Abstract
Background
Given high rates of HIV among Baltimore MSM, we examined characteristics associated with HIV prevalence and unrecognized HIV infection among Baltimore MSM at two time points.
Methods
Cross-sectional behavioral surveys and HIV testing in 2004–2005 and 2008 using venue-based sampling among adult Baltimore men at MSM-identified locations. MSM was defined as sex with a male partner in the past year. Bivariate and backwards stepwise regression identified characteristics associated with HIV and unrecognized infection.
Findings
HIV prevalence was 37.7% overall in 2004–2005 (n=645) and 37.5% in 2008 (n=448), 51.4% and 44.7% among Black MSM, and 12.9% and 18.3% among non-Hispanic White MSM. Compared to non-Hispanic White MSM, Black MSM were 4.0 times (95% C.I.: 2.3, 7.0) more likely to be HIV-positive in 2004–2005 and 2.5 times (95% C.I.: 1.5, 4.0) more likely in 2008. Prevalence of unrecognized HIV infection was 58.4% overall in 2004–2005 and 74.4% in 2008, 63.8% and 76.9% among Black MSM, and 15.4% and 47.4% among non-Hispanic White MSM. In adjusted models, unrecognized infection was significantly associated with minority race/ethnicity, younger age, and no prior year doctor visits in 2004–5 and with younger age and no prior year doctor visits in 2008.
Conclusion
High rates of HIV infection and substantial rates of unrecognized HIV infection among Baltimore MSM, particularly men of color and young men, require urgent public and private sector attention and increased prevention response.
Keywords: MSM, HIV prevalence, Unrecognized HIV infection, Baltimore, Racial disparity
Introduction
Since the late 1990s, the gay community’s early success in initiating risk reduction behavior change and slowing the rate of new cases of HIV/AIDS1–3 has been overshadowed by evidence of a resurgence of HIV/AIDS among men who have sex with men (MSM) in the United States. HIV infection rates among MSM have climbed steadily since the early 1990s, now accounting for more than half of new infections4. The rate of new HIV diagnosis among MSM is 44 times higher compared to non-MSM men5.
Throughout the MSM HIV/AIDS epidemic, Black men have been at particular risk. In 1986, nearly 15 percent of cumulative AIDS cases in homosexual and bisexual men occurred among Black men6. By the end of 2007, Black MSM comprised more than a quarter of the cumulative reported AIDS cases among MSM7 and 35% of new infections among MSM were among African-Americans8.
Maryland had the second highest estimated AIDS diagnosis rate in the United States in 20089. Baltimore is the location hardest hit by HIV/AIDS in Maryland, accounting for 78% of prevalence in the central region. MSM account for 24% of HIV/AIDS prevalence and represent the only transmission category in central Maryland for which HIV incidence is increasing10.
There are more new HIV infections among African-American MSM aged 13–29 than any other age or race/ethnic group8. In Maryland, 1 in 5 African-American MSM are estimated to be HIV positive, compared to 1 in 24 Hispanic/Latino MSM and 1 in 38 White MSM 11. The Young Men’s Survey showed that young Black MSM in Baltimore had the highest percent prevalence of HIV infection, HIV incidence12, and unrecognized HIV infection13 among seven participating cities.
The CDC National HIV Behavioral Surveillance System (NHBS) collects behavioral data among populations at high risk for HIV, including MSM, in selected U.S. areas with high HIV prevalence14. Recently released 2008 findings show that 19% of MSM are HIV positive and 44% of HIV positive men are unaware of their HIV status nationwide15. HIV prevalence and unrecognized HIV infection was highest among Black MSM. Among participating cities, HIV prevalence ranged from 6% in Atlanta to 39% in Baltimore. Unrecognized infection ranged from 15% in Seattle to 73% in Baltimore. These high rates of HIV and unrecognized infection among Baltimore MSM further bolster a need to understand social and behavioral correlates of HIV in this city.
Research among young MSM in Baltimore shows strong racial disparities in HIV infection 16,17 and HIV incidence 18. Older age, recent STD diagnosis, and high numbers of sex partners have also been associated with HIV among young Baltimore MSM 16. It is unknown whether these findings can be generalized to the larger community of MSM in Baltimore. Additionally, little is known about factors associated with unrecognized HIV infection in this population. The NHBS project, known as the Behavioral Surveillance Research (BESURE) Study in Baltimore, recruited MSM in 2005 and 2008. Using data from both BESURE MSM waves, the current paper examined the extent of racial disparity and correlates of HIV prevalence and awareness of HIV seropositivity at each time point.
Methods
Sampling Design and Recruitment
The BESURE Study (NHBS Baltimore) is an HIV infection and behavioral risk cross-sectional survey among populations at high risk for HIV. The methods and sampling for NHBS-MSM have been previously described in detail15,19. Two serial cross-sectional waves of venue-based data collection were conducted among MSM in Baltimore. The first was conducted between June 2004 and April 2005 and the second between July and October 2008. Study protocols for both waves were identical.
Formative research included focus groups with MSM and interviews with community informants and public health practitioners to identify current public and private venues (e.g., bars, clubs, businesses, events, neighborhood locations) frequently attended by Baltimore MSM and high-traffic day/time periods for recruitment. Sampling frames were subsequently constructed from the universe of venues and their corresponding day/time periods, and 15 or more venue-day-time periods (i.e., sampling events) were randomly selected and scheduled for recruitment each month. Sampling events averaged 18 per month in both waves.
During each sampling event, study recruiters consecutively approached men who crossed a predetermined intercept area at the venue and assessed eligibility. Eligible participants were: males 18 years or older, Baltimore-Towson metropolitan area residents, and had not previously participated in the current data collection wave; sexual identity or practice did not preclude men from being eligible. Eligible men completed study procedures either in a nearby mobile unit or in study offices at another scheduled time. All study procedures were anonymous. Consent was provided orally and documented in writing by trained interviewers. Consent for HIV testing was provided separately and not required for study participation in either wave. After completing informed consent procedures, participants were interviewed using a handheld computer-assisted standard questionnaire, provided a serum sample for HIV testing, received counseling and referral to prevention services, and received US$50.00 as reimbursement for their time. Follow-up appointments were scheduled within two weeks for HIV test results, post-test counseling, and referral to care or services as appropriate. The protocol and all study materials were reviewed and approved by the Maryland Department of Health and Mental Hygiene and the Johns Hopkins Bloomberg School of Public Health Institutional Review Boards.
Measures
The standardized questionnaire included demographics, HIV-related risk behaviors, medical history, and sexual identity. Race/ethnicity categories were non-Hispanic White, Black, Hispanic, Native American, Pacific Islander, Asian-American or of mixed race (i.e., participants who reported being of more than one racial/ethnic ancestry). Since small numbers of Hispanic, Native American, Pacific Islander, Asian-American, or mixed race participants precluded detailed analysis, a single category called ‘Other race’ was created in addition to categories for non-Hispanic White and Black. Age was categorized to reflect quartiles of the age distribution of study participants. Being currently homeless was examined as a binary variable, whereas education was reported as the level attained at the time of the survey.
Participants were asked about HIV risk behaviors over their lifetimes and in the prior year. Recent sexual risk behaviors included number of male sexual partners (categorized to reflect quartiles of number of partners), sex with men only or sex with men and women, and unprotected anal intercourse (UAI). UAI was defined as not using a condom during one or more sex acts in the prior 12 months and categorized as ‘No UAI’, UAI only with a main male sexual partner, or UAI with at least one casual or exchange (i.e., sex in exchange for money or goods) male sexual partner. Lifetime variables included having received a prior HIV test, having injected illicit drugs and having had an STD diagnosis. Behaviors in the past year included having used non-prescribed drugs and having visited a doctor’s office. Finally, participants reported whether they had no, public, or private insurance coverage.
Blood specimens with sufficient volume were tested for HIV-1 antibodies by the Maryland Department of Health and Mental Hygiene Laboratories Administration with a U.S. Food and Drug Administration-licensed enzyme immunoassay (EIA) (Sanofi Diagnostics Pasteur, Chaska, MN, U.S.A.). The Maryland DHMH lab confirmed repeatedly reactive samples using Western blot (WB) (Bio-Rad, Hercules, CA, U.S.A. or Epitope, Inc., Organon-Teknika Corporation, Durham, NC, U.S.A.). An HIV-seropositive individual was defined as having a reactive EIA with a positive WB confirmation. Three respondents with indeterminant test results were excluded from the analysis. Unrecognized HIV infection was defined as having a confirmed HIV positive BESURE Study test and either reporting a negative or an unknown prior HIV test result during the survey. This category also included three respondents who refused to report results of their most recent test in the second wave.
Statistical Methods
Sample characteristics between wave 1 and wave 2 were compared using the chi-square statistic. Analyses with HIV positivity as the outcome were restricted to the 645 men and 448 men who reported a same-sex experience within the past year in the first and second cross-sectional waves, respectively, and whose HIV test results were definitive. Analyses of unrecognized HIV infection were restricted to the 243 MSM and 168 MSM who tested HIV positive in the first and second cross-sectional waves, respectively. We assessed patterns of attendance at venues types included in the sampling frame. There was no association between frequency of venue attendance and the two outcomes of interest and data are presented and analyzed without weights.
Associations between demographic variables and HIV risk behaviors with HIV prevalence and HIV unrecognized infection were assessed using the chi-square statistic. Unadjusted prevalence ratios (PR) were calculated with corresponding 95% confidence intervals (95% CI) using SAS PROC GENMOD’s log-binomial regression capability with a binomial distribution and a logarithmic link function21. Variables that showed a significant association with HIV prevalence or with HIV unrecognized infection (p ≤ 0.05) were analyzed using the COPY method to directly estimate adjusted prevalence ratios (APR) with their corresponding 95% CI 22. Both unadjusted and adjusted PROC GENMOD analyses used the REPEATED statement to account for clustering by venue. The PR was deemed as a more appropriate measure of association and a better approximation of the relative risk since the frequency of both outcomes exceeded 15%23,24. We arrived at the most parsimonious model by removing variables that were insignificant (P > 0.05) using a backwards stepwise approach and as determined by the likelihood ratio test. QIC was used to assess model fit. All statistical analyses were performed using Statistical Analysis Software (SAS) version 9.1.
Results
Participant and sample characteristics
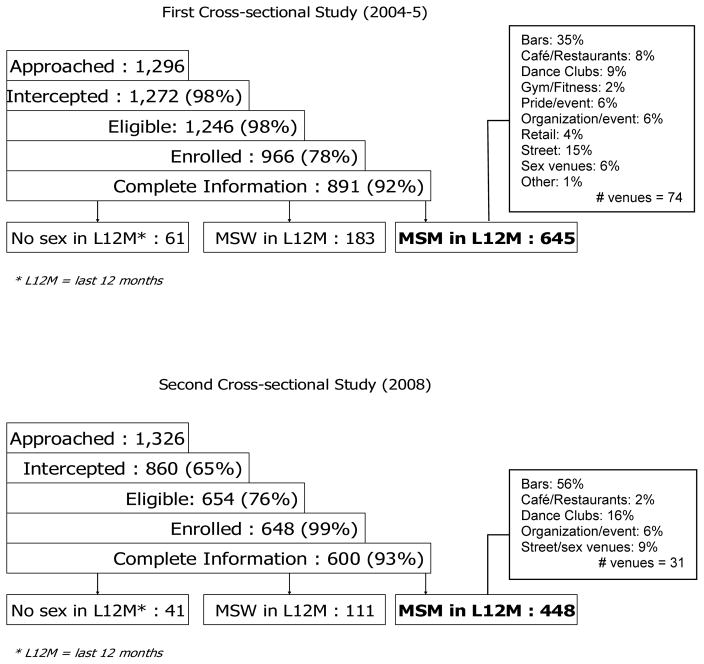
Figure 1 shows recruitment and venue information for both waves. During the first cross-sectional wave (2004–2005), 1,296 men were approached to participate. After eligibility and enrollment, 891 had complete survey and serologic information and 645 reported a same-sex experience in the year prior to the survey (Figure 1). Eligible participants were recruited from 74 venues with mean venue sample size of 8.3 (SD: 14.6; range 1–88). During the second cross-sectional wave (2008), 1,326 men were approached for participation. After eligibility and enrollment, 600 participants had complete survey and serologic information and 448 reported prior year same-sex experience (Figure 1). In wave 2, eligible participants were recruited from 31 venues with mean venue sample size of 13.6 (SD: 9.0; range 2–35).
Figure 1.
Recruitment for BESURE study among MSM in Baltimore, 2004–2005 and 2008
The demographic and HIV risk behavior composition of the two samples from the two waves differed (Table 1). Compared with the first cross-sectional wave, the second cross-sectional wave was more likely to enroll MSM who reported: Black race; being less than 24 years of age; homelessness; having had 2–3 male sexual partners in the past year; having had male sexual partners only; not having had UAI; using non-injection drugs in the past year; and lifetime drug injection. The two samples did not statistically differ in educational attainment, ever receiving an HIV test, ever receiving an STI diagnosis, health insurance status, or past year doctor visits.
Table 1.
Sample composition of MSM in Baltimore, 2004–2005 (n=645) and 2008 (n=448)
| Sample Characteristics | |||
|---|---|---|---|
|
| |||
| Socio-demographic and behavioral characteristics | Wave 1 (2004–2005) | Wave 2 (2008) | p-value |
| Total (Column %) | Total (Column %) | ||
| Total | 645 (100) | 448 (100) | |
|
| |||
| Socio-demographic | |||
| Race | |||
| White | 201 (31.16) | 104 (23.21) | 0.0040 |
| Black | 403 (62.48) | 320 (71.43) | 0.0021 |
| Other | 41 (6.36) | 24 (5.36) | 0.4920 |
| Age | |||
| < 24 years old | 163 (24.27) | 140 (31.25) | 0.0299 |
| 25–34 years old | 166 (25.74) | 135 (30.13) | 0.1095 |
| 35–44 years old | 197 (30.54) | 108 (24.11) | 0.0197 |
| > 45 years old | 119 (18.45) | 65 (14.51) | 0.0868 |
| Current Homeless | |||
| No | 625 (96.90) | 421 (93.97) | 0.019 |
| Yes | 20 (3.10) | 27 (6.03) | 0.019 |
| Education | |||
| High school education or less | 316 (48.99) | 217 (48.44) | 0.8568 |
| College or some college | 288 (44.65) | 203 (45.31) | 0.8288 |
| Graduate education | 41 (6.36) | 28 (6.25) | 0.9432 |
| Sexual Identity | |||
| Homosexual | 407 (63.10) | 302 (67.71) | 0.1164 |
| Bisexual | 205 (31.78) | 134 (30.04) | 0.5420 |
| Heterosexual or other | 33 (5.35) | 10 (2.24) | 0.0165 |
| Sexual Behaviors | |||
| #male sex partners in last 12 months | |||
| One (ref) | 165 (25.58) | 109 ( 24.33) | 0.6388 |
| 2 to 3 | 182 (28.22) | 154 (34.38) | 0.0300 |
| 4 to 8 | 149 (23.10) | 115 (25.67) | 0.3291 |
| 9 or more | 149 (23.10) | 70 (15.63) | 0.0024 |
| In past 12 months, any sex with: | |||
| Men only | 429 (66.51) | 339 (75.67) | 0.0011 |
| Men and women | 216 (33.49) | 109 (24.33) | 0.0011 |
| In past 12 months | |||
| No UAI | 304 (47.13) | 286 (63.84) | <.0001 |
| UAI with main partners only | 164 (25.43) | 80 (17.86) | 0.0031 |
| UAI with casual/exchange partners | 177 (27.44) | 82 (18.30) | 0.0005 |
| Drug Use Behaviors | |||
| Ever injected drugs | |||
| No | 534 (82.79) | 421 (93.97) | <.0001 |
| Yes | 111 (17.21) | 27 (6.03) | <.0001 |
| Non-injection drug use in last 12 months | |||
| No | 329 (51.01) | 184 (41.07) | 0.0012 |
| Yes | 316 (48.99) | 264 (58.93) | 0.0012 |
| Health History and Insurance | |||
| Ever been tested for HIV | |||
| No | 85 (13.18) | 43 (9.62) | 0.0722 |
| Yes | 560 (86.82) | 404 (90.38) | 0.0722 |
| Been told by MD had any STD: | |||
| No | 542 (84.03) | 361 (80.58) | 0.1516 |
| Yes | 103 (15.97) | 87 (19.42) | 0.1516 |
| Health insurance | |||
| No health insurance (ref) | 230 (36.28) | 164 (37.19) | 0.2767 |
| Public | 146 (23.03) | 84 (19.05) | 0.2767 |
| Private | 258 (40.69) | 193 (43.76) | |
| Visit a doctor’s office in last 12 months | |||
| No | 146 (22.64) | 87 (19.42) | 0.2017 |
| Yes | 499 (77.36) | 361 (80.58) | 0.2017 |
In the first cross-sectional wave, median age was 34 years (range: 18–69), 70% were of minority race, and more than half reported post-secondary education. Most reported being homosexual/gay (63%), never injecting illegal drugs (83%), no STD diagnosis (84%), and had been tested for HIV (87%). In the prior year, 67% had sex with men only and 74% had more than one same-sex partner. About half reported using non-injected illegal drugs of which marijuana (76%) was most common, followed by cocaine (47%), and crack cocaine (25%). Approximately 60% had some form of health insurance and 77% had visited a doctor in the past year. Median time since last HIV test was 276 days.
In the second cross-sectional study, median age was 30 (range: 18–72), 77% were of minority race, and more than half reported post-secondary education. Most reported being homosexual/gay (68%), never injecting drugs (94%), no STD diagnosis (81%), and having ever tested for HIV (90%). Three-quarters had sex with men only and the majority reported multiple male partners in the past year. Among the 59% who reported non-injected illegal drug use, marijuana was most common (89%), followed by cocaine (28%) and crack cocaine (20%). The majority had some form of health insurance and 81% had visited a doctor in the past year. Median time since last HIV test was 305 days.
Prevalence of HIV infection and associated factors
Prevalence of HIV infection by socioeconomic and behavioral characteristics is presented in Table 2 for both cross-sectional recruitment waves. In 2004–2005, 38% of participants tested HIV positive. HIV prevalence by race was 51% among Black MSM, 13% among non-Hispanic White MSM, and 24% among other MSM of color. Table 3 shows the results of univariate and multivariate analyses of prevalent HIV infection for both waves of data collection. In the first wave, minority race, being older than 24 years, having nine or more partners, STD diagnosis, having public health insurance, and a doctor’s visit in the prior year were significantly and independently associated with being HIV positive. Older MSM were 1.4–1.8 times more likely to be HIV positive compared to 18–24 year olds. Black MSM were approximately 3.7 times and other race MSM were 2.0 times more likely to be HIV positive than non-Hispanic White MSM.
Table 2.
Characteristics of BESURE MSM participants with HIV and unrecognized HIV infection, 2004–2005 and 2008
| Socio-demographic and behavioral characteristics | HIV positive
|
Unrecognized HIV infection
|
||
|---|---|---|---|---|
| 2004–2005 | 2008 | 2004–2005 | 2008 | |
| n (row %) | n (row %) | n (row %) | n (row %) | |
| Total | 243 (37.7) | 168 (37.5) | 142 (58.44) | 125 (74.40) |
| Socio-demographic | ||||
| Race Ethnicity | ||||
| Non-Hispanic White | 26 (12.94) | 19 (18.3) | 4 (15.38) | 9 (47.37) |
| Non-Hispanic Black | 207 (51.36) | 143 (44.7) | 132 (63.77) | 110 (76.92) |
| Other | 10 (24.39) | 6 (25.0) | 6 (60.00) | 6 (100.00) |
| Age | ||||
| < 24 years old | 41 (25.15) | 41 (29.3) | 36 (87.80) | 35 (85.37) |
| 25–34 years old | 53 (31.93) | 56 (41.5) | 36 (67.92) | 42 (75.00) |
| 35–44 years old | 99 (50.25) | 43 (39.8) | 51 (51.52) | 34 (79.07) |
| > 45 years old | 50 (42.02) | 28 (43.1) | 19 (38.00) | 14 (50.00) |
| Current Homeless | ||||
| No | 236 (37.76) | 157 (37.3) | 138 (58.47) | 117 (74.52) |
| Yes | 7 (35.00) | 11 (40.7) | 4 (57.14) | 8 (72.73) |
| Education | ||||
| High school education or less | 139 (43.99) | 91 (41.9) | 86 (61.87) | 68 (74.73) |
| College or some college | 98 (34.03) | 69 (34.0) | 54 (55.10) | 53 (76.81) |
| Graduate education | 6 (14.63) | 8 (28.6) | 2 (33.33) | 4 (50.00) |
| Sexual Identity | ||||
| Homosexual | 167 (41.03) | 119 (39.4) | 95 (56.89) | 86 (72.27) |
| Bisexual | 63 (30.73) | 47 (35.1) | 39 (61.90) | 38 (80.85) |
| Heterosexual or other | 13 (39.39) | 2 (20.0) | 8 (61.54) | 1 (50.00) |
| Sexual Behaviors | ||||
| Number of male sex partners last 12 months | ||||
| One (ref) | 52 (31.52) | 37 (33.9) | 22 (42.31) | 28 (75.68) |
| 2 to 3 | 72 (39.56) | 54 (35.1) | 49 (68.06) | 40 (74.07) |
| 4 to 8 | 51 (34.23) | 50 (43.5) | 32 (62.75) | 38 (76.00) |
| 9 or more | 68 (45.64) | 27 (38.6) | 39 (57.35) | 19 (70.37) |
| In past 12 months, any sex with: | ||||
| Men only | 175 (40.79) | 135 (39.8) | 97 (55.43) | 96 (71.11) |
| Men and women | 68 (31.48) | 33 (30.3) | 45 (66.18) | 29 (87.88) |
| In past 12 months | ||||
| No UAI | 108 (35.53) | 99 (34.6) | 58 (53.70) | 72 (72.73) |
| UAI with main partners only | 55 (33.54) | 26 (32.5) | 40 (72.73) | 21 (80.77) |
| UAI with casual/exchange partners | 80 (45.20) | 43 (52.4) | 44 (55.00) | 32 (74.42) |
| Drug Use Behaviors | ||||
| Ever injected drugs | ||||
| No | 199 (37.27) | 160 (38.0) | 120 (60.30) | 118 (73.75) |
| Yes | 44 (39.64) | 8 (29.6) | 22 (50.00) | 7 (87.50) |
| Injection drug use last 12 months | ||||
| No | 232 (38.60) | 167 (38.0) | 134 (58.01) | 124 (74.25) |
| Yes | 11 (25.00) | 1 (12.5) | 7 (63.64) | 1 (100) |
| Non-injection drug use last 12 months | ||||
| No | 122 (37.08) | 61 (33.2) | 67 (54.92) | 48 (78.69) |
| Yes | 121 (38.29) | 107 (40.5) | 75 (61.98) | 77 (71.96) |
| Health History and Insurance | ||||
| Ever been tested for HIV | ||||
| No | 30 (35.29) | 15 (34.9) | 30 (100.00) | 15 (100.00) |
| Yes | 213 (38.04) | 152 (37.6) | 112 (52.58) | 109 (71.71) |
| Been told by MD had any STD: | ||||
| No | 188 (34.76) | 124 (34.4) | 115 (61.50) | 96 (77.42) |
| Yes | 55 (53.40) | 44 (50.6) | 26 (47.27) | 29 (65.91) |
| Health insurance | ||||
| No health insurance (ref) | 73 (31.74) | 66 (40.2) | 54 (73.97) | 53 (80.30) |
| Public | 85 (58.22) | 40 (47.6) | 40 (47.06) | 29 (72.50) |
| Private | 79 (30.62) | 60 (31.1) | 44 (55.70) | 41 (68.33) |
| Visit a doctor’s office in last 12 months | ||||
| No | 38 (26.03) | 29 (33.3) | 34 (89.47) | 27 (93.10) |
| Yes | 205 (41.08) | 139 (38.5) | 108 (52.68) | 98 (70.50) |
Table 3.
Predictors of positive HIV status among MSM in Baltimore BESURE study 2004–2005 (n=645) and 2008 (n=448)
| Socio-demographic and behavioral characteristics | Wave 1 (2004–2005) HIV positive
|
Wave 2 (2008) HIV positive
|
||
|---|---|---|---|---|
| Unadjusted PR (95% CI) | Adjusted PR (95% CI)a | Unadjusted PR (95% CI) | Adjusted PR (95% CI)a | |
| Socio-demographic | ||||
| Race Ethnicity | ||||
| Non-Hispanic White | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Non-Hispanic Black | 3.97 (2.27–6.96) | 3.70 (2.24–6.12) | 2.45 (1.49–4.01) | 2.46 (1.53–3.96) |
| Other | 1.89 (0.95–3.76) | 1.95 (1.03–3.71) | 1.37 (0.53–3.56) | 1.32 (0.52–3. 35) |
| Age | ||||
| < 24 years old | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |
| 25–34 years old | 1.27 (0.97–1.66) | 1.37 (1.04–1.80) | 1.42 (0.96–2.08) | |
| 35–44 years old | 2.00 (1.53–2.61) | 1.76 (1.39–2.22) | 1.35 (0.95–1.95) | |
| > 45 years old | 1.67 (1.18–2.36) | 1.76 (1.39–2.22) | 1.47 (0.93–2.32) | |
| Current Homeless | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 0.93 (0.54–1.50) | 1.09 (0.63–1.89) | ||
| Education | ||||
| Graduate education | 1.0 (ref) | 1.0 (ref) | ||
| College or some college | 3.01 (1.52–5.95) | 1.19 (0.61–2.31) | ||
| High school education or less | 2.33 (1.11–4.88) | 1.47 (0.66–3.22) | ||
| Sexual Identity | ||||
| Homosexual | 1.0 (ref) | 1.0 (ref) | ||
| Bisexual | 0.75 (0.52–1.07) | 0.89 (0.66–1.20) | ||
| Heterosexual or other | 0.96 (0.68–1.35) | 0.51 (0.18–1.45) | ||
| Sexual Behaviors | ||||
| # of male sex partners last 12 months | ||||
| One (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |
| 2 to 3 | 1.26 (0.91–1. 72) | 1.23 (1.00–1.51) | 1.03 (0.78–1.36) | |
| 4 to 8 | 1.09 (0.73–1.60) | 1.12 (0.83–1.52) | 1.28 (0.96–1.72) | |
| 9 or more | 1.45 (1.12–1.87) | 1.31 (1.07–1.61) | 1.14 (0.67–1.93) | |
| In past 12 months, any sex with: | ||||
| Men only | 1.0 (ref) | 1.0 (ref) | ||
| Men and women | 0.77 (0.59–1.01) | 0.76 (0.53–1.10) | ||
| In past 12 months | ||||
| No UAI | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |
| UAI with main partners only | 0.94 (.75–1.19) | 0.94 (0.62–1.42) | 0.89 (0.57–1.38) | |
| UAI with casual/exchange partners | 1.27 (.94–1.72) | 1.51 (1.10–2.09) | 1.43 (1.05–1.95) | |
| Drug Use Behaviors | ||||
| Ever injected drugs | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 1.06 (0.75–1.52) | .78 (0.46–1.32) | ||
| Injection drug use last 12 months | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 0.65 (0.26–1.64) | 0.33 (0.05–2.23) | ||
| Non-injection drug use last 12 months | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 1.03 (0.85–1.25) | 1.23 (0.94–1.59) | ||
| Health History and Insurance | ||||
| Ever been tested for HIV | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 1.08 (0.76–1.54) | 1.07 (0.71–1.64) | ||
| Been told by MD had any STD: | ||||
| No | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Yes | 1.53 (1.26–1.88) | 1.23 (1.06–1.42) | 1.47 (1.14–1.89) | 1.47 (1.17 – 1.84) |
| Health insurance | ||||
| No health insurance (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |
| Public | 1.83 (1.15–2.39) | 1.32 (1.05–1.66) | 1.18 (0.93–1.51) | |
| Private | 0.96 (0.66–1.41) | 1.10 (0.81–1.50) | 0.77 (0.59–1.04) | |
| Visit a doctor’s office in last 12 months | ||||
| No | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |
| Yes | 1.58 (1.20–2.07) | 1.43 (1.03–1.96) | 1.16 (0.86–1.56) | |
All variables with p < .05 in the univariate analysis were included in the log-binomial model.
In the second wave, 38% of participants overall, 45% of Black, 18.3% of non-Hispanic White MSM, and 25% of other men of color tested HIV positive. HIV infection was significantly and independently associated with Black race, UAI with casual or exchange male partners in the prior year (compared to no UAI), and prior STI diagnosis. Risk of being HIV positive among Black MSM was 2.5 times higher than among non-Hispanic White MSM.
Prevalence of Unrecognized HIV infection and associated factors
Prevalence of unrecognized HIV infection by socioeconomic and behavioral factors is shown in Table 2 for both cross-sectional recruitment waves. In the first wave, 58% of HIV positive MSM were unaware of their HIV positive serostatus at the time of enrollment. Of these, 57% reported their most recent test was negative, 20% did not obtain the results of their most recent test, 3% had a recent indeterminant test, less than 1% had never been tested, and 20% did not know results of their most recent test. The proportion unrecognized HIV infection was higher among younger men, from 38% among those 45 years or older to 89% among those 18–24 years of age. By race, the proportion of unrecognized HIV infection ranged from 64% among Black men to 15% among non-Hispanic White participants.
Table 4 shows the results of univariate and multivariate analyses of characteristics associated with unrecognized HIV infection. In the first wave, unrecognized infection was higher among those who reported minority race/ethnicity, younger age, multiple partners, UAI with main partners, no STD diagnosis, no health insurance, and no doctor visit in the past year. In multivariate analysis, minority race/ethnicity, decreasing age, having 2–3 partners (compared to 1), having no health insurance (compared to private insurance) and not visiting a doctor in past year were significantly associated with unrecognized HIV infection (Table 3). Controlling for these factors, Black MSM were 4 times as likely and other MSM of color were 3.5 times as likely to have unrecognized infection than non-Hispanic White MSM.
Table 4.
Predictors of unrecognized HIV infection among HIV-positive MSM in Baltimore BESURE study 2004–2005 (n=142) and 2008 (n=125)
| Socioeconomic and behavioral characteristics | Wave 1 (2004–2005) Unrecognized HIV infection
|
Wave 2 (2008) Unrecognized HIV infection
|
||
|---|---|---|---|---|
| Unadjusted PR (95% CI) | Adjusted PR (95% CI)a | Unadjusted PR (95% CI) | Adjusted PR (95% CI)a | |
| Socioeconomic | ||||
| Race Ethnicity | ||||
| Non-Hispanic White | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Non-Hispanic Black | 4.14 (1.88–9.13) | 3.95 (1.92–8.15) | 1.62 (0.95–2.76) | 1.39 (.89–2.19) |
| Other | 3.90 (1.58–9.61) | 3.48 (1.68–7.21) | 2.11 (1.25–3.55) | 1.51 (.96–2.40) |
| Age | ||||
| < 24 years old | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 25–34 years old | 0.77 (0.63–0.96) | 0.88 (0.84–0.93) | 0.88 (0.70–1.11) | 0.92 (0.83–1.02) |
| 35–44 years old | 0.59 (0.48–0.72) | 0.75 (0.64–0.88) | 0.93 (0.73–1.18) | 0.92 (0.83–1.03) |
| > 45 years old | 0.43 (0.25–0.75) | 0.55 (0.37–0.84) | 0.59 (0.41–0.84) | 0.65 (0.45–0.93) |
| Current Homeless | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 0.98 (0.47–2.01) | 0.98 (0.70–1.36) | ||
| Education | ||||
| Graduate education | 1.0 (ref) | 1.0 (ref) | ||
| College or some college | 1.09 (0.88–1.34) | 1.53 (.82–2.87) | ||
| High school education or less | 1.08 (0.68–1.72) | 1.49 (0.82–2.73) | ||
| Sexual Identity | ||||
| Homosexual | 1.0 (ref) | 1.0 (ref) | ||
| Bisexual | 1.09 (0.86–1.38) | 1.12 (.98–1.28) | ||
| Heterosexual or other | 1.08 (0.69–1.70) | .69 (.17–2.83) | ||
| Sexual Behaviors | ||||
| # male sex partners last 12 mos | ||||
| One (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |
| 2 to 3 | 1.61 (1.12–2.31) | 1.48 (1.10–1.98) | .98 (0.78–1.23) | |
| 4 to 8 | 1.48 (1.00–2.18) | 1.30 (0.98–1.73) | 1.00 (0.79–1.27) | |
| 9 or more | 1.36 (0.93–1.97) | 1.30 (0.98–1.73) | .93 (0.68–1.27) | |
| In past 12 months, any sex with: | ||||
| Men only | 1.0 (ref) | 1.0 (ref) | ||
| Men and women | 1.19 (0.99–1.48) | 1.24 (1.08–1.41) | ||
| In past 12 months | ||||
| No UAI | 1.0 (ref) | 1.0 (ref) | ||
| UAI with main partners only | 1.35 (1.08.–1.70) | 1.11 (.88–.1.40) | ||
| UAI with casual/exchange partners | 1.02 (0.80–1.30) | 1.02 (.85–1.23) | ||
| Drug Use Behaviors | ||||
| Ever injected drugs | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | .83 (.59–1.16) | 1.19 (0.92–1.54) | ||
| Injection drug use last 12 months | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 1.10 (.75–1.60) | 1.17 (0.86–1.58) | ||
| Non-injection drug use last 12 months | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 1.13 (0.87–1.46) | 0.91 (0.79–1.05) | ||
| Health History and Insurance | ||||
| Been told by MD had any STD: | ||||
| No | 1.0 (ref) | 1.0 (ref) | ||
| Yes | 0.77 (0.61–.97) | 0.85 (0.67–1.10) | ||
| Health insurance | ||||
| No health insurance (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |
| Public | 0.64 (0.49–0.83) | 0.84 (0.68–1.03) | 0.91 (0.73–.99) | |
| Private | 0.75 (0.55–1.02) | 0.88 (0.84–0.93) | 0.90 ( 0.70–1.16) | |
| Visit doctor’s office last 12 mos | ||||
| No | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Yes | 0.59 (0.50–0.70) | 0.88 (0.84–0.93) | 0.76 (0.63–0.91) | 0.82 (0.71–0.94) |
All variables with p < .05 in the univariate analysis were included in the log-binomial model
In the second wave (2008), 74% of HIV positive participants were unaware of their HIV positive serostatus. Among these, 67% reported their most recent test was negative, 15% did not obtain the results of their most recent test, 4% had a recent indeterminant test, 0% had never been tested, 5.7% did not know results of their most recent test, and 2.4% refused to answer. Seventy-seven percent of Black MSM did not know they were HIV positive, compared to 47% of non-Hispanic White MSM. None of the six HIV positive men of other race/ethnicity were aware of their HIV status. Unrecognized infection was significantly higher among men of other race/ethnicity and who had both male and female partners, but significantly lower among men over 45, those with public insurance (compared to no insurance), and those who had seen a doctor in the past year. In multivariate analysis, men over 45 and those who had visited a doctor in the past year were 35% less likely and approximately 20% less likely, respectively, to be unaware of their HIV infection.
Discussion
These results show high prevalence of HIV infection and unrecognized HIV infection among MSM in Baltimore in 2005 and 2008, particularly among men of color and young men. Two CDC reports have compared HIV prevalence and unrecognized infection rates among MSM in U.S. cities. In 2005, MSM in Baltimore had the highest HIV prevalence and undiagnosed infection rates compared to their counterparts in Los Angeles, Miami, San Francisco, and New York25 and, in 2008, rates in Baltimore exceeded those of 20 other high prevalence cities15. A recent study estimated that racial disparities in MSM HIV infection were highest in Maryland compared to 16 other southern states (Maryland Rate Ratio=7.1, p<0.001 vs Total: 4.6, p<0.001) 11. Although it is possible that the NHBS HIV prevalence rankings are confounded by racial differences across cities, the current study confirms the disproportionate HIV burden borne by Black and other minority MSM in Baltimore. There is a very real possibility that the HIV epidemic among MSM may further expand, particularly given the high levels of unrecognized infection among young MSM and UAI among men unaware of their HIV infection.
Despite the different demographic and behavioral compositions of these two recruitment waves, overall HIV prevalence was consistent. These findings corroborate reports of high HIV prevalence among MSM from other cities24 and expand on prior reports of pronounced racial disparity in HIV among young Baltimore MSM 17,18. Recent attention to HIV resurgence among U.S. MSM may not fully account for the historically high HIV prevalence among African-American MSM as observed in Baltimore. HIV infection in these studies among adult MSM was much higher than the in 1996–2000 YMS study among young MSM in Baltimore, which reported 12% prevalence overall and 27% among non-Hispanic blacks16. Yet the similarity in demographic and behavioral correlates of infection between this study and the YMS findings suggests that there are persistent prevention needs in Baltimore.
Notably, the majority of men who tested HIV positive in both waves of data collection were not aware of their HIV status. Beginning in 2001, CDC recommended greater emphasis on finding undiagnosed HIV infections26 and later revised the recommendation to enhance testing in healthcare settings27. While the effectiveness and cost of this approach relative to other HIV testing policies has been debated28, the current study examined the burden of unrecognized HIV infection and racial disparities of unrecognized infections among MSM in Baltimore at two time points following these recommendations. The very high observed proportion of participants who were unaware of their HIV infection suggests that testing efforts are not adequately reaching MSM in Baltimore, particularly minority and young MSM, which in turn limits access to the benefits of HIV treatment and secondary prevention.
Known HIV infection was associated with a doctor visit, suggesting that clinical settings are feasible venues to target some MSM and may be effectively providing testing services. However, many primary healthcare providers miss opportunities to provide HIV testing29 and counseling30. Given the high HIV prevalence rates and multiple risk behaviors, a diverse portfolio of HIV behavioral interventions along with routine testing and counseling will be needed to adequately meet the challenges of the current epidemic. This study utilized a structured venue-based sampling method to recruit participants. A similar methodology for outreach HIV testing efforts and other prevention programming may be viable for increasing service availability for Baltimore MSM.
These findings are subject to numerous limitations. Temporal relationships cannot be determined due to the cross-sectional design in both waves and temporal trend comparisons are not definitive, as they may be due to true differences or the recruitment of different samples. Differential enrollment bias may have occurred between the two waves. Although the study protocols were identical, qualitative differences in implementation may have occurred. The venue universe differed between waves and this may also have contributed to differences between the 2004–5 and 2008 samples. Demographic and HIV risk behavior data were self-reported, and therefore subject to misreporting due to recall or social desirability, concerns about stigma, or cultural differences. Some men who knew their HIV status to be positive may have reported negative status due to perceived stigma or concern about study eligibility, although materials clearly described eligibility and reinforced anonymity and staff members were trained in rapport-building and cultural sensitivity. Lack of disclosure due to stigma concerns may also be a barrier to partner disclosure and a worthy target of prevention efforts. Sexual behavior and drug use measures were summary measures, which may limit interpretation. Findings may not be generalizable to MSM who do not frequent study recruitment venues, who only frequent less well-attended venues, or do not reside in the Baltimore-Towson metropolitan area. There may also be residual bias and underestimation of uncertainty because the data were not weighted by venue attendance patterns and likelihood of recruitment.
Despite limitations, these BESURE surveys provide a needed assessment of urban MSM and a useful foundation for future research and HIV prevention planning. That these two recruitment waves were demographically different but had similar epidemiological profiles suggests a broad need for prevention across the diverse population of Baltimore MSM. These surveys indicate that venue-based recruitment methods are feasible for reaching diverse MSM populations and may be useful for HIV testing and prevention programs. Given that demographic and behavioral characteristics did not alter the association between race/ethnicity and HIV status, it is likely that individual level explanations are insufficient to explain the observed disparities. Input from local community members, providers, and researchers points to the following to combat the strikingly pronounced epidemiological disparities observed here: culturally-sensitive health care and structural prevention approaches to reduce stigma and discrimination toward same-sex behavior and HIV infection; interventions that increase pro-condom use norms; prevention for positives; integration of prevention and medical care; and contextualized prevention strategies that address men who have sex with both women and men. It is imperative to implement interventions that are not only ethnically diverse but also ones that acknowledge and embrace the diversity of ethnic, sexual, and social identities and lifestyles among urban MSM.
Acknowledgments
Sources of support: Contracts to The Johns Hopkins University from the Maryland Department of Health and Mental Hygiene and by cooperative agreements between the Maryland Department of Health and Mental Hygiene and the Centers for Disease Control and Prevention.
The authors express their gratitude to the BESURE Study field staff, NHBS colleagues, and the men who participated in this study. Supported by contracts to The Johns Hopkins University from the Maryland Department of Health and Mental Hygiene and by cooperative agreements between the Maryland Department of Health and Mental Hygiene and the Centers for Disease Control and Prevention.
Footnotes
A preliminary analysis of a subset of participants in one of the two data collection waves reported here was presented at the Conference on Retroviruses and Opportunistic Infections, Denver, CO, February 5–8, 2006 in, entitled: “High HIV Prevalence and Incidence Observed among African-American Men who have Sex with Men in Baltimore: The Behavioral Surveillance Research (BESURE) Study”.
References
- 1.Martin JL, Garcia MA, Beatrice ST. Sexual behavior changes and HIV antibody in a cohort of New York City gay men. Am J Public Health. 1989;79(4):501–503. doi: 10.2105/ajph.79.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winkelstein W, Jr, Samuel M, Padian NS, et al. The San Francisco Men’s Health Study: III. Reduction in human immunodeficiency virus transmission among homosexual/bisexual men, 1982–86. Am J Public Health. 1987;77(6):685–689. doi: 10.2105/ajph.77.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkelstein W, Jr, Wiley JA, Padian NS, et al. The San Francisco Men’s Health Study: continued decline in HIV seroconversion rates among homosexual/bisexual men. Am J Public Health. 1988;78(11):1472–1474. doi: 10.2105/ajph.78.11.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. CDC analysis provides new look at disproportionate impact of HIV and syphilis among US gay and bisexual men. Atlanta, GA: CDC; 2010. [Accessed August 15, 2010]. Available at: http://www.cdc.gov/nchhstp/newsroom/msmpressrelease.html. [Google Scholar]
- 6.Centers for Disease Control and Prevention. AIDS Weekly Surveillance Report, December 29, 1986. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 7.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2005. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 8.Centers for Disease Control and Prevention. Subpopulation estimates from the HIV incidence surveillance system--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(36):985–989. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Diagnoses of HIV infection and AIDS in the United States and Dependent Areas, 2008. HIV Surveillance Report. 2008;20 [Google Scholar]
- 10.Maryland Department of Health and Mental Hygeine. HIV/AIDS in Central Maryland: An Epidemiologic Profile. Baltimore: Center for Surveillance and Epidemiology, Infectious Disease and Environmental Health Administration, Maryland Department of Health and Mental Hygiene; 2009. [Google Scholar]
- 11.Lieb S, Prejean J, Thompson DR, et al. HIV prevalence rates among men who have sex with men in the Southern United States: Population-based estimates by race/ethnicity. AIDS Behav. 2010 doi: 10.1007/s10461-010-9820-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. HIV incidence among young men who ave sex with men --- seven US cities, 1994–2000. MMWR Morb Mortal Wkly Rep. 2001;50(21):440–444. [PubMed] [Google Scholar]
- 13.MacKellar DA, Valleroy LA, Secura GM, et al. Unrecognized HIV infection, risk behaviors, and perceptions of risk among young men who have sex with men: opportunities for advancing HIV prevention in the third decade of HIV/AIDS. J Acquir Immune Defic Syndr. 2005;38(5):603–614. doi: 10.1097/01.qai.0000141481.48348.7e. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher KM, Sullivan PS, Lansky A, Onorato IM. Behavioral surveillance among people at risk for HIV infection in the U.S.: the National HIV Behavioral Surveillance System. Public Health Rep. 2007;122(Suppl 1):32–38. doi: 10.1177/00333549071220S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. The prevalence of Human Immunodeficiency Virus (HIV) and awareness of HIV infection among men who have sex with men – 21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(37) [PubMed] [Google Scholar]
- 16.Sifakis F, Hylton JB, Flynn C, et al. Prevalence of HIV infection and prior HIV testing among young men who have sex with men. The Baltimore Young Men’s Survey. AIDS Behav. 2010;14(4):904–12. doi: 10.1007/s10461-007-9317-5. [DOI] [PubMed] [Google Scholar]
- 17.Celentano DD, Sifakis F, Hylton J, et al. Race/ethnic differences in HIV prevalence and risks among adolescent and young adult men who have sex with men. J Urban Health. 2005;82(4):610–21. doi: 10.1093/jurban/jti124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sifakis F, Hylton JB, Flynn C, et al. Racial disparities in HIV incidence among young men who have sex with men: the Baltimore Young Men’s Survey. J Acquir Immune Defic Syndr. 2007;46(3):343–8. doi: 10.1097/QAI.0b013e31815724cc. [DOI] [PubMed] [Google Scholar]
- 19.MacKellar DA, Gallagher KM, Finlayson T, et al. Surveillance of HIV risk and prevention behaviors of men who have sex with men--a national application of venue-based, time-space sampling. Public Health Rep. 2007;122(Suppl 1):39–47. doi: 10.1177/00333549071220S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacKellar D, Valleroy L, Karon J, et al. The Young Men’s Survey: methods for estimating HIV seroprevalence and risk factors among young men who have sex with men. Public Health Rep. 1996;111(Suppl 1):138–144. [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 22.Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occupational and Environmental Medicine. 2008;65(7):501–506. doi: 10.1136/oem.2007.034777. [DOI] [PubMed] [Google Scholar]
- 23.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American Journal of Epidemiology. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 24.Zochetti C, Consonni D, Bertazzi P. Estimation of prevalence rate ratios from cross-sectional data. International Journal of Epidemiology. 1995;24:1064–1105. doi: 10.1093/ije/24.5.1064. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. HIV prevalence, unrecognized infection, and HIV testing among men who have sex with men--five U.S. cities, June 2004–April 2005. MMWR Morb Mortal Wkly Rep. 2005;54(24):597–601. [PubMed] [Google Scholar]
- 26.Janssen RS, Holtgrave DR, Valdiserri RO, et al. The serostatus approach to fighting the HIV epidemic: Prevention strategies for infected individuals. American Journal of Public Health. 2001;91(7):1019–1024. doi: 10.2105/ajph.91.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(14):1–17. [PubMed] [Google Scholar]
- 28.Holtgrave DR, Pinkerton SD. Can increasing awareness of HIV seropositivity reduce infections by 50% in the United States? J Acquir Immune Defic Syndr. 2007;44(3):360–363. doi: 10.1097/QAI.0b013e31802ea4dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liddicoat RV, Horton NJ, Urban R, et al. Assessing missed opportunities for HIV testing in medical settings. J Gen Intern Med. 2004;19(4):349–356. doi: 10.1111/j.1525-1497.2004.21251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao G, Branson BM, Anderson LA, Irwin KL. Do physicians provide counseling with HIV and STD testing at physician offices or hospital outpatient departments? AIDS. 2003;17(8):1243–1247. doi: 10.1097/00002030-200305230-00017. [DOI] [PubMed] [Google Scholar]