Abstract
Background: Malignant glioma is the most common brain cancer with devastating prognosis. Recurrence of malignant glioma following surgery is very common with few preventive and therapeutic options. Novel targets and therapeutic agents are constantly sought for better outcome. Our previous study established that inhibition of transient receptor potential melastatin 7 (TRPM7) channels resulted in significant decrease of human glioma cell growth and proliferation. As local anesthetic lidocaine has been shown to inhibit TRPM7 currents, we hypothesize that lidocaine may suppress glioma cell proliferation through TRPM7 channel inhibition. Methods: TRPM7 currents were recorded in rat C6 glioma cells using the whole cell patch clamp technique. Cell growth and proliferation were assessed under microscopic examination and biochemical assays. Results: Lidocaine inhibits TRPM7-like currents in a dose-dependent and reversible manner. At 1 and 3 mM, it inhibits ~30% and ~50% of TRPM7 currents. At these concentrations, it is effective in inhibiting the proliferation of C6 cells. As expected, the TRPM7 inhibitors gadolinium and 2-Aminoethoxydiphenyl borate have similar effects on TRPM7 currents and proliferation of C6 cells. Similar to its effect on C6 cells, lidocaine inhibits the proliferation of A172 cells, a human glioblastoma cell line. Conclusions: Lidocaine significantly inhibits the proliferation of glioma cells. The effect of lidocaine is mediated, at least in part, by inhibiting TRPM7 channels.
Keywords: Lidocaine, local anesthetic, TRPM7, glioma, proliferation
Introduction
Malignant glioma is the most common primary brain tumor with devastating prognosis [1]. Maximal resection is essential for the treatment but surgery itself is a risk factor for recurrence [2]. Recurrence of malignant glioma following surgery is very high with few preventive and therapeutic options. One major advance over last two decades is the development of temozolomide that increased the survival time only by about 2.6 months after surgery when adding to radiotherapy [3,4]. Even so, around 70% malignant glioma is not responsive to temozolomide [5]. Therefore, it is hoped that potential new targets and drugs are able to reduce the growth and proliferation of glioma cells and increase the survival rate of cancer patients.
The melastatin-like transient receptor potential 7 (TRPM7) is a member of TRPM family mediating the entry of Ca2+ and Mg2+ [6]. High expression of TRPM7 has been found in a number of human cancer tissue and cell lines, including head and neck cancer, breast cancer, ovarian cancer, prostate cancer and pancreatic cancer [7-11]. TRPM7 plays an important role in maintaining the homeostasis of Ca2+ and Mg2+ in cancer cells regulating the cell function and cycle [12]. Thus it appears to be an attractive target to suppress the cancer cell proliferation and potentially reduce the recurrence of cancer. Our previous studies have demonstrated that TRPM7 play an important role in the proliferation, migration and invasion of human malignant glioma cells [13], suggesting that TRPM7 might serve as a promising target for malignant glioma treatment.
Lidocaine is a widely used local anesthetic that can be administered to local or regional tissue, epidural or intrathecal space to induce nerve conduction block. It has been shown that lidocaine is neuroprotective against ischemia [14] and Zn2+ induced neurotoxicity [15]. Lidocaine is the only local anesthetic that can be administered intravenously. Intravenous infusion of lidocaine has been found safe and effective in reducing postoperative ileus and pain following colon resection [16]. We have found that lidocaine inhibits TRPM7 currents in HEK293 cells and cultured neurons [15]. A safe and beneficial profile of lidocaine makes it attractive to test its potential effect on cancer cell biology. In the current study, we demonstrated that lidocaine inhibits the proliferation of malignant glioma cells at least in part, by inhibiting TRPM7 channel currents.
Materials and methods
Cell culture
C6 rat glioma and A172 Human glioblastoma cell lines were cultured in Dulbecco’s modified Eagle’s medium (MEM, Sigma, St. Louis, MO) plus 10% fetal bovine serum, 50 units/ml penicillin, and 50 µg/ml streptomycin at 37°C. Cells were plated in 35 mm poly-L-ornithine coated dishes for electrophysiological recording and proliferation assay 2 days after plating.
Electrophysiology
Whole-cell patch clamp recordings were performed as described previously [15]. Data were acquired using an AXOPATCH 200B amplifier with pCLAMP 8.1 software and filtered at 2 kHz and digitized at 5 kHz using Digidata 1322A (Axon Instruments, Foster City, CA). Unless otherwise specified, cells were voltage-clamped at -60 mV. Patch electrodes were constructed from thin-walled borosilicate glass (1.5 mm diameter; WPI, Sarasota, FL) on a two-stage puller (PP83, Narishige, Tokyo, Japan). Pipettes had a resistance of 2-4 MW when filled with the intracellular solution (see below). For rapid changes of extracellular solutions, a multibarrel perfusion system (SF-77, Warner Instruments, Hamden, CT) was used. (WPI, Sarasota, FL). All experiments were performed at room temperature.
LDH assay
Lactate dehydrogenase (LDH) assay was performed as described [15]. At the end of the experiments, 0.5% Triton X-100 was used to permeabilize the cells for obtaining the maximal releasable LDH. After 30 min, 50 µl medium was collected from each well. 50 µl of assay mixture from Cytotoxicity Detection Kit (Roche Applied Science) was then added to each sample. Following 30 min incubation at room temperature in dark, the absorbance at 492 nm was read on a multi-well plate reader, subtracting background absorbance measured at 620 nm.
Solutions and chemicals
Standard extracellular solution contained (in mM): 140 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 20 HEPES, 10 glucose (pH 7.4 adjusted with NaOH; 320-335 mOsm). For divalent-free external solutions, CaCl2 and MgCl2 were removed with osmolarity adjusted with sucrose. Patch electrodes contained (in mM): 140 CsF, 10 HEPES, 1 CaCl2, 11 EGTA, 2 TEA (pH 7.3 adjusted with CsOH, 290-300 mOsm). 2-Aminoethoxydiphenyl borate (2-APB) was purchased from Calbiochem (San Diego, CA); gadolinium chloride was from Sigma.
Statistics
Data are expressed as mean ± S.E. Student’s t-test was employed as appropriate for the analysis of statistical significance.
Results
2-APB and Gd3+ inhibit TRPM7 currents and the proliferation of rat C6 glioma cells
The presence of functional TRPM7 channel in rat C6 glioma cells was confirmed by whole-cell patch clamp. Reducing the extracellular Ca2+ concentration from 2 to 0 mM evoked a non-desensitizing inward current (Figure 1A and 1C), which characteristic is consistent with our previous report of TRPM7 currents expressed in HEK-293 cells [15]. This current was inhibited by 2-APB and Gd3+ (Figure 1A and 1C), the nonspecific TRPM7 inhibitors, further confirming the presence of TRPM7 currents in rat C6 glioma cells. We then examined whether 2-APB and Gd3+ have inhibitory effect on the proliferation of C6 glioma cells. We found that the proliferation of C6 glioma cells were significantly suppressed by 2-APB and Gd3+ (Figure 1B and 1D), which findings are agreeable with our previous results in human glioma cells.
Figure 1.
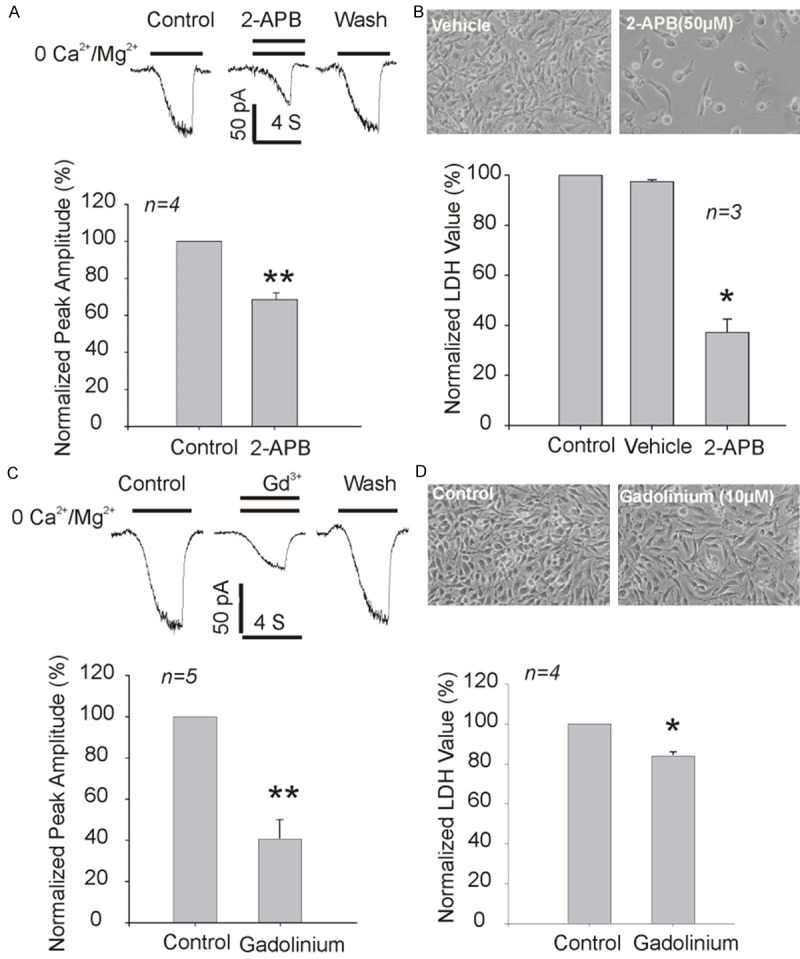
2-APB and Gadolinium inhibit TRPM7 currents and proliferation of C6 glioma cells. A: Representative traces and summary data showing the inhibition of TRPM7 currents by 50 µM 2-APB. B: Effects of 2-APB on the growth and proliferation of C6 glioma cells as measured by total LDH release. Data were from three independent experiments. C: Representative traces and summary data showing the inhibition of TRPM7 currents by 10 µM Gadolinium. D: Effects of Gadolinium on the growth and proliferation of C6 glioma cells as measured by total LDH release at 48 h. Data were from four independent experiments. *P<0.05 and **P<0.01, unpaired t-tests.
Lidocaine inhibited TRPM7 currents in C6 glioma cells
We further examined the effect of lidocaine on TRPM7 currents in C6 glioma cells. As expected, the evoked non-desensitizing inward current was blocked by lidocaine in a dose-dependent and reversible manner (Figure 2A). Around 20% and 50% of TRPM7 currents were inhibited by 1 and 3 mM lidocaine, respectively (Figure 2B).
Figure 2.
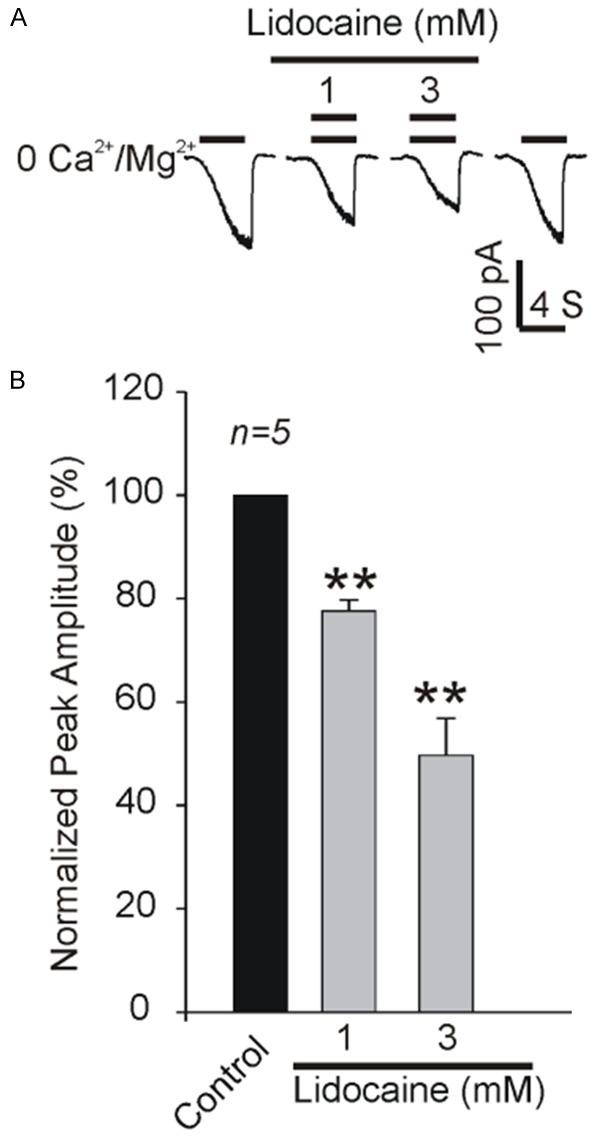
Inhibition of TRPM7 currents by lidocaine in C6 glioma cells. Representative traces (A) and summarized data (B) showing the inhibition of TRPM7 currents by 1 and 3 mM lidociane in C6 glioma cells. **P<0.01, n=5 cells.
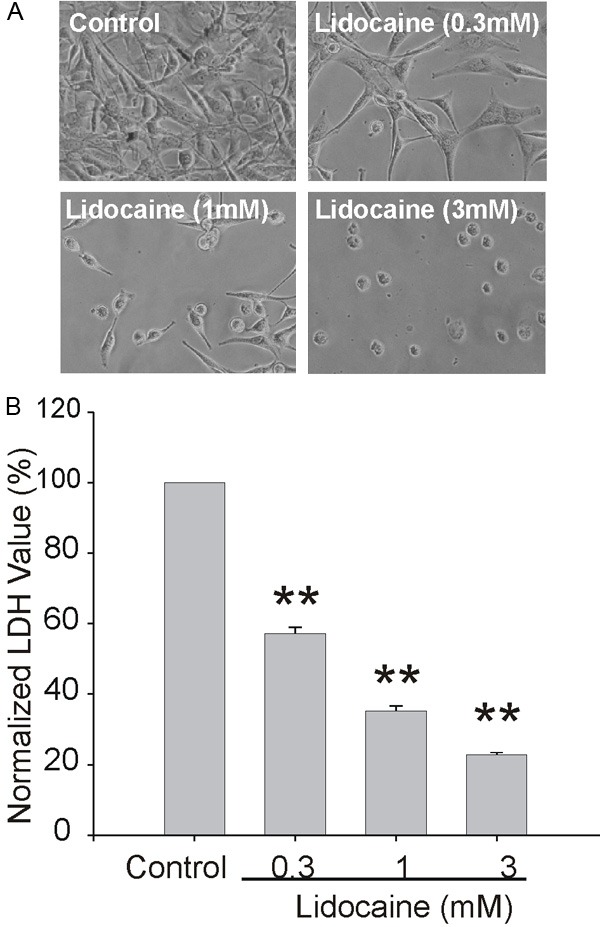
Lidocaine inhibited proliferation of C6 glioma cells
As TRPM7 plays an important role in glioma cell proliferation, we speculated that lidocaine might have an inhibitory effect on the proliferation of glioma cells by negative modulation of TRPM7 channel function. As expected, microscopic examination showed that lidocaine significantly suppressed the growth of C6 cells in a dose dependent fashion (Figure 3A). The inhibition of proliferation was supported by LDH assays, which showed 30% reduction of total LDH at 3 mM (Figure 3B).
Figure 3.
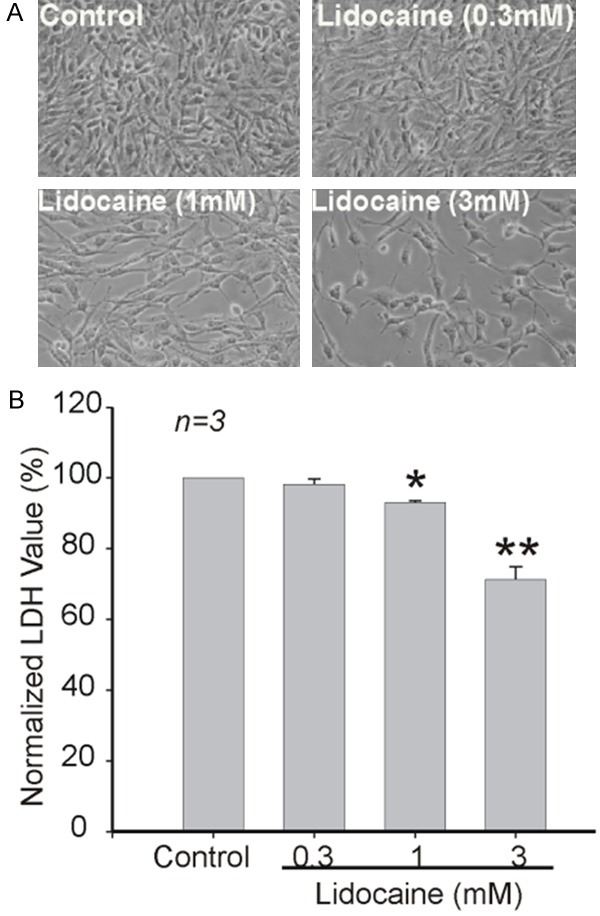
Inhibition of growth and proliferation of C6 glioma cells by lidocaine. A: Representative images showing the growth and proliferation of C6 glioma cells in the absence or presence of variable indicated concentrations of lidocaine for 48 h. B: Effects of lidocaine on the growth and proliferation of C6 glioma cells as measured by total LDH release. *P<0.05, **P<0.01. Data were from three independent experiments.
No voltage-gated Na+ and ASIC currents were recorded in C6 glioma cells
Lidocaine is a potent voltage-gated Na+ channel inhibitor and has recently been identified as an ASIC inhibitor [17]. ASIC was reported to play a role in some glioma cells [18]. The inhibition of lidocaine on those channels might contribute to its inhibition on the proliferation of C6 rat glioma cells. However, our findings show that there are no voltage-gated Na+ and ASIC currents in this cell line (Figure 4A and 4B). A small and non-desensitized current was induced by lower pH of 4.5, however, it was not inhibited by high concentration of amiloride, excluding the possibility of ASIC current (Figure 4B). In addition, amiloride has no significant inhibitory effect on the proliferation of C6 glioma cells (Figure 4C).
Figure 4.
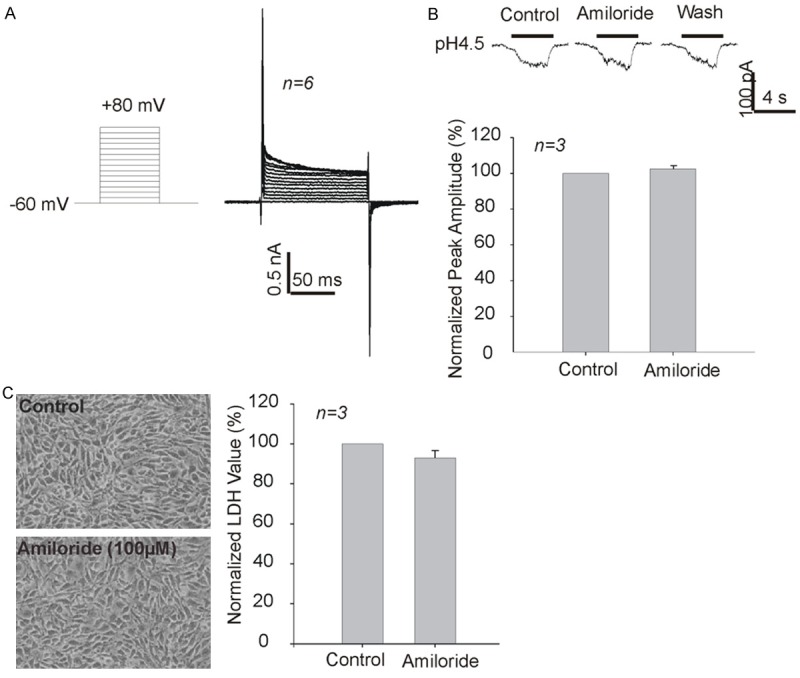
Lack of voltage-gated sodium channel currents and typical ASIC currents in C6 glioma cells. A: No voltage-gated sodium channel currents were recorded. B: No typical ASICs currents were recorded. The small non-desensitizing current induced by acid was insensitive to high concentration (100 uM) of ASIC blocker amiloride. C: Lack of effect by amiloride on the proliferation of C6 glioma cells as measured by total LDH release.
The inhibitory effect on glioma cell proliferation by lidocaine was further extended to human A 172 glioma cells. Visual examination under microscope indicated a significant inhibition occurred at 0.3 mM and the inhibition increased at higher concentration of 1 and 3 mM (Figure 5). Interestingly, A172 cells seem to be more sensitive to lidocaine treatment, suggesting that other potential mechanisms exist in this cell line.
Figure 5.
Effects of lidocaine on the growth and proliferation of A172 human glioma cells. A: Representative images showing the proliferation of A172 human glioma cells in the absence or presence of variable indicated concentrations of lidocaine for 48 h. B: Effects of lidocaine on the growth and proliferation of A172 human glioma cells as measured by total LDH release. **P<0.01.
Discussion
Most of glioma are malignant and account for 81% of malignant tumors in CNS and overall 29% of all primary brain and CNS tumors [1]. Glioblastoma (also known as glioblastoma multiforme, grade IV astrocytoma) is the most common form of glioma with dismal prognosis. Current standard of care for primary glioblastoma is surgical resection, combined with chemotherapy and radiation. In addition to chemotherapy with temozolomide, a number of target therapies on various growth hormones and intracellular pathways have been attempted with limited success [19]. Recurrence after surgical resection is inevitable, and estimated survival is only 15-18 months upon diagnosis and 5 year survival is only 5%.
TRPM7 has been shown to be overexpressed in a number of human cancer cells and mediate cell proliferation, migration, invasion and metastasis, including head and neck cancer cells [7], breast cancer cells [7,8], ovarian cancer [7,9], prostate cancer [10], and lung cancer [20]. Inhibition of TRPM7 currents or knockdown of TRPM7 expression by small interference RNA was able to suppress their malignant biological behaviors. Therefore, TRPM7 may serve as a prognostic marker and a potential therapeutic target [21].
In this study, we first confirmed the existence of TRPM7 channels in rat C6 cells and then tested the effects of lidocaine on TRPM7 currents and cell proliferation. Lidocaine inhibited the TRPM7 currents in a dose dependent manner. About 20% inhibition occurred at 1 mM and 50% inhibition at 3 mM, which was quite significant as two known TRPM7 inhibitors 2-APB (50 µM) and Gadolinium (10 µM) had about 30% inhibition and 60% inhibition respectively.
We further showed that lidocaine caused significant reduction in the proliferation of C6 cells. The role of TRPM7 in mediating the effect of lidocaine is supported by our results showing that both TRPM7 inhibitors exhibited a similar inhibitory effect on C6 cell proliferation. The suppressing effect of lidocaine on glioma cell proliferation was also demonstrated in A172 human glioma cells.
We have shown previously that lidocaine inhibits ASICs, another Na+ influx channels [17]. However, there was no typical ASICs current was recorded in C6 cells. In addition, ASIC inhibitor amiloride had no effects on the proliferation of C6 glioma cells. Thus, we can exclude the possibility that lidocaine suppresses the proliferation of glioma through its effect on ASICs.
Further study is needed to disclose the mechanism of lidocaine on TRPM7 channels. The action site appears to be extracellular, as our previous study showed that lidocaine analog QX-314 which does not cross cell membrane, was able to inhibit TRPM7 currents in cultured cortical neurons [15]. It may be possible to develop new local anesthetics with higher potency, efficacy, and specificity targeting TRPM7, which may have novel therapeutic applications.
Lidocaine is one of the most commonly used local anesthetics for topical, local and neuraxial anesthesia. It is routinely administered intravenously for attenuating sympathetic response during tracheal intubation. It has been used intravenously as anti-arrhythmia agent. At anti-arrhythmia dose, lidocaine was found to be neuroprotective against ischemia in rats [14,22]. Intravenous administration of lidocaine was found to reduce postoperative cognitive dysfunction in patients underwent coronary bypass surgery [23,24]. Systemic administration of lidocaine also reduced the perioperative opioid requirement [25], and effective in reducing postoperative ileus and pain following colon resection [16]. Clinical preparation of lidocaine usually ranges from 0.5% to 5%, e.g., 18.5 to 185 mM. The observed effective concentrations (0.3 mM-3 mM) might be reachable by direct application, however, we need to be cautious about interpreting our result in clinical application since it is more than ten times of the serum concentration 6-12 μM for 48 hours of lidocaine infusion24 and the toxic effects of lidocaine need to be considered. Structure modification of lidocaine to enhance the glioma suppressing effects might be a solution. Surgery is associated with related stress induced hormone and immune function change that renders patient vulnerable for recurrence [2]. Considering its devastating prognosis, even a small decrease of the recurrence of glioma would be significant. Application of a well-known local anesthetic with safe and beneficial profile during surgical resection of glioma might be beneficial with little risk.
In summary, we demonstrated that local anesthetic lidocaine significantly inhibited the proliferation of glioma cells, and indicated that the effect of lidocaine was mediated by inhibiting TRPM7 currents.
Acknowledgements
This work was supported by NIH grants R01NS066027, NIMHD S21MD000101, and U54NS08932. This work was supported by Guangdong Innovative and Entrepreneurial Research Team Program (No. 2013Y104).
Disclosure of conflict of interest
None.
References
- 1.Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(Suppl 4):iv1–63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldfarb Y, Ben Eliyahu S. Surgery as a risk factor for breast cancer recurrence and metastasis: mediating mechanisms and clinical prophylactic approaches. Breast Dis. 2006;26:99–114. doi: 10.3233/bd-2007-26109. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Venur VA, Peereboom DM, Ahluwalia MS. Current medical treatment of glioblastoma. Cancer Treat Res. 2015;163:103–15. doi: 10.1007/978-3-319-12048-5_7. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain MC. Temozolomide: therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev Neurother. 2010;10:1537–1544. doi: 10.1586/ern.10.32. [DOI] [PubMed] [Google Scholar]
- 6.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation. Cancer Res. 2007;67:10929–10938. doi: 10.1158/0008-5472.CAN-07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middelbeek J, Kuipers AJ, Henneman L, Visser D, Eidhof I, van Horssen R, Wieringa B, Canisius SV, Zwart W, Wessels LF, Sweep FC, Bult P, Span PN, van Leeuwen FN, Jalink K. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012;72:4250–4261. doi: 10.1158/0008-5472.CAN-11-3863. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Liao QJ, Zhang Y, Zhou H, Luo CH, Tang J, Wang Y, Tang Y, Zhao M, Zhao XH, Zhang QY, Xiao L. TRPM7 is required for ovarian cancer cell growth, migration and invasion. Biochem Biophys Res Commun. 2014;454:547–553. doi: 10.1016/j.bbrc.2014.10.118. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Sukumaran P, Varma A, Derry S, Sahmoun AE, Singh BB. Cholesterol-induced activation of TRPM7 regulates cell proliferation, migration, and viability of human prostate cells. Biochim Biophys Acta. 2014;1843:1839–1850. doi: 10.1016/j.bbamcr.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rybarczyk P, Gautier M, Hague F, Dhennin-Duthille I, Chatelain D, Kerr-Conte J, Pattou F, Regimbeau JM, Sevestre H, Ouadid-Ahidouch H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int J Cancer. 2012;131:E851–E861. doi: 10.1002/ijc.27487. [DOI] [PubMed] [Google Scholar]
- 12.Yee NS, Zhou W, Liang IC. Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer. Dis Model Mech. 2011;4:240–254. doi: 10.1242/dmm.004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leng TD, Li MH, Shen JF, Liu ML, Li XB, Sun HW, Branigan D, Zeng Z, Si HF, Li J, Chen J, Xiong ZG. Suppression of TRPM7 inhibits proliferation, migration, and invasion of malignant human glioma cells. CNS Neurosci Ther. 2015;21:252–261. doi: 10.1111/cns.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popp SS, Lei B, Kelemen E, Fenton AA, Cottrell JE, Kass IS. Intravenous antiarrhythmic doses of lidocaine increase the survival rate of CA1 neurons and improve cognitive outcome after transient global cerebral ischemia in rats. Neuroscience. 2011;192:537–49. doi: 10.1016/j.neuroscience.2011.06.086. [DOI] [PubMed] [Google Scholar]
- 15.Leng TD, Lin J, Sun HW, Zeng Z, O’Bryant Z, Inoue K, Xiong ZG. Local anesthetic lidocaine inhibits TRPM7 current and TRPM7-mediated zinc toxicity. CNS Neurosci Ther. 2015;21:32–39. doi: 10.1111/cns.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swenson BR, Gottschalk A, Wells LT, Rowlingson JC, Thompson PW, Barclay M, Sawyer RG, Friel CM, Foley E, Durieux ME. Intravenous lidocaine is as effective as epidural bupivacaine in reducing ileus duration, hospital stay, and pain after open colon resection: a randomized clinical trial. Reg Anesth Pain Med. 2010;35:370–376. doi: 10.1097/AAP.0b013e3181e8d5da. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Chu X, Maysami S, Li M, Si H, Cottrell JE, Simon RP, Xiong Z. Inhibition of acid sensing ion channel currents by lidocaine in cultured mouse cortical neurons. Anesth Analg. 2011;112:977–981. doi: 10.1213/ANE.0b013e31820a511c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem. 2003;278:15023–15034. doi: 10.1074/jbc.M300991200. [DOI] [PubMed] [Google Scholar]
- 19.Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82. doi: 10.1016/j.pharmthera.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Gao H, Chen X, Du X, Guan B, Liu Y, Zhang H. EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium. 2011;50:559–568. doi: 10.1016/j.ceca.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhou W, Guo S, Xiong Z, Liu M. Oncogenic role and therapeutic target of transient receptor potential melastatin 7 channel in malignancy. Expert Opin Ther Targets. 2014;18:1177–1196. doi: 10.1517/14728222.2014.940894. [DOI] [PubMed] [Google Scholar]
- 22.Lei B, Cottrell JE, Kass IS. Neuroprotective effect of low-dose lidocaine in a rat model of transient focal cerebral ischemia. Anesthesiology. 2001;95:445–451. doi: 10.1097/00000542-200108000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Wu X, Li J, Xiao F, Liu X, Meng M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95:1134–41. doi: 10.1097/00000539-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell SJ, Merry AF, Frampton C, Davies E, Grieve D, Mills BP, Webster CS, Milsom FP, Willcox TW, Gorman DF. Cerebral protection by lidocaine during cardiac operations: a followup study. Ann Thorac Surg. 2009;87:820–825. doi: 10.1016/j.athoracsur.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 25.McKay A, Gottschalk A, Ploppa A, Durieux ME, Groves DS. Systemic lidocaine decreased the perioperative opioid analgesic requirements but failed to reduce discharge time after ambulatory surgery. Anesth Analg. 2009;109:1805–1808. doi: 10.1213/ANE.0b013e3181be371b. [DOI] [PubMed] [Google Scholar]


