Abstract
Infectious diseases caused by bacteria, viruses or fungi are among the leading causes of death worldwide. The emergence of drug-resistance mechanisms, especially among bacteria, threatens the efficacy of all current antimicrobial agents, some of them already ineffective. As a result, there is an urgent need for new antimicrobial drugs. Host defense antimicrobial peptides (HDPs) are natural occurring and well-conserved peptides of innate immunity, broadly active against Gram-negative and Gram-positive bacteria, viruses and fungi. They also are able to exert immunomodulatory and adjuvant functions by acting as chemotactic for immune cells, and inducing cytokines and chemokines secretion. Moreover, they show low propensity to elicit microbial adaptation, probably because of their non-specific mechanism of action, and are able to neutralize exotoxins and endotoxins. HDPs have the potential to be a great source of novel antimicrobial agents. The goal of this review is to provide an overview of the advances made in the development of human defensins as well as the cathelicidin LL-37 and their derivatives as antimicrobial agents against bacteria, viruses and fungi for clinical use.
Keywords: defensins, cathelicidin, antibacterial, antiviral, antifungal, infectious diseases
Naturally occurring human antimicrobial peptides represent a potential source of new and safe antimicrobial drugs to treat infections with unmet medical needs.
INTRODUCTION
Host defense antimicrobial peptides (HDPs) are key components of the innate immunity system of multicellular organisms that play an essential role in the control of microbial infections. They exhibit a broad range of antimicrobial properties and a potential ability to avoid the emergence of resistance, often observed with other antimicrobial therapies. More than 2700 HDPs have been described so far from the six Kingdoms (bacteria, archaea, protists, fungi, plants and animals; http://aps.unmc.edu/AP/main.php) (Wang, Li and Wang 2016). They can be classified in four groups: (i) α-helical peptides, (ii) peptides containing β-sheet elements, (iii) peptides combining both α and β structures and (iv) peptides free of α and β structures and unusually rich in particular amino acids such as proline, arginine, tryptophan or histidine (Hancock and Lehrer 1998; Zhang and Sunkara 2014). Among HDPs, the two major classes of mammalian antimicrobial peptides are defensins and cathelicidins, being the linear peptide LL-37 the only member of this family present in humans (Guani-Guerra et al.2010).
Defensins consist of a combination of an α-helical domain linked to a two-stranded antiparallel β-sheet domains via three disulfide bonds, while LL-37 is a lineal peptide with an amphipatic α-helical structure (Sigurdardottir et al.2006; Wilmes and Sahl 2014). They are all cationic, small (29–42 amino acids) and amphipathic peptides displaying broad-spectrum and direct and indirect antimicrobial activity against Gram-positive and Gram-negative bacteria, fungi and viruses (Guani-Guerra et al.2010; Wilson, Wiens and Smith 2013; Wilmes and Sahl 2014). Defensins can be further subdivided into α-, β- and θ-defensins based on their gene structures and conserved disulfide bridges. The α- and β-defensins are linear peptides widely distributed in vertebrate species, while θ-defensins are cyclic peptides present only in certain non-human primates (Wilson, Wiens and Smith 2013). In humans, six different α-defensins, grouped in myeloid (HNP1-4) and enteric (HD5 and HD6), and 31 β-defensins (hBD) have been described so far, although the most studied at the level of their antimicrobial potential and spectrum are hBD1-4 (Scheetz et al.2002; Schutte et al.2002; Selsted and Ouellette 2005). As for the θ-defensins, their mRNA has been found in humans, but due to the presence of premature stop codons they are not translated. However, when those stop codons are avoided, the produced peptides, called retrocyclines, also present antimicrobial activities (Cole et al.2002; Doss et al.2012; Hooven et al.2012).
Besides their antimicrobial activity, some disadvantages for the use of these HDPs have been observed such as their susceptibility to proteolysis, their low activity in physiological conditions and the high cost of their production. However, their limited availability and the high cost of manufacture have prompted the production of HDPs by different approaches (Bai et al.2013; Luan et al.2014; Maiti et al.2014; Vernieri et al.2014; Tomisawa et al.2015). Consequently, the synthesis and evaluation of HDPs-derivatives, -analogs or -mimetics have already been reported with encouraging results, regarding their antimicrobial activity and feasible synthesis (Sigurdardottir et al.2006; Doss et al.2012; Scudiero et al.2013, 2015; Varney et al.2013; Wood et al.2013; Mathew and Nagaraj 2015a,b; Olli, Nagaraj and Motukupally 2015; Sharma, Mathew and Nagaraj 2015; Tripathi et al.2015). Other additional biological functions of the HDPs have been reported, such as the immunomodulatory and adjuvant functions that can help the control of infection and inflammation and wound-healing activity (Zasloff 2002; Wilmes and Sahl 2014; Mangoni, McDermott and Zasloff 2016).
In this review, we focus on the current state of knowledge regarding human defensins, including the rhesus θ-defensins also called RTD, and the cathelicidin LL-37, in terms of their antimicrobial efficacy and spectrum against bacteria, viruses and fungi, as well as the advances made in their development as antimicrobial agents for clinical use.
DIRECT MECHANISMS OF ACTION
HDPs are components of the ancient and non-specific innate immune system. Because of their cationic and amphipathic nature, many of the mechanisms of action of these antimicrobial peptides are intrinsically associated with their interaction with the anionic microbial membranes. It is noteworthy that the reported potency and mechanisms of action of the HDPs have been obtained in most of the cases under non-physiological conditions, since their activity is drastically reduced by the presence of polyanions and divalent cations found in body fluids (Bowdish et al.2005; Mansour, Pena and Hancock 2014). This observation casts serious doubts on the potential future application of these peptides in their actual formulation. However, an in-depth knowledge of their mechanisms of action, as well as their structure–activity relationship characteristics, may help to improve their pharmacokinetics by the modification of their structures to protect them from host degradation. Below is a summarized overview of the currently accepted antibacterial, antiviral and antifungal mechanisms of action of the main human HDPs.
Antibacterial mechanisms of action
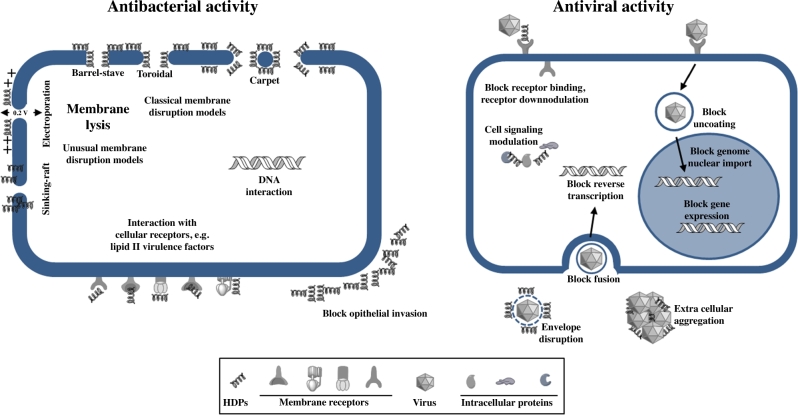
The negative charge generally presented at the bacterial cell surfaces, containing lipopolysacarides or teichoic acids among others acidic polymers, guarantees the accumulation of these cationic and hydrophobic antimicrobial peptides in both Gram-negative and Gram-positive bacteria, independently of their actual target of action. It had been proposed for many years that the permeabilization of the bacterial cell membranes was the sole mechanism of action of these peptides. However, nowadays we know that some of these peptides exert their antimicrobial effects through alternative modes of action against different bacterial targets (Table 1, Fig. 1).
Table 1.
Direct antibacterial activity of HDPs.
| HDPs | Target bacteria | Mechanisms of action | References |
|---|---|---|---|
| α-defensins | |||
| HNP1 | Clostridium difficile, Escherichia coli, Enterobacter aerogenes, Salmonella typhimurium, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Enterococcus faecalis, En. faecium, Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis, Bacillus cereus, Lysteria monocytogenes, Mycobacterium avium, M. intracellulare | Inhibit cell wall synthesis by binding to lipid II, membrane lysis | Ganz et al. (1985), Ogata et al. (1992), Turner et al. (1998), Wilmes et al. (2011), Varney et al. (2013), Furci et al. (2015) |
| HNP2 | E. coli, Enterob aerogenes, P. aeruginosa, S. aureus, B. cereus, M. avium, M. intracellulare | Membrane disruption, aggregation and fusion of vesicles | Ganz et al. (1985), Ogata et al. (1992), Ericksen et al. (2005), Pridmore et al. (2016) |
| HNP3 | E. coli, Enterob aerogenes, P. aeruginosa, S. aureus, B. cereus, M. avium, M. Intracellulare | Membrane disruption, pore formation | Ganz et al. (1985), Ogata et al. (1992), Ericksen et al. (2005) |
| HNP4 | E. coli, Enterob aerogenes, En. faecalis, S. aureus, B. cereus | Alters membrane permeabilization | Wilde et al. (1989), Ericksen et al. (2005) |
| HD5 | C. difficile, E. coli, Sa. typhimurium, En. faecalis | Membrane lysis, DNA interaction | Salzman et al. (2003), Furci et al. (2015), Mathew and Nagaraj (2015) |
| HD6 | Sa. typhimurium, Yersinia enterocolitica, Bifidobacterium adolescentes | Nanonets formation, enviroment-dependent bactericidal activity | Chu et al. (2012), Schroeder et al. (2015) |
| β-defensins | |||
| hBD1 | E. coli, Klebsiella pneumoniae, Salmonella enterica serovar Typhi, P. aeruginosa, S. aureus, Bifidobacterium spp., Lactobacillus spp. | Block epithelial invasion, membrane lysis | Schroder (1999), Kraemer et al. (2011), Schroeder et al. (2011), Maiti et al. (2014) |
| hBD2 | E. coli, P. aeruginosa, Acinetobacter baumannii, En. faecalis, En. faecium, S. aureus | Membrane lysis, disrupt sites of virulence factors | Sahl et al. (2005), Routsias et al. (2010), Kandaswamy et al. (2013), Maiti et al. (2014) |
| hBD3 | E. coli, K. pneumoniae, P. aeruginosa, S. maltophilia, A. baumannii, En. faecium, S. aureus, Streptococcus pneumoniae, Streptococcus pyogenes | Inhibit cell wall synthesis by binding to lipid II, membrane lysis | Sahl et al. (2005), Maisetta et al. (2006), Pazgier et al. (2006), Sass et al. (2010), Wilmes et al. (2011) |
| hBD4 | P. aeruginosa, A. baumannii, S. aureus, S. pneumoniae | Membrane lysis | Schneider et al. (2005), Supp et al. (2009) |
| θ-defensins | |||
| Retrocyclins | E. coli, P. aeruginosa, S. aureus, L. monocytogenes | Peptide-induced permeabilization/impairment of the cytoplasmic membrane | Cole et al. (2002), Beringer et al. (2016) |
| RTD | E. coli, P. aeruginosa, S. aureus | Membrane lysis | Tran et al. (2002), Tongaonkar et al. (2011), Tai et al. (2015), Beringer et al. (2016) |
| Cathelicidins | |||
| LL-37 | E. coli, K. pneumoniae, Sa. typhimurium, Proteus vulgaris, Haemophilus influenzae, Yersinia pestis, P. aeruginosa, S. maltophilia, A. baumannii, Enterococcus spp., Staphylococcus spp., Streptococcus spp., Bacillus spp., L. monocytogenes, Lactobacillus acidophilus, Propionibacterium acnes, Helicobacter pylori, Neisseria gonorrhoeae | Pores formation in bacterial membranes, interfering DNA transcription | Xhindoli et al. (2016) |
Figure 1.
Mechanisms of action of human defensins and cathelicidins.
As for the membrane-permeabilizing mechanism, different models of interaction have been proposed (Table 1): (i) Barrel-stave pore model, in which the HDPs form dimers or multimers that cross the membrane forming barrel-like channels (Matsuzaki et al.1991; Ben-Efraim and Shai 1997); (ii) Toroidal pore model, in which the peptide forms a monolayer by connecting the outer and the inner lipid layers in the pore (Mor and Nicolas 1994); (iii) Carpet model, where HDPs form a carpet-like structure covering the outer surface of the membrane acting like detergents disrupting the bacterial membrane (Oren and Shai 1998); (iv) other less frequent models are the sinking-raft and the molecular electroporation models, where the HDPs can either bind and sink into the structure of the membrane or create a sufficient electrostatic potential across the membrane to generate pores (Chan, Prenner and Vogel 2006; Dawson and Liu 2008).
Besides the pore formation in lipid membranes to kill bacteria, human HDPs can interact with additional targets to induce bacterial death. α-Defensin HNP1 has been proposed to target the bacterial membrane in the case of Escherichia coli; however, it exerts a different mechanism against Staphylococcus aureus through the inhibition of lipid II, a bacterial cell wall precursor (de Leeuw et al.2010). The main mechanism of action of the α-defensin HNP2 consists of the lysis of the bacterial membrane like for HNP3; however, it has also been reported for HNP2 to cause aggregation and fusion of vesicles as alternative mechanisms of actions (Ericksen et al.2005; Pridmore, Rodger and Sanderson 2016). HNP4, the less abundant α-defensin in the azurophilic granules of neutrophils, has proven to be 100 times more potent against E. coli than HNP1-3 targeting as well as the bacterial membrane (Wilde et al.1989; Ericksen et al.2005). In the same way, the α-defensin HD5, which is more efficient killing Gram-positive than Gram-negative bacteria, also presents as mechanism of action the permeabilization of the bacterial cell membrane. This subject is cause of considerable controversy, since the destabilization of model membranes has not been observed (de Leeuw et al.2010). In a recent work, Mathew and Nagaraj proposed the ability of HD5 to strongly bind DNA as the basis of its mechanism of action, suggesting that this interaction may inhibit essential processes associated with DNA replication, transcription or translation of important genes (Mathew and Nagaraj 2015). Finally, the α-defensin HD6 shows a characteristic mechanism of action in both Gram-negative and Gram-positive bacteria (Schroeder et al.2015). It has been showed that HD6 forms nanonets to capture these bacteria and to block physical contact with the epithelial cells, and therefore preventing bacterial invasion (Chu et al.2012).
As for the β-defensins, hBD1 has been proposed to act like a barrier protecting epithelial cells being infected by commensal bacteria (Schroeder et al.2011). The antibacterial activity of hBD1 seems to be lower than with other defensins; however, after reduction of its disulphide bridges, hBD1 becomes a more potent antibacterial agent (Schroeder et al.2011). Schroeder et al. found that in human skin epidermis reduced hBD1 is co-localized with thioredoxin, an ubiquitously expressed oxidoreductase, supporting the hypothesis that thioredoxin may act as a physiological mediator catalyzing reduction of hBD1 in human epithelia (Schroeder et al.2011). Moreover, another new antimicrobial activity of hBD1 is the induction of neutrophils to extrude extracellular traps that capture S. aureus in response to the presence of S. aureus-derived toxin (Kraemer et al.2011). hBD2 shares with most of HDPs the common mechanism of action, binding to the negatively charged cytoplasmic membranes, leading to a leakage of intracellular components and finally death (Sahl et al.2005). Another proposed mechanism of antimicrobial activity for hBD2, observed in Enterococcus faecalis, is the disruption at discrete foci, near nascent septal sites, of membrane-localized micro domains of secretion and sorting (Kandaswamy et al.2013). Regarding hBD3, two mechanisms of action have been described, the destabilization of the bacterial membrane and, as in the case of HNP1, the inhibition of lipid II leading to the perturbation of the biosynthesis machinery of the cell wall (Maisetta et al.2006; Sass et al.2010). For hBD4, the only mechanism of action described is the interaction with the bacterial membrane and the subsequent alteration of its integrity leading to bacterial death (Supp et al.2009). In studies carried out with S. aureus, E. coli and Pseudomonas aeruginosa, the θ-defensins, retrocyclines and RTD showed the same mechanism of action, peptide-induced permeabilization and impairment of the cytoplasmic membrane as these microorganisms (Tongaonkar et al.2011; Tai et al.2015; Beringer et al.2016). Finally, the main direct mechanisms of action proposed for the cathelicidin LL-37 have been the bacterial membrane disruption following the toroidal pore model previously commented on and the probable interference with internal targets such as DNA (Xhindoli et al.2015).
Antiviral mechanisms of action
The antiviral mechanisms of action showed by the HDPs are extremely variable and affect all the steps in the virus life cycle (Wilson, Wiens and Smith 2013) (Fig. 1). Being cationic and amphipathic peptides, the first interference is with the negatively charged lipid bilayer of encapsulated viruses. However, not all enveloped viruses are susceptible to these HDPs and their spectrum of activity can be extremely variable (Table 2). Only α-defensin HNP1 against herpes simplex virus type 1 (HSV-1) and β-defensin hBD2 against respiratory syncytial virus (RSV) have proven to act directly on the viral envelope to deactivate viruses (Daher, Selsted and Lehrer 1986; Kota et al.2008). In other cases, like HNP1or hBD2 and hBD3 against human immunodeficiency virus type 1 (HIV-1), although the binding association has been demonstrated, whether this interaction alters in some way the viral envelope has not been determined (Quinones-Mateu et al.2003; Seidel et al.2010).
Table 2.
Direct antiviral activity of HDPs.
| HDPs | Target virusa | Mechanisms of action | References |
|---|---|---|---|
| α-defensins | |||
| HNP1 | CMV, HIV-1, HSV-1/HSV-2, IAV, VSV, AAV, HAdV, HPV, PyV | Envelope disruption, block receptor binding, receptor downmodulation, extracellular aggregation, block fusion, block uncoating, block genome nuclear import, cell signaling modulation, block gene expression | Daher, Selsted and Lehrer (1986), Virella-Lowell et al. (2000), Buck et al. (2006), Hazrati et al. (2006), Salvatore et al. (2007), Dugan et al. (2008), Smith and Nemerow (2008), Doss et al. (2009), Smith et al. (2010), Wei et al. (2010), Demirkhanyan et al. (2012), Wilson, Wiens and Smith (2013) |
| HNP2 | HIV-1, HSV-1/HSV-2, IAV, AAV, HPV | Block receptor binding, receptor downmodulation, extracellular aggregation, block fusion, cell signaling modulation | Daher, Selsted and Lehrer (1986), Virella-Lowell et al. (2000), Buck et al. (2006), Hazrati et al. (2006), Salvatore et al. (2007), Doss et al. (2009) |
| HNP3 | HIV-1, HSV-1/HSV-2, IAV, HPV | Block receptor binding, receptor downmodulation, extracellular aggregation, block fusion, cell signaling modulation | Buck et al. (2006), Hazrati et al. (2006), Salvatore et al. (2007), Doss et al. (2009) |
| HNP4 | HIV-1, HSV-1/HSV-2, IAV, HPV | Block receptor binding, receptor downmodulation, cell signaling modulation | Wu et al. (2005), Hazrati et al. (2006), Salvatore et al. (2007), Doss et al. (2009) |
| HD5 | HSV-1/HSV-2, IAV, HAdV, HPV, PyV | Block receptor binding, induce viral aggregation, block uncoating, block genome nuclear import, DNA interaction, block gene expression | Buck et al. (2006), Hazrati et al. (2006), Dugan et al. (2008), Smith and Nemerow (2008), Doss et al. (2009), Smith et al. (2010), Mathew and Nagaraj (2015) |
| HD6 | HSV-1/HSV-2, IAV | Block receptor binding | Hazrati et al. (2006), Doss et al. (2009) |
| β-defensins | |||
| hBD1 | IAV, PyV | Block fusion | Doss et al. (2009) |
| hBD2 | HIV-1, HPIV-3, IAV, RSV, VZV, PyV | Envelope disruption, receptor downmodulation, block fusion, cell signaling modulation, block reverse transcription | Quinones-Mateu et al. (2003), Doss et al. (2009), Crack et al. (2012), Wilson, Wiens and Smith (2013) |
| hBD3 | HIV-1, HSV-1/HSV-2, IAV, VV | Envelope disruption, block receptor binding, receptor downmodulation, cell signaling modulation, block reverse transcription | Quinones-Mateu et al. (2003), Leikina et al. (2005), Howell, Streib and Leung (2007), Wilson, Wiens and Smith (2013) |
| hBD4 | No antiviral activity reported | – | – |
| θ-defensins | |||
| Retrocyclins | HSV-1/HSV-2, HIV-1, IAV | Block receptor binding, block fusion, induce viral aggregation | Yasin et al. (2004), Doss et al. (2009), Lehrer, Cole and Selsted (2012), Gwyer Findlay, Currie and Davidson (2013) |
| RTD | HSV-1/HSV-2 | Block receptor binding | Yasin et al. (2004), Lehrer, Cole and Selsted (2012), Gwyer Findlay, Currie and Davidson (2013) |
| Cathelicidins | |||
| LL-37 | RSV, IAV, VV, HSV-1, HAdV19, HIV-1, VZV | Envelope disruption, block receptor binding, block reverse transcription | Wong et al. (2011), Crack et al. (2012), Vandamme et al. (2012), Tripathi et al. (2013), Currie et al. (2016) |
Viruses: CMV, cytomegalovirus; HIV, human inmunodeficiency virus; HSV, herpes simplex virus; IAV, influenza A virus; VSV, vesicular stomatitis virus; AAV, adeno-associated virus; HAdV, human adenovirus; HPV, human papillomavirus; PyV, polyoma virus; HPIV, human parainfluenza virus; RSV, respiratory syncytial virus; VV, vaccinia virus; VZV, varicella zoster virus.
α- and θ-defensins HNP1-3 and HD5, RTD, retrocyclins and cathelicidin LL-37 show lectin-like activities; therefore, they are able to disrupt viral glycoprotein involved in binding and fusion of the viruses to host cells (Wang et al.2003; Wilson, Wiens and Smith 2013). HSV infection is blocked by the activity of α-, β- and θ-defensins HNP1-4, HD5, HD6, hBD3, retrocyclins, RTD and cathelicidin LL-37, mainly through the inhibition of the viral-binding and -penetration processes targeting HSV glycoprotein B (gB) and heparan sulphate (Hazrati et al.2006). Defensins HNP1-4, hBD2-3, retrocyclines and LL-37 can neutralize HIV-1 infection by blocking the viral life cycle at different steps. LL-37 has proven to block HIV-1 replication by inhibiting the reverse transcriptase activity (Bergman et al.2007; Wong et al.2011). The mentioned lectin nature of retrocyclins confers on them the ability to prevent HIV-1 entry into host cells, by blocking fusion through the binding to the viral gp120 and the cellular CD4 (cluster of differentiation 4) receptor (Munk et al.2003; Gallo et al.2006; Penberthy et al.2011). hBD2 and hBD3 inhibit HIV-1 infection in three main ways: direct inactivation of virions, modulation of the CXCR4 co-receptor and inhibiting viral replication (Quinones-Mateu et al.2003; Sun et al.2005; Weinberg, Quinones-Mateu and Lederman 2006). On the other hand, all HNP α-defensins, especially HNP1, can act in very different ways in the HIV-1 virus life cycle to block the infection. Their ability to bind the CD4 receptor and the HIV envelope protein gp120 inducing the downregulation of CD4 and CXCR4 co-receptor has been described. Moreover, recently it has been reported that the HNP1 binding to targets is necessary but not sufficient for blocking HIV-1 fusion, and an additional step, as for example defensin oligomerization, must occur to inhibit virus fusion (Demirkhanyan et al.2012). There are other mechanisms of action described for HNP1 to block HIV-1 infection, such as the blockage of internalization steps, the inhibition of gene expression, reverse transcription and integration by interfering protein kinase C (Hazrati et al.2006; Salvatore et al.2007; Doss et al.2009; Contreras et al.2012).
Influenza A virus (IAV) has been reported to be susceptible to the antiviral activity of α-, β- and θ-defensins HNP1-4, HD5-6 and hBD1-3, retrocyclins and cathelicidin LL-37 (Tripathi et al.2013; Wilson, Wiens and Smith 2013). LL-37 has been proposed to block IAV infection by disrupting the viral membrane, while α-defensins act either inhibiting the activity of the enzyme protein kinase C, necessary for IAV endosomal escape and the nuclear entry and viral trafficking, or inducing viral aggregation (Gwyer Findlay, Currie and Davidson 2013; Tripathi et al.2013). Inhibition of the virus fusion has been proposed as the mechanism of action for retrocyclines and hBDs to neutralize IAV infection (Leikina et al.2005).
Other mechanisms of action showed by HNP1-4, HD5 and LL-37 against the non-enveloped viruses, such as human papillomavirus (HPV) and human adenovirus (HAdV), have been reported. These peptides block the viral endosomal escape by the stabilization of the viral capsid (Buck et al.2006; Smith and Nemerow 2008). Moreover, HD5 and HNP1 have been proposed to induce aggregation of BK virus particles, preventing BK virus infection through inhibition of viral attachment to host cells (Dugan et al.2008; Wilson, Wiens and Smith 2013). RSV is another important virus, in terms of frequency and severity of disease, for which no optimal specific treatment exists. hBD2 and LL-37 are able to neutralize RSV through the destabilization of the membrane of the virion envelope and entry inhibition, although in this case the specific mechanism of action remains undetermined (Kota et al.2008; Currie et al.2013).
Antifungal mechanisms of action
The antifungal HDPs mechanisms of action have also been widely studied (Table 3). The α- and β-defensins have showed antifungal activity against different species of Candida such as Candida albicans, C. krusei, C. tropicalis and C. parapsilosis (Schroder and Harder 1999; Edgerton et al.2000; Joly et al.2004; Meyer et al.2004; Lu et al.2006; Vylkova et al.2006, 2007; Shi et al.2009; Song et al.2009; Schroeder et al.2011; Rizzo, Losacco and Carratelli 2013; Gacser et al.2014).
Table 3.
Direct antifungal activity of HDPs.
| HDP | Target fungi | Mechanisms of action | References |
|---|---|---|---|
| α-defensins | |||
| HNP1 | Candida albicans | Depletion of ATP | Edgerton et al. (2000), Vylkova et al. (2006) |
| HD6 | C. albicans | Block adhesion to host cells, suppression of biofilm formation | Chairatana, Chiang and Nolan (2016) |
| β-defensins | |||
| hBD1 | C. albicans | Membrane permeabilization | Vylkova et al. (2006), Vylkova et al. (2007), Krishnakumari, Rangaraj and Nagaraj (2009) |
| hBD2 | C. albicans | Binding to Ssa1/2, membrane permeabilization, depletion of ATP | Vylkova et al. (2006), Vylkova et al. (2007), Krishnakumari, Rangaraj and Nagaraj (2009), Chang et al. (2012) |
| hBD3 | C. albicans | Binding to Ssa1/2 and Xog1, membrane permeabilization, depletion of ATP | den Hertog et al. (2005), Tsai et al. (2011), Chang et al. (2012), Ordonez et al. (2014), Tsai et al. (2014), Scarsini et al. (2015), Durnas et al. (2016) |
| Cathelicidins | |||
| LL-37 | C. albicans, C. parapsilosis | Membrane lysis, genes and proteins modulation, block adhesion to host, binding to Xog1 | Sigurdardottir et al. (2006) |
The fungicidal mechanism of β-defensins has been shown to be a multifaceted process. hBD2 and hBD3 bind to Ssa1/2 proteins in the surface of C. albicans, causing membrane permeabilization (Vylkova et al.2006, 2007; Krishnakumari, Rangaraj and Nagaraj 2009). In a second step, hBD2 and hBD3 act on the energy metabolism by causing depletion of ATP and leading to cell death (Vylkova et al.2007). Alike hBD2 and hBD3, hBD1 has also been reported to cause the membrane destabilization of C. albicans leading to cell death (Krishnakumari, Rangaraj and Nagaraj 2009). Moreover, hBD3 can elevate the β-1,3-exoglucanase (Xog1) activity, resulting in reduced C. albicans adherence to abiotic surfaces (Chang et al.2012).
The fungicidal mechanism of action of α-defensins has been studied to a lesser extent than that of β-defensins. HNP-1, unlike HBD2 and HBD3, does not require C. albicans Ssa1/2 proteins for its antifungal activity but acts in the same way on the energy metabolism, causing depletion of intracellular ATP and increasing extracellular ATP concentrations to kill C. albicans (Edgerton et al.2000; Vylkova et al.2006). On the other hand, HD6 has been reported to block C. albicans adhesion to human intestinal epithelial cells and to suppress the invasion into these epithelial cells and biofilm formation (Chairatana, Chiang and Nolan 2016).
Caco-2 cells, oral and bronchial epithelial cells and Pneumocystis cells have been shown to produce basal levels of hBD2 upon induction by the presence of fungi providing a first line of protection against C. albicans, C. krusei, C. tropicalis, C. parapsilosis and Aspergillus fumigatus (Meyer et al.2004; Alekseeva et al.2009; Sun et al.2012; Gacser et al.2014). In esophagus and in engineered human oral mucosa tissues, C. albicans induce a massive upregulation of hBD2 and hBD3 but just a slightly increased expression of hBD-1 in the esophagus (Kiehne et al.2005; Steubesand et al.2009; Rouabhia et al.2011). Also, the expression of hBD2 and hBD3 by HeLa epithelial cells has been associated with the immune response effect of the probiotic strain Lactobacillus crispatus against C. albicans (Rizzo, Losacco and Carratelli 2013). Similarly, secretion of α-defensins HNP1-3 is significantly increased in whole blood and in esophagus after exposure to C. albicans (Kiehne et al.2005; Gacser et al.2014).
Cathelicidin LL-37 also shows antifungal activity against different Candida spp. (den Hertog et al.2005; Lopez-Garcia et al.2005; Ordonez et al.2014; Durnas et al.2016). Therefore, LL-37 can kill C. albicans rapidly by binding and destabilizing the cell membrane (den Hertog et al.2005; Ordonez et al.2014). It is suggested that LL-37 accumulate at high concentration near its target in the cell membrane of C. albicans, thus stimulating its killing (den Hertog et al.2005). Recently, Durnás et al. demonstrated that LL-37 causes extensive surface changes in the C. albicans cell membrane, leading to cell death (Tsai et al.2014; Durnas et al.2016). After LL-37 action, C. albicans cell membrane is fragmented into a series of adjacent vesicle-like structures of ∼100 nm, with exposed inner and outer leaflets (den Hertog et al.2005). Moreover, functional properties of the cytoplasmic membrane of C. albicans are affected, leading to the segregation of lipids and proteins, the release of vital components and the subsequent cell death (den Hertog et al.2005, 2006). Additional mechanisms of action have been described for LL-37, when small concentration of LL-37 has been used to not affect the membrane permeability and to render C. albicans vulnerable to the immune response (Ordonez et al.2014). Thus, Tsai et al. demonstrated that sub-lethal concentrations of LL-37 modulated the expression of genes involved in a variety of functions, including transport, regulation of biological processes, response to stress or chemical stimulus and pathogenesis (Tsai et al.2014).
As for the human defensins, LL-37 prevents the adhesion of C. albicans to abiotic and biotic surfaces, blocking the formation of biofilm on medical grade silicone and polystyrene (Tsai et al.2011a,b; Scarsini et al.2015). Also, LL-37 reduces the infectivity of C. albicans by inhibiting its adhesion to oral epidermoid and urinary bladder cells of female BALB/c mice (Tsai et al.2011b). This inhibitory effect on cell adhesion is mediated by its preferential binding to mannans, the main component of the C. albicans cell wall, and partially by its ability to bind chitin or glucans, which underlie the mannans layer (Tsai et al.2011b). In addition, the β-1,3-exoglucanase Xog1, responsible for C. albicans cell wall construction and maintenance, has been identified as a LL-37 receptor (Chang et al.2012). Thus, the interaction between Xog1 and LL-37 reduces Xog1 activity, interfering with cell wall remodeling and consequently impairs C. albicans adhesion (Tsai et al.2011; Chang et al.2012).
HOST-DIRECTED ANTIMICROBIAL ACTIVITY
In addition to their ability to exert direct antimicrobial activity over a broad spectrum of pathogens, human HDPs have the capacity to modulate the immune response to control infection and inflammation. Defensins as well as cathelicidin LL-37 act by regulating the production of inflammatory messengers, enhancing phagocytosis and intracellular killing of bacteria, stimulate antigen presentation and cell proliferation, induce and inhibit cytokines release and are potent chemotactic agents (Arnett and Seveau 2011; Yeung, Gellatly and Hancock 2011). Thus, hBD1 and hBD2 act as chemotactic factors for memory T cells and immature dendritic cells by direct binding to the chemokine receptor CCR6, whilst α-defensin HNP1-3 act by attracting monocytes, immature dendritic cells, and CD4+ and CD8+ T cells (Territo et al.1989; Yang et al.1999; Yang et al.2000). LL-37 has been described as inhibiting the activation of mitogen-activated protein kinases and Akt signal pathways, thus attenuating Staphylococcus aureus LTA-induced inflammatory effects by decreasing the pro-inflammatory cytokines levels in vitro (Ruan et al.2013). Moreover, LL-37 has shown its capacity to reduce endotoxin immunotoxicity through its binding to anionic molecules such as LPS (Cirioni et al.2006).
The growing interest generated by the immunomodulatory activities of these HDPs has led to the recent development of the so-called innate defense regulators (IDRs). IDRs are small and synthetic peptides derivate from natural HDPs, which conserve their immunomodulatory activity and lack direct antimicrobial activity (Scott et al.2007; Nijnik et al.2010). One of these regulators is IDR-1, a synthetic IDR constituted by 13 amino acids (KSRIVPAIPVSLL-NH2) that present protective activity against Gram-negative and Gram-positive bacteria entirely mediated by its immunomodulatory properties (Scott et al.2007; Hou et al.2013). IDR-1 has proven to have a protective effect in murine models of infection by methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE) and Salmonella enterica serovar typhimurium (Scott et al.2007; Hou et al.2013). This peptide was effective when administered locally and systemically, 48 h before to 6 h post-bacterial infection, enhancing the levels of monocyte chemokines by reducing the pro-inflammatory cytokines response, without obvious toxicities (Scott et al.2007). In the same way, an in vivo MRSA pneumonia study demonstrated the capacity of IDR-1 and LL-37 to restore pulmonary function while inhibiting inflammation and decreasing the release of inflammatory cytokines (Hou et al.2013). More recently, the novel IDR-1002 demonstrated higher in vitro and in vivo activities controlling bacterial infections compared to IDR-1(Nijnik et al.2010), with significant reductions in bacterial loads, in invasive S. aureus and Escherichia coli infection models, with IDR-1002 at a dose of 200 μg (Nijnik et al.2010). IDR-1018, the most advanced IDR synthetic peptide, and IDR-HH2 have also shown immunomodulatory activities in animal models of infection by multidrug-resistant (MDR) Mycobacterium tuberculosis, E. coli, MRSA and HSV (Wieczorek et al.2010; Steinstraesser et al.2012; Rivas-Santiago et al.2013a; Mansour, de la Fuente-Nunez and Hancock 2015). Moreover, the immunomodulatory activity of these IDRs has been also applied as co-adjuvant to improve the effectiveness of pertussis vaccine or as adjunctive therapy to treat malaria (Achtman et al.2012; Polewicz et al.2013). IDR-1018 has proven also a potent anti-biofilm activity, both preventing biofilm formation and on preformed biofilm, versus P. aeruginosa, E. coli, Acinetobacter baumannii, Klebsiella pneumoniae, MRSA, Sa. typhimurium and Burkholderia cenocepacia (Mansour, de la Fuente-Nunez and Hancock 2015; Wang et al. 2015b). The predicted mechanism of activity would involve the degradation of stress-induced nucleotides (p)ppGpp involved in biofilm formation and maintenance (de la Fuente-Nunez et al.2014).
Other immunoregulator is SGX94 (susquetide), a fully synthetic 5-amino acid peptide, analog of IDR-1, which targets the protein sequestome-1 (also called p62), a multifunctional protein involved in several cellular signaling pathways like autophagy, and in the activation of the nuclear factors kappa-B and erythroid 2-related factor 2 (Komatsu and Ichimura 2010; White 2012; North et al.2016). This peptide has demonstrated improvement of survival in murine models of infection, either by local and systemic infections and administered both prophylactically or therapeutically. Without a direct bactericidal activity, SGX94 was able to promote bacterial clearance of antibiotic-susceptible and antibiotic-resistant Gram-positive and Gram-negative bacteria (North et al.2016).
ANTIMICROBIAL ACTIVITY OF HDPs ANALOGS
Novel peptides have been designed based on the knowledge generated from the structure–activity relationship studies of natural HDPs to improve their antimicrobial activity, to differentiate their direct antimicrobial activity from their immunomodulatory effects and to decrease susceptibility to proteolytic hydrolysis by the cellular proteases. Smaller truncated peptides containing the minimal domain for activity, chimeric constructions as well as de novo designed sequences, bearing structural features that are crucial for the activity of natural peptides, have been developed and reported. Some of these analogs are active against bacteria, viruses and fungi (Tables 4, 5 and 6, respectively); thus, in this section, the different peptides are classified in the defensins and the cathelicidin LL-37 analogs.
Table 4.
Direct antibacterial activity of HDP derivatives and analogs.
| Original peptide/s | Peptide sequences | Target bacteria | References |
|---|---|---|---|
| α-defensins | |||
| HNP1 | AYRIPAIAGERRYGTIYQGRLWAF-CONH2 | Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus | Varkey and Nagaraj (2005) |
| IAGERRYGTIYQGRLWAF-CONH2 | |||
| IAAERRYATIYQARLWAF-CONH2 | |||
| HD6 | AFTHRRSYSTEYRYGTTVRGIRHRFL | E. coli, Salmonella enterica, P. aeruginosa, S. aureus | Mathew and Nagaraj (2015) |
| AFTHC2RRSYSTEYSYGTC2TV-NH2 | |||
| AFTRC2RRSYSTEYSYGTC2TV-NH2 | |||
| AFTRRRSYSTEYSYGTTV-NH2 | |||
| AFTrrrSYSTEYSYGTTV-NH2 | |||
| Myr-AFTRRRSYSTEYSYGTTV-NH2 | |||
| Myr-AFTrrrSYSTEYSYGTTV-NH2 | |||
| HD5 | ATCYCRGRCATRESLSGVCISGRLYRLCCR | E. coli, P. aeruginosa, S. aureus, Bacillus subtilis | Mathew and Nagaraj (2015), Wang et al. (2015) |
| ATYRTGRATRESLSGVEISGRLYRLR | |||
| ATYRTGRATRESLSGVEISGRLYRLR | |||
| Myr-ATYRTGRATRESLSGVEISGRLYRLR | |||
| Myr-ATYRTGRATRESLSGVEISGRLYRLR | |||
| Laur-ATYRTGRATRESLSGVEISGRLYRLR | |||
| Laur-ATYRTGRATRESLSGVEISGRLYRLR | |||
| β-defensins | |||
| hBD1/hBD3 | HYNCVSSGGQCLYSACPIFTKIQGTCYRGKAKCCK | E. coli, P. aeruginosa, Enterococcus faecalis | Scudiero et al. (2010) |
| KYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | |||
| HYNCVSSGGQCLYSACPIFTKIQGTCYRGKRKCCRRKK | |||
| KYYCRVRGGRCAVLSCPIFTKIQGTCSTRGRKCCRRKK | |||
| KYYCRVRGGRCAVLSCLPKEEQIGKCSTRGAKCCK | |||
| hBD1/hBD 3 | CPIFTKIQGTC———GG———-RRKK | E. coli, P. aeruginosa, En. faecalis | Scudiero et al. (2015) |
| hBD1/2/3 | AC3PIFTKIQGT—YRGKAK—C6K | E. coli, S. aureus | Krishnakumari, Singh and Nagaraj (2006) |
| FC3PRRYKQIGT—GLPGTK—C6K | |||
| SC3LPKEEQIGK—STRGRK—C6RRKK | |||
| hBD4 | ELDRIC1GYGTARRKKC1R | E. coli, P. aeruginosa, S. aureus | Sharma and Nagaraj (2012), Sharma, Mathew and Nagaraj (2015) |
| ELDRIGYGTARC1RKKC1R | |||
| ELDRIC1GYGTARC1RKKR | |||
| ELC1DRIC2GYGTARC1RKKC2R | |||
| ELC1C2DRIGC3YTARC2RKKRSC1C3L | |||
| KRSQEYRIGRC1PNTYAC1LKR ELDRIGYGTARRKKR | |||
| ELDRIUGYGTARURKKUR | |||
| LDRIUGYGTARURKKUR | |||
| ELDdRIGYGTAdRdRKKR | |||
| Myr-ELDRIGYGTARRKKR | |||
| Myr-ELDdRIGYGTAdRdRKKR | |||
| θ-defensins | |||
| Retrocyclin/hBD1 | AC1PIFTKIQGTYRGKAKRIGRRIC1 | E. coli, P. aeruginosa, Burkholderia cepacia, S. aureus, Staphylococcus epidermidis, Corynebacterium amycolatum | Olli, Nagaraj and Motukupally (2015) |
| Retrocyclins | GICRCICGKGICRCICGR | S. aureus, Gardnerella vaginalis | Lamers et al. (2011), Doss et al. (2012), Hooven et al. (2012) |
| CICRCIChaCICRCI | |||
| CIIRI(L)P(D)PIIIRCICILRL(L)P(D)PLILRCI | |||
| Cathelicidins | |||
| LL-37 | IGKKFKRIVKRIKKWLRKL | E. coli, Salmonella typhimurium, P. aeruginosa, Acinetobacter baumannii, S. aureus, S. epidermidis, B. subtilis | Sigurdardottir et al. (2006), Nan et al. (2012), Feng et al. (2013), Jacob et al. (2013) |
| GKEFKRIVQRIKDFLRNLVPR | |||
| KSKEKIGKEFKRIVQRIKDFLRNLVPRTES | |||
| KRIVQRIKDFLRNLVPRTES | |||
| KRIVQRIKDFLR |
Table 5.
Direct antiviral activity of HDP derivatives and analogs.
| Original peptide/s | Peptide sequences | Target virusa | References |
|---|---|---|---|
| α-defensins | |||
| HD5 | ATCYCRTGRCATRESLSGVCRISGRLYRLCCR | HSV-2, HIV-1 | Wang et al. (2013) |
| β-defensins | |||
| hBD1/hBD3 | HYNCVSSGGQCLYSACPIFTKIQGTCYRGKAKCCK KYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK HYNCVSSGGQCLYSACPIFTKIQGTCYRGKRKCCRRKK KYYCRVRGGRCAVLSCPIFTKIQGTCSTRGRKCCRRKK KYYCRVRGGRCAVLSCLPKEEQIGKCSTRGAKCCK | HSV-1 | Scudiero et al. (2010) |
| hBD1/hBD3 | KYYCRVRGGRCAVLSCPIFTKIQGTCSTRGRKCCRRKK | HSV-1 | Scudiero et al. (2013) |
| hBD1/hBD3 | CPIFTKIQGTC———GG———-RRKK | HSV-1 | Scudiero et al. (2015) |
| θ-defensins | |||
| Retrocyclins | GICRCICGKGICRCICGR | IAV, HIV | Doss et al. (2009), Doss et al. (2012), Wood et al. (2013) |
| ICRLIL(D)PPLRLIC | |||
| Cathelicidins | |||
| LL-37 | GIKEFKRIVQRIKDFLRNLVFKRIVQRIKDFLR | IAV, RSV, HIV | Wang, Watson and Buckheit (2008), Currie et al. (2013), Tripathi et al. (2015) |
| GIKEFKRIVQRIKDFLRNLV KIGKEFKRIVQRIKDFLRNLVP |
Viruses: HSV, herpes simplex virus; HIV, human inmunodeficiency virus; IAV, influenza A virus; RSV, respiratory syncytial virus.
Table 6.
Direct antifungal activity of HDPs derivatives and analogs.
| Original peptide/s | Peptide sequences | Target fungi | References |
|---|---|---|---|
| α-defensins | |||
| HNP2 | RRC1YC2RIPAC3IAGERRYGTC2IYQGRLWAFC3C1RR | Candida albicans | Raj, Antonyraj and Karunakaran (2000) |
| hBD1 | AC1PIFTKIQGTYRGRAKC1R AC1PDIFTKDIQGTYDRGDRAKC1R | C. albicans | Olli, Rangaraj and Nagaraj (2013) |
| β-defensins | |||
| hBD3 | SC1LPKEEQIGKSTRGRKC1RRKK SC1LPKEEQDIGKSTDRGDRKC1DR DRKR GKCSTRGRKCCRRKK | C. albicans | Olli, Rangaraj and Nagaraj (2013), Lim et al. (2016) |
| hBD4 | ELC1C2DRIGC3YTARC2RKKRSC1C3L | C. albicans | Sharma and Nagaraj (2012) |
| Cathelicidins | |||
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIK LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNL GKEFKRIVQRIKDFLRNLVPR | C. albicans, C. parapsilosis | Sigurdardottir et al. (2006) |
Defensins analogs
Different α-defensin analogs have been generated and evaluated in vitro. Derivatives of HNP1 without cysteines have been proved to maintain certain antibacterial activity (lethal concentrations ranging 1–23 μM), although at significantly higher concentrations than those observed with the natural peptide (0.8–1 μM), against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus (Varkey and Nagaraj 2005). The insertions of two basic residues, such as arginine, at both the N- and the C-terminal regions of HNP2 have showed to increase its polarity and cationic charge, and it has been reported to significantly enhance the antifungal activity against Candida albicans. In contrast, the addition of anionic residues, such as aspartic acid, at the N- and C-terminal fragments renders a totally inactive molecule (Raj, Antonyraj and Karunakaran 2000). Lineal analogs of HD5 and HD6 have also demonstrated an improved antibacterial activity against Gram-negative and Gram-positive bacteria, with the same concentrations range (2.5–12.5 μM) of the original peptides (Mathew and Nagaraj 2015a,b; Wang et al.2015). Mathew and Nagaraj demonstrated that the antimicrobial activity of α-defensin could be improved by the fatty acylation of the linear peptides, and that amino acids replacements and covalent modifications in selected regions of HD6 could generate antimicrobial peptides from the apparently inactive HD6 (Mathew and Nagaraj 2015a,b).
Regarding the β-defensins, several analogs of hBD1 and hBD3 have also showed promising results (Krishnakumari, Singh and Nagaraj 2006; Scudiero et al.2010, 2013). Krishnakumari et al. generated shorter peptides with a single disulfide bridge which had in vitro antimicrobial activity at the same range than their parental defensins (MIC, 15–23 μM) against E. coli and S. aureus (Sahl et al.2005; Krishnakumari, Singh and Nagaraj 2006). Another set of hBD1 and hBD3 analogs were generated by Scudiero et al. comprising different domain of these defensins (Scudiero et al.2010). These derivatives showed improved antibacterial activity against Enterococcus faecalis, E. coli and P. aeruginosa with MICs values in the range of 2.5–12.5 μM even at high NaCl concentrations, when it is well known that most of these HDPs are salt-sensitive (Scudiero et al.2010, 2013). These new hBD1/3 derivatives also showed enhanced activity against HSV-1, inhibiting its infectivity at concentrations of 50 μM and, again, some of them showed these antimicrobial activities in presence of high NaCl concentrations (Scudiero et al.2010, 2013). Scudiero et al. also proposed the internal region of hBD1 and the C-terminal region of hBD3 as being responsible for the antimicrobial activity of these peptides and so they designed an analog combining these two domains (Scudiero et al.2015). This novel peptide maintained the same antimicrobial activity against P. aeruginosa, E. coli and En. faecalis as well as against HSV-1, enhancing the stability in serum with respect to the parent defensins (Scudiero et al.2015).
The antimicrobial activity of hBD4 analogs has also been investigated, synthesizing shorter sequences spanning the N-terminal region of hBD4 and containing 1–3 disulfide bridges (Sharma and Nagaraj 2012). These new peptides showed enhanced antimicrobial activity against E. coli, P. aeruginosa and S. aureus compared to hBD4, which was higher as they kept the three disulfide bridges and decrease with a high concentration of NaCl. A new batch of derivatives was designed subsequently in an attempt to obtain antimicrobial active peptides from linearized segments of the N-terminal region of hBD4 (Sharma, Mathew and Nagaraj 2015). They showed that the introduction of α-aminoisobutyric acid (a helix-inducing amino acid) instead of cysteine did not favor antibacterial activity, while it improved antifungal activity (Sharma, Mathew and Nagaraj 2015). They found antibacterial activity when substituted L-arginine with D-arginine in the linear inactive peptide and activity was increased in the fatty acylated peptides (Sharma, Mathew and Nagaraj 2015).
C-terminal analogs of human hBD1 and hBD3, wherein lysines were selectively replaced by L- and D-arginines and L-isoleucine substituted with its D-enantiomer, exhibited antifungal activities by impairing C. albicans membrane permeability (Olli, Rangaraj and Nagaraj 2013). When hBD3 was shortened to only 15 amino acid at the C-terminal, higher activity against C. albicans was observed (Lim et al.2016). In contrast, single disulfide peptides spanning the C-terminal regions of hBD1, hBD2 and hBD3, called Phd1, Phd2 and Phd3, respectively, showed lower antifungal activity than their respective precursors against C. albicans (Krishnakumari, Rangaraj and Nagaraj 2009). Other analogs of hBD3 have been synthetized with native disulfide linkages, with non-native linkages or with replacement of all cysteine residues by aminobutyric acid creating linear peptides. Any of these peptides improved the antifungal activity of hBD3 against C. albicans (Hoover et al.2003). The introduction of three disulfide bridges in the N-terminal segment of hBD4 enhanced its antifungal activity by entering rapidly inside C. albicans without disrupting the membrane integrity (Sharma and Nagaraj 2012).
The C-terminal region of hBD1 containing a single disulfide bridge was responsible for the antibacterial activity of the peptide and was combined with the RIGRRIC region of retrocyclin in order to improve its antimicrobial activity (Olli, Nagaraj and Motukupally 2015). This chimeric defensin presented antibacterial activity at concentrations under 5 μM against wild-type strains of E. coli, P. aeruginosa and S. aureus and against MDR clinical strains of Corynebacterium amycolatum, S. aureus, S. epidermidis and P. aeruginosa (Olli, Nagaraj and Motukupally 2015). This hydrid peptide also showed antimicrobial activity with or without the disulfide bridge and, furthermore, it retained the activity in the presence of serum and NaCl, and it was not hemolytic in vitro. Moreover, it had valuable pharmacokinetics characteristics for the development of new therapeutic molecules (Olli, Nagaraj and Motukupally 2015).
Retrocylins, θ-defensins, have also been used to design and synthesize new and more potent antimicrobial peptides. The retrocyclin analog RC-101 has showed significant activity against clinical isolates of S. aureus at concentrations as low as 5 μM (Lamers et al.2011). As an antiviral agent, it has demonstrated exerting significantly more potent antiviral activity against IAV and HIV than retrocyclin-1, even presenting a very conservative modification in respect to this one (Arg → Lys) (Owen et al.2004; Doss et al.2009). RC-101 also proved to be active against the enfuvirtide-resistant HIV mutants V38A and V38+N126K, which highlighted its potential clinical importance as a fusion inhibitor (Wood et al.2013). In fact, RC-101 is being developed as an intravaginal microbicide to prevent sexually transmitted HIV-1 and bacterial vaginosis by Gardnerella vaginalis (Hooven et al.2012; Eade et al.2013).
Other examples of retrocyclin derivatives are hapivirins and diprovirins, cyclic peptides that incorporate key cationic and hydrophobic residues substitutions and synthetic amino acids (Doss et al.2012). These peptides were designed with the purpose of simplifying the structure of retrocyclins, making them easier to synthesize while improving or at least maintaining their antiviral activity against IAV. As a result, they obtained some variants of each derivative with great anti-IAV potency, showing significant reductions of viral load at concentrations ranging 0.4–3 μg mL−1, in the same range as retrocyclin 2 or RC-101 (Doss et al.2012). Those derivatives that showed the most potent antiviral activity were also tested for their potential antibacterial and antifungal activity. They found that diprovirins caused a much stronger inhibition of S. aureus and C. albicans growth than hapivirins, with in vitro activity concentrations between 1.25 and 20 μg mL−1, which highlighted their potential broad-spectrum activity (Doss et al.2012).
Cathelicidin LL-37 analogs
A number of shorter cathelicidin LL-37 variants have been generated during the last few years in order to improve its antimicrobial activity and reduce its toxicity (Wang, Watson and Buckheit 2008; Feng et al.2013; Jacob et al.2013; Tripathi et al.2015). The 21-amino acid fragment GKE displayed similar or higher antimicrobial activity against E. coli, P. aeruginosa, S. aureus, C. albicans and C. parapsilosis than the whole LL-37 at concentrations ranging 0.5–100 μM (Sigurdardottir et al.2006). GKE and LL-37 showed antimicrobial activity against E. coli independently of the physiological concentration of NaCl (±150 mM) and the presence of serum (99%) (Sigurdardottir et al.2006). Unlike LL-37, GKE was less toxic, showing a diminished hemolytic activity and lesser DNA-damage induction (Sigurdardottir et al.2006). KR-12 (residues 18–29) is the shorter fragment of LL-37 that has showed antimicrobial activity against for example, MDR Acinetobacter baumannii strains (Wang, Watson and Buckheit 2008; Feng et al.2013; Tripathi et al.2015). A series of KR-12 analogs have been designed showing improvements regarding its antimicrobial and antiendotoxic activities. Some of these analogs presented MICs of 4 μM against MRSA isolates (Jacob et al.2013). KS-30 and KR-20, other shorter fragments of LL-37, were also reported to exert antibacterial activity against MDR A. baumannii with MICs of 8 and 16 μg mL−1, respectively, and also to prevent biofilm formation (Feng et al.2013). Nan et al. generated another batch of analogs consisting in a series of Lys- and/or Leu-substituted derivatives based on the internal region 13–31 of LL-37 (IG-19) (Nan et al.2012). They found some of these IG-19 variants to present great in vitro antibacterial activity against both Gram-negative and Gram-positive bacteria, with MICs ranging 2–8 μM against E. coli, P. aeruginosa, Sa. typhimurium, Bacillus subtilis, S. epidermidis and S. aureus (Nan et al.2012).
Smaller derivatives of LL-37 have also showed antiviral activity, essentially against IAV, HIV and RSV (Wang, Watson and Buckheit 2008; Currie et al.2013; Tripathi et al.2013, 2015). The variant GI-20 presented greater anti-HIV activity than LL-37 with less toxicity, thus showing an improved therapeutic index (Wang, Watson and Buckheit 2008). This variant GI-20 also showed a notably anti-IAV activity (Tripathi et al.2015); the same authors have reported that both LL-37 and the fragment GI-20 had similar neutralizing activity against IAV but, surprisingly, against the influenza A(H1N1)pdm09 strain, LL-37 reduced its activity while GI-20 retained it (Tripathi et al.2015). RSV has been also showed to be susceptible to a central fragment of LL-37 (amino acids 12–33); this central fragment exerts an anti-RSV activity similar to that of the parental peptide at concentrations ranging 1–25 μg mL−1 (Currie et al.2013).
Similarly for defensins, a number of shorter variants have been generated during the last years in order to improve LL-37 antifungal activity. Truncated variants LL-25 and LL-31 have presented better antifungal activity than LL-37 due to their longer accumulation in the cell membrane of C. albicans (den Hertog et al.2006; Rapala-Kozik et al.2015). LL-25 also lacks the immunomodulatory properties of LL-37 (Rapala-Kozik et al.2015).
CLINICAL AND EXPERIMENTAL THERAPEUTIC STUDIES
Because of their small size and relatively simple structure, many HDPs have been produced and investigated in vivo in animal models of infection with promising results. In addition to their features as a novel generation of therapeutic agents, HDPs have also showed their utility as adjuvants agents in combination with classical antimicrobial drugs, neutralizing endotoxins and reducing mortality in animal model of infections (Zasloff 2002; Gordon, Romanowski and McDermott 2005; Mygind et al.2005). However, only very few of them have progressed to early clinical development and to the market (Zhang and Falla 2006). A few cationic peptides have been tested in clinical-efficacy trials, for indications such as skin infections and nasal carriers by MRSA (LTX-109), Clostridium difficile infections (LFF551 and surotomycin), oral candidiasis (PAC113), impetigo and diabetic foot ulcers and infections (Pexiganan), oral mucositis and ventilator-associated pneumonia (Iseganan), sepsis (Neuprex), catheter-associated infections (Omiganan), dental caries, targeting Streptococcus mutans (C16G2, a synthetic antimicrobial targeted peptide) or wound healing, among others (Zasloff 2002; Hancock and Sahl 2006; Eckert, Sullivan and Shi 2012; Thakur, Qureshi and Kumar 2012; Fox 2013; Mangoni, McDermott and Zasloff 2016).
Three major factors still prevent their clinical development and widespread use: stability, toxicity and cost (van 't Hof et al.2001). Some of these HDPs are salt-sensitive or serum-sensitive, which reduce significantly their antimicrobial activity and represent a serious problem for their systemic and topical uses. HDPs are relatively susceptible to proteolytic degradation. Upon systemic administration they are cleared quickly due to proteolytic degradation showing poor pharmacokinetics, all of which restrict their potential application (van 't Hof et al.2001). Thus, a number of different strategies have been applied to circumvent their reduced half-life, such as different ways of administration or chemical modifications in the peptides: introduction of D-amino acids, amidation at the N-terminus, design of peptidomimetics and introduction of synthetic aminoacids or peptic cyclation (Wiley and Rich 1993; Adessi and Soto 2002; Chongsiriwatana and Barron 2010).
This section is mainly devoted to providing a review of the in vivo direct-antimicrobial activity in animal models of infection of defensins and cathelicidin LL-37 and also a subsection for the revision of peptides used to treat infections indirectly by modulating the immune response.
Experimental in vivo defensins studies
Antibacterial studies
As experimental therapeutics, HDPs have been used in a number of animal models of infection. The efficacy of bacterium-derived recombinant human β-defensins hBD1-2 was tested in a murine model of Salmonella infection (Maiti et al.2014). Mice of all treatment groups were challenged intraperitoneally (i.p.) with an inoculum of Salmonella enterica serotype typhi containing 103 CFU, followed by i.p. administration of hBD1 (100 μg), hBD2 (100 μg) or a combination of hBD1/2 (2:1, 66 μg of hBD1 and 33 μg of hBD2; 1:1, 50 μg each). Mice mortality was monitored during 10 days to evaluate the efficacies of these therapeutic molecules. The authors concluded that the administration of these defensins, both alone and in combination, increased mice survival and decreased Sa. typhimurium load in peritoneal fluid, liver and spleen (Maiti et al.2014). Similar results were reported when the hBD1-2 combination was expressed and extracted from tobacco plants (Patro et al.2015).
Another study has evaluated the therapeutic efficacy of RTD-1 in a murine P. aeruginosa chronic lung infection model (Beringer et al.2016); nebulized treatment with RTD-1 (167 μg kg−1) resulting in a significant decreased in the P. aeruginosa load in lungs, as well as airway leucocytes and weight loss compared with untreated controls (Beringer et al.2016). RTD-1 was also evaluated in two animal models of peritoneal infection. An Escherichia coli murine peritonitis model was induced by a single i.p. injection of 8 × 108 CFU of log-phase E. coli K12 strain, and mice were treated immediately with a single subcutaneous injection of RTD-1 (5 mg kg−1), and monitored for 28 days (Schaal et al.2012); RTD-1 significantly increased mice survival in 30% after 22 days of bacterial challenge (Schaal et al.2012). In the same work, they performed a cecal-ligation/puncture (CLP) induced sepsis model, in which polymicrobial peritonitis is induced (Eskandari et al.1992), and 4 h following CLP surgery each animal received normal saline (controls) or 5 mg kg−1 of RTD-1 intravenously (i.v.); moreover, a separate group of mice was treated with RTD-1 (5 mg kg−1, i.v.), but the single administration was delayed 24 h after CLP surgery; in both experiments, animals were evaluated daily for 28 days (Schaal et al.2012). A single dose of RTD-1 significantly improved survival (80%) of mice infected i.p. with E. coli. Moreover, a single intravenous dose of RTD-1 administered 4 h after CLP surgery resulted in long-term survival (90%) by day 22 post-surgery (Schaal et al.2012). They also found that escalating doses of RTD-1 from 0.2 to 3.0 mg kg−1 over 14 days administered i.v. to two chimpanzees resulted in no adverse clinical effects, no detection of anti-RTD-1 antibodies, and normal metabolic panels and blood counts during the 60 days of the study (Schaal et al.2012).
Antiviral studies
A recombinant mouse β-defensin 3 proved also in vivo antiviral activity in a murine model of infection with a mouse-adapted IAV (Jiang et al.2012). Mice were challenged intranasally with a dose of 10× the lethal dose 50 (LD50) and were assigned to different treatment regimens. Therapy began 12 h after infection and was performed once a day for 3 weeks. The most effective regimen improved mice survival to 83.3% when the peptide was administered i.v. at a concentration of 10 mg kg−1 per day 3 weeks post-virus challenge (Jiang et al.2012).
HD5 and its derivatives have been also evaluated in a lethal murine model of HSV-2 (Wang et al.2013). All the mice were pretreated with progesterone subcutaneously (2 mg) and 5 days later each mouse was inoculated with a lethal dose of HSV-2 (105 PFU). Prior to or after viral inoculation, mice received an intravaginal instillation of the peptide HD5 or E21R-HD5 (HD5 derivative carrying arginine instead of glutamic acid at position 21) solutions (10 mg mL−1) as a prophylactic or therapeutic administration, while control mice received PBS alone (Wang et al.2013). Both HD5 and E21R-HD5 showed strong anti-HSV-2 activity either as prophylactic or therapeutic use, increasing the survival rates (60% and 70%, respectively) compared to non-treated controls (10%) (Wang et al.2013).
Experimental in vivo cathelicidin studies
Antibacterial studies
Cathelicidin LL-37 showed promising therapeutic effects in an experimental model of progressive pulmonary tuberculosis in mice infected by endotracheal instillation with 2.5 × 105 CFU of Mycobacterium tuberculosis, and treated by intratracheal instillation with LL-37 60 days post-bacterial challenge three times a week at dose of 1 mg kg−1 (Rivas-Santiago et al.2013). LL-37 generated significant 3–10-fold reductions of M. tuberculosis load in lungs, both against a drug-susceptible strain and a MDR strain, after 28–30 days of treatment.
In another study, a single systemic administration of LL-37 (1 mg kg−1) showed protection against lethal bacteremia in three different sepsis rat models by E. coli, two rat models of intraperitoneal infection and a CLP model, without differences among those treated with LL-37 or polymixin B (1 mg kg−1) (Cirioni et al.2006).
Antiviral studies
In a recent work by Currie et al., they demonstrated the antiviral activity of LL-37 in a murine model of pulmonary RSV infection (Currie et al.2016). Mice received 5.6 × 105 PFU of RSV by intranasal delivery and were treated intranasally with 10 μg per mouse of LL-37 in three different groups, either administered 1 h before infection, at the same time of the infection or 1 h post-infection, and further with a daily dose of 10 μg per mouse until day 6 post-infection. Concomitant delivery of LL-37 showed significant protective effects on weight loss and viral load while neither pretreatment nor delayed application of the peptide achieved protective effects (Currie et al.2016). These results may be explained because of the necessity of the pulmonary co-localization of LL-37 with RSV to exert its direct antiviral activity. Barlow et al. demonstrated that LL-37 (nebulized 1 day prior to and for 7 days post-infection with 10x LD50) provided significant protection against a lethal infection with influenza A(H1N1)/Puerto Rico/8/1934 by improving the animal survival rate and avoiding the weight loss at the same level as the well characterized influenza antiviral zanamivir (Barlow et al.2011).
Experimental in vivo studies based on HDPs immunoregulatory activities
Many HDPs have been evaluated in animal models of infection, and they have demonstrated improvement in infection outcomes, the recruitment of immune cells to the site of the infection or to influence the secretion of pro-inflammatory and anti-inflammatory cytokines by the modulation of the immune system (Zasloff 2002; Scott et al.2007; Steinstraesser et al.2008).
IDR-1 was evaluated in MRSA and VRE animal models of infection with satisfactory results in both cases (Scott et al.2007; Hou et al.2013). The peptide was effective reducing bacterial counts and mortality at concentrations ranging from 8 to 24 mg kg−1, and using different routes of administration in the thigh and intraperitoneal models (Scott et al.2007). In this case, the immunomodulatory activity of IDR-1 was related to the recruitment of monocytes and macrophages to the site of the infection, and also with the decrease of pro-inflammatory (TNF-α and IL-6) and the increase of anti-inflammatory (IL-10) cytokines, a perfect combination to control infections and inflammation at the same time (Scott et al.2007). In case of the intratracheal-induced MRSA murine pneumonia model, IDR-1 and LL-37 also showed a decrease in the production of TNF-α and IL-6 in bronchoalveolar lavage fluid and serum, and they could trigger the recruitment of lymphocytes to the site of the infection (Hou et al.2013). Moreover, both peptides showed a significant amelioration of the induced pneumonia according to the histopathology data, with the best result obtained at concentration of 0.8 mg kg−1 for LL-37 and 0.6 mg kg−1 for IDR-1 (Hou et al.2013). Another IDR peptide, IDR-1002, has shown an enhanced chemokine induction activity compared to IDR-1 that was confirmed in invasive S. aureus and E. coli infection models (Nijnik et al.2010). The i.p. administration of IDR-1002 at concentrations ranging from 20 to 100 μg mL−1 demonstrated stronger chemokine induction and protection than IDR-1, enhancing the recruitment of neutrophils and monocytes, although without increasing the levels of pro-inflammatory cytokines IL-6 and TNF-α (Nijnik et al.2010).
The immunomodulatory activities of IDR-1018 have been demonstrated to be effective reducing soft tissue infections and disrupting bacterial biofilm formation (Achtman et al.2012; Rivas-Santiago et al.2013; Mansour, de la Fuente-Nunez and Hancock 2015). It has proven together with IDR-HH2 to be effective reducing bacterial loads in a murine pneumonia model by M. tuberculosis leading to a reduction in lung inflammation (Rivas-Santiago et al.2013). It has also been effective in the treatment of S. aureus infections in several animal models like the murine model of implant infection or the murine and porcine wound healing models (Zasloff 2002; Steinstraesser et al.2012; Choe et al.2015). The mechanisms of action of IDR-1018 involve the downregulation of inflammatory cytokine expression (e.g. TNF-α) and upregulation of genes involved in the erythropoiesis, as well as reduction of pro-inflammatory mediators such as nitric oxide synthase or the regulation of chemokines and cytokines production by the modulation of macrophages and neutrophils differentiation and activation (Achtman et al.2012; Choe et al.2015).
As previously mentioned, the immunomodulatory activity of the synthetic peptide SGX94 has been evaluated in different animal models of infection and also in a Phase 1 and Phase 2 clinical studies (North et al.2016). Its safety and effectivity in clearing bacterial infections has been assayed in mice and rats, in models of peritoneal, skin, gastrointestinal and lung infections, against methicillin-sensitive S. aureus, MRSA, P. aeruginosa and antibiotic-resistant Burcholderia pseudomallei (North et al.2016). Despite the absence of direct antibacterial activity, SGX94 showed significant broad-spectrum antibacterial activity, sustained even in the absence of a continued exposure to the peptide (North et al.2016). Concentrations ranging 5 to 50 mg kg -1 were found to be efficacious controlling the infections whether administered therapeutically or prophylactically (North et al.2016). Importantly, and as it was previously described for IDR-1, the activity of SGX94 was not dependent on immunosuppression (Scott et al.2007). In clinical trials, Phase 1 and Phase 2 studies showed that SGX94 was involved in the modulation of pro- and anti-inflammatory cytokine levels, reducing the damage produced as a consequence of innate immune activation and, consequently, the number of registered infections was reduced in patients treated with SGX94 (Kudrimoti et al.2016; North et al.2016).
To date, in vivo studies of infection to evaluate the therapeutic efficacy of IG-19, a peptide derived from the LL-37, to control bacterial or virus infections have not been carried out. However, the immunomodulatory properties of this peptide in a murine model of collagen-induced arthritis have been demonstrated (Chow et al.2014); mice injected subcutaneously with IG-19 at 6 mg kg−1 per mouse every 48 h for 5 weeks did not show any significant adverse effect. More importantly, IG-19 showed a significant reduction on pro-inflammatory cytokine levels in serum, which together with its direct antibacterial activity makes it a candidate for future studies to evaluate its potential effect to control infections in vivo (Chow et al.2014).
PRODUCTION OF HDPs FOR BIOLOGICAL AND CLINICAL APPLICATIONS
The fascinating characteristics of these HDPs may prompt the development of a novel generation of antimicrobial drugs in the near future, with wider spectrum of activity, novel targets and mechanisms of action and less propensity for emergence of microbial resistances. However, there are some obstacles that should be first overcome regarding the optimization of their pharmacokinetic characteristics. Moreover, a great obstacle for a wider clinical and commercial application of these HDPs and their derivatives relies on the ability to find a scalable cost-efficient production method. The high cost of production of these antimicrobial peptides is due to several factors, such as the complexity of their secondary structure and also for the lack of suitable expression system (Table 7).
Table 7.
Comparison of recombinant expression systems.
| Plants | Mammalian cells | Bacteria | Yeast/algae | Baculovirus infected insects | |
|---|---|---|---|---|---|
| Production cost | Low | High | Low | Medium | High |
| Production speed | Low | Medium | Very high | High | Medium |
| Folding | Medium | Optimal | Poor | Poor | Medium |
| Glycosilation | Medium | Optimal | Poor | Poor | Medium |
| Expression level | >10 g kg−1 | <10 mg L−1 | 10–30 g L−1 | <1 g L−1 | 1–5 g L−1 |
| Protein yield | Very high | High | Medium | High | Very high |
| Scale-up-capacity | Very high | Very low | High | High | Medium |
| Storage | Easy | Difficult | Medium | Medium | Difficult |
| Government regulation | Difficult | Easy | Medium | Medium | Difficult |
The study of the structure and the antimicrobial activity of these HDPs require large amounts of correctly folded peptides, which are difficult to obtain from natural sources because of the low-yield concentrations. Previous studies have reported the chemical synthesis of α-defensins which is feasible but very costly (Dawson et al.2000; Raj et al.2000). The chemical synthesis of defensins has been performed so far using Boc chemistry for chain assembly and anhydrous hydrogen fluoride (HF) for deprotection and cleavage. However, this methodology is inefficient to generate great amounts of the peptide, and it is impractical for many laboratories due to the safety hazards and restrictions for HF (Wu et al.2004). Wu et al. reported the chemical synthesis of α-defensins HNP4, HD5 and HD6 using directly crude peptides which showed an overall yield of 10%–16% with high purity (Wu et al.2004). More recently, Vernieri et al. described a new methodology based on the use of the fluoromethyloxycarbonyl chloride (Fmoc) strategy for an optimized solid-phase synthesis of HD5 (Vernieri et al.2014). As a result, they obtained a correctly folded HD5, highly pure (> 95%) and with an overall yield of 15 mg per run.
Another approach that has been attempted in many occasions has been the use of recombinant techniques (Pazgier and Lubkowski 2006; Wang et al.2010; Chapnik et al.2012). The most commonly used host cell has been Escherichia coli mainly because it grows easily, fast and is inexpensive, and also because it is a well-established expression system (Pazgier and Lubkowski 2006; Tomisawa et al.2015). Disadvantages of the use of these recombinant strategies to produce HDPs are their potential toxicity to the host and their susceptibility to proteolytic degradation (Li 2009). This degradation could be prevented by expressing these peptides as fusion proteins in E. coli; however, there will always be a necessary extra step to remove the fusion protein tag either by enzymatic or chemical cleavage, with the potential risk of unfavorable degradation of the recombinant peptides (Huang et al.2006; Xu et al.2006). The peptide yield obtained by this methodology is highly variable with some authors reporting productivities of 1.70–2.68 g L−1 and other reaching concentrations around 100 mg L−1 with 74.7% purity (Xu et al.2006; Wang et al.2010). The formation of inclusion bodies as a novel way to prevent degradation and improve productivity has also been assayed with promising results compared to the conventional method (Tomisawa et al.2015).
Other host cells that have been used to improve the stability of the recombinant protein and the yield biosynthesis of these peptides are Saccharomyces cerevisiae, Pichia pastoris, a methylotrophic yeast, and Chlorella ellipsoidea. Pichia pastoris, an unicellular algae, has proven to be an effective system for expression of recombinant proteins, including HD5, hBD2, sheep BD1 or porcine BD2 among others plants and invertebrate defensins but with very variable yield of the purified peptides, ranging from 1 to 383.7 mg L−1 depending on each case (Hsu et al.2009; Zhao and Cao 2012; Peng et al.2014). Disadvantages of the yeast systems are the low stability of these peptides at 28°C and the proteolytic activity in the fermentation medium (Cipakova and Hostinova 2005). As well as with the yeast, the use of C. ellipsoidea, apart from the fact that it presents a high growth rate and easy control production, can be used together with the fodder in cattle industry, enabling the direct use of centrifugation and resulting cell-free medium without further peptide purification (Damaso et al.2003).
The baculovirus-infected insect cell system is the first choice for production of those cytosolic proteins that are unable to be produced in prokaryotic hosts, but for production of secreted mammalian proteins its use is more limited (Unger and Peleg 2012). There are two main limitations to the use of this platform; first of all the high cost, as expensive as mammalian cell culture, and secondly the requirement of viral transduction and all the extra steps associated with the use of viral stocks (Jarvis 2009). Notwithstanding, there have been some works reporting the production of defensins using this baculovirus-insect platform, the results of which suggest that the use of this platform could be considered as an option for high efficient production of recombinant defensins (Cai et al.2004; Fukushima et al.2013).
In general, mammalian cells are the preferred system to produce these recombinant peptides, mostly because their ability to produce proteins similar to those naturally occurring in human, and because the CHO cell line still represents the major mammalian host for protein production (Zhu 2012). Also, for this cell line, and that is something that has not changed since the first recombinant product approval, the preferred culture system is large-scale stirred tank bioreactors (Hacker, De Jesus and Wurm 2009). With the techniques employed nowadays, the improvements in media composition, and bioprocess developments, volumetric productivity can be achieved up to 1–5 g L−1 (Wurm 2004).
Transgenic plants as bioreactors have also been subject of study, because they are easy and cheap to grow and are able to express foreign proteins at high levels. Thus, they are a promising biofactory systems for HDPs. Advantages for the use of plant-based systems include their low costs of production compared to microbial and mammalian cell culture systems, minimum risks of product contamination with endotoxins or human pathogens and the mass-scale production of recombinant proteins, among others.
Several vaccine antigens and therapeutic proteins have been expressed in plants, specifically by integrating them in their chloroplast genomes (Arlen et al.2007, 2008; Farran et al.2008; Lee et al.2011). An advantage of the use of chloroplast, for example in the case of the tobacco plant, is the possibility of scaling up the production to up to 40 metric tons of biomass acre−1 per year, leading to potential yields up to 2 kg of peptides, enough for preclinical or clinical studies (Lee et al.2011). An alternative to the chloroplasts is the use of seeds as natural warehouse for proteins. Rice seeds have been considered good bio-factories due to their high yields (up to 2 metric tons acre−1 per year) and their easy handle (Stoger et al.2005). Thus, the production of cecropin A, a linear and cationic antimicrobial peptide from insects, has been recently reported using rice seeds as storage (Bundo et al.2014). These authors showed that the use of rice endosperm for production and accumulation of the peptide had no impact on seeds viability and presented an accumulation ranging from 10 to 100 ng per seed (Bundo et al.2014). As an additional advantage of this approach, the cecropin A produced in these plants conferred them resistance against bacterial and fungal pathogens, which has additional implications for plant protection (Bundo et al.2014; Merlin et al.2014). In summary, plants are promising bio-factories systems for HDPs, they have successfully been used for the production of different proteins for therapeutic applications, and the results obtained indicate that they can sustain the production of HDPs and potentially turn it into an optimized large-scale purification method for this aim (Merlin et al.2014).
CONCLUDING REMARKS
HDPs involve a wide group of potent and broad-spectrum antimicrobial molecules that present a number of attractive properties. Other than their broad-spectrum and direct activity against Gram-negative and Gram-positive bacteria (mostly hBD and HNP), virus (HD and HNP) and fungi (mostly hBD and LL-37), HDPs can exert immunomodulatory, wound healing and adjuvant functions. They can control infection and inflammation, present chemotactic signals for immune cells, by inducing cytokines and chemokines secretion and can neutralize exotoxins and endotoxins. In this way, IDRs, defensins derivatives lacking direct antimicrobial activity but able to induct immune response of the host, have emerged as potential new tools to fight against infections. In any case, these new strategies also present the advantage of generating low propensity to microbial adaptation, probably because of their non-specific mechanisms of action. This became a key factor considering the current emergency alert regarding the lack of therapeutic alternatives for MDR bacteria and viruses. Thus, the potential clinical use of HDPs and their derivatives represents a promising strategy for the treatment of infections caused by MDR bacteria and viruses. This fact is clearly reflected in the exponential growth in the number of research studies for the discovering of HDPs in recent years and in the interest of several companies worldwide on their development.
Unfortunately, some disadvantages including stability, susceptibility to proteolysis, low activity in physiological conditions, and high cost of production must be circumvented before these fascinating peptides can be introduced in the clinical practice. The highest in vitro direct antimicrobial activities of these HDPs are only achieved, in most of the cases, in absence of serum, which generates doubts as to whether their activity in vivo follows a microbial-directed or a host-directed mechanism. It seems mandatory to understand the structure-based activity of these peptides, to be able to isolate the function in charge of their reported direct antimicrobial activities and to design new derivatives with improved pharmacokinetics parameters. In the same way, the wide number of different physiological functions exerted by these HDPs has to be fully characterized to prevent future adverse side effects of their use as antimicrobial drugs. Some of these issues have been already improved and continuous work is ongoing to solve those still unsatisfactory issues with the aim of making HDPs viable therapeutic alternatives in the near future.
Although showing very low stability due to the presence of L-amino acids in their structure, IDR peptides show great potential because their activity is directed to the launch of a state of protection rather than acting directly on pathogens. Also, other advantages of the use of IDRs are the probable reduction in the emergence of resistance and, in addition, their potential use together with common antimicrobial drugs because of the observed additive effect when used in combination.
With the probability that these HDPs reach the market, it would be desirable not to repeat the same mistake we have made in the past with traditional antimicrobial drugs, that is to say, we must make a better use of these new generation of antimicrobial drugs to prevent the admittedly unlikely possibility of the emergence of resistant microorganisms due to the permanent exposure to HDPs.
Acknowledgments
The present work has been supported by Plan Nacional de I+D+i and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015/0001)—co-financed by European Development Regional Fund ‘A way to achieve Europe’ ERDF and the Spanish Adenovirus Network (AdenoNet, BIO2015-68990-REDT). YS has a Miguel Servet grant from the Instituto de Salud Carlos III; MEPI has a grant by the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, co-financed by the European Development Regional Fund (‘A way to achieve Europe’) and by the Spanish Network for Research in Infectious Diseases (grant REIPI RD16/0009); and JSC has a grant from the V Plan Propio de Investigación of the University of Seville, Spain.
Conflict of interest. None declared.
REFERENCES
- Achtman AH, Pilat S, Law CW et al. Effective adjunctive therapy by an innate defense regulatory peptide in a preclinical model of severe malaria. Sci Transl Med 2012;4:135ra164. [DOI] [PubMed] [Google Scholar]
- Adessi C, Soto C. Converting a peptide into a drug: strategies to improve stability and bioavailability. Curr Med Chem 2002;9:963–78. [DOI] [PubMed] [Google Scholar]
- Alekseeva L, Huet D, Femenia F et al. Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiol 2009;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlen PA, Falconer R, Cherukumilli S et al. Field production and functional evaluation of chloroplast-derived interferon-alpha2b. Plant Biotechnol J 2007;5:511–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlen PA, Singleton M, Adamovicz JJ et al. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect Immun 2008;76:3640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett E, Seveau S. The multifaceted activities of mammalian defensins. Curr Pharm Des 2011;17:4254–69. [DOI] [PubMed] [Google Scholar]
- Bai LL, Yin WB, Chen YH et al. A new strategy to produce a defensin: stable production of mutated NP-1 in nitrate reductase-deficient Chlorella ellipsoidea. PLoS One 2013;8:e54966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow PG, Svoboda P, Mackellar A et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One 2011;6:e25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Efraim I, Shai Y. The structure and organization of synthetic putative membranous segments of ROMK1 channel in phospholipid membranes. Biophys J 1997;72:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman P, Walter-Jallow L, Broliden K et al. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res 2007;5:410–5. [DOI] [PubMed] [Google Scholar]
- Beringer PM, Bensman TJ, Ho H et al. Rhesus theta-defensin-1 (RTD-1) exhibits in vitro and in vivo activity against cystic fibrosis strains of Pseudomonas aeruginosa. J Antimicrob Chemoth 2016;71:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdish DM, Davidson DJ, Lau YE et al. Impact of LL-37 on anti-infective immunity. J Leukocyte Biol 2005;77:451–9. [DOI] [PubMed] [Google Scholar]
- Buck CB, Day PM, Thompson CD et al. Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci USA 2006;103:1516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundo M, Montesinos L, Izquierdo E et al. Production of cecropin A antimicrobial peptide in rice seed endosperm. BMC Plant Biol 2014;14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai SH, Du J, Huang N et al. Transfection and expression of recombinant human beta-defensin-2 gene in insect cells. Sichuan Da Xue Xue Bao Yi Xue Ban 2004;35:832–5. [PubMed] [Google Scholar]
- Chairatana P, Chiang I-L, Nolan EM. Human α-defensin 6 self-assembly prevents adhesion and suppresses virulence traits of Candida albicans. Biochemistry 2017;56:1033–1041. [DOI] [PubMed] [Google Scholar]
- Chan DI, Prenner EJ, Vogel HJ. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta 2006;1758:1184–202. [DOI] [PubMed] [Google Scholar]
- Chang HT, Tsai PW, Huang HH et al. LL37 and hBD-3 elevate the beta-1,3-exoglucanase activity of Candida albicans Xog1p, resulting in reduced fungal adhesion to plastic. Biochem J 2012;441:963–70. [DOI] [PubMed] [Google Scholar]
- Chapnik N, Levit A, Niv MY et al. Expression and structure/function relationships of human defensin 5. Appl Biochem Biotech 2012;166:1703–10. [DOI] [PubMed] [Google Scholar]
- Choe H, Narayanan AS, Gandhi DA et al. Immunomodulatory peptide IDR-1018 decreases implant infection and preserves osseointegration. Clin Orthop 2015;473:2898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chongsiriwatana NP, Barron AE. Comparing bacterial membrane interactions of antimicrobial peptides and their mimics. Methods Mol Biol 2010;618:171–82. [DOI] [PubMed] [Google Scholar]
- Chow LN, Choi KY, Piyadasa H et al. Human cathelicidin LL-37-derived peptide IG-19 confers protection in a murine model of collagen-induced arthritis. Mol Immunol 2014;57:86–92. [DOI] [PubMed] [Google Scholar]
- Chu H, Pazgier M, Jung G et al. Human alpha-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science 2012;337:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipakova I, Hostinova E. Production of the human-beta-defensin using Saccharomyces cerevisiae as a host. Protein Pept Lett 2005;12:551–4. [DOI] [PubMed] [Google Scholar]
- Cirioni O, Giacometti A, Ghiselli R et al. LL-37 protects rats against lethal sepsis caused by gram-negative bacteria. Antimicrob Agents Ch 2006;50:1672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AM, Hong T, Boo LM et al. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci USA 2002;99:1813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras X, Mzoughi O, Gaston F et al. Protein kinase C-delta regulates HIV-1 replication at an early post-entry step in macrophages. Retrovirology 2012;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack LR, Jones L, Malavige GN et al. Human antimicrobial peptides LL-37 and human beta-defensin-2 reduce viral replication in keratinocytes infected with varicella zoster virus. Clin Exp Dermatol 2012;37:534–43. [DOI] [PubMed] [Google Scholar]
- Currie SM, Findlay EG, McHugh BJ et al. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS One 2013;8:e73659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SM, Gwyer Findlay E, McFarlane AJ et al. Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J Immunol 2016;196:2699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. J Virol 1986;60:1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaso MC, Almeida MS, Kurtenbach E et al. Optimized expression of a thermostable xylanase from Thermomyces lanuginosus in Pichia pastoris. Appl Environ Microb 2003;69:6064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson NF, Craik DJ, McManus AM et al. Chemical synthesis, characterization and activity of RK-1, a novel alpha-defensin-related peptide. J Pept Sci 2000;6:19–25. [DOI] [PubMed] [Google Scholar]
- Dawson RM, Liu CQ. Properties and applications of antimicrobial peptides in biodefense against biological warfare threat agents. Crit Rev Microbiol 2008;34:89–107. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Nunez C, Reffuveille F, Haney EF et al. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog 2014;10:e1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw E, Li C, Zeng P et al. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett 2010;584:1543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirkhanyan LH, Marin M, Padilla-Parra S et al. Multifaceted mechanisms of HIV-1 entry inhibition by human alpha-defensin. J Biol Chem 2012;287:28821–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog AL, van Marle J, van Veen HA et al. Candidacidal effects of two antimicrobial peptides: histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem J 2005;388:689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog AL, van Marle J, Veerman EC et al. The human cathelicidin peptide LL-37 and truncated variants induce segregation of lipids and proteins in the plasma membrane of Candida albicans. Biol Chem 2006;387:1495–502. [DOI] [PubMed] [Google Scholar]
- Doss M, Ruchala P, Tecle T et al. Hapivirins and diprovirins: novel theta-defensin analogs with potent activity against influenza A virus. J Immunol 2012;188:2759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss M, White MR, Tecle T et al. Interactions of alpha-, beta-, and theta-defensins with influenza A virus and surfactant protein D. J Immunol 2009;182:7878–87. [DOI] [PubMed] [Google Scholar]
- Dugan AS, Maginnis MS, Jordan JA et al. Human alpha-defensins inhibit BK virus infection by aggregating virions and blocking binding to host cells. J Biol Chem 2008;283:31125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnas B, Wnorowska U, Pogoda K et al. Candidacidal activity of selected ceragenins and human cathelicidin LL-37 in experimental settings mimicking infection sites. PLoS One 2016;11:e0157242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eade CR, Cole AL, Diaz C et al. The anti-HIV microbicide candidate RC-101 inhibits pathogenic vaginal bacteria without harming endogenous flora or mucosa. Am J Reprod Immunol 2013;69:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R, Sullivan R, Shi W. Targeted antimicrobial treatment to re-establish a healthy microbial flora for long-term protection. Adv Dent Res 2012;24:94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton M, Koshlukova SE, Araujo MW et al. Salivary histatin 5 and human neutrophil defensin 1 kill Candida albicans via shared pathways. Antimicrob Agents Ch 2000;44:3310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericksen B, Wu Z, Lu W et al. Antibacterial activity and specificity of the six human {alpha}-defensins. Antimicrob Agents Ch 2005;49:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari MK, Bolgos G, Miller C et al. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol 1992;148:2724–30. [PubMed] [Google Scholar]
- Farran I, Rio-Manterola F, Iniguez M et al. High-density seedling expression system for the production of bioactive human cardiotrophin-1, a potential therapeutic cytokine, in transgenic tobacco chloroplasts. Plant Biotechnol J 2008;6:516–27. [DOI] [PubMed] [Google Scholar]
- Feng X, Sambanthamoorthy K, Palys T et al. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides 2013;49:131–7. [DOI] [PubMed] [Google Scholar]
- Fox JL. Antimicrobial peptides stage a comeback. Nat Biotechnol 2013;31:379–82. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Iiyama K, Yamashita J et al. Production of small antibacterial peptides using silkworm-baculovirus protein expression system. Prep Biochem Biotechnol 2013;43:565–76. [DOI] [PubMed] [Google Scholar]
- Furci L, Baldan R, Bianchini V et al. New role for human alpha-defensin 5 in the fight against hypervirulent Clostridium difficile strains. Infect Immun 2015;83:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacser A, Tiszlavicz Z, Nemeth T et al. Induction of human defensins by intestinal Caco-2 cells after interactions with opportunistic Candida species. Microbes Infect 2014;16:80–85. [DOI] [PubMed] [Google Scholar]
- Gallo SA, Wang W, Rawat SS et al. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J Biol Chem 2006;281:18787–92. [DOI] [PubMed] [Google Scholar]
- Ganz T, Selsted ME, Szklarek D et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest 1985;76:1427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon YJ, Romanowski EG, McDermott AM. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res 2005;30:505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guani-Guerra E, Santos-Mendoza T, Lugo-Reyes SO et al. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol 2010;135:1–11. [DOI] [PubMed] [Google Scholar]
- Gwyer Findlay E, Currie SM, Davidson DJ. Cationic host defence peptides: potential as antiviral therapeutics. BioDrugs 2013;27:479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker DL, De Jesus M, Wurm FM. 25 years of recombinant proteins from reactor-grown cells - where do we go from here? Biotechnol Adv 2009;27:1023–7. [DOI] [PubMed] [Google Scholar]
- Hancock RE, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol 1998;16:82–88. [DOI] [PubMed] [Google Scholar]
- Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 2006;24:1551–7. [DOI] [PubMed] [Google Scholar]
- Hazrati E, Galen B, Lu W et al. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J Immunol 2006;177:8658–66. [DOI] [PubMed] [Google Scholar]
- Hooven TA, Randis TM, Hymes SR et al. Retrocyclin inhibits Gardnerella vaginalis biofilm formation and toxin activity. J Antimicrob Chemoth 2012;67:2870–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DM, Wu Z, Tucker K et al. Antimicrobial characterization of human beta-defensin 3 derivatives. Antimicrob Agents Ch 2003;47:2804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Zhang N, Yang J et al. Antimicrobial peptide LL-37 and IDR-1 ameliorate MRSA pneumonia in vivo. Cell Physiol Biochem 2013;32:614–23. [DOI] [PubMed] [Google Scholar]
- Howell MD, Streib JE, Leung DY. Antiviral activity of human beta-defensin 3 against vaccinia virus. J Allergy Clin Immunol 2007;119:1022–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KH, Pei C, Yeh JY et al. Production of bioactive human alpha-defensin 5 in Pichia pastoris. J Gen Appl Microbiol 2009;55:395–401. [DOI] [PubMed] [Google Scholar]
- Huang L, Wang J, Zhong Z et al. Production of bioactive human beta-defensin-3 in Escherichia coli by soluble fusion expression. Biotechnol Lett 2006;28:627–32. [DOI] [PubMed] [Google Scholar]
- Jacob B, Park IS, Bang JK et al. Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity. J Pept Sci 2013;19:700–7. [DOI] [PubMed] [Google Scholar]
- Jarvis DL. Baculovirus-insect cell expression systems. Methods Enzymol 2009;463:191–222. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Yang D, Li W et al. Antiviral activity of recombinant mouse beta-defensin 3 against influenza A virus in vitro and in vivo. Antivir Chem Chemoth 2012;22:255–62. [DOI] [PubMed] [Google Scholar]
- Joly S, Maze C, McCray PB et al. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol 2004;42:1024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandaswamy K, Liew TH, Wang CY et al. Focal targeting by human beta-defensin 2 disrupts localized virulence factor assembly sites in Enterococcus faecalis. P Natl Acad Sci USA 2013;110:20230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehne K, Brunke G, Meyer D et al. Oesophageal defensin expression during Candida infection and reflux disease. Scand J Gastroenterol 2005;40:501–7. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett 2010;584:1374–8. [DOI] [PubMed] [Google Scholar]
- Kota S, Sabbah A, Chang TH et al. Role of human beta-defensin-2 during tumor necrosis factor-alpha/NF-kappaB-mediated innate antiviral response against human respiratory syncytial virus. J Biol Chem 2008;283:22417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer BF, Campbell RA, Schwertz H et al. Novel anti-bacterial activities of beta-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog 2011;7:e1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumari V, Rangaraj N, Nagaraj R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob Agents Ch 2009;53:256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumari V, Singh S, Nagaraj R. Antibacterial activities of synthetic peptides corresponding to the carboxy-terminal region of human beta-defensins 1-3. Peptides 2006;27:2607–13. [DOI] [PubMed] [Google Scholar]
- Kudrimoti M, Curtis A, Azawi S et al. Dusquetide: A novel innate defense regulator demonstrating a significant and consistent reduction in the duration of oral mucositis in preclinical data and a randomized, placebo-controlled phase 2a clinical study. J Biotechnol 2016;239:115–25. [DOI] [PubMed] [Google Scholar]
- Lamers RP, Eade CR, Waring AJ et al. Characterization of the retrocyclin analogue RC-101 as a preventative of Staphylococcus aureus nasal colonization. Antimicrob Agents Ch 2011;55:5338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Li B, Jin S et al. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol J 2011;9:100–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Cole AM, Selsted ME. theta-Defensins: cyclic peptides with endless potential. J Biol Chem 2012;287:27014–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E, Delanoe-Ayari H, Melikov K et al. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat Immunol 2005;6:995–1001. [DOI] [PubMed] [Google Scholar]
- Li Y. Carrier proteins for fusion expression of antimicrobial peptides in Escherichia coli. Biotechnol Appl Bioc 2009;54:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM, Ahn KB, Kim C et al. Antifungal effects of synthetic human beta-defensin 3-C15 peptide. Restor Dent Endod 2016;41:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia B, Lee PH, Yamasaki K et al. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol 2005;125:108–15. [DOI] [PubMed] [Google Scholar]
- Lu Q, Jayatilake JA, Samaranayake LP et al. Hyphal invasion of Candida albicans inhibits the expression of human beta-defensins in experimental oral candidiasis. J Invest Dermatol 2006;126:2049–56. [DOI] [PubMed] [Google Scholar]
- Luan C, Xie YG, Pu YT et al. Recombinant expression of antimicrobial peptides using a novel self-cleaving aggregation tag in Escherichia coli. Can J Microbiol 2014;60:113–20. [DOI] [PubMed] [Google Scholar]
- Maisetta G, Batoni G, Esin S et al. In vitro bactericidal activity of human beta-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob Agents Ch 2006;50:806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti S, Patro S, Purohit S et al. Effective control of Salmonella infections by employing combinations of recombinant antimicrobial human beta-defensins hBD-1 and hBD-2. Antimicrob Agents Ch 2014;58:6896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni ML, McDermott AM, Zasloff M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp Dermatol 2016;25:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour SC, de la Fuente-Nunez C, Hancock RE. Peptide IDR-1018: modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J Pept Sci 2015;21:323–9. [DOI] [PubMed] [Google Scholar]
- Mansour SC, Pena OM, Hancock RE. Host defense peptides: front-line immunomodulators. Trends Immunol 2014;35:443–50. [DOI] [PubMed] [Google Scholar]
- Mathew B, Nagaraj R. Antimicrobial activity of human alpha-defensin 5 and its linear analogs: N-terminal fatty acylation results in enhanced antimicrobial activity of the linear analogs. Peptides 2015a;71:128–40. [DOI] [PubMed] [Google Scholar]
- Mathew B, Nagaraj R. Antimicrobial activity of human alpha-defensin 6 analogs: insights into the physico-chemical reasons behind weak bactericidal activity of HD6 in vitro. J Pept Sci 2015b;21:811–8. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Shioyama T, Okamura E et al. A comparative study on interactions of alpha-aminoisobutyric acid containing antibiotic peptides, trichopolyn I and hypelcin A with phosphatidylcholine bilayers. Biochim Biophys Acta 1991;1070:419–28. [DOI] [PubMed] [Google Scholar]
- Merlin M, Gecchele E, Capaldi S et al. Comparative evaluation of recombinant protein production in different biofactories: the green perspective. BioMed Res Int 2014;2014:136419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JE, Harder J, Gorogh T et al. Human beta-defensin-2 in oral cancer with opportunistic Candida infection. Anticancer Res 2004;24:1025–30. [PubMed] [Google Scholar]
- Mor A, Nicolas P. The NH2-terminal alpha-helical domain 1-18 of dermaseptin is responsible for antimicrobial activity. J Biol Chem 1994;269:1934–9. [PubMed] [Google Scholar]
- Munk C, Wei G, Yang OO et al. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res Hum Retroviruses 2003;19:875–81. [DOI] [PubMed] [Google Scholar]
- Mygind PH, Fischer RL, Schnorr KM et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005;437:975–80. [DOI] [PubMed] [Google Scholar]
- Nan YH, Bang JK, Jacob B et al. Prokaryotic selectivity and LPS-neutralizing activity of short antimicrobial peptides designed from the human antimicrobial peptide LL-37. Peptides 2012;35:239–47. [DOI] [PubMed] [Google Scholar]
- Nijnik A, Madera L, Ma S et al. Synthetic cationic peptide IDR-1002 provides protection against bacterial infections through chemokine induction and enhanced leukocyte recruitment. J Immunol 2010;184:2539–50. [DOI] [PubMed] [Google Scholar]
- North JR, Takenaka S, Rozek A et al. A novel approach for emerging and antibiotic resistant infections: Innate defense regulators as an agnostic therapy. J Biotechnol 2016;226:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Linzer BA, Zuberi RI et al. Activity of defensins from human neutrophilic granulocytes against Mycobacterium avium-Mycobacterium intracellulare. Infect Immun 1992;60:4720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olli S, Nagaraj R, Motukupally SR. A hybrid cationic peptide composed of human beta-defensin-1 and humanized theta-defensin sequences exhibits salt-resistant antimicrobial activity. Antimicrob Agents Ch 2015;59:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olli S, Rangaraj N, Nagaraj R. Effect of selectively introducing arginine and D-amino acids on the antimicrobial activity and salt sensitivity in analogs of human beta-defensins. PLoS One 2013;8:e77031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez SR, Amarullah IH, Wubbolts RW et al. Fungicidal mechanisms of cathelicidins LL-37 and CATH-2 revealed by live-cell imaging. Antimicrob Agents Ch 2014;58:2240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren Z, Shai Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers 1998;47:451–63. [DOI] [PubMed] [Google Scholar]
- Owen SM, Rudolph DL, Wang W et al. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res Hum Retroviruses 2004;20:1157–65. [DOI] [PubMed] [Google Scholar]
- Patro S, Maiti S, Panda SK et al. Utilization of plant-derived recombinant human beta-defensins (hBD-1 and hBD-2) for averting salmonellosis. Transgenic Res 2015;24:353–64. [DOI] [PubMed] [Google Scholar]
- Pazgier M, Hoover DM, Yang D et al. Human beta-defensins. Cell Mol Life Sci 2006;63:1294–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazgier M, Lubkowski J. Expression and purification of recombinant human alpha-defensins in Escherichia coli. Protein Expr Purif 2006;49:1–8. [DOI] [PubMed] [Google Scholar]
- Penberthy WT, Chari S, Cole AL et al. Retrocyclins and their activity against HIV-1. Cell Mol Life Sci 2011;68:2231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Wang A, Feng Q et al. High-level expression, purification and characterisation of porcine beta-defensin 2 in Pichia pastoris and its potential as a cost-efficient growth promoter in porcine feed. Appl Microbiol Biot 2014;98:5487–97. [DOI] [PubMed] [Google Scholar]
- Polewicz M, Gracia A, Garlapati S et al. Novel vaccine formulations against pertussis offer earlier onset of immunity and provide protection in the presence of maternal antibodies. Vaccine 2013;31:3148–55. [DOI] [PubMed] [Google Scholar]
- Pridmore CJ, Rodger A, Sanderson JM. The association of defensin HNP-2 with negatively charged membranes: A combined fluorescence and linear dichroism study. Biochim Biophys Acta 2016;1858:892–903. [DOI] [PubMed] [Google Scholar]
- Quinones-Mateu ME, Lederman MM, Feng Z et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 2003;17:F39–48. [DOI] [PubMed] [Google Scholar]
- Raj PA, Antonyraj KJ, Karunakaran T. Large-scale synthesis and functional elements for the antimicrobial activity of defensins. Biochem J 2000;347Pt 3:633–41. [PMC free article] [PubMed] [Google Scholar]
- Rapala-Kozik M, Bochenska O, Zawrotniak M et al. Inactivation of the antifungal and immunomodulatory properties of human cathelicidin LL-37 by aspartic proteases produced by the pathogenic yeast Candida albicans. Infect Immun 2015;83:2518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Santiago B, Castaneda-Delgado JE, Rivas Santiago CE et al. Ability of innate defence regulator peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis infections in animal models. PLoS One 2013a;8:e59119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Santiago B, Rivas Santiago CE, Castaneda-Delgado JE et al. Activity of LL-37, CRAMP and antimicrobial peptide-derived compounds E2, E6 and CP26 against Mycobacterium tuberculosis. Int J Antimicrob Ag 2013b;41:143–8. [DOI] [PubMed] [Google Scholar]
- Rizzo A, Losacco A, Carratelli CR. Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll-like receptors 2 and 4, interleukin 8 and human beta-defensins 2 and 3. Immunol Lett 2013;156:102–9. [DOI] [PubMed] [Google Scholar]
- Rouabhia M, Mukherjee PK, Lattif AA et al. Disruption of sphingolipid biosynthetic gene IPT1 reduces Candida albicans adhesion and prevents activation of human gingival epithelial cell innate immune defense. Med Mycol 2011;49:458–66. [DOI] [PubMed] [Google Scholar]
- Routsias JG, Karagounis P, Parvulesku G et al. In vitro bactericidal activity of human beta-defensin 2 against nosocomial strains. Peptides 2010;31:1654–60. [DOI] [PubMed] [Google Scholar]
- Ruan Y, Shen T, Wang Y et al. Antimicrobial peptide LL-37 attenuates LTA induced inflammatory effect in macrophages. Int Immunopharmacol 2013;15:575–80. [DOI] [PubMed] [Google Scholar]
- Sahl HG, Pag U, Bonness S et al. Mammalian defensins: structures and mechanism of antibiotic activity. J Leukocyte Biol 2005;77:466–75. [DOI] [PubMed] [Google Scholar]
- Salvatore M, Garcia-Sastre A, Ruchala P et al. alpha-Defensin inhibits influenza virus replication by cell-mediated mechanism(s). J Infect Dis 2007;196:835–43. [DOI] [PubMed] [Google Scholar]
- Salzman NH, Ghosh D, Huttner KM et al. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 2003;422:522–6. [DOI] [PubMed] [Google Scholar]
- Sass V, Schneider T, Wilmes M et al. Human beta-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect Immun 2010;78:2793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarsini M, Tomasinsig L, Arzese A et al. Antifungal activity of cathelicidin peptides against planktonic and biofilm cultures of Candida species isolated from vaginal infections. Peptides 2015;71:211–21. [DOI] [PubMed] [Google Scholar]
- Schaal JB, Tran D, Tran P et al. Rhesus macaque theta defensins suppress inflammatory cytokines and enhance survival in mouse models of bacteremic sepsis. PLoS One 2012;7:e51337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz T, Bartlett JA, Walters JD et al. Genomics-based approaches to gene discovery in innate immunity. Immunol Rev 2002;190:137–45. [DOI] [PubMed] [Google Scholar]
- Schneider JJ, Unholzer A, Schaller M et al. Human defensins. J Mol Med 2005;83:587–95. [DOI] [PubMed] [Google Scholar]
- Schroder JM. Epithelial antimicrobial peptides: innate local host response elements. Cell Mol Life Sci 1999;56:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell B 1999;31:645–51. [DOI] [PubMed] [Google Scholar]
- Schroeder BO, Ehmann D, Precht JC et al. Paneth cell alpha-defensin 6 (HD-6) is an antimicrobial peptide. Mucosal Immunol 2015;8:661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BO, Wu Z, Nuding S et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature 2011;469:419–23. [DOI] [PubMed] [Google Scholar]
- Schutte BC, Mitros JP, Bartlett JA et al. Discovery of five conserved beta -defensin gene clusters using a computational search strategy. P Natl Acad Sci USA 2002;99:2129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MG, Dullaghan E, Mookherjee N et al. An anti-infective peptide that selectively modulates the innate immune response. Nat Biotechnol 2007;25:465–72. [DOI] [PubMed] [Google Scholar]
- Scudiero O, Galdiero S, Cantisani M et al. Novel synthetic, salt-resistant analogs of human beta-defensins 1 and 3 endowed with enhanced antimicrobial activity. Antimicrob Agents Ch 2010;54:2312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudiero O, Galdiero S, Nigro E et al. Chimeric beta-defensin analogs, including the novel 3NI analog, display salt-resistant antimicrobial activity and lack toxicity in human epithelial cell lines. Antimicrob Agents Ch 2013;57:1701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudiero O, Nigro E, Cantisani M et al. Design and activity of a cyclic mini-beta-defensin analog: a novel antimicrobial tool. Int J Nanomed 2015;10:6523–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel A, Ye Y, de Armas LR et al. Cyclic and acyclic defensins inhibit human immunodeficiency virus type-1 replication by different mechanisms. PLoS One 2010;5:e9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol 2005;6:551–7. [DOI] [PubMed] [Google Scholar]
- Sharma H, Mathew B, Nagaraj R. Engineering of a linear inactive analog of human beta-defensin 4 to generate peptides with potent antimicrobial activity. J Pept Sci 2015;21:501–11. [DOI] [PubMed] [Google Scholar]
- Sharma H, Nagaraj R. Antimicrobial activity of human beta-defensin 4 analogs: insights into the role of disulfide linkages in modulating activity. Peptides 2012;38:255–65. [DOI] [PubMed] [Google Scholar]
- Shi Y, Song W, Feng ZH et al. Disinfection of maxillofacial silicone elastomer using a novel antimicrobial agent: recombinant human beta-defensin-3. Eur J Clin Microbiol 2009;28:415–20. [DOI] [PubMed] [Google Scholar]
- Sigurdardottir T, Andersson P, Davoudi M et al. In silico identification and biological evaluation of antimicrobial peptides based on human cathelicidin LL-37. Antimicrob Agents Ch 2006;50:2983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JG, Nemerow GR. Mechanism of adenovirus neutralization by Human alpha-defensins. Cell Host Microbe 2008;3:11–9. [DOI] [PubMed] [Google Scholar]
- Smith JG, Silvestry M, Lindert S et al. Insight into the mechanisms of adenovirus capsid disassembly from studies of defensin neutralization. PLoS Pathog 2010;6:e1000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Shi Y, Xiao M et al. In vitro bactericidal activity of recombinant human beta-defensin-3 against pathogenic bacterial strains in human tooth root canal. Int J Antimicrob Ag 2009;33:237–43. [DOI] [PubMed] [Google Scholar]
- Steinstraesser L, Hirsch T, Schulte M et al. Innate defense regulator peptide 1018 in wound healing and wound infection. PLoS One 2012;7:e39373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinstraesser L, Koehler T, Jacobsen F et al. Host defense peptides in wound healing. Mol Med 2008;14:528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steubesand N, Kiehne K, Brunke G et al. The expression of the beta-defensins hBD-2 and hBD-3 is differentially regulated by NF-kappaB and MAPK/AP-1 pathways in an in vitro model of Candida esophagitis. BMC Immunol 2009;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoger E, Ma JK, Fischer R et al. Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotechnol 2005;16:167–73. [DOI] [PubMed] [Google Scholar]
- Sun L, Finnegan CM, Kish-Catalone T et al. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol 2005;79:14318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WK, Lu X, Li X et al. Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur J Clin Microbiol 2012;31:2755–64. [DOI] [PubMed] [Google Scholar]
- Supp DM, Gardner J, Klingenberg JM et al. Antibiotic resistance in clinical isolates of Acinetobacter baumannii, Pseudomonas aeruginosa, and Staphylococcus aureus does not impact sensitivity to human beta defensin 4. Burns 2009;35:949–55. [DOI] [PubMed] [Google Scholar]
- Tai KP, Kamdar K, Yamaki J et al. Microbicidal effects of alpha- and theta-defensins against antibiotic-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Innate Immun 2015;21:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Territo MC, Ganz T, Selsted ME et al. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest 1989;84:2017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur N, Qureshi A, Kumar M. AVPpred: collection and prediction of highly effective antiviral peptides. Nucleic Acids Res 2012;40:W199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomisawa S, Sato Y, Kamiya M et al. Efficient production of a correctly folded mouse alpha-defensin, cryptdin-4, by refolding during inclusion body solubilization. Protein Expr Purif 2015;112:21–28. [DOI] [PubMed] [Google Scholar]
- Tongaonkar P, Tran P, Roberts K et al. Rhesus macaque theta-defensin isoforms: expression, antimicrobial activities, and demonstration of a prominent role in neutrophil granule microbicidal activities. J Leukocyte Biol 2011;89:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D, Tran PA, Tang YQ et al. Homodimeric theta-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J Biol Chem 2002;277:3079–84. [DOI] [PubMed] [Google Scholar]
- Tripathi S, Tecle T, Verma A et al. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J Gen Virol 2013;94:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S, Wang G, White M et al. Antiviral activity of the human cathelicidin, LL-37, and derived peptides on seasonal and pandemic influenza A viruses. PLoS One 2015;10:e0124706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PW, Cheng YL, Hsieh WP et al. Responses of Candida albicans to the human antimicrobial peptide LL-37. J Microbiol 2014;52:581–9. [DOI] [PubMed] [Google Scholar]
- Tsai PW, Yang CY, Chang HT et al. Characterizing the role of cell-wall beta-1,3-exoglucanase Xog1p in Candida albicans adhesion by the human antimicrobial peptide LL-37. PLoS One 2011a;6:e21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PW, Yang CY, Chang HT et al. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS One 2011b;6:e17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J, Cho Y, Dinh NN et al. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Ch 1998;42:2206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T, Peleg Y. Recombinant protein expression in the baculovirus-infected insect cell system. Methods Mol Biol 2012;800:187–99. [DOI] [PubMed] [Google Scholar]
- van 't Hof W, Veerman EC, Helmerhorst EJ et al. Antimicrobial peptides: properties and applicability. Biol Chem 2001;382:597–619. [DOI] [PubMed] [Google Scholar]
- Vandamme D, Landuyt B, Luyten W et al. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol 2012;280:22–35. [DOI] [PubMed] [Google Scholar]
- Varkey J, Nagaraj R. Antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines. Antimicrob Agents Ch 2005;49:4561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varney KM, Bonvin AM, Pazgier M et al. Turning defense into offense: defensin mimetics as novel antibiotics targeting lipid II. PLoS Pathog 2013;9:e1003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernieri E, Valle J, Andreu D et al. An optimized Fmoc synthesis of human defensin 5. Amino Acids 2014;46:395–400. [DOI] [PubMed] [Google Scholar]
- Virella-Lowell I, Poirier A, Chesnut KA et al. Inhibition of recombinant adeno-associated virus (rAAV) transduction by bronchial secretions from cystic fibrosis patients. Gene Ther 2000;7:1783–9. [DOI] [PubMed] [Google Scholar]
- Vylkova S, Li XS, Berner JC et al. Distinct antifungal mechanisms: beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob Agents Ch 2006;50:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vylkova S, Nayyar N, Li W et al. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob Agents Ch 2007;51:154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Chen F, Wang Y et al. Enhancement of antiviral activity of human alpha-defensin 5 against herpes simplex virus 2 by arginine mutagenesis at adaptive evolution sites. J Virol 2013;87:2835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Su Y, Wang S et al. High efficiency preparation of bioactive human alpha-defensin 6 in Escherichia coli Origami(DE3)pLysS by soluble fusion expression. Appl Microbiol Biot 2010;87:1935–42. [DOI] [PubMed] [Google Scholar]
- Wang C, Shen M, Gohain N et al. Design of a potent antibiotic peptide based on the active region of human defensin 5. J Med Chem 2015a;58:3083–93. [DOI] [PubMed] [Google Scholar]
- Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 2016;44:D1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Watson KM, Buckheit RW Jr. Anti-human immunodeficiency virus type 1 activities of antimicrobial peptides derived from human and bovine cathelicidins. Antimicrob Agents Ch 2008;52:3438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Cole AM, Hong T et al. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J Immunol 2003;170:4708–16. [DOI] [PubMed] [Google Scholar]
- Wang Z, de la Fuente-Nunez C, Shen Y et al. Treatment of oral multispecies biofilms by an anti-biofilm peptide. PLoS One 2015b;10:e0132512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Pazgier M, de Leeuw E et al. Trp-26 imparts functional versatility to human alpha-defensin HNP1. J Biol Chem 2010;285:16275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Quinones-Mateu ME, Lederman MM. Role of human beta-defensins in HIV infection. Adv Dent Res 2006;19:42–48. [DOI] [PubMed] [Google Scholar]
- White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 2012;12:401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek M, Jenssen H, Kindrachuk J et al. Structural studies of a peptide with immune modulating and direct antimicrobial activity. Chem Biol 2010;17:970–80. [DOI] [PubMed] [Google Scholar]
- Wilde CG, Griffith JE, Marra MN et al. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J Biol Chem 1989;264:11200–3. [PubMed] [Google Scholar]
- Wiley RA, Rich DH. Peptidomimetics derived from natural products. Med Res Rev 1993;13:327–84. [DOI] [PubMed] [Google Scholar]
- Wilmes M, Cammue BP, Sahl HG et al. Antibiotic activities of host defense peptides: more to it than lipid bilayer perturbation. Nat Prod Rep 2011;28:1350–8. [DOI] [PubMed] [Google Scholar]
- Wilmes M, Sahl HG. Defensin-based anti-infective strategies. International J Med Microbiol 2014;304:93–9. [DOI] [PubMed] [Google Scholar]
- Wilson SS, Wiens ME, Smith JG. Antiviral mechanisms of human defensins. J Mol Biol 2013;425:4965–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JH, Legowska A, Rolka K et al. Effects of cathelicidin and its fragments on three key enzymes of HIV-1. Peptides 2011;32:1117–22. [DOI] [PubMed] [Google Scholar]
- Wood MP, Cole AL, Ruchala P et al. A compensatory mutation provides resistance to disparate HIV fusion inhibitor peptides and enhances membrane fusion. PLoS One 2013;8:e55478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Cocchi F, Gentles D et al. Human neutrophil alpha-defensin 4 inhibits HIV-1 infection in vitro. FEBS Lett 2005;579:162–6. [DOI] [PubMed] [Google Scholar]
- Wu Z, Ericksen B, Tucker K et al. Synthesis and characterization of human alpha-defensins 4-6. J Pept Res 2004;64:118–25. [DOI] [PubMed] [Google Scholar]
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 2004;22:1393–8. [DOI] [PubMed] [Google Scholar]
- Xhindoli D, Morgera F, Zinth U et al. New aspects of the structure and mode of action of the human cathelicidin LL-37 revealed by the intrinsic probe p-cyanophenylalanine. Biochem J 2015;465:443–57. [DOI] [PubMed] [Google Scholar]
- Xhindoli D, Pacor S, Benincasa M et al. The human cathelicidin LL-37–A pore-forming antibacterial peptide and host-cell modulator. Biochim Biophys Acta 2016;1858:546–66. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhong Z, Huang L et al. High-level production of bioactive human beta-defensin-4 in Escherichia coli by soluble fusion expression. Appl Microbiol Biot 2006;72:471–9. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Chertov O et al. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukocyte Biol 2000;68:9–14. [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999;286:525–8. [DOI] [PubMed] [Google Scholar]
- Yasin B, Wang W, Pang M et al. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J Virol 2004;78:5147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung AT, Gellatly SL, Hancock RE. Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci 2011;68:2161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002;415:389–95. [DOI] [PubMed] [Google Scholar]
- Zhang G, Sunkara LT. Avian antimicrobial host defense peptides: from biology to therapeutic applications. Pharmaceuticals 2014;7:220–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Falla TJ. Antimicrobial peptides: therapeutic potential. Expert Opin Pharmacother 2006;7:653–63. [DOI] [PubMed] [Google Scholar]
- Zhao P, Cao G. Production of bioactive sheep beta-defensin-1 in Pichia pastoris. J Ind Microbiol Biotechnol 2012;39:11–7. [DOI] [PubMed] [Google Scholar]
- Zhu J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol Adv 2012;30:1158–70. [DOI] [PubMed] [Google Scholar]