Abstract
A small number of cardiac stem cells that express the c-Kit and Nanog biomarkers can be differentiated into myocardial cells, which suggests that these stem cells may be able to repair damage and provide an internal reserve for tissue regeneration. It is unknown, however, whether the levels of myocardial stem cells are altered after heart failure (HF), and whether HF affects the myocardial regenerative ability. In this study, to address this question, we developed a rat model of chronic HF induced by Adriamycin, and examined the morphological changes in c-Kit and Nanog-expressing stem cells in heart tissue of normal and HF rats. We further measured levels of c-Kit and Nanog expression in the hearts of HF vs. healthy control rats using immunohistochemistry, immunofluorescence, and semi-quantitative reverse transcription (RT)-PCR methods. c-Kit and Nanog were expressed in both normal and HF rats; c-Kit was mainly found in and around the epicardial region, whereas Nanog was primarily expressed in vascular endothelium of some myocardial cells and in stem cells. However, expression of both c-Kit and Nanog was significantly decreased in myocardial cells from HF rats, which may be reflective of reduced myocardial regeneration capacity. These findings indicate that HF not only seriously damages the heart muscle cells, but also the cardiac stem cells. This reduced pool of cardiac stem cells and their related factors is likely to be deleterious for tissue repair after myocardial injury.
Keywords: Myocardial cells, stem cells, immunohistochemistry, heart failure, c-Kit, Nanog, Adriamycin
Introduction
For decades, it was believed that the adult mammalian heart is a post-mitotic organ. However, recent studies have provided clear evidence that the adult heart has an intrinsic regenerative potential, suggesting the potential for cardiac stem cell therapy and heart regeneration. It was previously shown that a small number of stem cells expressing the c-Kit and Nanog proteins can be differentiated into myocardial cells in the normal heart [3]. This suggests that cardiac stem cells have the ability to repair damage, and that they provide an internal reserve for tissue regeneration [14].
c-Kit, also known as mast/stem cell growth factor receptor (SCFR) or CD117, is a receptor tyrosine kinase protein that is expressed on the surface of hematopoietic stem cells and other cell types and plays an important role in cell survival, proliferation, and differentiation [1]. Nanog is a transcription factor that is mainly expressed in undifferentiated stem cells. It is involved in the self-renewal of undifferentiated embryonic stem cells and is also the major phenotypic marker for multipotent stem cells [2]. We previously demonstrated an increased number of Nanog-expressing cells after myocardial infarction, at the edge of the infarction, contributing to the repair of injured tissue [4]. However, it is not yet clear whether myocardial stem cells retain regenerative ability after heart failure (HF).
Anticancer drugs, including anthracyclines such as doxorubicin (trade name, Adriamycin) [5] are cardiotoxic, causing a significant reduction in left ventricular ejection fraction and often resulting in congestive HF. Injection of Adriamycin specifically causes congestive HF in rats and is therefore widely used to generate HF rat models [5,6]. In this study, we generated a rat model of chronic Adriamycin-induced HF and determined the number of c-Kit- and Nanog-expressing stem cells in heart tissue, as well as the levels of mRNA transcript for each gene, in HF vs. control rats, to better understand the role of c-Kit and Nanog in determining potential for myocardial regeneration in congestive HF.
Materials and methods
Animal groups
The rat study protocols were approved by the Xinxiang Medical University Animal Study Committee (Reference No: 20130021). A total of 140 male, 10-week old, Sprague Dawley rats, weighing 240 ± 2.5 g, were provided by the Xinxiang Medical University Animal Center, and were divided into a normal control group (115 rats) and an HF group (25 rats).
Generation of rat model for congestive HF
HF was induced by injecting Adriamycin into the peritoneal cavity of 10 week-old male rats at a dosage of 4 mg/kg, once a week, for a total of 6 weeks. Control-group rats were injected with saline only, once a week, for a total of 6 weeks. Starting at 15 weeks of age, control and HF rats were subjected to examination every week for 4 weeks, continuously. Cardiac function for both groups was then measured by Color Doppler ultrasound. A left ventricular ejection fraction (EF%) <45% was designated as the cut-off criterion for HF.
Sampling
After cardiac function was determined, rats were anesthetized with 10% chloral hydrate anesthesia. The chests were opened, and the hearts were rapidly dissected; residual blood was washed away with ice-cold phosphate-buffered saline (PBS) buffer, and large blood vessels and surrounding connective tissue were removed with scissors. For each dissected heart, half of the organ was placed in a frozen pipe that was flash frozen in liquid nitrogen and transferred to a -80°C freezer for storage and subsequent analysis by western blotting and reverse transcription (RT-PCR). The other half was fixed in 4% paraformaldehyde for 12 h, followed by 20% and 30% sucrose gradient dehydration. This tissue was then frozen and sectioned at a thickness of 10 μm, using a Constant-cooling Freezing and Paraffin Microtome. Slides were subjected to Masson’s trichrome, immunohistochemical, and immunofluorescence staining.
Immunohistochemical staining
Frozen sections were immunostained with rabbit-anti-rat Nanog polyclonal antibody (1:200, Santa Cruz Biotechnology, Dallas, TX) and mouse anti-rat c-Kit monoclonal antibody (1:100, Santa Cruz Biotechnology). Hematoxylin counterstaining was then performed, and the sections were dehydrated and sealed with rhamsan gum for imaging. Immunohistochemistry diluent was used in place of the anti-Nanog and anti-c-Kit antibodies in negative controls.
Reverse transcription (RT)-PCR analysis
Myocardium tissue from five HF and five control rats was excised and grounded, and total RNA was isolated using the TRIzol Reagent (Life Technologies, Carlsbad, CA). Synthesis of cDNA was then performed using the SUPERSCRIPT Preamplification System for First-Strand cDNA Synthesis, according to manufacturer protocols (Life Technologies). PCR products were electrophoresed on agarose gels, and this was imaged and processed with ImageJ software (rsb.info.nih.gov/ij) for quantification and analysis. Levels of the c-kit and Nanog mRNA transcripts were normalized to β-actin. The PCR primers used to amplify target genes were as follows: c-kit: 5’-CTAGCCAGAGACATCAGGA, 3’-CCATAGGACCAGACATCAC; nanog 5’-AGAAGATGCGGACTGTGTTC, 3’-GCTCAGGTTCAGAATGGTAGA; β-actin 5’-TGCTTCCTCTTCTTCCCTGG, 3’-AAGATGGCTGGAAGAGGGTC. The relative mRNA expression ratios for c-kit, nanog, and β-actin were determined according to the integrated absorbance values.
Statistical analysis
Statistical analyses were performed using the Student t-test for data analysis. All data are presented as mean values ± standard deviation (Mean ± SD). P<0.05 was considered statistically significant.
Results
Validation of HF models by accessing cardiac function
After Adriamycin injection, the cardiac function of Adriamycin-injected rats (HF group) and age-matched normal control rats (Control group) was accessed by echocardiography measurements. The normal control rats showed normal heart function in each of the measured cardiac function parameters, including Left Ventricular Diastolic Dimension (LVDD, mm), Left Ventricular Systolic Dimension (LVSD, mm), Left Ventricular Ejection Fraction (LVEF%), and Left Ventricular Fractional Shortening (LVFS). Critically, these parameters were all significantly altered in rats from the HR group, as compared with the age-matched controls (Table 1); LVDD and LVSD were significantly higher (P<0.05) and LVEF% and LVFS were significantly lower (P<0.05), indicating left ventricular systolic dysfunction. Thus, these cardiac function tests validated that HF was successfully induced in rats by injection of Adriamycin.
Table 1.
Echocardiographic cardiac function in normal control and HF rats (Mean ± SD)
| Group | Age (Weeks) | After injection (weeks) | LVDD (mm) | LVSD (mm) | LVEF (%) | LV FS (%) |
|---|---|---|---|---|---|---|
| Ctrl | 15 | 5 | 5.7 ± 1.4 | 3.7 ± 1.1 | 70.7 ± 1.2 | 35.1 ± 1.3 |
| HF | 15 | 5 | 6.1 ± 1.4 | 4.0 ± 1.1 | 70.4 ± 0.9 | 35.0 ± 1.6 |
| Ctrl | 16 | 6 | 4.9 ± 1.5 | 3.2 ± 1.3 | 70.0 ± 1.4 | 34.4 ± 1.1 |
| HF | 16 | 6 | 5.8 ± 0.7 | 2.8 ± 1.8 | 65.3 ± 2.1 | 30.8 ± 1.9 |
| Ctrl | 17 | 7 | 5.0 ± 1.3 | 3.2 ± 1.4 | 72.5 ± 1.1 | 36.2 ± 1.2 |
| HF | 17 | 7 | 5.5 ± 1.9 | 4.1 ± 0.8 | 55.7±1.3 | 25.0 ± 1.7 |
| Ctrl | 18 | 8 | 4.0 ± 1.5 | 2.1 ± 1.4 | 70.9 ± 1.1 | 34.9 ± 1.1 |
| HF | 18 | 8 | 6.7 ± 0.5* | 5.7 ± 0.6* | 34.6 ± 9.1* | 14.8 ± 3.6* |
P<0.05.
Pathologic changes in the myocardium of rats with congestive HF
Using Masson’s trichrome staining, we found that in control rats, the myocardium tissue appeared normal, with little intercellular collagen fibers and few vacuoles within the subendocardial and subepicardial myocardial cells (Figure 1A and 1B). In contrast, the myocardium of HF rats showed displacement of cardiac myocytes, and some portions of the heart muscle were disrupted. Intercellular collagen fibers were also substantially increased in HF tissue, and the subendocardial and subepicardial myocardial cells contained numerous vacuoles of varying sizes and shapes (e.g. round and elongated) (Figure 1C and 1D).
Figure 1.
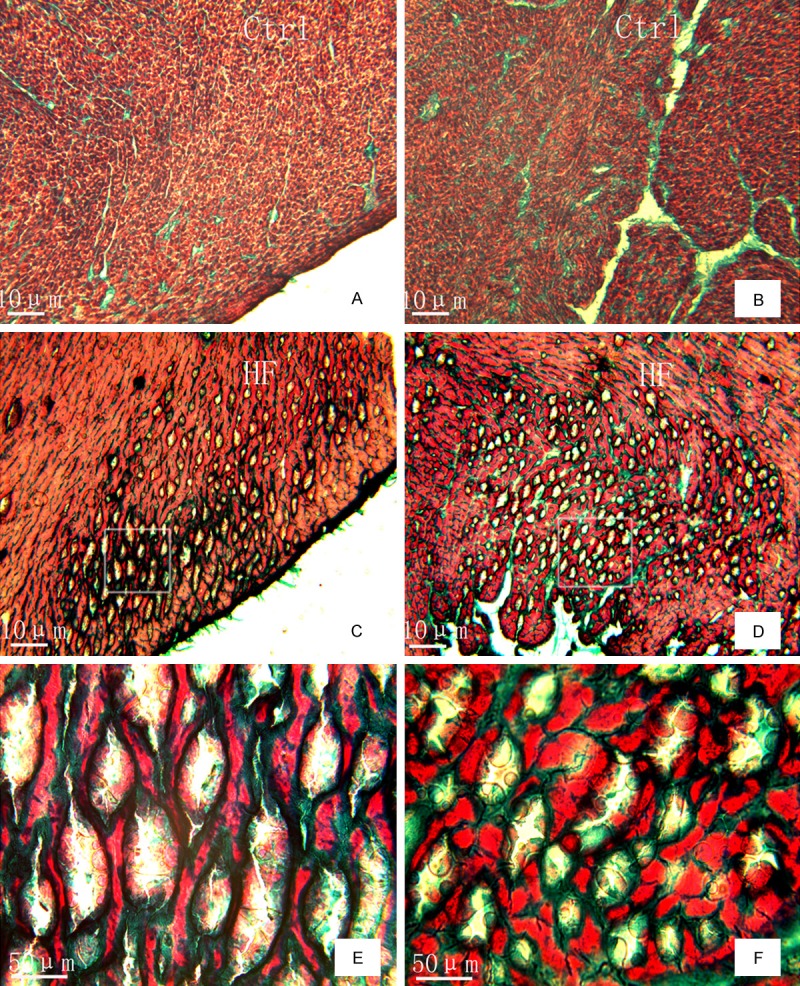
Pathological changes in the myocardial tissue of HF rats compared to normal control animals, as assessed by Masson’s trichrome staining. (A, C) Shows the subepicardial myocardium, (B, D) shows the subendocardial myocardium, and (E, F) are magnification of grams in (C and D). Scale bar: 10 µm.
Nanog and c-Kit protein expression in cardiac tissue from control and HF rats
Immunohistochemical staining revealed that, in normal myocardium, Nanog expression was observed in a small number of heart muscle cells, vascular endothelial cells, and small round stem cells. Nanog-expressing myocardial cells were either scattered or in clustered groups, and this protein was present in most vascular endothelial cells. The majority of Nanog-positive stem cells were found singly or in small groups associated with the small blood vessels (Figure 2A). In the myocardial tissue of rats with congestive HF, all three types of Nanog-positive cells were decreased, and positive cells were only present in the subendocardial and subepicardial myocardium (Figure 2B).
Figure 2.
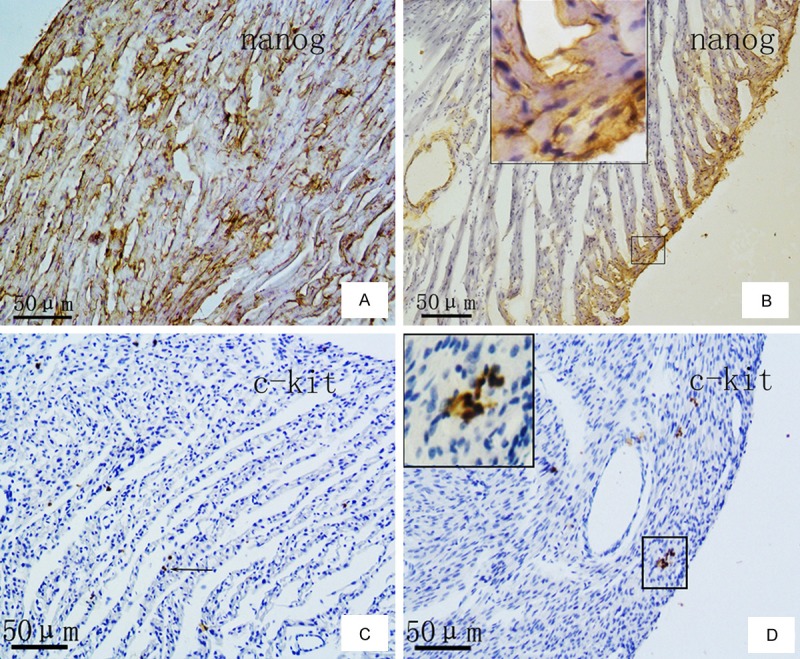
Expression of Nanog and c-Kit proteins in normal rat myocardial tissue (A, C) and in HF rat myocardial tissue (B, D) by immunohistochemical staining with anti-Nanog (A, B) and anti-c-Kit antibodies (C, D) and hematoxylin counterstaining. Scale bar: 50 µm.
In myocardial tissue from normal rats and those with congestive HF, c-Kit expression was mainly observed in small round or oval cells, which were clustered in small groups. No positive expression for c-Kit was detected in either cardiac muscle or endothelial cells (Figure 2C). Notably, when compared with normal myocardium, the number of the c-Kit positive cells was significantly reduced in the HF myocardium (Figure 2D).
Expression of c-kit and Nanog mRNA transcripts in HF and control rats
We performed RT-PCR to detect the c-kit and Nanog transcripts in mRNA from the cardiac tissue of control and HF rats, and found that the levels of both were significantly reduced in the HF myocardium compared to controls (Figure 3A). The relative expression levels of c-kit mRNA were significantly reduced about 50% in HF animals, as compared to controls (P=0.037) by quantification of the RT-PCR product image density. In addition, the relative expression levels of Nanog mRNA were also significantly reduced 22% in HF tissue vs. controls (P=0.018) (Figure 3B).
Figure 3.
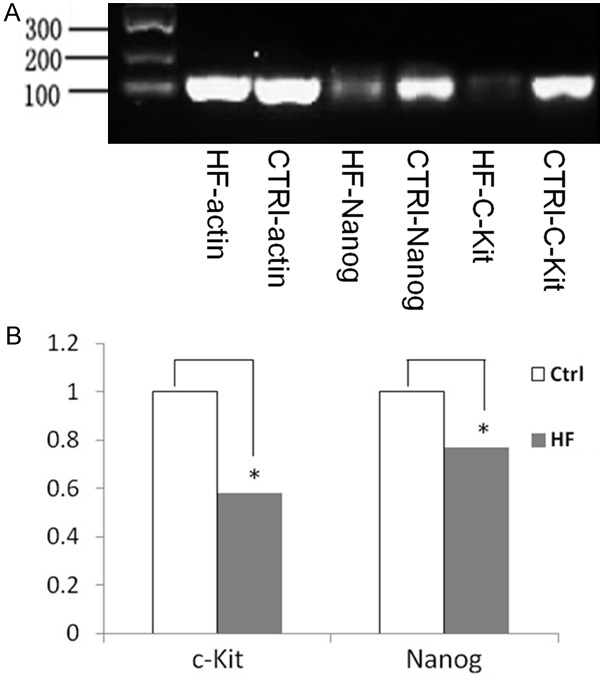
A. Expression levels of c-kit and nanog mRNAs in myocardial tissue from normal and HF rats by RT-PCR. B. Quantification of transcripts relative to β-actin demonstrates that, in HF rats, myocardial levels of c-kit and nanog mRNAs are significantly reduced 50% and 22% respectively, as compared to the levels found in normal controls control (*P<0.05).
Discussion
In this study, we have demonstrated that c-Kit and Nanog are expressed in the myocardial stem cells from both normal and HF rats. In addition, their expression was found to be significantly decreased in myocardial cells of HF rats, at both the RNA and protein levels, which may reduce the myocardial regeneration capacity after HF. These findings support the hypothesis that HF not only seriously damages the heart muscle cells, but also the cardiac stem cells.
Chronic HF is often the end stage or final outcome of many cardiovascular conditions; it has a high incidence and is associated with a high morbidity and mortality. Currently, the only curative option for HF treatment is a heart transplant. Even though the success rate for this procedure has been high, with the 5-year survival rate reaching 75%, this treatment option is limited due to severe shortage of heart donors. Therefore, options for chronic HF treatment remain limited.
It has been a long-held assertion that although mammalian embryonic heart cells can divide and mature, these proliferative abilities are lost soon after birth. However, in recent years, new data obtained using improved experimental techniques have shifted this paradigm, leading to significant progress in this field. In 2001, Beltrami et al. first observed mitosis in normal adult rat heart myocardial cells [7]. This has been supported by a growing body of research, demonstrating that adult myocardial cells can grow and divide, and isolated cardiac stem cells can be differentiated into myocardial cells, both in vitro and in vivo.
The c-Kit protein is an intrinsic stem cell biomarker that is present in embryonic, newborn, and adult mammalian heart stem cells [8]. It has been hypothesized that the number of the c-Kit-expressing cells may reflect the intrinsic cell developmental and/or physical stage of development [9]. Notably, c-Kit-positive cardiac stem cells are currently being used in clinical trials to treat heart disease, for example, as an infusion to promote tissue regeneration after myocardial infarction (CADUCEUS trial) [10] and to restore myocardial function in patients with ischemic heart failure (SCIPIO trial) [11]. The Nanog transcription factor was first described in 2003 that described an inner cell mass of the blastocyst containing primordial germ cells and embryonic stem cells (ESCs), both of which express this protein [12]. Nanog is mainly expressed in undifferentiated ESCs, as well as in some adult cells and tumors, and it is believed to helps maintain stem cell self-renewal, differentiation, and proliferative abilities. It can also be used to induce myocardial cells to re-enter the cell cycle [12]. In 2013, research from Senyo et al. suggested that Nanog-positive stem cells in the adult heart reach an annual turnover rate of 4%-10% [13], and it was subsequently suggested by Kajstura et al. that this rate can reach as high a 40% [14]. Collectively, these data suggest that cardiac stem cells expressing c-Kit and Nanog may play important roles in self-renewal and in the ability of the heart to repair itself after injury.
Most previously published reports have focused on the role of c-Kit- and Nanog-positive cells in the normal myocardium [7,15], and to date, how these cells are affected by acute myocardial infarction has not been reported. In this study, we compared the levels of c-Kit and Nanog expression in myocardial tissue from adult rats with Adriamycin-induced HF and healthy controls. We found that in HF, the number of both c-Kit and Nanog-positive cells was reduced, and consistent with this, the levels of their associated mRNA transcripts were also reduced in the myocardial tissue of adult HF rats. These findings indicate that HF not only seriously damages the heart muscle cells, but also appears to deplete the reserve of cardiac stem cells, which is likely to have deleterious effects on the ability to mediate repair after myocardial injury. This phenomenon may result from HF-induced changes in the myocardial environment that make it no longer suitable for stem cell growth and proliferation, or that trigger an unknown mechanism, which induces stem cell apoptosis. In addition, although it has been generally recognized that c-Kit- and Nanog-positive cells represent two different types of cardiac stem cells, this study showed a small number of cells co-expressing these two proteins by immunofluorescence colocalization, consistent with our previous report [16]. Further studies are warranted to determine whether this small population of cells co-expressing c-Kit and Nanog merely reflect the phenotypic diversity of known stem cells or whether they are, in fact, a distinct type of stem cell.
Acknowledgements
The work in this study was supported by grants from the National Natural Science Foundation of China (No: 30740069).
Disclosure of conflict of interest
None.
References
- 1.Edling CE, Hallberg B. c-Kit--a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol. 2007;39:1995–1998. doi: 10.1016/j.biocel.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 3.Tulloch NL, Pabon L, Murry CE. Get with the(re) programl: cardiovascular potential of skin-derived induced pluripotent stem cells. Circulation. 2008;118:472–475. doi: 10.1161/CIRCULATIONAHA.108.791442. [DOI] [PubMed] [Google Scholar]
- 4.Luo H, Li Q, Pramanik J, Luo J, Guo Z. Nanog expression in heart tissues induced by acute myocardial infarction. Histol Histopathol. 2014;29:1287–1293. doi: 10.14670/HH-29.1287. [DOI] [PubMed] [Google Scholar]
- 5.Li YL, Yang JY, Tang JM, Pan GD, Wang JN. Comparison of various chronic heart failure models induced by adriamycin. Chinese J Comp Med. 2006;16:93–96. [Google Scholar]
- 6.Rochette L, Guenancia C, Gudjoncik A, Hachet O, Zeller M, Cottin Y, Vergely C. Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci. 2015;36:326–348. doi: 10.1016/j.tips.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Eng J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 8.Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, Heffernan C, Menon MK, Scarlett CJ, Rashidianfar A, Biben C, Zoellner H, Colvin EK, Pimanda JE, Biankin AV, Zhou B, Pu WT, Prall OW, Harvey RP. Adult cardiac-resident MSC-like stem cells with aproepicardial origin. Cell Stem Cell. 2011;9:527–540. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellison GM, Galuppo V, Vicinanza C. Cardiac stem and progenitor cell identification: different markers for the same cell? Front Biosci (Schol Ed) 2010;2:641–652. doi: 10.2741/s91. [DOI] [PubMed] [Google Scholar]
- 10.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, rando-mised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial functionand viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang L, Guo ZK, Yan Q. Immortality gene Nanog advances. Med Rev. 2007;13:1292–1294. [Google Scholar]
- 13.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mam-malian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajstura J, Rota M, Cappetta D, Ogórek B, Arranto C, Bai Y, Ferreira-Martins J, Signore S, Sanada F, Matsuda A, Kostyla J, Caballero MV, Fiorini C, D’Alessandro DA, Michler RE, del Monte F, Hosoda T, Perrella MA, Leri A, Buchholz BA, Loscalzo J, Anversa P. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126:1869–1881. doi: 10.1161/CIRCULATIONAHA.112.118380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Liudmila Z, Hikmet NG, James N, Popovic S, Gaballa MA. Chronic heart failure is associated with transforming growth factor beta-dependent yield and functional decline in atrial explant-derived c-kit+ cells. J Am Heart Assoc. 2013;2:e000317. doi: 10.1161/JAHA.113.000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo ZK, Guo K, Luo HH, Mu LM, Li Q, Chang YQ. The expression analysis of nanog in the developing rat myocardial tissues. Cell Physiol Bioch. 2015;35:866–874. doi: 10.1159/000369744. [DOI] [PubMed] [Google Scholar]


