Abstract
Background
Understanding the genetic structure of island Anopheles gambiae populations is important for the current tactics in mosquito control and for the proposed strategy using genetically-modified mosquitoes (GMM). Genetically-isolated mosquito populations on islands are a potential site for testing GMM. The objective of this study was to determine the genetic structure of A. gambiae populations on the islands in Lake Victoria, western Kenya.
Methods
The genetic diversity and the population genetic structures of 13 A. gambiae populations from five islands on Lake Victoria and six villages from the surrounding mainland area in the Suba District were examined using six microsatellite markers. The distance range of sampling sites varied between 2.5 and 35.1 km.
Results
A similar level of genetic diversity between island mosquito populations and adjacent mainland populations was found. The average number of alleles per locus was 7.3 for the island populations and 6.8 for the mainland populations. The average observed heterozygosity was 0.32 and 0.28 for the island and mainland populations, respectively. A low but statistically significant genetic structure was detected among the island populations (FST = 0.019) and between the island and mainland populations (FST = 0.003). A total of 12 private alleles were found, and nine of them were from the island populations.
Conclusion
A level of genetic differentiation between the island and mainland populations was found. Large extent of gene flow between the island and mainland mosquito populations may result from wind- or human-assisted dispersal. Should the islands on Lake Victoria be used as a trial site for the release program of GMM, mosquito dispersal between the islands and between the island and the mainland should be vigorously monitored.
Background
Despite 50 years of malaria vector control efforts, malaria remains a major public health threat in tropical and subtropical countries [1-3]. In recent years, malaria has caused increased human mortality and morbidity as malaria epidemics have spread to areas where it was previously rare [4,5]. The current strategies for malaria control involve the treatment of infected individuals with antimalarial drugs to kill the parasites and vector management to reduce human-vector contacts via residual spraying and the use of insecticide-impregnated bednets. As demonstrated in multisite trials throughout Africa, the large-scale use of insecticide-treated bednets can reduce overall mortality by up to 30% [6] and morbidity in young children [7]. The emergence of insecticide resistance in mosquito vectors [8] and antimalarial drug resistance in Plasmodium [9] has significantly reduced the viability of many malaria control programs. An efficacious malaria vaccine will not be available in the near future [10].
One potential alternative malaria control strategy is based on the genetic disruption of mosquito vector competence [11-13]. This genetic control approach requires identification and cloning of parasite-inhibiting genes in the mosquito vectors, development of stable and efficient mosquito transformation tools and the development of strategies for spreading the parasite-inhibiting genes. Over the past several years, remarkable progress has been made in the development of mosquito germline transformation and in the identification of parasite-inhibiting molecules. For example, A. gambiae cell lines were successfully transformed with the Hermes element [14,15], and the Minos transposable element bearing an exogenous gene was efficiently integrated into the genome of Anopheles stephensi [16,17]. Genetic linkage maps have been constructed for A. gambiae [18], and genes conferring mosquito refractoriness to malaria parasites have been mapped [19]. Availability of complete A. gambiae genome sequences will greatly facilitate identification and cloning of parasite-inhibiting genes [20].
The success of the transgenic mosquito approach depends on the spread and even fixation of parasite-inhibiting genes into natural populations. Presently, releasing transgenic mosquitoes to the field is premature. Isolated islands have been suggested as an ideal natural site for testing transgenic mosquito release strategies and spatial spreading of transgenes [13,21,22]. Information on mosquito population genetic structure and gene flow on islands and the surrounding mainland area is critical. Using microsatellite markers, the A. gambiae population genetic structure in the African continent has been examined [23-28]. These studies revealed that the Great Rift Valley in East Africa is a substantial gene flow barrier for A. gambiae; however, no significant genetic structure was detected for mosquito populations between western Kenya and West Africa. The minimum area associated for a deme of A. gambiae in western or coastal Kenya is larger than 50 km [24]. Simard et al. [29] found a high degree of genetic differentiation of the Anopheles arabiensis populations from the high plateau of Madagascar and those from Réunion and Mauritius islands (FST ranges from 0.080 to 0.215). Population substructure was also detected on the island of São Tomé, West Africa [22].
The present study examined the genetic diversity and the population genetic structures of A. gambiae mosquitoes from five islands on Lake Victoria and the surrounding mainland in western Kenya. This information is valuable for selecting field sites to test transgene release strategies and evaluating the spread of transgenes in nature.
Materials and Methods
Study sites and mosquito collection
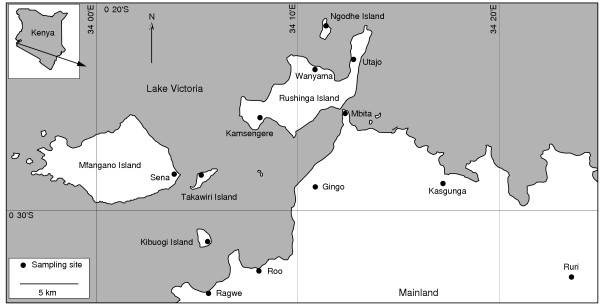
Anopheline female mosquitoes were collected from seven villages on five islands in Lake Victoria and from six villages in the mainland Suba District, western Kenya (Fig. 1). The sampled islands were Kibuogi, Mfangano (Sena village), Ngodhe, Takawiri and Rushinga. Mosquitoes were collected from three villages (Kamsengere, Utajo and Wanyama) on Rushinga Island and one village on each of the other islands. Mfangano Island is the largest and the most offshore (about 10 km away from the nearest mainland village). Rushinga Island is the most populated among the five islands and is connected to the mainland by a walkway. The islands are about 2.5–21.0 km apart. Also, five mainland villages (Ragwe, Roo, Gingo, Mbita and Kasunga) along the shore of Lake Victoria and one inland village (Ruri) about 11 km away from the lakeshore were selected. The distance between the islands and the mainland sites ranges from 4.9 to 35.1 km. Malaria on these islands and the mainland area is holoendemic, and A. gambiae mosquitoes are the major malaria vectors in this region [30].
Figure 1.
Map of study area showing the distribution of Anopheles gambiae populations on the Lake Victoria islands and the surrounding mainland area in Suba District, western Kenya.
At least 170 anopheline mosquitoes were collected from four to 28 houses within each village using the pyrethrum spray collection method [31]. Mosquitoes from Mbita, Kasgunga and Ruri were sampled in May 1997; collection in other villages was conducted in April and May 1999. A. gambiae sensu lato (s.l.) specimens were separated from other anophelines according to the identification key provided by Gillies and Coetzee [32] and then preserved in 95% ethanol and kept at -20°C until further analyzed.
PCR assay for species identification
PCR analysis was conducted for species identification using the rDNA-PCR method because individual species within the A. gambiae species complex cannot be identified by morphology alone [33]. About 100 A. gambiae s.l. females per village were tested. If the initial PCR testing failed to amplify for a sample, then the PCR analysis was repeated once or twice until successful amplification was achieved. If a sample could not be identified after three PCR amplifications, it was scored as unknown.
Microsatellite loci and genotyping
Six microsatellite markers were used for specimen genotyping, including AGXH1D1 and AGXH131 of Chromosome X, AG2H46 and AG2H79 of Chromosome 2, and AG3H29C and AG3H33C of Chromosome 3 [17,18,22,23]. Microsatellite analyses were conducted on 51–70 individuals per village (See the additional file: A table of sample size, allelic number, heterozygosities and breeding coefficient of 13 A. gambiae populations from the Lake Victoria islands and the surrounding mainland in western Kenya).
A Li-Cor Model 4200 Automated DNA Analyzer (Li-Cor Inc., Lincoln, NE) was used for gel electrophoresis. For the apparatus to detect PCR products, one primer in every pair of microsatellite primers must be fluorescently labelled. To reduce the cost associated with synthesis of fluorescently labelled primers, we used the "tailed primer" method [34,35]; that is, the forward primer for each microsatellite locus was synthesized with an additional 19 bp sequence (5' CACGACGTTGTAAAACGAC 3') added to the 5' end of the primer. A third primer with the same 19 bp sequence was directly labelled with the fluorescence and was used as the sole type of labelled primer for the detection of all microsatellite alleles. The tailed primer method reduced the cost of oligonucleotide synthesis by >80%. The 10 μl PCR reaction contained 1X Taq buffer, 0.2 mM dNTPs, 1.5 pmol forward and reverse primers, 1.5 pmol fluorescently labelled 19 bp sequences, 1.5 mM MgCl2, 1.0 μg BSA, 1.0 unit Taq polymerase and about 20 ng genomic DNA. Cycling conditions in a MJ Research PTC-220 thermocycler were 35–40 cycles of 94°C for 30 seconds, 55°C for 30 seconds and 72°C for 45 seconds. Allele sizes were determined using Gene ImagIR computer software [36]. The allele sizes used in the analysis were true allele sizes that have been adjusted for the 19 bp tail in the forward primer.
Data analysis
Microsatellite polymorphism was measured by the number of alleles and heterozygosity at each locus. Using the probability test available in the GENEPOP computer program [37], conformance with Hardy-Weinberg Equilibrium (HWE) was tested for each locus and population, and the Bonferroni correction was applied for multiple comparisons. The FIS statistics and probability test were used to determine whether distortion from HWE resulted from heterozygosity deficiency or excess using. Because the probability test is robust to low allele frequencies, rare alleles were not pooled. Variations in heterozygosity among the populations were analyzed following Weir's method [38], using the analysis of variance (ANOVA) with subpopulations, individuals, loci and interactions of loci, and individuals as factors. All factors were treated as random effects except loci. The Fisher exact test was performed to detect linkage disequilibrium for pair-wise loci in each population and the pooled population. Population genetic structure was examined with Wright's F-statistics (FST) using FSTAT 2.8 [39]. FST statistic appears to be more sensitive to detect intraspecific differentiation than RST [40,41]. The standard deviations of the F-statistics were obtained for each locus by a jackknife procedure over all the alleles and were used to test the statistical significance. Nei's unbiased genetic distances [42] were calculated for all pairs of populations based on microsatellite allele frequencies at six loci using TFPGA [43]. A dendrogram was created based on the pair-wise genetic distances using the unweighted pair group method with arithmetic mean (UPGMA). The bootstrap confidence values were generated by 1,000 permutations.
The isolation-by-distance model of population genetic structure was tested by linear regression of pair-wise FST/(1 - FST) against the natural logarithm of straight-line geographical distance between population pairs [44]. Statistical significance of the regression was tested using the Mantel test with 10,000 permutations [45].
Results
Population genetic variability
A moderate to high level of polymorphism was found in six loci across the 13 populations (See the additional file). The three populations from Rushinga Island had a similar number of alleles per locus (ANOVA, F = 0.02, df = 2, P > 0.05) and observed heterozygosities (F = 0.029, df = 2, P > 0.05). Among the island populations, the average observed number of alleles per locus was not significantly different (F = 0.08, df = 6, P > 0.05), but observed heterozygosity varied significantly (F = 4.52, df = 6, P < 0.01). The three populations on Rushinga Island, Kamsengere, Utajo and Wanyama, showed significantly lower heterozygosity than other islands. Similarly, the six mainland populations did not differ in the number of alleles per locus (F = 0.29, df = 5, P > 0.05), but they varied significantly in the observed heterozygosities (F = 5.45, df = 5, P < 0.01). In particular, the Ruri population had the highest observed heterozygosity (0.343), about two-fold higher than the Mbita population (See the additional file). Overall, there was no significant difference between the island and mainland populations in the number of alleles per locus (7.3 vs. 6.8; t = 0.67, df = 74, P > 0.05) and observed heterozygosities (0.32 vs. 0.28; t = 1.82, df = 74, P > 0.05). A total of 12 private alleles were identified, nine of them from the island populations.
A total of 14.1% loci (11 out of 78 tests) showed significant departure from Hardy-Weinberg equilibrium, all due to heterozygote deficiency. This was caused entirely by heterozygote deficiency in the locus AG2H46, a locus known for the presence of null alleles in western Kenyan A. gambiae populations [46]. The Fisher exact test revealed linkage disequilibrium in 13 out of 195 pairs of loci (6.7%; data not shown), suggesting a low level of linkage disequilibrium among the six loci scored.
Population genetic structure
A low, but significant, genetic structure was detected among the seven island and the six mainland populations (Table 1). The genetic differentiation in the seven island populations (FST = 0.019, P < 0.001) was almost twice as high as the six mainland populations (FST = 0.010, P < 0.001). Genetic differentiation between island and mainland populations was also small (FST = 0.010, P < 0.001). Pair-wise comparisons between all populations revealed that only seven pairs (Kibougo/Kamsengere, Kasgunga/Kamsengere, Takawiri/Ruri, Sena/Ruri, Utajo/Ruri, Ngodhe/Ruri and Ngodhe/Gingo) exhibited significant FST values, and six of them were between an island and a mainland population.
Table 1.
FST estimates of Anopheles gambiae populations on the islands of Lake Victoria and from surrounding mainland sites in western Kenya
| Locus | Among seven island populations | Among six mainland populations | Between island and mainland areas | Among all populations |
| AGXH1D1 | 0.082*** | 0.022*** | 0.002*** | 0.042*** |
| AGXH131 | 0.000 | 0.009*** | 0.005*** | 0.006*** |
| AG2H46 | 0.008*** | 0.006 | 0.006*** | 0.009*** |
| AG2H79 | 0.011*** | 0.011*** | 0.000 | 0.010*** |
| AG3H29C | 0.026*** | 0.008*** | 0.000 | 0.007*** |
| AG3H33C | 0.003*** | 0.009*** | 0.000 | 0.005*** |
| Overall | 0.019*** | 0.010*** | 0.003*** | 0.012*** |
* P < 0.05, ** P < 0.01, *** P < 0.001.
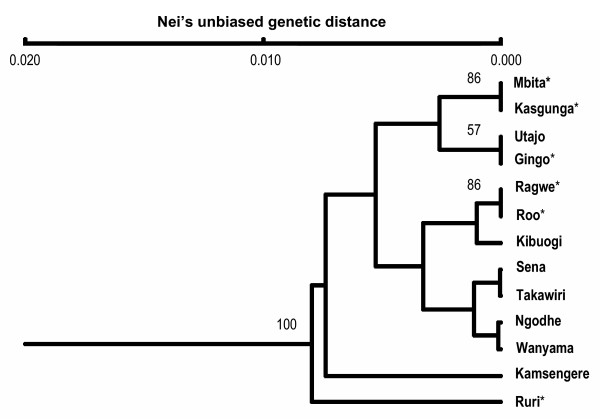
The Mantel test revealed a significant correlation between geographic distance and pair-wise FST/(1 - FST) (P < 0.001), suggesting that the population genetic structure of A. gambiae populations from the island and mainland is consistent with the isolation-by-distance model. When the Ruri population is removed from the analysis, the correlation was still statistically significant (P = 0.015). Therefore, the population genetic structure of our study populations is consistent with the isolation-by-distance model. The cluster analysis revealed that the Ruri population, located farther inland from the other populations, was out-grouped from other populations with a significant bootstrap value, while other mainland and island populations were intermixed with non-significant branch bootstrap values (Fig. 2).
Figure 2.
A UPGMA tree based on Nei's unbiased distance showing genetic divergence among Anopheles gambiae populations. The numbers above branches indicate those with >50% bootstrap support. The populations marked with an asterisk are the mainland populations.
Discussion
The present study demonstrated a similar level of genetic diversity between the island A. gambiae populations in the Lake Victoria and adjacent mainland populations in the Suba District, western Kenya. For the seven island populations, the average number of alleles at six microsatellite loci was 7.3 and the observed heterozygosity was 0.32. For the six mainland populations, the average number of alleles was 6.8 and the observed heterozygosity was 0.28. The population genetic diversity at most loci in this study was similar to other western Kenyan populations [23,47-49]. Compared with West Africa populations [23,27,48,49], lower heterozygosities, particularly at loci AG2H46, AG2H79 and AG3H33, were reported in this study, caused by fewer alleles detected in the studied populations. The comparable level of genetic diversity between island and mainland populations suggests that the island mosquito populations have a similar effective population size as the mainland populations, and they have not suffered severe genetic bottleneck during the previous vector control efforts. For each population, all loci except the AG2H46 locus did not show a significant deviation from Hardy-Weinberg equilibrium, suggesting that the microsatellite markers used in the study are not under strong selection and mosquito populations are in random mating. A heterozygote deficit at the locus AG2H46 was observed for all populations in this study. Heterozygote deficiency at the locus AG2H46 was also demonstrated in other western Kenya populations by Lehmann et al. [23,24]; the presence of null alleles as a result of mutations in the primer-annealing region was the cause.
A small but statistically significant genetic structure was detected for A. gambiae populations among the five islands in Lake Victoria (FST = 0.019) and among the six villages in the mainland in an area of approximately 40 × 20 km2 (FST = 0.010). The degree of genetic differentiation between the island populations in this study was less than for the island A. gambiae populations of São Tomé, western Africa (FST = 0.032) [22]. The lower FST estimates in the populations in this study were probably caused by shorter distance between islands (3–15 vs. 23–38 km) [22] and a lack of mountainous topography as gene flow barriers. The FST estimates for the mainland populations in this study were comparable to other studies on the western Kenya populations (FST = 0.0033) [24,27]. The genetic differentiation between island and mainland populations was small but statistically significant (FST = 0.003). Thus, there is a very small degree of genetic isolation between island and mainland populations. This estimation is consistent with the private allele distribution in the studied populations, in which nine of the 12 private alleles were from the island populations. Further evidence for a small degree of genetic differentiation between island and mainland populations is from pair-wise population comparisons in which six out of the seven pairs that exhibited significant genetic differentiation were between an island population and a mainland population.
The low level of genetic differentiation between island and mainland mosquito populations implies large gene flow between the two areas (83.1 migrants per generation). The normal flight range of A. gambiae is usually less than 1 km [50]. The distance to the lake shore of the mainland from the islands ranges from 2.5 to 15 km, farther than the normal flight range of the mosquitoes. Thus, mosquito migration is likely assisted by wind. Lindsay et al. [51] found that the spatial distribution of A. gambiae mosquitoes was related to the predominant wind direction at night, suggesting that wind assisted the dispersal of mosquitoes from their breeding site. A. gambiae have been shown to fly up to 7 km with the assistance of wind [52,53]. This distance is in the range for mosquitoes to disperse between the closest islands and between islands and their closest mainland in this study area. Mosquitoes may also use one island as a stepping-stone to extend their dispersal distance.
Mosquito migration may also be assisted by human activities. A study on Aedes polynesiensis populations from islands found no significant effect of geographic distance on the population genetic structure, but detected a significant correlation between gene flow and commercial traffic by planes and/or boats between islands [54]. The introduction of A. arabiensis to the Mascarene islands and Madagascar was thought to be caused by human transportation by steamship lines [55,56]. In Lake Victoria, small wooden boats may transport mosquito larvae between the islands and the mainland. A. gambiae larvae were collected at the bottom of a wooden fishing boat [57]. Rushinga Island in the study area was connected to the mainland by a walkway, and the island mosquito larvae could be moved to the mainland by vehicle transportation.
The results of this study of the population genetic stricture of island and mainland A. gambiae populations have implications for the ecological safety evaluation of the transgenic mosquito release program. During the initial field test of environmental safety and public health consequences by transgenic mosquito release, ideal sites would be islands that are totally genetically isolated from other islands and the mainland, with a sufficient number of human inhabitants and active malaria transmission on the island. Such an island may be extremely difficult to find, so islands with some genetic isolation from the mainland may have to be chosen. If so, the Lake Victoria islands could be used as field test sites; however, due to potential gene flow between the islands and between the islands and the mainland, mosquito dispersal between the islands and between the islands and the mainland should be vigorously monitored. After the release of the genetically modified mosquitoes, long-term monitoring programs should be launched to evaluate the spread of the transgenes to any unintended areas. In addition, methods to minimize the negative effects of transgene leak need to be developed prior to the field trial of transgene release [58].
Conclusions
This study showed that a low level of genetic differentiation existed between the island and mainland populations and no any genetically-isolated population was found among the 13 mosquito populations. If the islands on Lake Victoria were used as a trial site for the program to release genetically-modified mosquitoes, short-term and long-term mosquito dispersal between the islands and between the island and the mainland should be vigorously monitored.
Authors' Contributions
HC conducted species identification using PCR, microsatellite analyses and drafting the manuscript. NM was responsible for sample collection, and participated in species identification and drafting the manuscript. JB and GY supervised the study, and assisted data analysis and manuscript preparations.
Supplementary Material
A table of sample size, allelic number, heterozygosities and breeding coefficient of 13 A. gambiae populations from the Lake Victoria islands and the surrounding mainland in western Kenya.
Acknowledgments
Acknowledgments
We thank L. Carson and two anonymous reviewers for critical comments. This work was supported by WHO TDR grant A10429 and NIH grants D43 TW01505 and R01 AI50243.
Contributor Information
Hong Chen, Email: hongchen@buffalo.edu.
Noboru Minakawa, Email: minakawa@post.saga-med.ac.jp.
John Beier, Email: jbeier@med.miami.edu.
Guiyun Yan, Email: gyan@buffalo.edu.
References
- Spielman A, Kitron U, Pollack R. Time limitation and the role of research in the worldwide attempt to eradicate malaria. J Med Entomol. 1993;30:6–19. doi: 10.1093/jmedent/30.1.6. [DOI] [PubMed] [Google Scholar]
- Miller LH, Greenwood B. Malaria – a shadow in Africa. Science. 2002;298:121–122. doi: 10.1126/science.1078048. [DOI] [PubMed] [Google Scholar]
- Sachs JD. A new global effect to control malaria. Science. 2002;298:122–124. doi: 10.1126/science.1077900. [DOI] [PubMed] [Google Scholar]
- Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Highland malaria in Uganda: prospective analysis of an epidemic associated with El Niño. Trans R Soc Trop Med Hyg. 1999;93:480–487. doi: 10.1016/S0035-9203(99)90344-9. [DOI] [PubMed] [Google Scholar]
- Shanks GD, Biomondo K, Hay SI, Snow RW. Changing patterns of clinical malaria since 1965 among a tea estate population located in the Kenyan highlands. Trans R Soc Trop Med Hyg. 2000;94:253–255. doi: 10.1016/S0035-9203(00)90310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines J. Mosquito nets and insecticides for net treatment: a discussion of existing and potential distribution systems in Africa. Trop Med Int Health. 1996;1:616–632. doi: 10.1046/j.1365-3156.1996.d01-97.x. [DOI] [PubMed] [Google Scholar]
- Maxwell CA, Msuya E, Sudi M, Njunwa KJ, Carneiro IA, Curtis CF. Effect of community-wide use of insecticide-treated nets for 3–4 years on malarial morbidity in Tanzania. Trop Med Int Health. 2002;7:1003–1008. doi: 10.1046/j.1365-3156.2002.00966.x. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Field L, Vontas J. An overview of insecticide resistance. Science. 2002;298:96–97. doi: 10.1126/science.1078052. [DOI] [PubMed] [Google Scholar]
- Wellems TE. Plasmodium chloroquine resistance and the search for a replacement antimalarial drug. Science. 2002;298:124–126. doi: 10.1126/science.1078167. [DOI] [PubMed] [Google Scholar]
- Long CA, Hoffman SL. Malaria – from infants to genomics to vaccines. Science. 2002;297:345–347. doi: 10.1126/science.1074484. [DOI] [PubMed] [Google Scholar]
- James AA. Mosquito molecular genetics: the hands that feed bite back. Science. 1992;257:37–38. doi: 10.1126/science.1352413. [DOI] [PubMed] [Google Scholar]
- Collins FH, Besansky NJ. Vector biology and the control of malaria in Africa. Science. 1994;264:1874–1875. doi: 10.1126/science.8009215. [DOI] [PubMed] [Google Scholar]
- Alphey L, Beard CB, Billingsley P, Coetzee M, Crisanti A, Curtis C, Eggleston P, Godfray C, Hemingway J, Jacobs-Lorena M, James AA, Kafatos FC, Mukwaya LG, Paton M, Powell JR, Schneider W, Scott TW, Sina B, Sinden R, Sinkins S, Spielman A, Toure Y, Collins FH. Malaria control with genetically manipulated insect vectors. Science. 2002;298:119–121. doi: 10.1126/science.1078278. [DOI] [PubMed] [Google Scholar]
- Jasinskiene N, Coates CJ, Benedict MQ, Cornel AJ, Rafferty CS, James AA, Collins FH. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci U S A. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Eggleston P. Stable transformation of an Anopheles gambiae cell line mediated by the Hemes mobile genetic element. Insect Biochem Mol Biol. 1998;28:213–219. doi: 10.1016/S0965-1748(98)00020-4. [DOI] [Google Scholar]
- Catteruccia F, Nolan T, Blass C, Muller HM, Crisanti A, Kafatos FC, Loukeris TG. Toward Anopheles transformation: Minos element activity in anopheline cells and embryos. Proc Natl Acad Sci U S A. 2000;97:2157–2162. doi: 10.1073/pnas.040568397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catteruccia F, Nolan T, Loukeris TG, Blass C, Savakis C, Kafatos FC, Crisanti A. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature. 2000;405:959–962. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]
- Zheng L, Benedict MQ, Cornel AJ, Collins FH, Kafatos FC. An integrated genetic map of the African human malaria vector mosquito, Anopheles gambiae. Genetics. 1996;143:941–952. doi: 10.1093/genetics/143.2.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Cornel AJ, Wang R, Erfle H, Voss H, Ansorge W, Kafatos FC, Collins FH. Quantitative trait loci for refractoriness of Anopheles gambiae to Plasmodium cynomolgi B. Science. 1997;276:425–428. doi: 10.1126/science.276.5311.425. [DOI] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O'Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Scott TW, Takken W, Knols BGJ, Boete C. The ecology of genetically modified mosquitoes. Science. 2002;298:117–119. doi: 10.1126/science.298.5591.117. [DOI] [PubMed] [Google Scholar]
- Pinto J, Donnelly MJ, Sousa CA, Malta-Vacas J, Gil V, Ferreira C, Petrarca V, do Rosario VE, Charlwood JD. An island within an island: genetic differentiation of Anopheles gambiae in São Tomé, West Africa, and its relevance to malaria vector control. Heredity. 2003;91:407–414. doi: 10.1038/sj.hdy.6800348. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Hawley WA, Kamau L, Fontenille D, Simard F, Collins FH. Genetic differentiation of Anopheles gambiae populations from East and West Africa: comparision of microsatellite and allozyme loci. Heredity. 1996;77:192–208. doi: 10.1038/hdy.1996.124. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Besansky NJ, Hawley WA, Fahey TG, Kamau L, Collins FH. Microgeographic structure of Anopheles gambiae in western Kenya based on mtDNA and microsatellite loci. Mol Ecol. 1997;6:243–253. doi: 10.1046/j.1365-294X.1997.00177.x. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Hawley WA, Grebert H, Danga M, Atieli F, Collins FH. The Rift Valley complex as a barrier to gene flow for Anopheles gambiae in Kenya. J Hered. 1999;90:613–621. doi: 10.1093/jhered/90.6.613. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Licht M, Elissa N, Maega BT, Chimumbwa JM, Watsenga FT, Wondji CS, Simard F, Hawley HA. Population structure of Anopheles gambiae in Africa. J Hered. 2003;94:133–147. doi: 10.1093/jhered/esg024. [DOI] [PubMed] [Google Scholar]
- Kamau L, Lehmann T, William AH, Hawley WA, Orago ASS, Collins FH. Microgeographic genetic differentiation and Anopheles gambiae mosquitoes from Asembo Bay, western Kenya: A comparison with Kilifi in coastal Kenya. Am J Trop Med Hyg. 1998;58:64–69. doi: 10.4269/ajtmh.1998.58.64. [DOI] [PubMed] [Google Scholar]
- Donnelly MJ, Simard F, Lehmann T. Evolutionary studies of malaria vectors. Trends Parasitol. 2002;18:75–80. doi: 10.1016/S1471-4922(01)02198-5. [DOI] [PubMed] [Google Scholar]
- Simard F, Fontenille D, Lehmann T, Girod R, Brutus L, Gopaul R, Dournon C, Collins FH. High amounts of genetic differentiation between populations of the malaria vector Anopheles arabiensis from West Africa and Eastern outer islands. Am J Trop Med Hyg. 1999;60:1000–1009. doi: 10.4269/ajtmh.1999.60.1000. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Sonye G, Mogi M, Githeko A, Yan G. The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. J Med Entomol. 2002;39:833–841. doi: 10.1603/0022-2585-39.6.833. [DOI] [PubMed] [Google Scholar]
- World Health Organization Manual on practical entomology in malaria Part II Methods and techniques No 13 Geneva. 1975.
- Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region) Publication of the South African Institute for Medical Research No 55. 1987.
- Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Oetting WS, Armstrong CM, Ronan SM, Young TL, Sellers TA, King RA. Multiplexed short tandem repeat polymorphisms of the Weber 8A set of markers using tailed primers and infrared fluorescence detection. Electrophoresis. 1998;19:3079–3083. doi: 10.1002/elps.1150191806. [DOI] [PubMed] [Google Scholar]
- Sharakhov IV, Braginets O, Mbogo CN, Yan G. Isolation and characterization of trinucleotide microsatellites in African malaria mosquito Anopheles funestus. Mol Ecol Notes. 2001;1:289–292. 10.1046/j.1471-8278 .2001.00114.x. [Google Scholar]
- Scanalytics, Inc Gene Profiler User's Manual, Version 1098 Fairfax, VA. 1998.
- Raymond M, Rousset F. An exact test for population differentiation. Evolution. 1995;49:1280–1283. doi: 10.1111/j.1558-5646.1995.tb04456.x. [DOI] [PubMed] [Google Scholar]
- Weir BS. Genetic Data Analysis: Methods of Discrete Population Genetic Data. Sunderland, MA: Sinauer Associates, Inc; 1990. [DOI] [PubMed] [Google Scholar]
- Goudet J. FSTAT Version 1.2: A computer program to calculate F-statistics. J Hered. 1995;86:485. [Google Scholar]
- Slatkin L. A measure of population subdivision based on microsatellite allele frequencies. Genetics. 1995;139:457–462. doi: 10.1093/genetics/139.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balloux F, Lugon-Moulin N. The estimation of population differentiation with microsatellite markers. Mol Ecol. 2002;11:155–165. doi: 10.1046/j.0962-1083.2001.01436.x. [DOI] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978;89:583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MP. Tools for population genetic analyses (TFPGA) 1.3: A windows program for the analysis of allozyme and molecular population genetic data. Computer software distributed by author. 1997.
- Rousset F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics. 1997;145:1219–1228. doi: 10.1093/genetics/145.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. The detection of disease clustering and a generalized regression approach. Cancer Research. 1967;27:209–220. [PubMed] [Google Scholar]
- Lehmann T, Hawley WA, Collins FH. An evaluation of evolutionary constraints on microsatellite loci using null alleles. Genetics. 1996;144:1155–1163. doi: 10.1093/genetics/144.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzaro GC, Zheng L, Toure YT, Traore SF, Kafatos FC, Vernick KD. Microsatellite DNA and isozyme variability in a West African population of Anopheles gambiae. Insect Mol Biol. 1995;4:105–112. doi: 10.1111/j.1365-2583.1995.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Norris DE, Shurtleff AC, Toure YT, Lanzaro GC. Microsatellite DNA polymorphism and heterozygosity among field and laboratory populations of Anopheles gambiae s.s. (Diptera: Culicidae) J Med Entomol. 2001;38:336–340. doi: 10.1603/0022-2585-38.2.336. [DOI] [PubMed] [Google Scholar]
- Wondji C, Simard F, Fontenille D. Evidence for genetic differentiation between the molecular forms M and S within the Forest chromosomal form of Anopheles gambiae in an area of sympatry. Insect Mol Biol. 2002;11:11–19. doi: 10.1046/j.0962-1075.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- Costantini C, Li SG, Della Torre A, Sagnon N, Coluzzi M, Taylor CE. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a west African Sudan savanna village. Med Vet Entomol. 1996;10:203–19. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Schellenberg A, JR, Zeiler HA, Daly RJ, Salum FM, Wilkins HA. Exposure of Gambian children to Anopheles gambiae malaria vectors in an irrigated rice production area. Med Vet Entomol. 1995;9:50–8. doi: 10.1111/j.1365-2915.1995.tb00116.x. [DOI] [PubMed] [Google Scholar]
- De Meillon B. The Anopheline of the Ethiopian Region. Publication of the South African Institute for Medical Research. 1947.
- Gillies MT, De Meillon B. The Anophelinae of Africa South of the Sahara. 2. Publications of the South African Institute for Medical Research No. 54; 1968. [Google Scholar]
- Failloux AB, Raymond M, Ung A, Chevillon C, Pasteur N. Genetic differentiation associated with commercial traffic in the Polynesian mosquito, Aedes polynesiensis Marks 1951. Biol J Linnean Soc. 1997;60:107–118. doi: 10.1006/bijl.1996.0091. [DOI] [Google Scholar]
- Julvez J, Mouchet J, Ragavoodoo C. Epidémiologie historique du paludisme dans l'archipel des Mascareignes (Océan Indien) Ann Soc Belg Med Trop. 1990;70:249–261. [PubMed] [Google Scholar]
- Julvez J, Mouchet J. Le peuplement culicidien des îles du sud-ouest de l'Océan Indien. L'action de l'homme dans l'importation des espèces d'intérêt médical. Ann Soc Entomol Fr. 1994;30:391–401. [Google Scholar]
- Minakawa N, Seda P, Yan G. Influence of host and larval distribution on the abundance of African malaria vectors in western Kenya. Am J Trop Med Hyg. 2002;67:32–38. doi: 10.4269/ajtmh.2002.67.32. [DOI] [PubMed] [Google Scholar]
- Braig HR, Yan G. The spread of genetic constructs in natural insect populations. In: Letourneau KD, Burrows BE, editor. In Genetically Engineered Organisms: Assessing Environmental and Human Health Effects. Boston: CRC Press; 2001. pp. 251–314. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A table of sample size, allelic number, heterozygosities and breeding coefficient of 13 A. gambiae populations from the Lake Victoria islands and the surrounding mainland in western Kenya.