Abstract
Physical inactivity and dyslipidemia are considered risk factors for cardiovascular diseases. There are few studies evaluating the effects of physical exercise in small-caliber artery in a model that mimics familial hypercholesterolemia. The aim of this study was to examine the effect of exercise training, at moderate intensity, on metabolic parameters and iliac artery responsiveness in LDL-/- mice. Sedentary (SD) and trained (TR) mice performed AET (5 days/week, 60 minutes/day at 60-70% of maximum speed) during 8 weeks. Body weight gain (BWG), epididymal fat, blood glucose, total cholesterol and triglycerides were evaluated. Concentration-response curves to acetylcholine (ACh), sodium nitroprusside, phenylephrine and U46619 were obtained in isolated iliac artery. The production of nitric oxide (NO) and reactive oxygen species as well as the expression and activity of MMP-2 were assessed. AET was effective in preventing BWG and epididymal fat gain, whereas no changes were observed in glucose, total cholesterol and triglycerides levels. Improvement in responsiveness to ACh was found in TR (Emax = 85±3%) compared with SD group (Emax = 62±5%) without changes in the maximal vascular response or potency to SNP, PHE and U46619. The NO level was increased (10.8-fold) while ROS formation was decreased (3.7-fold) in iliac artery from TR, without changes in MMP-2 activity or its expression. AET was effective to improve endothelium-dependent relaxation that was accompanied by increased NO production and decreased ROS formation in iliac artery. The intensity of AET should be greater to modify metabolic disorders in this experimental model of dyslipidemia.
Keywords: Vascular reactivity, microvasculature, familial hypercholesterolemia, oxidative stress, physical exercise
Introduction
According to the World Health Organization more than 17.3 million deaths per year are attributed to cardio-metabolic diseases such as arterial hypertension, type 2 diabetes mellitus and atherosclerosis and it is expected an increase to over 23 million in 2030. Atherosclerosis is a long lasting disease with genetic and environmental background that contributes to the development of the atherosclerotic lesion and its progression. Physical inactivity, obesity, lipid profile alterations, smoking, excessive alcohol consumption, and mutation in LDL-cholesterol receptor are the main risk factors for this cardio-metabolic disease [1,2].
Endothelium dysfunction is a hallmark of atherosclerosis [3]. Indeed, previous studies have consistently demonstrated an impairment of relaxing responses in experimental model of atherosclerosis in different animal species including rats [4] mice [5] and pigs [6]. In the early stage of atherosclerosis, infiltration of small particles of LDL-cholesterol, reactive oxygen species (ROS) production and immune cells, particularly monocytes, which infiltrate into the arterial intima to phagocyte the oxidized LDL took place. Release of pro-inflammatory cytokines, increased metalloproteinases (MMP), mainly MMP-2 and MMP-9, cell growth factors resulting in proliferation of smooth muscle layer and endothelial cells damage complete this phase [7].
On the other hand, it is well known that aerobic physical training ameliorates vascular responses through shear stress [8]. Accordingly, the beneficial effects of physical training are by activation of mechanosensors present in endothelial cells that are coupled to complex biochemical signaling pathways, including Ras/MEK/ERK, c-Src, G proteins, ion channel, VE-cadherin, and PI3K/Akt, which in turn, regulate nitric oxide (NO) signaling pathway [9]. A number of studies has evaluated the effects of exercise training in low-density lipoprotein receptor deficient mice (LDLr-/-) using isolated aorta [10-13]. However, no studies exist examining the effects of exercise training in iliac artery in this experimental model of atherosclerosis.
The rationale for studying iliac artery are a) arteries with lower blood flow velocity are more susceptible to the development of atherosclerosis; b) in the late stage of atherosclerosis, plaque reduces the blood flow in the iliac artery, as they enter the lower limbs, causing intermittent claudication and critical limb ischemia; c) lower limb peripheral artery disease (PAD) usually is underdiagnosed, undertreated and poorly understood [14,15], and finally d) examining the early stage of atherosclerosis is a key strategy to preventing the complications of this disease in lower limb artery with a low cost. Therefore, the aim of this study was to examine the effect of exercise training, at moderate intensity, on vascular reactivity of the iliac artery and metabolic parameters in LDLr-/- mice fed high fat diet. To further evaluate the insight mechanisms by which exercise training would promote beneficial effects, we also measured NO and ROS production as well as the protein expression of MMP-2 in iliac artery from LDLr-/- mice.
Material and methods
Animals and experimental procedures
Twenty-eight week-old LDLr-/- mice were divided into sedentary (SD) and trained (TR). Animals were housed in polypropylene cages (41 × 34 × 30 cm), five animals in each on a 12 h light/dark cycle and had free access to water and high-fat diet (32% carbohydrate, 20% protein, 38% fat) providing 6.2 Kcal/gram (PragSoluções Biosciences, Jau, SP). Body weight and food intake were assessed weekly.
Physical exercise sessions were carried out during eight weeks for TR group. Mice ran on a treadmill during 60 minutes, five days a week, at 60% of their maximal speed which was determined using a maximal incremental test. This test consisted of run, beginning at 5 m/minute and every 3 minutes the speed was increased in 5 m/minute until exhaustion (determined when the animal touched the bottom of the bay, five times within one minute). The speed at which the mouse achieved exhaustion was considered as the maximal speed.
All procedures were reviewed and approved by the Ethics Committee on Animal Use in Research of University of Campinas (CEUA/UNICAMP protocol number 3537-1) in compliance with the Brazilian national law (CONCEA publication Nº 11.794, 2008).
Blood glucose, serum cholesterol and triglycerides
After 48 hours of the last exercise session, fasting blood glucose was determined using a drop of blood collected from the tail of animals and reactive test strips that were inserted into digital meter Accu-Check Advantage (Roche Diagnostics Corporation, Indianapolis, IN).
After this procedure, animals were anesthetized (2 g/kg urethane i.p.) and cardiac puncture was performed to exsanguination and diaphragm was ruptured. Blood samples were taken and serum was immediately separated by centrifugation (8,000 g), freeze (-80°C) and after used to determine total cholesterol and triglycerides by enzymatic colorimetric method (Roche Diagnostic GmbH, Mannheim, Germany). Epididymal fat was carefully removed and weight.
Concentration-response curves in isolated iliac artery
The iliac artery was carefully isolated and placed in freshly prepared ice-cold Krebs solution containing (mM): NaCl, 118; NaHCO3, 25; Glucose, 5.6; KCl, 4.7; KH2PO4, 1.2; MgSO4, 7; H2O, 1.1; and CaCl2, 2; H2O, 2.5. In the sequence, the iliac artery was cleaned of all adherent tissue and cut into rings of 2 mm; two wires (20 mm diameter each) were introduced through the lumen of segments and mounted in a small vessel myograph chamber Model 610 M-DMT (Danish Myo Technology, Aarhus, NA) with 5 ml Krebs solution at 37°C, pH 7.4 and continuously gassed with 95% O2 and 5% CO2 under a resting tension of 0 mN. After 15 minutes of equilibration period, rings were stretched to their optimal lumen diameter based on the internal circumference, wall length and wall tension using specific software for normalization (LabChart Pro-DMT Normalization Module, AD Instruments, Sydney, Australia). Normalization was performed by distending the vessel stepwise and measuring sets of micrometer readings of force [16,17]. Data acquisition was performed using PowerLab 8/30 (LabChart, version 7.0, AD Instruments, Sydney, Australia).
After 45 minutes of equilibration period, iliac rings were pre-contracted with KCl (80 mM) until reach maximal response and washed using Krebs solution. Cumulative concentration response curves to acetylcholine (ACh: 1 nM-300 μM) and sodium nitroprusside (SNP: 100 fM-3 μM) were done in rings pre-contracted with tromboxane A2 analogue (U46619) in a concentration that indices 50-80% of maximal response obtained with KCl (80 mM).
Contractile responses were also evaluated using cumulative concentration-response curves to phenylephrine (PHE: 1 nM-300 μM) added propranolol (100 nM) and to tromboxane A2 analogue (U46619: 100 pM-3 μM). The contractile response was determined is mN/mm.
Concentration-response data were evaluated for a fit to a logistics function in the form: E = Emax/((1+(10c/10x)n) + Ф), where E is the response; Emax is the maximum response that the agonist produces; c is the logarithm of the EC50, the concentration of agonist that produces half-maximal response; x is the logarithm of the concentration of agonist; the exponential term, n, is a curve fitting parameter that defines the slope of the concentration-response line, and Ф is the response observed in the absence of added agonist. Nonlinear regression analyses to determine the parameters Emax, log EC50 and n were done using GraphPad Prism (GraphPad Software, San Diego-CA, USA) with the constraint that Ф = zero.
NO production
NO production was evaluated using fluorescent dye 4,5-diaminofluorescein diacetate (DAF-2, Sigma-Aldrich CO, Saint Louis, MO) as previous described [17]. Iliac artery was embedded in OCT and transversal sections (10 µm) were obtained using a cryostat. Two section of each artery were incubated at 37°C for 30 minutes with 100 µl phosphate buffer (PBS, 0.1 M, pH = 7.4) added calcium (0, 45 mM). Then, fresh PBS containing DAF-2 (8 µM) was applied to each tissue section and the slices were incubated in a light-protected humidified chamber at 37°C for 25 min. After, one slice of each artery was stimulated with ACh (10 µM) during 15 minutes. Images were obtained with an optical microscope (Olympus BX60, Olympus, Center Valley, PA, USA) equipped with filter to fluorescein and camera (Olympus DP-72) using a 20 × objective. The fluorescence was quantified using Image J Software (National Institutes of Health, Bethesda, MD, USA). The value used was a mean obtained from seven different vessel areas. The results were expressed as the delta of ACh-stimulation integrative density minus basal integrative density.
ROS detection
The oxidative fluorescent dye dihydroethidium (DHE) was used to evaluate in situ ROS production, more specifically the anion superoxide [18]. Transverse iliac sections (10 µm) obtained in a cryostat were incubated at 37°C for 10 minutes with 100 µl PBS (0.1 M, pH = 7.4) added pentetic acid (DTPA, 10-4 M). Then, fresh PBS containing DHE (2 µM) was applied to each tissue section and the slices were incubated in a light-protected humidified chamber at 37°C for 30 minutes, in the presence or in the absence of superoxide dismutase (SOD, 2.5 µM). Images were obtained with an optical microscope (Olympus BX60, Olympus, Center Valley, PA, USA) equipped with filter to rhodamine and camera (Olympus DP-72) using a 20 × objective. The fluorescence was quantified using the Image J Software (National Institutes of Health, Bethesda, MD, USA). The value used was a mean obtained from seven different vessel areas. The results were expressed as the delta of basal integrative density minus SOD integrative density.
Activity and expression of MMP-2
Transverse iliac sections (5 µm) obtained in a cryostat were incubated with 50 µL of PBS (0.1 M, pH = 7.4) for 10 minutes. Then, buffer was removed and sections were incubated with 10 μl of DQ gelatin (1:10 in Tris 50 mM CaCl2 pH 7.4, Thermo Fisher Scientific) in a light-protected humidified chamber at 37°C for 60 minutes. All sections were then washed three times with PBS and incubated with 50 µL of MMP-2 antibody (1:200 dilution, MAB3308, Millipore) in a light-protected humidified chamber at room temperature for 60 minutes. Subsequently, all sections were washed using PBS and incubated with 50 µL rhodamine secondary antibody (1:200 dilution, Millipore) in a light-protected humidified chamber at room temperature for 60 minutes. Finally, 10 μl of 4% paraformaldehyde were applied to each section, and then maintained in a light-protected humidified chamber at 37°C for 20 minutes. Afterwards, the samples were washed three times with PBS and DAPI (1:1000 dilution, Life Technologies) was applied. Images were obtained with an optical microscope (Olympus BX50, Olympus, Center Valley, PA, USA) using filter to DQ gelatin, rhodamine and DAPI and equipped with camera (Olympus DP-72) using a 20 × objective. The fluorescence was quantified using the Image J Software. The value used was a mean obtained from seven different areas in the media layer. Negative controls were done using only PBS, only MMP-2 antibody and only rhodamine.
Statistical analysis
Data are presented as means ± standard error mean (SEM) of n experiments. GraphPad Instat version 3.0 was used to perform Kolmogorov and Smirnov normality test followed by unpaired t test (two-tail). The level of significance adopted was P<0.05.
Results
Body weight gain, epididymal fat, food intake and metabolic parameters
TR animals had lower body weight gain and epididymal fat pad as compared with SD, approximately 62 and 45%, respectively. On the other hand, no changes were found in blood glucose, total cholesterol and triglycerides levels after eight weeks of exercise training (Table 1).
Table 1.
Body weight values, epididymal fat mass and metabolic parameters in LDLr-/- mice from SD and TR groups
| SD | TR | |
|---|---|---|
| Initial body weight (g) | 23.2±0.3 | 23.3±0.6 |
| Final body weight (g) | 33.4±1.0 | 27.1±0.8* |
| Body weight gain (g) | 10.6±0.9 | 4.0±0.5* |
| Epididymal fat (g) | 1.1±0.1 | 0.6±0.1* |
| Food intake (g/mice/dia) | 3.5±0.1 | 3.6±0.1 |
| Food energy (Kcal/mice/day) | 21.5±0.5 | 22.2±0.5 |
| Glucose (mg/dL) | 164.6±10.0 | 151.6±7.4 |
| Total cholesterol (mg/dL) | 271.3±14.2 | 271.8±14.3 |
| Triglycerides (mg/dL) | 180.8±11.3 | 179.1±15.3 |
Data are expressed as mean ± SEM.
Different SD;
P<0.05 (unpaired t test).
Concentration-response curves in iliac artery
Endothelium-dependent relaxation evoked by ACh was 37% higher in TR than in SD mice, without changes in its potency (SD: 7.33±0.20 and TR: 7.28±0.12). Neither the maximal arterial responses (SD: 96.1±0.9% and TR: 92.3±2.7%) nor the potency to SNP (SD: 6.96±0.20 and TR: 6.68±0.28) were modified by exercise training (Figure 1A and 1B).
Figure 1.
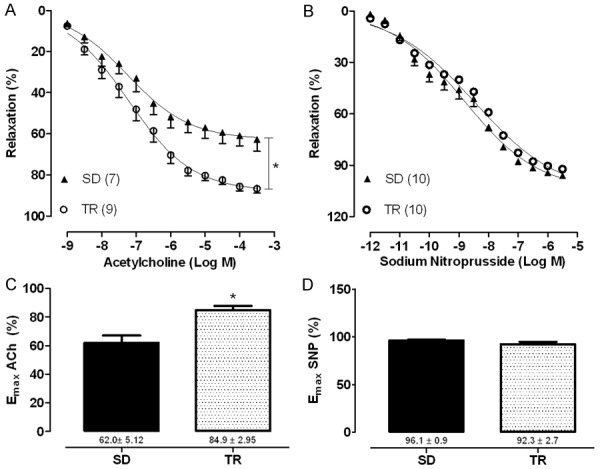
Concentration-response curve and maximal response to acetylcholine (ACh, A, C) and sodium nitroprusside (SNP, B, D) in iliac artery with intact endothelium in sedentary (SD) and trained (TR) groups. Data are expressed as mean ± SEM. Experimental n is represented in parentheses. *Different SD; P<0.05 (unpaired t test).
Regarding arterial contractile response to PHE, no differences were seen in both, the maximal responses (SD: 3.3±0.3 mN/mm and TR: 2.9±0.4 mN/mm) and the potency (SD: 6.23±0.06 and TR: 6.57±0.20) for this contractile agent. Similar results were observed by using U46619 (maximal response: SD: 3.8±0.3 mN/mm and TR: 3.3±0.3 mN/mm, and potency: SD: 8.02±0.09 and TR: 7.98±0.02) (Figure 2A and 2B).
Figure 2.
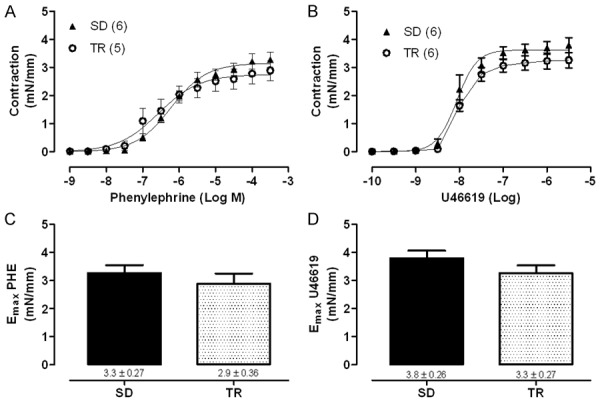
Concentration-response curves and maximal response to phenylephrine (PHE, A, C) and to the analogue of thromboxane A2 (U46619, B, D) in iliac artery with intact endothelium in sedentary (SD) and trained (TR) groups. Data are expressed as mean ± SEM. Experimental n is represented in parentheses.
In order to examine the insight mechanisms underlying the improvement in the relaxing responses found in trained LDLr-/- mice fed with high fat diet, NO and ROS production were analyzed in situ in the iliac arteries. Increased NO production (10.8-fold) was found in iliac artery from trained animals (Figure 3A and 3B) whereas ROS formation was significantly decreased, approximately 3.7-fold (Figure 3C and 3D).
Figure 3.
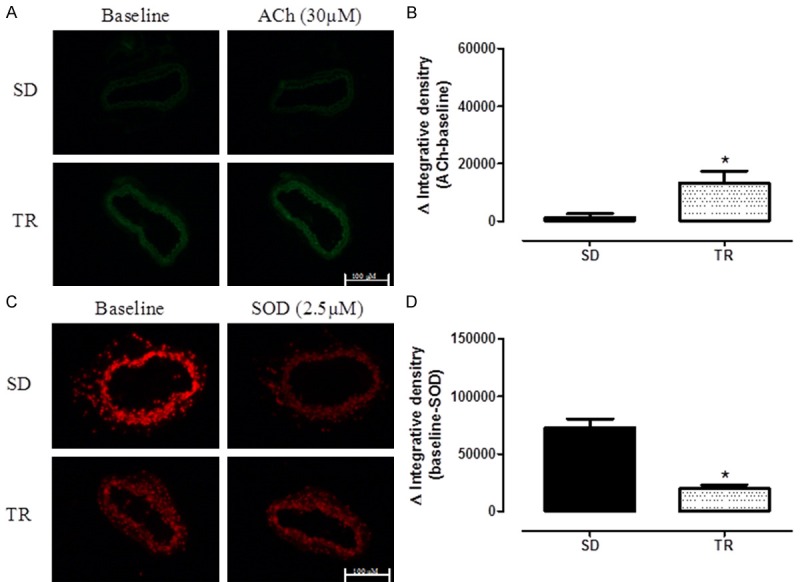
Representative/quantitative analysis for 4.5-diaminofluoresceína (DAF-2), a marker of NO production (A and B) and to DHE a marker of reactive oxygen species production (C and D) in iliac artery with intact endothelium in sedentary (SD, n = 8) and trained (TR, n = 7) groups. DAF-2 was done in absence (baseline) or presence of Acetylcholine (ACh, 30 μM). DHE was done in absence (baseline) or presence of SOD mimetic (2.5 μM). The value of fluorescence integrated density is a mean obtained from the measurement of 7 regions (randomly choose) of smooth muscle layer. Data are expressed as mean ± SEM. *Different SD; P<0.05 (unpaired test).
Given that MMP-2 plays a crucial effect on vascular remodeling in atherosclerosis and NO plays a protective action on vascular smooth muscle cells proliferation, MMP-2 activity and its expression were examined in iliac artery from TR LDLr-/- mice fed with high fat diet. Exercise training for eight weeks failed to cause any changes in both parameters of MMP-2 (Figure 4).
Figure 4.
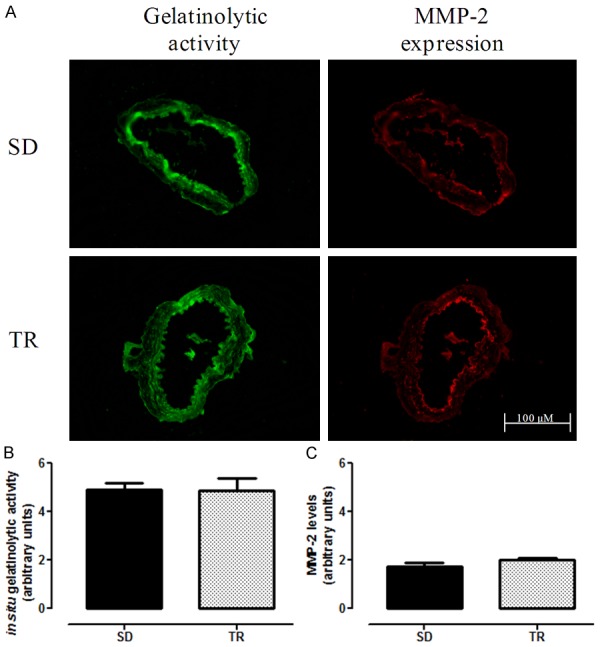
Representative/quantitative analysis for MMP-2 in iliac artery with intact endothelium in sedentary (SD, n = 8) and trained (TR, n = 7) groups. Gelatinolytic activity and MMP-2 expression (A), quantification of gelatinase activity (B) and quantification of MMP-2 expression (C). The value of fluorescence integrated density is a mean obtained from the measurement of 7 regions (randomly choose) of the media layer. Data are expressed as mean ± SEM.
Discussion
Familial hypercholesterolemia is a genetic disorder characterized by high levels of LDL-cholesterol and premature atherosclerotic cardiovascular disease [19]. The animal model used in this study mimics familial hypercholesterolemia as it lacks LDL receptor [20].
In the present study, AET was successful in reducing body weight gain and epididymal fat pad showing that high-fat diet associated with the practice of physical exercise was effective in changing both parameters without changing food intake. However, lipid profile and glucose were unaltered after AET. Accordingly, a previous study examining a similar volume of AET (8 weeks) failed to find changes in lipid profile even though the period for high-fat diet was longer as compared with our study [21]. On the other hand, studies using cholesterol-rich diets found beneficial effects of exercise training on lipid profile in the same mouse model [12,13]. Blood glucose was not affected by moderate exercise training during eight weeks. Indeed, a recent study using ob/ob mice shows that high-intensity exercise is more effective in increasing glucose transporters-4 and consequently glycemia reduction [22]. Collectively, these findings indicate that the intensity of exercise training should be higher to achieve metabolic changes in this particular animal model if fed with high-fat diet.
It is well known that vascular responses in LDLr-/- mice show impairment in endothelium-dependent relaxation [5,23]. However, most of these studies have examined the aortic rings in this experimental model [24-26], and no studies have examined the lower limb artery. In view of the fact that vascular responsiveness to acetylcholine, for example, is different among vascular beds [4,17] this study was the first to show iliac artery responsiveness in a model of familial hypercholesterolemia. Iliac artery is an important vascular bed in terms of blood supply of the lower limbs, thus studies using this vascular bed are extremely relevant in an attempt to prevent the high rate of mortality and morbidity as consequence of peripheral artery disease [15]. The relaxing response evoked by acetylcholine, an agent that elicits endothelium-dependent response was significantly attenuated in iliac artery from LDLr-/- sedentary mice (62%). On the other hand, trained animals exhibit maximal responses to acetylcholine about 82%. This improvement was associated with a marked increase in NO production as well as a lower ROS production in the isolated tissue. Thus, the beneficial effects of AET in this mice model was similar to those found in others isolated arteries ameliorating the NO signaling pathway and modulating the redox by exercise-induce shear stress [4,12].
Given that the MMPs play a crucial role on vascular remodeling and increased MMP activity is significantly observed in vasculature of atherosclerotic mice, contributing to plaques instability and rupture, leading to the development of cardiovascular diseases, [27] we examined the effects of AET on MMP-2 in iliac artery from LDLr-/-. Our study failed to find any changes in the protein expression of MMP-2 as well as its activity. Possibly, the lack of changes in this important MMP is due to the stage of atherosclerosis where changes in MMP are not yet established. In fact, previous studies investigating atherosclerotic lesions and plaque vulnerability were carried out for 12 weeks reaching up to 18 months in LDLr-/- mice fed with high-fat diet to detect changes in MMP expression [10,11,28,29]. However, one of objective of this study was to detect the early changes of atherosclerosis process and the possible effects of AET on lower limb artery.
In conclusion, AET was effective to improve endothelium-dependent relaxation that was accompanied by increased NO production and decreased ROS formation in iliac artery from LDLr-/- mice fed with high-fat diet. In addition, AET simultaneously to high-fat diet failed to change metabolic parameters showing that in this experimental model of dyslipidemia the intensity of AET should be greater to modify metabolic disorders.
Acknowledgements
We acknowledge the Brazilian Federal Government funding agencies CAPES and CNPq for financial support.
Disclosure of conflict of interest
None.
References
- 1.Schramm JMA, Oliveira AF, Leite IC, Valente JG, Gadelha AMJ, Portella MC, Campos MR. Epidemiological transition and the study of burden of disease in Brazil. Ciên e saúde coletiva. 2004;9:897–908. [Google Scholar]
- 2.Boudoulas KD, Triposciadis F, Geleris P, Boudoulas H. Coronary atherosclerosis: pathophysiologic basis for diagnosis and management. Prog Cardiovasc Dis. 2016;58:676–692. doi: 10.1016/j.pcad.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Cahill PA, Redmond EM. Vascular endothelium-gatekeeper of vessel health. Atherosclerosis. 2016;248:97–109. doi: 10.1016/j.atherosclerosis.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Moraes C, Davel AP, Rossoni LV, Antunes E, Zanesco A. Exercise training improves relaxation response and SOD-1 expression in aortic and mesenteric rings from high caloric diet-fed rats. BMC Physiol. 2008;8:12. doi: 10.1186/1472-6793-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabelo LA, Cortes SF, Alvarez-Leite JI, Lemos VS. Endothelium dysfunction in LDL receptor knockout mice: a role for H2O2 . Br J Pharmacol. 2003;138:1215–1220. doi: 10.1038/sj.bjp.0705164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodman CR, Ingram D, Bonagura J, Laughlin MH. Exercise training improves femoral artery blood flow responses to endothelium-dependent dilators in hypercholesterolemic pigs. Am J Physiol Heart Circ Physiol. 2006;290:H2362–H2368. doi: 10.1152/ajpheart.01026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuzawa Y, Lerman A. Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coron Artery Dis. 2014;25:713–724. doi: 10.1097/MCA.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanesco A, Antunes E. Effects of exercise training on the cardiovascular system: pharmacological approaches. Pharmacol Ther. 2007;114:307–317. doi: 10.1016/j.pharmthera.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 10.Napoli C, Williams-Ignarro S, De Nigris F, Lerman LO, Rossi L, Guarino C, Mansueto G, Di Tuoro F, Pignalosa O, De Rosa G, Sica V, Ignarro LJ. Long-term combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2004;101:8797–8802. doi: 10.1073/pnas.0402734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandran S, Penumetcha M, Merchant NK, Santanam N, Rong R, Parthasarathy S. Exercise reduces preexisting atherosclerotic lesions in LDL receptor knockout mice. Atherosclerosis. 2005;178:33–38. doi: 10.1016/j.atherosclerosis.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Meissner M, Lombardo E, Havinga R, Tietge UJ, Kuipers F, Groen AK. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis. 2011;218:323–329. doi: 10.1016/j.atherosclerosis.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Cardinot TM, Lima TM, Moretti AI, Koike MK, Nunes VS, Cazita PM, Krieger MH, Brum PC, Souza HP. Preventive and therapeutic moderate aerobic exercise programs convert atherosclerotic plaques into a more stable phenotype. Life Sci. 2016;153:163–70. doi: 10.1016/j.lfs.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Pedron C, Ristow AV, Filho JMC, Martin HC, Peixoto CC, Da Fonseca LMB. Endovascular treatment of iliac arteries occlusion. Radiol Bras. 2001;34:261–265. [Google Scholar]
- 15.Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678–692. doi: 10.4065/mcp.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Delbin MA, Davel AP, Couto GK, De Araújo GG, Rossoni LV, Antunes E, Zanesco A. Interaction between advanced glycation and products formation and vascular responses in femoral and coronary arteries from exercise rats. PLoS One. 2012;7:e53318. doi: 10.1371/journal.pone.0053318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wenceslau CF, Davel AP, Xavier FE, Rossoni LV. Long-term ouabain treatment impairs vascular function in resistance arteries. J Vasc Res. 2011;48:316–326. doi: 10.1159/000322576. [DOI] [PubMed] [Google Scholar]
- 19.Bell DA, Hooper AJ, Edwards G, Southwell L, Pang J, Van Bockxmeer FM, Watts GF, Burnett JR. Detecting familial hypercholesterolaemia in the community: impact of a telephone call from a chemical pathologist to the requesting general practitioner. Atherosclerosis. 2014;234:469–472. doi: 10.1016/j.atherosclerosis.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teodoro BG, Natali AJ, Fernandes SA, Silva LA, Pinho RA, Matta SL, Peluzio Mdo C. Improvements of atherosclerosis and hepatic oxidative stress are independent of exercise intensity in LDL-/- mice. J Atheroscler Thromb. 2012;19:904–911. doi: 10.5551/jat.11569. [DOI] [PubMed] [Google Scholar]
- 22.CCunha VN, de Paula Lima M, Motta-Santos D, Pesquero JL, de Andrade RV, de Almeida JA, Araujo RC, Grubert Campbell CS, Lewis JE, Simões HG. Role of exercise intensity on GLUT4 content, aerobic fitness and fasting plasma glucose in type 2 diabetic mice. Cell Biochem Funct. 2015;33:435–442. doi: 10.1002/cbf.3128. [DOI] [PubMed] [Google Scholar]
- 23.Langbein H, Hofmann A, Brunssen C, Goettsch W, Morawietz H. Impact of high-fat diet and voluntary running on body weight and endothelial function in LDL receptor knockout mice. Atherosclerosis. 2015;18:59–66. doi: 10.1016/j.atherosclerosissup.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Napoli C, Williams-Ignarro S, De Nigris F, Lerman LO, D’Armiento FP, Crimi E, Byrns RE, Casamassimi A, Lanza A, Gombos F, Sica V, Ignarro LJ. Physical training and metabolic supplementation reduce spontaneous atherosclerotic plaque rupture and prolong survival in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2006;103:10479–10484. doi: 10.1073/pnas.0602774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlotter F, Matsumoto Y, Mangner N, Schuler G, Linke A, Adams V. Regular exercise or changing diet does not influence aortic valve disease progression in LDLR deficient mice. PLoS One. 2012;7:e37298. doi: 10.1371/journal.pone.0037298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchon C, De Marco Ornelas E, Da Silva Viegas KA, Lacchini S, De Souza RR, Fonseca FL, Maifrino LB. Effects of moderate exercise on the biochemical, physiological, morphological and functional parameters of the aorta in the presence of estrogen deprivation and dyslipidemia: an experimental model. Cell Physiol Biochem. 2015;35:397–405. doi: 10.1159/000369705. [DOI] [PubMed] [Google Scholar]
- 27.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad and the ugly. Circ Res. 2002;22:251–262. [PubMed] [Google Scholar]
- 28.Momi S, Monopoli A, Alberti PF, Falcinelli E, Corazzi T, Conti V, Miglietta D, Ongini E, Minuz P, Gresele P. Nitric oxide enhances the anti-inflammatory and anti-atherogenic activity of atorvastatin in a mouse model of accelerated atherosclerosis. Cardiovasc Res. 2012;94:428–438. doi: 10.1093/cvr/cvs100. [DOI] [PubMed] [Google Scholar]
- 29.Hasan ST, Zingg JM, Kwan P, Noble T, Smith D, Meydani M. Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis. 2014;232:40–51. doi: 10.1016/j.atherosclerosis.2013.10.016. [DOI] [PubMed] [Google Scholar]


