Abstract
Essential oils are one of the most notorious natural products used for medical purposes. Combined with their popular use in dermatology, their availability, and the development of antimicrobial resistance, commercial essential oils are often an option for therapy. At least 90 essential oils can be identified as being recommended for dermatological use, with at least 1500 combinations. This review explores the fundamental knowledge available on the antimicrobial properties against pathogens responsible for dermatological infections and compares the scientific evidence to what is recommended for use in common layman's literature. Also included is a review of combinations with other essential oils and antimicrobials. The minimum inhibitory concentration dilution method is the preferred means of determining antimicrobial activity. While dermatological skin pathogens such as Staphylococcus aureus have been well studied, other pathogens such as Streptococcus pyogenes, Propionibacterium acnes, Haemophilus influenzae, and Brevibacterium species have been sorely neglected. Combination studies incorporating oil blends, as well as interactions with conventional antimicrobials, have shown that mostly synergy is reported. Very few viral studies of relevance to the skin have been made. Encouragement is made for further research into essential oil combinations with other essential oils, antimicrobials, and carrier oils.
1. Introduction
The skin is the body's largest mechanical barrier against the external environment and invasion by microorganisms. It is responsible for numerous functions such as heat regulation and protecting the underlying organs and tissue [1, 2]. The uppermost epidermal layer is covered by a protective keratinous surface which allows for the removal of microorganisms via sloughing off of keratinocytes and acidic sebaceous secretions. This produces a hostile environment for microorganisms. In addition to these defences, the skin also consists of natural microflora which offers additional protection by competitively inhibiting pathogenic bacterial growth by competing for nutrients and attachment sites and by producing metabolic products that inhibit microbial growth. The skin's natural microflora includes species of Corynebacterium, staphylococci, streptococci, Brevibacterium, and Candida as well as Propionibacterium [3–8].
In the event of skin trauma from injuries such as burns, skin thinning, ulcers, scratches, skin defects, trauma, or wounds, the skin's defence may be compromised, allowing for microbial invasion of the epidermis resulting in anything from mild to serious infections of the skin. Common skin infections caused by microorganisms include carbuncles, furuncles, cellulitis, impetigo, boils (Staphylococcus aureus), folliculitis (S. aureus, Pseudomonas aeruginosa), ringworm (Microsporum spp., Epidermophyton spp., and Trichophyton spp.), acne (P. acnes), and foot odour (Brevibacterium spp.) [3, 8–11]. Environmental exposure, for example, in hospitals where nosocomial infections are prominent and invasive procedures make the patient vulnerable, may also create an opportunity for microbial infection. For example, with the addition of intensive therapy and intravascular cannulae, S. epidermidis can enter the cannula and behave as a pathogen causing bloodborne infections. Noninfective skin diseases such as eczema can also result in pathogenic infections by damaging the skin, thus increasing the risk of secondary infection by herpes simplex virus and/or S. aureus [5, 8, 12].
Skin infections constitute one of the five most common reasons for people to seek medical intervention and are considered the most frequently encountered of all infections. At least six million people worldwide are affected by chronic wounds and up to 17% of clinical visits are a result of bacterial skin infections and these wounds are a frequent diagnosis for hospitalised patients. These are experienced daily and every doctor will probably diagnose at least one case per patient. Furthermore, skin diseases are a major cause of death and morbidity [8, 13, 14]. The healing rate of chronic wounds is affected by bacterial infections (such as S. aureus, E. coli, and P. aeruginosa), pain, inflammation, and blood flow, and thus infection and inflammation control may assist in accelerating healing [15–17].
Topical skin infections typically require topical treatment; however, due to the ability of microbes to evolve and due to the overuse and incorrect prescribing of the current available conventional antimicrobials, there has been emergence of resistance in common skin pathogens such as S. aureus resulting as methicillin-resistant Staphylococcus aureus (MRSA) and other such strains. Treatment has therefore become a challenge and is often not successful [8, 18, 19]. In some regions of the world, infections are unresponsive to all known antibiotics [20]. This threat has become so severe that simple ulcers now require treatment with systemic antibiotics [21]. A simple cut on the finger or a simple removal of an appendix could result in death by infection. The World Health Organization (WHO) has warned that common infections may be left without a cure as we are headed for a future without antibiotics [22]. Therefore, one of the solutions available is to make use of one of the oldest forms of medicine, natural products, to treat skin infections and wounds [18, 23].
Complementary and alternative medicines (CAMs) are used by 60–80% of developing countries as they are one of the most prevalent sources of medicine worldwide [24–27]. Essential oils are also one of the most popular natural products, with one of their main applications being for their use in dermatology [28–30]. In fact, of all CAMs, essential oils are the most popular choice for treating fungal skin infections [13, 31]. Their use in dermatology, in the nursing profession, and in hospitals has been growing with great popularity worldwide, especially in the United States and the United Kingdom [1, 27, 32–35]. Furthermore, the aromatherapeutic literature [1, 2, 26, 32, 36–43] identifies numerous essential oils for dermatological use, the majority of which are recommended for infections. This brought forth the question as to the efficacy of commercial essential oils against the pathogens responsible for infections. The aim of this review was to collect and summarise the in vivo, in vitro, and clinical findings of commercial essential oils that have been tested against infectious skin diseases and their pathogens and, in doing so, offer aromatherapists and dermatologists valuable information regarding the effectiveness of essential oils for dermatological infections.
The readily available aromatherapeutic literature has reported over 90 (Table 1) commercial essential oils that may be used for treating dermatological conditions [1, 2, 26, 32, 36–43]. An overview of the skin related uses can be seen in Figure 1. Essential oils are mostly used for the treatment of infections caused by bacteria, fungi, or viruses (total 62%). This is followed by inflammatory skin conditions (20%) such as dermatitis, eczema, and lupus and then general skin maintenance (18%) such as wrinkles, scars, and scabs, which are the third most common use of essential oils. Other applications include anti-inflammatory and wound healing applications (Figure 1). Of the 98 essential oils recommended for dermatological use, 88 are endorsed for treating skin infections. Of these, 73 are used for bacterial infections, 49 specifically for acne, 34 for fungal infections, and 16 for viral infections.
Table 1.
Essential oils used in dermatology.
| Scientific name | Common name | Dermatological use | Reference |
|---|---|---|---|
| Abies balsamea | Balsam (Peru, Canadian) | Burns ∗, cracks, cuts, eczema, rashes, sores, and wounds | [32] |
|
| |||
| Abies balsamea | Fir | Skin tonic | [36] |
|
| |||
| Acacia dealbata | Mimosa | Antiseptic, general care, oily conditions, and nourisher | [2, 32] |
|
| |||
| Acacia farnesiana | Cassie | Dry or sensitive conditions | [32] |
|
| |||
| Achillea millefolium | Yarrow | Acne, burns, chapped skin, cuts, dermatitis, eczema, healing agent, infections, inflammation, oily conditions, pruritus, rashes, scars, toner, sores, ulcers, and wounds | [32, 36, 40, 42] |
|
| |||
| Allium sativum | Garlic | Acne, antiseptic, fungal infections (ringworm), lupus, septic wounds, and ulcers | [32, 36] |
|
| |||
| Amyris balsamifera | Amyris | Inflammation | [36] |
|
| |||
| Anethum graveolens | Dill | Wound healing encouragement | [36] |
|
| |||
| Angelica archangelica | Angelica | Congested and dull conditions, fungal infections, inflammation, psoriasis, and tonic | [32, 36] |
|
| |||
| Aniba rosaeodora | Rosewood | Acne, congested conditions, cuts, damaged skin, dermatitis, general care, greasy and oily conditions, inflammation, psoriasis, scars, regeneration, sores, wounds, and wrinkles | [2, 32, 36, 37, 39, 41, 42] |
|
| |||
| Anthemis nobilis | Roman chamomile | Abscesses, acne, allergies, antiseptic, blisters, boils, burns, cleanser, cuts, dermatitis, eczema, foot blisters, general care, herpes, inflammation, insect bites and stings, nappy rash, nourisher, problematic skin, pruritus, psoriasis, rashes, rosacea, sores, sunburn, ulcers, and wounds | [2, 26, 32, 36–43] |
|
| |||
| Apium graveolens | Celery | Reducing puffiness and redness | [36] |
|
| |||
| Artemisia dracunculus | Tarragon | Infectious wounds | [36] |
|
| |||
| Betula alba | Birch (white) | Congested conditions, dermatitis, eczema, psoriasis, and ulcers | [32, 36] |
|
| |||
| Boswellia carteri | Frankincense/olibanum | Abscesses, acne, aged or dry and damaged complexions, antiseptic, bacterial infections, blemishes, carbuncles, dermatitis, disinfectant, eczema, fungal and nail infections, general care, healing agent, inflammation, oily conditions, psoriasis, problematic conditions, regeneration or rejuvenation, scars, sores, toner, tonic, ulcers, wounds, and wrinkles | [1, 2, 32, 36–43] |
|
| |||
| Bursera glabrifolia | Linaloe (copal) | Acne, conditioning, cuts, dermatitis, sores, and wounds | [32, 40] |
|
| |||
| Calendula officinalis | Marigold | Athlete's foot, burns, cuts, diaper rash, eczema, fungal infections, inflammation, oily and greasy conditions, and wounds | [26, 32, 39] |
|
| |||
| Cananga odorata | Ylang-ylang | Acne, balancing sebum, dermatitis, eczema, general care, greasy and oily conditions, insect bites, and toner | [2, 32, 36–38, 40, 42, 43] |
|
| |||
| Canarium luzonicum | Elemi | Aged and dry complexions, bacterial infections, balancing sebum, cuts, fungal infections, inflammation, sores, ulcers, wounds, and wrinkles | [32, 36, 40] |
|
| |||
| Carum carvi | Caraway | Acne, boils, infected wounds, oily conditions, and pruritus | [36] |
|
| |||
| Cedrus atlantica | Cedar wood | Acne, antiseptic, ∗bromodosis, cellulite, cracked skin, dandruff, dermatitis, eczema, eruptions, fungal infections, general care, genital infections, greasy and oily conditions, inflammation, insect bites and stings, psoriasis, scabs, and ulcers | [1, 2, 32, 36–39, 41–43] |
|
| |||
| Cinnamomum camphora | Camphor (white) | Acne, burns, inflammation, oily conditions, spots, and ulcers | [32, 36, 42] |
|
| |||
| Cinnamomum zeylanicum | Cinnamon | Antiseptic, gum and tooth care, warts, and wasp stings | [32, 36, 37, 41, 42] |
|
| |||
| Cistus ladanifer | Rock rose/Cistus/labdanum | Aged complexion, bacterial infections, bedsores, blocked pores, eczema, oily conditions, sores, ulcers, varicose ulcers, wounds, and wrinkles | [2, 32, 40] |
|
| |||
| Citrus aurantifolia | Lime | Acne, bacterial infections, boils, cellulite, congested or greasy and oily conditions, cuts, insect bites, pruritus, tonic, sores, ulcers, warts, and wounds | [2, 32, 36, 40–43] |
|
| |||
| Citrus aurantium var. amara | Neroli | Acne, aged and dry complexions, antiseptic, broken capillaries, cuts, dermatitis, eczema, general care, healing agent, psoriasis, scars, stretch marks, toner, tonic, thread veins, wounds, and wrinkles | [2, 26, 32, 36–43] |
|
| |||
| Citrus aurantium var. amara | Petitgrain | Acne, antiseptic, bacterial infections, balancing sebum, blemishes, greasy and oily conditions, ∗∗ hyperhidrosis, pimples, pressure sores, sensitive complexions, toner, tonic, and wounds | [1, 2, 32, 36, 37, 39–42] |
|
| |||
| Citrus bergamia | Bergamot | Abscesses, acne, antiseptic, athlete's foot, bacterial infections, blisters, boils, cold sores, deodorant, dermatitis, eczema, fungal infections, greasy and oily conditions, healing agent, inflammation, insect bites, pruritus, psoriasis, shingles, ulcers, viral infections (chicken pox, herpes, and shingles), and wounds | [2, 26, 32, 36, 37, 40–43] |
|
| |||
| Citrus limon | Lemon | Abscesses, acne, antiseptic, athlete's foot, blisters, boils, cellulite, corns, cuts, grazes, greasy and oily conditions, insect bites, mouth ulcers, rosacea, sores,ulcers, viral infections (cold sores, herpes, verrucae,and warts), and wounds | [1, 2, 26, 32, 36, 37, 39, 41–43] |
|
| |||
| Citrus paradisi | Grapefruit | Acne, antiseptic, cellulite improvement, cleanser, combination and problematic skin, congested and oily conditions, stretch marks, and toner | [1, 2, 32, 36, 37, 39–43] |
|
| |||
| Citrus reticulata | Mandarin | Acne, cellulite, congested and oily conditions, general care, healing agent, scars, stretch marks, and toner | [1, 32, 36–38, 40, 43] |
|
| |||
| Citrus sinensis | Orange | Acne, blocked pores, congested and oily conditions, dermatitis, dry and dull complexions, problematic skin, ulcers, and wrinkles | [1, 32, 36–38, 40–43] |
|
| |||
| Citrus tangerina | Tangerine | Acne, chapped skin, inflammation, oily conditions, rashes, stretch marks, and toner | [36, 40, 42] |
|
| |||
| Commiphora myrrha | Myrrh | Acne, antiseptic, athlete's foot, bacterial infections, bedsores, boils, cracked skin, cuts, dermatitis, eczema, fungal infections (athlete's foot, ringworm), healing agent, inflammation, scars, sores, ulcers, weeping wounds, and wrinkles | [1, 2, 26, 32, 36–43] |
|
| |||
| Coriandrum sativum | Coriander | Used to prevent the growth of odour causing bacteria | [37] |
|
| |||
| Cupressus sempervirens | Cypress | Acne, blocked pores, bromodosis, cellulite, cellulitis, deodorant, hyperhidrosis, oily conditions, rashes, rosacea, and wounds | [1, 2, 32, 36–38, 40–43] |
|
| |||
| Curcuma longa | Turmeric | Cuts, sores, and wounds | [40] |
|
| |||
| Cymbopogon citratus | Lemongrass | Acne, athlete's foot, bacterial infections, blocked or open pores, cellulite, fungal infections, hyperhidrosis, oily conditions, and toner | [2, 32, 36, 37, 41, 42] |
|
| |||
| Cymbopogon martinii | Palmarosa | Acne, bacterial infections, balancing sebum, damaged and dry complexions, dermatitis, eczema, fungal infections, oily conditions, pressure sores, psoriasis, scars, toner, tonic, sores, wounds, and wrinkles | [2, 32, 36–42] |
|
| |||
| Cymbopogon nardus | Citronella | Bromodosis, hyperhidrosis, oily conditions, and softener | [32, 36, 42] |
|
| |||
| Daucus carota | Carrot seed | Aged and dry complexions, carbuncles, dermatitis, eczema, inflammation, oily conditions, pruritus, psoriasis, rashes, scarring, toner, ulcers, vitiligo, weeping sores, wounds, and wrinkles | [2, 32, 36, 40, 42] |
|
| |||
| Dryobalanops aromatica | Borneol (Borneo Camphor) | Cuts and sores | [32] |
|
| |||
| Eucalyptus globulus | Eucalyptus | Abscesses, antiseptic, athlete's foot, bacterial dermatitis, bacterial infections, blisters, boils, burns, chicken pox, cleanser, congested conditions, cuts, fungal infections, general infections, herpes (cold sores), inflammation, insect bites, shingles, sores, ulcers, and wounds | [1, 26, 32, 36–39, 41–43] |
|
| |||
| Syzygium aromaticum | Clove | Acne, antiseptic, athlete's foot, burns, cuts, cold sores, fungal infections, lupus, sores, septic ulcers, and wounds | [32, 36, 37, 41, 42] |
|
| |||
| Ferula galbaniflua | Galbanum | Abscesses, acne, blisters, boils, cuts, inflammation, scar tissue improvement, toner, and wounds | [32, 36] |
|
| |||
| Foeniculum dulce | Fennel | Aged and wrinkled complexions, bromodosis, cellulite, cellulitis, congested, greasy, and oily conditions, cleanser, and tonic | [1, 32, 36, 37, 40–43] |
|
| |||
| Guaiacum officinale | Guaiacwood | Firming or tightening the skin | [36] |
|
| |||
| Helichrysum italicum | Immortelle/everlasting/Helichrysum | Abscesses, acne, athlete's foot, bacterial infections, boils, blisters, cell regeneration, cuts, damaged skin conditions, dermatitis, eczema, fungal infections (ringworm), inflammation, psoriasis, rosacea, scars, sores, ulcers, and wounds | [2, 32, 36, 40, 41] |
|
| |||
| Humulus lupulus | Hops | Dermatitis, ulcers, rashes, and nourisher | [32] |
|
| |||
| Hyssopus officinalis | Hyssop | Cuts, dermatitis, eczema, healing agent, inflammation, scars, sores, and wounds | [32, 36, 41] |
|
| |||
| Jasminum officinale | Jasmine | Aged and dry complexions, general care, inflammation, revitalization, oily conditions, and psoriasis | [2, 26, 32, 36, 37, 40] |
|
| |||
| Juniperus virginiana | Juniper | Acne, antiseptic, blocked pores, cellulite, congested and oily conditions, deodorant, eczema, dermatitis, general care, general infections, psoriasis, toner, ulcers, weeping eczema, and wounds | [1, 2, 32, 36, 37, 39, 41–43] |
|
| |||
| Juniperus oxycedrus | Cade | Cuts, dermatitis, eczema, sores, and spots | [32] |
|
| |||
| Kunzea ericoides | Kānuka | Athlete's foot | [40] |
|
| |||
| Laurus nobilis | Bay | Acne, fungal infections, inflammation, oily conditions, pressure sores, and varicose ulcers | [32, 36, 41] |
|
| |||
| Lavandula angustifolia | Lavender | Abscesses, acne, antiseptic, bacterial infections, blisters, boils, burns, carbuncles, cellulite, congested and oily conditions, cuts, deodorant, dermatitis, eczema, foot blisters, fungal infections (athlete's foot, ringworm), general care, healing agent, inflammation, insect bites and stings, pressure sores, pruritus, psoriasis, rosacea, scalds, scarring, sores, sunburn, ulcers,viral infections (chicken pox, cold sores, shingles,and warts), and wounds | [2, 26, 32, 36–43] |
|
| |||
| Lavandula flagrans | Lavandin | Acne, abscesses, boils, blisters, congested conditions, cuts, eczema, healing agent, inflammation, insect bites and stings, pressure sores, scalds, sores, and wounds | [32, 36, 41] |
|
| |||
| Lavandula spica | Lavender spike | Abscesses, acne, bacterial infections, blisters, boils, burns, congested and oily conditions, cuts, dermatitis, eczema, inflammation, fungal infections (athlete's foot, ringworm), pressure sores, psoriasis, sores, ulcers, and wounds | [32, 36, 41] |
|
| |||
| Leptospermum scoparium | Manuka | Acne, cuts, fungal infections (athlete's foot, ringworm), ulcers, and wounds | [2, 40] |
|
| |||
| Verbena officinalis | Verbena | Congested conditions and nourisher | [36] |
|
| |||
| Liquidambar orientalis | Sweetgum | Cuts, ringworm, sores, and wounds | [32] |
|
| |||
| Litsea cubeba | May Chang | Acne, dermatitis, greasy and oily conditions, and hyperhidrosis | [32, 36] |
|
| |||
| Melaleuca alternifolia | Tea tree | Abrasions, abscesses, acne, antiseptic, bacterial infections, blemishes, blisters, boils, burns, carbuncles, cuts, dandruff, fungal infections (athlete's foot, nails, ringworm, and tinea), inflammation, insect bites, oily conditions, rashes, sores, spots, sunburn, ulcers, viral infections (cold sores, chicken pox, herpes, shingles,and warts), and wounds | [1, 2, 26, 32, 36–43] |
|
| |||
| Melaleuca cajuputi | Cajuput | Acne, insect bites, oily conditions, psoriasis, and spots | [32, 36, 42] |
|
| |||
| Melaleuca viridiflora | Niaouli/Gomenol | Abscesses, acne, antiseptic, bacterial infections, blisters, boils, burns, chicken pox, congested and oily conditions, cuts, eruptions, healing agent, insect bites, psoriasis, sores, ulcers, and wounds | [2, 32, 36, 39–42] |
|
| |||
| Melissa officinalis | Melissa/lemon balm | Allergic reactions, cold sores, eczema, fungal infections, inflammation, insect stings, ulcers, and wounds | [1, 26, 32, 36, 41, 42] |
|
| |||
| Mentha piperita | Peppermint | Acne, antiseptic, blackheads, chicken pox, congested and greasy conditions, dermatitis, inflammation, pruritus, ringworm, scabies, softener, toner, and sunburn | [1, 2, 32, 36, 37, 41–43] |
|
| |||
| Mentha spicata | Spearmint | Acne, congested conditions, dermatitis, pruritus, scabs, and sores | [32, 36, 39, 42] |
|
| |||
| Myristica fragrans | Nutmeg | Hair conditioner | [36] |
|
| |||
| Myrocarpus fastigiatus | Cabreuva | Cuts, scars, and wounds | [32] |
|
| |||
| Myrtus communis | Myrtle | Acne, antiseptic, blemishes, blocked pores, bruises, congested and oily conditions, and psoriasis | [2, 32, 36, 40] |
|
| |||
| Nardostachys jatamansi | Spikenard | Eczema, inflammation, psoriasis, and sores | [32, 40] |
|
| |||
| Ocimum basilicum | Basil | Acne, antiseptic, congested conditions, insect bites, and wasp stings | [1, 36, 37, 39, 40, 42] |
|
| |||
| Origanum majorana | Marjoram | Bruises and fungal infections | [32, 36] |
|
| |||
| Origanum vulgare | Oregano | Athlete's foot, bacterial infections, cuts, eczema, fungal infections, psoriasis, warts, and wounds | [36, 41] |
|
| |||
| Pelargonium odoratissimum | Geranium | Acne, aged and dry complexions, bacterial infections, balancing sebum, burns, cellulite, chicken pox, congested and oily conditions, cracked skin, cuts, dermatitis, deodorant, eczema, fungal infections (athlete's foot, ringworm), general care, healing agent, herpes, impetigo, inflammation, measles, psoriasis, rosacea, shingles, problematic skin, sores, ulcers, and wounds | [2, 26, 32, 36–43] |
|
| |||
| Pelargonium roseum | Rose geranium | Aging and dry or wrinkled skin | [40] |
|
| |||
| Petroselinum sativum | Parsley | Bruises, scalp conditioning, and wounds | [36] |
|
| |||
| Pimpinella anisum | Anise | Infectious diseases | [36] |
|
| |||
| Pinus sylvestris | Pine | Antiseptic, bromodosis, congested conditions, cuts, eczema, hyperhidrosis, pruritus, psoriasis, and sores | [32, 36, 37, 41–43] |
|
| |||
| Piper nigrum | Black pepper | Bruises and fungal infections | [36, 42] |
|
| |||
| Pistacia lentiscus | Mastic | Abscesses, blisters, boils, cuts, ringworm, and wounds | [32] |
|
| |||
| Pistacia palaestina | Terebinth | Abscesses, blisters, boils, cuts, infectious wounds, ringworm, and sores | [32, 36] |
|
| |||
| Pogostemon patchouli | Patchouli | Abscesses, acne, chapped or damaged and cracked skin, dermatitis, cold sores, eczema, fungal infections (athlete's foot), general care, healing agent, impetigo, inflammation, oily conditions, pruritus, scalp disorders, scars, sores, tonic, stretch marks, and wounds | [1, 2, 32, 36–43] |
|
| |||
| Rosa damascena | Rose otto | Aging and dry conditions, bacterial infections, eczema, inflammation, toner, tonic, and wounds | [2, 38–41] |
|
| |||
| Rosa gallica | Rose | Broken capillaries, cuts, dry and aging conditions, burns, eczema, healing agent, inflammation, pruritus, psoriasis, scars, toner, tonic, stretch marks, sunburn, thread veins, and wrinkles | [26, 32, 36–38, 42, 43] |
|
| |||
| Rosmarinus officinalis | Rosemary | Acne, bacterial infections, balancing sebum, cellulite, congested and oily conditions, dandruff, dermatitis, dry scalp, eczema, general care, and rosacea | [1, 32, 36, 37, 39, 41, 42] |
|
| |||
| Salvia lavandulifolia | Spanish sage | Acne, antiseptic, bacterial infections, cellulite, cold sores, cuts, dermatitis, deodorant, hyperhidrosis, oily conditions, psoriasis, sores, and ulcers | [32, 36, 37, 41] |
|
| |||
| Salvia sclarea | Clary sage | Abscesses, acne, balancing sebum, blisters, boils, cell regeneration, dandruff, dermatitis, greasy and oily conditions, hyperhidrosis of the feet, inflammation, ulcers, and wrinkles | [1, 2, 32, 36, 40, 42] |
|
| |||
| Santalum album | Sandalwood | Acne, antiseptic, bacterial infections, boils, burns, chapped or damaged and dry conditions, eczema, fungal infections, general care, greasy and oily conditions, inflammation, pruritus, sunburn, and wounds | [1, 2, 26, 32, 36–39, 41–43] |
|
| |||
| Santolina chamaecyparissus | Santolina | Inflammation, pruritus, ringworm, scabs, verrucae, and warts | [36] |
|
| |||
| Styrax benzoin | Benzoin | Cracks, cuts, dermatitis, eczema, healing, inflammation, injured and irritated conditions, pruritus, sores, and wounds | [1, 2, 32, 36, 40, 42] |
|
| |||
| Tagetes minuta | Tagetes | Bacterial infections, fungal infections, inflammation, and viral infections (verrucae and warts) | [32, 36, 42] |
|
| |||
| Thymus vulgaris | Thyme | Abscesses, acne, antiseptic, blisters, burns, carbuncles, cellulitis, cuts, deodorant, dermatitis, eczema, fungal infections, oily conditions, sores, and wounds | [1, 32, 36, 37, 41, 42] |
|
| |||
| Tilia europaea | Linden Blossom | Blemishes, burns, freckles, softener, tonic, and wrinkles | [36] |
|
| |||
| Vetiveria zizanioides | Vetiver | Acne, antiseptic, balancing sebum, cuts, eczema, malnourished and aging skin, oily conditions, weeping sores, and wounds | [1, 2, 32, 36, 37, 41, 42] |
|
| |||
| Viola odorata | Violet | Acne, bruises, congested and oily conditions, eczema, inflammation, infections, ulcers, and wounds | [2, 32, 36, 40] |
|
| |||
| Zingiber officinale | Ginger | Bruises, carbuncles, and sores | [36] |
∗Conditions involved in dermatological infections are shown in italics.
∗∗A medical condition that causes excessive sweating.
Figure 1.
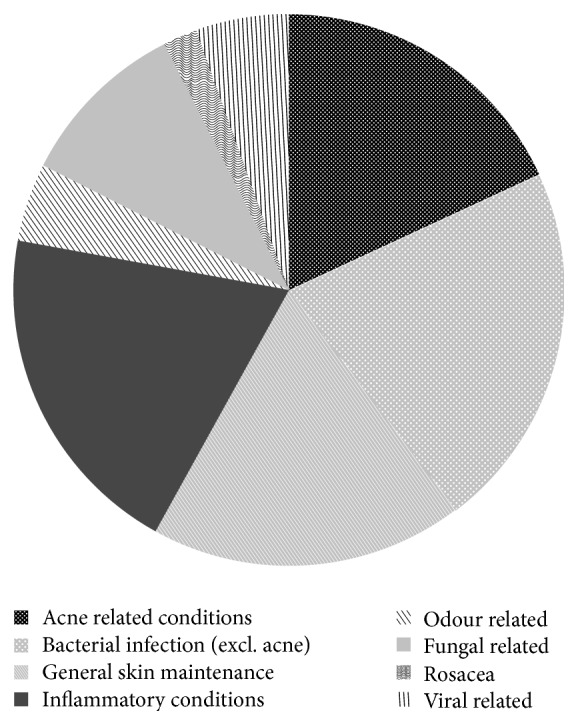
Summary of categorised dermatological conditions in which essential oils are used.
2. Materials and Methods
2.1. Searching Strategy/Selection of Papers
The aim of the comparative review was to identify the acclaimed dermatological commercial essential oils according to the aromatherapeutic literature and then compare and analyse the available published literature. This will serve as a guideline in selecting appropriate essential oils in treating dermatological infections. The analysed papers were selected from three different electronic databases: PubMed, ScienceDirect, and Scopus, accessed during the period 2014–2016. The filters used included either “essential oils”, “volatile oils”, or “aromatherapy” or the scientific or common name for each individual essential oil listed in Table 1 and the additional filters “antimicrobial”, “antibacterial”, “skin”, “infection”, “dermatology”, “acne”, “combinations”, “fungal infections”, “dermatophytes”, “Brevibacteria”, “odour”, “antiviral”, “wounds”, “dermatitis”, “allergy”, “toxicity”, “sentitisation”, or “phototoxicity”.
2.2. Inclusion Criteria
In order to effectively understand the possible implications and potential of essential oils, the inclusion criteria were broad, especially with this being the first review to collate this amount of scientific evidence with the aromatherapeutic literature. Inclusion criteria included the following:
Type of in vitro studies for bacterial and fungal pathogens by means of the microdilution assay, macrodilution assay, or the agar dilution assay
In vivo studies
Antiviral studies
Case reports
Animal studies
All clinical trials
2.3. Exclusion Criteria
Papers or pieces of information were excluded for the following reasons:
Lack of accessibility to the publication
If the incorrect in vitro technique (diffusion assays) was employed
Indigenous essential oils with no relevance to commercial oils
If they were in a language not understood by the authors of the review
Pathogens studied not relevant to skin disease
2.4. Data Analysis
The two authors (Ané Orchard and Sandy van Vuuren) conducted their own data extraction independently, after which critical analysis was applied. Information was extrapolated and recorded and comments were made. Observations were made and new recommendations were made as to future studies.
3. Results
3.1. Description of Studies
After the initial database search, 1113 reports were screened. Duplicates were removed, which brought the article count down to 513, after which the abstracts were then read and additional reports removed based on not meeting the inclusion criteria. A final number of 349 articles were read and reviewed. Of these, 143 were in vitro bacterial and fungal studies (individual oil and 45 combinations), two in vivo studies, 15 antiviral studies, 19 clinical trials, and 32 toxicity studies. The process that was followed is summarised in Figure 2.
Figure 2.
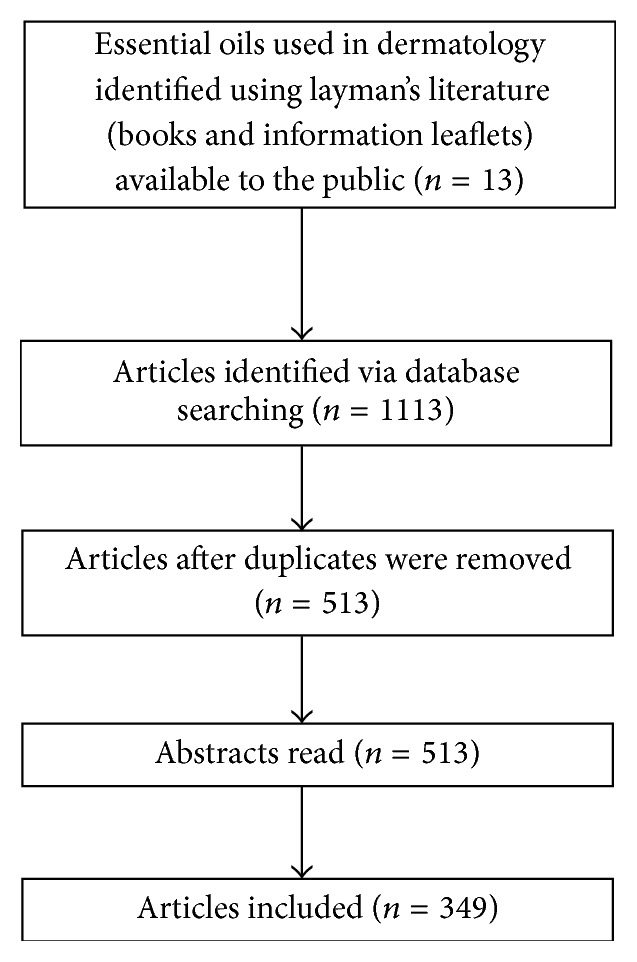
Flow diagram of the review approach.
3.2. Experimental Approaches
3.2.1. Chemical Analysis
Essential oils are complex organic (carbon containing) chemical entities, which are generally made up of hundreds of organic chemical compounds in combination that are responsible for the essential oil's many characteristic properties. These characteristics may include medicinal properties, such as anti-inflammatory, healing, or antimicrobial activities, but may also be responsible for negative qualities such as photosensitivity and toxicity [37].
Even with the high quality grade that is strived for in the commercial sector of essential oil production, it must be noted that it is still possible for essential oil quality to display discrepancies, changes in composition, or degradation. The essential oil composition may even vary between the same species [1, 44]. This may be due to a host of different factors such as the environment or location that the plants are grown in, the harvest season, which part of the plant was used, the process of extracting the essential oil, light or oxygen exposure, the storage of the oil, and the temperature the oil was exposed to [45–51].
Gas chromatography in combination with mass spectrometry (GCMS) is the preferred technique for analysis of essential oils [52]. This is a qualitative and quantitative chemical analysis method which allows for the assurance of the essential oil quality through the identification of individual compounds that make up an essential oil [1, 45, 53]. It has clearly been demonstrated that there is a strong correlation between the chemical composition and antimicrobial activity [51, 54, 55]. Understanding the chemistry of essential oils is essential for monitoring essential oil composition, which then further allows for a better understanding of the biological properties of essential oils. It is recommended to always include the chemical composition in antimicrobial studies [56].
3.3. Antimicrobial Investigations
Several methods exist that may be employed for antimicrobial analysis, with two of the most popular methods being the diffusion and the dilution methods [56–59].
3.3.1. Diffusion Method
There are two types of diffusion assays. Due to the ease of application, the disc diffusion method is one of the most commonly used methods [60]. This is done by applying a known concentration of essential oil onto a sterile filter paper disc. This is then placed onto agar which has previously been inoculated with the microorganism to be tested, or it is spread on the surface. If necessary, the essential oil may also be dissolved in an appropriate solvent. The other diffusion method is the agar diffusion method, where, instead of discs being placed, wells are made in the agar into which the essential oil is instilled. After incubation, antimicrobial activity is then interpreted from the zone of inhibition (measured in millimetres) using the following criteria: weak activity (inhibition zone ≤ 12 mm), moderate activity (12 mm < inhibition zone < 20 mm), and strong activity (inhibition zone ≤ 20 mm) [24, 60–62].
Although this used to be a popular method, it is more suitable to antibiotics rather than essential oils as it does not account for the volatile nature of the essential oils. Essential oils also diffuse poorly through an aqueous medium as they are hydrophobic. Thus, the results are less reliable as they are influenced by the ability of the essential oil to diffuse through the agar medium, resulting in variable results, false negatives, or a reduction in antimicrobial activity [24, 63]. The results have been found to vary significantly when tested this way and are also influenced by other factors such as disc size, amount of compound applied to the disc, type of agar, and the volume of agar [57, 59, 64–68]. It has thus been recommended that results are only considered where the minimum inhibitory concentration (MIC) or cidal concentration values have been established [65].
3.3.2. Dilution Methods
The dilution assays are reliable, widely accepted, and promising methods for determining an organism's susceptibility to inhibitors. The microdilution method is considered the “gold standard” [64, 68–70]. This is a quantitative method that makes it possible to calculate the MIC and allows one to understand the potency of the essential oil [68, 71]. With one of the most problematic characteristics of essential oils being their volatility, the microdilution technique allows for an opportunity to work around this problem as it allows for less evaporation due to the essential oil being mixed into the broth [67].
This microdilution method makes use of a 96-well microtitre plate under aseptic conditions where the essential oils (diluted in a solvent to a known concentration) are serially diluted. Results are usually read visually with the aid of an indicator dye. The microdilution results can also be interpreted by reading the optical density [72, 73]; however, the shortcoming of this method is that the coloured nature of some oils may interfere with accurate turbidimetric readings [74].
Activity is often classified differently according to the quantitative method followed. van Vuuren [56] recommended 2.00 mg/mL and less for essential oils to be considered as noteworthy, Agarwal et al. [75] regarded 1.00% and less, and Hadad et al. [76] recommended ≤250.00 μg/mL. On considering the collection of data and frequency of certain MIC values, this review recommends MIC values of ≤1.00 mg/mL as noteworthy.
The macrodilution method employs a similar method to that of the microdilution method, except that, instead of a 96-well microtitre plate being used, multiple individual test tubes are used. Although the results are still comparable, this is a time-consuming and a tedious method, whereas the 96-well microtitre plate allows for multiple samples to be tested per plate, allowing for speed, and it makes use of smaller volumes which adds to the ease of its application [77, 78]. The agar dilution method is where the essential oil is serially diluted, using a solvent, into a known amount of sterile molten agar in bottles or tubes and mixed with the aid of a solvent. The inoculum is then added and then the agar is poured into plates for each dilution and then incubated. The absence of growth after incubation is taken as the MIC [79–81].
3.3.3. The Time-Kill Method
The time-kill (or death kinetic) method is a labour intensive assay used to determine the relationship between the concentration of the antimicrobial and the bactericidal activity [82]. It allows for the presentation of a direct relationship in exposure of the pathogen to the antimicrobial and allows for the monitoring of a cidal effect over time [74]. The selected pathogen is exposed to the antimicrobial agent at selected time intervals and aliquots are then sampled and serially diluted. These dilutions are then plated out onto agar and incubated at the required incubation conditions for the pathogen. After incubation, the colony forming units (CFU) are counted. These results are interpreted from a logarithmic plot of the amount of remaining viable cells against time [74, 82, 83]. This is a time-consuming method; however, it is very useful for deriving real-time exposure data.
3.4. Summary of Methods
The variation in essential oil test methods makes it difficult to directly compare results [24, 58]. Numerous studies were found to employ the use of a diffusion method due to its acclaimed “ease” and “time saving” ability of the application. Researchers tend to use this as a screening tool whereby results displaying interesting outcomes are further tested using the microdilution method [84–87]. The shortcoming of this method is that firstly, due to the discussed factors affecting the diffusion methods, certain essential oils demonstrate no inhibition against the pathogen, and thus further studies with the oils are overlooked. Secondly, the active oils are then investigated further using the microdilution method. Therefore, the researchers have now doubled the amount of time required to interpret the quantitative data. Thirdly, the method may be believed to be a faster method if one considers the application; however, if one considers the preparation of the agar plates and their risk of contamination as well as the overall process of this method, there is very little saving of time and effort.
It is recommended to follow the correct guidelines as set out by the Clinical and Laboratory Standards Institute M38-A (CLSI) protocol [88] and the standard method proposed by the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing (AFST-EUCAST) [89] for testing with bacteria and filamentous fungi.
Other factors that may affect results and thus make it difficult to compare published pharmacological results of essential oils are where data is not given on the chemical composition, the microbial strain number, temperature and length of incubation, inoculum size, and the solvent used. The use of appropriate solvents helps address the factor of poor solubility of essential oils. Examples include Tween, acetone, dimethylformamide (DMF), dimethylsulfoxide (DMSO), and ethanol. Tween, ethanol, and DMSO have, however, been shown to enhance antimicrobial activity of essential oils [24, 53, 90]. Soković et al. [91] tested antimicrobial activity with ethanol as the solvent and Tween. When the essential oils were diluted with Tween, it resulted in a greater antifungal activity; however, Tween itself does not display its own antimicrobial activity [92]. Eloff [93] identified acetone as the most favourable solvent for natural product antimicrobial studies.
The inoculum is a representative of the microorganisms present at the site of infection [94]. When comparing different articles, the bacterial inoculum load ranges from 5 × 102 to 5 × 108 CFU/mL. The antibacterial activity is affected by inoculum size [62, 95–99]. If this concentration is too weak, the effect of the essential oils strengthens; however, this does not allow for a good representation of the essential oil's activity. If the inoculum is too dense, the effect of the essential oil weakens and the inoculum becomes more prone to cross contamination [100]. Future studies should aim to keep the inoculum size at the recommended 5 × 106 CFU/mL [99].
4. Pathogenesis of Wounds and Skin Infections and the Use of Essential Oils
The pathogenesis of the different infections that are frequently encountered in wounds and skin infections is presented in Table 2. A more in-depth analysis of essential oils and their use against these dermatological pathogens follows.
Table 2.
Pathogens responsible for infectious skin diseases.
| Skin disease | Anatomical structure affected by infection | Responsible pathogens | Reference |
|---|---|---|---|
| Bacterial infections | |||
| Abscesses | Skin and subcutaneous tissue | Staphylococcus aureus; methicillin-resistant S. aureus (MRSA) | [101] |
| Acne | Sebum glands | Propionibacterium acnes; S. epidermidis | [8, 102] |
| Actinomycosis | Skin and subcutaneous tissue | Actinomyces israelii | [5] |
| Boils/carbuncles and furuncles | Hair follicles | S. aureus | [8] |
| Bromodosis (foot odour) | Epidermis/cutaneous | Brevibacterium spp.; P. acnes | [6, 103] |
| Cellulitis | Subcutaneous fat | β-Hemolyticstreptococci; S. aureus; MRSA | [7, 8, 101] |
| Ecthyma | Cutaneous | S. aureus; Streptococcus pyogenes | [7] |
| Erysipelas | Dermis, intradermal | S. pyogenes | [8] |
| Erythrasma | Epidermis | Corynebacterium minutissimum | [5] |
| Folliculitis | Hair follicles | S. aureus; MRSA | [8, 101] |
| Impetigo | Epidermis | S. pyogenes; S. aureus | [8, 104, 105] |
| Periorbital cellulitis | Subcutaneous fat | Haemophilus influenzae | [106] |
| Surgical wounds | Skin, fascia, and subcutaneous tissue | Escherichia coli; Enterococcus spp.; Pseudomonas aeruginosa; S. aureus | [8] |
|
| |||
| Necrotizing infections | |||
| Necrotizing fasciitis | Skin, fascia, subcutaneous tissue, and muscle | S. pyogenes; anaerobic pathogens | [5, 8, 107] |
| Gas forming infections | Skin, subcutaneous tissue, and muscle | Gram-negative and various anaerobes | [5] |
| Gas gangrene | Skin, subcutaneous tissue, and muscle | Clostridium spp. (C. perfringens, C. septicum, C. tertium, C. oedematiens, and C. histolyticum) | [5, 8, 107] |
|
| |||
| Fungal infections | |||
| Candidal infections (intertrigo, balanitis, nappy rash, angular cheilitis, and paronychia) | Superficial skin | Candida albicans | [7] |
| Eumycetoma | Subcutaneous infection | Madurella mycetomatis | [108] |
| Dermatophytosis (tinea pedis/athlete's foot, tinea cruris, tinea capitis, tinea corporis, tinea manuum, and tinea unguium/onychomycosis) | Keratin layer, epidermis | Dermatophytes (Microsporum, Epidermophyton, and Trichophyton spp.) | [8] |
| Seborrheic dermatitis | Subcutaneous infection | Malasseziafurfur | [109] |
| Tinea/pityriasis versicolor | Superficial skin | M. furfur | [7, 110] |
|
| |||
| Viral infections | |||
| Herpes simplex | Mucocutaneous epidermidis | Herpes simplex virus (HSV) type 1, orofacial disease; HSV type 2, genital infection | [7] |
| Chicken pox | Mucocutaneous epidermidis | Varicella zoster | |
| Molluscum contagiosum | Prickle cells of epidermidis | Poxvirus | |
| Shingles | Mucocutaneous epidermidis | Herpes zoster | |
|
| |||
| Warts and verrucae | Epidermis | Human papillomavirus | [5, 7] |
4.1. Gram-Positive Bacteria
The Gram-positive bacterial cell wall is comprised of a 90–95% peptidoglycan layer that allows for easy penetration of lipophilic molecules into the cells. This thick lipophilic cell wall also results in essential oils making direct contact with the phospholipid bilayer of the cell membrane which allows for a physiological response to occur on the cell wall and in the cytoplasm [183, 184].
4.1.1. Staphylococcus aureus
Staphylococcus aureus is a common Gram-positive bacterium that can cause anything from local skin infections to fatal deep tissue infections. The pathogen is also found colonising acne and burn wounds [185–187]. Methicillin-resistant S. aureus (MRSA) is one of the most well-known and widespread “superbugs” and is resistant to numerous antibiotics [158]. Methicillin-resistant S. aureus strains can be found to colonise the skin and wounds of over 63%–90% of patients and have been especially infamous as being the dreaded scourge of hospitals for several years [22, 188–190]. Staphylococcus aureus has developed resistance against erythromycin, quinolones, mupirocin, tetracycline, and vancomycin [190–192].
Table 3 shows some of the antimicrobial in vitro studies undertaken on commercial essential oils and additional subtypes against this most notorious infectious agent of wounds. Of the 98 available commercial essential oils documented from the aromatherapeutic literature for use for dermatological infections, only 54 oils have been tested against S. aureus and even fewer against the resistant S. aureus strain. This is troubling, especially if one considers the regularity of S. aureus resistance. It should be recommended that resistant S. aureus strains always be included with every study.
Table 3.
Essential oil studies against S. aureus.
| Essential oila | Methodb | Species strainc | Solventd | Resulte | Main componentsf | Reference |
|---|---|---|---|---|---|---|
| Abies balsamea (fir/balsam) | MIC | S. aureus (ATCC 6538) | Acetone | 3.00 mg/mL | β-Pinene (31.00%), bornyl acetate (14.90%), δ-3-carene (14.20%) | [99] |
|
| ||||||
| Abies holophylla (Manchurian fir) | MIC | S. aureus (ATCC 25923) | 5% DMSO | 21.80 mg/mL | Bicyclo[2.2.1]heptan-2-ol (28.05%), δ-3-carene (13.85%), α-pinene (11.68%), camphene (10.41%) | [111] |
| S. aureus (ATCC 6538) | >21.80 mg/mL | |||||
| Abies koreana (Korean fir) | MIC | S. aureus (ATCC 25923) | 5% DMSO | 21.80 mg/mL | Bornyl ester (41.79%), camphene (15.31%), α-pinene (11.19%) | |
| S. aureus (ATCC 6538) | >21.80 mg/mL | |||||
|
| ||||||
| Achillea millefolium (yarrow) | MIC | S. aureus (ATCC 25923) | Tween 80 | 72.00 mg/mL | Eucalyptol (24.60%), camphor (16.70%), α-terpineol (10.20%) | [112] |
|
| ||||||
| Achillea setacea (bristly yarrow) | MIC | S. aureus (ATCC 25923) | Tween 80 | 4.50 mg/mL | Sabinene (10.80%), eucalyptol (18.50%) | [113] |
|
| ||||||
| Angelica archangelica (angelica), root | MIC | S. aureus (ATCC 6538) | Acetone | 1.75 mg/mL | α-Phellandrene (18.50%), α-pinene (13.70%), β-phellandrene (12.60%), δ-3-carene (12.1%) | [99] |
| Angelica archangelica (angelica), seed | 2.00 mg/mL | β-Phellandrene (59.20%) | ||||
|
| ||||||
| Anthemis aciphylla var. discoidea (chamomile), flowers | MIC | S. aureus (ATCC 6538) | DMSO | 1.00 mg/mL | α-Pinene (39.00%), terpinen-4-ol (32.10%) | [114] |
| Anthemis aciphylla var. discoidea (chamomile), aerial parts | 0.50 mg/mL | α-Pinene (49.40%), terpinen-4-ol (21.80%) | ||||
| Anthemis aciphylla var. discoidea (chamomile), leaves | Terpinen-4-ol (24.30%) | |||||
|
| ||||||
| Anthemis nobilis (chamomile) | MIC | S. aureus (ATCC 6538) | Acetone | 16.00 mg/mL | 2-Methylbutyl-2-methyl propanoic acid (31.50%), limonene (18.30%), 3-methylpentyl-2-butenoic acid (16.70%), isobutyl isobutyrate (10.00%) | [99] |
|
| ||||||
| Artemisia dracunculus (tarragon) | MIC | S. aureus (ATCC 6538) | Acetone | 3.00 mg/mL | Estragole (82.60%) | [99] |
|
| ||||||
| Backhousia citriodora (lemon myrtle) | ADM | S. aureus (NCTC 4163) | Tween 20 | 0.05% v/v | Geranial (51.40%), neral (40.90%) | [115] |
| MRSA (clinical isolate) | 0.20% v/v | |||||
|
| ||||||
| Boswellia carteri (frankincense) (9 samples) | MIC | S. aureus (ATCC 12600) | Acetone | 5.00–16.00 mg/mL | α-Pinene (4.80–40.40%), myrcene (1.60–52.40%), limonene (1.90–20.40%), α-thujene (0.30–52.40%), p-cymene (2.70–16.90%), β-pinene (0.30–13.10%) | [116] |
| Boswellia frereana (frankincense) (3 samples) | 4.00–12.00 mg/mL | α-Pinene (2.00–64.70%), α-thujene (0.00–33.10%), p-cymene (5.40–16.90%) | ||||
| Boswellia neglecta (frankincense) | 6.00 mg/mL | NCR | [117] | |||
| α-Pinene (43.40%), β-pinene (13.10%) | [116] | |||||
| Boswellia papyrifera (frankincense) | 1.50 mg/mL | NCR | [117] | |||
| Boswellia rivae (frankincense) | 2.50 mg/mL | |||||
| Boswellia sacra (frankincense) (2 samples) | 4.00–8.00 mg/mL | α-Pinene (18.30–28.00%), α-thujene (3.90–11.20%), limonene (11.20–13.10%) | [116] | |||
| Boswellia spp. (frankincense) (4 samples) | 6.00–9.30 mg/mL | α-Pinene (18.80–24.20%), limonene (11.70–19.00%) | ||||
| Boswellia thurifera (frankincense) | 10.00 mg/mL | α-Pinene (28.0%), limonene (14.6%) | ||||
|
| ||||||
| Cananga odorata (ylang-ylang) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | Bicyclosesquiphellandrene (19.50%), β-farnesene (13.90%) | [99] |
| Cananga odorata (ylang-ylang), heads | 4.00 mg/mL | Benzyl acetate (31.90%), linalool (27.00%), methyl benzoate (10.40%) | ||||
|
| ||||||
| Canarium luzonicum (elemi) | MIC | S. aureus (ATCC 6538) | Acetone | 3.00 mg/mL | Limonene (41.90%), elemol (21.60%), α-phellandrene (11.40%) | [99] |
|
| ||||||
| Carum carvi (caraway) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | Limonene (27.60%), carvone (67.50%) | [99] |
| S. aureus | DMSO | ≤1.00 μg/mL | DL-limonene (53.35%), β-selinene (11.08%), β-elemene (10.09%) | [118] | ||
|
| ||||||
| Caryophyllus aromaticus (clove) | ADM90 | S. aureus (ATCC 25923, 16 MRSA and 15 MSSA clinical isolates) | Tween 80 | 2.70 mg/mL | Eugenol (75.85%), eugenol acetate (16.38%) | [119] |
|
| ||||||
| Cinnamomum Cassia (cinnamon) | MIC | S. aureus | DMSO | ≤1.00 μg/mL | trans-Caryophyllene (17.18%), eugenol (14.67%), | [118] |
| Linalool L (14.52%), trans-cinnamyl acetate (13.85%), cymol (11.79%), cinnamaldehyde (11.25%) | ||||||
|
| ||||||
| Cinnamomum zeylanicum (cinnamon) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | Eugenol (80.00%) | [99] |
| MIC | S. aureus (ATCC 25923) | n.m. | 0.02 mg/mL | NCR | [85] | |
| ADM | 10% DMSO | 3.20 mg/mL | [80] | |||
| ADM90 | S. aureus (ATCC 25923, 16 MRSA and 15 MSSA clinical isolates) | Tween 80 | 0.25 mg/mL | Cinnamaldehyde (86.31%) | [119] | |
|
| ||||||
| Citrus aurantifolia (lime) | ADM | S. aureus (ATCC 25923) | 10% DMSO | 12.80 mg/mL | Cinnamaldehyde (52.42%) | [80] |
|
| ||||||
| Citrus aurantium (bitter orange), flowers | MIC | S. aureus (ATCC 25923) | 50% DMSO | 0.31 mg/mL | Limonene (27.50%), E-nerolidol (17.50%), α-terpineol (14.00%) | [120] |
| MIC | S. aureus (ATCC 6536) | 0.63 mg/mL | ||||
|
| ||||||
| Citrus aurantium (petitgrain) | MIC | S. aureus (ATCC 6536) | Acetone | 4.00 mg/mL | Linalyl acetate (54.90%), linalool (21.10%) | [99] |
|
| ||||||
| Citrus bergamia (bergamot) | MAC | S. aureus (ATCC 6538) | n.m. | 1.25 μL/mL | Bergamol (16.10%), linalool (14.02%), D-limonene (13.76%) | [62] |
|
| ||||||
| Citrus grandis (grapefruit) | MIC | S. aureus (ATCC 6538) | Acetone | 3.00 mg/mL | Limonene (74.80%) | [99] |
|
| ||||||
| Citrus medica limonum (lemon) | ADM | S. aureus (ATCC 25923) | 10% DMSO | >12.80 mg/mL | NCR | [80] |
| MIC | S. aureus (ATCC 6538) | Acetone | 3.00 mg/mL | [99] | ||
|
| ||||||
| Citrus sinensis (orange) | ADM | S. aureus (ATCC 25923) | 10% DMSO | >12.80 mg/mL | NCR | [80] |
| MAC | S. aureus (ATCC 9144) | 0.1% ethanol | 0.94 mg/L | [121] | ||
| MIC | S. aureus (ATCC 6538) | Acetone | 4.00 mg/mL | Limonene (93.20%) | [99] | |
|
| ||||||
| Commiphora guidotti (myrrh) | MIC | S. aureus (ATCC 12600) | Acetone | 1.50 mg/mL | (E)-β-Ocimene (52.60%), α-santalene (11.10%), (E)-bisabolene (16.00%) | [117] |
|
| ||||||
| Commiphora myrrha (myrrh) | MIC | S. aureus (ATCC 12600) | Acetone | 1.30 mg/mL | Furanogermacrene (15.90%), furanoeudesma-1,3-diene (44.30%) | [117] |
| S. aureus (ATCC 6538) | 2.00 mg/mL | Furanoeudesma-1,3-diene (57.70%), lindestrene (16.30%) | [117] | |||
|
| ||||||
| Coriandrum sativum (coriander), seed | MIC | S. aureus (7 clinical isolates) | 0.5% DMSO with Tween 80 | 0.16 mg/mL | NCR | [122] |
|
| ||||||
| Cupressus arizonica (smooth cypress), branches | MIC | S. aureus (ATCC 25923) | 10% DMSO | 1.50 μg/mL | α-Pinene (58.60%), δ-3-carene (15.60%) | [123] |
| Cupressus arizonica (smooth cypress),female cones | 2.95 μg/mL | α-Pinene (60.50%), δ-3-carene (15.30%) | ||||
| Cupressus arizonica (smooth cypress), leaves | 0.98 μg/mL | α-Pinene (20.00%), umbellulone (18.40%) | ||||
|
| ||||||
| Cupressus sempervirens (cypress) | MIC | S. aureus (ATCC 6538) | Acetone | 12.00 mg/mL | α-Pinene (41.20%), δ-3-carene (23.70%) | [99] |
|
| ||||||
| Cymbopogon giganteus (lemongrass) | MIC | S. aureus (ATCC 9144) | 0.5% ethanol | 2.10 mg/mL | Limonene (42.00%), trans-p-mentha-1(7),8-dien-2-ol (14.20%), cis-p-mentha-1(7),8-dien-2-ol (12.00%) | [124] |
|
| ||||||
| Cymbopogon citratus (lemongrass) | MIC | S. aureus (ATCC 9144) | 0.5% ethanol | 2.50 mg/mL | Geranial (48.10%), neral (34.60%), myrcene (11.00%) | [124] |
| S. aureus | DMSO | ≤1.00 μg/mL | Geranial (47.34%), β-myrcene (16.53%), Z-citral (8.36%) | [118] | ||
| MAC | S. aureus (MTCC 96) | Sodium taurocholate | 0.80–0.27 μL/mL | Citral (72.80%) | [125, 126] | |
| MIC | S. aureus (ATCC 6538) | Acetone | 1.67 mg/mL | Geranial (44.80%) | [99] | |
|
| ||||||
| Cymbopogon martinii (palmarosa) | MAC | S. aureus (MTCC 96) | Sodium taurocholate | 0.80 μL/mL | Geraniol (61.6%) | [125, 126] |
|
| ||||||
| Cymbopogon nardus (citronella) | MIC | S. aureus (ATCC 6538) | Acetone | 4.00 mg/mL | Citronellal (38.30%), geraniol (20.70%), citronellol (18.80%) | [99] |
|
| ||||||
| Daucus carota (carrot seed) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | Carotol (44.40%) | [99] |
|
| ||||||
| Eucalyptus camaldulensis (eucalyptus) | MAC | S. aureus (ATCC 25923) | Acetone | 3.90 μg/mL | 1,8-Cineol (54.37%), α-pinene (13.24%) | [127] |
| S. aureus (clinical isolate) | ||||||
|
| ||||||
| Eucalyptus globulus (eucalyptus) | MIC | S. aureus (ATCC 25923) | Tween 80 | 10.00 mg/mL | 1,8-Cineol (81.93%) | [128] |
| MRSA (ATCC 10442) | ||||||
| MRSA (MRSA USA 300) | ||||||
| MIC | S. aureus (ATCC 43387) | DMSO | 0.20% v/v | NCR | [129] | |
| MAC | S. aureus (MTCC 96) | Sodium taurocholate | 0.41 μL/mL | Cineole (23.20%) | [125, 126] | |
| ADM | MRSA (ATCC 33592) | Tween 20 | 85.60 μg/mL | Eucalyptol (47.20%), (+)-spathulenol (18.10%) | [81] | |
| S. aureus (ATCC 25922) | 51.36 μg/mL | |||||
| MRSA (14 clinical isolates) | 8.56–85.60 μg/mL | |||||
| MIC | S. aureus (ATCC 6538) | Acetone | 4.00 mg/mL | 1,8-Cineole (58.00%), α-terpineol (13.20%) | [99] | |
| MIC | S. aureus (ATCC 25923) | Acetone | 2.00 mg/mL | NCR | [130] | |
| MRSA (ATCC 33592) | 0.75 mg/mL | |||||
|
| ||||||
| Eucalyptus radiata (eucalyptus) | MIC | S. aureus (ATCC 25923) | Acetone | 2.00 mg/mL | 1,8-Cineole (65.7% ± 9.5), α-terpineol (12.8% ± 4.4) | [130] |
| MRSA (ATCC 33592) | 1.00–2.00 mg/mL | |||||
|
| ||||||
| Eucalyptus camaldulensis (eucalyptus) | MIC | S. aureus (ATCC 25923) | Acetone | 0.50 mg/mL | NCR | [130] |
| MRSA (ATCC 33592) | ||||||
|
| ||||||
| Eucalyptus citriodora (eucalyptus) | MIC | S. aureus (ATCC 25923) | Acetone | 1.00 mg/mL | NCR | [130] |
| MRSA (ATCC 33592) | ||||||
|
| ||||||
| Eucalyptus smithii (eucalyptus) | MIC | S. aureus (ATCC 25923) | Acetone | 2.00 mg/mL | NCR | [130] |
| MRSA (ATCC 33592) | ||||||
|
| ||||||
| Eucalyptus dives (eucalyptus) | MIC | S. aureus (ATCC 25923) | Acetone | 2.00 mg/mL | NCR | [130] |
| MRSA (ATCC 33592) | 1.00 mg/mL | |||||
|
| ||||||
| Eucalyptus intertexta (eucalyptus) | MIC | S. aureus (ATCC 29737) | 10% DSMO | 7.80 μg/mL | NCR | [131] |
|
| ||||||
| Eucalyptus largiflorens (eucalyptus) | MAC | S. aureus (ATCC 25923) | n.m. | 7.80 μg/mL | 1,8-Cineol (70.32%), α-pinene (15.46%) | [127] |
| S. aureus (clinical isolate) | ||||||
| MIC | S. aureus (ATCC 29737) | 10% DSMO | 250.00 μg/mL | NCR | [131] | |
|
| ||||||
| Eucalyptus melliodora (eucalyptus) | MAC | S. aureus (ATCC 25923) | n.m. | 3.90 μg/mL | 1,8-Cineol (67.65%), α-pinene (18.58%) | [127] |
| S. aureus (clinical isolate) | ||||||
|
| ||||||
| Eucalyptus polycarpa (eucalyptus) | MAC | S. aureus (ATCC 25923) | n.m. | 1.95 μg/mL | 1,8-Cineol (50.12%) | [127] |
| S. aureus (clinical isolate) | 3.90 μg/mL | |||||
|
| ||||||
| Foeniculum dulce (fennel) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | E-Anethole (79.10%) | [99] |
|
| ||||||
| Foeniculum vulgare (fennel) | MAC | S. aureus (ATCC 25923) | DMSO | >10.00 mg/mL | trans-Anethole (68.53%), estragole (10.42%) | [132] |
| Foeniculum vulgare (fennel) (6 samples) | MIC | S. aureus | ≤1.00 μg/mL | trans-Anethole (33.3%), DL-limonene (19.66%), carvone (12.03%) | [118] | |
| S. aureus (ATCC 28213) | 125.00–500.00 μg/mL | Fenchone (16.90–34.70%), estragole (2.50–66.00%), trans-anethole (7.90–77.70%) | [133] | |||
|
| ||||||
| Foeniculum vulgare Mill. ssp. vulgare (fennel), Aurelio | MAC | S. aureus (ATCC 25923) | Tween 20 | 50.00–100.00 μg/mL | Limonene (16.50–21.50%), (E)-anethole (59.80–66.00%) | [134] |
| Foeniculum vulgare Mill. ssp. vulgare (fennel), Spartaco | Limonene (0.20–17.70%), (E)-anethole (66.30–90.40%) | |||||
|
| ||||||
| Geranium dissectum (geranium) | MIC | S. aureus | DMSO | ≤1.00 μg/mL | β-Citronellol (25.45%), geraniol (13.83%) | [118] |
|
| ||||||
| Hyssopus officinalis (hyssop) | MIC | S. aureus (ATCC 6538) | Acetone | 3.00 mg/mL | Isopinocamphone (48.70%), pinocamphone (15.50%) | [99] |
|
| ||||||
| Juniperi aetheroleum (juniper) | MAC80 | S. aureus (ATCC 6538) | n.m. | 40.00% v/v | α-Pinene (29.17%), β-pinene (17.84%), sabinene (13.55%) | [135] |
| MAC80 | S. aureus (MFBF) | 15.00% v/v | ||||
|
| ||||||
| Juniperus communis (juniper), berry | MIC | S. aureus (ATCC 25923) | n.m. | 10.00 mg/mL | NCR | [85] |
| MIC | MRSA (15 clinical isolates) | Ethanol | >2.00% v/v | [136] | ||
|
| ||||||
| Juniperus excelsa (juniper), berries, Dojran | ADM | S. aureus (ATCC 29213) | 50% DSMO | >50.00% | α-Pinene (70.81%) | [87] |
| Juniperus excelsa (juniper), leaves, Dojran | 125.00% | α-Pinene (33.83%) | ||||
| Juniperus excelsa (juniper), leaves, Ohrid | 125.00% | Sabinene (29.49%) | ||||
|
| ||||||
| Juniperus officinalis (juniper), berry | MIC | S. aureus (ATCC 29213) | Tween 80 | 10.00 mg/mL | α-Pinene (39.76%) | [128] |
| Juniperus officinalis (juniper), berry | MRSA (clinical isolates) | 20.00 mg/mL | ||||
|
| ||||||
| Juniperus virginiana (juniper) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | Thujopsene (29.80%), cedrol (14.90%), α-cedrene (12.40%) | [99] |
| Juniperus virginiana (juniper), berries | 3.00 mg/mL | α-Pinene (20.50%), myrcene (13.70%), bicyclosesquiphellandrene (10.70%) | ||||
|
| ||||||
| Kunzea ericoides (Kānuka) | MAC | S. aureus (ATCC 6538) | Tween 80 | 0.25% v/v | α-Pinene (61.60%) | [137] |
| MRSA (clinical isolate) | 0.20% v/v | |||||
| MIC | S. aureus (ATCC 12600) | Acetone | 8.00 mg/mL | α-Pinene (26.2–46.7%), p-cymene (5.8–19.1%) | [138] | |
|
| ||||||
| Laurus nobilis (bay) | MIC | S. aureus (ATCC 6538) | Acetone | 0.83 mg/mL | Eugenol (57.20%), myrcene (14.30%), carvacrol (12.70%) | [99] |
|
| ||||||
| Lavandula angustifolia (lavender) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | Linalyl acetate (36.70%), linalool (31.40%), terpinen-4-ol (14.90%) | [99] |
| S. aureus (NCTC 6571) | 10% DSMO | 310.00 μg/mL | Linalool (25.10%), linalyl acetate (22.50%) | [139] | ||
| S. aureus (NCTC 1803) | 320.40 μg/mL | |||||
| MRSA (15 clinical isolates) | Ethanol | 0.50% v/v | NCR | [136] | ||
| S. aureus (ATCC 12600) | Acetone | 8.60 mg/mL | Linalool (30.80%), linalyl acetate (31.30%) | [140] | ||
| S. aureus (clinical strain and ATCC 6538) | Acetone | 2.00 mg/mL | Linalyl acetate (36.7%), linalool (31.4%), terpinen-4-ol (14.9%) | [99] | ||
| MRSA (clinical strain and 43300) | ||||||
| Methicillin-gentamicin-resistant S. aureus (MGRSA) (ATCC 33592) | ||||||
|
| ||||||
| Lavandula dentata (French lavender) | MIC | S. aureus (BNI 18) | 5% DMSO | 1.53 mg/mL | Camphor (12.40%) | [141] |
| Lavandula officinalis (lavender) | MIC | S. aureus | DMSO | ≤1.00 μg/mL | δ-3-Carene (17.14%), α-fenchene (16.79%), diethyl phthalate (13.84%) | [118] |
| Lavandula stoechas (French lavender) | MIC | S. aureus (STCC 976) | Tween 80 | 2.00 μL/mL | 10s,11s-Himachala-3(12),4-diene (23.62%), cubenol (16.19%) | [142] |
|
| ||||||
| Lavandula stoechas (French lavender), flower | MIC | MRSA (clinical isolate) | 20% DMSO | 31.25 μg/mL | α-Fenchone (39.20%) | [47] |
| Lavandula stoechas (French lavender), leaf | 125.00 μg/mL | α-Fenchone (41.90%), 1,8-cineole (15.60%), camphor (12.10%) | ||||
|
| ||||||
| Leptospermum scoparium (manuka) | MAC | S. aureus (ATCC 6538) | Tween 80 | 0.10% v/v | (−)-(E)-Calamenene (14.50%), leptospermone (17.60%) | [137] |
| MRSA (clinical isolate) | 0.05% v/v | |||||
| MIC | S. aureus (ATCC 12600) | Acetone | 4.00 mg/mL | Eudesma-4(14),11-diene (6.2–14.5%), α-selinene (5.90–13.5%), (E)-methyl cinnamate (9.2–19.5%) | [138] | |
|
| ||||||
| Litsea cubeba (May Chang) | MIC | S. aureus (ATCC 6538) | Acetone | 1.50 mg/mL | Geranial (45.60%), nerol (31.20%) | [99] |
|
| ||||||
| Matricaria chamomilla (German chamomile) | MIC | S. aureus (ATCC 6538) | Acetone | 1.50 mg/mL | Bisabolene oxide A (46.90%), β-farnesene (19.20%) | [99] |
|
| ||||||
| Matricaria recutita (German chamomile) | ADM90 | S. aureus (ATCC 25923, 16 MRSA and 15 MSSA clinical isolates) | Tween 80 | 26.50 mg/mL | Chamazulene (31.48%), α-bisabolol (15.71%), bisabolol oxide (15.71%) | [119] |
|
| ||||||
| Matricaria songarica (chamomile) | MIC | S. aureus (CCTCC AB91093) | Tween 80 | 50.00 μg/mL | E-β-Farnesene (10.58%), bisabolol oxide A (10.46%) | [143] |
|
| ||||||
| Melaleuca alternifolia (tea tree) | ADM | S. aureus (NCIM 2079) | Tween 80 | 1.00% | NCR | [79] |
| S. aureus (clinical isolate) | ||||||
| MAC | S. aureus (ATCC 6538) | Tween 80 | 0.25% v/v | α-Terpinene (11.40%), γ-terpinene (22.50%), terpinen-4-ol (35.20%) | [137] | |
| MRSA (clinical isolate) | 0.35% v/v | |||||
| MIC | S. aureus (ATCC 29213) | None used | 0.50% (v/v) | Terpinen-4-ol (40.00%), δ-terpinen (13.00%), p-cymene (13.00%) | [97] | |
| MRSA (98 clinical isolates) | n.m. | 512.00–2048.00 mg/L | NCR | [144] | ||
| S. aureus (NCIB 6571) | 1.00% v/v | [145] | ||||
| Coagulase-negative staphylococci (9 clinical isolates) | Polyoxyl 35 castor oil | 0.63–2.50% v/v | Terpinen-4-ol (>35.00%) | [146] | ||
| MRSA (10 clinical isolates) | 0.30–0.63% v/v | |||||
| 0.30% v/v | ||||||
| MRSA (15 clinical isolates) | Ethanol | 0.25% v/v | NCR | [136] | ||
| S. aureus (ATCC 12600) | Acetone | 8.60 mg/mL | Terpinen-4-ol (38.60%), γ-terpinene (21.60%) | [140] | ||
| MIC90 | S. aureus (NCTC 6571) | Tween 80 | 0.25% v/v | Terpinen-4-ol (35.70%) | [147] | |
| S. aureus (105 clinical isolates) | 0.12–0.50% v/v | |||||
| MIC | MRSA (60 clinical isolates, 29 mupirocin-resistant) | 0.25% | [148] | |||
| MIC | S. aureus (NCTC 8325) | n.m. | 0.50% (v/v) | Terpinen-4-ol (39.80%), γ-terpinene (17.80%) | [149] | |
| 0.25% (v/v) | [150] | |||||
| MIC90 | MRSA (100 clinical isolates) | Tween 80 | 0.16–0.32% | NCR | [151] | |
| MIC | S. aureus (69 clinical isolates) | Tween 80 | 0.12–0.50% v/v | Terpinen-4-ol (35.70%) | [152] | |
| ADM | S. aureus (NCTC 4163) | Tween 20 | 0.20% v/v | Terpinen-4-ol (42.80%), γ-terpinene (18.20%) | [115] | |
| MRSA (clinical isolate) | 0.30% v/v | |||||
| MIC | S. aureus (ATCC 6538) | Acetone | 8.00 mg/mL | Terpinen-4-ol (49.30%), γ-terpinene (16.90%) | [99] | |
| MAC | S. aureus (2 clinical isolates) | n.m. | 0.10–0.20% | Eucalyptol (70.08%) | [153] | |
| S. aureus (ATCC 25923) | 0.20% | |||||
| S. aureus (NCTC 9518) | 0.63–1.25% v/v | α-Pinene (11.95%), α-terpinene (14.63%), terpinen-4-ol (29.50%), p-cymene (17.74%) | [154] | |||
| α-Pinene (24.87%), α-terpinene (12.47%), terpinen-4-ol (28.59%) | ||||||
|
| ||||||
| Melaleuca cajuputi (cajuput) | MIC | S. aureus (ATCC 25923) | Tween 80 | 2.50 mg/mL | 1,8-Cineol (67.60%) | [128] |
| MRSA (ATCC 10442) | 5.00 mg/mL | |||||
| MRSA (clinical isolate) | 2.50 mg/mL | |||||
| MAC | S. aureus (ATCC 6538) | 0.20% v/v | 1,8-Cineole (55.50%) | [137] | ||
| MRSA (clinical isolate) | 0.30% v/v | |||||
|
| ||||||
| Melaleuca quinquenervia (niaouli) | MAC | S. aureus (ATCC 6538) | Tween 80 | 0.20% v/v | 1,8-Cineole (61.20%) | [137] |
| MRSA (clinical isolate) | 0.30% v/v | |||||
|
| ||||||
| Melaleuca viridiflora (niaouli) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | 1,8-Cineole (45.90%), α-terpinene (21.00%) | [99] |
|
| ||||||
| Melissa officinalis (lemon balm) | MIC | S. aureus (NCTC 6571) | 10% DSMO | 300.60 μg/mL | 1,8-Cineol (27.40%), α-thujone (16.30%), β-thujone (11.20%), borneol (10.40%) | [139] |
| MIC | S. aureus (NCTC 1803) | 330.30 μg/mL | ||||
|
| ||||||
| Mentha piperita (peppermint) | MIC | S. aureus (ATCC 12600) | Acetone | 11.90 mg/mL | Menthone (18.20%), menthol (42.90%) | [140] |
| S. aureus (ATCC 25923) | Tween 80 | 0.60 mg/mL | 1,8-Cineol (12.06%), menthone (22.24%), menthol (47.29%) | [128] | ||
| MRSA (ATCC 10442) | ||||||
| MRSA (clinical isolate) | ||||||
| S. aureus (ATCC 6538) | DMSO | 0.63–2.50 mg/mL | Menthol (27.50–42.30%), menthone (18.40–27.90%) | [155] | ||
| S. aureus | ≤1.00 μg/mL | Menthone (40.82%), carvone (24.16%) | [118] | |||
| MRSA (15 clinical isolates) | Ethanol | 0.50% v/v | NCR | [136] | ||
| S. aureus (ATCC 43387) | DMSO | 0.20% v/v | [129] | |||
| MAC | S. aureus (MTCC 96) | Sodium taurocholate | 1.66 μL/mL | Menthol (36.40%) | [125, 126] | |
| MIC | S. aureus (ATCC 9144) | 0.5% ethanol | 8.30 mg/mL | Menthol (39.30%), menthone (25.20%) | [156] | |
| MIC | S. aureus (ATCC 6538) | Acetone | 4.00 mg/mL | Menthol (47.50%), menthone (18.60%) | [99] | |
|
| ||||||
| Myrtus communis (myrtle) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | Myrtenyl acetate (28.20%), 1,8-cineole (25.60%), α-pinene (12.50%) | [99] |
| ADM | S. aureus (ATCC 6538) | Tween 20 | 2.80 mg/mL | NCR | [157] | |
| S. aureus (ATCC 29213) | ||||||
|
| ||||||
| Ocimum basilicum (basil) | MIC | S. aureus (ATCC 9144) | 0.5% ethanol | 2.50 mg/mL | Linalool (57.00%), eugenol (19.20%) | [156] |
| MAC | S. aureus (ATCC 6538) | n.m. | 1.25 μL/mL | Eugenol (62.60%), caryophyllene (21.51%) | [62] | |
| Tween 80 | 0.07 × 10−2% v/v | Linalool (54.95%), methyl chavicol (11.98%) | [158] | |||
| S. aureus (3 clinical strains) | Tween 80 | ((0.15–0.30) × 10)−2 % v/v | Linalool (54.95%), methyl chavicol (11.98%) | [158] | ||
| MIC | S. aureus (ATCC 6538) | Acetone | 1.50 mg/mL | Linalool (54.10%) | [99] | |
| MIC90 | S. aureus (ATCC 6538) | n.m. | 45.00 μg/mL | Methyl chavicol (46.90%), geranial (19.10%), neral (15.15%) | [159] | |
| MIC | S. aureus (ATCC 6538) | Tween 80 | 0.68–11.74 μg/mL | Linalool (30.30–58.60%) | [160] | |
|
| ||||||
| Origanum acutidens (Turkey oregano) | MIC | S. aureus (clinical isolate) | 10% DMSO | 125.00 μg/mL | Carvacrol (72.00%) | [161] |
| S. aureus (ATCC2913) | ||||||
|
| ||||||
| Origanum majorana (marjoram) | MIC | S. aureus (ATCC 43387) | DMSO | 0.05% v/v | NCR | [129] |
| S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | 1,8-Cineole (46.00%), linalool (26.10%) | [99] | ||
|
| ||||||
| Origanum microphyllum (oregano) | MIC | S. aureus (ATCC 25923) | Tween 80 | 6.21 mg/mL | Terpin-4-ol (24.86%), γ-terpinene (13.83%), linalool (10.81%) | [162] |
|
| ||||||
| Origanum scabrum (oregano) | MIC | S. aureus (ATCC 25923) | Tween 80 | 0.35 mg/mL | carvacrol (74.86%) | [162] |
|
| ||||||
| Origanum vulgare (oregano) | ADM | S. aureus (ATCC 6538) | 1% DMSO | 0.13% v/v | p-Cymene (14.60%), γ-terpinene (11.70%), thymol (24.70%), carvacrol (14.00%) | [163] |
| S. aureus (ATCC 25923) | ||||||
| S. aureus (ATCC 43300) | ||||||
| MRSA (22 isolates) | 0.06–0.13% v/v | |||||
| MIC | S. aureus (ATCC 6538) | n.m. | 575.00 mg/L | NCR | [164] | |
| MAC | 0.63.00 μL/mL | Carvacrol (30.17%), p-cymene (15.20%), γ-terpinen (12.44%) | [62] | |||
| MIC | S. aureus (ATCC 43387) | DMSO | 0.10% v/v | NCR | [129] | |
| ADM | S. aureus (ATCC 6538) | Tween 20 | 0.70 mg/mL | [157] | ||
| S. aureus (ATCC 29213) | ||||||
|
| ||||||
| Origanum vulgare subsp. hirtum (Greek oregano) | MIC | S. aureus (ATCC 25923) | 10% DMSO + Tween 80 | 170.70 μg/mL | Linalool (96.31%) | [165] |
| Origanum vulgare subsp. vulgare (oregano) | 106.70 μg/mL | Thymol (58.31%), carvacrol (16.11%), p-cymene (13.45%) | ||||
|
| ||||||
| Pelargonium graveolens (geranium) | ADM | S. aureus (ATCC 25923) | 10% DMSO | >12.80 mg/mL | NCR | [80] |
| S. aureus (ATCC 6538) | Tween 20 | 0.72 mg/mL | [157] | |||
| S. aureus (ATCC 29213) | ||||||
| MIC | S. aureus (strains isolated from skin lesions) | Ethanol | 0.25–1.50 mL/mL | Citronellol (26.70%), geraniol (13.40%) | [166] | |
| S. aureus (strains isolated postoperatively) | 0.50–2.25 mL/mL | |||||
| MRSA and MSSA (clinical strains) | 1.00 mL/mL | |||||
| S. aureus (ATCC 6538) | Acetone | 1.50 mg/mL | Citronellol (34.20%), geraniol (15.70%) | [99] | ||
|
| ||||||
| Perovskia abrotanoides (Russian sage) | MIC | S. aureus (ATCC 25923) | 10% DMSO | 8.00 μL/mL | Camphor (23.00%), 1,8-cineole (22.00%), α-pinene (12.00%) | [167] |
|
| ||||||
| Pimpinella anisum (anise) | MIC | S. aureus | DMSO | 125.00 μg/mL | NCR | [168] |
| ≤1.00 μg/mL | Anethole (64.82%) | [118] | ||||
|
| ||||||
| Pinus sylvestris (pine) | MIC | S. aureus (ATCC 6538) | Acetone | 4.00 mg/mL | Bornyl acetate (42.30%), camphene (11.80%), α-pinene (11.00%) | [99] |
|
| ||||||
| Piper nigrum (black pepper) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | β-Caryophyllene (33.80%), limonene (16.40%) | [99] |
|
| ||||||
| Pogostemon cablin (patchouli) | MIC | S. aureus (NCTC 6571) | 10% DSMO | 395.20 μg/mL | α-Guaiene (13.80%), α-bulnesene (17.10%), patchouli alcohol (22.70%) | [139] |
| S. aureus (NCTC 1803) | 520.00 μg/mL | |||||
|
| ||||||
| Pogostemon patchouli (patchouli) | MIC | S. aureus (ATCC 6538) | Acetone | 1.50 mg/mL | Patchouli alcohol (37.30%), α-bulnesene (14.60%), α-guaiene (12.50%) | [99] |
|
| ||||||
| Rosmarinus officinalis (rosemary) | MIC | S. aureus (ATCC 6538) | Tween 80 | 0.13% v/v | 1,8-Cineole (27.23%), α-pinene (19.43%), camphor (14.26%), camphene (11.52%) | [169] |
| S. aureus (NCTC 6571) | 10% DSMO | 305.30 μg/mL | 1,8-Cineol (29.2%), (+)-camphor (17.2%) | [139] | ||
| S. aureus (NCTC 1803) | 310.40 μg/mL | |||||
| MRSA (clinical isolate) | Tween 80 | 0.03% v/v | 1,8-Cineole (26.54%), α-pinene (20.14%), camphene (11.38%), camphor (12.88%) | [170] | ||
| S. aureus (MTCC 96) | n.m. | >11.00 mg/mL | NCR | [171] | ||
| S. aureus (ATCC 6538) | Hexane | 1.88–7.50 mg/mL | 1,8-Cineole (10.56–11.91%), camphor (16.57–16.89%), verbenone (17.43–23.79%) | [172] | ||
| ADM | S. aureus (ATCC 25923) | 10% DMSO | >12.80 mg/mL | NCR | [80] | |
| S. aureus (ATCC 6538) | Tween 20 | 5.60 mg/mL | [157] | |||
| S. aureus (ATCC 29213) | ||||||
| MIC | S. aureus (ATCC 12600) | Acetone | 6.20 mg/mL | 1,8-Cineole (41.40%), α-pinene (13.30%), camphor (12.40%) | [140] | |
| S. aureus (ATCC 43387) | DMSO | 0.20% v/v | NCR | [129] | ||
| S. aureus (ATCC 6538) | Acetone | 4.00 mg/mL | 1,8-Cineole (48.00%) | [99] | ||
| ADM90 | S. aureus (ATCC 25923, 16 MRSA and 15 MSSA clinical isolates) | Tween 80 | 8.60 mg/mL | Camphor (27.51%), limonene (21.01%), myrcene (11.19%), α-pinene (10.37%) | [119] | |
|
| ||||||
| Salvia bracteata (sage) | MIC | S. aureus (ATCC 25923) | 50.00 μg/mL | Caryophyllene oxide (16.60%) | [173] | |
| Salvia eremophila (sage) | MIC | S. aureus (ATCC 29737) | 10% DMSO | 8.00 μg/mL | Borneol (21.83%), α-pinene (18.80%), bornyl acetate (18.68%) | [174] |
| Salvia nilotica (sage) | ADM | S. aureus (ATCC 25923) | n.m. | 5.40 mg/mL | trans-Caryophyllene (10.90%) | [175] |
|
| ||||||
| Salvia officinalis (sage) | MIC | S. aureus (NCTC 6571) | 10% DSMO | 302.40 μg/mL | 1,8-Cineol (27.40%), α-thujone (16.30%), β-thujone (11.20%), borneol (10.40%) | [139] |
| S. aureus (NCTC 1803) | 324.30 μg/mL | |||||
| S. aureus (ATCC 43387) | DMSO | 0.20% v/v | NCR | [129] | ||
| ADM | S. aureus (ATCC 6538) | Tween 20 | 11.20 mg/mL | NCR | [157] | |
| S. aureus (ATCC 29213) | 5.60 mg/mL | |||||
| S. aureus (ATCC 25923) | n.m. | 7.50 mg/mL | [176] | |||
|
| ||||||
| Salvia ringens (sage) | MIC | S. aureus (ATCC 25923) | n.m. | NI | α-Pinene (12.85%), 1,8-cineole (46.42%) | [177] |
| Salvia rosifolia (sage) (3 samples) | MRSA | 20% DMSO | 125.00–1000.00 μg/mL | α-Pinene (15.70–34.80%), 1,8-cineole (16.60–25.10%), β-pinene (6.70–13.50%) | [178] | |
| Salvia rubifolia (sage) | S. aureus (ATCC 25923) | Tween 20 | 50.00 μg/mL | γ-Muurolene (11.80%) | [173] | |
|
| ||||||
| Salvia sclarea (clary sage) | MIC | S. aureus (11 MRSA and 16 MSSA) | Ethanol | 3.75–5.25 | Linalyl acetate (57.90%), linalool (12.40%) | [179] |
| S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | Linalyl acetate (72.90%), linalool (11.90%) | [99] | ||
|
| ||||||
| Santalum album (sandalwood) | MIC | S. aureus (ATCC 6538) | Acetone | 0.25 mg/mL | α-Santalol (32.10%) | [99] |
|
| ||||||
| Styrax benzoin (benzoin) | MIC | S. aureus (ATCC 6538) | Acetone | 2.00 mg/mL | cinnamyl alcohol (44.80%), benzene propanol (21.70%) | [99] |
|
| ||||||
| Syzygium aromaticum (clove) | MIC | S. aureus (ATCC 6538) | Tween 80 | 0.13% v/v | Eugenol (68.52%), β-caryophyllene (19.00%), 2-methoxy-4-[2-propenyl]phenol acetate (10.15%) | [169] |
| S. aureus | DMSO | ≤1.00 μg/mL | Eugenol (84.07%), isoeugenol (10.39%) | [118] | ||
| ADM | S. aureus (ATCC 25923) | 10% DMSO | >6.40 mg/mL | NCR | [80] | |
| MIC | S. aureus (ATCC 6538) | Acetone | 1.50 mg/mL | Eugenol (82.20%), eugenol acetate (13.20%) | [99] | |
|
| ||||||
| Tagetes minuta (Mexican marigold) | MIC90 | S. aureus (ATCC 6538) | n.m. | 67.00 μg/mL | Dihydrotagetone (33.90%), E-ocimene (19.90%), tagetone (16.10%) | [159] |
| Tagetes patula (French marigold) | MIC | S. aureus (ATCC 6538) | Acetone | 4.00 mg/mL | (E)-β-Ocimene (41.30%), E-tagetone (11.20%), verbenone (10.90%) | [99] |
|
| ||||||
| Thymbra spicata (thyme) | MIC | S. aureus (ATCC 29213) | Tween 80 | 2.25 mg/mL | Carvacrol (60.39%), γ-terpinene (12.95%) | [180] |
| Thymus capitatus (thyme) | S. aureus (ATCC 25923) | 900.00 μg/mL | p-Cymene (26.40%), thymol (29.30%), carvacrol (10.80%) | [181] | ||
| Thymus capitatus (thyme), commercial | α-Pinene (25.20%), linalool (10.30%), thymol (46.10%) | |||||
| Thymus herba-barona (thyme), Gennargentu | 225.00 μg/mL | Thymol (46.90%), carvacrol (20.60%) | ||||
| Thymus herba-barona (thyme), Limbara | 900.00 μg/mL | p-Cymene (27.60%), thymol (50.30%) | ||||
|
| ||||||
| Thymus hyemalis (thyme) (thymol, thymol/linalool, carvacrol chemotypes) | MAC | S. aureus (CECT 239) | 95% ethanol | <0.20–0.50 μL/mL | p-Cymene (16.00–19.80%), linalool (2.10–16.60%), thymol (2.90–43.00%), carvacrol (0.30–40.10%) | [61] |
|
| ||||||
| Thymus numidicus | ADM | S. aureus (ATCC 25923) | n.m. | 0.23 mg/mL | NCR | [176] |
| Thymus serpyllum (thyme) | S. aureus (ATCC 6538) | Tween 20 | 0.28 mg/mL | [157] | ||
| S. aureus (ATCC 29213) | 0.70 mg/mL | |||||
|
| ||||||
| Thymus vulgaris (thyme) | MIC | S. aureus | DMSO | 31.20 μg/mL | NCR | [168] |
| S. aureus (NCTC 6571) | 10% DSMO | 160.50 μg/mL | p-Cymene (17.90%), thymol (52.40%) | [139] | ||
| S. aureus (NCTC 1803) | 210.00 μg/mL | |||||
| S. aureus (ATCC 25923) | n.m. | 0.40 mg/mL | NCR | [85] | ||
| ADM | S. aureus (ATCC 433000) | Ethanol | 0.25 μL/mL | Thymol (38.1%), p-cymene (29.10%) | [182] | |
| S. aureus (2 multidrug-resistant clinical strains from hands) | 0.50 μL/mL | Thymol (38.1%), p-cymene (29.10%) | [182] | |||
| S. aureus (6 multidrug-resistant clinical strains from wounds) | 0.50–1.00 μL/mL | |||||
| S. aureus (4 multidrug-resistant clinical strains from ulcers) | 0.50–0.75 μL/mL | |||||
| S. aureus (multidrug-resistant clinical strain from abscesses) | 0.25 μL/mL | |||||
| MIC | S. aureus (ATCC 12600) | Acetone | 1.30 mg/mL | Thymol (47.20%), p-cymene (22.10%) | [140] | |
| MRSA (15 clinical isolates) | Ethanol | 0.50% v/v | NCR | [136] | ||
| ADM | MRSA (ATCC 33592) | Tween 20 | 18.50 μg/mL | Thymol (48.1%), p-cymene (15.60%), γ-terpinene (15.40%) | [81] | |
| S. aureus (ATCC 25922) | ||||||
| MRSA (14 clinical isolates) | 18.50–37.00 μg/mL | |||||
| MIC | S. aureus (ATCC 6538) | Acetone | 3.33 mg/mL | p-Cymene (39.90%), thymol (20.70%) | [99] | |
|
| ||||||
| Thymus vulgaris (thyme) (thymol chemotype) | MAC | S.aureus (CECT 239) | 95% ethanol | <0.20 μL/mL | p-Cymene (18.70%), thymol (57.70%) | [61] |
| Thymus zygis subsp. gracilis (thyme) (thymol and two linalool chemotypes) | <0.20–1.20 μL/mL | p-Cymene (0.50–11.20%), (E)-sabinene hydrate (0.20–18.20%), linalool (2.00–82.30%) | ||||
|
| ||||||
| Vetiveria zizanioides/Andropogon muricatus (vetiver) | MIC | S. aureus (ATCC 6538) | Acetone | 0.75 mg/mL | Zizanol (13.6%), β-vetirenene (7.2%) | [99] |
aScientific name (common name), part of plant (if applicable).
bMIC: microdilution method; MAC: macrodilution method; ADM: agar dilution method; CTA: contact time assay.
cAmerican Type Culture Collection, Rockville, USA (ATCC); Colección Espanõla de Cultivos Tipo (CECT); collection of microorganisms of the Department of Microbiology (MFBF); culture collection of antibiotics resistant microbes (CCRM); Eskişehir Osmangazi University, Faculty of Medicine, clinical isolate (OGU); Laboratorio de Microbiología, Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Mendoza, Argentina (LM); Microbial Type Culture Collection (MTCC); Mycology Laboratory (LM); National Center of Industrial Microorganisms (NCIM); National Collection of Type Cultures, London, Great Britain (NCTC); Spanish Collection of Type Cultures (STCC).
dDMSO concentration was not included; n.m.: not mentioned.
eNI: no inhibition.
fNCR: no composition results reported.
When considering the antimicrobial activity of the tested essential oils, it can be noted how the main compounds influence overall antimicrobial activity. Melaleuca alternifolia (tea tree), rich in terpinen-4-ol, showed noteworthy activity, and Anthemis aciphylla var. discoidea (chamomile) containing α-pinene and terpinen-4-ol displayed noteworthy activity (1.00 mg/mL) [114], whereas the essential oil predominantly containing terpinen-4-ol displayed an MIC value of 0.50 mg/mL. The Origanum spp. (Origanum scabrum and Origanum vulgare) were shown to display rather impressive antimicrobial activity, which appeared to predominantly be related to the amount of carvacrol [163]. Geraniol also appears to be a compound that influences antimicrobial activity against the staphylococci spp. as can be seen for Backhousia citriodora (lemon myrtle) and Cymbopogon martinii (palmarosa) (geraniol 61.6%) [125, 126]. Cymbopogon martinii, with lower levels of geranial (44.80%), showed moderate antimicrobial activity [99]. Mentha piperita (peppermint) had higher antimicrobial activity for oils with higher concentrations of menthol [128, 155]. Laurus nobilis (bay), Styrax benzoin, and Cinnamomum zeylanicum (cinnamon), each rich in eugenol, were found to have notable activity [99].
It is interesting to consider the essential oils investigated and to compare them to what is recommended in the aromatherapeutic literature. For example, Lavandula angustifolia (lavender) is recommended for abscesses, carbuncles, and wounds [2, 26, 32, 36–43], which all involve S. aureus; however, in vitro activity was found to discount this oil as an antimicrobial [99, 136, 139, 140]. The same could be said about essential oils such as Achillea millefolium (yarrow) [112], Anthemis nobilis (Roman chamomile) [99], Boswellia carteri (frankincense) [116], Citrus aurantifolia (lime) [80], Foeniculum vulgare (fennel) [132, 133], and Melissa officinalis (lemon balm) [139].
Some clinical studies included the evaluation of the effects of essential oils on malodorous necrotic ulcers of cancer patients. The use of an essential oil combination (mostly containing Eucalyptus globulus (eucalyptus)) resulted in a decrease in inflammation, reduction of the odour, and improved healing rates [193]. Edwards-Jones et al. [194] performed a clinical study with a wound dressing containing essential oils to decrease infection risk. Ames [195] found Melaleuca alternifolia (tea tree) to be effective in treating wounds; and Matricaria recutita (German chamomile) with L. angustifolia at a 50 : 50 ratio diluted in calendula oil was found to improve leg ulcers and pressure sores.
Methicillin-resistant S. aureus hinders the rate of wound healing, which may lead to chronic wounds [196]. Delayed wound healing has been proven to lead to psychological stress and social isolation [197, 198]. A randomised controlled trial, consisting of 32 patients (16 in control group, 16 in placebo group) with stage II and above MRSA-colonised wounds that were not responding to treatment, was undertaken where the control group was treated with a 10% topical M. alternifolia preparation and was found to effectively decrease colonising MRSA in 87.5% of patients and result in a 100% healing rate within 28 days [196]. These studies lead to the high recommendation of the incorporation of this essential oil combination in palliative care.
Methicillin-resistant S. aureus may potentially be carried and propagated by hospital staff and patients, which is an acknowledged risk for hospital-acquired infections [147, 189]. Therefore, successful decolonisation of MRSA from patients and good hygiene may improve the microbial load, number of reinfections, and ultimately therapeutic outcomes of patients [199]. A topical preparation containing M. alternifolia essential oil has been considered for assistance in eradicating MRSA in hospitals, due to its reported efficacy [200]. The largest randomised trial against MRSA colonisation included 224 patients where the control group was treated with 2% nasal mupirocin applied three times a day, 4% chlorhexidine gluconate soap used at least once a day, and 1% silver sulfadiazine cream applied to skin infections once a day. The study group was treated with 10% M. alternifolia oil nasal cream applied three times a day and 5% M. alternifolia oil body wash used at least once daily with a 10% M. alternifolia cream applied to skin infections. The results showed that 41% of patients in the study group were cleared as opposed to 49% of patients on the standard therapy [200]. A small three-day pilot study was designed by Caelli et al. [189] to observe whether daily washing with a 5% M. alternifolia oil would clear MRSA colonisation which may result in ICU patient outcome improvement [199]. The test group made use of 4% M. alternifolia nasal ointment and 5% M. alternifolia oil body wash and was compared to a conventional treatment consisting of 2% mupirocin nasal ointment and triclosan body wash. The test group overall was found to have more improvement at the infection site when compared to the control group. Although the pilot study was too small to be statistically significant, the researchers did find that the M. alternifolia oil performed better than the conventional treatment and was effective, nontoxic, and well tolerated [189]. Messager et al. [90] tested 5% M. alternifolia ex vivo in a formulation, where it again was proven to decrease the pathogenic bacteria on the skin. In another study, M. alternifolia oil was investigated to determine the influence on healing rates [201]. The patients were treated with water-miscible tea tree oil (3.30%) solution applied as part of the wound cleansing regimen. This study used this oil as a wash only three times a week which is not how this oil is prescribed and hence the results were not positive. A more accurate method of study was shown by Chin and Cordell [202], where M. alternifolia oil was used in a dressing for wound healing abilities. All patients, except for one, were found to have an accelerated healing rate of abscessed wounds and cellulitis. The concluding evidence shows that there is definitely potential for the use of M. alternifolia (tea tree) oil as an additional/alternative treatment to standard wound treatments [203].
The healing potential of Commiphora guidotti (myrrh) was investigated via excisions of rats. The authors could confidently report on an increased rate in wound contraction and candid wound healing activity that was attributed to the antimicrobial and anti-inflammatory effects of this oil [204]. Ocimum gratissimum (basil) was also found by Orafidiya et al. [205] to promote wound healing by eradicating the infectious pathogens and by inducing early epithelialisation and moderate clotting formation, thereby accelerating scab formation, contraction, and granulation.
From these studies, clearly, M. alternifolia has shown great promise against S. aureus. However, considering the potential of essential oils in clinical practice and comparing them to essential oils with promising in vitro activity, other oils such as Cymbopogon citratus (lemongrass), Santalum album (sandalwood), and Vetiveria zizanioides/Andropogon muricatus (vetiver) should in the future be paid the same amount of attention.
4.1.2. Pathogens Involved in Acne
Pathogens associated with acne include Propionibacterium acnes, Propionibacterium granulosum, and Staphylococcus epidermidis [206–208]. Methicillin-resistant S. epidermidis (MRSE) have become extensively problematic microorganisms in the recent years due to their antimicrobial resistance and P. acnes has developed resistance to tetracycline, erythromycin, and clindamycin. Both have also shown multidrug resistance, including against quinolones [158, 188, 206]. Table 4 displays the in vitro antimicrobial efficacies of commercial essential oils against bacteria involved in the pathogenesis of acne. When observing the number of commercial essential oils that are recommended for acne treatment, less than half of the commercial oils have actually focused on S. epidermidis, P. granulosum, and P. acnes. Overall, the acne pathogens have been sorely neglected in essential oil studies.
Table 4.
Antimicrobial efficacy of essential oils against pathogens associated with acne.
| Essential oila | Methodb | Species strainc | Solventd | Resulte | Main componentsf | Reference |
|---|---|---|---|---|---|---|
| Abies koreana (Korean fir) | MIC | S. epidermidis (antibiotic-susceptible strain SK4) | n.m. | 0.63 μL/mL | Bornyl acetate (30.40%), limonene (19.00%) | [208] |
| S. epidermidis (antibiotic-resistant strain SK9) | 0.31 μL/mL | |||||
| S. epidermidis (antibiotic-resistant strain SK19) | 5.00 μL/mL | |||||
| P. acnes (ATCC 3314) | 0.31 μL/mL | |||||
| P. acnes (antibiotic-resistant strain SKA 4) | ||||||
| P. acnes (antibiotic-resistant strain SKA 7) | 0.63 μL/mL | |||||
|
| ||||||
| Anthemis aciphylla var. discoidea (chamomile), flowers | MIC | S. epidermidis (ATCC 12228) | DMSO | 0.25 mg/mL | α-Pinene (39.00%), terpinen-4-ol (32.10%) | [114] |
| Anthemis aciphylla var. discoidea (chamomile), aerial parts | 0.13 mg/mL | α-Pinene (49.40%), terpinen-4-ol (21.80%) | ||||
| Anthemis aciphylla var. discoidea (chamomile), leaves | 0.06 mg/mL | Terpinen-4-ol (24.30%) | ||||
|
| ||||||
| Anthemis nobilis (chamomile) | MIC | P. acnes (CMCC 65002) | Tween 80 | 0.13% v/v | NCR | [209] |
|
| ||||||
|
Cananga odorata var. fruticosa (dwarf ylang-ylang) Cananga odorata (ylang-ylang) |
ADM | P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | >4.00% v/v | NCR | [210] |
|
| ||||||
| Cinnamomum burmannii (cinnamon stick) | MIC | S. epidermidis (16 clinical isolates) | 5% propylene glycol (PG) | 0.50–2.00% | Cinnamaldehyde | [211] |
| S. epidermidis strains RP62A (ATCC 35984) | 1.00% | |||||
| S. epidermidis (ATCC 12228) | 0.50% | |||||
|
| ||||||
| Cinnamomum zeylanicum (cinnamon) | MIC | P. acnes (CMCC 65002) | Tween 80 | 0.012% v/v | NCR | [209] |
|
| ||||||
| Citrus aurantium (bitter orange), flowers | MIC | S. epidermidis (ATCC 12228) | 50% DMSO | 1.25 mg/mL | Limonene (27.50%), E-nerolidol (17.5%), α-terpineol (14.00%) | [120] |
|
| ||||||
| Citrus medica limonum (lemon) | MIC | P. acnes (CMCC 65002) | Tween 80 | 0.25% v/v | NCR | [209] |
|
| ||||||
| Citrus natsudaidai (Japanese summer orange) | MIC | S. epidermidis (KCTC 3958) | 5% Tween 80 | 10.00 μL/mL | Limonene (81.60%) | [102] |
| P. acnes (ATCC 6919) | 0.31 μL/mL | |||||
|
| ||||||
| Citrus paradisi (grapefruit) | MIC | P. acnes (CMCC 65002) | Tween 80 | 0.25% v/v | NCR | [209] |
| MAC | S. epidermidis (KCTC 3958) | >50.00 μL/mL | Limonene (55.40–91.70%), myrcene (2.10–32.10%) | [212] | ||
|
| ||||||
| Citrus species (citrus) (14 spp.) | MAC | S. epidermidis (KCTC 3958) | Tween 80 | >50.00 μL/mL | Limonene (55.40–91.70%), myrcene (2.10–32.10%) | [212] |
| P. acnes (ATCC 6919) | 1.25–>50.00 μL/mL | |||||
|
| ||||||
| Coriandrum sativum (coriander) | ADM | P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | 1.00% v/v | NCR | [210] |
| Curcuma longa (turmeric) | >4.00% v/v | |||||
| Cymbopogon citratus (lemongrass) | 0.13% v/v | |||||
| Cymbopogon nardus (citronella) | ||||||
|
| ||||||
| Eucalyptus globulus (eucalyptus) | MIC | S. epidermidis (ATCC 14990) | Tween 80 | 10.00 mg/mL | 1,8-Cineol (81.93%) | [128] |
| S. epidermidis (RP62A) | 5% DSMO | 4.00 mg/L | NCR | [213] | ||
| S. epidermidis (clinical isolate TK1) | 8.00 mg/L | |||||
| P. acnes (DMST 14917) | n.m. | 9.38 mg/mL | p-Cymene (28.75%), γ-terpinene (44.60%) | [214] | ||
| ADM | P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | 4.00% v/v | NCR | [210] | |
|
| ||||||
| Eucalyptus intertexta (eucalyptus) | MIC | S. epidermidis (ATCC 12228) | 10% DSMO | 7.80 μg/mL | NCR | [131] |
| Eucalyptus largiflorens (eucalyptus) | 125.00 μg/mL | |||||
|
| ||||||
| Foeniculum vulgare (fennel), Aurelio | MAC | S. epidermidis (ATCC 12228) | Tween 20 | 50.00 μg/mL | Limonene (16.50–21.50%), (E)-anethole (59.80–66.00%) | [134] |
| Foeniculum vulgare (fennel), Spartaco | 25.00–50.00 μg/mL | Limonene (0.20–17.70%), (E)-anethole (66.30–90.40%) | ||||
| Foeniculum vulgare (fennel) (6 samples) | MIC | DMSO | 250.00–750.00 μg/mL | Fenchone (16.90–34.70%), estragole (2.50–66.00%), trans-anethole (7.90–77.70%) | [133] | |
|
| ||||||
| Jasminum grandiflora (jasmine) | MIC | P. acnes (CMCC 65002) | Tween 80 | 0.50% v/v | NCR | [209] |
| Jasminum sambac (jasmine) | ADM | P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | 2.00% v/v | [210] | |
|
| ||||||
| Juniperi aetheroleum (juniper) | MAC80 | S. epidermidis (MFBF) | n.m. | 40.00% v/v | α-Pinene (29.17%), β-pinene (17.84%), sabinene (13.55%) | [135] |
| Juniperus communis (juniper) | CTA | P. acnes (ATCC 6919) | PEG 200 | 2.00 mg/mL | α-Pinene (22.75%), β-myrcene (11.88%) | [215] |
| Juniperus officinalis (juniper), berry | MIC | S. epidermidis (ATCC 14990) | Tween 80 | 20.00 mg/mL | α-Pinene (39.76%) | [128] |
|
| ||||||
| Kunzea ericoides (Kānuka) | MAC | S. epidermidis (clinical isolate) | Tween 80 | 0.25% v/v | α-Pinene (61.60%) | [137] |
| MIC | S. epidermidis (ATCC 2223) | Acetone | 8.00 mg/mL | α-Pinene (26.2–46.7%), p-cymene (5.8–19.1%) | [138] | |
| MIC | P. acnes (ATCC 11827) | Acetone | 4.00 mg/mL | |||
|
| ||||||
| Lavandula angustifolia (lavender) | MIC | S. epidermidis (ATCC 2223) | Acetone | 6.20 mg/mL | Linalool (30.80%), linalyl acetate (31.30%) | [140] |
| S. epidermidis (antibiotic-susceptible strain SK4) | n.m. | 1.00 μL/mL | NCR | [208] | ||
| S. epidermidis (antibiotic-resistant strain SK9) | n.m. | 0.13 μL/mL | ||||
| S. epidermidis (antibiotic-resistant strain SK19) | n.m. | 1.00 μL/mL | ||||
| P. acnes (ATCC 3314) | n.m. | 0.25 μL/mL | ||||
| P. acnes (antibiotic-resistant strain SKA 4) | n.m. | 1.25 μL/mL | ||||
| P. acnes (antibiotic-resistant strain SKA 7) | n.m. | 0.25 μL/mL | ||||
| ADM | P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | 2.00% v/v | NCR | [210] | |
|
| ||||||
| Lavandula stoechas (French lavender), flower | MIC | S. epidermidis (ATCC 12228) | 20% DMSO | 250.00 μg/mL | α-Fenchone (39.20%) | [47] |
| Lavandula stoechas (French lavender), leaf | MIC | α-Fenchone (41.90%), 1,8-cineole (15.60%), camphor (12.10%) | ||||
| Lavandula stoechas (lavender) | MIC | P. acnes (CMCC 65002) | Tween 80 | 0.13% v/v | NCR | [209] |
|
| ||||||
| Leptospermum scoparium (manuka) | MAC | S. epidermidis (clinical isolate) | Tween 80 | 0.05% v/v | (−)-(E)-Calamenene (14.50%), leptospermone (17.60%) | [137] |
| MIC | S. epidermidis (ATCC 2223) | Acetone | 4.00 mg/mL | Eudesma-4(14),11-diene (6.20–14.50%), α-selinene (5.90–13.50%), (E)-methyl cinnamate (9.20–19.50%) | [138] | |
| P. acnes (ATCC 11827) | 1.00 mg/mL | |||||
|
| ||||||
| Melaleuca alternifolia (tea tree) | ADM | S. epidermidis (NCIM number 2493) | Tween 80 | 1.00% | NCR | [79] |
| S. epidermidis (clinical isolate) | ||||||
| MAC | S. epidermidis (clinical isolate) | 0.45% v/v | α-Terpinene (11.40%), γ-terpinene (22.50%), terpinen-4-ol (35.20%) | [137] | ||
| MIC | S. epidermidis (15 clinical isolates) | 0.12–1.00% v/v | Terpinen-4-ol (35.70%) | [152] | ||
| MIC | S. epidermidis (RP62A) | 5% DSMO | 2.00 mg/L | NCR | [213] | |
| S. epidermidis (clinical isolate TK1) | 16.00 mg/L | |||||
| MIC | S. epidermidis (ATCC 2223) | Acetone | 6.20 mg/mL | Terpinen-4-ol (38.60%), γ-terpinene (21.60%) | [140] | |
| MIC | S. epidermidis (antibiotic-susceptible strain SK4) | n.m. | 0.13 μL/mL | NCR | [208] | |
| S. epidermidis (antibiotic-resistant strain SK9) | 1.00 μL/mL | |||||
| S. epidermidis (antibiotic-resistant strain SK19) | ||||||
| MAC | P. acnes (MTCC 1951) | Tween 80 | 0.50% v/v | NCR | [79] | |
| P. acnes (32 clinical strains) | 0.25–0.50% | Terpinen-4-ol (35.70%) | [92] | |||
| ADM | P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | 1.00% v/v | NCR | [210] | |
| MIC | P. acnes (ATCC 3314) | n.m. | 0.25 μL/mL | NCR | [208] | |
| P. acnes (antibiotic-resistant strain SKA 4) | 2.50 μL/mL | |||||
| P. acnes (antibiotic-resistant strain SKA 7) | 0.25 μL/mL | |||||
| MAC | S. epidermidis (NCTC 11047) | n.m. | 0.63–1.25% v/v | α-Pinene (11.95%), α-terpinene (14.63%), terpinen-4-ol (29.5%), p-cymene (17.74%) | [154] | |
| P. acnes (NCTC 737) | 0.31–0.63% v/v | |||||
| S. epidermidis (NCTC 11047) | 0.63–1.25% v/v | α-Pinene (24.87%), α-terpinene (12.47%), terpinen-4-ol (28.59%) | ||||
| P. acnes (NCTC 737) | 0.31–0.63% v/v | |||||
|
| ||||||
| Melaleuca cajuputi (cajuput) | MAC | S. epidermidis (clinical isolate) | Tween 80 | 0.40% v/v | 1,8-Cineole (55.50%) | [137] |
| MIC | S. epidermidis (ATCC 14990) | Tween 80 | 10.00 mg/mL | 1,8-Cineol (67.60%) | [128] | |
|
| ||||||
| Melaleuca quinquenervia (niaouli) | MAC | S. epidermidis (clinical isolate) | tween 80 | 0.40% v/v | 1,8-Cineole (61.20%) | [137] |
|
| ||||||
| Mentha piperita (peppermint) | MIC | S. epidermidis (ATCC 14990) | Tween 80 | 1.25 mg/mL | 1,8-Cineol (12.06%), menthone (22.24%), menthol (47.29%) | [128] |
| S. epidermidis (NCTC 12228) | DMSO | 0.63–2.50 mg/mL | Menthol (27.50–42.30%), menthone (18.40–27.90%) | [155] | ||
| S. epidermidis (ATCC 2223) | Acetone | 6.20 mg/mL | Menthone (18.20%), menthol (42.90%) | [140] | ||
| S. epidermidis (antibiotic-susceptible strain SK4) | n.m. | 0.13 μL/mL | NCR | [208] | ||
| S. epidermidis (antibiotic-resistant strain SK9) | 0.50 μL/mL | |||||
| S. epidermidis (antibiotic-resistant strain SK19) | ||||||
| P. acnes (ATCC 3314) | 0.25 μL/mL | |||||
| P. acnes (antibiotic-resistant strain SKA 4) | 0.63 μL/mL | |||||
| P. acnes (antibiotic-resistant strain SKA 7) | 0.06 μL/mL | |||||
|
| ||||||
| Mentha spicata (spearmint) | MIC | P. acnes (CMCC 65002) | Tween 80 | 0.25% v/v | NCR | [209] |
|
| ||||||
| Ocimum americanum (hoary basil) | ADM | P. acnes (DMST 14916) | Polysorbate 80 | >5.00% v/v | Neral (27.20%), geraniol (32.00%) | [44] |
|
| ||||||
| Ocimum basilicum (basil) | MAC | S. epidermidis (2 clinical strains) | Tween 80 | (0.15–0.30) × 10−2% v/v | Linalool (54.95%), methyl chavicol (11.98%) | [158] |
| ADM | P. acnes (DMST 14916) | Polysorbate 80 | 2.00% v/v | Methyl chavicol (93.00%) | [44] | |
| P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | >4.00% v/v | NCR | [210] | ||
|
| ||||||
| Ocimum sanctum (holy basil) | ADM | P. acnes (DMST 14916) | Polysorbate 80 | 3.0% v/v | Eugenol (41.50%), methyl eugenol (11.80%), γ-caryophyllene (23.70%) | [44] |
| Ocimum tenuiflorum (holy basil) | P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | 2.00% v/v | NCR | [210] | |
|
| ||||||
| Origanum acutidens (Turkey oregano) | MIC | S. epidermis (A233) | 10% DMSO | 125.00 μg/mL | Carvacrol (72.0%) | [161] |
| Origanum microphyllum (oregano) | S. epidermidis (ATCC 12228) | Tween 80 | 5.32 mg/mL | Terpin-4-ol (24.86%), γ-terpinene (13.83%), linalool (10.81%) | [162] | |
| Origanum scabrum (oregano) | 0.38 mg/mL | Carvacrol (74.86%) | ||||
|
| ||||||
| Origanum vulgare (oregano) | ADM | S. epidermidis (ATCC 12228) | 1% DMSO | 0.13% v/v | p-Cymene (14.60%), γ-terpinene (11.70%), thymol (24.70%), carvacrol (14.00%) | [163] |
| ADM | S. epidermidis (21 clinical isolates) | 0.06–0.13% v/v | ||||
|
| ||||||
| Piper nigrum (black pepper) | ADM | P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | >4.00% v/v | NCR | [210] |
|
| ||||||
| Rosa centifolia (rose) | MIC | P. acnes (CMCC 65002) | Tween 80 | 0.03% v/v | NCR | [209] |
|
| ||||||
| Rosmarinus officinalis (rosemary) | MIC | S. epidermidis (ATCC 12228) | Tween 80 | 0.25% v/v | 1,8-Cineole (27.23%), α-pinene (19.43%), camphor (14.26%), camphene (11.52%) | [169] |
| S. epidermidis | 0.10% (v/v) | 1,8-Cineole (26.54%), α-pinene (20.14%), camphene (11.38%), camphor (12.88%) | [170] | |||
| S. epidermidis (MTCC 435) | n.m. | >11.00 mg/mL | NCR | [171] | ||
| S. epidermidis (ATCC 2223) | Acetone | 10.10 mg/mL | 1,8-Cineole (41.40%), α-pinene (13.30%), camphor (12.40%) | [140] | ||
|
| ||||||
| Salvia bracteata (sage) | MAC | S. epidermidis (ATCC 12228) | Tween 20 | 50.00 μg/mL | Caryophyllene oxide (16.6%) | [173] |
|
| ||||||
| Salvia eremophila (sage) | MIC | S. epidermidis (ATCC 12228) | 10% DMSO | 32.00 μg/mL | Borneol (21.83%), α-pinene (18.80%), bornyl acetate (18.68%) | [174] |
|
| ||||||
| Salvia nilotica (sage) | ADM | S. epidermidis (ATCC 12228) | n.m. | 5.50 mg/mL | trans-Caryophyllene (10.90%) | [175] |
|
| ||||||
| Salvia ringens (sage) | MIC | S. epidermidis (ATCC 12228) | n.m. | NI | α-Pinene (12.85%), 1,8-cineole (46.42%) | [177] |
|
| ||||||
| Salvia rosifolia (sage) (3 samples) | MIC | S. epidermidis (ATCC 12228) | 20% DMSO | 125.00–1000.00 μg/mL | α-Pinene (15.70–34.80%), 1,8-cineole (16.60–25.10%), β-pinene (6.70–13.50%) | [178] |
|
| ||||||
| Salvia rubifolia (sage) | MAC | S. epidermidis (ATCC 12228) | Tween 20 | 50.00 μg/mL | γ-Muurolene (11.80%) | [173] |
|
| ||||||
| Salvia sclarea (clary sage) | MIC | S. epidermidis (19 clinical isolates) | Ethanol | 4.50–6.25 | Linalyl acetate (57.90%), linalool (12.40%) | [179] |
|
| ||||||
| Syzygium aromaticum (clove) | MIC | S. epidermidis (ATCC 12228) | Tween 80 | 0.25% v/v | Eugenol (68.52%), β-caryophyllene (19.00%), 2-methoxy-4-[2-propenyl]phenol acetate (10.15%) | [169] |
| ADM | P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | NCR | [210] | ||
| MIC | P. acnes | n.m. | 0.31 mg/mL | DL-Limonene (61.60%) | [216] | |
|
| ||||||
| Thymus capitatus (thyme) | MIC | S. epidermidis (ATCC 12228) | Tween 80 | 900.00 μg/mL | p-Cymene (26.40%), thymol (29.30%), carvacrol (10.80%) | [181] |
| Thymus capitatus (thyme), commercial | α-Pinene (25.20%), linalool (10.30%), thymol (46.10%) | |||||
| Thymus herba-barona (thyme), Gennargentu | 450.00 μg/mL | Thymol (46.90%), carvacrol (20.6 0%) | ||||
| Thymus herba-barona (thyme), Limbara | 900.00 μg/mL | p-Cymene (27.60%), thymol (50.30%) | ||||
|
| ||||||
| Thymus quinquecostatus (thyme), Jeju | MAC | P. acnes (ATCC 6919) | n.m. | 0.50 mg/mL | p-Cymen-3-ol (50.41%), p-cymen-2-ol (24.06%), cymene (19.04%) | [207] |
| P. granulosum (ATCC 25564) | ||||||
|
| ||||||
| Thymus vulgaris (thyme) | MIC | S. epidermidis (ATCC 2223) | Acetone | 4.70 mg/mL | Thymol (47.20%), p-cymene (22.10%) | [140] |
| P. acnes (CMCC 65002) | Tween 80 | 0.02% v/v | NCR | [209] | ||
|
| ||||||
| Zingiber officinale (ginger) | MIC | P. acnes (CMCC 65002) | Tween 80 | 0.25% v/v | NCR | [209] |
|
| ||||||
| Zingiber officinale Roscoe (ginger) | ADM | P. acnes (DMST 4916, 14917, 4918, 21823, 21823, 21824) | 0.5% polysorbate 80 | >4.00% v/v | NCR | [210] |
aScientific name (common name), part of plant (if applicable).
bMIC: microdilution method; MAC: macrodilution method; ADM: agar dilution method; CTA: contact time assay.
cAmerican Type Culture Collection, Rockville, USA (ATCC); Colección Espanõla de Cultivos Tipo (CECT); collection of microorganisms of the Department of Microbiology (MFBF); culture collection of antibiotics-resistant microbes (CCRM); Eskişehir Osmangazi University, Faculty of Medicine, clinical isolate (OGU); Laboratorio de Microbiología, Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Mendoza, Argentina (LM); Microbial Type Culture Collection (MTCC); Mycology Laboratory (LM); National Center of Industrial Microorganisms (NCIM); National Collection of Type Cultures, London, Great Britain (NCTC); Spanish Collection of Type Cultures (STCC).
dDMSO concentration was not included; n.m.: not mentioned.
eNI: no inhibition.
fNCR: no composition results reported.
For Anthemis aciphylla var. discoidea (chamomile) 0.13–0.25 mg/mL, initially, it appeared that higher α-pinene and lower terpinen-4-ol showed higher antimicrobial activity. However, the sample with terpinen-4-ol predominantly as its main component displayed the best activity at 0.06 mg/mL. This makes α-pinene appear as an antimicrobial antagonist. Cinnamomum zeylanicum, Rosa centifolia (rose), L. angustifolia, and Syzygium aromaticum (clove) displayed noteworthy antimicrobial activity against both S. epidermidis and P. acnes. Only the latter two are, however, recommended in the aromatherapeutic literature for the treatment of acne. Leptospermum scoparium (manuka) showed noteworthy activity for both P. acnes and S. epidermidis; however, Tween 80 was used as a solvent, which may overexaggerate the antimicrobial activity. Another study also found L. scoparium to effectively inhibit P. acnes. As was seen against S. aureus, O. scabrum and O. vulgare also notably inhibited S. epidermidis. Unfortunately, these oils were not studied against P. acnes. Cymbopogon citratus was shown to effectively inhibit P. acnes; however, no data was available against S. epidermidis. Essential oils such as S. album, V. zizanioides, Viola odorata (violet), Citrus aurantium var. amara (petitgrain), and Citrus bergamia (bergamot) are a few that are recommended for the treatment of acne and other microbial infections [2, 26, 32, 36, 37, 40–43] in the aromatherapeutic literature that are yet to be investigated.
Some clinical studies have shown promising results. A four-week trial comparing O. gratissimum oil with 10% benzoyl peroxide and a placebo was conducted and was aimed at reducing acne lesions in students. The 2% and 5% O. gratissimum oils in the hydrophilic cetomacrogol base were found to reduce acne lesions faster than standard therapy, and they were well tolerated. The 5% preparation, despite being highly effective, caused skin irritation. Overall, O. gratissimum oil showed excellent potential in the management of acne as it was as effective as benzoyl peroxide, although it was less popular with patients due to the unpleasant odour [217].
Melaleuca alternifolia oil demonstrated in vitro antimicrobial and anti-inflammatory activity against P. acnes and S. epidermidis and is in fact the essential oil on which most clinical trials have been undertaken. Bassett et al. [218] performed one of the first rigorous single-blinded randomised (RCT) controlled trials consisting of 124 patients that assessed the efficacy of 5% M. alternifolia gel in comparison to 5% benzoyl peroxide lotion in the management of mild to moderate acne. Both treatments showed equal improvement in the acne lesions. Enshaieh et al. [219] evaluated the efficacy of 5% M. alternifolia on mild to moderate acne vulgaris. The 5% M. alternifolia oil was found to be effective in improving the number of papules in both inflammatory and noninflammatory acne lesions and was found to be more effective than the placebo. Proven efficacy has made M. alternifolia preparations popular in acne products.
Other oil studies included a gel formulation containing acetic acid, Citrus sinensis (orange), and Ocimum basilicum (sweet basil) essential oils, which was tested in acne patients. The combination of these antimicrobial essential oils and the keratolytic agent resulted in a 75% improvement in the rate of acne lesion healing [220].
If one examines the results displayed in Table 4, essential oils such as Anthemis aciphylla var. discoidea (chamomile), C. zeylanicum, Citrus aurantium (bitter orange), O. vulgare (oregano), and S. aromaticum displayed higher antimicrobial activity in vitro than M. alternifolia, yet these essential oils have to be investigated clinically.
4.1.3. Gram-Negative Bacteria
The Gram-negative bacterial cell wall consists of a 2-3 nm thick peptidoglycan layer (thinner than Gram-positive bacteria), which means that the cell wall consists of a very small percentage of the bacteria. The cell wall is further surrounded by an outer membrane (OM) which is comprised of a double layer of phospholipids that are linked to an inner membrane by lipopolysaccharides (LPS). This OM protects the bacteria from lipophilic particles; however, it makes them more vulnerable to hydrophilic solutes due to the abundance of porin proteins that serve as hydrophilic transmembrane channels [184, 221, 222].
Gram-negative pathogens present a serious threat with regard to drug resistance, especially Escherichia coli and Pseudomonas aeruginosa [190, 192]. These pathogens that are found to colonise wounds often cause multidrug resistance [166, 223]. β-Lactamase-positive E. coli is appearing frequently among nonhospital patients [224]. Pseudomonas aeruginosa is a regular cause of opportunistic nosocomial infections [187]. It is often involved in localised skin infections, green nail syndrome, and interdigital infection, colonises burn wounds, and may expand into a life-threatening systemic illness [225].
A number of essential oils display antimicrobial activity against E. coli and P. aeruginosa with the predominant studies having been done against E. coli (Table 5). The Gram-negative pathogens appear to be a lot more resistant to essential oil inhibition than the Gram-positive bacteria, but this a known fact.
Table 5.
Essential oil studies showing efficacy against Gram-negative pathogens associated with skin infections.
| Essential oila | Methodb | Species strainc | Solventd | Resulte | Main componentsf | Reference |
|---|---|---|---|---|---|---|
| Abies balsamea (fir) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | β-Pinene (31.00%), bornyl acetate (14.90%), δ-3-carene (14.20%) | [99] |
|
| ||||||
| Abies holophylla (Manchurian fir) | MIC | E. coli (ATCC 10536) | DMSO | 21.8 mg/mL | Bicyclo[2.2.1]heptan-2-ol (28.05%), δ-3-carene (13.85%), α-pinene (11.68%), camphene (10.41%) | [111] |
| E. coli (ATCC 25922) | ||||||
| E. coli (ATCC 33312) | ||||||
| P. aeruginosa (NCTC 10490) | >21.80 mg/mL | |||||
|
| ||||||
| Abies koreana (Korean fir) | MIC | E. coli (ATCC 10536) | DMSO | 21.8 mg/mL | Bornyl ester (41.79%), camphene (15.31%), α-pinene (11.19%) | [111] |
| E. coli (ATCC 25922) | ||||||
| E. coli (ATCC 33312) | 10.9 mg/mL | |||||
| P. aeruginosa (NCTC 10490) | >21.80 mg/mL | |||||
|
| ||||||
| Achillea millefolium (yarrow) | MIC | P. aeruginosa (ATCC 27853) | Tween 80 | NI | Eucalyptol (24.60%), camphor (16.70%), α-terpineol (10.20%) | [112] |
| E. coli (ATCC 25922) | ||||||
| Achillea setacea (bristly yarrow) | E. coli (ATCC 25922) | 0.5% Tween 80 | 72.00 mg/mL | Sabinene (10.80%), eucalyptol (18.50%) | [113] | |
| P. aeruginosa (ATCC 27853) | >72.00 mg/mL | |||||
| Achillea teretifolia (bristly yarrow) | E. coli (ATCC 25922) | 36.00 mg/mL | Eucalyptol (19.90%), camphor (11.10%), borneol (11.90%) | |||
| P. aeruginosa (ATCC 27853) | >72.00 mg/mL | |||||
|
| ||||||
| Angelica archangelica (angelica) root | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | α-Phellandrene (18.50%), α-pinene (13.70%), β-phellandrene (12.60%), δ-3-carene (12.10%) | [99] |
| Angelica archangelica (angelica) seed | β-Phellandrene (59.20%) | |||||
|
| ||||||
| Aniba rosaeodora (rosewood) | MIC | E. coli (ATCC 25922) | n.m. | 0.40 mg/mL | NCR | [85] |
|
| ||||||
| Anthemis aciphylla var. discoidea (chamomile), flowers | MIC | E. coli (ATCC 25922) | DMSO | 1.00 mg/mL | α-Pinene (39.00%), terpinen-4-ol (32.10%) | [114] |
| P. aeruginosa (ATCC 27853) | 0.25 mg/mL | |||||
|
| ||||||
| Anthemis aciphylla var. discoidea (chamomile), aerial parts | MIC | E. coli (ATCC 25922) | DMSO | 1.00 mg/mL | α-Pinene (49.40%), terpinen-4-ol (21.80%) | [114] |
| P. aeruginosa (ATCC 27853) | ||||||
|
| ||||||
| Anthemis aciphylla var. discoidea (chamomile), leaves | MIC | E. coli (ATCC 25922) | DMSO | 0.50 mg/mL | Terpinen-4-ol (24.30%) | [114] |
| P. aeruginosa (ATCC 27853) | ||||||
|
| ||||||
| Anthemis nobilis (chamomile) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | 2-Methylbutyl-2-methyl propanoic acid (31.50%), limonene (18.30%), 3-methylpentyl-2-butenoic acid (16.70%), isobutyl isobutyrate (10.00%) | [99] |
|
| ||||||
| Artemisia dracunculus (tarragon) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Estragole (82.60%) | [99] |
|
| ||||||
| Backhousia citriodora (lemon myrtle) | ADM | E. coli (NCTC 8196) | Tween 80 | 0.03% v/v | Geranial (51.40%), neral (40.90%) | [115] |
| P. aeruginosa (NCTC 6750) | 2.00% v/v | |||||
|
| ||||||
| Boswellia carteri (frankincense) (9 samples) | MIC | E. coli (ATCC 25922) | Acetone | 4.00–12.00 mg/mL | α-Pinene (4.80–40.40%), myrcene (1.60–52.40%), limonene (1.90–20.40%), α-thujene (0.30–52.40%), p-cymene (2.70–16.90%), β-pinene (0.30–13.10%) | [116] |
| Boswellia frereana (frankincense) (3 samples) | 4.00–6.00 mg/mL | α-Pinene (2.00–64.70%), α-thujene (0.00–33.10%), p-cymene (5.40–16.90%) | ||||
| Boswellia neglecta (frankincense) | E. coli (ATCC 8739) | Acetone | 3.00 mg/mL | NCR | [117] | |
| E. coli (ATCC 25922) | 6.00 mg/mL | α-Pinene (43.40%), β-pinene (13.10%) | [116] | |||
| P. aeruginosa (ATCC 27858) | 1.30 mg/mL | NCR | [117] | |||
| Boswellia papyrifera (frankincense) | E. coli (ATCC 8739) | 3.30 mg/mL | ||||
| P. aeruginosa (ATCC 27858) | 1.50 mg/mL | |||||
| Boswellia rivae (frankincense) | E. coli (ATCC 8739) | 3.00 mg/mL | ||||
| P. aeruginosa (ATCC 27858) | 1.00 mg/mL | |||||
| Boswellia sacra (frankincense) (2 samples) | E. coli (ATCC 25922) | 4.00–6.00 mg/mL | α-Pinene (18.30–28.00%), α-thujene (3.90–11.20%), limonene (11.20–13.10%) | [116] | ||
| Boswellia spp. (frankincense) (4 samples) | 6.00 mg/mL | α-Pinene (18.80–24.20%), limonene (11.70–19.00%) | ||||
| Boswellia thurifera (frankincense) | α-Pinene (28.00%), limonene (14.60%) | |||||
|
| ||||||
| Cananga odorata (ylang-ylang) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 3.00 mg/mL | Bicyclosesquiphellandrene (19.50%), β-farnesene (13.90%) | [99] |
| Cananga odorata (ylang-ylang), heads | 1.50 mg/mL | Benzyl acetate (31.90%), linalool (27.00%), methyl benzoate (10.40%) | ||||
|
| ||||||
| Canarium luzonicum (elemi) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Limonene (41.90%), elemol (21.60%), α-phellandrene (11.40%) | [99] |
|
| ||||||
| Carum carvi (caraway) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Limonene (27.60%), carvone (67.50%) | [99] |
| P. aeruginosa | DMSO | >16.00 μg/mL | DL-Limonene (53.35%), β-selinene (11.08%), β-elemene (10.09%) | [118] | ||
|
| ||||||
| Caryophyllus aromaticus (clove) | ADM90 | P. aeruginosa (ATCC 27853 and 15 clinical isolates) | Tween 80 | 3.00 mg/mL | Eugenol (75.85%), eugenyl acetate (16.38%) | [119] |
| E. coli (ATCC 25922 and 15 clinical isolates) | ||||||
|
| ||||||
| Cinnamomum cassia (cinnamon) | MIC | P. aeruginosa | DMSO | ≤1.00 μg/mL | trans-Caryophyllene (17.18%), eugenol (14.67%), linalool L (14.52%), trans-cinnamyl acetate, (13.85%), cymol (11.79%), cinnamaldehyde (11.25%) | [118] |
|
| ||||||
| Cinnamomum zeylanicum (cinnamon) | MAC | E. coli (ATCC 25922) | 75% ethanol | 200.00 mg/L | NCR | [231] |
| E. coli (10 clinical strains) | 400.00 mg/L | |||||
| ADM | E. coli | DMSO | 1.25 μL/mL | NCR | [232] | |
| E. coli (ATCC 25922) | 10% DMSO | >1.6 mg/mL | Cinnamaldehyde (52.42%) | [80] | ||
| MIC | P. aeruginosa (ATCC 27853) | 75% ethanol | 400.00 mg/L | NCR | [231] | |
| P. aeruginosa (clinical strain) | ||||||
| ADM | P. aeruginosa (ATCC 27853) | 10% DMSO | >0.80 mg/mL | Cinnamaldehyde (52.42%) | [80] | |
| MIC | Acetone | 1.50 mg/mL | Eugenol (80.00%) | [99] | ||
| ADM90 | P. aeruginosa (ATCC 27853 and 15 clinical isolates) | Tween 80 | 0.80 mg/mL | Cinnamaldehyde (86.31%) | [119] | |
| E. coli (ATCC 25922 and 15 clinical isolates) | 0.25 mg/mL | |||||
|
| ||||||
| Citrus aurantifolia (lime) | ADM | E. coli (ATCC 25922) | 10% DMSO | 6.40 mg/mL | NCR | [80] |
| P. aeruginosa (ATCC 27853) | ||||||
|
| ||||||
| Citrus aurantium (bitter orange), flowers | MIC | E. coli (ATCC 25922) | 10% DMSO | 1.25 mg/mL | Limonene (27.50%), α-terpineol (14.00%), E-nerolidol (17.50%), α-terpinyl acetate (11.7%) | [120] |
| E. coli (ATCC 8739) | ||||||
| P. aeruginosa (ATCC 9027) | 2.50 mg/mL | |||||
|
| ||||||
| Citrus aurantium (petitgrain) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Linalyl acetate (54.90%), linalool (21.10%) | [99] |
|
| ||||||
| Citrus bergamia (bergamot) | MAC | E. coli (ATCC 8739) | n.m. | 5.0 μL/mL | Bergamol (16.10%), linalool (14.02%), D-limonene (13.76%) | [62] |
|
| ||||||
| Citrus grandis (grapefruit) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 1.50 mg/mL | Limonene (74.80%) | [99] |
| ADM | E. coli | DMSO | 2.5 μL/mL | NCR | [232] | |
|
| ||||||
| Citrus medica limonum (lemon) | ADM | E. coli (ATCC 25922) | 10% DMSO | >6.4 mg/mL | NCR | [80] |
| P. aeruginosa (ATCC 27853) | 12.80 mg/mL | |||||
| MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | [99] | ||
|
| ||||||
| Citrus sinensis (orange) | ADM | E. coli (ATCC 25922) | 10% DMSO | >12.8 mg/mL | NCR | [80] |
| MAC | E. coli (ATCC 10536) | 0.1% ethanol | 1.875 mg/L | [121] | ||
| ADM | P. aeruginosa (ATCC 27853) | 10% DMSO | >12.80 mg/mL | [80] | ||
| MAC | P. aeruginosa (ATCC 15442) | 0.1% ethanol | 1.88 mg/mL | [121] | ||
| MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Limonene (93.20%) | [99] | |
|
| ||||||
| Commiphora guidotti (myrrh) | MIC | E. coli (ATCC 8739) | Acetone | 4.00 mg/mL | (E)-β-Ocimene (52.60%), α-santalene (11.10%), (E)-α-bisabolene (16.00%) | [117] |
| P. aeruginosa (ATCC 27858) | 1.40 mg/mL | |||||
| Commiphora myrrha (myrrh) | E. coli (ATCC 8739) | 1.00 mg/mL | Furanogermacrene (15.9%), furanoeudesma-1,3-diene (44.3%) | |||
| P. aeruginosa (ATCC 27858) | 0.50 mg/mL | |||||
| P. aeruginosa (ATCC 27858) | 4.00 mg/mL | Furanoeudesma-1,3-diene (57.70%), lindestrene (16.30%) | [99] | |||
|
| ||||||
| Coriandrum sativum (coriander), seed | MIC | E. coli (7 clinical isolates) | 0.5% DMSO with Tween 80 | 0.14 mg/mL | NCR | [122] |
| Cumin cymene (cumin) (6 samples) | 0.30 mg/mL | |||||
|
| ||||||
| Cupressus arizonica (smooth cypress) | MIC | P. aeruginosa (ATCC 27853) | 10% DMSO | 6.02–23.60 μg/mL | α-Pinene (20.00–60.50%), δ-3-carene (1.00–15.60%), umbellulone (0.80–18.40%) | [123] |
| Cupressus arizonica (smooth cypress), branches | E. coli (ATCC 25922) | 0.37 μg/mL | α-Pinene (58.60%), δ-3-carene (15.60%) | |||
| P. aeruginosa (ATCC 27853) | 11.80 μg/mL | |||||
| Cupressus arizonica (smooth cypress), female cones | E. coli (ATCC 25922) | 2.95 μg/mL | α-Pinene (60.50%), δ-3-carene (15.30%) | |||
| P. aeruginosa (ATCC 27853) | 6.02 μg/mL | |||||
| Cupressus arizonica (smooth cypress), leaves | E. coli (ATCC 25922) | 0.38 μg/mL | α-Pinene (20.00%), umbellulone (18.40%) | |||
| P. aeruginosa (ATCC 27853) | 23.60 μg/mL | |||||
| Cupressus sempervirens (cypress) | P. aeruginosa (ATCC 27853) | Acetone | 2.00 mg/mL | α-Pinene (41.20%), δ-3-carene (23.70%) | [99] | |
|
| ||||||
| Cymbopogon giganteus (lemongrass) | MIC | E. coli (CIP 105182) | 0.5% ethanol | 6.3 mg/mL | Limonene (42.00%), trans-p-mentha-1(7),8-dien-2-ol (14.20%), cis-p-mentha-1(7),8-dien-2-ol (12.00%) | [124] |
| P. aeruginosa (CRBIP 19.249) | 70.00 mg/mL | |||||
|
| ||||||
| Cymbopogon citratus (lemongrass) | MIC | E. coli (CIP 105182) | 0.5% ethanol | 10 mg/mL | Geranial (48.1%), neral (34.6%), myrcene (11.0%) | [124] |
| MAC | E. coli (clinical isolate VR 12 and MTCC 424) | Sodium taurocholate | 1.66–3.33 μL/mL | Citral (72.80%) | [125, 126] | |
| P. aeruginosa (MTCC 424 and clinical isolate VR 6) | 11.60–>20.00 μL/mL | |||||
| MIC | P. aeruginosa (CRBIP 19.249) | 0.5% ethanol | >80.00 mg/mL | Geranial (48.10%), neral (34.60%), myrcene (11.00%) | [124] | |
| P. aeruginosa (ATCC 27858) | Acetone | 1.50 mg/mL | Geranial (44.80%) | [99] | ||
| P. aeruginosa | DMSO | >16.00 μg/mL | Geranial (47.34%), β-myrcene (16.53%), Z-citral (8.36%) | [118] | ||
|
| ||||||
| Cymbopogon martinii (palmarosa) | MAC | E. coli (clinical isolate VR 12 and MTCC 424) | sodium taurocholate | 1.66–4.16 μL/mL | Geraniol (61.60%) | [125, 126] |
| P. aeruginosa (MTCC 424 and clinical isolate VR 6) | 8.33–>20.00 μL/mL | |||||
|
| ||||||
| Cymbopogon nardus (citronella) | MIC | P. aeruginosa (ATCC 27858) | acetone | 1.50 mg/mL | Citronellal (38.30%), geraniol (20.70%), citronellol (18.80%) | [99] |
| Daucus carota (carrot seed) | 3.00 mg/mL | Carotol (44.40%) | ||||
|
| ||||||
| Eucalyptus camaldulensis (eucalyptus) | ADM | E. coli | DMSO | 5.00 μL/mL | NCR | [232] |
| MIC | E. coli (ATCC 25922) | Acetone | 2.00 mg/mL | [130] | ||
| P. aeruginosa (ATCC 27853) | 1.00 mg/mL | |||||
|
| ||||||
| Eucalyptus globulus (eucalyptus) | ADM | E. coli (ATCC3428) | Tween 20 | 51.36 μg/mL | Eucalyptol (47.20%), (+)-spathulenol (18.10%) | [81] |
| MIC | E. coli (ATCC 25922) | Tween 80 | 10.00 mg/mL | 1,8-Cineol (81.93%) | [128] | |
| P. aeruginosa (ATCC 27853) | 10.00 mg/mL | [128] | ||||
| MAC | E. coli (clinical isolate and MTCC 424) | Sodium taurocholate | 1.66–3.33 μL/mL | Cineole (23.20%) | [125, 126] | |
| P. aeruginosa (MTCC 424 and clinical isolate VR 6) | 8.33–>20.00 μL/mL | |||||
| MIC | P. aeruginosa (ATCC 9027) | DMSO | 0.10% v/v | NCR | [129] | |
| P. aeruginosa (ATCC 27858) | Acetone | 3.00 mg/mL | 1,8-Cineole (58.00%), α-terpineol (13.20%) | [99] | ||
| E. coli (ATCC 25922) | 2.00 mg/mL | NCR | [130] | |||
| P. aeruginosa (ATCC 27853) | 1.00 mg/mL | |||||
| Eucalyptus radiata (eucalyptus) | E. coli (ATCC 25922) | Acetone | 2.00 mg/mL | 1,8-Cineole (65.7% ± 9.5), α-terpineol (12.8% ± 4.4) | [130] | |
| P. aeruginosa (ATCC 27853) | 1.00 mg/mL | [130] | ||||
| Eucalyptus citriodora (eucalyptus) | E. coli (ATCC 25922) | 2.00 mg/mL | NCR | [130] | ||
| P. aeruginosa (ATCC 27853) | 1.00 mg/mL | [130] | ||||
| Eucalyptus smithii (eucalyptus) | E. coli (ATCC 25922) | 2.00 mg/mL | NCR | [130] | ||
| P. aeruginosa (ATCC 27853) | 2.00 mg/mL | [130] | ||||
| Eucalyptus dives (eucalyptus) | E. coli (ATCC 25922) | 2.00 mg/mL | NCR | [130] | ||
| P. aeruginosa (ATCC 27853) | 1.00 mg/mL | [130] | ||||
| Eucalyptus intertexta (eucalyptus) | E.coli (ATCC 10536) | 10% DSMO | 15.6 μg/mL | NCR | [131] | |
| P. aeruginosa (ATCC 27853) | NI | |||||
| Eucalyptus largiflorens (eucalyptus) | E.coli (ATCC 10536) | |||||
| P. aeruginosa (ATCC 27853) | ||||||
|
| ||||||
| Foeniculum dulce (fennel) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 3.00 mg/mL | E-Anethole (79.10%) | [99] |
|
| ||||||
| Foeniculum vulgare (fennel) | MAC | E. coli (ATCC 25922) | DMSO | 0.25 mg/mL | trans-Anethole (68.53%), estragole (10.42%) | [132] |
| P. aeruginosa (ATCC 9027) | >10.00 mg/mL | |||||
| MIC | P. aeruginosa | >16.00 μg/mL | trans-Anethole (33.3%), DL-limonene (19.66%), carvone (12.03%) | [118] | ||
| Foeniculum vulgare (fennel) (6 samples) | MIC | P. aeruginosa (ATCC 27853) | 1000.00–>2000.00 μg/mL | Fenchone (16.90–34.70%), estragole (2.50–66.00%), trans-anethole (7.90–77.70%) | [133] | |
| E. coli (ATCC 25922) | 62.50–500 μg/mL | |||||
|
| ||||||
| Foeniculum vulgare Mill. ssp. vulgare (fennel), Aurelio | MAC | P. aeruginosa (ATCC 27853) | Tween 20 | >100.00 μg/mL | Limonene (16.50–21.50%), (E)-anethole (59.80–66.00%) | [134] |
| E. coli (ATCC 25922) | 50.00 μg/mL | |||||
| Foeniculum vulgare Mill. ssp. vulgare (fennel), Spartaco | E. coli (ATCC 25922) | 50.00–100.00 μg/mL | Limonene (0.20–17.70%), (E)-anethole (66.30–90.40%) | |||
| P. aeruginosa (ATCC 27853) | ≥100.00 μg/mL | |||||
|
| ||||||
| Geranium dissectum (geranium) | MAC | P. aeruginosa | DMSO | >16.00 μg/mL | β-Citronellol (25.45%), geraniol (13.83%) | [118] |
|
| ||||||
| Hyssopus officinalis (hyssop) | MAC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Isopinocamphone (48.70%), pinocamphone (15.50%) | [99] |
|
| ||||||
| Jasminum sambac (jasmine) | MAC | E. coli (MTCC 443) | Sodium taurocholate | 31.25 μL/mL | Linalool (59.00%), benzyl acetate (22.50%) | [233] |
|
| ||||||
| Juniperus excelsa (juniper), berries, Ohrid | ADM | E. coli (25927) | 50% DSMO | >50.00% | Sabinene (58.85%) | [87] |
| Juniperus officinalis (juniper), berry | MIC | E. coli (ATCC 25922) | Tween 80 | 20.00 mg/mL | α-Pinene (39.760%) | [128] |
| P. aeruginosa (ATCC 27853) | ||||||
| Juniperus virginiana (juniper) | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Thujopsene (29.80%), cedrol (14.90%), α-cedrene (12.40%) | [99] | |
| Juniperus virginiana (juniper), berries | α-Pinene (20.50%), myrcene (13.70%), bicyclosesquiphellandrene (10.70%) | |||||
|
| ||||||
| Kunzea ericoides (Kānuka) | MAC | E. coli (ATCC 11229) | Tween 80 | >2.00% v/v | α-pinene (61.60%) | [137] |
| P. aeruginosa (ATCC 15442) | >2.00% v/v | |||||
| MIC | P. aeruginosa (ATCC 9027) | Acetone | 4.00 mg/mL | α-Pinene (26.20–46.70%), p-cymene (5.80–19.10%) | [138] | |
|
| ||||||
| Laurus nobilis (bay) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.67 mg/mL | Eugenol (57.20%), myrcene (14.30%), chavicol (12.70%) | [99] |
|
| ||||||
| Lavandula angustifolia (lavender) | MIC | E. coli (ATCC 8739) | 10% DSMO | 730.1 μg/mL | Linalool (25.10%), linalyl acetate (22.50%) | [139] |
| E. coli (ampicillin-resistant NCTC 10418) | 722.2 μg/mL | |||||
| P. aeruginosa (NCTC 1662) | 1040.00 μg/mL | |||||
| E. coli (ATCC 11775) | Acetone | 6.20 mg/mL | Linalool (30.80%), linalyl acetate (31.30%) | [140] | ||
| P. aeruginosa (ATCC 9027) | 8.60 mg/mL | |||||
| P. aeruginosa (ATCC 27858) | 2.00 mg/mL | Linalyl acetate (36.70%), linalool (31.40%), terpinen-4-ol (14.90%) | [99] | |||
| Lavandula dentata (French lavender) | E. coli (BNI 2) | 5% DMSO | 2.20 mg/mL | Camphor (12.40%) | [141] | |
| Lavandula officinalis (lavender) | P. aeruginosa | DMSO | >16.00 μg/mL | δ-3-Carene (17.14%), α-fenchene (16.79%), diethyl phthalate (13.84%) | [118] | |
| Lavandula stoechas (French lavender) | E. coli (STCC 471) | Tween 80 | 8.00 μL/mL | 10s,11s-Himachala-3(12),4-diene (23.62%), cubenol (16.19%) | [142] | |
| Lavandula stoechas (French lavender), flower | E. coli (NRRL B-3008) | 20% DMSO | 250.00 μg/mL | α-Fenchone (39.20%) | [47] | |
| P. aeruginosa (NRRL B-23) | ||||||
| Lavandula stoechas (French lavender), leaf | E. coli (NRRL B-3008) | 250.00 μg/mL | α-Fenchone (41.90%), 1,8-cineole (15.60%), camphor (12.10%) | |||
| P. aeruginosa (NRRL B-23) | 500.00 μg/mL | |||||
|
| ||||||
| Leptospermum scoparium (manuka) | MAC | E. coli (ATCC 11229) | Tween 80 | >2.00% v/v | (−)-(E)-Calamenene (14.50%), leptospermone (17.60%) | [137] |
| P. aeruginosa (ATCC 15442) | 0.85% v/v | |||||
| MIC | P. aeruginosa (ATCC 9027) | Acetone | 4.00 mg/mL | Eudesma-4(14),11-diene (6.20–14.50%), α-selinene (5.90–13.50%), (E)-methyl cinnamate (9.20–19.50%) | [138] | |
|
| ||||||
| Litsea cubeba (May Chang) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 1.50 mg/mL | Geranial (45.60%), nerol (31.20%) | [99] |
| Matricaria chamomilla (German chamomile) | 4.00 mg/mL | Bisabolene oxide A (46.90%), β-farnesene (19.20%) | [99] | |||
|
| ||||||
| Matricaria recutita (German chamomile) | ADM90 | P. aeruginosa (ATCC 27853 and 15 clinical isolates) | Tween 80 | 54.40 mg/mL | Chamazulene (31.48%), α-bisabolol (15.71%), bisabolol oxide (15.71%) | [119] |
| E. coli (ATCC 25922 and 15 clinical isolates) | ||||||
|
| ||||||
| Matricaria songarica (chamomile) | MIC | E. coli (CCTCC AB91112) | Tween 80 | 100 μg/mL | E-β-Farnesene (10.58%), bisabolol oxide A (10.46%) | [143] |
| P. aeruginosa (CCTCC AB93066) | 200.00 μg/mL | |||||
|
| ||||||
| Melaleuca alternifolia (tea tree) | MIC | E. coli (ATCC 10536) | Tween 80 | 0.12% v/v | NCR | [147] |
| E. coli (110 clinical isolates) | 0.12–0.25% v/v | |||||
| E. coli (AG100) | n.m. | 0.25% (v/v) | Terpinen-4-ol (39.80%), γ-terpinene (17.80%) | [149, 150] | ||
| E. coli (ATCC 25922) | None used | 0.25% (v/v) | Terpinen-4-ol (40.00%), γ-terpinen (13.00%), p-cymene (13.00%) | [97] | ||
| E. coli (ATCC 11775) | Acetone | 3.70 mg/mL | Terpinen-4-ol (38.60%), γ-terpinene (21.60%) | [140] | ||
| MAC | E. coli (ATCC 11229) | Tween 80 | 0.25% v/v | α-Terpinene (11.40%), γ-terpinene (22.50%), terpinen-4-ol (35.20%) | [137] | |
| P. aeruginosa (ATCC 15442) | 1.00% v/v | |||||
| MIC | P. aeruginosa (NCTC 6749) | n.m. | 8.00% (v/v) | Terpinen-4-ol (39.80%), γ-terpinene (17.80%) | [150] | |
| P. aeruginosa (10 clinical isolates) | Tween 80 | 2.00–5.00% v/v | terpinen-4-ol (35.70%) | [152] | ||
| ADM | P. aeruginosa (NCTC 6750) | 0.5% Tween 20 | >2.00% v/v | Terpinen-4-ol (42.80%), γ-terpinene (18.20%) | [115] | |
| E. coli (NCTC 8196) | 0.20% v/v | [115] | ||||
| MIC | P. aeruginosa (NCIB 8295) | n.m. | 12.50% v/v | NCR | [145] | |
| MIC90 | P. aeruginosa (30 clinical isolates) | Tween 80 | 4.00% | Terpinen-4-ol (40.30%), γ-terpinene (19.70%) | [234] | |
| MIC | P. aeruginosa (ATCC 9027) | Acetone | 8.60 mg/mL | Terpinen-4-ol (38.60%), γ-terpinene (21.60%) | [140] | |
| MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Terpinen-4-ol (49.30%), γ-terpinene (16.90%) | [99] | |
|
| ||||||
| Melaleuca cajuputi (cajuput) | MIC | E. coli (ATCC 25922) | Tween 80 | 5.00 mg/mL | 1,8-Cineol (67.60%) | [128] |
| P. aeruginosa (ATCC 27853) | ||||||
| P. aeruginosa (ATCC 15442) | 1.90% v/v | 1,8-Cineole (55.50%) | [137] | |||
| E. coli (ATCC 11229) | 0.40% v/v | |||||
|
| ||||||
| Melaleuca quinquenervia (niaouli) | MIC | E. coli (ATCC 11229) | Tween 80 | 0.40% v/v | 1,8-Cineole (61.20%) | [137] |
| P. aeruginosa (ATCC 15442) | 1.90%v/v | |||||
| Melaleuca viridiflora (niaouli) | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | 1,8-Cineole (45.90%), α-terpinene (21.00%) | [99] | |
|
| ||||||
| Melissa officinalis (lemon balm) | MIC | P. aeruginosa (NCTC 1662) | 10% DSMO | 1000.30 μg/mL | Citronellal (20.50%), β-citronellol (11.50%), geraniol (17.00%) | [139] |
| E. coli (ATCC 8739) | 442.30 μg/mL | |||||
| E. coli (ampicillin-resistant NCTC 10418) | 567.40 μg/mL | |||||
| E. coli (ATCC 25922) | n.m. | 10.00 mg/mL | NCR | [85] | ||
|
| ||||||
| Mentha piperita (peppermint) | MIC | E. coli (ATCC 25922) | Tween 80 | 0.6 mg/mL | 1,8-Cineol (12.06%), menthone (22.24%), menthol (47.29%) | [128] |
| MAC | E. coli (clinical isolate and MTCC 424) | Sodium taurocholate | 1.66–2.50 μL/mL | Menthol (36.40%) | [125, 126] | |
| MIC | E. coli (ATCC 11775) | Acetone | 5.70 mg/mL | Menthone (18.20%), menthol (42.90%) | [140] | |
| E. coli (ATCC 25922) | DMSO | 1.25–2.50 mg/mL | Menthol (27.50–42.30%), menthone (18.40–27.90%) | [155] | ||
| n.m. | 3.20 mg/mL | NCR | [85] | |||
| P. aeruginosa (ATCC 27853) | Tween 80 | 20.00 mg/mL | 1,8-Cineol (12.06%), menthone (22.24%), menthol (47.29%) | [128] | ||
| P. aeruginosa | DMSO | ≤1.00 μg/mL | Menthone (40.82%), carvone (24.16%) | [118] | ||
| P. aeruginosa (ATCC 9027) | 0.10% v/v | NCR | [129] | |||
| MAC | P. aeruginosa (MTCC 424 and clinical isolate VR 6) | Sodium taurocholate | 10.00–>20.00 μL/mL | Menthol (36.40%) | [125, 126] | |
| MIC | P. aeruginosa (ATCC 9027) | Acetone | 8.60 mg/mL | Menthone (18.20%), menthol (42.90%) | [140] | |
| P. aeruginosa (ATCC 27853) | DMSO | 2.50–5.00 mg/mL | Menthol (27.50–42.30%), menthone (18.40–27.90%) | [155] | ||
| P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Menthol (47.50%), menthone (18.60%) | [99] | ||
| E. coli (CIP 105182) | Ethanol | 40.0 mg/mL | Menthol (39.30%), menthone (25.20%) | [156] | ||
| P. aeruginosa (CRBIP 19.249) | 0.5% ethanol | >80.00 mg/mL | ||||
|
| ||||||
| Myrtus communis (myrtle) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Myrtenyl acetate (28.20%), 1,8-cineole (25.60%), α-pinene (12.50%) | [99] |
| ADM | E. coli (ATCC 35218) | Tween 20 | 11.20 mg/mL | NCR | [157] | |
|
| ||||||
| Ocimum basilicum (basil) | MIC | E. coli (CIP 105182) | Ethanol | 8.30 mg/mL | Linalool (57.00%), eugenol (19.20%) | [156] |
| MAC | E. coli (ATCC 8739) | n.m. | 1.25 μL/mL | Eugenol (62.60%), caryophyllene (21.51%) | [62] | |
| ADM | E. coli (ATCC 25922) | 96% ethanol | 8.00 μL/mL | Estragole (86.4%) | [223] | |
| E. coli (ESBL+) (4 clinical strains from wounds) | 8.50–9.25 μL/mL | |||||
| E. coli (ESBL−) (4 clinical strains from wounds) | 10.00–11.50 μL/mL | |||||
| MIC | P. aeruginosa (CRBIP 19.249) | 0.5% ethanol | >80.00 mg/mL | Linalool (57.00%), eugenol (19.20%) | [156] | |
| MAC | P. aeruginosa (ATCC 9027) | Tween 80 | 0.0030% v/v | Linalool (54.95%), methyl chavicol (11.98%) | [158] | |
| P. aeruginosa (clinical isolate) | ||||||
| MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.67 mg/mL | Linalool (54.10%) | [99] | |
| MIC90 | E. coli (ATCC 8739) | n.m. | 160.00 μg/mL | Methyl chavicol (46.90%), geranial (19.10%), neral (15.15%) | [159] | |
| MIC | E. coli (ATCC 35210) | Tween 80 | 0.18–5.40 μg/mL | Linalool (30.30–58.60%) | [160] | |
| P. aeruginosa (ATCC 27853) | 0.11–11.74 μg/mL | |||||
|
| ||||||
| Origanum acutidens (Turkey oregano) | MIC | E. coli (A1) | 10% DMSO | 62.50 μg/mL | Carvacrol (72.00%) | [161] |
| P. aeruginosa (ATCC9027) | 125.00 μg/mL | |||||
| P. aeruginosa (ATCC27859) | 125.00 μg/mL | |||||
| Origanum majorana (marjoram) | P. aeruginosa (ATCC 9027) | DMSO | 0.05% v/v | NCR | [129] | |
| P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | 1,8-Cineole (46.00%), linalool (26.10%) | [99] | ||
| Origanum microphyllum (oregano) | P. aeruginosa (ATCC 227853) | Tween 80 | NI | Terpin-4-ol (24.86%), γ-terpinene (13.83%), linalool (10.81%) | [162] | |
| E. coli (ATCC 25922) | 3.35 mg/mL | |||||
| Origanum scabrum (oregano) | E. coli (ATCC 25922) | 0.28 mg/mL | Carvacrol (74.86%) | |||
| P. aeruginosa (ATCC 227853) | 1.27 mg/mL | |||||
|
| ||||||
| Origanum vulgare (oregano) | MAC | E. coli (ATCC 25922) | 75% ethanol | 200.00 mg/L | NCR | [231] |
| E. coli (10 clinical isolates) | 200.00–400.00 mg/L | |||||
| E. coli (ATCC 8739) | n.m. | 0.63 μL/mL | Carvacrol (30.17%), p-cymene (15.20%), γ-terpinen (12.44%) | [62] | ||
| ADM | E. coli (ATCC 35218) | Tween 20 | 0.70 mg/mL | NCR | [157] | |
| MIC | P. aeruginosa (ATCC 27853) | 75% ethanol | 800.00 mg/L | [231] | ||
| P. aeruginosa (clinical isolate) | 400.00 mg/L | |||||
| P. aeruginosa (ATCC 2730) | n.m. | 1648.00 mg/L | [164] | |||
| P. aeruginosa (ATCC 9027) | DMSO | 0.20% v/v | [129] | |||
| Origanum vulgare subsp. hirtum (Greek oregano) | MIC | E. coli (ATCC 25922) | 10% DMSO and Tween 80 | >512.00 μg/mL | Linalool (96.31%) | [165] |
| P. aeruginosa (ATCC 27853) | ||||||
| Origanum vulgare subsp. vulgare (oregano) | E. coli (ATCC 25922) | 213.30 μg/mL | Thymol (58.31%), carvacrol (16.11%), p-cymene (13.45%) | |||
| P. aeruginosa (ATCC 27853) | 256.00 μg/mL | |||||
|
| ||||||
| Pelargonium graveolens (geranium) | ADM | E. coli (ATCC 25922) | 10% DMSO | >6.40 mg/mL | NCR | [80] |
| E. coli (ATCC 35218) | Tween 20 | 5.60 mg/mL | [157] | |||
| MIC | E. coli (clinical strains isolated from wounds) | Ethanol | 3.00–3.75 mL/mL | Citronellol (26.70%), geraniol (13.40%) | [166] | |
| ADM | P. aeruginosa (ATCC 27853) | 10% DMSO | >12.80 mg/mL | NCR | [80] | |
| MIC | P. aeruginosa | Ethanol | 9.25–10.50 mL/mL. | Citronellol (26.70%), geraniol (13.40%) | [166] | |
|
| ||||||
| Pelargonium odoratissimum (geranium) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Citronellol (34.20%), geraniol (15.70%) | [99] |
|
| ||||||
| Perovskia abrotanoides (Russian sage) | MIC | E. coli (ATCC 8739) | 10% DMSO | >8.00 μL/mL | Camphor (23.00%), 1,8-cineole (22.00%), α-pinene (12.00%) | [167] |
| P. aeruginosa (ATCC 9027) | ||||||
|
| ||||||
| Pimpinella anisum (anise) | MIC | E. coli | DMSO | >500.00 μg/mL | NCR | [168] |
| P. aeruginosa | ||||||
| P. aeruginosa | >16.00 μg/mL | Anethole (64.82%) | [118] | |||
|
| ||||||
| Pinus sylvestris (pine) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Bornyl acetate (42.30%), camphene (11.80%), α-pinene (11.00%) | [99] |
| Piper nigrum (black pepper) | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | β-Caryophyllene (33.80%), limonene (16.40%) | [99] | |
|
| ||||||
| Pogostemon cablin (patchouli) | MIC | E. coli (ampicillin-resistant NCTC 10418) | 10% DSMO | 530.2 μg/mL | α-Guaiene (13.80%), α-bulnesene (17.10%), patchouli alcohol (22.70%) | [139] |
| E. coli (ATCC 8739) | 410.7 μg/mL | |||||
| P. aeruginosa (NCTC 1662) | 1200.00 μg/mL | |||||
| Pogostemon patchouli (patchouli) | P. aeruginosa (ATCC 27858) | Acetone | 2.00 mg/mL | Patchouli alcohol (37.30%), α-bulnesene (14.60%), α-guaiene (12.50%) | [99] | |
|
| ||||||
| Rosmarinus officinalis (rosemary) | MIC | E. coli (ATCC 8739) | Tween 80 | 0.25% v/v | 1,8-Cineole (27.23%), α-pinene (19.43%), camphor (14.26%), camphene (11.52%) | [169] |
| E. coli (ATCC 8739) | 10% DSMO | 733.70 μg/mL | 1,8-Cineol (29.20%), (+)-camphor (17.20%) | [139] | ||
| E. coli (ampicillin-resistant NCTC 10418) | 810.70 μg/mL | |||||
| E. coli (ATCC 8739) | Tween 80 | 0.03% (v/v) | 1,8-Cineole (26.54%), α-pinene (20.14%), camphene (11.38%), camphor (12.88%) | [170] | ||
| E. coli (MTCC 723) | n.m. | >11.00 mg/mL | NCR | [171] | ||
| E. coli (ATCC 8739) | Hexane | 0.47–3.75 mg/mL | α-Pinene (8.14–11.47)%, 1,8-cineole (10.56–11.91%), camphor (16.57–16.89%), verbenone (17.43–23.79%), bornyl acetate (9.19–11.62%) | [171] | ||
| ADM | E. coli (ATCC 25922) | 10% DMSO | >6.40 mg/mL | NCR | [80] | |
| E. coli (ATCC 35218) | Tween 20 | 11.20 mg/mL | [157] | |||
| MIC | E. coli (ATCC 11775) | Acetone | 4.50 mg/mL | 1,8-Cineole (41.40%), α-pinene (13.30%), camphor (12.40%) | [140] | |
| ADM | E. coli (ATCC 25922) | 96% ethanol | 18.50 μL/mL | 1,8-Cineole (46.40%), camphor (11.40%), α-pinene (11.00%) | [223] | |
| E. coli (ESBL+) (4 clinical strains from wounds) | 18.50–19.25 μL/mL | |||||
| E. coli (ESBL−) (4 clinical strains from wounds) | 18.25 μL/mL–20.0 μL/mL | |||||
| MIC | P. aeruginosa (ATCC 27853) | Tween 80 | 1.00%v/v | 1,8-Cineole (27.23%), α-pinene (19.43%), camphor (14.26%), camphene (11.52%) | [169] | |
| P. aeruginosa (NCTC 1662) | 10% DSMO | 1113.30 μg/mL | 1,8-Cineol (29.20%), (+)-camphor (17.20%) | [139] | ||
| P. aeruginosa (ATCC 27853) | Tween 80 | 0.10% v/v | 1,8-Cineole (26.54%), α-pinene (20.14%), camphene (11.38%), camphor (12.88%) | [170] | ||
| P. aeruginosa (MTCC 741) | n.m. | >11.00 mg/mL | NCR | [171] | ||
| P. aeruginosa (ATCC 9027) | DMSO | 0.20% v/v | [129] | |||
| ADM | P. aeruginosa (ATCC 27853) | 10% DMSO | >6.40 mg/mL | NCR | [80] | |
| MIC | P. aeruginosa (ATCC 9027) | Acetone | 6.20 mg/mL | 1,8-Cineole (41.40%), α-pinene (13.30%), camphor (12.40%) | [140] | |
| P. aeruginosa (ATCC 27858) | 2.00 mg/mL | 1,8-Cineole (48.00%) | [99] | |||
| ADM90 | P. aeruginosa (ATCC 27853 and 15 clinical isolates) | Tween 80 | 79.91 mg/mL | Camphor (27.51%), limonene (21.01%), myrcene (11.19%), α-pinene (10.37%) | [119] | |
| E. coli (ATCC 25922 and 15 clinical isolates) | 79.35 mg/mL | |||||
|
| ||||||
| Salvia bracteata (sage) | MIC | E. coli (ATCC 25922) | Tween 20 | >100.00 μg/mL | Caryophyllene oxide (16.60%) | [173] |
| MAC | P. aeruginosa (ATCC 27853) | |||||
|
| ||||||
| Salvia eremophila (sage) | MIC | E. coli (ATCC 10536) | 10% DMSO | 500.00 μg/mL | Borneol (21.83%), α-pinene (18.80%), bornyl acetate (18.68%) | [174] |
| P. aeruginosa (ATCC 27853) | NI | |||||
|
| ||||||
| Salvia nilotica (sage) | ADM | E. coli (ATCC 25922) | n.m. | NI | trans-Caryophyllene (10.90%) | [175] |
| P. aeruginosa (ATCC 227853) | 7.80 mg/mL | |||||
|
| ||||||
| Salvia officinalis (sage) | ADM | E. coli (ATCC 35218) | Tween 20 | 11.2 mg/mL | NCR | [157] |
| MIC | E. coli (ATCC 8739) | 10% DSMO | 475.0 μg/mL | 1,8-Cineol (27.40%), α-thujone (16.30%), β-thujone (11.20%), borneol (10.40%) | [139] | |
| E. coli (ampicillin-resistant NCTC 10418) | 548.0 μg/mL | |||||
| P. aeruginosa (NCTC 1662) | 1250.30 μg/mL | |||||
| P. aeruginosa (ATCC 9027) | DMSO | 0.20% v/v | NCR | [129] | ||
| ADM | P. aeruginosa (clinical strain) | n.m. | 7.50 mg/mL | NCR | [176] | |
| E. coli (ATCC 25922) | 3.75 mg/mL | |||||
| E. coli (clinical strain) | 7.50 mg/mL | |||||
|
| ||||||
| Salvia ringens (sage) | MIC | E. coli (ATCC 25922) | n.m. | 3.25 mg/mL | α-Pinene (12.85%), 1,8-cineole (46.42%) | [177] |
| P. aeruginosa (ATCC 227853) | 3.75 mg/mL | |||||
| Salvia rosifolia (sage) (3 samples) | E. coli (NRRL B 3008) | 20% DMSO | 250–1000 μg/mL | α-Pinene (15.70–34.80%), 1,8-cineole (16.60–25.10%), β-pinene (6.70–13.50%) | [178] | |
| P. aeruginosa (NRRL B 23) | 250.00–500.00 μg/mL | |||||
| Salvia rubifolia (sage) | E. coli (ATCC 25922) | Tween 20 | >100 μg/mL | γ-Muurolene (11.80%). | [173] | |
|
| ||||||
| Salvia sclarea (clary sage) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 3.50 mg/mL | Linalyl acetate (72.90%), linalool (11.90%) | [99] |
| Santalum album (sandalwood) | 0.50 mg/mL | α-Santalol (32.10%) | ||||
| Styrax benzoin (benzoin) | 3.00 mg/mL | Cinnamyl alcohol (44.80%), benzene propanol (21.70%) | ||||
|
| ||||||
| Syzygium aromaticum (clove) | MIC | E. coli (ATCC 8739) | Tween 80 | 0.50% v/v | Eugenol (68.52%), β-caryophyllene (19.00%), 2-methoxy-4-[2-propenyl]phenol acetate (10.15%) | [169] |
| ADM | E. coli (ATCC 25922) | 10% DMSO | >1.6 mg/mL | NCR | [80] | |
| MIC | P. aeruginosa (ATCC 27853) | Tween 80 | 0.13% v/v | Eugenol (68.52%), β-caryophyllene (19.00%), 2-methoxy-4-[2-propenyl]phenol acetate (10.15%) | [169] | |
| ADM | P. aeruginosa (ATCC 27853) | 10% DMSO | >1.60 mg/mL | NCR | [80] | |
| MIC | P. aeruginosa | DMSO | >16.00 μg/mL | Eugenol (84.07%), isoeugenol (10.39%) | [118] | |
| P. aeruginosa (ATCC 27858) | Acetone | 1.50 mg/mL | Eugenol (82.20%), eugenol acetate (13.20%) | [99] | ||
|
| ||||||
| Tagetes minuta (Mexican marigold) | MIC90 | E. coli (ATCC 8739) | n.m. | 165.00 μg/mL | Dihydrotagetone (33.90%), E-ocimene (19.90%), tagetone (16.10%) | [159] |
|
| ||||||
| Tagetes patula (French marigold) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 1.50 mg/mL | (E)-β-Ocimene (41.30%), E-tagetone (11.20%), verbenone (10.90%) | [99] |
|
| ||||||
| Thymus capitatus (thyme) | MIC | E. coli (ATCC 25922) | Tween 80 | 900.00 μg/mL | p-Cymene (26.40%), thymol (29.30%), carvacrol (10.80%) | [181] |
| P. aeruginosa (ATCC 27853) | >900.00 μg/mL | |||||
| Thymus capitatus (thyme), commercial | E. coli (ATCC 25922) | 900.00 μg/mL | α-Pinene (25.20%), linalool (10.30%), thymol (46.10%) | |||
| P. aeruginosa (ATCC 27853) | >900.00 μg/mL | |||||
| Thymus herba-barona (thyme), Gennargentu | E. coli (ATCC 25922) | 450 μg/mL | Thymol (46.90%), carvacrol (20.60%) | |||
| P. aeruginosa (ATCC 27853) | >900.00 μg/mL | |||||
| Thymus herba-barona (thyme), Limbara | E. coli (ATCC 25922) | 450 μg/mL | p-Cymene (27.60%), thymol (50.30%) | |||
| P. aeruginosa (ATCC 27853) | >900.00 μg/mL | |||||
|
| ||||||
| Thymus hyemalis (thymol, thymol/linalool, carvacrol chemotypes) (thyme) | MAC | E. coli (CECT 516) | 95% ethanol | <0.2–2.0 μL/mL | p-Cymene (16.00–19.80%), linalool (2.10–16.60%), thymol (2.90–43.00%), carvacrol (0.30–40.10%) | [61] |
|
| ||||||
| Thymus numidicus | ADM | P. aeruginosa (clinical strain) | n.m. | 0.47 mg/mL | NCR | [176] |
| E. coli (ATCC 25922) | 0.12 mg/mL | |||||
| E. coli (clinical strain) | 0.23 mg/mL | |||||
|
| ||||||
| Thymus schimperi (thyme) | ADM | E. coli | DMSO | 0.63 μL/mL | NCR | [232] |
| Thymus serpyllum (thyme) | E. coli (ATCC 35218) | Tween 20 | 0.70 mg/mL | [157] | ||
|
| ||||||
| Thymus vulgaris (thyme) | MIC | E. coli | DMSO | 62.50 μg/mL | NCR | [168] |
| E. coli (ATCC 8739) | 10% DSMO | 430.40 μg/mL | p-Cymene (17.90%), thymol (52.40%) | [139] | ||
| E. coli (ampicillin-resistant NCTC 10418) | 360.60 μg/mL | |||||
| E. coli (ATCC 25922) | n.m. | 0.30 mg/mL | NCR | [85] | ||
| ADM | E. coli (ATCC 25922) | Ethanol | 0.25 μL/mL | Thymol (38.10%), p-cymene (29.10%) | [182] | |
| E. coli (2 multidrug-resistant clinical strains from groin) | 0.25–0.50 μL/mL | |||||
| E. coli (7 multidrug-resistant clinical strains from wounds) | ||||||
| E. coli (multidrug-resistant clinical strain from abdominal cavity) | 0.50 μL/mL | |||||
| E. coli (2 multidrug-resistant clinical strains from ulcers) | 0.25–0.50 μL/mL | |||||
| E. coli (5 multidrug-resistant clinical strains from bedsores) | ||||||
| MIC | E. coli (ATCC 11775) | Acetone | 0.50 mg/mL | Thymol (47.20%), p-cymene (22.10%) | [140] | |
| ADM | E. coli (ATCC3428) | Tween 20 | 9.25 μg/mL | Thymol (48.10%), p-cymene (15.60%), γ-terpinene (15.40%) | [81] | |
| MIC | P. aeruginosa | DMSO | >500.00 μg/mL | NCR | [168] | |
| P. aeruginosa (NCTC 1662) | 10% DSMO | 1250.30 μg/mL | p-Cymene (17.90%), thymol (52.40%) | [139] | ||
| ADM | P. aeruginosa (ATCC 27853) | Ethanol | 0.50 μL/mL | Thymol (38.10%), p-cymene (29.10%) | [182] | |
| P. aeruginosa (multidrug-resistant clinical strain from toes) | 1.50 μL/mL | |||||
| P. aeruginosa (6 multidrug-resistant clinical strains from wounds) | 1.50–2.00 μL/mL | |||||
| P. aeruginosa (6 multidrug-resistant clinical strains from ulcers) | 2.00–2.50 μL/mL | |||||
| P. aeruginosa (6 multidrug-resistant clinical strains from bedsores) | 1.50–2.00 μL/mL | |||||
| MIC | P. aeruginosa (ATCC 9027) | Acetone | 8.6. mg/mL | Thymol (47.20%), p-cymene (22.10%) | [140] | |
| P. aeruginosa (ATCC 27858) | 2.00 mg/mL | p-Cymene (39.90%), thymol (20.70%) | [99] | |||
|
| ||||||
| Thymus vulgaris (thyme) (thymol chemotype) | MAC | E. coli (CECT 516) | 95% ethanol | 0.5 μL/mL | p-Cymene (18.70%), thymol (57.70%) | [61] |
| Thymus zygis subsp. gracilis (thyme) (thymol and two linalool chemotypes) | <0.2 μL/mL | p-Cymene (0.50–11.20%), (E)-sabinene hydrate (0.20–18.20%), linalool (2.00–82.30%) | ||||
|
| ||||||
| Vetiveria zizanioides/Andropogon muricatus (vetiver) | MIC | P. aeruginosa (ATCC 27858) | Acetone | 1.50 mg/mL | Zizanol (13.60%), β-vetirenene (7.20%) | [99] |
aScientific name (common name), part of plant (if applicable).
bMIC: microdilution method; MAC: macrodilution method; ADM: agar dilution method; CTA: contact time assay.
cAmerican Type Culture Collection, Rockville, USA (ATCC); Colección Espanõla de Cultivos Tipo (CECT); collection of microorganisms of the Department of Microbiology (MFBF); culture collection of antibiotics-resistant microbes (CCRM); Eskişehir Osmangazi University, Faculty of Medicine, clinical isolate (OGU); Laboratorio de Microbiología, Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Mendoza, Argentina (LM); Microbial Type Culture Collection (MTCC); Mycology Laboratory (LM); National Center of Industrial Microorganisms (NCIM); National Collection of Type Cultures, London, Great Britain (NCTC); Spanish Collection of Type Cultures (STCC).
dDMSO concentration was not included; n.m.: not mentioned.
eNI: no inhibition.
fNCR: no composition results reported.
Aniba rosaeodora (rosewood) was found to inhibit E. coli at an MIC value of 0.40 mg/mL. No GC-MS data was given [85]. Anthemis aciphylla var. discoidea (chamomile) also displayed notable inhibition against E. coli and P. aeruginosa; however, the highest activity was seen for the essential oil containing high levels of α-pinene (39.00%) and terpinen-4-ol (32.10%) [114]. Cinnamomum zeylanicum, with the main compound cinnamaldehyde, was shown to have inhibited these two Gram-negative pathogens at noteworthy MIC values [80]. Noteworthy activity was also reported for Commiphora myrrha (myrrh) and Thymus numidicus (thyme) [99]. Syzygium aromaticum and S. album were reported to effectively inhibit P. aeruginosa [99]; and Thymus vulgaris (thyme) inhibits E. coli (including multidrug-resistant strains) [182].
4.1.4. Other Bacterial Pathogens
Brevibacterium spp. form part of the Coryneform bacteria and are involved in foul body odour [3, 103]. Insufficient quantitative studies have been conducted using commercial essential oils to treat problems caused by these microorganisms, even though there have been some earlier studies using the diffusion assays against B. linen [226–228]. One quantitative study reported on the activity of Ziziphora persica against B. agri (125 μg/mL) and B. brevis (250 μg/mL), in addition to Ziziphora clinopodioides against B. agri (31.25 μg/mL) and B. brevis (125 μg/mL) [229]. In another study, essential oils of Kunzea ericoides (Kānuka) and L. scoparium were able to inhibit three species of Brevibacterium (MIC: 0.06–1.00 mg/mL) [138]. Clearly, the lack of attention to this neglected group of microorganisms warrants further attention, especially considering that, to the best of our knowledge, not one essential oil recommended for odour has been investigated against relevant pathogens in vitro.
The β-hemolytic Streptococcus (S. pyogenes) is a threatening pathogen that needs to be considered when investigating wound infections [166]. Group A Streptococcus (GAS) is usually involved in impetigo and necrotising fasciitis (“flesh-eating” disease). This pathogen has developed resistance to erythromycin, azithromycin, clarithromycin, clindamycin, and tetracycline [188, 190]. Group B Streptococcus is also involved in skin infections and has developed resistance to clindamycin, erythromycin, azithromycin, and vancomycin [190]. Periorbital cellulitis is a common occurrence in children and is caused by Haemophilus influenzae [106], and Clostridium spp. (C. perfringens, C. septicum, C. tertium, C. oedematiens, and C. histolyticum) are involved in gas green/gangrene infections. Table 6 summarises the antimicrobial activity of essential oils that have been studied and shown to have some in vitro efficacy against these pathogens. The lack of studies against S. pyogenes, C. perfringens, and H. influenza highlights the need to investigate these sorely neglected dermatologically important pathogens, especially since the few available studies have shown these organisms to be highly susceptible to essential oil inhibition. These are also pathogens that cause deeper skin infections, so, with the enhanced penetration offered by essential oils, they may prove beneficial.
Table 6.
Essential oil studies showing efficacy against other bacterial skin pathogens.
| Essential oila | Methodb | Species strainc | Solventd | Resulte | Main componentsf | Reference |
|---|---|---|---|---|---|---|
| Achillea millefolium (yarrow) | MIC | C. perfringens KUKENS-Turkey | Tween 80 | 4.50 mg/mL | Eucalyptol (24.60%), camphor (16.70%), α-terpineol (10.20%) | [112] |
| Achillea setacea (bristly yarrow) | 0.56 mg/mL | Sabinene (10.80%), eucalyptol (18.50%) | [113] | |||
| Achillea teretifolia (yarrow) | 0.28 mg/mL | Eucalyptol (19.90%), camphor (11.10%), borneol (11.90%) | ||||
|
| ||||||
| Eucalyptus globulus (eucalyptus) | MIC | S. pyogenes (ATCC 12344) | Tween 80 | 10.00 mg/mL | 1,8-Cineol (81.93%) | [128] |
| S. pyogenes (NHLS 8668) | 0.50 mg/mL | |||||
|
| ||||||
| Eucalyptus radiata (eucalyptus) | MIC | S. pyogenes (NHLS 8668) | Acetone | 0.50–1.00 mg/mL | 1,8-Cineole (65.7% ± 9.5), α-terpineol (12.8% ± 4.4) | [130] |
| Eucalyptus camaldulensis (eucalyptus) | Acetone | 0.50 mg/mL | ||||
| Eucalyptus citriodora (eucalyptus) | Acetone | 1.00 mg/mL | ||||
| Eucalyptus smithii (eucalyptus) | Acetone | 0.50 mg/mL | ||||
| Eucalyptus dives (eucalyptus) | Acetone | |||||
|
| ||||||
| Juniperus excelsa (juniper), berries, Dojran | ADM | S. pyogenes (clinical isolate) | 50% DSMO | >50.00% | α-Pinene (70.81%) | [87] |
| H. influenzae (clinical isolate) | 31.00 μL/mL | |||||
| Juniperus excelsa (juniper), berries, Ohrid | S. pyogenes (clinical isolate) | >50.00% | Sabinene (58.85%) | |||
| H. influenzae (clinical isolate) | ||||||
| Juniperus excelsa (juniper), leaves, Dojran | H. influenzae (clinical isolate) | >50.00% | α-Pinene (33.83%), cedrol (24.44%) | |||
| Juniperus excelsa (juniper), leaves, Ohrid | S. pyogenes (clinical isolate) | 125.00 μL/mL | Sabinene (29.49%), cis-thujone (26.20%), menth-2-en-1-ol (12.86%) | |||
| H. influenzae (clinical isolate) | ||||||
|
| ||||||
| Juniperus officinalis (juniper berry) | MIC | S. pyogenes (ATCC 12344) | Tween 80 | 20.00 mg/mL | α-Pinene (39.76%) | [128] |
|
| ||||||
| Kunzea ericoides (Kānuka) | MAC | C. diphtheriae (clinical isolate) | Tween 80 | 0.25% v/v | α-Pinene (61.60%) | [137] |
| C. minutissimus (clinical isolate) | 0.30% v/v | |||||
| MIC | S. pyogenes (ATCC 8668) | Acetone | 2.00 mg/mL | α-Pinene (26.2–46.7%), p-cymene (5.8–19.1%) | [138] | |
| B. brevis (ATCC 8246) | 1.00 mg/mL | |||||
| B. agri (ATCC 51663) | ||||||
| B. laterosporus (ATCC 64) | ||||||
|
| ||||||
| Leptospermum scoparium (manuka) | MAC | C. diphtheriae (clinical isolate) | Tween 80 | 0.05% v/v | (−)-(E)-Calamenene (14.50%), leptospermone (17.60%) | [137] |
| C. minutissimus (clinical isolate) | ||||||
| MIC | S. pyogenes (ATCC 8668) | Acetone | 1.00 mg/mL | Eudesma-4(14),11-diene (6.2–14.5%), α-selinene (5.90–13.5%), (E)-methyl cinnamate (9.2–19.5%) | [138] | |
| B. brevis (ATCC 8246) | ||||||
| B. agri (ATCC 51663) | 0.06 mg/mL | |||||
| B. laterosporus (ATCC 64) | 0.25 mg/mL | |||||
|
| ||||||
| Melaleuca alternifolia (tea tree) | MIC | Corynebacterium spp. (10 clinical isolates) | Tween 80 | 0.06–2% v/v | Terpinen-4-ol (35.70%) | [152] |
| MIC90 | S. pyogenes (15 clinical isolates) | 0.12% | [235] | |||
| MIC | C. diphtheriae (clinical isolate) | Tween 80 | 0.20% v/v | α-Terpinene (11.40%), γ-terpinene (22.50%), terpinen-4-ol (35.20%) | [137] | |
| C. minutissimus (clinical isolate) | ||||||
|
| ||||||
| Melaleuca cajuputi (cajuput) | MIC | S. pyogenes (ATCC 12344) | Tween 80 | 5.00 mg/mL | 1,8-Cineol (67.60%) | [128] |
| MAC | C. diphtheriae (clinical isolate) | 0.30% v/v | 1,8-Cineole (55.50%) | [137] | ||
| C. minutissimus (clinical isolate) | ||||||
|
| ||||||
| Melaleuca quinquenervia (niaouli) | MAC | C. diphtheriae (clinical isolate) | Tween 80 | 0.25% v/v | 1,8-Cineole (61.20%) | [137] |
| C. minutissimus (clinical isolate) | ||||||
|
| ||||||
| Mentha piperita (peppermint) | MIC | S. pyogenes (ATCC 12344) | Tween 80 | 5.00 mg/mL | 1,8-Cineol (12.06%), menthone (22.24%), menthol (47.29%) | [128] |
aScientific name (common name), part of plant (if applicable).
bMIC: microdilution method; MAC: macrodilution method; ADM: agar dilution method; CTA: contact time assay.
cAmerican Type Culture Collection, Rockville, USA (ATCC).
dDMSO concentration was not included; n.m.: not mentioned.
eNI: no inhibition.
fNCR: no composition results reported.
4.1.5. Fungal Infections: Yeasts
Yeasts may act as opportunistic pathogens and can result in infection if presented with the opportunity, the most common pathogen being Candida albicans. Candida spp. can cause candidiasis at several different anatomical sites [230]. Candida has started developing resistance to first-line and second-line antifungal treatment agents such as fluconazole [190]. Essential oils demonstrating noteworthy activity against this organism are shown in Table 7. Candida albicans has been quite extensively investigated and most oils used in dermatology have been tested against this pathogen.
Table 7.
Essential oils demonstrating noteworthy antimicrobial efficacy against C. albicans.
| Essential oila | Methodb | Species strainc | Solventd | Resulte | Main componentsf | Reference |
|---|---|---|---|---|---|---|
| Abies balsamea (fir) | MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | β-Pinene (31.00%), bornyl acetate (14.90%), δ-3-carene (14.20%) | [99] |
| Abies holophylla (Manchurian fir) | C. albicans (B02630) | DMSO | >2.20 mg/mL | Bicyclo[2.2.1]heptan-2-ol (28.05%), δ-3-carene (13.85%), α-pinene (11.68%), camphene (10.41%) | [111] | |
| Abies koreana (Korean fir) | Bornyl ester (41.79%), camphene (15.31%), α-pinene (11.19%) | |||||
|
| ||||||
| Achillea millefolium subsp. millefolium (yarrow) | MIC | C. albicans (ATCC 10239) | Tween 80 | 4.50 mg/mL | Eucalyptol (24.60%), camphor (16.70%), α-terpineol (10.20%) | [112] |
| Achillea setacea (bristly yarrow) | 1.12 mg/mL | Sabinene (10.80%), eucalyptol (18.50%) | [113] | |||
|
| ||||||
| Angelica archangelica (angelica) root | MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | α-Phellandrene (18.50%), α-pinene (13.70%), β-phellandrene (12.60%), δ-3-carene (12.10%) | [99] |
| Angelica archangelica (angelica) seed | β-Phellandrene (59.20%) | |||||
|
| ||||||
| Anthemis nobilis (chamomile) | MIC | C. albicans (ATCC 10231) | Acetone | 3.00 mg/mL | 2-Methylbutyl-2-methyl propanoic acid (31.50%), limonene (18.30%), 3-methylpentyl-2-butenoic acid (16.70%), isobutyl isobutyrate (10.00%) | [99] |
|
| ||||||
| Apium graveolens (celery) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 1.00% v/v | NCR | [236] |
| Artemisia dracunculus (tarragon) | MIC | Acetone | 2.00 mg/mL | Estragole (82.60%) | [99] | |
|
| ||||||
| Boswellia carteri (frankincense) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 1.00% v/v | NCR | [236] |
| Boswellia carteri (frankincense) (9 samples) | MIC | Acetone | 5.30–12.00 mg/mL | α-Pinene (4.80–40.40%), myrcene (1.60–52.40%), limonene (1.90–20.40%), α-thujene (0.30–52.40%), p-cymene (2.70–16.90%), β-pinene (0.30–13.10%) | [116] | |
| Boswellia frereana (frankincense) (3 samples) | 6.00–12.00 mg/mL | α-Pinene (2.00–64.70%), α-thujene (0.00–33.10%), p-cymene (5.40–16.90%) | ||||
| Boswellia neglecta (frankincense) | 1.80 mg/mL | NCR | [117] | |||
| 6.60 mg/mL | α-Pinene (43.40%), β-pinene (13.10%) | [116] | ||||
| Boswellia papyrifera (frankincense) | 1.40 mg/mL | NCR | [117] | |||
| Boswellia rivae (frankincense) | 4.00 mg/mL | |||||
| Boswellia sacra (frankincense) (2 samples) | 8.00 mg/mL | α-Pinene (18.30–28.00%), α-thujene (3.90–11.20%), limonene (11.20–13.10%) | [116] | |||
| Boswellia spp. (frankincense) (4 samples) | 6.00–8.00 mg/mL | α-Pinene (18.80–24.20%), limonene (11.70–19.00%) | ||||
| Boswellia thurifera (frankincense) | 6.00 mg/mL | α-Pinene (28.00%), limonene (14.60%) | ||||
|
| ||||||
| Cananga odorata (ylang-ylang) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 1.00% v/v | NCR | [236] |
| MIC | Acetone | 2.00 mg/mL | Bicyclosesquiphellandrene (19.50%), β-farnesene (13.90%) | [99] | ||
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.25–0.50% | NCR | [237] | |
| Cananga odorata (ylang-ylang) heads | MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | Benzyl acetate (31.90%), linalool (27.00%), methyl benzoate (10.40%) | [99] |
|
| ||||||
| Canarium luzonicum (elemi) | MIC | C. albicans (ATCC 10231) | Acetone | 3.00 mg/mL | Limonene (41.90%), elemol (21.60%), α-phellandrene (11.40%) | [99] |
|
| ||||||
| Carum carvi (caraway) | MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | Limonene (27.60%), carvone (67.50%) | [99] |
|
| ||||||
| Carum carvi (caraway) | MIC | C. albicans | DMSO | ≤1.00 μg/mL | DL-Limonene (53.35%), β-selinene (11.08%), β-elemene (10.09%) | [118] |
|
| ||||||
| Cedrus atlantica (cedar wood) | ADM90 | C. albicans | Tween 80 | >2.00% v/v | NCR | [236] |
|
| ||||||
| Cinnamomum camphora (camphor) | ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.50% | NCR | [237] |
|
| ||||||
| Cinnamomum cassia (cinnamon) | MAC80 | C. albicans (ATCC90029) | n.m. | 0.17 μL/mL | trans-Cinnamaldehyde (92.20%) | [238] |
|
| ||||||
| Cinnamomum cassia (cinnamon) | MIC | C. albicans | DMSO | ≤1.00 μg/mL | trans-Caryophyllene (17.18%), eugenol (14.67%), linalool L (14.52%), trans-cinnamyl acetate (13.85%), cymol (11.79%), cinnamaldehyde (11.25%) | [118] |
|
| ||||||
| Cinnamomum zeylanicum (cinnamon) | ADM | C. albicans | DMSO | 0.08 μL/mL | NCR | [232] |
| MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | Eugenol (80.00%) | [99] | |
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.01% | NCR | [237] | |
|
| ||||||
| Citrus aurantium (petitgrain) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 0.25% v/v | NCR | [236] |
| MIC | Acetone | 2.00 mg/mL | Linalyl acetate (54.90%), linalool (21.10%) | [99] | ||
|
| ||||||
| Citrus bergamia (bergamot) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 1.00% v/v | NCR | [236] |
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 1.00–2.00% | [237] | ||
|
| ||||||
| Citrus grandis (grapefruit) | MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | Limonene (74.80%) | [99] |
|
| ||||||
| Citrus medica limonum (lemon) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 2.00% v/v | NCR | [236] |
| ADM | C. albicans | DMSO | 2.50 μL/mL | [232] | ||
| MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | [99] | ||
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.50–1.00% | [237] | ||
|
| ||||||
| Citrus limon (lemon), aromatic art | MAC | C. albicans (clinical strain C31) | Tween 80 | 0.03% | Limonene (22.42%), isopropyl myristate (42.78%) | [239] |
| Citrus limon (lemon), Avicenna | 0.60% | Limonene (42.03%), β-pinene (15.15%) | ||||
| Citrus limon (lemon) -Vera Nord | Limonene (23.39%), trans-citral (15.52%), cis-citral (19.41%) | |||||
|
| ||||||
| Citrus sinensis (orange) | MAC | C. albicans (ATCC 10231) | 0.1% ethanol | 3.75 mg/L | NCR | [121] |
| MIC | Acetone | 2.00 mg/mL | Limonene (93.20%) | [99] | ||
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 1.00% | NCR | [237] | |
|
| ||||||
| Commiphora guidotti (myrrh) | MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | (E)-β-Ocimene (52.60%), α-santalene (11.10%), (E)-bisabolene (16.00%) | [117] |
| Commiphora myrrha (myrrh) | 1.50 mg/mL | Furanogermacrene (15.90%), furanoeudesma-1,3-diene (44.30%) | ||||
| 4.00 mg/mL | Furanoeudesma-1,3-diene (57.70%), lindestrene (16.30%) | [99] | ||||
|
| ||||||
| Coriandrum sativum (coriander) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 0.25% v/v | NCR | [236] |
| MIC | C. albicans (CBS 562 and 4 clinical isolates) | n.m. | 0.02–0.06 mg/mL | Decanal (10.97%), 1-decanol (15.30%), 2-dodecenol (11.26%) | [240] | |
|
| ||||||
| Cupressus sempervirens (cypress) | MIC | C. albicans (ATCC 10231) | Acetone | 4.00 mg/mL | α-Pinene (41.20%), δ-3-carene (23.70%) | [99] |
|
| ||||||
| Cymbopogon citratus (lemongrass) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 0.12% v/v | NCR | [236] |
| ADM | C. albicans (SP-14) | Sodium taurocholate | 5.00 μL/mL | Citral (72.80%) | [125, 126] | |
| MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | Geranial (44.80%) | [99] | |
| MIC | C. albicans | DMSO | ≤1.00 μg/mL | Geranial (47.34%), β-myrcene (16.53%), Z-citral (8.36%) | [118] | |
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.06% | NCR | [237] | |
|
| ||||||
| Cymbopogon martinii (palmarosa) | MIC | C. albicans (CBS 562 and 4 clinical isolates) | n.m. | 0.06–0.25 mg/mL | NCR | [240] |
| ADM | C. albicans (SP-14) | Sodium taurocholate | 2.00 μL/mL | Geraniol (61.60%) | [125, 126] | |
| C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.12–0.15% | NCR | [237] | ||
| C. albicans (clinical samples) | 0.08% | [75] | ||||
|
| ||||||
| Cymbopogon nardus (citronella) | ADM90 | C. albicans (ATCC10231) | Tween 80 | 0.25% v/v | NCR | [236] |
| MIC | Acetone | 0.75 mg/mL | Citronellal (38.30%), geraniol (20.70%), citronellol (18.80%) | [99] | ||
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.50–1.00% | NCR | [237] | |
|
| ||||||
| Cymbopogon winterianus (citronella grass) | MIC | C. albicans (CBS 562 and 4 clinical isolates) | n.m. | 0.50–1.00 mg/mL | NCR | [240] |
|
| ||||||
| Daucus carota (carrot seed) | MIC | C. albicans (ATCC10231) | Acetone | 3.00 mg/mL | Carotol (44.40%) | [99] |
| MAC | 2% DSMO | 1.25–2.50 μL/mL | Sabinene (28.30–33.80%), limonene (6.50–11.80%), elemicin (6.20–26.00%) | [241] | ||
| ≥20.00 μL/mL (v/v) | β-Bisabolene (17.6%), carotol (25.1), 11αH-himachal-4-en-1β-ol (21.6%) | [242] | ||||
| 5.00–10.00 μL/mL (v/v) | β-Bisabolene (51.00%), (E)-methyl isoeugenol (10.00%) | |||||
| α-Pinene (37.9%), geranyl acetate (15%) | ||||||
| >20.00 μL/mL (v/v) | Geranyl acetate (65.00%) | |||||
|
| ||||||
| Eucalyptus camaldulensis (eucalyptus) | ADM | C. albicans | DMSO | 5.00 μL/mL | NCR | [232] |
|
| ||||||
| Eucalyptus fruticetorum (eucalyptus) | ADM90 | C. albicans (ATCC10231) | Tween 80 | 1.00% v/v | NCR | [236] |
|
| ||||||
| Eucalyptus globulus (eucalyptus) | ADM | C. albicans (clinical samples) | Tween 20 | 0.05% | NCR | [75] |
| MIC90 | C. albicans (ATCC 90028) | Tween 80 | 10.00 mg/mL | 1,8-Cineol (81.93%) | [128] | |
| ADM | C. albicans (SP-14) | Sodium taurocholate | 5.00 μL/mL | Cineole (23.20%) | [125, 126] | |
| MIC | C. albicans (ATCC 10231) | Acetone | 1.50 mg/mL | 1,8-Cineole (58.00%), α-terpineol (13.20%) | [99] | |
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 1.50% | NCR | [237] | |
| MIC | C. albicans (ATCC 10231) | Acetone | 1.00 mg/mL | NCR | [130] | |
|
| ||||||
| Eucalyptus radiata (eucalyptus) | MIC | C. albicans (ATCC 10231) | Acetone | 1.00 mg/mL | 1,8-Cineole (65.7% ± 9.5), α-terpineol (12.8% ± 4.4) | [130] |
| Eucalyptus camaldulensis (eucalyptus) | 0.50 mg/mL | NCR | ||||
| Eucalyptus citriodora (eucalyptus) | 1.00 mg/mL | |||||
| Eucalyptus smithii (eucalyptus) | ||||||
| Eucalyptus dives (eucalyptus) | ||||||
|
| ||||||
| Eucalyptus intertexta (eucalyptus) | MAC | C. albicans (ATCC 10231) | 10% DSMO | 7.80 μg/mL | NCR | [131] |
| Eucalyptus largiflorens (eucalyptus) | 125.00 μg/mL | NCR | ||||
|
| ||||||
| Eugenia caryophyllus (clove) | ADM | C. albicans (clinical samples) | Tween 20 | 0.33% | NCR | [75] |
| Foeniculum vulgare (fennel) | MIC | C. albicans | DMSO | ≤1.00 μg/mL | trans-Anethole (33.3%), DL-limonene (19.66%), carvone (12.03%) | [118] |
| Foeniculum dulce (fennel) | MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | E-Anethole (79.10%) | [99] |
|
| ||||||
| Hyssopus officinalis (hyssop) | MIC | C. albicans (ATCC 10231) | Acetone | 1.00 mg/mL | Isopinocamphone (48.70%), pinocamphone (15.50%) | [99] |
|
| ||||||
| Geranium dissectum (geranium) | MIC | C. albicans | DMSO | ≤1.00 μg/mL | β-Citronellol (25.45%), geraniol (13.83%) | [118] |
|
| ||||||
| Jasminum nudiflorum (jasmine) | ADM | C. albicans (clinical samples) | Tween 20 | >3.00% | NCR | [75] |
| Juniperi aetheroleum (juniper) | MAC80 | C. albicans (MFBF) | n.m. | 1.00% v/v | α-Pinene (29.17%), β-pinene (17.84%), sabinene (13.55%) | [135] |
| Juniperus chinensis (Chinese juniper) | ADM | C. albicans (clinical samples) | Tween 20 | 2.00% | NCR | [75] |
| Juniperus communis (juniper berry) | MIC90 | C. albicans (ATCC 90028) | Tween 80 | 20.00 mg/mL | α-Pinene (39.76%) | [128] |
| Juniperus communis (juniper) | ADM90 | C. albicans (ATCC10231) | 2.00% v/v | NCR | [236] | |
| Juniperus communis ssp. alpina (juniper), berries | MAC | C. albicans (clinical strain D5) | 2% DMSO | 1.25–2.50 μL/mL | α-Pinene (77.40%) | [243] |
| Juniperus communis subsp. alpina (juniper) | C. albicans (ATCC 10231) | 5.00–10.00 μL/mL | ||||
| 2.50 μL/mL | Sabinene (26.20%), α-pinene (12.90%), limonene (10.40%) | [244] | ||||
| Juniperus virginiana (juniper) | MIC | C. albicans (ATCC 10231) | Acetone | 1.50 mg/mL | Thujopsene (29.80%), cedrol (14.90%), α-cedrene (12.40%) | [99] |
| Juniperus virginiana (juniper), berries | 2.00 mg/mL | α-Pinene (20.50%), myrcene (13.70%), bicyclosesquiphellandrene (10.70%) | ||||
| Juniperus virginiana (juniper) | ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 3.00% | NCR | [237] |
| Juniperus turbinata (juniper), berries | MAC | C. albicans (ATCC 10231) | 2% DMSO | 10.00–20.00 μL/mL | α-Pinene (66.70%) | [243] |
| C. albicans (clinical strain D5) | 5.00 μL/mL | |||||
| Juniperus turbinata (juniper), leaf | C. albicans (ATCC 10231) | 2% DMSO | 5.00 μL/mL | α-Pinene (48.20%), β-phellandrene (23.10%) | ||
| C. albicans (clinical strain D5) | 1.25 μL/mL | |||||
| Juniperus oxycedrus (cade juniper) | ADM | C. albicans (clinical samples) | Tween 20 | NI | NCR | [75] |
|
| ||||||
| Juniperus oxycedrus ssp. oxycedrus (cade), leaf | MAC | C. albicans (ATCC 10231) | 2% DMSO | 1.25–25.00 μL/mL | α-Pinene (65.50%) | [243] |
| C. albicans (clinical strain D5) | 0.32–0.64 μL/mL | |||||
| Juniperus oxycedrus ssp. oxycedrus (cade), berries | C. albicans (ATCC 10231) | 2% DMSO | 10.00–20.00 μL/mL | α-Pinene (54.70)%, myrcene (17.80%), germacrene D (10.40%) | ||
| C. albicans (clinical strain D5) | 5.00 μL/mL | |||||
|
| ||||||
| Kunzea ericoides (Kānuka) | MAC | C. albicans (ATCC 10231) | Tween 80 | >2.00% v/v | α-Pinene (61.60%) | [137] |
| MIC | C. albicans (ATCC 10231) | Acetone | 4.00 mg/mL | α-Pinene (26.20–46.70%), p-cymene (5.80–19.10%) | [138] | |
| Laurus nobilis (bay) | 0.75 mg/mL | Eugenol (57.20%), myrcene (14.30%), chavicol (12.70%) | [99] | |||
|
| ||||||
| Lavandula angustifolia (lavender) | ADM | C. albicans (clinical samples) | Tween 20 | >3.00% | NCR | [75] |
| MIC | C. albicans (ATCC 10231) | Acetone | 5.70 mg/mL | Linalool (30.80%), linalyl acetate (31.30%) | [140] | |
| 3.00 mg/mL | Linalyl acetate (36.70%), linalool (31.40%), terpinen-4-ol (14.90%) | [99] | ||||
| ADM90 | C. albicans (ATCC 10231) | Tween 80 | 0.50% v/v | NCR | [236] | |
| 0.25% v/v | ||||||
|
| ||||||
| Lavandula officinalis (lavender) | MIC | C. albicans (ATCC 10231) | DMSO | ≤1.00 μg/mL | δ-3-Carene (17.14%), α-fenchene (16.79%), diethyl phthalate (13.84%) | [118] |
|
| ||||||
| Lavandula pedunculata (French lavender) | MAC | C. albicans (ATCC 10231) | 2% DMSO | 2.50 μL/mL | 1,8-Cineole (2.40–55.50%), fenchone (1.30–59.70%), camphor (3.60–48.00%) | [245] |
| Lavandula stoechas (French lavender) | Fenchone (37.00%) and camphor (27.30%) | [246] | ||||
| Lavandula viridis (yellow lavender) | 1,8-Cineole (34.50%–42.2%), camphor (13.40%) | [247] | ||||
|
| ||||||
| Leptospermum scoparium (manuka) | MAC | C. albicans (ATCC 10231) | Tween 80 | >2.00% v/v | (−)-(E)-Calamenene (14.50%), leptospermone (17.60%) | [137] |
| MIC | Acetone | 8.00 mg/mL | Eudesma-4(14),11-diene (6.2–14.5%), α-selinene (5.90–13.5%), (E)-methyl cinnamate (9.2–19.5%) | [138] | ||
|
| ||||||
| Litsea cubeba (May Chang) | MIC | C. albicans (ATCC 10231) | Acetone | 6.00 mg/mL | Geranial (45.60%), nerol (31.20%) | [99] |
|
| ||||||
| Matricaria chamomilla (German chamomile) | MIC | C. albicans (ATCC 10231) | Acetone | 0.50 mg/mL | Bisabolene oxide A (46.90%), β-farnesene (19.20%) | [99] |
| ADM | C. albicans (clinical samples) | Tween 20 | NI | NCR | [75] | |
|
| ||||||
| Melaleuca alternifolia (tea tree) | ADM | C. albicans (clinical samples) | Tween 20 | 0.73% | NCR | [75] |
| MIC | C. albicans (KEM H5) | n.m. | 0.13% (v/v) | Terpinen-4-ol (39.80%), γ-terpinene (17.80%) | [149, 150] | |
| ADM90 | C. albicans (ATCC10231) | Tween 80 | 0.25% v/v | NCR | [236] | |
| ADM | 0.20% v/v | Terpinen-4-ol (42.80%), γ-terpinene (18.20%) | [115] | |||
| MIC | C. albicans (NCYC 854) | n.m. | 0.25% v/v | NCR | [145] | |
| C. albicans (fluconazole- and itraconazole-susceptible isolates) | Tween 80 | 0.06–0.50% v/v | Terpinen-4-ol (42.35%), γ-terpinene (20.65%) | [248] | ||
| C. albicans (fluconazole- and/or itraconazole-resistant isolates; six isolates were cross-resistant) | 0.25–0.50% v/v | |||||
| ADM | C. albicans (NRRL y-12983) | n.m. | 3.50 mg/mL | NCR | [249] | |
| C. albicans (ATCC 14053) | ||||||
| C. albicans (NRRL y-869) | ||||||
| C. albicans (NRRL y-22077) | 1.75 mg/mL | |||||
| C. albicans (ATCC 10231) | 3.50 mg/mL | |||||
| MIC | C. albicans (NRRL 12983) | 4.73 mg/mL | γ-Terpinene (16.30%), terpinen-4-ol (30.30%) | [61] | ||
| C. albicans (ATCC 14053) | ||||||
| C. albicans (ATCC 90028) | ||||||
| C. albicans (NRRL 22077) | 2.30 mg/mL | |||||
| C. albicans (ATCC 10231) | 4.73 mg/mL | |||||
| C. albicans (ATCC 10231) | Acetone | 3.70 mg/mL | Terpinen-4-ol (38.60%), γ-terpinene (21.60%) | [140] | ||
| MAC | C. albicans (ATCC 10231) | Tween 80 | 0.30% v/v | α-Terpinene (11.40%), γ-terpinene (22.50%), terpinen-4-ol (35.20%) | [137] | |
| MIC | C. albicans (ATCC 10231) | Acetone | 1.50 mg/mL | Terpinen-4-ol (49.30%), γ-terpinene (16.90%) | [99] | |
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.12–0.25% | NCR | [237] | |
|
| ||||||
| Melaleuca cajuputi (cajuput) | MIC90 | C. albicans (ATCC 90028) | Tween 80 | 2.50 mg/mL | 1,8-Cineol (67.60%) | [128] |
| MAC | C. albicans (ATCC10231) | 0.40% v/v | 1,8-Cineole (55.50%) | [137] | ||
|
| ||||||
| Melaleuca quinquenervia (niaouli) | MAC | C. albicans (ATCC10231) | Tween 80 | 0.40% v/v | 1,8-Cineole (61.20%) | [137] |
|
| ||||||
| Melaleuca viridiflora (niaouli) | MIC | C. albicans (ATCC10231) | Acetone | 1.75 mg/mL | 1,8-Cineole (45.90%), α-terpinene (21.00%) | [99] |
|
| ||||||
| Mentha piperita (peppermint) | ADM90 | C. albicans (ATCC10231) | Tween 80 | 0.50% v/v | NCR | [236] |
| MIC90 | C. albicans (ATCC 90028) | 0.30 mg/mL | 1,8-Cineol (12.06%), menthone (22.24%), menthol (47.29%) | [128] | ||
| ADM | C. albicans (SP-14) | Sodium taurocholate | 5.00 μL/mL | Menthol (36.40%) | [125, 126] | |
| MIC | C. albicans (ATCC 10231) | Acetone | 2.40 mg/mL | Menthone (18.20%), menthol (42.90%) | [140] | |
| ADM | C. albicans (clinical isolate) | Tween 20 | 0.08% | NCR | [75] | |
| MIC | C. albicans (clinical isolate) | DMSO | 0.31–0.63 mg/mL | Menthol (27.50–42.30%), menthone (18.40–27.90%) | [155] | |
| C. albicans | ≤1.00 μg/mL | Menthone (40.82%), carvone (24.16%) | [118] | |||
| C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | Menthol (47.50%), menthone (18.60%) | [99] | ||
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.25–0.30% | NCR | [237] | |
| Mentha pulegium (peppermint) | MIC | C. albicans (ATCC 10231) | 10% DMSO | 1.00 μL/mL | Piperitone (38.00%), piperitenone (33.00%) | [250] |
| Mentha rotundifolia (peppermint), Beja | C. albicans | Tween 80 | 0.80% v/v | β-Caryophyllene (26.67%), germacrene D (12.31%) | [50] | |
| Mentha rotundifolia (peppermint), Bizerte | Pulegone (32.09%), piperitenone oxide (17.28%), 5-acetyl thiazole (11.26%) | |||||
|
| ||||||
| Mentha spicata (spearmint) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 0.12% v/v | NCR | [236] |
|
| ||||||
| Myrtus communis (myrtle) | MIC | C. albicans (ATCC 10231) | Acetone | 1.50 mg/mL | Myrtenyl acetate (28.20%), 1,8-cineole (25.60%), α-pinene (12.50%) | [99] |
| Myrtus nivellei (Sahara myrtle) | MAC | C. albicans (ATCC 10231) | 2% DMSO | 1.25–2.50 μg/mL | 1,8-Cineole (37.50%), limonene (25.00%) | [251] |
|
| ||||||
| Ocimum basilicum var. minimum (basil) | MIC | C. albicans (ATCC 11006) | DMSO | NI | Linalool (440%), 1,8-cineole (15.50%) | [252] |
| Ocimum americanum (basil) | 5000.00 μg/mL | 1,8-Cineole (25.90%), (Z)-methyl cinnamate (29.40%) | ||||
| Ocimum basilicum var. purpurascens (basil) | Linalool (41.50%), α-muurolol (11.80%) | |||||
| Ocimum micranthum (basil) | 625.00 μg/mL | Eugenol (64.11%), β-caryophyllene (14.30%) | ||||
| Ocimum selloi | 1250.00 μg/mL | Linalool (16.8%), anethole (52.2%) | ||||
|
| ||||||
| Ocimum basilicum (basil) | MIC | C. albicans | n.m. | 30.00 μg/μL | Estragole (45.80%), linalool (24.20%) | [253] |
| ADM90 | C. albicans (ATCC10231) | Tween 80 | 0.50% v/v | NCR | [236] | |
| ADM | C. albicans (clinical samples) | Tween 20 | 1.50% | [75] | ||
| MIC | C. albicans (ATCC 10231) | Acetone | 1.00 mg/mL | Linalool (54.10%) | [99] | |
| MIC90 | n.m. | 95.00 μg/mL | Methyl chavicol (46.90%), geranial (19.10%), neral (15.15%) | [159] | ||
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 1.00% | NCR | [237] | |
|
| ||||||
| Ocimum gratissimum (African basil) | MIC | C. albicans (clinical isolate) | n.m. | 750.00 μg/mL | Eugenol (67.00%) | [254] |
|
| ||||||
| Ocimum sanctum (holy basil) | MIC90 | C. albicans (37 clinical isolates (5 resistant to fluconazole)) | 10% DMSO | 0.10–0.24 μL/mL | Methyl chavicol (44.63%), linalool (21.84%) | [255] |
| C. albicans (ATCC 90028) | 0.25 μL/mL | |||||
| C. albicans (ATCC 10261) | 0.20 μL/mL | |||||
| C. albicans (ATCC 44829) | ||||||
| ADM | C. albicans (clinical samples) | Tween 20 | 0.48% | NCR | [75] | |
|
| ||||||
| Origanum majorana (marjoram) | MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | 1,8-Cineole (46.00%), linalool (26.10%) | [99] |
|
| ||||||
| Origanum acutidens (Turkey oregano) | MIC | C. albicans (A117) | 10% DMSO | 125.00 μg/mL | Carvacrol (72.00%) | [161] |
|
| ||||||
| Origanum microphyllum (oregano) | MIC | C. albicans | Tween 80 | 3.23 mg/mL | Terpinen-4-ol (24.86%), γ-terpinene (13.83%), linalool (10.81%) | [162] |
|
| ||||||
| Origanum vulgare (oregano) | MIC | C. albicans | n.m. | 2.00 μg/μL | Carvacrol (61.30%), thymol (13.90%) | [253] |
| ADM90 | C. albicans (ATCC10231) | Tween 80 | 0.12% v/v | NCR | [236] | |
| ADM | C. albicans (NRRL y-12983) | n.m. | 0.70 mg/mL | NCR | [249] | |
| C. albicans (ATCC 14053) | ||||||
| C. albicans (NRRL y-869) | ||||||
| C. albicans (NRRL y-22077) | ||||||
| C. albicans (ATCC 10231) | ||||||
| MIC | C. albicans (NRRL 12983) | Cymene (25.00%), cymenol (58.6.00%) | [61] | |||
| C. albicans (ATCC 14053) | ||||||
| C. albicans (ATCC 90028) | ||||||
| C. albicans (NRRL 22077) | ||||||
| C. albicans (ATCC 10231) | ||||||
|
| ||||||
| Origanum vulgare subsp. hirtum (Greek oregano) | MAC | C. albicans (ATCC 10239) | 10% DMSO + Tween 80 | 85.30 μg/mL | Linalool (96.31%) | [165] |
| Origanum vulgare subsp. vulgare (oregano) | 128.00 μg/mL | Thymol (58.31%), carvacrol (16.11%), p-cymene (13.45%) | ||||
|
| ||||||
| Pelargonium graveolens (geranium) | ADM | C. albicans (NRRL y-12983) | n.m. | 0.70 mg/mL | NCR | [249] |
| C. albicans (ATCC 14053) | ||||||
| C. albicans (NRRL y-869) | ||||||
| C. albicans (NRRL y-22077) | ||||||
| C. albicans (ATCC 10231) | ||||||
| MIC | C. albicans (NRRL 12983) | n.m. | 0.12 mg/mL | Citronellol (47.30%) | [61] | |
| C. albicans (ATCC 14053) | ||||||
| C. albicans (ATCC 90028) | ||||||
| C. albicans (NRRL 22077) | ||||||
| C. albicans (ATCC 10231) | n.m. | 0.06 mg/mL | ||||
| C. albicans (ATCC 10231) | Acetone | 0.75 mg/mL | Citronellol (34.20%), geraniol (15.70%) | [99] | ||
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.12% | NCR | [237] | |
|
| ||||||
| Perovskia abrotanoides (Russian sage) | MIC | C. albicans (ATCC 10231) | 10% DMSO | 8.00 μL/mL | Camphor (23.00%), 1,8-cineole (22.00%), α-pinene 12.00% | [167] |
|
| ||||||
| Pimenta racemosa (West Indian bay) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 0.12% v/v | NCR | [236] |
|
| ||||||
| Pimpinella anisum (anise) | MAC83 | C. albicans | n.m. | 1.00% v/v | Anethole, anisaldehyde, linalool | [256] |
| MIC | DMSO | ≤1.00 μg/mL | Anethole (64.82%) | [118] | ||
|
| ||||||
| Pinus sylvestris (pine) | MIC | C. albicans (ATCC 10231) | Acetone | 1.50 mg/mL | Bornyl acetate (42.30%), camphene (11.80%), α-pinene (11.00%) | [99] |
|
| ||||||
| Piper nigrum (black pepper) | MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | β-Caryophyllene (33.80%), limonene (16.40%) | [99] |
|
| ||||||
| Pogostemon patchouli (patchouli) | MIC | C. albicans (ATCC 10231) | Acetone | 1.50 mg/mL | Patchouli alcohol (37.30%), α-bulnesene (14.60%), α-guaiene (12.50%) | [99] |
|
| ||||||
| Ricinus officinalis (rose) | ADM | C. albicans (clinical samples) | Tween 20 | >3.00% | NCR | [75] |
|
| ||||||
| Rosa gallica (rose) | ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 1.00–2.00% | NCR | [237] |
|
| ||||||
| Rosmarinus officinalis (rosemary) | MIC | C. albicans (ATCC 10231) | Tween 80 | 0.25% v/v | 1,8-Cineole (27.23%), α-pinene (19.43%), camphor (14.26%), camphene (11.52%) | [169] |
| 0.10% v/v | 1,8-Cineole (26.54%), α-pinene (20.14%), camphene (11.38%), camphor (12.88%) | [170] | ||||
| C. albicans (MTCC 1637) | n.m. | 5.50 mg/mL | NCR | [171] | ||
| C. albicans (10 antifungal-resistant isolates) | 2.75–5.50 mg/mL | |||||
| C. albicans (ATCC 10231) | Acetone | 5.70 mg/mL | 1,8-Cineole (41.40%), α-pinene (13.30%), camphor (12.40%) | [140] | ||
| 2.00 mg/mL | 1,8-Cineole (48.00%) | [99] | ||||
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 1.00% | NCR | [237] | |
|
| ||||||
| Salvia eremophila (sage) | MIC | C. albicans (ATCC 10231) | 10% DMSO | 13.00 μg/mL | Borneol (21.83%), α-pinene (18.80%), bornyl acetate (18.68%) | [174] |
| Salvia officinalis (sage) | ADM90 | Tween 80 | 0.50% v/v | NCR | [236] | |
|
| ||||||
| Salvia ringens (sage) | MIC | C. albicans | n.m. | 0.75 mg/mL | α-Pinene (12.85%), 1,8-cineole (46.42%) | [177] |
|
| ||||||
| Salvia rosifolia (sage) (3 samples) | MIC | C. albicans | 20% DMSO | 500.00 μg/mL | α-Pinene (15.70–34.80%), 1,8-cineole (16.60–25.10%), β-pinene (6.70–13.50%) | [178] |
|
| ||||||
| Salvia sclarea (clary sage) | MIC | C. albicans (13 clinical isolates and 1 reference strain ATCC 10231) | 1% DMSO | 128.00–256.00 μg/mL | Linalyl acetate (56.88%), linalool (20.75%) | [257] |
| C. albicans (ATCC 10231) | Acetone | 0.88 mg/mL | Linalyl acetate (72.90%), linalool (11.90%) | [99] | ||
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 2.00% | NCR | [237] | |
|
| ||||||
| Santalum album (sandalwood) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 0.06% v/v | NCR | [236] |
| MIC | Acetone | 2.00 mg/mL | α-Santalol (32.10%) | [99] | ||
|
| ||||||
| Santolina chamaecyparissus (santolina) | MIC | C. albicans (CBS 562 and 4 clinical isolates) | n.m. | 0.25–>1.00 mg/mL | NCR | [240] |
|
| ||||||
| Styrax benzoin (benzoin) | MIC | C. albicans (ATCC 10231) | Acetone | 2.00 mg/mL | Cinnamyl alcohol (44.80%), benzene propanol (21.70%) | [99] |
|
| ||||||
| Syzygium aromaticum (clove) | ADM90 | C. albicans (ATCC 10231) | Tween 80 | 0.12% v/v | NCR | [236] |
| MIC | C. albicans (ATCC 10231) | Tween 80 | 0.13% v/v | Eugenol (68.52%), β-caryophyllene (19.00%), 2-methoxy-4-[2-propenyl]phenol acetate (10.15%) | [169] | |
| DMSO | ≤1.00 μg/mL | Eugenol (84.07%), isoeugenol (10.39%) | [118] | |||
| MAC | C. albicans (ATCC 10231) | DMSO | 0.64 μg/mL | Eugenol (85.30%) | [258] | |
| C. albicans (clinical isolate D5) | ||||||
| C. albicans (clinical isolate D1) | ||||||
| MIC | C. albicans (ATCC 10231) | Acetone | 0.50 mg/mL | Eugenol (82.20%), eugenol acetate (13.20%) | [99] | |
| ADM | C. albicans (ATCC 10231 and 3 clinical isolates) | Tween 20 | 0.12% | NCR | [237] | |
|
| ||||||
| Tagetes minuta (Mexican marigold) | MIC90 | C. albicans (ATCC 10231) | n.m. | 115.00 μg/mL | Dihydrotagetone (33.90%), E-ocimene (19.90%), tagetone (16.10%) | [159] |
| Tagetes patula (French marigold) | MIC | Acetone | 2.00 mg/mL | (E)-β-Ocimene (41.30%), E-tagetone (11.20%), verbenone (10.90%) | [99] | |
|
| ||||||
| Thymus broussonetii (thyme) | MAC | C. albicans (CCMM L4) | n.m. | 0.25 mg/mL | Thymol (39.64%), carvacrol (21.31%), borneol (20.13%) | [259] |
|
| ||||||
| Thymus capitatus (thyme) | MIC | C. albicans (ATCC 10231) | Tween 80 | 450.00 μg/mL | p-Cymene (26.40%), thymol (29.30%), carvacrol (10.80%) | [181] |
| Thymus capitatus (thyme), commercial | α-Pinene (25.20%), linalool (10.30%), thymol (46.10%) | |||||
|
| ||||||
| Thymus herba-barona (thyme) | MAC | C. albicans (ATCC 10231) | 2% DMSO | 0.32 μL/mL | Carvacrol (54.00%), thymol (30.20%) | [246] |
|
| ||||||
| Thymus herba-barona (thyme), Limbara | MIC | C. albicans (ATCC 10231) | Tween 80 | 450.00 μg/mL | p-Cymene (27.60%), thymol (50.30%) | [181] |
| Thymus herba-barona (thyme), Gennargentu | 225.00 μg/mL | Thymol (46.90%), carvacrol (20.60%) | ||||
| Thymus magnus (thyme) | C. albicans (KCCM 11282) | Ethanol and Tween 80 | 0.39 mg/mL | Thymol (39.80%) | [260] | |
|
| ||||||
| Thymus maroccanus (thyme) | MAC | C. albicans (CCMM L4) | n.m. | 0.25 mg/mL | Carvacrol (89.15%) | [259] |
| Thymus mastichina subsp. mastichina (thyme) | C. albicans (ATCC 10231, H37, M1) | n.m. | 1.25–2.50 μL/mL | 1,8-Cineole (67.40%) | [261] | |
|
| ||||||
| Thymus quinquecostatus (thyme) | MIC | C. albicans (KCCM 11282) | Ethanol and Tween 80 | 0.39 mg/mL | Thymol (41.70%), γ-terpinene (16.00%) | [260] |
|
| ||||||
| Thymus schimperi (thyme) | ADM | C. albicans | DMSO | 0.16 μL/mL | NCR | [232] |
|
| ||||||
| Thymus vulgaris (thyme) | MIC | C. albicans | n.m. | 4.00 μg/μL | Thymol (47.90%) | [253] |
| MAC | C. albicans (ATCC 10231, H37, M1) | 0.16–0.32 μL/mL | Carvacrol (70.30%), p-cymene (11.70%) | [261] | ||
| MIC | C. albicans (ATCC 10231) | Acetone | 2.40 mg/mL | Thymol (47.20%), p-cymene (22.10%) | [140] | |
| 1.00 mg/mL | p-Cymene (39.90%), thymol (20.70%) | [99] | ||||
|
| ||||||
| Thymus x viciosoi (thyme) | MAC | C. albicans (clinical isolates M1, D5), C. albicans (ATCC 10231) | 1% DMSO | 0.04–0.64 μL/ mL | Carvacrol (30.0 0%), thymol (18.00 %), p-cymene (19.0 0%) | [262] |
| Thymus zygis subsp. sylvestris (thyme) | C. albicans (ATCC 10231) | 2% DMSO | 0.32–1.25 μL/mL | p-Cymene (11.00–17.00%), γ-terpinene (3.80–11.50%), linalool (3.50–30.00%), geraniol (0.10–19.80%), thymol (5.20–23.80%), carvacrol (1.30–25.00%), geranyl acetate (0.50–20.80%) | [263] | |
| Thymus zygis subsp. zygis (thyme) | C. albicans (ATCC 10231, H37, M1) | n.m. | NI | Thymol (39.60%), p-cymene (21.20%) | [261] | |
|
| ||||||
| Vetiveria zizanioides/Andropogon muricatus (vetiver) | ADM | C. albicans (clinical samples) | Tween 20 | NI | NCR | [75] |
| MIC | C. albicans (ATCC 10231) | Acetone | 1.75 mg/mL | Zizanol (13.60%), β-vetirenene (7.20%) | [99] | |
|
| ||||||
| Zingiber officinalis (ginger) | ADM | C. albicans (clinical samples) | Tween 20 | 3.00% | NCR | [75] |
| C. albicans (ATCC 10231 and 3 clinical isolates) | [237] | |||||
aScientific name (common name), part of plant (if applicable).
bMIC: microdilution method; MAC: macrodilution method; ADM: agar dilution method, CTA: contact time assay.
cAmerican Type Culture Collection, Rockville, USA (ATCC); Colección Espanõla de Cultivos Tipo (CECT); collection of microorganisms of the Department of Microbiology (MFBF); culture collection of antibiotics-resistant microbes (CCRM); Eskişehir Osmangazi University, Faculty of Medicine, clinical isolate (OGU); Laboratorio de Microbiología, Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Mendoza, Argentina (LM); Microbial Type Culture Collection (MTCC); Mycology Laboratory (LM); National Center of Industrial Microorganisms (NCIM); National Collection of Type Cultures, London, Great Britain (NCTC); Spanish Collection of Type Cultures (STCC).
dDMSO concentration was not included; n.m.: not mentioned.
eNI: no inhibition.
fNCR: no composition results reported.
Cymbopogon citratus, C. martinii, L. nobilis, M. piperita, P. graveolens, Santolina chamaecyparissus (santolina), and Thymus spp. are essential oils recommended in the aromatherapeutic literature for the treatment of fungal infections that have in vitro evidence confirming the effectiveness as antifungals. Cananga odorata (ylang-ylang), Cinnamomum cassia (cinnamon), C. zeylanicum, Coriandrum sativum (coriander), Cymbopogon nardus (citronella), Matricaria chamomilla (German chamomile), and S. benzoin also displayed in vitro noteworthy activity; however, these are interestingly not recommended in the aromatherapeutic literature.
In an in vivo study, L. angustifolia was found to effectively inhibit growth of C. albicans isolated from 20 patients, which was comparative to the inhibition observed by clotrimazole [272].
4.1.6. Fungal Infections: Dermatophytes
Infection with these organisms results in dermatophytosis, which affects the skin, nails, or hair [230, 273, 274]. There is a 10–20% risk of a person acquiring a dermatophyte infection [29], and although the symptoms do not necessarily pose a threat, the treatment is costly and onerous due to resistance and side effects [29]. Essential oils present an excellent option for treating superficial human fungal infections, especially when one is confronted with the effective antifungal results found in previous studies (Table 8). This is encouraging considering the difficulty and challenges faced in treating these infections.
Table 8.
Essential oils against dermatophytes and other yeasts.
| Essential oila | Methodb | Species strainc | Solventd | Resulte | Main componentsf | Reference |
|---|---|---|---|---|---|---|
| Apium nodiflorum (celery), aerial parts, Italy | MAC | E. floccosum (clinical strain FF9) | 2% DMSO | 0.16 μL/mL | Dillapiole (70.80%), limonene (14.40%) | [230] |
| T. mentagrophytes (clinical strain FF7) | ||||||
| M. canis (clinical strain FF1) | 2% DMSO | 0.04 μL/mL | ||||
| T. rubrum (CECT 2794) | 0.16 μL/mL | |||||
| M. gypseum (CECT 2908) | 0.08 μL/mL | |||||
| T. mentagrophytes var. interdigitale (CECT 2958) | 0.16 μL/mL | |||||
| T. verrucosum (CECT 2992) | 0.32 μL/mL | |||||
|
| ||||||
| Apium nodiflorum (celery), aerial parts, Portugal | MAC | E. floccosum (clinical strain FF9) | 2% DMSO | 0.08 μL/mL | Myristicin (29.10%), dillapiole (22.50%), limonene (16.70%) | [230] |
| T. mentagrophytes (clinical strain FF7) | 0.16 μL/mL | |||||
| M. canis (clinical strain FF1) | 0.04 μL/mL | |||||
| T. rubrum (CECT 2794) | 2% DMSO | 0.08 μL/mL | ||||
| M. gypseum (CECT 2908) | ||||||
| T. mentagrophytes var. interdigitale (CECT 2958) | 2% DMSO | 0.16 μL/mL | ||||
| T. verrucosum (CECT 2992) | 0.32 μL/mL | |||||
|
| ||||||
| Cedrus atlantica (cedar wood) | MIC | T. erinacei (KCCM 60411) | Tween 80 | 2.0 mg/mL | NCR | [264] |
| T. mentagrophytes (KCCM 11950) | 1.00 mg/mL | |||||
| T. rubrum (ATCC 6345) | ||||||
| T. schoenleinii (KCCM 60477) | ||||||
| T. soudanense (KCCM 60448) | 0.25 mg/mL | |||||
| T. tonsurans (KCCM 11866) | 0.50 mg/mL | |||||
|
| ||||||
| Cinnamomum zeylanicum (cinnamon) | ADM | Trichophyton spp. (nail isolate) | DMSO | 0.31 μL/mL | NCR | [232] |
| Trichophyton spp. (scalp isolate) | 0.16 μL/mL | |||||
| Microsporum spp. | ||||||
|
| ||||||
| Citrus bergamia (bergamot) | MIC | T. erinacei (KCCM 60411) | Tween 80 | 4.00 mg/mL | NCR | [264] |
| T. mentagrophytes (KCCM 11950) | 2.00 mg/mL | |||||
| T. rubrum (ATCC 6345) | 1.00 mg/mL | |||||
| T. schoenleinii (KCCM 60477) | 2.00 mg/mL | |||||
| T. soudanense (KCCM 60448) | 0.50 mg/mL | |||||
| T. tonsurans (KCCM 11866) | 1.00 mg/mL | |||||
| T. mentagrophytes (20 isolates) | Tween 80 | 0.16–1.30% v/v | NCR | [265] | ||
| T. rubrum (18 isolates) | 0.16–0.63% v/v | |||||
| T. interdigitale (15 isolates) | 0.31–1.30% v/v | |||||
| T. tonsurans (2 isolates) | 2.50% v/v | |||||
| M. canis (24 isolates) | 0.16–0.63% v/v | |||||
| M. gypseum (1 isolate) | 2.50% v/v | |||||
| E. floccosum (12 isolates) | 0.16–0.31% v/v | |||||
|
| ||||||
| Citrus limon (lemon) | MIC | M. canis (11 clinical isolates) | Prunus dulcis (sweet almond oil) | 4.60–7.50% | Limonene (59.20%), β-pinene (13.70%), γ-terpinene (10.80%) | [266] |
| ADM | Trichophyton spp. (nail isolate) | DMSO | 2.50 μL/mL | NCR | [232] | |
| Trichophyton spp. (scalp isolate) | ||||||
| Microsporum spp. | 1.25 μL/mL | |||||
|
| ||||||
| Cymbopogon citratus (lemongrass) | MIC | T. erinacei (KCCM 60411) | Tween 80 | 0.25 mg/mL | NCR | [264] |
| T. mentagrophytes (KCCM 11950) | <0.13 mg/mL | |||||
| T. rubrum (ATCC 6345) | ||||||
| T. schoenleinii (KCCM 60477) | ||||||
| T. soudanense (KCCM 60448) | ||||||
| T. tonsurans (KCCM 11866) | ||||||
| ADM | T. mentagrophytes (SP-12) | Sodium taurocholate | 0.25 μg/mL | Citral (72.80%) | [125, 126] | |
|
| ||||||
| Cymbopogon martinii (palmarosa) | ADM | T. mentagrophytes (SP-12) | Sodium taurocholate | 1.50 μg/mL | Geraniol (61.60%) | [125, 126] |
|
| ||||||
| Cymbopogon winterianus (citronella) | MIC90 | T. rubrum (15 LM strains), T. rubrum strain (ATCC 1683) | Tween 80 | 312.00 μg/mL | NCR | [84] |
|
| ||||||
| Daucus carota (carrot seed) | MAC | E. floccosum (clinical isolate FF9) | 2% DSMO | 0.32–0.64 μL/mL | Sabinene (28.30–33.80%), limonene (6.50–11.80%), elemicin (6.20–26.00%) | [241] |
| T. mentagrophytes (clinical isolate FF7) | 0.16–0.64 μL/mL | |||||
| M. canis (clinical isolate FF1) | 2% DSMO | 0.32–0.64 μL/mL | ||||
| T. rubrum (CECT 2794) | ||||||
| M. gypseum (CECT 2905) | ||||||
| E. floccosum (clinical strain FF9) | DMSO | 1.25 μL/mL (v/v) | α-Pinene (37.90%), geranyl acetate (15.00%) | [242] | ||
| T. mentagrophytes (clinical strain FF7) | ||||||
| M. canis (clinical strain FF1) | ||||||
| T. rubrum CECT 2794 | ||||||
| M. gypseum (CECT 2908) | ||||||
| E. floccosum (clinical strain FF9) | DMSO | 0.64 μL/mL (v/v) | Geranyl acetate (65.00%) | |||
| T. mentagrophytes (clinical strain FF7) | ||||||
| M. canis (clinical strain FF1) | ||||||
| T. rubrum CECT 2794 | ||||||
| M. gypseum (CECT 2908) | ||||||
| E. floccosum (clinical strain FF9) | DMSO | 0.16–0.32 μL/mL (v/v) | β-Bisabolene (17.60%), carotol (25.10%), 11αH-himachal-4-en-1β-ol (21.60%) | |||
| T. mentagrophytes (clinical strain FF7) | DMSO | 0.32 μL/mL (v/v) | ||||
| M. canis (clinical strain FF1) | ||||||
| T. rubrum CECT 2794 | ||||||
| M. gypseum (CECT 2908) | ||||||
| E. floccosum (clinical strain FF9) | DMSO | 0.64 μL/mL (v/v) | β-Bisabolene (51.00%), (E)-methyl isoeugenol (10.00%) | |||
| T. mentagrophytes (clinical strain FF7) | ||||||
| M. canis (clinical strain FF1) | ||||||
| T. rubrum CECT 2794 | ||||||
| M. gypseum (CECT 2908) | ||||||
|
| ||||||
| Eucalyptus camaldulensis (river red gum eucalyptus) | ADM | Trichophyton spp. (nail isolate) | DMSO | 5.00 μL/mL | NCR | [232] |
| Trichophyton spp. (scalp isolate) | 2.50 μL/mL | |||||
| Microsporum spp. | 5.00 μL/mL | |||||
|
| ||||||
| Eucalyptus globulus (eucalyptus) | ADM | T. mentagrophytes (SP-12) | Sodium taurocholate | 0.25 μg/mL | Cineole (23.20%) | [125, 126] |
| MIC | T. erinacei (KCCM 60411) | Tween 80 | 0.25 mg/mL | NCR | [264] | |
| T. mentagrophytes (KCCM 11950) | ||||||
| T. rubrum (ATCC 6345) | <0.13 mg/mL | |||||
| T. schoenleinii (KCCM 60477) | 0.25 mg/mL | |||||
| T. soudanense (KCCM 60448) | ||||||
| T. tonsurans (KCCM 11866) | <0.13 mg/mL | |||||
| MIC | M. canis (ATCC 32903) | Tween 80: DMSO | 500.00 μg/mL | 1,8-Cineole (72.20%) | [267] | |
| M. gypseum (ATCC 14683) | 1000.00 μg/mL | |||||
| T. mentagrophytes (ATCC 9533) | 250.00 μg/mL | |||||
| T. mentagrophytes (ATCC 11480) | <7.80 μg/mL | |||||
| T. mentagrophytes (ATCC 11481) | 125.00 μg/mL | |||||
| T. rubrum (CCT 5507) | 62.50 μg/mL | |||||
|
| ||||||
| Illicium verum (star anise) | MIC | M. canis (11 clinical isolates) | Prunus dulcis (sweet almond oil) | 1.00–5.00% | E-anethole (93.70%) | [266] |
|
| ||||||
| Juniperi aetheroleum (juniper) | MAC80 | M. gypseum (MFBF) | n.m. | 2.00% v/v |
α-Pinene (29.17%), β-pinene (17.84%), sabinene (13.55%) |
[135] |
| T. mentagrophytes (MFBF) | 1.00% v/v | |||||
| T. rubrum (MFBF) | 0.39% v/v | |||||
|
| ||||||
| Juniperus communis (juniper) | MIC | T. erinacei (KCCM 60411) | Tween 80 | 0.50 mg/mL | NCR | [264] |
| T. mentagrophytes (KCCM 11950) | 4.00 mg/mL | |||||
| T. rubrum (ATCC 6345) | 1.00 mg/mL | |||||
| T. schoenleinii (KCCM 60477) | 2.00 mg/mL | |||||
| T. soudanense (KCCM 60448) | 0.50 mg/mL | |||||
| T. tonsurans (KCCM 11866) | 2.00 mg/mL | |||||
|
| ||||||
| Juniperus communis ssp. alpina (juniper), berries | MAC | E. floccosum (clinical strain FF9) | 2% DMSO | 1.25 μL/mL | α-Pinene (77.40%) | [243] |
| T. rubrum (clinical strain FF5) | ||||||
| T. mentagrophytes (clinical strain FF7) | ||||||
| M. canis (clinical strain FF1) | ||||||
| M. gypseum (clinical strain FF3) | ||||||
|
| ||||||
| Juniperus communis subsp. alpina (juniper) | MAC | E. floccosum (clinical strain FF9) | 2% DMSO | 0.64 μL/mL | Sabinene (26.20%), α-pinene (12.90%), limonene (10.40%) | [244] |
| T. rubrum (clinical strain FF5) | 0.32 μL/mL | |||||
| T. mentagrophytes (clinical strain FF7) | 0.64 μL/mL | |||||
| M. canis (clinical strain FF1) | 0.32 μL/mL | |||||
| M. gypseum (clinical strain FF3) | 0.64 μL/mL | |||||
| T. mentagrophytes var. interdigitale (CECT 2958) | 1.25 μL/mL | |||||
| T. verrucosum (CECT 2992) | 2.5 μL/mL | |||||
|
| ||||||
| Juniperus turbinata (juniper), berries | MAC | E. floccosum (clinical strain FF9) | 2% DMSO | 0.64 μL/mL | α-Pinene (66.70%) | [243] |
| T. rubrum (clinical strain FF5) | 1.25 μL/mL | |||||
| T. mentagrophytes (clinical strain FF7) | ||||||
| M. canis (clinical strain FF1) | 0.32 μL/mL | |||||
| M. gypseum (clinical strain FF3) | 1.25 μL/mL | |||||
| Juniperus turbinata (juniper), leaf | MAC | E. floccosum (clinical strain FF9) | 2% DMSO | 0.64 μL/mL | α-Pinene (48.20%), β-phellandrene (23.10%) | |
| T. rubrum (clinical strain FF5) | 0.64–1.25 μL/mL | |||||
| T. mentagrophytes (clinical strain FF7) | 1.25 μL/mL | |||||
| M. canis (clinical strain FF1) | 0.64–1.25 μL/mL | |||||
| M. gypseum (clinical strain FF3) | 1.25 μL/mL | |||||
| Juniperus oxycedrus ssp. oxycedrus (cade), leaf | MAC | E. floccosum (clinical strain FF9) | 2% DMSO | 0.08–0.16 μL/mL | α-Pinene (65.50%) | |
| T. rubrum (clinical strain FF5) | 0.08 μL/mL | |||||
| T. mentagrophytes (clinical strain FF7) | 0.16 μL/mL | |||||
| M. canis (clinical strain FF1) | 0.08 μL/mL | |||||
| M. gypseum (clinical strain FF3) | 0.16 μL/mL | |||||
| Juniperus oxycedrus ssp. oxycedrus (cade), berries | MAC | E. floccosum (clinical strain FF9) | 2% DMSO | 0.32 μL/mL | α-Pinene (54.70%), myrcene (17.80%), germacrene D (10.30%) | |
| T. rubrum (clinical strain FF5) | ||||||
| T. mentagrophytes (clinical strain FF7) | ||||||
| M. canis (clinical strain FF1) | ||||||
| M. gypseum (clinical strain FF3) | ||||||
|
| ||||||
| Kunzea ericoides (Kānuka) | MAC | E. floccosum (clinical isolate) | Tween 80 | 1.10% v/v | α-Pinene (61.60%) | [137] |
| T. rubrum (clinical isolate) | ||||||
|
| ||||||
| Lavandula angustifolia (lavender) | MIC | T. erinacei (KCCM 60411) | Tween 80 | 0.50 mg/mL | NCR | [264] |
| T. mentagrophytes (KCCM 11950) | 2.00 mg/mL | |||||
| T. rubrum (ATCC 6345) | 0.50 mg/mL | |||||
| T. schoenleinii (KCCM 60477) | 2.00 mg/mL | |||||
| T. soudanense (KCCM 60448) | 0.25 mg/mL | |||||
| T. tonsurans (KCCM 11866) | 1.00 mg/mL | |||||
|
| ||||||
| Lavandula pedunculata (French lavender) | MAC | T. mentagrophytes (clinical strains isolated FF7) | 2% DMSO | 0.64–1.25 μL/mL | 1,8-Cineole (2.40–55.50%), fenchone (1.30–59.70%), camphor (3.60–48.00%) | [245] |
| T. rubrum (CECT 2794) | 0.32–1.25 μL/mL | |||||
| E. floccosum (clinical strains isolated FF9) | 0.32–0.64 μL/mL | |||||
| M. canis (clinical strains isolated FF1) | 0.32–1.25 μL/mL | |||||
| M. gypseum (CECT 2905) | 0.64–2.50 μL/mL | |||||
|
| ||||||
| Lavandula stoechas (Spanish lavender) | MAC | E. floccosum (clinical isolate FF9) | 2% DMSO | 0.32 μL/mL | Fenchone (37.00%), camphor (27.30%) | [246] |
| T. mentagrophytes var. interdigitale (CECT 2958) | 0.64 μL/mL | |||||
| T. mentagrophytes (clinical isolate FF7) | ||||||
| M. canis (clinical isolate FF1) | ||||||
| T. rubrum (CECT 2794) | ||||||
| T. verrucosum (CECT 2992) | ||||||
| M. gypseum (CECT 2908) | ||||||
|
| ||||||
| Lavandula viridis (yellow lavender) | MAC | E. floccosum (clinical isolate FF9) | 2% DMSO | 0.32–0.64 μg/mL | 1,8-Cineole (34.50% and 42.20%), camphor (13.40%) | [247] |
| T. mentagrophytes var. interdigitale (CECT 2958) | 0.32 μg/mL | |||||
| T. mentagrophytes (clinical isolate FF7) | 0.32–0.64 μg/mL | |||||
| M. canis (clinical isolate FF1) | 0.32 μg/mL | |||||
| T. rubrum (CECT 2794) | ||||||
| T. verrucosum (CECT 2992) | 0.64 μg/mL | |||||
| M. gypseum (CECT 2908) | 0.32 μg/mL | |||||
|
| ||||||
| Leptospermum scoparium (manuka) | MAC | E. floccosum (clinical isolate) | Tween 80 | 0.40% v/v | (−)-(E)-Calamenene (14.50%), leptospermone (17.60%) | [137] |
| T. rubrum (clinical isolate) | 0.30% v/v | |||||
|
| ||||||
| Melaleuca alternifolia (tea tree) | MIC | M. furfur (10 clinical isolates) | Tween 80 | 0.03–0.12% v/v | NCR | [268] |
| M. sympodialis (10 clinical isolates) | (0.16 × 10−1)–0.12% v/v | |||||
| E. floccosum | (0.08 × 10−1)–0.03% v/v | |||||
| M. canis | (0.04 × 10−1)–0.03% v/v | |||||
| M. gypseum | (0.16 × 10−1)–0.03% v/v | |||||
| T. interdigitale | (0.08 × 10−1)–0.03% v/v | |||||
| T. mentagrophytes | (0.08 × 10−1)–0.06% v/v | |||||
| T. rubrum | (0.08 × 10−1)–0.03% v/v | |||||
| T. tonsurans | (0.04 × 10−1)–(0.16 × 10−1)% v/v | |||||
| T. erinacei (KCCM 60411) | tween 80 | 0.50 mg/mL | NCR | [264] | ||
| T. mentagrophytes (KCCM 11950) | 1.00 mg/mL | |||||
| T. rubrum (ATCC 6345) | ||||||
| T. schoenleinii (KCCM 60477) | 2.00 mg/mL | |||||
| T. soudanense (KCCM 60448) | 8.00 mg/mL | |||||
| T. tonsurans (KCCM 11866) | 1.00 mg/mL | |||||
| MAC | Madurella mycetomatis (34 clinical isolates) | DMSO | (0.08 × 10−1)–0.25% v/v | NCR | [108] | |
| E. floccosum (clinical isolate) | Tween 80 | 0.70% v/v | α-Terpinene (11.40%), γ-terpinene (22.50%), terpinen-4-ol (35.20%) | [137] | ||
| T. rubrum (clinical isolate) | 0.60% v/v | |||||
|
| ||||||
| Melaleuca cajuputi (cajuput) | MAC | E. floccosum (clinical isolate) | Tween 80 | 0.60% v/v | 1,8-Cineole (55.50%) | [137] |
| T. rubrum (clinical isolate) | ||||||
|
| ||||||
| Melaleuca quinquenervia (niaouli) | MAC | E. floccosum (clinical isolate) | Tween 80 | 0.60% v/v | 1,8-Cineole (61.20%) | [137] |
| T. rubrum (clinical isolate) | ||||||
|
| ||||||
| Mentha piperita (peppermint) | ADM | T. mentagrophytes (SP-12) | Sodium taurocholate | 3.00 μg/mL | Menthol (36.40%) | [125, 126] |
| MIC | Dermatophytes clinical isolates (M. canis, E. floccosum, T. rubrum, T. mentagrophytes, T. tonsurans) | Tween 80 | 1.00–2.50 μL/mL | Menthol (37.40%), menthyl acetate (17.40%), menthone (12.70%) | [91] | |
|
| ||||||
| Mentha spicata (spearmint) | MIC | Dermatophytes clinical isolates (M. canis, E. floccosum, T. rubrum, T. mentagrophytes, T. tonsurans) | Tween 80 | 0.075–2.25 μL/mL | Carvone (49.50%), menthone (21.90%) | [91] |
| MIC90 | M. furfur (clinical isolate) | 1% DMSO | 125.00 μg/mL | NCR | [269] | |
|
| ||||||
| Myrtus nivellei (Sahara myrtle) | MAC | E. floccosum (clinical isolate FF9) | 2% DMSO | 0.64 μL/mL | 1,8-Cineole (37.50%), limonene (25.00%) | [251] |
| T. mentagrophytes (clinical isolate FF7) | 1.25 μL/mL | |||||
| M. canis (clinical isolate FF1) | 0.64 μL/mL | |||||
| T. mentagrophytes var. interdigitale (CECT 2958) | 1.25 μL/mL | |||||
| T. rubrum (CECT 2794) | 0.64 μL/mL | |||||
| T. verrucosum (CECT 2992) | 1.25 μL/mL | |||||
| M. gypseum (CECT 2908) | ||||||
|
| ||||||
| Ocimum basilicum (basil) | ADM | T. mentagrophytes | n.m. | 8.30 μg/μL | Estragole (45.80%), linalool (24.20%) | [253] |
| T. tonsurans | 8.00 μg/μL | |||||
| T. rubrum | 8.30 μg/μL | |||||
| E. floccosum | 15.00 μg/μL | |||||
| M. canis | 15.20 μg/μL | |||||
|
| ||||||
| Ocimum gratissimum (African basil) | ADM | M. gypseum (5 clinical isolates) | Tween 80 + DMSO | 250.00 μg/mL | NCR | [270] |
| T. rubrum (10 clinical isolates) | 250.00 μg/mL | |||||
| T. mentagrophytes (10 clinical isolates) | 250.00 μg/mL | |||||
| M. canis (5 clinical isolates) | 500.00 μg/mL | |||||
|
| ||||||
| Origanum vulgare (oregano) | ADM | T. mentagrophytes | n.m. | 1.00 μg/μL | Carvacrol (61.30%), thymol (13.90%) | [253] |
| T. tonsurans | ||||||
| T. rubrum | 1.20 μg/μL | |||||
| E. floccosum | 2.00 μg/μL | |||||
| M. canis | ||||||
| M. canis (11 clinical isolates) | Prunus dulcis (sweet almond oil) | 0.03–0.05% | p-Cymene (14.30%), γ-terpinene (11.20%), thymol (45.00%) | [266] | ||
|
| ||||||
| Pelargonium graveolens (geranium) | MIC | T. erinacei (KCCM 60411) | Tween 80 | 0.50 mg/mL | NCR | [264] |
| T. mentagrophytes (KCCM 11950) | ||||||
| T. rubrum (ATCC 6345) | ||||||
| T. schoenleinii (KCCM 60477) | ||||||
| T. soudanense (KCCM 60448) | 0.25 mg/mL | |||||
| T. tonsurans (KCCM 11866) | 0.50 mg/mL | |||||
|
| ||||||
| Pimpinella anisum (anise fruits) | MAC80 | T. rubrum | n.m. | 0.20% v/v | Anethole, anisaldehyde, linalool | [256] |
| T. mentagrophytes | 0.78% v/v | |||||
| M. canis | 0.10% v/v | |||||
| M. gypseum | 0.20% v/v | |||||
|
| ||||||
| Pogostemon patchouli (patchouli) | MIC | T. erinacei (KCCM 60411) | Tween 80 | 8.00 mg/mL | NCR | [264] |
| T. mentagrophytes (KCCM 11950) | >32.00 mg/mL | |||||
| T. rubrum (ATCC 6345) | 2.00 mg/mL | |||||
| T. schoenleinii (KCCM 60477) | ||||||
| T. soudanense (KCCM 60448) | 0.50 mg/mL | |||||
| T. tonsurans (KCCM 11866) | 8.00 mg/mL | |||||
|
| ||||||
| Rosmarinus officinalis (rosemary) | MIC | T. erinacei (KCCM 60411) | Tween 80 | 4.00 mg/mL | NCR | [264] |
| T. mentagrophytes (KCCM 11950) | 8.00 mg/mL | |||||
| T. rubrum (ATCC 6345) | ||||||
| T. schoenleinii (KCCM 60477) | 4.00 mg/mL | |||||
| T. soudanense (KCCM 60448) | 0.50 mg/mL | |||||
| T. tonsurans (KCCM 11866) | 8.00 mg/mL | |||||
| T. rubrum | n.m. | 1.38 mg/mL | NCR | [171] | ||
| M. gypseum | 2.75 mg/mL | |||||
| M. canis (11 clinical isolates) | Prunus dulcis (sweet almond oil) | 2.34–7.50% | 1,8-Cineole (27.50%), α-pinene (23.40%) | [266] | ||
| MIC90 | M. furfur (clinical isolate) | 1% DMSO | 260.00 μg/mL | NCR | [269] | |
|
| ||||||
| Syzygium aromaticum (clove) | MAC | E. floccosum (clinical isolate FF9) | DMSO | 0.16 μg/mL | Eugenol (85.30%) | [258] |
| T. rubrum (clinical isolate FF5) | ||||||
| T. mentagrophytes | 0.100 μg/mL | |||||
| (clinical isolate FF7) | ||||||
| M. canis (clinical isolate FF1) | 0.08–0.16 μg/mL | |||||
| M. gypseum | 0.16 μg/mL | |||||
| (clinical isolate FF3) | ||||||
|
| ||||||
| Thymus herba-barona (thyme) | MAC | E. floccosum (clinical isolate FF9) | 2% DMSO | 0.16 μL/mL | Carvacrol (54.00%), thymol (30.20%) | [246] |
| T. mentagrophytes var. interdigitale (CECT 2958) | ||||||
| T. mentagrophytes (clinical isolate FF7) | ||||||
| M. canis (clinical isolate FF1) | ||||||
| T. rubrum (CECT 2794) | ||||||
| T. verrucosum (CECT 2992) | ||||||
| M. gypseum (CECT 2908) | ||||||
|
| ||||||
| Thymus magnus (thyme) | MIC | T. rubrum (ATCC 6345) | Ethanol + Tween 80 | 0.09 μg/mL | Thymol (39.80%) | [260] |
|
| ||||||
| Thymus quinquecostatus (thyme) | MIC | T. rubrum (ATCC 6345) | Ethanol + Tween 80 | 0.04 μg/mL | Thymol (41.70%), γ-terpinene (16.00%), p-cymene (13.00%) | [260] |
|
| ||||||
| Thymus schimperi (thyme) | ADM | Trichophyton spp. (nail isolate) | DMSO | 0.31 μL/mL | NCR | [232] |
| Trichophyton spp. (scalp isolate) | 0.08 μL/mL | |||||
| Microsporum spp. | ||||||
|
| ||||||
| Thymus serpyllum (thyme) | MIC | M. canis (11 clinical isolates) | Prunus dulcis (sweet almond oil) | 0.025–0.10% | Carvacrol (72.00%) | [266] |
|
| ||||||
| Thymus tosevii (thyme) | MIC | Dermatophytes clinical isolates (M. canis, E. floccosum, T. rubrum, T. mentagrophytes, T. tonsurans) | Tween 80 | (0.25–0.75) × 10−1 μL/mL | Geranyl acetate (17.90%), α-terpinyl acetate (12.30%), carvacrol (12.80%), thymol (10.40%), cis-myrtanol (11.20%) | [91] |
|
| ||||||
| Thymus vulgaris (thyme) | MIC | T. erinacei (KCCM 60411) | Tween 80 | 0.50 mg/mL | NCR | [264] |
| T. mentagrophytes (KCCM 11950) | 1.00 mg/mL | |||||
| T. rubrum (ATCC 6345) | ||||||
| T. schoenleinii (KCCM 60477) | 0.50 mg/mL | |||||
| T. soudanense (KCCM 60448) | ||||||
| T. tonsurans (KCCM 11866) | 1.00 mg/mL | |||||
| T. mentagrophytes | n.m. | 2.20 μg/μL | thymol (47.90%) | [253] | ||
| T. tonsurans | ||||||
| T. rubrum | 2.00 μg/μL | |||||
| E. floccosum | 4.00 μg/μL | |||||
| M. canis | 2.20 μg/μL | |||||
| Dermatophytes clinical isolates (M. canis, E. floccosum, T. rubrum, T. mentagrophytes, T. tonsurans) | Tween 80 | 0.03–0.50 μL/mL | Thymol (48.90%), p-cymene (19.00%) | [91] | ||
| T. rubrum (clinical isolate) | 72.00 μg/mL | Thymol (44.71%), γ-terpinene (26.01%), α-cymene (21.22%) | [271] | |||
|
| ||||||
| Thymus zygis subsp. sylvestris (thyme) | MAC |
T. rubrum (CECT 2794) T. mentagrophytes (clinical isolate FF7) M. canis (clinical isolate FF1) M. gypseum (CECT 2908) |
2% DMSO | 0.16–0.32 μL/mL | p-Cymene (11.00–17.00%), γ-terpinene (3.80–11.50%), linalool (3.50–30.0%), geraniol (0.10–19.80%), thymol (5.20–23.80%), carvacrol (1.30–25.00%), geranyl acetate (0.50–20.80%) | [263] |
aScientific name (common name), part of plant (if applicable).
bMIC: microdilution method; MAC: macrodilution method; ADM: agar dilution method; CTA: contact time assay.
cAmerican Type Culture Collection, Rockville, USA (ATCC), Colección Espanõla de Cultivos Tipo (CECT), collection of microorganisms of the Department of Microbiology (MFBF), Korean Culture Center of Microorganisms (KCCM).
dDMSO concentration was not included; n.m.: not mentioned.
eNI: no inhibition.
fNCR: no composition results reported.
The ability of topical formulations to penetrate the skin is crucial for the effective treatment of subcutaneous infections [108]. Melaleuca alternifolia oil has displayed in vitro activity against M. mycetomatis and M. furfur, proving its potential in treating eumycetoma, pityriasis, and seborrheic dermatitis, not only because of its antifungal activity, but also because of its ability to penetrate the skin due to its main compound (terpinen-4-ol) [108, 109, 275, 276].
Onychomycosis is generally resilient to topical treatment of any kind; thus, there is a poor cure rate. It is usually treated systemically due to its infrequency in responding to topical treatments [277, 278]. With onychomycosis being the most frequent cause of nail disease, Buck et al. [279] aimed to treat onychomycosis in clinical trials whereby 60% of patients were treated with M. alternifolia oil and 61% of patients were treated with 1% clotrimazole. There was only a 1% difference between the two study groups. What would be interesting for future studies is to determine what the results would be when testing the same treatments against resistant strains.
Tinea pedis is often treated topically, which presents an opportunity for essential oil use [280]. Melaleuca alternifolia oil was evaluated in two trials for treating tinea pedis. In the first trial by Tong et al. [281], the patients were treated with either a 10% M. alternifolia oil in sorbolene, 1% tolnaftate, or a placebo (sorbolene). The patients on M. alternifolia oil treatment had a mycological cure rate of 30%. Mycological cure rates of 21% were seen in the placebo group and of 85% in patients receiving tolnaftate, proving the essential oil to not be as effective. The second trial tested two solutions of 25% and 50% M. alternifolia oil in ethanol and polyethylene glycol. This was compared to a placebo containing only the vehicle in a double-blinded randomised controlled trial [282]. The placebo group showed a clinical response in 39% of patients. Melaleuca alternifolia oil test groups showed a 72% improvement. A higher concentration of the oil is thus required for treating this type of infection.
In spite of the dermatophytes showing susceptibility to essential oils, there are few studies dedicated to these pathogens. One would expect more essential oil treatments considering the difficulty in treating these infections which require expensive prolonged treatment. An essential oil with superior activity certainly warrants further investigation, particularly as essential oils work well on skin surfaces and are shown to display good penetration capabilities [283, 284]. Madurella mycetomatis and Malassezia furfur are sorely neglected pathogens in research. Possibly their fastidious nature acts as a barrier for further research. As far as clinical studies are concerned, essential oils against fungal pathogens have also been neglected. Only M. alternifolia oil has been clinically studied extensively with investigations incorporating onychomycosis, tinea pedis, and dandruff [275, 279, 281, 282, 285]. It would be interesting to observe the antidermatophytic property of essential oils that have shown to be noteworthy in vitro antifungal activity such as for Apium nodiflorum (celery), Cedrus atlantica (cedar wood), C. citratus, Juniperus oxycedrus ssp. oxycedrus (cade), Pelargonium graveolens (geranium), S. aromaticum, and Thymus spp.
5. Essential Oil Combinations
Other than the use of oils within carrier oils, most essential oils are used in blends or combinations of two or more oils [32]. These blends are considered to be an art where the oils are carefully selected and combined with the intention of holistically healing the “whole” individual according to his/her symptoms. The goal of blending is to create a synergistic therapeutic effect where the combination of essential oils is greater than the sum of the individual oil [37, 40, 286]. The beneficial value of synergy has been notorious and used since antiquity [74]. Synergy can be achieved if the compounds in the oil are able to affect different target sites, or they may interact with one another to increase solubility thereby enhancing bioavailability [287–289]. Mechanisms that can lead to pharmacological synergy are (1) multitarget effect where multiple target sites of the bacterial cell are affected; (2) solubility and bioavailability enhancement; (3) the mechanism where the essential oil may inhibit the mutation mechanism of bacteria to the antimicrobial; or (4) the mechanism where the essential oil may inhibit the efflux pump of bacteria, thus allowing for the antimicrobial to accumulate inside the bacteria [11, 288, 290]. The goal is for a multitargeted treatment to decrease pathogen mutation and thus retard the development of resistance. The combined formulation also has the potential to decrease toxicity and adverse side effects by lowering the required dose [290–292]. This is not an infallible method, however, as even the combined penicillin with clavulanic acid has become prone to resistance [293, 294].
When blends are created, the intention is to create therapeutic synergy [2, 26, 32]. The reasoning for the combinations is to produce a forceful blend that has more than one mode of action. For example, in the treatment of abscesses, C. bergamia and L. angustifolia may be used in combination. C. bergamia is used for its antiseptic properties and L. angustifolia for antiseptic and anti-inflammatory effects. Anthemis nobilis is also often used for anti-inflammatory effects [2, 26, 32, 37]. The theory is sound and not too far off considering that numerous essential oils have been proven to possess additional pharmacological properties. For example, P. graveolens is known for antiseptic and anti-inflammatory properties. It is often used for the ability to balance sebum secretions and clear oily and sluggish skin [295]. Eucalyptus globulus (eucalyptus) may be used for its proven antimicrobial and anti-inflammatory activity [296, 297]. Often used on acne prone skin because of its antiseptic properties is L. angustifolia [298, 299]. Anthemis nobilis is believed to ease inflammation and L. angustifolia assists with healing and regeneration [25]. Citrus aurantium (neroli) flower oil has displayed antioxidant activity [120], and the main component of M. alternifolia (terpinen-4-ol) has the ability to hinder tumour necrosis factor (TNF), interleukin-1, interleukin-8, and interleukin-10, and prostaglandin E2 [300]. The anti-inflammatory activity of C. bergamia has been proven by several studies in vitro or on animal models [301, 302]. This supports the theory behind therapeutic synergy; however, the mistaken belief that any essential oil blend will result in synergy is not fully accurate [33]. It is a complex area, because although a certain combination may have a synergistic therapeutic effect, it does not necessarily translate into antimicrobial synergy and this needs further investigation.
By reviewing the aromatherapeutic literature [1, 2, 26, 32, 36–43], at least 1500 possible combinations (made up of two oils) could be identified for dermatology alone. This brings forth the question as to the antimicrobial effect of the overall combination. After all, if essential oils are to be investigated as options to curb antimicrobial resistance, the aim of combination therapy should be to broaden the spectrum of the antimicrobial activity and prevent development of additional resistance occurring [96]. The risk of resistance emerging against essential oils should not be disregarded because suboptimal doses of essential oils may impact these phenomena [303]. Sublethal concentration exposure to M. alternifolia has been proven to result in slightly lowered bacterial susceptibility to M. alternifolia and a larger decrease in susceptibility to conventional antimicrobials. The study concluded that essential oil products containing sublethal concentrations may result in stress-hardened (mutated) S. aureus isolates and possible treatment failure [146]. This highlights that although therapeutic synergy is strived for, these must still be verified in a controlled environment [288].
Studies have proven that essential oils, whether in combination with other essential oils [99] or in combination with conventional antimicrobials [304], can initiate a synergistic antimicrobial effect. This effect, however, is limited to the studied pathogen [290]. de Rapper et al. [99] demonstrated that even when essential oils displayed synergistic blends against one pathogen, the same could not be said against other pathogens. This highlights how the assumption should not be made that all synergistic blends are the same against all pathogens.
The fractional inhibitory concentration index (∑FIC or FICI) is the commonly accepted mathematical method employed to interpret interactions in 1 : 1 combinations [74]. ∑FIC is determined from the sum of all individual FICs of each of the test agents within the combination [305]. This then allows for the determination of their individual interactions in the combination [306]. The results are interpreted as synergistic (∑FIC ≤ 0.5), additive (∑FIC > 0.5–1.0), indifferent ∑FIC (>1.0 ≤ 4.0), or antagonistic (∑FIC > 4.0) [74]. Although using ∑FIC calculations is an easy method, it is not without its limitation. When examining 1 : 1 ratios between two essential oils, it is assumed that half the concentration will only offer half the effect. This is not necessarily the case between agents, as two agents may not have the same dose response at the same concentrations [307]. An interactive assessment of the different ratio combinations is mostly carried out using the isobole method [308, 309]. This method allows for more accurate valuation of the combination contribution made by each agent on a mathematical level line where all points are collected on a surface that lies at a specific value [288, 305, 310]. There are, however, other complex methods that can also be used [311, 312].
5.1. Essential Oils in Combination with Other Essential Oils
Although combinations are frequently mentioned in aromatherapy to treat skin ailments, only a handful of studies documenting essential oil combinations were found against skin pathogens (Table 9). The combination studies are predominantly limited to S. aureus, P. aeruginosa, C. albicans, and, to a lesser extent, E. coli. Even fewer studies were found against the dermatophytes and acne pathogens. This is rather abysmal considering the amount of combinations and the regularity of their use. An interesting observation was made even in an early study [316], where it was shown that synergy found in the 1 : 1 combinations was apparent irrespective of the poor efficacy displayed by the individual oils. This indicates that essential oils do not necessarily have to be combined based purely on independent noteworthy antimicrobial activity.
Table 9.
Essential oil with essential oil combinations against skin pathogens.
| Essential oil | Species strain | FICa | Resultb | Reference |
|---|---|---|---|---|
| Aniba rosaeodora (rosewood) + Thymus vulgaris (thyme) | E. coli (ATCC 25922) | 0.23 | S | [85] |
|
| ||||
| Boswellia papyrifera (frankincense) + Commiphora myrrha (myrrh) | E. coli (ATCC 8739) | 0.65 | A | [117] |
| C. albicans (ATCC 10231) | 1.21 | I | ||
| S. aureus (ATCC 12600) | 0.82 | A | ||
| P. aeruginosa (ATCC 27858) | 0.77 | |||
|
| ||||
| Boswellia neglecta (frankincense) + Commiphora guidotti (myrrh) | E. coli (ATCC 8739) | 1.46 | I | [117] |
| C. albicans (ATCC 10231) | 0.59 | A | ||
| S. aureus (ATCC 12600) | 2.5 | I | ||
| P. aeruginosa (ATCC 27858) | 1.04 | |||
| E. coli (ATCC 8739) | 0.67 | A | ||
|
| ||||
| Boswellia neglecta (frankincense) + Commiphora myrrha (myrrh) | C. albicans (ATCC 10231) | 1.19 | I | [117] |
| S. aureus (ATCC 12600) | 3.65 | |||
| P. aeruginosa (ATCC 27858) | 0.6 | A | [117] | |
|
| ||||
| Boswellia papyrifera (frankincense) + Commiphora guidotti (myrrh) | E. coli (ATCC 8739) | 0.91 | A | [117] |
| C. albicans (ATCC 10231) | 1.21 | I | ||
| S. aureus (ATCC 12600) | 0.5 | S | ||
| P. aeruginosa (ATCC 27858) | 0.91 | A | ||
|
| ||||
| Boswellia rivae (frankincense) + Commiphora guidotti (myrrh) | E. coli (ATCC 8739) | 1.3 | I | [117] |
| C. albicans (ATCC 10231) | 1.38 | |||
| S. aureus (ATCC 12600) | 2 | |||
| P. aeruginosa (ATCC 27858) | 1.2 | |||
|
| ||||
| Boswellia rivae (frankincense) + Commiphora myrrha (myrrh) | E. coli (ATCC 8739) | 0.67 | A | [117] |
| C. albicans (ATCC 10231) | 2.14 | I | ||
| S. aureus (ATCC 12600) | 1.27 | |||
| P. aeruginosa (ATCC 27858) | 0.58 | A | ||
|
| ||||
| Cinnamomum zeylanicum (cinnamon) + Syzygium aromaticum (clove) | S. aureus (ATCC 29213) | 1.8 | I | [313] |
| E. coli (ATCC 29252) | 4.2 | An | ||
|
| ||||
| Cinnamomum zeylanicum (cinnamon) + Thymus vulgaris (thyme) | S. aureus (ATCC 25923) | 0.26 | S | [85] |
|
| ||||
| Cymbopogon citratus (lemongrass) + Cymbopogon giganteus (lemongrass) | E. coli (CIP 105182) | 0.5 | S | [124] |
| S. aureus (ATCC 9144) | 0.4 | |||
|
| ||||
| Cuminum cyminum (cumin) + Coriandrum sativum (coriander) seed | S. aureus (7 clinical isolates) | 0.5 | S | [122] |
| E. coli (7 clinical isolates) | ||||
|
| ||||
| Juniperus communis (juniper berry) + Thymus vulgaris (thyme) | S. aureus (ATCC 25923) | 0.74 | A | [85] |
|
| ||||
| Lavandula angustifolia (lavender) + Angelica archangelica (angelica) root | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 1.07 | I | ||
| P. aeruginosa (ATCC 27858) | 0.67 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Anthemis nobilis (chamomile) | C. albicans (ATCC 10231) | 0.33 | S | [99] |
| S. aureus (ATCC 6538) | 0.84 | A | ||
| P. aeruginosa (ATCC 27858) | 0.54 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Citrus aurantium (petitgrain) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 1.13 | I | ||
| P. aeruginosa (ATCC 27858) | 0.51 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Citrus grandis (grapefruit) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 1.67 | I | ||
| P. aeruginosa (ATCC 27858) | 0.52 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Citrus sinensis (orange) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 0.38 | |||
| P. aeruginosa (ATCC 27858) | 0.51 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Citrus medica limonum (lemon) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 2.5 | I | ||
| P. aeruginosa (ATCC 27858) | 0.52 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Abies balsamea (fir) | C. albicans (ATCC 10231) | 0.63 | A | [99] |
| S. aureus (ATCC 6538) | 2.5 | I | ||
| P. aeruginosa (ATCC 27858) | 0.52 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Andropogon muricatus (vetiver) | C. albicans (ATCC 10231) | 0.45 | S | [99] |
| S. aureus (ATCC 6538) | 0.92 | A | ||
| P. aeruginosa (ATCC 27858) | 1.02 | I | ||
|
| ||||
| Lavandula angustifolia (lavender) + Angelica archangelica (angelica) seed | C. albicans (ATCC 10231) | 0.83 | A | [99] |
| S. aureus (ATCC 6538) | 2 | I | ||
| P. aeruginosa (ATCC 27858) | 0.75 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Artemisia dracunculus (tarragon) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 1.67 | I | ||
| P. aeruginosa (ATCC 27858) | 0.51 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Cananga odorata (ylang-ylang) | C. albicans (ATCC 10231) | 1.25 | I | [99] |
| S. aureus (ATCC 6538) | 1.5 | |||
| P. aeruginosa (ATCC 27858) | 1.02 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Cananga odorata heads (ylang-ylang) | C. albicans (ATCC 10231) | 0.83 | A | [99] |
| S. aureus (ATCC 6538) | 1.13 | I | ||
| P. aeruginosa (ATCC 27858) | 1.02 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Canarium luzonicum (elemi) | C. albicans (ATCC 10231) | 0.25 | S | [99] |
| S. aureus (ATCC 6538) | 3.33 | I | ||
| P. aeruginosa (ATCC 27858) | 0.53 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Carum carvi (caraway) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 1 | A | ||
| P. aeruginosa (ATCC 27858) | 0.56 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Cinnamomum zeylanicum (cinnamon) | C. albicans (ATCC 10231) | 0.4 | S | [99] |
| S. aureus (ATCC 6538) | 0.5 | |||
| P. aeruginosa (ATCC 27858) | 0.53 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Commiphora myrrha (myrrh) | C. albicans (ATCC 10231) | 0.29 | S | [99] |
| S. aureus (ATCC 6538) | 1 | A | ||
| P. aeruginosa (ATCC 27858) | 1.03 | I | ||
|
| ||||
| Lavandula angustifolia (lavender) + Cupressus sempervirens (cypress) | C. albicans (ATCC 10231) | 0.15 | S | [99] |
| S. aureus (ATCC 6538) | 0.58 | A | ||
| P. aeruginosa (ATCC 27858) | 0.53 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Cymbopogon citratus (lemongrass) | C. albicans (ATCC 10231) | 6.67 | An | [99] |
| S. aureus (ATCC 6538) | 0.55 | A | ||
| P. aeruginosa (ATCC 27858) | 0.52 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Cymbopogon nardus (citronella) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 0.75 | A | ||
| P. aeruginosa (ATCC 27858) | 0.53 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Daucus carota (carrot seed) | C. albicans (ATCC 10231) | 0.5 | S | [99] |
| S. aureus (ATCC 6538) | ||||
| P. aeruginosa (ATCC 27858) | 0.56 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Eucalyptus globulus (eucalyptus) | C. albicans (ATCC 10231) | 0.38 | S | [99] |
| S. aureus (ATCC 6538) | 1.5 | I | ||
| P. aeruginosa (ATCC 27858) | 0.53 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Foeniculum dulce (fennel) | C. albicans (ATCC 10231) | 0.45 | S | [99] |
| S. aureus (ATCC 6538) | 2 | I | ||
| P. aeruginosa (ATCC 27858) | 0.52 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Hyssopus officinalis (hyssop) | C. albicans (ATCC 10231) | 0.33 | S | [99] |
| S. aureus (ATCC 6538) | 1.67 | I | ||
| P. aeruginosa (ATCC 27858) | 0.52 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Juniperus virginiana (juniper) | C. albicans (ATCC 10231) | 0.5 | S | [99] |
| S. aureus (ATCC 6538) | ||||
| P. aeruginosa (ATCC 27858) | 0.55 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Juniperus virginiana berries (juniper) | C. albicans (ATCC 10231) | 0.21 | S | [99] |
| S. aureus (ATCC 6538) | 1.25 | I | ||
| P. aeruginosa (ATCC 27858) | 0.52 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Laurus nobilis (bay) | C. albicans (ATCC 10231) | 0.83 | A | [99] |
| S. aureus (ATCC 6538) | 1.7 | I | ||
| P. aeruginosa (ATCC 27858) | 0.6 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Litsea cubeba (May Chang) | C. albicans (ATCC 10231) | 0.19 | S | [99] |
| S. aureus (ATCC 6538) | 1.17 | I | ||
| P. aeruginosa (ATCC 27858) | 0.52 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Matricaria chamomilla (German chamomile) | C. albicans (ATCC 10231) | 1.17 | I | [99] |
| S. aureus (ATCC 6538) | ||||
| P. aeruginosa (ATCC 27858) | 0.54 | A | ||
| Lavandula angustifolia (lavender) + Melaleuca alternifolia (tea tree) | C. albicans (ATCC 10231) | 0.5 | S | [99] |
| S. aureus (ATCC 6538) | 0.63 | A | ||
| P. aeruginosa (ATCC 27858) | 0.51 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Melaleuca viridiflora (niaouli) | C. albicans (ATCC 10231) | 0.9 | A | [99] |
| S. aureus (ATCC 6538) | 2 | I | ||
| P. aeruginosa (ATCC 27858) | 0.51 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Mentha piperita (peppermint) | C. albicans (ATCC 10231) | 0.63 | A | [99] |
| S. aureus (ATCC 6538) | 0.75 | |||
| P. aeruginosa (ATCC 27858) | 0.51 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Myrtus communis (myrrh) | C. albicans (ATCC 10231) | 0.5 | S | [99] |
| S. aureus (ATCC 6538) | 4 | An | ||
| P. aeruginosa (ATCC 27858) | 0.51 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Ocimum basilicum (basil) | C. albicans (ATCC 10231) | 0.67 | A | [99] |
| S. aureus (ATCC 6538) | 0.58 | |||
| P. aeruginosa (ATCC 27858) | 0.63 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Origanum majorana (marjoram) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 4 | An | ||
| P. aeruginosa (ATCC 27858) | 0.52 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Pelargonium odoratissimum (geranium) | C. albicans (ATCC 10231) | 1.04 | I | [99] |
| S. aureus (ATCC 6538) | 1.17 | |||
| P. aeruginosa (ATCC 27858) | 0.52 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Pinus sylvestris (pine) | C. albicans (ATCC 10231) | 0.5 | S | [99] |
| S. aureus (ATCC 6538) | 0.75 | A | ||
| P. aeruginosa (ATCC 27858) | 1 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Piper nigrum (black pepper) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 1 | A | ||
| P. aeruginosa (ATCC 27858) | 0.57 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Pogostemon patchouli (patchouli) | C. albicans (ATCC 10231) | 0.5 | S | [99] |
| S. aureus (ATCC 6538) | 1.17 | I | ||
| P. aeruginosa (ATCC 27858) | 0.51 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Rosmarinus officinalis (rosemary) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 0.75 | A | ||
| P. aeruginosa (ATCC 27858) | 0.51 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Salvia sclarea (clary sage) | C. albicans (ATCC 10231) | 0.73 | A | [99] |
| S. aureus (ATCC 6538) | 1 | |||
| P. aeruginosa (ATCC 27858) | 0.51 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Santalum album (sandalwood) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 2.25 | I | ||
| P. aeruginosa (ATCC 27858) | 0.51 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Styrax benzoin (benzoin) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 1 | A | ||
| P. aeruginosa (ATCC 27858) | 0.58 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Syzygium aromaticum (clove) | C. albicans (ATCC 10231) | 0.58 | A | [99] |
| S. aureus (ATCC 6538) | 1.17 | I | ||
| P. aeruginosa (ATCC 27858) | 0.53 | A | ||
|
| ||||
| Lavandula angustifolia (lavender) + Tagetes patula (French marigold) | C. albicans (ATCC 10231) | 0.42 | S | [99] |
| S. aureus (ATCC 6538) | 0.75 | A | ||
| P. aeruginosa (ATCC 27858) | 0.51 | |||
|
| ||||
| Lavandula angustifolia (lavender) + Thymus vulgaris (thyme) | C. albicans (ATCC 10231) | 0.67 | A | [99] |
| S. aureus (ATCC 6538) | 0.4 | S | ||
| P. aeruginosa (ATCC 27858) | 0.51 | A | ||
|
| ||||
| Melaleuca alternifolia (tea tree) + Backhousia citriodora (lemon myrtle) | S. aureus | n.m. | I | [115] |
| E. coli | ||||
| P. aeruginosa | ||||
| C. albicans | A | |||
|
| ||||
| Melissa officinalis (lemon balm) + Thymus vulgaris (thyme) | E. coli (ATCC 25922) | 0.34 | S | [85] |
|
| ||||
| Mentha piperita (peppermint) + Ocimum basilicum (basil) | E. coli (CIP 105182) | 0.29 | S | [156] |
| S. aureus (ATCC 9144) | 0.36 | |||
|
| ||||
| Mentha piperita (peppermint) + Thymus vulgaris (thyme) | E. coli (ATCC 25922) | 0.55 | A | [85] |
|
| ||||
| Ocimum basilicum (basil) + Citrus bergamia (bergamot) | S. aureus (ATCC 6538) | 0.38 | S | [62] |
|
| ||||
| Origanum vulgare (oregano) + Cinnamomum zeylanicum (cinnamon) | E. coli (ATCC 25922) | n.m. | A | [231] |
| E. coli (10 clinical isolates, 2 extended-spectrum β-lactamase producing) | ||||
| P. aeruginosa (ATCC 27853) | I | |||
| P. aeruginosa (clinical isolate) | A | |||
|
| ||||
| Origanum vulgare (oregano) + Citrus bergamia (bergamot) | S. aureus (ATCC 6538) | 0.38 | S | [62] |
|
| ||||
| Origanum vulgare (oregano) + Melissa officinalis (lemon balm) | P. aeruginosa (ATCC 27853) | 1.38 | I | [314] |
| E. coli (ATCC 25922) | 1.17 | |||
|
| ||||
| Origanum vulgare (oregano) + Ocimum basilicum (basil) | E. coli (ATCC 8739) | 0.75 | A | [62] |
| S. aureus (ATCC 6538) | 0.38 | S | ||
| P. aeruginosa (ATCC 27853) | 1 | A | [314] | |
| E. coli (ATCC 25922) | ||||
|
| ||||
| Origanum vulgare (oregano) + Origanum majorana (marjoram) | P. aeruginosa (ATCC 27853) | 1.75 | I | [314] |
| E. coli (ATCC 25922) | 0.83 | A | ||
|
| ||||
| Origanum vulgare (oregano) + Rosmarinus officinalis (rosemary) | P. aeruginosa (ATCC 27853) | 1.5 | I | [314] |
| E. coli (ATCC 25922) | 1.83 | |||
|
| ||||
| Origanum vulgare (oregano) + Salvia triloba (sage) | P. aeruginosa (ATCC 27853) | 1.5 | I | [314] |
| Origanum vulgare (oregano) + Thymus vulgaris (thyme) | P. aeruginosa (ATCC 27853) | 0.88 | A | [314] |
| Origanum vulgare (oregano) + Rosmarinus officinalis (rosemary) | S. aureus (5 clinical isolates) | 0.5 | S | [315] |
| Salvia officinalis (sage) + Thymus numidicus (thyme) | E. coli (clinical strain) | 1.03 | I | [176] |
|
| ||||
| Syzygium aromaticum (clove) + Rosmarinus officinalis (rosemary) | S. aureus (ATCC 6538) | n.m. | A | [169] |
| S. epidermidis (ATCC 12228) | ||||
| C. albicans (ATCC 10231) | ||||
| P. aeruginosa (ATCC 27853) | I | |||
| E. coli (ATCC 8739) | A | |||
|
| ||||
| Thymus vulgaris (thyme) + Pimpinella anisum (anise) | E. coli | n.m. | A | [168] |
| P. aeruginosa | S | |||
| S. aureus | A | |||
an.m.: not mentioned.
bS: synergy; A: additive; I: indifference; An: antagonism.
One of the largest studies on combinations was done by de Rapper et al. [99], where 45 essential oils were combined with L. angustifolia, which is one of the most popular essential oils used in combination. What could be observed was that there was no predictive pattern as to what the combined FIC index would be. There were a few synergistic interactions, most of which against C. albicans and some antagonism; however, the majority of the combinations resulted in an indifferent or additive interaction. A study investigated the antimicrobial activity of the popular commercial product containing essential oils (Olbas). The individual essential oils were tested separately and then in the combined product [128]. The combination of the four oils showed no further enhancement in the antimicrobial. The combination of Syzygium aromaticum (clove) and Rosmarinus officinalis (rosemary) has also displayed synergy against C. albicans, at ratios of 1 : 5, 1 : 7, and 1 : 9 [169]. Synergy was observed with a combination of commercially popular L. angustifolia and M. alternifolia essential oils against dermatophytes T. rubrum and T. mentagrophytes var. interdigitale in various combinations [303]. Unfortunately, only a few essential oil combinations have been investigated in clinical settings.
Essential oil combinations have proven efficacy in clinical settings. L. angustifolia and Matricaria recutita (German chamomile) were investigated in a small trial involving eight patients with chronic leg ulcers. Five received a 6% mixture of the two essential oils mixed in Vitis vinifera (grape seed) carrier oil, and three received conventional wound care. It was noted that four of the five patients in the control group had complete healing of the wounds with the fifth patient making progress towards a recovery [317]. Another successful essential oil combination included L. angustifolia, Artemisia vulgaris (mugwort), and Salvia officinalis (sage) in treating chronic wounds such as venous ulcers, pressure sores, skin tears, and abrasions. It was speculated that the essential oils had increased circulation and vascular permeability resulting in accelerated angiogenesis [318]. An in vivo study by Mugnaini et al. [266] made use of a mixture composed of 5% O. vulgare, 5% R. officinalis, and 2% Thymus serpyllum (Breckland thyme), diluted in Prunus dulcis (sweet almond), and this was topically administered on M. canis lesions. A 71% success rate in treatment was observed.
5.2. Essential Oils in Combination with Conventional Antimicrobials
In an effort to prevent resistance and increase antimicrobial efficacy against multidrug-resistant bacteria, the combination of essential oils with antibiotics has been investigated [182, 319–321]. Certain studies are based on the assumption that the antimicrobial and essential oils attack at different sites of the pathogen [304], while others believe this is due to the increase in chemical complexity, together with the added advantage of enhanced skin penetration by the essential oil components [322], or the hope that the essential oils will improve antibiotic diffusion across the bactericidal cell membranes and/or inhibit the Gram-negative efflux pump [323]. Conventional medication in combination with essential oils (bought over the counter or shelves) is also common among patients [183]; therefore, unknowingly, they may be causing enhancement or failure.
Table 10 displays the studies validating the improvement of antimicrobial activity from the combined use of antimicrobials with essential oils. The majority of the studies have shown essential oils to enhance antimicrobial activity of antibiotics and antifungals [81, 346, 347]. Origanum vulgare oil displayed synergy (FICs 0.4–0.5) when combined with doxycycline, florfenicol, or sarafloxacin against an ESBL producing E. coli [324]. This presents a possible solution for β-lactamase antibiotic-resistant bacteria. Origanum vulgare essential oils were investigated and shown to improve the activity of β-lactam antibiotics against both Gram-positive and Gram-negative β-lactamase-producing bacteria [77, 324]. Helichrysum italicum (everlasting) (2.5%) reduced the multidrug resistance of Gram-negative bacteria, E. coli and P. aeruginosa, to chloramphenicol [320].
Table 10.
Essential oil studies demonstrating synergistic interactions in combination with conventional antimicrobials.
| Antimicrobial | Essential oil | Microbial species studied | IFICa | Resultb | Reference |
|---|---|---|---|---|---|
| Amoxicillin | Origanum vulgare (oregano) | E. coli | 0.75 | A | [324] |
| Elettaria cardamomum (cardamom) | S. aureus (ATCC 25923) | 1.06–2.00 | I | [325] | |
| MRSA (clinical isolate) | 0.56–1.11 | A-I | |||
| 0.61–1.50 | |||||
|
| |||||
| Amphotericin B | Melaleuca alternifolia (tea tree) | C. albicans (NRRL y-12983, ATCC 14053, NRRL y-869, NRRL y-22077, ATCC 10231) | 0.13–1.75 | S | [249] |
| Origanum vulgare (oregano) | 0.03–0.35 | ||||
| Pelargonium graveolens (geranium) | 0.04–0.18 | ||||
| Thymus broussonetii (thyme) | C. albicans (CCMM L4) | 0.37 | S | [259] | |
| Thymus maroccanus (thyme) | 0.49 | ||||
| Thymus vulgaris (thyme) | C. albicans (ATCC 90029) | n.m. | [326] | ||
|
| |||||
| Ampicillin | Cinnamomum verum (cinnamon) | E. coli | 0.75 | A | [327] |
| Lavandula angustifolia (lavender) | 2 | I | |||
| Melaleuca alternifolia (tea tree) | 0.75 | A | |||
| Mentha piperita (peppermint) | E. coli | 1 | A | [328] | |
| Origanum majorana (marjoram) | 0.63 | [327] | |||
|
| |||||
| Carbenicillin | Cinnamomum verum (cinnamon) | E. coli | 2 | I | [327] |
| Lavandula angustifolia (lavender) | |||||
| Melaleuca alternifolia (tea tree) | E. coli | 0.56 | A | [327] | |
| Mentha piperita (peppermint) | 0.75 | ||||
| Origanum majorana (marjoram) | E. coli | 1.06 | I | [327] | |
|
| |||||
| Cefazolin | Cinnamomum verum (cinnamon) | E. coli | 0.63 | A | [327] |
| Lavandula angustifolia (lavender) | E. coli | 2 | I | [327] | |
| Melaleuca alternifolia (tea tree) | E. coli | 1.5 | |||
| Mentha piperita (peppermint) | E. coli | 2 | I | [327] | |
| Origanum majorana (marjoram) | E. coli | ||||
|
| |||||
| Cefixime | Thymus broussonetii (thyme) | E. coli | 0.5 | S | [329] |
| P. aeruginosa | |||||
| S. aureus | |||||
| Thymus maroccanus (thyme) | E. coli | 0.5 | S | [329] | |
| P. aeruginosa | 0.75 | A | |||
| S. aureus | 0.18 | S | |||
|
| |||||
| Ceftazidime | Cinnamomum verum (cinnamon) | E. coli (J53 pMG321) | 2 | I | [327] |
| Lavandula angustifolia (lavender) | E. coli (J53 pMG321) | 1 | A | [327] | |
| Melaleuca alternifolia (tea tree) | |||||
| Mentha piperita (peppermint) | E. coli (J53 pMG321) | 2 | I | [327] | |
| Origanum majorana (marjoram) | |||||
|
| |||||
| Ceftiofur | Origanum vulgare (oregano) | E. coli | 0.63 | A | [324] |
| Ceftriaxone | |||||
|
| |||||
| Cefuroxime | Cinnamomum verum (cinnamon) | E. coli | 2 | I | [327] |
| Lavandula angustifolia (lavender) | 0.53 | A | |||
| Melaleuca alternifolia (tea tree) | 1.5 | I | |||
| Mentha piperita (peppermint) | 0.56 | A | |||
| Origanum majorana (marjoram) | 0.63 | ||||
|
| |||||
| Chlorhexidine | Cinnamomum burmannii (cinnamon) | S. epidermidis (clinical isolate) | 0.3 | S | [211] |
| S. epidermidis (clinical isolate 64) | 0.35 | ||||
| S. epidermidis (clinical isolate) | 0.3 | ||||
| S. epidermidis strains (ATCC 35984) | 0.15 | ||||
| S. epidermidis (ATCC 12228) | 0.45 | ||||
|
| |||||
| Chlorhexidine digluconate | Eucalyptus globulus (eucalyptus) | S. epidermidis (RP62A) | 2 | I | [213] |
| S. epidermidis (clinical isolate) | |||||
| Melaleuca alternifolia (tea tree) | S. epidermidis (RP62A) | ||||
| Melaleuca alternifolia (tea tree) | S. epidermidis (clinical isolate TK1) | ||||
|
| |||||
| Ciprofloxacin | Melaleuca alternifolia (tea tree) | S. aureus | 1.58–7.70 | I-An | [304] |
| Mentha piperita (peppermint) | 0.75–1.40 | A-I | |||
| Pelargonium graveolens (geranium) | S. aureus (ST2) | 0.38 | S | [330] | |
| Rosmarinus officinalis (rosemary) | S. aureus | 1.03–1.30 | I | [304] | |
| Thymus broussonetii (thyme) | E. coli | 0.37 | S | [329] | |
| P. aeruginosa | 0.14 | ||||
| S. aureus | 0.5 | ||||
| Thymus maroccanus (thyme) | E. coli | 0.12 | S | [329] | |
| P. aeruginosa | 0.15 | ||||
| S. aureus | 0.26 | ||||
| Thymus vulgaris (thyme) | S. aureus | 0.80–2.59 | A-I | [304] | |
| Elettaria cardamomum (cardamom) | S. aureus (ATCC 25923) | 0.62–1.12 | A-I | [325] | |
| MRSA (clinical isolate) | 1.01–2 | I | |||
| 1.01–1.50 | |||||
| Salvia officinalis (sage) | E. coli (ATCC 25922) | 1.03 | I | [176] | |
|
| |||||
| Doxycycline | Origanum vulgare (oregano) | E. coli | 0.38 | S | [324] |
| Erythromycin | Mentha piperita (peppermint) | E. coli | 1 | A | [328] |
|
| |||||
| Florfenicol | Origanum vulgare (oregano) | E. coli | 0.38 | S | [324] |
|
| |||||
| Fluconazole | Ocimum sanctum (holy basil) | C. albicans (29 clinical isolates) | 0.24–0.50 | S | [255] |
| C. albicans (3 clinical isolates) | 0.63–0.93 | A | |||
| C. albicans (ATCC 90028) | 0.48 | S | [255] | ||
| C. albicans (ATCC 10261) | 0.47 | ||||
| C. albicans (ATCC 44829) | 0.48 | ||||
| Thymus broussonetii (thyme) | C. albicans (CCMM L4) | 0.3 | S | [259] | |
| Thymus maroccanus (thyme) | 0.27 | ||||
| Thymus vulgaris (thyme) | T. rubrum (clinical isolate) | 0.25 | [271] | ||
|
| |||||
| Gentamicin | Cinnamomum burmannii (cinnamon) | S. epidermidis (clinical isolate 46) | 1.5 | I | [211] |
| S. epidermidis (clinical isolate 64) | 0.23 | S | |||
| S. epidermidis (clinical isolate 236) | 0.15 | ||||
| S. epidermidis (ATCC 35984) | 1.1 | I | |||
| S. epidermidis (ATCC 12228) | 1.2 | ||||
| Melaleuca alternifolia (tea tree) | MRSA (2 clinical isolates) | n.m. | I | [144] | |
| Mentha piperita (peppermint) | E. coli | 1.25 | [328] | ||
| Thymus broussonetii (thyme) | E. coli | 0.37 | S | [329] | |
| P. aeruginosa | 0.28 | ||||
| S. aureus | 0.5 | ||||
| Thymus maroccanus (thyme) | E. coli | 0.28 | S | [329] | |
| P. aeruginosa | 0.18 | ||||
| S. aureus | 0.5 | ||||
|
| |||||
| Kanamycin | Origanum vulgare (oregano) | E. coli | 1.5 | I | [324] |
|
| |||||
| Ketoconazole | Pelargonium graveolens (geranium) | T. erinacei (KCCM 60411) | 0.56 | A | [264] |
| T. schoenleinii (KCCM 60477) | 0.31 | S | [264] | ||
| T. soudanense (KCCM 60448) | 0.18 | ||||
| Ocimum sanctum (holy basil) | C. albicans (26 clinical isolates) | 0.25–0.50 | S | [255] | |
| C. albicans (6 clinical isolates) | 0.52–0.71 | A | |||
| C. albicans (ATCC 90028) | 0.42 | S | [255] | ||
| C. albicans (ATCC 10261) | 0.41 | ||||
| C. albicans (ATCC 44829) | 0.5 | ||||
| Thymus magnus (thyme) | T. rubrum (ATCC 6345) | 0.37 | S | [260] | |
| Thymus quinquecostatus (thyme) | 0.35 | ||||
|
| |||||
| Levofloxacin | Origanum vulgare (oregano) | E. coli | 0.5 | S | [324] |
| Lincomycin | 0.75 | A | |||
|
| |||||
| Lysostaphin | Melaleuca alternifolia (tea tree) | MRSA (2 clinical isolates) | n.m. | I | [144] |
| Maquindox | Origanum vulgare (oregano) | E. coli | 0.5 | S | [324] |
|
| |||||
| Meropenem | Cinnamomum verum (cinnamon) | E. coli (clinical isolate) | 0.75 | A | [327] |
| Lavandula angustifolia (lavender) | 1.5 | I | |||
| Melaleuca alternifolia (tea tree) | 1 | A | |||
| Mentha piperita (peppermint) | 0.26 | S | |||
| Origanum majorana (marjoram) | 1 | A | |||
|
| |||||
| Mupirocin | Melaleuca alternifolia (tea tree) | MRSA (2 clinical isolates) | n.m. | I | [144] |
|
| |||||
| Norfloxacin | Pelargonium graveolens (geranium) | E. coli (ATCC 35218) | 0.57 | A | [157] |
| S. aureus (ATCC 6538) | 0.37 | S | [157] | ||
| S. aureus (ATCC 29213) | 0.38 | ||||
|
| |||||
| Nystatin | Melaleuca alternifolia (tea tree) | C. albicans (ATCC 14053) | >0.5 | A | [61] |
| Origanum vulgare (oregano) | C. albicans (ATCC 14053) | 0.04 | S | [61] | |
| 0.04–0.35 | |||||
| Pelargonium graveolens (geranium) | 0.01–0.06 | ||||
|
| |||||
| Oxytetracycline | Mentha piperita (peppermint) | E. coli | 0.5 | S | [328] |
|
| |||||
| Piperacillin | Cinnamomum verum (cinnamon) | E. coli (J53 R1) | 0.5 | S | [327] |
| Lavandula angustifolia (lavender) | 0.26 | ||||
| Melaleuca alternifolia (tea tree) | E. coli (J53 R1) | 0.56 | A | [327] | |
| Mentha piperita (peppermint) | 0.31 | S | |||
| Origanum majorana (marjoram) | 0.75 | A | |||
|
| |||||
| Polymycin | Origanum vulgare (oregano) | E. coli | 0.75 | A | [324] |
|
| |||||
| Pristinamycin | Thymus broussonetii (thyme) | E. coli | 0.37 | S | [329] |
| P. aeruginosa | 0.75 | A | |||
| S. aureus | 0.5 | S | [329] |
||
| E. coli | |||||
| P. aeruginosa | 0.75 | A | [329] | ||
| S. aureus | 0.62 | ||||
|
| |||||
| Sarafloxacin | Origanum vulgare (oregano) | E. coli | 0.38 | S | [324] |
|
| |||||
| Tobramycin | Melaleuca alternifolia (tea tree) | E. coli (ATCC 25922) | 0.37 | S | [97] |
| S. aureus (ATCC 29213) | 0.62 | A | |||
|
| |||||
| Triclosan | Cinnamomum burmannii (cinnamon) | S. epidermidis (clinical isolate 46) | 1.2 | I | [211] |
| S. epidermidis (clinical isolate 64) | 1.5 | ||||
| S. epidermidis (clinical isolate 236) | 1.2 | ||||
| S. epidermidis (ATCC 35984) | 0.03 | S | [211] | ||
| S. epidermidis (ATCC 12228) | 0.06 | ||||
|
| |||||
| Vancomycin | Melaleuca alternifolia (tea tree) | MRSA (2 clinical isolates) | >0.50 | A | [144] |
an.m.: not mentioned.
bS: synergy; A: additive; I: indifference; An: antagonism.
Four community-associated methicillin-resistant S. aureus (CA-MRSA) isolates were used to compare benzethonium chloride 0.2% with M. alternifolia and T. vulgaris combination with conventional antimicrobials (neomycin with polymyxin B sulphate and polymyxin B sulphate with gramicidin). The essential oil-antibiotic combination was found to be more effective than conventional medicines on their own [348]. In another study, however, where M. piperita, M. alternifolia, T. vulgaris, and R. officinalis were each individually combined with amphotericin B against C. albicans, antagonism was observed [304], indicating that there may still be risks present when combining essential oils with antimicrobials. Cinnamomum cassia showed potentiation of amphotericin B activity against C. albicans. The increased activity was attributed to the essential oil because synergy increased with an increase in essential oil concentration; however, antagonism was observed for combinations with a lower concentration of essential oil [238].
Although there have been some studies in vitro on essential oil combinations with antibiotics and antifungals, little attention has been paid to in vivo studies or clinical trials. Syed et al. [285] tested a 2% butenafine hydrochloride combination with a 5% M. alternifolia oil cream in a clinical trial, consisting of 60 patients, treating toenail onychomycosis. The control group showed an 80% cure rate compared to 0% by the placebo group containing M. alternifolia alone, allowing the study to conclude clinical effectiveness of butenafine hydrochloride and M. alternifolia in combination. However, in order to determine whether the same could be said for butenafine, a control group should have also been allowed for this product to allow for comparison.
6. Antiviral Studies
Viral infections are a worldwide threat, firstly due to the lack of effective treatments available and secondly due to resistance [333]. Essential oils are a potential source for novel medicines in this regard [30]. Certain essential oils have previously displayed antiviral activity [30, 334], with the best viral inhibitors specifically acting on the steps involved in viral biosynthesis. These work by inhibiting viral replication, thereby limiting viral progeny production [30]. It is advantageous that the viral replication cycle consists of a complex sequence of different steps because it increases the chance of interference from antiviral agents [30].
Less than half of the essential oils recommended for skin infections have been studied for antiviral activity. Table 11 records the readily available studies. The most studied virus is the herpes simplex virus (HSV) and the most studied essential oil is M. alternifolia.
Table 11.
Essential oil studies showing efficacy against viral pathogens associated with skin infections.
| Essential oil | Typea | CC50b | IC50c | SId | [EO]e | Reference |
|---|---|---|---|---|---|---|
| Citrus limon (lemon) | HSV-1 | n.d. | n.m. | n.m. | 1.00% | [331] |
| Cupressus sempervirens (cypress) | >1.00% | |||||
| Cupressus sempervirens ssp. pyramidalis (cypress) | >1000.00 µg/mL | >1000.00 µg/mL | >1 | n.a. | [332] | |
| Cymbopogon citratus (lemongrass) | n.d. | n.m. | n.m. | 0.10% | [331] | |
| Eucalyptus caesia (eucalyptus) | 0.2540% | 0.01% | 38.81 | n.a. | [333] | |
|
| ||||||
| Eucalyptus globulus (eucalyptus) | HSV-1 | n.d. | n.m. | n.m. | 1.00% | [331] |
| HSV-1/HSV-2 | 0.0300% | 0.0090/0.0080% | 38.81 | n.a. | [334] | |
|
| ||||||
| Hyssopus officinalis (hyssop) | HSV-1/HSV-2 | 0.0075% | 0.001/0.0006% | 7.5/13 | n.a. | [335, 336] |
| HSV-1 | 0.0075% | 0.00% | 75 | [337] | ||
|
| ||||||
| Illicium verum (anise) | HSV-1 | 160.00 µg/mL | 40.00 µg/mL | 4 | n.a. | [338] |
| HSV-2 | 0.0160% | 0.00% | 5 | [336] | ||
|
| ||||||
| Juniperus communis (juniper) | HSV-1 | n.d. | n.m. | n.m. | >1.00% | [331] |
| Juniperus oxycedrus ssp. oxycedrus (juniper) | 1000.00 µg/mL | 200.00 µg/mL | 5 | n.a. | [332] | |
| Laurus nobilis (bay) | 500.00 µg/mL | 60.00 µg/mL | 8.3 | |||
| Lavandula latifolia (lavender) | n.d. | n.m. | n.m. | 1.00% | [331] | |
|
| ||||||
| Leptospermum scoparium (manuka) | HSV-1/HSV-2 | 28.80 µg/mL | 0.96 µg/mL/0.58 µg/mL | 30/50 | n.a. | [339] |
| Matricaria recutita (chamomile) | HSV-1 | 30.00 µg/mL | 0.30 µg/mL | 100 | [338] | |
| HSV-2 | 0.0030% | 0.00% | 20 | [336] | ||
|
| ||||||
| Melaleuca alternifolia (tea tree) | HSV-1/HSV-2 | 0.0250% | >0.0250% | >1 | n.a. | [340] |
| HSV-1 | 568.40 µg/mL | 13.20 µg/mL | 44 | [341] | ||
| n.d. | n.m. | n.m. | 1.00% | [331] | ||
| HSV-1/HSV-2 | 0.0060% | 0.0009/0.0008% | 7/7.5 | n.a. | [334] | |
|
| ||||||
| Melissa officinalis (lemon balm) | HSV-1/HSV-2 | 0.0030% | 0.0004%/0.00008% | 7.5/37.5 | n.a. | [342] |
|
| ||||||
| Mentha piperita (peppermint) | HSV-1 | n.d. | n.m. | n.m. | 1.00% | [331] |
| HSV-1/HSV-2 | 0.0140% | 0.0020/0.0008% | 7/17.5 | n.a. | [343] | |
|
| ||||||
| Mentha suaveolens (apple mint) | HSV-1 | 343.60 µg/mL | 5.10 µg/mL | 67 | n.a. | [341] |
| Ocimum basilicum album (basil) | n.d. | n.m. | n.m. | >1.00% | [331] | |
| Origanum majorana (marjoram) | 1.00% | |||||
|
| ||||||
| Pinus mugo (dwarf pine) | HSV-2 | 0.0160% | 0.00% | 5 | n.a. | [335] |
| HSV-1 | 40.00 µg/mL | 7.00 µg/mL | 6 | [338] | ||
| Pistacia palaestina (terebinth) | 500.00 µg/mL | 500.00 µg/mL | 1 | [332] | ||
|
| ||||||
| Rosmarinus officinalis (rosemary) | HSV-1 | 0.2580% | 0.01% | 46.12 | n.a. | [333] |
| n.d. | n.m. | n.m. | 1.00% | [331] | ||
|
| ||||||
| Salvia officinalis (sage) | HSV-1 | >1000.00 µg/mL | >1000.00 µg/mL | >1 | n.a. | [332] |
| Santalum album (sandalwood) | HSV-1/HSV-2 | 0.0015% | 0.0002/0.0005% | 7.5/3 | [335, 336] | |
| 60.00 µg/mL | 2.005 µg/mL/>60.00 µg/mL | 2.4/>1 | [344] | |||
| HSV-1 | 0.0015% | 0.00% | 7 | [337] | ||
|
| ||||||
| Santolina insularis (santolina) | HSV-1/HSV-2 | 112.00 µg/mL | 0.88/0.70 µg/mL | 127/160 | n.a. | [345] |
|
| ||||||
| Thymus vulgaris (thyme) | HSV-1/HSV-2 | 0.0070% | 0.0010/0.0007% | 10/14 | n.a. | [335, 336] |
| HSV-1 | 0.0070% | 0.00% | 7 | [337] | ||
| Zingiber officinale (ginger) | HSV-1/HSV-2 | 0.0040% | 0.0002/0.0001% | 20/40 | [335, 336] | |
| HSV-1 | 0.0040% | 0.00% | 20 | [337] | ||
aHSV: herpes simplex virus, type 1 or 2.
bCC: cytotoxic concentration.
cIC: inhibitory concentration.
dSelectivity index > 4.
eEssential oil concentration at 100% plaque reduction.
Antiviral studies encompass an extensive process where the cytotoxicity and antiviral activity need to be determined. Antiviral activity is usually tested via the plaque reduction assay on Vero (African green monkey kidney cells) cells infected with the virus. This assay determines the effective concentration inhibiting 50% of virus growth (IC50). The selective indicator or selectivity index is calculated with the equation of CC50/IC50. An essential oil with a SI value greater than four is considered suitable as an antiviral agent [332, 333]. Besides the criteria being made for the SI, no criteria for the IC50 have been made. According to the results reviewed, an IC50 value of less than 0.0010% or 1.00 μg/mL should be considered as noteworthy.
Essential oils recommended in the aromatherapeutic literature, with supporting in vitro evidence, include Citrus limon (lemon), Lavandula latifolia (lavender), M. piperita, Santolina insularis (santolina), M. alternifolia, E. globulus, and S. officinalis. Of these oils, the latter three are not ideally suited for antiviral use against HSV-1, due firstly to the IC50 values being weaker than what is recommended (less than 0.0010% or 1.00 μg/mL) and due to their low selectivity index (below 4) [331, 332, 334, 340, 341]. Essential oils still to be studied according to the literature include C. zeylanicum, C. bergamia, Pelargonium odoratissimum (geranium), and Tagetes minuta (Mexican marigold).
In a small pilot study, consisting of 18 patients undergoing treatment of recurrent herpes labialis, a 6% M. alternifolia oil gel applied five times daily was compared to a placebo gel [349]. Reepithelialisation occurred after nine days for the test group compared to the placebo group where reepithelialisation occurred only after 12.5 days. Millar and Moore [350], undertook a case study of a patient with six reoccurring warts (human papillomavirus) after countless treatments with 12% w/w salicylic acid and lactic acid (4% w/w) for several weeks. Alternative treatment consisted of 100% topical M. alternifolia oil applied each evening straight after bathing and prior to bedtime. After five days, a significant reduction in wart size was observed, and, after an additional seven days, all warts were cleared, with complete reepithelialisation of the infected areas and no recurrence. The main shortfall of the two studies is the small sample size. It should also be recommended that any trial involving viral pathogens include a one-, two-, and six-month follow-up after the discontinuation of treatment, the reason being due to the tendency of viral pathogens remaining dormant for an extended period. It can then be observed how effective the essential oil is for long-term effects.
The nonenveloped (such as HPV) viruses have thus far been shown to be more resilient to essential oils [30] compared to the enveloped viruses (HSV) which are more susceptible to essential oils that could dissolve the lipid membrane [5]. Essential oil studies against viruses are clearly lacking. The most studied virus is HSV, which is one of the most prevalent viruses [351], and the most studied essential oil is M. alternifolia. Although numerous studies have proven efficacy of tea tree oil, the problem with a few of the studies is that these were compared to a placebo, which is expected to display poor activity.
Although these studies demonstrate some antiviral activity, other viral pathogens (e.g., varicella zoster, herpes zoster, human papillomavirus, and Molluscum contagiosum) associated with skin infections have clearly been neglected and warrant further study.
7. Essential Oil Toxicity
Plants used for therapeutic purposes are normally assumed to be safe and free of toxicity. This misconception is mainly due to the long-term usage of medicinal plants for the treatment of diseases based on basic knowledge accumulated and shared from generation to generation over many centuries. However, scientific studies and reports have highlighted the toxic effects of essential oils used to treat skin ailments, which are known to produce adverse effects such as allergic contact dermatitis, skin irritation, or photosensitization [300]. Phenols and aldehyde containing oils may often cause irritation [352]. Furanocoumarin containing essential oils (such as C. bergamia) have been proven to induce phototoxicity [353–355]. The evidence based review on botanicals in dermatology by Reuter et al. [18] identifies certain medicinal plants which have been used for dermatological purposes, which have also reported toxic effects. These include C. bergamia and M. recutita. Mentha piperita oil has been reported to cause dermal irritation [356]. Prashar et al. [357] have shown in an in vitro study that L. angustifolia oil and linalool (one of the main compounds) are cytotoxic to human fibroblast and endothelial cells [357]. There have also been a few case reports on L. angustifolia use resulting in contact dermatitis [358–360].
Stonehouse and Studdiford [361] determined that nearly 5% of patients that use M. alternifolia oil will experience allergic contact dermatitis. Centred on a patch test study of 311 volunteers, it was determined that neat 5% tea tree oil can cause irritancy (mean irritancy score of 0.25) [362]. In contrast, however, the study of 217 patients from a dermatology clinic, subjected to a patch test with 10% M. alternifolia oil, showed no irritation [363]. Two additional studies tested the M. alternifolia in patch tests at concentrations of 5% and 10%; 0.15–1.8% of patients experienced allergic contact dermatitis [364, 365]. However, considering that patch tests exaggerate real-world product use [366, 367], they do not necessarily give a good indication of products containing the essential oils. This is evident in the discussed clinical trials using M. alternifolia oil where only mild reactions were observed [189, 200, 219, 281, 282, 285]. Increasing the oil concentration to 25–100%, however, resulted in an increased risk of contact dermatitis in 2–8% of patients [275, 279]. Several additional reports exist reporting contact dermatitis and one systemic hypersensitivity reaction, from the use of M. alternifolia [368–371].
As the prospective use of these essential oils may be for topical application, it is necessary to test toxicity against skin fibroblasts and human skin cell lines F1-73 [115]. Backhousia citriodora oil at a concentration of 1.00% showed low toxicity to human skin cells and skin fibroblasts [115], whereas neat B. citriodora oil and citral were shown to be toxic to human skin cells (F1-73) and skin fibroblasts [115]. Thymus quinquecostatus, when tested against fibroblast cells for cytotoxicity, showed low cytotoxicity at concentrations below 12.5 μg/mL in fibroblast cells and thus may be suitable for topical treatment [207]. Mentha piperita is one of the most popularly used essential oils [372]; however, there have been reports that M. piperita oil can cause both dermal irritations [356]. A review by Reichling et al., containing more information regarding essential oil toxicity, is available [30].
8. Conclusion
Of all the skin pathogens studied, dermatophytes were found to be the most sensitive to essential oil inhibition, followed by the yeast C. albicans and then Gram-positive bacteria (anaerobes more than aerobes), with Gram-negative bacteria being the most resistant, especially P. aeruginosa [168, 181]. The most frequently studied organisms are E. coli, P. aeruginosa, C. albicans, and S. aureus. However, less attention has been paid to pathogens such as S. epidermidis, H. influenzae, S. pyogenes, P. acnes, Clostridium spp., Brevibacterium spp., and the dermatophytes. The reason for this may be due to the difficulty in performing such studies on fastidious pathogens and the lack of a perceived threat. Furthermore, many of these pathogens are slow growing and, combined with the volatile nature of oils, may prove difficult in retaining the oil with the pathogen during the incubation period. Where possible, resistant strains should be included in essential oil studies, along with the reference strain [56, 147]. Antiviral studies should extend to the neglected viruses. These should also report on which part of the cycle the inhibition occurred. The focus should be directed towards the aromatherapeutic recommendation of the essential oil and the responsible pathogens connected to the type of infection, together with the inclusion of the microorganism strain number, the solvent, essential oil composition, and the reason for testing. This is especially relevant for combination studies where it is ill advised to just randomly test different combinations.
Regardless of the frequency of the therapeutic claims made for essential oils and the proven in vitro activity, most evidence of the therapeutic efficacy of aromatherapy has been published in books about aromatherapy and not in peer-reviewed journals. A few clinical trials have emerged, but their results are rarely confirmed completely to substantiate essential oil effectiveness. More rigorous clinical trials would establish confidence from the medical professionals [352].
Besides the antimicrobial activities, toxicity studies are also recommended using skin fibroblasts for sensitivity, as the use is topical. The toxicological effects of essential oils are important facets that need to be addressed. Discernment also needs to be applied as certain sensitivity studies may have been done on rabbit skin; however, human skin has been found to be more sensitive to irritants [115].
Further essential oil combinations need to be studied, along with the reason for the combination selection. Whether the interaction is synergistic, additive, indifferent, or antagonistic, each interaction is a valuable result. If antagonism is not reported, it will not be known to avoid those combinations, which in turn will result in their continuous use, which may eventually lead to resistance to the essential oils themselves. Including synergistic results will allow for these essential oil combinations to be used more frequently in practice. The inclusion of additive and indifferent interactions is also vital in order to report essential oil combinations already studied. This will prevent unnecessary duplication of combination research and confirm essential oil combinations that have useful antimicrobial activity. This research will provide an insight into the understanding of these combinations which could allow for newer directives for integrating essential oils into mainstream medicine. Although essential oil combinations with other essential oils and with antimicrobials have started gaining some attention, there is still a gap in the research with regard to carrier oils. Essential oils are seldom used directly on the skin because direct use onto the skin can cause irritation [26, 38]. Therefore, essential oils are blended with carrier oils before they are applied to the skin. This raises the question as to whether or not the carrier oils influence the overall antimicrobial activity of the essential oils. Gemeda et al. [373] tested the antimicrobial activity of essential oils mixed in different hydrophilic and lipophilic bases. They found better effects in hydrophilic bases than in lipophilic bases. This study confirmed that the base may have an influence on the antimicrobial activity; however, carrier oils in combination have to the best of our knowledge not been studied further.
Essential oils, such as M. alternifolia, are often used in subinhibitory concentrations in commercial products such as shampoos, shower gels, and creams to enhance commercial selling point of a greener product or improve fragrance or desire for the product [202]. This in itself can cause resistance. Therefore, although essential oils are showing promise, the use of essential oils in subinhibitory concentrations in cosmetics and other dermatological formulations may weaken the efficacies of the essential oils as antiseptics, as was shown by Nelson [136]. This highlights the need to insure that there is sufficient evidence supporting aromatherapeutic combinations not only for therapeutics, but also in commercial products.
Resistant strains such as P. aeruginosa, MRSA, and methicillin-resistant S. epidermidis (MRSE) have become extensively problematic microorganisms in the recent years due to their antimicrobial resistance [158], and, as such, including these organisms in screening studies is becoming more and more important.
For viral studies, one needs to consider that genuine antiviral potential is seen for those essential oils that display activity after absorption into the host cell's nucleus because this is where viral DNA replicates by using viral DNA polymerase [30].
Clinical trial and ex vivo studies should consider regular essential oil dosing, instead of once daily, or every several days, application. According to the aromatherapeutic literature, essential oils are generally applied two to three times a day. The reason may be due to the volatile nature resulting in essential oil evaporation. Thus, in order to give credit to essential oil use, application studies should consider timed dosages.
Finally, M. alternifolia is the most studied of all commercial essential oils. However, many other oils have shown better antimicrobial activity. It is time essential oil researchers give just as much attention to oils such as C. zeylanicum, L. scoparium, O. vulgare, S. album, and S. aromaticum in the hope of increasing the global knowledge of essential oils used on the skin.
Acknowledgments
The authors are thankful to the bursary funding from NRF.
Competing Interests
The authors declare no competing interests regarding the publication of this paper.
References
- 1.Clarke S. Essential Chemistry for Aromatherapy. London, UK: Churchill Livingstone; 2008. [Google Scholar]
- 2.Farrer-Halls G. The Aromatherapy Bible: The Definitive Guide to Using Essential Oils. London, UK: Bounty Books; 2011. [Google Scholar]
- 3.Dixon B. Cheese, toes, and mosquitoes. British Medical Journal. 1996;312(7038):p. 1105. doi: 10.1136/bmj.312.7038.1105. [DOI] [Google Scholar]
- 4.Mims C. A., Playfair J., Roitt I., Wakelin D., Williams R. Medical Microbiology. Detroit, Mich, USA: Mosby; 1998. [Google Scholar]
- 5.Bannister B. A., Begg N. T., Gillepsie S. H. Infectious Disease. New York, NY, USA: Blackwell Science; 2000. [Google Scholar]
- 6.Wilson M. Microbial Inhabitants of Humans, their Ecology and Role in Health and Disease. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- 7.Cevasco N. C., Tomecki K. J. Common skin infections. Disease Management Project 2012, 2013, http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/dermatology/common-skin-infections.
- 8.Dryden M. S. Complicated skin and soft tissue infection. Journal of Antimicrobial Chemotherapy. 2010;65(supplement 3):iii35–iii44. doi: 10.1093/jac/dkq302. [DOI] [PubMed] [Google Scholar]
- 9.Davis C. P. Normal flora. In: Baron S., editor. Medical Microbiology. chapter 6. Galveston, Tex, USA: University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- 10.Stulberg D. L., Penrod M. A., Blatny R. A. Common bacterial skin infections. American Family Physician. 2002;66(1):119–124. [PubMed] [Google Scholar]
- 11.Hemaiswarya S., Kruthiventi A. K., Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15(8):639–652. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood D., Slack R., Peutherer J., Barer M. Medical Microbiology, A Guide to Microbial Infections: Pathogenesis, Immunity, Laboratory Diagnosis and Control. Philadelphia, Pa, USA: Churchill Livingstone; 2007. [Google Scholar]
- 13.Millikan L. E. Complementary medicine in dermatology. Clinics in Dermatology. 2002;20(5):602–605. doi: 10.1016/S0738-081X(02)00263-8. [DOI] [PubMed] [Google Scholar]
- 14.Ranzato E., Martinotti S., Burlando B. Wound healing properties of jojoba liquid wax: an in vitro study. Journal of Ethnopharmacology. 2011;134(2):443–449. doi: 10.1016/j.jep.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Iossifova T., Kujumgiev A., Ignatova A., Vassileva E., Kostova I. Antimicrobial effects of some hydroxycoumarins and secoiridoids from Fraxinus ornus bark. Pharmazie. 1994;49(4):298–299. [PubMed] [Google Scholar]
- 16.Halcón L., Milkus K. Staphylococcus aureus and wounds: a review of tea tree oil as a promising antimicrobial. American Journal of Infection Control. 2004;32(7):402–408. doi: 10.1016/j.ajic.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Menke N. B., Ward K. R., Witten T. M., Bonchev D. G., Diegelmann R. F. Impaired wound healing. Clinics in Dermatology. 2007;25(1):19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Reuter J., Merfort I., Schempp C. M. Botanicals in dermatology: an evidence-based review. American Journal of Clinical Dermatology. 2010;11(4):247–267. doi: 10.2165/11533220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Walsh F. Golden Age' of Antibiotics ‘Set to End’. BBC News Website; 2014. [Google Scholar]
- 20.Udwadia Z. F., Amale R. A., Ajbani K. K., Rodrigues C. Totally drug-resistant tuberculosis in India. Clinical Infectious Diseases. 2012;54(4):579–581. doi: 10.1093/cid/cir889. [DOI] [PubMed] [Google Scholar]
- 21.Dryden M. S., Andrasevic A. T., Bassetti M., et al. A European survey of antibiotic management of methicillin-resistant Staphylococcus aureus infection: current clinical opinion and practice. Clinical Microbiology and Infection. 2010;16(1):3–30. doi: 10.1111/j.1469-0691.2010.03135.x. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher J. Analysis: Antibiotic Apocalypse. BBC News; 2013. [Google Scholar]
- 23.Bodeker G. C., Ryan T. J., Ong C.-K. Traditional approaches to wound healing. Clinics in Dermatology. 1999;17(1):93–98. doi: 10.1016/S0738-081X(98)00056-X. [DOI] [PubMed] [Google Scholar]
- 24.Nakatsu T., Lupo A. T., Jr., Chinn J. W., Jr., Kang R. K. L. Biological activity of essential oils and their constituents. Studies in Natural Products Chemistry. 2000;21:571–631. doi: 10.1016/S1572-5995(00)80014-9. [DOI] [Google Scholar]
- 25.Buckle J. Clinical Aromatherapy: Essential Oils in Practice. New York, NY, USA: Churchill Livingston; 2003. [Google Scholar]
- 26.Evans M. Natural Healing: Remedies & Therapies. London, UK: Hermes House; 2010. [Google Scholar]
- 27.Lee M. S., Choi J., Posadzki P., Ernst E. Aromatherapy for health care: an overview of systematic reviews. Maturitas. 2012;71(3):257–260. doi: 10.1016/j.maturitas.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Neuwinger H. D. African Traditional Medicine—A Dictionary of Plant Use and Applications. Stuttgart, Germany: Medpharm; 2000. [Google Scholar]
- 29.Bajpai V. K., Yoon J. I., Kang S. C. Antifungal potential of essential oil and various organic extracts of Nandina domestica Thunb. against skin infectious fungal pathogens. Applied Microbiology and Biotechnology. 2009;83(6):1127–1133. doi: 10.1007/s00253-009-2017-5. [DOI] [PubMed] [Google Scholar]
- 30.Reichling J., Schnitzler P., Suschke U., Saller R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties-an overview. Research in Complementary Medicine. 2009;16(2):79–90. doi: 10.1159/000207196. [DOI] [PubMed] [Google Scholar]
- 31.More evidence is needed to confirm the benefits and risks of many botanicals in dermatology. Drugs & Therapy Perspectives. 2011;27(7):24–26. doi: 10.2165/11206640-000000000-00000. [DOI] [Google Scholar]
- 32.Lawless J. The Illustrated Encyclopedia of Essential Oils: The Complete Guide to the Use of Oils in Aromatherapy and Herbalism. Rockport, Mass, USA: Element Books; 1995. [Google Scholar]
- 33.Lis-Balchin M. Essential oils and ‘aromatherapy’: their modern role in healing. The Journal of the Royal Society for the Promotion of Health. 1997;117(5):324–329. doi: 10.1177/146642409711700511. [DOI] [PubMed] [Google Scholar]
- 34.Stevensen C. J. Aromatherapy in dermatology. Clinics in Dermatology. 1998;16(6):689–694. doi: 10.1016/S0738-081X(98)00058-3. [DOI] [PubMed] [Google Scholar]
- 35.Maddocks-Jennings W., Wilkinson J. M. Aromatherapy practice in nursing: literature review. Journal of Advanced Nursing. 2004;48(1):93–103. doi: 10.1111/j.1365-2648.2004.03172.x. [DOI] [PubMed] [Google Scholar]
- 36.Sellar W. The Directory of Essential Oils. London, UK: C. W. Daniel Company; 1992. [Google Scholar]
- 37.Curtis S. Essential Oils. London, UK: Aurum Press; 1996. [Google Scholar]
- 38.Harding J. A Guide to Essential Oils. Bath, UK: Parragon; 2002. [Google Scholar]
- 39.Ark Creative. Just Aromatherapy. Valencia, Calif, USA: Top That! Publishing; 2005. [Google Scholar]
- 40.Harding J. The Essential Oils Handbook. London, UK: Duncan Baird; 2008. [Google Scholar]
- 41.Kovac M. A Quick Guide to Essential Oils. Ljubljana, Slovenia: Aromadelavnice s.p.; 2011. [Google Scholar]
- 42.Meadowbank. Ailments leaflet-find an essential oil for your ailment, 2012.
- 43.Burgess. Burgess and Finch Aromatherapy: Patient Leaflet. 2013. [Google Scholar]
- 44.Viyoch J., Pisutthanan N., Faikreua A., Nupangta K., Wangtorpol K., Ngokkuen J. Evaluation of in vitro antimicrobial activity of Thai basil oils and their micro-emulsion formulas against Propionibacterium acnes. International Journal of Cosmetic Science. 2006;28(2):125–133. doi: 10.1111/j.1467-2494.2006.00308.x. [DOI] [PubMed] [Google Scholar]
- 45.Gonçalves M. J., Vicente A. M., Cavaleiro C., Salgueiro L. Composition and antifungal acticvity of the essential oil of Mentha cervia from Portugal. Natural Product Research. 2007;21(10):867–871. doi: 10.1080/14786410701482244. [DOI] [PubMed] [Google Scholar]
- 46.Deriu A., Zanetti S., Sechi L. A., et al. Antimicrobial activity of Inula helenium L. essential oil against Gram-positive and Gram-negative bacteria and Candida spp. International Journal of Antimicrobial Agents. 2008;31(6):588–590. doi: 10.1016/j.ijantimicag.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Kirmizibekmez H., Demirci B., Yeşilada E., Başer K. H. C., Demirci F. Chemical composition and antimicrobial activity of the essential oils of Lavandula stoechas L. ssp. stoechas growing wild in Turkey. Natural Product Communications. 2009;4(7):1001–1006. [PubMed] [Google Scholar]
- 48.Nguyen H., Campi E. M., Jackson W. R., Patti A. F. Effect of oxidative deterioration on flavour and aroma components of lemon oil. Food Chemistry. 2009;112(2):388–393. doi: 10.1016/j.foodchem.2008.05.090. [DOI] [Google Scholar]
- 49.Turek C., Stintzing F. C. Impact of different storage conditions on the quality of selected essential oils. Food Research International. 2012;46(1):341–353. doi: 10.1016/j.foodres.2011.12.028. [DOI] [Google Scholar]
- 50.Riahi L., Elferchichi M., Ghazghazi H., et al. Phytochemistry, antioxidant and antimicrobial activities of the essential oils of Mentha rotundifolia L. in Tunisia. Industrial Crops and Products. 2013;49:883–889. doi: 10.1016/j.indcrop.2013.06.032. [DOI] [Google Scholar]
- 51.Calo J. R., Crandall P. G., O'Bryan C. A., Ricke S. C. Essential oils as antimicrobials in food systems—a review. Food Control. 2015;54:111–119. doi: 10.1016/j.foodcont.2014.12.040. [DOI] [Google Scholar]
- 52.Lockwood G. B. Techniques for gas chromatography of volatile terpenoids from a range of matrices. Journal of Chromatography A. 2001;936(1-2):23–31. doi: 10.1016/S0021-9673(01)01151-7. [DOI] [PubMed] [Google Scholar]
- 53.Lahlou M. Methods to study the phytochemistry and bioactivity of essential oils. Phytotherapy Research. 2004;18(6):435–448. doi: 10.1002/ptr.1465. [DOI] [PubMed] [Google Scholar]
- 54.van Vuuren S. F. The antimicrobial activity and essential oil composition of medicinal aromatic plants used in African traditional healing [Ph.D. thesis] Johannesburg, South Africa: University of Witwatersrand; 2007. [Google Scholar]
- 55.Elaissi A., Rouis Z., Mabrouk S., et al. Correlation between chemical composition and antibacterial activity of essential oils from fifteen Eucalyptus species growing in the Korbous and Jbel Abderrahman arboreta (North East Tunisia) Molecules. 2012;17(3):3044–3057. doi: 10.3390/molecules17033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Vuuren S. F. Antimicrobial activity of South African medicinal plants. Journal of Ethnopharmacology. 2008;119(3):462–472. doi: 10.1016/j.jep.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 57.Janssen A. M., Scheffer J. J. C., Svendsen A. B. Antimicrobial activity of essential oils: a 1976–1986 literature review. Aspects of the test methods. Planta Medica. 1987;53(5):395–398. doi: 10.1055/s-2006-962755. [DOI] [PubMed] [Google Scholar]
- 58.Rios J. L., Recio M. C., Villar A. Screening methods for natural products with antimicrobial activity: a review of the literature. Journal of Ethnopharmacology. 1988;23(2-3):127–149. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 59.Pauli A., Schilcher H. In Vitro antimicrobial activities of essential oils monographed in the European Pharmacopoeia. In: Baser K. H. C., Buchbauer G., editors. Handbook of Essential Oils: Science, Technology, and Applications. Boca Raton, Fla, USA: CRC Press, Taylor & Francis Group; 2010. pp. 353–547. [Google Scholar]
- 60.Xiangwei Z., Xiaodong W., Peng N., Yang Z., Jiakuan C. Chemical composition and antimicrobial activity of the essential oil of Sagittaria trifolia. Chemistry of Natural Compounds. 2006;42(5):520–522. doi: 10.1007/s10600-006-0203-6. [DOI] [Google Scholar]
- 61.Rota M. C., Herrera A., Martínez R. M., Sotomayor J. A., Jordán M. J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control. 2008;19(7):681–687. doi: 10.1016/j.foodcont.2007.07.007. [DOI] [Google Scholar]
- 62.Lv F., Liang H., Yuan Q., Li C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Research International. 2011;44(9):3057–3064. doi: 10.1016/j.foodres.2011.07.030. [DOI] [Google Scholar]
- 63.Saïdana D., Mahjoub M. A., Boussaada O., et al. Chemical composition and antimicrobial activity of volatile compounds of Tamarix boveana (Tamaricaceae) Microbiological Research. 2008;163(4):445–455. doi: 10.1016/j.micres.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 64.Pauli A., Kubeczka K. H. Evaluation of inhibitory data of essential oil constituents obtained with different microbiological testing methods. In: Franz C. H., Mathe A., Buchbauer G., editors. Essential Oils: Basic and Applied Research. Carol Stream, Ill, USA: Allured Publishing Corporation; 1997. pp. 33–36. [Google Scholar]
- 65.Kalemba D., Kunicka A. Antibacterial and antifungal properties of essential oils. Current Medicinal Chemistry. 2003;10(10):813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 66.Ríos J. L., Recio M. C. Medicinal plants and antimicrobial activity. Journal of Ethnopharmacology. 2005;100(1-2):80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 67.Cos P., Vlietinck A. J., Berghe D. V., Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. Journal of Ethnopharmacology. 2006;106(3):290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Lang G., Buchbauer G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour and Fragrance Journal. 2012;27(1):13–39. doi: 10.1002/ffj.2082. [DOI] [Google Scholar]
- 69.Lambert R. J. W., Pearson J. Susceptibility testing: accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. Journal of Applied Microbiology. 2000;88(5):784–790. doi: 10.1046/j.1365-2672.2000.01017.x. [DOI] [PubMed] [Google Scholar]
- 70.Seow Y. X., Yeo C. R., Chung H. L., Yuk H.-G. Plant essential oils as active antimicrobial agents. Critical Reviews in Food Science and Nutrition. 2014;54(5):625–644. doi: 10.1080/10408398.2011.599504. [DOI] [PubMed] [Google Scholar]
- 71.Varela N. P., Friendship R., Dewey C., Valdivieso A. Comparison of Agar Dilution and E-test for antimicrobial susceptibility testing of Campylobacter coli isolates recovered from 80 Ontario swine farms. The Canadian Journal of Veterinary Research. 2008;72(2):168–174. [PMC free article] [PubMed] [Google Scholar]
- 72.Beale A. S., Sutherland R. Measurement of Combined Antibiotic Action in Antibiotics: Assessment of Antimicrobial Activity and Resistance. London, UK: Academic Press; 1983. [Google Scholar]
- 73.Dufour M., Simmonds R. S., Bremer P. J. Development of a method to quantify in vitro the synergistic activity of ‘natural’ antimicrobials. International Journal of Food Microbiology. 2003;85(3):249–258. doi: 10.1016/s0168-1605(02)00544-5. [DOI] [PubMed] [Google Scholar]
- 74.van Vuuren S. F., Viljoen A. M. Plant-based antimicrobial studies—methods and approaches to study the interaction between natural products. Planta Medica. 2011;77(11):1168–1182. doi: 10.1055/s-0030-1250736. [DOI] [PubMed] [Google Scholar]
- 75.Agarwal V., Lal P., Pruthi V. Effect of plant oils on Candida albicans. Journal of Microbiology, Immunology and Infection. 2010;43(5):447–451. doi: 10.1016/s1684-1182(10)60069-2. [DOI] [PubMed] [Google Scholar]
- 76.Hadad M., Zygadlo J. A., Lima B., et al. Chemical composition and antimicrobial activity of essential oil from Baccharis grisebachii hieron (asteraceae. Journal of the Chilean Chemical Society. 2007;52(2):1186–1189. [Google Scholar]
- 77.Nascimento A. M. A., Brandão M. G. L., Oliveira G. B., Fortes I. C. P., Chartone-Souza E. Synergistic bactericidal activity of Eremanthus erythropappus oil or β-bisabolene with ampicillin against Staphylococcus aureus. Antonie van Leeuwenhoek. 2007;92(1):95–100. doi: 10.1007/s10482-006-9139-x. [DOI] [PubMed] [Google Scholar]
- 78.Teke G. N., Elisée K. N., Roger K. J. Chemical composition, antimicrobial properties and toxicity evaluation of the essential oil of Cupressus lusitanica Mill. leaves from Cameroon. BMC Complementary and Alternative Medicine. 2013;13, article 130 doi: 10.1186/1472-6882-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biju S. S., Ahuja A., Khar R. K., Chaudhry R. Formulation and evaluation of an effective pH balanced topical antimicrobial product containing tea tree oil. Pharmazie. 2005;60(3):208–211. [PubMed] [Google Scholar]
- 80.Prabuseenivasan S., Jayakumar M., Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complementary and Alternative Medicine. 2006;6, article 39 doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tohidpour A., Sattari M., Omidbaigi R., Yadegar A., Nazemi J. Antibacterial effect of essential oils from two medicinal plants against Methicillin-resistant Staphylococcus aureus (MRSA) Phytomedicine. 2010;17(2):142–145. doi: 10.1016/j.phymed.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Tam V. H., Schilling A. N., Nikolaou M. Modelling time-kill studies to discern the pharmacodynamics of meropenem. Journal of Antimicrobial Chemotherapy. 2005;55(5):699–706. doi: 10.1093/jac/dki086. [DOI] [PubMed] [Google Scholar]
- 83.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. International Journal of Food Microbiology. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 84.de Oliveira Pereira F., Wanderley P. A., Viana F. A. C., de Lima R. B., de Sousa F. B., de Oliveira Lima E. Growth inhibition and morphological alterations of Trichophyton rubrum induced by essential oil from Cymbopogon winterianus Jowitt ex Bor. Brazilian Journal of Microbiology. 2011;42(1):233–242. doi: 10.1590/s1517-83822011000100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kon K., Rai M. Antibacterial activity of Thymus vulgaris essential oil alone and in combination with other essential oils. Nusantara Bioscience. 2012;4(2):50–56. doi: 10.13057/nusbiosci/n040202. [DOI] [Google Scholar]
- 86.Cherrat L., Espina L., Bakkali M., García-Gonzalo D., Pagán R., Laglaoui A. Chemical composition and antioxidant properties of Laurus nobilis L. and Myrtus communis L. essential oils from Morocco and evaluation of their antimicrobial activity acting alone or in combined processes for food preservation. Journal of the Science of Food and Agriculture. 2014;94(6):1197–1204. doi: 10.1002/jsfa.6397. [DOI] [PubMed] [Google Scholar]
- 87.Sela F., Karapandzova M., Stefkov G., Cvetkovikj I., Kulevanova S. Chemical composition and antimicrobial activity of essential oils of Juniperus excelsa Bieb. (Cupressaceae) grown in R. Macedonia. Pharmacognosy Research. 2015;7(1):74–80. doi: 10.4103/0974-8490.147212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wayne P. A. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard. CLSI Document. 2008;(M38-A2, vol. 22, no. 16)
- 89.Cuenca-Estrella M., Moore C. B., Barchiesi F., et al. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST) Clinical Microbiology and Infection. 2003;9(6):467–474. doi: 10.1046/j.1469-0691.2003.00592.x. [DOI] [PubMed] [Google Scholar]
- 90.Messager S., Hammer K. A., Carson C. F., Riley T. V. Effectiveness of hand-cleansing formulations containing tea tree oil assessed ex vivo on human skin and in vivo with volunteers using European standard EN 1499. The Journal of Hospital Infection. 2005;59(3):220–228. doi: 10.1016/j.jhin.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 91.Soković M. D., Vukojević J., Marin P. D., Brkić D. D., Vajs V., Van Griensven L. J. L. D. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules. 2009;14(1):238–249. doi: 10.3390/molecules14010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carson C. F., Riley T. V. Susceptibility of Propionibacterium acnes to the essential oil of Melaleuca alternifolia. Letters in Applied Microbiology. 1994;19(1):24–25. doi: 10.1111/j.1472-765x.1994.tb00894.x. [DOI] [Google Scholar]
- 93.Eloff J. N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica. 1998;64(8):711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 94.König C., Simmen H.-P., Blaser J. Reply (to Morrissey and George, 1999) Journal of Antimicrobial Chemotherapy. 1999;43(3):p. 425. doi: 10.1093/jac/43.3.425. [DOI] [Google Scholar]
- 95.Morrissey I., George J. T., Konig C., Simmen H.-P., Blaser J. The effect of the inoculum size on bactericidal activity. Journal of Antimicrobial Chemotherapy. 1999;43(3):423–425. doi: 10.1093/jac/43.3.423. [DOI] [PubMed] [Google Scholar]
- 96.Lambert R. J. W. Susceptibility testing: Inoculum size dependency of inhibition using the Colworth MIC technique. Journal of Applied Microbiology. 2000;89(2):275–279. doi: 10.1046/j.1365-2672.2000.01105.x. [DOI] [PubMed] [Google Scholar]
- 97.D'Arrigo M., Ginestra G., Mandalari G., Furneri P. M., Bisignano G. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine. 2010;17(5):317–322. doi: 10.1016/j.phymed.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 98.de Souza E. L., de Barros J. C., de Oliveira C. E. V., da Conceição M. L. Influence of Origanum vulgare L. essential oil on enterotoxin production, membrane permeability and surface characteristics of Staphylococcus aureus. International Journal of Food Microbiology. 2010;137(2-3):308–311. doi: 10.1016/j.ijfoodmicro.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 99.de Rapper S., Kamatou G., Viljoen A., van Vuuren S. The in vitro antimicrobial activity of Lavandula angustifolia essential oil in combination with other aroma-therapeutic oils. Evidence-Based Complementary and Alternative Medicine. 2013;2013:10. doi: 10.1155/2013/852049.852049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Remmal A., Bouchikhi T., Rhayour K., Ettayebi M., Tantaoui-Elaraki A. Improved method for the determination of antimicrobial activity of essential oils in agar medium. Journal of Essential Oil Research. 1993;5(2):179–184. [Google Scholar]
- 101.Pulido Pérez A., Baniandrés Rodríguez O., Ceballos Rodríguez M. C., Mendoza Cembranos M. D., Campos Domínguez M., Suárez Fernández R. Skin infections caused by community-acquired methicillin-resistant Staphylococcus aureus: clinical and microbiological characteristics of 11 cases. Actas Dermo-Sifiliograficas. 2014;105(2):150–158. doi: 10.1016/j.ad.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 102.Kim S.-S., Baik J. S., Oh T.-H., Yoon W.-J., Lee N. H., Hyun C.-G. Biological activities of Korean Citrus obovoides and Citrus natsudaidai essential oils against acne-inducing bacteria. Bioscience, Biotechnology and Biochemistry. 2008;72(10):2507–2513. doi: 10.1271/bbb.70388. [DOI] [PubMed] [Google Scholar]
- 103.Rennie P. J., Gower D. B., Holland K. T., Mallet A. I., Watkins W. J. The skin microflora and the formation of human axillary odour. International Journal of Cosmetic Science. 1990;12(5):197–207. doi: 10.1111/j.1467-2494.1990.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 104.Barton L. L., Friedman A. D. Impetigo: a reassessment of etiology and therapy. Pediatric Dermatology. 1987;4(3):185–188. doi: 10.1111/j.1525-1470.1987.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 105.Pereira L. B. Impetigo—review. Anais Brasileiros de Dermatologia. 2014;89(2):293–299. doi: 10.1590/abd1806-4841.20142283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stevens D. L., Bisno A. L., Chambers H. F., et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clinical Infectious Diseases. 2005;41(10):1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 107.Miller L. G., Perdreau-Remington F., Rieg G., et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. The New England Journal of Medicine. 2005;352(14):1445–1453. doi: 10.1056/nejmoa042683. [DOI] [PubMed] [Google Scholar]
- 108.Van de Sande W. W. J., Fahal A. H., Riley T. V., Verbrugh H., van Belkum A. In vitro susceptibility of Madurella mycetomatis, prime agent of Madura foot, to tea tree oil and artemisinin. Journal of Antimicrobial Chemotherapy. 2007;59(3):553–555. doi: 10.1093/jac/dkl526. [DOI] [PubMed] [Google Scholar]
- 109.Wananukul S., Chindamporn A., Yumyourn P., Payungporn S., Samathi C., Poovorawan Y. Malassezia furfur in infantile seborrheic dermatitis. Asian Pacific Journal of Allergy and Immunology. 2005;23(2-3):101–105. [PubMed] [Google Scholar]
- 110.Gupta A. K., Nicol K. A. Ciclopirox 1% shampoo for the treatment of seborrheic dermatitis. International Journal of Dermatology. 2006;45(1):66–69. doi: 10.1111/j.1365-4632.2004.02331.x. [DOI] [PubMed] [Google Scholar]
- 111.Lee J.-H., Hong S.-K. Comparative analysis of chemical compositions and antimicrobial activities of Essential oils from Abies holophylla and Abies koreana. Journal of Microbiology and Biotechnology. 2009;19(4):372–377. doi: 10.4014/jmb.0811.630. [DOI] [PubMed] [Google Scholar]
- 112.Candan F., Unlu M., Tepe B., et al. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae) Journal of Ethnopharmacology. 2003;87(2-3):215–220. doi: 10.1016/s0378-8741(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 113.Ünlü M., Daferera D., Dönmez E., Polissiou M., Tepe B., Sökmen A. Compositions and the in vitro antimicrobial activities of the essential oils of Achillea setacea and Achillea teretifolia (Compositae) Journal of Ethnopharmacology. 2002;83(1-2):117–121. doi: 10.1016/s0378-8741(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 114.Hüsnü K. H., Demirci B., Iscan G., et al. The essential oil constituents and antimicrobial activity of Anthemis aciphylla BOISS. var. discoidea BOISS. Chemical and Pharmaceutical Bulletin. 2006;54(2):222–225. doi: 10.1248/cpb.54.222. [DOI] [PubMed] [Google Scholar]
- 115.Hayes A. J., Markovic B. Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food and Chemical Toxicology. 2002;40(4):535–543. doi: 10.1016/s0278-6915(01)00103-x. [DOI] [PubMed] [Google Scholar]
- 116.van Vuuren S. F., Kamatou G. P. P., Viljoen A. M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. South African Journal of Botany. 2010;76(4):686–691. doi: 10.1016/j.sajb.2010.06.001. [DOI] [Google Scholar]
- 117.de Rapper S., van Vuuren S. F., Kamatou G. P. P., Viljoen A. M., Dagne E. The additive and synergistic antimicrobial effects of select frankincense and myrrh oils-a combination from the pharaonic pharmacopoeia. Letters in Applied Microbiology. 2012;54(4):352–358. doi: 10.1111/j.1472-765x.2012.03216.x. [DOI] [PubMed] [Google Scholar]
- 118.Tarek N., Hassan H. M., AbdelGhani S. M., Radwan I., Hammouda O., El-Gendy A. O. Comparative chemical and antimicrobial study of nine essential oils obtained from medicinal plants growing in Egypt. Beni-Suef University Journal of Basic and Applied Sciences. 2014;3(2):149–156. doi: 10.1016/j.bjbas.2014.05.009. [DOI] [Google Scholar]
- 119.Barbosa L. N., da Silva Probst I., Andrade B. F. M. T., et al. In vitro antibacterial and chemical properties of essential oils including native plants from Brazil against pathogenic and resistant bacteria. Journal of Oleo Science. 2015;64(3):289–298. doi: 10.5650/jos.ess14209. [DOI] [PubMed] [Google Scholar]
- 120.Hsouna A. B., Hamdi N., Halima N. B., Abdelkafi S. Characterization of essential oil from Citrus aurantium L. flowers: antimicrobial and antioxidant activities. Journal of Oleo Science. 2013;62(10):763–772. doi: 10.5650/jos.62.763. [DOI] [PubMed] [Google Scholar]
- 121.Yangui T., Bouaziz M., Dhouib A., Sayadi S. Potential use of Tunisian Pituranthos chloranthus essential oils as a natural disinfectant. Letters in Applied Microbiology. 2009;48(1):112–117. doi: 10.1111/j.1472-765x.2008.02499.x. [DOI] [PubMed] [Google Scholar]
- 122.Bag A., Chattopadhyay R. R. Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS ONE. 2015;10(7, article e131321) doi: 10.1371/journal.pone.0131321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chéraif I., Ben Jannet H., Hammami M., Khouja M. L., Mighri Z. Chemical composition and antimicrobial activity of essential oils of Cupressus arizonica Greene. Biochemical Systematics and Ecology. 2007;35(12):813–820. doi: 10.1016/j.bse.2007.05.009. [DOI] [Google Scholar]
- 124.Bassolé I. H. N., Lamien-Meda A., Bayala B., et al. Chemical composition and antimicrobial activity of Cymbopogon citratus and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine. 2011;18(12):1070–1074. doi: 10.1016/j.phymed.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 125.Pattnaik S., Subramanyam V. R., Kole C. R. Antibacterial and antifungal activity of ten essential oils in vitro. Microbios. 1996;86(349):237–246. [PubMed] [Google Scholar]
- 126.Pattnaik S., Subramanyam V. R., Bapaji M., Kole C. R. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios. 1997;89(358):39–46. [PubMed] [Google Scholar]
- 127.Panahi Y., Sattari M., Babaie A. P., et al. The essential oils activity of Eucalyptus polycarpa, E. largiflorence, E. malliodora and E. camaldulensis on Staphylococcus aureus. Iranian Journal of Pharmaceutical Research. 2011;10(1):43–48. [PMC free article] [PubMed] [Google Scholar]
- 128.Hamoud R., Sporer F., Reichling J., Wink M. Antimicrobial activity of a traditionally used complex essential oil distillate (Olbas® Tropfen) in comparison to its individual essential oil ingredients. Phytomedicine. 2012;19(11):969–976. doi: 10.1016/j.phymed.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 129.Patrone V., Campana R., Vittoria E., Baffone W. In vitro synergistic activities of essential oils and surfactants in combination with cosmetic preservatives against Pseudomonas aeruginosa and Staphylococcus aureus. Current Microbiology. 2010;60(4):237–241. doi: 10.1007/s00284-009-9531-7. [DOI] [PubMed] [Google Scholar]
- 130.Mahumane G. D., van Vuuren S. F., Kamatou G., Sandasi M., Viljoen A. M. Chemical composition and antimicrobial activity of Eucalyptus radiata leaf essential oil, sampled over a year. Journal of Essential Oil Research. 2016;28(6):475–488. doi: 10.1080/10412905.2016.1175386. [DOI] [Google Scholar]
- 131.Safaei-Ghomi J., Ahd A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens andEucalyptus intertexta. Pharmacognosy Magazine. 2010;6(23):172–175. doi: 10.4103/0973-1296.66930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Diao W.-R., Hu Q.-P., Zhang H., Xu J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.) Food Control. 2014;35(1):109–116. doi: 10.1016/j.foodcont.2013.06.056. [DOI] [Google Scholar]
- 133.Mota A. S., Rosário Martins M., Lopes V. R., et al. Antimicrobial activity and chemical composition of the essential oils of Portuguese Foeniculum vulgare fruits. Natural Product Communications. 2015;10(4):673–676. [PubMed] [Google Scholar]
- 134.Senatore F., Oliviero F., Scandolera E., et al. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell] Fitoterapia. 2013;90:214–219. doi: 10.1016/j.fitote.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 135.Pepeljnjak S., Kosalec I., Kalodera Z., Blažević N. Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae) Acta Pharmaceutica. 2005;55(4):417–422. [PubMed] [Google Scholar]
- 136.Nelson R. R. S. In-vitro activities of five plant essential oils against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. Journal of Antimicrobial Chemotherapy. 1997;40(2):305–306. doi: 10.1093/jac/40.2.305. [DOI] [PubMed] [Google Scholar]
- 137.Christoph F., Kaulfers P.-M., Stahl-Biskup E. A comparative study of the in vitro antimicrobial activity of tea tree oils s.I. with special reference to the activity of β-triketones. Planta Medica. 2000;66(6):556–560. doi: 10.1055/s-2000-8604. [DOI] [PubMed] [Google Scholar]
- 138.van Vuuren S. F., Docrat Y., Kamatou G. P. P., Viljoen A. M. Essential oil composition and antimicrobial interactions of understudied tea tree species. South African Journal of Botany. 2014;92:7–14. doi: 10.1016/j.sajb.2014.01.005. [DOI] [Google Scholar]
- 139.Hussain A. I., Anwar F., Nigam P. S., et al. Antibacterial activity of some Lamiaceae essential oils using resazurin as an indicator of cell growth. LWT—Food Science and Technology. 2011;44(4):1199–1206. doi: 10.1016/j.lwt.2010.10.005. [DOI] [Google Scholar]
- 140.Van Vuuren S. F., Viljoen A. M. A comparative investigation of the antimicrobial properties of indigenous South African aromatic plants with popular commercially available essential oils. Journal of Essential Oil Research. 2006;18:66–71. [Google Scholar]
- 141.Mothana R. A., Alsaid M. S., Hasoon S. S., Al-Mosaiyb N. M., Al-Rehaily A. J., Al-Yahya M. A. Antimicrobial and antioxidant activities and gas chromatography mass spectrometry (GC/MS) analysis of the essential oils of Ajuga bracteosa Wall. ex Benth. and Lavandula dentata L. growing wild in Yemen. Journal of Medicinal Plants Research. 2012;6(15):3066–3071. [Google Scholar]
- 142.Cherrat L., Espina L., Bakkali M., Pagán R., Laglaoui A. Chemical composition, antioxidant and antimicrobial properties of Mentha pulegium, Lavandula stoechas and Satureja calamintha Scheele essential oils and an evaluation of their bactericidal effect in combined processes. Innovative Food Science and Emerging Technologies. 2014;22:221–229. doi: 10.1016/j.ifset.2013.12.016. [DOI] [Google Scholar]
- 143.Jian-Yu S., Zhu L., Tian Y.-J. Chemical composition and antimicrobial activities of essential: oil of Matricaria songarica. International Journal of Agriculture and Biology. 2012;14(1):107–110. [Google Scholar]
- 144.LaPlante K. L. In vitro activity of lysostaphin, mupirocin, and tea tree oil against clinical methicillin-resistant Staphylococcus aureus. Diagnostic Microbiology and Infectious Disease. 2007;57(4):413–418. doi: 10.1016/j.diagmicrobio.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 145.Low W. L., Martin C., Hill D. J., Kenward M. A. Antimicrobial efficacy of silver ions in combination with tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. International Journal of Antimicrobial Agents. 2011;37(2):162–165. doi: 10.1016/j.ijantimicag.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 146.McMahon M. A. S., Tunney M. M., Moore J. E., Blair I. S., Gilpin D. F., McDowell D. A. Changes in antibiotic susceptibility in staphylococci habituated to sub-lethal concentrations of tea tree oil (Melaleuca alternifolia) Letters in Applied Microbiology. 2008;47(4):263–268. doi: 10.1111/j.1472-765x.2008.02420.x. [DOI] [PubMed] [Google Scholar]
- 147.Carson C. F., Hammer K. A., Riley T. V. Broth micro-dilution method for determining the susceptibility of Escherichia coli and Staphylococcus aureus to the essential oil of Melaleuca alternifolia (tea tree oil) Microbios. 1995;82(332):181–185. [PubMed] [Google Scholar]
- 148.Carson C. F., Cookson B. D., Farrelly H. D., Riley T. V. Susceptibility of methicillin-resistant Staphylococcus aureus to the essential oil of Melaleuca alternifolia. Journal of Antimicrobial Chemotherapy. 1995;35(3):421–424. doi: 10.1093/jac/35.3.421. [DOI] [PubMed] [Google Scholar]
- 149.Cox S. D., Mann C. M., Markham J. L., et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil) Journal of Applied Microbiology. 2000;88(1):170–175. doi: 10.1046/j.1365-2672.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 150.Cox S. D., Mann C. M., Markham J. L. Interactions between components of the essential oil of Melaleuca alternifolia. Journal of Applied Microbiology. 2001;91(3):492–497. doi: 10.1046/j.1365-2672.2001.01406.x. [DOI] [PubMed] [Google Scholar]
- 151.Elsom G. K. F., Hide D. Susceptibility of methicillin-resistant Staphylococcus aureus to tea tree oil and mupirocin. Journal of Antimicrobial Chemotherapy. 1999;43(3):427–428. doi: 10.1093/jac/43.3.427. [DOI] [PubMed] [Google Scholar]
- 152.Hammer K. A., Carson C. F., Riley T. V. Susceptibility of transient and commensal skin flora to the essential oil of Melaleuca alternifolia (tea tree oil) American Journal of Infection Control. 1996;24(3):186–189. doi: 10.1016/s0196-6553(96)90011-5. [DOI] [PubMed] [Google Scholar]
- 153.Falci S. P. P., Teixeira M. A., das Chagas P. F., et al. Antimicrobial activity of Melaleuca sp. oil against clinical isolates of antibiotics resistant Staphylococcus aureus. Acta Cirurgica Brasileira. 2015;30(6):401–406. doi: 10.1590/s0102-865020150060000005. [DOI] [PubMed] [Google Scholar]
- 154.Raman A., Weir U., Bloomfield S. F. Antimicrobial effects of tea tree oil and its major components on Staphylococcus aureus, Staphylococcus epidermidis and Propionibacterium acnes. Letters in Applied Microbiology. 1995;21(4):242–245. doi: 10.1111/j.1472-765x.1995.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 155.Işcan G., Kirimer N., Kürkcüoglu M., Başer K. H. C., Demirci F. Antimicrobial screening of Mentha piperita essential oils. Journal of Agricultural and Food Chemistry. 2002;50(14):3943–3946. doi: 10.1021/jf011476k. [DOI] [PubMed] [Google Scholar]
- 156.Bassolé I. H. N., Lamien-Meda A., Bayala B., et al. Composition and antimicrobial activities of lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules. 2010;15(11):7825–7839. doi: 10.3390/molecules15117825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rosato A., Vitali C., De Laurentis N., Armenise D., Antonietta Milillo M. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine. 2007;14(11):727–732. doi: 10.1016/j.phymed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 158.Opalchenova G., Obreshkova D. Comparative studies on the activity of basil-an essential oil from Ocimum basilicum L.-against multidrug resistant clinical isolates of the genera Staphylococcus, Enterococcus and Pseudomonas by using different test methods. Journal of Microbiological Methods. 2003;54(1):105–110. doi: 10.1016/s0167-7012(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 159.Shirazi M. T., Gholami H., Kavoosi G., Rowshan V., Tafsiry A. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of Tagetes minuta and Ocimum basilicum essential oils. Food Science and Nutrition. 2014;2(2):146–155. doi: 10.1002/fsn3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Beatović D., Krstić-Milošević D., Trifunović S., et al. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Records of Natural Products. 2015;9(1):62–75. [Google Scholar]
- 161.Sökmen M., Serkedjieva J., Daferera D., et al. In vitro antioxidant, antimicrobial, and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum acutidens. Journal of Agricultural and Food Chemistry. 2004;52(11):3309–3312. doi: 10.1021/jf049859g. [DOI] [PubMed] [Google Scholar]
- 162.Aligiannis N., Kalpoutzakis E., Mitaku S., Chinou I. B. Composition and antimicrobial activity of the essential oils of two Origanum species. Journal of Agricultural and Food Chemistry. 2001;49(9):4168–4170. doi: 10.1021/jf001494m. [DOI] [PubMed] [Google Scholar]
- 163.Nostro A., Blanco A. R., Cannatelli M. A., et al. Susceptibility of methicillin-resistant Staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiology Letters. 2004;230(2):191–195. doi: 10.1016/s0378-1097(03)00890-5. [DOI] [PubMed] [Google Scholar]
- 164.Lambert R. J. W., Skandamis P. N., Coote P. J., Nychas G.-J. E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. Journal of Applied Microbiology. 2001;91(3):453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- 165.Sarikurkcu C., Zengin G., Oskay M., Uysal S., Ceylan R., Aktumsek A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Industrial Crops and Products. 2015;70:178–184. doi: 10.1016/j.indcrop.2015.03.030. [DOI] [Google Scholar]
- 166.Sienkiewicz M., Poznańska-Kurowska K., Kaszuba A., Kowalczyk E. The antibacterial activity of geranium oil against Gram-negative bacteria isolated from difficult-to-heal wounds. Burns. 2014;40(5):1046–1051. doi: 10.1016/j.burns.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 167.Mahboubi M., Kazempour N. The antimicrobial activity of essential oil from Perovskia abrotanoides karel and its main components. Indian Journal of Pharmaceutical Sciences. 2009;71(3):343–347. doi: 10.4103/0250-474x.56016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Al-Bayati F. A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. Journal of Ethnopharmacology. 2008;116(3):403–406. doi: 10.1016/j.jep.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 169.Fu Y., Zu Y., Chen L., et al. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytotherapy Research. 2007;21(10):989–994. doi: 10.1002/ptr.2179. [DOI] [PubMed] [Google Scholar]
- 170.Jiang Y., Wu N., Fu Y.-J., et al. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environmental Toxicology and Pharmacology. 2011;32(1):63–68. doi: 10.1016/j.etap.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 171.Luqman S., Dwivedi G. R., Darokar M. P., Kalra A., Khanuja S. P. S. Potential of Rosemary oil to be used in drug-resistant infections. Alternative Therapies in Health and Medicine. 2007;13(5):54–59. [PubMed] [Google Scholar]
- 172.Okoh O. O., Sadimenko A. P., Afolayan A. J. Comparative evaluation of the antibacterial activities of the essential oils of Rosmarinus officinalis L. obtained by hydrodistillation and solvent free microwave extraction methods. Food Chemistry. 2010;120(1):308–312. doi: 10.1016/j.foodchem.2009.09.084. [DOI] [Google Scholar]
- 173.Cardile V., Russo A., Formisano C., et al. Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. Journal of Ethnopharmacology. 2009;126(2):265–272. doi: 10.1016/j.jep.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 174.Ebrahimabadi A. H., Mazoochi A., Kashi F. J., Djafari-Bidgoli Z., Batooli H. Essential oil composition and antioxidant and antimicrobial properties of the aerial parts of Salvia eremophila Boiss. from Iran. Food and Chemical Toxicology. 2010;48(5):1371–1376. doi: 10.1016/j.fct.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 175.Vagionas K., Ngassapa O., Runyoro D., Graikou K., Gortzi O., Chinou I. Chemical analysis of edible aromatic plants growing in Tanzania. Food Chemistry. 2007;105(4):1711–1717. doi: 10.1016/j.foodchem.2007.05.029. [DOI] [Google Scholar]
- 176.Adrar N., Oukil N., Bedjou F. Antioxidant and antibacterial activities of Thymus numidicus and Salvia officinalis essential oils alone or in combination. Industrial Crops and Products. 2016;88:112–119. doi: 10.1016/j.indcrop.2015.12.007. [DOI] [Google Scholar]
- 177.Tzakou O., Pitarokili D., Chinou I. B., Harvala C. Composition and antimicrobial activity of the essential oil of Salvia ringens. Planta Medica. 2001;67(1):81–83. doi: 10.1055/s-2001-10627. [DOI] [PubMed] [Google Scholar]
- 178.Özek G., Demirci F., Özek T., et al. Gas chromatographic-mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm., and evaluation for biological activity. Journal of Chromatography A. 2010;1217(5):741–748. doi: 10.1016/j.chroma.2009.11.086. [DOI] [PubMed] [Google Scholar]
- 179.Sienkiewicz M., Głowacka A., Poznańska-Kurowska K., Kaszuba A., Urbaniak A., Kowalczyk E. The effect of clary sage oil on Staphylococci responsible for wound infections. Advances in Dermatology and Allergology. 2015;32(1):21–26. doi: 10.5114/pdia.2014.40957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ünlü M., Vardar-Ünlü G., Vural N., Dönmez E., Özbaş Z. Y. Chemical composition, antibacterial and antifungal activity of the essential oil of Thymbra spicata L. from Turkey. Natural Product Research. 2009;23(6):572–579. doi: 10.1080/14786410802312316. [DOI] [PubMed] [Google Scholar]
- 181.Cosentino S., Tuberoso C. I. G., Pisano B., et al. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Letters in Applied Microbiology. 1999;29(2):130–135. doi: 10.1046/j.1472-765x.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- 182.Sienkiewicz M., Łysakowska M., Denys P., Kowalczyk E. The antimicrobial activity of thyme essential oil against multidrug resistant clinical bacterial strains. Microbial Drug Resistance. 2012;18(2):137–148. doi: 10.1089/mdr.2011.0080. [DOI] [PubMed] [Google Scholar]
- 183.Alviano D. S., Alviano C. S. Plant extracts: search for new alternatives to treat microbial diseases. Current Pharmaceutical Biotechnology. 2009;10(1):106–121. doi: 10.2174/138920109787048607. [DOI] [PubMed] [Google Scholar]
- 184.Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6(12):1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nishijima S., Kurokawa I., Katoh N., Watanabe K. The bacteriology of acne vulgaris and antimicrobial susceptibility of Propionibacterium aches and Staphylococcus epidermidis isolated from acne lesions. The Journal of Dermatology. 2000;27(5):318–323. doi: 10.1111/j.1346-8138.2000.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 186.Guggenheim M., Zbinden R., Handschin A. E., Gohritz A., Altintas M. A., Giovanoli P. Changes in bacterial isolates from burn wounds and their antibiograms: a 20-year study (1986–2005) Burns. 2009;35(4):553–560. doi: 10.1016/j.burns.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 187.Solórzano-Santos F., Miranda-Novales M. G. Essential oils from aromatic herbs as antimicrobial agents. Current Opinion in Biotechnology. 2012;23(2):136–141. doi: 10.1016/j.copbio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 188.Espersen F. Resistance to antibiotics used in dermatological practice. The British Journal of Dermatology. 1998;139(53):4–8. doi: 10.1046/j.1365-2133.1998.1390s3004.x. [DOI] [PubMed] [Google Scholar]
- 189.Caelli M., Porteous J., Carson C. F., Heller R., Riley T. V. Tea tree oil as an alternative topical decolonization agent for methicillin-resistant Staphylococcus aureus. The Journal of Hospital Infection. 2000;46(3):236–237. doi: 10.1053/jhin.2000.0830. [DOI] [PubMed] [Google Scholar]
- 190.CDC. Antibiotic Resistance Threats in the United States. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 191.Cookson B. D. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. Journal of Antimicrobial Chemotherapy. 1998;41(1):11–18. doi: 10.1093/jac/41.1.11. [DOI] [PubMed] [Google Scholar]
- 192.Boucher H. W., Talbot G. H., Bradley J. S., et al. Bad bugs, no drugs: no ESKAPE! An update from the infectious diseases society of America. Clinical Infectious Diseases. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 193.Warnke P. H., Sherry E., Russo P. A. J., et al. Antibacterial essential oils in malodorous cancer patients: clinical observations in 30 patients. Phytomedicine. 2006;13(7):463–467. doi: 10.1016/j.phymed.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 194.Edwards-Jones V., Buck R., Shawcross S. G., Dawson M. M., Dunn K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns. 2004;30(8):772–777. doi: 10.1016/j.burns.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 195.Ames D. Aromatic wound care in a health care system: a report from the United States. International Journal of Clinical Aromatherapy. 2006;3(2):3–8. [Google Scholar]
- 196.Lee R. L. P., Leung P. H. M., Wong T. K. S. A randomized controlled trial of topical tea tree preparation for MRSA colonized wounds. International Journal of Nursing Sciences. 2014;1(1):7–14. doi: 10.1016/j.ijnss.2014.01.001. [DOI] [Google Scholar]
- 197.Detillion C. E., Craft T. K. S., Glasper E. R., Prendergast B. J., DeVries A. C. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29(8):1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 198.Vurnek M., Weinman J., Whiting C., Tarlton J. Skin immune markers: psychosocial determinants and the effect on wound healing. Brain, Behavior, and Immunity. 2006;20(3):73–74. doi: 10.1016/j.bbi.2006.04.115. [DOI] [Google Scholar]
- 199.Thompson G., Blackwood B., McMullan R., et al. A randomized controlled trial of tea tree oil (5%) body wash versus standard body wash to prevent colonization with methicillin-resistant Staphylococcus aureus (MRSA) in critically ill adults: research protocol. BMC Infectious Diseases. 2008;8(1, article 161) doi: 10.1186/1471-2334-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dryden M., Dailly S., Crouch M. A randomized, controlled trial of tea tree topical preparations versus a standard topical regimen for the clearance of MRSA colonization. The Journal of Hospital Infection. 2004;56(4):283–286. doi: 10.1016/j.jhin.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 201.Edmondson M., Newall N., Carville K., Smith J., Riley T. V., Carson C. F. Uncontrolled, open-label, pilot study of tea tree (Melaleuca alternifolia) oil solution in the decolonisation of methicillin-resistant Staphylococcus aureus positive wounds and its influence on wound healing. International Wound Journal. 2011;8(4):375–384. doi: 10.1111/j.1742-481x.2011.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chin K. B., Cordell B. The effect of tea tree oil (Melaleuca alternifolia) on wound healing using a dressing model. Journal of Alternative and Complementary Medicine. 2013;19(12):942–945. doi: 10.1089/acm.2012.0787. [DOI] [PubMed] [Google Scholar]
- 203.Blackwood B., Thompson G., Mcmullan R., et al. Tea tree oil (5%) body wash versus standard care (johnson's baby softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. Journal of Antimicrobial Chemotherapy. 2013;68(5):1193–1199. doi: 10.1093/jac/dks501. [DOI] [PubMed] [Google Scholar]
- 204.Gebrehiwot M., Asres K., Bisrat D., Mazumder A., Lindemann P., Bucar F. Evaluation of the wound healing property of Commiphora guidottii Chiov. ex. Guid. BMC Complementary and Alternative Medicine. 2015;15(1):282–292. doi: 10.1186/s12906-015-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Orafidiya L. O., Agbani E. O., Abereoje O. A., Awe T., Abudu A., Fakoya F. A. An investigation into the wound-healing properties of essential oil of Ocimum gratissimum linn. Journal of Wound Care. 2003;12(9):331–334. doi: 10.12968/jowc.2003.12.9.26537. [DOI] [PubMed] [Google Scholar]
- 206.Scheinfeld N. S. Acne: a review of diagnosis and treatment. Pharmacy and Therapeutics. 2007;32(6):340–347. [Google Scholar]
- 207.Oh T. H., Kim S.-S., Yoon W.-J., et al. Chemical composition and biological activities of Jeju Thymus quinquecostatus essential oils against Propionibacterium species inducing acne. Journal of General and Applied Microbiology. 2009;55(1):63–68. doi: 10.2323/jgam.55.63. [DOI] [PubMed] [Google Scholar]
- 208.Yoon W.-J., Kim S.-S., Oh T.-H., Lee N. H., Hyun C.-G. Abies koreana essential oil inhibits drug-resistant skin pathogen growth and lps-induced inflammatory effects of murine macrophage. Lipids. 2009;44(5):471–476. doi: 10.1007/s11745-009-3297-3. [DOI] [PubMed] [Google Scholar]
- 209.Zu Y., Yu H., Liang L., et al. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules. 2010;15(5):3200–3210. doi: 10.3390/molecules15053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Luangnarumitchai S., Lamlertthon S., Tiyaboonchai W. Antimicrobial activity of essential oils against five strains of Propionibacterium acnes. Mahidol University Journal of Pharmaceutical Sciences. 2007;34(1–4):60–64. [Google Scholar]
- 211.Nuryastuti T., Van Der Mei H. C., Busscher H. J., Iravati S., Aman A. T., Krom B. P. Effect of cinnamon oil on icaA expression and biofilm formation by Staphylococcus epidermidis. Applied and Environmental Microbiology. 2009;75(21):6850–6855. doi: 10.1128/aem.00875-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Baik J. S., Kim S. S., Lee J. A., et al. Chemical composition and biological activities of essential oils extracted from Korean endemic citrus species. Journal of Microbiology and Biotechnology. 2008;18(1):74–79. [PubMed] [Google Scholar]
- 213.Karpanen T. J., Worthington T., Hendry E. R., Conway B. R., Lambert P. A. Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis. Journal of Antimicrobial Chemotherapy. 2008;62(5):1031–1036. doi: 10.1093/jac/dkn325. [DOI] [PubMed] [Google Scholar]
- 214.Athikomkulchai S., Watthanachaiyingcharoen R., Tunvichien S., et al. The development of anti-acne products from Eucalyptus globulus and Psidium guajava oil. Journal of Health Research. 2008;22(3):109–113. [Google Scholar]
- 215.Gavini E., Sanna V., Sharma R., et al. Solid lipid microparticles (SLM) containing Juniper oil as anti-acne topical carriers: preliminary studies. Pharmaceutical Development and Technology. 2005;10(4):479–487. doi: 10.1080/10837450500299727. [DOI] [PubMed] [Google Scholar]
- 216.Fu Y., Chen L., Zu Y., et al. The antibacterial activity of clove essential oil against Propionibacterium acnes and its mechanism of action. Archives of Dermatology. 2009;145(1):86–88. doi: 10.1001/archdermatol.2008.549. [DOI] [PubMed] [Google Scholar]
- 217.Orafidiya L. O., Agbani E. O., Oyedele A. O., Babalola O. O., Onayemi O. Preliminary clinical tests on topical preparations of Ocimum gratissimum linn leaf essential oil for the treatment of acne vulgaris. Clinical Drug Investigation. 2002;22(5):313–319. doi: 10.2165/00044011-200222050-00005. [DOI] [Google Scholar]
- 218.Bassett I. B., Pannowitz D. L., Barnetson R. S. C. A comparative study of tea-tree oil versus benzoylperoxide in the treatment of acne. Medical Journal of Australia. 1990;153(8):455–458. doi: 10.5694/j.1326-5377.1990.tb126150.x. [DOI] [PubMed] [Google Scholar]
- 219.Enshaieh S., Jooya A., Siadat A. H., Iraji F. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: a randomized, double-blind placebo-controlled study. Indian Journal of Dermatology, Venereology and Leprology. 2007;73(1):22–25. doi: 10.4103/0378-6323.30646. [DOI] [PubMed] [Google Scholar]
- 220.Matiz G., Osorio M. R., Camacho F., Atencia M., Herazo J. Effectiveness of antimicrobial formulations for acne based on orange (Citrus sinensis) and sweet basil (Ocimum basilicum L) essential oils. Biomedica. 2012;32(1):125–133. doi: 10.1590/S0120-41572012000100014. [DOI] [PubMed] [Google Scholar]
- 221.Vaara M. Agents that increase the permeability of the outer membrane. Microbiological Reviews. 1992;56(3):395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Griffin S. G., Wyllie S. G., Markham J. L. Role of the outer membrane of Eschericia coli AG100 and Pseudomonas aeruginosa NCTC 6749 and resistance/susceptibility to monoterpenes of similar chemical structure. Journal of Essential Oil Research. 2001;13(5):380–386. doi: 10.1080/10412905.2001.9712240. [DOI] [Google Scholar]
- 223.Sienkiewicz M., Łysakowska M., Pastuszka M., Bienias W., Kowalczyk E. The potential of use basil and rosemary essential oils as effective antibacterial agents. Molecules. 2013;18(8):9334–9351. doi: 10.3390/molecules18089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Velasco C., Romero L., Martínez J. M. R., Rodríguez-Baño J., Pascual A. Analysis of plasmids encoding extended-spectrum β-lactamases (ESBLs) from Escherichia coli isolated from non-hospitalised patients in Seville. International Journal of Antimicrobial Agents. 2007;29(1):89–92. doi: 10.1016/j.ijantimicag.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 225.Wu D. C., Chan W. W., Metelitsa A. I., Fiorillo L., Lin A. N. Pseudomonas skin infection: clinical features, epidemiology, and management. American Journal of Clinical Dermatology. 2011;12(3):157–169. doi: 10.2165/11539770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 226.Deans S. G., Ritchie G. Antibacterial properties of plant essential oils. International Journal of Food Microbiology. 1987;5(2):165–180. doi: 10.1016/0168-1605(87)90034-1. [DOI] [Google Scholar]
- 227.Piccaglia R., Marotti M., Giovanelli E., Deans S. G., Eaglesham E. Antibacterial and antioxidant properties of Mediterranean aromatic plants. Industrial Crops and Products. 1993;2(1):47–50. doi: 10.1016/0926-6690(93)90010-7. [DOI] [Google Scholar]
- 228.Dorman H. J. D., Deans S. G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. Journal of Applied Microbiology. 2000;88(2):308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 229.Ozturk S., Ercisli S. Antibacterial activity and chemical constitutions of Ziziphora clinopodioides. Food Control. 2007;18(5):535–540. doi: 10.1016/j.foodcont.2006.01.002. [DOI] [Google Scholar]
- 230.Maxia A., Falconieri D., Piras A., et al. Chemical composition and antifungal activity of essential oils and supercritical CO2 extracts of Apium nodiflorum (L.) Lag. Mycopathologia. 2012;174(1):61–67. doi: 10.1007/s11046-011-9519-2. [DOI] [PubMed] [Google Scholar]
- 231.Becerril R., Nerín C., Gómez-Lus R. Evaluation of bacterial resistance to essential oils and antibiotics after exposure to oregano and cinnamon essential oils. Foodborne Pathogens and Disease. 2012;9(8):699–705. doi: 10.1089/fpd.2011.1097. [DOI] [PubMed] [Google Scholar]
- 232.Nasir M., Tafess K., Abate D. Antimicrobial potential of the Ethiopian Thymus schimperi essential oil in comparison with others against certain fungal and bacterial species. BMC Complementary and Alternative Medicine. 2015;15(1, article 260) doi: 10.1186/s12906-015-0784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rath C. C., Devi S., Dash S. K., Mishra R. K. Antibacterial potential assessment of Jasmine essential oil against E. coli. Indian Journal of Pharmaceutical Sciences. 2008;70(2):238–241. doi: 10.4103/0250-474x.41465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Papadopoulos C. J., Carson C. F., Hammer K. A., Riley T. V. Susceptibility of Pseudomonads to Melaleuca alternifolia (tea tree) oil and components. Journal of Antimicrobial Chemotherapy. 2006;58(2):449–451. doi: 10.1093/jac/dkl200. [DOI] [PubMed] [Google Scholar]
- 235.Carson C. F., Hammer K. A., Riley T. V. In-vitro activity of the essential oil of Melaleuca alternifolia against Streptococcus spp. Journal of Antimicrobial Chemotherapy. 1996;37(6):1177–1178. doi: 10.1093/jac/37.6.1177. [DOI] [PubMed] [Google Scholar]
- 236.Hammer K. A., Carson C. F., Riley T. V. In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. Journal of Antimicrobial Chemotherapy. 1998;42(5):591–595. doi: 10.1093/jac/42.5.591. [DOI] [PubMed] [Google Scholar]
- 237.Devkatte A. N., Zore G. B., Karuppayil S. M. Potential of plant oils as inhibitors of Candida albicans growth. FEMS Yeast Research. 2005;5(9):867–873. doi: 10.1016/j.femsyr.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 238.Giordani R., Regli P., Kaloustian J., Portugal H. Potentiation of antifungal activity of amphotericin B by essential oil from Cinnamomum cassia. Phytotherapy Research. 2006;20(1):58–61. doi: 10.1002/ptr.1803. [DOI] [PubMed] [Google Scholar]
- 239.Białoń M., Krzyśko-Łupicka T., Koszałkowska M., Wieczorek P. P. The influence of chemical composition of commercial lemon essential oils on the growth of Candida strains. Mycopathologia. 2014;177(1-2):29–39. doi: 10.1007/s11046-013-9723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Furletti V. F., Teixeira I. P., Obando-Pereda G., et al. Action of Coriandrum sativum L. essential oil upon oral Candida albicans biofilm formation. Evidence-Based Complementary and Alternative Medicine. 2011;2011:9. doi: 10.1155/2011/985832.985832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tavares A. C., Gonçalves M. J., Cavaleiro C., et al. Essential oil of Daucus carota subsp. halophilus: composition, antifungal activity and cytotoxicity. Journal of Ethnopharmacology. 2008;119(1):129–134. doi: 10.1016/j.jep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 242.Maxia A., Marongiu B., Piras A., et al. Chemical characterization and biological activity of essential oils from Daucus carota L. subsp. carota growing wild on the Mediterranean coast and on the Atlantic coast. Fitoterapia. 2009;80(1):57–61. doi: 10.1016/j.fitote.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 243.Cavaleiro C., Pinto E., Gonçalves M. J., Salgueiro L. Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus and Candida strains. Journal of Applied Microbiology. 2006;100(6):1333–1338. doi: 10.1111/j.1365-2672.2006.02862.x. [DOI] [PubMed] [Google Scholar]
- 244.Cabral C., Francisco V., Cavaleiro C., et al. Essential oil of Juniperus communis subsp. alpina (Suter) Čelak needles: chemical composition, antifungal activity and cytotoxicity. Phytotherapy Research. 2012;26(9):1352–1357. doi: 10.1002/ptr.3730. [DOI] [PubMed] [Google Scholar]
- 245.Zuzarte M., Gonçalves M. J., Cavaleiro C., Dinis A. M., Canhoto J. M., Salgueiro L. R. Chemical composition and antifungal activity of the essential oils of Lavandula pedunculata (MILLER) CAV. Chemistry and Biodiversity. 2009;6(8):1283–1292. doi: 10.1002/cbdv.200800170. [DOI] [PubMed] [Google Scholar]
- 246.Zuzarte M., Gonçalves M. J., Cavaleiro C., et al. Antifungal and anti-inflammatory potential of Lavandula stoechas and Thymus herba-barona essential oils. Industrial Crops and Products. 2013;44:97–103. doi: 10.1016/j.indcrop.2012.11.002. [DOI] [Google Scholar]
- 247.Zuzarte M., Gonçalves M. J., Cavaleiro C., et al. Chemical composition and antifungal activity of the essential oils of Lavandula viridis l'her. Journal of Medical Microbiology. 2011;60(5):612–618. doi: 10.1099/jmm.0.027748-0. [DOI] [PubMed] [Google Scholar]
- 248.Mondello F., De Bernardis F., Girolamo A., Salvatore G., Cassone A. In vitro and in vivo activity of tea tree oil against azole-susceptible and -resistant human pathogenic yeasts. Journal of Antimicrobial Chemotherapy. 2003;51(5):1223–1229. doi: 10.1093/jac/dkg202. [DOI] [PubMed] [Google Scholar]
- 249.Rosato A., Vitali C., Piarulli M., Mazzotta M., Argentieri M. P., Mallamaci R. In vitro synergic efficacy of the combination of Nystatin with the essential oils of Origanum vulgareand Pelargonium graveolens against some Candida species. Phytomedicine. 2009;16(10):972–975. doi: 10.1016/j.phymed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 250.Mahboubi M., Haghi G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. Journal of Ethnopharmacology. 2008;119(2):325–327. doi: 10.1016/j.jep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 251.Bouzabata A., Bazzali O., Cabral C., et al. New compounds, chemical composition, antifungal activity and cytotoxicity of the essential oil from Myrtus nivellei Batt. & Trab., an endemic species of Central Sahara. Journal of Ethnopharmacology. 2013;149(3):613–620. doi: 10.1016/j.jep.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 252.Vieira P. R. N., de Morais S. M., Bezerra F. H. Q., Ferreira P. A. T., Oliveira I. R., Silva M. G. V. Chemical composition and antifungal activity of essential oils from Ocimum species. Industrial Crops and Products. 2014;55:267–271. doi: 10.1016/j.indcrop.2014.02.032. [DOI] [Google Scholar]
- 253.Bozin B., Mimica-Dukic N., Simin N., Anackov G. Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. Journal of Agricultural and Food Chemistry. 2006;54(5):1822–1828. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- 254.Nakamura C. V., Ishida K., Faccin L. C., et al. In vitro activity of essential oil from Ocimum gratissimum L. against four Candida species. Research in Microbiology. 2004;155(7):579–586. doi: 10.1016/j.resmic.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 255.Amber K., Aijaz A., Immaculata X., Luqman K. A., Nikhat M. Anticandidal effect of Ocimum sanctum essential oil and its synergy with fluconazole and ketoconazole. Phytomedicine. 2010;17(12):921–925. doi: 10.1016/j.phymed.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 256.Kosalec I., Pepeljnjak S., Kuatrak D. Antifungal activity of fluid extract and essential oil from anise fruits (Pimpinella anisum L., Apiaceae) Acta Pharmaceutica. 2005;55(4):377–385. [PubMed] [Google Scholar]
- 257.Hristova Y., Gochev V., Wanner J., Jirovetz L., Schmidt E., Girova T. Chemical composition and antifungal activity of essential oil of Salvia sclarea L. from Bulgaria against clinical isolates of Candida species. Journal of Bioscience and Biotechnology. 2013;2(1):39–44. [Google Scholar]
- 258.Pinto E., Vale-Silva L., Cavaleiro C., Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. Journal of Medical Microbiology. 2009;58, part 11:1454–1462. doi: 10.1099/jmm.0.010538-0. [DOI] [PubMed] [Google Scholar]
- 259.Saad A., Fadli M., Bouaziz M., Benharref A., Mezrioui N.-E., Hassani L. Anticandidal activity of the essential oils of Thymus maroccanus and Thymus broussonetii and their synergism with amphotericin B and fluconazol. Phytomedicine. 2010;17(13):1057–1060. doi: 10.1016/j.phymed.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 260.Shin S., Kim J.-H. Antifungal activities of essential oils from Thymus quinquecostatus and T. magnus. Planta Medica. 2004;70(11):1090–1092. doi: 10.1055/s-2004-832654. [DOI] [PubMed] [Google Scholar]
- 261.Pina-Vaz C., Rodrigues A. G., Pinto E., et al. Antifungal activity of Thymus oils and their major compounds. Journal of the European Academy of Dermatology and Venereology. 2004;18(1):73–78. doi: 10.1111/j.1468-3083.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 262.Vale-Silva L. A., Gonçalves M. J., Cavaleiro C., Salgueiro L., Pinto E. Antifungal activity of the essential oil of Thymus x viciosoi against Candida, Cryptococcus, Aspergillus and dermatophyte species. Planta Medica. 2010;76(9):882–888. doi: 10.1055/s-0029-1240799. [DOI] [PubMed] [Google Scholar]
- 263.Gonçalves M. J., Cruz M. T., Cavaleiro C., Lopes M. C., Salgueiro L. Chemical, antifungal and cytotoxic evaluation of the essential oil of Thymus zygis subsp. sylvestris. Industrial Crops and Products. 2010;32(1):70–75. doi: 10.1016/j.indcrop.2010.03.005. [DOI] [Google Scholar]
- 264.Shin S., Lim S. Antifungal effects of herbal essential oils alone and in combination with ketoconazole against Trichophyton spp. Journal of Applied Microbiology. 2004;97(6):1289–1296. doi: 10.1111/j.1365-2672.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- 265.Sanguinetti M., Posteraro B., Romano L., et al. In vitro activity of Citrus bergamia (bergamot) oil against clinical isolates of dermatophytes. Journal of Antimicrobial Chemotherapy. 2007;59(2):305–308. doi: 10.1093/jac/dkl473. [DOI] [PubMed] [Google Scholar]
- 266.Mugnaini L., Nardoni S., Pinto L., et al. In vitro and in vivo antifungal activity of some essential oils against feline isolates of Microsporum canis. Journal de Mycologie Médicale. 2012;22(2):179–184. doi: 10.1016/j.mycmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 267.Baptista E. B., Zimmermann-Franco D. C., Lataliza A. A. B., Raposo N. R. B. Chemical composition and antifungal activity of essential oil from Eucalyptus smithii against dermatophytes. Revista da Sociedade Brasileira de Medicina Tropical. 2015;48(6):746–752. doi: 10.1590/0037-8682-0188-2015. [DOI] [PubMed] [Google Scholar]
- 268.Hammer K. A., Carson C. F., Riley T. V. In vitro activities of ketoconazole, econazole, miconazole, and Melaleuca alternifolia (tea tree) oil against Malassezia species. Antimicrobial Agents and Chemotherapy. 2000;44(2):467–469. doi: 10.1128/aac.44.2.467-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Khosravi A. R., Shokri H., Fahimirad S. Efficacy of medicinal essential oils against pathogenic Malassezia sp. isolates. Journal of Medical Mycology. 2016;26(1):28–34. doi: 10.1016/j.mycmed.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 270.Silva M. R. R., Oliveira J. G., Jr., Fernandes O. F. L., et al. Antifungal activity of Ocimum gratissimum towards dermatophytes. Mycoses. 2005;48(3):172–175. doi: 10.1111/j.1439-0507.2005.01100.x. [DOI] [PubMed] [Google Scholar]
- 271.Khan M. S. A., Ahmad I., Cameotra S. S. Carum copticum and Thymus vulgaris oils inhibit virulence in Trichophyton rubrum and Aspergillus spp. Brazilian Journal of Microbiology. 2014;45(2):523–531. doi: 10.1590/s1517-83822014000200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Behmanesh F., Pasha H., Sefidgar A. A., et al. Antifungal effect of lavender essential oil (Lavandula angustifolia) and clotrimazole on Candida albicans: an in vitro study. Scientifica. 2015;2015:5. doi: 10.1155/2015/261397.261397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Woodfolk J. A. Allergy and dermatophytes. Clinical Microbiology Reviews. 2005;18(1):30–43. doi: 10.1128/CMR.18.1.30-43.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Croxtall J. D., Plosker G. L. Sertaconazole: a review of its use in the management of superficial mycoses in dermatology and gynaecology. Drugs. 2009;69(3):339–359. doi: 10.2165/00003495-200969030-00009. [DOI] [PubMed] [Google Scholar]
- 275.Satchell A. C., Saurajen A., Bell C., Barnetson R. S. Treatment of dandruff with 5% tea tree oil shampoo. Journal of the American Academy of Dermatology. 2002;47(6):852–855. doi: 10.1067/mjd.2002.122734. [DOI] [PubMed] [Google Scholar]
- 276.Gupta A. K., Nicol K., Batra R. Role of antifungal agents in the treatment of seborrheic dermatitis. American Journal of Clinical Dermatology. 2004;5(6):417–422. doi: 10.2165/00128071-200405060-00006. [DOI] [PubMed] [Google Scholar]
- 277.Weitzman I., Summerbell R. C. The dermatophytes. Clinical Microbiology Reviews. 1995;8(2):240–259. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Carson C. F., Hammer K. A., Riley T. V. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clinical Microbiology Reviews. 2006;19(1):50–62. doi: 10.1128/cmr.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Buck D. S., Nidorf D. M., Addino J. G. Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. Journal of Family Practice. 1994;38(6):601–605. [PubMed] [Google Scholar]
- 280.Hammer K. A., Carson C. F., Riley T. V. In vitro activity of Melaleuca alternifolia (tea tree) oil against dermatophytes and other filamentous fungi. Journal of Antimicrobial Chemotherapy. 2002;50(2):195–199. doi: 10.1093/jac/dkf112. [DOI] [PubMed] [Google Scholar]
- 281.Tong M. M., Altman P. M., Barnetson R. S. Tea tree oil in the treatment of tinea pedis. Australasian Journal of Dermatology. 1992;33(3):145–149. doi: 10.1111/j.1440-0960.1992.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 282.Satchell A. C., Saurajen A., Bell C., Barnetson R. S. C. Treatment of interdigital tinea pedis with 25% and 50% tea tree oil solution: a randomized, placebo-controlled, blinded study. The Australasian Journal of Dermatology. 2002;43(3):175–178. doi: 10.1046/j.1440-0960.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- 283.Warnke P. H., Becker S. T., Podschun R., et al. The battle against multi-resistant strains: renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. Journal of Cranio-Maxillofacial Surgery. 2009;37(7):392–397. doi: 10.1016/j.jcms.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 284.Karpanen T. J., Conway B. R., Worthington T., Hilton A. C., Elliott T. S. J., Lambert P. A. Enhanced chlorhexidine skin penetration with eucalyptus oil. BMC Infectious Diseases. 2010;10, article 278 doi: 10.1186/1471-2334-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Syed T. A., Qureshi Z. A., Ali S. M., Ahmad S., Ahmad S. A. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Tropical Medicine and International Health. 1999;4(4):284–287. doi: 10.1046/j.1365-3156.1999.00396.x. [DOI] [PubMed] [Google Scholar]
- 286.Bensouilah J., Buck P. Aromadermatology: Aromatherapy in the Treatment and Care of Common Skin Conditions. Abingdon, UK: Radcliffe Publishing; 2006. [Google Scholar]
- 287.Wagner H. Multitarget therapy—the future of treatment for more than just functional dyspepsia. Phytomedicine. 2006;13(supplement 1):122–129. doi: 10.1016/j.phymed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 288.Wagner H., Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16(2-3):97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 289.Suliman S., van Vuuren S. F., Viljoen A. M. Validating the in vitro antimicrobial activity of Artemisia afra in polyherbal combinations to treat respiratory infections. South African Journal of Botany. 2010;76(4):655–661. doi: 10.1016/j.sajb.2010.07.003. [DOI] [Google Scholar]
- 290.Cottarel G., Wierzbowski J. Combination drugs, an emerging option for antibacterial therapy. Trends in Biotechnology. 2007;25(12):547–555. doi: 10.1016/j.tibtech.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 291.Li R. C., Schentag J. J., Nix D. E. The fractional maximal effect method: a new way to characterize the effect of antibiotic combinations and other nonlinear pharmacodynamic interactions. Antimicrobial Agents and Chemotherapy. 1993;37(3):523–531. doi: 10.1128/aac.37.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Inui T., Wang Y., Deng S., Smith D. C., Franzblau S. G., Pauli G. F. Counter-current chromatography based analysis of synergy in an anti-tuberculosis ethnobotanical. Journal of Chromatography A. 2007;1151(1-2):211–215. doi: 10.1016/j.chroma.2007.01.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Blazquez J., Baquero M.-R., Canton R., Alos I., Baquero F. Characterization of a new TEM-type beta-lactamase resistant to clavulanate, sulbactam, and tazobactam in a clinical isolate of Escherichia coli. Antimicrobial Agents and Chemotherapy. 1993;37(10):2059–2063. doi: 10.1128/aac.37.10.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Enright M. C., Robinson D. A., Randle G., Feil E. J., Grundmann H., Spratt B. G. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA) Proceedings of the National Academy of Sciences of the United States of America. 2002;99(11):7687–7692. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kapoor S., Saraf S. Topical herbal therapies an alternative and complementary choice to combat acne. Research Journal of Medicinal Plant. 2011;5(6):650–669. doi: 10.3923/rjmp.2011.650.669. [DOI] [Google Scholar]
- 296.Grassmann J., Hippeli S., Dornisch K., Rohnert U., Beuscher N., Elstner E. F. Antioxidant properties of essential oils: possible explanations for their anti-inflammatory effects. Arzneimittel-Forschung. 2000;50(2):135–139. [PubMed] [Google Scholar]
- 297.Trivedi N. A., Hotchandani S. C. A study of the antimicrobial activity of oil of Eucalyptus. Indian Journal of Pharmacology. 2004;36(2):93–94. [Google Scholar]
- 298.Boelens M. H. Chemical and sensory evaluation of lavandula oils. Perfumer and Flavorist. 1995;20(3):23–51. [Google Scholar]
- 299.Cavanagh H. M. A., Wilkinson J. M. Biological activities of lavender essential oil. Phytotherapy Research. 2002;16(4):301–308. doi: 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- 300.Pazyar N., Yaghoobi R., Bagherani N., Kazerouni A. A review of applications of tea tree oil in dermatology. International Journal of Dermatology. 2013;52(7):784–790. doi: 10.1111/j.1365-4632.2012.05654.x. [DOI] [PubMed] [Google Scholar]
- 301.Karaca M., Özbek H., Him A., Tütüncü M., Akkan H. A., Kaplanoglu V. Investigation of anti-inflammatory activity of bergamot oil. European Journal of General Medicine. 2007;4(4):176–179. [Google Scholar]
- 302.Forlot P., Pevet P. Bergamot (Citrus bergamia Risso et Poiteau) essential oil: biological properties, cosmetic and medical use. A review. Journal of Essential Oil Research. 2012;24(2):195–201. doi: 10.1080/10412905.2012.659527. [DOI] [Google Scholar]
- 303.Cassella S., Cassella J. P., Smith I. Synergistic antifungal activity of tea tree (Melaleuca alternifolia) and lavender (Lavandula angustifolia) essential oils against dermatophyte infection. International Journal of Aromatherapy. 2002;12(1):2–15. doi: 10.1054/ijar.2001.0127. [DOI] [Google Scholar]
- 304.Van Vuuren S. F., Suliman S., Viljoen A. M. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Letters in Applied Microbiology. 2009;48(4):440–446. doi: 10.1111/j.1472-765X.2008.02548.x. [DOI] [PubMed] [Google Scholar]
- 305.Boucher A. N., Tam V. H. Mathematical formulation of additivity for antimicrobial agents. Diagnostic Microbiology and Infectious Disease. 2006;55(4):319–325. doi: 10.1016/j.diagmicrobio.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 306.Berenbaum M. C. A method for testing for synergy with any number of agents. Journal of Infectious Diseases. 1978;137(2):122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 307.Lambert R. J. W., Lambert R. A model for the efficacy of combined inhibitors. Journal of Applied Microbiology. 2003;95(4):734–743. doi: 10.1046/j.1365-2672.2003.02039.x. [DOI] [PubMed] [Google Scholar]
- 308.Williamson E. M. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8(5):401–409. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]
- 309.Bell A. Antimalarial drug synergism and antagonism: mechanistic and clinical significance. FEMS Microbiology Letters. 2005;253(2):171–184. doi: 10.1016/j.femsle.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 310.Tallarida R. J. An overview of drug combination analysis with isobolograms. The Journal of Pharmacology and Experimental Therapeutics. 2006;319(1):1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- 311.Wing-Shing Cheung D., Koon C.-M., Ng C.-F., et al. The roots of Salvia miltiorrhiza (Danshen) and Pueraria lobata (Gegen) inhibit atherogenic events: a study of the combination effects of the 2-herb formula. Journal of Ethnopharmacology. 2012;143(3):859–866. doi: 10.1016/j.jep.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 312.Lewis R., Guha R., Korcsmaros T., Bender A. Synergy Maps: exploring compound combinations using network-based visualization. Journal of Cheminformatics. 2015;7, article 36 doi: 10.1186/s13321-015-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Goñi P., López P., Sánchez C., Gómez-Lus R., Becerril R., Nerín C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chemistry. 2009;116(4):982–989. doi: 10.1016/j.foodchem.2009.03.058. [DOI] [Google Scholar]
- 314.Gutierrez J., Barry-Ryan C., Bourke P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. International Journal of Food Microbiology. 2008;124(1):91–97. doi: 10.1016/j.ijfoodmicro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 315.Honório V. G., Bezerra J., Souza G. T., et al. Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1,8-cineole. Frontiers in Microbiology. 2015;6, article 1223 doi: 10.3389/fmicb.2015.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Geda A. K. Antibacterial activity of essential oils and their combination. Fat Science and Technology. 1995;97(12):458–460. doi: 10.1002/lipi.2700971207. [DOI] [Google Scholar]
- 317.Hartman D., Coetzee J. C. Two US practitioners' experience of using essential oils for wound care. Journal of Wound Care. 2002;11(8):317–320. doi: 10.12968/jowc.2002.11.8.26432. [DOI] [PubMed] [Google Scholar]
- 318.Guba R. Wound healing. International Journal of Aromatherapy. 1999;9(2):67–74. doi: 10.1016/s0962-4562(98)80022-2. [DOI] [Google Scholar]
- 319.Shin S., Kang C.-A. Antifungal activity of the essential oil of Agastache rugosa Kuntze and its synergism with ketoconazole. Letters in Applied Microbiology. 2003;36(2):111–115. doi: 10.1046/j.1472-765x.2003.01271.x. [DOI] [PubMed] [Google Scholar]
- 320.Lorenzi V., Muselli A., Bernardini A. F., et al. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrobial Agents and Chemotherapy. 2009;53(5):2209–2211. doi: 10.1128/AAC.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.de Carvalho Nilo Bitu V., Fecundo H. D. T. F., Coutinho H. D. M., et al. Chemical composition of the essential oil of Lippia gracilis Schauer leaves and its potential as modulator of bacterial resistance. Natural Product Research. 2014;28(6):399–402. doi: 10.1080/14786419.2013.867343. [DOI] [PubMed] [Google Scholar]
- 322.Harris R. Synergism in the essential oil world. International Journal of Aromatherapy. 2002;12(4):179–186. doi: 10.1016/S0962-4562(02)00083-8. [DOI] [Google Scholar]
- 323.Bolla J.-M., Alibert-Franco S., Handzlik J., et al. Strategies for bypassing the membrane barrier in multidrug resistant Gram-negative bacteria. FEBS Letters. 2011;585(11):1682–1690. doi: 10.1016/j.febslet.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 324.Si H., Hu J., Liu Z., Zeng Z.-L. Antibacterial effect of oregano essential oil alone and in combination with antibiotics against extended-spectrum β-lactamase-producing Escherichia coli. FEMS Immunology and Medical Microbiology. 2008;53(2):190–194. doi: 10.1111/j.1574-695x.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 325.Gradinaru A. C., Aprotosoaie A. C., Trifan A., Spac A., Brebu M., Miron A. Interaction between cardamom essential oil and conventional antibiotics against Staphylococcus aureus clinical isolates. Farmacia. 2014;62(6):1214–1222. [Google Scholar]
- 326.Giordani R., Regli P., Kaloustian J., Mikaïl C., Abou L., Portugal H. Antifungal effect of various essential oils against Candida albicans. Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phytotherapy Research. 2004;18(12):990–995. doi: 10.1002/ptr.1594. [DOI] [PubMed] [Google Scholar]
- 327.Yap P. S. X., Lim S. H. E., Hu C. P., Yiap B. C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine. 2013;20(8-9):710–713. doi: 10.1016/j.phymed.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 328.Schelz Z., Molnar J., Hohmann J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia. 2006;77(4):279–285. doi: 10.1016/j.fitote.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 329.Fadli M., Saad A., Sayadi S., et al. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection-bacteria and their synergistic potential with antibiotics. Phytomedicine. 2012;19(5):464–471. doi: 10.1016/j.phymed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 330.Malik T., Singh P., Pant S., Chauhan N., Lohani H. Potentiation of antimicrobial activity of ciprofloxacin by pelargonium graveolens essential oil against selected uropathogens. Phytotherapy Research. 2011;25(8):1225–1228. doi: 10.1002/ptr.3479. [DOI] [PubMed] [Google Scholar]
- 331.Minami M., Kita M., Nakaya T., Yamamoto T., Kuriyama H., Imanishi J. The inhibitory effect of essential oils on herpes simplex virus type-1 replication in vitro. Microbiology and Immunology. 2003;47(9):681–684. doi: 10.1111/j.1348-0421.2003.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 332.Loizzo M. R., Saab A. M., Tundis R., et al. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chemistry & Biodiversity. 2008;5(3):461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Gavanji S., Sayedipour S. S., Larki B., Bakhtari A. Antiviral activity of some plant oils against herpes simplex virus type 1 in Vero cell culture. Journal of Acute Medicine. 2015;5(3):62–68. doi: 10.1016/j.jacme.2015.07.001. [DOI] [Google Scholar]
- 334.Schnitzler P., Schön K., Reichling J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Die Pharmazie. 2001;56(4):343–347. [PubMed] [Google Scholar]
- 335.Koch C. Antivirale effekte ausgewahlter atherischer Ole auf behullte Viren unter besonderer berucksichtiging des herpes simplex virus typ 1 und 2 [Dissertation] Universitat Heidelberg; 2005. [Google Scholar]
- 336.Koch C., Reichling J., Schneele J., Schnitzler P. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine. 2008;15(1-2):71–78. doi: 10.1016/j.phymed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 337.Schnitzler P., Koch C., Reichling J. Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrobial Agents and Chemotherapy. 2007;51(5):1859–1862. doi: 10.1128/AAC.00426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Koch C., Reichling J., Schnitzler P. Essential oils inhibit the replication of herpes simples virus type 1 (HSV-1) and type 2 (HSV-2) In: Preedy V. R., Watson R. R., editors. Botanical Medicine in Clinical Practice. Wallingsford, UK: CABI; 2008. pp. 192–197. [Google Scholar]
- 339.Reichling J., Koch C., Stahl-Biskup E., Sojka C., Schnitzler P. Virucidal activity of a β-triketone-rich essential oil of Leptospermum scoparium (manuka oil) against HSV-1 and HSV-2 in cell culture. Planta Medica. 2005;71(12):1123–1127. doi: 10.1055/s-2005-873175. [DOI] [PubMed] [Google Scholar]
- 340.Garozzo A., Timpanaro R., Bisignano B., Furneri P. M., Bisignano G., Castro A. In vitro antiviral activity of Melaleuca alternifolia essential oil. Letters in Applied Microbiology. 2009;49(6):806–808. doi: 10.1111/j.1472-765x.2009.02740.x. [DOI] [PubMed] [Google Scholar]
- 341.Civitelli L., Panella S., Marcocci M. E., et al. In vitro inhibition of herpes simplex virus type 1 replication by Mentha suaveolens essential oil and its main component piperitenone oxide. Phytomedicine. 2014;21(6):857–865. doi: 10.1016/j.phymed.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 342.Schnitzler P., Schuhmacher A., Astani A., Reichling J. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomedicine. 2008;15(9):734–740. doi: 10.1016/j.phymed.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 343.Schuhmacher A., Reichling J., Schnitzler P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine. 2003;10(6-7):504–510. doi: 10.1078/094471103322331467. [DOI] [PubMed] [Google Scholar]
- 344.Benencia F., Courrèges M. C. Antiviral activity of sandalwood oil against Herpes simplex viruses-1 and -2. Phytomedicine. 1999;6(2):119–123. doi: 10.1016/S0944-7113(99)80046-4. [DOI] [PubMed] [Google Scholar]
- 345.De Logu A., Loy G., Pellerano M. L., Bonsignore L., Schivo M. L. Inactivation of HSV-1 and HSV-2 and prevention of cell-to-cell virus spread by Santolina insularis essential oil. Antiviral Research. 2000;48(3):177–185. doi: 10.1016/s0166-3542(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 346.Mahboubi M., Bidgoli F. G. In vitro synergistic efficacy of combination of amphotericin B with Myrtus communis essential oil against clinical isolates of Candida albicans. Phytomedicine. 2010;17(10):771–774. doi: 10.1016/j.phymed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 347.Mahboubi M., Ghazian Bidgoli F. Antistaphylococcal activity of Zataria multiflora essential oil and its synergy with vancomycin. Phytomedicine. 2010;17(7):548–550. doi: 10.1016/j.phymed.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 348.Bearden D. T., Allen G. P., Christensen J. M. Comparative in vitro activities of topical wound care products against community-associated methicillin-resistant Staphylococcus aureus. Journal of Antimicrobial Chemotherapy. 2008;62(4):769–772. doi: 10.1093/jac/dkn272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Carson C. F., Ashton L., Dry L., Smith D. W., Riley T. V. Melaleuca alternifolia (tea tree) oil gel (6%) for the treatment of recurrent herpes labialis. Journal of Antimicrobial Chemotherapy. 2001;48(3):450–451. doi: 10.1093/jac/48.3.450. [DOI] [PubMed] [Google Scholar]
- 350.Millar B. C., Moore J. E. Successful topical treatment of hand warts in a paediatric patient with tea tree oil (Melaleuca alternifolia) Complementary Therapies in Clinical Practice. 2008;14(4):225–227. doi: 10.1016/j.ctcp.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 351.Edris A. E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytotherapy Research. 2007;21(4):308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 352.Raines D. Wound care. In: Price S., Price L., editors. Aromatherapy for Health Professionals. 4th. chapter 10. London, UK: Churchill Livingstone; 2012. [Google Scholar]
- 353.Clark S. M., Wilkinson S. M. Phototoxic contact dermatitis from 5-methoxypsoralen in aromatherapy oil. Contact Dermatitis. 1998;38(5):289–290. doi: 10.1111/j.1600-0536.1998.tb05752.x. [DOI] [PubMed] [Google Scholar]
- 354.Cocks H., Wilson D. Letter to the editor. Burns. 1998;24(1):p. 82. doi: 10.1016/s0305-4179(97)00102-2. [DOI] [PubMed] [Google Scholar]
- 355.Kaddu S., Kerl H., Wolf P. Accidental bullous phototoxic reactions to bergamot aromatherapy oil. Journal of the American Academy of Dermatology. 2001;45(3):458–461. doi: 10.1067/mjd.2001.116226. [DOI] [PubMed] [Google Scholar]
- 356.Nair B. Final report on the safety assessment of Mentha piperita (peppermint) oil, Mentha piperita (peppermint) leaf extract, Mentha piperita (peppermint) leaf, and Mentha piperita (peppermint) leaf water. International Journal of Toxicology. 2001;20:61–73. [PubMed] [Google Scholar]
- 357.Prashar A., Locke I. C., Evans C. S. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Proliferation. 2004;37(3):221–229. doi: 10.1111/j.1365-2184.2004.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Bleasel N., Tate B., Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australasian Journal of Dermatology. 2002;43(3):211–213. doi: 10.1046/j.1440-0960.2002.00598.x. [DOI] [PubMed] [Google Scholar]
- 359.Trattner A., David M., Lazarov A. Occupational contact dermatitis due to essential oils. Contact Dermatitis. 2008;58(5):282–284. doi: 10.1111/j.1600-0536.2007.01275.x. [DOI] [PubMed] [Google Scholar]
- 360.Boonchai W., Iamtharachai P., Sunthonpalin P. Occupational allergic contact dermatitis from essential oils in aromatherapists. Contact Dermatitis. 2007;56(3):181–182. doi: 10.1111/j.1600-0536.2007.01024.x. [DOI] [PubMed] [Google Scholar]
- 361.Stonehouse A., Studdiford J. Allergic contact dermatitis from tea tree oil. Consultant. 2007;47(8):p. 781. [Google Scholar]
- 362.Aspres N., Freeman S. Predictive testing for irritancy and allergenicity of tea tree oil in normal human subjects. Exogenous Dermatology. 2003;2(5):258–261. doi: 10.1159/000078694. [DOI] [Google Scholar]
- 363.Veien N. K., Rosner K., Skovgaard G. L. Is tea tree oil an important contact allergen? Contact Dermatitis. 2004;50(6):378–379. doi: 10.1111/j.0105-1873.2004.0350f.x. [DOI] [PubMed] [Google Scholar]
- 364.Rutherford T., Nixon R., Tam M., Tate B. Allergy to tea tree oil: retrospective review of 41 cases with positive patch tests over 4.5 years. Australasian Journal of Dermatology. 2007;48(2):83–87. doi: 10.1111/j.1440-0960.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- 365.Pirker C., Hausen B. M., Uter W., et al. Sensitization to tea tree oil in Germany and Austria. A multicenter study of the German Contact Dermatitis Group. Journal of the German Society of Dermatology. 2003;1(8):629–634. doi: 10.1046/j.1610-0387.2003.03727.x. [DOI] [PubMed] [Google Scholar]
- 366.Robinson M. K., Gerberick G. F., Ryan C. A., McNamee P., White I. R., Basketter D. A. The importance of exposure estimation in the assessment of skin sensitization risk. Contact Dermatitis. 2000;42(5):251–259. doi: 10.1034/j.1600-0536.2000.042005251.x. [DOI] [PubMed] [Google Scholar]
- 367.Gerberick G. F., Robinson M. K., Felter S. P., White I. R., Basketter D. A. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45(6):333–340. doi: 10.1034/j.1600-0536.2001.450603.x. [DOI] [PubMed] [Google Scholar]
- 368.Van der Valk P., De Groot A., Bruynzeel D., Coenraads P., Weijland J. Allergic contact eczema due to'tea tree'oil. Nederlands Tijdschrift voor Geneeskunde. 1994;138(16):823–825. [PubMed] [Google Scholar]
- 369.Mozelsio N. B., Harris K. E., McGrath K. G., Grammer L. C. Immediate systemic hypersensitivity reaction associated with topical application of Australian tea tree oil. Allergy and Asthma Proceedings. 2003;24(1):73–75. [PubMed] [Google Scholar]
- 370.Lippert U., Walter A., Hausen B., Fuchs T. 127 Increasing incidence of contact dermatitis to tea tree oil. Journal of Allergy and Clinical Immunology. 2000;105(1):p. S43. doi: 10.1016/s0091-6749(00)90558-x. [DOI] [Google Scholar]
- 371.Bhushan M., Beck M. H. Allergic contact dermatitis from tea tree oil in a wart paint. Contact Dermatitis. 1997;36(2):117–118. doi: 10.1111/j.1600-0536.1997.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 372.Herro E., Jacob S. E. Mentha piperita (Peppermint) Dermatitis. 2010;21(6):327–329. doi: 10.2310/6620.2011.10080. [DOI] [PubMed] [Google Scholar]
- 373.Gemeda N., Urga K., Tadele A., Lemma H., Melaku D., Mudie K. Antimicrobial activity of topical formulation containing Eugenia caryophyllata L. (Krunfud) and Myritus communis L. (Ades) essential oils on selected skin disease causing microorganisms. Ethiopian Journal of Health Sciences. 2008;18(3):101–107. [Google Scholar]


