Abstract
Human RNA methyltransferase BCDIN3D is overexpressed in breast cancer cells, and is related to the tumorigenic phenotype and poor prognosis of breast cancer. Here, we show that cytoplasmic tRNAHis is the primary target of BCDIN3D in human cells. Recombinant human BCDIN3D, expressed in Escherichia coli, monomethylates the 5΄-monophosphate of cytoplasmic tRNAHis efficiently in vitro. In BCDN3D-knockout cells, established by CRISPR/Cas9 editing, the methyl moiety at the 5΄-monophosphate of cytoplasmic tRNAHis is lost, and the exogenous expression of BCDIN3D in the knockout cells restores the modification in cytoplasmic tRNAHis. BCIDN3D recognizes the 5΄-guanosine nucleoside at position −1 (G−1) and the eight-nucleotide acceptor helix with the G−1–A73 mis-pair at the top of the acceptor stem of cytoplasmic tRNAHis, which are exceptional structural features among cytoplasmic tRNA species. While the monomethylation of the 5΄-monophosphate of cytoplasmic tRNAHis affects neither the overall aminoacylation process in vitro nor the steady-state level of cytoplasmic tRNAHisin vivo, it protects the cytoplasmic tRNAHis transcript from degradation in vitro. Thus, BCDIN3D acts as a cytoplasmic tRNAHis-specific 5΄-methylphosphate capping enzyme. The present results also suggest the possible involvement of the monomethylation of the 5΄-monophosphate of cytoplasmic tRNAHis and/or cytoplasmic tRNAHis itself in the tumorigenesis of breast cancer cells.
INTRODUCTION
Bicoid-interacting protein 3 (BCDIN3) was initially isolated as a protein that interacts with the homeodomain-containing transcription factor, Bicoid, in Drosophila (1,2). BCDIN3 contains an S-(5΄-adenosyl)-l-methionine (AdoMet) binding motif, and is homologous to a conserved family of eukaryotic protein methyltransferases acting on RNA-binding proteins (2,3). BCDIN3 is required for embryonic development in Drosophila (4). Subsequent studies revealed that the human BCDIN3 homolog monomethylates the 5΄-γ-phosphate of 7SK RNA, a non-coding RNA involved in regulating the activity of the positive-acting transcription elongation factor, P-TEFb, a cyclin-dependent kinase complex (5,6). Monomethylation of the 5΄-γ-phosphate of 7SK RNA protects 7SK from degradation and controls the activity of P-TEFb; therefore, BCDIN3 was renamed methylphosphate capping enzyme, MePCE (7,8).
The BCDIN3 Domain containing protein, BCDIN3D, is conserved from worm to human (9). The biological properties and function of BCDIN3D have not been characterized. BCDIN3D mRNA is overexpressed in human breast cancer cells, and the elevated expression of BCDIN3D is related to poor prognosis in breast cancer (10,11). Recently, it was reported that BCDIN3D dimethylates the 5΄-monophosphate of specific precursor miRNAs (pre-miRNAs), such as the tumor suppressor miR145 (12–14), in MCF-7 breast cancer cells (9). Dimethylation of the 5΄-monophosphate of the pre-miRNA negatively regulates the subsequent processing by Dicer in vitro (9,15), and results in the downregulated expression of the mature miRNA form. The shRNA-mediated depletion of BCDIN3D also reportedly results in the suppression of the tumorigenic phenotype of MDA-MB-231 breast cancer cells. Thus, BCDIN3D was proposed to promote the cellular invasion of breast cancer cells, by downregulating the expression of tumor suppressor miRNAs through the dimethylation of the 5΄-monophosphate of the corresponding pre-miRNAs (9).
The mechanisms by which BCDIN3D recognizes specific pre-miRNAs and negatively regulates the expression of matured miRNAs in breast cancer cells are not well understood. To identify other possible target RNAs that are methylated by BCDIN3D, and to clarify the mechanism by which BCDIN3D recognizes specific RNAs, we analyzed the BCDIN3D-binding RNAs in human HEK293T cells.
Here, we report that cytoplasmic histidyl tRNA (tRNAHis) is the primary target of BCDIN3D in HEK293T cells. We show that cytoplasmic tRHAHis tightly binds to BCDIN3D in vivo, and the 5΄-monophosphate of cytoplasmic tRNAHis is monomethylated by BCDIN3D in vitro and in vivo. BCDIN3D monomethylates tRNAHis more efficiently than pre-miR145 by over two orders of magnitude in vitro, and never dimethylates the 5΄-monophosphates of tRNAHis and pre-miR145. BCDIN3D recognizes the unique structures within cytoplasmic tRNAHis—the additional guanosine residue at position −1 (G−1) and the eight-nucleotide acceptor helix with the G−1–A73 mis-pair, which are exceptional features among cytoplasmic tRNA species. The monomethylation of the 5΄-phosphate of cytoplasmic tRNAHis protects it from degradation in vitro. Therefore, BCDIN3D acts as a cytoplasmic tRNAHis-specific 5΄-methylphosphate capping enzyme. The involvement of the 5΄-monophosphate methylation of cytoplasmic tRNAHis and/or tRNAHis itself in the tumorigenic phenotype of breast cancer cells is discussed.
MATERIALS AND METHODS
BCDINS3D-expressing stable cell lines
HEK293T and Flp-InTM T-RExTM-293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS, under a humidified 5% CO2 atmosphere at 37°C. The stable human cell line expressing streptavidin binding protein (SBP)-fused BCDIN3D (SBP-BCDIN3D) was established by Flp Recombinase-Mediated Integration (Flp-In system, Invitrogen, Japan), according to the manufacturer's instructions. The synthetic cDNA encoding human BCDIN3D was purchased from Eurofin Genomics, Japan, and was cloned into the pcDNA5/FRT/TO-Flag-HA-SBP vector (kind gift from Dr. Yamashita at Yokohama City University, Medical School). The resultant BCDIN3D expression plasmid, pcDNA5-SBP-BCDIN3D, was transfected into the host Flp-InTM T-RExTM-293 cells, using Lipofectamine 2000 (Invitrogen, Japan). The cell line harboring pcDNA5-SBP-BCDIN3D was selected with hygromycin (100 μg/ml) and blasticidin (10 μg/ml) for two weeks, according to the manufacturer's instructions.
Preparation of SBP-BCDIN3D-associating RNAs
The stable cell lines expressing SBP-BCDIN3D were expanded to ten 10 cm plates, and SBP-BCDIN3D expression was induced by a treatment with 500 ng/ml of doxycycline for 48 h. The cell extracts were prepared with cell lysis buffer, containing 10 mM Tris–Cl, pH 8.0, 150 mM KCl, 10% (v/v) glycerol, 1% (v/v) Triton X-100, 5 mM DTT, 0.5 mM PMSF and complete protease cocktail (Roche, Japan) (buffer-A). SBP-BCDIN3D was purified using streptavidin beads (GE Healthcare, Japan). The beads were washed with buffer-A, and SBP-BCDIN3D was eluted by buffer-A containing 2 mM biotin (Sigma-Aldrich, Japan). The SBP-BCDIN3D associating RNAs were prepared using ISOGEN II (Nippon Gene, Japan), and separated by 10% (w/v) polyacrylamide gel electrophoresis under denaturing conditions, and the gel was stained with SYBR-Gold Nucleic Acid Stain (Invitrogen, Japan).
Mass spectrometric analysis of RNAs
The gel-purified RNA was digested by RNases, and the resultant RNA fragments were analyzed by capillary LC-nano ESI-mass spectrometry, as described previously (16,17). The gel-purified RNA-x (0.1 μg) was digested with RNase T1 (Epicentre) or RNase A (Ambion) and analyzed by an LTQ Orbitrap mass spectrometer (ThermoFisher Scientific, Japan), with a nano-electrosprayer connected to a splitless nanoflow high pressure liquid chromatography system (DiNa, KYA Technologies).
Expression and purification of BCDIN3D in E. coli
The DNA encoding human BCDIN3D was cloned between the Nde I and Xho I sites of the pET15b vector (Novergen, Japan). Escherichia coli BL21(DE3) cells were transformed by the plasmid and grown in LB medium containing 50 μg/ml ampicillin at 37°C until the A600 reached 0.8, and the expression of histidine-tagged BCDIN3D was induced by the addition of IPTG (isopropyl-β-d thiogalactopyranoside) at a final concentration of 0.1 mM for 12 h at 20°C. The cells were harvested, sonicated in buffer containing 50 mM Tris–Cl, pH 7.0, 500 mM NaCl, 5 mM β-mercaptoethanol, 10 mM imidazole and 10% (v/v) glycerol (buffer-B), and centrifuged at 100 000 g for 1 h at 4°C. The clear supernatant was applied to a Ni2+-NTA agarose column (QIAGEN, Japan) equilibrated with buffer-B. The column was washed with buffer-B, and the protein was eluted with buffer containing 50 mM Tris–Cl, pH 7.0, 500 mM NaCl, 5 mM β-mercaptoethanol and 400 mM imidazole. The protein was further purified by chromatography on Hi-Trap Q and Hi-Trap Heparin columns (GE Healthcare, Japan). Finally, the protein was purified by chromatography on a Hi-Load 16/60 Superdex 200 column (GE Healthcare, Japan), in buffer containing 25 mM Tris–Cl, pH 7.0, 200 mM NaCl and 10 mM β-mercaptoethanol, concentrated to 3 mg/ml and stored at −80°C. The catalytically inactive BCDIN3D protein with the D72A/G74A mutations was also prepared as described above.
In vitro methylation assay
In vitro methylation of RNAs was carried out by the trichloroacetic acid (TCA) precipitation of reaction products. For standard assays using 14C-S-adenosylmethionine (AdoMet), a reaction mixture (80 μl volume) containing 50 mM Tris–Cl, pH 7.9, 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, 2 mM DTT, 5 % (v/v) glycerol, 2 μM RNA, 50 μM [14C]-AdoMet (40 mCi/mmol, PerkinElmer, Japan), and 0.1 μM BCDIN3D was incubated at 37°C. An aliquot (10 μl) was withdrawn at the indicated time from the reaction solution and spotted onto a Whatman 3MM filter (GE Healthcare, Japan) that was pre-soaked with cold 10% (w/v) TCA. After the filters were washed with cold 10% (w/v) TCA, they were washed with ethanol and dried. The filters were suspended in Ultima Gold liquid scintillation cocktail (PerkinElmer, Japan) in vials, and the radioactivities on the filters were quantified with a liquid scintillation counter (Beckman Coulter). To visualize the 14C-methylated RNA products, after the reaction, the RNAs were purified by phenol–chloroform treatment, followed by ethanol precipitation, and separated by 10% (v/v) polyacrylamide gel electrophoresis under denaturing conditions. The gel was dried and exposed to an imaging plate for 16 h, and the intensities of the methylated RNAs were quantified with a BASS2000 imager (Fujifilm, Japan). For kinetic analysis, the specific activity of 3H-AdoMet (55 Ci/mmol, PerkinElmer, Japan) was adjusted to 1.1 Ci/mol (500 μM) with non-radiolabeled AdoMet (Sigma-Aldrich, Japan), and used for the assays. Reaction mixtures (10 μl), containing 50 mM Tris–Cl, pH 7.9, 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, 2 mM DTT, 5 % (v/v) glycerol, 50 μM [3H]-AdoMet (1.1 Ci /mmol), 0.1 μM BCDIN3D, and various concentrations of RNAs (0.125–15 μM), were incubated at 37°C for 10 min. RNA substrates (pre-miR145, tRNAHis, tRNAPhe and their variants) were synthesized, using plasmids encoding the respective template DNA sequences downstream of the T7 promoter, by T7 RNA polymerase in the presence of excess GMP, and were purified by 10% (w/v) polyacrylamide gel electrophoresis under denaturing conditions. The RNA mini-helices corresponding to the top-half of cytoplasmic tRNAHis, with 5΄-monophosphate and its variants (Supplementary Table S1), were purchased from FASMAC, Japan.
Construction of BCDIN3D knockout cells by CRISPR/Cas9
BCDIN3D knockout cells were generated by the CRISPR-Cas9 system (18). Oligonucleotides encoding sgRNAs (sgRNA1 or sgRNA2), which target exon 1 of the BCDIN3D gene, were cloned into the pX330 (Addgene plasmid 42230) vector (19). The nucleotide sequences of the oligonucleotides encoding the sgRNAs are shown in Supplementary Table S1. HEK293T cells were seeded into 24-well plates at a density of 250 000 cells per well, and were used for transfection. Cells were transfected with 300 ng of PX330 containing the sgRNA sequence, 100 ng of pLL3.7-puro and 100 ng of pEGFP-N1, using Lipofectamine 3000 (Life Technologies, Japan) according to the manufacturer's protocol. After 24 h, the transfection efficiency was estimated from the fraction of fluorescent cells, and transfected cells were selected by adding 5 μg/ml of puromycin (Sigma Aldrich) for 5 days. After 5 days, the cells were diluted to obtain isolated clones. The isolated HEK293T cells were further transfected with PX330 containing the sgRNA sequence, to enhance the possibility of obtaining BCDIN3D knockout cells, and isolated clones were obtained as described above. The knockouts of the BCDIN3D gene were confirmed by DNA sequencing of PCR fragments spanning the target sites, and the lack of BCDIN3D expression in the isolated cells was confirmed by western blotting using an anti-BCDIN3D antibody (Sigma Aldrich). Cytoplasmic tRNAHis species were isolated from total tRNA fractions, prepared from HEK293T and BCDIN3D knockout cells, by the solid-phase hybridization method (20–22). The 3΄-biotinylated oligonucleotide probe, with the sequence complementary to the 3΄-terminal 30 nucleotides of cytoplasmic tRNAHis (5΄-CCGTGACTCGGATTCGAACCGAGGTTGCTG-biotin-3΄), was purchased from Eurofins, Japan. The isolated tRNAHis species were further purified by 10% (v/v) polyacrylamide gel electrophoresis under denaturing conditions.
Measurement of cell growth
For measurement of cell growth, HEK293T, KO1 and KO2 cells were seeded in a 96-well plate (2500 cells/ well; 100 μl medium) and cultured at 37°C. Cell proliferation was measured using a CellTiter-Blue Cell Viability Assay (Promega, Japan), according to the manufacturer's instructions. At the indicated times (1, 2, 3 and 4 days), the CellTiter-Blue Reagent (20 μl/well) was added, and the cells were incubated for 1 h before recording the fluorescence (560Ex/590Em), using a GloMax Multi-Detection System (Promega, Japan).
In vitro aminoacylation assay
The tRNAHis transcript with the 5΄-monophospate was methylated by BCDIN3D in vitro, and the resultant tRNAHis with a 5΄-monomethylphosphate was gel-purified. The fraction of methylated tRNAHis (tRNAHis_pmG-1) was found to be more than 99%, as revealed by the LC–mass spectrometric analysis. The DNA encoding human histidine tRNA synthetase (HisRS) lacking the C-terminal three amino acids (C507I508C509) was cloned between the Nde I and Xho I sites of the pET15b vector (Novagen, Japan). The hexa-histidine-tagged HRS was expressed in Escherichia coli BL21(DE3) cells, and purified as described above. Reaction solutions containing 50 mM Tris–Cl, pH 7.6, 100 mM KCl, 10 mM MgCl2, 8 mM DTT, 2.5 mM ATP, 60 μM l-[14C]-histidine (320 mCi/mmol, PerkinElmer, Japan), tRNAHis variants (0–7.5 μM) and 5 nM HisRS were incubated at 37°C for 10 min. The reaction solutions were spotted onto Whatman 3MM filters (GE Healthcare, Japan) pre-soaked with cold 10% (w/v) TCA. The filters were washed with cold 10% (w/v) TCA, and then with ethanol and dried. The radioactivities on the filters were quantified as described above.
Northern blotting
Total RNAs or smaller RNAs (shorter than 200 nucleotides) from human cells were purified with the Isogen-II (Nippon Gene, Japan) or NucleoSpin miRNA (Takara, Japan) reagent, according to the manufacturer's protocol. The RNAs (2.5–10 μg) were separated by 10% (v/v) polyacrylamide gel electrophoresis under denaturing conditions, and blotted onto a Hybond-N+ membrane (GE Healthcare, Japan) using a Trans-Blot SD semi-dry Electrophoretic Transfer Cell (Bio-Rad, Japan), according to the manufacturer's protocol. Hybridization was performed overnight at 55°C in PerfectHyb Hybridization solution (Toyobo, Japan), using 5΄-32P-labeled oligo DNA probes. The membrane was washed three times in 2 × standard saline citrate (SSC) and 0.1% (w/v) SDS at 55°C for 20 min. The DNA sequences used as specific probes are shown in Supplementary Table S1.
Quantitative PCR
Smaller RNAs (shorter than 200 nucleotides) from human cells were purified, as described above. The expression level of mature miR145 relative to that of U6 snRNA was quantified by using a TaqMan MicroRNA Assay (Applied Biosystems, Japan). The RNA (10 ng) was used for reverse transcription (RT, 15 μl reaction solution) with a Taqman MicroRNA Reverse Transcription kit (Applied Biosystems, Japan). After RT, qPCR was performed using 0.75 μl of the RT solution with Taqman Universal Master mix II (Applied Biosystems, Japan, 10 μl reaction solution). The expression level of pre-miR145 relative to that of U6 snRNA was quantified by qPCR, using pre-miR145-specific primers (Supplementary Table S1). For RT (10 μl reaction solution), 10 ng RNA was incubated with Primescript RT kit (Perfect Real Time (Takara, Japan) reagents. After RT, qPCR was performed using 1 μl of RT solution and SYBR Premix Ex Taq II (Takara, Japan, 20 μl reaction solution).
In vitro decay of tRNAs
The tRNAHis transcript (1 μg) was dephosphorylated by bacterial alkaline phosphatase (BAP; Takara, Japan), and was 5΄-32P-labeled by T4 polynucleotide kinase (PNK; Toyobo, Japan) and γ-[32P]-ATP (3000 Ci/mmol, PerkinElmer, Japan). The 5΄-32P labeled tRNAHis (5΄p-tRNAHis) was methylated by recombinant BCDIN3D, resulting in the synthesis of 5΄mp-RNAHis. The 5΄p-tRNAHis and 5΄mp-tRNAHis were further purified by acrylamide gel electrophoresis under denaturing conditions. The cytoplasmic fraction of HEK293T cells was prepared with NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Japan), according to the manufacturer's protocol, and the concentration of proteins in the extracts was quantified by a Bradford assay (Bio-Rad, Japan), using BSA as the standard. The reaction mixtures (90 μl), containing 20 mM Tris–Cl, pH 8.0, 100 mM KCl, 3.2 mM MgCl2, 1mM DTT, 140 μg cytoplasmic protein and 10 000 cpm 5΄-ptRNAHis (or 5΄-mptRNAHis), were incubated at 37°C. At the indicated times (0, 10, 20, 30, 60, 90, 120 and 150 min), an aliquot (10 μl) was withdrawn from the solution, and the RNA was purified by phenol-extraction and ethanol-precipitated. The RNAs were separated by 10% (w/v) polyacrylamide gel electrophoresis under denaturing conditions. The intensities of the intact RNA were quantified with a BASS2000 imager (Fujifilm, Japan).
RESULTS
Cytoplasmic tRNAHis associates with BCDIN3D in vivo
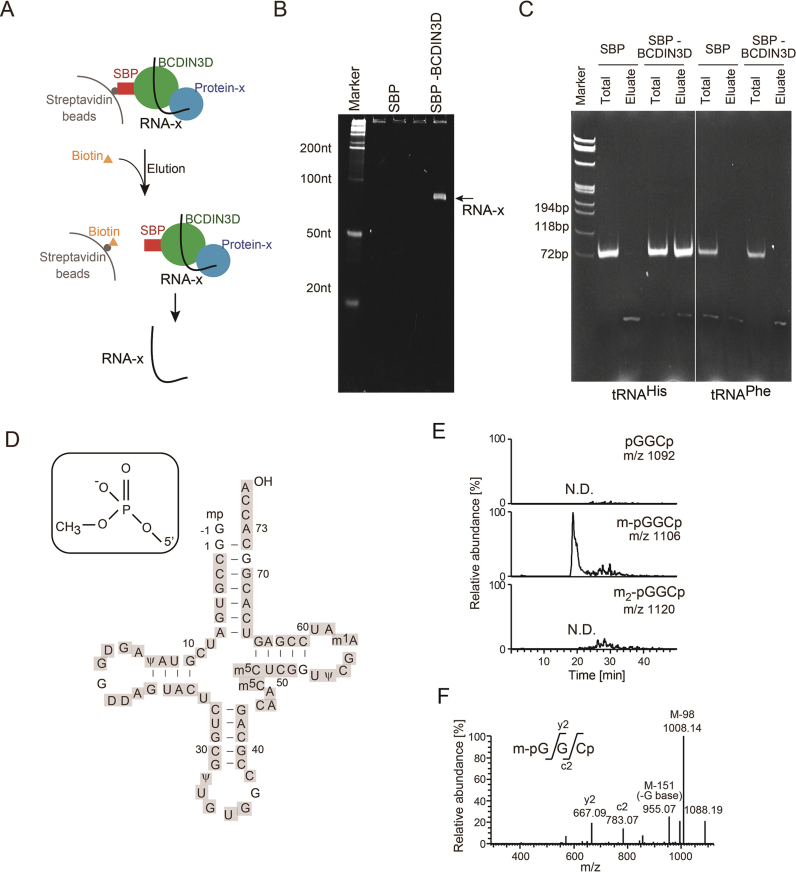
To identify RNA species that interact with BCDIN3D, a HEK293T cell line stably expressing streptavidin binding protein (SBP)-tagged BCDIN3D (SBP-BCDIN3D) was established. The SBP-BCDIN3D in the cell extracts was bound to streptavidin beads, and SBP-BCDIN3D was eluted from the beads by biotin (Figure 1A). The electrophoretic analysis of the RNA species that co-purified with SBP-BCDIN3D revealed a distinct RNA with a 70–80 nucleotide length (named RNA-x) that co-purified with SBP-BCDIN3D, but not with the control SBP (Figure 1B). This suggested that RNA-x specifically binds to BCDIN3D or BCDIN3D-associating proteins.
Figure 1.
Cytoplasmic tRNAHis binds to BCDIN3D or its associated protein(s) and has 5΄-monomethylmonophosphate. (A) Schematic presentation of the isolation of RNAs interacting with BCDIN3D. (B) Electrophoretic analysis of the RNA fraction co-purified with SBP-BCDIN3D (SBP-BCDIN3D, right lane), and control SBP (SBP, left lane) from cell extracts. The arrow indicates RNA-x specifically bound to BCDIN3D or its associated protein(s). (C) RT-PCR of RNAs interacting with BCDIN3D. Total RNAs from SBP-BCDIN3D- or SBP-expressing cells or RNAs co-purified with SBP-BCDIN3D or SBP were subjected to RT-PCR, using tRNAHis- (left) or tRNAPhe- (right) specific primers. (D) Nucleotide sequence of human cytoplasmic tRNAHis (23). LC/MS analysis of RNase T1-digested fragments of RNA-x identified cytoplasmic tRNAHis (Supplementary Figure S1A–C). The fragments cover the sequences of cytoplasmic tRNAHis (grey-shaded). (E) LC/MS analysis of RNase A-digested fragments of RNA-x. Identification of the molecular mass corresponding to 5΄pmG-1-G1-C2 p (pmG: guanosine 5΄-monomethyl monophosphate; m/z 1,106) of cytoplasmic tRNAHis (Supplementary Figure S1D). (F) Collision-induced dissociation (CID) spectrum of the RNA fragment of 5΄pmG-1-G1-C2 p in (E), showing that a methyl-group is attached to the 5΄-monophosphate of tRNAHis.
Over thirty years ago, human and fruit fly cytoplasmic histidyl tRNAs (tRNAHiss) were reported to have a 5΄-monomethylmonophosphate (23,24), and BCDIN3D is localized in the cytoplasm in human cells (9). Thus, we presumed that RNA-x is a cytoplasmic tRNAHis. RT-PCR of the RNA fractions that co-purified with BCDIN3D, using primers specific to cytoplasmic tRNAHis, indicated that the cytoplasmic tRNAHis co-purified with BCDIN3D, but not with the control SBP from the cell extracts (Figure 1C). DNA sequencing of the RT-PCR product, amplified using tRNAHis-specific primers, confirmed that cytoplasmic tRNAHis indeed co-purified with BCDIN3D. In contrast, cytoplasmic tRNAPhe did not co-purify with BCDIN3D. RT-PCRs also confirmed that neither tRNAHis nor tRNAPhe was co-purified with SBP. Thus, RNA-x was identified as cytoplasmic tRNAHis.
To substantiate the results, RNA-x was digested with RNase T1 and subjected to capillary LC-nano ESI-MS analysis (16,25). The series of RNA fragments were definitively assigned to the entire sequence of cytoplasmic tRNAHis with post-transcriptional modifications (23) (Figure 1D, Supplementary Figure S1A–C). To detect the 5΄ terminal fragment of tRNAHis, RNA-x was digested with RNase A. An RNA fragment with a molecular mass corresponding to the 5΄-terminal trimer with monomethylation, 5΄-mpGGCp (mp: monomethylmonophosphate; m/z 1,106), of cytoplasmic tRNAHis was identified (Figure 1D, E, Supplementary Figure S1D). No RNA fragment with a molecular mass corresponding to either the unmethylated fragment (5΄-pGGCp, m/z 1092) or the dimethylated fragment (5΄-m2pGGCp; m/z 1120) was detected. Collision-induced dissociation (CID) spectrometric analysis of the fragment confirmed the presence of a methyl-group at the 5΄-terminal monophosphate of cytoplasmic tRNAHis (Figure 1F), as previously reported (23). Together, our results showed that cytoplasmic tRNAHis binds to BCDIN3D or its associated proteins, and has a 5΄-monomethylmonophosphate. These results prompted us to analyze cytoplasmic tRNAHis as a substrate of BCDIN3D.
BCDIN3D monomethylates the 5΄-phosphate of tRNAHisin vitro
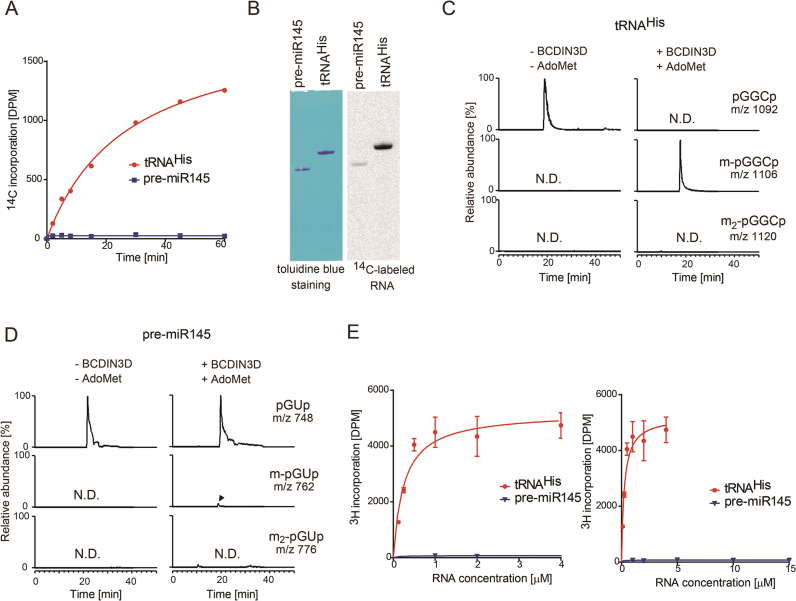
To examine whether BCDIN3D can methylate the 5΄-monophosphate of cytoplasmic tRNAHisin vitro, BCDIN3D was overexpressed in E. coli, purified and tested for its activity, using a cytoplasmic tRNAHis transcript as the substrate and S-(5΄-adenosyl)-L-methionine (AdoMet) as the methyl-group donor. The in vitro methylation assay demonstrated that wild-type BCDIN3D transfers the methyl group of AdoMet to tRNAHis efficiently in a time-dependent manner (Figure 2A), under standard conditions. The catalytically-inactive mutant protein, BCDIN3D-D72A/G74A, does not transfer the methyl group of AdoMet tRNAHis at all (Supplementary Figure S2A and B). This excludes the possibility that the observed methylation of tRNAHis by BCDIN3D is due to contamination by other methyltransferases in E. coli. Recently, BCDIN3D was reported to dimethylate the 5΄-monophosphates of a specific group of miRNA precursors (pre-miRNAs), such as pre-miR145. The dimethylation of the 5΄-phosphate of pre-miR145 negatively regulates the following Dicer processing (9). However, the methyl group of AdoMet is not significantly transferred to pre-miR145, as compared with tRNAHis, under the tested conditions (Figure 2A).
Figure 2.
Monomethylation of 5΄-monophosphate of tRNAHis by BCDIN3D in vitro. (A) In vitro methylation of tRNAHis and pre-miR145 transcripts. Time courses of methyl-group transfer from AdoMet to tRNAHis and pre-miR145 transcripts under standard conditions (2 μM RNA substrate and 0.1 μM recombinant BCDIN3D). (B) In vitro methylation of tRNAHis and pre-miR145 with a higher concentration of BCDIN3D (1 μM) at 37°C for 2 h. After the reaction, the RNA was separated by 10% (v/v) polyacrylamide gel electrophoresis under denaturing conditions. The gel was stained with toluidine blue (left), dried and exposed to a BASS2000 imaging plate (Fujifilm, Japan) for 12 hours (right). Mass spectrometric analysis of the RNase A-digested products of BCDIN3D-treated. (C) tRNAHis and (D) pre-miR145. (E) The steady state kinetics of methylation of tRNAHis and pre-miR145. Reaction mixtures containing various concentrations of tRNAHis (0.125–4 μM; left) and pre-miR145 (1–15 μM; right) were incubated at 37°C for 10 min. The bars in the graphs are SD of more than two independent experiments.
Under conditions with larger amounts of recombinant BCDIN3D and a prolonged incubation time, a slight amount of the methyl group is transferred to pre-miR145, as detected with a phosphorimager, but the efficiency is much lower than that of tRNAHis (∼1%, Figure 2B). LC-mass spectrometric analyses of RNaseA-treated reaction products confirmed that the 5΄-monophosphate of tRNAHis is fully monomethylated, and the 5΄-monophosphate of the pre-miRNA transcript is monomethylated with lower efficiency. Neither tRNAHis nor pre-miR145 was dimethylated in vitro (Figure 2C and D).
The steady-state kinetics analyses of the methylation of the 5΄-monophosphates of tRNAHis and pre-miR145 provided estimated Km values for tRNAHis and pre-miR145 of 0.25 μM and >>15 μM, respectively (Figure 2E), and the Vmax value of pre-miR145 was much lower than that of tRNAHis. Therefore, cytoplasmic tRNAHis is a much better substrate of BCDIN3D than pre-miR145, by over two to three orders of magnitude, in vitro. Thus, the primary target of BCDIN3D is cytoplasmic tRNAHis, rather than pre-miRNAs, and BCDIN3D has monomethylation activity acting on the 5΄-monophosphate of cytoplasmic tRNAHis.
BCDIN3D monomethylates the 5΄-phosphate of tRNAHisin vivo
To determine whether the monomethylation of the 5΄-monophosphate of cytoplasmic tRNAHis occurs under normal physiological conditions, cytoplasmic tRNAHis was purified from HEK293T cells, using an oligonucleotide probe complementary to cytoplasmic tRNAHis. The purified cytoplasmic tRNAHis was analyzed by LC-mass spectrometry (Supplementary Figure S3). Quantification of the methylation of the 5΄-monophosphate of cytoplasmic tRNAHis confirmed that 99% of the cytoplasmic tRNAHis has the 5΄-monomethylmonophosphate. This suggests that, under normal physiological conditions, the 5΄-monophsphate of cytoplasmic tRNAHis is almost fully monomethylated.
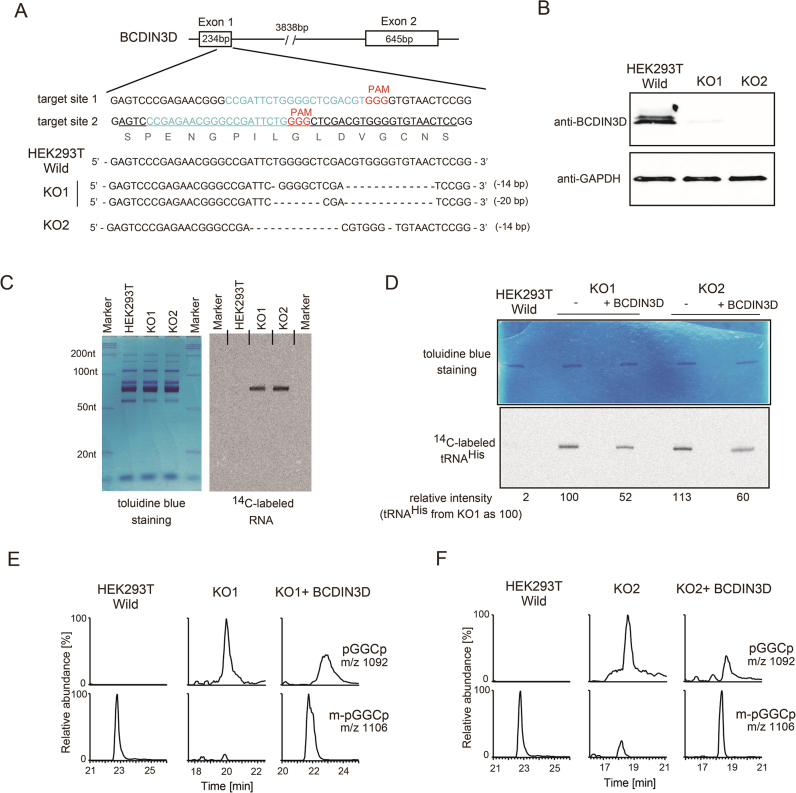
Next, to examine the monomethylation of the 5΄-monophosphate of tRNAHis by BCDIN3D in vivo, the BCDIN3D gene in the HEK293T genome was knocked out, using the clustered regulatory interspaced short palindromic repeat CRISPR/Cas9 system (19,26). Two knockout cell lines were obtained (KO1 and KO2). Both KO cell lines have nucleotide deletions in exon-1 of the BCDIN3D gene, which lead to the frame-shift mutation of the BCDIN3D mRNA (Figure 3A). The absence of BCDIN3D protein expression in the knockout cells was confirmed by western blotting (Figure 3B). The knockout of BCDIN3D slightly impairs the growth of HEK293T cells (Supplementary Figure S4A).
Figure 3.
BCDIN3D monomethylates the 5΄-monophosphate of tRNAHisin vivo. (A) Schematic representation of the location of guide RNAs (gRNA1 and gRNA2) targeting the BCDIN3D gene locus. The nucleotide sequence of the region surrounding the target site (upper), and those of the regions surrounding the targeted deletion sites in knockout cells (KO1 and KO2, lower) for cleavage by CRISPR/Cas9. (B) Expression of the BCDIN3D protein in wild-type HEK293T cells and BCDIN3D knockout cells (KO1 and KO2). The BCDIN3D protein was detected by western blotting, using an anti-BCDIN3D antibody. GAPDH expression was used as a positive control. (C) Small RNA fractions were prepared from wild-type HEK293T cells and BCDIN3D KO cells (KO1 and KO2). The RNAs were subjected to in vitro methylation by BCDIN3D, as in Figure 2B, using 5 μg small RNA fractions, and the reaction products were separated by 10% (v/v) polyacrylamide gel electrophoresis under denaturing conditions. The gel was stained with toluidine blue and dried (left). The 14C-labeled RNAs were detected with a phosphorimager (right). (D) In vitro methylations of tRNAHis species isolated from HEK293T, KO1 and KO2 cells, and those of tRNAHis species from rescued KO1 and KO2 cells with exogenous expression of BCDIN3D (KO1+BCDIN3D and KO2+BCDIN3D). tRNAHis species after the reaction were separated by polyacrylamide gel electrophoresis, and the gel was stained with toluidine blue (upper). The 14C-labeled tRNAHis species were detected with a phosphorimager (lower), and the relative 14C-band intensities were calculated. The intensity of tRNAHis from KO1 was designated as 100. (E, F) LC/MS analysis of tRNAHis isolated from KO1 and KO2. The RNase A-digested fragments of tRNAHis were analyzed by LC/MS. The 5΄-mpG-1G1C2p is not detected in tRNAHis from KO1 and KO2. The 5΄-mpG-1G1C2p was partially restored by the exogenous expression of BCDIN3D in the KO cells (KO1+BCDIN3D and KO2+BCDIN3D).
Small RNA fractions were prepared from wild-type HEK293T, KO1 and KO2 cells (Figure 3C, left), and subjected to in vitro methylation by recombinant BCDIN3D. While the RNA fractions from HEK293T cells were not significantly methylated, those from the KO1 and KO2 cells were methylated (Figure 3C, right). The length of the 14C-labeled RNA corresponds to that of tRNAs, and no other RNA smaller or larger than the tRNA fractions was 14C-labeled. The cytoplasmic tRNAHis was further purified from the total tRNA fractions of wild-type HEK293T and KO cells, and subjected to in vitro methylation using recombinant BCDIN3D (Figure 3D). The tRNAHis purified from HEK293T cells was barely methylated by BCDIN3D in vitro, confirming that most of the cytoplasmic tRNAHis fraction from the cells bears the 5΄-monomethylmonophosphate in vivo (Supplementary Figure S3). In contrast, the cytoplasmic tRNAHis species purified from the KO cells are methylated in vitro. These results suggest that BCDIN3D is responsible for the methylation of tRNAHisin vivo. Analyses by qPCR also confirmed that the expression levels of the 7SK-specific methyltransferase, BCDIN3, in BCDIN3D-knockout cells are not affected (Supplementary Figure S4B). This excludes the possibility that BCDIN3 is involved in the methylation of tRNAHisin vivo.
The tRNAHis species from rescued KO cells, in which BCDIN3D was exogenously expressed, are also methylated, but the efficiencies are about half of those of tRNAHis from KO cells. This suggests that BCDIN3D methylates cytoplasmic tRNAHisin vivo. The exogenous expression levels of SBP-BCDIN3D in KO1 and KO2 were much higher than the endogenous expression of BCDIN3D in wild-type HEK293T cells (Supplementary Figure S5). Thus, the modest effect (∼ 50%) of the exogenous expression of BCDIN3D in KO cells on the methylation is probably due to the transfection efficiencies of the BCDIN3D plasmid in the KO cells. The cytoplasmic tRNAHis species isolated from these cells were further analyzed by LC-mass spectrometry (Figure 3E, F). The results confirmed that, in KO cells, the cytoplasmic tRNAHis lacks the 5΄-monomethylmonophosphate, and only the 5΄-monophosphate form of tRNAHis is observed. Furthermore, in the rescued KO cells, tRNAHis with the 5΄-monomethylmonophosphate is restored. Thus, BCDIN3D is responsible for the monomethylation of the 5΄-monophosphate of cytoplasmic tRNAHisin vivo.
Recognition of cytoplasmic tRNAHis by BCDIN3D
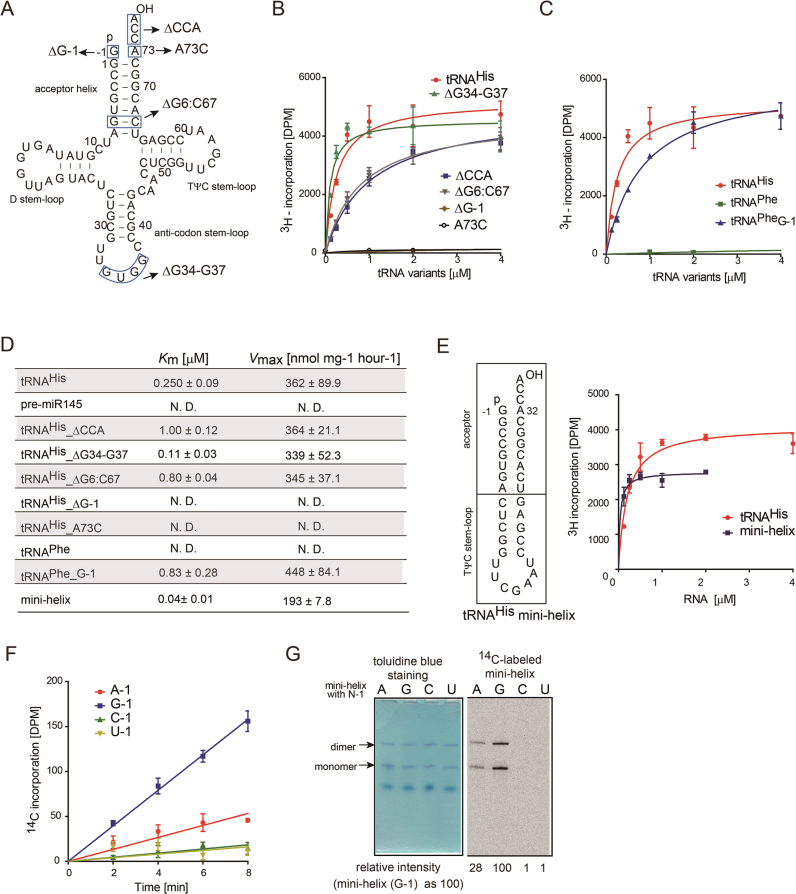
Human cytoplasmic tRNAHis has unique features, among cytoplasmic tRNA species. After the 5΄-leader is removed from the precursor tRNAHis transcript by an endonuclease, an extra guanosine residue (G−1) is attached to the 5΄-end by tRNAHis guanyltransferase (Thg) (27). As a result, the mature cytoplasmic tRNAHis has an eight-nucleotide acceptor helix with a protruding C74C75A76 sequence, but G−1 does not base-pair with the A73 discriminator nucleotide (Figure 1D). In contrast, all other canonical cytoplasmic tRNA species have a seven-nucleotide acceptor helix.
To evaluate the substrate requirement for the methylation of cytoplasmic tRNAHis by BCDIN3D, mutation(s) or deletion was introduced into the tRNAHis transcript (Figure 4A–D), and the steady-state kinetics were analyzed. The tRNAHis mutant lacking G-1 (tRNAHis_ΔG−1) and that with the A73C mutation (tRNAHis_A73C) were quite poor substrates for BCDIN3D, suggesting that the G−1 and G−1–A73 mis-pair are required for the methylation of the 5΄-monophosphate of tRNAHis (Figure 4B). The methylation efficiencies of tRNAHis mutants lacking either C74C75A76 (tRNAHis_ΔCCA) or the G6:C67 base pair in the acceptor stem (tRNAHis_ΔG6-C67) were reduced to ∼30% of that of wild-type tRNAHis. The reduced methylation efficiencies arise from the lower affinities of the mutant tRNAs (Figure 4B, D): the Km values of tRNAHis_ΔCCA and tRNAHis _ΔG6:C67 are increased by factors of four and three as compared to that of wild-type tRNAHis, respectively (Figure 4D). In contrast, the methylation efficiency of the tRNAHis mutant lacking the anticodon loop (tRNAHis_Δ32–35) is actually slightly better than that of wild-type tRNAHis. Furthermore, while the wild-type tRNAPhe transcript does not function as a substrate for BCDIN3D, the introduction of an extra G (G−1) at the 5΄ end of tRNAPhe (tRNAPhe_G−1) increases the methylation efficiency to ∼40% of that of tRNAHis (Figure 4C, D). In addition, the Vmax value is almost the same as that of tRNAHis.
Figure 4.
tRNAHis recognition by BCDIN3D. (A) Nucleotide sequences of the human cytoplasmic tRNAHis transcript and its variants used for biochemical analyses. (B) The steady-state kinetics of the methylation of the 5΄-monophosphate of tRNAHis variants. Reaction mixtures containing various concentrations of tRNAHis and its variants [(tRNAHis_ΔG-1, tRNAHis_ΔCCA, tRNAHis_ΔG6:C67, and tRNAHis_Δ32-35; 0.125–4 μM) and (tRNAHis_ΔG−1, tRNAHis_A73C; 1–15 μM)] were incubated at 37°C for 10 min. (C) The steady-state kinetics of human cytoplasmic tRNAPhe and its variant. Reaction mixtures containing various concentrations of tRNAPhe (1–15 μM) and its variant (tRNAPhe_G−1: 0.125–4 μM) were incubated at 37°C for 10 min. (D) Summary of kinetic parameters. N.D. (Not Determined). (E) Mini-helix of tRNAHis (left). The steady-state kinetics of the methylation of the 5΄-monophosphate of the tRNAHis mini-helix with G−1. Reaction mixtures containing various concentrations of tRNAHis and tRNAHis mini-helix (0.125–2 μM) were incubated at 37°C for 10 min (right). (F) Time courses of methyl-group transfer from AdoMet to tRNAHis mini-helix variants (G−1, A−1, C−1 and U−1) under standard conditions (1 μM RNA substrate and 0.1 μM recombinant BCDIN3D). (G) In vitro methylation of tRNAHis mini-helix variants at 37°C for 2 h. After the reaction, the RNA was purified by phenol–chloroform treatment and separated by 10% (v/v) polyacrylamide gel electrophoresis under denaturing conditions. The gel was stained with toluidine blue (left), dried and exposed to a BASS2000 imaging plate (Fujifilm, Japan) for 12 h (right). The bars in the graphs in (B), (C), (E) and (F) are SD of three independent experiments.
Moreover, the tRNAHis mini-helix is also methylated efficiently. Thus, the top half of tRNAHis, especially the acceptor helix, is recognized by BCDIN3D (Figure 4E). The nucleotide preference at position -1 of the tRNAHis mini-helix by BCDIN3D was also analyzed, using tRNAHis mini-helix variants (Figure 4F, G). The tRNAHis mini-helices with A-1, C−1 and U−1 were methylated less efficiently than the wild-type tRNAHis mini-helix with G−1. The methylation efficiencies of tRNAHis mini-helices with A−1, C−1 and U−1 are 28, 1 and 1%, respectively, of that of the tRNAHis mini-helix with G−1, at the reaction end points (Figure 4F, G). Thus, the order of nucleotide preference at position −1 of the tRNAHis mini-helix is G−1 > A−1 >> C−1, U−1. In addition to the G−1–A73 mis-pair at the top of the acceptor helix of tRNAHis, the guanine base at position −1 would be recognized by BCDIN3D at the methylation step of the 5΄-monophosphate of tRNAHis.
Except for tRNAHis, there are no other cytoplasmic tRNAs with an eight-nucleotide acceptor helix and a mis-pair at the top of the acceptor stem. Thus, BCDIN3D recognizes these unique structural features of the acceptor helix of cytoplasmic tRNAHis, and discriminates tRNAHis from other tRNA species.
Effects of 5΄-phosphate methylation of tRNAHis on aminoacylation
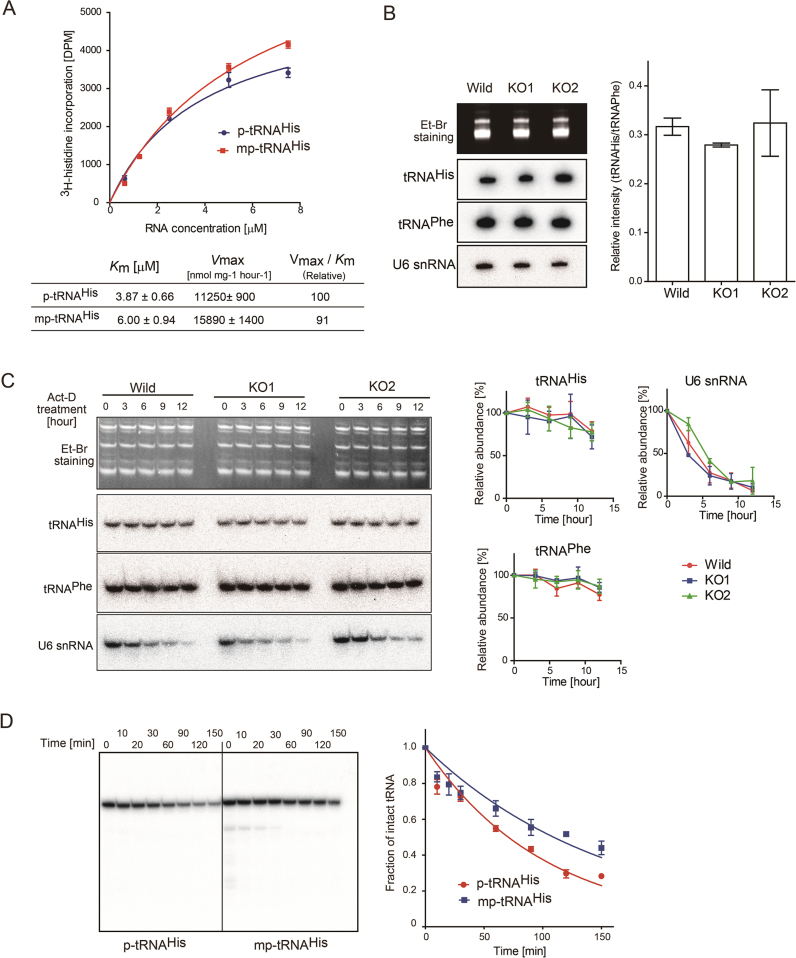
The effects of the methyl group at the 5΄-monophosphate of tRNAHis on the aminoacylation by histidine-tRNA synthetase (HisRS) were examined in vitro, and the steady-state kinetic parameters were estimated (Figure 5A). The Km value of 5΄mp-tRNAHis (tRNAHis with 5΄-monomethylmonophosphate) is about 1.5-fold of that of 5΄p-tRNAHis (tRNAHis with 5΄-monophosphate). The Vmax value of 5΄mp-tRNAHis is about 1.5-fold of that of 5΄p-tRNAHis. These results suggest that the introduction of the methyl-group on the 5΄-monophosphate reduces the affinity of tRNAHis toward HisRS, and increases the turnover of HisRS. The increased Km of 5΄mp-tRNAHis is consistent with biochemical and recent structural analyses of the complex of eubacterial HisRS and tRNAHis (28,29). In the structures, the 5΄-monophosphate of tRNAHis is strictly recognized by HisRS by hydrogen bonds. The reduced negative charge of the 5΄-monophosphate by methylation would decrease the affinity of tRNAHis toward HisRS, but enhance the structural changes of tRNAHis (or enzyme) required for the catalysis. However, since the overall aminoacylation efficiencies (Vmax/Km value) are almost the same between 5΄p-tRNAHis and 5΄mp-tRNAHis (Figure 5A), the BCDIN3D activity is not indispensable for the aminoacylation process.
Figure 5.
Stability of tRNAHis with 5΄-monomethylmonophosphate. (A) Aminoacylation of p-tRNAHis and mp-tRNAHis by histidine tRNA synthetase in vitro. The steady-state kinetics parameters were calculated. (B) Steady-state levels of tRNAHis species in wild-type (HEK293T) and BCDIN3D knockout cells (KO1 and KO2). The amounts of tRNAHis in wild-type HEK293, KO1 and KO2 cells were analyzed by northern blotting. Quantification of the ratios of the band intensities of tRNAHis and tRNAPhe. (C) In vivo stabilities of tRNAHis. Wild-type HEK293T and KO cells were treated with actinomycin-D (Act-D) for 12 h. The stabilities of tRNAHis from the cells were analyzed by northern blotting, and quantified. The intensities of the bands of tRNAHis (or tRNAPhe or U6 snRNA) at zero time were designated as 1.0, and the relative amount of tRNAHis (or tRNAPhe or U6 snRNA) was quantified. (D) The in vitro decay of tRNAHis species with 5΄-monomethylmonophosphate (5΄pm-tRNAHis) and with 5΄-monophosphate (5΄p-tRNAHis) was performed using cytoplasmic extracts, and the amounts of intact tRNAHis species were quantified. The intensities of the bands of intact 32P-tRNAHis at zero time were designated as 1.0, and the relative amounts of tRNAHis were quantified. Bars in graphs in (A)–(D) are SD of more than three independent experiments.
Effects of 5΄-phosphate methylation of tRNAHis on its stability in vivo and in vitro
To elucidate the function of the 5΄-monomethylmonophosphate of cytoplasmic tRNAHis in terms of its stability in vivo, the steady-state levels of tRNAHis species in wild-type HEK293T and BCDIN3D knockout (KO1 and KO2) cells were analyzed by northern blotting. The results demonstrated that the methylation of the 5΄-phosphate does not affect the steady state level of tRNAHisin vivo (Figure 5B). Furthermore, to examine the stability of tRNAHisin vivo, the KO cells were treated with actinomycin-D to inhibit global transcription. Under the assay conditions, the U6 snRNA degraded in a time-dependent manner over the twelve-hour period, in wild-type HEK293T and KO cells. The cytoplasmic tRNAHis species from KO cells were stable over twelve hours after the actinomycin-D treatment, and no significant rapid decay of tRNAHis in the KO cells was observed, as compared with tRNAHis from wild-type HEK293T cells (Figure 5C).
However, an in vitro decay assay using cytoplasmic extracts revealed that tRNAHis with the 5΄-monophosphate (5΄p-tRNAHis) is more rapidly degraded than tRNAHis with the 5΄-monomethylated phosphate (5΄mp-tRNAHis) (Figure 5D). The half-lives of 5΄p-tRNAHis and 5΄mp-tRNAHis are estimated to be approximately 71 and 109 min, respectively. This result suggests that the 5΄-monomethylmonophosphate of tRNAHis protects it from degradation or dephosphorylation. Since BCDIN3D is localized in the cytoplasm (9), 5΄ to 3΄-exonucleases and/or phosphatases would be cooperatively involved in the degradation of 5΄p-tRNAHis. The differences in the stabilities of tRNAHis between the in vivo and in vitro analyses are discussed below.
DISCUSSION
In this study, we identified cytoplasmic tRNAHis as a target of BCDIN3D. Our results showed that human cytoplasmic tRNAHis tightly binds to BCDIN3D in vivo (Figure 1B), and has a 5΄-monomethymonophosphate (Figure 1E, F), as previously reported (23). Human BCDIN3D efficiently monomethylates the 5΄-monophosphate of cytoplasmic tRNAHisin vitro (Figure 2A), and BCDIN3D is responsible for the modification in HEK293T cells (Figure 3C–E). BCDIN3D recognizes the unique features of cytoplasmic tRNAHisin vitro (Figure 4B, C). In particular, BCDIN3D recognizes G−1 itself and the eight-nucleotide acceptor helix with the G−1–A73 mis-pair at the top of the helix (Figure 4E, F). Except for cytoplasmic tRNAHis, there is no other cytoplasmic tRNA species with these unique structural features. Thus, BCDIN3D discriminates tRNAHis from other tRNA species, and can act as a tRNAHis-specific 5΄-monophosphate monomethyltransferase. It is not clear whether G−1 of tRNAHis forms non-Watson–Crick hydrogen-bonds with A73. A variety of non-Watson–Crick G:A pairings have been found in RNA structures and RNA–protein complex structures (30). The involvement of G−1:A73 non-Watson–Crick hydrogen-bonds in the methylation of the 5΄-monophosphate of tRNAHis by BCDIN3D would be clarified by the structural analysis of BCDIN3D complexed with tRNAHis.
Recently, it was reported that human BCDIN3D dimethylates the 5΄-monophosphate of a specific group of pre-miRNAs, such as pre-miR145 and pre-miR23b, in vitro (9). However, our in vitro results revealed that BDIN3D monomethylates the 5΄-monophosphate of cytoplasmic tRNAHis more efficiently than the 5΄-monophosphate of pre-miR145 by over two orders of magnitude (Figure 2B, E), and neither cytoplasmic tRNAHis nor pre-miR145 is dimethylated under our assay conditions (Figure 2C, D). In the previous report (9), the methylation efficiency of the 5΄-monophosphate of pre-miR145 was calculated to be <1% of the input pre-miR145 substrate at the reaction end point. The low methylation efficiency of pre-miR145 is consistent with our results (Figure 2B, E).
In BCDIN3D knockout cells derived from HEK293T cells, the expression level of mature miR145 is not up-regulated, as compared with that in wild-type HEK293T cells (Supplementary Figure S6A). In addition, the expression of BCDIN3D in HEK293T cells does not downregulate the expression of mature miR145 (Supplementary Figure S6B). These observations are distinct from those in a recent report showing that, in MCF-7 breast cancer cells, the knock-down of BCDIN3D by siRNA results in the upregulation of mature miR145 and the downregulation of pre-miR145 (9). The expression level of the BCDIN3D protein in HEK293T cells is higher than that in MCF-7 breast cancer cells (Supplementary Figure S7A). Since almost all of the cytoplasmic tRNAHis is methylated even in MCF-7 cells, as in HEK293T and HeLa cells (Supplementary Figure S7B), the enzymatic activity and amount of BCDIN3D in MCF-7 cells are sufficient to methylate the 5΄-phosphate of cytoplasmic tRNAHis. These observations suggest the absence of a direct co-relation between the expression levels of the BCDIN3D protein and miR145. Our in vitro results confirmed that BCDIN3D never dimethylates the 5΄-monophosphate of either tRNAHis or pre-miR145 (Figure 2). A recent report showed that synthetic pre-miR145 with a 5΄-dimethylphosphate is poorly processed by Dicer in vitro, while synthetic pre-miR145 with a 5΄-monomethylphosphate is processed by Dicer as efficiently as pre-miR145 with a 5΄-phosphate (9). Thus, it is unlikely that miR145 expression is controlled by BCDIN3D, and the 5΄-phosphate of pre-miR145 would not be dimethylated by BCDIN3D in HEK293T cells.
Collectively, the primary target of BCDIN3D is cytoplasmic tRNAHis, rather than pre-miRNAs, and BCDIN3D has monomethylation activity acting on the 5΄-monophosphate of RNAs. Under specific conditions or in certain biological processes, the pre-miRNA might be methylated efficiently, and unknown regulatory factors specific to breast cancer cells might enhance the efficient pre-miRNA (di)methylation process in vivo. The in vivo mechanism of 5΄-monophosphate dimethylation of specific pre-miRNAs, such as pre-miR145, in breast cancer cells awaits further studies.
The monomethylation of the 5΄-monophosphate of cytoplasmic tRNAHis increases both the Km value of tRNAHis toward HisRS and Vmax, and the overall aminoacylation efficiencies by HisRS in vitro are not affected (Figure 5A). The steady-state levels of cytoplasmic tRNAHis in HEK293T and BCDIN3D-knockout cells are not significantly different (Figure 5B, C). In human cells, under normal conditions, most tRNAs exist as aminoacyl-tRNAs and are protected by binding to elongation factor 1A (31,32). This may explain the similar stabilities of tRNAHis in the wild-type and KO cells in vivo (Figure 5B, C). In contrast, the in vitro stability assay using cytoplasmic cell extracts showed that the 5΄-monomethylation of the 5΄-phosphate of the tRNAHis transcript protects it from degradation (Figure 5D). Since the tRNAHis transcript lacks the other post-transcriptional modifications (Figures 1D, 4A), it would be less stable than the native tRNAHis, and thus the effect of the 5΄-monomethylation of the 5΄-phosphate of tRNAHis on its stability could be observed in vitro.
In the cytoplasm, 5΄ to 3΄ exonucleases, such as XRN1 requiring a 5΄-monophosphate (33,34), might degrade tRNAHis lacking a methylated 5΄-monophosphate. However, there were no significant differences in the stabilities of tRNAHis transcripts with or without a methyl-group at the 5΄-phosphate, upon the treatment of tRNAHis transcripts with recombinant yeast XRN1 (Supplementary Figure S8). Since the 5΄-monomethylated phosphate of RNA is more resistant to the phosphatase, the dephosphorylation of the 5΄-phosphate of tRNAHis by some phosphatases and some other exonucleases in the extract would cooperatively degrade the tRNAHis with a 5΄-monophosphate. The G−1–A73 mis-pair at the top of the tRNAHis acceptor helix would allow these enzymes to access the 5΄-end of tRNAHis. The identification of the enzymes responsible for the degradation of tRNAHis awaits further studies.
The involvement of the 5΄-monomethylation of cytoplasmic tRNAHis by BCDIN3D in the tumorigenic phenotype of breast cancer cells still remains elusive. Recent studies have shown that the expression levels of specific tRNAs, such as initiator tRNAMet, are elevated in breast cancer cells (35). The upregulation of specific tRNAs, such as tRNAGluUUC and tRNAArgCCG, reportedly stabilizes mRNAs containing the corresponding codons and enhances the translation in highly metastatic breast cancer cells (36). However, the steady state level of cytoplasmic tRNAHis is not affected by the knockdown of BCDIN3D in HEK293T cells (Figure 5B, C). Recent studies have also provided evidence that tRNA fragments (tRFs), produced under stress conditions, participate in various cellular functions beyond their established roles in protein synthesis (37,38). In breast and prostate cancers, specific tRNAs are cleaved by angiogenin, an RNaseA-type nuclease, and tRNA halves are abundantly expressed in a sex hormone-dependent manner (39). Interestingly, about 27% of the tRNA halves are the 5΄-half of tRNAHis, while about 60% are the 5΄-half of tRNALys. These tRNA halves have been shown to promote the proliferation of breast and prostate cancer cells, by unknown mechanisms. In human and mouse cells, 3΄- or 5΄-terminal tRFs (3΄-tRF or 5΄-tRF) reportedly accumulate in an asymmetric manner (40), and these tRFs associate with Ago2 and downregulate their target genes by cleaving their transcripts (41). The 3΄-tRF, but not the 5΄-tRF, derived from cytoplasmic tRNAHis is complementary to human endogeneous retroviral sequences in the genome (41). It would be interesting to determine whether the 5΄-monomethylation of the 5΄-monophosphate of tRNAHis controls the expression of tRNA halves and tRFs derived from tRNAHis in breast cancer cells or under specific biological or stress conditions. Future work will clarify whether the methylation of the 5΄-phosphate of tRNAHis by BCDIN3D is involved in the tumorigenic phenotypes of breast cancer and other cancers.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Yuka Fujimoto and Azusa Hamada for technical assistance.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Funding Program for Next Generation World-Leading Researchers of JSPS [to K.T.]; Grants-in-Aid for Scientific Research (A) [to K.T.] from JSPS and a Grant-in-Aid for Scientific Research on Innovative Areas from Ministry of Education, Science, Sports and Culture of Japan [to K.T., T.S.]; Takeda Science Foundation, Astellas Foundation for Research on Metabolic Disorders and Hamaguchi Foundation for the Advancement of Biochemistry [to K.T.]. Funding for open access charge: Grant-in-Aid for Scientific Research on Innovative Areas from Ministry of Education, Science, Sports, and Culture of Japan [to K.T.].
Conflict of interest statement. None declared.
REFERENCES
- 1. Driever W., Nusslein-Volhard C.. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989; 337:138–143. [DOI] [PubMed] [Google Scholar]
- 2. Zhu W., Hanes S.D.. Identification of drosophila bicoid-interacting proteins using a custom two-hybrid selection. Gene. 2000; 245:329–339. [DOI] [PubMed] [Google Scholar]
- 3. Cosgrove M.S., Ding Y., Rennie W.A., Lane M.J., Hanes S.D.. The Bin3 RNA methyltransferase targets 7SK RNA to control transcription and translation. Wiley Interdiscipl. Rev. RNA. 2012; 3:633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh N., Morlock H., Hanes S.D.. The Bin3 RNA methyltransferase is required for repression of caudal translation in the Drosophila embryo. Dev. Biol. 2011; 352:104–115. [DOI] [PubMed] [Google Scholar]
- 5. Diribarne G., Bensaude O.. 7SK RNA, a non-coding RNA regulating P-TEFb, a general transcription factor. RNA Biol. 2009; 6:122–128. [DOI] [PubMed] [Google Scholar]
- 6. Peterlin B.M., Brogie J.E., Price D.H.. 7SK snRNA: a noncoding RNA that plays a major role in regulating eukaryotic transcription. Wiley Interdiscipl. Rev. RNA. 2012; 3:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jeronimo C., Forget D., Bouchard A., Li Q., Chua G., Poitras C., Thérien C., Bergeron D., Bourassa S., Greenblatt J. et al. . Systematic analysis of the protein interaction network for the human transcription machinery reveals the identity of the 7SK capping enzyme. Mol. Cell. 2007; 27:262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shuman S. Transcriptional networking cap-tures the 7SK RNA 5΄-γ-methyltransferase. Mol. Cell. 2007; 27:517–519. [DOI] [PubMed] [Google Scholar]
- 9. Xhemalce B., Robson S.C., Kouzarides T.. Human RNA methyltransferase BCDIN3D regulates microRNA processing. Cell. 2012; 151:278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu R., Wang X., Chen G.Y., Dalerba P., Gurney A., Hoey T., Sherlock G., Lewicki J., Shedden K., Clarke M.F.. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N. Engl. J. Med. 2007; 356:217–226. [DOI] [PubMed] [Google Scholar]
- 11. Yao L., Chi Y., Hu X., Li S., Qiao F., Wu J., Shao Z.M.. Elevated expression of RNA methyltransferase BCDIN3D predicts poor prognosis in breast cancer. Oncotarget. 2016; 7:53895–53902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sachdeva M., Zhu S., Wu F., Wu H., Walia V., Kumar S.. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. U.S.A. 2009; 106:3207–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi B., Sepp-Lorenzino L., Prisco M., Linsley P., deAngelis T., Baserga R.. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J. Biol. Chem. 2007; 282:32582–32590. [DOI] [PubMed] [Google Scholar]
- 14. Spizzo R., Nicoloso M.S., Lupini L., Lu Y., Fogarty J., Rossi S., Zagatti B., Fabbri M., Veronese A., Liu X. et al. . miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-[alpha] in human breast cancer cells. Cell Death Differ. 2009; 17:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park J.E., Heo I., Tian Y., Simanshu D.K., Chang H., Jee D., Patel D.J., Kim V.N.. Dicer recognizes the 5΄ end of RNA for efficient and accurate processing. Nature. 2011; 475:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki T., Ikeuchi Y., Noma A., Suzuki T., Sakaguchi Y.. Mass spectrometric identification and characterization of RNA-modifying enzymes. Methods Enzymol. 2007; 425:211–229. [DOI] [PubMed] [Google Scholar]
- 17. Soma A., Ikeuchi Y., Kanemasa S., Kobayashi K., Ogasawara N., Ote T., Kato J., Watanabe K., Sekine Y., Suzuki T.. An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Mol. Cell. 2003; 12:689–698. [DOI] [PubMed] [Google Scholar]
- 18. Pyzocha N.K., Ran F.A., Hsu P.D., Zhang F.. RNA-guided genome editing of mammalian cells. Methods Mol. Biol. 2014; 1114:269–277. [DOI] [PubMed] [Google Scholar]
- 19. Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A. et al. . Multiplex genome engineering using CRISPR/Cas systems. Science (New York, N.Y.). 2013; 339:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsurui H., Kumazawa Y., Sanokawa R., Watanabe Y., Kuroda T., Wada A., Watanabe K., Shirai T.. Batchwise purification of specific tRNAs by a solid-phase DNA probe. Anal. Biochem. 1994; 221:166–172. [DOI] [PubMed] [Google Scholar]
- 21. Tomita K., Ueda T., Ishiwa S., Crain P.F., McCloskey J.A., Watanabe K.. Codon reading patterns in Drosophila melanogaster mitochondria based on their tRNA sequences: a unique wobble rule in animal mitochondria. Nucleic Acids Res. 1999; 27:4291–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomita K., Ueda T., Watanabe K.. The presence of pseudouridine in the anticodon alters the genetic code: A possible mechanism for assignment of the AAA lysine codon as asparagine in echinoderm mitochondria. Nucleic Acids Res. 1999; 27:1683–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosa M.D., Hendrick J.P. Jr., Lerner M.R., Steitz J.A., Reichlin M.. A mammalian tRNAHis-containing antigen is recognized by the polymyositis-specific antibody anti-Jo-1. Nucleic Acids Res. 1983; 11:853–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cooley L., Appel B., Soll D.. Post-transcriptional nucleotide addition is responsible for the formation of the 5΄ terminus of histidine tRNA. Proc. Natl. Acad. Sci. U.S.A. 1982; 79:6475–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohira T., Suzuki T.. Precursors of tRNAs are stabilized by methylguanosine cap structures. Nat. Chem. Biol. 2016; 12:648–655. [DOI] [PubMed] [Google Scholar]
- 26. Sander J.D., Joung J.K.. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotech. 2014; 32:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gu W., Jackman J.E., Lohan A.J., Gray M.W., Phizicky E.M.. tRNAHis maturation: an essential yeast protein catalyzes addition of a guanine nucleotide to the 5΄ end of tRNAHis. Genes Dev. 2003; 17:2889–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fromant M., Plateau P., Blanquet S.. Function of the extra 5΄-phosphate carried by histidine tRNA. Biochemistry. 2000; 39:4062–4067. [DOI] [PubMed] [Google Scholar]
- 29. Tian Q., Wang C., Liu Y., Xie W.. Structural basis for recognition of G-1-containing tRNA by histidyl-tRNA synthetase. Nucleic Acids Res. 2015; 43:2980–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hermann T., Westhof E.. Non-Watson-Crick base pairs in RNA-protein recognition. Chem. Biol. 1999; 6:R335–R343. [DOI] [PubMed] [Google Scholar]
- 31. Petrushenko Z.M., Budkevich T.V., Shalak V.F., Negrutskii B.S., El'skaya A.V.. Novel complexes of mammalian translation elongation factor eEF1A.GDP with uncharged tRNA and aminoacyl-tRNA synthetase. Implications for tRNA channeling. Eur. J. Biochem. / FEBS. 2002; 269:4811–4818. [DOI] [PubMed] [Google Scholar]
- 32. Negrutskii B.S., El'skaya A.V.. Eukaryotic translation elongation factor 1 alpha: structure, expression, functions, and possible role in aminoacyl-tRNA channeling. Progr. Nucleic Acid Res. Mol. Biol. 1998; 60:47–78. [DOI] [PubMed] [Google Scholar]
- 33. Chernyakov I., Whipple J.M., Kotelawala L., Grayhack E.J., Phizicky E.M.. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5΄-3΄ exonucleases Rat1 and Xrn1. Genes Dev. 2008; 22:1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu J., Hopper A.K.. Healing for destruction: tRNA intron degradation in yeast is a two-step cytoplasmic process catalyzed by tRNA ligase Rlg1 and 5΄-to-3΄ exonuclease Xrn1. Genes Dev. 2014; 28:1556–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pavon-Eternod M., Gomes S., Rosner M.R., Pan T.. Overexpression of initiator methionine tRNA leads to global reprogramming of tRNA expression and increased proliferation in human epithelial cells. RNA (New York, N.Y.). 2013; 19:461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goodarzi H., Nguyen H.C., Zhang S., Dill B.D., Molina H., Tavazoie S.F.. Modulated expression of specific tRNAs Drives gene expression and cancer progression. Cell. 2016; 165:1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gebetsberger J., Polacek N.. Slicing tRNAs to boost functional ncRNA diversity. RNA Biol. 2013; 10:1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sobala A., Hutvagner G.. Transfer RNA-derived fragments: origins, processing, and functions. Wiley Interdiscipl. Rev. RNA. 2011; 2:853–862. [DOI] [PubMed] [Google Scholar]
- 39. Honda S., Loher P., Shigematsu M., Palazzo J.P., Suzuki R., Imoto I., Rigoutsos I., Kirino Y.. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc. Natl. Acad. Sci. U.S.A. 2015; 112:E3816–E3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kumar P., Mudunuri S.B., Anaya J., Dutta A.. tRFdb: a database for transfer RNA fragments. Nucleic Acids Res. 2015; 43:D141–D145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Z., Ender C., Meister G., Moore P.S., Chang Y., John B.. Extensive terminal and asymmetric processing of small RNAs from rRNAs, snoRNAs, snRNAs, and tRNAs. Nucleic Acids Res. 2012; 40:6787–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.