Abstract
Infrared thermography (IRT) is a real-time non-contact diagnostic tool with a broad potential for neurosurgical applications. Here we describe the intraoperative use of this technique in a single patient with newly diagnosed glioblastoma multiforme (GBM). An 86-year-old female was admitted in the clinic with a 2-month history of slowly progressing left-sided paresis. Neuroimaging studies demonstrated an irregular space-occupying process consistent with a malignant glioma in the right fronto-temporo-insular region. An elective surgical intervention was performed by using 5-aminolevulinic acid fluorescence (BLUE 400, OPMI) and intraoperative IRT brain mapping (LWIR, 1.25 mRad IFOV, 0.05°C NETD). After dura opening, the cerebral surface appeared inconspicuous. However, IRT revealed a significantly colder area (Δt° 1.01°C), well corresponding to the cortical epicenter of the lesion. The underlying tumor was partially excised and the histological result was GBM. Intraoperative IRT seems to be a useful technique for subcortical convexity brain tumor localization. Further studies with a large number of patients are needed to prove the reliability of this method in GBM surgery.
Keywords: Glioblastoma multiforme, Neuroimaging, Infrared thermography, 5-aminolevulinic acid
Introduction
Infrared thermography (IRT) has a potential for wide application in the experimental and clinical medicine [1, 2]. The reliability of the method in surgery of malignant brain tumors, however, is not well established. Here we report on the intraoperative use of this technique in a single patient with newly diagnosed glioblastoma multiforme (GBM).
Case Description
An 86-year-old female was admitted to the clinic with a 2-month history of slowly progressing left-sided paresis. Neuroimaging studies demonstrated an irregular space-occupying process consistent with a malignant glioma in the right fronto-temporo-insular region (Fig. 1). An elective surgical intervention was performed by using 5-aminolevulinic acid fluorescence (Gliolan®; BLUE 400, OPMI) and intraoperative IRT brain mapping (LWIR, 1.25 mRad IFOV, 0.05°C NETD). After the dura opening, the cerebral surface appeared seemingly normal (Fig. 2a). However, the ultraviolet light exposure revealed a moderate fluorescence in the region of the Silvian fissure (Fig. 2b), and the IRT observation demonstrated a significantly colder area (Δt° 1.01°C) in the limited field of the middle frontal gyrus, well corresponding to the epicenter of the lesion (Fig. 2c). The underlying tumor was approached directly only by using the fused brain topography and thermal imaging data. A partial excision of the lesion was performed, and the histological result was GBM (Fig. 3). No additional neurological deficit appeared on the next day after the intervention, and the computed tomography scan of the patient was rated as postoperatively normal.
Fig. 1.
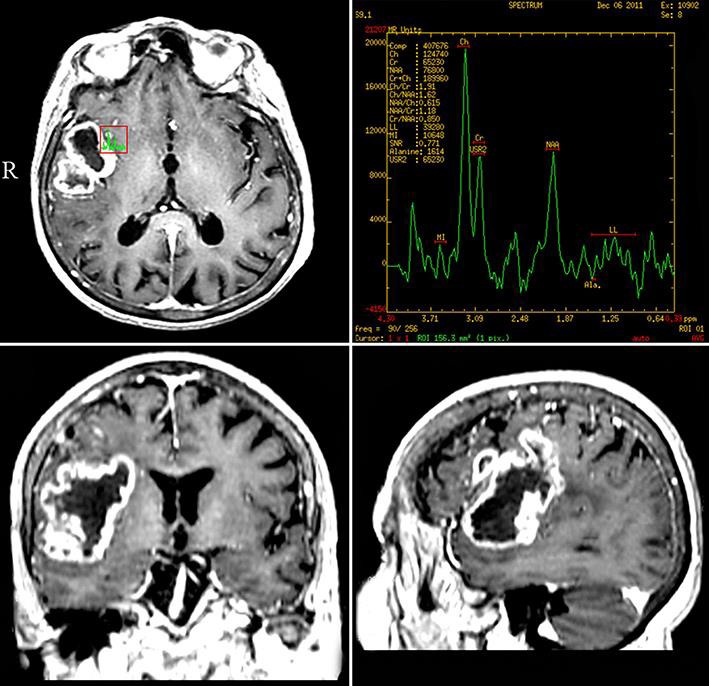
Preoperative neuroimaging of the patient showing an infiltrating right fronto-temporo-insular tumor with irregular contrast enhancement.
Fig. 2.
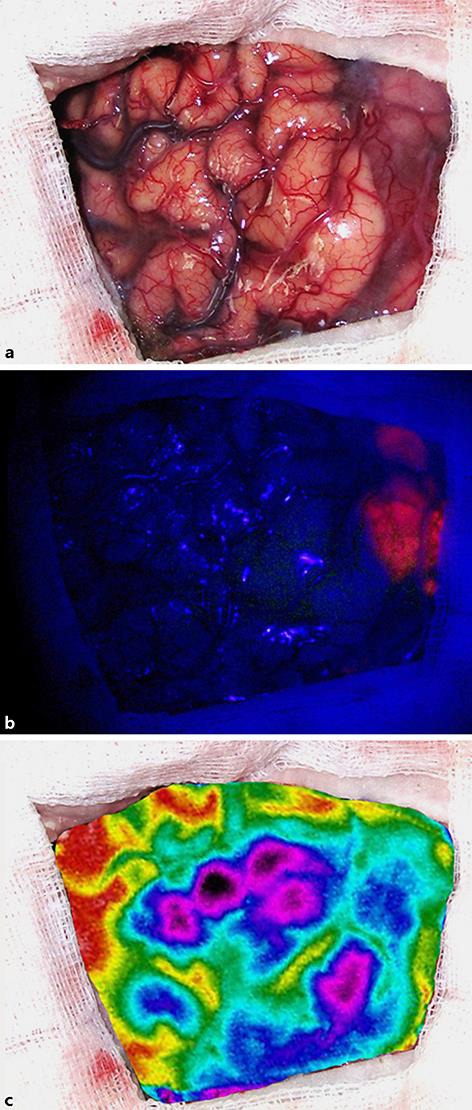
Intraoperative view of the patient's cerebral surface with the naked eye (a), under blue light exposure (b), and by using IRT brain mapping (c).
Fig. 3.
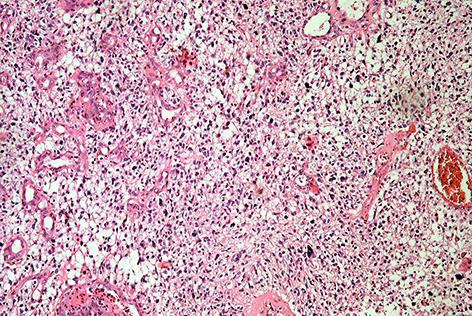
Conventional histological analysis of the lesion revealing prominent cellular polymorphism, microvascular proliferation, and pseudopalisading necroses. HE. ×100.
Discussion
The surgical approach in cases with newly diagnosed GBM depends on the anatomical localization of the tumor and the neurological status of the patient. Choosing the most direct and safest way to access the lesion is one of the major tasks of the operator during the intervention [3]. In order to facilitate the work of the neurosurgeon, a number of different imaging and electrophysiological procedures have been developed, each of them, however, presenting one or more significant and well-known disadvantages [4, 5]. In this regard, the ideal technique should be safe for the patient, fast and easy to perform, with a high level of sensitivity and specificity.
IRT is an imaging method with a broad potential for neurosurgical applications. It is a non-contact diagnostic tool able to provide real-time functional and structural information of the patient's brain [6]. The use of this technique in surgery of malignant brain tumors has been previously reported. Nevertheless, the number of cases is generally small [7] and some of the expectations may not be warranted based on the experience available so far [8].
Intrinsic malignant gliomas have a lower temperature in comparison with the surrounding normal brain matter, and this finding has been confirmed in both animal and human models [9, 10]. The two major factors that may contribute to hypothermia in these cases are (1) decreased metabolism of the surrounding and overlying tissues, caused by the perifocal edema and (2) low density in tumor microvessels, due to presence of concomitant necrosis [11]. In our case, IRT mapping was able to detect a significantly colder area in the limited field of the brain surface, well corresponding to the cortical epicenter of the underlying lesion.
Intraoperative 5-aminolevulinic acid fluorescence is a well-recognized method for achieving gross-total resection in cases with newly diagnosed GBM. In some instances, even when the cerebral surface appears seemingly normal, switching to blue excitation light may allow discrimination of subcortical tumor extensions, providing a valuable guide for initial approach [12]. By using this method in our patient, moderate fluorescence was observed only in the region of the Sylvian fissure, which is, however, an inadvisable point for entering into the lesion in this particular case.
Conclusion
Intraoperative IRT seems to be a useful technique for subcortical convexity brain tumor localization. Further studies with a large number of patients are needed to prove the reliability of this method in GBM surgery.
Statement of Ethics
The authors have no ethical conflicts to disclose. Patient consent was graciously obtained for the publication needs.
Disclosure Statement
The authors declare that there are no conflicts of interest.
Acknowledgement
The authors wish to thank the Stay Foundation, Sofia, Bulgaria (www.stay.eu.com), for supporting this research project.
References
- 1.Saxena K, Willital H. Infrared thermography: experience from a decade of pediatric imaging. Eur J Pediatr. 2008;67:757–764. doi: 10.1007/s00431-007-0583-z. [DOI] [PubMed] [Google Scholar]
- 2.Shevelev I, Tsicalov E, Gorbach A, Budko K, Sharaev G. Thermoimaging of the brain. J Neurosci Methods. 1993;46:49–57. doi: 10.1016/0165-0270(93)90140-m. [DOI] [PubMed] [Google Scholar]
- 3.Hentschel S, Lang F. Current surgical management of glioblastoma. Cancer J. 2003;9:113–125. doi: 10.1097/00130404-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Feigl GC, Ritz R, Moraes M, Klein J, Ramina K, Gharabaghi A, Krischek B, Danz S, Bornemann A, Liebsch M, Tatagiba MS. Resection of malignant brain tumors in eloquent cortical areas: a new multimodal approach combining 5-aminolevulinic acid and intraoperative monitoring. J Neurosurg. 2010;113:352–357. doi: 10.3171/2009.10.JNS09447. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RD, Stacey RJ. The impact of new imaging technologies in neurosurgery. Surgeon. 2008;6:344–349. doi: 10.1016/s1479-666x(08)80006-6. [DOI] [PubMed] [Google Scholar]
- 6.Gorbach AM, Heiss J, Kufta C, Sato S, Fedio P, Kammerer WA, Solomon J, Oldfield EH. Intraoperative infrared functional imaging of human brain. Ann Neurol. 2003;54:297–309. doi: 10.1002/ana.10646. [DOI] [PubMed] [Google Scholar]
- 7.Koga H, Mori K, Ono H, Kuwahara M, Matsuse E. Intraoperative regional thermography during surgery of brain tumors. Neurol Med Chir. 1987;27:1033–1038. doi: 10.2176/nmc.27.1033. [DOI] [PubMed] [Google Scholar]
- 8.Kateb B, Yamamoto V, Yu C, Grundfest W, Gruen J. Infrared thermal imaging: a review of the literature and case report. Neuroimage. 2009;47((suppl 2)):T154–T162. doi: 10.1016/j.neuroimage.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 9.Ecker R, Goerrss S, Meyer F, Cohen-Gadol A, Britton J, Levine J. Vision of the future: initial experience with real-time intraoperative high resolution dynamic infrared imaging (DIRI) J Neurosurg. 2002;97:1460–1471. doi: 10.3171/jns.2002.97.6.1460. [DOI] [PubMed] [Google Scholar]
- 10.Shevelev I. Temperature topography of the brain cortex: thermoencephaloscopy. Brain Topogr. 1992;5:77–85. doi: 10.1007/BF01129034. [DOI] [PubMed] [Google Scholar]
- 11.Gorbach A, Heiss J, Kopylev L, Oldfield E. Intraoperative infrared imaging of brain tumors. J Neurosurg. 2004;101:960–969. doi: 10.3171/jns.2004.101.6.0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonn J, Stummer W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: practical use, risks, and pitfalls. Clin Neurosurg. 2008;55:20–26. [PubMed] [Google Scholar]


