Abstract
Purpose of review
Cervical disc replacement (CDR) is a surgical option for appropriately indicated patients, and high success rates have been reported in the literature. Complications and failures are often associated with patient indications or technical variables, and the goal of this review is to assist surgeons in understanding these factors.
Recent findings
Several investigations have been published in the last 5 years supporting the use of CDR in specific patient populations. CDR has been shown to be comparable or favorable to anterior cervical discectomy and fusion in several meta-analyses and mid-term follow-up studies.
Summary
CDR was developed as a technique to preserve motion following a decompression procedure while minimizing several of the complications associated with fusion and posterior cervical spine procedures. Though success with cervical fusion and posterior foraminotomy has been well documented in the literature, high rates of mid- and long-term complications have been clearly established. CDR has also been associated with several complications and challenges with regard to surgical technique, though improvements in implant design have lead to an increase in utilization. Several devices currently exist and vary in terms of material, design, and outcomes. This review paper discusses indications, surgical technique, and technical pearls and reviews the CDR devices currently available.
Keywords: Cervical disc arthroplasty, Disc replacement, Anterior cervical discectomy and fusion (ACDF), Radiculopathy, Myelopathy, Cervical degenerative disc disease
Introduction
Spine surgeons and patients are confronted with multiple surgical options when managing cervical pathology which has not responded to conservative treatment. Anterior cervical discectomy and fusion (ACDF), posterior laminoforaminotomy, laminoplasty, and laminectomy with fusion have been supported by well-designed mid- and long-term studies [1–4]. These surgical procedures provide successful means of relieving cervical radiculopathy and arresting the progression of cervical myelopathy, though each has associated complications which may lead to high rates of recurrence, adjacent segment degeneration (ASD), or need for revision surgery [2, 5–7, 8••].
These variables in addition to growing patient demands, financial pressures, and longer life expectancy have fueled the development of motion preservation procedures such as cervical disc replacement (CDR). CDR emerged in the 1960s as a means of minimizing the biomechanical stress placed on adjacent levels following a fusion procedure; however, these early devices were fraught with high complication rates and poor results [9, 10]. Decades of technological advancements have lead to the current devices available to spine surgeons, some of which have produced excellent mid-term results [11–12, 13••, 14–15 ]. Cadaveric studies have supported these findings from a biomechanical standpoint, and several investigations have shown superior patient outcomes, higher patient satisfaction, less implant related complications, and lower reoperation rates in comparing CDR with ACDF [8, 14, 16–18].
Regardless, CDR is associated with several technical considerations, which spine surgeons should understand during preoperative planning as these may increase complications and failure rates without a comprehensive surgical strategy [19, 20]. Despite CDR being a motion preservation procedure, several studies have shown higher than expected rates of heterotopic ossification (HO) and ASD following CDR [18, 19, 21–24]. These findings support the need for comprehensive review papers to assist spine surgeons in counseling patients and proficiently performing CDR. We feel that with properly indicated patients, meticulous preoperative planning, and sound surgical technique, CDR offers an excellent surgical option for many patients.
Patient indications
Current indications for CDR (Table 1) include patients with underlying cervical degenerative disc disease with the clinical presentation of cervical radiculopathy or myelopathy, which has been unresponsive to conservative treatment [25], [41]. Commonly, patients present with cervical disc pathology in conjunction with coexisting spondylosis or foraminal stenosis which may complicate this decision. Patients without dynamic instability (>3.5 mm translation on flexion-extension lateral radiographs) are potential candidates for CDR as are patients without circumferential spinal cord compression such as seen in patients with severe spondylosis or with diffuse ossification of the posterior longitudinal ligament (OPLL). Patients with a high amount of axial neck pain due to facet arthropathy should also be counseled appropriately before proceeding with CDR as these symptoms may not improve and when severe arthropathy is contributing to instability, CDR may not be the optimal surgical option.
Table 1.
Indications: ACDF vs. CDR
| Indications | ACDF | CDA |
|---|---|---|
| Central or foraminal compression | + | + |
| Single level | + | + |
| Multilevel | + | ± |
| Retrovertebral compression (i.e., congenital stenosis, ossification of posterior longitudinal ligament) | + | − |
| Spondyloarthropathies (ankylosing spondylitis) | + | − |
| Underlying instability (i.e., prior cervical laminectomy, pre-op instability) | + | − |
| Poor bone quality (i.e., osteoporosis, metabolic bone disease) | + | − |
| Infection | + | − |
| Known malignancy | + | − |
| Allergy to device material | − | − |
Indications are in addition to cervical myeloradiculopathy with clinical symptoms and radiographic evidence that has failed conservative therapy
Some authors have recommended a disc height of >3 mm for adequate disc space access and removal [26]. Placing an oversized implant into a collapsed disc space can potentially place excessive forces through the facet joints and lead to worsening of axial neck pain [26]. Patients with a kyphotic deformity of >15° should also be approached with caution as severe kyphotic deformity will typically coexist with posterior spinal pathology (ligamentum flavum hypertrophy, facet capsule thickening, etc.). The goal of CDR is to preserve motion, not to recreate motion, thus awareness of the cervical spinal segment in question should be taken into consideration during preoperative planning.
Other contraindications to CDR include active infection, known malignancy, inflammatory spondyloarthropathy, allergy to implant materials, and metabolic bone disease (osteoporosis, renal osteodystrophy, etc.) [25]. Osteoporosis is a contraindication for CDR as the risk of implant subsidence and migration is higher [27]. Other approach-related concerns such as patients whom have undergone prior anterior neck surgery should undergo comprehensive evaluations by an otolaryngologist to assess vocal cord integrity and avoid iatrogenic nerve injury.
Complications
Given the similarity in approach between ACDF and CDR, there are many shared short-term complications, though mid- and long-term complications vary between these two cohorts. The short-term complications include dysphagia, dysphonia, bleeding, recurrent laryngeal nerve injury, esophageal injury, tracheal injury, dural tear, hematoma, and spinal cord injury [28–30]. With less implant prominence on the posterior esophagus, the incidence of dysphagia and esophageal symptoms may be less following CDR [31]. Skeppholm et al. utilized the dysphagia short questionnaire (DSQ) to find that at 2-year follow-up, patients that had undergone CDR had more favorable scores compared to ACDF patients [31]. Less common complications of CDR that have been reported in the literature include internal carotid artery dissection, paralysis from posterior implant dislocation, and solid intervertebral body fusion [32–34].
The mid- and long-term complications of CDR most commonly reviewed in the literature are ASD, implant related failures, and HO. Patients and surgeons should be aware that the significance of these findings is characterized by the incidence of symptomatic pathology, not in an isolated radiographic finding. Cases of implant migration and subsidence have been reported and often relate to technical variables and patient factors (Fig. 1a–d). Rates of HO following CDR range between 7.3 and 69.2%. [21, 22, 35, 36]. Yi et al. found gender, male sex, and implant type to be statistically significant factors in the development of HO, with ProDisc-C having the highest rate followed by Mobi-C then Bryan [23]. A study looking at the impact of HO on patient outcomes showed no statistical difference in terms of VAS neck pain, VAS arm pain, and NDI score when compared to a control group, though ROM was less in patients who developed HO [18].
Fig. 1.
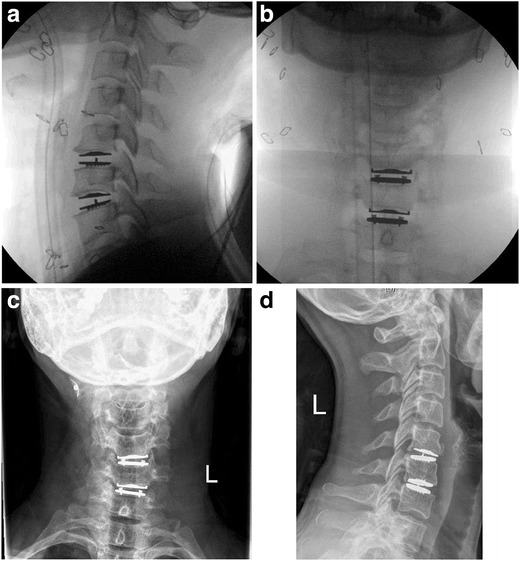
a-d. a and b Intraoperative floursocopy following C5–7 CDA in a 41-year-old male with two level degenerative disc disease. c and d 1 year postoperative visit showing implant subsidence
Nunley et al. showed that postoperative ASD occurred in about 15% of patients and preoperative osteopenia and lumbar disc degeneration were significant risk factors [37]. This is in comparison to Robertson et al. who reported rates of 0% in those undergoing CDR and 7% for ACDF patients; however, plain radiograph was the only measure used in assessment [7]. In a large institutional review, Lee et al. found similar rates of ASD (2.9%) at follow-up regardless of whether patients underwent ACDF, CDR, or posterior foraminotomy [20].
Design
Artificial cervical disks come with a moderate degree of variation, mostly in terms of variable bearing design, materials, implantation techniques, and articulation type. There are three different types of bearing designs: constrained, semi-constrained, and unconstrained. A constrained device is defined as a bearing that has a physical stop device that is within the normal physiologic range of motion. This allows for greater stability of the operated joint, thus decreasing the shear forces experienced by the facet joints, but it also leads to a more challenging procedure as more stress is placed on the bone-implant connection and exact placement is necessary in order to replicate the natural axis of rotation. On the other end of the spectrum are unconstrained devices, which do not possess a physical stop, allowing for increased mobility at the cost of decreased stability. While device placement and bone-implant forces are not of as high of concern, more demand is placed on adjacent facet joints. Semi-constrained devices fall in the middle, possessing physical stops that are outside of the normal physiologic range of motion [38].
There are three main material types for cervical disc devices: stainless steel, cobalt, and titanium. Stainless steel is rarely used, as it prevents the future use of magnetic resonance imaging. Cobalt and titanium are most commonly used, as they have shown high rates of long-term success in their use for other arthroplastic devices. Another critical aspect of success is determined by the strength of the implant-bone fusion. Several techniques have been developed and taken advantage of to increase the likelihood of successful fusion. Surface features including keels, spikes, wire mesh, increased porosity, and screw fixations as well as specialized coatings of plasma-sprayed titanium, aluminum oxide, hydroxyapatite, and calcium phosphate are strategies that are frequently implemented. Articulation type is defined based on the number of rotational centers present. Ball-and-socket articulation allows for rotation around one individual point, whereas saddle articulation offers more than one center of rotation where movement can occur [38].
Technique and technical pearls
The patient is placed on the operating table in the supine position with the neck in the neutral position. The patient’s head is secured in place with tape across the forehead to maintain a neutral position, and his or her shoulders are secured down with tape to allow for proper visualization with fluoroscopy. The endplates should be examined on lateral fluoroscopy to visualize that they are positioned in a parallel position (Fig. 2). Hyperflexion can lead to a lordotic malalignment and hyperextension can lead to a kyphotic malalignment. Neutral axial rotation can be assessed by using the AP radiograph and visualizing the spinous processes in an equidistant position from the pedicles [39–41].
Fig. 2.
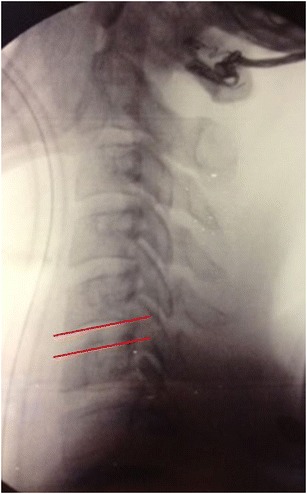
Parallel end plates obtained on fluoroscopy
The Smith-Robinson approach is utilized, which is described in detail elsewhere in the literature [25] [41]. Distraction pins are placed into the vertebral bodies directly superior and inferior to the disc space. Typically, the pins are placed in the midline of the vertebral body on the AP view, though if a keeled arthroplasty is being utilized, they should be placed slightly eccentrically to avoid interfering with the keel on the implant. The discectomy and decompression are then performed and often the foraminotomies are more extensive than performed in a typical ACDF. Once the discectomy is completed, the uncovertebral joints are decompressed bilaterally. Full resection of the uncinate can lead to intersegmental instability, and we typically perform a partial resection until the corresponding foramina is adequately decompressed. We typically release the posterior longitudinal ligament to allow for a thorough assessment of the posterior and posterolateral decompression. Care is taken to remove all osteophytes though we recommend minimizing the use of a high-speed burr during osteophyte removal and end plate preparation. Typically, we utilize handheld instruments (Kerrison rongeurs and curretes) for this stage of the procedure to minimize the potential for HO across the disc space. If a burr is needed during this stage, aggressive irrigation should also be utilized to remove all resulting bone dust [39–41].
Trials are then used to determine proper implant size in all dimensions (height, width, and depth) and ensure proper alignment with the guidance of fluoroscopic imaging. We prefer using the largest possible size implant in the anteroposterior and mediolateral planes (Fig. 2a–d). Once the proper implant size has been selected, it is placed in the disc space on lateral fluoroscopy and confirmed on the AP view. Of note, devices with keels require additional carving before insertion of the implant. Fluoroscopic imaging is used once again to ensure proper placement has occurred. Closure is standard for the Smith-Robinson approach with care taken to avoid electrocautery on the anterior surface of the vertebral bodies as it may lead to the formation of HO. Slight variations do exist between devices and will be discussed below [39] [40] [41].
Specific implant characteristics
Bryan artificial disc
The Bryan artificial disc (Fig. 3) is a semi-constrained, biarticulating device. It is a single unit that is made up of three components, two titanium alloy end plates and a polyurethane center. The external surface (in contact with the vertebral body) of the end plates has a titanium porous surface to produce bony ingrowth and stability. An anterior stop is also present on both end plates, which helps to prevent posterior dislocation into the vertebral canal and is also utilized during insertion of the implant. The polyurethane core is also surrounded by a saline-filled sheath that becomes a pseudocapsule over time with the goal of replicating the cushioning effect of the natural vertebral disc [42].
Fig. 3.

Bryan artificial disc and lateral radiograph
Slight variation in operative technique occurs with this device. First, a concave surface must be formed into the vertebral end plates in order to place the implant. Then, a jig is used to accurately locate the axial center of the vertebral body, using the midline between the uncovertebral joints as a guide. A milling structure is then anchored to the vertebral body, using the identified central point, in order for proper implant positioning. The device is available in five different diameters [42].
When assessing the long-term, 10-year outcomes for Bryan artificial disks, Zhao et al. found neurologic improvement in patients with myelopathy using the modified Japanese Orthopaedic Association (mJOA) system and radiculopathy using the NDI and VAS [43]. These authors also found a decrease in range of motion in the sagittal plane from 7.8 to 4.7° at 10-year follow-up with an ASD rate of 47.6%. Sixty-nine percent of patients developed some degree of HO, and only two of them developed recurrent radiculopathy due to the HO and went on to undergo reoperation [43]. A meta-analysis by Zhu et al. found no statistical difference in NDI scores when comparing those undergoing Bryan CDR to ACDF, but when looking further out they found favorable NDI scores in the CDR group. In the same study, range of motion was also better in the CDR group [44].
DISCOVER artificial disc
The DISCOVER artificial disc is a ball-and-socket device made up of two titanium alloy end plates, is an ultra-high-molecular-weight polyethylene center, and is an unconstrained device. The end plates are trapezoidal to match the natural geometry of the cervical vertebra, lined with 1-mm teeth and coated with porous titanium plasma spray and hydroxyapatite. The center contacts the end plates with a spherical surface, allowing for motion in all directions [38].
Skeppholm et al. found both ACDF and the Discover artificial disc replacement to provide statistically significant decreases in their respective NDI scores at 2-year follow-up; when compared to one another, no statistical difference was found [45]. EQ-5D was used to assess health-related quality of life, and the Discover group had a statistically significant lower score when compared to the ACDF group using an intention to treat analysis.
Mobi-C artificial disc
The Mobi-C artificial disc (Fig. 4) is constructed from three individual components: two end plates and one ultra-high-molecular-weight polyethylene center piece. The end plates are made from cobalt, chromium, 29 molybdenum ISO 5832–12 alloy, each being lined with one row of teeth on the lateral edge of their external surface and coated with a titanium and hydroxyapatite spray. The internal contact surface of the inferior end plate is spherical, while that of the superior end plate is flat. The core centers itself on the inferior plate, laterally inhibited by two stops, and continuously re-centers itself as the top plate moves, allowing for five independent degrees of motion, two of which are translational and three rotational. Following joint decompression, no further modeling of the vertebral bodies is necessary and the posterior longitudinal ligament can be spared. After confirming proper implant placement, compression of the joint space is performed using the distraction pins in order to obtain proper purchase between the implant and vertebral bodies [28].
Fig. 4.
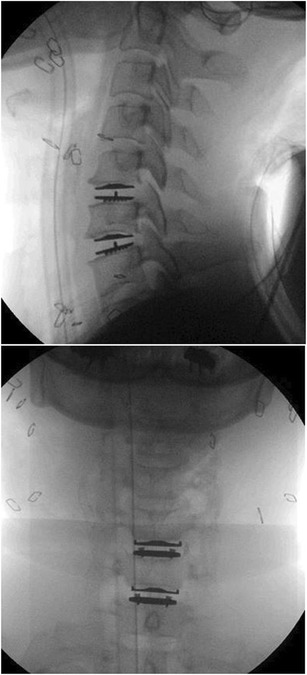
Mobi-C Lateral and AP Radiographs
A study comparing CDR with Mobi-C to ACDF found that on several measures the Mobi-C’s results were better when compared to the ACDF group, and on the remaining measures the Mobi-C group was found to be non-inferior to the ACDF group. Specifically, the Mobi-C group was found to have significantly lower rates of device-related revision surgery and a lower percentage of ASD [46]. In the two-level arm of the original Investigational Device Exemption trial, Mobi-C has shown significantly favorable outcomes over ACDF with regard to NDI, return to work time, reoperation rates, and negative radiographic changes. This is the first device to receive FDA insurance approvals for two-level cervical pathology.
Radcliff et al., looked specifically at 5-year results for adjacent two-level disease and found a greater mean mprovement in NDI pain in the Mobi-C group compared to the ACDF group; similar trends were seen when looking at VAS neck pain, VAS arm pain, patient satisfaction, SF-12 Physical Component Summary (PCS) scores, secondary surgeries, and overall success rate. The ACDF group also showed higher rates of index level and adjacent level reoperations and decreased range of motion compared to baseline. Similar rates of neurologic adverse events and gait dysfunction were observed between the two groups, showing that both procedures are safe [12].
Prestige artificial disc
The Prestige artificial disc is unconstrained and made from two separate pieces composed of metal, making this a “metal-on-metal” device. There are two variations: the ST model, stainless steel, and the LP model, titanium. They also differ on their method of bone implantation, with the ST fixating to the vertebral body via screws and the LP via two rails on the external surface of both end plates that are coated with titanium plasma spray. When placing this device, it is important to ensure that adequate vertebral body end plate surface is covered by the device. Upon attaining proper alignment, the ST model requires screw placement [47].
Gornet et al. showed that both ACDF and Prestige CDR were associated with a significant improvement in NDI score, neck pain, and arm pain. There were not significant differences in these variables when comparing the two groups [48].
ProDisc-C artificial disc
The ProDisc-C artificial disc is a two-piece device that functions as a ball-and-socket joint and is a semi-constrained device. The end plates are a cobalt chrome alloy with porous external surfaces. Attached to the internal surface of the inferior plate is a convex ultra-high-molecular-weight insert, which articulates with the concave inner surface of the superior end plate. Rotation is allowed in all three axes with this device, and translation is restricted. The external surfaces of both the superior and inferior implant end plates are coated with a titanium plasma spray and possess slotted keels. Given the presence of the keels and porous external end plate surfaces, minimal end plate preparation is required [49].
Zigler et al. reports no statistical difference in the change of NDI score at 2 or 5 years when comparing ProDisc-C to ACDF, though both statistically improved [50]. Similar outcomes were found for the SF-36 survey, patient satisfaction, neurologic function, and device-related adverse events. A higher percent of improvement was found in the ProDisc-C group when looking at VAS scores at 5 years, however. When looking at flexion-extension ROM, the ProDisc-C group remained similar to pre-op values at post-op years two and five, whereas ROM was decreased in the ACDF group.
CerviCore artificial disc
CerviCore artificial disc is a two-piece semi-constrained device made entirely of cobalt-chromium-molybdenum. The external surface of each base is coated with titanium plasma spray and has two fins, each containing three spikes. This artificial disk has a saddle bearing design, which allows for two separate centers of rotation. The first is located in the vertebral body directly inferior to the device, functioning as the center of rotation for flexion-extension. The center of rotation for lateral bending is located in the vertebral body directly superior to the implant. An anterior stop is also present to prevent posterior dislocation of the device. A template with vertical processes is inserted into the disc, using the vertical processes to properly align with the midline. Fluoroscopic imaging is then used to confirm that the template placement is in the midline which is then marked. Channels must then be drilled and chiseled in order to accommodate the fins of the implant end plates [29]. A cadaveric study showed that when comparing CerviCore to fusion, the CerviCore group ROM was more comparable to the control group, except when looking at axial rotation [51].
SECURE-C artificial disc
The SECURE-C artificial disc is a semi-constrained device with three separate pieces that are inserted into the disc space as one. There are two cobalt chrome alloy end plates and an ultra-high-molecular-weight polyethylene sliding center. The end plates are porous with a plasma spray coating and serrated keels that allow for a press fit and immediate stabilization. The superior and inferior surfaces of the center piece have different shapes, being spherical and cylindrical, respectively. This allows for anteroposterior sliding, resulting in more physiologic loading and a moving instantaneous axis of rotation in the sagittal plane. Contrary to other devices, when performing the discectomy, the lateral annulus is left intact. Vertebral end plates are also left intact, unless osteophytes or spondylosis requires correction [52]. Vaccaro et al. reported superiority in overall success (as defined by several variables including NDI scores, device failures, absence of major complications, and radiographic findings) when comparing SECURE-C to ACDF. Similar results were observed when comparing patient satisfaction at 24 months. The SECURE-C group also showed non-inferiority for NDI score, VAS neck pain, VAS arm pain, and neurologic status [53].
M6 artificial disc
The M6 artificial disc is a single-piece device with titanium alloy end plates and a complex center piece. There are three keels present on the external surface of each end plate. The external surfaces are also coated with porous titanium. The center piece is a polycarbonate urethane polymeric material, which functions as the nucleus pulposus, surrounded by a polyethylene woven fiber construct, functioning as the annulus fibrosis. A polymer sheath surrounds the entire center piece, preventing tissue ingrowth and debris entry, and this implant allows for motion in all six degrees of freedom [54]. Reyes-Sanchez et al. showed a significant decrease in NDI scores in patient receiving the M6 cervical disc implant, with single-level patients having better results than two-level patients. Similar results were found for both arm and neck pain. Global range of motion was found to be similar when compared to pretreatment values for patients at 24 months post-op [55].
Future directions
With progress towards establishing successful outcomes with CDR, further well-designed studies comparing different types of CDR and non-industry funded studies will aid the spine surgeon in selecting the optimal device for each patient. Several meta-analyses have been performed recently to review outcomes and data points with regard to multiple different types of CDR compared with ACDF, though most of the included index studies are the original IDE investigations which may have inherent bias [13••, 18]. Luo et al. compared CDR to ACDF for treatment of single-level disease and found no significant difference in improvement of NDI scores at 2 years [13••]. However, improvements in the VAS neck pain and VAS arm pain scores were significant. The opposite was observed when looking at neurological success, as the ACDF group was slightly better, though when comparing ROM between the two groups, the CDR group was significantly better. Fewer secondary surgical procedures occurred in the CDR group as well [13••]. Similarly, Hu et al. performed a meta-analysis and found that CDR was favorable to ACDF with regard to patient-reported outcomes, adverse events, and ASD [56].
There has been growing interest in designing an intervertebral implant that more closely mimics the biomechanical properties of a healthy human disc [57]. As disc material is viscoelastic, the stress-strain relationship varies with the frequency and duration of the applied load. The normal coordination of intervertebral and facet joint motion allows for complex three-dimensional motion (coronal, axial, and sagittal) through varying degrees of compression or distraction. Therefore, disc arthroplasty should closely replicate these characteristics with regard to the quality and quantity of motion in addition to shock absorption and stiffness. The Axiomed disc arthroplasty is a one-piece viscoelastic implant bonded to two endplates, which may offer benefits over earlier implant designs as it has shown to closely mimic human disc biomechanics in early preclinical and clinical studies [57, 58••, 59]. Further long-term clinical outcomes studies are needed.
Conclusion
In the last two decades, significant advancements in motion preservation technology have been made and CDR has gained popularity as a result of this progress. The potential to overcome complications associated with fusion procedures such as ASD and pseudarthrosis may improve patient outcomes, decrease reoperation rates, and minimize the financial burden on the healthcare system. Currently, several reliable implants for CDR are available that can vary greatly and a comprehensive understanding of each device is paramount to the spine surgeon. Continuing to improve upon current arthroplasty technology may provide a reliable and less invasive treatment option with preservation of motion.
Compliance with ethical standards
Conflict of interest
Kris Radcliff reports personal fees from Globus Medical, personal fees from Depuy, personal fees from Stryker, personal fees from MEdtronic, personal fees from Orthopedic Sciences, Inc., personal fees from Nuvasive, personal fees from 4 Web MEdical, other from LDR Medical (now Zimmer), outside the submitted work, and shareholder status Rothman Institute.
All the other authors declare no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Motion Preserving Spine Surgery
Contributor Information
Dante Leven, Email: danteleven@gmail.com.
Joshua Meaike, Email: Joshua.meaike@icahn.mssm.edu.
Kris Radcliff, Email: radcliffk@gmail.com.
Sheeraz Qureshi, Email: sheerazqureshimd@gmail.com.
References
Papers of particular interest, published recently, have been highlighted as: • of Importance •• Of major importance
- 1.Edwards CC, 2nd, Heller JG, Murakami H. Corpectomy versus laminoplasty for multilevel cervical myelopathy: an independent matched-cohort analysis. Spine (Phila Pa 1976) 2002;27(11):1168–1175. doi: 10.1097/00007632-200206010-00007. [DOI] [PubMed] [Google Scholar]
- 2.Bohlman HH, Anderson PA. Anterior decompression and arthrodesis of the cervical spine: long-term motor improvement. Part I—improvement in incomplete traumatic quadriparesis. J Bone Joint Surg Am. 1992;74(5):671–682. doi: 10.2106/00004623-199274050-00006. [DOI] [PubMed] [Google Scholar]
- 3.Seng C, Tow BP, Siddiqui MA, Srivastava A, Wang L, Yew AK, Yeo W, Khoo SH, Balakrishnan NM, Bin Abd Razak HR, Chen JL, Guo CM, Tan SB, Yue WM. Surgically treated cervical myelopathy: a functional outcome comparison study between multilevel anterior cervical decompression fusion with instrumentation and posterior laminoplasty. Spine J. 2013;13(7):723–731. doi: 10.1016/j.spinee.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 4.Bydon M, Mathios D, Macki M, de la Garza-Ramos R, Sciubba DM, Witham TF, Wolinsky JP, Gokaslan ZL, Bydon A. Long-term patient outcomes after posterior cervical foraminotomy: an analysis of 151 cases. J Neurosurg Spine. 2014;21(5):727–731. doi: 10.3171/2014.7.SPINE131110. [DOI] [PubMed] [Google Scholar]
- 5.Radhakrishnan K, Litchy WJ, O’Fallon WM, Kurland LT. Epidemiology of cervical radiculopathy. A population-based study from Rochester, Minnesota, 1976 through 1990. Brain. 1994;117(Pt 2):325–335. doi: 10.1093/brain/117.2.325. [DOI] [PubMed] [Google Scholar]
- 6.Matsunaga S, Kabayama S, Yamamoto T, Yone K, Sakou T, et al. Strain on intervertebral discs after anterior cervical decompression and fusion. Spine (Phila Pa 1976) 1999;24(7):670–675. doi: 10.1097/00007632-199904010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine. 2005;3(6):417–423. doi: 10.3171/spi.2005.3.6.0417. [DOI] [PubMed] [Google Scholar]
- 8.Zhong ZM, Zhu SY, Zhuang JS, Wu Q, Chen JT. Reoperation after cervical disc arthroplasty versus anterior cervical discectomy and fusion: a meta-analysis. Clin Orthop Relat Res. 2016;474(5):1307–1316. doi: 10.1007/s11999-016-4707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reitz H, Joubert MJ. Intractable headache and cervico-brachialgia treated by complete replacement of cervical intervertebral discs with a metal prosthesis. S Afr Med J. 1964;38:881–884. [PubMed] [Google Scholar]
- 10.Fernström U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand Suppl. 1966;357:154–159. [PubMed] [Google Scholar]
- 11.Delamarter RB, Zigler J. Five-year reoperation rates, cervical total disc replacement versus fusion, results of a prospective randomized clinical trial. Spine (Phila Pa 1976) 2013;38(9):711–717. doi: 10.1097/BRS.0b013e3182797592. [DOI] [PubMed] [Google Scholar]
- 12.Radcliff K, Coric D, Albert T. Five-year clinical results of cervical total disc replacement compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: a prospective, randomized, controlled, multicenter investigational device exemption clinical trial. J Neurosurg Spine. 2016;25(2):213–224. doi: 10.3171/2015.12.SPINE15824. [DOI] [PubMed] [Google Scholar]
- 13.Luo J, Huang S, Gong M, Dai X, Gao M, Yu T, Zhou Z, Zou X. Comparison of artificial cervical arthroplasty versus anterior cervical discectomy and fusion for one-level cervical degenerative disc disease: a meta-analysis of randomized controlled trials. Eur J Orthop Surg Traumatol. 2015;25(Suppl 1):S115–S125. doi: 10.1007/s00590-014-1510-4. [DOI] [PubMed] [Google Scholar]
- 14.Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, Tay B, Darden B. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9(4):275–286. doi: 10.1016/j.spinee.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Davis RJ, Nunley PD, Kim KD, Hisey MS, Jackson RJ, Bae HW, Hoffman GA, Gaede SE, Danielson GO, 3rd, Gordon C, Stone MB. Two-level total disc replacement with Mobi-C cervical artificial disc versus anterior discectomy and fusion: a prospective, randomized, controlled multicenter clinical trial with 4-year follow-up results. J Neurosurg Spine. 2015;22(1):15–25. doi: 10.3171/2014.7.SPINE13953. [DOI] [PubMed] [Google Scholar]
- 16.Puttlitz CM, Rousseau MA, Xu Z, Hu S, Tay BK, et al. Intervertebral disc replacement maintains cervical spine kinetics. Spine (Phila Pa 1976) 2004;29(24):2809–2814. doi: 10.1097/01.brs.0000147739.42354.a9. [DOI] [PubMed] [Google Scholar]
- 17.DiAngelo DJ, Foley KT, Morrow BR, Schwab JS, Song J, et al. In vitro biomechanics of cervical disc arthroplasty with the ProDisc-C total disc implant. Neurosurg Focus. 2004;17(3) [PubMed] [Google Scholar]
- 18.Zhou HH, Qu Y, Dong RP, Kang MY, Zhao JW. Does heterotopic ossification affect the outcomes of cervical total disc replacement? A meta-analysis Spine (Phila Pa 1976) 2015;40(6):E332–E340. doi: 10.1097/BRS.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 19.Verma K, Gandhi SD, Maltenfort M, Albert TJ, Hilibrand AS, Vaccaro AR, Radcliff KE. Rate of adjacent segment disease in cervical disc arthroplasty versus single-level fusion: meta-analysis of prospective studies. Spine (Phila Pa 1976) 2013;38(26):2253–2257. doi: 10.1097/BRS.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 20.Lee JC, Lee SH, Peters C, Riew KD. Risk-factor analysis of adjacent-segment pathology requiring surgery following anterior, posterior, fusion, and nonfusion cervical spine operations: survivorship analysis of 1358 patients. J Bone Joint Surg Am. 2014;96(21):1761–1767. doi: 10.2106/JBJS.M.01482. [DOI] [PubMed] [Google Scholar]
- 21.Wu JC, Huang WC, Tsai HW, Ko CC, Fay LY, Tu TH, Wu CL, Cheng H. Differences between 1- and 2-level cervical arthroplasty: more heterotopic ossification in 2-level disc replacement: clinical article. J Neurosurg Spine. 2012;16(6):594–600. doi: 10.3171/2012.2.SPINE111066. [DOI] [PubMed] [Google Scholar]
- 22.Yi S, Kim KN, Yang MS, Yang JW, Kim H, Ha Y, Yoon DH, Shin HC. Difference in occurrence of heterotopic ossification according to prosthesis type in the cervical artificial disc replacement. Spine (Phila Pa 1976) 2010;35(16):1556–1561. doi: 10.1097/BRS.0b013e3181c6526b. [DOI] [PubMed] [Google Scholar]
- 23.Yi S, Shin DA, Kim KN, Choi G, Shin HC, Kim KS, Yoon DH. The predisposing factors for the heterotopic ossification after cervical artificial disc replacement. Spine J. 2013;13(9):1048–1054. doi: 10.1016/j.spinee.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 24.Nunley PD, Jawahar A, Kerr EJ, 3rd, Gordon CJ, Cavanaugh DA, Birdsong EM, Stocks M, Danielson G. Factors affecting the incidence of symptomatic adjacent-level disease in cervical spine after total disc arthroplasty: 2- to 4-year follow-up of 3 prospective randomized trials. Spine (Phila Pa 1976) 2012;37(6):445–451. doi: 10.1097/BRS.0b013e31822174b3. [DOI] [PubMed] [Google Scholar]
- 25.Buchowski J, Riew K: Primary indications and disc space preparation for cervical disc arthroplasty. In: Yue J, editors. Motion preservation surgery of the spine: advanced techniques and controversies. Saunders/Elsevier; 2008.
- 26.Ding D, Shaffrey ME. Cervical disk arthroplasty: patient selection. Clin Neurosurg. 2012;59:91–97. doi: 10.1227/NEU.0b013e31826b6fbe. [DOI] [PubMed] [Google Scholar]
- 27.Goffin J. Complications of cervical disc arthroplasty. Semin Spine Surg. 2006;18:87–97. doi: 10.1053/j.semss.2006.03.010. [DOI] [Google Scholar]
- 28.Beaurin J, Bernard P, Dufour T, Fuentes J, Hovorka I, Huppert J, Steib J, Vital J. Mobi-C. In: Yue J, editor. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 29.Stieber J, Fischgrund J, Abitbol J. The cervicore cervical intervertebral disc replacement. In: Yue J, editor. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 30.Fassett D, Jeyamohan S, Vaccaro A, Whang P. Mobi-C. In: Yue J, editor. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 31.Skeppholm M, Olerud C. Comparison of dysphagia between cervical artificial disc replacement and fusion: data from a randomized controlled study with two years of follow-up. Spine (Phiila Pa 1976) 2013;38(24):E1507–E1510. doi: 10.1097/BRS.0b013e3182a516ef. [DOI] [PubMed] [Google Scholar]
- 32.Loret JE, Francois P, Papagiannaki C, Cottier JP, Terrier LM, Zemmoura I. Internal carotid artery dissection after anterior cervical disc replacement: first case report and literature review of vascular complications of the approach. Eur J Orthop Surg Traumatol. 2013;23(Suppl 1):S107–S110. doi: 10.1007/s00590-013-1228-8. [DOI] [PubMed] [Google Scholar]
- 33.Viezens L, Schaefer C, Beyerlein J, Thietje R, Hansen-Algenstaedt N. An incomplete paraplegia following the dislocation of an artificial cervical total disc replacement. J Neurosurg Spine. 2013;18(3):255–259. doi: 10.3171/2013.1.SPINE12691. [DOI] [PubMed] [Google Scholar]
- 34.Heary RF, Goldstein IM, Getto KM, Agarwal N. Solid radiographic fusion with a nonconstrained device 5 years after cervical arthroplasty. J Neurosurg Spine. 2014;21(6):951–955. doi: 10.3171/2014.8.SPINE14101. [DOI] [PubMed] [Google Scholar]
- 35.Mehren C, Suchomel P, Grochulla F, Barsa P, Sourkova P, Hradil J, Korge A, Mayer HM. Heterotopic ossification in total cervical artificial disc replacement. Spine (Phila Pa 1976) 2006;31(24):2802–2806. doi: 10.1097/01.brs.0000245852.70594.d5. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Wang X, Bai W, Shen X, Yuan W. Prevalence of heterotopic ossification after cervical total disc arthroplasty: a meta-analysis. Eur Spine J. 2012;21(4):674–680. doi: 10.1007/s00586-011-2094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunley PD, Jawahar A, Cavanaugh DA, Gordon CR, Kerr EJ, 3rd, Utter PA. Symptomatic adjacent segment disease after cervical total disc replacement: re-examining the clinical and radiological evidence with established criteria. Spine J. 2013;13(1):5–12. doi: 10.1016/j.spinee.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 38.Orndorff D, Poelstra K, Albert T. Discover artificial cervical disc. In: Yue J, editor. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 39.Coric D, Oberer D. Cervical approaches. In: Yue J, editor. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 40.Pearson A, Albert T: Cervical and cervicothoracic instrumentation. In: Patel V, editors. Spine surgery basics. Springer; 2014.
- 41.Grochulla F: Anterior cervical discectomy and fusion. In: Vieweg U, Grochulla F, editors. Manual of spine surgery. Springer; 2012.
- 42.Sasso R, Martin L. The Bryan artificial disc. In: Yue J, editor. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 43.Zhao Y, Zhang Y, Sun Y, Pan S, Zhou F, Liu Z. Application of cervical arthroplasty with Bryan cervical disc: 10-year follow-up results in China. Spine (Phila Pa 1976) 2016;41(2):111–115. doi: 10.1097/BRS.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, Tian Z, Zhu B, Zhang W, Li Y, Zhu Q. Bryan cervical disc arthroplasty versus anterior cervical discectomy and fusion for treatment of cervical disc diseases: a meta-analysis of prospective, randomized controlled trials. Spine (Phila Pa 1976) 2016;41(12):E733–E741. doi: 10.1097/BRS.0000000000001367. [DOI] [PubMed] [Google Scholar]
- 45.Skeppholm M, Lindgren L, Henriques T, Vavruch L, Löfgren H, Olerud C. The Discover artificial disc replacement versus fusion in cervical radiculopathy—a randomized controlled outcome trial with 2-year follow-up. Spine J. 2015;15(6):1284–1294. doi: 10.1016/j.spinee.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 46.Hisey MS, Zigler JE, Jackson R, Nunley PD, Bae HW, Kim KD, Ohnmeiss DD. Prospective, randomized comparison of one-level Mobi-C cervical total disc replacement vs. anterior cervical discectomy and fusion: results at 5-year follow-up. Int J Spine Surg. 2016;10:10. doi: 10.14444/3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traynelis V. The prestige cervical disc. In: Yue J, editor. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 48.Gornet MF, Burkus JK, Shaffrey ME, Nian H, Harrell FE., Jr Cervical disc arthroplasty with prestige LP disc versus anterior cervical discectomy and fusion: seven-year outcomes. Int J Spine Surg. 2016;10:24. doi: 10.14444/3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delamarter R, Pradhan B. ProDisc-C total cervical disc replacement. In: Yue J, editor. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 50.Zigler JE, Delamarter R, Murrey D, Spivak J, Janssen M. ProDisc-C and anterior cervical discectomy and fusion as surgical treatment for single-level cervical symptomatic degenerative disc disease: five-year results of a Food and Drug Administration study. Spine (Phila Pa 1976) 2013;38(3):203–209. doi: 10.1097/BRS.0b013e318278eb38. [DOI] [PubMed] [Google Scholar]
- 51.Colle KO, Butler JB, Reyes PM, Newcomb AG, Theodore N, Crawford NR. Biomechanical evaluation of a metal-on-metal cervical intervertebral disc prosthesis. Spine J. 2013;13(11):1640–1649. doi: 10.1016/j.spinee.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 52.Rushton S, Marzluff J, McConnel J. SECURE-C cervical artificial disc. In: Yue J, editors. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 53.Vaccaro A, Beutler W, Peppelman W, Marzluff JM, Highsmith J, Mugglin A, DeMuth G, Gudipally M, Baker KJ. Clinical outcomes with selectively constrained SECURE-C cervical disc arthroplasty: two-year results from a prospective, randomized, controlled, multicenter investigational device exemption study. Spine (Phila Pa 1976) 2013;38(26):2227–2239. doi: 10.1097/BRS.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 54.Reyes-Sanchez A, Patwardhan A, Block J. The M6 artificial cervical disc. In: Yue J, editor. Motion preservation surgery of the spine: advanced techniques and controversies. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 55.Reyes-Sanchez A, Miramontes V, Olivarez LM, Aquirre AA, Quiroz AO, Zarate-Kalfopulos B. Initial clinical experience with a next-generation artificial disc for the treatment of symptomatic degenerative cervical radiculopathy. SAS J. 2010;4(1):9–15. doi: 10.1016/j.esas.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu Y, Lv G, Ren S, Johansen D. Mid- to long-term outcomes of cervical disc arthroplasty versus anterior cervical discectomy and fusion for treatment of symptomatic cervical disc disease: a systematic review and meta-analysis of eight prospective randomized controlled trials. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0149312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benzel E, Lieberman I, Ross ER, Linovitz RJ, Kuras J, Zimmers K. Mechanical characterization of a viscoelastic disc for lumbar total disc replacement. J of Med Devices. 2011;5:1–7. doi: 10.1115/1.4003536. [DOI] [Google Scholar]
- 58.Rischke B, Ross R, Jollenbeck B, Zimmers K, Defibaugh N. Preclinical and clinical experience with a viscoelastic total disc replacement. SciVerse ScienceDirect Journal. 2011;5:97–107. doi: 10.1016/j.esas.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rischke B, Zimmers K, Smith E. Viscoelastic disc arthroplasty provides superior back and leg pain relief in patients with lumbar disc degeneration compared to anterior lumbar interbody fusion. Int J Spine Surgery. 9(26):1–8. [DOI] [PMC free article] [PubMed]


