Abstract
Purpose of the review
The purposes of this study are to examine the literature within the past 5 years regarding osteoporosis and offer a discussion on new topics and controversies.
Recent findings
Patient compliance with therapy remains an issue.
The effectiveness of Vitamin D and calcium are being called into question
Atypical femur fractures have been associated with bisphosphonate and denosumab use. Treatment is both surgical and pharmaceutical.
A multidisciplinary approach to osteoporotic fractures is important and having some form of fracture liaison service (FLS) improves the efficacy of osteoporotic care and decreases secondary fractures.
Screening for osteoporosis remains low.
Ultrasound may be cost-effective for diagnosis.
Summary
Understanding of osteoporosis has come a long way in the medical community, but the translation to the lay community has lagged behind. Patients often take a laissez-faire attitude toward osteoporosis that can affect compliance. Information read by patients often focuses on complications, such as atypical femur fractures and myocardial infarctions. It is essential for providers to be able to discuss these issues with patients. Newer medications and more cost-effective diagnostic tests exist, but availability may be limited. FLS are effective, but the most cost-effective model for therapy still eludes us. Areas for further investigation include FLS models, the effectiveness of vitamin supplementation, and more ubiquitous and cost-effective diagnostic tools.
Keywords: Osteoporosis review, Osteoporosis controversies, Osteoporosis treatment, Osteoporosis management, Vitamin D and calcium supplementation
Introduction
Osteoporosis is a medical disease with many ramifications for orthopedic surgeons. Prior to modern imaging, the diagnosis was often made only after a person sustained a fragility fracture. Dual energy X-ray absorptiometry (DXA) allowed diagnosis prior to a fracture occurring and gave providers the chance to initiate appropriate treatment. Ongoing discussion regarding prevention and screening eventually led to programs such as “Own the Bone,” a national web-based program that focused on post fracture osteoporotic care and prevention [1]. The FRAX tool allows clinicians to calculate risk for future fractures for their patients and can be used to help tailor treatment [2]. The medical community has made great strides in terms of osteoporosis diagnosis and care; however, not all of that has translated to the patients. The idea that osteoporosis is just a normal part of the aging process has been difficult to overcome. This article will review the recent updates and advances regarding: patient education challenges, atypical femur fractures, vitamin supplementation, medication advances, post fracture management including the fracture liaison service (FLS), and screening/diagnostic modalities.
New challenges
Compliance
Historically, challenges regarding osteoporotic care were related more to the complications that arose from fragility fractures than from the acute treatment itself. However, as advances have been made in the diagnosis of the disease and preventative treatment, these challenges have shifted toward patient compliance and complications associated with the treatment. There are many reasons for poor patient compliance including the regimen and side effects of the medication [3], the failure to effectively transition care [4], the understanding of the diagnosis [5–7], and the availability of information. Convincing patients to take their medication to prevent what they believe is a normal aging process can be difficult [5]. Rather than trying to prevent fractures with evaluation and management, patients take their chances with sustaining a fracture. Patients must realize that the morbidity associated with osteoporosis affects more people and carries more disability-adjusted life years lost than most cancers [8]. Furthermore, the mortality and morbidity of sustaining a hip fracture is well established with roughly a 30% 1-year mortality rate in the elderly [9, 10]. This lack of urgency regarding osteoporosis even exists in the medical community. If a patient has already sustained a fracture, stressing to them the risk of a secondary fracture, including the associated mortality and morbidity, is an important part of the conversation [11–13]. Patient compliance and understanding has been linked, in previous studies, to the quality of physician explanations and the physician-patient relationship [14]. Physicians’ perception of patient compliance is also usually over-estimated [8], so continuing to have an open dialog regarding the disease and its management is important.
Available information
The internet provides a significant amount of information for patients, however, processing this knowledge can often be difficult, and the way the information is presented may be misleading. Studies have examined the release of information regarding complications and the compliance with medications. One in particular examined the use of bisphosphonates over several years and found that each time a complication of the medication was announced, the use of the medicine declined and internet searches on the topic increased [15]. The release of complications by the FDA has also been shown to negatively impact the use of bisphosphonates [5, 16]. The decrease in the prescription of anti-resorptive agents is concerning, as the baseline initiation of therapy is already low [16•]. It should be emphasized to patients that bisphosphonate therapy is still the first line prescription medication for osteoporosis, and that the risks of fracture far outweigh the risks of complications associated with the medication [17]. Staying up to date and aware of potential complications is important for providers, as is being able to have a conversation with patients regarding these recent advances in a relatable way. Offering a reliable source of information to the patient is also an important step in patient compliance and satisfaction with treatment.
Atypical femur fractures
Atypical femur fractures (AFF) (Fig. 1) are associated with prolonged use of bisphosphonates and have now become a recognized complication of the drug [18–20] . While the mechanism is unclear, it is likely related to abnormal bone healing associated with microfractures in the high stress area of the subtrochanteric femur and limited bone turnover due to the anti-resorptive agent [20]. This is an important complication to recognize, as treatment is often difficult and there is a higher nonunion rate compared to typical femur fractures [21, 22]. Prior to a complete fracture, a consistent finding is “beaking” of the lateral femoral cortex in the subtrochanteric region (Fig. 1) [23]. In addition, patients will often have prodromal vague pain in the hip and upper thigh prior to fracture, making it an important question to ask when patients have been on bisphosphonates for an extended period of time. It is also important to recognize that AFF may occur bilaterally, so imaging of the contralateral side is indicated with all recognized atypical femur fractures or patients with lateral cortical beaking (Fig. 1) [19]. While the nonunion rate for AFF may be higher, recent literature has suggested that teriparatide may decrease this nonunion rate [24–26].
Fig. 1.
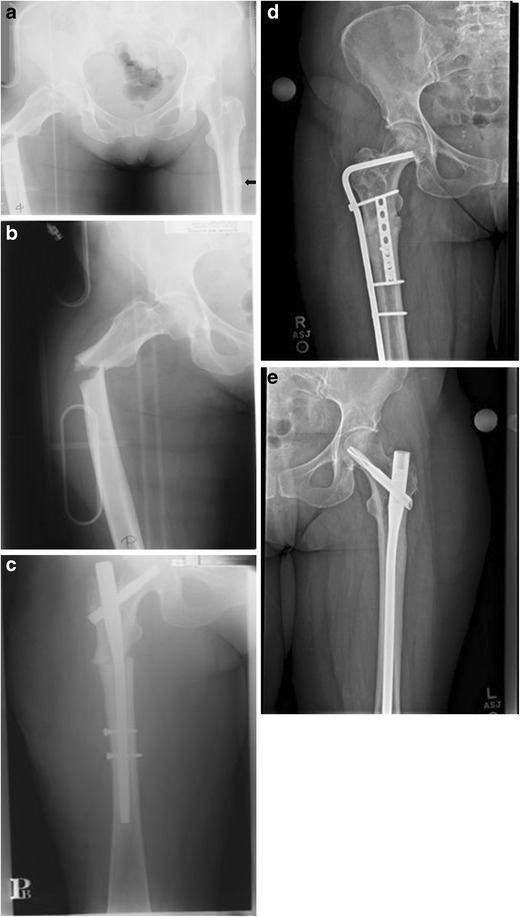
a, b Preoperative images of a patient on chronic antiresorptive therapy presenting to an outside facility with an AFF. Notice the atypical fracture pattern and contralateral beaking (arrow). c Images after fracture fixation with cephalomedullary device of the right femur only. Pt eventually presented with a nonunion of the right femur and left hip pain d, e After revision open reduction and internal fixation of the right femur, the left side underwent a prophylactic cephalomedullary nailing
It is important to note that the risk-benefit ratio remains in favor of taking a bisphosphonate and they remain the first line of therapy for osteoporosis. However, this positive risk-benefit ratio only remains for 5 years of oral and 3 years of intravenous bisphosphonate therapy [20, 27, 28•]. After that time, benefits may no longer outweigh risks. This has prompted the idea of a 2- to 3-year “drug holiday” to prevent the occurrence of AFF and the associated morbidity [20, 27•]. Treatment of AFF currently follows the same algorithm as the treatment of typical subtrochanteric femur fractures—i.e., appropriate reduction followed by a cephalomedullary nail. While healing may be delayed, it is typically reliable [29].
Management of the Disease
Post-fracture management
While recent efforts have focused on preventing fragility fractures and treating osteoporosis, a fracture remains the only true “symptom” of osteoporosis and is likely the most common reason for diagnosis. Many models exist on how to manage osteoporosis once a fracture occurs. Some of these models focus on a primary care or emergency medical physician evaluating and initiating osteoporosis therapy while others focus on orthopedic surgeons [30•]. There is literature that supports improved compliance and adherence when orthopedic surgeons take the lead, at least initially [30•, 31]. Since long-term care after the sentinel fracture is less likely to occur with an orthopedic surgeon, the concept of a fracture liaison service (FLS) developed. This service usually consists of a physician extender with physician oversight. The goal of the service is to identify an osteoporotic fracture, initiate an appropriate work-up and treatment, and ensure an appropriate long-term management of the disease. FLS improves the initiation of treatment for osteoporosis, prolongs adherence to treatment, and decreases secondary fracture risk with an overall cost-savings to the healthcare system through continuity of care [32–38]. While the perfect model has not yet been determined, hospitals and physicians should push for a FLS regardless of final make-up of the team, as several studies support even a limited FLS as being effective from both a cost and a re-fracture standpoint [39, 40].
An orthogeriatric service is a multi-disciplinary service consisting of an orthopedic surgeon and a geriatric specialist that coordinates the surgical and medical care of elderly patients after an orthopedic injury, usually a hip fracture. This service is usually protocol-driven and is effective at decreasing mortality and improving morbidity; however, this multi-disciplinary approach has been inconclusive when it comes to decreasing the incidence of secondary fractures, decreasing length of stay, and decreasing time to surgery [41–43]. Although the patients may be discharged on appropriate osteoporosis medications, long-term follow-up is lacking. Since inpatient osteoporosis evaluation is not covered by Medicare and coordinating long-term post-discharge bone health care is more difficult, others advocate utilizing both an orthogeriatric service and an FLS to help improve long-term bone health. Data shows that this is effective at decreasing mortality, decreasing secondary fracture risk, and is cost-effective [44, 45, 32, 46]. However, the specific model for an orthogeriatric, FLS, or combined approach has yet to be established.
Supplementation
Calcium and vitamin D supplementation is still a staple for the treatment and management of osteoporosis. There is some variation in recommended daily values depending on the source, but the range for Vitamin D is typically 600–800 international units per day (IU/d), with calcium ranging from 1000 to 1200 mg/day for men and women over 50 [47, 48]. A relatively cheap, over the counter medication with a traditionally safe profile has made the supplement easy to prescribe and manage. Even after a fracture has occurred, it is usually the supplementation that is both prescribed and adhered to by patients, as opposed to pharmaceuticals [49]. While a recent meta-analysis continues to support the use of calcium and Vitamin D [50•], some recent literature has called into question both the dosing of calcium and efficacy of the supplementation, with some even suggesting that vitamin D may be all that’s necessary [51–53]. Other studies have shown that only a small increase of bone mineral density (BMD) is noted with calcium supplementation and that it is not enough to prevent a fracture [54, 55]. Others question the risk profile, stating that calcium should not be routinely used due to gastrointestinal, renal, and cardiac-related side effects [56]. With most of the dose recommendations based on studies several decades old, perhaps it is time to re-evaluate this issue.
A recent study made a significant link between calcium supplementation and cardiovascular disease (CVD)—specifically arteriosclerosis [57•]. However, the study failed to establish a link between calcium supplementation and myocardial infarction (MI) or other adverse clinical manifestations. While the study brings up an important issue to consider, there is currently no correlation with an adverse clinical outcome. Other studies are needed to assess the risk-benefit ratio between potential MI and osteoporotic fractures, with many already failing to find a link [58–61]. One of the major issues with the study associating calcium supplementation and arteriosclerosis was how it was presented to the public. A recent news article regarding the study inferred to the general public that the supplements could lead to a “heart attack” [62•]. Based on previous literature regarding release of information to the public and medications, this could lead to a decrease in compliance for calcium supplementation when prescribed and could undermine patient-physician relationships. It is important to be able to address these issues with patients to help mitigate their concerns and both improve their compliance and maintain their trust. It should also be noted that the link between supplementation and CVD is controversial, with the National Osteoporosis Foundation and the American Society for Preventative Cardiology recently releasing a joint position statement, and press release, on the lack of evidence between the two [63•].
Recent advent in medicine
For years, the first line in therapy for osteoporosis has remained bisphosphonates. However, associated complications such as osteonecrosis of the jaw, bowel/esophageal complications, and atypical femur fractures, combined with the regimen required for effective treatment made patient compliance challenging [16•, 3, 64–66]. While newer, less-frequent dosing and intravenous regimens, as well as improved side effect profiles, may have led to better patient compliance, it still remains relatively low [67, 68, 5]. Cost remains an issue concerning newer medications, with the more cost-effective drugs often carrying greater side effects and a more difficult dosing regimen.
Teriparatide
Teriparatide, a recombinant form of parathyroid hormone (PTH), binds to PTH receptors on osteoblasts and produces an anabolic effect. Teriparatide has recently become a drug of choice for the treatment of osteoporosis for many providers. The daily dosing and need for injection does not offer an improvement in ease of use over bisphosphonates; however, the side effects are minimal. Cost has remained the major issue, making the drug difficult to prescribe ubiquitously. Forteo® (Eli Lilly, Indianapolis, IN), currently costs $3100 for a 4-week supply (August 2016). Standard therapy is a once-daily injection, and because long-term health effects are relatively unknown, the drug is not currently indicated for use greater than 2 years. Of note, the manufacturer, Eli-Lilly (Indianapolis, IN), has raised the price of Forteo® by 9–15% biannually for the past 3 years [69]. With teriparatide being the only current anabolic drug on the market, the incentive for making the drug more affordable is likely limited. The drug is contraindicated in patients with diseases such as Paget’s or history of metastatic bone disease due to an increased risk of osteosarcoma and exacerbation of skeletal metastasis, respectively. [70, 71]. It is currently indicated in postmenopausal women with osteoporosis and an increased fracture risk having failed or not able to tolerate other forms of osteoporosis therapy, including bisphosphonates [20].
Abaloparatide
Abaloparatide is a medication that recently completed phase three clinical trials. Its function is through selective activation of the parathyroid hormone type 1 receptor. It has a daily dosing regimen as well; however, it is only anabolic and does not carry any of the catabolic effects that teriparatide potentially offers [72]. In a recent study, the drug was more effective than both placebo and teriparatide in the prevention of new osteoporotic vertebral fractures and had a lower incidence of hypercalcemia when compared with teriparatide [73•]. While cost of the drug is not available, the availability of the drug may create a more competitive, affordable market.
Denosumab
Denosumab is a direct inhibitor of RANKL [74]. It only affects osteoclasts. Denosumab has been shown to be effective in the treatment of osteoporosis and prevention of osteoporotic fractures [75, 20, 76]. Its current indications include postmenopausal women with osteoporosis, women receiving aromatase inhibitor therapy (prevents the conversion of androgens to estrogens) for breast cancer treatment with an increased fracture risk, men with osteoporosis and men at high risk for fracture who receive androgen-deprivation therapy for non-metastatic prostate cancer [20]. A recent trial showed a significant reduction in vertebral and non-vertebral fractures and denosumab was as effective as zolendronate [77, 78]. It is a biannual injection, making patient compliance easier as well. Like teriparatide, the cost of the drug can be high, up to $1100 per treatment, but assistance programs are available. While the drug is effective at decreasing the risk of osteoporotic fractures, it has been shown to increase the risk of infection [79•, 80, 81]. Another interesting complication of Denosumab is its association with osteonecrosis of the jaw and potential association with atypical femur fractures that are typically linked to bisphosphonates [80–83]. This may be explained by the fact that while the mechanism of action of Denosumab and bisphosphonates are different, they both inhibit osteoclasts. Combination therapy with Denosumab and Teriparatide has shown to increase bone mineral density significantly more than with either therapy alone [84–86]
Screening
Clinical/laboratory screening
The idea of pre-screening patients who are at risk for osteoporosis is often discussed. Many other diseases that carry the morbidity similar to osteoporosis have several laboratory and clinical tests available to help guide treatment, yet preselecting patients to receive a DXA or medications for osteoporosis is often limited or underutilized. The FRAX score (https://www.shef.ac.uk), while carrying some limitations and having a lower sensitivity and specificity for younger patients [87], is validated, readily available, and easily administered to patients. However, the actual utilization of FRAX is poor, with the USA performing 11,807 calculations per 1 million people over the age of 50 [88]. Furthermore, the utilization of any screening method for osteoporosis is less than 27% for patients ages 65–79, with percentages worsening with both younger and older patient populations [89]. Utilization of prescreening methods in a clinical setting could easily increase among physicians, with many other questionnaires and tests available. With the risk of decreased bone density starting at the age of 50, it would be appropriate to start screening patients at age 50 to maximize the benefit and cost-effectiveness of screening and fracture prevention [90].
As opposed to imaging, which is used to diagnose osteoporosis, a laboratory work-up is traditionally used to exclude secondary causes of the disease, lacking an ability to accurately diagnose the disease itself. Many potential laboratory markers have been used, including evaluation of thyroid and parathyroid hormones and testosterone. Hypomagnesemia has recently been evaluated as a possible risk factor for osteoporosis [91]. Bone turnover markers have been used as a way of monitoring osteoporosis treatment [92, 93]. However, the topic remains controversial and standardization of laboratory marker levels, which markers to use, and monitoring intervals remain issues [94, 95]. Other markers of interest are genetic in nature. Recent investigations have evaluated the association between genes and a person’s resistance or susceptibility to osteoporosis [96, 97].
Diagnostic modalities
The diagnosis of osteoporosis is generally based on the use of DXA. DXA scans are a measure of the bone mineral density, as X-ray absorption is directly related to calcium content within the tissues [98]. While a full discussion of DXA scans is beyond this article, the diagnosis is based upon the standard deviation from the normal 30-year-old woman. A standard deviation 2.5 or greater below normal confirms osteoporosis with a full breakdown available in Table 1. Some of the current recommendations for BMD testing, according to the US Preventive Services Task Force, include testing women 65 years of age and older, or those 64 and under with a risk profile equal to that of a 65 year old. There is some variation by source in terms of testing, but most agree that testing women 65 years and older, and men over 70, is prudent [99•]. While the normal baseline in the T-score value is for women, there are studies that show it can be applied to men with equal efficacy [99•, 100]. Several limitations to DXA scans have been documented, including previous fracture, osteoarthritis, osteomalacia, metal implants, and collection and interpretation of results [101, 98]. Even with its limitations, it has become the gold standard for diagnosing osteoporosis. Quantitative computed tomography (QCT) overcomes some of these limitations, with the exception of metal artifact, and has the benefit of being the only study that provides a true measurement of bone density [102, 103]. However, the radiation dose is higher, the cost greater, and there is poor quality control as CT scanners must be calibrated for the measurement. Several other methods are available, with most using a form of radiograph or CT scan. Recently, more evidence supports the use of ultrasound in the diagnosis of osteoporosis, which carries the benefit of no radiation exposure, is readily available, and is cost-effective for an office setting [103–106]. However, its cost-effectiveness when compared to DXA has been called into question and it may serve as a more appropriate tool for pre-screening patients prior to DXA or where DXA is not readily available [103, 107]. The combination of FRAX with ultrasound shows promise in both diagnosing osteoporosis and avoiding DXA scans, but additional studies are needed [104].
Table 1.
Diagnostic thresholds for osteoporosis
| Disease | T Score on DXA |
|---|---|
| Normal | T Score ≥−1 SD |
| Osteopenia | T Score between −1 and −2.5 |
| Osteoporosis | T Score ≤−2.5 |
| Severe osteoporosis | T Score ≤−2.5 w/a fragility fracture |
Adapted from osteoporosis: the evolution of a diagnosis [108]
Conclusion
The management and understanding of osteoporosis has made great strides over the years, but there is still a long way to go. Patient screening, education, and medical compliance are all areas that can easily be improved by physicians. The disease crosses many specialties, and a team approach with interdisciplinary communication is important. Bone health remains an area of medicine with many unanswered questions that can be easily explored. The development of FLS and orthogeriatric services has led to many improvements in overall patient care, but the appropriate model in each setting remains unknown. Developing more cost-effective and ubiquitous screening methods, as well as making medication regimens and side effect profiles more manageable, will be important issues moving forward. As our understanding of the disease grows, perhaps the most important issue lies in the physician-patient relationship. Recognizing osteoporosis and its risk factors, as well as maintaining an open dialog with patients, should be a priority of all physicians. Managing patient expectations and the breadth of information available will be an ongoing and significant problem that will largely fall on the physician to not only initiate the conversation, but also determine the patient’s level of understanding of osteoporosis and its associated morbidity and mortality.
Compliance with ethics guidelines
Conflict of interest
Brett D. Crist reports other from Arthrex, personal fees from Globus, other from Orthopaedic Implant Company, other from Amedica, personal fees from Acelity/KCI, personal fees from Johnson/Johnson DePuy, outside the submitted work.
Kyle M Schweser declares that he has no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Orthopaedic Health Policy
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Tosi LL, Gliklich R, Kannan K, Koval KJ. The American Orthopaedic Association’s “own the bone” initiative to prevent secondary fractures. J Bone Joint Surg Am. 2008;90(1):163–173. doi: 10.2106/JBJS.G.00682. [DOI] [PubMed] [Google Scholar]
- 2.FRAX. University of Sheffield. http://www.shef.ac.uk/FRAX/.
- 3.Anagnostis P, Karras SN. Compliance with osteoporosis medications—an underestimated determinant of anti-fracture efficacy. Curr Med Res Opin. 2015;31(5):1015–1016. doi: 10.1185/03007995.2015.1027186. [DOI] [PubMed] [Google Scholar]
- 4.Arora VM, Prochaska ML, Farnan JM, D’Arcy MJ, Schwanz KJ, Vinci LM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–391. doi: 10.1002/jhm.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khosla S, Shane E. A crisis in the treatment of osteoporosis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31(8):1485–1487. doi: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- 6.Sujic R, Gignac MA, Cockerill R, Beaton DE. Factors predictive of the perceived osteoporosis-fracture link in fragility fracture patients. Maturitas. 2013;76(2):179–184. doi: 10.1016/j.maturitas.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Majumdar SR, McAlister FA, Johnson JA, Weir DL, Bellerose D, Hanley DA, et al. Critical impact of patient knowledge and bone density testing on starting osteoporosis treatment after fragility fracture: secondary analyses from two controlled trials. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25(9):2173–2179. doi: 10.1007/s00198-014-2728-z. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JR, Cai Q, Wade SW, Stolshek BS, Adams JL, Balasubramanian A, et al. Osteoporosis medication adherence: physician perceptions vs. patients utilization. Bone. 2013;55(1):1–6. doi: 10.1016/j.bone.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith T, Pelpola K, Ball M, Ong A, Myint PK. Pre-operative indicators for mortality following hip fracture surgery: a systematic review and meta-analysis. Age Ageing. 2014;43(4):464–471. doi: 10.1093/ageing/afu065. [DOI] [PubMed] [Google Scholar]
- 10.Kannegaard PN, van der Mark S, Eiken P, Abrahamsen B. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing. 2010;39(2):203–209. doi: 10.1093/ageing/afp221. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda S. Hip fracture—epidemiology, management and liaison service. Practice of the secondary fracture prevention of the proximal femoral fracture by the osteoporosis liaison service. Clinical calcium. 2015;25(4):551–558. [PubMed] [Google Scholar]
- 12.Sale JE, Beaton D, Bogoch E. Secondary prevention after an osteoporosis-related fracture: an overview. Clin Geriatr Med. 2014;30(2):317–332. doi: 10.1016/j.cger.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE, Jr, McLellan A, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res Off J Am Soc Bone Miner Res. 2012;27(10):2039–2046. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 14.Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long-term statin therapy? Current atherosclerosis reports. 2013;15(1):291. doi: 10.1007/s11883-012-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996-2012: an ecological analysis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015;30(12):2179–2187. doi: 10.1002/jbmr.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SC, Kim DH, Mogun H, Eddings W, Polinski JM, Franklin JM, et al. Impact of the U.S. Food and Drug Administration’s safety-related announcements on the use of bisphosphonates after hip fracture. J Bone Miner Res Off J Am Soc Bone Miner Res. 2016;31(8):1536–1540. doi: 10.1002/jbmr.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gambacciani M, Levancini M. Management of postmenopausal osteoporosis and the prevention of fractures. Panminerva Med. 2014;56(2):115–131. [PubMed] [Google Scholar]
- 18.Lee S, Yin RV, Hirpara H, Lee NC, Lee A, Llanos S, et al. Increased risk for atypical fractures associated with bisphosphonate use. Fam Pract. 2015;32(3):276–281. doi: 10.1093/fampra/cmu088. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly E, Saleh A, Unnanuntana A, Lane JM. Atypical femoral fractures: epidemiology, etiology, and patient management. Current opinion in supportive and palliative care. 2012;6(3):348–354. doi: 10.1097/SPC.0b013e3283552d7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philipp LM. Osteoporosis and pathologic bone. In: Ricci WM, Ostrum RF, editors. Orthopaedic. Knowledge Update: Trauma; 2016. [Google Scholar]
- 21.Molvik H, Khan W. Bisphosphonates and their influence on fracture healing: a systematic review. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26(4):1251–1260. doi: 10.1007/s00198-014-3007-8. [DOI] [PubMed] [Google Scholar]
- 22.Yue B, Ng A, Tang H, Joseph S, Richardson M. Delayed healing of lower limb fractures with bisphosphonate therapy. Ann R Coll Surg Engl. 2015;97(5):333–338. doi: 10.1308/003588415X14181254789321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhadada SK, Sridhar S, Muthukrishnan J, Mithal A, Sharma DC, Bhansali A, et al. Predictors of atypical femoral fractures during long term bisphosphonate therapy: a case series & review of literature. Indian J Med Res. 2014;140(1):46–54. [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda F, Kurinomaru N, Hijioka A. Weekly teriparatide for delayed unions of atypical Subtrochanteric femur fractures. Biol Ther. 2014;4(1–2):73–79. doi: 10.1007/s13554-014-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Im GI, Lee SH, et al. Journal of bone metabolism. 2015;22(4):183–189. doi: 10.11005/jbm.2015.22.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyakoshi N, Aizawa T, Sasaki S, Ando S, Maekawa S, Aonuma H, et al. Healing of bisphosphonate-associated atypical femoral fractures in patients with osteoporosis: a comparison between treatment with and without teriparatide. J Bone Miner Metab. 2015;33(5):553–559. doi: 10.1007/s00774-014-0617-3. [DOI] [PubMed] [Google Scholar]
- 27.Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res Off J Am Soc Bone Miner Res. 2016;31(1):16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2013;28(8):1729–1737. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res. 2014;472(9):2728–2734. doi: 10.1007/s11999-013-2963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller AN, Lake AF, Emory CL. Establishing a fracture liaison service: an orthopaedic approach. J Bone Joint Surg Am. 2015;97(8):675–681. doi: 10.2106/JBJS.N.00957. [DOI] [PubMed] [Google Scholar]
- 31.Beaton DE, Sujic R, McIlroy Beaton K, Sale J, Elliot-Gibson V, Bogoch ER. Patient perceptions of the path to osteoporosis care following a fragility fracture. Qual Health Res. 2012;22(12):1647–1658. doi: 10.1177/1049732312457467. [DOI] [PubMed] [Google Scholar]
- 32.Ruggiero C, Zampi E, Rinonapoli G, Baroni M, Serra R, Zengarini E, et al. Fracture prevention service to bridge the osteoporosis care gap. Clin Interv Aging. 2015;10:1035–1042. doi: 10.2147/CIA.S76695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Judge A, Javaid MK, Leal J, Hawley S, Drew S, Sheard S et al. Health Services and Delivery Research. Models of care for the delivery of secondary fracture prevention after hip fracture: a health service cost, clinical outcomes and cost-effectiveness study within a region of England. Southampton (UK): NIHR Journals Library Copyright (c) Queen’s Printer and Controller of HMSO 2016. This work was produced by Judge et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.; 2016.
- 34.Ozalp B, Aspray TJ. Orthogeriatric medicine and fracture liaison going from strength to strength. Age Ageing. 2016;45(2):180–181. doi: 10.1093/ageing/afw008. [DOI] [PubMed] [Google Scholar]
- 35.Huntjens KM, van Geel TA, van den Bergh JP, van Helden S, Willems P, Winkens B, et al. Fracture liaison service: impact on subsequent nonvertebral fracture incidence and mortality. J Bone Joint Surg Am. 2014;96(4) doi: 10.2106/JBJS.L.00223. [DOI] [PubMed] [Google Scholar]
- 36.Aizer J, Bolster MB. Fracture liaison services: promoting enhanced bone health care. Curr Rheumatol Rep. 2014;16(11):455. doi: 10.1007/s11926-014-0455-2. [DOI] [PubMed] [Google Scholar]
- 37.Eekman DA, van Helden SH, Huisman AM, Verhaar HJ, Bultink IE, Geusens PP, et al. Optimizing fracture prevention: the fracture liaison service, an observational study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25(2):701–709. doi: 10.1007/s00198-013-2481-8. [DOI] [PubMed] [Google Scholar]
- 38.Yates CJ, Chauchard MA, Liew D, Bucknill A, Wark JD. Bridging the osteoporosis treatment gap: performance and cost-effectiveness of a fracture liaison service. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2015;18(2):150–156. doi: 10.1016/j.jocd.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Fraser M. Using nurse-led liaison to prevent further fractures. Nurs Times. 2014;110(27):12–14. [PubMed] [Google Scholar]
- 40.Yong JH, Masucci L, Hoch JS, Sujic R, Beaton D. Cost-effectiveness of a fracture liaison service—a real-world evaluation after 6 years of service provision. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27(1):231–240. doi: 10.1007/s00198-015-3280-1. [DOI] [PubMed] [Google Scholar]
- 41.Henderson CY, Shanahan E, Butler A, Lenehan B, O’Connor M, Lyons D, et al. Dedicated orthogeriatric service reduces hip fracture mortality. Ir J Med Sci. 2016 doi: 10.1007/s11845-016-1453-3. [DOI] [PubMed] [Google Scholar]
- 42.Wyller TB, Watne LO, Torbergsen A, Engedal K, Frihagen F, Juliebo V, et al. The effect of a pre- and post-operative orthogeriatric service on cognitive function in patients with hip fracture. The protocol of the Oslo Orthogeriatrics Trial BMC geriatrics. 2012;12:36. doi: 10.1186/1471-2318-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49–e55. doi: 10.1097/BOT.0b013e3182a5a045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leal J, Gray AM, Hawley S, Prieto-Alhambra D, Delmestri A, Arden NK, et al. Cost-effectiveness of Orthogeriatric and fracture liaison service models of Care for hip Fracture Patients: a population-based study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2016 doi: 10.1002/jbmr.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawley S, Javaid MK, Prieto-Alhambra D, Lippett J, Sheard S, Arden NK, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing. 2016;45(2):236–242. doi: 10.1093/ageing/afv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giles M, Van Der Kallen J, Parker V, Cooper K, Gill K, Ross L, et al. A team approach: implementing a model of care for preventing osteoporosis related fractures. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22(8):2321–2328. doi: 10.1007/s00198-010-1466-0. [DOI] [PubMed] [Google Scholar]
- 47.Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National Academies Collection: Reports funded by National Institutes of Health. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US) National Academy of Sciences. 2011. [PubMed]
- 48.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pro-Risquez A, Harris SS, Song L, Rudicel S, Barnewolt B, Dawson-Hughes B. Calcium supplement and osteoporosis medication use in women and men with recent fractures. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2004;15(9):689–694. doi: 10.1007/s00198-004-1668-4. [DOI] [PubMed] [Google Scholar]
- 50.Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27(1):367–376. doi: 10.1007/s00198-015-3386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harvey NC, Biver E, Kaufman JM, Bauer J, Branco J, Brandi ML, et al. The role of calcium supplementation in healthy musculoskeletal ageing : an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF) Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016 doi: 10.1007/s00198-016-3773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reid IR. Should we prescribe calcium supplements for osteoporosis prevention? Journal of bone metabolism. 2014;21(1):21–28. doi: 10.11005/jbm.2014.21.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cesareo R, Iozzino M, D’Onofrio L, Terrinoni I, Maddaloni E, Casini A, et al. Effectiveness and safety of calcium and vitamin D treatment for postmenopausal osteoporosis. Minerva Endocrinol. 2015;40(3):231–237. [PubMed] [Google Scholar]
- 54.Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ (Clinical research ed) 2015;351:h4183. doi: 10.1136/bmj.h4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolland MJ, Leung W, Tai V, Bastin S, Gamble GD, Grey A, et al. Calcium intake and risk of fracture: systematic review. BMJ (Clinical research ed) 2015;351:h4580. doi: 10.1136/bmj.h4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reid IR, Bristow SM, Bolland MJ. Calcium supplements: benefits and risks. J Intern Med. 2015;278(4):354–368. doi: 10.1111/joim.12394. [DOI] [PubMed] [Google Scholar]
- 57.• Anderson J, Kruszka B, Delany J, He K, Burke G, Alonso A et al. Calcium Intake From Diet and Supplements and the Risk of Coronary Artery Calcification and its Progression Among Older Adults: 10-Year Follow-Up of the Multi-Ethnic Study of Atherosclerosis (MESA). Journal of the American Heart Association. 2016; 5(10). Significant link between calcium and atherosclerosis, but no link to MI. [DOI] [PMC free article] [PubMed]
- 58.Raffield LM, Agarwal S, Hsu FC, de Boer IH, Ix JH, Siscovick D, et al. The association of calcium supplementation and incident cardiovascular events in the multi-ethnic study of atherosclerosis (MESA) Nutrition, metabolism, and cardiovascular diseases: NMCD. 2016;26(10):899–907. doi: 10.1016/j.numecd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial) J Clin Endocrinol Metab. 2012;97(2):614–622. doi: 10.1210/jc.2011-1309. [DOI] [PubMed] [Google Scholar]
- 60.Lewis JR, Calver J, Zhu K, Flicker L, Prince RL. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5-year RCT and a 4.5-year follow-up. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(1):35–41. doi: 10.1002/jbmr.176. [DOI] [PubMed] [Google Scholar]
- 61.Chung M, Tang AM, Fu Z, Wang DD, Newberry SJ. Calcium intake and cardiovascular disease risk: an updated systematic review and meta-analysis. Ann Intern Med. 2016;165(12):856–866. doi: 10.7326/M16-1165. [DOI] [PubMed] [Google Scholar]
- 62.• Pesce N. Calcium supplements could give you a heart attack. New York Daily News. 2016. http://www.nydailynews.com/life-style/health/calcium-supplements-give-heart-attack-article-1.2828098. Recent news article discussing the link between calcium and heart disease. A good representation of how medical research can be presented to the public.
- 63.Kopecky SL, Bauer DC, Gulati M, Nieves JW, Singer AJ, Toth PP, et al. Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann Intern Med. 2016;165(12):867–868. doi: 10.7326/M16-1743. [DOI] [PubMed] [Google Scholar]
- 64.Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O. Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26(10):2401–2411. doi: 10.1007/s00198-015-3253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Boven JF, de Boer PT, Postma MJ, Vegter S. Persistence with osteoporosis medication among newly-treated osteoporotic patients. J Bone Miner Metab. 2013;31(5):562–570. doi: 10.1007/s00774-013-0440-2. [DOI] [PubMed] [Google Scholar]
- 66.Lee S, Glendenning P, Inderjeeth CA. Efficacy, side effects and route of administration are more important than frequency of dosing of anti-osteoporosis treatments in determining patient adherence: a critical review of published articles from 1970 to 2009. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22(3):741–753. doi: 10.1007/s00198-010-1335-x. [DOI] [PubMed] [Google Scholar]
- 67.Kishimoto H, Maehara M. Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch Osteoporos. 2015;10:231. doi: 10.1007/s11657-015-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Devine J, Trice S, Finney Z, Yarger S, Nwokeji E, Linton A, et al. A retrospective analysis of extended-interval dosing and the impact on bisphosphonate compliance in the US military health system. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23(4):1415–1424. doi: 10.1007/s00198-011-1729-4. [DOI] [PubMed] [Google Scholar]
- 69.Kolata G. Osteoporosis, a disease with few treatment options. New York Times: May Soon Have One More; 2016. [Google Scholar]
- 70.Medical L. Forteo. http://www.forteo.com/?WT.srch=1&WT.mc_id=frtodtcsem_ggl_br_br-dtcbrandcore_43700008875295794_e&srcid=frtodtcsem_ggl_br_br-dtcbrandcore_43700008875295794_e&gclid=CIrP9pH07NACFVMvgQodTnEDEQ#isi.
- 71.Chen YC, Sosnoski DM, Mastro AM. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast cancer research: BCR. 2010;12(6):215. doi: 10.1186/bcr2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Isogai Y, Takao-Kawabata R, Takakura A, Sugimoto E, Nakazono O, Ikegaki I, et al. Early effects of single and low-frequency repeated administration of teriparatide, hPTH(1-34), on bone formation and resorption in ovariectomized rats. Calcif Tissue Int. 2015;97(4):412–420. doi: 10.1007/s00223-015-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316(7):722–733. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- 74.Miller PD. A review of the efficacy and safety of denosumab in postmenopausal women with osteoporosis. Therapeutic advances in musculoskeletal disease. 2011;3(6):271–282. doi: 10.1177/1759720X11424220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papapoulos S, Lippuner K, Roux C, Lin CJ, Kendler DL, Lewiecki EM, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM extension study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26(12):2773–2783. doi: 10.1007/s00198-015-3234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott LJ. Denosumab: a review of its use in postmenopausal women with osteoporosis. Drugs Aging. 2014;31(7):555–576. doi: 10.1007/s40266-014-0191-3. [DOI] [PubMed] [Google Scholar]
- 77.Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, et al. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab. 2016;101(8):3163–3170. doi: 10.1210/jc.2016-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheedy KC, Camara MI, Camacho PM. Comparison of the efficacy, adverse effects, and cost of zoledronic acid and denosumab in the treatment of osteoporosis. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2015;21(3):275–279. doi: 10.4158/EP14106.OR. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Z, Chen C, Zhang J, Ji X, Liu L, Zhang G, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7(5):2113–2122. [PMC free article] [PubMed] [Google Scholar]
- 80.Bone HG, Chapurlat R, Brandi ML, Brown JP, Czerwinski E, Krieg MA, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab. 2013;98(11):4483–4492. doi: 10.1210/jc.2013-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katsarelis H, Shah NP, Dhariwal DK, Pazianas M. Infection and medication-related osteonecrosis of the jaw. J Dent Res. 2015;94(4):534–539. doi: 10.1177/0022034515572021. [DOI] [PubMed] [Google Scholar]
- 82.Farooki A, Fornier M, Boland P. Atypical femur fractures associated with use of bisphosphonates and denosumab. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26(4):819–820. doi: 10.1093/annonc/mdv014. [DOI] [PubMed] [Google Scholar]
- 83.Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(1):1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 84.Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet (London, England) 2013;382(9886):50–56. doi: 10.1016/S0140-6736(13)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsai JN, Uihlein AV, Burnett-Bowie SM, Neer RM, Derrico NP, Lee H, et al. Effects of two years of teriparatide, denosumab, or both on bone microarchitecture and strength (DATA-HRpQCT study) J Clin Endocrinol Metab. 2016;101(5):2023–2030. doi: 10.1210/jc.2016-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai JN, Uihlein AV, Burnett-Bowie SA, Neer RM, Zhu Y, Derrico N, et al. Comparative effects of teriparatide, denosumab, and combination therapy on peripheral compartmental bone density, microarchitecture, and estimated strength: the DATA-HRpQCT study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015;30(1):39–45. doi: 10.1002/jbmr.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bansal S, Pecina JL, Merry SP, Kennel KA, Maxson J, Quigg S, et al. US preventative services task force FRAX threshold has a low sensitivity to detect osteoporosis in women ages 50-64 years. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26(4):1429–1433. doi: 10.1007/s00198-015-3026-0. [DOI] [PubMed] [Google Scholar]
- 88.Kanis JA, Johansson H, Oden A, Cooper C, McCloskey EV. Worldwide uptake of FRAX. Arch Osteoporos. 2014;9:166. doi: 10.1007/s11657-013-0166-8. [DOI] [PubMed] [Google Scholar]
- 89.Gillespie CW, Morin PE. Trends and disparities in osteoporosis screening among women in the United States, 2008-2014: declines in utilization among women 50 to 64 years old and persistent underutilization among women 65 and older. Am J Med. 2016 doi: 10.1016/j.amjmed.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 90.Office of the Surgeon G. Reports of the Surgeon General. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville (MD): Office of the Surgeon General (US); 2004. [PubMed]
- 91.Zheng J, Mao X, Ling J, He Q, Quan J, Jiang H. Association between serum level of magnesium and postmenopausal osteoporosis: a meta-analysis. Biol Trace Elem Res. 2014;159(1–3):8–14. doi: 10.1007/s12011-014-9961-3. [DOI] [PubMed] [Google Scholar]
- 92.Naylor KE, Jacques RM, Paggiosi M, Gossiel F, Peel NF, McCloskey EV, et al. Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: the TRIO study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27(1):21–31. doi: 10.1007/s00198-015-3145-7. [DOI] [PubMed] [Google Scholar]
- 93.Lee J, Vasikaran S. Current recommendations for laboratory testing and use of bone turnover markers in management of osteoporosis. Annals of laboratory medicine. 2012;32(2):105–112. doi: 10.3343/alm.2012.32.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burch J, Rice S, Yang H, Neilson A, Stirk L, Francis R, et al. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health technology assessment (Winchester, England) 2014;18(11):1–180. doi: 10.3310/hta18110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cavalier E, Bergmann P, Bruyere O, Delanaye P, Durnez A, Devogelaer JP, et al. The role of biochemical of bone turnover markers in osteoporosis and metabolic bone disease: a consensus paper of the Belgian bone Club. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27(7):2181–2195. doi: 10.1007/s00198-016-3561-3. [DOI] [PubMed] [Google Scholar]
- 96.Luo Y, Hu Z, Hao J, Jiang W, Shen J, Zhao J. Significant associations between the A163G and G1181C polymorphisms of the osteoprotegerin gene and risk of osteoporosis, especially in postmenopausal women: a meta-analysis. Genetic testing and molecular biomarkers. 2014;18(3):211–219. doi: 10.1089/gtmb.2013.0420. [DOI] [PubMed] [Google Scholar]
- 97.Yu KH, Tang J, Dai CQ, Yu Y, Hong JJ. COL1A1 gene -1997G/T polymorphism and risk of osteoporosis in postmenopausal women: a meta-analysis. Genetics and molecular research: GMR. 2015;14(3):10991–10998. doi: 10.4238/2015.September.21.11. [DOI] [PubMed] [Google Scholar]
- 98.Kanis JA. Assessment of Osteoporosis at the Primary Health-Care Level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Disease. In: University of Sheffield U, editor.: University of Sheffield; 2007.
- 99.Nordin C. Screening for osteoporosis: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2011;155(4):276. doi: 10.7326/0003-4819-155-4-201108160-00021. [DOI] [PubMed] [Google Scholar]
- 100.Nayak S, Greenspan SL. Cost-effectiveness of osteoporosis screening strategies for men. J Bone Miner Res Off J Am Soc Bone Miner Res. 2016;31(6):1189–1199. doi: 10.1002/jbmr.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garg MK, Kharb S. Dual energy X-ray absorptiometry: pitfalls in measurement and interpretation of bone mineral density. Indian journal of endocrinology and metabolism. 2013;17(2):203–210. doi: 10.4103/2230-8210.109659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Link TM. Osteoporosis imaging: state of the art and advanced imaging. Radiology. 2012;263(1):3–17. doi: 10.1148/radiol.12110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pisani P, Renna MD, Conversano F, Casciaro E, Muratore M, Quarta E, et al. Screening and early diagnosis of osteoporosis through X-ray and ultrasound based techniques. World journal of radiology. 2013;5(11):398–410. doi: 10.4329/wjr.v5.i11.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karjalainen JP, Riekkinen O, Toyras J, Jurvelin JS, Kroger H. New method for point-of-care osteoporosis screening and diagnostics. Osteoporos Int. 2016;27(3):971–977. doi: 10.1007/s00198-015-3387-4. [DOI] [PubMed] [Google Scholar]
- 105.Hoiberg MP, Rubin KH, Hermann AP, Brixen K, Abrahamsen B. Diagnostic devices for osteoporosis in the general population: a systematic review. Bone. 2016;92:58–69. doi: 10.1016/j.bone.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 106.Chin KY, Ima-Nirwana S. Calcaneal quantitative ultrasound as a determinant of bone health status: what properties of bone does it reflect? Int J Med Sci. 2013;10(12):1778–1783. doi: 10.7150/ijms.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mueller D, Gandjour A. Cost effectiveness of ultrasound and bone densitometry for osteoporosis screening in post-menopausal women. Applied health economics and health policy. 2008;6(2–3):113–135. doi: 10.1007/BF03256127. [DOI] [PubMed] [Google Scholar]
- 108.Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. J Intern Med. 2015;277(6):650–661. doi: 10.1111/joim.12369. [DOI] [PubMed] [Google Scholar]


