Abstract
Human telomeric RNA has been identified as a key component of the telomere machinery. Recently, the growing evidence suggests that the telomeric RNA forms G-quadruplex structures to play an important role in telomere protection and regulation. In the present studies, we developed a 19F NMR spectroscopy method to investigate the telomeric RNA G-quadruplex structures in vitro and in living cells. We demonstrated that the simplicity and sensitivity of 19F NMR approach can be used to directly observe the dimeric and two-subunits stacked G-quadruplexes in vitro and in living cells and quantitatively characterize the thermodynamic properties of the G-quadruplexes. By employing the 19F NMR in living cell experiment, we confirmed for the first time that the higher-order G-quadruplex exists in cells. We further demonstrated that telomere RNA G-quadruplexes are converted to the higher-order G-quadruplex under molecular crowding condition, a cell-like environment. We also show that the higher-order G-quadruplex has high thermal stability in crowded solutions. The finding provides new insight into the structural behavior of telomere RNA G-quadruplex in living cells. These results open new avenues for the investigation of G-quadruplex structures in vitro and in living cells.
INTRODUCTION
RNA structure influences the functions of nearly all classes of RNAs (1–3), including RNA G-quadruplexes, which are four-stranded RNA structures that have emerged as potential targets for drug design because of their biological importance (4–6). For example, RNA G-quadruplexes were recently reported to cause protein-dependent oncogene translation in cancer and neurodegenerative diseases (7,8). Recently, we and other groups demonstrated that human telomere RNA, a newly found telomeric repeat-containing RNA (9,10), forms G-quadruplex structures (11–14). We also found that telomere RNA G-quadruplex structures play an important role in providing a protective structure for telomere ends (15). Recently, some studies have also suggested that telomere RNA G-quadruplexes may form polymorphic higher-order G-quadruplexes comprising two stacked G-quadruplex subunits (16,17). However, direct evidence for human telomeric RNA higher-order G-quadruplexes existing in cells has not yet been obtained. Therefore, a more effective chemical approach for obtaining structural information on telomere RNA G-quadruplexes in living cells is desired.
In the present study, we employed fluorine-19 (19F) NMR spectroscopy of oligonucleotides with fluorine labels to further investigate the structural basis of telomere RNA G-quadruplexes in vitro and in living cells. 19F NMR has been successfully used to investigate important conformations of nucleic acids due to the high sensitivity of the 19F chemical shift to the environment, 100% natural abundance of 19F, absence of any natural background signals in RNA and cells and relative simplicity of 19F NMR spectra (18–32).
Thus, using the simplicity and sensitivity of 19F NMR spectroscopy, we directly observed the two-subunits stacked telomere RNA G-quadruplex in living cells, providing the in cell evidence for the presence of the higher-order G-quadruplex in human RNA at first time. The in-cell result suggested that molecularly crowded environment in cells promotes the higher-order G-quadruplex structure formation even at low RNA concentration. It is known that biomolecules function in a crowded intracellular environment. Molecular crowding could affect the structure, stability and conformational transition of biomolecules (33–35). We further demonstrated that telomere RNA G-quadruplexes are converted to the higher-order G-quadruplex under molecular crowding condition and further shown that the higher-order G-quadruplex has high thermal stability in crowded solutions. These results provide valuable information for understanding the structure and function of human telomere RNA.
MATERIALS AND METHODS
Sample preparation
RNA was incorporated a six-ethyl linker and 3,5 bis(trifluoromethyl)phenyl moiety at the 5΄ terminal on a 1.0 μmol scale using an automatic DNA/RNA synthesizer using solid-phase phosphoramidite chemistry. After automated synthesis, the oligomer was detached from the support, deprotected and purified by RP-HPLC using an appropriate linear gradient of 50 mM ammonium formate in H2O and 50 mM ammonium formate in 1:1 acetonitrile/H2O. The oligomer was desalted by NAP10 column (GE Healthcare) and identified by a matrix-assisted laser desorption/ionization-time-of flight mass spectrometer (MALDI-TOFMS) on an autoflex III smart beam mass spectrometer (negative mode), Calcd. 4376.58, Found. 4379.88.
CD measurement
Circular dichroism (CD) spectra were measured using a Jasco model J-820 CD spectrophotometer. The spectra were recorded using a 1 cm path length cell. Samples were prepared by heating the oligonucleotides at 95°C for 5 min and gradually cooling to room temperature. Solutions for CD spectra were prepared as 0.3 ml samples at a 10 μM strand concentration in the presence of 50 mM KCl, 10 mM Tris-HCl buffer (pH 7.0).
In vitro 19F-NMR spectroscopy
RNA samples of 0.2–5.0 mM were dissolved in 150 μl of designed solvent which containing 10 mM Tris-HCl buffer (pH 7.0) and 50 mM KCl. 19F NMR spectra were measured at a frequency of 376.05 MHz on a Bruker AVANCE 400 MHz spectrometer and were referenced relative to external CF3COOH (−75.66 ppm). Experimental parameters were as follows: 19F excitation pulse 15.0 μs, spectral width 89.3 kHz, acquisition time 0.73 s, relaxation delay 1 s, number of scans 64 or 512 or 2048, an exponential window function with lb = 0.3 Hz was used, no zerofilling was used. At temperature-dependent experiment, the sample is kept for 10 min at each temperature.
In-cell 19F-NMR spectroscopy
In-cell NMR sample was prepared by direct microinjection 50 nl aliquot of the stock solution (3 and 5 mM of 12-mer RNA) into the oocyte cell (∼150 and ∼250 μM intracellular concentration). Approximately 150 Xenopus laevis oocytes were injected by using an IM-30 Electric Microinjector (NARISHIGE, Tokyo). After injection, the oocytes were transferred to a disposable dish, washed carefully with oocyte stocking buffer (15 mM Tris-HCl (pH7.6), 88 mM NaCl, 1 mM KCl, 0.4 mM CaCl2, 0.3 mM Ca(NO3)2, 0.8 mM MgCl2 and 2.4 mM NaHCO3). The injected oocytes were transferred to a Shigemi tube (Shigemi 5 mm Symmetrical NMR microtube) and kept in an oocyte stocking buffer containing 10% of D2O. Sample for measurement in lysate was prepared as follows: after the in-cell NMR measurements, oocytes were mechanically crushed and used for NMR measurement.
RESULTS AND DISCUSSION
In vitro 19F NMR analysis of telomere RNA G-quadruplexes
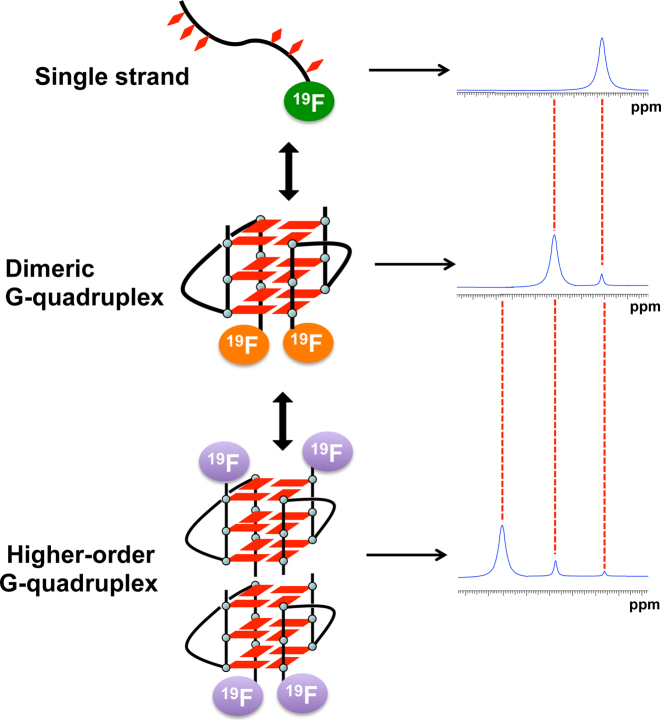
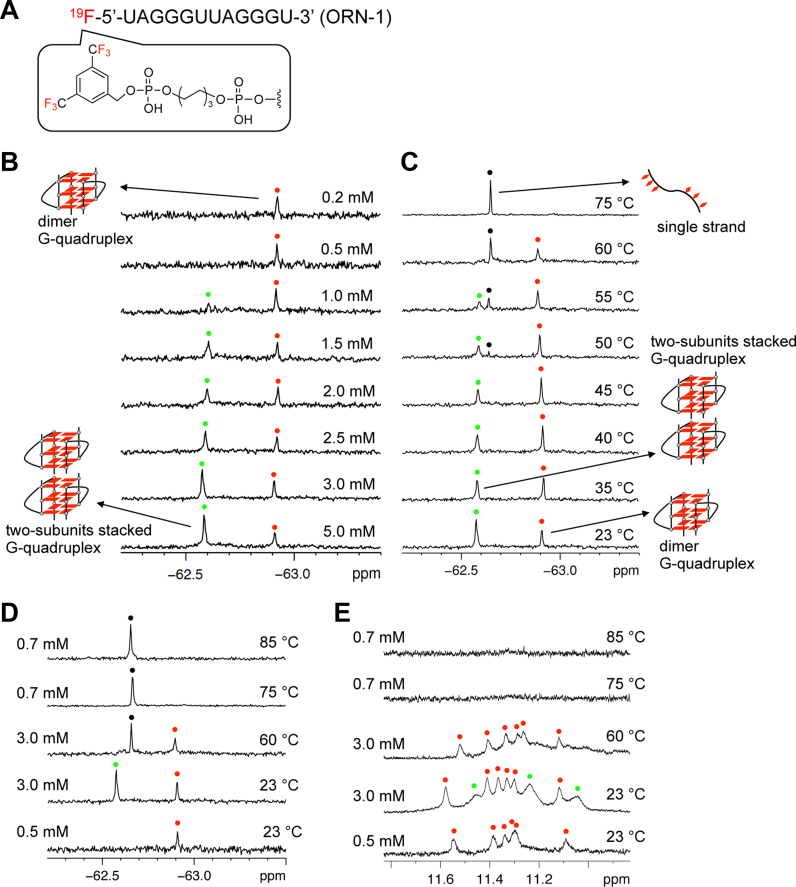
The present study is based on the concept that 19F NMR signals are strongly dependent on the structural environment of the 19F label. Thus, it should be possible to distinguish different RNA structures of the same sequence by the corresponding resonances of the different structures, such as single strands and G-quadruplexes (Figure 1). To achieve this goal, a 3,5-bis(trifluoromethyl)benzene moiety was introduced into 5΄ termini of oligonucleotide using phosphoramidite chemistry (Figure 2A and Supplementary Figures S1–S6). The 19F labeling of RNA with six equivalent 19F atoms was expected to afford the high 19F intensity. First, we examined the conformation of the oligonucleotide ORN-1 with the 19F label at 5΄ termini by CD spectroscopy. The CD spectrum of ORN-1 in the presence of K+ at 20°C showed a positive band at 265 nm and a negative band at 240 nm (Supplementary Figure S7), which are the characteristic CD signatures of a parallel dimeric G-quadruplex structure of RNA consistent with previously reported (11–14), suggesting that the 19F label at 5΄ termini does not affect the folding of G-quadruplex. Next, we performed a concentration-dependent experiment to investigate the structural behavior of ORN-1 for the formation of RNA G-quadruplex by 19F NMR (Figure 2B). The 19F NMR spectrum was firstly obtained at 0.2 mM RNA concentration. One signal was observed at −62.91 ppm in the presence of 50 mM KCl, indicating dimer G-quadruplex formation consistent with CD result. As shown in Figure 2B, a new signal appears as the RNA concentration increases (−62.57 ppm). The new signal is clearly observed at an RNA concentration of 1.5 mM, and at 3.0 and 5.0 mM its intensity becomes remarkably greater than that of the initial peak. Each 19F NMR signal arises as a result of the unique fluorine environment; thus, the presence of the two signals confirms the existence of two conformers of the telomere RNA. Accordingly, the two signals were assigned to the dimeric and two-subunits stacked G-quadruplexes in accord with previous studies that suggested two RNA G-quadruplex subunits are most likely to form a two-subunits stacked G-quadruplex (16,17).
Figure 1.
Concept for the detection different RNA structures by a 19F label. Three 19F resonances of different chemical shifts are expected according to single-stranded, dimeric G-quadruplex and two-subunits stacked G-quadruplex.
Figure 2.
19F NMR and 1H NMR spectra of 19F labeled RNA. (A) Chemical structure of 19F labeled telomere RNA bearing 3,5 bis(trifluoromethyl)phenyl group at the 5΄ terminal. (B) 19F NMR spectra of 19F labeled telomere RNA at different concentrations. The peaks of the dimeric and two-subunits stacked G-quadruplex are marked with red and green spots, respectively. Concentrations of RNA indicated on the right. (C)19F NMR of 19F labeled RNA at different temperatures. Red and green spots indicated dimer and two-subunits stacked G-quadruplex. The peaks of single strand are marked with black spots. Temperatures indicated on the right. Condition: 3 mM RNA in 50 mM KCl and 10 mM Tris-HCl buffer (pH 7.0). The sample is kept for 10 min at each temperature. (D) 19F NMR of 19F labeled RNA at different temperatures and concentrations. The peaks of dimer G-quadruplex are red spots. The peak of two-subunits stacked G-quadruplex is green spot. The peaks of single strand are marked with black spots. (E) 1H imino proton NMR of 19F labeled RNA corresponding to 19F NMR at different temperatures and concentrations. The peaks characteristic of the dimeric and two-subunits stacked G-quadruplex are marked with red and green spots, respectively.
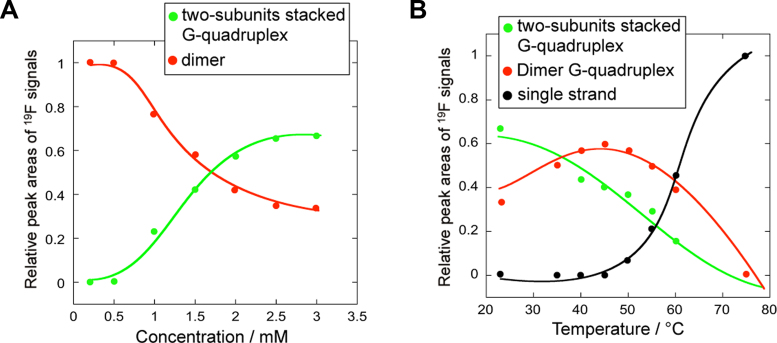
As shown in Figure 3A, profiles of the relative peak areas of the 19F resonance signals versus concentration revealed that a higher RNA concentration promotes the formation of the two-subunits stacked G-quadruplex, suggesting that the of stacking two G-quadruplex subunits is a concentration-dependent manner.
Figure 3.
19F NMR shift versus concentration and temperature profiles. (A) Profiles of the relative peak areas of the 19F resonance signals versus concentration. Dimer and two-subunits stacked G-quadruplex conversions followed by 19F NMR spectroscopy. (B) Profiles of the relative peak areas of the 19F resonance signals versus temperature. Two-subunits stacked G-quadruplex/dimer G-quadruplex/single strand conversions followed by 19F NMR spectroscopy. To obtain the relative peak signal of each conformation, the total value of the relative peak signal for three conformations was estimated to be 1.0. Plotting the values of relative peak signal against temperature results in two melting curves for the two-subunits stacked Gquadruplex and dimer G-quadruplex.
A temperature-dependent experiment was then performed to confirm the assignment of the two signals observed in the 19F NMR spectrum (Figure 2C). As the temperature increased from 23°C to 75°C, the intensity of the signal at −62.57 ppm for the two-subunits stacked G-quadruplex decreased, while that of the signal at −62.91 ppm for the dimeric G-quadruplex increased, indicating the conversion of the two-subunits stacked G-quadruplex to the dimeric G-quadruplex at higher temperatures. Upon heating to 50°C, a new peak at –62.64 ppm corresponding to the unfolded single strand appeared, and at 75°C, only this peak remained with a strong intensity, whereas the peaks of the dimeric G-quadruplex (at −62.91 ppm) and two-subunits stacked G-quadruplex (at −62.57 ppm) completely disappeared. This result indicated that at high temperatures, both the dimer and two-subunits stacked G-quadruplexes unfolded to a single strand.
To further confirm that two subunit G-quadruplexes stack together to form a two-subunits stacked G-quadruplex structure, 1H imino proton NMR analysis was performed. Figure 2E shows the spectrum for a 0.5 mM RNA solution. Six peaks assignable to the dimeric G-quadruplex are observed at 11.0–12.0 ppm that correspond to the peak of the dimeric G-quadruplex in the 19F NMR spectrum (Figure 2D) and are consistent with the results of previous studies (11–14). In the 1H NMR spectrum of a 3 mM RNA solution at 23°C, new signals were observed in addition to major ones (Figure 2E), which was in agreement with the presence of two signals for the dimeric and two-subunits stacked G-quadruplexes in the 19F NMR spectrum (Figure 2D) and suggested that the new signals were due to the two-subunits stacked G-quadruplex structure. Upon heating to 60°C, the new signals disappeared and only the dimeric G-quadruplex peaks were detected in the 1H NMR spectrum (Figure 2E). These observations agreed well with the 19F NMR spectrum at 60°C (Figure 2D), in which signal for the dimeric G-quadruplex appeared. Although signal for the unstructured single strand was observed at 19F NMR at 60°C (Figure 2D), the unstructured single strand does not exhibit any resonance peaks at 11.0–12.0 ppm of the 1H NMR because this region only corresponds to the imino protons of the G-quartet. After complete denaturation (75°C and 85°C), the signals for the dimeric and two-subunits stacked G-quadruplexes were not observed in the 1H NMR spectrum, which is in accordance with the 19F NMR spectrum, in which only the peak for the unstructured single strand was observed.
Encouraged by the ability to use 19F NMR spectroscopy to monitor the conformational transition behavior of the RNA telomere via observation of pronounced changes in the 19F resonances as a function of temperature, the melting process was characterized by plotting the relative peak areas of the 19F resonance signals at various temperatures (Figure 3B). The Tm values for conversion of the two-subunits stacked G-quadruplex to dimeric G-quadruplex and finally to the single strand were estimated to be 52.4°C and 67.8°C, respectively, suggesting stable two-subunits stacked G-quadruplex formation. Importantly, using the corresponding 19F signal curve, a highly precise Tm value was obtained for the two-subunits stacked G-quadruplex, which is not easy to do using other methods, such as CD and ultraviolet spectroscopy, because CD and ultraviolet spectra could not discriminate the higher-order G-quadruplex among different structures.
The presence of resonances for different conformations in the same spectrum at different temperatures allowed quantitative characterization of the thermodynamics of the process (Table 1, and Supplementary Figures S8–S10). The enthalpy change (ΔH) indicates that the stacking interaction of two G-quadruplex subunits is of an enthalpic origin (ΔH = −61.8 kJ/mol). Although conversion from the dimeric G-quadruplex to the single strand involved an unfavorable entropic gain (ΔS = −764.9 J/mol K), large enthalpic contributions to the stability of the G-quadruplex were attributed to the formation of three core G-tetrads (ΔH = −260.6 kJ/mol). Accordingly, the ΔH value for the formation of a single G-tetrad was estimated assuming that the three G-tetrads contribute equally (ΔH = −86.9 kJ/mol = −260.6 ÷ 3). Notably, the ΔH value (ΔH = −61.8 kJ/mol) for the stacking interaction of two G-quadruplex subunits was close to that for the formation of the single G-tetrad, reflecting the significant contribution of the stacking interaction of the two G-quadruplex subunits to the stability of the two-subunits stacked G-quadruplex. These data provide the first evidence that the stacking interactions between two G-quadruplex subunits provide nearly the same energetic contribution as a single G-tetrad.
Table 1. Thermodynamic parameters for RNA conformational transition.
| RNA conformational transition | −ΔH (kJ/mol) | −ΔS (J/mol K) |
|---|---|---|
| Two-subunits G-quadruplex → Dimeric G-quadruplex | 61.8 | 186.5 |
| Dimeric G-quadruplex → Single strand | 260.6 | 764.9 |
aThe thermodynamic parameters were determined from van't Hoff plots (Supplementary Data). The experimental errors for enthalpy (ΔH) and entropy (ΔS) were ±5 kJ/mol and ±10 J/mol K, respectively.
In-cell 19F NMR analysis of telomere RNA G-quadruplexes
Although in vitro19F NMR spectroscopic methods have made very valuable contributions to our understanding of human telomere RNA structures, in vivo observations of RNA G-quadruplex structures are required to gain better insight into the structural basis of their functions inside cells. Indeed, direct evidence for the formation of higher-order G-quadruplexs by human telomere RNA within cells has not yet been obtained.
Recently, developments in NMR technology have made it an effective method for the investigation of biological macromolecules in living cells (in-cell NMR) (36–39). However, the stronger background in the cellular environment often leads to complicated or poor-quality in-cell NMR spectra. For example, 1H imino proton NMR analysis of a telomere DNA G-quadruplex in the cellular environment yields a low-resolution spectrum, thus distinguishing the resonance signals for molecules of interest from the background noise due to the other molecules present in cells remains a challenge (40).
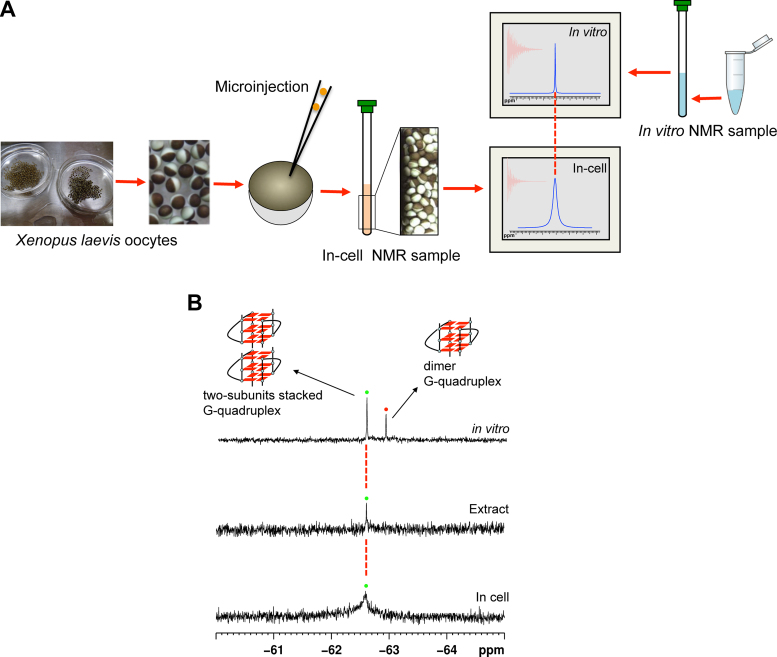
To overcome these limitations, in-cell 19F NMR spectroscopy was employed to investigate the structural features of human telomere RNA in living cells. Because there is no natural intracellular concentration of fluorine, fluorinated oligonucleotides do not suffer from high background signals. The in-cell NMR study was performed by injecting 19F-labeled telomere RNA into Xenopus laevis oocytes and detecting their in-cell conformations using 19F NMR spectroscopy. The strategy used for in-cell 19F NMR spectroscopy is shown in Figure 4A. Nuclei of Xenopus oocytes are transferred directly by inserting the glass nuclear transfer pipette into the centre of the animal pole of the oocyte (41,42). The reference in vitro spectrum was compared to the in-cell 19F-NMR spectrum, enabling reliable determination of the intracellular telomere RNA conformation. Figure 4B shows a comparison of the in vitro and in-cell NMR spectra for the RNA in the pure form (top panel) and upon oocyte injection (bottom panel). Only one signal was observed in the bottom panel NMR spectrum, for which the chemical shift is identical to that observed for the corresponding higher-order G-quadruplex in the in vitro19F NMR spectrum. We further performed an in-cell experiment using low concentration RNA and showed that only one signal, which is same as the chemical shift with high RNA concentration (Supplementary Figure S11), indicating that the two-subunits stacked G-quadruplex structure is still formed at relatively low RNA concentrations. These results demonstrate that the higher-order G-quadruplex structure is present in living cells. The line width of the signal increased in the in-cell spectrum compared to that in the in vitro spectrum, partially because of the higher viscosity of the cellular environment (43,44). NMR signals depend on whether the molecule of interest is freely available for tumbling in solution. In general, molecules display small tumbling rates due to their sizes, intermolecular interactions, intramolecular interactions with subsets of residues, and the high viscosity environments of intracellular materials, leading to fast relaxation and broad NMR lines with reduced overall signal intensities. In addition, an inherent sample inhomogeneity due to the in-cell environment is also is the cause of the broad line width of in-cell signal (43,44). A control experiment in oocyte stocking buffer at 0.5 mM RNA concentration was performed to confirm that the formation of high order G-quadruplex does not result from the surrounding oocyte stocking buffer of in-cell experiment. As shown in Supplementary Figure S12, the chemical shift in oocyte stocking buffer is same with dimeric G-quadruplex, indicating that the NMR signal of higher-order G-quadruplex results from inside the cells rather than the surrounding oocyte stocking buffer.
Figure 4.
In-cell 19F NMR of telomere RNA G-quadruplex. (A) Schematic overview of in-cell 19F NMR experiments. Xenopus laevis oocytes are sorted and collected for microinjections. For in-cell 19F NMR applications in Xenopus oocytes, telomere RNA sample was injected into the oocyte cells. Comparison with the position of reference in vitro spectrum provides a reliable determination of intracellular telomere RNA conformation. (B) Comparison of 19F NMR spectra of in vitro sample of telomere RNA (up) with Xenopus egg lysates (middle) and in Xenopus oocytes (bottom).
For the further investigation of the influence of molecular crowding, cellular lysates represent a good molecular crowding since they provide a native-like condition. We crushed the oocytes containing the injected RNA and recorded 19F NMR spectrum. Notably, the NMR spectral pattern for the lysate of the telomere RNA is similar to those observed for both the corresponding in vitro and in-cell samples (Figure 4B), indicating higher-order G-quadruplex formation in a cell-like condition.
Therefore, by using 19F NMR spectroscopy, it was demonstrated that the telomere RNA G-quadruplexes preferentially adopt a stacked two-subunits G-quadruplex conformation rather than remaining as single-unit G-quadruplex in living cells. These findings provide the first insights into the structures of telomere RNA G-quadruplexes in the cellular environment.
19F NMR analysis of telomere RNA G-quadruplexes in molecular crowding conditions
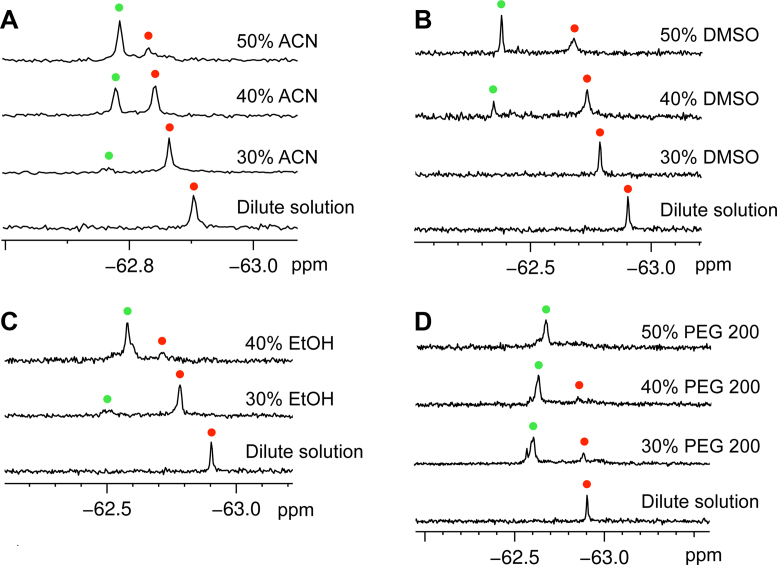
It is known that an intracellular environment was occupied with biological molecules of the cellular volume. A crowded environment (molecular crowding) has reported to influence the structural transition and thermodynamic stability of DNA G-quadruplexes (45,46). Recently, it is reported that ligands can specifically distinguish DNA multimeric G-quadruplexes from monomeric ones under molecular crowding conditions (47,48). To test whether the higher-order RNA G-quadruplex structure is promoted by the crowded environment, we used 19F NMR spectroscopy to investigate the structural feature of telomere RNA G-quadruplex in K+-containing crowded conditions, simulated by different cosolutes. We performed a concentration-dependent experiment to investigate the effect of molecular crowding conditions on the RNA G-quadruplex in crowded solution simulated by three organic solvents acetonitrile (ACN), dimethyl sulfoxide (DMSO) and ethanol (EtOH) at different concentrations (45,46). In the dilute solution (50 mM KCl water solution), one signal was observed at −62.91 ppm, indicating a dimeric parallel G-quadruplex formation, which is consistent with previously reported results (11–14). In crowded solutions of ACN, DMSO and EtOH, a new signal appeared when increasing the volume of cosolutes as compared with the dilute solution (Figure 5A–C). Furthermore, we employed a widely used cosolute PEG 200 to mimic steric crowing, showing the same result that one new signal appears (Figure 5D). This new signal was clearly observed, and its intensity increased remarkably at a cosolute volume of 40–50% (v/v) in four different types of crowded solutions. Each 19F NMR signal arises as a result of the unique fluorine environment; thus, the presence of the two signals confirms the existence of two conformers of the telomere RNA. Therefore, we assigned the new signal to the two-subunits stacked G-quadruplex structure based on the previous reports that molecular crowding could induce the formation of a multimer complex (44,49–54). It is notable that although the PEG 200 and organic solvents have different dielectric constant, polarity and other properties, all of these promote the formation of stacked dimeric G-quadruplex. Recently, several studies demonstrated the dehydration of organic solvents leads to the formation of parallel DNA G-quadruplex from other species (55–57). To combine our results and previous studies, we suggested that the exclusion effect and/or dehydration effect can promote the formation of high-order RNA G-quadruplex.
Figure 5.
19F NMR of telomere RNA G-quadruplex in molecular crowding conditions. (A–D) 19F NMR spectra of 12-mer telomere RNA (0.5 mM) in crowded solutions induced by (A) ACN, (B) DMSO, (C) EtOH and (D) PEG 200. 19F NMR spectra were recorded under 30% (v/v), 40% (v/v) and 50% (v/v) of ACN, DMSO and PEG 200. The crowding condition of EtOH was only simulated by the addition of 30% (v/v) and 40% (v/v) EtOH, due to 50% (v/v) EtOH leading to a RNA precipitation. Red and green spots indicated the dimeric G-quadruplex and two-subunits stacked G-quadruplex. 19F NMR reference spectrum of 19F labeled 12-mer telomere RNA in dilute solution is shown in each case for comparison.
To further understand the effect of molecular crowding on RNA G-quadruplex structure, we measured the specific NMR signals by diluting high concentration RNA to 0.5 mM in diluted solution and molecular crowded condition. We found the transition of two-subunits stacked G-quadruplex to dimeric G-quadruplex is fast in diluted solution, while two-subunits stacked G-quadruplex is still stabilized at ∼0.5 mM under molecular crowded condition (Supplementary Figure S13), indicating that the observation of high-order G-quadruplex in living cells was due to the molecular crowding environment.
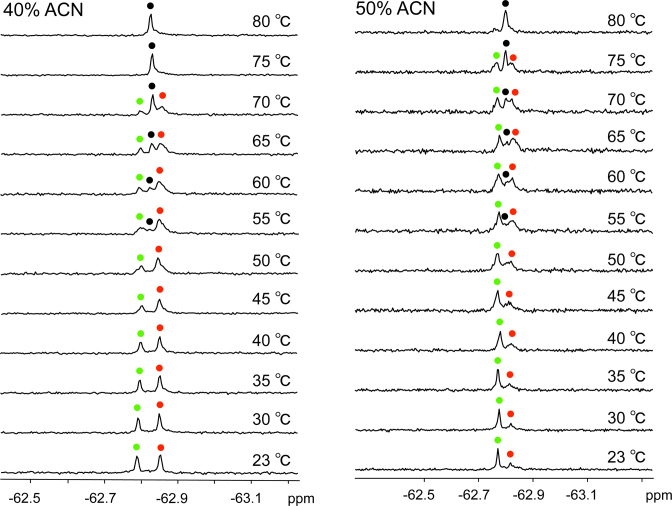
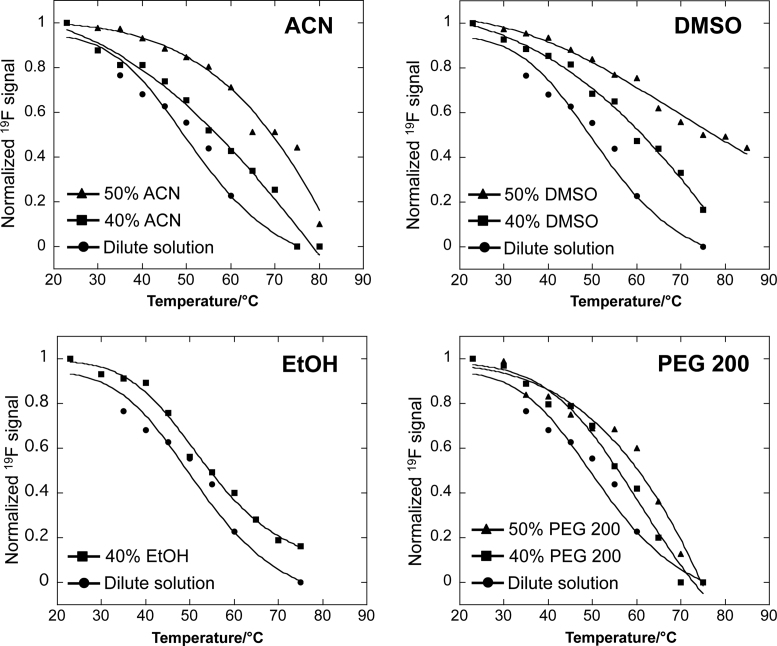
A temperature-dependent experiment was then performed to further confirm the assignment of the two signals that were observed in the 19F NMR spectrum. Figure 6 shows that as the temperature increased from 23°C to 80°C in crowded solution of 40% and 50% (v/v) ACN, the peak intensity of the higher-order G-quadruplex decreased, whereas that of dimeric G-quadruplex increased, indicating the conversion of the higher-order to the dimeric G-quadruplex. In addition, upon heating to 55°C, a new peak corresponding to the unfolded single strand appeared and, at 80°C, only this peak remained with a strong intensity, whereas the peaks of the dimeric G-quadruplex and higher-order G-quadruplex nearly disappeared. These results indicate that at high temperatures, both G-quadruplexes unfolded to an unstructured single strand. Notably, the peak of the higher-order G-quadruplex in 50% (v/v) ACN had a higher intensity than that in 40% (v/v) ACN and still appeared at 75°C but not in 40% (v/v) ACN, indicating a higher thermal stability of the higher-order conformation in higher ratio of cosolutes. NMR data (Supplementary Figure S14) showed that a similar conformational transition could also occur in DMSO, PEG 200 and EtOH molecular crowding conditions under increased temperature. These observations suggest that molecular crowding induced the conformational transition from a dimeric G-quadruplex to a higher-order G-quadruplex structure. The results of Tm value show that molecular crowding substantially stabilizes the higher-order G-quadruplex structure in all types of crowded solutions, leading to an increase in Tm (ΔTm = 2.6–23.8°C) as compared with the dilute solution (Figure 7 and Table 2). A greater stabilization of the higher-order G-quadruplex may result from the structural conversion of RNA G-quadruplex that is induced by the crowded solutions.
Figure 6.
A temperature-dependent experiment of telomere RNA G-quadruplex in molecular crowding conditions. NMR spectra were recorded in crowded solution simulated by 40% (v/v) and 50% (v/v) of ACN. Condition: 0.5 mM RNA in 50 mM KCl and 10 mM Tris-HCl buffer (pH 7.0). The sample is kept for 10 min at each temperature. Red and green spots indicated the dimeric G-quadruplex and two-subunits stacked G-quadruplex. The peaks of single strand are marked with black spots. Temperatures indicated on the right.
Figure 7.
Melting profiles of the relative peak areas of the 19F resonance signals versus temperature. The profiles were obtained by plotting the normalized two-subunits stacked G-quadruplex relative peak areas of the 19F resonance signals in NMR spectra (Figure 2C, Figure 6 and Supplementary Figure S14) as a function of temperature.
Table 2. T m for higher-order G-quadruplex in dilute and different crowded solutions.
| Conditions | T m (°C) | ΔTm (°C) |
|---|---|---|
| Dilute solution | 52.4 | − |
| 40% ACN | 58.6 | 6.2 |
| 50% ACN | 69.5 | 17.1 |
| 40% DMSO | 61.5 | 9.1 |
| 50% DMSO | 76.2 | 23.8 |
| 40% PEG 200 | 56.8 | 4.4 |
| 50% PEG 200 | 61.9 | 9.5 |
| 40% EtOH | 55.0 | 2.6 |
aThe experimental errors for Tm were ±0.5°C.
3 mM RNA in diluted solution and 0.5 mM RNA in molecular crowding solution.
CONCLUSIONS
First, we showed that the simplicity and sensitivity of 19F NMR spectroscopy effectively enables structural studies of telomere RNA G-quadruplexes in vitro. Using 19F NMR spectroscopy, we directly observed the two-subunits stacked G-quadruplex, demonstrating that the two-subunits stacked G-quadruplex is formed predominantly at relatively high RNA concentration. Based on the sharp and clear 19F NMR signals, we determined the thermodynamic parameters of the telomere RNA G-quadruplexes. We demonstrated that the stacking interactions of two G-quadruplex subunits provide the primary energetic contribution to the stability of higher-order RNA G-quadruplex at a level similar to that of a single G-tetrad. These results enable a better understanding of the formation of higher-order RNA G-quadruplexes.
Next, because there is no natural intracellular concentration of fluorine in cells, there is no background noise in in-cell 19F NMR spectra. Therefore, 19F NMR spectroscopy is an ideal tool for studying RNA G-quadruplex structures in living cells. We demonstrated that telomere RNA G-quadruplexes preferentially adopt a two-subunits stacked G-quadruplex in living cells using 19F NMR spectroscopy. To our knowledge, this study is the first direct observation of higher-order RNA G-quadruplex in a cellular environment and provides new insight into the structural behavior of telomere RNA G-quadruplexes in living cells. Our results open new avenues for the investigation of G-quadruplex structures in vitro and in living cells.
Finally we demonstrated that molecular crowding conditions simulated by ACN, EtOH, DMSO and PEG 200 promoted the formation of two-subunits stacked G-quadruplex and stabilized the higher-order structure, which provided unequivocal evidence for the formation of higher-order G-quadruplex structure in living cells.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a Grant-in-Aid for Scientific Research (B) (26288083) and a Grant-in-Aid for Research Activity Start-up (25888019) from the Ministry of Education, Science, Sports, Culture and Technology (Japan). This work was also supported by grants from the Takeda Science Foundation.
Footnotes
Present address: Takashi Sakamoto, Faculty of Systems Engineering, Wakayama University, 930 Sakaedani, Wakayama, Japan.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Grant-in-Aid for Scientific Research (B) and Research Activity Start-up from Ministry of Education, Science, Sports, Culture and Technology (Japan) [26288083 and 25888019]; Takeda Science Foundation. Funding for open access charge: Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Science, Sports, Culture and Technology (Japan) [26288083].
Conflict of interest statement. None declared.
REFERENCES
- 1. Kertesz M., Wan Y., Mazor E., Rinn J.L., Nutter R.C., Chang H.Y., Segal E.. Genome-wide measurement of RNA secondary structure in yeast. Nature. 2010; 467:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wan Y., Kertesz M., Spitale R.C., Segal E., Chang H.Y.. Understanding the transcriptome through RNA structure. Nat. Rev. Genet. 2011; 12:641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding Y., Tang Y., Kwok C.K., Zhang Y., Bevilacqua P.C., Assmann S.M.. In vivo genome-wide profiling of RNA secondary structure reveals novel regulatory features. Nature. 2014; 505:696–700. [DOI] [PubMed] [Google Scholar]
- 4. Collie G.W., Parkinson G.N.. The application of DNA and RNA G-quadruplexes to therapeutic medicines. Chem. Soc. Rev. 2011; 40:5867–5892. [DOI] [PubMed] [Google Scholar]
- 5. Xu Y. Chemistry in human telomere biology: structure, function and targeting of telomere DNA/RNA. Chem. Soc. Rev. 2011; 40:2719–2740. [DOI] [PubMed] [Google Scholar]
- 6. Balk B., Maicher A., Dees M., Klermund J., Luke-Glaser S., Bender K., Luke B.. Telomeric RNA-DNA hybrids affect telomere-length dynamics and senescence. Nat. Struct. Mol. Biol. 2013; 20:1199–1205. [DOI] [PubMed] [Google Scholar]
- 7. Haeusler A.R., Donnelly C.J., Periz G., Simko E.A., Shaw P.G., Kim M.S., Maragakis N.J., Troncoso J.C., Pandey A., Sattler R. et al. . C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature. 2014; 507:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolfe A.L., Singh K., Zhong Y., Drewe P., Rajasekhar V.K., Sanghvi V.R., Mavrakis K.J., Jiang M., Roderick J.E., Van der Meulen J. et al. . RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature. 2014; 513:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azzalin C.M., Reichenbach P., Khoriauli L., Giulotto E., Lingner J.. Telomeric repeat-containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007; 318:798–801. [DOI] [PubMed] [Google Scholar]
- 10. Schoeftner S., Blasco M.A.. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat. Cell Biol. 2008; 10:228–236. [DOI] [PubMed] [Google Scholar]
- 11. Xu Y., Kaminaga K., Komiyama M.. G-quadruplex formation by human telomeric repeats-containing RNA in Na+ solution. J. Am. Chem. Soc. 2008; 130:11179–11184. [DOI] [PubMed] [Google Scholar]
- 12. Martadinata H., Phan A.T.. Structure of propeller-type parallel-stranded RNA G-quadruplexes, formed by human telomeric RNA sequences in K+ solution. J. Am. Chem. Soc. 2009; 131:2570–2578. [DOI] [PubMed] [Google Scholar]
- 13. Xu Y., Ishizuka T., Kimura T., Komiyama M.. A U-tetrad stabilizes human telomeric RNA G-quadruplexstructure. J. Am. Chem. Soc. 2010; 132:7231–7233. [DOI] [PubMed] [Google Scholar]
- 14. Xu Y., Suzuki Y., Ito K., Komiyama M.. Telomeric repeat-containing RNA structure in living cells. Proc. Natl. Acad. Sci. U.S.A. 2010; 107:14579–14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu Y., Ishizuka T., Yang J., Ito K., Katada H., Komiyama M., Hayashi T.. Oligonucleotide models of telomeric DNA and RNA form a Hybrid G-quadruplex structure as a potential component of telomeres. J. Biol. Chem. 2012; 287:41787–41796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collie G.W., Parkinson G.N., Neidle S., Rosu F., De Pauw E., Gabelica V.. Electrospray mass spectrometry of telomeric RNA (TERRA) reveals the formation of stable multimeric G-quadruplex structures. J. Am. Chem. Soc. 2010; 132:9328–9334. [DOI] [PubMed] [Google Scholar]
- 17. Martadinata H., Phan A.T.. Structure of human telomeric RNA (TERRA): stacking of two G-quadruplex blocks in K+ solution. Biochemistry. 2013; 52:2176–2183. [DOI] [PubMed] [Google Scholar]
- 18. Zhou L., Rajabzadeh M., Traficante D.D., Cho B.P.. Conformational heterogeneity of arylamine-modified DNA: 19F NMR evidence. J. Am. Chem. Soc. 1997; 119:5384–5389. [Google Scholar]
- 19. Hammann C., Norman D.G., Lilley D.M.. Dissection of the ion-induced folding of the hammerhead ribozyme using 19F NMR. Proc. Natl. Acad. Sci. U.S.A. 2001; 98:5503–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scott L.G., Geierstanger B.H., Williamson J.R., Hennig M.. Enzymatic synthesis and 19F NMR studies of 2-fluoroadenine-substituted RNA. J. Am. Chem. Soc. 2004; 126:11776–11777. [DOI] [PubMed] [Google Scholar]
- 21. Olsen G.L., Edwards T.E., Deka P., Varani G., Sigurdsson S.T., Drobny G.P.. Monitoring tat peptide binding to TAR RNA by solid-state 31P-19F REDOR NMR. Nucleic Acids Res. 2005; 33:3447–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hennig M., Scott L.G., Sperling E., Bermel W., Williamson J.R.. Synthesis of 5-fluoropyrimidine nucleotides as sensitive NMR probes of RNA structure. J. Am. Chem. Soc. 2007; 129:14911–14921. [DOI] [PubMed] [Google Scholar]
- 23. Barhate N.B., Barhate R.N., Cekan P., Drobny G., Sigurdsson S.T.. A nonafluoro nucleoside as a sensitive 19F NMR probe of nucleic acid conformation. Org. Lett. 2008; 10:2745–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graber D., Moroder H., Micura R.. 19F NMR spectroscopy for the analysis of RNA secondary structure populations. J. Am. Chem. Soc. 2008; 130:17230–17231. [DOI] [PubMed] [Google Scholar]
- 25. Kiviniemi A., Virta P.. Characterization of RNA invasion by 19F NMR spectroscopy. J. Am. Chem. Soc. 2010; 132:8560–8562. [DOI] [PubMed] [Google Scholar]
- 26. Sakamoto T., Hayakawa H., Fujimoto K.. Development of a potassium ion sensor for 19F magnetic resonance chemical shift imaging based on fluorine-labeled thrombin aptamer. Chem. Lett. 2011; 40:720–721. [Google Scholar]
- 27. Fauster K., Kreutz C., Micura R.. 2΄-SCF3 uridine-a powerful label for probing structure and function of RNA by 19F NMR spectroscopy. Angew. Chem. Int. Ed. 2012; 51:13080–13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lombes T., Moumne R., Larue V., Prost E., Catala M., Lecourt T., Dardel F., Micouin L., Tisne C.. Investigation of RNA-ligand interactions by 19F NMR spectroscopy using fluorinated probes. Angew. Chem. Int. Ed. 2012; 51:9530–9534. [DOI] [PubMed] [Google Scholar]
- 29. Chen H., Viel S., Ziarelli F., Peng L.. 19F NMR: a valuable tool for studying biological events. Chem. Soc. Rev. 2013; 42:7971–7982. [DOI] [PubMed] [Google Scholar]
- 30. Tanabe K., Tsuda T., Ito T., Nishimoto S.. Probing DNA mismatched and bulged structures by using 19F NMR spectroscopy and oligodeoxynucleotides with an 19F-labeled nucleobase. Chem. Eur. J. 2013; 19:15133–15140. [DOI] [PubMed] [Google Scholar]
- 31. Granqvist L., Virta P.. 4΄-C-[(4-trifluoromethyl-1H-1,2,3-triazol-1-yl)methyl]thymidine as a sensitive 19F NMR sensor for the detection of oligonucleotide secondary structures. J. Org. Chem. 2014; 79:3529–3536. [DOI] [PubMed] [Google Scholar]
- 32. Zhao C., Devany M., Greenbaum N.L.. Measurement of chemical exchange between RNA conformers by 19F NMR. Biochem. Biophys. Res. Commun. 2014; 453:692–695. [DOI] [PubMed] [Google Scholar]
- 33. Zimmerman S.B., Trach S.O.. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991; 222:599–620. [DOI] [PubMed] [Google Scholar]
- 34. Ellis R.J., Minton A.P.. Cell biology: join the crowd. Nature. 2003; 425:27–28. [DOI] [PubMed] [Google Scholar]
- 35. Zhou H.X., Rivas G., Minton A.P.. Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 2008; 37:375–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sakai T., Tochio H., Tenno T., Ito Y., Kokubo T., Hiroaki H., Shirakawa M.. In-cell NMR spectroscopy of proteins inside Xenopus laevis oocytes. J. Biomol. NMR. 2006; 36:179–188. [DOI] [PubMed] [Google Scholar]
- 37. Serber Z., Selenko P., Hansel R., Reckel S., Lohr F., Ferrell J.E. Jr, Wagner G., Dotsch V.. Investigating macromolecules inside cultured and injected cells by in-cell NMR spectroscopy. Nat. Protoc. 2006; 1:2701–2709. [DOI] [PubMed] [Google Scholar]
- 38. Selenko P., Serber Z., Gadea B., Ruderman J., Wagner G.. Quantitative NMR analysis of the protein G B1 domain in Xenopus laevis egg extracts and intact oocytes. Proc. Natl. Acad. Sci. U.S.A. 2006; 103:11904–11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sakakibara D., Sasaki A., Ikeya T., Hamatsu J., Hanashima T., Mishima M., Yoshimasu M., Hayashi N., Mikawa T., Walchli M. et al. . Protein structure determination in living cells by in-cell NMR spectroscopy. Nature. 2009; 458:102–105. [DOI] [PubMed] [Google Scholar]
- 40. Hansel R., Foldynova-Trantirkova S., Lohr F., Buck J., Bongartz E., Bamberg E., Schwalbe H., Dotsch V., Trantirek L.. Evaluation of parameters critical for observing nucleic acids inside living Xenopus laevis oocytes by in-cell NMR spectroscopy. J. Am. Chem. Soc. 2009; 131:15761–15768. [DOI] [PubMed] [Google Scholar]
- 41. Allen T.D., Rutherford S.A., Murray S., Sanderson H.S., Gardiner F., Kiseleva E., Goldberg M.W., Drummond S.P.. A protocol for isolating Xenopus oocyte nuclear envelope for visualization and characterization by scanning electron microscopy (SEM) or transmission electron microscopy (TEM). Nat. Protoc. 2007; 2:1166–1172. [DOI] [PubMed] [Google Scholar]
- 42. Halley-Stott R.P., Pasque V., Astrand C., Miyamoto K., Simeoni I., Jullien J., Gurdon J.B.. Mammalian nuclear transplantation to Germinal Vesicle stage Xenopus oocytes-a method for quantitative transcriptional reprogramming. Methods. 2010; 51:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Selenko P., Wagner G.. Looking into live cells with in-cell NMR spectroscopy. J. Struct. Biol. 2007; 158:244–253. [DOI] [PubMed] [Google Scholar]
- 44. Hansel R., Lohr F., Foldynova-Trantirkova S., Bamberg E., Trantirek L., Dotsch V.. The parallel G-quadruplex structure of vertebrate telomeric repeat sequences is not the preferred folding topology under physiological conditions. Nucleic Acids Res. 2011; 39:5768–5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu H., Gu X., Nakano D., Miyoshi D., Sugimoto N.. Beads-on-a-string structure of long telomeric DNAs under molecular crowding conditions. J. Am. Chem. Soc. 2012; 134:20060–20069. [DOI] [PubMed] [Google Scholar]
- 46. Heddi B., Phan A.T.. Structure of human telomeric DNA in crowded solution. J. Am. Chem. Soc. 2011; 133:9824–9833. [DOI] [PubMed] [Google Scholar]
- 47. Huang X.X., Zhu L.N., Wu B., Huo Y.F., Duan N.N., Kong D.M.. Two cationic porphyrin isomers showing different multimeric G-quadruplex recognition specificity against monomeric G-quadruplexes. Nucleic Acids Res. 2014; 42:8719–8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang Q., Liu Y.C., Kong D.M., Guo D.S.. Tetraphenylethene derivatives with different numbers of positively charged side arms have different multimeric G-quadruplex recognition specificity. Chem. Eur. J. 2015; 21:13253–13260. [DOI] [PubMed] [Google Scholar]
- 49. Minton A.P. Excluded volume as a determinant of macromolecular structure and reactivity. Biopolymers. 1981; 20:2093–2120. [Google Scholar]
- 50. Zimmerman S.B., Minton A.P.. Macromolecular crowding: biochemical, biophysical, and physiological consequences Annu. Rev. Biophys. Biomol. Struct. 1993; 22:27–65. [DOI] [PubMed] [Google Scholar]
- 51. Wenner J.R., Bloomfield V.A.. Crowding effects on EcoRV kinetics and binding. Biophys. J. 1999; 77:3234–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Davis-Searles P.R., Saunders A.J., Erie D.A., Winzor D.J., Pielak G.J.. Interpreting the effects of small uncharged solutes on protein-folding equilibria. Annu. Rev. Biophys. Biomol. Struct. 2001; 30:271–306. [DOI] [PubMed] [Google Scholar]
- 53. Minton A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2011; 276:10577–10580. [DOI] [PubMed] [Google Scholar]
- 54. Miyoshi D., Karimata H., Sugimoto N.. Drastic effect of a single base difference between human and tetrahymena telomere sequences on their structures under molecular crowding conditions. Angew. Chem. Int. Ed. 2005; 44:3740–3744. [DOI] [PubMed] [Google Scholar]
- 55. Buscaglia R., Miller M.C., Dean W.L., Gray R.D., Lane A.N., Trent J.O., Chaires J.B.. Polyethylene glycol binding alters human telomere G-quadruplex structure by conformational selection. Nucleic Acids Res. 2013; 41:7934–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dhakal S., Cui Y., Koirala D., Ghimire C., Kushwaha S., Yu Z., Yangyuoru P.M., Mao H.. Structural and mechanical properties of individual human telomeric G-quadruplexes in molecularly crowded solutions. Nucleic Acids Res. 2013; 41:3915–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miller M.C., Buscaglia R., Chaires J.B., Lane A.N., Trent J.O.. Hydration is a major determinant of the G-Quadruplex stability and conformation of the human telomere 3΄ sequenc of d(AG3(TTAG3)3). J. Am. Chem. Soc. 2010; 132:17105–17107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.