Abstract
The trial-unique, delayed nonmatching-to-location (TUNL) task is a recently developed behavioral task that measures spatial working memory and a form of pattern separation in touchscreen-equipped operant conditioning chambers. Limited information exists regarding the neurotransmitters and neural substrates involved in the task. The present experiments tested the effects of systemic and intracranial injections of NMDA receptor antagonists on the TUNL task. After training, male Long Evans rats systemically injected with the competitive NMDA receptor antagonist CPP (10 mg/kg) had impaired accuracy regardless of the degree of stimuli separation or length of delay between the sample and test phases. Injections of Ro 25-6981 (6 or 10 mg/kg), an antagonist selective for GluN2B subunit-containing NMDA receptors, did not affect accuracy on the task. Direct infusion of the competitive NMDA receptor antagonist AP5 into mPFC or dmSTR reduced overall accuracy on the TUNL task. These results demonstrate that TUNL task performance depends on NMDA receptors within the mPFC and dmSTR.
The trial-unique, delayed nonmatching-to-location (TUNL) task, performed in touchscreen-equipped operant conditioning chambers, allows for assessment of spatial working memory and spatial pattern separation in rodents. The TUNL task is adapted from touchscreen-based tests for humans such as the Cambridge Neuropsychological Test Automated Battery spatial working memory task (Bussey et al. 2012). In TUNL, working memory is assayed by delayed responding to a novel location following presentation of a sample stimulus. During the task, pattern separation, the cognitive ability that enables similar patterns to be distinguished, is assessed by varying the separation (or distance) between the sample and choice stimuli. Thus, the TUNL task assesses a form of spatial pattern separation (Bussey et al. 2012; Kumar et al. 2015; Hurtubise et al. 2017). Given that the stimuli are presented at a variety of locations, rodents performing the TUNL task show fewer mediating responses to bridge the delay phase (Talpos et al. 2010), a problem that can confound simpler delayed nonmatching to location tasks.
Delineating the neural substrates underlying performance of the TUNL task may increase our understanding of the circuitry involved in working memory and pattern separation. Working memory impairments are observed in the TUNL task following NMDA receptor blockade with systemic administration of broad antagonists but not a selective GluN2B subunit-containing NMDA receptor antagonist (Kumar et al. 2015; Hurtubise et al. 2017). Rats with mPFC or hippocampal lesions have reduced working memory performance in TUNL (Talpos et al. 2010; McAllister et al. 2013) and the continuous TUNL task variant (Hvoslef-Eide et al. 2015; Oomen et al. 2015), while pattern separation was only impaired in rats with hippocampal lesions (Talpos et al. 2010). The prefrontal cortex projects to the striatum and interactions between these areas contribute to attention and working memory in a number of behavioral tasks (O'Reilly 2006). NMDA receptors within the prefrontal cortex and striatum also contribute to working memory in a number of tasks (Smith-Roe et al. 1999; Wang et al. 2013; Monaco et al. 2015). However, the role of NMDA receptors within the prefrontal cortex and striatum has yet to be assessed in TUNL. In particular, the dorsomedial striatum (dmSTR) receives glutamatergic projections from the prelimbic area of the prefrontal cortex and may be involved in working memory (Smith-Roe et al. 1999). Thus, we used systemic injections of the NMDA receptor antagonists CPP or GluN2B-containing NMDA receptor antagonist Ro 25-6981 to examine spatial working memory and spatial pattern separation on the TUNL task. We also used direct brain infusions of the NMDA receptor antagonist AP5 into the mPFC or the dmSTR to examine the role of NMDA receptors within these areas during TUNL.
Adult male Long Evans rats (n = 40; Charles River, Quebec, Canada) were individually housed in clear plastic cages in a colony room on a 12 h light–dark cycle (lights on at 07:00). Except for several days after arrival and surgery, rats were restricted to 85% of their free feeding weight. Throughout the experiment, rats had ad libitum access to water. Experiments were conducted in accordance with the standards of the Canadian Council on Animal Care and were approved by the University of Saskatchewan Animal Research Ethics Board. TUNL training and testing followed previously published procedures (Oomen et al. 2013; Hurtubise et al. 2017). Briefly, experiments were conducted in touchscreen-equipped operant conditioning chambers (Lafayette Instruments). The touchscreen was covered with a black polycarbonate mask with 14 square windows arranged in a 7 × 2 pattern (2 cm × 2 cm), which allowed the rats to contact the touchscreen only in areas where stimuli can be presented. Below the 14 windows is a spring-loaded “response shelf” that the rat presses down to contact the touchscreen thereby making a selection. Rats were habituated to the chambers and subsequently trained to touch an illuminated square stimulus presented pseudorandomly in one of the windows to receive a food reward. Pretraining comprised four stages: Initial Touch, Must Touch, Must Initiate, and Punish Incorrect as described in Hurtubise et al. (2017). A standard trial of the TUNL task comprised a sample phase, during which a sample stimulus is presented in one of the 14 possible locations on the screen, and a test phase, during which two stimuli are presented (one in the sample location (incorrect) and the other in the new (correct) location). A touch to the correct location resulted in delivery of a food reward and a 20-sec ITI before the next trial, but a touch to the incorrect location resulted in a 5-sec time-out and then correction trial(s). Correction trials followed the same process as normal trials, except that the same sample and test locations from the previous incorrect trial were repeated until the correct choice was selected. During initial TUNL acquisition, rats received up to 42 trials in 60 min and then were subsequently trained on sessions with 84 trials until they attained 80% correct on trials with large separations between stimuli (minimum of four unilluminated squares between sample and test stimuli). After TUNL acquisition was completed, treatments were administered and sessions were conducted as during training with correction trials included. Dependent measures assessed on treatment days included overall accuracy (percentage of correct on selection trials), accuracy on large (minimum of four unilluminated squares between sample and test stimuli) and small (one or two unilluminated squares between stimuli) separation selection trials, accuracy on 2- and 6-sec delay selection trials, number of selection trials (total correct and incorrect trials), number of correction trials, total trials (selection trials + correction trials), and response latencies for correct trials, incorrect trials, and reward collection.
In the CPP and Ro 25-6981 experiments, two 30-min blocks of a 2- and 6-sec delay were presented in a counterbalanced order. Rats (n = 8) were injected (i.p.) with either vehicle (Veh; saline) or CPP (10 mg/kg) 30 min before TUNL testing (Whitlock et al. 2006). A second group of rats (n = 16) were injected (i.p) with either Veh (20% DMSO; 80% H2O) or Ro 25-6981 (6 mg/kg or 10 mg/kg) (Howland and Cazakoff 2010; Davies et al. 2013a). For intracranial infusions, a third group of naïve rats (n = 16) were implanted with guide cannula (23 Ga) bilaterally to target the mPFC (AP + 3.00 mm; ML ± 0.70; DV-3.20 from bregma) and dmSTR (AP + 0.80 mm; ML ± 2.20; DV-3.40) using previously described procedures (Davies et al. 2013a,b). One rat died during surgery and another rat did not learn the task adequately and was excluded from testing. Therefore, 14 rats received brain infusions and were tested on the TUNL task using trials with a 2-sec delay as the systemic treatments did not reveal delay-dependent effects of NMDA receptor antagonism. Rats were habituated to the infusion procedure on three separate days during the week before infusions were administered (Davies et al. 2013a,b). Custom made needles (30 Ga stainless steel tubing) linked via PE-50 tubing to an infusion pump (PHD 2000, Harvard Apparatus) were inserted 1 mm past the end of the cannula (final DV coordinates, mPFC: −4.20 mm; dmSTR: −4.40 mm from bregma). Drugs were infused over 1 min and the infusion needles remained in place for 1 min after the infusion to allow diffusion of the drug. Rats were tested 5 min following brain infusions. AP5 (1.0 µL of a 30 mM solution) was infused bilaterally into mPFC or dmSTR (Winters et al. 2010; Baker and Ragozzino 2014). After testing, rats were perfused with saline. Brains were then removed, stored in a 10% formalin–10% sucrose solution until sectioning on a sliding microtome. Infusion sites were determined with reference to a rat brain atlas (Paxinos and Watson 1997) following cresyl violet staining. Behavioral data were recorded using ABET II Touch software (Lafayette) and analyzed using the Statistical Package for the Social Sciences. Descriptive values are reported as means ± standard error of the mean. Comparisons were performed using paired t-tests or repeated-measures ANOVAs and post hoc analyses were completed using Tukey HSD. Statistical tests were considered significant when P < 0.05.
Similar to previous studies that used NMDA receptor antagonists (Kumar et al. 2015; Hurtubise et al. 2017), systemic NMDA receptor blockade with CPP impaired accuracy (Fig. 1A; t(7) = 3.52, P = 0.01) regardless of separation (Fig. 1B; main effect of treatment: F(1,7) = 14.37, P = 0.01) or delay (Fig. 1C; main effect of treatment: F(1,7) = 8.71, P = 0.02). Analyses of treatment, stimuli separation, and delay revealed a main effect of treatment (Fig. 1D; F(1,7) = 14.37, P = 0.01), delay (F(1,7) = 7.13, P = 0.03), and an interaction between stimuli separation and delay (F(1,7) = 6.58, P = 0.04) but no main effect of stimuli separation or other interactions. CPP increased correction trials (Fig. 1F; t(7) = −3.18, P = 0.02) but did not affect the number of selection trials (Fig. 1E), total trials (Fig. 1G), or latencies (Fig. 1H). Ro 25-6981 did not affect accuracy (Fig. 2A). Significant main effects of separation (Fig. 2B; F(1,15) = 14.86, P = 0.002) and delay (Fig. 2C; F(1,15) = 38.89, P < 0.001) were noted. Analyses of treatment, stimuli separation, and delay revealed main effects of delay (Fig. 2D; F(1,14) = 31.26, P < 0.001), stimuli separation (F(1,14) = 12.15, P = 0.004), and a delay by stimuli separation interaction (F(1,14) = 6.09, P = 0.03), but not a main effect of treatment or other interactions. Ro 25-6981 did not affect selection trials (Fig. 2E), although Ro 25-6981 (10 mg/kg) increased correction trials (Fig. 2F; F(2,30) = 3.66, P = 0.04; post hoc P < 0.05). Both doses of Ro 25-6981 increased total trials relative to Veh (Fig. 2G; F(2,30) = 6.28, P = 0.01; post hoc P < 0.05). Latencies were unaffected by Ro 25-6981 (Fig. 2H).
Figure 1.
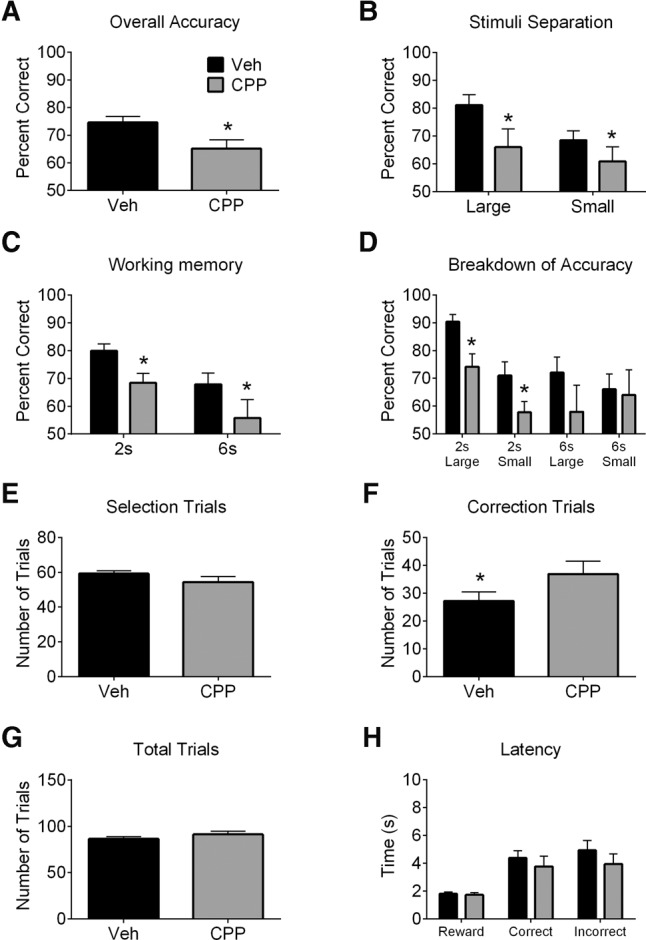
Performance of rats in the TUNL task following CPP (10 mg/kg) or vehicle (Veh) treatment. Overall accuracy (percent correct) on selection trials was reduced following CPP treatment (A) regardless of stimuli separation (B) or delay (C). (D) When trials were broken down by both stimuli separation and delay, significant main effects of treatment and delay emerged (see Results for details). (E) CPP treatment did not affect the number of selection trials but significantly increased the number of correction trials (F). (G,H) CPP treatment did not affect the number of total trials (selection trials + correction trials) or the reward latency, correct response latency, or incorrect response latency. Values are plotted as mean ± standard error of the mean. Asterisks indicate a significant effect of treatment.
Figure 2.
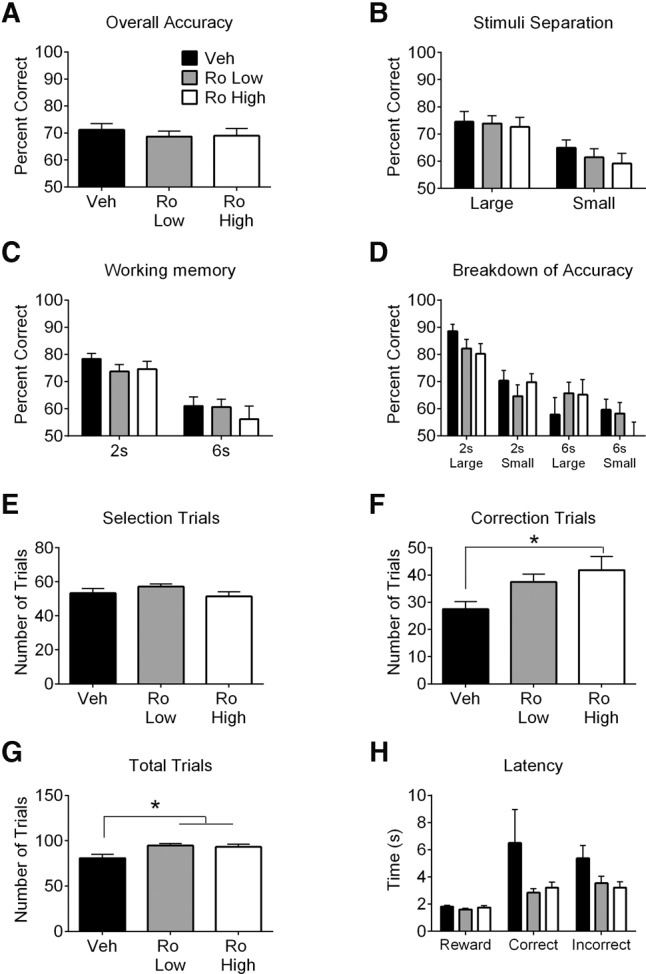
Performance of rats in the TUNL task following Ro 25-6981 (Ro Low = 6 mg/kg or Ro high = 10 mg/kg) or vehicle (Veh) treatment. Ro 25-6981 did not affect accuracy (percent correct) on selection trials (A) regardless of stimuli separation (B) or delay (C). (D) Ro 25-6981 did not affect accuracy when trials were considered separately by stimuli separation and delay. Ro 25-6981 treatment did not affect the number of selection trials (E) but increased correction trials relative to Veh treatment (F). (G) Ro 25-6981 (Ro Low and Ro High) increased total trials relative to Veh treatment. (H) Ro 25-6981 did not affect reward response latency, correct response latency, or incorrect response latency. Values are plotted as mean ± standard error of the mean. Asterisks indicate a significant effect of treatment.
Infusions of AP5 into mPFC impaired accuracy (Fig. 3A; t(13) = 3.56, P = 0.01). When trials were analyzed according to separation (Fig. 3B), significant main effects of stimuli separation (F(1,13) = 52.59, P < 0.001) and treatment (F(1,13) = 5.83, P = 0.03) were noted with no interaction. In addition, intra-mPFC AP5 infusions reduced selection trials (Fig. 3C; t(13) = 4.92, P < 0.001) and total trials (Fig. 3E; t(13) = 3.02, P = 0.01). The number of correction trials was not significantly influenced by AP5 infusions into mPFC (Fig. 3D). Latencies were unaffected by AP5 infusions into mPFC (Fig. 2F). AP5 infused into dmSTR impaired overall accuracy (Fig. 3A; t(13) = 2.16, P = 0.049). A main effect of stimuli separation (Fig. 3B; F(1,13) = 14.46, P = 0.002) was observed on days when dmSTR infusions were performed, although main effects of treatment (F(1,13) = 3.67, P = 0.078) and the treatment by separation interaction were not significant. Infusions of AP5 into dmSTR also decreased selection trials (Fig. 3C; t(13) = 2.86, P = 0.01) and increased the number of correction trials (Fig. 3D; t(13) = 2.67, P = 0.02). Total trials (Fig. 3E) and latencies (Fig. 3F) were unaffected by AP5 infusions into dmSTR.
Figure 3.
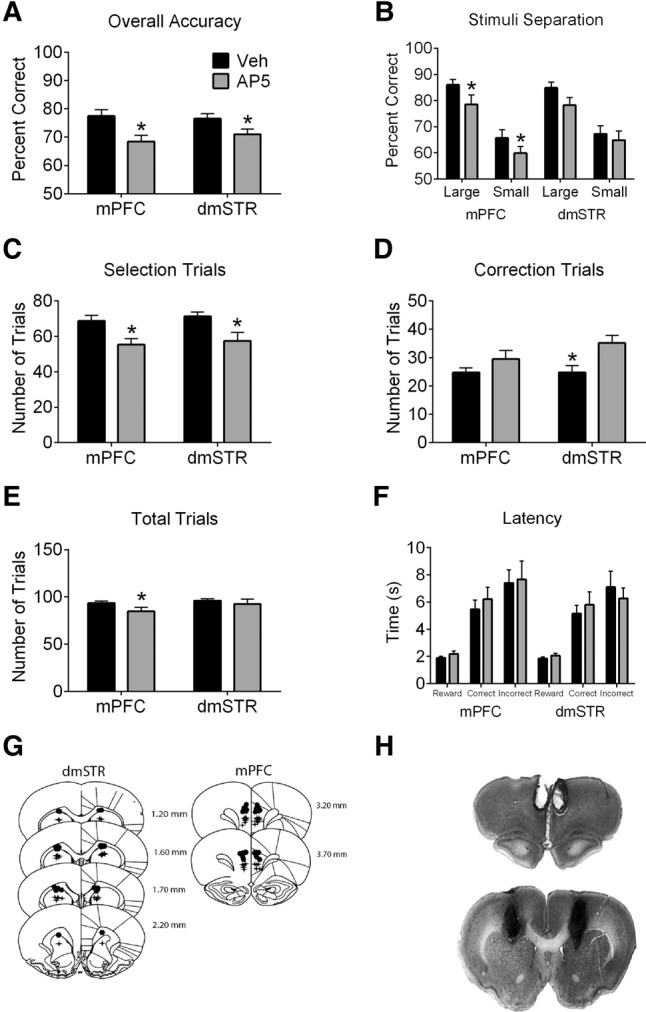
Performance of rats in the TUNL task following AP5 (1 µL of a 30 mM solution) or vehicle (Veh) infusions into mPFC or dmSTR. (A) Accuracy (percent correct) on selection trials was reduced following AP5 infusions into either site. (B) Accuracy on large and small separations was reduced with AP5 infusions into mPFC but not dmSTR. (C) AP5 infusions reduced the number of selection trials regardless of infusion site. (D) AP5 infused into dmSTR, but not mPFC, increased correction trials relative to Veh treatment. (E) AP5 treatment in mPFC, but not dmSTR, reduced the number of total trials (selection trials + correction trials). (F) AP5 did not affect reward latency, correct response latency, or incorrect response latency. (G) Representative infusion sites in the mPFC and dmSTR. Values are plotted as mean ± standard error of the mean. Circles show the locations of the ventral aspect of the guide cannulae and crosses show location of the infusion site. Numbers refer to the anterior–posterior location of plates relative to bregma. (H) A photomicrograph of representative placements from rats included in the present experiment (mPFC: top; dmSTR: bottom). Asterisks indicate a significant effect of treatment.
The present results show that systemic administration of CPP, but not Ro 25-6981, impairs accuracy on the TUNL task. These results confirm previous findings with MK-801 that TUNL is NMDA receptor dependent (Kumar et al. 2015; Hurtubise et al. 2017). In the present study and Hurtubise et al. (2017), correction trials were increased following NMDA receptor blockade. These results are consistent with other studies showing that broad NMDA receptor antagonists disrupt working memory in a variety of tasks (Li et al. 1997; Doyle et al. 1998; Moghaddam and Adams 1998; Aura and Riekkinen Jr. 1999; MacQueen et al. 2011; Rushforth et al. 2011; Smith et al. 2011; Davies et al. 2013a; Galizio et al. 2013). Interestingly, Ro 25-6981 increased correction trials in the present experiment without significant effects on task accuracy. In Kumar et al. (2015), no effects were found of the GluN2B-containing NMDA receptor antagonist CP 101-606 on the TUNL task, although correction trials were not used. Ro 25-6981 also failed to have effects in other working memory studies using operant delayed-match-to-position tasks (Doyle et al. 1998; Smith et al. 2011). However, systemic blockade of GluN2B-containing NMDA receptors impaired working memory capacity on the odor span task (Davies et al. 2013a) and GluN2B-containing NMDA receptors in monkey prefrontal cortex are important for the persistent neural firing observed during the delay phase of a working memory task (Wang et al. 2013). GluN2B-containing NMDA receptors on adult-born granule cells within the dentate gyrus also contribute to contextual discrimination in similar environments, a form of pattern separation (Kheirbek et al. 2012). In the current experiment, there was not a significant separation by treatment interaction following Ro 25-6981 suggesting that GluN2B subunit-containing NMDA receptors do not contribute to spatial pattern separation in the TUNL task. Kumar et al. (2015) showed similar findings for spatial pattern separation in the TUNL task using CP 101-606.
Local infusions of AP5 into either mPFC or dmSTR also impaired task accuracy. To the best of our knowledge, these findings are novel. Although the role of the mPFC in TUNL has been described previously (McAllister et al. 2013), the importance of NMDA receptors in mPFC has not been shown. In addition, no manipulations of dmSTR during TUNL have been reported. As the dmSTR receives substantial glutamatergic projections from the prefrontal cortex, it is logical to expect its involvement in the TUNL task. Others have shown that dmSTR is involved in working memory as lesions of the dmSTR impair working memory in a delayed-match-to-sample task (DeCoteau et al. 2004; Kesner and Gilbert 2006) and a t-maze task (Moussa et al. 2011). Smith-Roe et al. (1999) found impaired performance in a working memory task on the radial arm maze when NMDA receptors were blocked within dmSTR. NMDA receptor blockade with AP5 in the prelimbic cortex or dmSTR also impaired the ability to switch a response choice for an entire trial block in a behavioral flexibly task (Baker and Ragozzino 2014).
The face validity of the TUNL task is high relative to the CANTAB visual spatial working memory task. Human patients with PFC lesions are impaired on the CANTAB spatial working memory task (Chase et al. 2008), and in bipolar patients, enlarged caudate volumes are correlated with poorer performance (Kozicky et al. 2013). Therefore, PFC and striatum appear essential for visual spatial working memory. Our experiments provide back translation, from humans to rodents, and neurotransmitter specificity since we show that NMDA receptors within the mPFC and dmSTR are necessary for TUNL performance. Therefore, increasing the activation of NMDA receptors in the prefrontal cortex and striatum may result in new therapeutics for disorders such as schizophrenia, which are associated with frontal-striatal dysfunction and working memory impairment (Pantelis et al. 1997).
Acknowledgments
Special thanks for Dr. Wendie N. Marks and Ms. Nadine K. Zabder for help with the figures and Jillian K Catton for assisting with data collection. D.A.D., J.L.H., and Q.G. performed experiments. D.A.D. designed the experiment, analysed the data, constructed the figures, and wrote the manuscript. J.G.H. designed the experiment, analyzed the data, and wrote the manuscript. This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada to J.G.H. D.A.D. was supported by a Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarship.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.044750.116.
References
- Aura J, Riekkinen P Jr. 1999. Blockade of NMDA receptors located at the dorsomedial prefrontal cortex impairs spatial working memory in rats. Neuroreport 10: 243–248. [DOI] [PubMed] [Google Scholar]
- Baker PM, Ragozzino ME. 2014. Contralateral disconnection of the rat prelimbic cortex and dorsomedial striatum impairs cue-guided behavioral switching. Learn Mem 21: 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. 2012. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology 62: 1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Clark L, Sahakian BJ, Bullmore ET, Robbins TW. 2008. Dissociable roles of prefrontal subregions in self-ordered working memory performance. Neuropsychologia 46: 2650–2661. [DOI] [PubMed] [Google Scholar]
- Davies DA, Greba Q, Howland JG. 2013a. GluN2B-containing NMDA receptors and AMPA receptors in medial prefrontal cortex are necessary for odor span in rats. Front Behav Neurosci 7: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DA, Molder JJ, Greba Q, Howland JG. 2013b. Inactivation of medial prefrontal cortex or acute stress impairs odor span in rats. Learn Mem 20: 665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoteau WE, Hoang L, Huff L, Stone A, Kesner RP. 2004. Effects of hippocampus and medial caudate nucleus lesions on memory for direction information in rats. Behav Neurosci 118: 540–545. [DOI] [PubMed] [Google Scholar]
- Doyle KM, Feerick S, Kirkby DL, Eddleston A, Higgins GA. 1998. Comparison of various N-methyl-D-aspartate receptor antagonists in a model of short-term memory and on overt behaviour. Behav Pharmacol 9: 671–681. [DOI] [PubMed] [Google Scholar]
- Galizio M, Deal M, Hawkey A, April B. 2013. Working memory in the odor span task: effects of chlordiazepoxide, dizocilpine (MK801), morphine, and scopolamine. Psychopharmacology (Berl) 225: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland JG, Cazakoff BN. 2010. Effects of acute stress and GluN2B-containing NMDA receptor antagonism on object and object-place recognition memory. Neurobiol Learn Mem 93: 261–267. [DOI] [PubMed] [Google Scholar]
- Hurtubise JL, Marks WM, Davies DA, Catton JK, Baker GB, Howland JG. 2017. MK-801-induced impairments on the trail-unique, delayed nonmatching-to-location task in rats: effects of acute sodium nitroprusside. Psychopharmacology (Berl) 234: 211–222. [DOI] [PubMed] [Google Scholar]
- Hvoslef-Eide M, Oomen CA, Fisher BM, Heath CJ, Robbins TW, Saksida LM, Bussey TJ. 2015. Facilitation of spatial working memory performance following intra-prefrontal cortical administration of the adrenergic α1 agonist phenylephrine. Psychopharmacology (Berl) 232: 4005–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE. 2006. The role of medial caudate nucleus, but not the hippocampus, in a matching-to-sample task for a motor response. Eur J Neurosci 23: 1888–1894. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. 2012. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci 32: 8696–8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicky JM, Ha TH, Torres IJ, Bond DJ, Honer WG, Lam RW, Yatham LN. 2013. Relationship between frontostriatal morphology and executive function deficits in bipolar I disorder following a first manic episode: data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM). Bipolar Disord 15: 657–668. [DOI] [PubMed] [Google Scholar]
- Kumar G, Olley J, Steckler T, Talpos J. 2015. Dissociable effects of NR2A and NR2B NMDA receptor antagonism on cognitive flexibility but not pattern separation. Psychopharmacology (Berl) 232: 3991–4003. [DOI] [PubMed] [Google Scholar]
- Li HB, Matsumoto K, Yamamoto M, Watanabe H. 1997. NMDA but not AMPA receptor antagonists impair the delay-interposed radial maze performance of rats. Pharmacol Biochem Behav 58: 249–253. [DOI] [PubMed] [Google Scholar]
- MacQueen DA, Bullard L, Galizio M. 2011. Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiol Learn Mem 95: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister KA, Saksida LM, Bussey TJ. 2013. Dissociation between memory retention across a delay and pattern separation following medial prefrontal cortex lesions in the touchscreen TUNL task. Neurobiol Learn Mem 101: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. 1998. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281: 1349–1352. [DOI] [PubMed] [Google Scholar]
- Monaco SA, Gulchina Y, Gao WJ. 2015. NR2B subunit in the prefrontal cortex: a double-edged sword for working memory function and psychiatric disorders. Neurosci Biobehav Rev 56: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa R, Poucet B, Amalric M, Sargolini F. 2011. Contributions of dorsal striatal subregions to spatial alternation behavior. Learn Mem 18: 444–451. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, Bussey TJ, Saksida LM. 2013. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc 8: 2006–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Hvoslef-Eide M, Kofink D, Preusser F, Mar AC, Saksida LM, Bussey TJ. 2015. A novel 2- and 3-choice touchscreen-based continuous trial-unique nonmatching-to-location task (cTUNL) sensitive to functional differences between dentate gyrus and CA3 subregions of the hippocampus. Psychopharmacology (Berl) 232: 3921–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC. 2006. Biologically based computational models of high-level cognition. Science 314: 91–94. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barnes TR, Nelson HE, Tanner S, Weatherley L, Owen AM, Robbins TW. 1997. Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain 120(Pt 10): 1823–1843. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1997. The rat brain in stereotaxic coordinates. Elsevier Academic Press, Amsterdam. [Google Scholar]
- Rushforth SL, Steckler T, Shoaib M. 2011. Nicotine improves working memory span capacity in rats following sub-chronic ketamine exposure. Neuropsychopharmacology 36: 2774–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, Malik N, Tricklebank M. 2011. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology (Berl) 217: 255–269. [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Sadeghian K, Kelley AE. 1999. Spatial learning and performance in the radial arm maze is impaired after N-methyl-D-aspartate (NMDA) receptor blockade in striatal subregions. Behav Neurosci 113: 703–717. [DOI] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ. 2010. Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol Learn Mem 94: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NG, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. 2013. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77: 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. 2006. Learning induces long-term potentiation in the hippocampus. Science 313: 1093–1097. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bartko SJ, Saksida LM, Bussey TJ. 2010. Muscimol, AP5, or scopolamine infused into perirhinal cortex impairs two-choice visual discrimination learning in rats. Neurobiol Learn Mem 93: 221–228. [DOI] [PubMed] [Google Scholar]


