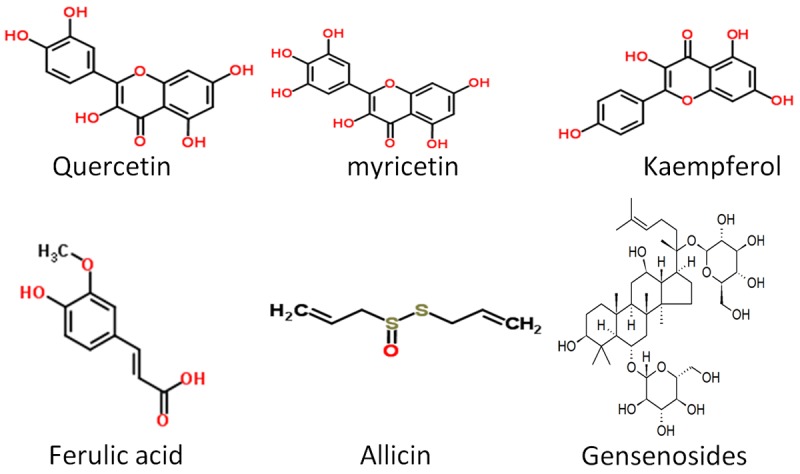
Structure of some compounds with antioxidant and anti-inflammatory.
The potentials of flavonoids in the treatment of CVD have been reported. The possible mechanism includes its ability to ameliorate/stop endothelial dysfunction [80], reduce LDL oxidation and inhibition of platelet aggregation [80]. They concluded by correlating the superlative cardioprotective activity of T. arjuna mainly to the antioxidant constituents.
The cardio protective potentials of Ocimum basilicum have been studied. O. basilicum belong to the lamiaceae family. Studies have revealed the essential oil of the plant is composed of interesting terpenoids. Phytochemical reports reveal the presence of triterpenoids, polyphenols, and steroids. Studies by Fathiazad et al. [17], reported an ameliorative effects of the plant extract on the changes in blood pressure observed in the isoprotenol induced cardio toxicity. The plant extract prevent the necrosis and fibrosis observed in the iso-induced group. Acute phase of myocardial necrosis induced by isoprotenol has been link with generation of cytotoxic reactive oxygen species. The maintenance of cardiac membrane integrity was reported, and the membrane integrity was assayed through the degree of lipid peroxidation, which was measured from the malondialdehyde (MDA) level in serum and myocardial homogenates and found out that the extract significantly lowered the MDA level that was elevated with isoprotenol treatment. They related the cardioprotective potential of Ocimum basilicum to the antioxidant potential of flavonoids and phenolics, which were reported present in the plant. The radical scavenging activity of the plant correlates to high content of these compounds. Harnafi et al. [81] in a previous research reported plasma lipid lowering effect for the plant and thus might be important in the treatment of hyperlipidemia and atherosclerosis.
Many of the medicinal properties of Allium sativum (garlic) are associated with its antioxidant properties [82]. Increase in the production of nitric oxide by garlic acid has been reported [83,84] thus prevention of endothelial dysfunction in models [86]. Shackebaei et al. [85] reported the protective effects of garlic juice in reperfusion, in this study; Gallic juice showed a significant vasodilator effect on the coronary vessels therefore increased the coronary flow in the test group before ischemia and during reperfusion. In addition, the authors studied the extent of reperfusion injury from the release of a marker intracellular enzyme. Observed was the lower concentration of lactate dehydrogenase (LDH) release in the test group reflecting significant protection against endothelial dysfunction and prevention of anaerobic metabolism within the heart muscle cells. The pharmacological activity observed in their studies could be because of antioxidant activity and vaso-regulatory effect. They identified Allicin (Figure 4) as the bioactive compound responsible for the cardioprotective activity of garlic.
Phenolic compounds and flavonoids in Evolvulus alsinoides with lipid peroxidation, antioxidant, and free radical scavenging properties were link to cardio protective activity in isoprotenol-induced myocardial infarction [34]. Observed was the decreased release of enzymes from cardiac cell fractions that could possibly infer a membrane stabilizing effect with near normal retention of clinical marker enzymes activities in the serum and cardiac tissue of experimental animals [34]. Membrane lipid peroxidation by free radicals is known to compromise membrane integrity.
Conclusively, the antioxidant role played by phenolics and flavonoids reported in various research resulted in the inhibition of platelet aggregation, restoration of vascular function, inhibition of lipid peroxidation, membrane stabilization, restoration of antioxidant enzyme and inhibition of reactive oxygen species. A similar mechanism reported by Mohanty et al. [86] in a combination treatment research he conducted for Withania somnifera, Curcuma longa and Ocimum sanctum. Previous investigation has also reported the cardioprotective activity of Ginkgo biloba and Ocinum sanctum. In both plant, the cardioprotective activity was attributed to the antioxidant activities associated with the presence of flavonoids and phenolics. The report also associated reduced glutathione augmenting effect, elevated antioxidant enzyme level and the inhibition of lipid peroxidation [87,88] to these compounds in both plant. Panda and Naik [16] validated the cardio protective claim for these bioactive compounds in the two medicinal plants when he combined Ginkgo biloba and Ocinum sanctum treatment in isoprotenol induced myocardial necrosis in rats, suggesting the possibility of similar mechanism previously reported, although no significant synergistic effect was observed in the combination treatment.
The high radical scavenging activity in phenolics and flavonoids is associated to their structure activity relationship. The high reactivity of the hydroxyl group of flavonoids is responsible for their free radical scavenging activity [16].
Anti-inflammatory role
Inflammation is a complex biological response of vascular tissues to harmful stimuli [89]. It is also protective in nature in the attempt by organism to remove injurious stimuli and initiate healing process [90]. Inflammation response involves cell activation and the release of inflammatory mediators [89]. Inflammation activation has been implicated as an important pathway in the pathogenesis and progression of CVD including chronic heart failure [27,91] and their mediators can serve as relevant markers of disease severity [92]. Markers such as interleukins, cytokines are reported to create inflammatory condition in the arterial wall [93]. Other inflammation factors are leukocyte adhesion molecules and chemokines [94]. Risk factors including hypercholesterolemia can lead to inflammatory response within the microvasculature, which is reflected, by endothelial cell activation, leukocyte recruitment, rolling and adherence as well as platelet activation and adhesion [44,45]. This is preventable by NO availability in the vasculature. However, diminished availability of NO result in vascular dysfunction and diminished anti-inflammatory properties of the endothelial cell [9].
Wallace [95] in his review reported the health benefit of diets rich in natural bioactive compounds especially the contributory role in maintaining and improving cardiovascular health. He reported flavonoids, a member of the polyphenols as potential candidate to protect against CVD.
Several medicinal plants have been reported with anti-inflammatory activity. Kadian and Parle [96] in a review reported the anti-inflammatory activity of Ocimum sanctum. The plant was reported to possess cardio protective effect as well as anti-hypertensive effect and the mechanism for this pharmacological efficacy is by inhibiting specific inflammatory molecules [97]. Singh and Majumdar [98] suggested the possible mechanism for the anti-inflammatory activity of linolenic acid present in Ocimum sanctum to be by blocking both the cyclooxygenase and lipooxygenase pathways of arachidonate metabolism. Arachidonic acid-derived lipid mediators are intimately involved in inflammation and are biosynthesized by pathways dependent on cyclooxygenase (COX) and lipoxygenase (LOX) enzymes.
The anti-inflammatory effect of Gensenosides (Figure 4), a triterpene dammarane-type saponins was reported by Xing et al. [99]. According to the research, a combination of the Chinese herbal medicines Ginseng Radix Et Rhizoma Rubra (the root of Panax ginseng C.A.Mey.), Ophiopogonis Radix (the root of Ophiopogon japonicas (L.f) Ker-Gawl), and Schisandrae Chinensis Fructus (the fructus of Schisandra chinensis (Turcz.) Baill) termed YQFM could suppress the expressions of inflammatory mediators. A constituent of one of the main bioactive components, ginsenosides according to the research contributed to the measured anti-inflammatory effects. The three herbs of YQFM were reported to be significant in the enhancement of myocardial contractility, blood-vessel dilation, anti-lipid peroxidation, and anti-inflammatory effect [100-104].
Conclusion
With cardiovascular disease assuming an increasing role as a major cause of mobility and mortality and the limitations in the use of conventional drugs in its treatment, more intensive approach is required in the current Epidemiological, clinical and experimental studies of compounds that are able to suppress free radical generation, augment endogenous antioxidant and maintain the integrity of the vasculature in medicinal plants. Critical analysis of the roles of bioactive compounds as observed in this review will be important as a guide to identify potential compounds in plant and consequently their characterization. Aside the commonly reported phenolics and flavonoids, other compounds with great potentials in treating cardiovascular disease and their roles should be elucidated.
Acknowledgements
The authors appreciate Dr. A. Adetutu for his mentorship and tutorship and the Department of Biochemistry, Ladoke Akintola University of Technology, Ogbomoso, Oyo State. Nigeria for giving us the platform to carry out this study.
Disclosure of conflict of interest
None.
Authors’ contribution
PI conceived the idea, PI, IA, WH and TO outline the content, WH and IA search the internet, PI wrote the manuscript. All author read and approved the final manuscript for submission.
References
- 1.Mayakrishnan V, Kannappan P, Abdullah N, Ali A. Cardioprotective activity of polysaccharides derived from marine algae: an overview. Trends Food Sci Technol. 2013:1–7. [Google Scholar]
- 2.Olorunnisola OS, Bradley G, Afolayan AJ. Ethnobotanical information on plants used for the management of cardiovascular diseases in Nkonkobe municipality, South Africa. J Med Plant Res. 2011;5:4256–4260. [Google Scholar]
- 3.Krisela S, editor. The heart and stroke foundation South Africa heart disease in South Africa media data document. 2007. http://www.heartfoundation.co.za/docs/heartmonth/HeartDiseaseinSA.pdf.
- 4. WHO: Cardiovascular disease http://www.who.int/cardiovascular_diseases/en/ (2013). Accessed 27 March 2015.
- 5.Thompkinson D, Bhavana V, Kanika P. Dietary approaches for management of cardio vascular health-a review. J Food Sci Technol. 2012;51:2318–2330. doi: 10.1007/s13197-012-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Lüscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Endothelial function and dysfunction. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the working group on endothelins and endothelial factors of the European society of hypertension. J Hypertens. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Vogiatzi G, Tousoulis D, Stefanadis C. The role of oxidative stress in atherosclerosis hellenic. J Cardiol. 2009;50:402–409. [PubMed] [Google Scholar]
- 8.Liu VW, Huang PL. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stapleton PA, Goodwill AG, James ME, Brock RW, Frisbee JC. Hypercholesterolemia and microvascular dysfunction: interventional strategies. J Inflamm. 2010;7:1–10. doi: 10.1186/1476-9255-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen HM, Schou M, Goetze JP, Faber J, Frystyk J, Flyvbjerg A, Kistorp C. Body mass index in chronic heart failure: association with biomarkers of neurohormonal activation, inflammation and endothelial dysfunction. BMC Cardiovasc Disord. 2013;13:80. doi: 10.1186/1471-2261-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton PA, Goodwill AG, James ME, D’Audiffret AC, Frisbee JC. Differential impact of familial hypercholesterolemia and combined hyperlipidemia on vascular wall and network remodeling in mice. Microcirculation. 2010;17:47–58. doi: 10.1111/j.1549-8719.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viswanatha GL, Vaidya S, Ramesh C, Krishnadas N, Rangappa S. Antioxidant and antimutagenic activities of bark extract of Terminalia arjuna . Asian Pac J Trop Med. 2010;3:965–970. [Google Scholar]
- 14.Saman H, Khalil-ur-R , Zahoor-ul-hassan D, Nazish J, Mansoor H, Zafar IK, Kafeel A, Khalid M, Ehsan EV. Cardioprotective effect of gemmotherapeutically treated withania somnifera against chemically induced myocardial injury Pak. J Bot. 2010;42:1487–1499. [Google Scholar]
- 15.Jahan N, Rahman K, Ali S. Cardioprotective and antilipidemic potential of cyperus rotundus in chemically induced cardiotoxicity. Int J Agric Biol. 2012;14:989–992. [Google Scholar]
- 16.Panda VS, Naik SR. Evaluation of cardioprotective activity of ginkgo biloba and Ocimum sanctumin rodents. Altern Med Rev. 2009;14:161–171. [PubMed] [Google Scholar]
- 17.Fathiazad F, Matlobi A, Khorrami A, Hamedeyazdan S, Soraya H, Hammami M, Maleki-Dizaji N, Garjan A. Phytochemical screening and evaluation of cardioprotective activity of ethanolic extract of Ocimum basilicum L. (basil) against isoproterenol induced myocardial infarction in rats. Daru. 2012;20:87. doi: 10.1186/2008-2231-20-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojha S, Bharti S, Sharma AK, Rani N, Bhatia J, Kumari S, Arya DS. Effect of Inula racemosa root extract on cardiac function and oxidative stress against isoproterenol induced myocardial infarction. Indian J Biochem Biophys. 2011;48:22–28. [PubMed] [Google Scholar]
- 19.Sangwan RS, Chaurasiya ND, Misra LN, Lal P, Uniyal GC, Sharma R, Sangwan NS, Suri KA, Oazi GN, Tuli R. Phytochemical variability in commercial herbal products and preparations of withania somnifera (Ashwagandha) Current Science. 2004;86:461–465. [Google Scholar]
- 20.Hasler CM. Functional foods: their role in disease prevention and health promotion. Food Tech. 1998;52:63–70. [Google Scholar]
- 21.Suroowan S, Mahomoodally F. Common phyto-remedies used against cardiovascular diseases and their potential to induce adverse events in cardiovascular patients. Clinical Phytoscience. 2015;1:1–13. [Google Scholar]
- 22.Rajalakshmy I, Ramya P, Kavimani S. Cardioprotective medicinal plants-A review. International Journal of Pharmaceutical Invention. 2011;1:24–41. [Google Scholar]
- 23.Xin YF, Wan LL, Peng JL, Guo C. Alleviation of acute doxorubicin induced cardiotoxicity by lycium barbarum polysaccharides through the suppression of oxidative stress. Food Chem Toxicol. 2011;49:259–264. doi: 10.1016/j.fct.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Luscher TF, Vanhoutte PM. The endothelium: modulator of cardiovascular function. Boca Raton, FL, USA: CRC press; 1990. pp. 1–215. [Google Scholar]
- 25.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 26.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libby P. Vascular biology of atherosclerosis: overview and state of art. Am J Cardiol. 2003;91:3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- 28.Navab M, Ananthramaiah GM, Reddy ST, Van-Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Puddu GM, Cravero E, Arnone G, Muscari A, Puddu P. Molecular aspects of atherogenesis: new insights and unsolved questions. J Biomed Sci. 2005;12:839–853. doi: 10.1007/s11373-005-9024-z. [DOI] [PubMed] [Google Scholar]
- 30.Puddu P, Puddu GM, Cravero E, Pascalis S, Muscari A. The emerging role of cardiovascular risk factor-induced mitochondrial dysfunction in atherogenesis. J Biomed Sci. 2009;16:1–9. doi: 10.1186/1423-0127-16-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thygesen K, Alpert JS, White HD. “Universal definition of myocardial infarction”. Eur Heart J. 2007;28:2525–38. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 32.Radhika S, Smila KH, Muthezhilan R. Cardioprotective activity of hybanthus enneaspermus (Linn.) on isoproterenol induced rats. Indian Journal of Fundamental and Applied Life Sciences. 2011;1:90–97. [Google Scholar]
- 33.Mohale DS, Dewani AP, Saoji AN, Khadse CD. Antihyperlipidemic activity of isolated constituents from lagenaria siceraria in albino rats. Int J Green Pharm. 2008;2:104–107. [Google Scholar]
- 34.Sudhakumari Anil KH, Aamir J, Manish J, Muralidhar ST. Cardioprotective effects in methanolic extract of Evolvulus alsinoides linn on isoproterenol-induced myocardial infarction in albino rats. International Journal of Basic Medical Sciences and Pharmacy (IJBMSP) 2012;2:53–57. [Google Scholar]
- 35.Levick JR. An introduction to cardiovascular physiology. 4th edition. London, UK: Oxford University Press Inc; 2003. [Google Scholar]
- 36.Chien KR, Han A, Sen A, Buja LM, Willerson JT. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ Res. 1984;54:313–322. doi: 10.1161/01.res.54.3.313. [DOI] [PubMed] [Google Scholar]
- 37.Shug AL, Thomsen JH, Folts JD, Bittar N, Klein MI, Koke JR, Huth PJ. Changes in tissue levels of carnitine and other metabolites during myocardial ischemia and anoxia. Arch Biochem Biophys. 1978;187:25–33. doi: 10.1016/0003-9861(78)90003-6. [DOI] [PubMed] [Google Scholar]
- 38.Toth PP. Making a case for quantitative assessment of cardiovascular risk. J Clin Lipidol. 2007;1:234–41. doi: 10.1016/j.jacl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–44. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 40.Dilaveris P, Giannopoulos G, Riga M, Synetos A, Stefanadis C. Beneficial effects of statins on endothelial dysfunction and vascular stiffness. Curr Vasc Pharmacol. 2007;5:227–237. doi: 10.2174/157016107781024091. [DOI] [PubMed] [Google Scholar]
- 41.Lakshmi SV, Padmaja G, Kuppusamy P, Kutala VK. Oxidative stress in cardiovascular disease. Indian J Biochem Biophys. 2009;46:421–440. [PubMed] [Google Scholar]
- 42.Simionescu M. Implications of early structural-functional changes in the endothelium for vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:266–274. doi: 10.1161/01.ATV.0000253884.13901.e4. [DOI] [PubMed] [Google Scholar]
- 43.Harrison DG. Endothelial function and oxidant stress. Clin Cardiol. 1997;20:2–7. [PubMed] [Google Scholar]
- 44.Scalia R, Appel JZ, Lefer AM. Leukocyte-endothelium interaction during the early stages of hypercholesterolemia in the rabbit: role of P-selectin, ICAM-1, and VCAM-1. Arterioscler Thromb Vasc Biol. 1998;18:1093–1100. doi: 10.1161/01.atv.18.7.1093. [DOI] [PubMed] [Google Scholar]
- 45.Stokes KY, Calahan L, Russell JM, Gurwara S, Granger DN. Role of platelets in hypercholesterolemia-induced leukocyte recruitment and arteriolar dysfunction. Microcirculation. 2006;13:377–388. doi: 10.1080/10739680600745877. [DOI] [PubMed] [Google Scholar]
- 46.Stokes KY, Cooper D, Tailor A, Granger DN. Hypercholesterolemia promotes inflammation and microvascular dysfunction: role of nitric oxide and superoxide. Free Radic Biol Med. 2002;33:1026–1036. doi: 10.1016/s0891-5849(02)01015-8. [DOI] [PubMed] [Google Scholar]
- 47.Roberts WC. Preventing and arresting coronary atherosclerosis. Am Heart J. 1995;130:580–600. doi: 10.1016/0002-8703(95)90369-0. [DOI] [PubMed] [Google Scholar]
- 48.Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004;3:913–925. doi: 10.1038/nrd1548. [DOI] [PubMed] [Google Scholar]
- 49.Mahsa Z, Komal KJ, Mehrdad Z, Syed B. Cardioprotective effect of the root extract of Hemidesmus indicus against doxorubicin-induced oxidative stress in mice. Der Pharmacia Lettre. 2013;5:334–339. [Google Scholar]
- 50.Sakthivel K, Palani S, Santhosh K, Devi K, Kumar BS. Phytoconstituents analysis by GC-MS, cardioprotective and antioxidant activity of Buchanania axillaris against doxorubicin-induced cardio toxicity in albino rats. International Journal of Pharmaceutical Studies and Research. 2010;1:34–48. [Google Scholar]
- 51.Yousefi K, Soraya H, Fathiazad F, Khorrami A, Hamedeyazdan S, Maleki-Dizaji N, Garjani A. Cardioprotective effect of methanolic extract of Marribum vulgare L. On isoprotenol-induced acute myocardial infarction in rats. Indian J Exp Biol. 2013;51:653–660. [PubMed] [Google Scholar]
- 52.El-Sayed EM, Abd El-azeem AS, Afify AA, Shabana MH, Ahmed HH. Cardioprotective effects of Curcuma longa L. Extracts against doxorubicin-induced cardiotoxicity in rats. J Med Plant Res. 2011;5:4049–4058. [Google Scholar]
- 53.Wattanapitayakul SK, Bauer JA. Oxidative pathways in cardiovascular disease roles, mechanisms, and therapeutic implications. Pharmacol Ther. 2001;89:187–206. doi: 10.1016/s0163-7258(00)00114-5. [DOI] [PubMed] [Google Scholar]
- 54.Finkel T. Signal transduction by ROS in non-phagocytic cells. J leukoc Biol. 1999;65:337–340. doi: 10.1002/jlb.65.3.337. [DOI] [PubMed] [Google Scholar]
- 55.Sizuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 56.Wolin MS. Interactions of oxidants with vascular signaling systems. Artrioscler throm vasc. Biol. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 57.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 58.Touyz RM, Schiffrin EL. Ang 11-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension. 1999;34:976–982. doi: 10.1161/01.hyp.34.4.976. [DOI] [PubMed] [Google Scholar]
- 59.Zafari AM, Ushio-fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase derived H2O2 in angiotensin 11-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 60.Gozin A, Franzini E, Andrieu V, Da Costa L, Rollet-Labelle E, Pasquier C. Reactive oxygen species activate focal adhesion kinase, paxillin and p130cas tyrosine phosphorylation in endothelial cells. Free Radic Biol Med. 1998;25:1021–1032. doi: 10.1016/s0891-5849(98)00134-8. [DOI] [PubMed] [Google Scholar]
- 61.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Cann B. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156:274–85. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 62.Victor VM, Rocha M, Solá E, Bañuls C, Garcia-Malpartida K, Hernández-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. 2009;15:2988–3002. doi: 10.2174/138161209789058093. [DOI] [PubMed] [Google Scholar]
- 63.Vepa S, Scribner WM, Parinandi NL, English D, Garcia JG, Natarajan V. Hydrogen peroxide stimulates tyrosine phosphorylation of focal adhesion kinase in vascular endothelial cells. Am J Physiol. 1999;277:150–158. doi: 10.1152/ajplung.1999.277.1.L150. [DOI] [PubMed] [Google Scholar]
- 64.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. The ageing endothelium, cardiovascular risk and disease in man. Exp Physiol. 2008;94:317–321. doi: 10.1113/expphysiol.2008.043356. [DOI] [PubMed] [Google Scholar]
- 65.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Ann Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 66.Freedman JE, Sauter R, Battinelli EM, Ault K, Knowles C, Huang PL, Loscalzo J. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ Res. 1999;84:1416–1421. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- 67.Radomski MW, Palmer RM, Moncada S. Modulation of platelet aggregation by an L-arginine-nitric oxide pathway. Trends Pharmacol Sci. 1991;12:87–88. doi: 10.1016/0165-6147(91)90510-y. [DOI] [PubMed] [Google Scholar]
- 68.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, Huang PL, Scalia R. Leukocyte-endothelial cell interactions in nitric oxide synthase deficient mice. Am J Physiol. 1999;276:1943–1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 69.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosz R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 71.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Hertog MG, Feskens EJ, Hollam PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart diseases: the Zutphen elderly study. Lancet. 1993;342:1007–1020. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 73.Yamagishi SI, Edelstein D, Du XL, Brownlee M. Hyperglycaemia potenciates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001;50:1491–1494. doi: 10.2337/diabetes.50.6.1491. [DOI] [PubMed] [Google Scholar]
- 74.Nabavi SF, Nabavi SM, Ebrahimzadeh MA, Eslami SH, Jafari N, Hajizadeh M. The protective effect of curcumin against sodium fluoride-induced oxidative stress in rat heart. Arch Biol Sci Belgrade. 2011;63:563–569. [Google Scholar]
- 75.Bartsch H, Nair J. Ultrasensitive and specific detection method for exocyclic DNA adducts markers for lipid peroxidation and oxidative stress. Toxicology. 2000;153:105–114. doi: 10.1016/s0300-483x(00)00307-3. [DOI] [PubMed] [Google Scholar]
- 76.Halliwell B, Guttteridge JM. Free radicals in biology and medicine. 2nd edition. Oxford, London: Oxford university press (Clarendon); 2007. [Google Scholar]
- 77.Sheweita SA, Abd El-Gabar M, Bastawy M. Carbon tetrachloride induced changes in the activity of phase II drug metabolizing enzyme in the liver of male rats: role of antioxidants. Toxicology. 2001;165:217–224. doi: 10.1016/s0300-483x(01)00429-2. [DOI] [PubMed] [Google Scholar]
- 78.Nandave M, Mohanty I, Nag TC, Ojha SK, Mittal R, Kumari S, Arya DS. “Cardioprotective response to chronic administration of vitamin E in isoproterenol induced myocardial necrosis: hemodynamic, biochemical and ultrastructural studies”. Indian J Clin Biochem. 2007;22:22–28. doi: 10.1007/BF02912876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurosawa T, Itoh F, Nozaki A, Nakano Y, Katsuda S, Osakabe N, Tsubone H, Kondo K, Itakura H. Suppressive effects of cacao liquor polyphenols (CLP) on LDL oxidation and the development of atherosclerosis in Kurosawa and Kusanagi hypercholesterolemic rabbits. Atherosclerosis. 2005;179:237–246. doi: 10.1016/j.atherosclerosis.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 80.Keevil JG, Osman HE, Reed JD, Folts JD. Grape juice, but not orange juice or grape fruit juice, inhibits human platelet aggregation. J Nutr. 2000;130:53–56. doi: 10.1093/jn/130.1.53. [DOI] [PubMed] [Google Scholar]
- 81.Harnafi H, Serghini CH, Bouanani NH, Aziz M, Amrani S. Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chem. 2008;108:205–212. [Google Scholar]
- 82.Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17:97–106. doi: 10.1002/ptr.1281. [DOI] [PubMed] [Google Scholar]
- 83.Maslin DJ, Brown CA, Das I, Zhang XH. Nitric oxide-a mediator of the effects of garlic? Biochem Soc Trans. 1997;25:408S. doi: 10.1042/bst025408s. [DOI] [PubMed] [Google Scholar]
- 84.Sooranna SR, Hirani J, Das I. Garlic can induce both GTP cyclohydrolase and nitric oxide synthase activity in choriocarcinoma cells. Biochem Soc Trans. 1995;23:543. doi: 10.1042/bst023543s. [DOI] [PubMed] [Google Scholar]
- 85.Shackebaei D, Ghazvineh S, Godini A, Pilehvarian A, Reshadat S. Cardioprotective effect of garlic juice on the isolated rat heart in ischemia-reperfusion. Journal of Medicinal Plants. 2010;9:71–79. [Google Scholar]
- 86.Mohanty IR, Gupta SK, Arya DS, Mohanty N, Deshmukh Y. Medicinal herbs can play significant role in attenuation of ischemia and reperfusion. Injury J Homeop Ayurv Med. 2013;2:1–11. [Google Scholar]
- 87.Naik SR, Pilgaonkar VW, Panda VS. Evaluation of antioxidant activity of ginkgo biloba phytosomes in rat brain. Phytother Res. 2006;20:1013–1016. doi: 10.1002/ptr.1976. [DOI] [PubMed] [Google Scholar]
- 88.Uma DP, Ganasoundari A, Vrinda B, Srinivasan KK, Unnikrishnan MK. Radiation 18. Protection by the Ocimum flavonoids orientin and vicenin: mechanisms of action. Radiat Res. 2000;154:455–460. doi: 10.1667/0033-7587(2000)154[0455:rpbtof]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 89.Anosike CA, Obidoa O, Ezeanyika LU. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (solanum aethiopicum) DARU. 2012;20:2–7. doi: 10.1186/2008-2231-20-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferrero-Miliani L, Nielson OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker-Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss HP, Tschöpe C, Van Bilsen M, Zannad F, McMurray J, Shah AM. Inflammation as a therapeutic target in heart failure? A scientific statement from the tanslational research committee of the heart failure association of the European society of cardiology. Eur J Heart Fail. 2009;11:119–129. doi: 10.1093/eurjhf/hfn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev. 2010;15:331–341. doi: 10.1007/s10741-009-9140-3. [DOI] [PubMed] [Google Scholar]
- 93.Chang HL, Jong-Hoon K. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Candia AM, Villacorta J, Mesquita ET. Immune-inflammatory activation in heart failure. Arquivos Brasileiros de Cardiologia. 2007;89:183–190. doi: 10.1590/s0066-782x2007001500009. [DOI] [PubMed] [Google Scholar]
- 95.Wallace TC. Anthocyanins in cardiovascular disease. American society for nutrition. Adv Nutr. 2011;2:1–7. doi: 10.3945/an.110.000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kadian R, Parle M. Therapeutic potential and phytopharmacology of tulsi. Int J of Pharm Life Sci (IJPLS) 2012;3:1858–1867. [Google Scholar]
- 97.Pandey G, Madhuri S. Pharmacological activities of Ocimum sanctum (Tulsi): a review. Int J Pharmaceutical Sci Rev Res. 2010;5:61–66. [Google Scholar]
- 98.Singh S, Majumdar DK. Evaluation of anti-inflammatory activity of fatty acids of Ocimum sanctum fixed oil. Indian J Exp Biol. 1997;35:380–383. [PubMed] [Google Scholar]
- 99.Xing L, Jiang M, Dong L, Gao J, Hou Y, Bai G, Luo G. Cardioprotective effects of the YiQiFuMai injection and isolated compounds on attenuating chronic heart failure via NF-κB inactivation and cytokine suppression. J Ethnopharmacol. 2013;148:239–245. doi: 10.1016/j.jep.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 100.Kou J, Sun Y, Lin Y, Cheng Z, Zheng W, Yu B, Zu Q. Anti-inflammatory activities of aqueous extract from radix ophiopogon japonicus and its two constituents. Biol Pharm Bull. 2005;28:1234–1238. doi: 10.1248/bpb.28.1234. [DOI] [PubMed] [Google Scholar]
- 101.Lee DC, Lau AS. Effects of Panax ginseng on tumor necrosis factor-alpha mediated inflammation: a mini-review. Molecules. 2011;16:2802–2816. doi: 10.3390/molecules16042802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oh SY, Kim YH, Bae DS, Um BH, Pan CH, Kim CY, Lee HJ, Lee JK. Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of schisandra chinensis. Biosci Biotechnol Biochem. 2010;74:285–291. doi: 10.1271/bbb.90597. [DOI] [PubMed] [Google Scholar]
- 104.Tian YQ, Yu BY, Kou JP. Progress in pharmacological actions of Ophiopogon japonicus . The Chinese Academic Medical Magazine of Organisms. 2004;1671:1–5. [Google Scholar]
- 105.National Cholesterol Education Program (NCEP) Second report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel II) Bethesda, Md.: National Heart, Lung, and Blood Institute; 1993. (NIH publication no. 93-3095) [Google Scholar]
- 106.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 107.Steinberg D, Witztum JL. Lipoproteins, lipoprotein, oxidation, and atherogenesis. Philadelphia, Pa, USA: WB Saunders; 1999. [Google Scholar]
- 108.Keaney JF. Atherosclerosis: from lesion formation to plaque activation and endothelial dysfunction. Mol Aspects Med. 2000;21:99–166. doi: 10.1016/s0098-2997(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 109.Shamala S, Baskaran G, Noor AS, Siti AA, Mohd YS. Anti-artherosclerotic effects of plant flavonoids. Bio Med Research International. 2014;2014:1–11. [Google Scholar]
- 110.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180:1332–9. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 111.Ross R. Rous-Whipple Award Lecture. Atherosclerosis: a defence mechanism gone awry. Am J Pathol. 1993;143:987–1002. [PMC free article] [PubMed] [Google Scholar]
- 112.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 113.Lee V, Randhawa AK, Singal PK. Adriamycin-induced myocardial dysfunction in vitro is mediated by free radicals. Am J Physiol. 1991;261:H989–995. doi: 10.1152/ajpheart.1991.261.4.H989. [DOI] [PubMed] [Google Scholar]
- 114.Gurvinder S, Anu ST, Aji A, Beena B, Ashok M, Riru V, Shiv KA, Shivesh J, Rama M, Anand CB. Protective effects of Terminalia arjuna against doxorubicin-induced cardiotoxicity. J Ethnopharmacol. 2008;117:123–129. doi: 10.1016/j.jep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 115.Vandana SP, Suresh RN. Evaluation of cardioprotective activity of ginkgo biloba and Ocimum sanctum in rodents. Altern Med Rev. 2009;14:161–171. [PubMed] [Google Scholar]
- 116.Angeloni C, Spencer JP, Leoncini E, Biagi PL, Hrelia S. Role of Quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie. 2007;89:73–82. doi: 10.1016/j.biochi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 117.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi S, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 118.You JS, Pan TL, Lee YS. Protective effects of danshen (Salvia miltiorrhiza) on adriamycin-induced cardiac and hepatic toxicity in rats. Phytother Res. 2007;21:1146–52. doi: 10.1002/ptr.2225. [DOI] [PubMed] [Google Scholar]


