Abstract
G-quadruplexes (G4s) are secondary structures of nucleic acids that epigenetically regulate cellular processes. In the human immunodeficiency lentivirus 1 (HIV-1), dynamic G4s are located in the unique viral LTR promoter. Folding of HIV-1 LTR G4s inhibits viral transcription; stabilization by G4 ligands intensifies this effect. Cellular proteins modulate viral transcription by inducing/unfolding LTR G4s. We here expanded our investigation on the presence of LTR G4s to all lentiviruses. G4s in the 5′-LTR U3 region were completely conserved in primate lentiviruses. A G4 was also present in a cattle-infecting lentivirus. All other non-primate lentiviruses displayed hints of less stable G4s. In primate lentiviruses, the possibility to fold into G4s was highly conserved among strains. LTR G4 sequences were very similar among phylogenetically related primate viruses, while they increasingly differed in viruses that diverged early from a common ancestor. A strong correlation between primate lentivirus LTR G4s and Sp1/NFκB binding sites was found. All LTR G4s folded: their complexity was assessed by polymerase stop assay. Our data support a role of the lentiviruses 5′-LTR G4 region as control centre of viral transcription, where folding/unfolding of G4s and multiple recruitment of factors based on both sequence and structure may take place.
Introduction
Lentiviruses are a genus of viruses that infect a broad range of mammalians, causing severe diseases mainly characterized by immunological and neurological deficiencies. They belong to the Retroviridae family: as such, they are characterized by a ssRNA genome that, once retrotranscribed by the viral reverse trascriptase enzyme, integrates into the host cell chromosome in the provirus form. The provirus can then undergo a productive replicative cycle or remain in a dormant state known as “latency”. Among lentiviruses, the Human Immunodeficiency Virus 1 (HIV-1) was first characterized in 19831, 2 when it was proposed as the causative agent of the acquired immunodeficiency syndrome (AIDS). HIV-2, SIV (Simian Immunodeficiency virus) and FIV (Feline Immunodeficiency virus), together with HIV-1, are lentiviruses responsible for severe and often fatal acquired immunodeficiency syndrome-like diseases. Other lentiviruses, i.e. Visna/Maedi virus, equine infectious anemia virus (EIAV) and caprine arthritis/encephalitis virus (CAEV) cause different pathologies, such as neurological disorders, anemia and wasting or arthritis and encephalitis. Lentiviruses that infect cattle, such as Bovine Immunodeficiency virus (BIV) and Jembrana Disease Virus (JDV), result in further pathophysiologic events that range from asymptomatic to systemic acute diseases3. Despite the wide range of clinical effects of lentiviral infections and the high divergence in nucleotide (nt) composition of their genome4, all lentiviruses are similar in structure, genome organization, and mode of replication. Importantly, effective progression of the viral cycle relies on the proper function of the long terminal repeat (LTR): even if the LTR region varies in terms of length and composition, it always originates from the multi-step reverse transcription process, which makes 3 main LTR regions, namely U3, R and U5. Once integrated, the 5′-LTR, and in particular its U3 region which is characterized by transcription factor binding sites, serves as unique viral promoter5. Each lentivirus has peculiar cis-acting regulatory sequences that are both essential for promoter activity and different from each other6. For example, the HIV-1 LTR is composed of 3 main sub-regions: the core, where GC-rich binding sites for Sp1 are located; the enhancer, just upstream of the core, containing binding sites for NF-κB; a modulatory region that comprises binding sites for several transcription factors, including C/EBP factors.
In HIV-1, formation of multiple G-quadruplex (G4) structures in the viral and proviral genome7, 8, and in particular in the LTR promoter9, 10, has been reported. G4s are nucleic acids secondary structures that may form in single-stranded G-rich sequences under physiological conditions11–13. Four Gs bind via Hoogsteen-type hydrogen bonds base-pairing to yield G-quartets, which stack to form the G4. The presence of K+ cations specifically supports G4 formation and stability14–16. In eukaryotes G4s have been shown to be involved in key regulatory roles, including transcriptional regulation of gene promoters and enhancers, translation, chromatin epigenetic regulation, DNA recombination9, 13, 17–19. Expansion of G4-forming motifs has been associated with relevant human neurological disorders20–26. Formation of G4s in vivo has been consolidated by the discovery of cellular proteins that specifically recognize G4s27, 28 and the development of G4-specific antibodies29, 30.
The presence of G4s has been recently reported in viruses31, such as SARS coronavirus32, human papilloma, Zika, Ebola and hepatitis C genomes33–36, Epstein–Barr virus37, 38 and herpes simplex virus 139, 40.
In HIV-1, functionally significant G4s have been implicated in pathogenic mechanisms7–10, 27. Formation of G4s in the U3 region of the 5′-LTR, the unique viral promoter, resulted in down-modulation of viral transcription. Further inhibition of viral transcription was achieved using G4 ligands41, 42 or by cellular proteins27. One LTR G4, LTR-IV, acted as a modulator of the dynamic G4s within the LTR region43.
Taking into account the strong evidence of a G4-mediated regulatory mechanism in the HIV-1 promoter, we here aimed at investigating the presence of putative G4 folding sequences (PQSs) in the LTR region of all other lentiviruses, phylogenetically correlate them and analyse their actual G4 formation. We showed that all primate lentiviruses have sequences capable of folding into G4 in the U3 region of their LTR promoter; as in HIV-1, the presence of G4s correlates with that of transcription factors binding sites, in particular Sp1 and NFκB. Our data indicate the 5′-LTR G4 region as a crucial control centre for viral transcription that was maintained during evolution of lentiviruses.
Results
Putative G-quadruplex forming sequences are present in the 5′-LTR of both human and non-human primate lentiviruses
To check if the G4 forming region observed in the 5′-LTR of the HIV-1 provirus was a conserved feature of lentiviruses, we investigated the presence of putative G4 forming sequences (PQS) in the LTRs of all known lentiviruses. The viruses belonging to the lentivirus genus can be grouped according to their 5 host types. The primate group, which our reference HIV-1 group M belongs to, comprises viruses that naturally infect both human (HIV-1 and HIV-2) and non-human primates (Simian Immunodeficiency Virus, SIV) mainly belonging to the Cercopithecidae family44. The SIV sub-group is the most abundant and composed of viruses isolated from 41 different primate species. Among these, 29 SIV genomes that include the entire LTR region are available: these were considered in our analysis (Fig. 1 and Supporting Table S1). The ovine-caprine group includes 3 viruses: Visna/Maedi virus, Caprine Arthritis Encephalitis Virus (CAEV) and Ovine Lentivirus (OL); the bovine group consists of Bovine Immunodeficiency Virus (BIV) and Jembrana Disease Virus (JDV); the equine group is represented by Equine Infectious Anemia Virus (EIAV); the feline group comprises Feline Immunodeficiency Virus (FIV) that naturally infects members of the Felidae family (Supporting Table S1).
Figure 1.

PQS analysis within the LTR region of lentiviruses (our reference HIV-1 group M is at the top of the list). GGG tracts are shown in red and bold; bulged tracts (e.g. GXGG that do not overlap with adjacent GGG tracts) are shown in red, bold and italics. Lower case letters that specify the SIV strain refer to the simian type they were first identified from (e.g. SIVcpz from chimpanzee or Pan troglodytes troglodytes, SIVsm from sooty mangabey monkey or Cercocebus atys). A complete list of PQS in all lentiviruses along with references and host species is provided in Supporting Table S1.
Analysis of PQS was initially performed using the online-based algorithm software (QGRS Mapper). The search was limited to 3-stacked tetrads G4s because these are the most abundant stable G4s within eukaryotic promoter regions45 and these were found in the HIV-1 group M LTR9. The following G4 pattern was thus investigated: GGG≥3N0–12GGG≥3N0–12GGG≥3N0–12GGG≥3, where N is a 0–12 nt-long loop sequence. This initial analysis allowed to identify the main G4 forming regions, which were next manually analysed in terms of G-tracts, loop composition and size. This step was necessary to include G4s with a single-nt bulge; in fact, this type of G4 has been previously found in the HIV-1 LTR-IV G443. Two-stacked tetrads G4 structures were excluded from the analysis. Results are summarized in Fig. 1 and Supporting Table S1.
PQSs were found in the 5′-LTR regions of 97% viruses of the primate group (32 viruses over 33 analysed viruses). Interestingly, most G4 sequences were located in the U3 region of the viral LTRs, as previously found in the HIV-1 group M LTR9. Within the bovine group, JDV presented one LTR PQS formed by 4 tracts of 3 or more Gs, 1 GG tract and additional single Gs that could also be involved in G4 formation. Conversely, BIV and viruses of the equine, feline and ovine-caprine groups lacked the possibility to form three-stacked tetrad G4s in the LTR (Supporting Table S1). Some of these viruses presented sequences compatible with formation of two-stacked tetrad G4s or G4s involving multiple bulges (Supporting Table S2). These sequences were not further considered due to their low intrinsic stability and lack of preferred conserved location.
In the primate group, the identified LTR PQSs highly varied both in length (from 27 to 89 nts) and base composition, and were all characterized by several G-runs that could in principle form multiple overlapping G4s. The HIV-2, SIVcol, SIVcpz, SIVmac, SIVsm and SIVstm genomes, however, shared peculiar features with our reference HIV-1 group M LTR, such as length and the potential to form at least 3 stable G4 structures. In fact, the LTR PQSs of these viruses were all characterized by 4–6 tracts of 3 or more Gs, with additional GG tracts and interspersed Gs. Notably, SIVcpz LTR PQS was composed of 6 GGG tracts, 2 GG tracts and 1 G base that could form a single-nt bulge G4, exactly as we previously reported for the HIV-1 group M LTR9, 43. The only exception in the SIV group is SIVmnd2, the LTR PQS of which displayed multiple G-runs with the potential of atypical G4 folding, such as three-stacked tetrad G4s with 2-nt bulges. PQSs of HIV-1 groups N and O were also quite different, being shorter (43 nts) and with unique G-patterns (Fig. 1). Interestingly, in the primate group the full-length LTR sequence is only moderately conserved: the pairwise sequence similarity of all possible sequence pairs is reasonably low (mean = 56.70%, st.dev. = 6.25%, median = 55.59%) (Supporting Fig. 1a). In contrast, the possibility to form G4 is conserved even if the G4 pattern is different among strains (Supporting Fig. 1b).
Putative G-quadruplex forming sequences of lentiviruses significantly overlap with Sp1 binding sites
We noted that most of the primate LTR G-rich sequences displayed a conserved sequence (GGGACTTTCC) located at the 5′-end of the PQS (Table 1) that corresponded to one NFκB binding site (consensus sequence 5′-GGGRNNYYCC-3′, R = A or G, N = any nt, and Y = C or T)46. The JDV PQS also partially overlapped with a NFκB binding site at its 3′ end (Table 1). In addition, the HIV-1 LTR G4s were associated to three Sp1 binding sites (consensus sequence KGGGCGGRRY, K = G or T, R = A or G and Y = C or T)47. Since Sp1 binding sites for only a very few viruses are available in the literature, the establishment of a straightforward correlation between LTR G4s and Sp1 was not possible. We thus used the online software PhysBinder48 to predict Sp1 binding regions in the LTR of all lentiviruses with relevant PQSs. In the primate lentiviruses, 30 out of 32 subgroups displayed Sp1 binding sites that overlapped with the PQS (Table 1). HIV-1 N and SIVwrc were two exceptions: the former was the only genome that displayed three NFκB binding sites overlapping with the PQS and no Sp1 binding site; the latter was the only genome that lacked both NFκB and Sp1 binding sites. All other subgroups presented 1–3 Sp1 binding sites associated to 0–2 NFκB binding sites, indicating a strong correlation between G4 and Sp1.
Table 1.
Transcription factor binding sites in 5′-LTR PQS.
| Group | Lentivirus | Transcription factor binding sites in LTR PQS | Ref |
|---|---|---|---|
| Primate | HIV-1 M | GGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGGCCTGGGCGGGACTGGGGAGTGG | 49 |
| SIVcpz | GGGACTTTCCAAGGGACGTTCCAAGGGGGTGGGTCAGGGCGGAACAGGGCGTGG | ||
| HIV-2 | GGGACTTTCCAGAAGGGGCTGTAACCAAGGGAGGGACATGGGAGGAGCTGGTGGGG | 49 | |
| SIVmac | GGGACTTTCCACAAGGGGATGTTACGGGGAGGTACTGGGGAGGAGCCGGTCGGG | 50 | |
| SIVsm | GGGACTTTCCACAAGGGGCTGTCATGGGGAGGTACTGGGGAGGAGCTGGCTGG | ||
| SIVstm | GGGACTTTCCACAAGGGGCTGTAACAGGGGAGGTACTGGGAGGAACTGGTGGGG | ||
| HIV-1 N | GGGACTTTACACATGGGGACTTTCCGCCGGGGACTTTCCAGGG | ||
| HIV-1 O | GGGACTTTCCAGTGGGAGGGACAGGGGGCGGTTCGGGGAGTGG | ||
| SIVgor | GGGACTTTCCGTGGAGGAAAGTCCCCGGGGGCGGAACTGGGAGGAGCAGGGGAGTGG | ||
| SIVcol | GGGACTTTCCGTTCGGGACTTTCCAAGTTGGGAGGGACCTGGGCGGAGGGAAGGG | ||
| SIVmne | GGGACTTTCCATAAGGGGATGTCATGGGGGGGTACTGGGGAGGAGCTGGTCGGG | ||
| SIVver | GGGACTTTCCAGCACGGGACTTTCC AAGGCGGGACATGGGCGGTACGGGGAGTGG | ||
| SIVwcm | GGGACTTCTAGCGGGACTTTCC AGGCGGTCATGGCGGTACGGGAGTGG | ||
| SIVrcm | GGGACTTTCCACTGGCGCCTGCGCGCTGGTGTAAGGGACTTTCCAGACTGACGTGGGAGGGGGGTGTGG | ||
| SIVgrv | GCGGTTGGGACTTTCCGCCAGGGACTTTCCACAGTGGGTGGATCGGAGGCGGTACAGGGGCGGTACTGGGAGTGG | ||
| SIVtal | GGGACTTTCCACGTTGCTAAGGCAACGGGGGACGGACTGGGGCGGGGAGCGGGAGGAGTTGGGAGTGG | ||
| SIVlhoest | GGGACTTTCCAGGACGGGCGGGGGAGG | ||
| SIVmnd-1 | GGGACTTTCCAAACAGGGAGGGGGAGG | ||
| SIVsun | GGGACTTTCCGGACAGGGAGGGGGAGG | ||
| SIVpat | GGGACTTCCCAGGGTGGAGACTGGGCGGTACTGGGAGTGG | 51 | |
| SIVagm sab1 | GGGACTTTCC AGGGTGGAGACTGGGCGGTACTGGGAGTGG | ||
| SIVagm tan1 | GGGACTTTCC AGGGTGGTGCGAGGGCGGTACTTGGGAGTGG | ||
| SIVmus1 | GGGACTTTCCAGTCACCATGACTACGGGGCCCGGTTGCTGAGGCAATCGGGGCGGACTCGTGGGTGGGACTGGGCGGTACTGGGAGTGG | ||
| SIVmus3 | GGGACTTTCCAGTTACCATGACTACGGGGCTGGTTGCTGAGGCAACCAGGGCGGACTCGTGGGTGGGACTGGGAGGAACGGGGAGTGG | ||
| SIVdeb | GGGGAGGGCCTGGGTGGTACGGGGAGTGG | ||
| SIVsyk | GGCCCAGGGGAGGAGCCTGGGCGGGGGAAGG | ||
| SIVwrc | GGGACATTGGGAGGAGACTGGGAGGTGCCTTGTGG | ||
| SIVden | GGGCGGACTCAGGGGAGGGCCTGGGAGGTCTCTGGG | ||
| SIVgsn | GGGCGGACTCGTGGGTGGGACTGGGAGGCCGGGAGTGG | ||
| SIVdrl | GTGGCAGGGACTTTCCAGGGTGACGTGGGTTGGGGGAGTGG | ||
| SIVmon | GGGGCCCGGTTGCTGCGGCAACCGGGCGGTCCAAAGGACTTGGTGGGTGGACCCGGGGAGTGG | ||
| SIVmus2 | GGGGCCCGGTTGCCGCAGCAACCGGGGCGGACTCAGGGCGGACTGGGAGGGACCTGAGAGTGG | ||
| Bovine | JDV | GGGGAGAAAGGGAACAGGTGGGGACGACCGGG(ACCTTTCC) |
Binding sites for NFκB are in bold. Sp1 binding sites reported in the literature are indicated in italics and are underlined. Sp1 binding sites predicted by Physbinder are underlined.
5′-LTR PQSs are highly conserved among lentivirus isolates
The relevance of the identified PQSs within the viral context was established by assessing the degree of base conservation among different isolates of the same virus strain (Supporting Table S3). Only viruses with more than 5 complete LTR sequences and from different strains were considered. An extremely high degree of G-base conservation, especially within G-tracts, was found for almost all 5′-LTR PQSs (generally higher than 70% and in most cases higher than 90%) (Fig. 2). In particular, the potentiality to form G4 was maintained in all viruses.
Figure 2.
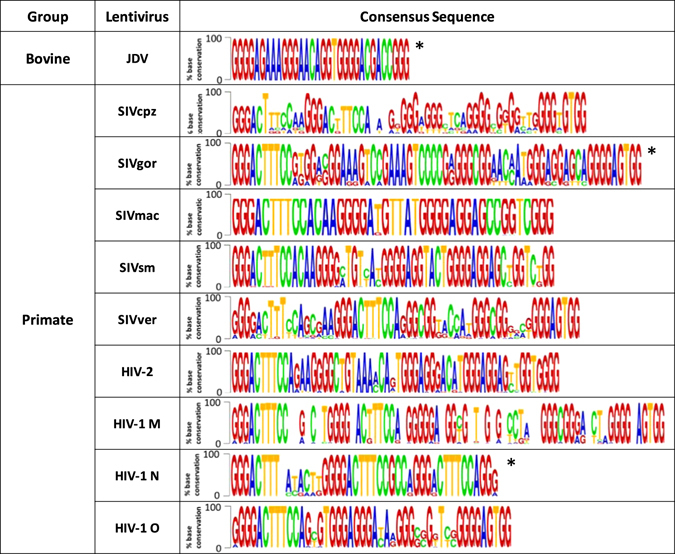
Base conservation of G4 sequences among isolates of lentiviruses. *Consensus sequence obtained by the alignment of 6–10 sequences. All other consensus sequences were obtained by alignment of more than 10 sequences. Lentiviruses with identified PQSs that are not present in this table had lower than 5 complete reported LTR sequences.
The 5′-LTR PQSs are present in phylogenetically related lentiviruses
Because the identified PQGs were quite diverse among viruses infecting different hosts, with the few exceptions highlighted above, we checked the level of sequence identity/variation among lentiviruses that occurred during evolution. To this end, we built a phylogenetic tree based on the alignment of the pol gene sequence of lentiviruses. The pol sequence-based tree was chosen because it displayed higher bootstrap support than the LTR-based tree: it showed some minor differences in the branching order with the LTR-based tree, but it correctly grouped the non-primate lentiviruses outside the primate group52 and resulted consistent with previously reported phylogenetic analysis53. This phylogenetic analysis confirmed that the host-based groups (Fig. 1 and Supporting Table S1) were correctly assigned since phylogenetically divergent.
The primate group, comprising our reference virus HIV-1 group M (symbol * in Fig. 3), included viruses that naturally infect both human (HIV-1 and HIV-2) and non-human primates (SIVs). An interesting feature here was the crossover of HIV and SIV lineages that indicates multiple cross-species transmission from simian to humans: in particular, SIVcpz from chimpanzee or Pan troglodytes troglodytes was the most closely related to HIV-1 group M, whereas SIVsm from sooty mangabey monkey or Cercocebus atys was closer to HIV-254. HIV-1 groups M and N originated from independent jumps from chimpanzee55 and HIV-1 group O from the western gorilla subspecies Gorilla gorilla 56, resulting in HIV-1 strains with very different pathogenic potential: HIV-1 group M causes the well-known AIDS pandemic, groups N and O cause a milder disease and have been limited to a few individuals mostly in Cameroon57, 58. This phylogenetic analysis on one hand further supports the central role of G4 formation in the LTR, since this feature was maintained in multiple and unrelated cross-species transmission from a simian ancestor strain, and it explains why all primate lentiviruses display PQSs in the same LTR U3 region; on the other it gives reason of the substantial differences in PQS among strains.
Figure 3.
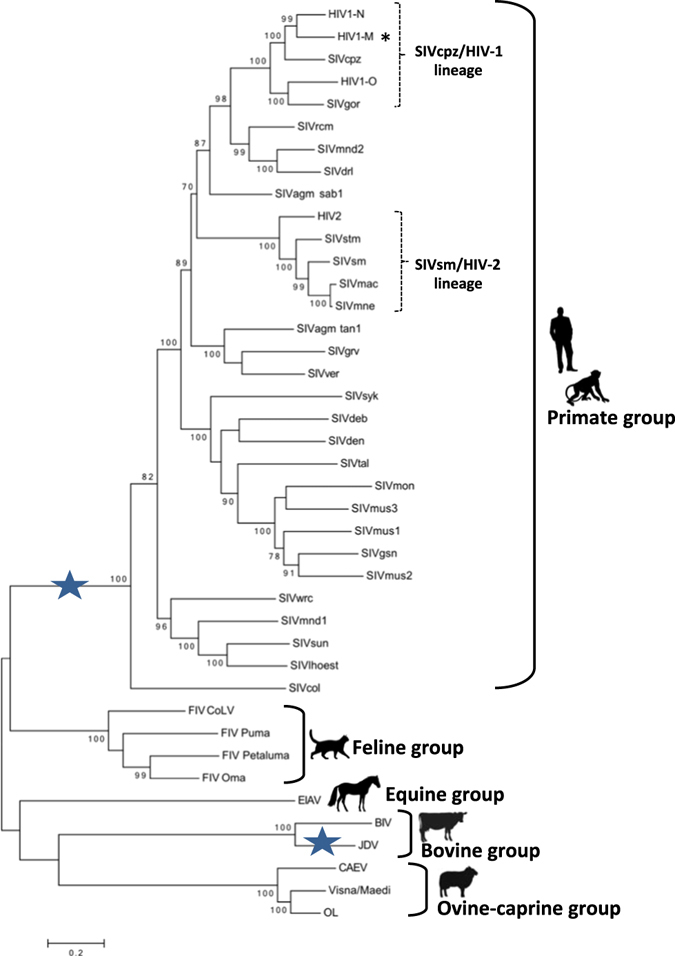
Phylogenetic tree of lentiviruses based on the pol gene. Blue stars correspond to the presence of PQSs in the corresponding group of lentiviruses. The symbol * indicates our reference virus HIV-1 group M. Human and animal silhouettes were obtained at http://www.freepik.com and http://www.flaticon.com/ (http://www.freepik.com/free-vector/business-team-outlines-pack_831669.htm#term=human; http://www.flaticon.com/free-icon/monkey_47138; http://www.freepik.com/free-vector/cat-silhouettes-set_718091.htm#term=cat; http://www.flaticon.com/free-icon/horse-standing-black-shape_35907; http://www.freepik.com/free-vector/cows-and-bull-silhouettes_788343.htm; http://www.freepik.com/free-vector/pack-of-farm-animal-silhouettes_1058750.htm#term=sheep&page=1&position=29).
Interestingly, only JDV of the bovine group displayed LTR PQSs, even if it was phylogenetically more distant to primate lentiviruses than the feline group, which lacked three-stacked tetrad LTR PQSs (blue stars in Fig. 3).
The identified 5′-LTR PQSs can fold into G4 structures
The actual ability of PQSs to form G4 structures was next investigated by Taq polymerase stop assay. Representative PQSs from the primate group and the unique JDV PQSs were analysed to cover the widest phylogenetic distance: PQSs from the SIVcpz/HIV-1 lineage (our reference HIV-1 group M, SIVcpz, HIV group N, HIV group O and SIVgor), the SIVsm/HIV-2 lineage (HIV-2, SIVmac and SIVsm) and SIVlhoest, SIVwrc, SIVsyk and SIVagm sab1, which are progressively closer to HIV-1 M in the phylogenetic tree, were selected.
The chosen sequences were investigated in the absence/presence of K+ to establish G4 formation, and in the presence of the G4 ligand BRACO-19 (200 nM) to assess ligand-induced G4 formation, which has been reported in the case of HIV-1 group M9. In the presence of K+ and BRACO-19, premature stop sites appeared in most of the selected PQSs (Fig. 4a) and corresponded to the most 3′-G-tract involved in G4 formation (Fig. 4b). In all cases, G4-specific stops were more pronounced in the presence of the G4 ligand (compare lanes + with lanes B in Fig. 4a) indicating that these G4s can be stabilized and in some cases induced by specific G4 ligands, consistently with previously reported data9. Sequences where no G4-related stops were obtained during the Taq polymerase elongation step at 47 °C, i.e. HIV N and SIVwrc (Fig. 4a), were further analysed at elongation at 37 °C to solve thermodynamically less stable secondary structures. In these conditions, these two sequences folded in G4s as well, as indicated by specific stops at the main G-tracts (Fig. 4a,b). Only SIVlhoest PQS folded in a unique G4 species, while all other tested sequences folded in 2 or 3 different G4s (Fig. 4a,b). Interestingly, PQSs of HIV-1 group M and SIVcpz not only were similar in terms of number of G-runs (Fig. 1, Table 1 and Supporting Table S1), conserved (Fig. 2) and phylogenetically related (Fig. 3), but were both also able to fold in 3 very similar and mutually exclusive G4 structures (Fig. 4a). Indeed, the predicted G4 species of SIVcpz fully coincided with those of HIV-1 group M9, 43 (Fig. 4b). PQSs in the 5′-LTR of HIV-1 group O and group N were also able to fold in G4 even if HIV-1 group N G4s were probably less stable because they could be visualized only at the lower Taq polymerase elongation temperature. Unfortunately, it was not possible to investigate SIVgor G4s by Taq Polymerase Stop assay because an extremely stable premature stop that did not correspond to a G4 structure was present (Fig. 4a,b). This stop was probably due to a hairpin-like secondary structure with 10 base pairs, as predicted by a web-based DNA tool (https://www.idtdna.com/calc/analyzer). As for viruses in the SIVsm/HIV-2 lineage, the behaviour of HIV-2, SIVmac and SIVsm G4s were extremely similar and in accordance to their relatedness in terms of sequence (Fig. 1) and evolutionary history (Fig. 3): they could effectively fold in at least two different G4s, the major one of which was located at the 3′-end of the sequence (Fig. 4b) and mainly induced by the addition of BRACO-19 (Fig. 4a).
Figure 4.

Taq polymerase stop assay of lentiviral G4 sequences. Oligonucleotides bearing PQSs were folded in the absence (−) or presence (+) of KCl. KCl-treated samples were further incubated with the G4 ligand BRACO-19 (B). Oligonucleotides were used as templates in a Taq polymerase reaction at 47 °C or 37 °C as indicated. Symbols * indicate premature stop site at G bases in gel images (a) and in the corresponding G4 sequences (b). Symbols ^ indicate stop sites independent of G4 folding. G-bases are shown in bold.
Discussion
The G4 cluster has been previously shown to be a fine modulator of HIV-1 group M transcription: in particular, when HIV-1 LTR G4s fold, transcription is inhibited9. Formation of HIV-1 LTR G4s is modulated by interaction with different cellular proteins, i.e. nucleolin and hnRNPA2/B1, which further inhibit or release transcription by inducing or unfolding G4s, respectively27, 59. The existence of cellular proteins that specifically interact with the HIV-1 G4 system is a powerful indication of the actual formation of LTR G4s in vivo.
A further indication comes from the present work, showing that almost all primate lentiviruses and JDV, a cattle infective virus, display G4 forming regions in the U3 region of their 5′-LTR promoter. These regions and their ability to fold into G4 are extremely well conserved. The LTR G4s are also evolutionary related: on one hand the most similar LTR G4 regions (i.e. HIV-1 group M and SIVcpz, HIV-2 and SIVmac/SIVsm) belong to viruses that are phylogenetically the closest; on the other, LTR G4s in primate lentiviruses, while sharing the possibility to form G4, are diverse in sequence, possibly because of the early divergence of the different lineages from a common ancestor (Fig. 3).
A further note of interest is that, while the feline lentiviruses that are phylogenetically closer to the primate lentiviruses do not possess naturally folding G4 regions, the more distantly related JDV of the bovine group do. In general, however, these latter G-rich regions form low complexity G4s, in contrast to primate G4s that are multiple, overlapping and thus mutually exclusive9. We have suggested that the HIV-1 group M LTR G4 complexity is necessary for the tuning of G4 modulation: in particular, initial evidence indicated that the least stable HIV-1 G4, LTR-IV, may be required to release the inhibitory activity of the most stable HIV-1 G4, LTR-III43. It is thus possible that the primate LTR G4s are more evolutionary progressed and control viral transcription in a G4-based high-complexity mechanism.
Our phylogenetic analysis showed that LTR G4s have evolved independently of the common ancestor in the primate and bovine group. This fact indicates that the presence of a G4-based transcription control must be beneficial to the overall virus biology so that it has been selected during lentivirus evolution. In addition, 6 out of 9 viruses that lacked the presence of three-stacked tetrad G4s, displayed sufficiently clustered G tracts to allow the formation of less stable two-stacked tetrad G4s (Supporting Table S2). This might be indication of the initial evolution towards more stable G4s also in these viruses.
Based on the evidence that cellular proteins are required for the LTR G4 modulatory mechanism27, a further possible explanation for LTR G4 diversity in lentiviruses is that the selection of G4 in the LTR promoter has been driven by the presence/absence of the necessary host co-factors. This would explain the LTR G4s host-specificity observed in the present work.
In this direction, we have found a significant correlation between the presence of G4s and Sp1 binding sites in the LTR promoter of lentiviruses. Sp1 is a ubiquitous transcription factor that has been shown to be the main driver of HIV-1 basal transcription through binding to the three sites in the U3 region of the 5′-LTR60. Beside the duplex, Sp1 is able to bind DNA in its G4 conformation, both in eukaryotic cells61 and HIV-18. Our data are in line with the previously reported association between G4s and Sp1 binding sites in human genes62. The reported suppression of viral gene expression when Sp1 binding to the HIV-1 5′-LTR is disrupted by cellular proteins or gene editing63, 64 may further support a key role played by G4s as regulatory elements of viral transcription.
One or more NFκB sites were also generally present upstream of the G4-forming region. In HIV-1 group M, we have previously shown that the sequence comprising NFκB, which could in principle fold into an additional overlapping G4, does not fold in vitro in the presence of K+ or G4 ligands9. Considering the degree of conservation of this sequence just upstream of the Sp1 binding sites and the G4 folding region, we suggest two possibilities: i) the recruitment of NFκB is necessary for processes that occur at the downstream G4 region; ii) there are additional cellular factors that induce G4 folding at this region. The former hypothesis is supported by the reported interaction of NFκB and Sp1 in an orientation and position-dependent manner65, which, based on our present observations, may rely on G4 folding/unfolding equilibria. The latter hypothesis is supported by the observation that nucleolin, the major reported LTR G4 binding protein27, preferentially binds regions that form low stability G4s66. The effect of G4-inducing proteins is expected to be more pronounced in less intrinsically stable G4s regions, such as the NFκB binding site, and thus biologically more significant.
On the whole, even if lentiviruses are characterized by a rapid evolution rate, they present a G-rich region in the 5′-LTR that is evolutionary very conserved in terms of structure, but not of sequence. This feature is shared with other key viral elements, such as the Lys-tRNA primer-binding site (PBS) that is required to start reverse transcription67. Thus, the use of structural conserved elements in a mechanosensor-regulated mechanism appears a theme commonly exploited by lentiviruses to control crucial viral steps. A similar G4/iMotif mechanism has been recently proposed in the promoter of the c-myc oncogene68.
In conclusion, we propose the 5′-LTR G4 region of lentiviruses as a control centre of viral transcription, where alternate folding/unfolding of the G4s and multiple recruitment of factors based on both sequence and structure may take place (Fig. 5).
Figure 5.
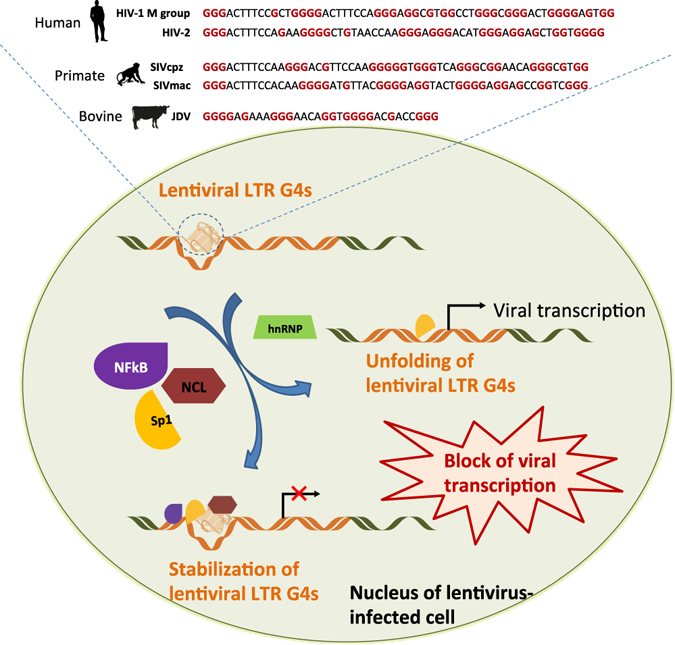
Model of transcription regulation in lentiviruses based on the 5′-LTR G4s. Sp1 and NFkB are transcription factors, NCL stands for the cellular protein nucleolin27, hnRNP stands for heterogeneous nuclear ribonucleoproteins. Human and animal silhouettes were obtained at http://www.freepik.com and http://www.flaticon.com/ (http://www.freepik.com/free-vector/business-team-outlines-pack_831669.htm#term = human; http://www.flaticon.com/free-icon/monkey_47138; http://www.freepik.com/free-vector/cows-and-bull-silhouettes_788343.htm).
Materials and Methods
G4 analysis of the lentivirus LTR Region
The LTR region of lentiviruses was analysed by QGRS Mapper (http://bioinformatics.ramapo.edu/QGRS/index.php) for prediction of G4 forming sequences. The following restrictions were applied: maximum length 45 nt; minimum G-group size 3 nt; loop size 0–12 nt.
Analysis of sequence conservation of lentiviral LTRs and G4 patterns within the primate group
Complete LTR sequences, when available, were extracted from lentiviral strains belonging to the primate group. A multiple alignment was built using USEARCH69, followed by a manual editing to correct artefacts due to the low similarity among sequences (Supplementary Figure S1). The global sequence similarity of the alignment was calculated by averaging the percentage of similarity of all possible pairwise comparisons.
Base conservation analysis of predicted G4 forming sequences
Predicted G4 forming sequences were further analysed in terms of base conservation by aligning sequences from Pubmed or from the HIV database (http://www.hiv.lanl.gov/) using USEARCH69. Accession numbers of the whole set of sequences were reported in Supplementary Table S3. The conservation analysis was performed only on lentiviruses with more than 5 sequences available in databases. LOGO representation of base conservation was obtained by the WebLogo software70.
Prediction of transcription factor binding sites
The prediction of Sp1 binding sites in putative G4 forming sequences were performed by the web-based tool PhysBinder using the model HSA0000031.1 [SP1] with the Max. F-measure threshold (2 × True Positives/(2 × True Positives + False Positives + False Negatives)48.
Molecular phylogenetic analysis of lentiviruses
The evolutionary history was inferred by using the Maximum Likelihood method based on the General Time Reversible mode71. The analysis involved 41 nucleotide sequences of the pol gene extracted from different lentiviruses (relative accession numbers in Table 1), which were multiple aligned with clustalW72. The percentage of trees in which the associated taxa clustered together is shown next to the branches (for values > = 70 on 500 bootstrap replicates73) (Fig. 3). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. All positions containing gaps and missing data were eliminated. There were a total of 2380 positions in the final dataset. Evolutionary analyses were conducted in MEGA674.
Taq Polymerase Stop Assay
Taq polymerase stop assay was performed as previously described9. Briefly, the 5′-end labeled primer was annealed to its template (Supporting Information, Table S1) in lithium cacodylate buffer in the presence or absence of KCl 100 mM and by heating at 95 °C for 5 min and gradually cooling to room temperature. Where specified, samples were incubated with BRACO-19 (200 nM). Primer extension was conducted with 2 U of AmpliTaq Gold DNA polymerase (Applied Biosystem, Carlsbad, California, USA) at 47 °C or 37 °C for 30 min. Reactions were stopped by ethanol precipitation, primer extension products were separated on a 15% denaturing gel, and finally visualized by phosphorimaging (Typhoon FLA 9000).
Electronic supplementary material
Acknowledgements
This work was supported by the Bill and Melinda Gates Foundation (GCE grant numbers OPP1035881, OPP1097238) and the European Research Council (ERC Consolidator grant 615879) to SNR. Funding for open access charge: Bill and Melinda Gates Foundation.
Author Contributions
R.P. performed the analysis of the presence, conservation and phylogenetic relation of PQS in lentiviruses, the Taq polymerase stop assay and wrote the manuscript; E.L. performed the phylogenetic analysis on the pol gene and the conservation analysis of lentiviral LTRs and G4 patterns within the primate group; G.P. commented on the manuscript; S.N.R. conceived of the work and wrote the manuscript. All authors analysed the data and reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02291-1
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barre-Sinoussi F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Gallo RC, et al. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS) Science. 1983;220:865–867. doi: 10.1126/science.6601823. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S, Patil SS, Sood R. Bovine immunodeficiency virus: a lentiviral infection. Indian J Virol. 2013;24:332–341. doi: 10.1007/s13337-013-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sala M, Wain-Hobson S. Are RNA viruses adapting or merely changing? J Mol Evol. 2000;51:12–20. doi: 10.1007/s002390010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira LA, Bentley K, Peeters A, Churchill MJ, Deacon NJ. A compilation of cellular transcription factor interactions with the HIV-1 LTR promoter. Nucleic Acids Res. 2000;28:663–668. doi: 10.1093/nar/28.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements JE, Zink MC. Molecular biology and pathogenesis of animal lentivirus infections. Clin Microbiol Rev. 1996;9:100–117. doi: 10.1128/cmr.9.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrone R, et al. Formation of a unique cluster of G-quadruplex structures in the HIV-1 Nef coding region: implications for antiviral activity. PLoS One. 2013;8:e73121. doi: 10.1371/journal.pone.0073121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piekna-Przybylska D, Sullivan MA, Sharma G, Bambara RA. U3 region in the HIV-1 genome adopts a G-quadruplex structure in its RNA and DNA sequence. Biochemistry. 2014;53:2581–2593. doi: 10.1021/bi4016692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrone R, et al. A dynamic G-quadruplex region regulates the HIV-1 long terminal repeat promoter. J Med Chem. 2013;56:6521–6530. doi: 10.1021/jm400914r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amrane S, et al. Topology of a DNA G-quadruplex structure formed in the HIV-1 promoter: a potential target for anti-HIV drug development. J Am Chem Soc. 2014;136:5249–5252. doi: 10.1021/ja501500c. [DOI] [PubMed] [Google Scholar]
- 11.Burge S, Parkinson GN, Hazel P, Todd AK, Neidle S. Quadruplex DNA: sequence, topology and structure. Nucleic Acids Res. 2006;34:5402–5415. doi: 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel DJ, Phan AT, Kuryavyi V. Human telomere, oncogenic promoter and 5′-UTR G-quadruplexes: diverse higher order DNA and RNA targets for cancer therapeutics. Nucleic Acids Res. 2007;35:7429–7455. doi: 10.1093/nar/gkm711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes D, Lipps HJ. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Research. 2015;43:8627–8637. doi: 10.1093/nar/gkv862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell NH, Neidle S. G-quadruplexes and metal ions. Met Ions Life Sci. 2012;10:119–134. doi: 10.1007/978-94-007-2172-2_4. [DOI] [PubMed] [Google Scholar]
- 15.Lane AN, Chaires JB, Gray RD, Trent JO. Stability and kinetics of G-quadruplex structures. Nucleic Acids Res. 2008;36:5482–5515. doi: 10.1093/nar/gkn517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sen D, Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature. 1990;344:410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Liu C, Geng Y, Zhu G. Topology of a G-quadruplex DNA formed by C9orf72 hexanucleotide repeats associated with ALS and FTD. Sci Rep. 2015;5:16673. doi: 10.1038/srep16673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holder IT, Hartig JS. A matter of location: influence of G-quadruplexes on Escherichia coli gene expression. Chem Biol. 2014;21:1511–1521. doi: 10.1016/j.chembiol.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Maizels N. G4-associated human diseases. EMBO Rep. 2015;16:910–922. doi: 10.15252/embr.201540607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry M, Loeb LA. The fragile X syndrome d(CGG)n nucleotide repeats form a stable tetrahelical structure. Proc Natl Acad Sci USA. 1994;91:4950–4954. doi: 10.1073/pnas.91.11.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fratta P, et al. C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Sci Rep. 2012;2:1016. doi: 10.1038/srep01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisette JF, Montagna DR, Mihailescu MR, Wolfe MS. A G-rich element forms a G-quadruplex and regulates BACE1 mRNA alternative splicing. J Neurochem. 2012;121:763–773. doi: 10.1111/j.1471-4159.2012.07680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor JP. Neurodegenerative diseases: G-quadruplex poses quadruple threat. Nature. 2014;507:175–177. doi: 10.1038/nature13067. [DOI] [PubMed] [Google Scholar]
- 24.Haeusler AR, et al. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature. 2014;507:195–200. doi: 10.1038/nature13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov P, et al. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc Natl Acad Sci USA. 2014;111:18201–18206. doi: 10.1073/pnas.1407361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sket P, et al. Characterization of DNA G-quadruplex species forming from C9ORF72 G4C2-expanded repeats associated with amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Neurobiol Aging. 2015;36:1091–1096. doi: 10.1016/j.neurobiolaging.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Tosoni E, et al. Nucleolin stabilizes G-quadruplex structures folded by the LTR promoter and silences HIV-1 viral transcription. Nucleic Acids Res. 2015;43:8884–8897. doi: 10.1093/nar/gkv897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu J, et al. Biological Function and Medicinal Research Significance of G-Quadruplex Interactive Proteins. Curr Top Med Chem. 2015;15:1971–1987. doi: 10.2174/1568026615666150515150803. [DOI] [PubMed] [Google Scholar]
- 29.Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat Chem. 2013;5:182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson A, et al. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res. 2014;42:860–869. doi: 10.1093/nar/gkt957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metifiot M, Amrane S, Litvak S, Andreola ML. G-quadruplexes in viruses: function and potential therapeutic applications. Nucleic Acids Res. 2014;42:12352–12366. doi: 10.1093/nar/gku999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan, J. Z. et al. The SARS-Unique Domain (SUD) of SARS Coronavirus Contains Two Macrodomains That Bind G-Quadruplexes. Plos Pathogens5, doi:ARTN e1000428 10.1371/journal.ppat.1000428 (2009). [DOI] [PMC free article] [PubMed]
- 33.Tluckova K, et al. Human papillomavirus G-quadruplexes. Biochemistry. 2013;52:7207–7216. doi: 10.1021/bi400897g. [DOI] [PubMed] [Google Scholar]
- 34.Wang SR, et al. A highly conserved G-rich consensus sequence in hepatitis C virus core gene represents a new anti-hepatitis C target. Sci Adv. 2016;2:e1501535–e1501535. doi: 10.1126/sciadv.1501535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleming AM, Ding Y, Alenko A, Burrows CJ. Zika Virus Genomic RNA Possesses Conserved G-Quadruplexes Characteristic of the Flaviviridae Family. ACS Infect Dis. 2016;2:674–681. doi: 10.1021/acsinfecdis.6b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SR, et al. Chemical Targeting of a G-Quadruplex RNA in the Ebola Virus L Gene. Cell Chem Biol. 2016;23:1113–1122. doi: 10.1016/j.chembiol.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Murat P, et al. G-quadruplexes regulate Epstein-Barr virus-encoded nuclear antigen 1 mRNA translation. Nat Chem Biol. 2014;10:358–364. doi: 10.1038/nchembio.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norseen J, Johnson FB, Lieberman PM. Role for G-quadruplex RNA binding by Epstein-Barr virus nuclear antigen 1 in DNA replication and metaphase chromosome attachment. J Virol. 2009;83:10336–10346. doi: 10.1128/JVI.00747-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Artusi S, et al. The Herpes Simplex Virus-1 genome contains multiple clusters of repeated G-quadruplex: Implications for the antiviral activity of a G-quadruplex ligand. Antiviral Res. 2015;118:123–131. doi: 10.1016/j.antiviral.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Artusi S, et al. Visualization of DNA G-quadruplexes in herpes simplex virus 1-infected cells. Nucleic Acids Res. 2016;44:10343–10353. doi: 10.1093/nar/gkw968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrone R, et al. Anti-HIV-1 activity of the G-quadruplex ligand BRACO-19. J Antimicrob Chemother. 2014;69:3248–3258. doi: 10.1093/jac/dku280. [DOI] [PubMed] [Google Scholar]
- 42.Perrone R, et al. Synthesis, Binding and Antiviral Properties of Potent Core-Extended Naphthalene Diimides Targeting the HIV-1 Long Terminal Repeat Promoter G-Quadruplexes. J Med Chem. 2015;58:9639–9652. doi: 10.1021/acs.jmedchem.5b01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Nicola, B. et al. Structure and possible function of a G-quadruplex in the long terminal repeat of the proviral HIV-1 genome. Nucleic Acids Res, doi:10.1093/nar/gkw432 (2016). [DOI] [PMC free article] [PubMed]
- 44.Peeters, M. & Courgnaud, V. In HIV sequence compendium. (ed. B. Foley C. Kuiken, E. Freed, B. Hahn, B. Korber, P. Marx, F. McCutchan, J. W. Mellors and S. Wolinsky) 2–23 (2002).
- 45.Balasubramanian S, Hurley LH, Neidle S. Targeting G-quadruplexes in gene promoters: a novel anticancer strategy? Nat Rev Drug Discov. 2011;10:261–275. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, et al. Identification of a novel nuclear factor-kappaB sequence involved in expression of urokinase-type plasminogen activator receptor. Eur J Biochem. 2000;267:3248–3254. doi: 10.1046/j.1432-1327.2000.01350.x. [DOI] [PubMed] [Google Scholar]
- 47.Song J, et al. Two consecutive zinc fingers in Sp1 and in MAZ are essential for interactions with cis-elements. J Biol Chem. 2001;276:30429–30434. doi: 10.1074/jbc.M103968200. [DOI] [PubMed] [Google Scholar]
- 48.Broos S, et al. PhysBinder: Improving the prediction of transcription factor binding sites by flexible inclusion of biophysical properties. Nucleic Acids Res. 2013;41:W531–534. doi: 10.1093/nar/gkt288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tong-Starksen SE, Welsh TM, Peterlin BM. Differences in transcriptional enhancers of HIV-1 and HIV-2. Response to T cell activation signals. J Immunol. 1990;145:4348–4354. [PubMed] [Google Scholar]
- 50.Pohlmann S, Floss S, Ilyinskii PO, Stamminger T, Kirchhoff F. Sequences just upstream of the simian immunodeficiency virus core enhancer allow efficient replication in the absence of NF-kappaB and Sp1 binding elements. J Virol. 1998;72:5589–5598. doi: 10.1128/jvi.72.7.5589-5598.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bibollet-Ruche F, et al. Simian immunodeficiency virus infection in a patas monkey (Erythrocebus patas): evidence for cross-species transmission from African green monkeys (Cercopithecus aethiops sabaeus) in the wild. J Gen Virol. 1996;77(Pt 4):773–781. doi: 10.1099/0022-1317-77-4-773. [DOI] [PubMed] [Google Scholar]
- 52.Benachenhou F, Blikstad V, Blomberg J. The phylogeny of orthoretroviral long terminal repeats (LTRs) Gene. 2009;448:134–138. doi: 10.1016/j.gene.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Gifford RJ, et al. A transitional endogenous lentivirus from the genome of a basal primate and implications for lentivirus evolution. Proc Natl Acad Sci USA. 2008;105:20362–20367. doi: 10.1073/pnas.0807873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rambaut A, Posada D, Crandall KA, Holmes EC. The causes and consequences of HIV evolution. Nat Rev Genet. 2004;5:52–61. doi: 10.1038/nrg1246. [DOI] [PubMed] [Google Scholar]
- 55.Keele BF, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Heuverswyn F, et al. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature. 2006;444:164–164. doi: 10.1038/444164a. [DOI] [PubMed] [Google Scholar]
- 57.Ayouba A, et al. HIV-1 group O infection in Cameroon, 1986 to 1998. Emerg Infect Dis. 2001;7:466–467. doi: 10.3201/eid0703.017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaguchi J, et al. HIV-1 Group N: evidence of ongoing transmission in Cameroon. AIDS Res Hum Retroviruses. 2006;22:453–457. doi: 10.1089/aid.2006.22.453. [DOI] [PubMed] [Google Scholar]
- 59.Scalabrin M, et al. The cellular protein hnRNP A2/B1 enhances HIV-1 transcription by unfolding LTR promoter G-quadruplexes. Sci. Rep. 2017;7:45244. doi: 10.1038/srep45244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones KA, Kadonaga JT, Luciw PA, Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986;232:755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- 61.Raiber EA, Kranaster R, Lam E, Nikan M, Balasubramanian S. A non-canonical DNA structure is a binding motif for the transcription factor SP1 in vitro. Nucleic Acids Res. 2012;40:1499–1508. doi: 10.1093/nar/gkr882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Todd AK, Neidle S. The relationship of potential G-quadruplex sequences in cis-upstream regions of the human genome to SP1-binding elements. Nucleic Acids Res. 2008;36:2700–2704. doi: 10.1093/nar/gkn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turrini F, et al. HIV-1 transcriptional silencing caused by TRIM22 inhibition of Sp1 binding to the viral promoter. Retrovirology. 2015;12:104. doi: 10.1186/s12977-015-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qu D, et al. The variances of Sp1 and NF-kappaB elements correlate with the greater capacity of Chinese HIV-1 B′-LTR for driving gene expression. Sci Rep. 2016;6:34532. doi: 10.1038/srep34532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perkins ND, et al. A cooperative interaction between NF-kappa B and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993;12:3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lago, S., Tosoni, E., Nadai, M., Palumbo, M. & Richter, S. N. The cellular protein nucleolin preferentially binds long-looped G-quadruplex nucleic acids. Biochim Biophys Acta, doi:10.1016/j.bbagen.2016.11.036 (2016). [DOI] [PMC free article] [PubMed]
- 67.Berkhout B. Structure and function of the human immunodeficiency virus leader RNA. Prog Nucleic Acid Res Mol Biol. 1996;54:1–34. doi: 10.1016/S0079-6603(08)60359-1. [DOI] [PubMed] [Google Scholar]
- 68.Sutherland, C., Cui, Y., Mao, H. & Hurley, L. H. A Mechanosensor Mechanism Controls the G-Quadruplex/i-Motif Molecular Switch in the MYC Promoter NHE III1. J Am Chem Soc, doi:10.1021/jacs.6b09196 (2016). [DOI] [PubMed]
- 69.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 70.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nei, M. & Kumar, S. Molecular Evolution and Phylogenetics. (Oxford University Press, New York, 2000).
- 72.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 73.Felsenstein J. Confidence-Limits on Phylogenies - an Approach Using the Bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 74.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


