Abstract
Cardio-oncology is a rapidly growing field aimed at minimizing the effects of cardiovascular morbidity and mortality in cancer survivors. To meet this aim, patients are assessed at baseline to define their risk of cardiotoxicity and then followed closely during and after chemotherapy to assess for early signs or symptoms of cardiovascular disease. Cardiac imaging, and in particular, transthoracic echocardiography, plays an essential role in the baseline assessment and serial follow-up of cardio-oncology patients. The objectives of this paper are to review the mechanisms of cardiotoxicity of several common chemotherapeutic agents associated with an increased risk for left ventricular systolic dysfunction and to outline recommendations regarding the baseline assessment and serial follow-up of cardio-oncology patients with a focus on the role of echocardiography.
Keywords: cardio-oncology, strain, echocardiography, cardiotoxicity, chemotherapy
Introduction
Cardio-oncology is an emerging field that takes a team-based approach with cardiologists, oncologists, and hematologists working together for the prevention, early detection and management of cardiovascular disease in cancer patients throughout all stages of cancer therapy and in the survivorship period. The field is rapidly growing due to the recognition that many agents that are effective cancer therapies leave survivors at heightened risk for cardiovascular disease. Since the original call to arms regarding the need for dedicated systems to care for these patients in 2010, cardio-oncology clinics have developed around the world, and the demand for cardio-oncology clinics is only going to continue to grow as cancer survivorship increases (1, 2). There are an estimated 15.5 million cancer survivors living in the United States and 2 million cancer survivors living in the United Kingdom and these numbers are predicted to grow (3, 4).
Chemotherapy and radiation therapy can put patients at risk for a variety of cardiovascular complications including heart failure, coronary artery disease, peripheral vascular disease, thromboembolism, pericardial disease and valvular heart disease. Cancer patients receiving therapy with known cardiac risk require close monitoring during and after treatment. Echocardiography plays an essential role in the early detection of many of these cardiac complications. The objectives of this review are to define the mechanisms of cardiotoxicity of common chemotherapeutic agents associated with an increased risk for left ventricular systolic dysfunction and to outline an approach to the clinical assessment and management of patients at risk for cardiotoxicity from these agents, with a focus on the role of echocardiography.
A pair of illustrative cases
The first case is of a childhood cancer survivor who received treatment for acute myeloid leukemia at age 13 months with a regimen that included an anthracycline. Unfortunately, she did not have routine cardiac follow-up in the survivorship period. She presented in her 20 s with dyspnea. On further inquiry, she had been experiencing exertional dyspnea and poor exercise tolerance for over a decade. She was diagnosed with heart failure with a left ventricular ejection fraction (LV EF) of 35% (Videos 1 and 2). Her clinical course was complicated by recurrent atrial fibrillation and inappropriate defibrillator shocks. Due to progressive heart failure, she received a left ventricular assist device and later a heart transplant. She died at the age of 36 years.
Parasternal long axis view from the transthoracic echocardiogram of a 25 year old woman who was a survivor of childhood cancer demonstrating left ventricular hypokinesis. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0013/video-1.
Download Video 1 (1.2MB, avi)
Apical four chamber view from the transthoracic echocardiogram of a 25 year old woman who was a survivor of childhood cancer demonstrating left ventricular hypokinesis. View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0013/video-2.
Download Video 2 (1MB, avi)
The second case demonstrates the benefit of cardiac surveillance. A 57-year-old female was referred to a cardio-oncology clinic in the midst of therapy for breast cancer. She had completed 4 cycles of chemotherapy with cyclophosphamide and doxorubicin (an anthracycline) and 3 months of trastuzumab (of a planned 12-month course). She was asymptomatic, but there had been a change on surveillance echocardiograms. Her LV EF had decreased from 60% at baseline (Video 3) to 49% (Video 4) at the time of cardio-oncology referral and her global longitudinal strain (GLS) had changed from −20% to −17% in the same interval (Fig. 1A and B). Given these changes and mild tachycardia, she was started on carvedilol. On follow-up echocardiograms her LV EF improved initially to 55% and then to 61% (Video 5). Her GLS also normalized (Fig. 1C). She was able to complete her prescribed course of trastuzumab.
Figure 1.
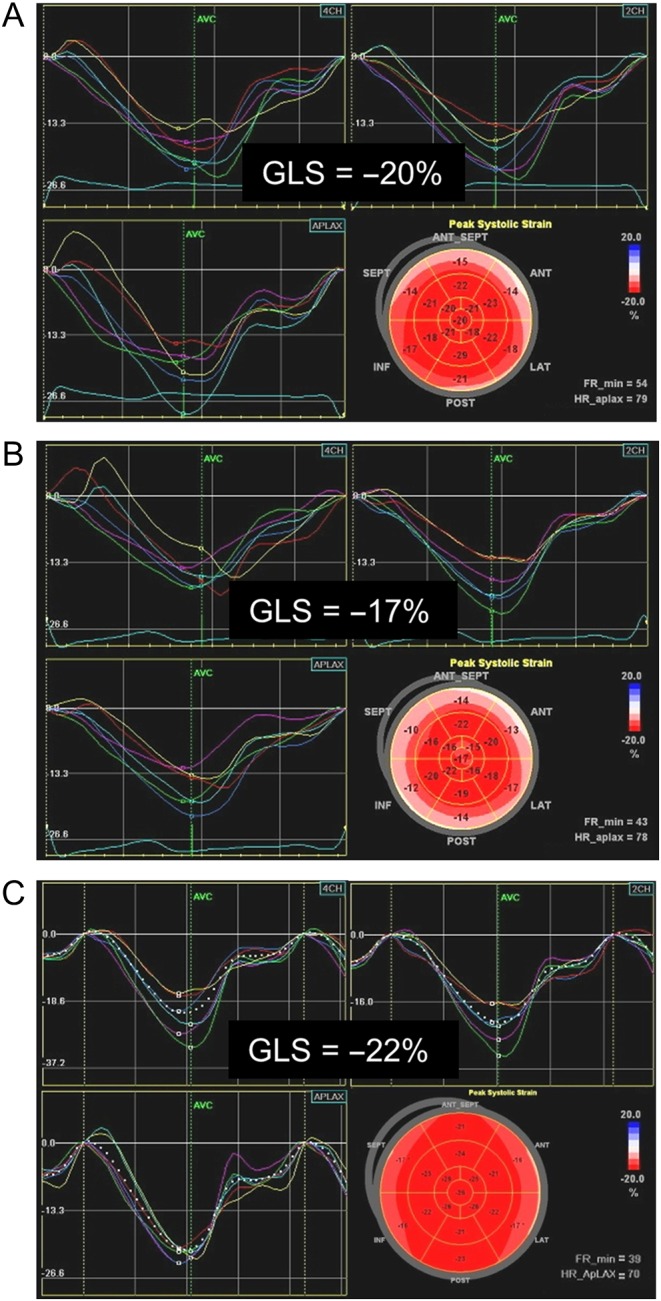
Global longitudinal strain before (A), during (B) and after (C) chemotherapy with trastuzumab.
Parasternal long axis view from the pre-treatment transthoracic echocardiogram of a 57 year old woman who subsequently received trastuzumab for breast cancer. A normal leftventricular ejection fraction of 60% is demonstrated. View Video 3 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0013/video-3.
Download Video 3 (764.1KB, avi)
Parasternal long axis view from the transthoracic echocardiogram of a 57 year old woman obtained after 3 months of trastuzumab therapy for breast cancer. A mildly reduced left ventricular ejection fraction of 49% is demonstrated. View Video 4 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0013/video-4.
Download Video 4 (888.3KB, avi)
Parasternal long axis view from the echocardiogram of a 57 year old woman after completion of trastuzumab for breast cancer. A normal left ventricular ejection fraction of 61% is demonstrated. View Video 5 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0013/video-5.
Download Video 5 (790.5KB, avi)
Cancer therapeutics-related cardiac dysfunction
Cardiotoxicity in the form of left ventricular dysfunction will be the primary focus of this review. It has historically been defined by changes in LV EF. Most recently, cancer therapeutics-related cardiac dysfunction (CTRCD) was defined as a drop in LV EF of ≥5% in symptomatic patients or a drop in LV EF of ≥10% to an EF of <53% in asymptomatic patients (5). Two types of CTRCD have been described. Type 1 is an irreversible, dose-dependent toxicity that results from ultra-structural changes in the myocardium. It is typified by anthracycline cardiotoxicity (6, 7). Anthracyclines are a class of chemotherapeutic agents frequently used in the treatment of breast cancer, lymphoma, leukemia, and sarcoma, which include the following agents: doxorubicin, daunorubicin, idarubicin and epirubicin. Mitoxantrone is a closely related agent that can also result in type I CTRCD. Anthracyclines inhibit the function of topoisomerase 2B in cardiomyocytes leading to DNA double strand breaks, mitochondrial dysfunction, the development of reactive oxygen species and damage to or death of cardiomyocytes (8). Patients who have received a cumulative dose of >250–300 mg/m2 of doxorubicin or its equivalent are considered to be at high risk of developing CTRCD; however, there is individual variability with some patients developing cardiotoxicity at lower cumulative doses (6, 9, 10).
Anthracycline cardiotoxicity most frequently presents as either early-onset chronic progressive (within the first year after completion of chemotherapy) or late-onset chronic progressive (greater than 1 year after completion of therapy) left ventricular systolic dysfunction, which is usually irreversible. Clinically, patients present with dilated cardiomyopathy. Those with late-onset chronic progressive cardiotoxicity can present as long as 1–2 decades after completion of cancer therapy. Much less commonly, anthracycline cardiotoxicity can present acutely as left ventricular systolic dysfunction immediately after administration of a dose of anthracycline and may be reversible (9).
Type 2 CTRCD is largely reversible, not dose dependent, and does not cause ultra-structural changes in the myocardium. It is typified by trastuzumab cardiotoxicity. Trastuzumab is most commonly used in the treatment of breast cancer. Importantly, because type 2 toxicity is usually reversible, re-challenge with the offending drug is usually well tolerated after initiating cardiac protective therapy (7, 11). Trastuzumab causes cardiac dysfunction through its target, human epidermal growth factor receptor 2 (HER2). Inhibition of HER2 on cardiomyocytes blocks an important signaling pathway involved in the prevention of dilated cardiomyopathy when the myocardium is under stress (7, 12). This mechanism explains why patients who receive an anthracycline and trastuzumab for breast cancer treatment are at higher risk for CTRCD than those who receive either agent alone (13). Other chemotherapeutic agents that have been associated with a risk of type II CTRCD include lapatinib, pertuzumab, imatinib, sorafenib, sunitinib, bevacizumab and bortezomib (5, 9).
In addition to CTRCD, vascular disease (coronary and/or peripheral) is the other major long-term cardiovascular risk associated with chemotherapy and radiation therapy. A similar schema of type 1 and type 2 toxicity has been proposed for vascular toxicity related to chemotherapeutic agents. In this schema, type 1 vascular toxicity is associated with persistent risk of vascular events even after drug discontinuation. Type 1 vascular toxicity is typified by the tyrosine kinase inhibitors nilotinib and ponatinib. Tyrosine kinase inhibitors have also been associated with new or worsening hypertension. Type 2 vascular toxicity is associated with risk while the patient is receiving chemotherapy, but the risk returns to baseline after therapy is complete. Type 2 toxicity is typified by 5-fluorouracil (14). A complete review of vascular toxicity is beyond the scope of this article; however, it is an important aspect of cardio-oncology and providers caring for these patients may be interested in one of the several recently published articles on the subject (14, 15).
Risk factors for cancer therapeutics-related cardiac dysfunction
A multi-hit hypothesis has been proposed to explain CTRCD. By this hypothesis, multiple insults to the myocardium increase the risk of developing cardiotoxicity. These insults to the myocardium can be in the form of pre-existing cardiovascular disease (coronary artery disease, heart failure and arrhythmias) or cardiovascular risk factors (age, hypertension, diabetes mellitus and hyperlipidemia) (11, 16). Use of multiple different chemotherapeutic agents with potential for cardiotoxicity and/or the combination of chemotherapy and radiation therapy increase the risk for cardiotoxicity. In addition to the previously mentioned agents, cyclophosphamide and paclitaxel (which are frequently used in combination with anthracyclines) have been associated with an increased risk of cardiotoxicity (9). The risk of anthracycline cardiotoxicity is also increased by female gender, administration of higher single doses, bolus dose administration, higher cumulative doses and the specific agent administered (6, 9, 17). In a meta-analysis of 55 randomized controlled trials, epirubicin and liposomal doxorubicin were associated with a lower risk of clinical and subclinical cardiotoxicity than doxorubicin; mitoxantrone was associated with a lower risk of clinical cardiotoxicity, but not of subclinical cardiotoxicity, when compared to doxorubicin (18).
Baseline patient assessment
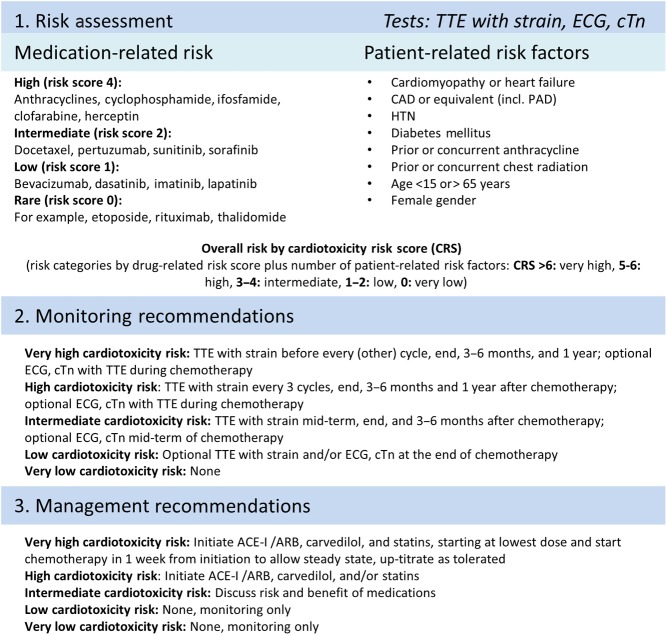
In a cardio-oncology clinic, the patient assessment begins before the start of chemotherapy by estimating the risk for cardiotoxicity. Two factors need to be taken into consideration when assessing the risk: (1) the risk associated with the specific chemotherapy and/or radiation therapy they will be receiving and (2) the risk related to their co-existing cardiac risk factors, age and sex. These factors can be used to generate a risk score (Fig. 2) (11). In patients assessed to be at high risk, cardioprotective medical therapy with an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB), a beta blocker, and/or a statin should be considered before the initiation of chemotherapy (9, 11). In patients at intermediate risk, it is also reasonable to have a discussion with the patient about the potential risks vs benefits of initiating cardioprotective medical therapy before chemotherapy (11). These agents have all been associated with a decreased incidence of cardiotoxicity, albeit in relatively small heterogeneous studies (different trial designs, cancer types and treatment regimens) (19, 20, 21, 22). Dexrazoxane is another cardioprotective agent that can be used to reduce the risk of anthracycline cardiotoxicity in patients with metastatic breast cancer if a cumulative dose of 300 mg/m2 of doxorubicin has been reached and ongoing anthracycline-based chemotherapy is recommended (9, 19).
Figure 2.
Outline of a proposed model for risk assessment, monitoring and management of patients undergoing chemotherapy. ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; cTn, cardiac troponin; ECG, electrocardiogram; HTN, hypertension; PAD, peripheral arterial disease; TTE, transthoracic echocardiogram. Reprinted from Mayo Clinic Proceedings, Volume 89(9), Joerg Herrmann, MD, Amir Lerman, MD, Nicole P. Sandhu, MD, PhD, Hector R. Villarraga, MD, Sharon L. Mulvagh, MD, and Manish Kohli MD, 2014, pages 1287–1306, with permission from Elsevier.
All patients receiving potentially cardiotoxic therapy, regardless of their calculated risk, should have optimization of cardiac risk factors prior to the initiation of chemotherapy. This includes optimizing blood pressure, lipids and blood sugar; smoking cessation and encouraging compliance with dietary and exercise recommendations for primary prevention of cardiovascular disease (23). Additionally, all patients require close follow-up throughout their course of chemotherapy and thereafter to monitor for signs of cardiotoxicity.
Serial cardiac monitoring
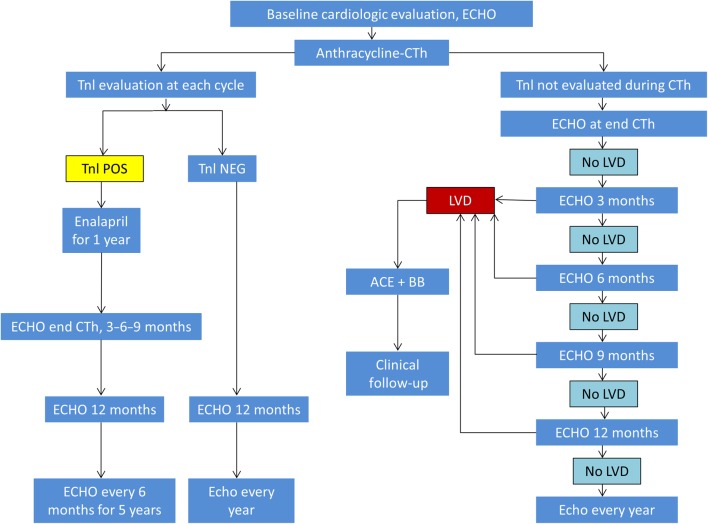
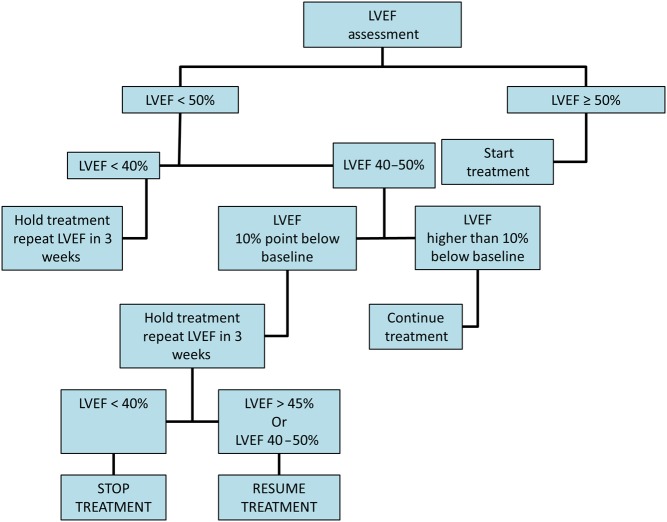
The European Society of Medical Oncology published algorithms for the serial cardiac monitoring of patients at risk for type 1 and type 2 cardiotoxicity (9). Additionally, the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE) published a consensus statement in 2014 on the multimodality imaging assessment of patients during and after cancer therapy (5). These algorithms all begin with a baseline assessment of LV EF. Echocardiography is frequently used in the serial assessment of LV EF in patients undergoing chemotherapy because of its widespread availability and the absence of radiation exposure. However, cardiac magnetic resonance imaging and gated radionucleotide angiography can also be used for serial assessment of cardiac function. Whichever technique is chosen for LV EF monitoring in a given patient, it is important that the same technique is utilized for that patient’s follow-up imaging. If the baseline EF is abnormal, then cardiology consultation should be obtained before initiating therapy. If the baseline LV EF is normal, then the timing of subsequent imaging follow-up depends on whether the patient is receiving an agent associated with type I toxicity (Fig. 3) or type 2 toxicity (Fig. 4) and upon whether or not biomarkers are also being utilized for cardiac monitoring (Fig. 3) (9). If evidence of cardiotoxicity is detected at any stage, then cardiology consultation should be obtained and therapy for left ventricular dysfunction (ACEi or ARB and beta blocker) initiated if not previously started. Patients experiencing type II toxicity can usually resume the offending chemotherapeutic agent if the LV EF improves with medical therapy (Fig. 4) (9). There are limited and conflicting data to support the use of medical therapy for type II CTRCD (20, 24). However, until further data are available, it is our practice to do so as it may promote the recovery of ventricular function and reduce the risk for subsequent ventricular dysfunction after chemotherapy is resumed. In patients who experience a type I toxicity, however, avoidance of further exposure to the offending agent should be recommended and alternative chemotherapeutic agents considered, if clinically indicated.
Figure 3.
Algorithm for cardiac monitoring in patients receiving anthracyclines. ECHO, transthoracic echocardiogram; CTh, chemotherapy; Tnl, troponin; POS, positive; NEG, negative; LVD, left ventricular dysfunction; ACE, angiotensin-converting enzyme inhibitor; BB, beta blocker. Reprinted from: G. Curigliano, D. Cardinale, T. Suter, G. Plataniotis, E. de Azambuja, M.T. Sandri, C. Criscitiello, A. Goldhirsch, C. Cipolla & F. Roila, Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines, Annals of Oncology, 2012, Volume 23 (supplement 7), pages vii155–vii166, by permission of Oxford University Press.
Figure 4.
Algorithm for cardiac monitoring in patients receiving trastuzumab. LVEF, left ventricular ejection fraction. Reprinted from: G. Curigliano, D. Cardinale, T. Suter, G. Plataniotis, E. de Azambuja, M.T. Sandri, C. Criscitiello, A. Goldhirsch, C. Cipolla & F. Roila, Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines, Annals of Oncology, 2012, Volume 23 (supplement 7), pages vii155–Vii166, by permission of Oxford University Press.
Early detection of subclinical cardiotoxicity
One major limitation to the use of LV EF to monitor for cardiac dysfunction is that changes in LV EF usually occur at a later stage when significant toxicity has already occurred. To minimize the risk of irreversible cardiomyopathy, the goal is to identify signs of toxicity as early as possible so medical therapy can be initiated. In patients with anthracycline toxicity, earlier initiation of medical therapy is associated with an increased likelihood of subsequent improvement in LV EF (25). Defining methods to detect cardiotoxicity before it manifests as a decline in the LV EF or symptomatic heart failure is an area of active investigation. Some of the most promising techniques to date have been various parameters of left ventricular strain and strain rate. GLS is of particular interest because it can be incorporated into a clinical echocardiographic examination relatively efficiently with currently available technology. A recent systematic review identified 6 studies that examined the prognostic value of changes in GLS for predicting subsequent declines in LV EF and/or clinical heart failure. The individual studies were small (36–81 patients) and utilized varying definitions of a significant change in GLS to define subclinical LV dysfunction in patients being treated with trastuzumab and/or an anthracycline. However, subclinical LV dysfunction as defined by GLS was found to have a sensitivity ranging from 65% to 86% and a specificity ranging from 73% to 95% for predicting subsequent declines in LV EF or heart failure (26). Longitudinal strain rate, radial strain and strain rate and circumferential strain and strain rate have also been shown to be early predictors of cardiotoxicity (26, 27).
The EACVI and ASE recommend assessing GLS as a routine component of clinical echocardiograms in patients at risk for type 1 or type 2 cardiotoxicity (5). A relative percentage decrease in GLS >15% is indicative of subclinical LV dysfunction. A relative decrease <8% is consistent with no evidence of subclinical LV dysfunction (5). A relative decrease in GLS between 8% and 15% is a gray zone and closer follow-up should be considered to see if there is a trend toward further decrease in GLS at the next assessment. A clinical trial is ongoing to determine whether using changes in GLS to guide the initiation of cardioprotective therapy improves clinical outcomes (Strain surveillance during chemotherapy for improving Cardiovascular Outcomes (SUCCOUR) Trial; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366020; accessed 12/20/2016). Although we await data from this trial, cardiac protective therapy with an ACEi or ARB and/or a beta blocker is recommended in our cardio-oncology clinic if subclinical LV dysfunction develops. Subclinical LV dysfunction is also an indication for cardio-oncology consultation if the patient is not already being followed by cardiology.
Cardiac biomarkers may play a complementary role to cardiac imaging in monitoring patients for cardiotoxicity. Elevations in cardiac troponin I (TnI) either early (checked with each cycle of chemotherapy) or late (one month after completion of last cycle of chemotherapy) have been shown to be predictive of LV EF reduction and cardiac events (28). The greatest value of TnI may be the high negative predictive value, such that patients who do not have an elevation in TnI have a very low cardiac event rate and perhaps need less-frequent surveillance imaging. The ESMO outlines two pathways for cardiac monitoring during therapy with an anthracycline (Fig. 3). One pathway involves only cardiac imaging. The second pathway utilizes TnI and cardiac imaging (9). Further study is needed to determine the optimal time at which to assess cardiac troponin after chemotherapy and to determine the level of cardiac troponin to be used as a threshold to predict increased cardiac risk (5). This process is complicated by variability in normal troponin values across laboratories and assays. Natriuretic peptides have also been studied as potential biomarkers for early detection of cardiotoxicity. However, the results have been mixed as to whether elevation in natriuretic peptides predicts subsequent cardiac dysfunction (5).
Stress echocardiography is also being studied for a potential role in the detection of subclinical LV dysfunction. Although resting LV EF and strain may be within normal limits, patients who are undergoing or who have previously undergone chemotherapy may have a reduced cardiac reserve, which could potentially be detected with exercise or pharmacologic stress (5). Studies looking at stress echocardiography in cancer survivors vs healthy controls have shown mixed results with regard to whether there is evidence of reduced cardiac reserve in cancer survivors (29, 30, 31, 32). However, when dobutamine stress echocardiograms were assessed prospectively during high-dose chemotherapy for breast cancer, a fall in cardiac reserve was found to be predictive of a subsequent decline in the LV EF (33). This is an area of ongoing investigation, and further study is needed before incorporation into clinical practice can be routinely recommended.
Long-term cardiovascular monitoring
Survivorship programs managed by Oncology or Hematology help coordinate long-term follow-up for cancer survivors. Cardio-Oncology clinics should work with such survivorship programs to assure patients who received potentially cardiotoxic therapies receive optimal cardiac follow-up. Unfortunately, limited data are available to guide long-term cardiac follow-up recommendations after potentially cardiotoxic chemotherapy. A yearly history and physical exam to assess for early signs of cardiovascular disease is recommended in a recent expert consensus document from the ASE and ESCVI. However, routine cardiac imaging after completion of chemotherapy is not recommended if the LV EF and GLS are normal 6 months after completion of chemotherapy and there are no additional factors increasing an individual’s risk (5). The ESMO guidelines recommend assessment of LV EF 12 months and 18 months after the initiation of potentially cardiotoxic therapy and further assessment of LV EF after treatment as clinically indicated. They also recommend the assessment of cardiac function 4 years and 10 years after completion of anthracycline therapy in patients who received >240 mg/m2 of doxorubicin or >360 mg/m2 of epirubicin (9).
Patients who develop type 1 cardiotoxicity require long-term treatment in accordance with heart failure management guidelines (34, 35). Long-term echocardiographic follow-up is not required for patients who only received an agent associated with a risk for type II cardiotoxicity if they did not develop cardiotoxicity during the course of their therapy (11). The optimal cardiac therapy and duration of cardiac therapy for patients who develop type II cardiotoxicity is unknown (9, 11).
Radiation therapy and cardiovascular toxicity
Patients who have received radiation therapy are at risk of long-term cardiovascular toxicity including radiation-induced heart disease (valvular disease, pericardial disease, myocardial disease and coronary artery disease) and peripheral artery disease depending on the field of radiation. Risk factors for radiation-induced heart disease include anterior or left chest radiation, cumulative radiation dose >30 Gy, radiation fraction dose >2 Gy/day, age <50 years, tumor in or near the heart, lack of shielding, concomitant chemotherapy particularly with an anthracycline, smoking and medical comorbidities (diabetes mellitus, hypertension, hyperlipidemia and obesity) (36). The ESCVI and ASE expert consensus statement recommendations for long-term follow-up after radiation therapy suggest a yearly physical examination to assess for symptoms or signs of radiation heart disease or peripheral vascular disease, which if present should prompt further evaluation. Individuals are considered to be at high risk for radiation heart disease if they received radiation to the anterior chest or the left side of the chest and have one additional risk factor. In asymptomatic patients, a transthoracic echocardiogram is recommended 5 years after exposure in high-risk individuals and 10 years after exposure in all others. Subsequently, transthoracic echocardiograms are recommended for reassessment every 5 years. Additionally, a cardiac stress test is recommended after 5 years in high-risk individuals, and after 10 years, in all others to assess for coronary artery disease related to radiation vasculopathy (36).
Conclusion
Cardio-oncology is a growing field that aims to optimize the cardiovascular care received by cancer patients before, during and after chemotherapy and/or radiation therapy. Cardiovascular disease can cause significant morbidity in cancer survivors. The goal is to decrease this morbidity by early risk factor modification, serial monitoring with imaging and/or biomarkers, cardioprotective medical therapy and optimal medical therapy for cardiotoxicity when it occurs. Further study is ongoing and greatly needed to define the optimal methods to achieve these aims with regard to both surveillance methods and timing of surveillance as well as to optimal medical therapies for prevention and treatment of type I and type II CTRCD.
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/ERP-17-0013.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. 2010. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies are clinicians responding optimally? Journal of the American College of Cardiology 56 1644–1650. ( 10.1016/j.jacc.2010.07.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. 2010. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. Journal of the National Cancer Institute 102 14–25. ( 10.1093/jnci/djp440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. 2016. Cancer treatment and survivorship statistics. CA: A Cancer Journal for Clinicians 66 271–289. ( 10.3322/caac.21349) [DOI] [PubMed] [Google Scholar]
- 4.Maddams J, Brewster D, Gavin A, Steward J, Elliott J, Utley M, Moller H. 2009. Cancer prevalence in the United Kingdom: estimates for 2008. British Journal of Cancer 101 541–547. ( 10.1038/sj.bjc.6605148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, et al. 2014. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal: Cardiovascular Imaging 15 1063–1093. ( 10.1093/ehjci/jeu192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewer MS, Ewer SM. 2010. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nature Reviews Cardiology 7 564–575. ( 10.1038/nrcardio.2010.121) [DOI] [PubMed] [Google Scholar]
- 7.Ewer MS, Lippman SM. 2005. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. Journal of Clinical Oncology 23 2900–2902. ( 10.1200/JCO.2005.05.827) [DOI] [PubMed] [Google Scholar]
- 8.Vejpongsa P, Yeh ET. 2014. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. Journal of the American College of Cardiology 64 938–945. ( 10.1016/j.jacc.2014.06.1167) [DOI] [PubMed] [Google Scholar]
- 9.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F, et al. 2012. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Annals of Oncology 23 (Supplement 7) vii155–vii166. ( 10.1093/annonc/mds293) [DOI] [PubMed] [Google Scholar]
- 10.Yeh JM, Nohria A, Diller L. 2014. Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects. Annals of Internal Medicine 160 661–671. ( 10.7326/M13-2266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. 2014. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clinic Proceedings 89 1287–1306. ( 10.1016/j.mayocp.2014.05.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negro A, Brar BK, Lee KF. 2004. Essential roles of Her2/erbB2 in cardiac development and function. Recent Progress in Hormone Research 59 1–12. ( 10.1210/rp.59.1.1) [DOI] [PubMed] [Google Scholar]
- 13.Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, et al. 2012. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. Journal of the National Cancer Institute 104 1293–1305. ( 10.1093/jnci/djs317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann J. 2016. Tyrosine kinase inhibitors and vascular toxicity: impetus for a classification system? Current Oncology Reports 18 33 ( 10.1007/s11912-016-0514-0) [DOI] [PubMed] [Google Scholar]
- 15.Iliescu CA, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, Hakeem A, Toutouzas KP, Leesar MA, Marmagkiolis K. 2016. SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of india, and sociedad Latino Americana de Cardiologia intervencionista). Catheterization and Cardiovascular Interventions 87 E202–E223. ( 10.1002/ccd.26379) [DOI] [PubMed] [Google Scholar]
- 16.Herrmann J, Lerman A. 2014. An update on cardio-oncology. Trends in Cardiovascular Medicine 24 285–295. ( 10.1016/j.tcm.2014.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkova M, Russell R., 3rd 2011. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Current Cardiology Reviews 7 214–220. ( 10.2174/157340311799960645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, Jones A. 2010. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 10 337 ( 10.1186/1471-2407-10-337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalam K, Marwick TH. 2013. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. European Journal of Cancer 49 2900–2909. ( 10.1016/j.ejca.2013.04.030) [DOI] [PubMed] [Google Scholar]
- 20.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff-Brenkenhoff F, Bratland A, Storas TH, et al. 2016. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. European Heart Journal 37 1671–1680. ( 10.1093/eurheartj/ehw022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chotenimitkhun R, D’Agostino R, Jr, Lawrence JA, Hamilton CA, Jordan JH, Vasu S, Lash TL, Yeboah J, Herrington DM, Hundley WG. 2015. Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. Canadian Journal of Cardiology 31 302–307. ( 10.1016/j.cjca.2014.11.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Liu ZZ, Liu YY, Zheng ZD, Liang XF, Han YL, Xie XD. 2013. Preventive effect of low-dose carvedilol combined with candesartan on the cardiotoxicity of anthracycline drugs in the adjuvant chemotherapy of breast cancer. Zhonghua Zhong Liu Za Zhi 35 936–940. [PubMed] [Google Scholar]
- 23.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, et al. 2014 2013. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129 S76–S99. ( 10.1161/01.cir.0000437740.48606.dl) [DOI] [PubMed] [Google Scholar]
- 24.Bosch X, Rovira M, Sitges M, Domenech A, Ortiz-Perez JT, de Caralt TM, Morales-Ruiz M, Perea RJ, Monzo M, Esteve J. 2013. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). Journal of the American College of Cardiology 61 2355–2362. ( 10.1016/j.jacc.2013.02.072) [DOI] [PubMed] [Google Scholar]
- 25.Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. 2010. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. Journal of the American College of Cardiology 55 213–220. ( 10.1016/j.jacc.2009.03.095) [DOI] [PubMed] [Google Scholar]
- 26.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. 2014. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. Journal of the American College of Cardiology 63 2751–2768. ( 10.1016/j.jacc.2014.01.073) [DOI] [PubMed] [Google Scholar]
- 27.Narayan HK, French B, Khan AM, Plappert T, Hyman D, Bajulaiye A, Domchek S, DeMichele A, Clark A, Matro J, et al. 2016. Noninvasive measures of ventricular-arterial coupling and circumferential strain predict cancer therapeutics-related cardiac dysfunction. JACC: Cardiovascular Imaging 9 1131–1141. ( 10.1016/j.jcmg.2015.11.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, et al. 2004. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 109 2749–2754. ( 10.1161/01.CIR.0000130926.51766.CC) [DOI] [PubMed] [Google Scholar]
- 29.Weesner KM, Bledsoe M, Chauvenet A, Wofford M. 1991. Exercise echocardiography in the detection of anthracycline cardiotoxicity. Cancer 68 435–438. () [DOI] [PubMed] [Google Scholar]
- 30.Khouri MG, Hornsby WE, Risum N, Velazquez EJ, Thomas S, Lane A, Scott JM, Koelwyn GJ, Herndon JE, Mackey JR, et al. 2014. Utility of 3-dimensional echocardiography, global longitudinal strain, and exercise stress echocardiography to detect cardiac dysfunction in breast cancer patients treated with doxorubicin-containing adjuvant therapy. Breast Cancer Research and Treatment 143 531–539. ( 10.1007/s10549-013-2818-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryerson AB, Border WL, Wasilewski-Masker K, Goodman M, Meacham L, Austin H, Mertens AC. 2015. Assessing anthracycline-treated childhood cancer survivors with advanced stress echocardiography. Pediatric Blood and Cancer 62 502–508. ( 10.1002/pbc.25328) [DOI] [PubMed] [Google Scholar]
- 32.Hamada H, Ohkubo T, Maeda M, Ogawa S. 2006. Evaluation of cardiac reserved function by high-dose dobutamine-stress echocardiography in asymptomatic anthracycline-treated survivors of childhood cancer. Pediatrics International 48 313–320. ( 10.1111/j.1442-200X.2006.02210.x) [DOI] [PubMed] [Google Scholar]
- 33.Civelli M, Cardinale D, Martinoni A, Lamantia G, Colombo N, Colombo A, Gandini S, Martinelli G, Fiorentini C, Cipolla CM. 2006. Early reduction in left ventricular contractile reserve detected by dobutamine stress echo predicts high-dose chemotherapy-induced cardiac toxicity. International Journal of Cardiology 111 120–126. ( 10.1016/j.ijcard.2005.07.029) [DOI] [PubMed] [Google Scholar]
- 34.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013. 20136 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 62 e147–e239. ( 10.1016/j.jacc.2013.05.019) [DOI] [PubMed] [Google Scholar]
- 35.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 37 2129–2200. ( 10.1093/eurheartj/ehw128) [DOI] [PubMed] [Google Scholar]
- 36.Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, Cosyns B, Coucke P, Dulgheru R, Edvardsen T, et al. 2013. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Journal of the American Society of Echocardiography 26 1013–1032. ( 10.1016/j.echo.2013.07.005) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parasternal long axis view from the transthoracic echocardiogram of a 25 year old woman who was a survivor of childhood cancer demonstrating left ventricular hypokinesis. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0013/video-1.
Download Video 1 (1.2MB, avi)
Apical four chamber view from the transthoracic echocardiogram of a 25 year old woman who was a survivor of childhood cancer demonstrating left ventricular hypokinesis. View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0013/video-2.
Download Video 2 (1MB, avi)
Parasternal long axis view from the pre-treatment transthoracic echocardiogram of a 57 year old woman who subsequently received trastuzumab for breast cancer. A normal leftventricular ejection fraction of 60% is demonstrated. View Video 3 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0013/video-3.
Download Video 3 (764.1KB, avi)
Parasternal long axis view from the transthoracic echocardiogram of a 57 year old woman obtained after 3 months of trastuzumab therapy for breast cancer. A mildly reduced left ventricular ejection fraction of 49% is demonstrated. View Video 4 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0013/video-4.
Download Video 4 (888.3KB, avi)
Parasternal long axis view from the echocardiogram of a 57 year old woman after completion of trastuzumab for breast cancer. A normal left ventricular ejection fraction of 61% is demonstrated. View Video 5 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0013/video-5.
Download Video 5 (790.5KB, avi)



 This work is licensed under a
This work is licensed under a