Abstract
Pre-mRNA alternative splicing and alternative polyadenylation have been implicated to play important roles during eukaryotic gene expression. However, much remains unknown regarding the regulatory mechanisms and the interactions of these two processes in plants. Here we focus on an Arabidopsis gene OXT6 (Oxidative Tolerant-6) that has been demonstrated to encode two proteins through alternative splicing and alternative polyadenylation. Specifically, alternative polyadenylation at Intron-2 of OXT6 produces a transcript coding for AtCPSF30, an Arabidopsis ortholog of 30 kDa subunit of the Cleavage and Polyadenylation Specificity Factor. On the other hand, alternative splicing of Intron-2 generates a longer transcript encoding a protein named AtC30Y, a polypeptide including most part of AtCPSF30 and a YT521B domain. To investigate the expression outcome of OXT6 in plants, a set of mutations were constructed to alter the splicing and polyadenylation patterns of OXT6. Analysis of transgenic plants bearing these mutations by quantitative RT-PCR revealed a competition relationship between these two processes. Moreover, when both splice sites and poly(A) signals were mutated, polyadenylation became the preferred mode of OXT6 processing. These results demonstrate the interplay between alternative splicing and alternative polyadenylation, and it is their concerted actions that define a gene’s expression outcome.
Introduction
Alternative splicing (AS) and alternative polyadenylation (APA) of pre-mRNAs allow organisms to increase their coding potential and regulate gene expression. Recent studies with transcriptome-wide analyses using RNA sequencing have revealed that both AS and APA are highly pervasive in plants: at least 61% of Arabidopsis genes1 and about 48% of rice genes2 undergo alternative splicing, while 70% of Arabidopsis genes3 and around 50% of rice genes4 have at least one alternative poly(A) site. Though the mechanisms of AS and APA are less well studied in plants, several conserved cis-elements have been identified. Analysis of the 5′ and 3′ splice sites in all known introns of Arabidopsis and rice indicates that these sites are very similar to that of humans, where 5′ splice site contains a conserved GT dinucleotide and 3′ splice site contains a conserved AG dinucleotide5, 6. Meanwhile, three classes of poly(A) signals in plant pre-mRNA have been described: the near-upstream element (NUE), the far-upstream element (FUE), and the poly(A) site along with its flanking U-rich element named Cleavage Element (CE)7. Growing evidence indicates that splicing and polyadenylation interplay with each other and take place co-transcriptionally8. Previous studies have shown that mutation of the polyadenylation signal can impair the in vitro splicing of proximal but not the 3′-most exons9, while weak 5′ splice site and large intron size can promote the usage of composite exon poly(A) sites10. A recent study shows that riboswitch controls the plant gene THIC expression by splicing and alternative polyadenylation, indicating a competitive nature between these two processes11. All these results demonstrate that splicing and polyadenylation are highly coupled events, however, much remains to be learned regarding the regulatory mechanisms of AS and APA in plants, particularly the interrelationship between these two events.
The focus of this study is a gene in Arabidopsis named OXT6 (Oxidative Tolerant-6; gene locus ID At1g30460). This gene encodes two mRNAs, which produce two proteins AtCPSF30 and AtC30Y through APA and AS, respectively (Fig. 1). It is a unique phenomenon in plants since its homologue in yeast and animals lacks the AtC30Y homologue coding capacity12, thus OXT6 provides us a good model to investigate the interaction between AS and APA in plants. Intron-2 of OXT6 contains multiple poly(A) sites. When one of these sites is used, AtCPSF30 transcript is generated (Fig. 1), which encodes an Arabidopsis ortholog of 30 kDa subunit of the Cleavage and Polyadenylation Specificity Factor (CPSF), serving as a hub of an extensive network of protein-protein interactions in plant mRNA polyadenylation13. AtCPSF30 also contains a number of domains for calmodulin binding, nuclease activity, and redox sensing14, and may serve as a core to sense some environmental stimuli15. On the other hand, if alternative splicing removes Intron-2, a larger transcript AtC30Y is produced, which can be translated into a 68 kDa protein containing all but the C-terminal 13 of its 250 amino acids of AtCPSF30 and an additional domain YT521-B (Fig. 1). The function of AtC30Y is less well understood, but the domain YT521-B has been firstly reported as a member in the family of mammalian splicing-associated proteins16, 17. The oxt6 mutant, where a T-DNA insertion in the first exon of the OXT6 gene disrupts the expression of both transcriptions, displays a reduced sensitivity to oxidative stress12, a number of developmental and physiological alterations18, and some altered disease responses19, suggesting the fact that OXT6 gene is involved in plant’s survival. Interestingly, a study using high-through-put sequencing methods revealed that over 90% of the poly(A) sites in the oxt6 mutant were altered20, indicating a regulatory role of OXT6 in plant mRNA alternative polyadenylation. Taken together, these results suggest that OXT6 can involve in a number of important biological functions such as plant development, hormonal and oxidative stress responses potentially through regulating mRNA 3′ end processing. In this study we show that mutations of the splice sites and/or poly(A) signals that are critical to pre-mRNA processing can significantly change the AS and/or APA patterns of OXT6. Through the analysis of a set of mutations, a dynamic interplay among alternative splicing, alternative polyadenylation, and gene expression was revealed.
Figure 1.
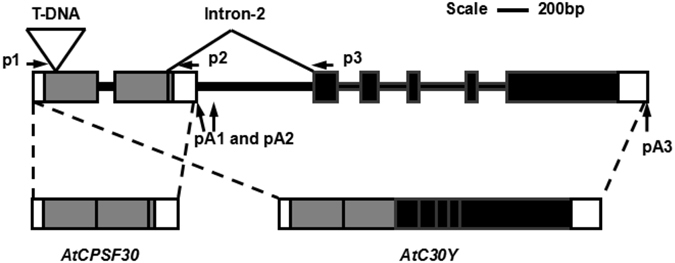
The structure of the OXT6 gene and RNA transcripts. Exons are indicated in gray or black boxes, UTRs are indicated in white boxes, and introns are indicated in thick lines. The position of the T-DNA insertion in the oxt6 mutant is indicated above Exon-1. The polyadenylation regions inside Intron-2 (pA1 and pA2) and 3′ UTR (pA3) are indicated with arrows. Positions of primers used for quantitative RT-PCR are labeled above the OXT6 gene. Primers p1 and p2 are used to detect AtCPSF30 transcript, while primers p1 and p3 are used to detect AtC30Y transcript. This figure is adapted from14 with modifications.
Results
Alternative splicing (AS) and alternative polyadenylation (APA) profiles of the OXT6 gene
To define the AS and APA sites, we first confirmed the splice sites and poly(A) signals as well as the AS and APA patterns of OXT6 gene in wild type plants. Intron-2 of OXT6 contains typical splice site, where 5′ splice site starts with GT and 3′ splice site starts with AG, as described previously14. To detect the poly(A) sites and poly(A) signals in both Intron-2 and 3′ UTR of OXT6, 3′ RACE was employed on both AtCPSF30 and AtC30Y transcripts. Two close polyadenylation regions named pA1 and pA2 were detected in Intron-2, and one more (named pA3) was detected in the 3′ UTR of OXT6 transcripts (Fig. 1 and Fig. 2). Sequencing analysis confirmed that pA1 and pA2 regions were located at approximately from 920 bp to 1050 bp of OXT6 (start from ATG), containing 10 poly(A) sites (from 23 sequenced transcripts or clones). The pA3 region is located at about from 4000 bp to 4060 bp of OXT6, containing 21 poly(A) sites (from 83 clones). All three regions contain presumed NUE signals and groups of poly(A) sites (Fig. 2b). Interestingly, 4 new poly(A) sites (from 7 clones) were detected in Exon-7 of OXT6 (Fig. 2a), which was not reported before. Taken together, both classical splice sites and poly(A) sites/signals were identified in Intron-2 and 3′ UTR of the OXT6 transcript for further testing.
Figure 2.
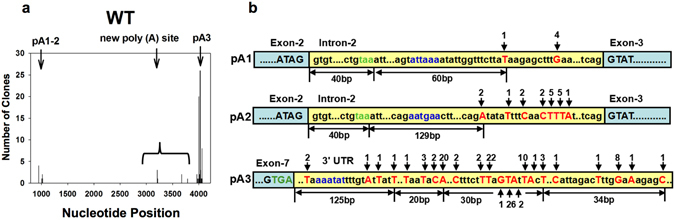
Poly(A) sites and Poly(A) signals of OXT6 in wild type plants detected by 3′ RACE. (a) Poly(A) sites detected by 3′ RACE performed with wild type plants. Nucleotide position of OXT6 gene (start from ATG) is listed at X-axis, and number of clones polyadenylated at the specific nucleotide is marked by vertical bars at Y-axis. (b) Illustrations of poly(A) sites and presumed NUE signals of pA1, pA2 and pA3 in wild type plants. Poly(A) sites [the last nucleotide before the poly(A) tail] are indicated in red; NUEs are indicated in blue; and stop codons are in green. Number of clones sequenced for each poly(A) site is labeled above or below the arrow. Intron-2 and 3′ UTR are shaded in yellow while exons are shaded in blue. Dots (…) represent omitted nucleotide sequences (not drawn in scale).
Mutations of splice sites and poly(A) signals change the profiles of AS and APA of OXT6
To test the interaction between AS and APA, the full length OXT6 gene was cloned into a vector, subsequently a set of site-directed mutations were introduced at splice sites and/or poly (A) signals as identified above. The 5′ splice site of Intron-2 was mutated from AG/gtgt to AG/atgc (lower case nucleotides indicate intron sequence, uppercase indicates exon, /indicates splice site), and the 3′ splice site was mutated from tttcag/GT to gatatc/GT (Fig. 3a). The pA1 and pA2 regions were mutated in combination (pA1-2): the presumed pA1 NUE signal were mutated from attaaa to ggcgcc, and a 36 bp of pA2 region was deleted, including the presumed pA2 NUE signal and poly(A) sites (Fig. 3a). A 78 bp of pA3 region, including presumed pA3 NUE signal and poly(A) sites, was also deleted (Fig. 3a). Several mutations were combined to generate double/triple mutations. A total of seven OXT6 mutation constructs were generated (Fig. 3b) and subcloned into the binary vector pMDC123. The latter was introduced into the Agrobacterium strain GV3101 and transformed into the oxt6 mutant background by the floral dip method to generate transgenic plants (Table 1). In addition, genomic DNA of AtCPSF30 and AtC30Y without any mutation were also introduced into the oxt6 mutant as complementary controls named as C30G and C30YG, respectively (Fig. 3b and Table 1).
Figure 3.
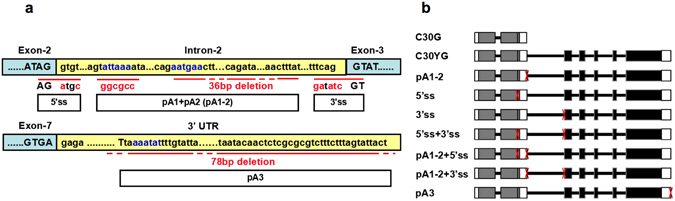
Mutagenesis of the OXT6 gene. (a) Illustrations of the 5′ss, 3′ss, pA1-2 and pA3 mutations. Intron-2 and 3′ UTR are shaded in yellow while exons are shaded in blue. Dots (…) represent omitted nucleotide sequences (not drawn in scale). The presumed NUE are indicated in blue, and the mutated nucleotides are indicated in red. (b) Illustrations of the complementary controls and seven OXT6 mutation constructs. Mutated regions are marked with red “Xs”.
Table 1.
OXT6 mutation constructs and transgenic plants used.
| Mutant name | Transgenic plants | Mutation target |
|---|---|---|
| pA1-2 | oxt6:: OXT6-pA1-2 | Poly(A) sites of AtCPSF30 in Intron-2 |
| 5′ss | oxt6:: OXT6-5′ss | 5′ splice site of Intron-2 |
| 3′ss | oxt6:: OXT6-3′ss | 3′ splice site of Intron-2 |
| 5′ss + 3′ss | oxt6:: OXT6-5′ss + 3′ss | 5′ and 3′ splice sites of Intron-2 |
| pA1-2 + 5′ss | oxt6:: OXT6-pA1-2 + 5′ss | Poly(A) sites of AtCPSF30 and 5′ splice site of Intron-2 |
| pA1-2 + 3′ss | oxt6:: OXT6-pA1-2 + 3′ss | Poly(A) sites of AtCPSF30 and 3′ splice site of Intron-2 |
| pA3 | oxt6:: OXT6-pA3 | Poly(A) site of full-length AtC30Y |
| C30G | oxt6:: C30G | No mutation |
| C30YG | oxt6:: C30YG | No mutation |
Rapid Amplification of cDNA 3′ Ends (3′ RACE) analyses performed on total RNA isolated from pA1-2 transgenic plants revealed an increased fraction of transcripts that are polyadenylated at a new poly(A) region adjacent to the pA1-2 region (Fig. 4, lane 4). This observation was confirmed by sequencing analyses of the 3′ RACE products, where 24 poly(A) sites (44 clones) were detected (Fig. 5a). 11 cryptic poly(A) sites (17 clones) were found in Intron-2 downstream of the previous pA1-2 region, 8 poly(A) sites (20 clones) were detected in pA3 region, and 3 new poly(A) sites (3 clones) were observed in a cryptic poly(A) region in Exon-7 (Fig. 5a). 3′ RACE performed on pA3 transgenic plants revealed 9 cryptic poly(A) sites (19 clones) downstream of the previous pA3 region, 13 ploy (A) sties (20 clones) in the pA1-2 region, and 3 new poly(A) sties (4 clones) in Exon-7 (Fig. 5d). These results indicated that mutations on poly(A) signals can change the poly(A) site selection on both Intron-2 and 3′ UTR of the OXT6 gene, however in most cases the AtCPSF30 and the AtC30Y transcripts can still be generated using cryptic poly(A) sites. 3′ RACE performed on 5′ss transgenic plants revealed 17 poly(A) sites, 9 of which located in the pA1-2 region (23 clones), 7 in the pA3 region (16 clones), and 1 in Exon-7 (1 clone) (Fig. 5b). Mutation on 3′ splice site results in 10 poly(A) sites in the pA1-2 region (19 clones), 7 in the pA3 region (19 clones), and 2 in Exon-7 (6 clones) (Fig. 5c). These data suggest that mutations on splice sites can also affect the poly(A) site selection on Intron-2.
Figure 4.
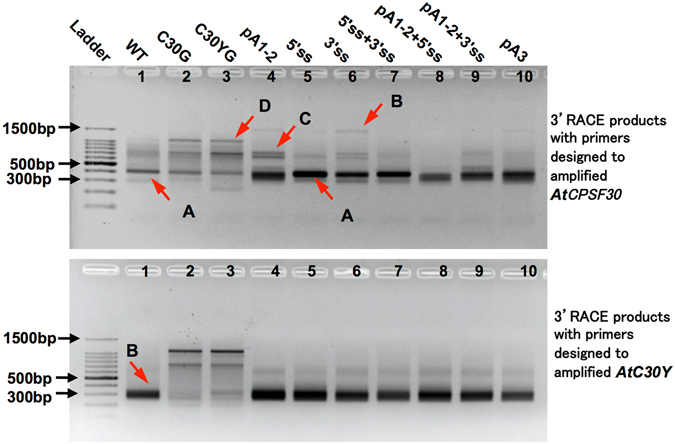
3′ RACE gel image of OXT6 gene in wild type plant, complementary controls and OXT6 mutant transgenic lines. Gel pictures of 3′ RACE performed with total RNA isolated from wild type (WT), complementary controls and OXT6 mutant transgenic lines with primers designed to amplify AtCPSF30 or AtC30Y transcript. Bands marked by A, B, C are transcripts polyadenylated at pA1-2 region, pA3 region, and a new polyadenylation region, respectively. Band marked by D is a nonspecific amplification.
Figure 5.
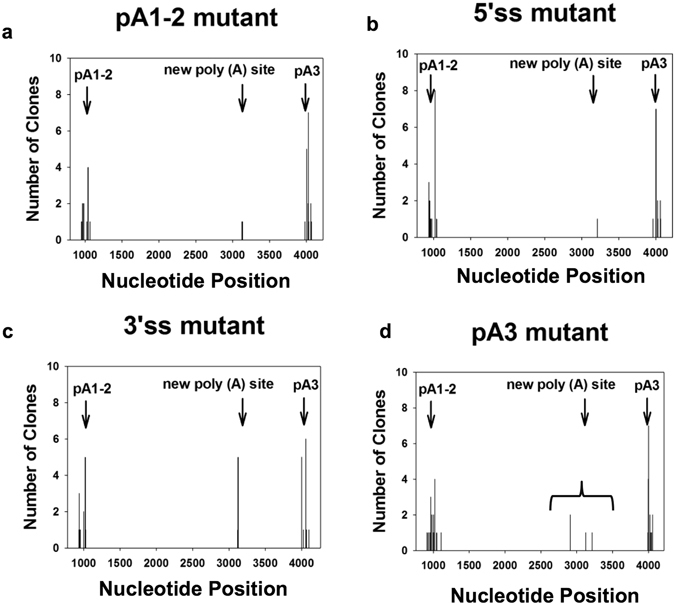
Poly(A) sites detected in OXT6 mutant transgenic lines. (a–d) Poly(A) sites detected by 3′RACE performed with pA1-2, 5′ss, 3′ss and pA3 transgenic plants. Nucleotide position of OXT6 gene (start from ATG) is listed at X-axis, and number of clones polyadenylated at the specific nucleotide is marked by vertical bars at Y-axis.
In order to detect the splicing patterns in mutant plants, total RNA of transgenic plants bearing mutations on 3′ splice site and/or 5′ splice site were isolated, reverse transcribed into cDNA, and inserted into vectors for sequencing validation. A cryptic 5′ splice site that was 73 bp upstream of the original 5′ splice site, and a cryptic 3′ splice site that was 52 bp downstream of the original 3′ splice site were identified (Fig. 6a). In transgenic plants with 5′ splice site mutation, 71.4% of 3′ splice site joint with the cryptic 5′ splice site (N = 7) (Fig. 6b), while in transgenic plants with 3′ splice site mutation, 80% of 5′ splice site joint with the cryptic 3′ splice site (N = 5) (Fig. 6b). In both cases, the open reading frame of OXT6 gene was shifted, though the splicing of the downstream intron (Intron-3) was not affected. These results indicated that mutation on splice sites could alter the splicing pattern of Intron-2; however, Intron-2 can still be removed albeit with altered exon sequences.
Figure 6.
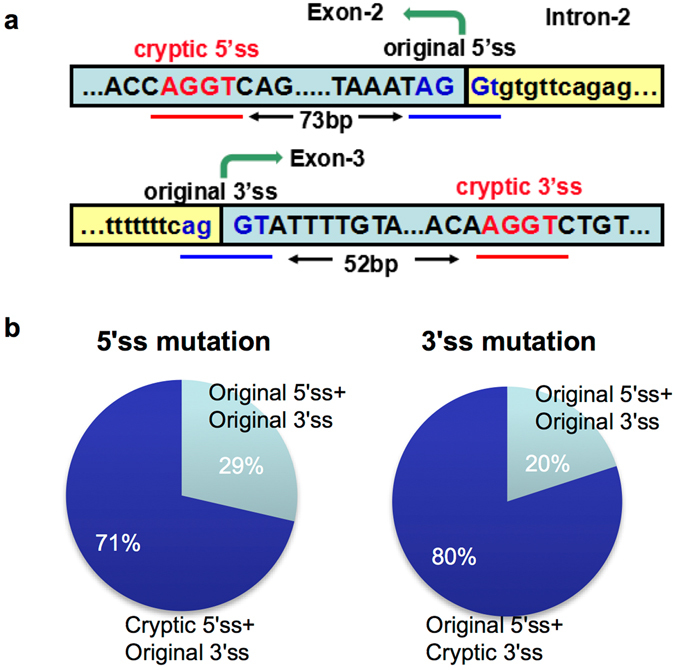
Cryptic splice sites and splicing pattern detected in oxt6 mutant transgenic lines. (a) Cryptic 5′ and 3′ splice sites of Intron-2 in OXT6 gene detected in transgenic lines with splice site mutations. Original splice sites are indicated in blue while cryptic splice sites are indicated in red. Original exons 2 and 3 are marked by green arrows. (b) Splicing pattern detected in transgenic lines with splice site mutations. N = 7 for clones with mutation in 5′ splice site (5′ss). N = 5 for clones with mutation in 3′ splice site (3′ss).
Competitive relationship between AS and APA in Intron-2 of the OXT6 gene
Since mutations on splice sites and poly(A) signals could change the splicing and polyadenylation patterns of the OXT6 gene, we then asked if these mutations were interacting with each other. Besides comparing the expression level of the two transcripts, AtCPSF30 and AtC30Y, the relationship between polyadenylation and splicing of Intron-2 could be drawn by comparing the ratio between the two transcripts. We reasoned that if the relationship between polyadenylation and splicing is competition, mutations on polyadenylation signals should promote the splicing process of Intron-2, leading to an increase of the AtC30Y transcripts and therefore increase the ratio of AtC30Y/AtCPSF30. Similarly, mutations on splice sites should stimulate the poly(A) site uses within Intron-2, generating more AtCPSF30 transcripts and decrease the ratio of AtC30Y/AtCPSF30. If the relationship between polyadenylation and splicing is cooperation, the result should be opposite.
To test these possibilities, total RNA was isolated from WT control, C30G and C30YG controls, as well as seven mutant transgenic lines (T2 generation). Expression profiles of AtCPSF30 and AtC30Y, as well as the ratio between these two transcripts were analyzed by quantitative RT-PCR (Fig. 7). Primers specifically designed to detect AtCPSF30 and AtC30Y transcripts even with the usage of cryptic poly(A) sites or cryptic splice sites were labeled in Fig. 1. Gene expression analyses revealed that in the wild type, the expression level of AtC30Y is about 3.3 fold more than that of AtCPSF30, which is consistent with our previous RNA blotting and protein immunoblot results14. Though the expression level of both AtCPSF30 and AtC30Y increased in the C30YG control, the ratio between the two transcripts (which is 4.4) was comparable with that of wild type. No AtC30Y transcript was detected in the C30G line as expected. Interestingly, mutations on poly(A) signals in Intron-2 lead to significantly increased AtC30Y expression and mildly increased AtCPSF30 expression, which rose the ratio between AtC30Y and AtCPSF30 to 23.6, indicating a promotion of splicing in the OXT6 gene processing (Fig. 7). On the contrary, mutations on both 3′ and 5′ splice sites resulted in dramatic increased AtCPSF30 expression, but the AtC30Y transcripts were almost non-detectable, suggesting a drastic switch from splicing to polyadenylation (Fig. 7). These results clearly demonstrated a competitive nature between APA and AS in Intron-2 processing. When one process was suppressed, the other became dominant and thus led to a higher expression level of the corresponding transcript.
Figure 7.
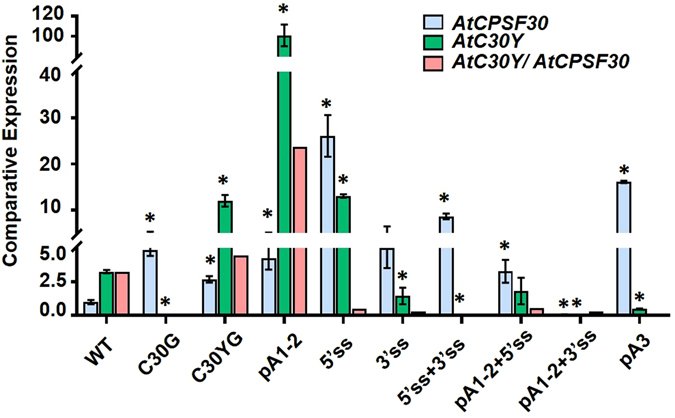
Comparative expression of AtCPSF30, AtC30Y and the ratio between these two transcripts detected by quantitative RT-PCR. The experiment was performed on at least three biological repeats from T2 generation, and representative results were shown. Bars represent means +/− SD. Two-tail t-test results are indicated by (*) with p < 0.05. The significant differences were all compared to the wild type (WT).
Interestingly, mutation on a single splice site resulted in various results. Mutation on 5′ splice site led to increased expression of both AtC30Y and AtCPSF30 transcripts, and the ratio between them was decreased to 0.5 (Fig. 7). This result suggested that both splicing and polyadenylation of Intron-2 were promoted with a weak 5′ splice site, and the processing of OXT6 gene shifted to the polyadenylation mode. On the other hand, mutation on 3′ splice site resulted in dramatically decreased expression of AtC30Y transcripts, but the expression of AtCPSF30 was not significantly changed (Fig. 7), suggesting that a weak 3′ splice site can significantly impair the splicing of Intron-2, but the process of polyadenylation was not effectively promoted. In conclusion, our data revealed a competitive relationship between APA and AS in Intron-2 processing, and suggested that weakened 5′ splice site could promote both polyadenylation and splicing events while weakened 3′ splice site majorly inhibited Intron-2 removal.
When both AS and APA signals are weak, APA dominants
Then we asked what happened if the strength of both splice sites and poly(A) signals were weakened. One possibility is that a new balance between AS and APA would be established and the transcripts ratio would be restored to wild type. However, surprisingly, in the mutants of both splice site and polyadenylation signals, a shift from AS to APA was observed (Fig. 7). The ratio between AtC30Y and AtCPSF30 decreased to 0.56 and 0.28, respectively. It is worth noting that the shift from AS to APA was consistent and comparable with that of the single splicing mutations (compare pA1-2 + 5′ss to 5′ss, or pA1-2 + 3′ss to 3′ss in Fig. 7). Particularly, when 3′ splice site was mutated, the splicing process was significantly impaired regardless of the mutation on poly(A) signals. These data suggest that, mutations on splice sites have a more severe effect than that of polyadenylation signals. In another word, when both AS and APA signals are weak, APA becomes a preferred mode.
Finally, mutations in 3′-UTR (pA3) led to inhibited expression of AtC30Y, but dramatically increased expression of AtCPSF30 (Fig. 7), suggesting a competitive relationship between two independent polyadenylation events at Intron-2 and 3′-UTR. Alternatively, due to reduced polyadenylation activity at the 3′-UTR, splicing of Intron-2 was also decreased (potentially via a feedback regulation), promoting polyadenylation at Intron-2.
Discussion
In our study we found that in the OXT6 gene, mutations on poly(A) signals in Intron-2 promoted the splicing of the intron while mutations on splice sites stimulated Intron-2 polyadenylation. When both splice site and poly(A) signal were compromised, polyadenylation became the preferred mode. This is the first paper that test the interrelationship between AS and APA in plants, and clearly suggests a competition relationship between these two processes during the OXT6 gene expression.
The expression outcome of OXT6 may be determined by several features, including the strength of the splice sites and the poly(A) signals, the presence of potential auxiliary cis-elements, as well as the stability of the two transcripts21. Intron-2 contains typical splice sites GT-AG, though no highly conserved poly(A) signals (e.g. AAUAAA) were found, some potential signals that were tested to be authentic NUE in other genes were detected. However, it has been reported that a number of cis-elements situated in both exons and introns may act as enhancers or silencers in the regulation of alternative mRNA processing21, 22. Since we only mutated splice sites and the major poly(A) signal NUE, the ratio between different OXT6 transcripts might also result from the dynamic interactions between trans-acting factors binding to the potential cis-elements. Further experimentation is required to reveal such potential signals. In addition, we cannot exclude the possibility that the usage of cryptic poly(A)/splice sites might give rise to transcripts with altered RNA stability. For example, transcripts containing premature stop codon might be eliminated by nonsense-mediated decay. However, according to our data, some transgenic lines such as pA1-2 and 5′ss showed increased expression level of both AtCPSF30 and AtC30Y transcripts (Fig. 7), which should rarely happen if the transcripts were targeted by nonsense-mediated decay. Though the stabilities of these transcripts might be altered by using cryptic poly(A)/splice sites, our results suggested that these changes did not mask the shift between AS and APA modes. To better address this issue, a half-life measurement of different OXT6 transcripts should be applied in the future.
The interplay between AS and APA may also reflect a self-regulatory expression of the OXT6 gene. Previous study shows that AtCPSF30, which is generated from APA of the OXT6 gene, serves as a hub during plant pre-mRNA polyadenylation, around which other subunits assemble into a large complex15. Therefore, the APA products of the OXT6 gene can promote the assembly of the polyadenylation complex, which might in turn stimulate the APA process and increase the expression level of AtCPSF30. On the other hand, the YT521-B domain of AtC30Y, which is generated from AS of the OXT6 gene, has been indicated to associate with splicing16, 17. Therefore, the AS product of the OXT6 gene may potentially involve in the splicing mechanism, and affect the expression level of AtC30Y itself, too. This predicated self-regulated mechanism suggests potential different mechanisms of plant mRNA polyadenylation.
In conclusion, our studies for the first time reveal the interrelationship of alternative splicing and alternative polyadenylation in plants. We demonstrated a competition relationship between splicing and polyadenylation during the OXT6 gene expression, revealed different effects on splice site and poly(A) signal mutation, and suggested a potential distinct role of the OXT6 gene in plant mRNA 3′ processing.
Materials and Methods
Isolation of the oxt6 mutant
The oxt6 mutant bearing a transfer-DNA (T-DNA) insertion in the first exon of OXT6 was identified from a collection of Arabidopsis thaliana (ecotype Columbia) from the Arabidopsis Biological Resource Center (Columbus, Ohio, USA) as previously described12.
Plasmid construction and plant transformation
The full length OXT6 gene (including 2.3 kb of the native promoter) was cloned into Gateway vector pENTR/D-TOPO (Invitrogen Inc.) to generate pENT-C30ASG. Site-directed mutagenesis was performed on the OXT6 gene with a set of oligonucleotides (Supplementary Table S1) according to the manufacturer’s instructions (QuickChange® II XL Site-Directed Mutagenesis Kit, Stratagene, Inc.). These OXT6 mutation constructs were converted into the Gateway compatible binary vector pMDC123 using the LR reaction23. The binary vectors were then introduced into the GV3101 Agrobacterium strain and transformed into oxt6 mutant background by the floral dip method as previously described24. Arabidopsis plants were grown at 24 °C in a 16 hrs light/8 hrs dark cycle. First (T1) and second (T2) generation of transgenic plants were selected on 1/1000 BASTA (ARBICO Organics, Inc.).
Detection of OXT6 polyadenylation sites
Rapid Amplification of cDNA 3′ Ends (3′ RACE) was conducted as previously described3. Total RNA was extracted from leaves of four-week wild type or T2 transgenic plants and reverse transcribed into cDNA. Two rounds of PCR analyses were performed using one reverse primer [oligo d(T) adaptor] and two forward primers (C30F-1 and C30F-2 for AtCPSF30, C30YF-1 and C30YF-2 for AtC30Y) (Supplementary Table S1). The second round PCR products were separated by 2% agarose gel electrophoresis. Bands with different sizes were cut and purified by gel extraction kit (Omega) and cloned into pGEM®-T easy vector (Promega) respectively. For each single mutant, more than 40 clones were selected for sequencing and polyadenylation sites analyses.
Gene expression analyses
Total RNA was extracted from leaves of four-week wild type or T2 transgenic plants and cDNA was synthesized from 1 μg of total RNA using SuperScript-III Reverse Transcriptase (Invitrogen Inc.) and an oligo-d(T) with adapter primer. Quantitative RT-PCR was performed on an iCycler (Bio-Rad Inc.) using SYBR green PCR master mix (Bio-Rad Inc.). The expression of TIP41-LIKE (At4g34270) was used as an internal reference gene25. For each transgenic line, at least three independent plants were examined. Primers used are listed in Supplementary Table S1.
Electronic supplementary material
Acknowledgements
The authors thank Ruqiang Xu for initial cloning of the full length OXT6 gene and David Martin for language editing. The project was made possible in part by a grant from the U.S. NSF (IOS-154173), a grant from Chinese Ministry of Science and Technology (2016YFE0108800), and funds from the Fujian 100 Talent Plan all to Q.Q.L.
Author Contributions
Q.Q.L. and Z.L. designed the experiments. Z.L., W.L., and M.L. performed the experiments and data analysis. Q.Q.L. and Z.L. wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Qingshun Q. Li and Zhaoyang Liu contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02215-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marquez Y, Brown JW, Simpson C, Barta A, Kalyna M. Transcriptome survey reveals increased complexity of the alternative splicing landscape in Arabidopsis. Genome research. 2012;22:1184–1195. doi: 10.1101/gr.134106.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu T, et al. Function annotation of the rice transcriptome at single-nucleotide resolution by RNA-seq. Genome research. 2010;20:1238–1249. doi: 10.1101/gr.106120.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, et al. Genome-wide landscape of polyadenylation in Arabidopsis provides evidence for extensive alternative polyadenylation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12533–12538. doi: 10.1073/pnas.1019732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen Y, et al. Genome level analysis of rice mRNA 3′-end processing signals and alternative polyadenylation. Nucleic acids research. 2008;36:3150–3161. doi: 10.1093/nar/gkn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy AS. Alternative splicing of pre-messenger RNAs in plants in the genomic era. Annual review of plant biology. 2007;58:267–294. doi: 10.1146/annurev.arplant.58.032806.103754. [DOI] [PubMed] [Google Scholar]
- 6.Reddy AS, Marquez Y, Kalyna M, Barta A. Complexity of the alternative splicing landscape in plants. The Plant cell. 2013;25:3657–3683. doi: 10.1105/tpc.113.117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing D, Li QQ. Alternative polyadenylation and gene expression regulation in plants. Wiley interdisciplinary reviews. RNA. 2011;2:445–458. doi: 10.1002/wrna.59. [DOI] [PubMed] [Google Scholar]
- 8.Proudfoot NJ, Furger A, Dye MJ. Integrating mRNA processing with transcription. Cell. 2002;108:501–512. doi: 10.1016/S0092-8674(02)00617-7. [DOI] [PubMed] [Google Scholar]
- 9.Niwa M, MacDonald CC, Berget SM. Are vertebrate exons scanned during splice-site selection? Nature. 1992;360:277–280. doi: 10.1038/360277a0. [DOI] [PubMed] [Google Scholar]
- 10.Tian B, Pan Z, Lee JY. Widespread mRNA polyadenylation events in introns indicate dynamic interplay between polyadenylation and splicing. Genome research. 2007;17:156–165. doi: 10.1101/gr.5532707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wachter A, et al. Riboswitch control of gene expression in plants by splicing and alternative 3′ end processing of mRNAs. Plant cell. 2007;19:3437–3450. doi: 10.1105/tpc.107.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, et al. A polyadenylation factor subunit implicated in regulating oxidative signaling in Arabidopsis thaliana. PloS one. 2008;3:e2410. doi: 10.1371/journal.pone.0002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt AG, et al. Arabidopsis mRNA polyadenylation machinery: comprehensive analysis of protein-protein interactions and gene expression profiling. BMC genomics. 2008;9:220. doi: 10.1186/1471-2164-9-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delaney KJ, et al. Calmodulin interacts with and regulates the RNA-binding activity of an Arabidopsis polyadenylation factor subunit. Plant physiology. 2006;140:1507–1521. doi: 10.1104/pp.105.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt AG. The Arabidopsis polyadenylation factor subunit CPSF30 as conceptual link between mRNA polyadenylation and cellular signaling. Current opinion in plant biology. 2014;21C:128–132. doi: 10.1016/j.pbi.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Imai Y, Matsuo N, Ogawa S, Tohyama M, Takagi T. Cloning of a gene, YT521, for a novel RNA splicing-related protein induced by hypoxia/reoxygenation. Brain research. Molecular brain research. 1998;53:33–40. doi: 10.1016/S0169-328X(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann AM, Nayler O, Schwaiger FW, Obermeier A, Stamm S. The interaction and colocalization of Sam68 with the splicing-associated factor YT521-B in nuclear dots is regulated by the Src family kinase p59(fyn) Molecular biology of the cell. 1999;10:3909–3926. doi: 10.1091/mbc.10.11.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, et al. Integration of developmental and environmental signals via a polyadenylation factor in Arabidopsis. PloS one. 2014;9:e115779. doi: 10.1371/journal.pone.0115779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruggeman Q, et al. The Polyadenylation Factor Subunit CLEAVAGE AND POLYADENYLATION SPECIFICITY FACTOR30: A Key Factor of Programmed Cell Death and a Regulator of Immunity in Arabidopsis. Plant physiology. 2014;165:732–746. doi: 10.1104/pp.114.236083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas PE, et al. Genome-wide control of polyadenylation site choice by CPSF30 in Arabidopsis. Plant cell. 2012;24:4376–4388. doi: 10.1105/tpc.112.096107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladd AN, Cooper TA. Finding signals that regulate alternative splicing in the post-genomic era. Genome biology. 2002;3:reviews0008. doi: 10.1186/gb-2002-3-11-reviews0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian B, Hu J, Zhang H, Lutz CS. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic acids research. 2005;33:201–212. doi: 10.1093/nar/gki158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brand L, et al. A versatile and reliable two-component system for tissue-specific gene induction in Arabidopsis. Plant physiology. 2006;141:1194–1204. doi: 10.1104/pp.106.081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing D, Zhao H, Xu R, Li QQ. Arabidopsis PCFS4, a homologue of yeast polyadenylation factor Pcf11p, regulates FCA alternative processing and promotes flowering time. Plant journal. 2008;54:899–910. doi: 10.1111/j.1365-313X.2008.03455.x. [DOI] [PubMed] [Google Scholar]
- 25.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant physiology. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


