Abstract
There is lack of data on the specific benefit of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors (NET) of pulmonary origin. This dual- centre study aimed to assess outcome and toxicity of standardized PRRT with 177Lu-octreotate in a patient population of advanced pulmonary NET of grade 1-2. We retrospectively assessed 22 consecutively patients treated with 4 intended cycles at 3 monthly intervals (mean activity per cycle 7.8±0.68 GBq). In a median follow-up period of 54 months, no significant nephrotoxicity (≥ grade 3) was observed. Reversible hematotoxicity (grade 3) occurred in 3 patients (13.6%). Treatment response consisted of partial response in 6 (27.3%), stable disease in 9 (40.9%), and progressive disease in 7 (31.8%) patients. Median progression-free survival (PFS) and overall survival (OS) was 27 (95% CI, 9-45) and 42 months (95% CI, 25-59), respectively. High hepatic tumor load (> 50%) and high plasma chromogranin A (> 600 ng/mL) were negative baseline predictors for PFS and OS on univariate analysis, CgA remained significant on multivariate analysis (PFS, P=0.011; OS, P=0.026). Disease progression despite PRRT was associated with shorter survival (median OS 15 vs 53 mo, P<0.001). Despite a higher incidence of treatment failure compared to NET of other origins, the observed substantial and sustained disease stabilization (median PFS of 27 mo, disease control rate of > 2/3 of pts) indicates considerable efficacy of 177Lu-octreotate in pulmonary NET.
Keywords: Peptide receptor radionuclide therapy, PRRT, 177Lu-octreotate, pulmonary neuroendocrine tumors, NET
Introduction
Well-differentiated neuroendocrine tumors (NET) of the respiratory tract, i.e. pulmonary NET, are rare malignancies with a serotonin-secreting potential comprising typical (low grade) and atypical (intermediate grade) forms [1]. Patients with metastatic pulmonary NET have generally worse prognosis compared to patients with metastatic NETs of other origins [2]. Furthermore, serotonin-related hypersecretion syndrome with intractable flushing may impact the quality of life in advanced metastatic stages. Treatment with somatostatin analogs is the recommended first-line therapy for unresectable disease with significant anti-secretory and suggested anti-proliferative, but very little cytoreductive capacities. Effective treatment options for somatostatin analog-refractory patients with uncontrolled functional symptoms or progressive metastatic disease are limited [3,4]. Various cytotoxic chemotherapy regimens and novel targeted treatments have shown only minor survival benefit with significant toxicity in a considerable portion of patients [5,6].
Peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin analogs is a highly effective treatment for metastatic NET with outstanding treatment tolerance and low toxicity [7-9]. However, data regarding the outcome of pulmonary NET after PRRT have been mainly driven form subgroup analyses of 90Y-based treatment in NET of different origins [10-15]. The only available report restricted to patients with pulmonary NET has described the outcome in a heterogeneous cohort treated with different compounds (177Lu-octreotate, 90Y-DOTATOC, or the combination) [16]. Although the inter-individual diversity of the treatment schemes hampered accurate comparative analyses, 177Lu-octreotate seemed superior to 90Y-DOTATOC [16]. In this dual-centre evaluation we aimed to assess the outcome and toxicity of PRRT with 177Lu-octreotate in a well-characterized patient population with metastatic well-differentiated pulmonary NET (G1/2) and to explore potential predictors for impaired survival.
Material and methods
We retrospectively analyzed a consecutive cohort of 22 patients (16 men, 6 women; mean age 63 y; range, 42-74) with distant metastatic, unresectable pulmonary NET (stage IV), who underwent PRRT with 177Lu-octreotate [17]. Other inclusion criteria for PRRT were sufficient tumor uptake, i.e. ≥ liver uptake on baseline receptor imaging, a glomerular filtration rate of > 30 ml/min/1.73 m2, a WBC count ≥2000/mm3, haemoglobin ≥8 g/dl and platelets ≥75000/mm3. Treatment decisions were made on a multi-disciplinary basis. 17 patients (77.3%) had documented morphological tumor progression within 12 months before initiation of PRRT. The remaining 5 patients (22.7%) showed uncontrolled functional symptoms despite biotherapy with somatostatin analog medication. 10 patients (45.5%) had functioning tumors with serotonin hypersecretion-related symptoms. 8 patients (36.4%) had extensive hepatic tumor burden, i.e. metastatic replacement of > 50% of total liver volume according to pretreatment CT/MRI. Metastatic sites included the liver in 19 (86.4%), lymph nodes in 10 (45.5%), bone in 15 (68.2%) and other organs in 6 (27.3%) patients. Previous treatments were comprised of biotherapy (n=16, 72.7%), surgery (n=14, 63.6%), chemotherapy (n=7, 31.8 %), and locoregional treatment (n=1, 4.6%). The study was performed in accordance with the Declaration of Helsinki and with national regulations. The local committee on ethics approved this retrospective study, and all patients signed written informed consent.
PRRT
PRRT was performed by standard protocol with administration of a mean activity of 7.8±0.68 GBq (211±18 mCi) 177Lu-octreotate per treatment cycle, aimed at four courses and standard intervals of 3 months (10-14 weeks). The 177Lu (IDB Holland, Baarle-Nassau, Netherlands) had a specific activity of approximately 100-160 GBq/μmol at the time of administration. The peptide labeling was performed to obtain an apparent specific activity of about 54 GBq/μmol (ratio of activity to the total amount of peptide) [18,19]. Nephroprotection was implemented with standard amino acid co-infusion according to the Rotterdam protocol (lysine 2.5% and arginine 2.5% in 1 L 0.9% NaCl; infusion of 250 ml/h). As described in previous reports, tumor uptake was classified according to the 177Lu-octreotate therapy scan of the first PRRT cycle (grade 3, > kidney/spleen; grade 2, > liver; grade 1 ~liver) [20,21]. This was done to standardize the uptake score for the entire cohort, since some patients underwent somatostatin receptor scintigraphy (eg. Octreoscan) at baseline whereas others received PET imaging with 68Ga labeled somatostatin analogs.
Response and toxicity assessment
Hematological parameters were determined prior to each treatment course, in 2-4 weeks intervals between the courses, 8-12 weeks after the last course of PRRT and in 3 monthly intervals in the further follow-up. Toxicity was recorded using the Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Symptomatic response in functioning tumors (n=10) was assessed according to the change in the intensity and frequency of tumor-specific symptoms and was categorized into complete resolution, partial resolution (≥50% reduction), no significant change, and progression of functional symptoms. Restaging was performed 3 months after termination of PRRT. Imaging consisted of CT and/or MRI according to the baseline imaging modality. Follow-up imaging was performed in 6-month intervals after the first restaging. In order to facilitate comparison with other studies, tumor response was classified according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.
Survival assessment and statistical analysis
Survival analysis was performed using the Kaplan-Meier curve method. Progression free survival (PFS) was censored at the start of another anti-proliferative treatment. Univariate analysis using the log-rank test was performed for each baseline factor. Multivariate analysis (Cox proportional-hazards model) was performed with those variables showing significance on univariate analysis (log-rank test). All tests were performed with a significance level of P<0.05. The statistical software package SPSS (version 20; SPSS Inc., Chicago/ IL, USA) was used to analyze the data.
Results
22 patients underwent PRRT with 177Lu-octreotate. The mean cumulative activity per patient was 27.2±5.9 GBq. 177Lu-octreotate administrations were well tolerated without any serious acute adverse events. In 4 patients (18.1%), treatment was stopped after 1 (n=1) or 2 (n=3) cycles because of a documented disease progression. Relevant hematotoxicity (grade 3) was observed 3-10 weeks after at least one of the administrations in 3 patients (13.6%). Blood count values returned to the normal range in all patients, the mean time to complete bone marrow recovery was 19 months after termination of PRRT (range 9-22 months). No significant nephrotoxicity (≥ grade 3) was observed during the follow-up and the mean relative change of GFR was -1% per year. No other relevant toxicities or treatment related deaths were observed. 3 of 10 patients (30%) with functional symptoms had complete and 7 patients (70%) partial resolution of symptoms after treatment. The observed treatment response according to RECIST 1.1 criteria consisted of partial response (PR) in 6 (27.3%), stable disease (SD) in 9 (40.9%), and progressive disease (PD) in 7 (31.8%) patients. An example of a patient with partial response to PRRT is illustrated in Figure 1. Disease control rate (DCR) was 68.1% and patients with high hepatic tumor load (> 50%), neuron-specific enolase (NSE) plasma levels > 15 ng/ml and chromogranin A (CgA) plasma levels > 600 ng/ml tended to fail treatment (i.e. progress despite PRRT). The baseline characteristics of the study population and the associated DCR are given in Table 1.
Figure 1.

Partial remission illustrated by 68Ga-DOTATOC PET/CT before (A) and 3 months after (B) PRRT in a patient with metastatic pulmonary NET. From left to right: Maximum-intensity-projection PET images (coronal view), fused PET-CT and unfused CT images.
Table 1.
Patient characteristics and disease control rate (DCR) according to RECIST 1.1 (SD and PR)
| Variable | N | DCR (%) | P |
|---|---|---|---|
| Total | 22 | 15 (68.2) | |
| Age | |||
| ≤ 65 y | 14 | 10 (71.4) | 0.512 |
| > 65 y | 8 | 5 (62.5) | |
| Ki-67 index | |||
| ≤ 2% | 9 | 8 (88.9) | 0.101 |
| 3-20% | 13 | 7 (46.7) | |
| Hepatic tumor burden | |||
| ≤ 50% | 14 | 12 (85.7) | 0.052 |
| > 50% | 8 | 3 (35.7) | |
| Tracer uptake | |||
| ≤ grade 2 | 10 | 6 (60) | 0.652 |
| > grade 2 | 12 | 9 (75) | |
| Progression at baseline | |||
| No | 5 | 4 (80) | 1.0 |
| Yes | 17 | 11 (64.7) | |
| Presence of BMs | |||
| No | 7 | 5 (71.4) | 1.0 |
| Yes | 15 | 10 (66.7) | |
| Functionality | |||
| Non-functional | 12 | 8 (66.7) | 1.0 |
| Functional | 10 | 7 (70.0) | |
| CgA | |||
| ≤ 600 ng/ml | 7 | 7 (100) | 0.051 |
| > 600 ng/ml | 15 | 8 (53.3) | |
| NSE | |||
| ≤ 15 ng/ml | 9 | 9 (100) | 0.017 |
| > 15 ng/ml | 13 | 6 (46.2) | |
| Typical/Atypical | |||
| Typical | 5 | 4 (80) | 1.0 |
| Atypical | 17 | 11 (64.7) |
CgA, chromogranin A; NSE, neuron-specific enolase; BMs, bone metastases.
Median PFS of the entire cohort was 27 months (95% CI, 9-45; Figure 2A). The baseline variables associated with shorter PFS in the univariate analysis were CgA > 600 ng/ml (P=0.006) and hepatic tumor load > 50% (P=0.008). As shown in Table 2 high CgA levels remained a significant predictor of earlier progression on multivariate analysis with cox proportional-hazards model: HR 4.4 (95%-CI, 1.4-13.6), P=0.011. The median follow-up period was 54 months (range 5-75) and 9 patients were still alive at the time of analysis. The median OS of the entire cohort was 42 months (95% CI, 25-59; Figure 2B). Treatment failure, i.e. disease progression despite PRRT, was associated with shorter survival: median OS of 15 mo (95% CI, 12-18) vs. 53 mo (95% CI, N/A), P<0.001 (Figure 3). The analysis of various baseline factors for potential contribution to OS is shown in Table 3. High hepatic tumor load (P=0.009) and high CgA levels (P=0.006) again were the only risk factors for impaired survival on univariate analysis; CgA > 600 ng/ml remained the only independent predictor of shorter OS on multivariate analysis: (HR 10.4 (95%-CI, 1.3-72.8), P=0.026). Kaplan-Meier curves (PFS and OS) of patients stratified by CgA and hepatic tumor burden are shown in Figure 4. Survival outcome of patients with typical and atypical tumors were similar (P=0.323). Patients with documented pretreatment morphologic tumor progression had comparable outcome to that of the remaining patients (P=0.570).
Figure 2.

Progression-free (A) and overall (B) survival of the entire patient cohort. The median PFS and OS were 27 months (95% CI, 9-45) and 42 months (95% CI, 25-59), respectively.
Table 2.
Uni- and multivariate analyses for potential factors associated with progression-free survival after PRRT
| Variable | PFS (95% CI) | Univariate analyses | Multivariate analysis | |
|---|---|---|---|---|
|
|
||||
| p | HR (95% CI) | p | ||
| Total | 27 (9-45) | |||
| Age | ||||
| ≤ 65 y | 23 (5-41) | 0.171 | ||
| > 65 y | 20 (1-72) | |||
| Ki-67 index | ||||
| ≤ 2% | 29 (22-36) | 0.276 | ||
| > 2% | 14 (1-44) | |||
| Hepatic tumor burden | ||||
| ≤ 50% | 8 (5-11) | 0.008 | 1.9 (0.7-5.7) | 0.224 |
| > 50% | 32 (29-35) | |||
| Tracer uptake | ||||
| ≤ grade 2 | 23 (1-50) | 0.703 | ||
| > grade 2 | 26 (1-51) | |||
| Presence of BMs | ||||
| No | 26 (1-52) | 0.446 | ||
| Yes | 31 (12-50) | |||
| Functionality | ||||
| No | 31 (6-56) | 0.268 | ||
| Yes | 23 (7-38) | |||
| CgA | ||||
| ≤ 600 ng/ml | 42 (27-56) | 0.006 | 4.4 (1.4-13.6) | 0.011 |
| > 600 ng/ml | 16 (6-26) | |||
| NSE | ||||
| ≤ 15 ng/ml | 35 (29-40) | 0.088 | ||
| > 15 ng/ml | 9 (1-17) | |||
| Typical/atypical histology | ||||
| Typical | 29 (9-49) | 0.758 | ||
| Atypical | 26 (6-47) | |||
CgA, chromogranin A; NSE, neuron-specific enolase; BMs, bone metastases.
Figure 3.
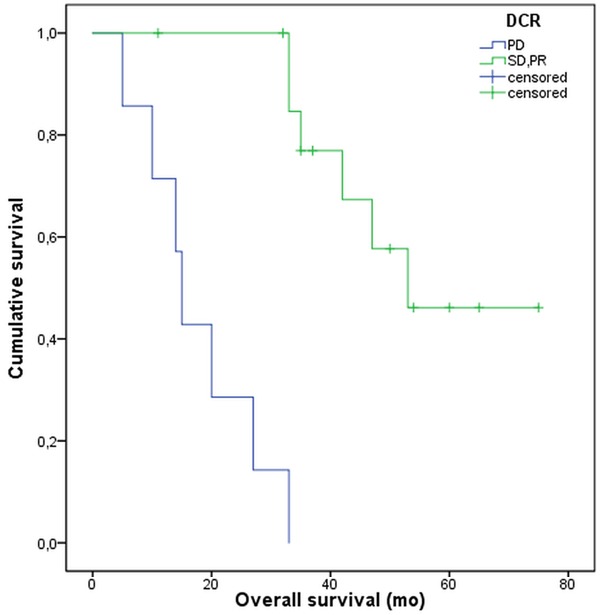
Kaplan-Meier curves illustrating impaired survival of patients failing treatment, i.e. having progressive disease despite PRRT. The median OS of these patients was 15 mo (95% CI, 12-18) vs. 53 mo (95% CI, N/A) in the remaining patients (P<0.001).
Table 3.
Overall survival according to various baseline factors
| Variable | OS (95% CI) | Univariate analyses | Multivariate analysis | |
|---|---|---|---|---|
|
|
||||
| p | HR (95% CI) | P | ||
| Total | 42 (24-60) | |||
| Age | ||||
| ≤ 65 y | 42 (29-60) | 0.883 | ||
| > 65 y | 33 (N/A) | |||
| Ki-67 index | ||||
| ≤ 2% | 53 (N/A) | 0.082 | ||
| > 2% | 33 (11-55) | |||
| Hepatic tumor burden | ||||
| ≤ 50% | NR | 0.009 | 2.3 (0.7-8.1) | 0.183 |
| > 50% | 27 (9-45) | |||
| Tracer uptake | ||||
| ≤ grade 2 | 33 (17-49) | 0.569 | ||
| > grade 2 | 47 (34-60) | |||
| Progression at baseline* | ||||
| Yes | NR | 0.570 | ||
| No | 42 (26-58) | |||
| Bone metastases | ||||
| Yes | 33 (27-39) | 0.710 | ||
| No | 53 (16-90) | |||
| Functionality | ||||
| Non-functional | NR | 0.306 | ||
| Functional | 33 (14-52) | |||
| CgA | ||||
| ≤ 600 ng/ml | NR | 0.006 | 10.4 (1.3-72.8) | 0.026 |
| > 600 ng/ml | 33 (23-43) | |||
| NSE | ||||
| ≤ 15 ng/ml | 47 (34-60) | 0.266 | ||
| > 15 ng/ml | 33 (11-55) | |||
| Histology | ||||
| Typical | 42 (24-60) | 0.323 | ||
| Atypical | 35 (N/A) | |||
Documented pretreatment morphologic tumor progression within 12 months before initiation of PRRT;
NR, not reached.
Figure 4.

Progression-free survival (above) and overall survival (below) stratified by pre-treatment plasma levels of chromogranin A (CgA, cut-off 600 ng/ml; left) and hepatic tumor burden (liver volume replacement, cut-off 50%; right).
Discussion
This retrospective dual-centre analysis provides efficacy results including explicit progression-free survival and overall survival of PRRT in a patient cohort with advanced well-differentiated pulmonary NET after failing standard treatment with somatostatin analogs. Despite the small patient number (n=22) these findings seem important as they convey the idea on the persistent anti-proliferative activity of 177Lu-octreotate in this specific NET entity at an advanced stage. Achievement of disease-control in more than 2/3 of the patient cohort and long-term stabilization with a median PFS of 27 months is very promising in the context of lacking established treatment alternatives.
Effective treatment options for patients with uncontrolled functionality (carcinoid syndrome) or tumor progression in metastatic pulmonary NET of well-differentiated histology are very limited. Previous investigations with various chemotherapy agents, either as mono- or combined-therapy, were generally discouraging. Best results have been observed after treatment of 13 patients (10 typical and 3 atypical carcinoids) with oral temozolomide achieving PR in 4 (31%) patients and disease stabilization in 8 (62%) patients [22]. Novel targeted treatments with tyrosine-kinase inhibitors such as sunitinib or inhibitor of mammalian target of rapamycin everolimus were also associated with limited efficacy in patients with non-pancreatic NET [23-26]. In a recent trial on patients with non-functional neuroendocrine tumors of lung or gastrointestinal origin (RADIANT4), everolimus could prolong the PFS in 203 patients including 63 patients with pulmonary NET to less than 15 months [27]. Particular efficacy in pulmonary NET, however, has only been reported in an earlier study on 33 patients and showed a statistically insignificant trend towards longer PFS (13.6 mo) under everolimus compared to 11 patients who received placebo (5.6 mo) [6].
Data supporting the efficacy of PRRT with radiolabelled somatostatin analogs in metastatic NET is accumulating [20,21,28-33] (Table 4). However, the particular benefit in patients with pulmonary NET, especially after 177Lu-based PRRT, is unclear. In a large study on more than 1000 patients with NET of different origins treated with 90Y-DOTATOC, 84 patients with pulmonary NET had a median OS of 40 months (95% CI, 31-50) with no PFS data available [10]. In a retrospective study on 59 patients with advanced NET, 13 patients with pulmonary NET were treated with 90Y-DOTATOC and/or 177Lu-DOTATOC. The reported objective response rate was 62% according to somatostatin receptor imaging; no further information regarding the characteristics and survival outcome of this subgroup was reported [34]. In a study on 16 patients with metastatic forgut NET (n=9 pulmonary, 5 gastric, and 2 of thymic origin) treated with177Lu-octreotate 8 patients with pulmonary NET showed at least disease stabilization with a median time to progression of 31 months. Similar to our observations treatment outcome did not differ between atypical and typical pulmonary NETs [12].
Table 4.
Overview of published PRRT results in pulmonary NET
| Author | Year/Type | Radioligand | n | ORR (%) | DCR (%) | PFS (mo) | OS (mo) |
|---|---|---|---|---|---|---|---|
| van Essen | 2007/RS | 177Lu-DOTATATE | 9 | 55 | 89 | 31 | NA |
| Pfeifer | 2011/RS | 90Y-DOTATOC/177Lu-DOTATOC | 6 | 17 | 67 | NA | NA |
| Imhof | 2011/RS | 90Y-DOTATOC | 84 | 29 | NA | NA | 40 |
| Filice | 2012/RS | 90Y-DOTATOC/177Lu-DOTATATE | 13 | 62 | 85 | NA | NA |
| Mariniello | 2016/RS | 90Y-DOTATOC/177Lu-DOTATATE | 114 | 13 | 67 | 28 | 59* |
| Sabet | 2017/RS | 177Lu-DOTATATE | 22 | 27 | 68 | 27 | 42 |
RS, retrospective study; ORR, objective response rate; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; NA, not available;
Estimated overall survival. Note the lacking survival data from larger reports on primarily mixed NET cohorts.
The only available study restricted to patients with pulmonary NET has described the outcome in a heterogeneous cohort treated with 177Lu-octreotate, 90Y-DOTATOC, or the combination of both compounds over more than a decade [16]. Less favorable histological characteristics of the patients treated with 90Y-DOTATOC and inter-individual diversity of the treatment schemes hampered an accurate comparative analysis of the therapeutic compounds. However, 177Lu-octreotate seemed superior to 90Y-DOTATOC. DCR of 75% was observed in patients treated with 177Lu-octreotate administered at various therapeutic dosages [16]. Similarly encouraging DCR of 68.2% was seen in our cohort. The objective response rate of 27% also corresponds to the results of the largest published study on PRRT with 177Lu-octreotate in GEP NET (n=310) by Kwekkeboom et al., reporting an overall response rate of 23% in patients with carcinoid tumors of different origins (n=188) [35]. Consistent with previous reports [36-38] a high level of plasma chromogranin A (> 600 ng/mL) was associated with worsened outcome in our cohort and remained the only independent risk factor for impaired PFS and OS (Tables 2, 3).
Small population size and the retrospective nature inevitably impact on the strength of our conclusions; especially comparisons with historical data should be interpreted with particular caution. We tried to characterize the study population in very detailed manner including all the important variables of potential influence on outcome in order to make comparative considerations possible. Therefore, the potency of therapeutic benefit for the patients seems to be perceptible from the large discrepancy between our findings and the reported data from other modalities.
Disclosure of conflict of interest
None.
References
- 1.Naalsund A, Rostad H, Strom EH, Lund MB, Strand TE. Carcinoid lung tumors--incidence, treatment and outcomes: a population-based study. Eur J Cardiothorac Surg. 2011;39:565–569. doi: 10.1016/j.ejcts.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 3.Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B, Salazar R Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–176. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 4.Oberg K, Hellman P, Kwekkeboom D, Jelic S Group EGW. Neuroendocrine bronchial and thymic tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v220–222. doi: 10.1093/annonc/mdq191. [DOI] [PubMed] [Google Scholar]
- 5.Kulke MH, Stuart K, Enzinger PC, Ryan DP, Clark JW, Muzikansky A, Vincitore M, Michelini A, Fuchs CS. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J. Clin. Oncol. 2006;24:401–406. doi: 10.1200/JCO.2005.03.6046. [DOI] [PubMed] [Google Scholar]
- 6.Fazio N, Granberg D, Grossman A, Saletan S, Klimovsky J, Panneerselvam A, Wolin EM. Everolimus plus octreotide long-acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest. 2013;143:955–962. doi: 10.1378/chest.12-1108. [DOI] [PubMed] [Google Scholar]
- 7.Sabet A, Ezziddin K, Pape UF, Reichman K, Haslerud T, Ahmadzadehfar H, Biersack HJ, Nagarajah J, Ezziddin S. Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with (177)Lu-octreotate. Eur J Nucl Med Mol Imaging. 2014;41:505–510. doi: 10.1007/s00259-013-2601-x. [DOI] [PubMed] [Google Scholar]
- 8.Sabet A, Ezziddin K, Pape UF, Ahmadzadehfar H, Mayer K, Poppel T, Guhlke S, Biersack HJ, Ezziddin S. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2013;54:1857–1861. doi: 10.2967/jnumed.112.119347. [DOI] [PubMed] [Google Scholar]
- 9.Sabet A, Haslerud T, Pape UF, Sabet A, Ahmadzadehfar H, Grunwald F, Guhlke S, Biersack HJ, Ezziddin S. Outcome and toxicity of salvage therapy with 177Lu-octreotate in patients with metastatic gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:205–210. doi: 10.1007/s00259-013-2547-z. [DOI] [PubMed] [Google Scholar]
- 10.Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, Macke HR, Rochlitz C, Muller-Brand J, Walter MA. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA] -TOC in metastasized neuroendocrine cancers. J. Clin. Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 11.Pfeifer AK, Gregersen T, Gronbaek H, Hansen CP, Muller-Brand J, Herskind Bruun K, Krogh K, Kjaer A, Knigge U. Peptide receptor radionuclide therapy with Y-DOTATOC and (177)Lu-DOTATOC in advanced neuroendocrine tumors: results from a Danish cohort treated in Switzerland. Neuroendocrinology. 2011;93:189–196. doi: 10.1159/000324096. [DOI] [PubMed] [Google Scholar]
- 12.van Essen M, Krenning EP, Bakker WH, de Herder WW, van Aken MO, Kwekkeboom DJ. Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with foregut carcinoid tumours of bronchial, gastric and thymic origin. Eur J Nucl Med Mol Imaging. 2007;34:1219–1227. doi: 10.1007/s00259-006-0355-4. [DOI] [PubMed] [Google Scholar]
- 13.Bushnell DL Jr, O’Dorisio TM, O’Dorisio MS, Menda Y, Hicks RJ, Van Cutsem E, Baulieu JL, Borson-Chazot F, Anthony L, Benson AB, Oberg K, Grossman AB, Connolly M, Bouterfa H, Li Y, Kacena KA, LaFrance N, Pauwels SA. 90Yedotreotide for metastatic carcinoid refractory to octreotide. J. Clin. Oncol. 2010;28:1652–1659. doi: 10.1200/JCO.2009.22.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cwikla JB, Sankowski A, Seklecka N, Buscombe JR, Nasierowska-Guttmejer A, Jeziorski KG, Mikolajczak R, Pawlak D, Stepien K, Walecki J. Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): a phase II study. Ann Oncol. 2010;21:787–794. doi: 10.1093/annonc/mdp372. [DOI] [PubMed] [Google Scholar]
- 15.Sowa-Staszczak A, Pach D, Kunikowska J, Krolicki L, Stefanska A, Tomaszuk M, Buziak-Bereza M, Mikolajczak R, Matyja M, Gilis-Januszewska A, Jabrocka-Hybel A, Trofimiuk M, Hubalewska-Dydejczyk A. Efficacy and safety of 90Y-DOTATATE therapy in neuroendocrine tumours. Endokrynol Pol. 2011;62:392–400. [PubMed] [Google Scholar]
- 16.Mariniello A, Bodei L, Tinelli C, Baio SM, Gilardi L, Colandrea M, Papi S, Valmadre G, Fazio N, Galetta D, Paganelli G, Grana CM. Longterm results of PRRT in advanced bronchopulmonary carcinoid. Eur J Nucl Med Mol Imaging. 2016;43:441–452. doi: 10.1007/s00259-015-3190-7. [DOI] [PubMed] [Google Scholar]
- 17.Rindi G. The ENETS guidelines: the new TNM classification system. Tumori. 2010;96:806–809. doi: 10.1177/030089161009600532. [DOI] [PubMed] [Google Scholar]
- 18.Breeman WA, De Jong M, Visser TJ, Erion JL, Krenning EP. Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur J Nucl Med Mol Imaging. 2003;30:917–920. doi: 10.1007/s00259-003-1142-0. [DOI] [PubMed] [Google Scholar]
- 19.Breeman WA, van der Wansem K, Bernard BF, van Gameren A, Erion JL, Visser TJ, Krenning EP, de Jong M. The addition of DTPA to [177Lu-DOTA0,Tyr3] octreotate prior to administration reduces rat skeleton uptake of radioactivity. Eur J Nucl Med Mol Imaging. 2003;30:312–315. doi: 10.1007/s00259-002-1054-4. [DOI] [PubMed] [Google Scholar]
- 20.Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Al Zreiqat A, Willinek W, Biersack HJ, Sabet A. Outcome of peptide receptor radionuclide therapy with (177)Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:925–933. doi: 10.1007/s00259-013-2677-3. [DOI] [PubMed] [Google Scholar]
- 21.Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grunwald F, Guhlke S, Biersack HJ, Sabet A. Predictors of longterm outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2014;55:183–190. doi: 10.2967/jnumed.113.125336. [DOI] [PubMed] [Google Scholar]
- 22.Ekeblad S, Sundin A, Janson ET, Welin S, Granberg D, Kindmark H, Dunder K, Kozlovacki G, Orlefors H, Sigurd M, Oberg K, Eriksson B, Skogseid B. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986–2991. doi: 10.1158/1078-0432.CCR-06-2053. [DOI] [PubMed] [Google Scholar]
- 23.Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Tye L, Huang X, Li JZ, Baum CM, Fuchs CS. Activity of sunitinib in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2008;26:3403–3410. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 24.Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, Jacobs C, Mares JE, Landgraf AN, Rashid A, Meric-Bernstam F. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J. Clin. Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duran I, Kortmansky J, Singh D, Hirte H, Kocha W, Goss G, Le L, Oza A, Nicklee T, Ho J, Birle D, Pond GR, Arboine D, Dancey J, Aviel-Ronen S, Tsao MS, Hedley D, Siu LL. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95:1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavel ME, Hainsworth JD, Baudin E, Peeters M, Horsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, Oberg K, Van Cutsem E, Yao JC. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 27.Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME Rad001 in Advanced Neuroendocrine Tumours FTSG. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–77. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabet A, Dautzenberg K, Haslerud T, Aouf A, Sabet A, Simon B, Mayer K, Biersack HJ, Ezziddin S. Specific efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur J Nucl Med Mol Imaging. 2015;42:1238–1246. doi: 10.1007/s00259-015-3041-6. [DOI] [PubMed] [Google Scholar]
- 29.Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, Lepensky C, Kwekkeboom DJ, Baum RP, Krenning EP, Modlin IM. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. doi: 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- 30.Ezziddin S, Opitz M, Attassi M, Biermann K, Sabet A, Guhlke S, Brockmann H, Willinek W, Wardelmann E, Biersack HJ, Ahmadzadehfar H. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2011;38:459–466. doi: 10.1007/s00259-010-1610-2. [DOI] [PubMed] [Google Scholar]
- 31.Sabet A, Khalaf F, Yong-Hing CJ, Sabet A, Haslerud T, Ahmadzadehfar H, Guhlke S, Grunwald F, Biersack HJ, Ezziddin S. Can peptide receptor radionuclide therapy be safely applied in florid bone metastases? A pilot analysis of late stage osseous involvement. Nuklearmedizin. 2014;53:54–59. doi: 10.3413/Nukmed-0614-13-08. [DOI] [PubMed] [Google Scholar]
- 32.Sabet A, Biersack HJ, Ezziddin S. Advances in peptide receptor radionuclide therapy. Semin Nucl Med. 2016;46:40–46. doi: 10.1053/j.semnuclmed.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Ezziddin S, Sabet A, Heinemann F, Yong-Hing CJ, Ahmadzadehfar H, Guhlke S, Holler T, Willinek W, Boy C, Biersack HJ. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with (177)Lu-octreotate. J Nucl Med. 2011;52:1197–1203. doi: 10.2967/jnumed.111.090373. [DOI] [PubMed] [Google Scholar]
- 34.Filice A, Fraternali A, Frasoldati A, Asti M, Grassi E, Massi L, Sollini M, Froio A, Erba PA, Versari A. Radiolabeled somatostatin analogues therapy in advanced neuroendocrine tumors: a single centre experience. J Oncol. 2012;2012:320198. doi: 10.1155/2012/320198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO, Krenning EP. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3] octreotate: toxicity, efficacy, and survival. J. Clin. Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence B, Gustafsson BI, Kidd M, Pavel M, Svejda B, Modlin IM. The clinical relevance of chromogranin a as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:111–134. viii. doi: 10.1016/j.ecl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427–2443. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 38.Jensen EH, Kvols L, McLoughlin JM, Lewis JM, Alvarado MD, Yeatman T, Malafa M, Shibata D. Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol. 2007;14:780–785. doi: 10.1245/s10434-006-9148-z. [DOI] [PubMed] [Google Scholar]


